Open Access Article
Open Access ArticleSolvometallurgical recovery of antimony from waste polyvinyl chloride plastic and co-extraction of organic additives†
Jeroen Spooren *
*
Waste Recycling Technologies, Materials & Chemistry Unit, Flemish Institute for Technological Research, VITO N.V., Boeretang 200, B-2400 Mol, Belgium. E-mail: jeroen.spooren@vito.be
First published on 6th January 2025
Abstract
Antimony is a critical raw material in Europe wherein for 43% of its market share it is applied in the form of antimony trioxide as a fire retardant in plastics. Currently, antimony recycling from waste plastics does not take place and has been scarcely studied. In this work, a process was developed to extract antimony from a soft PVC material and recover it as Sb4Cl2O5. Antimony was extracted by means of an ethanolic hydrochloric acid solution, prepared by diluting fuming HCl with ethanol to achieve the desired concentration. The addition of an organic solvent, such as ethanol, is known to enhance the chloride ion activity and promote metal chloride complex formation. This study confirms that the use of aqueous ethanol as a solvent increased the solvation of antimony at moderate temperature (i.e. 80 °C) as opposed to aqueous HCl solutions. The optimised leaching process showed high antimony extraction yields (94%) in the presence of an aqueous ethanol solution containing 4 M HCl at 80 °C for 4 h. Furthermore, the addition of the organic solvent ethanol to the reaction mixture caused organic additives to be co-extracted from the PVC (71% di-n-octyl phenyl phosphate, 51% di-iso-nonylphthalate, 76% 2-ethylhexyl diphenyl phosphate, 30% 9-octadecanamide and 15% butylated hydroxytoluene). The pregnant leaching solution was subsequently distilled to recover ethanol and washed with n-hexane to recover the extracted organic additives. Finally, water addition to the obtained solution led to the precipitation of 95% of antimony from the solution as Sb4Cl2O5 with a high purity (≥99.8%). The residual PVC was not degraded and could be suitable for recycling.
Introduction
Inorganic and organic compounds are applied as additives in plastics, such as polyvinyl chloride (PVC), to obtain desired mechanical and physical properties.1,2 Antimony trioxide (ATO) is one such inorganic additive that is used as a flame retardant in synergy with organic flame retardants.3 Antimony is considered by the European Commission to be a critical raw material due to its economic importance and supply risks.4 Although 43% of the total antimony demand in Europe is as a flame retardant, antimony is currently not recovered from plastic products and thus disperses in products derived from plastic recycling, in incineration ashes and in landfills.5 Research efforts to recover antimony from waste plastics mainly focused on the destruction of polymers, by decomposition, pyrolysis, or incineration, to subsequently recover antimony from the produced gasses or within the obtained solid residues.6 Direct hydrometallurgical extraction of antimony from waste plastics has been scarcely studied. Such studies focused on antimony extraction from poly(acrylonitrile butadiene styrene) (ABS) plastics. Acid extraction was studied under harsh conditions at 175 °C by microwave heating and showed that extraction proceeded best according to 18 M H2SO4 > 12 M HCl > 6 M HCl, whereas in aqua regia no antimony was extracted.7 The highest antimony extraction yields were in the range of 21.2–21.5%. Alkaline (20 g per L NaOH) leaching yielding 87% of antimony extraction was performed under harsh hydrothermal conditions at 220 °C for 2 h in the presence of Na2S to form soluble SbS33−.7,8 Whereas another approach consisted of co-dissolving the polymer in a solution of 0.5 M sodium hydrogen tartrate in DMSO for 20 h at 100–108 °C, after which the polymer reprecipitated and 48% of the antimony remained dissolved in the lixiviant.9 In the latter two cases, the ABS polymer was recovered and was suitable for recycling.HCl leaching of Sb2O3 generally occurs via the formation of SbCl3, according to eqn (1), which is soluble in concentrated hydrochloric acid solutions through the formation of soluble chloride complexes following eqn (2).10 Thermodynamic restrictions would require to operate at high temperatures (>130 °C) to leach antimony (Fig. S1 in the ESI†). This implies working under hydrothermal conditions in closed reactor systems, which are difficult to implement at industrial scale. Furthermore, operating under hydrothermal conditions at elevated temperatures could possibly lead to dechlorination of the PVC polymer.11
| Sb2O3 + 6HCl → 2SbCl3 + 3H2O | (1) |
| SbCln(3−n) + Cl− ⇌ SbCln+1(2−n) | (2) |
The formation of antimony chloride complexes according to the reaction equilibrium in eqn (2) is governed by the Cl− activity according to eqn (3),
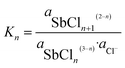 | (3) |
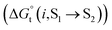 , which is related to the ionic activity according to eqn (4)
, which is related to the ionic activity according to eqn (4)
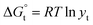 | (4) |
| Sb(H2O)h3+ + nCl(H2O)l− ⇌ SbCln(H2O)m(3−n) + (h + l − m)H2O | (5) |
It was shown that the addition of ethanol to a chlorine aqueous solution containing metal cations increased both the cation and chloride anion activity and promote the formation of metal chloro-complexes.15 Furthermore, the solubility product is thermodynamically related to the product of the individual ionic activities and the solvent medium effect can thus be exploited in, amongst others, metallurgic extraction systems.
A recent study showed that the chlorine reactivity towards antimony extraction from a waste lead-rich dross enhanced in a hydrochloric acid water–ethanol mixture.16 Indeed, hydrochloric acid extraction in the presence of alcohols has also been shown to be more efficient than aqueous HCl extraction for scheelite,17 sea nodules18,19 and zinc ferrite.20 In all cases, the hydrochloric acid concentration and reaction temperature could be significantly lowered, and the metal leaching efficiency was increased compared to aqueous systems. Solvometallurgy, whereby at least 50% of the aqueous solvent is replaced by an organic solvent, recently gained additional interest as it could offer benefits such as more selective leaching, reduced acid consumption and fewer purification steps.21 Furthermore, the presence of an organic solvent could also give rise to the improved dissolution of organic molecules. For example, soil remediation with a hydrochloric acid solution in ethyl-acetate/ethanol/water led to the simultaneous extraction of heavy metals and organic pollutants.22 Whereas, at increasing ethanol concentrations in water, plasticizer extraction from PVC plastics takes place.23,24 Interestingly, these studies also reported that at increased ethanol concentrations, above 8%, in water, liquid transfer into the PVC took place, which further increased with increasing temperature. Cano et al. studied the extraction of adipate and phthalate plasticizers from a PVC paste by means of microwave-assisted extraction in alcohols, such as, methanol and ethanol.25 In ethanol at an extraction temperature of 120 °C for 10 min, 78–88% of di-2-ethylhexyl adipate (DEHA) was extracted. In the same study, microwave-assisted extraction with methanol was optimised showing already effective extractions at mild temperatures and short times (e.g., 55–75% DEHA at 40 °C and 5 min to 10 min, respectively). At the optimised temperature of 120 °C for 10 min in methanol high yields of phthalate extraction (103% di-2-ethylhexyl, 93% diisononyl and 95% diisodecyl phthalate) were achieved.
The extraction of additives from plastics is a complex process that is governed by chemical and physical processes.2 On the one hand, the solubility of the additive molecule in the chosen solvent system plays an important role and can be predicted for organic additives via solubility theories, such as the Hansen theory.26 This theory is commonly used and provides solubility parameters based on the three interactions non-polar/dispersion, polar and hydrogen bonding that allow to calculate the distance between the solubility parameters of an additive/polymer and solvent to estimate the suitability of a solvent to dissolve or swell a certain polymer or additive.2,26 On the other hand, diffusivity is an important physical process that allows a molecule to effectively move from one medium (the polymer) to another (the solvent). Additionally, diffusivity of the solvent into the polymer structure is also required to enhance the interaction with additives. Diffusion in polymer systems is complex and depends on many factors such as morphology, pore size and swelling of the polymer, as well as the volatility and concentration of solutes, their crystallinity, surface energy differences, temperature etc.27 An important parameter of diffusivity in polymers is the glass transition temperature (Tg), above which in rubbery polymers small molecules typically have a steady-state diffusion according Fick's law, whereas below Tg in glassy polymers unsteady-state diffusion (non-Fickian motion) occurs.2 Specific models have been developed to theoretically estimate the diffusion of moieties in polymers and are described in literature which the reader is advised to consult.2,28
In this work, it was hypothesized that leaching waste PVC with an alcoholic chloridic acid solution could improve antimony leaching at mild temperatures and extract both metal-containing inorganic additives as well as organic additives. These hypotheses were experimentally tested, and the developed process was optimised to achieve a high antimony extraction and recovery yield.
Materials and methods
Materials
An antimony-containing soft PVC sheet material was used for this study (Fig. S2, ESI†). Initial leaching experiments applied the PVC sheet which was cut into uniform flakes of 1 cm × 1 cm. Whereas, for the final validation experiment the same PVC sheet was cryogenic milled to <4 mm by submerging the material in liquid nitrogen prior to treatment with a Fritsch Pulverisette cutting mill that was equipped with a 4 mm sieve.Hydrochloric acid (37 wt% HCl in water) and n-hexane (SupraSolv®, ≥98.0%) were purchased from Merck KGaA (Darmstadt, Germany), while ethanol (absolute) and 2-propanol (technical grade, ≥98%) from VWR Chemicals (Fontenay-sous-Bois, France). For precipitation reactions the chemicals antimony chloride (SbCl3, ACS reagent), diethyl phthalate (99.5%), and dioctyl phthalate (≥99.5%) were acquired Merck KGaA (Darmstadt, Germany), and 2-ethylhexyl diphenyl phosphate from VWR Chemicals (Fontenay-sous-Bois, France). All chemicals were used as received without any further purification.
Leaching optimisation experiments
For leaching in aqueous HCl solutions, 2 g of PVC samples was brought into PTFE containers with 20 mL of an aqueous hydrochloric acid solution. The PTFE containers were sealed, and then shaken horizontally at 225 rpm for 2 h in a water bath at the set reaction temperature. After the leaching process, the containers were removed from the water bath and cooled to room temperature. The leaching experiments with ethanolic HCl solutions were carried out in a round-bottom flask equipped with a reflux condenser. The hydrochloric acid solutions were prepared by diluting an aqueous 37 wt% HCl solution with pure ethanol to obtain the desired HCl concentration. Such solution is in the below text also referred to as ethanolic HCl solution. The reaction mixture in the flask was heated for 2 h in a water bath at a set reaction temperature. After the leaching process, the round-bottom flasks were cooled down to room temperature. After leaching the reduction potential and the pH of the liquid phase were measured and the lixiviant was separated from the solid residue by vacuum filtration using a 0.45 μm mixed cellulose ester membrane filter from Whatman. The PVC residues were dried at 40 °C and subsequently analysed by X-ray fluorescence (XRF), whereas the filtrates were conserved through dilution with a 5 vol% nitric acid solution for subsequent analysis by inductively coupled plasma atomic emission spectroscopy (ICP-OES) Since ICP-OES analysis of the liquid fraction was carried out with aqueous standards, the ethanol was removed from the lixiviants by rotary evaporation prior to analysis. The obtained aqueous fractions were then diluted to 20 mL with ultra-pure water. Subsequently, 5 vol% nitric acid was added for preservation. All experiments were carried out in duplicate.The extraction efficiency, expressed in %, of an extracted element into the leachate solution was calculated according to
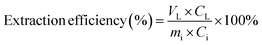 | (6) |
Antimony recovery optimisation
A bigger scale leaching experiment was performed in duplicate in a 1 L round-bottom flask, placed in a heating mantle and equipped with a reflux condenser. The flask was filled with 50 g of PVC sample (1 × 1 cm flakes) and 500 mL of 4 M HCl ethanolic solution. The reaction mixture was heated to 80 °C for 5 h without stirring. After cooling to room temperature and filtration, the recovered leaching solution was divided in two equal fractions of the same volume (235 mL); FR1 and FR2. Ethanol was removed from FR1 through rotary evaporation (130 mbar, 55 °C). Whereas FR2 was not distilled. Subsequently, MilliQ water was added stepwise to a known volume of FR1 and FR2 by means of a graduated burette at room temperature and under constant stirring to improve mixing and uniform particle formation. The pH of the solution was measured after each water addition step. Water was added until the formation of a precipitate was observed, after which the solution was left to stir for an additional 1 h. Next, the slurry obtained for FR1 was filtered over a 0.45 μm mixed cellulose ester membrane filter (Whatman) to recover the precipitate. Whereas, for the FR2 solution the solid was recovered through centrifugation (5 min, 3500 rpm). The solid residues were dried at 40 °C in a nitrogen atmosphere until constant mass. The obtained liquid fractions were analysed by ICP-OES, the PVC solid resides by ICP-OES after acid digestion, and the precipitates by XRD and ICP-OES after acid digestion.The recovery yield, expressed in %, of a precipitated element from the solution was calculated according to
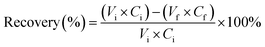 | (7) |
Antimony hydrolysis precipitation in the presence of organic additives
In a beaker 10 g per L SbCl3 was dissolved in 12 M HCl in the presence of diethyl phthalate, dioctyl phthalate or 2-ethylhexyl diphenyl phosphate, respectively, were executed. The organic additives were added in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with antimony. The solution was stirred at room temperature while gradually MilliQ water was added to the solution until precipitation occurred. The slurry was left to stir for an additional 10 min. Next, filtration and washing with water over a Whatman cellulose filter was performed to recover the precipitate which was subsequently dried at 40 °C. The dried precipitate was analysed by XRD, ICP-OES and CHNS analysis.
1 molar ratio with antimony. The solution was stirred at room temperature while gradually MilliQ water was added to the solution until precipitation occurred. The slurry was left to stir for an additional 10 min. Next, filtration and washing with water over a Whatman cellulose filter was performed to recover the precipitate which was subsequently dried at 40 °C. The dried precipitate was analysed by XRD, ICP-OES and CHNS analysis.
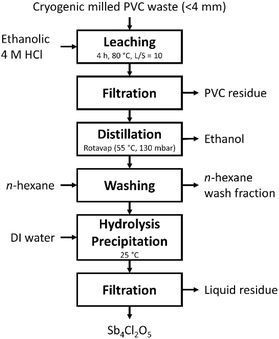
Process validation experiment
A cryogenic-milled PVC sample (50 g) was brought in a 1 L round bottom flask to which 500 mL of a 4 M HCl ethanolic solution was added. The flask was equipped with a condenser and heated by a temperature-controlled heating mantle to 80 °C and left to react for 4 h under mild stirring (150 rpm). After cooling to room temperature, the reaction mixture was filtered over a Whatman cellulose filter to remove the solid PVC residue. The PVC residue was washed over the filter with additional ethanol that was added to the collected filtrate. The PVC residue was dried at 50 °C and subsequently analysed by TGA-DSC, GC MS and ICP-OES (after acid digestion). Ethanol was removed from the filtrate by rotary evaporation (150 mbar, 55 °C). Subsequently, the aqueous lixiviant was washed twice with 50 mL n-hexane in a separatory funnel. To the washed aqueous leaching solution, known volumes of MilliQ water were stepwise added under constant stirring until a precipitate was observed. After which, an additional 100 mL of MilliQ water was added and left to stir for an additional 1 h. The precipitate was filtered off over a Whatman membrane filter (0.45 μm) and the filtrate was collected for ICP-OES analysis. The precipitate was dried at 50 °C and subsequently analysed by XRD, XRF and ICP-OES (after acid digestion). The experiment was performed in duplicate.Analysis methods
A PerkinElmer Avio 500 optical emission spectrometer with prepfast was used for the ICP-OES analysis. The aqueous samples were stabilised with 5 vol% HNO3 prior to ICP-OES analysis. Solid samples underwent acid digestion prior to ICP-OES analysis, whereby the sample was dissolved in an HNO3/H2O2/HCl solution and microwave digested under high pressure at 250 °C for 180 minutes. X-ray fluorescence (XRF) analysis on solid samples were performed by a Thermo Scientific Niton XL3t GOLDD+ handheld XRF analyzer. The reported elemental compositions in this work are average values for duplicate experiments and error values are given by the standard deviation.For the thermogravimetric- and differential scanning calorimetry analysis of PVC samples a NETZSCH STA 449 F3 Jupiter simultaneous thermal analyzer was used. X-Ray Diffraction (XRD) using a PANalytical Empyrean Diffractometer (Co anode) was performed to determine the crystallographic phase composition of the obtained precipitates. HighScore Plus software was used for qualitative analyses of the diffractograms. The CHNS measurements involves a high-temperature combustion at 1150 °C under oxygen atmosphere and thermal conductivity detection. The oxygen concentration analysis relies on the conversion of sample oxygen to carbon monoxide through high-temperature pyrolysis at 1450 °C and thermal conductivity detection. The CHNS measurements were performed with the Vario EL Cube Element Analyser (Elementar) and the oxygen measurement was conducted with a rapid OXY Cube Element Analyser (Elementar). The presence of organic additives was measured and quantified by means of GC MS. Measurements were performed on Trace DSQ GC MS (Thermo Fisher Scientific) equipped with a VF1701 30 m; 0.25 mm 0.25 μm column. Helium flow was set to 1 mL min−1. Injection was 1 μl with a split ration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 at an injector temperature of 250 °C. The Oven program was set as: 60 °C to 280 °C at 20 °C min−1. Hold for 5 min, to 320 °C at 20 °C min−1 and hold again for 5 min. The mass range was set from 50–650 amu at a scan rate of 1000. The samples were spiked with D4 di-n-butylphthalate as internal standard. SEM analysis was performed on cryogenic-milled PVC sample and PVC residue after leaching by a FEI NOVA NANOSEM 450 platform, equipped with a BRUKER QUANTAX 200 SDD detector for EDX analysis. Before electron microscopy, the samples were embedded in Epofix resin, polished and Pt-coated. Thermodynamic modelling was performed by means of HSC Chemistry v8 software.
50 at an injector temperature of 250 °C. The Oven program was set as: 60 °C to 280 °C at 20 °C min−1. Hold for 5 min, to 320 °C at 20 °C min−1 and hold again for 5 min. The mass range was set from 50–650 amu at a scan rate of 1000. The samples were spiked with D4 di-n-butylphthalate as internal standard. SEM analysis was performed on cryogenic-milled PVC sample and PVC residue after leaching by a FEI NOVA NANOSEM 450 platform, equipped with a BRUKER QUANTAX 200 SDD detector for EDX analysis. Before electron microscopy, the samples were embedded in Epofix resin, polished and Pt-coated. Thermodynamic modelling was performed by means of HSC Chemistry v8 software.
Results and discussion
Table 1 reports the average elemental composition of the PVC sample, whereby the antimony content accounted for 3.8 wt%. A detailed analyses by GC-MS confirmed the presence of 2-ethylhexyl diphenyl phosphate (7188 μg g−1), also known with the commercial name Phosflex 362®, and di-iso-nonylphthalate (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 364 μg g−1). Furthermore, the antioxidant butylated hydroxytoluene (5893 μg g−1) was also detected.
364 μg g−1). Furthermore, the antioxidant butylated hydroxytoluene (5893 μg g−1) was also detected.
| Element | Concentration (mg kg−1) | Element | Concentration (mg kg−1) |
|---|---|---|---|
| Al | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 6100 500 ± 6100 |
Pb | 680 ± 11 |
| Ca | 172 ± 18 | S | 540 ± 88 |
| Cr | 157 ± 19 | Sb | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 1500 200 ± 1500 |
| K | 170 ± 37 | Si | 1720 ± 270 |
| Mg | 95.6 ± 9.8 | Sn | 542 ± 77 |
| Na | 1220 ± 170 | Zn | 356 ± 40 |
| P | 1130 ± 170 |
The material was analysed for its N, C, H and S content, which were 0.20 wt%, 42.84 wt%, 6.37 wt% and 0.23 wt%, respectively. Finally, the chloride content was 22 wt%. The comparison between the H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C molar ratio of fresh and treated PVC provides an indicative measure of its dechlorination rate, as H
C molar ratio of fresh and treated PVC provides an indicative measure of its dechlorination rate, as H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ≈ 1.5 for pure PVC and 2 or 1 for its polyol or polyene dechlorinated products, respectively.29 The H
C ≈ 1.5 for pure PVC and 2 or 1 for its polyol or polyene dechlorinated products, respectively.29 The H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
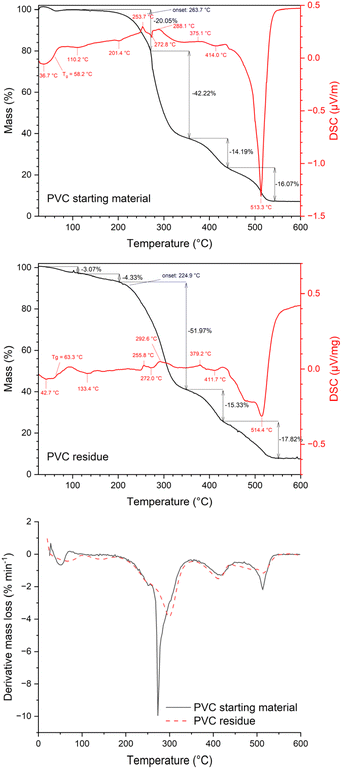
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C molar ratio of the investigated starting material equalled 1.8. TGA-DSC measurements of the PVC sample in air showed a glass transition temperature at 58.2 °C (vide infra). Typically, thermal decomposition of PVC occurs in two stages, whereby in the first stage (250 °C – 350 °C) HCl loss occurs due to the reaction of chlorine radicals from –C–Cl bonds and hydrogen radicals from adjacent C–H groups.30 In the second stage (350–520 °C) the polyene backbone degrades, showing a strong exothermic peak indicating the pyrolysis of the material.
C molar ratio of the investigated starting material equalled 1.8. TGA-DSC measurements of the PVC sample in air showed a glass transition temperature at 58.2 °C (vide infra). Typically, thermal decomposition of PVC occurs in two stages, whereby in the first stage (250 °C – 350 °C) HCl loss occurs due to the reaction of chlorine radicals from –C–Cl bonds and hydrogen radicals from adjacent C–H groups.30 In the second stage (350–520 °C) the polyene backbone degrades, showing a strong exothermic peak indicating the pyrolysis of the material.
Ethanolic HCl leaching systems were tested and compared to aqueous leaching systems at 50 °C and 80 °C in 2 M, 4 M and 6 M HCl (Fig. 1).
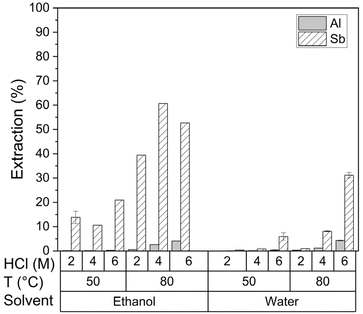 | ||
| Fig. 1 Leachability of aluminium and antimony at 50 °C and 80 °C in aqueous and ethanolic HCl solutions of different concentrations at L/S = 10 for 2 h. | ||
The ethanolic systems yielded a significant increase in antimony extraction at both tested temperatures. On average the measured pH of the lixiviants after leaching was ∼0.4 lower for the ethanolic solutions compared to that of the aqueous solutions with the same HCl concentrations (Table S1 in the ESI†). Antimony extraction by ethanolic HCl leaching drastically increased when increasing the temperature from 50 °C to 80 °C. Hereby it should be noted that according to differential scanning calorimetry the glass transition temperature of the material was estimated to be 58.2 °C, above which diffusion of the lixiviant into the polymer matrix is expected to enhance.2,27 Whereas, at 80 °C more antimony was extracted in 4 M ethanolic HCl solution than in 6 M. Hereby it needs to be noted that the HCl solution was prepared by dilution of an aqueous 12 M HCl solution with ethanol. Thus, the ethanol:water volume ratio in the 6 M HCl solution is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas for 4 M HCl it is 2
1, whereas for 4 M HCl it is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Upon an increased ethanol addition the standard Gibbs energy of transfer
1. Upon an increased ethanol addition the standard Gibbs energy of transfer 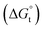 of the chloride anion solute increases from 4.9 kJ mol−1 at a 20% ethanol molar ratio aqueous solution to 9.2 kJ mol−1 for a 40% molar ratio.14 Therefore, it is plausible that at 6 M ethanolic HCl, the chloride activity is lower than at 4 M ethanolic HCl due to a much lower ethanol addition. Also, as the ionic activity is directly related to the thermodynamics of the solubility product, as well as chloride complexation reactions, it needs to be noted that the antimony solubility in an ethanolic HCl solution seems to be thermodynamically favoured at a lower temperature than in an aqueous HCl solution.31 Noteworthy, aluminium did not demonstrate an increase in leachability when switching from an aqueous to an ethanolic HCl solution. Thus, the antimony leaching selectivity, with respect to aluminium, improved and was optimal in an ethanolic HCl solution of 4 M at L/S = 10, 80 °C for 2 h. The obtained PVC residues after leaching maintained the original shape and colour but were observed to be less flexible. The chloride content of the PVC dropped slightly from 22 wt% in the original sample to 18–21 wt% in the residues and the H
of the chloride anion solute increases from 4.9 kJ mol−1 at a 20% ethanol molar ratio aqueous solution to 9.2 kJ mol−1 for a 40% molar ratio.14 Therefore, it is plausible that at 6 M ethanolic HCl, the chloride activity is lower than at 4 M ethanolic HCl due to a much lower ethanol addition. Also, as the ionic activity is directly related to the thermodynamics of the solubility product, as well as chloride complexation reactions, it needs to be noted that the antimony solubility in an ethanolic HCl solution seems to be thermodynamically favoured at a lower temperature than in an aqueous HCl solution.31 Noteworthy, aluminium did not demonstrate an increase in leachability when switching from an aqueous to an ethanolic HCl solution. Thus, the antimony leaching selectivity, with respect to aluminium, improved and was optimal in an ethanolic HCl solution of 4 M at L/S = 10, 80 °C for 2 h. The obtained PVC residues after leaching maintained the original shape and colour but were observed to be less flexible. The chloride content of the PVC dropped slightly from 22 wt% in the original sample to 18–21 wt% in the residues and the H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio decreased from 1.8 to 1.3–1.7, respectively. No clear trend was observed for the change in chloride content and H
C ratio decreased from 1.8 to 1.3–1.7, respectively. No clear trend was observed for the change in chloride content and H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio of the residues as a function of reaction temperature or HCl concentration in the ethanolic lixiviant. Thus, no apparent degradation of the PVC took place.
C ratio of the residues as a function of reaction temperature or HCl concentration in the ethanolic lixiviant. Thus, no apparent degradation of the PVC took place.
Next, 50 g of PVC was leached in 500 mL of an ethanolic 4 M HCl solution at 80 °C for 5 h. A mass loss of 4.8 ± 0.7 wt% was recorded for the PVC residue. About 67% of antimony was extracted, as well as significant percentages of phosphorous (64%), sulphur (28%), tin (56%) and zinc (33%) (Table 2).
| Element | Composition (mg kg−1) | Extraction efficiency (%) |
|---|---|---|
| Al | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 5700 000 ± 5700 |
15 ± 16 |
| Ca | 144 ± 13 | 20 ± 7 |
| Cr | 136 ± 1 | 17.8 ± 0.1 |
| K | 160 ± 170 | 11 ± 95 |
| Mg | 64 ± 30 | 37 ± 29 |
| Na | 1001 ± 93 | 22 ± 7 |
| P | 426 ± 15 | 64 ± 1 |
| Pb | 710 ± 0.0 | 0.7 ± 0.8 |
| S | 407 ± 5 | 28 ± 1 |
| Sb | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 3700 400 ± 3700 |
67 ± 9 |
| Si | 1570 ± 520 | 13 ± 28 |
| Sn | 252 ± 12 | 56 ± 2 |
| Zn | 250 ± 14 | 33 ± 4 |
The solid residue contained 20 ± 1 wt% chloride and had a H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C molar ratio of 1.45 ± 0.07, indicating that the PVC did not decompose. This was corroborated by comparing the thermogravimetric and calorimetric profiles of the PVC residue with those of the original PVC sample (Fig. 2). Although the TGA and DSC curves of the starting material and PVC residue showed similar trends, indicating that the PVC polymer did not degrade during leaching, the onset temperature of thermal degradation was different for the original sample (i.e. 263.7 °C) and the residue (i.e. 224.9 °C) due to the presence or absence, respectively, of the antimony-based flame retardant.
C molar ratio of 1.45 ± 0.07, indicating that the PVC did not decompose. This was corroborated by comparing the thermogravimetric and calorimetric profiles of the PVC residue with those of the original PVC sample (Fig. 2). Although the TGA and DSC curves of the starting material and PVC residue showed similar trends, indicating that the PVC polymer did not degrade during leaching, the onset temperature of thermal degradation was different for the original sample (i.e. 263.7 °C) and the residue (i.e. 224.9 °C) due to the presence or absence, respectively, of the antimony-based flame retardant.
The obtained pregnant leaching solution was observed to be turbid and was split in two fractions, labelled FR1 and FR2. For FR1 ethanol was removed through distillation upon which the colour of the solution turned yellowish with an oily phase being observed floating on top of the aqueous phase (Fig. S3 in the ESI†). This indicated that co-extraction of organic additives could have occurred. FR2 was not distilled.
The distilled FR1 (pH = −1.46) and non-distilled FR2 (pH = −1.14) were subsequently treated by gradually adding water, with the aim to precipitate antimony oxychlorides via hydrolysis of SbCl3 (eqn (8) and (9)).
| SbCl3 + H2O → SbOCl + 2HCl | (8) |
| 4SbOCl + H2O → Sb4Cl2O5 + 2HCl | (9) |
A precipitate was formed when reaching a pH of 0.06 for FR1 and a pH of 0.50 for FR2. For the distilled FR1, precipitation occurred at about 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Vwater
1 Vwater![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VFR1 addition and the pH remained stable over time. The recovered precipitate was rich in antimony (57 wt%) along with 0.44 wt% phosphorous and 0.18 wt% tin as the main impurities (Table 3). The recovery rate from solution was high for Sb (92%), S (84%) and Sn (94%), and in the range of 11–40% for the other investigated elements. However, given the low concentration of most elements other than antimony in the leachate, their final concentration in the precipitate remained rather low. Whereas, for FR2 the pH varied when the solution was left to stand over time and the formed precipitate tended to redissolve. Hence, a considerable amount of water needed to be added (i.e. 6.2
VFR1 addition and the pH remained stable over time. The recovered precipitate was rich in antimony (57 wt%) along with 0.44 wt% phosphorous and 0.18 wt% tin as the main impurities (Table 3). The recovery rate from solution was high for Sb (92%), S (84%) and Sn (94%), and in the range of 11–40% for the other investigated elements. However, given the low concentration of most elements other than antimony in the leachate, their final concentration in the precipitate remained rather low. Whereas, for FR2 the pH varied when the solution was left to stand over time and the formed precipitate tended to redissolve. Hence, a considerable amount of water needed to be added (i.e. 6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Vwater
1 Vwater![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VFR2, whereby VFR2 ≫ VFR1). Eventually a small amount of precipitate was recovered that had an oily appearance and redissolved upon washing with n-hexane. Therefore, it was concluded that removal of ethanol is necessary to allow for hydrolysis precipitation of antimony.
VFR2, whereby VFR2 ≫ VFR1). Eventually a small amount of precipitate was recovered that had an oily appearance and redissolved upon washing with n-hexane. Therefore, it was concluded that removal of ethanol is necessary to allow for hydrolysis precipitation of antimony.
| Element | Concentration (mg kg−1) | Recovery (%) |
|---|---|---|
| Al | 76 ± 90 | 20 ± 34 |
| Ca | 26 ± 9 | n.a. |
| Cr | <5 | 23 ± 35 |
| K | 125 ± 160 | n.a. |
| Mg | <10 | 14 ± 51 |
| Na | <10 | 11 ± 35 |
| P | 4400 ± 140 | 40 ± 8 |
| Pb | 113 ± 24 | 27 ± 29 |
| S | 468 ± 32 | 84 ± 9 |
| Sb | 568![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 95 000 ± 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
92 ± 1 |
| Si | 118 ± 54 | n.a. |
| Sn | 1800 ± 0.0 | 94 ± 1 |
| Zn | <10 | 19 ± 33 |
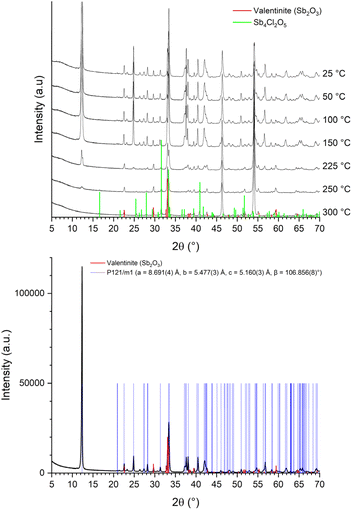
The X-ray diffractogram of the FR1 precipitate indicated the presence of Sb2O3 (valentinite), as well as a phase which could not be assigned to a known crystal phase of the ICDD Powder Diffraction database in the X'Pert PANalytical High Score Plus software. In situ X-ray diffraction upon heating of the sample showed a phase change at 225 °C whereat Sb4Cl2O5 formed, indicating the presence of antimony and chloride in the unknown phase (Fig. 3, top).
Furthermore, the FR1 precipitate contained 0.42 wt% of phosphorous, indicating that 2-ethylhexyl diphenyl phosphate or a derivative thereof could be present. The former has a decomposition temperature of about 240 °C,32 which coincides with the phase change in the temperature range 225–250 °C (Fig. 3, top). Antimony is known to form a myriad of organic complexes, including those with phthalate and phosphate molecules.33,34 To validate if the unknown crystal phase could be an organic antimony complex, 3 hydrolysis precipitation reactions of a 10 g per L SbCl3 solution in 12 M HCl in the presence of either diethyl phthalate, dioctyl phthalate and 2-ethylhexyl diphenyl phosphate were executed. The organic additives were present in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with antimony. Upon addition of water to the said solutions, precipitation took place upon reaching a pH of about 0.32–0.51. Subsequently the recovered precipitates were analysed by powder XRD (Fig. S4 in the ESI†) and elemental analyses (Table 4). In the presence of phthalates crystalline Sb4Cl2O5 precipitated. Whereas, in the presence of 2-ethylhexyl diphenyl phosphate the same unknown crystal phase formed along with Sb4Cl2O5. Noteworthily; preferential orientation of the crystals during powder diffraction led to intense orientation peaks including the (h00) peaks of the Sb4Cl2O5 phase. Additionally, this latter precipitate contained alongside antimony and chloride also phosphorous, carbon and hydrogen, with a derived C
1 molar ratio with antimony. Upon addition of water to the said solutions, precipitation took place upon reaching a pH of about 0.32–0.51. Subsequently the recovered precipitates were analysed by powder XRD (Fig. S4 in the ESI†) and elemental analyses (Table 4). In the presence of phthalates crystalline Sb4Cl2O5 precipitated. Whereas, in the presence of 2-ethylhexyl diphenyl phosphate the same unknown crystal phase formed along with Sb4Cl2O5. Noteworthily; preferential orientation of the crystals during powder diffraction led to intense orientation peaks including the (h00) peaks of the Sb4Cl2O5 phase. Additionally, this latter precipitate contained alongside antimony and chloride also phosphorous, carbon and hydrogen, with a derived C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P molar ratio of 20
P molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is near the molar composition of 2-ethylhexyl diphenyl phosphate (i.e., C20H27O4P) (Table 4). Thus, an organometallic complex was formed between antimony, chloride(s) and 2-ethylhexyl diphenyl phosphate. Refinement of the obtained diffraction pattern leads to a monoclinic crystal system with a primitive unit cell that likely could be assigned to the P121/m1 space group (a = 8.691 Å, b = 5.477 Å, c = 5.160 Å, β = 106.856°) (Fig. S4 bottom in the ESI†). The X-ray diffraction pattern shows a clear orientation of the (h00) peaks of this space group, like the alignment of the Sb4Cl2O5 phase. Future elucidation of the obtained crystal structure through single crystal diffraction is required. When transposing the peak positions of the refined space group to the diffraction pattern of the FR1 precipitate (Fig. 3, bottom), an agreement with peak positions of the unknown phase is observed, confirming that it contains the same mineral as in the artificial precipitate.
1, which is near the molar composition of 2-ethylhexyl diphenyl phosphate (i.e., C20H27O4P) (Table 4). Thus, an organometallic complex was formed between antimony, chloride(s) and 2-ethylhexyl diphenyl phosphate. Refinement of the obtained diffraction pattern leads to a monoclinic crystal system with a primitive unit cell that likely could be assigned to the P121/m1 space group (a = 8.691 Å, b = 5.477 Å, c = 5.160 Å, β = 106.856°) (Fig. S4 bottom in the ESI†). The X-ray diffraction pattern shows a clear orientation of the (h00) peaks of this space group, like the alignment of the Sb4Cl2O5 phase. Future elucidation of the obtained crystal structure through single crystal diffraction is required. When transposing the peak positions of the refined space group to the diffraction pattern of the FR1 precipitate (Fig. 3, bottom), an agreement with peak positions of the unknown phase is observed, confirming that it contains the same mineral as in the artificial precipitate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with antimony
1 molar ratio with antimony
| Organic additive | Diethyl phthalate | Dioctyl phthalate | 2-Ethylhexyl diphenyl phosphate |
|---|---|---|---|
| Sb (mg kg−1) | 645![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 21 000 ± 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
680![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 14 000 ± 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
565![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 7100 000 ± 7100 |
| P (mg kg−1) | <75 | <75 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 0 000 ± 0 |
| Cl (mg kg−1) | 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| C (wt%) | 7.5 | 1.8 | 13.9 |
| H (wt%) | 0.9 | 0.4 | 1.7 |
The above findings demonstrated that co-extraction of organic additives from PVC took place during ethanolic HCl leaching. These coextracted organic compounds need to be removed from solution prior to antimony precipitation through hydrolysis to obtain a pure Sb4Cl2O5 product. Therefore, a process was experimentally tested that considered (i) the need to have a fine-grained PVC sample to increase the antimony extraction yield and (ii) the need to remove co-extracted organic additives from the lixiviant prior to antimony recovery to avoid interferences and contaminations during antimony precipitation. Furthermore, the removal of organic additives by means of cheap and readily available solvents, such as water and ethanol, and under mild reaction conditions has a great potential towards the upgrading of plastic waste to allow for a closed-loop recycling of plastics.2 During the leaching step 50 g of cryogenic-milled PVC (<4 mm) was leached for 4 h at 80 °C in an ethanolic 4 M HCl solution with L/S = 10. The subsequent step involved filtration to separate the PVC residue from the pregnant leach solution. GC MS analyses were performed on the solid PVC leach residue to quantify the extraction of organic additives from the PVC. During ethanolic HCl leaching 76 ± 9% of 2-ethylhexyl diphenyl phosphate was removed and 71 ± 3% of di-n-octyl phenyl phosphate. Whereas butylated hydroxytoluene and 9-octadecanamide were extracted with lower efficiencies of 15% and 30%, respectively. The extraction efficiency of di-iso-nonylphthalate was 51%. After leaching ethanol was distilled off from the pregnant leach solution and the remaining aqueous solution was washed with n-hexane to remove the co-extracted organic additives. Subsequent hydrolysis of antimony in the washed HCl aqueous solution (WAS) yielded a precipitate at about 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Vwater
1 Vwater![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VWAS addition when a pH = 0.282 ± 0.072 was reached. The X-ray diffractogram of the obtained precipitate showed diffraction peaks of the Sb4Cl2O5 phase (space group P21/c (a = 6.24 Å, b = 5.11 Å, c = 13.53 Å, β = 97.20°)) (Fig. 4).
VWAS addition when a pH = 0.282 ± 0.072 was reached. The X-ray diffractogram of the obtained precipitate showed diffraction peaks of the Sb4Cl2O5 phase (space group P21/c (a = 6.24 Å, b = 5.11 Å, c = 13.53 Å, β = 97.20°)) (Fig. 4).
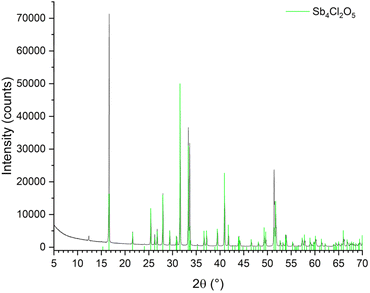 | ||
| Fig. 4 X-ray diffractogram of the obtained precipitated. All observed diffraction peaks can be assigned to the Sb4Cl2O5 mineral phase. | ||
Chemical analyses of the precipitate showed a high purity (≥99.8%) and the recovery rate of antimony from solution was 95% (Table 5). The formation of Sb4Cl2O5 under the studied conditions, i.e., in the presence of a high chloride concentration and at low pH, is in line with previous studies which predicted that the antimony chloride hydrolysis reaction would indeed yield Sb4Cl2O5.35 The same authors showed that the antimony hydrolysis reaction is influenced by the pH and chloride anion concentration and that SbOCl cannot be formed according to theoretic modelling and through experimental verification.35
| Element | Leachate | Precipitate | |
|---|---|---|---|
| Extraction efficiency (%) | Concentration (mg kg−1) | Recovery (%) | |
| Al | 39.1 ± 7.1 | 28.5 ± 7.8 | 4.5 |
| Ca | 66.9 ± 5.1 | 11.3 ± 1.1 | 3.4 |
| Cr | 34 ± 50 | <5 | 3.0 |
| K | 65 ± 40 | 118 ± 45 | n.d. |
| Mg | 60.8 ± 7.0 | <10 | 4.5 |
| Na | 64.7 ± 5.8 | <10 | 3.0 |
| P | 61.6 ± 6.5 | 240 ± 180 | 19 |
| Pb | 63.0 ± 8.4 | 440 ± 240 | 7.9 |
| S | 45.0 ± 5.9 | 65.0 ± 5.7 | n.d. |
| Sb | 92.5 ± 0.7 | 768![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 60 000 ± 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
95 |
| Si | 44 ± 73 | 150 ± 60 | 1.9 |
| Sn | 49 ± 17 | 44 ± 10 | 20 |
| Zn | 57 ± 36 | <10 | 0.1 |
| Cl | n.a. | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 11 000 ± 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n.a. |
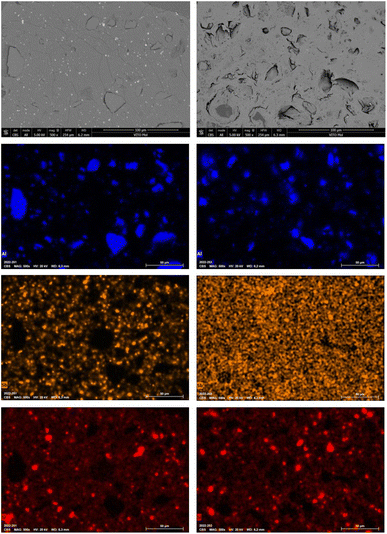
TGA-DSC analyses of the final PVC residue gave similar temperature profiles as those of the starting material (Fig. S5 in the ESI†) and the molar H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio equalled 1.7, indicating that the PVC did not decompose during leaching. Whereas, upon comparison of SEM-EDX analyses of the starting material with those of leached PVC it is shown that small antimony containing grains were removed, whereas larger aluminium containing grains and intermediate sized silicon containing grains remained in the plastic (Fig. 5).
C ratio equalled 1.7, indicating that the PVC did not decompose during leaching. Whereas, upon comparison of SEM-EDX analyses of the starting material with those of leached PVC it is shown that small antimony containing grains were removed, whereas larger aluminium containing grains and intermediate sized silicon containing grains remained in the plastic (Fig. 5).
Conclusions
In this work a process was developed that could extract 94% of antimony from a cryogenically milled soft plastic material through ethanolic HCl leaching at a mild temperature of 80 °C in 4 M HCl at L/S = 10 for 4 h (Fig. 6). Subsequently, ethanol was recovered through distillation and co-extracted organic additives were removed from solution by an n-hexane washing step. From the resulting pregnant leach solution 95% of the antimony was precipitated by a hydrolysis reaction through water addition to form a pure (>99.8%) Sb4Cl2O5 crystal phase with very low amounts of impurities (Al, P, Pb, Si and Sn). The obtained PVC residue after ethanolic HCl leaching was deprived of antimony and the organic additives 2-ethylhexyl diphenyl phosphate and di-isononylphthalate were also extracted for 76% and 51%, respectively. Also, other organic additives were observed to be coextracted with varying efficiencies (i.e., 15% butylated hydroxytoluene, 30% 9-octadecanamide). The extraction efficiency of di-iso-nonylphthalate was 51%. The PVC polymer remained intact during the leaching process.The improved antimony leachability at a milder temperature (i.e., 80 °C) in an ethanolic HCl solution as compared to in aqueous HCl (>130 °C) can be ascribed to a combination of factors. Ethanol–water solutions are known to better diffuse into PVC plastics than water, allowing for the reagent to interact with the additive that is dispersed in the PVC matrix. Also, the solvent medium effect on ionic species has a direct influence on their activity in solution, whereby ethanol addition to a HCl solution is known to promote the formation of metal chloride complexes which will lead to better solvation of such complexes.
