Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A persistent concealed non-Kekulé nanographene: synthesis and in situ characterization†
Muhammad
Imran‡
 ab,
Lin
Yang‡
ab,
Jin-Jiang
Zhang
b,
Zhen-Lin
Qiu
a,
Yubin
Fu
ab,
Lin
Yang‡
ab,
Jin-Jiang
Zhang
b,
Zhen-Lin
Qiu
a,
Yubin
Fu
 ab,
Noel
Israel
ab,
Noel
Israel
 c,
Evgenia
Dmitrieva
c,
Evgenia
Dmitrieva
 c,
Andrea
Lucotti
c,
Andrea
Lucotti
 d,
Gianluca
Serra
d,
Matteo
Tommasini
d,
Gianluca
Serra
d,
Matteo
Tommasini
 d,
Ji
Ma
d,
Ji
Ma
 *e and
Xinliang
Feng
*e and
Xinliang
Feng
 *ab
*ab
aCenter for Advancing Electronics Dresden (cfaed) & Faculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: xinliang.feng@tu-dresden.de
bMax Planck Institute of Microstructure Physics Weinberg 2, 06120 Halle, Germany
cLeibniz Institute for Solid State and Materials Research, 01069 Dresden, Germany
dDipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci 32, 20133 Milano, Italy
eCollege of Materials Science and Opto-Electronic Technology & Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Science, 100049 Beijing, P. R. China. E-mail: maji@ucas.ac.cn
First published on 20th December 2024
Abstract
Concealed non-Kekulé (CNK) nanographenes have recently gained attention as promising non-Kekulé model systems due to their distinctive antiferromagnetic electronic spins, which offer potential applications in spintronics and quantum information science. However, synthesizing CNK nanographenes in solution remains a significant challenge because of their strong biradical character and high reactivity. In this study, we report the successful synthesis of a novel CNK nanographene with two phenalene units fused in a cis configuration to perylene (c-CNK), which exhibits persistent stability under ambient conditions, with a half-life (t1/2) of 59 minutes. The formation of c-CNK is confirmed using in situ UV-Vis-NIR spectroscopy, Raman spectroscopy, and high-resolution mass spectrometry. The open-shell character of c-CNK is supported by electron paramagnetic resonance (EPR) spectroscopy by observing an isotropic signal with a g-value of 2.0026. Quantum chemical simulations reveal a high biradical character (y0 = 0.97) and a singlet open-shell ground state with a small singlet–triplet energy gap (ΔES–T) of 0.4 kcal mol−1. This work presents a solution synthesis of a next-generation concealed non-Kekulé nanographene with intrinsic antiferromagnetic electronic spins, highlighting its potential as a promising material for future quantum technologies.
Introduction
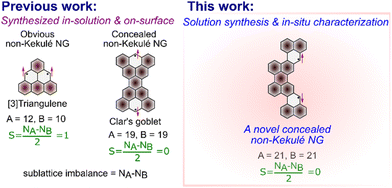
π-Magnetism in fused polycyclic hydrocarbons, commonly known as nanographenes (NGs), has recently attracted significant attention in various scientific fields including chemistry, condensed matter physics, and materials science.1–11 Among them, non-Kekulé NGs are particularly interesting owing to their unique arrangement of π-electrons such that a Kekulé structure is impossible without leaving unpaired electron(s).12 Phenalene, composed of three fused benzene rings, is the smallest non-Kekulé NG and has been extensively studied since its first isolation in crystalline form back in 1999 by Kubo.13 However, large members of the non-Kekulé family have remained elusive due to their high reactivity even at low temperatures resulting in air oxidation or sigma dimerization.14 In a recent work,3 triangulene derivatives have been achieved both in situ and in the crystalline form separately through solution synthesis by sufficient steric protection enabling kinetic stability.15,16 Overall, less effort has been directed towards the solution synthesis of non-Kekulé NGs, owing to the challenges in isolation and purification along with high risks of air oxidation or decomposition of these hard-earned final π-magnetic molecules.Non-Kekulé NGs can be categorized into (i) obvious non-Kekulé NGs and (ii) concealed non-Kekulé NGs based on the difference in their sublattice and net spin in their lowest energy ground state. In contrast to the obvious non-Kekulé NGs, concealed non-Kekulé NGs (CNKs) have two unpaired electronic spins aligned anti-parallel (anti-ferromagnetic) to each other in the ground state (Fig. 1). The topological frustration of π-bonds in a CNK renders it impossible to assign a classical Kekulé structure without leaving unpaired electrons, driving the system into a magnetically non-trivial open-shell ground state.17–19 CNK-NGs mostly remained an interesting subject of mathematical chemistry owing to their structural peculiarity.20 For example, the smallest CNK can be possible in a special arrangement of eleven fused benzene rings, resulting in only 8 possible arrangements.
