Open Access Article
Open Access ArticleThe coiled-coil protein carrier structure affects the activation of certain endocytosis pathways†
Ken-Ichi Sano *ab and
Yuta Nomatab
*ab and
Yuta Nomatab
aDepartment of Applied Chemistry, Faculty of Fundamental Engineering, Nippon Institute of Technology, 4-1 Gakuendai, Miyashiro, Saitama 345-8501, Japan. E-mail: kisano@nit.ac.jp
bGraduate School of Environmental Symbiotic System Major, Nippon Institute of Technology, 4-1 Gakuendai, Miyashiro, Saitama 345-8501, Japan
First published on 10th January 2025
Abstract
Coiled-coil protein carrier (CCPC) 140 is a rigid and anisotropically structured cationic coiled-coil artificial protein that has displayed up to a 1000 times higher level of cellular internalization activity than that of unstructured cell-penetrating peptides. Previous studies have demonstrated that CCPC 140's rigid and anisotropic structural properties and cationic surface properties are important for its superior cellular internalization activity. In this study, we investigated whether each physicochemical characteristic of CCPC 140 effectively contributed to activating the cellular internalization pathway. By evaluating CCPC 140's ability to penetrate glycosaminoglycan (GAG)-lacking cells, the activation of GAG-dependent endocytosis by electrostatic interactions between cationic CCPC 140 and anionic GAGs has been found to play a major role in CCPC 140's superior cellular internalization activity. Using endocytosis inhibitors, it was revealed that the GAG-binding-dependent activation of caveola-mediated endocytosis plays a role in cellular internalization, which requires rigid and anisotropic structural properties, not the cationic properties of CCPC 140. Macropinocytosis is a common route of cellular internalization. However, CCPC 140's rigid and anisotropic structural properties activate macropinocytosis, but this does not involve the Rho-family GTPase-dependent macropinocytosis pathway.
1 Introduction
The efficient intracellular delivery of therapeutic molecules using peptides has been achieved by mimicking viral infections. The first cell-penetrating peptide (CPP) was found in the human immunodeficiency virus TAT protein.1,2 Heretofore, considerable variations in CPPs have been detected in natural proteins, and artificial peptides have been designed to exhibit cell-penetrating activity.3–5 Most CPPs are highly cationic because inter-ionic interactions between the positively charged CPPs and the negatively charged proteoglycans and phospholipids of the plasma membrane's extracellular domains are thought to be important for cellular internalization.6–9In particular, the binding of glycosaminoglycans (GAGs) to proteoglycans has been reported as a trigger for the internalization of most CPPs by endocytosis.3,6–14 CPPs, such as Tat and oligo-arginine, exhibit largely decreasing cellular internalization activity against the Chinese hamster ovary (CHO)-derived pgsA-745 cell line, which lacks GAGs on its cell surface.10–13 The cellular internalization of CPPs is not limited to endocytosis, but can also occur through direct penetration, that is, translocation.6–9,15,16 However, most CPPs show a dramatic reduction in the extent of cell penetration when endocytosis is suppressed.13,15–19 These studies indicate that GAG-dependent endocytosis is the most effective pathway of intracellular delivery using CPPs. However, high concentrations of CPPs, in most cases 5–10 μM, are required for endocytic activation. The requirement for the high concentration and cooperative cellular internalization of CPPs is explained by the accumulation and formation of clusters on GAGs, which trigger the activation of endocytosis.6,8,20
Asbestos and carbon nanotubes, being rigid and anisotropic (fibrous) nanomaterials, show remarkably effective cellular internalization.21–23 However, they are highly toxic in the long term because of their non-biodegradability.21,24–26 In a previous study, we hypothesized that an artificial protein having a highly cationic surface and rigid and anisotropic structure would show superior cellular internalization activity.27 Long-term toxicity should have been avoided since the protein was biodegradable. Therefore, we designed and produced an artificial protein, coiled-coil protein carrier (CCPC) 140 (“140” refers to the number of amino acid residues in the polypeptide chain of CCPC. Fig. 1A), with a structured frame derived from the filamentous protein tropomyosin, the entire molecule of which consists of a two-stranded parallel α-helical coiled-coil structure.27 Similar to tropomyosin, CCPC 140 has an anisotropic structure, with a 2 nm diameter and 20 nm length (Fig. 1B).27–30 The α-helical coiled-coil primary structure shows a heptad-repeat amino acid sequence with each position assigned a to g. The amino acids at positions b, c, and f are exposed outside the coiled-coil structure, and these amino acids define the surface properties of coiled-coil proteins.31,32 CCPC 140 was designed to have 53.3% basic amino acids, lysine and arginine at positions b, c, and f, and a calculated isoelectric point of 10.6.27 Thus, the surface of CCPC 140 was highly cationic. Physicochemical studies on tropomyosin have revealed that its persistence length is presumed to be longer than its actual length.33 This accounts for the rigid and anisotropic structure of CCPC 140.
