Copper(II)-catalyzed synthesis of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles via four-component cascade annulation†
Hong
Xu‡
ab,
Jie
Liao‡
a,
Fei
Ren
a,
Chuan-Rong
Zhou
a,
Xiao-Zhuo
Liu
a,
Yao
Xiao
a,
Dong-Wei
Hang
a,
Fuyu
Li
 ab,
Bei
Wang
*a and
Ji-Yu
Wang
ab,
Bei
Wang
*a and
Ji-Yu
Wang
 *ab
*ab
aDepartment of Chemistry, Xihua University, Chengdu 610041, P. R. China. E-mail: wangbei1004@163.com; Jiyuwang@cioc.ac.cn
bAsymmetric Synthesis and Chiraltechnology Key Laboratory of Sichuan Province, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, P. R. China
First published on 29th October 2024
Abstract
A copper-catalyzed four-component cascade annulation is realized to access novel sulfonyl-functionalized quinone-fused cyclopenta[b]indoles with high step economy and broad substrate scope, and 34 examples are constructed in one pot with up to 88% yield. This process includes sulfonyl radical triggered tandem cyclization by selective 1,1,2-trifunctionalization of terminal acetylene.
Introduction
Cyclopenta[b]indoles, a class of the most important carbazole analogues, are common motifs in natural products and biologically active molecules, exhibiting various biological activities (Fig. 1).1–3 For example, paspaline has good antibacterial and insecticidal activities, MK-0524 was a D2 (PGD2) receptor (DP) antagonist, yeuhchukene has antifertility activity. Fused quinones are also an important class of organic compounds that are widely used in biomedicine, probes, materials and other fields because of their unique biological activity and electron transfer properties.4,5 However, there are few reports on quinone-fused cyclopenta[b]indole derivatives. Developing practical and scalable synthesis strategies to construct novel quinone-fused cyclopenta[b]indole derivatives is desirable.Multi-component reactions can rapidly and selectively realize the synthesis of a single complex structure product from three or more compounds, which plays an important role in pesticide chemistry, natural medicine chemistry, etc.6,7 Sulfones are an important class of organic compounds due to their unique biological and pharmacological activity.8 In recent years, numerous transition-metal-catalyzed/visible-light-mediated sulfonyl radical triggered synthetic methods have been developed for accessing highly functionalized sulfones from sodium sulfinates, sulfonyl chlorides and sulfonic acids.9–20
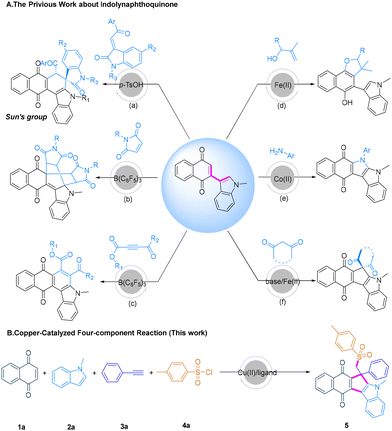
Indolyl naphthoquinone is an important synthon for accessing nonclassical conjugated dienes, which can undergo synergistic cyclization, simple mono-functionalization and tandem difunctionalization. In recent years, our group and the Sun group have achieved the Diels–Alder reaction of indolyl naphthoquinone with activated alkenes and alkynes to generate multiple quinone-fused carbazole derivatives (Scheme 1A, (a)–(c)).21–23 Some of the compounds present high fluorescence quantum yields and could be used as colorimetric sensors to identify carbonates. Through the mono-functionalization of the conjugated diene, Fe(III) catalyzed radical reduction to give dihydronaphthofuran (Scheme 1A, (d)) and merging oxidative coupling and cascade palladium-catalyzed intramolecular oxidative cyclization to give spiro polycyclic N-heterocycles or indolecarbazoles were respectively developed in 2021 and 2024.24,25 Moreover, successive electrophilic addition/C–N bond coupling or successive electrophilic addition/radical addition were realized to provide various quinone-fused pyrrole[b]indoles or quinone-fused cyclopenta[b]indoles (Scheme 1A, (e) and (f)), and they exhibited good photophysical properties and were promising as fluorescent probes to selectively identify methanesulfonic acid or Type I photosensitizers.26,27 However, no research on sulfonyl substituted fused-quinones has been reported. Herein, we realized the construction of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles through a four-component reaction from quinone 1a, indole 2a, alkyne 3a and 4-methylbenzenesulfonyl chloride 4a (Scheme 1B).