Early synthetic efforts towards CNK-NGs by Eric Clar date back to 1972 which resulted in the unsuccessful realization of targeted molecules.21 The representative bow-tie-shaped CNK-NG known as Clar's Goblet was successfully achieved by our group in 2020 in collaboration with Fasel et al., through a combined in-solution and on-surface synthesis approach.22 The discovery of antiferromagnetically coupled spins in a CNK (Clar's Goblet), with an effective exchange parameter (Jeff) of 23 meV, exceeding the dissipation limit, has sparked interest in their potential for fault-tolerant spin-logic operations near room temperature for quantum computing technologies. Surface-assisted synthesis has undoubtedly become a promising alternative for producing challenging molecular systems like non-Kekulé NGs.4,23–28 However, this approach encounters several fundamental limitations, including low yield, poor selectivity, limited scalability, and the necessity for a planar structure. As a result, there is an increasing need to develop robust solution synthesis methods and design structures that offer both scalability and enhanced stability.
Herein, we report the solution synthesis of a novel nonplanar CNK nanographene named c-CNK with very high biradical character (y0 = 0.97) by building two phenalene units in a cis configuration at the 3,10-positions of the perylene core through multi-step synthesis (Fig. 1). The key precursor 2H-c-CNK is achieved by the installation of naphthalene with a benzylic ester functionality at the 3,10-positions of dibromoperylene via double Suzuki–Miyaura coupling. The final c-CNK is synthesized through oxidative dehydrogenation of the key precursor 2H-c-CNK by treatment with tetrachloro-1,4-benzoquinone (TCBQ). The two mesityl substituents at high spin positions of the CNK provide optimum steric protection, ensuring a reasonable stability with a half-life (t1/2) of 59 min in solution under ambient conditions. The in situ formation of c-CNK is confirmed by UV-Vis-NIR spectroscopy, high-resolution (HR) matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and Raman spectroscopy. c-CNK shows a narrow optical energy gap (Eopt.) of 1.07 eV as revealed by UV-Vis-NIR absorption data. The open-shell character of c-CNK is supported by electron paramagnetic resonance (EPR) spectroscopy. This study provides a synthetic approach for accomplishing novel CNK systems where two spins are anti-ferromagnetically coupled in a single molecule in the ground state.
