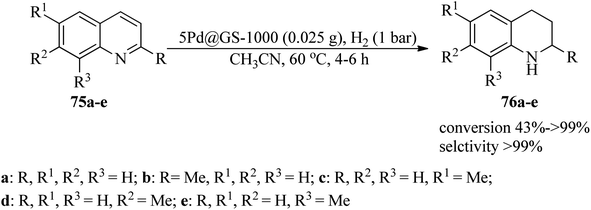Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
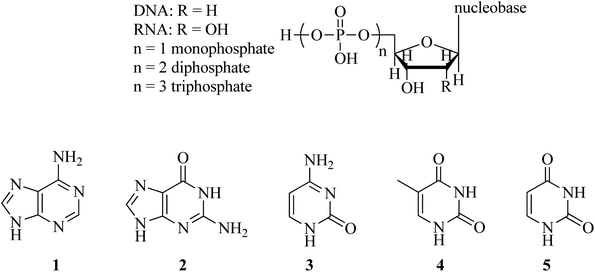
Applications of catalytic systems containing DNA nucleobases (adenine, cytosine, guanine, and thymine) in organic reactions
Zahra Khademi and
Kobra Nikoofar *
*
Department of Organic Chemistry, Faculty of Chemistry, Alzahra University, P.O. Box 1993891176, Tehran, Iran. E-mail: k.nikoofar@alzahra.ac.ir; kobranikoofar@yahoo.com; Fax: +982188041344; Tel: +982188041344
First published on 31st January 2025
Abstract
In recent years, nucleobases have attracted special attention because of their abundant resources and multiple interaction sites, which enable them to interact with and functionalize other molecules. This review focuses on the catalytic activities of each of the four main nucleobases found in deoxyribonucleic acid (DNA) in various organic reactions. Based on the studies, most of the nucleobases act as heterogeneous catalytic systems. The authors hope their assessment will help chemists and biochemists to propose new procedures for utilizing nucleobases as catalysts in various organic synthetic transformations. The review covers the corresponding literature published till the end of August 2023.
1. Introduction
Nucleobases, such as adenine (A, 1), guanine (G, 2), cytosine (C, 3), thymine (T, 4), and uracil (U, 5), are the fundamental units of the genetic code and have been found in DNA (Scheme 1). Nucleosides are made up of a sugar ring (2′-deoxyribose for DNA and ribose for RNA) and a nucleobase. Nucleotides contain a nucleoside and at least one phosphate unit.The bases A, T, C, and G are found in deoxyribonucleic acid (DNA), while A, U, C, and G are present in RNA. It is widely known that A binds to T (or U), while G pairs with C through hydrogen bonding. Due to the capability of nucleobases to form base pairs and stack upon one another, they are capable of forming long-chain helical frameworks, such as those found in DNA and RNA.1–4 Overall, the nucleobases can interact with each other as well as with other organic/inorganic small molecules.1
Adenine and guanine have a fused-ring structure derived from purine; thus, they are classified as purine bases. On the other hand, cytosine, uracil, and thymine consist of a simple heterocyclic aromatic ring derived from pyrimidine, and hence, they are called pyrimidine bases.5,6
Adenine was first synthesized as a white powder by Oro in 1960.7 This compound was produced by heating concentrated ammonium cyanide (NH4CN, 1–15 M) at 27 °C to 100 °C for several days, followed by the elimination of a black polymer through centrifugation and the reaction of the supernatant with HCl.8
Thymine was first isolated by Kossel and Neumann in 1893 from calf thymus glands, followed by its first synthesis through hydrolysis of the related nucleoside derived from natural sources. In the early 1900s, Fischer presented a synthetic procedure starting from urea, but a more applicable method utilized methylisothiourea instead of urea in the condensation reaction with ethyl formyl propionate in water to form the pyrimidine intermediate, which was then subjected to acidic hydrolysis to afford thymine, with methanethiol as a by-product.9
In 1984, cytosine was isolated through the hydrolysis of calf thymus tissue. Its structure was identified in 1903, and it was first synthesized from 2-ethylthiopyrimidin-4(3H)-one.10
The first isolation of guanine was reported in 1846 by Unger from the excreta of sea birds as a mineral. Subsequently, between 1882 and 1906, the guanine structure was discovered, and the transformation of uric acid into guanine was revealed.11,12
Uracil was originally identified in 1900 by Ascoli and then isolated via hydrolysis of yeast nuclein.13 This compound was also detected in bovine thymus and spleen.14
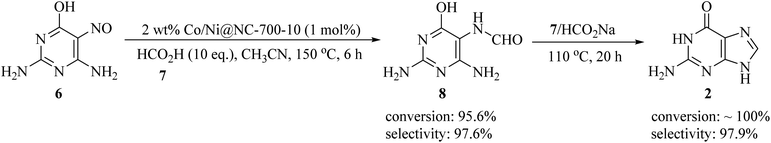
Because of the widespread utility of nucleobases, many studies have been published on different synthesis methods for nucleobases. For example, in 2022, Wang et al. prepared a nitrogen-doped carbon-based Co/Ni bimetallic catalyst (2 wt% Co/Ni@NC-700-10) using chitosan as the nitrogen and carbon source. It was found to be effective for guanine formation, utilizing 2,4-diamino-5-nitroso-6-hydroxypyrimidine (DANHP, 6) as starting material. In the first step, the reaction was initiated via the one-pot reductive N-formylation of 6 with formic acid (7) using 2 wt% Co/Ni@NC-700-10 in acetonitrile media at 150 °C to obtain 2,4-diamino-5-formyl-6-hydroxypyrimidine (DAFHP, 8) with excellent conversion (95.6%) and selectivity (97.6%), which after isolation underwent a cyclization reaction in the presence of formic acid (7) and sodium formate at 110 °C to achieve guanine (2). Based on the resulting HPLC spectra, the conversion of 8 was about 100% with an excellent selectivity of 97.9% (Scheme 2).15
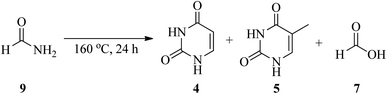
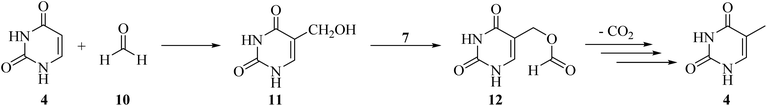
In 2021, an article entitled “Prebiotic route to thymine from formamide-a combined experimental–theoretical study” focused on the catalyst-free conversion of uracil to thymine through the thermolysis of formamide (9) at 160 °C within 24 h. This reaction progressed in the presence of formic acid (7) as a key molecule formed via the hydrolysis of 9 (Scheme 3). It is important that the disproportionation of formic acid (7) resulted in the formation of CO2 and formaldehyde (10), and the latter compound plays an essential role in the hydroxymethylation of uracil16 in the first step of the transformation procedure. The reaction was followed by the esterification of the hydroxyl group of 5-hydroxymethyluracil (11) to give 12, which finally rearranged to thymine (4) upon the removal of CO2 (Scheme 4).17,18
In 2020, Yadav et al. reported a review article entitled “Chemistry of abiotic nucleotide synthesis”, which pointed to the prebiotic synthesis of nucleobases from HCN derivatives, such as formamide and urea19 or by other sources, with the exception of HCN and formamide.20 In 2018, a review entitled “Origins of building blocks of life: a review” reported various methods for the synthesis of nucleobases under simulated prebiotic conditions.21
In addition to the above-mentioned methods, the nucleobases could be generated under various conditions. For example, the Nelson group in 2001 studied the synthesis of cytosine and uracil from urea and cyanoacetaldehyde at 100 °C under dry-down conditions and in solution at 4 °C and −20 °C.22 In 2004, Orgel prepared adenine via the photochemical conversion of the tetramer of hydrogen cyanide in eutectic solution to 4-amino-5-cyano-imidazole.23 Cleaves et al. in 2006 reported a simple prebiotic procedure to obtain cytosine and uracil upon freezing in solution.24 Menor-Salvan et al. in 2009 synthesized cytosine and uracil from cyanoacetylene/cyanoacetaldehyde and frozen urea solution under a methane/nitrogen atmosphere.25 In 2007, they also obtained some purine bases through the spark activation of an atmosphere of methane, nitrogen and hydrogen in the presence of an aqueous aerosol.26
Formamide (which could be obtained from hydrogen cyanide and water) has received potential pre-genetic and pre-metabolic interest and is sometimes considered the origin of life.27
Owing to the critical role of formamide in the prebiotic synthesis of nucleobases, numerous studies have been performed utilizing formamide as a possible source of nucleobases under different experimental conditions.28 Barks et al. in 2010 reported the catalyst-free synthesis of adenine and guanine from UV-irradiated formamide solutions.29 In addition, several mineral and metal oxide catalysts were utilized to obtain various DNA-nucleobases from formamide. Saladino et al. in 2001 reported the catalytic (in the presence of CaCO3, silica, alumine, kaolin, and zeolite-Y) formation from neat formamide.30 Some other catalytic systems to obtain DNA-nucleobases from the formamide substrate are: TiO2,31 montmorillonites,32 cosmic-dust analogues,33 iron–sulfur and iron–copper–sulfur minerals,34 zirconia,35 common rock-forming minerals (such as silicates, and carbonates),36 and iron oxides/hydroxides (hematite, goethite, and akaganeite).37 In 2022, Nejdl et al. utilized ZnCd QDs upon UV irradiation in prebiotic liquid formamide to obtain some nucleobases with increased yields.38 Oba et al. in 2019 achieved DNA-nucleobases (cytosine, uracil, thymine, and adenine) in interstellar ice analogues composed of simple molecules, including H2O, CO, NH3, and CH3OH after exposure to ultraviolet photons, followed by thermal processes.39 In 2013, the preparation of adenine form formamide was considered a self-catalytic mechanism in an abiotic approach.40
A considerable number of enzymes consist of nucleobase motifs.41 As some enzymes or enzyme complexes require several cofactors, they utilize nucleotide cofactors, including a purine base (usually adenine) binding site.42 Adenine is an inextricable part of enzyme cofactors and second messenger systems, such as NAD+, FADH2, and cAMP, which are essential for certain catalytic reactions and biochemical processes. In addition, a crucial catalytic role of the adenine moiety is also observed in group II intron catalysis and at the ribosomal peptidyltransferase center.43
Nucleobases and their derivatives reveal widespread biological and therapeutic activities in medicinal chemistry as notable pharmacophores,44,45 antituberculosis agents,46 kinase or cyclin-dependent kinase inhibitors,47,48 antibiotics and biofilm inhibitors,49 HIV viral capsid inhibitor,50 antiviral (such as Human Immunodeficiency Virus and Hepatitis Virus),51–53 antineoplastic agents,54 and A3 adenosine receptor antagonists and ligands.55
Nucleobases possess a wide range of applications in different branches of science and technology (with various roles such as organic/bio-catalyst, reagent, substrate, ligand, capping agent, and promoter) such as polymer preparation and/or modifications,56–58 medicinal chemistry and therapeutic investigations,59 in vitro and/or in vivo drug delivery60,61 nanomaterials and nanotechnology,62–65 electrochemistry and electrochemical sensors,66–70 supramolecular chemistry,71–73 metal–organic frameworks (MOFs) chemistry,74–76 heterogeneous catalysts and/or catalytic systems,77–80 batteries and energy-storage,81–83 oxidation/reduction chemistry,84–87 and biodiesel synthesis.88 In 1999, some Cu(adenine)2 complexes displayed the promotion of O2 production from H2O2 through the disproportionation of hydrogen peroxide into oxygen and water.89 Adenine-functionalized conjugated polymer PF6A-DBTO2 demonstrated high photocatalytic activity with hydrogen evolution from water.90
The plentiful resources of nucleobases and numerous interaction sites, for instance, hydrogen bonding, π–π stacking, and van der Waals forces, enable them to interact and functionalize other molecules.91 Based to these properties, the authors have reviewed the literature reports about the catalytic activity of nucleobases in various organic transformations. It must be mentioned that the number of reports for applications of adenine, cytosine, guanine, and thymine as a sole catalyst or part of the catalytic systems is few, so the catalytic role of each of the four DNA nucleobases id investigated in each part as below.
2. Catalytic activity of adenine in various organic transformations
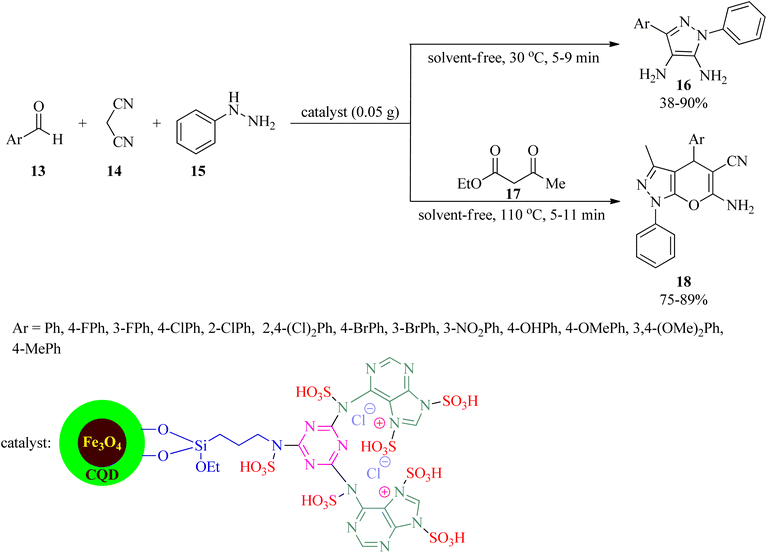
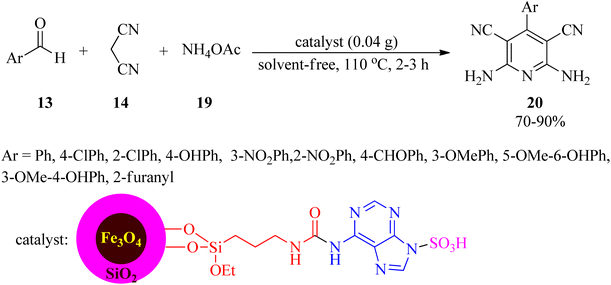
Owing to the key role of pyrazole motif in various fields of chemistry and biology,92–95 Ahmadi et al. in 2023 presented a novel, applicable, and efficacious technique to prepare 5-amino-1,3-diphenyl-1H-pyrazole 4-carbonitrile and pyrano[2,3-c]pyrazoles utilizing [Fe3O4@CQD@Si(OEt)(CH2)3NH@CC@A@SO3H]+Cl− catalyst under solvent-free conditions. Firstly, the catalyst was synthesized via coating the Fe3O4 magnetic nanoparticles with carbon quantum dots (CQD), followed by surface modification with (3-propylamine)-triethoxysilane, functionalization by cyanuric chloride (CC) and adenine, and subsequent functionalization with chlorosulfonic acid. Then, 5-amino-1,3-diphenyl-1H-pyrazole 4-carbonitrile (16) was obtained via the condensation of equimolar amounts of aromatic aldehydes (13) with malononitrile (14) and phenyl hydrazine (15) at 30 °C in low to excellent yields (38–90%) and very short reaction times (5–9 min). On the other hand, the one-pot four-component condensation reaction of 13, 14, 15, and ethyl acetoacetate (17) realized pyrano[2,3-c]pyrazoles (18) within short reaction times in good yields (Scheme 5). The magnetic separability and recoverability of the catalyst were examined within 5 runs without notable activity loss.96In 2022, Sadri et al. established a straightforward, convenient, and useful method for the production of 2,6-diamino-4-arylpyridine-3,5-dicarbonitrile (20) via the one-pot pseudo-four-component condensation reaction of aldehydes (13) with 14, and ammonium acetate (19) utilizing a nanomagnetic catalyst coated with adenine and sulfonic acid (Fe3O4@SiO2@(CH2)3NHCO-adenine sulfonic acid), as a facile, cost-effective, recyclable, and reusable catalyst, under solvent-free conditions at 110 °C (Scheme 6). Mild reaction conditions, easy catalyst preparation, simple purification and isolation of the products (no chromatographical techniques) are some of the noteworthy features of this process.97
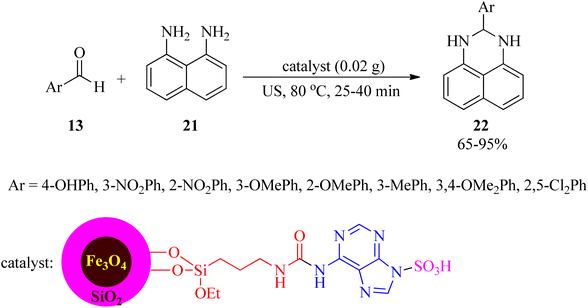
The same research group also subjected this catalyst for the synthesis of 2,3-dihydro-2-aryl-1H-perimidines (22) using aryl aldehydes (13) and 1,8-diamino naphthalene (21) under ultrasonic irradiation and solvent-free conditions (Scheme 7).98 The recovery and reusability test of the nanostructure was successful within 4 runs.
