Open Access Article
Open Access ArticleSelenium-based nanomaterials: green and conventional synthesis methods, applications, and advances in dye degradation
Nilmadhab Roy
a,
Nivedya T.
a,
Priyankar Paira
 *a and
Rinku Chakrabarty
*a and
Rinku Chakrabarty
 *b
*b
aDepartment of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore, Tamil Nadu, India. E-mail: priyankar.paira@vit.ac.in
bDepartment of Chemistry, Alipurduar University, Alipurduar, West Bengal, India. E-mail: rckncs@gmail.com
First published on 29th January 2025
Abstract
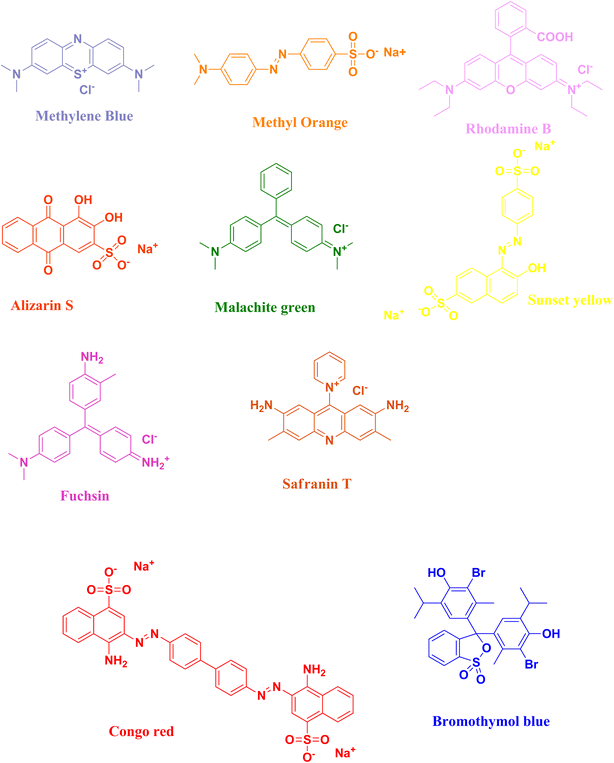
The rapidly expanding industrialization and global increase in economic activities have drawn attention to the concerning accumulation of waste. The textile industry plays a significant role in environmental pollution, especially in and water pollution. Harmful dyes used during the fabrication process are mixed with water bodies through sewage or wastewater ejected from industrial factories. These toxic dyes are not only applied in textile industries but also used in other industries like pharmaceutical companies and rubber manufacturing. Therefore, scientists have adopted alternative techniques for the degradation of organic dyes because of eliminating the drawbacks from the traditionally used techniques. Catalytic degradation of organic dyes with the help of a safe and easy nanocatalyst is one of the best alternatives. Accordingly, the use of biomaterials or waste materials offers an easy, cost-effective and eco-friendly approach for the synthesis of such nanocatalysts. Several nanocatalysts have been used for the degradation of dyes present in industrial wastewater. The well-known semi-conductor selenium has several important properties, viz., optoelectronic, photovoltaic, thermoconductivity, and anisotropy, and has drawn significant research attention for its catalytic application in dye degradation. Considering all these points, selenium nanoparticles synthesized via green techniques provide the best possible alternative catalyst for the degradation of organic dyes in industrial wastewater. The current review covers various aspects of the biosynthesis of selenium nanoparticles; their application as a catalyst for the degradation of harmful organic dyes, viz., methylene blue, methyl orange, rhodamine B, alizarin S, malachite green, sunset yellow, fuchsin, safranin T, Congo red, and bromothymol blue; and their mechanism for the degradation process. This review will also shed light on the importance of using green chemistry towards the synthesis of selenium nanoparticles and different biosynthesis procedures and explores all aspects of the interesting catalytic activity towards the dye degradation mechanism. Hence this article will be beneficial to both industrialists and acdemicians bridging the gap between industrial and academic sceintists.
1. Introduction
The significant increase in industrialization, along with the growing population and environmental pollution, has become a major global concern.1,2 Globally, the prevention and reduction of pollution have become major challenges. A considerable amount of wastewater is discharged into water bodies every day. The dyes present within the wastewater generated by the effluents of the various industries show cytotoxic, neurogenic, teratogenic and carcinogenic effects.3,4 Thus, the treatment of water through different techniques such as sedimentation, reverse osmosis, anaerobic treatment, membrane filtration, adsorption, filtration, ionization, ion exchange, and degradation using bacteria, fungi, etc. is mandatory to eliminate these harmful organic dyes and other waste materials present in the industrial effluents.5,6 All these physical, chemical and biological methods for the removal of dyes are generally costly, and the concomitant production of toxic sludge is an another concern. The effectiveness of these treatments is sometimes questionable because these stable organic synthetic dyes are very resilient towards the biodegradation process that occurs naturally with the help of microorganisms.7 Synthetic organic dyes present in wastewater have complex chemical structures, which makes them resistant towards removal techniques generally applied by physical and chemical methods.1 Thus, metallic nanocatalysts are considered as effective candidates for catalysing the dye degradation.8Nanoscience is a branch of science that deals with materials at the nanoscale. Metal nanoparticles have gained considerable attention due their diversified applications in the field of research catalysis, sensors, medicine, optics, electronics, etc.9,10 They are also effective in toxic gas sensing and the removal of heavy metals.11–14 Different varieties of metal nanoparticles, e.g., copper, zinc, silver, gold, iron, platinum, palladium, titanium, magnesium, etc., have been synthesized, and their biological applications, e.g., anticancer, antifungal and antimicrobial, have been studied extensively.15 The presence of a large surface-to-volume ratio is a special characteristic feature of the metallic nanoparticles, which makes them a strong candidate for application in the optical and chemical fields.16 Two methods are generally employed for the synthesis of nanomaterials: top-down method and the bottom-up approach. The top-down approach is mainly applicable to physical techniques like ball-milling method, vapour deposition and laser ablation. By comparison, the bottom-up approach includes chemical techniques like pyrolysis, sol–gel method, microwave irradiation, solvothermal method, etc., and biological techniques like synthesis using plant extracts, fungi, microorganisms, etc.17 The use of chemical and physical methods is less common nowadays because of their harmful side effects. Rather, the biological synthesis procedure is more cost-effective, safe, simple and environmentally friendly.18 In the green synthesis approach, emphasis is given on the reduction or decreased use of chemicals, especially harmful ones. Furthermore, the production of less toxic products is taken into account, along with the twelve principles of green chemistry.
Natural organic compounds, including various types of polyphenols, alkaloid, terpenoids, etc., present in plants act as both reducing and capping agents during the synthesis of nanomaterials using the green technique. Different parts of plants, fungi, and microorganisms are used for this purpose. The biomolecules present in the plant extract get adsorbed on the surface of the produced nanoparticles, reducing the possible toxicity of the nanomaterials.19 In many cases, more stable and less toxic nanomaterials are generated using the green technique compared to other methods.20
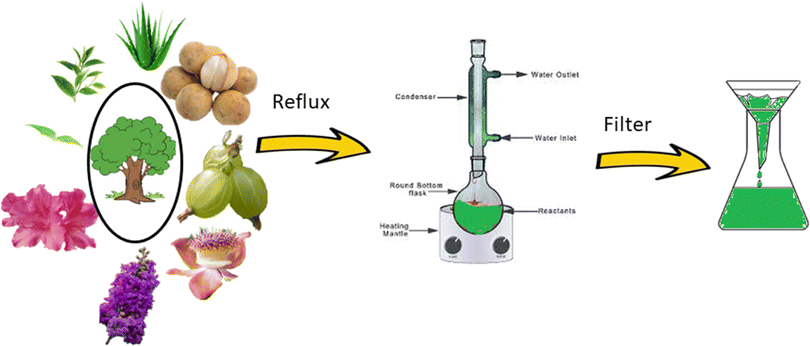
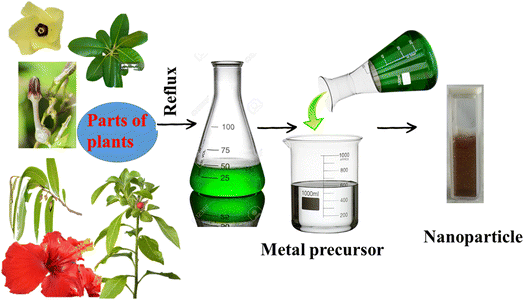
Based on the green technique, the use of fungi or plant extract has recently become quite popular. The process described in Fig. 1 shows the step in which parts of the plants are used without further modification. In this process, parts of a particular plant are collected, washed properly, chopped, air-dried, and weighed. A water extraction is performed in a round-bottom flask under refluxing condition, followed by cooling and filtration. The extract is then used for the synthesis, from the corresponding metal salt as precursor (Fig. 2).