Results and discussion
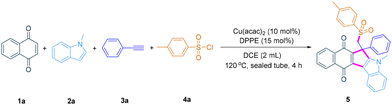
We commenced our study in the presence of Cu(acac)2 (10 mol%) and DPPE (15 mol%), 4-methylbenzenesulfonyl chloride 4a was used as the sulfonyl radical source to to stirred with quinone 1a, indole 2a and alkyne 3a in 2 mL of DCE in a sealed tube at 120 °C for 4 hours, successfully affording sulfonyl-functionalized quinone-fused cyclopenta[b]indole 5 in 49% yield (Table 1, entry 1 and Table S1†). Then the reaction parameters are extensively screened to identify the optimized conditions. The reaction cannot proceed at all without Cu(acac)2 (Table 1, entry 2), whereas without the addition of DPPE, the yield of the product decreases significantly (Table 1, entry 3). Multiple catalysts were screened (Table S2†), such as copper salts (Table 1, entries 4 and 5), cobalt salts, nickel salts and iron salts, among which Cu(acac)2 shows better efficiency. A series of ligands were investigated (Table S3†); BINAP provides a higher yield than DPPE (Table 1, entry 6), although other N-, O-, and P-ligands can also give the target product. Among the screening of solvents (Table S4†), only DCE and CH3CN provided 5 in an acceptable yield, while DCE was better than CH3CN. The ratio of substrates was also screened (Table S5†). Optimal conditions are as shown in entry 6 in Table 1 and the reaction delivers the product in 56% yield. Moreover, our group is interested in the development of copper-catalyzed asymmetric syntheses of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles, and preliminary exploration of chiral ligands was attempted (Table S6†), but only very low enantioselectivity was realized. Therefore, this work mainly focuses on copper-catalyzed four-component cascade cyclization to rapidly synthesise racemic sulfonyl-functionalized quinone-fused cyclopenta[b]indoles and further experimental exploration of enantioselectivity will be carried out in the laboratory.| Entry | Variation | Yield (%) |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), 3a (0.6 mmol), 4a (0.6 mmol), Cu(acac)2 (10 mol%), DPPE (15 mol%), DCE (2 mL), T = 120 °C, t = 4 h. b Yield refers to the isolated product. c N.D. means not detected. | ||
| 1 | None | 49 |
| 2 | Without Cu(acac)2 | N.D. |
| 3 | Without DPPE | 35 |
| 4 | CuCl2·2H2O instead of Cu(acac)2 | 38 |
| 5 | CuBr2 instead of Cu(acac)2 | 29 |
| 6 | BINAP instead of DPPE | 56 |
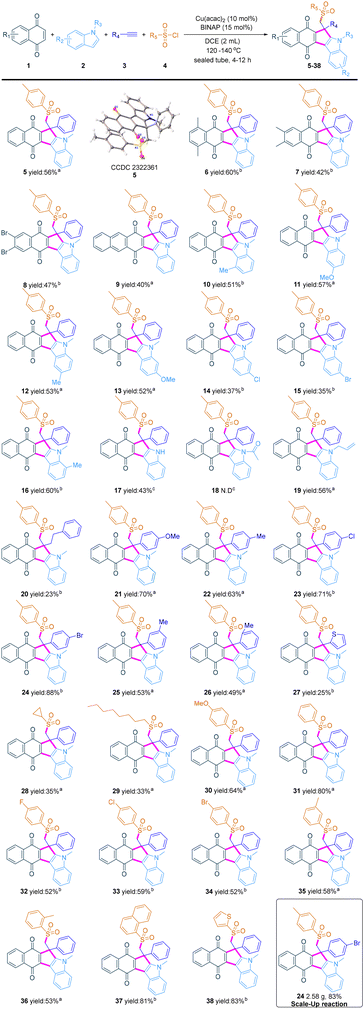
After the reaction conditions were optimized, we turned our attention to evaluating the substrate scope of the copper-catalyzed four-component cascade reaction. The transformation features wide functional group tolerance and the results are shown in Scheme 2. Several naphthoquinones such as 5,8-dimethylnaphthalene-1,4-dione, 6,7-dimethylnaphthalene-1,4-dione, 6,7-dibromonaphthalene-1,4-dione and 1,4-anthraquinone were tested, furnishing a variety of fused-quinones in moderate to good yields (5–9). Then, a variety of indoles with