Results and discussion
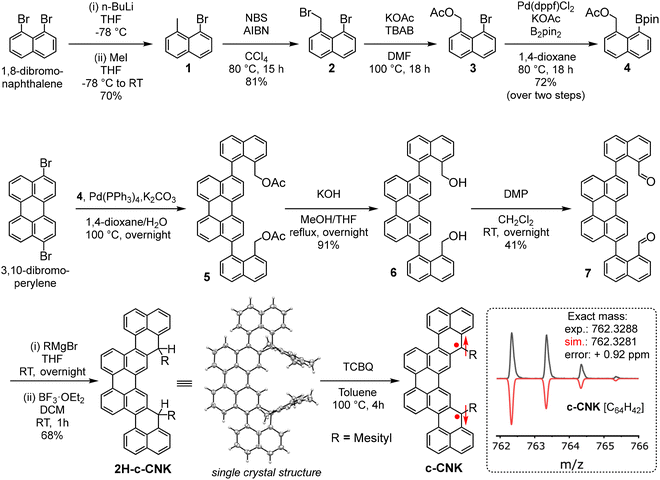
To realize the synthesis of c-CNK, we conceived a synthetic route as illustrated in Scheme 1. Firstly, the initial precursor 4 was synthesized via 4-step synthesis by using commercially available 1,8-dibromonaphthalene as the starting material. Compound 1 was synthesized by selective lithiation using n-BuLi followed by electrophilic addition of methyl iodide at low temperature. Next, benzylic bromination was performed on 1 using N-bromosuccinimide in carbon tetrachloride solvent at 80 °C to achieve compound 2.Subsequently, compound 2 was reacted with potassium acetate at 100 °C to obtain 3. Finally, palladium catalyzed borylation was performed on 3 to get the precursor 4 with a boronic ester functionality for subsequent double Suzuki–Miyaura coupling with 3,10-dibromoperylene. Next, the key precursor 2H-c-CNK was achieved in four steps. After successful double Suzuki–Miyaura coupling, 3,10-dibromoperylene afforded compound 5 with a benzylic ester functionality which was subjected to hydroxylation with KOH without further purification to afford compound 6 in 72% yield over two steps. Compound 6 was oxidized by Dess–Martin oxidation to obtain compound 7 in 41% yield. Afterward, compound 7 was treated with an excess of 2-mesitylmagnesium bromide to produce the diol in situ, which was then subjected to Friedel–Crafts alkylation promoted by BF3·OEt2 to afford the dihydro-precursor 2H-c-CNK with a yield of 68% in two steps. Notably, the key precursor 2H-c-CNK was confirmed by single crystal X-ray diffraction (XRD) analysis, MALDI-TOF, and NMR spectroscopy (Fig. S4†). All of the intermediates were purified and fully characterized by HR MALDI-TOF mass spectrometry and NMR (1H and 13C) spectroscopy.
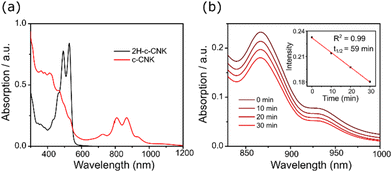
We further monitored the oxidative dehydrogenation reaction of the precursor 2H-c-CNK using different oxidation conditions. The most successful results were obtained when tetrachloro-1,4-benzoquinone (TCBQ) was used as an oxidant in a toluene solution of 2H-c-CNK at 100 °C as evident from UV-Vis-NIR spectroscopy and MALDI-TOF mass analysis of the reaction mixture. HR MALDI-TOF mass analysis of the reaction mixture in positive mode revealed that the intense signal at m/z = 764.3451 of the precursor 2H-c-CNK ([M+]), calculated for C64H44 as 764.3443, disappeared and a new signal emerged at m/z = 762.3288 (Scheme 1), which is fully consistent with the expected molecular mass of c-CNK ([M+]), calculated for C64H42 as 762.3281, validating the successful formation of the targeted c-CNK. Notably, the isotopic distribution observed for the obtained c-CNK agrees with the simulated pattern for C64H42. Due to the high biradical character and high reactivity of c-CNK, conventional structural characterization by NMR spectroscopy and single-crystal structural analysis or attempts to isolate and purify c-CNK did not succeed. Therefore, we turned to in situ UV-Vis-NIR spectroscopy which is a powerful tool to characterize open-shell NGs due to the characteristic low energy absorption bands in the NIR region associated with SOMO to LUMO transitions. As shown in Fig. 2a, the dihydro-precursor 2H-c-CNK only shows UV-Vis absorption below λ = 560 nm. In contrast, the UV-Vis-NIR absorption spectrum of c-CNK displays the characteristic low-energy absorption bands with absorption wavelengths (λ) extending up to 1150 nm resulting from electronic excitation from SOMO to LUMO with vibrionic features related to absorption bands at 940 nm, 866 nm, 809 nm, and 727 nm. A narrow optical energy gap (Eopt.) of 1.07 eV is revealed by UV-Vis-NIR absorption data of c-CNK. The spectral changes accompanied by colour changes from yellow to greenish-brown can obviously be attributed to the in situ formation of c-CNK.