Selenium is a trace micronutrient and an essential element for most living organisms. The presence of selenium in lower concentrations within the human body may affect the immune system. Selenium is found to be present in certain functional proteins, which are involved in many important activities like promoting anticancer activities within the body and enhancing the immune system. Se increases the antimicrobial effects of the body by reacting with the peroxides present in the membrane and producing oxygen-free radicals.21 Se is a good semiconductor, possessing superior thermoconductivity, high photoconductivity, and anisotropy. Selenium nanoparticles have important applications as catalysts in organic synthesis and semiconducting materials.22 It has widespread application in areas utilizing optoelectronic and photovoltaic properties, owing to the photocatalytic nature of selenium nanoparticles. Selenium is known to possess comparable size and similar properties to other nonmetals like phosphorous. It has excellent stability in air and moisture. It has strong metal coordination properties. Its unique properties, like photoconductivity and semiconducting nature, make it different among other chalcogens, also known as the oxygen family. Its melting point and boiling point do not follow the trend of this family.23 Due to its distinct physical and chemical properties, there has been a remarkable upsurge of interest in the biogenic synthesis of selenium nanoparticles and their widespread application.24 During the biogenic synthesis of selenium nanoparticles, Se(IV) is converted to Se(0). Selenium nanoparticles produced using the green technique are found to possess important biological properties, like anti-microbial, anticancer, anti-fungal properties. It acts as a nutritional supplement, and is extensively used in drug delivery.25,26 It has also been known to exhibit superior biodegradability and biocompatibility compared to other metal nanoparticles synthesized using biomaterials.27 Se nanoparticles have strong affinity towards certain metals. This property has been utilized for the adsorption of heavy pollutant metals like Cd(II), Cu(II), Hg(0), Zn(II), etc. Amorphous Se and crystalline Se possess a bandgap of ∼1.8 eV, which makes it an ideal photocatalyst for the degradation of organic pollutant dyes.
Amidst previous attempts21 to only summarize the catalytic applications of biosynthesized selenium nanoparticles, a review of the synthesis, detailed mechanism, apllications and all other related topics is required under one umbrella is required to organize myriad information regarding selenium nanoparticle, which will be helpful for researchers for further studies. Emphasis has been given on the synthetic procedure, including the source of the metal nanoparticle in the current review article.
In this review, the degradation of organic pollutant dyes [methylene blue, methyl orange, rhodamine B, alizarin S, malachite green, sunset yellow, fuchsin, safranin T, Congo red, bromothymol blue] (Fig. 3) using biosynthesized selenium nanoparticles will be discussed with their plausible mechanism of action (Tables 1–9). Due to the intense dark colour of the organic dyes, researchers have studied the degradation process by keeping track of their optical properties, which can easily be studied via the absorption properties of the organic dyes with the help of UV-Vis spectroscopy. The surface plasmon resonance vibration of selenium nanoparticles appears in the 250–500 nm range. The λmax value of selenium nanoparticles appears at ∼270 nm, as reported by various research groups.
2. Degradation of organic dyes
2.1 Methylene blue
Methylene blue is a synthetic organic blue-coloured dye, and is highly water-soluble. Methylene blue is used as a dyeing agent in industries, like the paper industry, cotton industry, etc., as well as in medication. The dye, methylthioninium chloride or methylene blue is used as a chemotherapeutic agent, and in the treatment of shock states. It is used for treating methemoglobinemia, staining lymph nodes, treating vasoplegia, and anaphylaxis. It is also used as a disinfectant, biological stain, optical probe in the biophysical systems, pH and redox indicator, and for the determination of anionic surfactants. Methylene blue is a highly pollutant dye having carcinogenic and mutagenic properties. Exposure to excess amounts of methylene blue causes serious damage to the nervous system. Furthermore, it causes irritation to the eyes, respiratory infections, affects the digestive system, and can cause vomiting and nausea.28,29 Therefore, methylene blue is a serious threat to living organisms. Its removal from aqueous media is necessary.The reduction of methylene blue is generally carried out using either nanoparticles as catalyst using sodium borohydride as a reducing agent or using only metallic nanoparticles as a photocatalyst (Table 1).
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | Yeast | Na2SeO4 | 170–240 | Rod | Visible | — | 31 |
| 2 | W. somnifera | H2SeO3 | 45–90 | Spherical | Sunlight | — | 32 |
| 3 | Sodium formaldehyde sulfoxylate | SeO2 | 100–200 | Rod | Visible | H2O2 | 33 |
| 4 | F. benghalensis | Se powder, sodium sulphide | 45–95 | Spherical | UV | — | 34 |
| 5 | M. purpureus | Na2SeO3 | 46.58 | Round | Sunlight | — | 35 |
| 6 | C. bulbosa tuber | H2SeO3 | 55.9 | Spherical | Halogen lamp | — | 36 |
| 7 | G. wightii, AA | Na2SeO3 | 80 | Spherical | Sunlight | — | 37 |
| 8 | A. paradoxum | Na2SeO3 | 37.5 | Semi-spherical | — | NaBH4 | 38 |
| 9 | H. esculentus | Na2SeO3 | 62 | Spherical | — | NaBH4 | 39 |
| 10 | H. rosa-sinensis | NiCl3, Se powder | 26 | Spherical | Visible | — | 40 |
| 11 | Nyctanthes arbor-tristis L. | Na2SeO3 | 44.57 | Spherical | — | — | 41 |
The plausible mechanism of the reduction of methylene blue using sodium borohydride as a reducing agent and selenium nanoparticles as catalyst is delineated in Fig. 4. Methylene blue dye shows its absorption maximum at ∼660 nm, which is slowly decreased with time in the presence of the reducing agent sodium borohydride. However, in the presence of the selenium nanoparticle catalyst, the time for the reduction of methylene blue decreases and the blue-coloured dye is converted into its colourless leuco form. Here, the synthesized selenium nanoparticle acts as in electron relay during the whole reduction process. The selenium nanoparticle generally acts as an electron donor and the dye molecule acts as an electron acceptor.
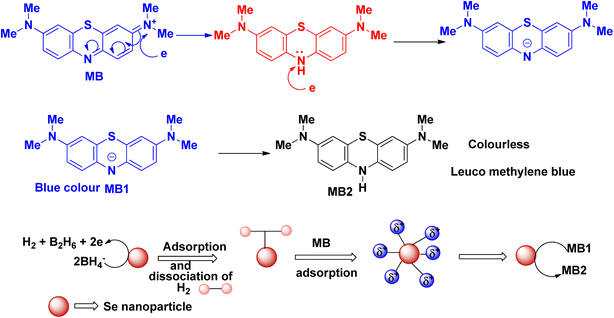 | ||
| Fig. 4 Schematic representation of the plausible mechanism for the reduction of methylene blue dye in the presence of selenium nanoparticles. | ||
The detailed mechanism of the photocatalytic degradation of methylene blue has been established by Kalaycıoğlu et al.30 for the CeO2 nanoparticle. Metal nanoparticles (NPs) absorb the photons and reach the excited state. During this process, the photoexcited electron in the conduction band and photogenerated hole in the valence band are produced (eqn (1)). Methylene blue (MB) is excited to directly produce the reactive intermediate, and is then oxidized by photogenerated holes (eqn (2)).
| NP + hν → NP* + e* (photoexcited electron) + hν (photogenerated hole) | (1) |
| hν + MB → MB+ → oxidation of MB | (2) |
Indirectly, it has been shown that the oxygen and water are adsorbed on the surface of the nanoparticle used as catalyst. Then, they react with the photoexcited electron and photogenerated hole to produce reactive oxygen species (eqn (3) and (4)). Methylene blue, in the presence of a hydroxyl radical and superoxide ion produced in situ, is converted to carbon dioxide and a water molecule (eqn (5)).
| O2 + e* → ˙O2− | (3) |
| H2O + hν → ˙OH + H+ | (4) |
| MB → CO2 + H2O | (5) |
The blue colour of methylene blue originates from the π electron transfer from the N and S atoms. The λmax of methylene blue appears due to this transition. In the structure of methylene blue, the positively charged S atom is attacked by the electrons, followed by the opening of the aromatic ring (Fig. 5). During this process, after the reduction process, the electron transfer between the S and N atoms is diminished and the whole solution becomes colourless.
Goud et al. synthesized rod-shaped selenium nanoparticles using fermented yeast broth from agricultural waste, and characterized the product by different spectroscopic techniques. Yeast fermented broth was derived from agricultural waste. This fermented yeast has been used as a reducing agent for synthesis purposes. Addition of this broth to the aqueous solution of sodium selenite yielded stable selenium nanoparticles. The fabricated nanoparticles had a size of 170–240 nm. For the decomposition study, 50 mg of biosynthesized catalyst were incorporated into 50 μL of the dye solution, methylene blue, and irradiated under visible light for 3 h. For this purpose, they set up a visible annular-type photoreactor with a tungsten lamp of 300 W. They also studied the use of their nanoparticles for biological applications, and showed that their catalyst is effective as an anticancer and antibacterial agent.31
Venkatesan and co-workers32 have synthesized amorphous and spherical-shaped selenium nanoparticles having a diameter in the range of 45–90 nm using Withania somnifera extract. Selenous acid was used as the starting material. Suitably washed and chopped leaves were boiled in a beaker with distilled water, followed by filtration and centrifugation to obtain the extract, which was then added to a sodium selenite solution and stirred at room temperature for the completion of the reaction. The nascent nanoparticles were centrifuged and washed with water and acetone, then suspended in PBS with the help of ultrasonication at neutral pH, and centrifuged again to obtain red pellets of selenium nanoparticles. The band gap of the nanoparticles was determined, and shown to be higher than that of the commercial selenium powder and bulk selenium. Decomposition of methylene blue was studied under sunlight after 30 minutes. The synthesized selenium nanoparticles also possess antioxidant, antimicrobial and antiproliferative properties.