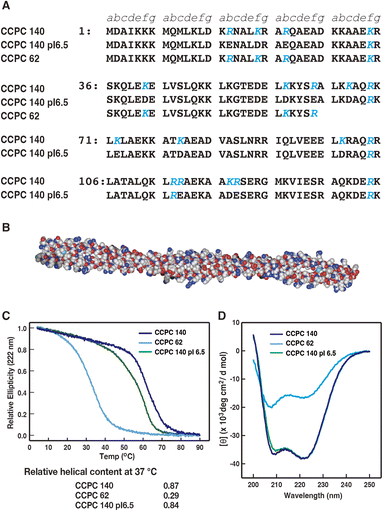 | ||
| Fig. 1 (A) Amino acid sequence of CCPC 140, CCPC 140 pI 6.5 and CCPC 62 (adapted with permission from ref. 34. Copyright 2015 American Chemical Society). The positions of the heptad repeat are described as a to g. Letters in blue represent substituted amino acids from human skeletal muscle α-tropomyosin. (B) Designation of CCPC 140 using the SWISS-MODEL Repository,28,29 and drowned space-filling model using Waals (Altif Laboratories, Tokyo, Japan). (C) Thermal unfolding profiles of CCPC 140, CCPC 140 pI 6.5, and CCPC 62. Relative helical contents are calculated as shown in the Materials and methods. (D) Circular dichroism spectra of CCPC 140, CCPC 140 pI 6.5, and CCPC 62 at 37 °C. | ||
Investigating the cellular internalization activity of fluorescently labeled CCPC 140 against several tumor-derived human cell lines, such as HeLa cells, Hep3B, A549, and K562, showed its superior cellular internalization.27 Regarding the HeLa cells, CCPC 140 was detected in all cells at a concentration as low as 3.1 nM.27 Higher concentrations are usually required for the internalization of CPPs by endocytosis, as described above.6–15 This indicates that the cellular internalization activity of cationic, rigid, and anisotropically structured CCPC 140 was approximately 1000 times greater than that of cationic but unstructured CPPs.27 Due to the high cellular internalization activity of CCPC 140, it may reduce the administered concentration of anticancer drugs with IC50s in the tens of μM range (unpublished result).
We also created CCPC 140 variants with noncationic surfaces. Their isoelectric points were 6.5 and 8.6, named CCPC 140 pI 6.5 and CCPC 140 pI 8.6, respectively.34 Compared to CCPC 140, a 100 times higher concentration of the CCPC 140 pI variants was required for cellular internalization.34 We also evaluated the effect of the structural rigidity of CCPC 140 on cellular internalization.27 A deletion variant of CCPC 62 having 62 amino acids per chain could not maintain its coiled-coil structure at 37 °C, the experimental temperature of a cellular internalization assay, because of thermal fluctuation.27 The cellular internalization activity of CCPC 62 is much lower than that of CCPC 140.27,35 At the effective concentration, the activity of CCPC 62 is slightly superior but almost comparable to that of other CPPs.27 In contrast, LZ-CCPC 62, a CCPC 62 variant with the same number of positively charged residues at the same positions, has an α-helical coiled-coil structure formation at 37 °C; it exhibits cellular internalization activity equal to that of CCPC 140.35 From the study of a series of deletion variants of LZ-CCPCs, an aspect ratio at 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or more was required for the superior cellular internalization of the cationic coiled-coil proteins.35
1 or more was required for the superior cellular internalization of the cationic coiled-coil proteins.35
Thus, we addressed the factors necessary for the superior cellular internalization of CCPC 140 based on the physicochemical properties of the molecule. Although the subcellular localization of DDS carriers is a critical issue in intracellular DDS research, that of CCPC 140 is still unknown. Previous studies have shown that the mechanism underlying the superior cellular internalization activity of CCPC 140 relies on the activation of endocytosis, but the details of this are unclear.27 This work, however, focuses on the first step of CCPC 140 entry into the cell. We clarified the endocytic pathways activated by each physicochemical property, including the rigid and anisotropic structural properties and cationic surface properties, of CCPC 140.
2 Materials and methods
2.1. Preparation of CCPC 140 and its derivatives
The preparation of CCPC 140 and its derivatives has been described in our previous reports.27,34,35 Purified CCPC 140 and its derivatives were checked by SDS-PAGE. Protein concentrations were determined using the micro-burette method.362.2. Circular dichroism measurements
Thermal denaturation of the protein was evaluated using a JASCO J-820 circular dichroism (CD) spectropolarimeter equipped with a CTU-100 Peltier temperature controller. The sample was placed in a 1 mm quartz cuvette. Temperature-dependent CD values and far-UV CD spectra were obtained as previously described.35 The relative helical content of the CCPC 140 and CCPC variants was evaluated with the following equation:| Relative helical content = ([θ222 nm]temp − [θ222 nm]4 °C)/([θ222 nm]90 °C − [θ222 nm]4 °C) |
2.3. In vitro cell penetration assay
CCPC 140 and its derivatives were labeled with Alexa Fluor 532 (Life Technologies, California, USA) as previously described.35 Labeled CCPC protein concentrations were determined using the micro-burette method,36 and the concentration of the conjugated Alexa Fluor 532 dye was determined at an absorbance of 532 nm. Then, the efficiency of the fluorescently labeled recombinant proteins was estimated by their division.In vitro cell penetration assays were carried out according to our previous reports.35 In brief, two CHO cell lines, CHO-K1 (JCRB cell bank JCRB9018) and pgsA-745 (ATCC CRL-2242),37 were used in this study and were obtained from the JCRB cell bank and ATCC, respectively. Both cell lines used for the assays had a passage number less than 15. The cells were maintained in Ham's F-12 nutrient mix medium (Gibco, Thermo Fisher) supplemented with 10% fetal calf serum (FCS) and penicillin–streptomycin (Wako-Fujifilm) at 37 °C under humidification with 5% CO2-containing air.