The in situ generated c-CNK was persistent in solution under ambient conditions, as shown in Fig. 2b. Time-depended UV-Vis-NIR absorption spectra of c-CNK were obtained to test its stability. The spectroscopic results reveal that the intensity of the NIR absorption bands diminishes with the concomitant increase of the peaks in the visible region suggesting the possible oxidative degradation of c-CNK. During the stability test, the solution of compound c-CNK in dry dichloromethane was exposed to ambient air and light. By plotting the change in absorption intensity with the exposure time of ambient conditions, according to the linear regression and extrapolation to % change = 0.50, the half-life time (t1/2) of c-CNK was estimated to be 59 min.
Next, EPR spectroscopy was used to examine the paramagnetic nature of c-CNK first at 293 K. The open-shell character of c-CNK is supported by observing a strong isotropic signal with a g-value of 2.0026 in the EPR spectrum in deuterated dichloromethane (Fig. 3a). The isotropic EPR signal arises from efficient spin delocalization and weak spin–spin coupling, as further supported by DFT calculations. We further performed variable-temperature EPR measurements, which show an increase in the signal intensity by increasing the temperature from 225 to 310 K (Fig. 3b and S2a†). This behaviour is typical of singlet biradicals and is the result of the thermal population of triplet states due to a smaller singlet–triplet energy gap.29 In addition, at low temperatures, intermolecular interactions within the perylene framework lead to enhanced π–π stacking, resulting in singlet state formation due to spin–spin coupling between the interacting molecules resulting in pronounced decrease in the EPR signal.30 The radical is not stable at high temperatures and the EPR signal intensity decreases over time (Fig. S2b†); the latter is in agreement with time-dependent UV-Vis-NIR spectroscopic measurements. Overall, these results confirm the singlet biradical character of the c-CNK generated in situ in dichloromethane solution.
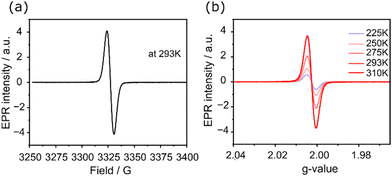 | ||
| Fig. 3 (a) EPR spectrum of c-CNK measured at 293 K in d-dichloromethane solution. (b) Variable temperature EPR spectra of c-CNK in d-dichloromethane solution. | ||
To further support the successful synthesis of c-CNK, we measured the Raman spectra, which is also an important tool for in situ investigation of redox reactions in NGs.31 We compared the experimental results with DFT calculation outcomes, as shown in Fig. 4a. The best experimental conditions for Raman spectroscopy of these compounds were reached with the excitation wavelength of 405 nm. Based on the UV-Vis-NIR absorption spectra of 2H-c-CNK and c-CNK (Fig. 2a), the excitation at 405 nm wavelength yields resonance Raman conditions. In the DFT calculation of the resonance Raman spectra of c-CNK and 2H-c-CNK, we obtain the best agreement with the experiments considering excitation wavelengths in the range of 405–415 nm (Fig. S3†). Understandably, these values are not identical to the experimental excitation wavelength (405 nm) because of the differences between the local molecular conditions of the sample and those of the isolated molecular model, which lacks intermolecular interactions and crystal packing effects. Nevertheless, all the spectra show good agreement with their computational counterparts. The precursor 2H-c-CNK and the in situ generated c-CNK were analyzed with excitation at 405 nm. For the precursor 2H-c-CNK, the experimental Raman bands at 1357 and 1383 cm−1 are assigned to the collective CC stretching modes of the graphene moiety. We also assign the intense feature at 1582 cm−1, as well as the shoulder at 1607 cm−1, to G modes. In the spectrum of c-CNK, we assign the band at 1383 cm−1 to collective CC stretching in the phenalene moiety and the peak at 1582 cm−1, together with its shoulder at 1607 cm−1, to the G mode of the perylene moiety. It is remarkable that the positions of the strongest features in the Raman spectrum of 2H-c-CNK and c-CNK do not differ much, in agreement with DFT results. Indeed, the analysis of the nuclear displacements of the strongest modes in the G and D regions reveals similar displacement patterns in the two molecules, irrespective of the dehydrogenation. The presence/absence of the two hydrogen atoms in 2H-c-CNK and c-CNK, respectively, has a weak influence on the collective vibrational dynamics of these CC stretching modes.