The green approach has been adopted by Xia et al.33 to synthesize trigonometric selenium nanowires with the diameter ranging between 100–200 nm, and their potential application as a catalyst for the reduction of methylene blue dye was studied. A Xe lamp of 300 W was used for the visible light source. Selenium dioxide was used as the starting material, and sodium formaldehyde sulfoxylate was used as the reducing agent for the synthesis. The bandgap determined for the synthesized selenium nanoparticle was slightly lower than that of the bulk selenium. Degradation of methylene blue was carried out with the oxidation process using H2O2 as an oxidizing agent, and it took 3 h to nearly complete the degradation process.
Spherical-shaped polycrystalline selenium nanoparticles have been synthesized by Tripathi and co-workers34 using Ficus benghalensis leaf extract from selenium metal powder and sodium sulphide having particle sizes in the range of 45–95 nm. The leaves were chopped and boiled in distilled water, followed by filtration through a muslin cloth to obtain the bioextract. Selenium sulphide was synthesized separately from the reaction of selenium and sodium sulphide under heating condition. Further reaction of the plant extract with sodium selenite solution afforded the desired nanoparticles, which was indicated by the dark reddish orange colour. The bandgap calculated for their synthesized nanoparticles is higher than that of the commercial Se powder, which affects their quantum confinement and the effect of the capping agents present on the surface of the metal nanoparticle. The degradation of methylene blue under UV light irradiation was studied, achieving ∼58% degradation after 40 minutes. The degradation process follows first-order kinetics with the calculated rate constant of 0.02162 s−1.
Another research group synthesized highly stable single-phase crystalline selenium nanoparticles through the green technique using Monascus purpureus ATCC16436 fungi extract from sodium selenite as a starting material at room temperature with a zeta potential value of 24.01 mV. The application of the biogenic synthesized selenium nanoparticles towards the degradation of organic dye, methylene blue, was successfully carried out with sunlight at the catalyst concentration of 4 mg mL−1. This catalyst also possesses antioxidant, antimicrobial and anticancer activities.35
Ceropegia bulbosa tuber extract was used by Cittrarasu et al. with selenous acid to synthesize uniformly spherical selenium nanoparticles, which was fully characterized using PXRD, FE-SEM, EDX, HRTEM, DLS, etc. The C. bulbosa tuber extract was initially obtained from distilled water, diluted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio, and used for the synthesis. The diluted extract was added to a 40 mM solution of selenous acid solution, and was stirred for another 24 h. After completion of the reaction, which is indicated by the change of colour to a brilliant ruby red, the product was further purified by washing with distilled water several times after centrifugation. The obtained solid was dried in a refrigerator for further studies. The synthesized selenium nanoparticles can degrade 98% of methylene blue dye through photocatalysis in 80 min. The synthesized nanoparticles have been proven to exhibit antimicrobial, antimosquitocidal and cytotoxic activity.36
9 ratio, and used for the synthesis. The diluted extract was added to a 40 mM solution of selenous acid solution, and was stirred for another 24 h. After completion of the reaction, which is indicated by the change of colour to a brilliant ruby red, the product was further purified by washing with distilled water several times after centrifugation. The obtained solid was dried in a refrigerator for further studies. The synthesized selenium nanoparticles can degrade 98% of methylene blue dye through photocatalysis in 80 min. The synthesized nanoparticles have been proven to exhibit antimicrobial, antimosquitocidal and cytotoxic activity.36
Goniothalamus wightii extract was used by Santhosh and co-workers37 to synthesize selenium nanoparticles through green technique. Sodium selenite was used as the precursor of selenium metal nanoparticles of average size distribution at 80 nm. Leaves of the plants were collected from Kalakad Mundanthurai Tiger Reserve Forest, Tirunelveli, India, and were washed, dried and ground to obtain the powder form of the leaves. The powder was then soaked in distilled water for half an hour, and filtered using a Whatman-1 filter paper for the plant extract. This plant extract was then added to the solution of sodium selenite, followed by the addition of ascorbic acid for 24 h to obtain an orange-coloured solution, which was then centrifuged and dried at 60 °C in a hot air oven to obtain selenium nanoparticles in powder form. The produced nanoparticles were characterized through different spectroscopic techniques, viz., PXRD, SEM, EDX, TEM, etc. Sunlight was then used for the photocatalytic degradation of methylene blue in 150 minutes. This synthesized nanoparticle possesses anticancer and radical scavenging properties.
Allium paradoxum aqueous extract was used by Alizadeh and colleagues38 very recently for the green synthesis of semi-spherical shaped selenium nanoparticles with an average size of 37.5 nm. Sodium selenite was used as the precursor for selenium metal. Common spectroscopic techniques like PXRD, SEM, EDX, TEM were applied for the characterization of the synthesized nanoparticles. Degradation of methylene blue dye using sodium borohydride as a reducing agent was carried out over a reasonable time period. It followed the pseudo-first order kinetics, and the rate constant values were calculated. It also possesses antibacterial, antioxidant, anticancer, antifungal, and iron-chelating properties.
Hibiscus esculentus L. extract has been proven by Ebrahimzadeh et al.39 to be effective for the biosynthesis of hexagonal crystals of spherical-shaped selenium nanoparticles having a size of 62 nm. Sun dried plant pieces of Hibiscus esculentus L. were added to distilled water, and treated with heat and sonication for the preparation of the extract. The obtained filtrate was added to the sodium selenite solution, which was stirred under heating at ∼50 °C for two days until the solution changed to a red colour. It was then centrifuged and underwent several washing with distilled water and methanol, followed by drying in a hot air oven at ∼60 °C to afford the selenium nanoparticles. Colloidal selenium nanoparticles were used for the degradation of methylene blue using sodium borohydride as a reducing agent in 21 minutes. This degradation process follows pseudo first order kinetics, and the rate constant value calculated for this process was found to be 0.1023 min−1.
Velayutham et al.40 used Hibiscus rosa-sinensis extract for the biosynthesis of spherical shaped selenium based nanoparticle having particle size of 24 nm using the hydrothermal process. Dried chopped leaves of Hibiscus rosa-sinensis was boiled for 2 h to get the extract, which was then added to an equimolar mixture of selenium powder and nickel chloride. The whole reaction mixture was then stirred and put into a thermal reactor at 120 °C for 24 h, followed by centrifugation and calcination at 500 °C for 2 h. The collected precipitate was washed and dried to afford NiSe nanoparticles. The produced nanoparticle has a bandgap of 1.74 eV, which is helpful for the generation of the photoexcited electron in the conduction band and photogenerated hole. The synthesized nanoparticle is active towards the degradation of methylene blue. It also exhibits good antibacterial properties against the well-known bacteria, Staphylococcus aureus and Escherichia coli.
Satpathy and coworkers have reported the synthesis of selenium nanoparticles using the extract of leaves of a plant having medicinal value, Nyctanthes arbor-tristis L., which is also known as tree of sorrow. Washed and dried leaves were stirred, and the aqueous solution was heated at 60 °C for half an hour, followed by centrifugation and filtration using a Whatman filter paper. The extract was then added to the solution of sodium selenite, and incubated for 24 h at 37 °C. The obtained solid was centrifuged, washed and dried at 80 °C. The average size of the nanoparticle was 44.57 nm with a spherical shape. These synthesized nanoparticles possess antioxidant, antimicrobial and anti-biofilm activities. A cytotoxicity study showed that the synthesized selenium nanoparticle possesses minimal toxicity. It was found to be effective in the photocatalytic degradation of the environmentally toxic dye methylene blue in 80 minutes.41
2.2 Methyl orange
Methyl orange is a sulphonated anionic azo dye. It is a yellow powder with the melting point of >300 °C. It is highly soluble in warm water, and sparingly soluble in water at ambient temperature. It is also an acid–base indicator. It shows a red colour in acidic medium, and is orange in colour in basic medium. The high chemical stability of methyl orange dye makes it less biodegradable, and thereby increases its toxicity to a certain extent.42Methyl orange shows its absorption maxima at ∼460 nm. The absorption maxima decrease during the photocatalytic degradation process using selenium nanoparticles as the catalyst. The mechanism for the photocatalytic degradation (Fig. 6) is similar to that described earlier in the case of methylene blue.
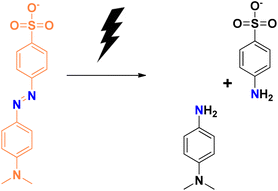
Degradation of methyl orange in the presence of the reducing agent sodium borohydride using selenium nanoparticles follows similar mechanistic pathways, and is shown in Fig. 7. Methyl orange is reduced to its corresponding colourless form. The addition of hydrogen atoms occurs at the azo group, which then eventually gets dissociated into small molecules in some cases.