For the cell penetration assay, the cells were plated at a density of 5 × 104 cells per well in a 24-well plate. After 16–20 h incubation, the medium was replaced with 500 μl of Ham's F-12 nutrient mix medium supplemented with 10% FCS, penicillin–streptomycin, and fluorescently labeled CCPC 140 and its derivatives and incubated for 2 h. The inhibitors nystatin, jasplakinolide, and toxin B were added at final concentrations of 50 μg mL−1, 10 ng mL−1, and 50 nM, respectively. Neuraminidase was added at a final concentration of 50 mU mL−1 30 min prior to addition of CCPC 140 and its derivatives. The effect of both CCPCs and these inhibitors on the cell proliferation was assessed by the WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetra-zolio]-1,3-benzene disulfonate) (Takara, Japan) assay. CCPCs and Inhibitors were added at the same concentration as that used in the cell penetration assay. The cells were then incubated for 2 h and assayed using a WST-1 kit.
For the fluorescence-activated cell sorting (FACS) assay, the cells were treated with 500 μl of 0.25% w/v Trypsin-1 mM EDTA-4Na (Wako-Fujifilm) and incubated for 1–2 min; the trypsin solution was removed, and protease activity was stopped by the addition of 500 μl of Ham's F-12 nutrient mix medium supplemented with 10% FCS. The cells were detached from the 24-well plate by gentle pipetting and collected by centrifugation at 1500g for 2 min at 20–25 °C. The cells were washed twice with 500 μl of PBS and suspended in 200 μl of PBS, and the fluorescent intensity from each cell was measured using a FACS Moxiflow instrument (ORFLO, Ketchum, Idaho, USA).
For microscopic observation, after two hours of incubation of fluorescently labeled CCPC 140 and its derivatives in the medium, the cells were washed twice with 500 μl PBS, and 500 μl DMEM/F-12 nutrient mixture medium (Gibco) without phenol red and supplemented with 10% FCS was added. The cells were observed using an Evos M5000 imaging system (Thermo Fisher Scientific).
3 Results and discussion
3.1. Evaluation of structural stability of CCPC 140 and its variants
First, we obtained the thermal denaturation profiles and CD far-UV spectra at a cell-penetration experimental temperature of 37 °C for CCPC 140 and its derivatives using CD spectroscopy (Fig. 1C and D). The results agreed with those of our previous studies.27,34 The thermal melting profiles revealed that CCPC 140 and CCPC 140 pI 6.5 still maintained a high α-helical content at 37 °C (0.87 and 0.84, respectively), but the structure of CCPC 62 was almost lost (0.29) because of thermal fluctuation. The far-UV CD spectra profiles of CCPC 140 and CCPC 140 pI 6.5 also demonstrate the maintenance of their α-helical coiled-coil structure at the experimental temperature. In conclusion, both CCPC 140 and CCPC 140 pI 6.5 displayed a rigid and anisotropic structure during the cell penetration assay, and CCPC 62 did not form a coiled-coil structure but did form a partially folded α-helix.3.2. Effect of GAGs on the cellular internalization activities of CCPC 140 and its variants
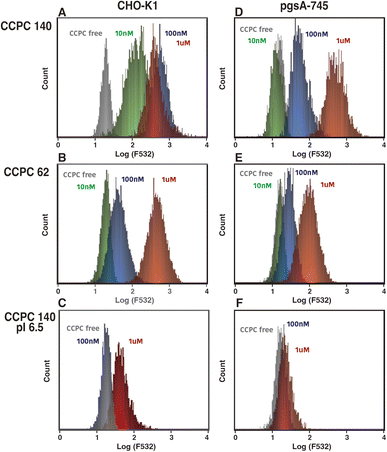
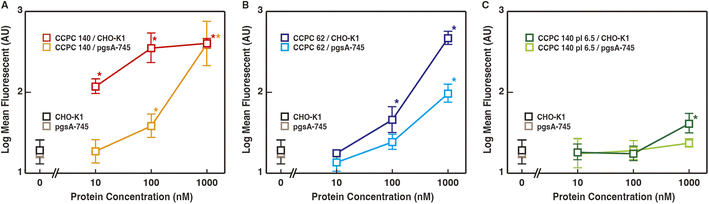
The binding to GAGs of proteoglycans has been shown to trigger CPPs and viral internalization by endocytosis. We investigated the effect of GAG binding on CCPC 140 and its variant cellular internalization activities (Fig. 2–4). The CHO-derived pgsA-745 strain lacks GAGs37,38 and is often used to evaluate the GAG-binding-triggered cell penetration of CPPs and viruses.10–13,39–41 To compare the cellular internalization activity of CCPC 140, uncationic CCPC 140 pI 6.5, and partially folded CCPC 62, we evaluated the effect of both the electrostatic interactions between CCPCs and GAGs and the structural rigidity and anisotropy of CCPCs on cellular internalization.CCPC 140 and its derivatives were fluorescently labeled with Alexa Fluor 532. The labeled CCPC 140 and its derivatives were adjusted to a molar ratio of 1.8–2.1 (two polypeptide chains reduced to one molecule), which was confirmed by the protein concentration and fluorophore absorbance. One possible effect on CCPC molecule by labeling is a decrease in primary amine at the surface of CCPCs (mainly side chain of lysine). This can cause decreasing pI of CCPCs. To minimize the effect of labeling on CCPCs structure and functions, almost one fluorophores bound on each CCPC polypeptide chain. The labeled CCPC 140 and its derivatives were added to both wild-type CHO-K1 with GAGs and pgsA-745 cultures at concentrations ranging from 10 nM to 1 μM, and the cellular uptake of the labeled CCPC 140 and its derivatives was examined using fluorescence microscopy and FACS (Fig. 2, 3 and S1†). Generally, cationic polymers are known to show cytotoxicity concentration dependent manner, we have confirmed that no significant cytotoxicity was observed at the concentrations of CCPCs used in this study (Fig. S2†). We detected a significant fluorescence signal from the cells 2 h after we added CCPC 140 to CHO-K1 cells at a final concentration of 10 nM (Fig. 2, S1,† and 4A). These observations agree with those made in our previous experiments using human cancer-derived cell lines.27 The FACS data showed that the fluorescent signals from each cell were saturated with more than 100 nM CCPC 140 in the CHO-K1 cells (Fig. 3A). Although we did not detect significant fluorescence signals from pgsA-745 at 10 nM CCPC 140, 100 nM and 1 μM CCPC 140 were sufficient to detect significant fluorescence signals from pgsA-745 (Fig. 4A). This tendency was also seen in the partially folded but cationic CCPC 62 (Fig. 3B). The cellular internalization activity of CCPC 62 was much lower than that of CCPC 140 but was also affected by the GAGs. These results agree with previous reports using unstructured but cationic CPPs.12–15 In the case of the structured but noncationic CCPC 140 pI 6.5, significant fluorescent signals were detected only with 1 μM addition to CHO-K1 (Fig. 3C).
These results show that the activation of GAG-dependent endocytosis plays a major role in the superior cellular internalization activity of CCPC 140, similar to CPP cell penetration. Moreover, as indicated by previous studies, this activation occurs through electrostatic interactions between the cationic CCPCs and acidic GAGs. However, the rigid and anisotropic structural properties of CCPC 140 can enhance the induction of GAG-dependent endocytosis to a much larger extent than those of unstructured cationic CPPs and partially folded CCPC 62. CPPs and CCPC 62, accumulation and cluster formation on GAGs are required to activate GAGs-dependent endocytosis.6,8,20 On the other hand, the activation of GAGs-dependent endocytosis by CCPC 140 would require only a few molecules at most.
Interestingly, adding 1 μM CCPC 140 to pgsA-745 allowed cellular internalization to reach the saturation level of the fluorescence signal. For CCPC 140 at a high concentration (1 μM), CCPC 140-activated GAG-independent endocytosis can compensate for the loss of GAG-dependent endocytosis. Sialic acid and hyaluronan binding-mediated endocytosis are considered candidates for GAG-independent endocytosis.12,15,42
3.3. Effect of neraminidase treatment on the cellular internalization activities of CCPC 140 and its variants
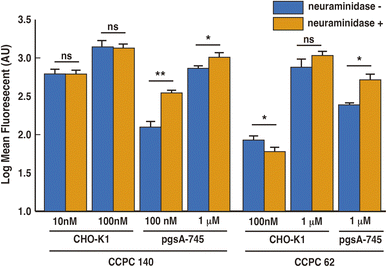
Previous studies showed that the cellular internalization activity of unstructured CPPs was reduced by the removal of sialic acid from cell surfaces.12,15 Neuraminidase catalyses the hydrolysis of sialic acid. To evaluate the effect of sialic acid binding on the cellular internalization of CCPC 140 and CCPC 62, we investigated the effect of sialic acid removal on the cellular internalization ability of CCPCs (Fig. 4).We found a significant increase in the cellular internalization activity by adding CCPC 140 and CCPC 62 to pgsA-745 cells with neuraminidase treatment. In the absence of GAGs on the cellular surface, sialic acid seems to display an inhibitory potency against both structured and partially folded CCPCs. On the other hand, in the presence of GAGs, the neuraminidase treatment had no effect on the cellular internalization activity of CCPC 140, whereas an inhibitory effect was observed on the cellular internalization activity of CCPC 62 (Fig. 4). Sialic acid itself has an inhibitory effect on the cellular internalization activity, but for unstructured CPPs and partially folded CCPC 62, it may act to recruit CPPs to GAGs due to its acidic properties. Also, it was found that the binding of CCPC 140 to GAGs does not require the recruitment by sialic acid because of its structural properties.
3.4. Cellular internalization mechanisms of CCPC 140 and its variants
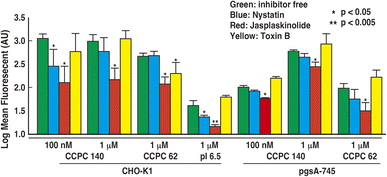
There was considerable variation in the activation of endocytic pathways. Previous CPP studies have indicated that CPPs activate multiple endocytosis pathways through many routes and can penetrate cells. Using endocytosis inhibitors, we attempted to clarify which endocytic pathways were activated by each physicochemical characteristic of CCPC 140. We refer to the experimental conditions used to measure the significant fluorescent signals in Fig. 3, and also evaluate the cellular internalization activities using FACS (Fig. 5). We have confirmed that there the endocytosis inhibitors had no effect on the cell viability under the conditions of the current experiment (Fig. S3†).Endocytic pathways include various mechanisms involving endocytosis and macropinocytosis. Nystatin breaks lipid raft structures and abolishes membrane raft endocytosis.43–46 Thus, nystatin acts as an inhibitor of caveola-mediated endocytosis. CCPC 140 and CCPC 140 pI 6.5 displayed significantly decreased cellular internalization of CHO-K1 cells with the addition of nystatin (Fig. 5). This decrease in the cellular internalization activity of CCPC 140 was not observed at a concentration of 1 μM. It was also not observed when CCPC 140 was administrated to GAG-free pgsA-745 cells. In contrast, the cellular internalization activity of partially folded CCPC 62 was not significantly affected with either CHO-K1 or pgsA-745 cells (Fig. 5).
On one hand, macropinocytosis often plays a much more important role in the cellular internalization of CPPs than endocytosis does.6–8,11–13 Jasplakinolide is known to completely inhibit macropinocytosis by inhibiting actin cytoskeleton reconstitution.47,48 Jasplakinolide inhibited the cellular internalization activity of CCPC 140 and its variants under all the conditions tested in our experiments (Fig. 5). Toxin B also inhibits macropinocytosis through the inhibition of Rho family GTPase activity.49,50 The cellular intenalization ability of CCPC 62 with CHO-K1 cells appeared to be affected by the addition of toxin B (Fig. 5). This decrease in the cellular internalization activity of CCPC 62 was not observed with pgsA-745 cells. In contrast, the cellular internalization activity of CCPC 140 and CCPC 140 pI 6.5 was unaffected by toxin B in CHO-K1 and pgsA-745 cells (Fig. 5).
The cellular internalization pathway of CCPC 140 seems to involve macropinocytosis more than caveolae endocytosis when comparing the degree of inhibition of cellular internalization activity of CCPC 140. In addition, macropinocytosis is a common route for the cellular internalization of CPPs, CCPC 140, and its variants.6–8,11–13 Previous studies have shown that CPPs binding to proteoglycans, including cell-surface GAGs, trigger the activation of Rac1, a Rho family GTPase.13,51 Following actin remodeling induced by Rac1, CPPs are internalized into cells through macropinocytosis.13,51 Although structured and partially folded CCPCs are internalized through macropinocytosis, the signaling pathway leading to actin remodeling activation differs from that associated with structured CCPCs and partially folded CCPC 62 (Fig. 6). The rigid and anisotropic structures of CCPCs, CCPC 140, and CCPC 140 pI 6.5 do not significantly affect the activation of Rho family GTPase-dependent macropinocytosis. In addition, the activation of macropinocytosis by the structured CCPCs does not seem to depend on the interaction between the cationic surface of CCPC 140 and GAGs.
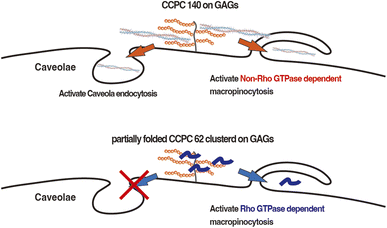 | ||
| Fig. 6 Schematic illustration of the activation pathways of endocytosis utilized by CCPC 140 and partially folded CCPC 62. | ||
It has been reported that unstructured short CPPs, such as octa-arginine and TAT, do not effect cellular internalization with the addition of nystatin.11,45,46 Nystatin's effect on the cellular internalization of CCPC 140 was only seen when 100 nM CCPC 140 was administered to CHO-K1 cells (Fig. 5). At higher concentrations (1 μM), nystatin's effect was not seen, possibly because the other endocytic pathways activated by CCPC 140 can be fully compensate nystatin's effect. However, this does not mean that the caveolae endocytosis pathway is not important for the cellular internalization of CCPC 140. The caveolae endocytosis pathway can be an important endocytic pathway at low concentrations of CCPC 140. The most significant feature of CCPC 140 is its ability to internalize cells efficiently, even when administered at low concentrations.
It has also been reported that proline-rich CPPs use caveola-mediated endocytosis for internalization.52 Proline-rich CPPs tend to form fibrillar structures by self-assembly.52 Therefore, we speculate that caveola-mediated endocytosis is a common route for the cellular internalization of both fibrillar proline-rich CPPs and CCPC 140. Furthermore, CPP–cargo protein conjugates and a PepFact14/DNA conjugate displayed cellular internalization via caveolae endocytosis.18,19,53–55 In the PepFact14/DNA conjugate, a tightly formed peptide/DNA polyion complex might work as a structural factor for the activation of caveola-mediated endocytosis.50 In the case of CPP–cargo protein conjugates, a portion of the cargo protein also becomes a factor for activating caveola-mediated endocytosis.18,19,53,54 Thus, we consider that low-concentration CCPC 140-activated GAG-binding-dependent activation of caveola-mediated endocytosis is due to the anisotropic structure of CCPC 140 (Fig. 6).53,54
4 Conclusions
We previously reported an artificial protein, CCPC 140, which displays superior cellular internalization activity because of its cationic surface properties and rigid and anisotropic structure. The activation of GAG-dependent endocytosis by electrostatic interactions between CCPC 140 and GAGs plays a major role in the superior cellular internalization activity of CCPC 140 and CPPs. Sialic acid itself has an inhibitory effect on the cellular internalization activity, but for unstructured CPPs and partially folded CCPC 62, it may act to recruit CPPs and CCPC 62 to GAGs due to its acidic properties. Macropinocytosis is a common route for the cellular internalization of CCPC 140 and CPPs, but structured CCPCs do not seem to depend on the activation of Rho-family GTPase-induced macropinocytosis. The GAG-binding-dependent activation of caveola-mediated endocytosis plays a role in CCPC 140 cellular internalization. Further studies on biophysical processes will provide important knowledge for the efficient intracellular delivery of pharmaceutical molecules, and control over the activity and endocytic pathways of carriers can be optimized.Data availability
All data used in this study were presented in the manuscript. Raw data available upon request to K. S.Author contributions
K. S. planned, designed, and performed the experiments, carried out analysis and interpretation of data, and wrote the manuscript. Y. N. assisted in the protein preparation and the cell penetration assay. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by a JSPS KAKENHI grant awarded to K.-I. S. (20K05285 and 24K08605), the Network Joint Research Center for Materials, and a special research grant from the Nippon Institute of Technology awarded to K.-I. S. We thank the Edanz Group (https://www.edanzediting.com/ac) and MDPI English Editing service for editing this manuscript.References
- M. Green and P. M. Loewenstein, Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein, Cell, 1988, 55, 1179 CrossRef CAS PubMed
.
- A. D. Frankel and C. O. Pabo, Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus, Cell, 1988, 55, 1189 CrossRef CAS PubMed
.
- D. M. Copolovici, K. Langel, E. Eriste and Ü. Langel, Cell-Penetrating Peptides: Design, Synthesis, and Applications, ACS Nano, 2014, 8, 1972 CrossRef CAS PubMed
.
- M. Zorko and Ü. Langel, Cell-Penetrating Peptides, Methods Mol. Biol., 2022, 2383, 3 CrossRef CAS PubMed
.
- P. Agrawal, S. Bhalla, S. S. Usmani, S. Singh and K. Chaudhary, et al., CPPsite 2.0: A Repository of Experimentally Validated Cell Penetrating Peptides, Nucleic Acids Res., 2016, 44, D1098 CrossRef CAS PubMed
.
- A. Walrant, S. Cardon, F. Burlina and S. Sagan, Membrane Crossing and Membranotropic Activity of Cell-Penetrating Peptides: Dangerous Liaisons?, Acc. Chem. Res., 2017, 50, 2968 CrossRef CAS PubMed
.
- S. Futaki and I. Nakase, Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization, Acc. Chem. Res., 2017, 50, 2449 CrossRef CAS PubMed
.
- I. Ruseska and A. Zimmer, Internalization Mechanisms of Cell-Penetrating Peptides, Beilstein J. Nanotechnol., 2020, 11, 101 CrossRef CAS PubMed
.
- F. Madani, S. Lindberg, Ü. Langel, S. Futaki and A. Gräslund, Mechanisms of Cellular Uptake of Cell-Penetrating Peptides, J. Biophys., 2011, 2011, 414729 Search PubMed
.
- M. E. Favretto, R. Wallbrecher, S. Schmidt, R. van de Putte and R. Brock, Glycosaminoglycans in the Cellular Uptake of Drug Delivery Vectors - Bystanders or Active Players?, J. Contr. Release, 2014, 180, 81 CrossRef CAS PubMed
.
- J. P. Richard, K. Melikov, H. Brooks, P. Prevot, B. Lebleu and L. V. Chernomordik, Cellular Uptake of Unconjugated TAT Peptide Involves Clathrin-Dependent Endocytosis and Heparan Sulfate Receptors, J. Biol. Chem., 2005, 280, 15300 CrossRef CAS PubMed
.
- J. M. Gump, R. K. June and S. F. Dowdy, Revised Role of Glycosaminoglycans in TAT Protein Transduction Domain-Mediated Cellular Transduction, J. Biol. Chem., 2010, 285, 1500 CrossRef CAS PubMed
.
- I. Nakase, A. Tadokoro, N. Kawabata, T. Takeuchi, H. Katoh, K. Hiramoto, M. Negishi, M. Nomizu, Y. Sugiura and S. Futaki, Interaction of Arginine-Rich Peptides with Membrane-Associated Proteoglycans Is Crucial for Induction of Actin Organization and Macropinocytosis, Biochemistry, 2007, 46, 492 CrossRef CAS PubMed
.
- H. L. Amand, H. A. Rydberg, L. H. Fornander, P. Lincoln, B. Nordén and E. K. Esbjörner, Cell Surface Binding and Uptake of Arginine- and Lysine-Rich Penetratin Peptides in Absence and Presence of Proteoglycans, Biochim. Biophys. Acta, 2012, 1818, 2669 CrossRef PubMed
.
- C. Y. Jiao, D. Delaroche, F. Burlina, I. D. Alves, G. Chassaing and S. Sagan, Translocation and Endocytosis for Cell-Penetrating Peptide Internalization, J. Biol. Chem., 2009, 284, 33957 CrossRef CAS PubMed
.
- K. Kardani, A. Milani, S. H. Shabani and A. Bolhassani, Cell Penetrating Peptides: The Potent Multi-cargo Intracellular Carriers, Expert Opin. Drug Deliv., 2019, 16, 1227 CrossRef CAS PubMed
.
- G. Drin, S. Cottin, E. Blanc, A. R. Rees and J. Temsamani, Studies on the Internalization Mechanism of Cationic Cell-Penetrating Peptides, J. Biol. Chem., 2003, 278, 31192 CrossRef CAS PubMed
.
- I. M. Kaplan, J. S. Wadia and S. F. Dowdy, Cationic TAT Peptide Transduction Domain Enters Cells by Macropinocytosis, J. Contr. Release, 2005, 102, 247 CrossRef CAS PubMed
.
- I. Nakase, M. Niwa, T. Takeuchi, K. Sonomura, N. Kawabata, Y. Koike, M. Takehashi, S. Tanaka, K. Ueda, J. C. Simpson, A. T. Jones, Y. Sugiura and S. Futaki, Cellular Uptake of Arginine-Rich Peptides: Roles for Macropinocytosis and Actin Rearrangement, Mol. Ther., 2004, 10, 1011 CrossRef CAS PubMed
.
- A. Ziegler and J. Seelig, Binding and Clustering of Glycosaminoglycans: a Common Property of Mono- and Multivalent Cell-Penetrating Compounds, Biophys. J., 2008, 94, 2142 CrossRef CAS PubMed
.
- K. Donaldson, F. A. Murphy, R. Duffin and C. A. Poland, Asbestos, Carbon Nanotubes and the Pleural Mesothelium: A Review of the Hypothesis regarding the Role of Long Fibre Retention in the Parietal Pleura, Inflammation and Mesothelioma, Part. Fibre Toxicol., 2010, 7, 5 CrossRef PubMed
.
- P. N. Yaron, B. D. Holt, P. A. Short, M. Lösche, M. F. Islam and K. N. Dahl, Single Wall Carbon Nanotubes Enter Cells by Endocytosis and Not Membrane Penetration, J. Nanobiotechnol., 2011, 9, 45 CrossRef CAS PubMed
.
- J. Du, J. Jin, M. Yan and Y. Lu, Synthetic Nanocarriers for Intracellular Protein Delivery, Curr. Drug Metab., 2012, 13, 82 CrossRef CAS PubMed
.
- S. Sharifi, S. Behzadi, S. Laurent, M. L. Forrest, P. Stroeve and M. Mahmoudi, Toxicity of Nanomaterials, Chem. Soc. Rev., 2012, 41, 2323–2343 RSC
.
- S. Marchesan, K. Kostarelos, A. Bianco and M. Prato, The Winding Road for Cabon nanotubes in Nanomedicine, Mater. Today, 2015, 18, 12 CrossRef CAS
.
- P. M. Costa, M. Bourgognon, J. T. Wang and K. T. Al-Jamal, Functionalised Carbon Nanotubes: from Intracellular Uptake and Cell-Related Toxicity to Systemic Brain Delivery, J. Contr. Release, 2016, 241, 200 CrossRef CAS PubMed
.
- N. Nakayama, K. Hagiwara, Y. Ito, K. Ijiro, Y. Osada and K. Sano, Superior Cell Penetration by a Rigid and Anisotropic Synthetic Protein, Langmuir, 2015, 31, 2826 CrossRef CAS PubMed
.
- S. Bienert, A. Waterhouse, T. A. de Beer, G. Tauriello, G. Studer, L. Bordoli and T. Schwede, The SWISS-MODEL Repository-New Features and Functionality, Nucleic Acids Res., 2017, 45, D313 CrossRef CAS PubMed
.
- A. Waterhouse, M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, R. Lepore and T. Schwede, SWISS-MODEL: Homology Modelling of Protein Structures and Complexes, Nucleic Acids Res., 2018, 46, W296 CrossRef CAS PubMed
.
- F. G. Whitby and G. N. Phillips Jr, Crystal Structure of Tropomyosin at 7 Angstroms Resolution, Proteins, 2000, 38, 49 CrossRef CAS
.
- J. Sodek, R. S. Hodges, L. B. Smillie and L. Jurasek, Amino-Acid Sequence of Rabbit Skeletal Tropomyosin and Its Coiled-Coil Structure, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 3800 CrossRef CAS PubMed
.
- A. D. McLachlan and M. Stewart, Tropomyosin Coiled-Coil Interactions: Evidence for an Unstaggered Structure, J. Mol. Biol., 1975, 98, 293 CrossRef CAS PubMed
.
- J. Howard, Mechanics of the Cytoskeleton, in Mechanics of Motor Proteins and the Cytoskeleton, Sinauer Associates, Inc., Sunderland, MA, 2001, p. 135 Search PubMed
.
- N. Nakayama, K. Hagiwara, Y. Ito, K. Ijiro, Y. Osada and K. Sano, Noncationic Rigid and Anisotropic Coiled-Coil Proteins Exhibit Cell-Penetration Activity, Langmuir, 2015, 31, 8218 CrossRef CAS PubMed
.
- N. Nakayama, S. Takaoka, M. Ota, K. Takagaki and K. Sano, Effect of the Aspect Ratio of Coiled-Coil Protein Carriers on Cellular Uptake, Langmuir, 2018, 34, 14286 CrossRef CAS PubMed
.
- R. F. Itzhaki and D. M. Gill, A Micro-biuret Method for Estimating Proteins, Anal. Biochem., 1964, 9, 401 CrossRef CAS PubMed
.
- J. D. Esko, T. E. Stewart and W. H. Taylor, Animal Cell Mutants Defective in Glycosaminoglycan Biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 3197 CrossRef CAS PubMed
.
- J. D. Esko, Genetic Analysis of Proteoglycan Structure, Function and Metabolism, Curr. Opin. Cell Biol., 1991, 3, 805 CrossRef CAS PubMed
.