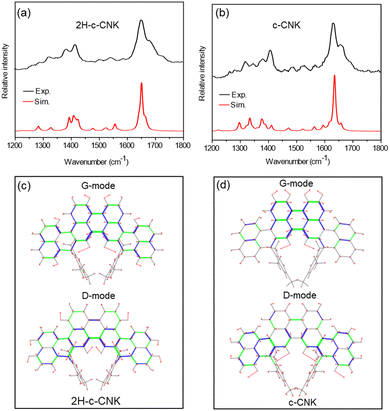 | ||
| Fig. 4 Top panel: experimental (black lines) and simulated (red lines) Raman spectra of (a) 2H-c-CNK and (b) c-CNK. Each spectrum has been normalized to unity. The simulated spectrum of 2H-c-CNK was computed with 410 nm excitation and that of c-CNK with 406 nm excitation resonance. Bottom panel: representation of the nuclear displacements assigned to the strongest G and D peaks observed in the experimental spectra of (c) 2H-c-CNK and (d) c-CNK; the green and blue segments represent stretching and contracting bonds, respectively, and the red lines represent nuclear displacement vectors (see the starred modes in Tables S1 and S2† for details). | ||
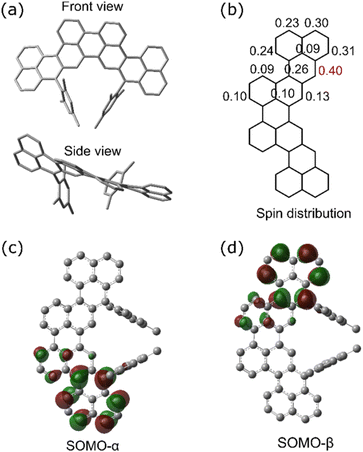
Quantum chemical simulations were performed to gain a deeper understanding of the electronic structure of c-CNK. DFT calculations were performed using the Gaussian 09 package. The geometry of the CNK was relaxed at the UB3LYP/6-311G level of theory. The DFT-optimized structures of compound c-CNK have a slightly curved backbone geometry with the mesityl substitutions perpendicular to the core structure (Fig. 5a). A comparison of the optimized singlet and triplet ground states of c-CNK shows that the singlet state, where the two electronic spins are antiparallel, is the most stable state. The calculated singlet–triplet energy gap (ΔEST) is small with a value of −0.42 kcal mol−1. The biradical character (y0) was calculated using UB3LYP/6-31G, according to the occupation numbers of the lowest singly occupied natural orbitals (SOMO) and lowest singly unoccupied natural orbitals (LUMO). The calculated high biradical yield (y0) of 0.97 agrees with the non-Kekulé structure with two unpaired spins.32 Furthermore, the spin density distribution of c-CNK reveals that spin is mostly concentrated over the phenalene units with the high spin density at the carbon centers attached to the mesityl-substituents (Fig. 5b). The calculated singly occupied molecular orbital (SOMO) profiles of the α and β electrons are separated from each other (Fig. 5c and d), which is typical of open-shell NGs.