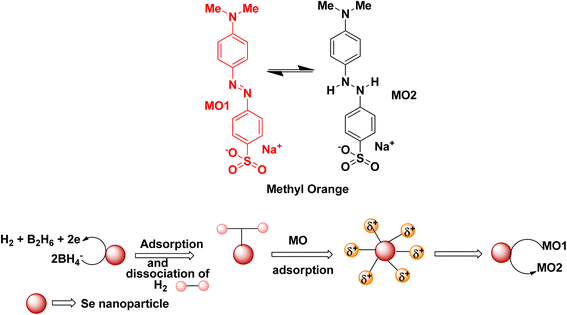 | ||
| Fig. 7 Schematic representation of the plausible mechanism for the reduction of methyl orange dye in the presence of selenium nanoparticles. | ||
Ahluwalia and co-workers synthesized selenium nanoparticles using Bacillus sp., taking sodium selenite as the starting material. Here, selenium nanoparticles were used as the cocatalyst. It improved the activity of ZnS through photocatalytic pathways. A mixture of selenium solution and Bacillus sp. was incubated on a rotary shaker for 48 h at 120 rpm. Centrifugation was followed by repeated washing with ethanol, yielding selenium nanoparticles, which were then used for wet impregnation method with ZnS nanoparticles. The reaction mixture was stirred overnight, followed by calcination to yield the final nanocomposite. It was shown to be effective for the degradation of methyl orange dye. The selenium nanocomposite synthesized through green technique, applying calcination at ∼200 °C, has been fully characterized through PXRD, SEM, TEM, etc., and showed an average particle size of ∼40–80 nm. The degradation of methyl orange dye followed pseudo-first order kinetics and the calculated rate constant value was 0.00842 min−1 (Table 2).43
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | Bacillus sp. | Na2SeO3, ZnNO3, L-cysteine | 40–80 | Cluster form | UV | Calcination | 43 |
2.3 Rhodamine B
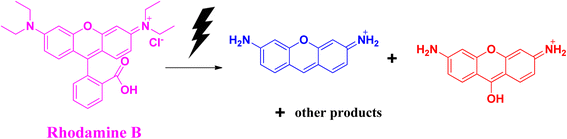
Rhodamine B is considered to be one of the most toxic dyes present in wastewater from textile industries. It is highly stable and nonbiodegradable in nature. In the UV-vis spectra, the λmax appears at ∼550 nm. This cationic dye is highly soluble in water. It is also highly carcinogenic in nature, and has mutagenic and neurotoxic properties. Excess exposure to rhodamine B dye can cause respiratory infections, vision problem, and hemolysis. This dye can cause liver and kidney diseases. Death of planktons has resulted from the presence of this toxic dye in water bodies. Due to the mixing of rhodamine B dye in the water bodies, pollution occurs that affects light transmission. It prevents the penetration of sunlight into the water bodies, thereby reducing the dissolved oxygen level in the water bodies and affecting the aquatic life. Like other dyes, rhodamine B dye is also very stable and resistant to other biological decomposition processes.44,45The photocatalytic degradation of rhodamine B (Fig. 8) and the degradation of rhodamine B using sodium borohydride as a reducing agent with the help of selenium nanoparticles (Fig. 9) follow similar mechanistic pathways as that shown in the case of methylene blue, where it is converted into its colourless form (Table 3).
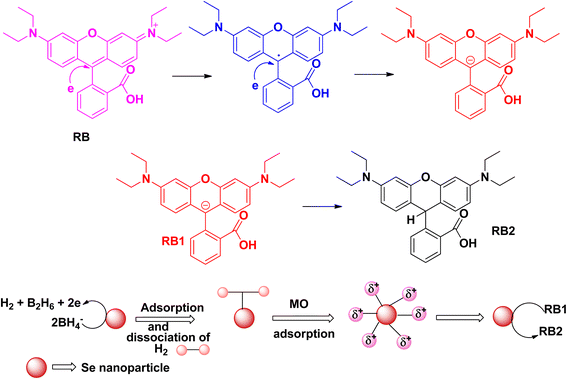 | ||
| Fig. 9 Schematic representation of the plausible mechanism for the reduction of rhodamine B dye in the presence of selenium nanoparticles. | ||
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | Sulfite reductase in Lysinibacillus sp. ZYM-1 | Na2SeO3 | 100–200 | Cubic and spherical | Visible | H2O2 | 46 |
| 2 | Chitosan Justicia adhatoda | Na2SeO3 | 87–152 | — | — | — | 47 |
| 3 | GA, AA | Na2SeO3 | 20–24 | — | UV-A | — | 24 |
| 4 | Chitosan, AA | Na2SeO3 | 20–27 | Rod | UV | — | 48 |
Che et al. used sulfite reductase enzyme in the Lysinibacillus sp. ZYM-1 for the synthesis of selenium nanoparticles. Sodium selenite has been used by this research group to afford cubic to spherical-shaped nanoparticles having the size of ∼100–200 nm. The addition of the marine sediment slurry into sodium selenite, and incubation for 7 days at ∼30 °C yielded a red-coloured product. It was found that upon increasing the concentration of sodium selenite, the morphology of the synthesized selenium nanoparticles drastically changed from a cubic to spherical shape. Photocatalytic degradation of rhodamine B dye was studied under visible light using the biogenic synthesized selenium nanoparticles in the presence of the oxidizing agent H2O2 after 300 min. The rate constants determined for the spherical and triangular nanoparticles were determined, and the calculated values were 0.0048 min−1 and 0.0011 min−1, respectively. A plausible mechanism for the degradation process has been determined using LC-HRMS.46
The Britto research group prepared selenium nanoparticles by green technique. They utilized Justicia adhatoda leaf extract from the sodium selenite precursor. The leaves of the plant were ground using a mortar and pestle by adding water, and the supernatant liquid obtained by centrifugation was added to the sodium selenite solution for the preparation of the selenium nanoparticles, which was then allowed to react for an additional 24 h and incubated under dark conditions. They also prepared chitosan–selenium nanoparticles. Chitosan in acetic acid solution was added to the aqueous solution of sodium selenite, followed by the addition of ascorbic acid to obtain chitosan–selenium nanoparticles. The Langmuir isotherm model was used to study the degradation process of rhodamine B dye using chitosan–selenium nanoparticles. The process was studied by varying the different conditions, like the absorbent material, temperature, dye concentration, pH, etc. The adsorption process was utilized in this research study. The adsorbent limit was reported as 34.5 mg g−1 for the rhodamine B dye.47
Velayati and coworkers produced selenium nanoparticles from gum Arabic. Sodium selenite was used as the precursor material of the selenium nanoparticle. An aqueous solution of sodium selenite and gum Arabic mixture was heated at ∼80 °C for 24 h to obtain the desired selenium nanoparticles, which was obtained after centrifugation and subsequent washing. The used biomaterial can act as both reducing and capping agent. Various spectroscopic techniques have been used to characterize the synthesized hexagonal selenium nanoparticles with an average size ∼20–24 nm, including UV-vis, FTIR, PXRD, EDX, FESEM, and TEM. A photocatalytic study was carried out under UV-A light. It was shown that 85% degradation of rhodamine B was completed after 135 min. The reaction kinetics were assumed to be pseudo-first order, and the value of the rate constant was calculated accordingly.24
The same research group used the biopolymer, chitosan, for the synthesis of highly crystalline hexagonal rod-shaped selenium nanoparticles with a size of ∼20–27 nm. The photocatalytic degradation of rhodamine B was achieved under UV light after 120 min. The biosynthesized selenium nanoparticle is proven to exhibit potential anticancer activity on the cancer cell lines tested, viz., HT-29 and CT-26.48
2.4 Alizarin red
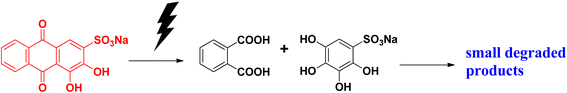
Alizarin S (or alizarin red S) or 1,2-dihydroxyanthraquinone-3-sulphonate is a red-coloured dye. It has been widely used as a synthetic dye for dying textile fabrics. Alizarin red dye was first extracted from the roots of the madder genus plant. It is the first synthesized dye. This dye is used as a staining agent in biological research. Studies have shown that it has toxic effects if it comes in contact with the skin or is swallowed. It is carcinogenic and mutagenic in nature. It affects the respiratory system and can damage eyes. Alizarin red S absorbs at the λmax of 271 nm.Alizarin red S can be degraded under a photocatalytic pathway to form a dicarboxylic acid and dihydroxy compound, which are then further dehydrated and form small degraded products that are eco-friendly in nature (Fig. 10).
Haewon et al. synthesized trigonal-shaped selenium nanoparticles from the leaf extract of Tectona grandis with an average size of ∼45 nm. The leaves were washed, air-dried, smashed, and the extract was prepared using distilled water. Then, the sodium selenite solution was added to the leaf extract solution, and kept in a hydrothermal oven for 9 h at 180 °C. After centrifugation, the obtained solid product was subjected to calcination for 1 h at 300 °C. Their green synthesized selenium nanoparticle is proven to be active against the toxic industrial dye alizarin red. A recyclability study proved that the synthesized nanomaterial is active after five consecutive cycles. The biosynthesized selenium nanoparticle is active against alizarin red dye; it takes 90 minutes to achieve 87% degradation. This nanoparticle is found to be active as a catalyst for use in the photocatalytic degradation of dyes, like crystal violet, reactive black and rhodamine B. The research group used sunlight for the photocatalytic degradation technique (Table 4).49
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | Tectona grandis | Na2SeO3 | 45 | Trigonal | Sunlight | — | 49 |
2.5 Malachite green
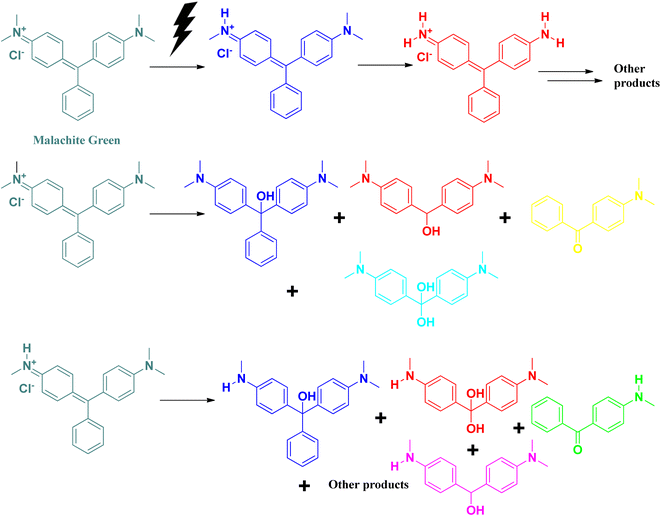
A triphenylmethane-based cationic dye, malachite green is widely used worldwide in the aquaculture industry for the treatment of fungal and protozoan infections among aquatic animals. Its excess use and high solubility in water contribute to its water pollutant nature. Like rhodamine B, malachite green also affects the dissolved oxygen level in water bodies by preventing the transmission of sunlight into the water surface.50,51 It affects the photosynthesis process of algae present in the aquatic system. It absorbs at 618 nm, which can be detected by UV-vis spectroscopic technique. Excess exposure to malachite green dye can damage the nervous system, cause eye irritation, hamper breathing, and affect the kidney and brain. It is carcinogenic in nature.50,51Photocatalytic degradation of malachite green follows similar pathways, as in the case of methylene blue (Fig. 11).
Aspergillus terreus pure cells were used for the synthesis of spherical-shaped selenium nanoparticles having a size of ∼10–100 nm by Saied and coworkers, where Aspergillus terreus acts as a bioreactor. Calcination at 200 °C for 24 h furnished the powdered selenium nanoparticles. The studies were successfully carried out for the degradation of 89% of rhodamine B for 240 min under sunlight. It also displayed weak antifungal effects, but strong antibacterial properties (Table 5).52
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | Aspergillus terreus | Na2SeO3 | 10–100 | Spherical | Sunlight | — | 52 |
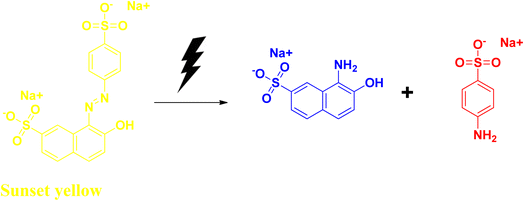
2.6 Sunset yellow
Sunset yellow is an azo dye that is widely used in confectionaries, including candies, some dairy products, soft drinks, chocolates, etc. It has some adverse side effects like allergies. It affects the respiratory system, liver, kidney, and digestive system, and has cytotoxic effects. Due to its structural stability, it is resistant to biodegradation. At acidic pH values, sunset yellow exhibits the λmax value at 480 nm. It shows its absorption peak at λmax at ∼480 nm.53–55Hassanien et al. synthesized selenium nanoparticles with the help of the bioextract, starting from sodium selenite salt. They have used Moringa oleifera extract to obtain spherical-shaped selenium nanoparticles with an average size ranging between 23 and 35 nm. Addition of the plant extract to the sodium selenite solution changes the colour from a light yellow to a brick red colour, indicating the formation of the selenium nanoparticle. The reaction mixture was further allowed to incubate at room temperature for another 24 h. The bandgap value for their synthesized nanoparticles was also determined, and was found to be 2.3 eV, which was higher than that of the bulk selenium and commercial selenium powder. The higher band gap can be attributed to its quantum size effects. Sunset yellow dye degradation (Fig. 12) was studied using their synthesized nanomaterial under UV light irradiation, as well as under solar light irradiation at slightly acidic pH range, and the dye degradation was successful after 10 h. This degradation process was reported to take place via pseudo-first order kinetics. The rate constant was determined from the equation applicable for the pseudo-first order reaction, and was found to be 0.173 min−1 (Table 6).56
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | M. oleifera | Na2SeO3 | 23–35 | Spherical | UV, solar | — | 56 |
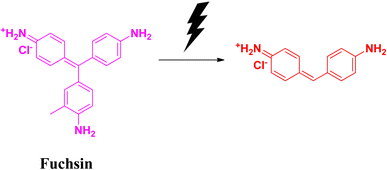
2.7 Fuchsin
Fuchsin is a dye that is basic in nature. It belongs to the triarylmethane family. It is highly flammable in nature. Fuchsin dye is popularly used in textile industries for the dyeing of clothes, leather, silk fibre, etc. This dye also possesses fungicidal, bactericidal and anaesthetic properties. Its high stability is attributed to its non-biodegradability issues. It is carcinogenic and affects the digestive system, as well as the respiratory system. In spite of its many positive sides, fuchsin is considered as a water pollutant. Therefore, the removal of fuchsin from the aqueous system is a big challenge.57–59Badreah and coworkers synthesized semi-spherical selenium nanoparticles having a diameter ranging between 8 and 22.5 nm. The photocatalytic degradation of fuchsin dye was successful after 34 min when the catalyst dose was 10 mg. The effects of changing the concentration of catalyst on the degradation efficiency were studied under visible light irradiation. When a significant amount of selenium nanoparticles was used, the degradation (Fig. 13) process was found to follow the pseudo-first order kinetics. This biosynthesized selenium nanoparticle is effective towards the generation of hydroxyl radicals, which is responsible to improve the degradation process leading to the removal of contaminant dyes ultimately from the wastewater effluents (Table 7).60
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | SeNPs, AA, polyvinyl alcohol | Na2SeO3 | 8–22.5 | Semi-spherical | Visible | — | 60 |
2.8 Safranin T
Safranin T is an important class of basic dye, belongs to the phenazinium dye family. This azine dye has widespread application as a synthetic dye in research as a spectroscopic analysis tool and as an indicator.61,62 This reddish brown-coloured powder is highly soluble in water. The cationic charge in the structure of safranin T facilitates its permeability into proteins and DNA molecules, thereby making it more toxic.63In the degradation of safranin T in the presence of the reducing agent sodium borohydride, adsorption on the surface of the nanomaterial is an important step. It follows a similar mechanism to that of methylene blue, and is depicted in Fig. 14.
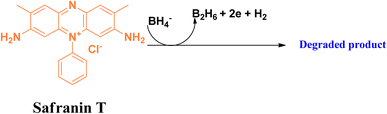 | ||
| Fig. 14 Probable degradation of safranin T in the presence of the reducing agent sodium borohydride. | ||
Xia et al. utilized the Escherichia coli strain for the biogenic synthesis of irregular sphere-shaped selenium nanoparticles. The E. coli culture was treated with sodium selenite and incubated for 36 h. The suspension was washed in distilled water and sonicated. Centrifugation yielded selenium nanoparticles, which were suspended again in 80% sucrose, and then centrifuged and washed with distilled water to obtain the pure product. A detailed study revealed that changing the pH of the system changed the zeta potential value. Safranin T absorbs at 520 nm, which gradually decreases during the degradation process with the biosynthesized selenium nanocatalyst. With increasing pH, the efficiency of safranin T dye degradation was increased. It was found that the optimum pH was 10 for safranin T dye degradation, which was achieved after 25 minutes. Temperature also has a significant effect on the dye removal efficiency of the synthesized nano-catalyst. The adsorption technique was used to determine the dye removal efficiently, which was 1911.0 mg g−1 for safranin T dye. Pseudo-second order kinetics was followed. Reusability of the biocatalyst is an important characteristic. It was demonstrated that the catalyst could be reused for more than five dye removal cycles with the help of 200 mM NaCl. This selenium nanoparticle could be used simultaneously for the degradation of Congo red dye as well as methylene blue dye, and was found to be very effective (Table 8).25
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | E. coli | Na2SeO3 | 60–105 | Irregular spheres | — | Adsorption | 25 |
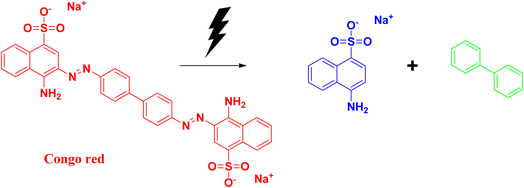
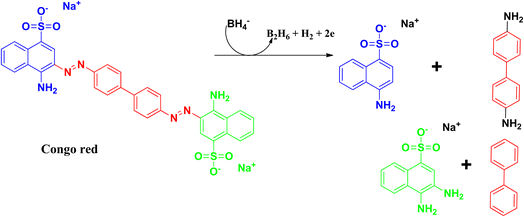
2.9 Congo red
Congo red is a synthetic azo dye. Congo red absorbs at a λmax value of 497 nm. The stable aromatic nature of the dye makes it a more potent bio-pollutant. Congo red is carcinogenic in nature, and requires number of years to be decomposed. Due to its toxicity, it has been banned in a number of countries. Therefore, it is very urgent to treat wastewater containing Congo red dye to eradicate this pollution issue.64,65The degradation mechanism of Congo red follows similar pathways as that of methylene blue, both through the photodegradation pathways and reduction pathways using sodium borohydride as reducing agent. The breaking and reduction of the azo bond occur during this process. During the photocatalysis process, the aromatic structure is destroyed in the final stage, followed by the production of CO2 and H2O, thereby removing the colour and the toxicity of the dye. The products that can be formed after the degradation pathways have been delineated in Fig. 15 and 16.25,66–70
Xia and coworkers synthesized selenium nanoparticles from sodium selenite, and used the E. coli extract for the synthesis process. Details have been discussed earlier in the case of safranin T dye. Congo red has a λmax at 437 nm, which decreases during the degradation process. The optimum pH for degradation was observed at pH 5, and it took 35 min for the whole degradation process. The adsorption capacity was found to be 1577.7 mg g−1, which was about 6% higher than that of the commonly used bio-based materials. Recyclability and kinetics are similar to that in the previously discussed dye, safranin T.25
Turna et al. synthesized selenium nanoparticles of 9.969 nm from leaf waste of Prunus armeniaca L., by obtaining the leaf extract with distilled water, followed by filtration and treatment with sodium selenite. Different spectroscopic techniques were utilized for characterizing the adsorption of toxic dye Congo red. Two different kinetics models and four different isotherm models were studied. It was proven that their adsorption phenomena followed pseudo-second order kinetics, and the Freundlich isotherm model was the best fit for their study with R2 values of 0.996. The calculated maximum adsorption capacity was 96.59 mg Congo red per g of the synthesized nanocatalyst.71
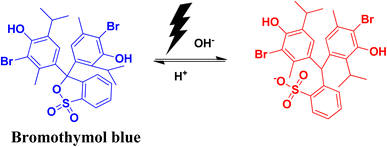
2.10 Bromothymol blue
Bromothymol blue is a widely used pH indicator. It is used for the identification of carbonic acid in liquid packaged items. Its important use is in the detection of fungal asparaginase enzyme and in many biological applications, including the rupture of membrane in cases of obstetrics and in gynecology. A change in pH has an impact on the colour of bromothymol blue. Changing the pH from acidic to basic pH causes the solution to change from yellow to blue colour. The λmax in the UV-vis spectra also shows a shift from 427 nm to 602 nm. Excess exposure to this dye can have adverse health effects, like infection in eyes and skin. It can also affect the functioning of the respiratory system.72,73The photocatalytic degradation of bromothymol blue produces the aromatic degraded product shown in Fig. 17.
Ameri and coworkers reported on the synthesis of selenium nanoparticles using the bioextract of Streptomyces griseobrunneus. Cultivation of the bacterial strain in CG broth medium was treated with SeO2 for 5 days, followed by centrifugation and grinding in liquid nitrogen with the help of a mortar and pestle. Finally, ultrasonication and centrifugation yielded the desired selenium nanoparticles. Selenium dioxide was used as the starting material to produce trigonal selenium. Photocatalytic degradation of bromothymol blue in buffer solution was completed in 60 min for under UV irradiation via oxidation with H2O2 (Table 9).74
| Sl no. | Biosources used | Precursor taken | Size (nm) | Shape | Irradiation | Condition | References |
|---|---|---|---|---|---|---|---|
| 1 | S. griseobrunneus | SeO2 | 73.8 | Spherical | UV | H2O2 | 74 |
3. Conclusion
In the last few decades, increased interest has emerged in the field of biogenic synthesis of nanoparticles using plant extracts or microorganisms. The green technique has snatched more attention due to its cost-effectiveness, availability, non-toxicity, and safe nature. The high surface-to-volume ratio and tuneable surface plasmon resonance make the nanoparticles more effective towards the catalytic activity for the degradation of dyes present in the effluents from various industries. Among the different types of nanoparticles used for the application of dye degradation and other biomedical applications, selenium nanoparticles possess unique characteristics. Furthermore, Se nanoparticles exhibit superior biodegradability and biocompatibility. The tremendous escalation in industrialization has resulted in increased water pollution, which not only affects human life, but also disturbs aquatic ecosystems. Physical, chemical and biological methods have been widely used to degrade these detrimental dyes for many years. However, among all of the methods, degradation of organic dyes using nanoparticles as a catalyst has gained much attention and is found to be more effective. Degradation of organic dyes by reduction method using sodium borohydride, or by using photocatalytic pathways, is quite common and effective. The end products are generally environmentally friendly products, thereby reducing and eliminating the toxic nature of the dyes. This review can shed light on the special features of selenium nanoparticles, its biogenic synthesis, and crucial application in the degradation of toxic pollutant dyes present in wastewater. Their mechanism of degradation and other related information have been discussed here. Synthesis of selenium nanoparticles through green techniques and its important applications as a catalyst in dye degradation, viz., the degradation of methylene blue, methyl orange, rhodamine B, alizarin S, malachite green, sunset yellow, fuchsin, safranin T, Congo red, and bromothymol blue, reported by different research groups, have been summarized here. A number of research groups have reported on the biogenic synthesis of selenium nanoparticles, and extensively studied their dye degradation properties. In spite of the important properties of selenium nanoparticles, this field is still largely unexplored. Along with this, thorough studies on issues like scalability and recyclability can augment the efficiency of the selenium particles to become an effective catalyst. More biogenic sources may be explored, which can improve the synthesis process, including the reduction of the synthesis time and the nanoparticle production yield. We hope that this review will be helpful for academic researchers and industrial scientists, thereby bridging the gap between academia and the industry. Further extensive work can be done on the degradation of other reactive dyes present in industrial effluents that have not been covered yet by the research groups.Data availability
No new data were generated in this review.Conflicts of interest
There are no known conflicts to declare.Acknowledgements
The authors, N. R., N. T. and P. P., are grateful to the Department of Science and Technology, Government of India for supporting the work through the DST-SERB CRG project grant (CRG/2021/002267), VIT University for VIT SEED funding, and DST New Delhi for DST-FIST project. R. C. would like to acknowledge the Department of Chemistry, Alipurduar University, West Bengal, India. R. C. acknowledges the SEED research grant (no. APDU/Reg./R. P./Notice/001/2024 dated 29.02.2024) of Alipurduar University for financial support.References
- T. B. Mekonnen, An overview on the photocatalytic degradation of organic pollutants in the presence of cerium oxide (CEO2) based nanoparticles: a review, Nanoscience and Nanometrology, 2021, 7, 14–26 CrossRef
.
- R. Gusain, K. Gupta, P. Joshi and O. P. Khatri, Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: a comprehensive review, Adv. Colloid Interface Sci., 2019, 272, 102009 CrossRef CAS PubMed
.
- R. A. Tohamy, S. S. Ali, F. Li, K. M. Okasha, Y. A. G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu and J. Sun, Ecotoxicol. Environ. Saf., 2022, 231, 113160 CrossRef PubMed
.
- S. Marimuthu, A. J. Antonisamy, S. Malayandi, K. Rajendran, P.-C. Tsai, A. Pugazhendhi and V. K. Ponnusamy, Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity, J. Photochem. Photobiol., B, 2020, 205, 111823 CrossRef CAS PubMed
.
- C. Wang, A. Yediler, D. Lienert, Z. Wang and A. Kettrup, Ozonation of an azo dye C.I. Remazol Black 5 and toxicological assessment of its oxidation products, Chemosphere, 2003, 52, 1225–1232 CrossRef CAS PubMed
.
- V. K. Gupta, R. Jain, A. Mittal, M. Mathur and S. Sikarwar, Photochemical degradation of the hazardous dye safranin-T using TiO2 catalyst, J. Colloid Interface Sci., 2007, 309, 464–469 CrossRef CAS PubMed
.
- A. Pandey, P. Shukla and P. K. Srivastava, Remediation of dyes in water using green synthesized nanoparticles (NPs), Int. J. Plant Env., 2020, 6, 68–84 CrossRef
.
- S. Joseph and B. Mathew, Green Synthesis of Silver Nanoparticles Using Datura metel Flower Extract Assisted by Ultrasound Method and Its Antibacterial Activity, J. Mol. Liq., 2015, 204, 184–191 CrossRef CAS
.