Sustainability aspects of the developed process include the lower reaction temperatures compared to aqueous leaching systems, the possibility to cycle ethanol in the process which lowers chemical consumption and wastewater generation and the possibility to recover the critical raw material antimony, as well as organic additives and obtain a cleaned PVC polymer that can be further recycled. Future research is needed to optimise the organic additive extraction and recovery and test the PVC polymer recyclability after the proposed treatment. Additionally, alternative solvent-acid or -base systems can be explored to widen the applicability to other plastic waste materials. The above proposed solvometallurgical co-extraction route provides the potential to remove valuable or unwanted (legacy) additives to enhance the recyclability of waste plastic.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Jeroen Spooren: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, writing – original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author thanks Mr Bo Peeraer and Mr Warre Van Dun for their help with the execution of experiments, Mrs Myrjam Mertens for XRD analyses, Mrs Anne-Marie De Wilde for performing TGA-DSC analyses and Dr Jan Jordens for his help with GC MS measurements. The experiments and data described in this work are subjected of patent application EP22211261.7 filed on December 2, 2022 ‘‘A method for the recycling of post-consumer plastic materials” by J. Spooren.References
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 344, 179–199 CrossRef PubMed.
- S. Ugduler, K. M. Van Geem, M. Roosen, E. I. P. Delbeke and S. De Meester, Waste Manag., 2020, 104, 148–182 CrossRef PubMed.
- A. Sevenster, J. Mater. Cycles Waste Manag., 2012, 14, 281–285 CrossRef.
- DG GROW – European Commission, Study on the Critical Raw Materials for the EU 2023, 2023 Search PubMed.
- European Commission, Study on the EU's List of Critical Raw Materials (2020) – Critical Raw Materials Factsheets, 2020 Search PubMed.
- D. Dupont, S. Arnout, P. T. Jones and K. Binnemans, J. Sustain. Metall., 2016, 2, 79–103 CrossRef.
- A. Alassali, C. Picuno, H. Samara, S. Diedler, S. Fiore and K. Kuchta, Sustainability, 2019, 11, 4021 CrossRef.
- L. Zhan, X. Zhao, Z. Ahmad and Z. Xu, Chemosphere, 2020, 245, 125684 CrossRef PubMed.
- S. Tostar, E. Stenvall, A. Boldizar and M. R. S. Foreman, Waste Manage., 2013, 33, 1478–1482 CrossRef.
- J. Milne, Can. J. Chem., 1975, 53, 888–893 CrossRef.
- J. Poerschmann, B. Weiner, S. Woszidlo, R. Koehler and F. D. Kopinke, Chemosphere, 2015, 119, 682–689 CrossRef.
- E. H. Oelkers, D. M. Sherman, K. V. Ragnarsdottir and C. Collins, Chem. Geol., 1998, 151, 21–27 CrossRef.
- C. Kalidas, G. Hefter and Y. Marcus, Chem. Rev., 2000, 100, 819–852 CrossRef.
- Y. Marcus, Chem. Rev., 2007, 107, 3880–3897 CrossRef CAS PubMed.
- G. Senanayake and D. M. Muir, Metall. Trans. B, 1990, 21, 439–448 CrossRef.
- T. Palden, L. Machiels, M. Regadio and K. Binnemans, ACS Sustain. Chem. Eng., 2021, 9, 5074–5084 CrossRef CAS.
- S. Özdemir and İ. Girgin, Miner. Eng., 1991, 4, 179–184 CrossRef.
- R. K. Jana, D. D. N. Singh and S. K. Roy, Mater. Trans., 1993, 34, 593–598 CrossRef CAS.
- R. K. Jana, D. D. N. Singh and S. K. Roy, Hydrometallurgy, 1995, 38, 289–298 CrossRef CAS.
- R. Nadirov and G. Karamyrzayev, Trans. Soc. Min., Metall., Explor., 2022, 39, 1743–1751 Search PubMed.
- K. Binnemans and P. T. Jones, J. Sustain. Met., 2017, 3, 570–600 CrossRef.
- C. E. Choong, K. T. Wong, S. Y. Yoon, H. Kim, M. Shin, Y.-Y. Chang, J.-K. Yang, S.-H. Kim, B.-H. Jeon, Y. Yoon and M. Jang, J. Clean. Prod., 2021, 278, 123425 CrossRef.
- D. Messadi and J. M. Vergnaud, J. Appl. Polym. Sci., 1982, 27, 3945–3955 CrossRef.
- Q. Yu and A. P. S. Selvadurai, Polym. Degrad. Stab., 2005, 89, 109–124 CrossRef.
- J. M. Cano, M. L. Marin, A. Sanchez and V. Hernandis, J. Chromatogr. A, 2002, 963, 401–409 CrossRef PubMed.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, Taylor & Francis Group, Boca Raton, 2nd edn, 2012 Search PubMed.
- S. C. George and S. Thomas, Prog. Polym. Sci., 2001, 26, 985–1017 CrossRef.
- J. Brandsch, P. Mercea, M. Ruter, V. Tosa and O. Piringer, Food Addit. Contam., 2002, 19(Suppl), 29–41 CrossRef PubMed.
- J. Yu, L. S. Sun, C. Ma, Y. Qiao and H. Yao, Waste Manage., 2016, 48, 300–314 CrossRef.
- W. Zhang, H. Wu, N. Zhou, X. Cai, Y. Zhang, H. Hu, Z. Feng, Z. Huang and J. Liang, J. Inorg. Organomet. Polym. Mater., 2021, 31, 3842–3856 CrossRef.
- G. Senanayake and D. M. Miur, Metall. Trans. B, 1990, 21B, 439–448 CrossRef.
- D. N. Brook, M. J. Crookes, P. Quarterman and J. Burns, Environmental Risk Evaluation Report: 2-Ethylhexyl Diphenyl Phosphate (CAS No. 1241-94-7), Environmental Agency, 2009 Search PubMed.
- V. V. Sharutin, A. I. Poddel'sky and O. K. Sharutina, Russ. J. Coord. Chem., 2020, 46, 663–728 CrossRef CAS.
- V. V. Sharutin, O. K. Sharutina and V. S. Senchurin, Russ. J. Inorg. Chem., 2014, 59, 947–950 CrossRef CAS.
- Q.-H. Tian, Y.-T. Xin, L. Yang, X.-H. Wang and X.-Y. Guo, Trans. Nonferrous Metals Soc. China, 2016, 26, 2746–2753 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (File type, PDF) containing 5 figures showing: the Gibbs free energy as function of temperature of the antimony trioxide dissolution reaction in HCl, the PVC starting material, a photo of FR1, XRD diffractograms of hydrolysis precipitates of SbCl3 in the presence of organic molecules, and a TGA-DSC diagram of the PVC after ethanolic HCl leaching; and 1 table showing the measured pH in the aqueous and ethanolic HCl lixiviants after leaching. See DOI: https://doi.org/10.1039/d4ra07240e |
| This journal is © The Royal Society of Chemistry 2025 |