Conclusions
In summary, we have successfully synthesized a next-generation persistent concealed non-Kekulé NG via multi-step solution synthesis. The synthesis of c-CNK is verified using in situ UV-Vis-NIR spectroscopy, Raman spectroscopy, and MALDI-TOF mass spectrometry, supported by quantum chemical calculations. Mesityl substitution at the high-spin positions imparts moderate kinetic stability, resulting in a half-life (t1/2) of 59 minutes under ambient conditions. Continuous-wave EPR analysis confirms the presence of unpaired electrons with a g-value of 2.0026. These results introduce a novel persistent concealed non-Kekulé NG, offering valuable insights for the design of CNK structural motifs having antiferromagnetic unpaired spins, with implications in the field of spintronics. Our ongoing work focuses on next-generation derivatives of c-CNK with enhanced kinetic stability through steric modifications, which will enable further exploration of their properties.Author contributions
All authors have given approval to the final version of the manuscript. MI and LY contributed equally to this work.Data availability
The data supporting this article have been included as part of the ESI.† The crystallographic data for 2H-c-CNK have been deposited at the CCDC under 2390595.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the EU Graphene Flagship (Graphene Core 3, 881603), EIC-2022-Pathfinder Open (ATYPIQUAL, 101099098), the Center for Advancing Electronics Dresden (cfaed), H2020-EU.1.2.2.-FET Proactive Grant (LIGHT-CAP, 101017821) and the DFG-SNSF Joint Switzerland-German Research Project (EnhanTopo, 429265950). The authors gratefully acknowledge the GWK support for funding this project by providing computing time through the Center for Information Services and HPC (ZIH) at TU Dresden. MI is thankful to the Alexander von Humboldt Foundation for generous postdoctoral fellowship support.References
- W. Zeng and J. Wu, Open-Shell Graphene Fragments, Chem, 2021, 7(2), 358–386 Search PubMed
.
- J. Liu and X. Feng, Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives, Angew. Chem., Int. Ed., 2020, 59(52), 23386–23401 CrossRef PubMed
.
- D. G. de Oteyza and T. Frederiksen, Carbon-Based Nanostructures as a Versatile Platform for Tunable π-Magnetism, J. Phys.: Condens. Matter, 2022, 34(44), 443001 CrossRef PubMed
.
- S. Song, J. Su, M. Telychko, J. Li, G. Li, Y. Li, C. Su, J. Wu and J. Lu, On-Surface Synthesis of Graphene Nanostructures with π-Magnetism, Chem. Soc. Rev., 2021, 50(5), 3238–3262 RSC
.
- T. Kubo, Phenalenyl-Based Open-Shell Polycyclic Aromatic Hydrocarbons, Chem. Rec., 2015, 15(1), 218–232 CrossRef PubMed
.
- H. Miyoshi, S. Nobusue, A. Shimizu and Y. Tobe, Non-Alternant Non-Benzenoid Kekulenes: The Birth of a New Kekulene Family, Chem. Soc. Rev., 2015, 44(18), 6560–6577 RSC
.
- Y. Morita, S. Suzuki, K. Sato and T. Takui, Synthetic Organic Spin Chemistry for Structurally Well-Defined Open-Shell Graphene Fragments, Nat. Chem., 2011, 3(3), 197–204 CrossRef PubMed
.
- F. Lombardi, A. Lodi, J. Ma, J. Liu, M. Slota, A. Narita, W. K. Myers, K. Müllen, X. Feng and L. Bogani, Quantum Units from the Topological Engineering of Molecular Graphenoids, Science, 2019, 366(6469), 1107–1110 CrossRef PubMed
.