- A. Schröfel, G. Kratošová, I. Šafařík, M. Šafaříková, I. Raška and L. M. Shor, Acta Biomater., 2014, 10, 4023–4042 CrossRef PubMed
.
- M. S. S. Danish, L. L. Estrella-Pajulas, I. M. Alemaida, M. L. Grilli, A. Mikhaylov and T. Senjyu, Green synthesis of silver oxide nanoparticles for photocatalytic environmental remediation and biomedical applications, Metals, 2022, 12, 769 CrossRef CAS
.
- M. S. S. Danish, L. L. Estrella, I. M. A. Alemaida, A. Lisin, N. Moiseev, M. Ahmadi, M. Nazari, M. Wali, H. Zaheb and T. Senjyu, Photocatalytic applications of metal oxides for sustainable environmental remediation, Metals, 2021, 11, 80 CrossRef CAS
.
- G. M. Nair, T. Sajini and B. Mathew, Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications, Talanta, 2022, 5, 100080 CrossRef
.
- M. S. S. Danish, A. Bhattacharya, D. Stepanova, A. Mikhaylov, M. L. Grilli, M. Khosravy and T. Senjyu, A systematic review of metal oxide applications for energy and environmental sustainability, Metals, 2020, 10, 1604 CrossRef CAS
.
- R. Su, C. Xie, S. I. Alhassan, S. Huang, R. Chen, S. Xiang, Z. Wang and L. Huang, Oxygen reduction reaction in the field of water environment for application of nanomaterials, J. Nanomater., 2020, 10, 1719 CrossRef CAS PubMed
.
- G. Gahlawat and A. R. Choudhury, A review on the biosynthesis of metal and metal salt nanoparticles by microbes, RSC Adv., 2019, 9, 12944–12967 RSC
.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chemistry and properties of nanocrystals of different shapes, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed
.
- N. Abid, A. M. Khan, S. Shujait, K. Chaudhary, M. Ikram, M. Imran, J. Haider, M. Khan, Q. Khan and M. Maqbool, Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: a review, Adv. Colloid Interface Sci., 2021, 300, 102597 CrossRef PubMed
.
- A. Sargazi, A. Barani and M. H. Majd, Synthesis and apoptotic efficacy of biosynthesized silver nanoparticles using acacia luciana flower extract in MCF-7 breast cancer cells: activation of bak1 and bclx for cancer therapy, BioNanoScience, 2020, 10, 683–689 CrossRef
.
- T. A. J. de Souza, L. R. R. Souza and L. P. Franchi, Silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity, Ecotoxicol. Environ. Saf., 2019, 171, 691–700 CrossRef PubMed
.
- J. M. Galúcio, S. G. B. de Souza, A. A. Vasconcelos, A. K. O. Lima, K. S. da Costa, H. de Campos Braga and P. S. Taube, Synthesis, characterization, applications, and toxicity of green synthesized nanoparticles, Curr. Pharm. Biotechnol., 2022, 23, 420–443 Search PubMed
.
- A. Barani, S. R. Alizadeh and M. A. Ebrahimzadeh, A Comprehensive Review on Catalytic Activities of Green-Synthesized Selenium Nanoparticles on Dye Removal for Wastewater Treatment, Water, 2023, 15, 3295 CrossRef CAS
.
- H. Joshi, K. N. Sharma, A. K. Sharma, O. Prakash and A. K. Singh, Graphene oxide grafted with Pd17Se15 nano-particles generated from a single source precursor as a recyclable and efficient catalyst for C–O coupling in O-arylation at room temperature, Chem. Commun., 2013, 49, 7483 RSC
.
- S. Singh, S. Mahala, N. Bhuvanesh and H. Joshi, Pd(II) NCSe–pincer complexes for regioselective cross-dehydrogenative coupling of arylthiophenes with hetero(arenes), Catal. Sci. Technol., 2025 10.1039/d4cy01198h
.
- M. Velayati, H. Hassani, Z. Sabouri, A. Mostafapour and M. Darroudi, Biosynthesis of Se-Nanorods using Gum Arabic (GA) and investigation of their photocatalytic and cytotoxicity effects, Inorg. Chem. Commun., 2021, 128, 108589 CrossRef CAS
.
- X. Xia, Z. Zhou, S. Wu, D. Wang, S. Zheng and G. Wang, Adsorption removal of multiple dyes using biogenic selenium nanoparticles from an Escherichia coli strain overexpressed selenite reductase CsrF, Nanomaterials, 2018, 8, 234 CrossRef PubMed
.
- M. Banerjee and D. Rajeswari V, Green synthesis and anti-biofilm effect on Drosophila melanogaster of Selenium nanoparticles from Vitis vinifera for photocatalytic degradation and different biological applications, Vietnam J. Chem., 2024, 1–20 Search PubMed
.
- C. Santhosh, B. Balasubramanian, P. Vino, M. Viji, C. Rejeeth, S. Kannan, H. Ullah, K. R. Rengasamy, M. Daglia and A. Maruthupandian, Biofabricated selenium nanoparticles mediated from Goniothalamus wightii gains biomedical applications and photocatalytic degrading ability, J. King Saud Univ., Sci., 2022, 34, 102331 CrossRef
.
- M. I. Din, R. Khalid, J. Najeeb and Z. Hussain, Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies—a critical review, J. Clean. Prod., 2021, 298, 126567 CrossRef CAS
.
- M. Oz, D. E. Lorke, M. Hasan and G. A. Petroianu, Cellular and molecular actions of Methylene Blue in the nervous system, Med. Res. Rev., 2011, 31, 93–117 CrossRef CAS PubMed
.
- Z. Kalaycıoğlu, B. Ö. Uysal, Ö. Pekcan and F. B. Erim, Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide, ACS Omega, 2023, 8, 13004–13015 CrossRef PubMed
.
- K. G. Goud, N. K. Veldurthi, M. Vithal and G. Reddy, Characterization and evaluation of biological and photocatalytic activities of selenium nanoparticles synthesized using yeast fermented broth, J. Mater. NanoSci., 2016, 3, 33–40 Search PubMed
.
- V. Alagesan and S. Venugopal, Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities, BioNanoScience, 2019, 9, 105–116 CrossRef
.
- Z. M. Xia, Y. N. Liu, Z. Huang, L. Z. Qin, H. Lin and Q. Li, A facile green approach for synthesis of selenium nanowires with visible light photocatalytic properties, J. Nanosci. Nanotechnol., 2019, 19, 156–162 CrossRef CAS PubMed
.
- R. M. Tripathi, P. Hameed, R. P. Rao, N. Shrivastava, J. Mittal and S. Mohapatra, Biosynthesis of highly stable fluorescent selenium nanoparticles and the evaluation of their photocatalytic degradation of dye, BioNanoScience, 2020, 10, 389–396 CrossRef
.
- E.-S. R. El-Sayed, H. K. Abdelhakim and A. S. Ahmed, Solid-state fermentation for enhanced production of selenium nanoparticles by gamma-irradiated Monascus purpureus and their biological evaluation and photocatalytic activities, Bioprocess Biosyst. Eng., 2020, 43, 797–809 CrossRef CAS PubMed
.
- V. Cittrarasu, D. Kaliannan, K. Dharman, V. Maluventhen, M. Easwaran, W. C. Liu, B. Balasubramanian and M. Arumugam, Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities, Sci. Rep., 2021, 11, 1032 CrossRef CAS PubMed
.
- C. Santhosh, B. Balasubramanian, P. Vino, M. Viji, C. Rejeeth, S. Kannan, H. Ullah, K. R. Rengasamy, M. Daglia and A. Maruthupandian, Biofabricated selenium nanoparticles mediated from Goniothalamus wightii gains biomedical applications and photocatalytic degrading ability, J. King Saud Univ., Sci., 2022, 34, 102331 CrossRef
.
- S. R. Alizadeh, M. Seyedabadi, M. Montazeri, B. A. Khan and M. A. Ebrahimzadeh, Allium paradoxum extract mediated green synthesis of SeNPs: assessment of their anticancer, antioxidant, iron chelating activities, and antimicrobial activities against fungi, ATCC bacterial strains, Leishmania parasite, and catalytic reduction of methylene blue, Mater. Chem. Phys., 2023, 296, 127240 CrossRef CAS
.
- M. A. Ebrahimzadeh, M. Moradsomarein, F. S. Lalerdi and S. R. Alizadeh, Biogenic synthesis of selenium nanoparticles using Hibiscus esculentus L. extract: catalytic degradation of organic dye and its anticancer, antibacterial and antifungal activities, Eur. J. Chem., 2023, 14, 144–154 CrossRef CAS
.
- L. Velayutham, C. Parvathiraja, D. C. Anitha, K. Mahalakshmi, M. Jenila, J. K. Gupta, S. M. Wabaidur, M. R. Siddiqui, S. Aftab and W.-C. Lai, Antibacterial and photocatalytic dye degradation activities of green synthesized nise nanoparticles from hibiscus rosa-sinensis leaf extract, Water, 2023, 15, 1380 CrossRef CAS
.