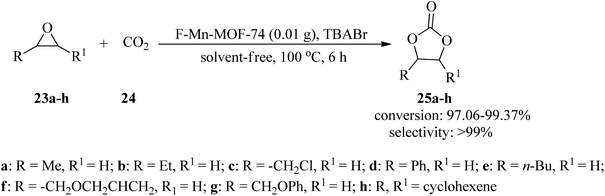
In 2020, a highly effective, applicable, and green technique was presented for the formation of cyclic carbonates (25a–h) through the cycloaddition reaction of epoxides (23a–h) with carbon dioxide (24, 10 bar pressure) by flower-like manganeses confined metal–organic framework (F-Mn-MOF-74) as a catalyst and tetrabutylammonium bromide (TBABr) as a co-catalyst under solvent-free conditions at 100 °C within 6 h with excellent conversion and selectivity. In this research, in order to prepare F-Mn-MOF-74, adenine was applied as an alkali source and competitive ligand in comparison to the synthetic Mn-MOF-74. It was found that the spherical F-Mn-MOF-74 catalyst, along with TBABr, disclosed excellent catalytic performance in the cyclic esterification reaction. It should be noted that the catalytic system proceeded with the transformation of cyclohexene oxide with CO2 (1 MP) at 160 °C within 24 h to produce the corresponding cyclic carbonate in moderate conversion (51.98%) and excellent selectivity (92.65%) (Scheme 8).99 Similarly, this transformation occurred at 80 °C in 8 h utilizing two new adenine-based Zn-(II)/Cd(II)-MOFs, namely, [Zn2(H2O)(stdb)2(5H-A)(9H-A)2]n (PNU-21) and [Cd2(Hstdb)(stdb)(8H-A)(A)]n (PNU-22), including auxiliary dicarboxylate ligand (stdb = 4,4′-stilbenedicarboxylate). The catalysts were characterized through different techniques, such as single-crystal X-ray diffraction (SXRD), which disclosed 2D and 3D rigid and robust building blocks for PNU-21 and PNU-22, respectively, along with coordinately unsaturated metal surroundings. It should be mentioned that the presence of unsaturated Zn and Cd metals and basic N atoms in both catalysts converted them into acid–base efficient binary catalysts for the preparation of the corresponding products. The results demonstrated the better utility of PNU-21 (11–96%) in comparison to PNU-22 (8–85%).100
In 2023, a new adenine-functionalized dendritic fibrous nanosilica (DAD) was synthesized by functionalizing the surface of dendritic fibrous nanosilica (DFNS) with adenine using a bifunctional isocyanate crosslinker ((EtO)3Si(CH2)3NCO). This adenine-functionalized dendritic fibrous nanosilica was used as a bifunctional catalyst for CO2 fixation in order to react with epoxide (23) to achieve cyclic carbonates (25). The reaction proceeded in the presence of TBAB (tetrabutylammonium bromide) co-catalyst under solvent-free conditions.101 In 2005, the cycloaddition of CO2 to epoxides (epichlorohydrin, propene oxide, and styrene oxide) to afford cyclic carbonates (25) occurred in the presence of adenine-modified Ti-SBA-15 catalysts. In addition, carbamates were synthesized through the reaction of alkyl/aryl amines, CO2, and n-butyl bromide. In the synthesis of cyclic carbonates, the reaction proceeded without any additional co-catalysts like N,N-dimethylaminopyridine (DMAP) or quaternary ammonium salts. The process using the present catalyst system avoids hazardous substances like phosgene or isocyanate and progresses at low temperatures and pressures.102
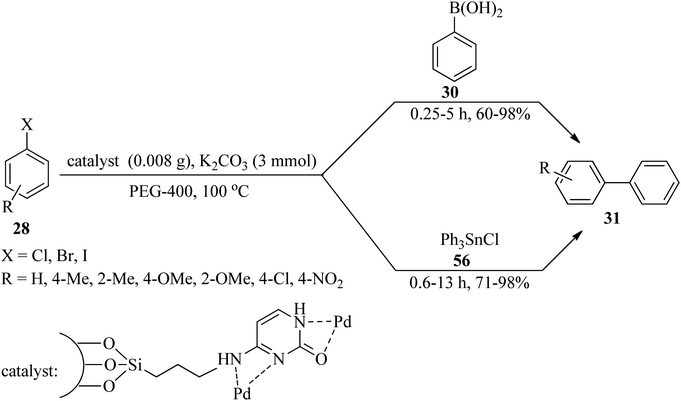
Rigid copper on adenine-coated boehmite nanoparticles (Cu-adenine@boehmite) was prepared by Ghorbani-Choghamarani et al. in 2019, which catalyzed various organic transformations, such as the selective oxidation of sulfides (26) to sulfoxides (27) by hydrogen peroxide as an oxidant under solvent-free conditions at room temperature (Scheme 9a). On the basis of the resulting data, diverse aromatic, aliphatic, and heterocyclic sulfides indicated no significant difference in the reaction yields, turnover numbers (TON), and turnover frequency (TOF) values. Generally, the aromatic sulfides prolong the reaction times. It is noteworthy that this transformation was not accompanied by forming sulfone as the by-product.103 The adenine-containing catalyst also promoted the aqua-mediated formation of biphenyls (31) through the Suzuki C–C coupling reaction of aryl halides (28) with sodium tetraphenylborate (29) or phenylboronic acid derivatives (30) (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios, respectively) utilizing sodium carbonate (Na2CO3) as a base at 80 °C (Scheme 9b). Notably, the coupling of with aryl iodides was performed in shorter reaction times than other aryl halides (chloride and bromide). The selectivity of the catalyst was examined via the utilization of 1-bromo-4-chlorobenzene in the coupling reaction with phenylboronic acid and sodium tetraphenyl borate. The results affirmed that the chloro functional group remained intact and the Suzuki reaction occurred on the bromo functional group as the only product. The formation of polyhydroquinolines (33) in aqueous media via the four-component condensation reaction of benzaldehyde (13), ethyl acetoacetate (17), ammonium acetate (19), and dimedone (32) in a 1
1 molar ratios, respectively) utilizing sodium carbonate (Na2CO3) as a base at 80 °C (Scheme 9b). Notably, the coupling of with aryl iodides was performed in shorter reaction times than other aryl halides (chloride and bromide). The selectivity of the catalyst was examined via the utilization of 1-bromo-4-chlorobenzene in the coupling reaction with phenylboronic acid and sodium tetraphenyl borate. The results affirmed that the chloro functional group remained intact and the Suzuki reaction occurred on the bromo functional group as the only product. The formation of polyhydroquinolines (33) in aqueous media via the four-component condensation reaction of benzaldehyde (13), ethyl acetoacetate (17), ammonium acetate (19), and dimedone (32) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, utilizing Cu-adenine@boehmite, were also performed successfully (Scheme 9c).103
1 molar ratio, utilizing Cu-adenine@boehmite, were also performed successfully (Scheme 9c).103
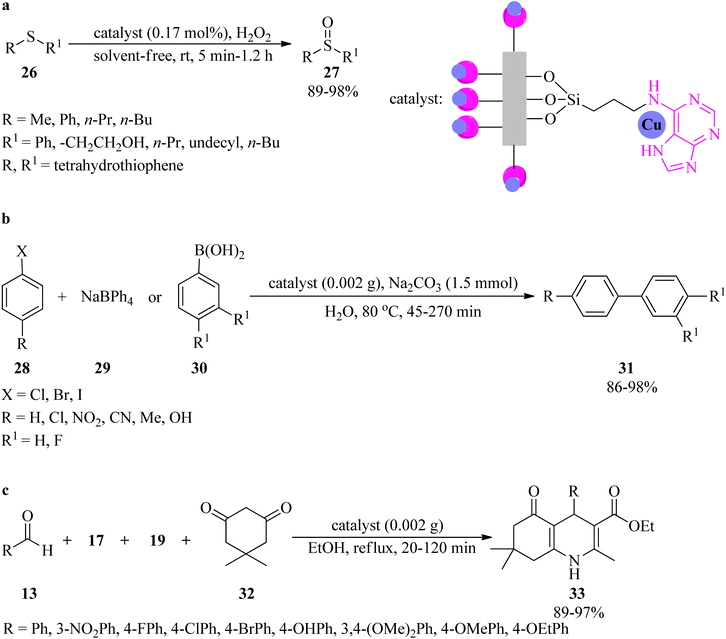 | ||
| Scheme 9 Various organic reactions in the presence of Cu-adenine@boehmite; (a) selective oxidation of sulfides to sulfoxides, (b) Suzuki coupling, and (c) synthesis of polyhydroquinolines. | ||
In 2018, Tamoradi et al. designed and characterized a zirconium complex of adenine coated on mesoporous silica MCM-41 (Zr-adenine-MCM-41) as a non-toxic and thermally stable nanocatalyst to accelerate the synthesis of symmetrical sulfides (26) via the dimethyl sulfoxide-mediated reaction of aryl halides (28) with sulfur (34) in equimolar amounts (Scheme 10a).104 As illustrated in Scheme 10b, the oxidative coupling reaction of thiols (35) was also accomplished to afford disulfides (36) using catalytic amounts of Zr-adenine-MCM-41 by H2O2 as the oxidative reagent under solvent-free conditions at room temperature in 89–98% yields and very short reaction times.104 Solvent-less oxidation of diverse kinds of sulfides (26) to their corresponding sulfoxides (27) was also performed in the presence of MCM-41-adenine-Zr (0.004 g) at ambient temperatures using H2O2 oxidant (0.4 mL) in short reaction times (5–65 min) and excellent yields (89–97%).104 The recovery and the reusability test of Zr-adenine-MCM-41 demonstrated good results for the 3-run synthesis of sulfides and 6-cycles for the oxidation of sulfides and oxidative coupling of thiols. The observation described negligible leaching of zirconium from MCM-41-adenine-Zr.
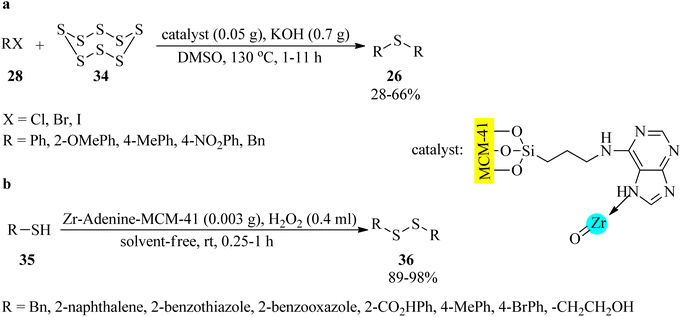 | ||
| Scheme 10 (a) Synthetic route to symmetrical sulfides, and (b) oxidative coupling reaction of thiols to disulfides in the presence of Zr-adenine-MCM-41. | ||
Tamoradi et al., in 2017, prepared a novel and green catalyst through the immobilization of Ni on magnetite nanoparticles coated with adenine (Fe3O4-adenine-Ni). The activity of the nanostructure was examined for the oxidation of sulfides (26) to sulfoxides (27) and oxidative coupling of thiols (35) to their corresponding disulfides (36) in the presence of H2O2 oxidant (0.5 mL). The reactions were performed at ambient temperature under solvent-free conditions and ethanol media to achieve the desired products in short reaction times (12–70 min, 25–90 min) and excellent efficacy (94–98%, 87–97%).105 The synthesis of polyhydroquinolines (33) via the refluxing ethanol-mediated four-component reaction of benzaldehydes (13), ethyl acetoacetate (17), ammonium acetate (19), and dimedone (32) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, utilizing Fe3O4-adenine-Ni (0.05 g) was also performed successfully (145–255 min, 91–97%).105
1 molar ratio, utilizing Fe3O4-adenine-Ni (0.05 g) was also performed successfully (145–255 min, 91–97%).105
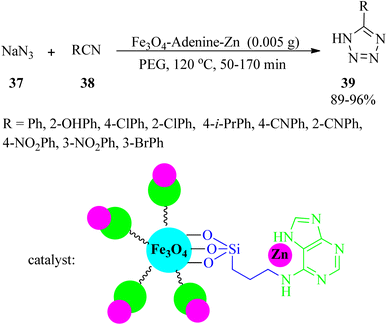
Zinc(II)-adenine complex functionalized on magnetite nanoparticles (Fe3O4-adenine-Zn) was synthesized and characterized in 2017. Its catalytic efficacy was tested for the synthesis of 5-substituted tetrazoles (39) via the reaction of sodium azide (37) with benzonitriles (38) in a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Scheme 11).106
1 molar ratio (Scheme 11).106
The catalyst was also utilized to promote the solvent-free oxidation of sulfides (26) to sulfoxides (27) using H2O2 oxidant in 15–130 min with excellent yields (89–98%). The oxidative coupling of thiols (35) to their corresponding disulfides (36) also occurred in the presence of Fe3O4-adenine-Zn in ethyl acetate media at room temperature within 40–130 min by 86–99%.106 A wide range of thiols (aliphatic, aromatic, and heterocyclic) were successfully subjected to the mentioned oxidative reactions. This catalyst could be recovered easily and reused at least six times without significant loss of its catalytic activity.