a range of functionalities were shown to be compatible. Many valuable functional groups such as methyl, methoxy, chloro, bromo, and allyl at 1-, 4-, 5-, 6-, and 7-positions of indole were well tolerated and afforded the corresponding compounds 10–16 and 19. In particular, the 1H-indole gives the fused-quinone (17) in a significantly reduced yield and the target product (18) of N-acetylindole is not detected by TLC, which may be caused by a decrease in the electron cloud density at the 2-position of indole. Next, various functionalized terminal alkynes were examined. 4-Phenyl-1-butyne can be suitable for this reaction under appropriate conditions, but the product (20) can be obtained only in 23% yield. Phenylacetylene with different substituents, such as methoxy, methyl, chlorine, and bromine, reacts smoothly to afford the target products in 49–88% yields (21–26). Among them, ortho- and meta-substituted ethynyltoluene afford the products in lower yields probably due to steric effects. In addition, this reaction is also suitable for heteroaromatic terminal alkynes, and the product 2-ethynylthiophene 27 is isolated in 25% yield. Finally, a variety of substituted sulfonyl chlorides were tested to probe the efficacy of the four-component reaction. To our delight, alkyl sulfonyl chlorides were readily converted, affording products (28 and 29) in moderate yields. Electron-donating substituents, electron-neutral substituents, and electron-withdrawing functional groups (fluoro, chloro and bromo) were readily converted to the desired compounds (30–36) in 52–80% yields. Moreover, naphthalene sulfonyl chloride and thiophene sulfonyl chloride could also be transformed into products 37 and 38 in good yields. In addition, when the reaction was scaled up to 5 mmol, the yield of compound 24 did not decrease significantly, indicating that the reaction has the prospect of industrialization.
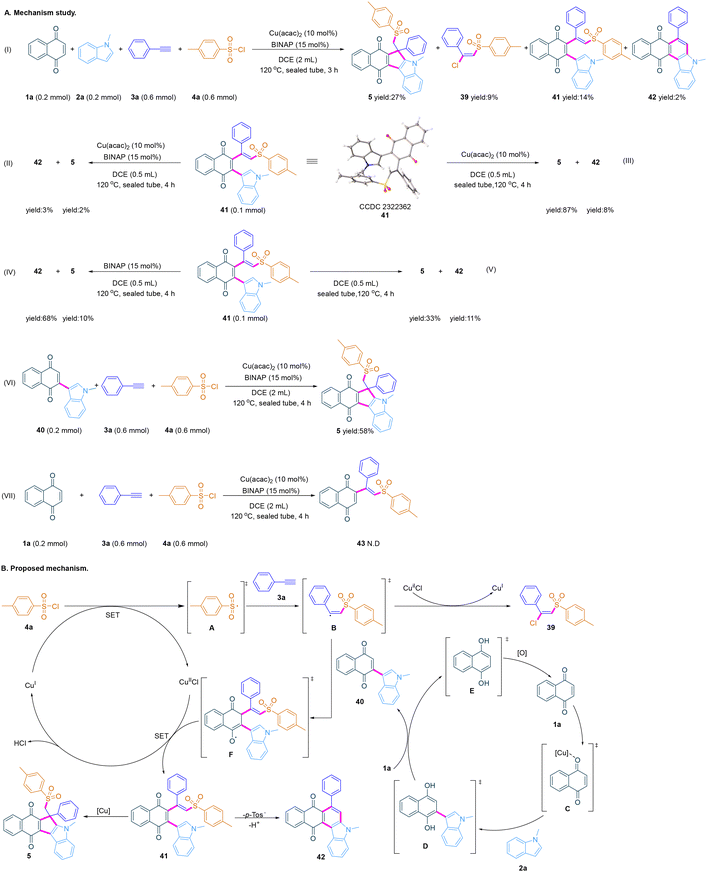
To investigate the mechanism of the four-component annulation, several control experiments were performed (Scheme 3A). Initially, when quinone 1a, indole 2a, alkyne 3a and 4-methylbenzenesulfonyl chloride 4a were stirred under the standard conditions for 3 hours, not only by-products (E)-1-((2-chloro-2-phenylvinyl)sulfonyl)-4-methylbenzene 39 and 5-methyl-7-phenyl-5H-naphtho[2,3-c]carbazole-8,13-dione 42 were isolated, but also (E)-2-(1-methyl-1H-indol-3-yl)-3-(1-phenyl-2-tosylvinyl)naphthalene-1,4-dione 41 was isolated, further confirmed by X-ray crystal structure analysis (eqn (I)), which converts smoothly to the annulation product 5 in good yield in the presence of Cu(acac)2 (eqn (1) of Scheme 3A). The results of the experiment given in eqn (II) to eqn (V) show that the intramolecular cyclization of the olefin intermediate 41 to the sulfonyl-functionalized quinone-fused cyclopenta[b]indole 5 is a Lewis acid-catalyzed thermodynamic cyclization. The presence of metals and ligands will inhibit this process (eqn (II) and eqn (III)). Only in the presence of a ligand, intermediate 41 is more easily converted into the quinone-fused carbazole 42 (eqn (IV)). The results of the experiment given in eqn (VI) and eqn (VII) suggest that the four-component annulation includes a radical addition process of intermediates 40, 3a and 4a, and not 1a, 3a and 4a.