- T. Kubo, Syntheses and Properties of Open-Shell π-Conjugated Molecules, Bull. Chem. Soc. Jpn., 2021, 94(9), 2235–2244 CrossRef
.
- S. K. Pal, M. E. Itkis, F. S. Tham, R. W. Reed, R. T. Okley and R. C. Haddon, Resonating Valence-Bond Ground State in a Phenalenyl-Based Neutral Radical Conductor, Science, 2005, 309(5732), 281–284 CrossRef PubMed
.
- I. Žutić, J. Fabian and S. Das Sarma, Spintronics: Fundamentals and Applications, Rev. Mod. Phys., 2004, 76(2), 323–410 Search PubMed
.
- D. Gatteschi, L. Bogani, A. Cornia, M. Mannini, L. Sorace and R. Sessoli, Molecular Magnetism, Status and Perspectives, Solid State Sci., 2008, 10(12), 1701–1709 CrossRef
.
- K. Goto, T. Kubo, K. Yamamoto, K. Nakasuji, K. Sato, D. Shiomi, T. Takui, M. Kubota and T. Kobayashi, A Stable Neutral Hydrocarbon Radical: Synthesis, Crystal Structure, and Physical Properties, J. Am. Chem. Soc., 1999, 778(6), 1619–1620 CrossRef
.
- Y. Li, K.-W. Huang, Z. Sun, R. D. Webster, Z. Zeng, W. Zeng, C. Chi, K. Furukawa and J. Wu, A Kinetically Blocked 1,14:11,12-Dibenzopentacene: A Persistent Triplet Diradical of a Non-Kekulé Polycyclic Benzenoid Hydrocarbon, Chem. Sci., 2014, 5(5), 1908–1914 Search PubMed
.
- S. Arikawa, A. Shimizu, D. Shiomi, K. Sato and R. Shintani, Synthesis and Isolation of a Kinetically Stabilized Crystalline Triangulene, J. Am. Chem. Soc., 2021, 143(46), 19599–19605 CrossRef
.
- L. Valenta, M. Mayländer, P. Kappeler, O. Blacque, T. Šolomek, S. Richert and M. Juríček, Trimesityltriangulene: A Persistent Derivative of Clar's Hydrocarbon, Chem. Commun., 2022, 58(18), 3019–3022 RSC
.
- X. Guo, F. Zhang, J. Brunvoll, B. N. Cyvin and S. J. Cyvin, Concealed Non-Kekuléan Benzenoids, J. Chem. Inf. Comput. Sci., 1995, 35(2), 226–232 CrossRef
.
- R. Ortiz, R. A. Boto, M. Melle-franco and J. Fernández-Rossie, Exchange Rules for Diradical π-Conjugated Hydrocarbons, Nano Lett., 2019, 19(9), 5991–5997 CrossRef PubMed
.
- S. Gil-Guerrero, M. Melle-Franco, Á. Peña-Gallego and M. Mandado, Clar Goblet and Aromaticity Driven Multiradical Nanographenes, Chem. – Eur. J., 2020, 26(68), 16138–16143 CrossRef
.
- S. J. Cyvin, J. Brunvoll and B. N. Cyvin, The Hunt for Concealed Non-Kekuléan Polyhexes, J. Math. Chem., 1990, 4(1), 47–54 CrossRef
.
- E. Clar and C. C. Mackay, Circobiphenyl and the Attempted Synthesis of 1:14, 3:4, 7:8, 10:11-Tetrabenzoperopyrene, Tetrahedron, 1972, 28(24), 6041–6047 CrossRef
.
- S. Mishra, D. Beyer, K. Eimre, S. Kezilebieke, R. Berger, O. Gröning, C. A. Pignedoli, K. Müllen, P. Liljeroth, P. Ruffieux, X. Feng and R. Fasel, Topological Frustration Induces Unconventional Magnetism in a Nanographene, Nat. Nanotechnol., 2020, 15(1), 22–28 CrossRef
.