- S. Satpathy, L. L. Panigrahi, P. Samal, K. K. Sahoo and M. Arakha, Biogenic synthesis of selenium nanoparticles from Nyctanthes arbor-tristis L. and evaluation of their antimicrobial, antioxidant and photocatalytic efficacy, Heliyon, 2024, 10, e32499 CrossRef CAS PubMed
.
- A. Aljuaid, M. Almehmadi, A. A. Alsaiari, M. Allahyani, O. Abdulaziz, A. Alsharif, J. A. Alsaiari, M. Saih, R. T. Alotaibi and I. Khan, g-C3N4 Based photocatalyst for the efficient photodegradation of toxic methyl orange dye: Recent modifications and future perspectives, Molecules, 2023, 28, 3199 CrossRef CAS PubMed
.
- S. Ahluwalia, N. T. Prakash, R. Prakash and B. Pal, Improved degradation of methyl orange dye using bio-co-catalyst Se nanoparticles impregnated ZnS photocatalyst under UV irradiation, Chem. Eng. J., 2016, 306, 1041–1048 CrossRef CAS
.
- Z. M. Saigl, Various adsorbents for removal of rhodamine b dye: a review, Indones. J. Chem., 2021, 21, 1039–1056 CrossRef CAS
.
- Y.-H. Chiu, T.-F. M. Chang, C.-Y. Chen, M. Sone and Y.-J. Hsu, Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts, Catalysts, 2019, 9, 430 CrossRef CAS
.
- L. Che, Y. Dong, M. Wu, Y. Zhao, L. Liu and H. Zhou, Characterization of selenite reduction by Lysinibacillus sp. ZYM-1 and photocatalytic performance of biogenic selenium nanospheres, ACS Sustain. Chem. Eng., 2017, 5, 2535–2543 CrossRef CAS
.
- J. Britto, P. Barani, M. Vanaja, E. Pushpalaksmi, J. J. Samraj and G. Annadurai, Adsorption of dyes by chitosan-selenium nanoparticles: recent developments and adsorption mechanisms, Nat. Environ. Pollut. Technol., 2021, 20, 467–479 CAS
.
- M. Velayati, H. Hassani, Z. Sabouri, A. Mostafapour and M. Darroudi, Green-based biosynthesis of Se nanorods in chitosan and assessment of their photocatalytic and cytotoxicity effects, Environ. Technol. Innov., 2022, 27, 102610 CrossRef CAS
.
- H. Byeon, V. P. Devi, A. Murugan, A. Tonk, K. Haribabu, H. Patil and V. G. A. G. Vincy, Evaluating the potential capability of selenium nanoparticles on degradation of hazardess textile dye using environmental friendly approach, Global NEST J., 2024, 26, 05633 CAS
.
- N. P. Raval, P. U. Shah and N. K. Shah, Malachite green “a cationic dye” and its removal from aqueous solution by adsorption, Appl. Water Sci., 2017, 7, 3407–3445 CrossRef CAS
.
- S. Srivastava, R. Sinha and D. Roy, Toxicological effects of malachite green, Aquat. Toxicol., 2004, 66, 319–329 CrossRef CAS PubMed
.
- E. Saied, A. E. Mekky, A. A. Al-Askar, A. F. Hagag, A. A. El-bana, M. Ashraf, A. Walid, T. Nour, M. M. Fawzi and A. A. Arishi, Aspergillus terreus-mediated selenium nanoparticles and their antimicrobial and photocatalytic activities, Crystals, 2023, 13, 450 CrossRef CAS
.
- R. S. Aliabadi and N. O. Mahmoodi, Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red, J. Clean. Prod., 2018, 179, 235–245 CrossRef CAS
.
- S. Fazeli, B. Sohrabi and A. R. Tehrani-Bagha, The study of Sunset Yellow anionic dye interaction with gemini and conventional cationic surfactants in aqueous solution, Dyes Pigm., 2012, 95, 768–775 CrossRef CAS
.
- K. Rovina, P. P. Prabakaran, S. Siddiquee and S. M. Shaarani, Methods for the analysis of Sunset Yellow FCF (E110) in food and beverage products—a review, TrAC, Trends Anal. Chem., 2016, 85, 47–56 CrossRef CAS
.
- R. Hassanien, A. A. Abed-Elmageed and D. Z. Husein, Eco-friendly approach to synthesize selenium nanoparticles: photocatalytic degradation of sunset Yellow Azo Dye and anticancer activity, ChemistrySelect, 2019, 4, 9018–9026 CrossRef CAS
.
- A. G. Ibrahim, A. Z. Sayed, H. A. El-Wahab and M. M. Sayah, Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye, Int. J. Biol. Macromol., 2020, 159, 422–432 CrossRef CAS PubMed
.
- R. Jain, S. Mendiratta, L. Kumar and A. Srivastava, Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye, Curr. Res. Green Sustainable Chem., 2021, 4, 100086 CrossRef CAS
.
- M. El Haddad, Removal of Basic Fuchsin dye from water using mussel shell biomass waste as an adsorbent: equilibrium, kinetics, and thermodynamics, J. Taibah Univ. Sci., 2016, 10, 664–674 CrossRef
.
- B. A. Al Jahdaly, N. S. Al-Radadi, G. M. Eldin, A. Almahri, M. Ahmed, K. Shoueir and I. Janowska, Selenium nanoparticles synthesized using an eco-friendly method: dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness, J. Mater. Res. Technol., 2021, 11, 85–97 CrossRef CAS
.
- S. C. Karadeniz, B. Isik, V. Ugraskan and F. Cakar, Agricultural Lolium perenne seeds as a low-cost biosorbent for safranine T adsorption from wastewater: isotherm, kinetic, and thermodynamic studies, Phys. Chem. Earth, Parts A/B/C, 2023, 129, 103338 CrossRef
.
- V. K. Gupta, A. Mittal, R. Jain, M. Mathur and S. Sikarwar, Adsorption of safranin-T from wastewater using waste materials—activated carbon and activated rice husks, J. Colloid Interface Sci., 2006, 303, 80–86 CrossRef CAS PubMed
.
- H. Wan, H. Chen, Y. Chu, X. Ju and H. Jiang, Structure characterization and optical properties investigation of the four main components of the classical phenazinium dye safranin O, Analyst, 2019, 144, 7149–7156 RSC
.
- N. P. Raval, P. U. Shah and N. K. Shah, Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review, Environ. Sci. Pollut. Res., 2016, 23, 14810–14853 CrossRef CAS PubMed
.
- M. Harja, G. Buema and D. Bucur, Recent advances in removal of Congo Red dye by adsorption using an industrial waste, Sci. Rep., 2022, 12, 6087 CrossRef CAS PubMed
.
- H. Tahir and M. Saad, Using dyes to evaluate the photocatalytic activity, Interface Sci. Technol., 2021, 32, 125–224 Search PubMed
.
- V. Selvaraj, T. S. Karthika, C. Mansiya and M. Alagar, An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications, J. Mol. Struct., 2021, 1224, 129195 CrossRef CAS
.
- C. Chen, C. Lu, Y. Chung and J. Jan, UV light induced photodegradation of malachite green on TiO2 nanoparticles, J. Hazard. Mater., 2007, 141, 520–528 CrossRef CAS PubMed
.
- R. Abu-Zurayk, A. Khalaf, H. A. Abbas, R. A. Nasr, T. S. Jamil and A. Al Bawab, Photodegradation of carbol fuchsin dye using an Fe2-xCuxZr2-xWxO7 photocatalyst under visible-light irradiation, Catalysts, 2021, 11, 1473 CrossRef CAS
.
- S. K. Ray, D. Dhakal and S. W. Lee, Insight into malachite green degradation, mechanism and pathways by morphology-tuned-NiMoO4 photocatalyst, J. Photochem. Photobiol., 2018, 94, 552–563 CrossRef CAS PubMed
.
- T. Turna, A. Solmaz and A. Baran, Green Synthesis Study: Adsorption of Congo Red Dye with Selenium Nanoparticles Obtained from Prunus Armeniaca L. Leaf Waste, ChemistrySelect, 2024, 9, e202403106 CrossRef CAS
.
- S. M. Ibrahim, A. F. Al-Hossainy, B. Saha and M. Abd El-Aal, Removal of bromothymol blue dye by the oxidation method using KMnO4: accelerating the oxidation reaction by Ru (III) catalyst, J. Mol. Struct., 2022, 1268, 133679 CrossRef CAS
.
- E. O. Dada, I. A. Ojo, A. O. Alade, T. J. Afolabi, M. O. Jimoh and M. O. Dauda, Biosorption of bromo-based dyes from wastewater using low-cost adsorbents: a review, Journal of Scientific Research and Reports, 2020, 26, 34–56 CrossRef
.
- A. Ameri, M. Shakibaie, A. Ameri, M. A. Faramarzi, B. Amir-Heidari and H. Forootanfar, Photocatalytic decolorization of bromothymol blue using biogenic selenium nanoparticles synthesized by terrestrial actinomycete Streptomyces griseobrunneus strain FSHH12, Desalin. Water Treat., 2016, 57, 21552–21563 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |