Open Access Article
Open Access ArticleVisible-light-induced radical cascade sulfonylation/cyclization towards indole-fused pyridine derivatives†
Qin Du *a,
Ruohan Panb,
Yu Chenb and
Yucai Tang
*a,
Ruohan Panb,
Yu Chenb and
Yucai Tang b
b
aChangde Hospital, Xiangya School of Medicine, Central South University (The First People's Hospital of Changde City), Changde 415000, China. E-mail: duqin1009@163.com
bCollege of Chemistry and Materials Engineering, Hunan University of Arts and Science, Engineering Technology Research Center of Key Preparation Technology of Biomedical Polymer Materials, Changde 415000, China
First published on 2nd January 2025
Abstract
Indole-fused pyridines are an important motif in pharmaceuticals and functional molecules. A visible-light induced Ru(bpy)3Cl2·6H2O catalyzed radical cascade sulfonylation/cyclization strategy for the synthesis of indole-fused pyridine derivatives was developed. Diverse indole-fused pyridines bearing different functional groups were obtained in moderate to good yields. Compared with previous work, the easily accessible starting materials, molecular nitrogen as byproduct, and eco-friendly visible light as an energy source all make this transformation more sustainable and practical.
Introduction
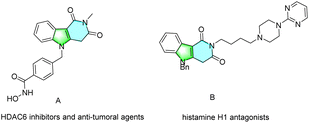
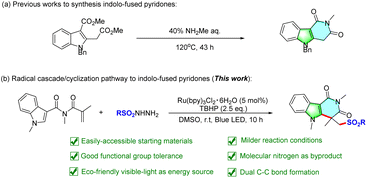
Indole-fused heterocycles are widely found in a large number of natural products, drug molecules, and artificial functional materials.1,2 Among them, indole-fused pyridines have attracted huge attention from medicinal chemists because of their biological activities and medicinal value. For example, compound a acts as an HDAC6 inhibitor and anti-tumoral agent, and compound b is a histamine H1 antagonist (Fig. 1).3 Therefore, developing efficient pathways to construct indole-fused pyridine compounds is of great importance. By viewing the literature, synthetic methods to construct indole-fused pyridines remain rare, only Mita and co-workers reported a cyclization reaction of a doubly carboxylated compound with methylamine to provide the corresponding indole-fused pyridine products in 2018 (Scheme 1a).4 However, the high reaction temperature, inaccessible starting materials, and long reaction time remain major shortcomings of this methodology, which may impede its wide application in more complex organic syntheses. Thus, the exploration of novel and more efficient strategies to synthesize indole-fused pyridine derivatives from cheap and readily available starting materials is still highly desirable.In recent years, the photoinduced radical cascade reaction has emerged as an appealing platform for multiple bond formation because of the inexhaustible, mild and inexpensive nature of the visible-light.5 In this context, the photoinduced direct C–H functionalization of N-acrylamide provides a versatile strategy for the synthesis of various functionalized nitrogen heterocycles.6,7 Commonly, these reactions are initiated by radical addition to unsaturated bonds and then followed by cyclization reaction. Very recently, we also developed a method of visible-light-induced organic dye-catalyzed alkoxycarbonylation/cyclization of 2-aryl indoles with methyl carbazates to obtain diverse indolo[2,1-a]isoquinolin-6(5H)-ones.8 On the other hand, sulfone-containing molecules are widely applied in medicinal chemistry and agrochemistry because of their remarkable anticancer and antibacterial activities.9 The sulfones can also serve as versatile building blocks in organic synthesis.10 Considering the importance of indole-fused pyridines and sulfones synthesis, it was hypothesized that indole-derived N-acrylamide may act as a radical acceptor via a similar addition/cyclization process to prepare a variety of indole-fused pyridines. Herein we report a novel and practical protocol for the synthesis of sulfonated indole-fused pyridines via visible-light initiated tandem reaction of N-methacryloyl-N,1-dimethyl-1H-indole-3-carboxamide with sulfonylhydrazides under mild reaction conditions (Scheme 1b).11 Compared with previous work, the reported reaction exhibits several advantages, such as easily-accessible starting materials, good functional group tolerance, molecular nitrogen as byproduct and eco-friendly visible-light as energy source, which make the present methodology more attractive and practical.
Results and discussion
We initiated our hypothesis by first exploring the model reaction of N-methacryloyl-N,1-dimethyl-1H-indole-3-carboxamide (1a) with 4-methylbenzenesulfonohydrazide (2a) under different reaction conditions (Table 1). After extensive evaluation of various photocatalyst, such as Eosin B, Fluorescein, Rose Bengal and Na2-Eosin Y, Ru(bpy)3Cl2·6H2O was found to be the best and provided indole-fused pyridine 3a in 65% yield (entries 1–5). Subsequently, several solvents, such as dichloromethane (DCM), DMF, THF, EtOH, CH3CN, 1,2-dichloroethane (DCE), CH3OH and 1,4-dioxane, were screened. In most of these cases, a trace of or lower yield of 3a was observed (entries 6–9, 11 and 12). While 1,2-dichloroethane (DCE) was used as a solvent, product 3a could be isolated in 57% yield (entry 10). Decreasing the loading of 2a leads to a reduction in the yield of 3a (entry 13). In contrast, increasing the amount of 4-methylbenzenesulfonohydrazide (2a) and oxidant TBHP to 2.5 equivalent, an enhancement in yield of 3a was observed (73%, entry 14). Finally, several control experiments were conducted. When the reaction was performed under without irradiation, or in the absence of photocatalyst, or oxidant, the reaction did not proceed at all, no desired product 3a was detected (entries 15–17). These results indicates that both the visible-light, photocatalyst and oxidant play a crucial role in the reaction.| Entry | Photocatalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.25 mmol), 2a (0.5 mmol, 2.0 eq.), photocatalyst (5 mol%) TBHP (2.0 eq., 70% aqueous solution) and DMSO (2 mL) were stirred under 10 W blue LED (460–465 nm) irradiation at room temperature under air for 10 h.b Isolated yield.c 1.5 eq. of 2a was used.d 2.5 eq. of 2a and 2.5 eq. of TBHP were used.e Without 10 W blue LED (460–465 nm) irradiation.f Without photocatalyst.g Without TBHP. | |||
| 1 | Eosin B | DMSO | 25 |
| 2 | Fluorescein | DMSO | 38 |
| 3 | Rose Bengal | DMSO | 48 |
| 4 | Na2-Eosin Y | DMSO | 46 |
| 5 | Ru(bpy)3Cl2·6H2O | DMSO | 65 |
| 6 | Ru(bpy)3Cl2·6H2O | DCM | 35 |
| 7 | Ru(bpy)3Cl2·6H2O | DMF | 15 |
| 8 | Ru(bpy)3Cl2·6H2O | THF | 0 |
| 9 | Ru(bpy)3Cl2·6H2O | CH3CN | 10 |
| 10 | Ru(bpy)3Cl2·6H2O | DCE | 57 |
| 11 | Ru(bpy)3Cl2·6H2O | CH3OH | 23 |
| 12 | Ru(bpy)3Cl2·6H2O | 1,4-Dioxane | 40 |
| 13c | Ru(bpy)3Cl2·6H2O | DMSO | 57 |
| 14d | Ru(bpy)3Cl2·6H2O | DMSO | 73 |
| 15e | Ru(bpy)3Cl2·6H2O | DMSO | 0 |
| 16f | Ru(bpy)3Cl2·6H2O | DMSO | 0 |
| 17g | Ru(bpy)3Cl2·6H2O | DMSO | 0 |
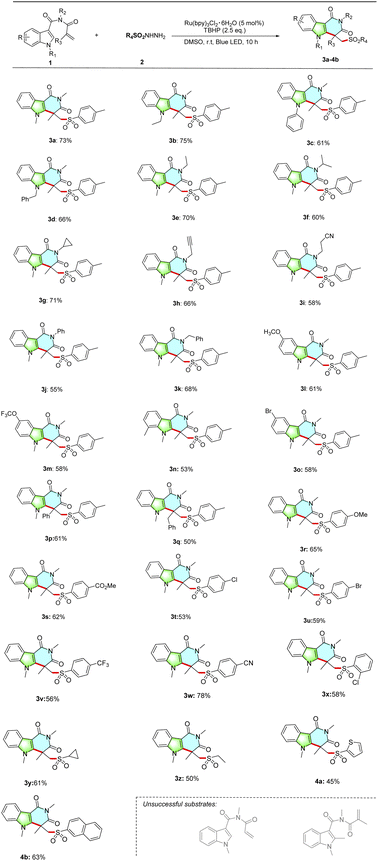
With the optimized condition in hand, we next investigated the substrate scope of N-methacryloyl-1H-indole-3-carboxamides with sulfonohydrazides (Table 2). The effect of N-protecting groups on the indole ring were first examined. It was found that a variety of N-methacryloyl-1H-indole-3-carboxamides bearing different groups at R1 position, such as methyl, ethyl, phenyl and benzyl, were compatible with the reaction conditions, giving the desired indole-fused pyridine products 3a–3d in 61–75% yields, respectively. The amide group bearing different substituents at R2 position was then evaluated. We were pleased to discover that a variety of aliphatic groups at R2-position including ethyl, isopropyl, cyclopropyl, propargyl, cyanoethyl, phenyl and benzyl were well compatible with the transformation (3e–3k). It is worth noting that alkynyl and cyano were retained in the reaction, which were suitable for potential further functionalization. Moreover, substrates bearing different groups at the C5 and C7 position of the indole ring were found to be well compatible under standard conditions, delivering the desired products in moderate to good yields (3l, 61%; 3m, 58%; 3n, 53%; 3o, 58%, respectively). The reaction also worked well when R3 position was substituted by phenyl or benzyl group, generating the target products 3p and 3q in 61% and 50% yields, respectively. Finally, the feasibility of a variety of substituted sulfonylhydrazides in this reaction system were tested. To our delight, sulfonylhydrazides bearing different substituents at aromatic ring such as OMe, CO2Me, Cl, Br, CF3 and CN could all participate in the reaction efficiently to deliver the corresponding products 3r–3x in 53–78% yields. Moreover, the alkyl-, thiophene- and naphthalene-substituted sulfonylhydrazides were also tolerated, giving the products 3y–4b in moderate to good yields. However, the use of N-acryloyl-N,1-dimethyl-1H-indole-3-carboxamide and N-methacryloyl-N,1,2-trimethyl-1H-indole-3-carboxamide as reactants, no desired products were detected.
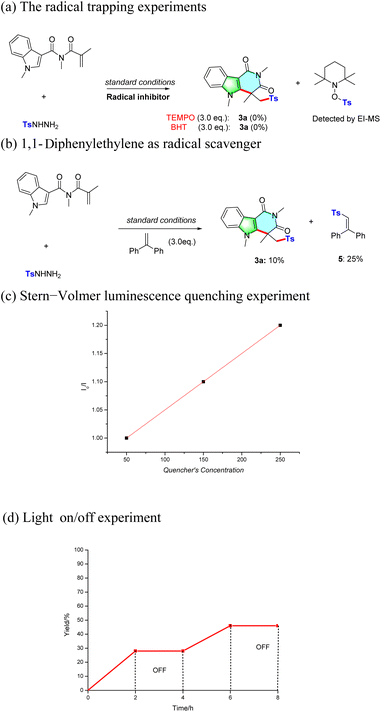
In order to understand the mechanism of this protocol, several control experiments were further conducted. To verify if the radical pathway is involved in the present transformation, 3.0 equiv. of radical scavenger [2,2,6,6-tetramethylpiperidoxyl (TEMPO) or butylated hydroxytoluene (BHT)] was added to the reaction. The formation of 3a was completely inhibited and the TEMPO-Ts adduct was detected by EI-MS (Scheme 2a). Furthermore, the addition of 3.0 equiv. of 1,1-diphenylethylene as the probe afforded adduct 5 in 25% yield (Scheme 2b). These results suggested that the sulfonyl radical may be an intermediate of this photocatalytic transformation. To gain insight into the photocatalytic cycle, a Stern–Volmer luminescence quenching experiment was further conducted by mixing Ru(bpy)3Cl2·6H2O with different concentrations of TBHP. It was observed that the excited-state *[Ru(bpy)3]2+ was quenched by the addition of TBHP, suggesting a strong interaction between the photocatalyst and TBHP (Scheme 2c). Finally, an on/off light experiment was conducted. The formation of product 3a was totally suppressed in the absence of light, demonstrating that the continuous light radiation was indispensable for this transformation and the radical chain reaction pathway is not involved in the present transformation (Scheme 2d).
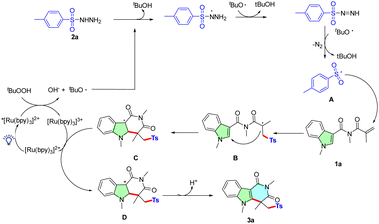
Based on the results of mechanistic studies and previously reported results,6,7,11–14 a plausible reaction mechanism is depicted in Scheme 3. First, visible-light irradiation of photocatalyst [Ru(bpy)3]2+ forms the excited state species *[Ru(bpy)3]2+. Then, the single electron transfer (SET) from *[Ru(bpy)3]2+ to oxidant t-BuOOH forms radical t-BuO˙, along with the oxidation of *[Ru(bpy)3]2+ to [Ru(bpy)3]3+. Next, the radical t-BuO˙ abstract hydrogen atoms from sulfonylhydrazide to generate sulfonyl radical with the release of molecular nitrogen. Subsequently, sulfonyl radical attacks the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of 1a, followed by intramolecular cyclization to afford radical intermediate C. Intermediate C was further oxidized to carbocation D by [Ru(ppy)3]3+ via SET process. Finally, the deprotonation of intermediate D affording the desired product 3a.
C bond of 1a, followed by intramolecular cyclization to afford radical intermediate C. Intermediate C was further oxidized to carbocation D by [Ru(ppy)3]3+ via SET process. Finally, the deprotonation of intermediate D affording the desired product 3a.
Conclusions
In conclusion, we have developed a novel radical cascade cyclization strategy for the synthesis of indole-fused pyridines by using Ru(bpy)3Cl2·6H2O as photocatalyst and TBHP as oxidant. With visible light irradiation, a variety of indole-fused pyridine derivatives bearing different functional groups were obtained in moderate to good yields. Compared with previous work, the easily-accessible starting materials, molecular nitrogen as byproduct and eco-friendly visible-light as energy source make this transformation more sustainable and practical, and may find applications in organic, materials and pharmaceutical chemistry in future.Experimental section
General information
All reagents and solvents were purchased from commercial suppliers and used without purifications. TLC was performed on silica gel plates (200–300 mesh) using UV light (254/365 nm) for detection and column chromatography was performed on silica gel (200–300 mesh). The 1H NMR and 13C NMR spectra were recorded at 25 °C in CDCl3 at 400 and 100 MHz, respectively, with TMS as the internal standard. Chemical shifts (δ) are expressed in ppm and coupling constants J are given in Hz. All reactions were performed on the photoreaction instrument (WP-TEC-1020SL), which are purchased from WATTCAS, China. High resolution mass spectra (HRMS) were obtained on a TOF MS instrument with ESI source. Melting points were measured with X-4B digital point apparatus and not corrected. All the starting materials are known compounds.General experimental procedure for the synthesis of 3a–4b
To a stirred solution of 1 (0.25 mmol) in DMSO (2.0 mL) were added 2 (2.5 eq., 0.625 mmol), Ru(bpy)3Cl2·6H2O (5 mol%) and TBHP (70% aqueous solution, 2.5 eq., 0.625 mmol). The resulting mixture was stirred in air under a 10 W blue LEDs (460–465 nm) and irradiated for 10 h. The temperature was maintained at 20–25 °C when the LED light was on. After the reaction was complete, the reaction mixture was diluted with a brine solution (25 mL) and extracted with EtOAc (30 mL × 3). The combined organic phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by flash column chromatography to afford the desired products 3a–4b.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its ESI†].Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support from the Scientific Research Foundation of Hunan Provincial Education Department (23B0650) is greatly appreciated.References
- (a) M. Till and M. R. Prinsep, J. Nat. Prod., 2009, 72, 796 CrossRef CAS PubMed; (b) A. AlQathama and J. M. Prieto, Nat. Prod. Rep., 2015, 32, 1170 RSC; (c) G. D. Cuny, N. P. Ulyanova, D. Patnaik, J. F. Liu, X. Lin, K. Auerbach, S. S. Ray, J. Xian, M. A. Glicksman, R. L. Stein and J. M. G. Higgins, Bioorg. Med. Chem. Lett., 2012, 22, 2015 CrossRef CAS; (d) G. Abbiati, E. M. Beccalli, G. Broggini and C. Zoni, J. Org. Chem., 2003, 68, 7625 CrossRef CAS.
- (a) Z. Shi, Y. Cui and N. Jiao, Org. Lett., 2010, 12, 2908 CrossRef CAS PubMed; (b) C. B. Cui, H. Kakeya and H. J. Osada, Tetrahedron, 1996, 52, 12651 CrossRef CAS; (c) A. Jossang, P. Jossang, H. A. Hadi, T. Sevenet and B. Bodo, J. Org. Chem., 1991, 56, 6527 CrossRef CAS; (d) J. S. Shi, J. X. Yu, X. P. Chen and R. X. Xu, Acta Pharmacol. Sin., 2003, 24, 97 CAS; (e) J. M. Polonsky, M. A. Merrien, T. Prange and C. Pascard, J. Chem. Soc., Chem. Commun., 1980, 13, 601 RSC.
- (a) M. A. Abou-Gharbia, US Pat. US4748247A, 1988; (b) Z. Wang and L. Li, WO2013/078544 2013.
- T. Mita, S. Ishii, Y. Higuchi and Y. Sato, Org. Lett., 2018, 20, 7603 CrossRef CAS.
- For selected reviews and reports, see: (a) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (c) H. Tan, H. Li, W. Ji and L. Wang, Angew. Chem., Int. Ed., 2015, 54, 8374 CrossRef CAS; (d) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS; (e) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034 CrossRef CAS; (f) C. Tian, Q. Wang, X. Wang, G. An and G. Li, J. Org. Chem., 2019, 84, 14241 CrossRef CAS PubMed; (g) G. E. M. Crisenza and P. Melchiorre, Nat. Commun., 2020, 11, 803 CrossRef PubMed; (h) H. Tian, H. Yang, C. Tian, G. An and G. Li, Org. Lett., 2020, 22, 7709 CrossRef CAS; (i) L. Candish, K. D. Collins, G. C. Cook, J. J. Douglas, A. Gómez-Suárez, A. Jolit and S. Keess, Chem. Rev., 2022, 122, 2907 CrossRef CAS PubMed.
- S.-M. Xu, J.-Q. Chen, D. Liu, Y. Bao, Y.-M. Liang and P.-F. Xu, Org. Chem. Front., 2017, 4, 1331 RSC.
- (a) Y. Liu, Z. Chen, Q.-L. Wang, P. Chen, J. Xie, B.-Q. Xiong, P.-L. Zhang and K.-W. Tang, J. Org. Chem., 2020, 85, 2385 CrossRef CAS; (b) P. Zhang, S. Shi, X. Gao, S. Han, J. Lin and Y. Zhao, Org. Biomol. Chem., 2019, 17, 2873 RSC; (c) Y. Liu, Q.-L. Wang, C.-S. Zhou, B.-Q. Xiong, P.-L. Zhang, C. Yang and K.-W. Tang, J. Org. Chem., 2018, 83, 2210 CrossRef CAS PubMed; (d) Y. Liu, Q.-L. Wang, B.-Q. Xiong, P.-L. Zhang, C.-A. Yang, Y.-X. Gong, J. Liao and Q. Zhou, Synlett, 2018, 29, 2396 CrossRef CAS.
- C. Y. Du, Y. C. Tang, J. L. Duan, B. Y. Yang, Y. P. He, Q. Zhou and X. W. Liu, Chin. J. Org. Chem., 2023, 43, 4268 CrossRef CAS.
- (a) T. M. Williams, T. M. Ciccarone and S. C. MacTough, et al., J. Med. Chem., 1993, 36, 1291 CrossRef CAS PubMed; (b) J. B. McMahon, R. J. Gulakowski and O. S. Weislow, et al., Antimicrob. Agents Chemother., 1993, 37, 754 CrossRef CAS PubMed; (c) M. Artico, R. Silvestri and S. Massa, et al., J. Med. Chem., 1996, 39, 522 CrossRef CAS PubMed; (d) N. Neamati, A. Mazumder and H. Zhao, et al., Antimicrob. Agents Chemother., 1997, 41, 385 CrossRef CAS.
- (a) A. N. R. Alba, X. Companyo and R. Rios, Chem. Soc. Rev., 2010, 39, 2018 RSC; (b) C. Cassani, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2009, 48, 5694 CrossRef CAS; (c) M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixao and K. A. Jorgensen, Angew. Chem., Int. Ed., 2010, 49, 2668 CrossRef CAS PubMed; (d) V. Sikervar, J. C. Fleet and P. L. Fuchs, Chem. Commun., 2012, 48, 9077 RSC; (e) V. Sikervar, J. C. Fleet and P. L. Fuchs, J. Org. Chem., 2012, 77, 5132 CrossRef CAS PubMed.
- During the preparation of this Letter, a similar visible-light-initiated radical cascade reaction for the synthesis of diverse fused Indolo-pyridones was reported by Guan's group, D.-Y. Yang, L. Liu, J.-Y. Gu, Y.-H. He and Z. Guan, J. Org. Chem., 2021, 86, 18042 CrossRef CAS PubMed , by comparison, our method uses cheap Ru(bpy)3Cl2·6H2O as photocatalyst, stable sulfonylhydrazide as reactants and under neutral reaction conditions. Moreover, our method is easier to operation than their work..
- (a) H.-C. Li, K. Sun, X. Li, S.-Y. Wang, X.-L. Chen, S.-Q. He, L.-B. Qu and B. Yu, J. Org. Chem., 2021, 86, 9055 CrossRef CAS; (b) Y. Pan, X. Gong, R. Hao, S. Zeng, J. Xu, Z. Shen and W. Huang, Asian J. Org. Chem., 2022, 11, e202100766 CrossRef CAS; (c) X.-Y. Hu, H.-F. Xu, Q. Chen, Y.-L. Pan and J.-Z. Chen, Org. Biomol. Chem., 2021, 19, 10376 RSC; (d) Y.-L. Wei, J.-Q. Chen, B. Sun and P.-F. Xu, Chem. Commun., 2019, 55, 5922 RSC; (e) H. Cui, C. Ni and C. Zhang, J. Org. Chem., 2021, 86, 15835 CrossRef CAS PubMed; (f) Y. Luo, T. Tian, Y. Nishihara, L. Lv and Z. Li, Chem. Commun., 2021, 57, 9276 RSC; (g) J.-R. Zhang, H.-Y. Liu, T. Fan, Y.-Y. Chen and Y.-L. Xu, Adv. Synth. Catal., 2021, 363, 497 CrossRef.
- K. Zhang, L. Qiao, J. Xie, Z. Lin, H. Li, P. Lu and Y. Wang, J. Org. Chem., 2021, 86, 9552 CrossRef CAS PubMed.
- K. Sun, Q. Y. Lv, X. L. Chen, L. B. Qu and B. Yu, Green Chem., 2021, 23, 232 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08000a |
| This journal is © The Royal Society of Chemistry 2025 |