On the basis of our mechanistic studies and reported literature,9–20,28–36 a plausible mechanism is proposed with substrates 1a, 2a, 3a and 4a (Scheme 3B). Initially, indolylquinone 40 is generated from the Michael addition of quinone 1a and indole 2a with the conversion of [CuII] species to [CuI] species. Then through a SET pathway, sulfonyl chloride 4a is converted to sulfonyl radical A with the in situ-formed [CuIICl] species, which then undergoes selective radical addition of the alkyne to afford the alkene radical intermediate B. Next, a small amount of intermediate B can abstract Cl˙ from [CuIICl] species to give the by-product (E)-1-((2-chloro-2-phenylvinyl) sulfonyl)-4-methylbenzene 39, and most of intermediate B follow a radical addition reaction with indolylquinone 40 to afford intermediate F, which is then converted to intermediate 41 through SET by the in situ-formed [CuI] species. Finally, driven by [Cu] species, most of intermediate 41 undergoes nucleophilic addition and 1,3-hydrogen migration (HAT) to obtain sulfonyl-functionalized quinone-fused cyclopenta[b]indole 5, while small amounts of quinone-fused carbazole 42 is provided by 6-π electron cyclization and demethylsulfonyl group of intermediate 41.
Conclusion
In summary, we conveniently constructed novel sulfonyl-functionalized quinone-fused cyclopenta[b]indoles by copper-catalyzed four-component cascade cyclization from naphthoquinone, indole, terminal alkyne and sulfonyl chloride. The reaction has good functional group tolerance and can be successfully scaled up to the gram level. Mechanistic studies indicated that this process was sulfonyl radical triggered tandem cyclization through selective 1,1,2-trifunctionalization of terminal acetylene with the construction of four new C–C bonds in one pot. Related photophysical properties and bioactivities of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles will be further explored in the laboratory.Data availability
Methods and experimental procedures, crystal data, compound characterization data, and NMR spectra can be found in the ESI.† All relevant data are within the manuscript and its ESI.† CCDC experimental crystal structure determination: 2322361, 2322362.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Sichuan Science and Technology Program (No. 2023NSFSC1977 ).References
- S. C. Munday-Finch, A. L. Wilkins and C. O. Miles, Isolation of Lolicine A, Lolicine B, Lolitriol, and Lolitrem N from Loliumperenne Infected with Neotyphodium lolii and Evidence for the Natural Occurrence of 31-Epilolitrem N and 31-Epilolitrem F, J. Agric. Food Chem., 1998, 46, 590–598 CrossRef CAS.
- C. F. Sturino, G. O'Neill, N. Lachance, M. Boyd, C. Berthelette, M. Labelle, L. Li, B. Roy, J. Scheigetz, N. Tsou, Y. Aubin, K. P. Bateman, N. Chauret, S. H. Day, J.-F. Lévesque, C. Seto, J. H. Silva, L. A. Trimble, M.-C. Carriere, D. Denis, G. Greig, S. Kargman, S. Lamontagne, M.-C. Mathieu, N. Sawyer, D. Slipetz, W. M. Abraham, T. Jones, M. McAuliffe, H. Piechuta, D. A. Nicoll-Griffith, Z. Wang, R. Zamboni, R. N. Young and K. M. Metters, Discovery of a Potent and Selective Prostaglandin D2 Receptor Antagonist, [(3R,)-4-(4-Chloro- benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic Acid (MK-0524), J. Med. Chem., 2007, 50, 794–806 CrossRef CAS.
- Y. C. Kong, K. H. Ng, K. H. Wat, A. Wong, I. F. Saxena, K. F. Cheng, P. P. H. But and H. T. Chang, Yuehchukene, a novel anti-implantation indole alkaloid from Murraya paniculata, Planta Med., 1985, 51, 304–307 CrossRef CAS PubMed.
- K. Matsumoto, T. Choshi, M. Hourai, Y. Zamami, K. Sasaki, T. Abe, M. Ishikura, N. Hatae, N. Iwamura, S. Tohyama, J. Nobuhirom and S. Hibino, Concise synthesis of carbazole-1,4-quinones and evaluation of their antiproliferative activity against HCT-116 and HL-60 cells, Med. Chem. Lett., 2012, 22, 4762–4764 CrossRef CAS PubMed.
- P. Cai, F. Kong, M. E. Ruppen, G. Glasier and G. T. Carter, Hygrocins A and B, Naphthoquinone Macrolides from Streptomyces hygroscopicus, J. Nat. Prod., 2005, 68, 1736–1742 CrossRef CAS.
- A. Dömling, W. Wang and K. Wang, Chemistry and biology of multicomponent reactions, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed.
- B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Small heterocycles in multicomponent reactions, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS.
- D. C. Meadows and J. Gervay-Hague, Vinyl sulfones: Synthetic preparations and medicinal chemistry applications, Med. Res. Rev., 2006, 26, 793–814 CrossRef CAS PubMed.
- S. Tripathi, M. Kumar, A. Shivhare, R. Kant, M. M. Deshmukh and A. K. Srivastava, Palladium(II)-Catalyzed Decarboxylative Difunctionalization of Alkynoic Acids To Access (E)-β-Sulfonylacrylamides and DFT Study, Org. Lett., 2023, 25, 6638–6642 CrossRef CAS PubMed.
- P. Chen, L. Tian, X. Ji, G.-J. Deng and H. Huang, Copper-Catalyzed 1, 2-Sulfonyletherification of 1, 3-Dienes, Org. Lett., 2024, 26, 2939–2944 CrossRef CAS PubMed.
- D. Bhatt, H. Y. Kim and K. Oh, Chemodivergent Sulfonylation of Enynones via Ionic and Radical Addition Modes of Sulfinic Acids, Org. Lett., 2023, 25, 5697–5701 CrossRef CAS.
- S. Dong, Z. Tian, J. Wang, L. He and J. Li, Organozinc pivalates for modular Cobalt-Catalyzed carbosulfonylation of 1,3-Enynes, J. Catal., 2023, 425, 350–358 CrossRef CAS.
- L. Wang, R. Ma, J. Sun, G. Zheng and Q. Zhang, NHC and visible light-mediated photoredox co-catalyzed 1, 4-sulfonylacylation of 1, 3-enynes for tetrasubstituted allenyl ketones, Chem. Sci., 2022, 13, 3169–3175 RSC.
- Y. Zhang, C. Zhao, C. Ma, Z. Cai, S. Trienes and L. Ackermann, Photocatalytic C−C Cleavage of Methylenecyclobutanes for γ,δ–Unsaturated Aldehydes by Strain Release, Angew. Chem., Int. Ed., 2023, 62, e202300166 CrossRef CAS.
- C.-S. Dong, W.-Y. Tong, P. Ye, N. Cheng, S. Qu and B. Zhang, Phenanthroline-initiated anti-selective hydrosulfonylation of unactivated alkynes with sulfonyl chlorides, ACS Catal., 2023, 13, 6983–6993 CrossRef CAS.
- A. Hossain, S. Engl, E. Lutsker and O. Reiser, Visible-Light-Mediated Regioselective Chlorosulfonylation of Alkenes and Alkynes: Introducing the Cu(II) Complex [Cu(dap)Cl2] to Photochemical ATRA Reactions, ACS Catal., 2019, 9, 1103–1109 CrossRef CAS.
- C.-Y. Zhang, J. Zhu, S.-H. Cui, X.-Y. Xie, X.-D. Wang and L. Wu, Visible-light-induced 1, 4-hydroxysulfonylation of vinyl enynes with sulfonyl chlorides: The bridge of chloride linking water and enynes, Org. Lett., 2021, 23, 3530–3535 CrossRef CAS.
- D. Bhatt, K. Miyake, S. Nakamura, H. Y. Kim and K. Oh, Photoredox-Catalyzed 1,4-Peroxidation–Sulfonylation of Enynones: A Three-Component Radical Coupling Approach for the Synthesis of Highly Functionalized Allenes, Org. Lett., 2024, 26, 2955–2959 CrossRef CAS.
- Y. Zhang and E. Vessally, Direct halosulfonylation of alkynes: an overview, RSC Adv., 2021, 11, 33447–33460 RSC.
- J. Ma, J. Li, Q. Meng and X. Zeng, Advances on the Radical Sulfonation of Alkynes, Chin. J. Org. Chem., 2023, 43, 2040–2052 CrossRef CAS.
- C. Yan, J. Sun, Y. Han and C.-G. Yan, Water Modulated Diastereoselective Synthesis of cis/trans-Spiro[indoline-3,6′-naphtho[2,3-c]carbazoles], J. Org. Chem., 2021, 86, 9263–9279 CrossRef CAS PubMed.
- B. Wang, H. Xu, H. Zhang, G.-M. Zhang, F.-Y. Li, S. He, Z.-C. Shi and J.-Y. Wang, B (C6F5)3-catalyzed three-component tandem reaction to construct novel polycyclic quinone derivatives: synthesis of a carbonate salt chromogenic chemosensor, Org. Chem. Front., 2021, 8, 6670–6677 RSC.
- H. Xu, B. Wang, F.-Y. Li and J.-Y. Wang, B(C6F5)3-Catalyzed [4 + 2] Cyclization Strategy: Synthesis and Photophysical Properties of 5H-Naphtho[2,3-c]carbazole-8,13-dione Derivatives, J. Org. Chem., 2023, 88, 2703–2713 CrossRef CAS.
- H. Zhang, B. Wang, H. Xu, F.-Y. Li and J.-Y. Wang, Synthesis of naphthodihydrofurans via an iron(III)-catalyzed reduction radical cascade reaction, Org. Chem. Front., 2021, 8, 6019–6025 RSC.
- Y. Dong, M.-F. Lan, Y.-Q. Lin, L. Chen, C.-M. Wu, Z.-F. Wang, Z.-C. Shi, G.-W. Deng and B. He, J. Org. Chem., 2024, 89, 6474–6488 CrossRef CAS PubMed.
- Y. Dong, J. Yang, H. Zhang, X.-Y. Zhan, S. He, Z.-C. Shi, X.-M. Zhang and J.-Y. Wang, Cobalt-Catalyzed Cycloamination: Synthesis and Photophysical Properties of Polycyclic N-Heterocycles, Org. Lett., 2020, 22, 5151–5156 CrossRef CAS PubMed.
- H. Xu, X.-C. Liu, B. Wang, F.-Y. Li, D.-W. Huang, Y. Xiao, N. Ma, Y.-H. Zhang and J.-Y. Wang, Iron(II)-catalyzed annulation to construct novel quinone-fused cyclopenta [2,1-b] indoles: a promising type I photosensitizer, Org. Chem. Front., 2024, 11, 4119–4124 RSC.
- D. Wu, M. Jiang, J.-J. Wang and W. Yu, Copper-Catalyzed Sulfonylation/Cyclization of Pent-4-ynamides toward Sulfonyl-Functionalized Pyrrol-2-ones, Org. Lett., 2023, 25, 2073–2077 CrossRef CAS.
- M. Zhong, Y. Gagn and T. O. Hope, Copper–Photocatalyzed Hydroboration of Alkynes and Alkenes, Angew. Chem., 2021, 133, 14619–14624 CrossRef.
- T. He, B. Li, L.-C. Liu, J. Wang, W.-P. Ma, G.-Y. Li, Q.-W. Zhang and W. He, Copper–Catalyzed Trifunctionalization of Alkynes: Rapid Formation of Oxindoles Bearing Geminal Diboronates, Chem. – Eur. J., 2019, 25, 966–970 CrossRef CAS PubMed.
- J. Yan, H. W. Cheo, W. K. Teo, X. Shi, H. Wu, S. B. Idres, L.-W. Deng and J. Wu, A radical smiles rearrangement promoted by neutral eosin Y as a direct hydrogen atom transfer photocatalyst, J. Am. Chem. Soc., 2020, 142, 11357–11362 CrossRef CAS.
- Y. Dong, H. Zhang, J. Yang, S. He, Z.-C. Shi, X.-M. Zhang and J.-Y. Wang, B(C6F5)3-Catalyzed C–C Coupling of 1,4-Naphthoquinones with the C-3 Position of Indole Derivatives in Water, ACS Omega, 2019, 4, 21567–21577 CrossRef CAS.
- K. T. P. Adarsh, S. Pandaram and A. Ilangovan, Iron-mediated site-selective oxidative C–H/C–H cross-coupling of aryl radicals with quinones: synthesis of β-secretase-1 inhibitor B and related arylated quinones, Org. Chem. Front., 2019, 6, 3244–3251 RSC.
- Q. Wang, B. Wang, H. Deng, Y. Shangguan, Y. Lin, Y. Zhang, Z. Zhang, Y. Xiao, H. Guo and C. Zhang, Silver-Catalyzed Three-Component Difunctionalization of Alkenes via Radical Pathways: Access to CF3-Functionalized Alkyl-Substituted 1,4-Naphthoquinone Derivatives, J. Org. Chem., 2019, 84, 1006–1014 CrossRef CAS PubMed.
- D. R. Sutherland, M. Veguillas, C. L. Oates and A. L. Lee, Metal-, photocatalyst-, and light-free, late-stage c–h alkylation of heteroarenes and 1, 4-quinones using carboxylic acids, Org. Lett., 2018, 20, 6863–6867 CrossRef CAS.
- S. Suresh, H.-S. Chien, C.-H. Chen, H.-Y. Tsai, D.-R. Chung, V. Kavala and C.-F. Yao, Regioselective Synthesis of Polysubstituted Carbazoles from Indoles and Simple 1, 4−Dicarbonyl Compounds, J. Org. Chem., 2023, 88, 17505–17510 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2322361 and 2322362. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01560f |
| ‡ Hong Xu and Jie Liao contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), 3a (0.6 mmol), 4a (0.6 mmol), Cu(acac)2 (10 mol%), BINAP (15 mol%), DCE (2 mL),
Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), 3a (0.6 mmol), 4a (0.6 mmol), Cu(acac)2 (10 mol%), BINAP (15 mol%), DCE (2 mL),