5-Substituted 1H-tetrazoles (39) were also obtained through the reaction of sodium azide (37) and benzonitrile (38) in a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
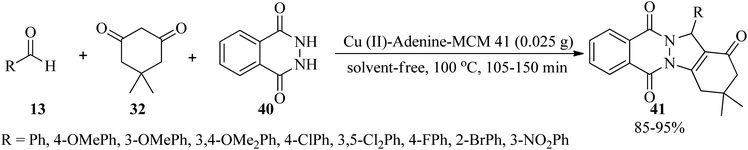
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of a Cu(II) complex supported in MCM-41 channels modified with adenine (Cu(II)-adenine-MCM-41 catalyst, 35 mg) in PEG-400 at 130 °C in 3–20 min and 70–92% yield.107 1H-indazolo[1,2-b]phthalazine-triones (41) were also obtained through the solvent-free reaction of various benzaldehydes (13), dimedone (32), and phthalhydrazide (40) in a 1
1 molar ratio in the presence of a Cu(II) complex supported in MCM-41 channels modified with adenine (Cu(II)-adenine-MCM-41 catalyst, 35 mg) in PEG-400 at 130 °C in 3–20 min and 70–92% yield.107 1H-indazolo[1,2-b]phthalazine-triones (41) were also obtained through the solvent-free reaction of various benzaldehydes (13), dimedone (32), and phthalhydrazide (40) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 100 °C (Scheme 12).107 The catalyst is a Cu(II)-Schiff-base complex supported on MCM-41, which was successfully prepared via the post-grafting method.
1 molar ratio at 100 °C (Scheme 12).107 The catalyst is a Cu(II)-Schiff-base complex supported on MCM-41, which was successfully prepared via the post-grafting method.
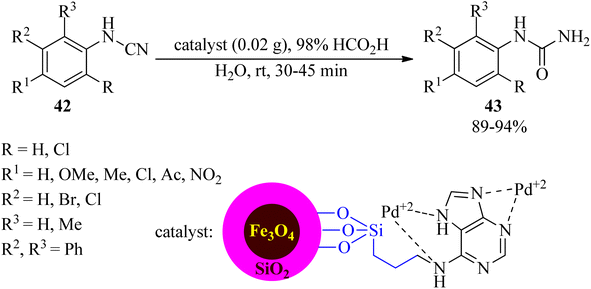
In 2023, Khorram Abadi et al. examined the hydrolysis of cyanamides (42) by formic acid in water media in the presence of palladium(II)-adenine complex coated on the surface of a silica-modified magnetic catalyst by employing 3-chloropropyltrimethoxysilane (CPTMS) as a linker and (Fe3O4@SiO2@CPTMS@A@Pd), as a new, efficient, and recyclable heterogeneous nanocatalyst at room temperature to obtain N-mono-substituted ureas (43) in high yields and short reaction times (Scheme 13). The catalytic system progressed well with all kinds of aryl cyanamides containing electron-donating and electron-withdrawing substituents.108 The catalyst was effective for five consecutive runs. The role of formic acid is probably to protonate the –CN group to increase its polarity. Also, it could protonate water to give H3O+ and HCOO− to assist Pd in hydrolyzing CN to CONH2.
In 2009, Goswami and Das developed an environmentally friendly approach for the diastereoselective synthesis of Mannich base products (46) via a three-component Mannich-type reaction of benzaldehydes (13), cyclohexanone (44), and anilines (45), utilizing adenine as an aminocatalyst (20 mol%) and H2O2 (30%, 4 μL) as additive in a mixture of water and ethanol (1/4) media at room temperature in 4–10 h in good to excellent yields (80–95%). It is significant that anilines, with electron-donating groups, resulted in the major generation of anti-products (46a) while the electron-withdrawing substituents (such as nitro and iodo) attained syn-form (46b) as the main products (Scheme 14a).109 It is necessary to mention that the condensation of cyclohexanone (47) and aniline, with both electron-donating and electron-withdrawing benzaldehydes, occurred successfully in high to excellent yields (75–93%). The stereochemical outcome appears to be unaffected, with the anti-isomer being the main product. Remarkably, the reaction with aliphatic aldehydes was unsuccessful. Replacing cyclohexanone with 2-butanone (47), as an unsymmetrical acyclic ketone, yielded two regioisomers 48 and 49, in which product 49 produced a mixture of diastereomers where the anti-diastereomers were the major products (Scheme 14b).109 In this transformation, the reactivity of 4-butanone was lower than that of cyclohexanone and 40 mol% of organocatalyst was needed for completion. The absence of column chromatography for majority of the compounds partially overcomes the major problem of epimerization of the Mannich products.110
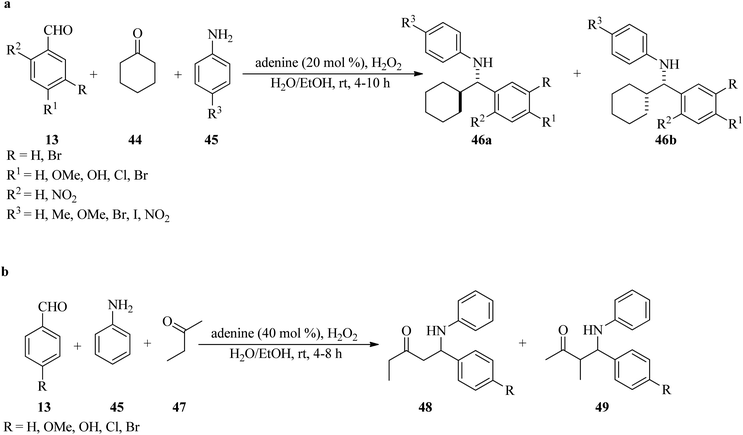 | ||
| Scheme 14 Adenine-mediated Mannich type reaction of (a) cyclohexanone, amines, and various benzaldehydes, and (b) 2-butanone, amines, and aldehydes. | ||
In 2020, the rigid palladium-adenine complex on modified boehmite nanoparticles (Pd-adenine@boehmite) was introduced as a beneficial, recoverable, and reusable heterogeneous nanocatalyst for the aqua-mediated preparation of biphenyls (31) through the Suzuki coupling reaction of aryl halides (28) with sodium tetraphenylborate (29) or phenylboronic acid derivatives (30) utilizing sodium carbonate (Na2CO3) as the base at 80 °C within 0.5–4 h in high to excellent yields (85–96%).111 Notably, the resulting TOF values for aryl halides affirmed their reactivity as PhI > PhBr > PhCl. Furthermore, aryl halides bearing an electron-withdrawing group were more reactive. Sodium tetraphenylborate or phenylboronic acid derivatives have no significant influence on the yields. In addition, the [3 + 2] cycloaddition reaction of sodium azide (37) with benzonitriles (38) in PEG at 120 °C using Pd-adenine@boehmite resulted in 5-substituted tetrazoles (39) in 0.3–21 h and good to excellent yields (85–96%). Both electron-donating and electron-withdrawing benzonitriles resulted in products with good yields and appropriate TOF numbers.111
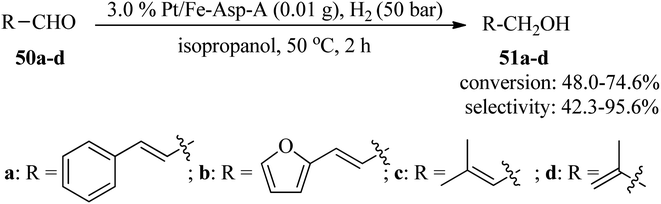
Cinnamyl alcohol is a significant chemical intermediate utilized in medicines, flavorings, and food additives. Cinnamyl alcohol is mainly produced through the selective hydrogenation of cinnamaldehyde.112–114 Hence, Tang et al., in 2022, employed an amorphous Pt/Fe-Asp-A nanocatalyst for chemoselective hydrogenation of cinnamaldehydes. Adenine was utilized as a reinforcing agent to improve the chemical stability and selectivity of the Fe-L-aspartic (Fe-Asp) coordination material, which was modified through the loading of platinum nanoparticles on its surface. Finally, the resultant amorphous 3.0% Pt/Fe-Asp-A nanocatalyst catalyzed the reaction of cinnamaldehyde (50a) with hydrogen (H2) at 20 bar in the presence of isopropanol at 50 °C to furnish cinnamyl alcohol (51a) in a 2 h period with excellent selectivity (91.2%) and conversion (93.1%). The amorphous 3.0% Pt/Fe-Asp-A nanocatalyst also exhibited good performance to promote various α,β-unsaturated aldehydes (50a–d) under similar conditions with 48.0–74.6% conversion and 42.3–95.6% selectivity (Scheme 15). The recyclability and reusability of the synthesized nanocatalyst have been successfully investigated in up to ten runs without any considerable loss in catalytic activity.115
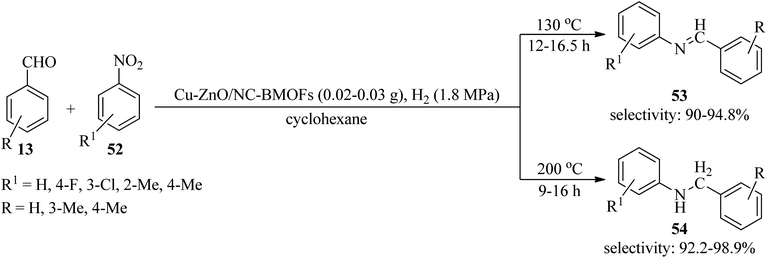
In 2021, a bimetallic CuZn-MOFs was generated via a facile solvothermal method utilizing adenine biomolecule as an organic linker. Moreover, a ZnO/nitrogen-doped carbon composite immobilized Cu catalyst (Cu-ZnO/NC-BMOFs) from CuZn-MOFs was obtained through a one-pot pyrolysis procedure. Then, its catalytic activity was examined by the hydrogenation/amination tandem reaction of benzaldehydes (13) and substituted nitroarenes (52) in cyclohexane via two pathways: (a) production of imines (53) at 130 °C in 12–16.5 h with high selectivity (90–94.8%), (b) generation of secondary amines (54) at 200 °C with high activity and high selectivity (92.2–98.9%) (Scheme 16). Comparatively, the Cu/ZnO/NC-IWI catalyst, prepared by incipient wetness impregnation, promoted the hydrogenation/amination reaction of nitrobenzene with benzaldehyde to give N-benzylaniline as compared to the CuZn-MOFs, and the conversion of nitrobenzene and selectivity toward N-benzylaniline was lower (49.6% and 10.9%). High selectivities, good stability, recyclability, and reusability of the catalyst are some of the advantages of this method.116
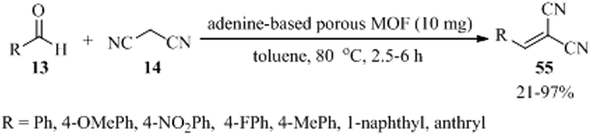
A novel adenine-based porous MOF, named [H2N(CH3)2]·[Zn4(L)1.5(ad)3(H2O)2].4DMF (denoted as JUC-188, DMF = N,N-dimethylformamide), was prepared utilizing tetracarboxylic acid organic ligand, namely 5,5′-(1,3,6,8-tetraoxobenzo[Imn][3,8] phenanthroline-2,7-diyl)bis-1,3-benzenedicarboxylic acid (H4L), and adenine (ad) as organic linker. H4L and ad are both successfully connected to Zn(II) ions. There are three different inorganic clusters in JUC-188, including ZnO2N2, Zn2O2N6, and ZnO5N clusters. The Knoevenagel condensation of different aldehydes (13) and malononitrile (14), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 molar ratio, in the presence of JUC-188 as a solid catalyst has been examined successfully (Scheme 17).117 In the case of small aldehydes, the results are satisfactory. But for 1-naphthaldehyde and 9-anthracenecarboxaldehyde, the yields are very low, even with prolonged reaction times of 6 h with 43% and 21% yields, respectively. So, the catalyst is referred to as size-selective. Furthermore, it showed that adenine ligands can be applied to construct MOFs with the Lewis basic sites (–NH2) as heterogeneous catalysts.
1.1 molar ratio, in the presence of JUC-188 as a solid catalyst has been examined successfully (Scheme 17).117 In the case of small aldehydes, the results are satisfactory. But for 1-naphthaldehyde and 9-anthracenecarboxaldehyde, the yields are very low, even with prolonged reaction times of 6 h with 43% and 21% yields, respectively. So, the catalyst is referred to as size-selective. Furthermore, it showed that adenine ligands can be applied to construct MOFs with the Lewis basic sites (–NH2) as heterogeneous catalysts.
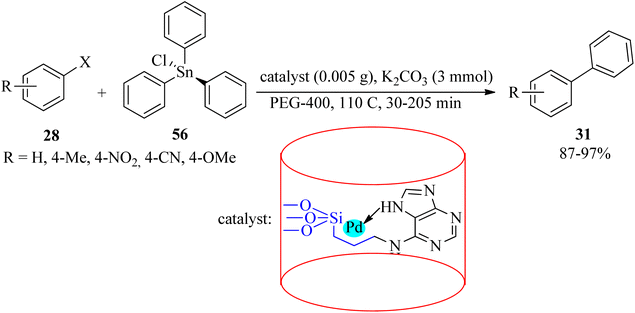
In 2019, palladium anchored on the surface of adenine-modified mesoporous silica was used to obtain SBA-15@adenine-Pd. Its efficacy was examined in the Stille cross-coupling reaction of different aryl halides (28) with triphenyltin chloride (56) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 molar ratio in PEG-400 media at 110 °C (Scheme 18).118
0.5 molar ratio in PEG-400 media at 110 °C (Scheme 18).118
The mesoporous silica-anchored SBA-15@adenine-Pd also promoted the Suzuki coupling of equimolar amounts of various aryl halides (28) with phenylboronic acid (30) in hot (110 °C) PEG-400 media in the presence of K2CO3 to obtain the corresponding biphenyl adducts (31) in 30–160 min and 83–98% yield.118 SBA-15@adenine-Pd was also effective for the synthesis of symmetrical sulfides (27) via the reaction of aryl halides (28) and sulfur (34) in DMSO at 130 °C in the presence of KOH (0.5 g) to afford the desired products within 80–610 min by 43–79%.118 The prepared catalyst separation could be easily achieved through filtration and drying after each run and can be reused for several consecutive cycles without a considerable decrease in its catalytic activity in all three types of the mentioned reactions.
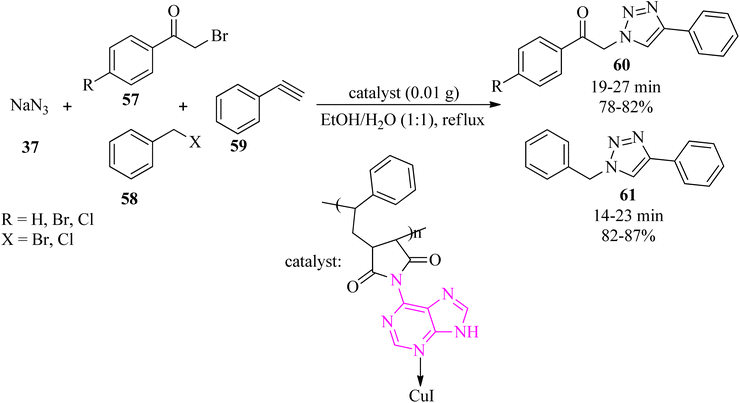
Novel adenine-based nano Cu(I) polymers were obtained via the immobilization of Cu(I) nanoparticles on modified poly(styrene-co-maleic anhydride) by adenine (Af-SMA-CuI). Its efficacy was successfully examined in the regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles (60)/(61) via the click reaction of sodium azide (37), α-haloketones (57)/alkyl halide (58), and alkyne (59), in a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 molar ratio, to give the corresponding products (60)/(61) in satisfactory yields (78–87%). The copper content in the catalyst was determined to be 23.83% (w/w), and each gram of the heterogeneous catalyst includes 1.25 mmol of copper (Scheme 19).119
1:1 molar ratio, to give the corresponding products (60)/(61) in satisfactory yields (78–87%). The copper content in the catalyst was determined to be 23.83% (w/w), and each gram of the heterogeneous catalyst includes 1.25 mmol of copper (Scheme 19).119
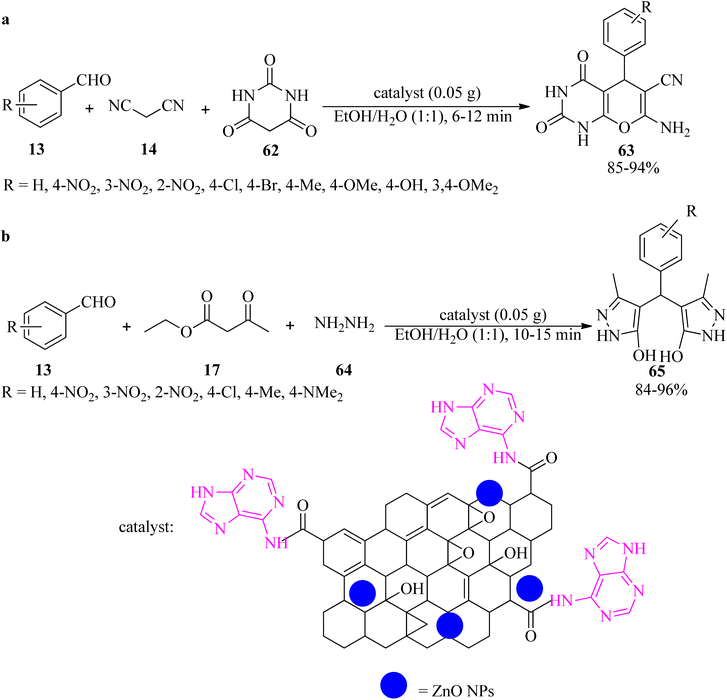
In 2020, the adenine-grafted carbon-modified amorphous ZnO nanocatalyst (ZnO@AC) was derived from garment industry waste (waste cotton cloth). It promoted the synthesis of pyrano[2,3-d]pyrimidines (63) via the reaction of aldehydes (13), malononitrile (14), and barbituric acid (62) in EtOH/H2O at ambient temperature (Scheme 20a).120 The catalyst is also effective for the domino-type synthesis of bis(pyrazol-5-ole) derivatives (65) via the pseudo-five-component reaction of aromatic aldehydes (13), ethyl acetoacetate (17), and hydrazine hydrate (64), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, at room temperature (Scheme 20b). The photocatalytic evaluation of ZnO@AC was performed on the methyl orange (MO) dye under UV light, with 87.3% degradation efficiency in 75 min. Moreover, the catalyst was recyclable and could be reused for up to eight runs, making it more sustainable.120
2 molar ratio, at room temperature (Scheme 20b). The photocatalytic evaluation of ZnO@AC was performed on the methyl orange (MO) dye under UV light, with 87.3% degradation efficiency in 75 min. Moreover, the catalyst was recyclable and could be reused for up to eight runs, making it more sustainable.120
3. Catalytic activity of guanine in two- and multi-component reactions
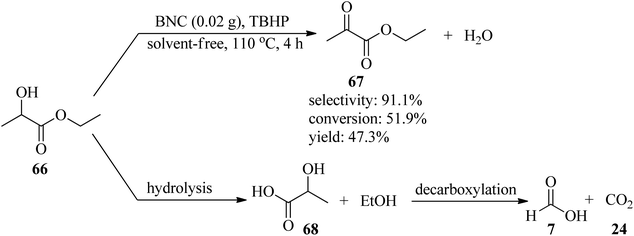
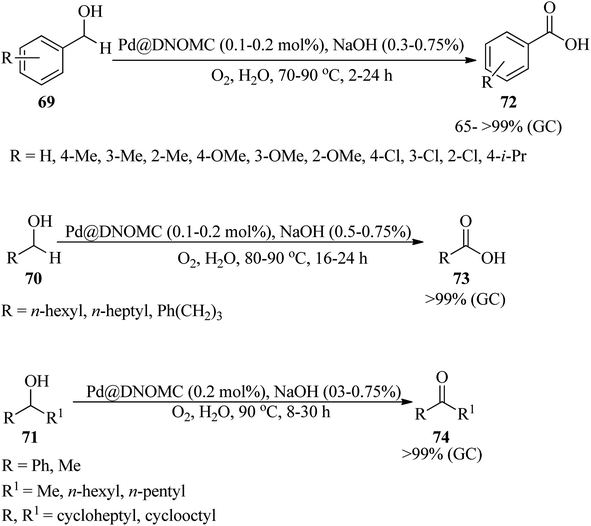
In 2012, a cost-effective, metal-free, and template-free methodology was presented for the generation of boron (B) and nitrogen (N) co-doped carbon nanosheets (BNC), utilizing biomolecule guanine as the carbon (C) and N sources and boric acid as the B precursor. In the obtained BNC, guanine forms the G-quartet unit cells and expands to a larger planar network structure through multiple hydrogen bonds as both C and N sources. Subsequently, the resultant BNC catalyzed a liquid phase selective oxidation of ethyl lactate (EL, 66) to ethyl pyruvate (EP, 67) by tert-butyl hydroperoxide (TBHP) as an oxidant under solvent-free conditions at 110 °C for 4 h with excellent selectivity (91.1%) and moderate conversion (51.9%) and yield (47.3%). This transformation was accompanied by the production of ethanol and lactic acid (68) as by-products through hydrolysis, which could be attributed to the instability of the ester functional group at elevated temperatures. Then, decarboxylation of the resulting lactic acid yielded formic acid (7) and CO2 (24) (Scheme 21). On the other hand, the selective reduction of nitrobenzene (52) in the presence of hydrazine hydrate (64) under solvent-free conditions at 100 °C within 4 h afforded aniline (45) with excellent selectivity (95.5%), conversion (97.9%), and yield (93.5%). It is worth noting that in order to affirm the usefulness of the protocol, the oxidation and reduction reactions were carried out under catalyst-free conditions and in the presence of different catalysts such as graphene, nitrogen-doped carbon (NC), oxidized carbon nanotubes (oCNT), BNC-1 and BNC-2 (containing lower boric acid, and lower amounts of B, N and O), and according to the resulting data, the utilization of BNC was more efficient.121 The authors claimed that the guanine biomolecule leads to the formation of a graphitic structure during carbonization.In 2022, Alizadeh et al. reported for the first time the synthesis of DES-derived nitrogen-rich ordered mesoporous carbon (DNOMC) with a three-dimensional cubic framework utilizing highly ordered mesoporous silica KIT-6 (arising from pluronic P123, n-butanol, and (EtO)4Si) and a deep eutectic solvent (DES) bearing choline chloride and D-glucose as starting materials. The reaction progressed via the addition of guanine and urea as nitrogen sources to the resultant DES and subsequent carbonization by the KIT-6 template. The resulting DNOMC was employed as a powerful and efficacious support for the stabilization of palladium nanoparticles (Pd@DNOMC). Subsequently, the catalytic system proceeded via the aqua-mediated aerobic oxidation of diverse primary and secondary benzylic alcohols (69) as well as cyclic and acyclic aliphatic alcohols (70/71) to the corresponding carboxylic acids (72/73) (in 65 → 99% yields) and ketones (74) (>99% yields) within 2–30 h at 70 °C by NaOH in the presence of molecular oxygen (Scheme 22). High yields, low cost, easily available substrates, experimental accessibility, recoverability, and reusability of the catalyst for up to ten runs without any considerable loss of efficiency are some of the advantages of this strategy. In this research, the hot filtration test showed that the catalyst works through a boomerang-type catalyst route.122
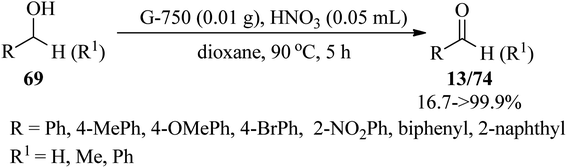
In 2023, Li et al. expressed a simple and metal-free procedure to synthesize in situ nitrogen-doped nanosheets through the pyrolysis of guanine at 750 °C, in which the guanine was chosen as the C and N precursor. The resulting N-doped nanocarbons (G-750) displayed a two-dimensional (2D) structure and high surface areas, which gave rise to excellent catalytic performance in the selective oxidation of benzyl alcohols (69) into benzaldehydes (13) or ketones (74) in 16.7%→99.9% yields by nitric acid as oxidant in 1,4-dioxane at 90 °C within 5 h (Scheme 23). It must be mentioned that graphitic nitrogen, pyridine nitrogen, and hydroxyl on the surface of nanocarbons play a key role in the reaction, in which the transformation of benzyl alcohol into benzaldehyde is mostly attributed to the concentration of hydroxyl groups, whereas the ratio of graphitic nitrogen and pyridine nitrogen has an important influence on the selectivity of benzaldehyde. Hence, the reactivity of benzyl alcohols bearing electron-donating groups was better than the alcohols with electron-withdrawing substituents, which could be ascribed to the inactivation of hydroxyl groups in catalytic reactions by the electron-withdrawing group.123
In 2019, Ng et al. prepared a novel palladium-guanine-reduced graphene oxide nanocomposite (Pd/rGOG) via an easy, scalable, one-pot microwave-assisted method, which introduced guanine to the reduced graphene oxide-supported palladium via non-covalent functionalization. The abundant amino, amide, and imino functional groups of guanine are considered to be the anchoring sites, which allow the uniform distribution of palladium nanoparticles (of various shapes, such as triangular, rectangular, circular, and diamond). The Pd/rGOG is an efficient catalyst for methanol oxidation. In addition, the guanine is revealed to be catalytically active toward the methanol oxidation reaction, serving as a second catalyst.124
Hu et al. successfully designed a sustainable strategy for the synthesis of highly active and stable catalysts of N-doped and N/S-doped carbon nanosheet-coated palladium nanoparticles utilizing guanine or guanine sulfate as the nitrogen and carbon precursor, named Pd@G-1000 and Pd@GS-1000, respectively. The catalytic application was examined in the hydrogenation reaction of quinolones (75) under H2 (1 bar) in CH3CN solvent at 60 °C (Scheme 24). The 5Pd@GS1000 catalyst indicated considerably improved activity with >99% conversion for 1,2,3,4-tetrahydroquinoline (76a) in comparison with 5Pd@G-1000 with 63% conversion.125
In 2021, Saberi et al. prepared and characterized a novel nanocomposite via guanine embedded on the surface of a silica-modified magnetic catalyst, using 3-chloropropyltrimethoxysilane (CPTMS) as a linker (Fe3O4@SiO2@CPTMS@guanine) and then its catalytic activity was investigated for the preparation of 2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitriles (81) through the one-pot three-component reaction of equimolar amounts of 1,3-dicarbonyls (77), reactive methylene derivatives (14, 78, 79), and isatins (80) in aqueous media at 70 °C in 81–99% for 20 min. The magnetite nanoparticles (2 mol%) also catalyzed the aqua-mediated synthesis of substituted dihydro-2-oxopyrroles (83) by the one-pot multi-component reaction of 14, 1,3-dicarbonyls (77), and bis(isatin) derivatives (82), in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, at 70 °C (Scheme 25). Mild reaction conditions, easy separation of the catalyst from the reaction mixture by an external magnet, recyclability and reusability of Fe3O4@SiO2@CPTMS@guanine to promote the reaction for ten runs without any appreciable loss of efficiency, easy work-up, and excellent yields are some of the advantages of this procedure.126
1 molar ratio, at 70 °C (Scheme 25). Mild reaction conditions, easy separation of the catalyst from the reaction mixture by an external magnet, recyclability and reusability of Fe3O4@SiO2@CPTMS@guanine to promote the reaction for ten runs without any appreciable loss of efficiency, easy work-up, and excellent yields are some of the advantages of this procedure.126
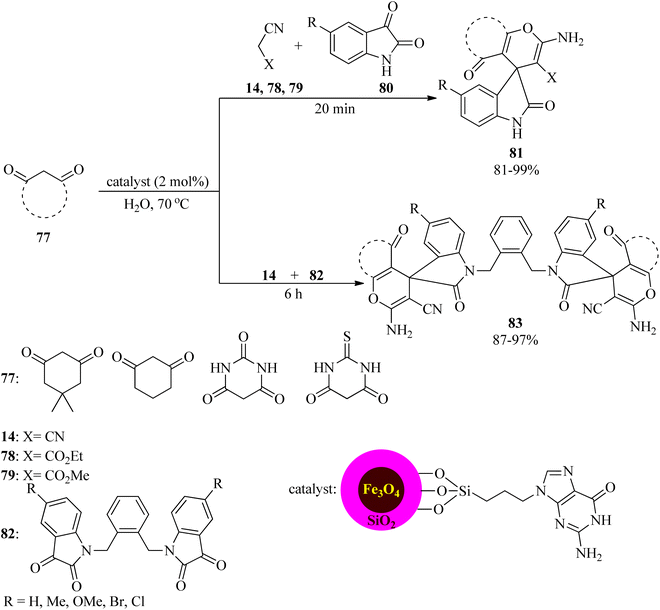 | ||
| Scheme 25 Preparation of 2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitriles and substituted dihydro-2-oxopyrroles. | ||
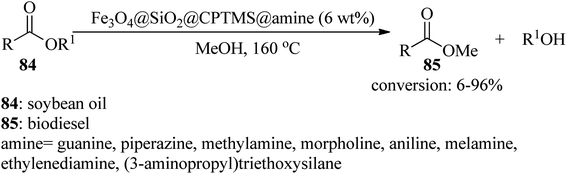
In 2018, Farzaneh et al. produced several Fe3O4@SiO2@CPTMS@amine nanocomposites utilizing various amines, such as guanine, piperazine, methylamine, morpholine, aniline, ethylenediamine, 3-aminopropyltriethoxysilane, and melamine to afford biodiesel (85) with 6–96% conversions by the trans-esterification reaction of soybean oil (84) with methanol, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 molar ratio, at 160 °C for 3 h (Scheme 26). According to the resulting data, guanine and melamine gave the highest and lowest yields of the product, respectively, which is ascribed to the amine basicity of the catalyst.127
36 molar ratio, at 160 °C for 3 h (Scheme 26). According to the resulting data, guanine and melamine gave the highest and lowest yields of the product, respectively, which is ascribed to the amine basicity of the catalyst.127
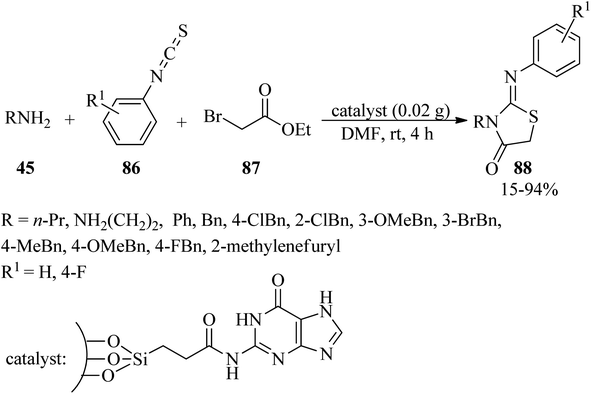
Due to the remarkable pharmaceutical activities of the heterocycles possessing thiazolidin-4-one motif, such as the antitubercular, antimicrobial, anticonvulsant, anticancer, antiprotozoal, and anti-inflammatory activities,128,129 Pathak and Gupta synthesized 2-iminothiazolidin-4-one derivatives (88) via the one-pot three-component annulation of amines (45), aryl isothiocyanates (86), and ethyl bromoacetate (87) utilizing guanine-coated SBA-16 (SBA-16@G) as an efficacious, practical, recyclable and reusable heterogeneous solid base catalyst in DMF at room temperature (Scheme 27). In this research, in order to probe the scope and limitations of the mentioned process, the reaction was performed using various aromatic and aliphatic amines such as aniline, benzylamines, ethylenediamine and propylamine. It was found that the reaction with benzylamines progressed faster. Remarkably, ethylenediamine and propylamine provided moderate yields (45% and 65%, respectively), while aniline gave poor yields (15%).130
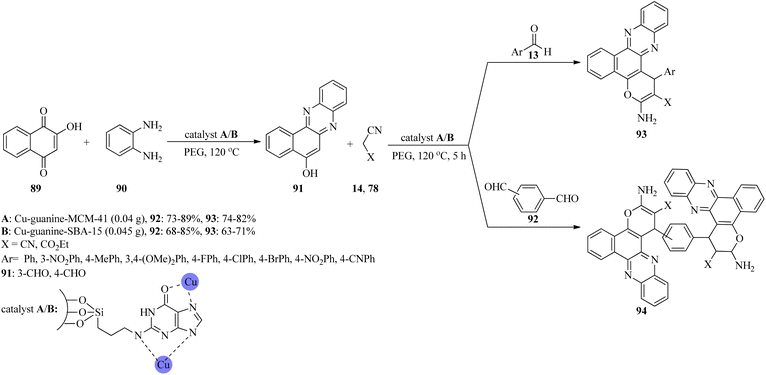
In 2020, novel copper(II) ion complexes of guanine anchored on MCM-41 and SBA-15 (Cu-guanine-MCM-41 and Cu-guanine-SBA-15) channels were found to catalyze the generation of benzo[c]pyrano[3,2-a]phenazines (93) and bis-benzo[c]pyrano[3,2-a]phenazines (94) via the one-pot domino four-component reactions. These reactions began through the domino condensation reaction of 2-hydroxy-1,4-naphthoquinone (89) with benzene-1,2-diamine (90) in the presence of PEG at 120 °C to form phenazine (91), as the orange solid within 10 min, which was then treated with aldehydes (13, 92) and alkylmalonates (14, 78) to achieve the adducts 93/94 (Scheme 28). The reaction works well with aldehydes containing electron-donating groups. Furthermore, malononitrile resulted in higher yields in comparison to ethyl cyanoacetate. The results showed the yield was better utilizing Cu-guanine-MCM-41 (73–89%). Some of the products revealed antimicrobial activity against the growth of Staphylococcus aureus.131
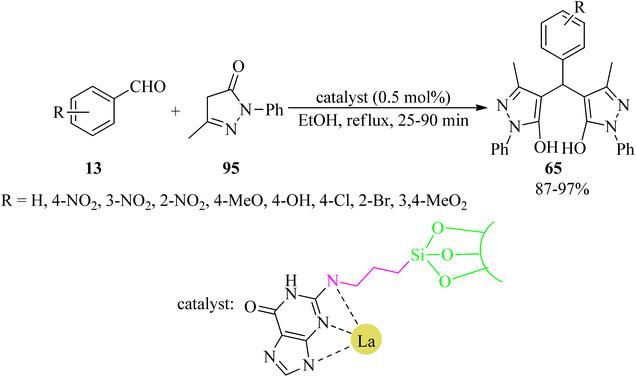
In 2020, Nikoorazm et al. obtained a novel lanthanum(III) organometallic complex through a guanine-La complex embedded in SBA-15 (La-guanine@SBA-15). The heterogeneous mesoporous nanocatalyst was utilized for the one-pot, multi-component tandem Knoevenagel condensation-Michael addition–cyclization reactions in order to prepare a series of benzo[a]pyrano[2,3-c]phenazines (93) via the domino reaction of 89, 90, 14/78, and aldehydes (13), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, in refluxing ethanol in 2.5–5 h with 87–99% yields. La-guanine@SBA-15 was also effective to obtain 4,4′-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) derivatives (65) via the reaction of aldehydes (13) and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (95), in a 2
1 molar ratio, in refluxing ethanol in 2.5–5 h with 87–99% yields. La-guanine@SBA-15 was also effective to obtain 4,4′-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) derivatives (65) via the reaction of aldehydes (13) and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (95), in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Scheme 29).132 In addition, this nanocatalyst was easily recovered, using simple filtration, and reused several times without significant loss of catalytic efficiency (confirmed by SEM and FT-IR techniques). Moreover, the leaching, heterogeneity and stability of La-guanine@SBA-15 were studied using the hot filtration test and ICP techniques.
1 molar ratio (Scheme 29).132 In addition, this nanocatalyst was easily recovered, using simple filtration, and reused several times without significant loss of catalytic efficiency (confirmed by SEM and FT-IR techniques). Moreover, the leaching, heterogeneity and stability of La-guanine@SBA-15 were studied using the hot filtration test and ICP techniques.
MCM-41-supported nanoscale guanine covered by Zr(IV) was prepared using the sol–gel method (Zr-guanine-MCM-41). This mesoporous nanostructure mediated the tandem chemoselective pseudo four-component reaction of 2-hydroxy-1,4-naphthoquinone (89), 90, and aldehydes (13), in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in PEG at 100 °C to produce benzo[α]benzo[6,7] chromeno[2,3-c]phenazines (96). The preparation of spiro[benzo[a]benzo[6,7] chromeno[2,3-c]phenazine] derivatives (98) was also performed through the pseudo-four-component reaction of 2-hydroxy-1,4-naphthoquinone (89), 90, isatins (80)/ninhydrin (97), effectively. These reactions progressed via the Schiff-base condensation reaction of 89 with 90 to generate the corresponding 91, which was treated subsequently with excess amounts of 89 and 13 or 80/97 (Scheme 30). Significantly, the reactivity of aldehydes, including electron-withdrawing groups, was better. The catalytic system also progressed well with aldehydes (13) and 3-methyl-1-phenyl-5-pyrazolone (95), in a 1
1 molar ratio in PEG at 100 °C to produce benzo[α]benzo[6,7] chromeno[2,3-c]phenazines (96). The preparation of spiro[benzo[a]benzo[6,7] chromeno[2,3-c]phenazine] derivatives (98) was also performed through the pseudo-four-component reaction of 2-hydroxy-1,4-naphthoquinone (89), 90, isatins (80)/ninhydrin (97), effectively. These reactions progressed via the Schiff-base condensation reaction of 89 with 90 to generate the corresponding 91, which was treated subsequently with excess amounts of 89 and 13 or 80/97 (Scheme 30). Significantly, the reactivity of aldehydes, including electron-withdrawing groups, was better. The catalytic system also progressed well with aldehydes (13) and 3-methyl-1-phenyl-5-pyrazolone (95), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, to furnish bis(pyrazolyl)methanes (65) in refluxing ethanol for 10–60 min in excellent yields (83–99%). Both electron-donating and electron-withdrawing aldehydes gave the products in good yields, but the reaction time of the aryl aldehydes containing electron-donating groups was longer.133
2 molar ratio, to furnish bis(pyrazolyl)methanes (65) in refluxing ethanol for 10–60 min in excellent yields (83–99%). Both electron-donating and electron-withdrawing aldehydes gave the products in good yields, but the reaction time of the aryl aldehydes containing electron-donating groups was longer.133
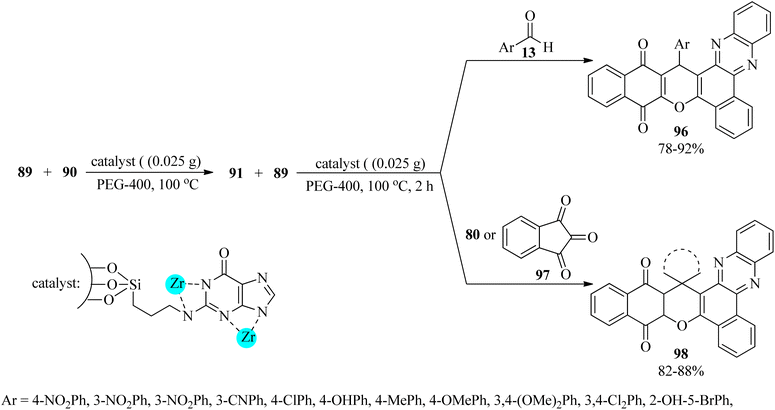 | ||
| Scheme 30 Preparation procedure of benzo[α]benzo6,7 chromeno[2,3-c] phenazines and spiro[benzo[a]benzo6,7 chromeno[2,3-c]phenazine] derivatives. | ||
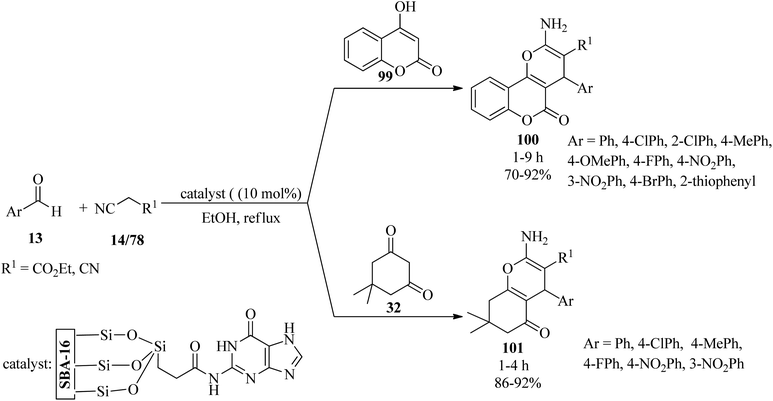
In 2019, Gupta et al. synthesized guanine-functionalized mesoporous silica [SBA-16-G] with a surface area and basicity of 524 m2 g−1 and 3.230 mmol g−1, respectively. Its catalytic activity was explored in the synthesis of a series of pyran-annulated heterocyclic compounds 100/101 from a one-pot three-component reaction of aromatic aldehydes (13), malononitrile (14)/ethyl cyanoacetate (78), and C–H activated acidic compounds (dimedone (32)/4-hydroxycoumarin (99)) (Scheme 31).134 The special features of the protocol are a simple work-up procedure (no chromatography), recyclability up to four times, and the environmental acceptability of the catalyst due to metal-free catalysis.
In 2020, Nikoofar and Shahriyari prepared a novel bio-based core–shell organic–inorganic nanohybrid by embedding aspartic acid-guanine IL on the hydroxylated nanosilica surface (nano[(Asp-Gua)IL@PEG-SiO2]), as a versatile nanostructure hybrid for the synthesis of 2,3-dihydroquinazolin-4(1H)-one-derivatives (104) via the one-pot pseudo-five-component reaction of aldehydes (13), 1,4-benznendiamine (102) and isatoic anhydride (103) at 70 °C in aqueous media (Scheme 32a). In addition, the peptide-like tricarboxamides (107) were also achieved using the pseudo-five-component condensation of aromatic aldehydes (13), aromatic amines (45), tert-butyl isocyanide (105), and Meldrum's acid (106) under green solventless conditions (Scheme 32b).135
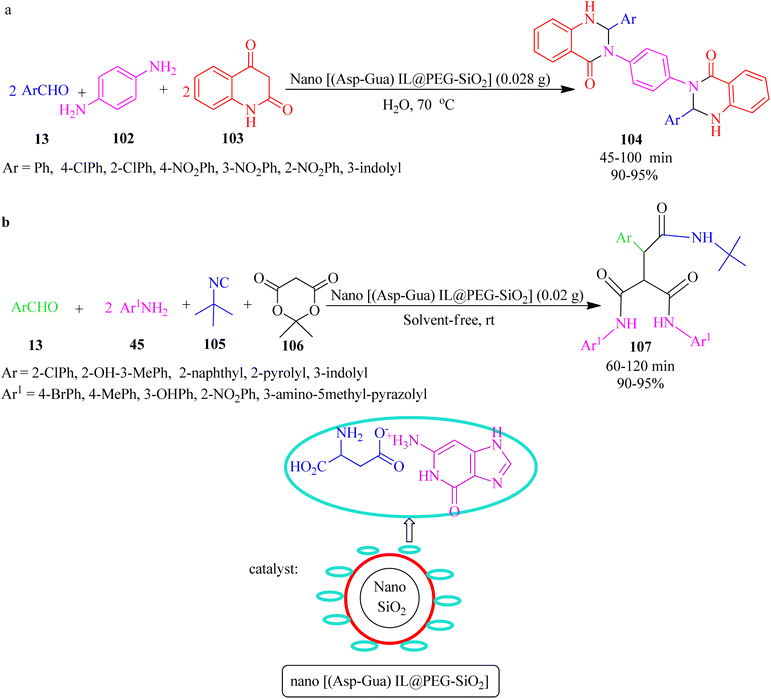 | ||
| Scheme 32 Synthesis of (a) bis(2,3-dihydroquinazolin-4(1H)-one) derivatives and (b) tricarboxamides. | ||
4. Catalytic activity of cytosine in two- and multi-component reactions
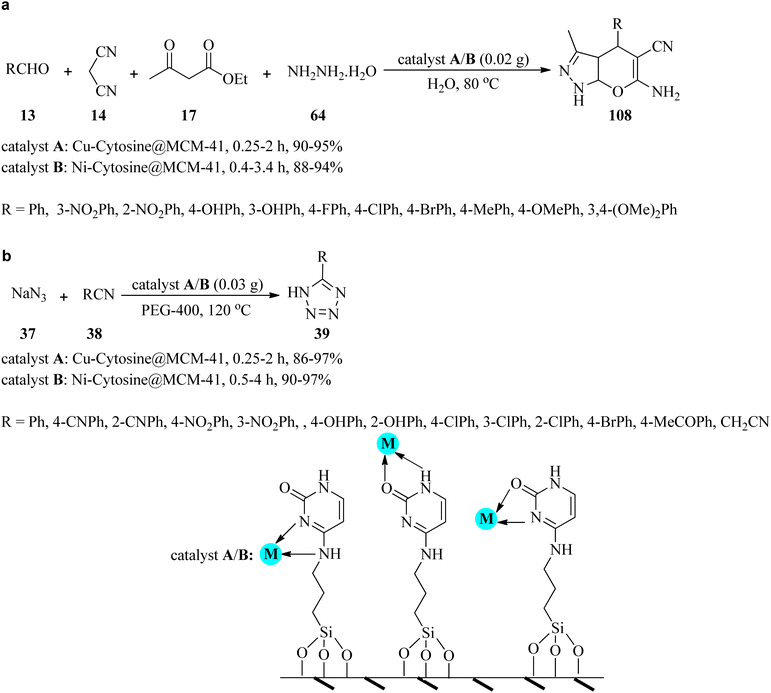
Due to the significant biological and pharmacological activities of pyranopyrazoles and tetrazoles,136–138 in 2020, Nikoorazm et al. presented an efficient and facile method for the preparation of pyranopyrazoles (108) and 5-substituted-1H-tetrazoles (39) by two catalytic systems, which are copper or nickel complexes of guanine confined in the mesoporous silica (Cu-cytosine@MCM-41 and Ni-cytosine@MCM-41). Pyranopyrazoles (108) were produced through the four-component condensation of equimolar amounts of aldehydes (13), 14, 17 and hydrazine hydrate (64) in water at 80 °C (Scheme 33a). On the other hand, the [3 + 2] cycloaddition of sodium azide (37) with nitriles (38) was accomplished by heating in polyethylene glycol (PEG-400) at 120 °C to give 5-substituted-1H-tetrazoles (39) (Scheme 33b). Based on the resulting data, both electron-donating and electron-withdrawing substituents on aldehydes and benzonitriles showed no significant effect on the reaction yields and the TOF. It was found that in both reactions, Cu-cytosine@MCM-41 had a great influence in decreasing the reaction time. Furthermore, the mesoporous framework of these catalysts was affirmed using nitrogen adsorption–desorption isotherms. Short reaction times, excellent yields, recyclability, and reusability of the synthesized nanocatalyst are some of the advantages of this process.139In 2019, Gholamian and Hajjami synthesized and characterized a Pd-cytosine complex stabilized on functionalized hexagonal mesoporous silica (HMS-CPTMS-Cy-Pd) as a novel, recyclable, and reusable catalyst. Its catalytic activity was investigated for the preparation of biphenyls via two pathways: (a) the Suzuki–Miyaura cross-coupling reaction of equimolar amounts of aryl halides (28) with phenylboronic acid (30) utilizing K2CO3 in PEG-400 at 100 °C gave rise to biphenyl compounds (31) in moderate to excellent yields (60–98%) within 0.25–5 h; (b) Stille reaction of aryl halides (28) and triphenyltin chloride (56), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 molar ratio, under similar conditions yielded 31 (Scheme 34). The recyclability and reusability of the catalyst, experimental simplicity, short reaction times, and high yields are some of the advantages of this procedure.140
0.5 molar ratio, under similar conditions yielded 31 (Scheme 34). The recyclability and reusability of the catalyst, experimental simplicity, short reaction times, and high yields are some of the advantages of this procedure.140
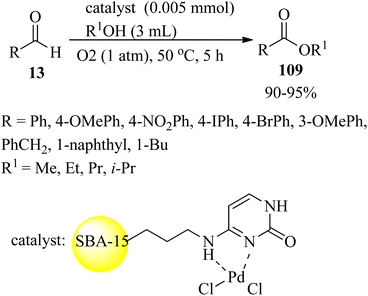
In 2021, Rajabi et al. constructed a cytosine palladium complex supported on ordered mesoporous silica (Pd-Cyt@SBA-15) that was utilized as a reusable nanocatalyst for the one-pot oxidative esterification of aldehydes (13). Diverse aliphatic, aromatic, and unsaturated aldehydes underwent oxidative transformation to the corresponding esters (109) in the presence of oxygen in large turnover numbers (Scheme 35). The Pd-Cyt@SBA-15 nanocatalyst demonstrated excellent reusability and stability up to ten times without loss of significant reactivity.141
Palladium on cytosine supported on SBA-15 nanoreactors (Pd-Cyt@SBA-15) also accelerated the formation of biphenyls (31) via the Suzuki–Miyaura reaction of various aryl chlorides (28) with boronic acid (30) in the presence of K2CO3 (1.5 mmol) base in a water/isopropanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) media at 60 °C in 88–98% yield within 6 h. Both electron-donating and electron-withdrawing aryl chlorides provided the corresponding products in excellent yields.142
1) media at 60 °C in 88–98% yield within 6 h. Both electron-donating and electron-withdrawing aryl chlorides provided the corresponding products in excellent yields.142
In 2017, Rajabi et al. designed and identified novel highly-ordered periodic mesoporous silica cytosine functionalized nanomaterials (Cyt@SBA-15) for the synthesis of α,β-unsaturated dicyanides (55) through the Knoevenagel condensation of 14 with aldehydes (13)/ketones (74) in ethanol at room temperature in a 1 h period with 88–99% yields. It was found that the aldehydes bearing electron-withdrawing substituents gave the related products in excellent yields, which could probably be ascribed to the higher reactivity of their carbonyl moiety. The catalytic system also indicated a good performance for less reactive ketones, in comparison with aldehydes, to furnish the corresponding compounds in 88–92% yields. Mild reaction conditions, low catalyst loading, recyclability and reusability of the catalyst, high yields, and short reaction times are some of the main advantages of this strategy.143
5. Catalytic activity of thymine in two- and multi-component reactions
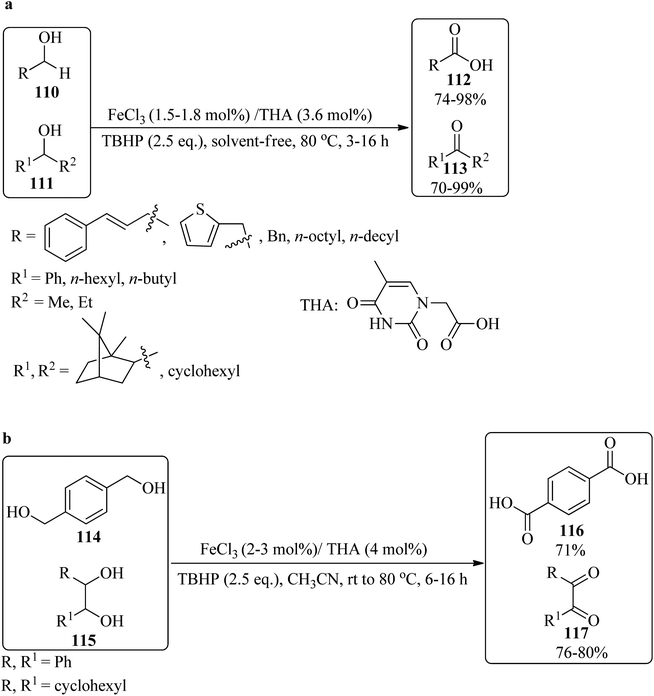
In 2010, Al-Hunaiti et al. produced thymine iron(III) (THA/FeCl3) from the in situ reaction of anhydrous FeCl3 (1.8 mol%) with thymine-1-acetate (THA, 3.6 mol%). The oxidation reaction of diverse alcohols was performed under solvent-free conditions at 80 °C by TBHP as an oxidant. The reaction was performed on various primary/secondary alcohols (110/111), which were selectively oxidized to their corresponding carboxylic acids (112)/ketones (113), respectively (Scheme 36a). On the other hand, various diols, including 1,4-dibenzylic diol (114) and internal/cyclic vic-diols (115) (such as diphenyl ethanediol and 1,2-cyclohexanediol), were selectively transformed to phenyl dicarboxylic acid (116) and diketones (117), respectively (Scheme 36b). Mild reaction conditions, inexpensiveness, experimental simplicity, environmentally friendly, and high yields are advantages of this strategy.1446. Conclusions
In this review, several types of nanocatalysts containing nucleobases have been presented. The sections have been classified according to the reactions catalyzed by each of the bases (A, G, C, T). Based on the overall results, the employment of nucleobases in the preparation of nanocatalysts, such as the metal-free and template-free in situ nitrogen-doped nanosheets, led to elevated catalytic performance, for instance, good stability (mechanical and/or thermal), high selectivity, high surface-to-volume ratio, clean energy production, eco-friendly, atom efficacy, time economic exploitation, and minimum chemical waste generation (green technology). Detailed examinations in the form of a literature survey revealed that due to the numerous interaction sites in adenine and guanine, and most organic reactions were carried out by multilayered nanocatalysts, including the immobilization of the mentioned nucleobases. The authors hope that this review article will guide general readers to attain an intense interest in utilizing the four main DNA nucleobases as promoters in various organic transformations. Due to the unique characteristics of these biochemical nucleobases, their activity in the field of green chemistry and medicinal/biochemical studies will definitely expand greatly in the future.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- S. Sivakova and S. J. Rowan, Nucleobases as supramolecular motifs, Chem. Soc. Rev., 2005, 34, 9–21 RSC.
- W. Saenger, Principles of Nucleic Acid Structures, Springer, New York, 1984 Search PubMed.
- V. N. Soyfer and V. N. Potaman, Triple-Helical Nucleic Acids, Springer, New York, 1995 Search PubMed.
- G. Michael Blackburn, M. J. Gait, D. Loakes and D. M. Williams, Nucleic Acids in Chemistry and Biology, RSC publishing, Cambridge, Uk, 3rd edn, 2006 Search PubMed.
- T. L. V. Ulbricht, Purines, Pyrimidines and Nucleotides and the Chemistry of Nucleic Acids, Pergamon, 2016 Search PubMed.
- M. P. Callahan, Pyrimidine base, in Encyclopedia of Astrobiology, ed. M. Gargaud, et al., Springer, Berlin, Heidelberg, pp. , pp. 1391–1392 Search PubMed.
- J. Oro, Synthesis of adenine from ammonium cyanide, Biochem. Biophys. Res. Commun., 1960, 2, 407–412 CrossRef.
- J. Oro and A. P. Kimball, Synthesis of purines under possible primitive earth conditions. I. adenine from hydrogen cyanide, Arch. Biochem. Biophys., 1961, 94, 217–227 CrossRef CAS PubMed.
- H. I. Wheeler and H. F. Merriam, On the condensation-products of the pseudothioureas: synthesis of uracil, thymine, and similar compounds, Am. Chem. J., 1903, 29, 478–492 CAS.
- A. Kossel and H. Z. Steudel, Weitere untersuchungen über das cytosin, Physiol. Chem., 1903, 38, 49–59 CrossRef CAS.
- B. Unger, Bemerliungen zu obiger no1iz. justus, Liebigs Ann. Chem., 1846, 58, 18–20 CrossRef.
- W. Traube, Ueber eine neue synthese des guanins und xanthins, Ber. Dtsch. Chem. Ges., 1900, 33, 1371–1383 CrossRef.
- A. Ascoli, Ueber ein neues spaltungsprodukt des hefenucleins, Hoppe Seyler's Z. Physiol. Chem., 1901, 31, 161–214 CrossRef.
- D. J. Brown, R. F. Evans, W. B. Cowden and M. D. Fenn, The pyrimidines, in Chemistry of Heterocyclic Compounds, ed. D. J. Brown, Wiley, New York, 1950, vol. 52 Search PubMed.
- K. Wang, P. Liu, D. Shi, H.-y. Zhang, Y. Zhang and J. Zhao, Nitrogen-doped carbon supported Co/Ni bimetallic catalyst for selectively reductive N-formylation of nitroso in guanine synthesis, Catal. Lett., 2022, 152, 2812–2822 CrossRef CAS.
- M. Robertson and S. Miller, Prebiotic Synthesis of 5-substituted uracils: A bridge between the RNA world and the DNA-protein world, Science, 1995, 268, 702–705 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, F. Ciciriello, G. Costanzo and E. Di Mauro, Formamide chemistry and the origin of informational polymers, Chem. Biodivers., 2007, 4, 694–720 CrossRef CAS PubMed.
- L. Petera, K. Mrazikova, L. Nejdl, K. Zemankova, M. Vaculovicova, A. Pastorek, S. Civis, P. Kubelik, A. Heays, G. Cassone, J. Sponer, M. Ferus and J. Sponer, Prebiotic route to thymine from formamide-A combined experimental-theoretical study, Molecules, 2021, 26, 2248 CrossRef CAS PubMed.
- R. Saladino, G. Botta, M. Delfino and E. Di Mauro, Meteorites as catalysts for prebiotic chemistry, Chem.–Eur. J., 2013, 19, 16916–16922 CrossRef CAS PubMed.
- M. Yadav, R. Kumar and R. Krishnamurthy, Chemistry of abiotic nucleotide synthesis, Chem. Rev., 2020, 120, 4766–4805 CrossRef CAS PubMed.
- N. Kitadai and S. Maruyama, Origins of building blocks of life: A review, Geosci. Front., 2018, 9, 1117–1153 CrossRef CAS.
- K. E. Nelson, M. P. Robertson, M. Levy and S. L. Miller, Concentration by evaporation and the prebiotic synthesis of cytosine, Origins Life Evol. Biospheres, 2001, 31, 221–229 CrossRef CAS PubMed.
- L. E. Orgel, Prebiotic adenine revisited: eutectics and photochemistry, Origins Life Evol. Biospheres, 2004, 34, 361–369 CrossRef CAS PubMed.
- H. J. Cleaves, K. E. Nelson and S. L. Miller, The prebiotic synthesis of pyrimidines in frozen solution, Naturwissenschaften, 2006, 93, 228–231 CrossRef CAS PubMed.
- C. Menor-Salvan, D. M. Ruiz-Bermejo, M. I. Guzman, S. Osuna-Esteban and S. Veintemillas-verdaguer, synthesis of pyrimidines and triazines in ice: implications for the prebiotic chemistry of nucleobases, Chem.–Eur. J., 2009, 15, 4411–4418 CrossRef CAS PubMed.
- M. Ruiz-Bermejo, C. Menor-Salvan, S. Osuna-Esteban and S. Veintemillas-Verdaguer, Prebiotic microreactors: a synthesis of purines and dihydroxy compounds in aqueous aerosol, Origins Life Evol. Biospheres, 2007, 37, 123–142 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, S. Pino, G. Costanzo and E. Di Mauro, Formamide and the origin of life, Phys. Life Rev., 2012, 9, 84–104 CrossRef PubMed.
- R. Saladino, G. Botta, S. Pino, G. Costanzo and E. Di Mauro, Genetics first or metabolism first? The formamide clue, Chem. Soc. Rev., 2012, 41, 5526–5565 RSC.
- H. L. Barks, R. Buckley, G. A. Grieves, E. Di Mauro, N. V. Hud and T. M. Orlando, Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: relaxation of the requirements for prebiotic purine nucleobase formation, ChemBioChem, 2010, 11, 1240–1243 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, G. Costanzo, R. Negri and E. Di Mauro, A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide: implications for the origin of life, Bioorg. Med. Chem., 2001, 9, 1249–1253 CrossRef CAS PubMed.
- R. Saladino, U. Ciambecchini, C. Crestini, G. Costanzo, R. Negri and E. Di Mauro, One-pot TiO2-catalyzed synthesis of nucleic bases and acyclonucleosides from formamide: implications for the origin of life, ChemBioChem, 2003, 4, 514–521 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, U. Ciambecchini, F. Ciciriello, G. Costanzo and E. Di Mauro, Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites, ChemBioChem, 2004, 5, 1558–1566 CrossRef CAS PubMed.
- R. Saladino, C. Crestini, V. Neri, J. R. Brucato, L. Colangeli, F. Ciciriello, E. Di Mauro and G. Costanzo, Synthesis and degradation of nucleic acid components by formamide and cosmic dust analogues, ChemBioChem, 2005, 6, 1368–1374 CrossRef CAS PubMed.
- R. Saladino, V. Neri, C. Crestini, G. Costanzo, M. Graciotti and E. Di Mauro, Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals, J. Am. Chem. Soc., 2008, 130, 15512–15518 CrossRef CAS PubMed.
- R. Saladino, V. Neri, C. Crestini, G. Costanzo, M. Graciotti and E. Di Mauro, The role of the formamide/zirconia system in the synthesis of nucleobases and biogenic carboxylic acid derivatives, J. Mol. Evol., 2010, 71, 100–110 CrossRef CAS PubMed.
- R. M. Hazen and D. A. Sverjensky, Mineral surfaces, geochemical complexities, and the origins of life, Cold Spring Harbor Perspect. Biol., 2010, 2, a002162 Search PubMed.
- U. Shanker, B. Bhushan and G. Bhattacharjee and Kamaluddin, Formation of nucleobases from formamide in the presence of iron oxides: Implication in chemical evolution and origin of life, Astrobiology, 2011, 11, 225–233 CrossRef CAS PubMed.
- L. Nejdl, L. Petera, J. Šponer, K. Zemánková, K. Pavelicová, A. Knížek, V. Adam, M. Vaculovičová, O. Ivanek and M. Ferus, Quantum dots in peroxidase-like chemistry and formamide-based hot spring synthesis of nucleobases, Astrobiology, 2022, 22, 541–551 CrossRef CAS PubMed.
- Y. Oba, Y. Takano, H. Naraoka, N. Watanabe and A. Kouchi, Nucleobase synthesis in interstellar ices, Nat. Commun., 2019, 10, 4413 CrossRef PubMed.
- J. Wang, J. Gu, M. T. Nguyen, G. Springsteen and J. Leszczynski, From formamide to adenine: a self-catalytic mechanism for an abiotic approach, J. Phys. Chem. B, 2013, 117, 14039–14045 CrossRef CAS PubMed.
- V. Gaded and R. Anand, Nucleobase deaminases: a potential enzyme system for new therapies, RSC Adv., 2018, 8, 23567–23577 RSC.
- Ch. Ellouze, T. Selmane, H.-K. Kim, E. Tuite, B. NordeÂn, K. Mortensen and M. Takahashi, Difference between active and inactive nucleotide cofactors in the effect on the DNA binding and the helical structure of RecA filament dissociation of RecA±DNA complex by inactive nucleotides, Eur. J. Biochem., 1999, 262, 88–94 CrossRef CAS PubMed.
- S. Verma, A. K. Mishra and J. Kumar, The many facets of adenine: coordination, crystal patterns, and catalysis, Acc. Chem. Res., 2010, 43, 79–91 CrossRef CAS PubMed.
- A. Conejo-García, O. Cruz-López, V. Gómez-Pérez, F. Morales, M. E. García-Rubiño, M. Kimatrai, M. C. Núñez and J. M. Campos, Synthesis of purine derivatives as scaffolds for a diversity of biological activities, Curr. Org. Chem., 2010, 14, 2463–2482 CrossRef.
- D. Ramesh, B. G. Vijayakumar and T. Kannan, Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders, Eur. J. Med. Chem., 2020, 207, 112801 CrossRef CAS PubMed.
- A. Singh, C. Biot, A. Viljoen, C. Dupont, L. Kremer, K. Kumar and V. Kumar, 1H-1,2,3-triazole-tethered uracil-ferrocene and uracil-ferrocenylchalcone conjugates: Synthesis and antitubercular evaluation, Chem. Biol. Drug Des., 2017, 89, 856–861 CrossRef CAS PubMed.
- L. Harmse, N. Gray, P. Schultz, S. Leclerc, D. C. Meijer and I. Havli, Structure-activity relationships and inhibitory effects of various purine derivatives on the in vitro growth of Plasmodium falciparum, Biochem. Pharmacol., 2001, 62, 341–348 CrossRef CAS PubMed.
- L. Meijer and E. Raymond, Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials, Acc. Chem. Res., 2003, 36, 417–425 CrossRef CAS PubMed.
- A. E. J. Yssel, J. Vanderleyden and H. P. Steenackers, Repurposing of nucleoside- and nucleobase-derivative drugs as antibiotics and biofilm inhibitors, J. Antimicrob. Chemother., 2017, 72, 2156–2170 CrossRef CAS PubMed.
- D. Ramesh, A. K. Mohanty, A. De, B. G. Vijayakumar, A. Sethumadhavan, S. K. Muthuvel, M. Mani and T. Kannan, Uracil derivatives as HIV-1 capsid protein inhibitors: design, in silico, in vitro and cytotoxicity studies, RSC Adv., 2022, 12, 17466–17480 RSC.
- P. A. Bonnet and R. K. Robins, Modulation of leukocyte genetic expression by novel purine nucleoside analogs. A new approach to antitumor and antiviral agents, J. Med. Chem., 1993, 36, 635–653 CrossRef CAS PubMed.
- D. Ramesh, B. G. Vijayakumar and T. Kannan, Advances in nucleoside and nucleotide analogues in tackling human immunodeficiency virus and hepatitis virus infections, ChemMedChem, 2021, 16, 1403–1419 CrossRef CAS PubMed.
- C. Wang, Z. Song, H. Yu, K. Liu and X. Man, Adenine: an important drug scaffold for the design of antiviral agents, Acta Pharm. Sin. B, 2015, 5, 431–441 CrossRef CAS PubMed.
- E. Badawey and T. Kappe, Synthesis of some new imidazo[1,2-a]pyrimidin-5(1H)-ones as potential antineoplastic agents, J. Heterocycl. Chem., 1995, 32, 1003–1006 CrossRef CAS.
- D. K. Tosh, K. A. Jacobson and L. S. Jeong, Nucleoside-based A3 adenosine receptor antagonists as drug candidates, Drugs Future, 2009, 34, 0043 CrossRef CAS.
- G. Nogueira, A. Favrelle, M. Bria, J. P. Ramalho, P. J. Mendes, A. Valente and P. Zinck, Adenine as an organocatalyst for the ring-opening polymerization of lactide: scope, mechanism and access to adenine-functionalized polylactide, React. Chem. Eng., 2016, 1, 508–520 RSC.
- Y. Wang, S. Zhao, Y. Zhang, W. Chen, S. Yuan, Y. Zhou and Z. Huang, Synthesis of graphitic carbon nitride with large specific surface area via copolymerizing with nucleobases for photocatalytic hydrogen generation, Appl. Surf. Sci., 2019, 463, 1–8 CrossRef CAS.
- H. Yang and W. Xi, Nucleobase-containing polymers: structure, synthesis, and applications, Polymers, 2017, 9, 666 CrossRef PubMed.
- F. Bahrami, F. Panahi, F. Daneshgar, R. Yousefi, M. B. Shahsavani and A. Khalafi-Nezhad, Synthesis of new α-aminophosphonate derivatives incorporating benzimidazole, theophylline and adenine nucleobases using Lcysteine functionalized magnetic nanoparticles (LCMNP) as magnetic reusable catalyst: Evaluation of their anticancer properties, RSC Adv., 2016, 6, 5915–5924 RSC.
- F. De Castro, E. Stefàno, F. P. Fanizzi, R. Di Corato, P. Abdalla, F. Luchetti, M. G. Nasoni, R. Rinaldi, M. Magnani, M. Benedetti and A. Antonelli, Compatibility of nucleobases containing Pt(II) complexes with red blood cells for possible drug delivery applications, Molecules, 2023, 28, 6760 CrossRef CAS PubMed.
- X. Tan, F. Jia, P. Wang and K. Zhang, Nucleic Acid-based drug delivery strategies, J. Controlled Release, 2020, 323, 240–252 CrossRef CAS PubMed.
- M.-A. Morikawa, M. Yoshihara, T. Endo and N. Kimizuka, ATP as building blocks for the self-assembly of excitonic nanowires, J. Am. Chem. Soc., 2005, 127, 1358–1359 CrossRef CAS PubMed.
- A. Terrón, J. J. Fiol, A. García-Raso, M. Barceló-Oliver and V. Moreno, Biological recognition patterns implicated by the formation and stability of ternary metal ion complexes of low-molecular-weight formed with amino acid/peptides and nucleobases/nucleosides, Coord. Chem. Rev., 2007, 251, 1973–1986 CrossRef.
- F. Pu, J. Ren and X. Qu, Nucleobases, nucleosides, and nucleotides: versatile biomolecules for generating functional nanomaterials, Chem. Soc. Rev., 2018, 47, 1285–1306 RSC.
- L. Stefan and D. Monchaud, Applications of guanine quartets in nanotechnology and chemical biology, Nat. Rev. Chem, 2019, 3, 650–668 CrossRef.
- M. Hocek and M. Fojta, Nucleobase modification as redox DNA labelling for electrochemical Detection, Chem. Soc. Rev., 2011, 40, 5802–5814 RSC.
- Y. Xing, Y. Zhang, X. Zhu, C. Wang, T. Zhang, F. Cheng, J. Qu and W. J. G. M. Peijnenburg, A highly selective and sensitive electrochemical sensor for tetracycline resistant genes detection based on the non-covalent interaction of graphene oxide and nucleobase, Sci. Total Environ., 2024, 906, 167615 CrossRef CAS PubMed.
- J. Zhou, H. Han and J. Liu, Nucleobase, nucleoside, nucleotide, and oligonucleotide coordinated metal ions for sensing and biomedicine applications, Nano Res., 2022, 15, 71–84 CrossRef CAS.
- B. Huang, X. Hu, Y. Liu, W. Qi and Z. Xie, Biomolecule-derived N/S co-doped CNT-graphene hybrids exhibiting excellent electrochemical activities, J. Power Sources, 2019, 414, 408–417 CrossRef.
- B. Huang, Y. Liu, X. Huang and Z. Xie, Multiple heteroatom-doped few-layer carbons for the electrochemical oxygen reduction reaction, J. Mater. Chem. A, 2018, 6, 22277–22286 RSC.
- X. Du, J. Zhou and B. Xu, Supramolecular Hydrogels made of basic biological building blocks, Chem.–Asian J., 2014, 9, 1446–1472 CrossRef CAS PubMed.
- S. Sivakova and S. J. Rowan, Nucleobases as supramolecular motifs, Chem. Soc. Rev., 2005, 34, 9–21 RSC.
- J. Pascual-Colino, G. Beobide, O. Castillo, P. Lodewyckx, A. Luque, S. Pérez-Yáñez, P. Román and L. F. Velasco, Adenine nucleobase directed supramolecular architectures based on ferrimagnetic heptanuclear copper(II) entities and benzenecarboxylate anions, J. Inorg. Biochem., 2020, 202, 110865 CrossRef CAS PubMed.
- Y. Tian, H. Wu, A. Hanif, Y. Niu, Y. Yin, Y. Gu, Z. Chen, Q. Gu, Y. H. Ng, J. Shang, L. Li and M. Liu, N-doped graphitic carbon encapsulating cobalt nanoparticles derived from novel metal–organic frameworks for electrocatalytic oxygen evolution reaction, Chin. Chem. Lett., 2023, 34, 108056 CrossRef CAS.
- J. Chen, T. Gong, Q. Hou, J. Li, L. Zhang, D. Zhao, Y. Luo, D. Zheng, T. Li, S. Sun, Z. Cai, Q. Liu, L. Xie, M. Wu, A. A. Alshehri and X. Sun, Co/N-doped carbon nanospheres derived from an adenine-based metal organic framework enabled high-efficiency electrocatalytic nitrate reduction to ammonia, Chem. Commun., 2022, 58, 13459–13462 RSC.
- H. Guo, Q. Feng, J. Zhu, J. Xu, Q. Li, S. Liu, K. Xu, C. Zhang and T. Liu, Cobalt nanoparticle-embedded nitrogen-doped carbon/carbon nanotube frameworks derived from metal-organic framework for tri-functional ORR, OER and HER electrocatalysis, J. Mater. Chem. A, 2019, 7, 3664–3672 RSC.
- B. Huang, Y. Li, X. Guan and Z. Xie, Nucleobases-derived carbon materials: Synthesis and application in heterogeneous catalysis, FlatChem, 2022, 35, 100415 CrossRef CAS.
- S. A. Abbas, J. T. Song, Y. C. Tan, K. M. Nam, J. Oh and K.-D. Jung, Synthesis of nickel single-atom catalyst based on ni-n4-xcx active sites for highly efficient CO2 reduction utilizing gas diffusion electrode, ACS Appl. Energy Mater., 2020, 3, 8739–8745 CrossRef CAS.
- Y. Liu, X. Hu, B. Huang and Z. Xie, Surface engineering of Rh catalysts with N/S-codoped carbon nanosheets toward high-performance hydrogen evolution from seawater, ACS Sustain. Chem. Eng., 2019, 7, 18835–18843 CrossRef CAS.
- Y. Kobayashi, R. Tanahashi, Y. Yamaguchi, N. Hatae, M. Kobayashi, Y. Ueno and M. Yoshimatsu, Ni–Pd catalyzed cyclization of sulfanyl 1,6-diynes: synthesis of 1’-homonucleoside analogs, J. Org. Chem., 2017, 82, 2436–2449 CrossRef CAS PubMed.
- M. Ma, L. Liu, H. Xu, X. Yang, H. Wang, X. Lu, P. Yang, P. Wu and L. Liao, Molten salt-mediated synthesis of porous N-doped carbon as an efficient ORR electrocatalyst for zinc–air batteries, New J. Chem., 2023, 47, 2279–2285 RSC.
- B. Huang, Y. Liu, M. Xia, J. Qiu and Z. Xie, Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries, Sustainable Energy Fuels, 2020, 4, 559–570 RSC.
- B. Huang, Y. Liu and Z. Xie, Two dimensional nanocarbons from biomass and biological molecules: Synthetic strategies and energy related applications, J. Energy Chem., 2021, 54, 795–814 CrossRef CAS.
- B. Huang, Y. Liu, Q. Wei and Z. Xie, Three-dimensional mesoporous graphene-like carbons derived from biomolecule exhibiting high-performance oxygen reduction activity, Sustainable Energy Fuels, 2019, 3, 2809–2818 RSC.
- B. Huang, Y. Liu, Q. Guo, Y. Fang, M.-M. Titirici, X. Wang and Z. Xie, Porous carbon nanosheets from biological nucleobase precursor as efficient pH-independent oxygen reduction electrocatalyst, Carbon, 2020, 156, 179–186 CrossRef CAS.
- Y. Liu, B. Huang, X. Zhang, X. Huang and Z. Xie, In-situ fabrication of nitrogen-doped carbon nanosheets containing highly dispersed single iron atoms for oxygen reduction reaction, J. Power Sources, 2019, 412, 125–133 CrossRef CAS.
- Y. Li, F. Hou, X. Sun, Z. Xiao, X. Zhang and G. Li, Toward an understanding of capping molecules on Pt nanoparticles for hydrogenation: the key role of hydroxyl groups, ChemistrySelect, 2019, 4, 3505–3514 CrossRef CAS.
- Q. Li, Y. Chen, S. Bai, X. Shao, L. Jiang and Q. Li, Immobilized lipase in bio-based metal-organic frameworks constructed by biomimetic mineralization: A sustainable biocatalyst for biodiesel synthesis, Colloids Surf., B, 2020, 188, 110812 CrossRef CAS PubMed.
- F. Bruston, J. Vergne, L. Grajcar, B. Drahi, R. Calvayrac, M.-H. Baron and M.-C. Maurel, Copper-adenine catalyst for O2 production from H2O2, Biochem. Biophys. Res. Commun., 1999, 263, 672–677 CrossRef CAS PubMed.
- R. Li, X. Zhang, C. Zhang, J. Lu, J.-C. Wang, C.-X. Cui, X. Yang, F. Huang, J.-X. Jiang and Y. Zhang, Adenine-functionalized conjugated polymer as an efficient photocatalyst for hydrogen evolution from water, Int. J. Hydrogen Energy, 2022, 47, 29771–29780 CrossRef CAS.
- R. M. C. Dawson, D. C. Elliott, W. H. Elliott and K. M. Jones, Data for Biochemical Research, Oxford University Press, Oxford, 3rd edn, 1986 Search PubMed.
- K. Karrouchi, S. Radi, Y. Ramli, J. Taoufik, Y. N. Mabkhot, F. A. Al-aizari and M. Ansar, Synthesis and pharmacological activities of pyrazole derivatives: A review, Molecules, 2018, 23, 134 CrossRef PubMed.
- F. E. Bennani, L. Doudach, Y. Cherrah, Y. Ramli, Kh. Karrouchi, M. Ansar and M. E. A. Faouzi, Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line, Bioorg. Chem., 2020, 97, 103470 CrossRef CAS PubMed.
- M. A. Elsherif, A. S. Hassan, G. O. Moustafa, H. M. Awad and N. M. Morsy, Antimicrobial evaluation and molecular properties prediction of pyrazolines incorporating benzofuran and pyrazole moieties, J. Appl. Pharm. Sci., 2020, 10, 37–43 CrossRef CAS.
- O. Ebenezer, M. Shapi and J. A. Tuszynski, A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives, Biomedicines, 2022, 10, 1124 CrossRef CAS PubMed.
- E. Ahmadi, A. Khazaei and T. Akbarpour, [Fe3O4@CQD@Si(OEt)(CH2)3NH@CC@Ad@SO3H]+Cl−: As a new, efficient, magnetically separable and reusable heterogeneous solid acid catalyst for the synthesis of 5-amino-1,3-diphenyl-1H-pyrazole 4-carbonitril and pyrano[2,3-c]pyrazole derivatives, Res. Chem. Intermed., 2023, 49, 2099–2122 CrossRef CAS.
- Z. Sadri, F. K. Behbahani and E. Keshmirizadeh, One-pot multicomponent synthesis of 2,6-diamino-4-arylpyridine-3,5-dicarbonitriles using prepared nanomagnetic Fe3O4@SiO2@(CH2)3NHCOadenine sulfonic acid, Inorg. Nano-Met. Chem., 2022, 54, 839–848 CrossRef.
- Z. Sadri, F. K. Behbahani and E. Keshmirizadeh, Synthesis and characterization of a novel and reusable adenine based acidic nanomagnetic catalyst and its application in the preparation of 2-substituted-2,3-dihydro -1H-perimidines under ultrasonic irradiation and solvent-free condition, Polycyclic Aromat. Compd., 2023, 43, 1898–1913 CrossRef CAS.
- C. Feng, S. Qiao, Y. Guo, Y. Xie, L. Zhang, N. Akram, S. Li and J. Wang, Adenine-assisted synthesis of functionalized F-Mn-MOF-74 as an efficient catalyst with enhanced catalytic activity for the cycloaddition of carbon dioxide, Colloids Surf., A, 2020, 597, 124781 CrossRef CAS.
- Y. Rachuri, J. F. Kurisingal, R. K. Chitumalla, S. Vuppala, Y. Gu, J. Jang, Y. Choe, E. Suresh and D.-W. Park, Adenine-based Zn(II)/Cd(II) metal-organic frameworks as efficient heterogeneous catalysts for facile CO2 fixation into cyclic carbonates: a DFT-supported study of the reaction mechanism, Inorg. Chem., 2019, 58, 11389–11403 CrossRef CAS PubMed.
- N. Guha, S. Krishnan, S. Gupta and D. K. Rai, Adenine-Functionalized Dendritic fibrous nanosilica as bifunctional catalyst for CO2 fixation into cyclic carbonate, ChemNanoMat, 2023, 9, e202200519 CrossRef CAS.
- R. Srivastava, D. Srinivas and P. Ratnasamy, CO2 activation and synthesis of cyclic carbonates and alkyl/aryl carbamates over adenine-modified Ti-SBA-15 solid catalysts, J. Catal., 2005, 233, 1–15 CrossRef CAS.
- A. Ghorbani-Choghamarani, P. Moradi and B. Tahmasbi, Modification of boehmite nanoparticles with Adenine for the immobilization of Cu(II) as organic-inorganic hybrid nanocatalyst in organic reactions, Polyhedron, 2019, 163, 98–107 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, Synthesis of new zirconium complex supported on MCM-41 and its application as an efficient catalyst for synthesis of sulfides and the oxidation of sulfur containing compounds, Appl. Organomet. Chem., 2018, 32, e4340 CrossRef.
- T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Ni(II)-Adenine complex coated Fe3O4 nanoparticles as high reusable nanocatalyst for the synthesis of polyhydroquinoline derivatives and oxidation reactions, Appl. Organomet. Chem., 2018, 32, e3974 CrossRef.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, Fe3O4-Adenine-Zn: as a novel, green and magnetically recoverable catalyst for the synthesis of 5-substituted tetrazoles and oxidation of sulfur containing compounds, New J. Chem., 2017, 41, 11714–11721 RSC.
- M. Nikoorazm, A. Ghorbani-Choghamaranai, M. Khanmoradi and P. Moradi, Synthesis and characterization of Cu(II)-Adenine-MCM-41 as stable and efficient mesoporous catalyst for the synthesis of 5-substituted 1H-tetrazoles and 1H-indazolo[1,2-b]phthalazine-triones, J. Porous Mater., 2018, 25, 1831–1842 CrossRef CAS.
- V. Khorram Abadi, D. Habibi, S. Heydari and M. M. Gilan, An adenine-based palladium complex: a capable heterogeneous magnetic nano-catalyst for the green synthesis of the ureas in aqueous media, J. Iran. Chem. Soc., 2023, 20, 1985–1996 CrossRef CAS.
- P. Goswami and B. Das, Adenine as aminocatalyst for green synthesis of diastereoselective Mannich products in aqueous medium, Tetrahedron Lett., 2009, 50, 2384–2388 CrossRef CAS.
- W. Notz, F. Tanaka and C. F. Barbas, Enamine-based organocatalysis with proline and diamines: the development of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels-Alder reactions, Acc. Chem. Res., 2004, 37, 580–591 CrossRef CAS PubMed.
- B. Tahmasbi, A. Ghorbani-Choghamarani and P. Moradi, Palladium fabricated on boehmite as an organic-inorganic hybrid nanocatalyst for the C-C cross coupling and chomoselective cycloaddition reactions, New J. Chem., 2020, 44, 3717–3727 RSC.
- X. Wang, X. Liang, P. Geng and Q. Li, Recent advances in selective hydrogenation of cinnamaldehyde over supported metal-based catalysts, ACS Catal., 2020, 10, 2395–2412 CrossRef CAS.
- X. Lan and T. Wang, Highly selective catalysts for the hydrogenation of unsaturated aldehydes: a review, ACS Catal., 2020, 10, 2764–2790 CrossRef CAS.
- P. Gallezot and D. Richard, Selective hydrogenation of α,β-unsaturated aldehydes, Catal. Rev., 1998, 40, 81–126 CrossRef CAS.
- Y. Tang, K. Cui, Y. Li, H. Li, Y. Lei, J. Chen, W. Xiong and G. Yuan, Mild-temperature chemoselective hydrogenation of cinnamaldehyde over amorphous Pt/Fe-Asp-A nanocatalyst with enhanced stability, Colloids Surf., A, 2022, 654, 130106 CrossRef CAS.
- W. She, J. Wang, X. Li, J. Li, G. Mao, W. Li and G. Li, Bimetallic CuZn-MOFs derived Cu-ZnO/C catalyst for reductive amination of nitroarenes with aromatic aldehydes tandem reaction, Appl. Surf. Sci., 2021, 569, 151033 CrossRef CAS.
- Sh. Zhang, H. He, F. Sun, N. Zhao, J. Du, Q. Pan and G. Zhu, A novel adenine-based zinc(II) metal-organic framework featuring the Lewis basic sites for heterogeneous catalysis, Inorg. Chem. Commun., 2017, 79, 55–59 CrossRef CAS.
- T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, SBA-15@adenine–Pd: a novel and green heterogeneous nanocatalyst in Suzuki and Stille reactions and synthesis of sulfides, J. Porous Mater., 2019, 26, 121–131 CrossRef CAS.
- M. M. Heravi, S. J. Mahdizade, M. Esfandiari and E. Hashemi, Experimental and computational studies on catalytic activity of novel adenine-based nano Cu(I) polymers in regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles, J. Inorg. Organomet. Polym., 2018, 28, 767–776 CrossRef CAS.
- B. Chowhan, M. Gupta and N. Sharma, Fabrication and characterization of adenine-grafted carbonmodified amorphous ZnO with enhanced catalytic activity, Appl. Organomet. Chem., 2020, e6013 CrossRef CAS.
- G. Wang, P. Wang, X. Zhang, Q.-H. Wei, S. Wu and Z. Xie, Nucleobase derived boron and nitrogen co-doped carbon nanosheets as efficient catalyst for selective oxidation and reduction reactions, Nanoscale, 2020, 12, 7797–7803 RSC.
- S. Z. Alizadeh, B. Karimi and H. Vali, From deep eutectic solvents to nitrogen-rich ordered mesoporous carbons: a powerful host for the immobilization of palladium nanoparticles in the aerobic oxidation of alcohols, ChemCatChem, 2022, 14, e202101621 CrossRef.
- S. Li, Y. Ke, X. Zhang, S. Wu, Y. Chen and Z. Xie, Guanine-derived nitrogen-doped carbon nanosheets for selective oxidation of benzyl alcohol, Diam. Relat. Mater., 2023, 132, 109642 CrossRef CAS.
- J. C. Ng, C. Y. Tan, B. H. Ong, A. Matsuda, W. J. Basirun, W. K. Tan, R. Singh and B. K. Yap, Novel palladium-guanine-reduced graphene oxide nanocomposite as efficient electrocatalyst for methanol oxidation reaction, Mater. Res. Bull., 2019, 112, 213–220 CrossRef CAS.
- X. Hu, Y. Chen, B. Huang, Y. Liu, H. Huang and Z. Xie, Pd-supported N/S-codoped graphene-like carbons boost quinoline hydrogenation activity, ACS Sustainable Chem. Eng., 2019, 7, 11369–11376 CrossRef CAS.
- D. Saberi, M. Bashkar, A. Rezaei, Z. Azizi and K. Niknam, Guanine base stabilized on the magnetic nanoparticles as recyclable catalyst “on water” for the synthesis of spirooxindole derivatives, J. Organomet. Chem., 2021, 948, 121912 CrossRef CAS.
- F. Farzaneh, Z. Mohammadi and Z. Azarkamanzad, Immobilized different amines on modified magnetic nanoparticles as catalyst for biodiesel production from soybean oil, J. Iran. Chem. Soc., 2018, 15, 1625–1632 CrossRef CAS.
- N. Trotsko, Antitubercular properties of thiazolidin-4-ones-A review, Eur. J. Med. Chem., 2021, 215, 113266 CrossRef CAS PubMed.
- D. Mech, A. Kurowska and N. Trotsko, The Bioactivity of thiazolidin-4-ones: A short review of the most recent studies, Int. J. Mol. Sci., 2021, 2, 11533 CrossRef PubMed.
- R. Gupta and D. D. Pathak, Synthesis of 2-iminothiazolidin-4-ones using guanine functionalized SBA-16 as a solid base catalyst, Tetrahedron Lett., 2021, 85, 153497 CrossRef CAS.
- M. Nikoorazm and M. Khanmoradi, Application of Cu(II)-guanine complexes anchored on SBA-15 and MCM-41 as efficient nanocatalysts for one-pot, four-component domino synthesis of phenazine derivatives and investigation of their antimicrobial behavior, Catal. Lett., 2020, 150, 2823–2840 CrossRef CAS.
- M. Nikoorazm, M. Khanmoradi and M. Mohammadi, Guanine-La complex supported onto SBA-15: a novel efficient heterogeneous mesoporous nanocatalyst for onepot, multi-component tandem Knoevenagel condensation-Michael addition-cyclization reactions, Appl. Organomet. Chem., 2020, e5504 CrossRef CAS.
- M. Nikoorazm, M. Mohammadi and M. Khanmoradi, Zirconium@guanine@MCM-41 nanoparticles: An efficient heterogeneous mesoporous nanocatalyst for one-pot, multicomponent tandem Knoevenagel condensation-Michael addition–cyclization Reactions, Appl. Organomet. Chem., 2020, 34, e5704 CrossRef CAS.
- R. Gupta, S. Layek and D. D. Pathak, Synthesis and characterization of guanine-functionalized mesoporous silica [SBA-16-G]: a metal-free and recyclable heterogeneous solid base catalyst for synthesis of pyran-annulated heterocyclic compounds, Res. Chem. Intermed., 2019, 5, 1619–1637 CrossRef.
- K. Nikoofar and F. Shahriyari, Novel bio-based core-shell organic-inorganic nanohybrid from embedding aspartic acid-guanine ionic liquid on the hydroxylated nano silica surface (nano [(Asp-Gua) IL@PEG-SiO2]): A versatile nanostructure for the synthesis of bis(2,3-dihydroquinazolin-4(1H)-one) derivatives and tricarboxamides under green media, Polyhedron, 2020, 179, 114361 CrossRef.
- C. G. Neochoritis, T. Zhao and A. Dömling, Tetrazoles via multicomponent reactions, Chem. Rev., 2019, 119, 1970–2042 CrossRef CAS PubMed.
- I. Sarosh, K. Shumaila, A. Aliza, A. Shazia, Kh. Ansa, M. G. Mark and A. N. Muhammad, An overview of synthetic routes of pharmaceutically important pyranopyrazoles, Curr. Med. Chem., 2022, 29, 6288–6333 CrossRef PubMed.
- C. Gao, L. Chang, Z. Xu, X.-F. Yan, C. Ding, F. Zhao, X. Wu and L.-S. Feng, Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents, Eur. J. Med. Chem., 2019, 163, 404–412 CrossRef CAS PubMed.
- M. Nikoorazm, B. Tahmasbi, S. Gholami and P. Moradi, Copper and nickel immobilized on cytosine@MCM-41: as highly efficient, reusable and organic–inorganic hybrid nanocatalysts for the homoselective synthesis of tetrazoles and pyranopyrazoles, Appl. Organomet. Chem., 2020, 34, e5919 CrossRef CAS.
- F. Gholamian and M. Hajjami, Synthesis of Pd immobilized on functionalized hexagonal mesoporous silica (HMS–CPTMS–Cy–Pd) for coupling Suzuki–Miyaura and Stille reactions, Polyhedron, 2019, 170, 649–658 CrossRef CAS.
- F. Rajabi, C. H. Chia, M. Sillanpää, L. G. Voskressensky and R. Luque, Cytosine palladium complex supported on ordered mesoporous silica as highly efficient and reusable nanocatalyst for one-pot oxidative esterification of aldehydes, Catalysts, 2021, 11, 1482 CrossRef CAS.
- F. Rajabi, F. Fayyaza, R. Bandyopadhyay, F. Ivars-Barcelo, A. R. Puente-Santiago and R. Luque, Cytosine palladium hybrid complex immobilized on SBA-15 as efficient heterogeneous catalyst for the aqueous Suzuki-Miyaura coupling, ChemistrySelect, 2018, 3, 6102–6106 CrossRef CAS.
- F. Rajabi, F. Fayyaz and R. Luque, Cytosine-functionalized SBA-15 mesoporous nanomaterials: synthesis, characterization and catalytic applications, Microporous Mesoporous Mater., 2017, 253, 64–70 CrossRef CAS.
- A. Al-Hunaiti, T. Niemi, A. Sibaouih, P. Pihko, M. Leskelä and T. Repo, Solvent free oxidation of primary alcohols and diols using thymine iron(III) catalyst, Chem. Commun., 2010, 46, 9250–9252 RSC.
| This journal is © The Royal Society of Chemistry 2025 |