- L. K. Hallak, P. L. Collins, W. Knudson and M. E. Peeples, Iduronic Acid-Containing Glycosaminoglycans on Target Cells Are Required for Efficient Respiratory Syncytial Virus Infection, Virology, 2000, 271, 264 CrossRef CAS PubMed
.
- C. M. Leistner, S. Gruen-Bernhard and D. Glebe, Role of Glycosaminoglycans for Binding and Infection of Hepatitis B Virus, Cell. Microbiol., 2008, 10, 122 CAS
.
- T. M. Clausen, D. R. Sandoval, C. B. Spliid, J. Pihl and H. R. Perrett, et al., SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2, Cell, 2020, 183, 1043 CrossRef CAS PubMed
.
- B. Zhou, J. A. Weigel, L. Fauss and P. H. Weigel, Identification of the Hyaluronan Receptor for Endocytosis (HARE), J. Biol. Chem., 2000, 275, 37733 CrossRef CAS PubMed
.
- K. G. Rothberg, J. E. Heuser, W. C. Donzell, Y.-S. Ying, J. R. Glenney and R. G. W. Anderson, Caveolin, a protein component of caveolae membrane coats, Cell, 1992, 68, 673–682 CrossRef CAS PubMed
.
- H. A. Anderson, Y. Chen and L. C. Norkin, Bound Simian Virus 40 Translocates to Caveolin-Enriched Membrane Domains, and Its Entry Is Inhibited by Drugs That Selectively Disrupt Caveolae, Mol. Biol. Cell, 1996, 7, 1825 CrossRef CAS PubMed
.
- I. Mäger, K. Langel, T. Lehto, E. Eiríksdóttir and Ü. Langel, The Role of Endocytosis on the Uptake Kinetics of Luciferin-Conjugated Cell-Penetrating Peptides, Biochim. Biophys. Acta, 2012, 1818, 502 CrossRef PubMed
.
- C. K. Payne, S. A. Jones, C. Chen and X. Zhuang, Internalization and Trafficking of Cell Surface Proteoglycans and Proteoglycan-Binding Ligands, Traffic, 2007, 8, 389–401 CrossRef CAS PubMed
.
- M. R. Bubb, A. M. Senderowicz, E. A. Sausville, K. L. Duncan and E. D. Korn, Jasplakinolide, a Cytotoxic Natural Product, Induces Actin Polymerization and Competitively Inhibits the Binding of Phalloidin to F-Actin, J. Biol. Chem., 1994, 269, 14869 CrossRef CAS PubMed
.
- A. S. Desai, M. R. Hunter and A. N. Kapustin, Using Macropinocytosis for Intracellular Delivery of Therapeutic Nucleic Acids to Tumour Cells, Philos. Trans. R. Soc. Lond. B Biol. Sci., 2019, 374, 20180156 CrossRef CAS PubMed
.
- P. Sehr, G. Joseph, H. Genth, I. Just and E. Pick, Aktories, Glucosylation and ADP Ribosylation of Rho Proteins: Effects on Nucleotide Binding, GTPase Activity, and Effector Coupling, Biochemistry, 1998, 37, 5296 CrossRef CAS PubMed
.
- W. P. Ciesla Jr and D. A. Bobak, Clostridium difficile Toxins A and B Are Cation-Dependent UDP-Glucose Hydrolases with Differing Catalytic Activities, J. Biol. Chem., 1998, 273, 16021 CrossRef CAS PubMed
.
- S. Gerbal-Chaloin, C. Gondeau, G. Aldrian-Herrada, F. Heitz, C. Gauthier-Rouvière and G. Divita, First Step of the Cell-Penetrating Peptide Mechanism Involves Rac1 GTPase-Dependent Actin-Network Remodelling, Biol. Cell, 2007, 99, 223–238 CrossRef CAS PubMed
.
- S. Pujals and E. Giralt, Proline-Rich, Amphipathic Cell-Penetrating Peptides, Adv. Drug Deliv. Rev., 2008, 60, 473 CrossRef CAS PubMed
.
- J. S. Wadia, R. V. Stan and S. F. Dowdy, Transducible TAT-HA Fusogenic Peptide Enhances Escape of TAT-Fusion Proteins after Lipid Raft Macropinocytosis, Nat. Med., 2004, 10, 310 CrossRef CAS PubMed
.
- A. Fittipaldi, A. Ferrari, M. Zoppé, C. Arcangeli, V. Pellegrini, F. Beltram and M. Giacca, Cell Membrane Lipid Rafts Mediate Caveolar Endocytosis of HIV-1 Tat Fusion Proteins, J. Biol. Chem., 2003, 278, 34141 CrossRef CAS PubMed
.
- K. L. Veiman, I. Mäger, K. Ezzat, H. Margus, T. Lehto, K. Langel, K. Kurrikoff, P. Arukuusk, J. Suhorutšenko, K. Padari, M. Pooga, T. Lehto and Ü. Langel, PepFect14 Peptide Vector for Efficient Gene Delivery in Cell Cultures, Mol. Pharm., 2013, 10, 199 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: microscopic observations of the cellular internalization of CCPCs. Fig. S2: cytotoxicity of endocytic inhibitors using in this study. See DOI: https://doi.org/10.1039/d4ra07763f |
| This journal is © The Royal Society of Chemistry 2025 |