- E. Turco, A. Bernhardt, N. Krane, L. Valenta, R. Fasel, M. Juríček and P. Ruffieux, Observation of the Magnetic Ground State of the Two Smallest Triangular Nanographenes, JACS Au, 2023, 3(5), 1358–1364 CrossRef PubMed
.
- X. Su, C. Li, Q. Du, K. Tao, S. Wang and P. Yu, Atomically Precise Synthesis and Characterization of Heptauthrene with Triplet Ground State, Nano Lett., 2020, 20(9), 6859–6864 CrossRef PubMed
.
- S. Song, A. Pinar Solé, A. Matěj, G. Li, O. Stetsovych, D. Soler, H. Yang, M. Telychko, J. Li, M. Kumar, Q. Chen, S. Edalatmanesh, J. Brabec, L. Veis, J. Wu, P. Jelinek and J. Lu, Highly Entangled Polyradical Nanographene with Coexisting Strong Correlation and Topological Frustration, Nat. Chem., 2024, 16(6), 938–944 CrossRef
.
- N. Pavliček, A. Mistry, Z. Majzik, N. Moll, G. Meyer, D. J. Fox and L. Gross, Synthesis and Characterization of Triangulene, Nat. Nanotechnol., 2017, 12(4), 308–311 CrossRef PubMed
.
- S. Mishra, D. Beyer, K. Eimre, J. Liu, R. Berger, O. Gröning, C. A. Pignedoli, K. Müllen, R. Fasel, X. Feng and P. Ruffieux, Synthesis and Characterization of π-Extended Triangulene, J. Am. Chem. Soc., 2019, 141(27), 10621–10625 CrossRef PubMed
.
- J. Su, M. Telychko, P. Hu, G. Macam, P. Mutombo, H. Zhang, Y. Bao, F. Cheng, Z.-Q. Huang, Z. Qiu, S. J. R. Tan, H. Lin, P. Jelínek, F.-C. Chuang, J. Wu and J. Lu, Atomically Precise Bottom-up Synthesis of π-Extended [5]Triangulene, Sci. Adv., 2019, 5, 4–10 Search PubMed
.
- F. Wu, J. Ma, F. Lombardi, Y. Fu, F. Liu, Z. Huang, R. Liu, H. Komber, D. I. Alexandropoulos, E. Dmitrieva, T. G. Lohr, N. Israel, A. A. Popov, J. Liu, L. Bogani and X. Feng, Benzo-Extended Cyclohepta[Def]Fluorene Derivatives with Very Low-Lying Triplet States, Angew. Chem., Int. Ed., 2022, 61(23), e202202170 CrossRef PubMed
.
- K. Sahara, M. Abe, H. Zipse and T. Kubo, Duality of Reactivity of a Biradicaloid Compound with an o-Quinodimethane Scaffold, J. Am. Chem. Soc., 2020, 142(11), 5408–5418 CrossRef PubMed
.
- M. R. Ajayakumar, J. Ma, A. Lucotti, K. S. Schellhammer, G. Serra, E. Dmitrieva, M. Rosenkranz, H. Komber, J. Liu, F. Ortmann, M. Tommasini and X. Feng, Persistent Peri-Heptacene: Synthesis and In situ Characterization, Angew. Chem., Int. Ed., 2021, 60(25), 13853–13858 CrossRef
.
- Y. Li, W.-K. Heng, B. S. Lee, N. Aratani, J. L. Zafra, N. Bao, R. Lee, Y. M. Sung, Z. Sun, K.-W. Huang, R. D. Webster, J. T. López Navarrete, D. Kim, A. Osuka, J. Casado, J. Ding and J. Wu, Kinetically Blocked Stable Heptazethrene and Octazethrene: Closed-Shell or Open-Shell in the Ground State?, J. Am. Chem. Soc., 2012, 134(36), 14913–14922 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2390595. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo02019g |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |