Open Access Article
Open Access ArticleAlum sludge-driven electro-phytoremediation in constructed wetlands: a novel approach for sustainable nutrient removal†
Daryoush Sanaei a,
Amir Mirshafiee
a,
Amir Mirshafiee *bc and
Amir Adibzadehbc
*bc and
Amir Adibzadehbc
aStudent Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran. E-mail: daryoushsanaei@gmail.com
bHealth Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran. E-mail: a_mirsh1@yahoo.com
cDepartment of Environmental Health Engineering, Faculty of Health, Baqiyatallah University of Medical Sciences, Tehran, Iran
First published on 29th January 2025
Abstract
In addition to their advantages as promising methods for wastewater treatment, CWs exhibit poor performance in terms of N and P removal efficiency in the effluent of wastewater treatment plants. By focusing on this issue, we designed CWs integrated with a biochar-doped activated carbon cloth (ACC) electrode and alum sludge from water treatment plants as a substrate to achieve concomitant organic matter and nutrient removal efficiency. Compared with the use of one layer of alum sludge in CWs (CWs-C3) with ACC electrodes inserted in two layers, which uses one layer of alum sludge, a significant improvement in removal efficiency was achieved (96% for COD; 89% for TN; and 77% for TP). The findings revealed that the application of potential accompanied by the insertion of a cathode ACC electrode into the first layer of alum sludge was beneficial for completing nitrification and facilitating denitrification in the cathode and anode regions, respectively, resulting in increased removal of organic matter and nutrients. Further evaluation revealed that the TN-TP synergetic removal mechanism was influenced by the use of Fe2+ as an electron donor and as a driving force for the development of autotrophic denitrifying bacteria to increase nitrate reduction. Additionally, the formation of FePO4 and AlPO4 and their adsorption through the interaction of FeOOH and AlOOH with phosphate constitute the main removal mechanism for TP in wastewater. Another reason for the increased removal efficiency in the CW-C3 reactor was the greater abundance and microbial diversity effectuated by the application of potential in the anode and cathode regions. In summary, a promising strategy for simultaneously promoting organic matter and nutrients and utilizing CWs on a large scale and in practical applications was proposed.
1. Introduction
The enormous and varied production of wastewater engendered by rapid economic and population growth, development urbanization and industrialization, in addition to the increasing utilization of fossil resources as both carbon and energy sources, has led to crucially rising issues across the globe. Harmful environmental discharge, water crises, energy scarcity, climate change, and global warming are consequences of the abovementioned issues.1 The generated wastewaters can be treated chemically or biologically using various methods to limit their adverse effects on the environment and natural resources. Most applied technologies require further sophisticated processes to remove or mitigate side products and sludge before being discharged into the environment.2 Additionally, wastewater treatment facilities that involve carbon-based and nutrient removal not only lead to the production of greenhouse gases (GHGs) but also indirectly contribute to energy consumption by various equipment and sludge handling and treatment methods.3Notably, nutrients such as nitrate and phosphorus, which are major components of wastewater, can cause excessive plant growth and eutrophication through entry into the environment and water bodies owing to incomplete treatment. This can further lead to the need for chemical or biological processes for mitigation at the standard level and increase capital and operational costs and energy consumption.4 To limit clean water resources, the ability to enter plastics and other pollutants in the environment, GHG and energy consumption, and pervasive mismanaging of wastewater-treated wastes, the development of environmentally friendly technology and an aspiration of clean water and energy have led to an increasing emphasis on less carbonization and less energizing wastewater treatment and infrastructure.5
The nature-based solution coupled with advanced technologies such as constructed wetlands (CWs) is a state-of-the-art technology performed worldwide, particularly for treating different types of wastewaters at scales ranging from small and rural communities to urban and industrial applications.6 As a green, eco-friendly, low-cost and easy maintenance method for treating wastewater, CWs are widely attracted and widely applied worldwide. This study aims to enhance the effectiveness of CWs by addressing the limitations of existing methods and introducing novel substrates and configurations to improve nutrient and organic matter removal. As this technology has all the hallmarks of ideal and effective wastewater treatment, Marwa et al.7 compared the wastewater treatment performances of CWs with two types of configurations (vertical flow and horizontal flow). They concluded that in terms of the removal rates of BOD, COD, TSS, TN, TP and NH4, both configurations of CWs were attained with sparingly higher removal efficiencies of 77% and 68% in the case of vertical flow than in the case of horizontal flow. The study revealed that the type of substrate, retention time and plant species can be considered the most dominant variables controlling CW performance. Moreover, CWs can be performed for particular wastewater with organic or inorganic pollutants. For example, Fazila Younas et al.8 evaluated the utilization of CWs to treat the tannery industry, which contains high concentrations of chromium (Cr). They reported that the dominant parameters for achieving a high removal rate of Cr via CWs are wetland plants and the type of substrate used as media. The Cr removal efficiency reached 80 to 99% when all types of configurations of CWs were used. However, the horizontal flow and vertical flow modes do not consider actual operating conditions, unlike the free water surface CW mode.
Conventional CWs, from a treatment perspective, have poor N and P removal performance in wastewater effluent and do not have enough time to degrade organic matter in wastewater as much as other methods can.9 Thus, to meet the requirements of improved treatment efficiency, various strategies, such as the introduction of novel and effective substrates in single or multilayer systems and different plants, the establishment of a microbial community in a fixed bed, and the integration of other wastewater treatment technologies, are widely recommended and investigated.10 A study was performed to successfully remediate phosphorus from wastewater via a novel composite substrate containing brick waste, tile waste and gravel in CWs.11 The total phosphorus and phosphate removal efficiencies from domestic wastewater were reported to be 72% and 78%, respectively. Additionally, the coupling of CWs with other systems for improving the performance of bioremediation of organic matter and nutrients has been investigated, particularly in the last few decades.12 Among many examples, Mengni Tao et al.13 utilized high-density polyethylene (HDPE) fillers as effective substrates coupled with microbial fuel cells (MFCs) for simultaneous bioelectricity generation and enhanced nitrogen removal for the treatment of low-carbon wastewater. A 97.55% total nitrogen removal and bioelectricity generation with a power density of 2.22 mW m−2 were achieved with the addition of both HDPE fillers and Acorus calamus biomass in coupled CWs-MFCs.
Considering the nature and concentration of organic matter/nutrients, an evaluation of the performance of CWs with water alum sludge with a carbon cloth electrode doped with biochar for improving the performance of CWs for nutrient and organic matter removal from domestic wastewater was performed. The application of alum sludge as a cost-effective substrate in this study demonstrated a uniform dispersion of metal species. This substrate proved to be highly effective in removing organic matter, nitrogen (N), and phosphorus (P), attributed to the abundant hydroxide sites that facilitate interactions and accelerate redox reactions. Furthermore, the incorporation of a biochar-coated carbon cloth electrode, alongside current application, resulted in significant removal rates. This success is due to the creation of regions within the substrate that fostered microbial growth and provided an ideal medium for biofilm formation. The findings indicated that the enhanced growth and biofilm formation observed in this study led to lower rates of nitrification-denitrification under these conditions, compared to those without an electrode. Consequently, the stable Fe–Al–N (or P) cycle within the alum sludge substrate, combined with the formation of active zones near the electrodes, accelerated biofilm formation and achieved efficient removal of nitrogen and phosphorus.
2. Materials and methods
2.1. Chemicals
| Parameters | units | Mean ± SD | Measuring method |
|---|---|---|---|
| pH | — | 6.9 ± 0.2 | pH meter (model 111) |
| VSS/SS | % | 0.27 ± 0.25 | In all analytical procedures for determination of suspended solids (SS), weighed filters are used for sample filtration, the filters are dried at approximately 105 °C after filtration, cooled in a desiccator to room temperature and the weight of the loaded filter is determined |
| A sample is filtered through a glass fiber filter with 1.5 micron openings. The filter is dried in an oven at 103 °C for 24 hours (TSS), and then placed in a 550 °C furnace for 1 hour (VSS)14 | |||
| TOC | g kg−1 | 116 ± 19 | Shimadzu TOC-VCSH total organic carbon analyzer |
| BET | g m−2 | 122 ± 39 | The N2 adsorption/desorption measurements with the use of an ASAP 2020 micromeritics instrument |
| Total P | g kg−1 | 1.4 ± 1.1 | Analyzed following method 365.1 (ref. 15) |
| Ca | g kg−1 | 15.6 ± 6.2 | One gram of each dried residual was digested with 30 mL of HCl, 10 mL HNO3, 5 mL HF, and 2 mL HClO4 on a hotplate and analyzed with ICP-AES (Varian, Vista Pro) according to method 6010C16 |
| Total Al | g kg−1 | 155 ± 18 | One gram of each dried residual was digested with 30 mL of HCl, 10 mL HNO3, 5 mL HF, and 2 mL HClO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) on a hotplate and analyzed with ICP–AES (Varian, Vista Pro) according to method 6010C16 on a hotplate and analyzed with ICP–AES (Varian, Vista Pro) according to method 6010C16 |
| Total Fe | g kg−1 | 6.2 ± 2.5 | One gram of each dried residual was digested with 30 mL of HCl, 10 mL HNO3, 5 mL HF, and 2 mL HClO4 on a hotplate and analyzed with ICP-AES (Varian, Vista Pro) according to method 6010C16 |
| SO42− | g kg−1 | 7.8 ± 0.28 | Sulfate results were measured using spectrophotometry (DR – 4000, Hach Co., Coveland Co.) |
| Cl− | g kg−1 | 16.4 ± 0.55 | Determined as described in ref. 17 |
| Moisture content | % | 34.59 ± 1.5 | Alum sludge samples were dried in an oven at 103 ± 2 °C for 72 h to determine the moisture content |
Carbon cloth (CC) was used as an electrode and was purchased from Redox Kala, Iran. To remove impurities, the carbon cloth (2 × 2 cm) was soaked and sonicated in acetone, ethanol, or 0.2 M HCl for 30 min each. After sonication, the CC was rinsed with DW several times and then dried in air at room temperature. To increase the stability and strongly bind the biochar on the carbon cloth electrode, CC was activated for 1 h at 100 °C in a vacuum oven to prepare activated carbon cloth (ACC) before use. To coat ACC with biochar, an ink material was prepared by dispersing 100 mg of biochar in a mixture of 50 μL of 5% Nafion solution and 500 μL of isopropyl alcohol: water mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 V/V), and the mixture was sonicated in an ultrasonic bath for 30 min to form homogeneous ink. The resulting ink was drop-cast on an ACC electrode (2 × 2 cm) via the air brushing method. The prepared electrode-coated biochar was dried in air overnight. The amount of biochar loaded on the ACC was 45 mg cm−2. Copper wire was used to connect the electrode of the cathode and anode, and a power supply was applied to provide the required current.
1 V/V), and the mixture was sonicated in an ultrasonic bath for 30 min to form homogeneous ink. The resulting ink was drop-cast on an ACC electrode (2 × 2 cm) via the air brushing method. The prepared electrode-coated biochar was dried in air overnight. The amount of biochar loaded on the ACC was 45 mg cm−2. Copper wire was used to connect the electrode of the cathode and anode, and a power supply was applied to provide the required current.
2.2. CWs setup and operation
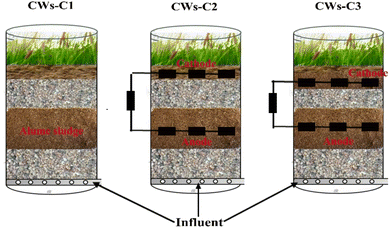
The CWs were constructed from cylindrical Plexiglas with a diameter of 5 cm and height of 38 cm with an outlet pipe at the bottom (Fig. 1). A stainless-steel mesh was placed at the bottom of the column just 1.5–2 cm above the outlet pipe to prevent the substrate from being removed from the column. The synthetic wastewater with the physicochemical properties given in Table 2 was introduced to the CWs in down to up flow mode.| Parameters | Units | Value |
|---|---|---|
| COD | mg L−1 | 500 ± 11.2 |
| TP | mg L−1 | 5 ± 0.81 |
| NH4+ | mg L−1 | 20 ± 0.58 |
| NO3− | mg L−1 | 30 ± 1.1 |
| Loading rate | m3 m−2 d−1 | 0.2 |
| HRT | d | 2 |
| pH | — | 7.1 ± 0.21 |
| Applied potential | V | 0.2–1 |
| C/N ratio | — | 1 to 15 |
| DO | mg L−1 | 2.22–3.14 |
Three columns were constructed with a total working height of 30 cm and were labeled CWs-C1 to CWs-C3. The column of CWs-C1 contained media with four layers (up to down): 2 cm of sand (1.5–2.5 mm), 5 cm of fine gravel as the top layer (3–5 mm), 13 cm of alum sludge as the middle layer (5–7 mm), and 7 cm of coarse gravel as the bottom layer (8–12 mm); the column of CWs-C2 had the same media patterns as those of CWs-C1 except for the insertion of two ACC electrode anodes and cathodes within the alum sludge layer and sand layer, respectively; and the CW-C3 column contained the same layer patterns as those of columns CWs-C2 except for two alum sludge layers in the column: 1 cm of sand, 4 cm of first alum sludge, 8 cm of coarse gravel, 7 cm later alum sludge, and 5 cm of coarse gravel with the insertion of the ACC cathode electrode and anode electrode, respectively, in the first and second alum layers. To deliver uniform synthetic wastewater into the columns, a fine bubble shower with a peristaltic pump was used. The applied potentials for columns of CWs-C2 and CWs-C3 were adjusted in the range of 0.2 to 1 V vs. Ag/AgCl. All the columns were planted with lentil plants (Lens culinaris). The plants were first seeded in sterile Petri dishes, and after they were grown and germinated, they were transplanted into columns. The fine sands of the CWs-C1 and CWs-C2 columns and first alum sludge layer in the column of CWs-C3 are aerated through fine diffusers with an aquarium pump to actively allow oxygen from the atmosphere in the mentioned layers and prevent further limitation of oxygen in those layers. A perforated hole was created on the opposite side of the influent aeration to prevent the diffusion of oxygen to other layers. Artificial sunlight was used to expose the reactors to simulated sunlight at an ambient temperature of nearly 25 °C. The system was operated continuously for 70 days. After this period, no significant removal of parameters was observed. Prior to this, a start-up period of two weeks was implemented to allow for acclimatization and the development of microorganisms over the media within the systems. All reactors were operated at a loading rate of 0.2 m3 m−2 d−1 with an HRT of 2 days.
In this study, by assuming the formation of biofilms on ACC and alum sludge and other possible layers via the application of an external current to stimulate and accelerate the growth of microorganisms and alum sludge, an inoculation and cultivation step was performed for two weeks. In this phase, all the columns were inoculated with a mixture of anaerobic and aerobic activated sludge from a wastewater treatment plant as the initial culture medium, which was placed in Tehran, Iran. The mixture of sludge was mixed with synthetic wastewater at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (V/V) and pumped into the reactor. The synthetic wastewater prepared in this study contained the following components: glucose, 0.502 g L−1; NH4Cl, 0.520 g L−1; NaNO3, 0.090 g L−1; NaCl, 0.031 g L−1; K2HPO4, 0.033 g L−1; and 5 mL of wolf vitamin solution and 10 mL wolf mineral solutions (more details about the prepared recipe are given in the ESI (Table S1†). The average values (mg L−1) of chemical oxygen demand (COD), total phosphorous (TP), ammonium nitrogen (NH4+–N), and nitrate-nitrogen (NO3) were 500, 5, 20, and 30, respectively. The initial pH of the synthetic wastewater was adjusted to 7–7.3 by adding an appropriate 50 mM phosphate buffer solution (Table S2†). Before the formal operation experiment started, the CWs were inoculated for two weeks (cultivation) to provide a suitable adaptation microenvironment for microbial and plant growth within the CWs, which was refreshed every 2 days. The operation experiments were run with the same composition and different C/N ratios, ranging from 2 to 15.
10 (V/V) and pumped into the reactor. The synthetic wastewater prepared in this study contained the following components: glucose, 0.502 g L−1; NH4Cl, 0.520 g L−1; NaNO3, 0.090 g L−1; NaCl, 0.031 g L−1; K2HPO4, 0.033 g L−1; and 5 mL of wolf vitamin solution and 10 mL wolf mineral solutions (more details about the prepared recipe are given in the ESI (Table S1†). The average values (mg L−1) of chemical oxygen demand (COD), total phosphorous (TP), ammonium nitrogen (NH4+–N), and nitrate-nitrogen (NO3) were 500, 5, 20, and 30, respectively. The initial pH of the synthetic wastewater was adjusted to 7–7.3 by adding an appropriate 50 mM phosphate buffer solution (Table S2†). Before the formal operation experiment started, the CWs were inoculated for two weeks (cultivation) to provide a suitable adaptation microenvironment for microbial and plant growth within the CWs, which was refreshed every 2 days. The operation experiments were run with the same composition and different C/N ratios, ranging from 2 to 15.
2.3. Sample collection and analysis
To evaluate the effects of inserting anode and cathode electrodes in CW systems, water samples from influent and effluent reactors were collected daily. The collected samples were passed through a 0.45 μm membrane and then preserved at 4 °C before analysis. The COD, NO3−–N, NH4+–N, and TP contents were analyzed via the colorimetric method with a UV/VIS spectrophotometer according to standard methods.18 The pH, electrical conductivity, temperature, and dissolved oxygen (DO) content were measured in situ. All experimental measurements were performed in triplicate, and the results are expressed as average values.The biofilm formed on the electrodes was observed by scanning electron microscopy (SEM) at the end of the experiment. The samples were cut into small portions and prepared before observation as follows: the electrodes were fixed in 2% glutaraldehyde (GA) or 2.5% paraformaldehyde solutions overnight at 4 °C. Dehydration of the samples was then performed with 40, 50, 60, 70, 80, 90, 100, or 100% (v/v) ethanol for 20 min. Finally, the dehydrated samples were dried in air overnight and then coated with a thin layer of palladium–gold to obtain SEM images.19
2.4. Characterization of biochar and alum sludge
A scanning electron microscope (SEM) coupled with EDAX (Hitachi, S-3500 N) was used to investigate the surface morphology and elemental distribution, respectively. The types of bonding and functional groups present in the biochar and alum sludge were determined via an IR spectrometer in the range of 4000–600 cm−1. The specific surface area and porosity were studied via BET analysis (Table S3†).2.5. Microbial community analysis
The formed biofilms on the electrodes inserted into substrates in CWs-C2 and CWs-C3 reactors were rinsed using saline phosphate buffer (SPB) after collecting at the end of operation experiments and after centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, were stored at −80 °C. The samples were submitted to Faculty of the Division of Cell and Molecular Biology, University of Tehran, Iran for DNA extraction and Illumina MiSeq high-throughput sequencing using Illumina platform PE250 model. The microbial community and their structures were analysed and clustered into the operational taxonomic units (OTUs) by performing the UPARSE software at 97% similarity (UPARSE OTU clustering (https://www.drive5.com). Data with the confidence threshold of 0.7 was considered according to the Silva database. Lastly, the microbial composition of samples was estimated according to obtained information from OTUs at the taxonomic level.
000 rpm for 10 min, were stored at −80 °C. The samples were submitted to Faculty of the Division of Cell and Molecular Biology, University of Tehran, Iran for DNA extraction and Illumina MiSeq high-throughput sequencing using Illumina platform PE250 model. The microbial community and their structures were analysed and clustered into the operational taxonomic units (OTUs) by performing the UPARSE software at 97% similarity (UPARSE OTU clustering (https://www.drive5.com). Data with the confidence threshold of 0.7 was considered according to the Silva database. Lastly, the microbial composition of samples was estimated according to obtained information from OTUs at the taxonomic level.
2.6. Statistical analysis
SPSS 22.0 was used to analyze the obtained data, and Origin Pro 2021 was used to plot the data.3. Results
3.1. Effects of applied potential and evaluation of pollutant removal in CWs
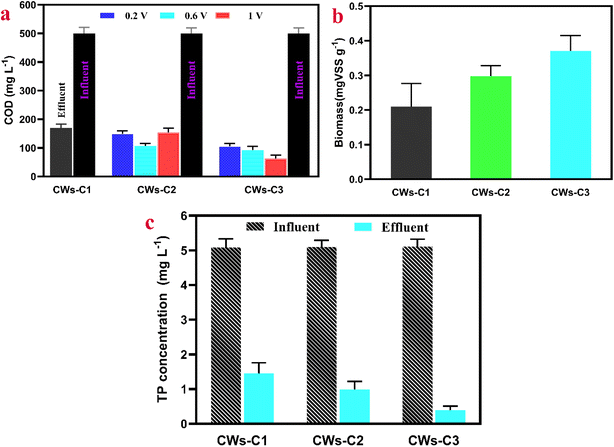
By placing electrodes into the substrates of CWs and applying potential, CWs behave differently than those without electrodes and those with two alum sludge substrates. This behavior can be observed for organic compounds such as COD removal in CWs. When the CW-C1 reactor was operated without an electrode, the average removal of COD was 68%. COD removal was significantly enhanced by the coupling electrode and applied potential, as shown in Fig. 2a, for the CW-C2 and CW-C3 systems at different applied potentials, with significant differences. The CW-C2 reactor with electrodes inserted into a single layer of alum sludge reached average COD removal rates of 70%, 78%, and 69.3% when the applied potential was increased to 0.2 V, 0.6 V, and 1 V vs. Ag/AgCl, respectively. These results confirmed that the application of potential was able to induce the acclimation of the electrode surface biofilm.20 Moreover, applying a potential can increase the electron affinity of the anode electrode,21 which in turn leads to an electromotive force that drives more and significantly more electrons to flow into the anode and enhances the growth of microorganisms and the degradation of organic matter (Fig. S1†). However, the COD removal values decreased to 70% and 69.3%, respectively, when the CW-C2 reactor was operated at lower (0.2 V vs. Ag/AgCl) and higher (1 V vs. Ag/AgCl) potentials than the ideal 0.6 V vs. Ag/AgCl potential. It can be posited that anode potential values greater than 0.6 V vs. Ag/AgCl (here, study) generated a very thin anode biofilm with low microbial density, which may be demolished due to lower stability, whereas anode potential values less than 0.6 V vs. Ag/AgCl (this study) can preferentially choose specific microorganisms, such as Geobacteraceae-like cells, and form a high density of cells and very thick biofilms.22 An appropriate anode biofilm thickness and microbial density and diverse biofilms could be achieved by applying 0.6 V vs. Ag/AgCl anodic potential.Compared with CWs-C2, CWs-C3 reactors with two layers of alum sludge and inserting ACC cathode and anode electrodes into the first and second alum layers (from perspective up to down), respectively, exhibit a higher COD removal rate when the applied potential is increased to 1 V vs. Ag/AgCl. As shown in Fig. 2a, CWs-C3 experienced a higher average COD removal of 87.11% at an applied potential of 1 V vs. Ag/AgCl than 80% and 74% at 0.6 and 0.2 V vs. Ag/AgCl, respectively. The insertion of two layers of alum sludge in CWs can significantly decrease organic matter degradation, which is consistent with other reported studies.23 The inclusion of two alum sludge layers can act as an active and adsorptive layer to induce organic matter to be removed collectively from wastewater via various mechanisms, such as adsorption, chemical oxidation or reduction, hydrolysis, and microbial degradation.24 By inserting a cathode electrode into alum sludge first and, with respect to the benefit of alum sludge, the coexistence of electroactive and aerobic bacteria over the cathode electrode and the formation of microbial biofilms with increased occupancy and variety can be posited as one of the reasons for the greater potential for achieving higher removal rates. The measured biomass results (Fig. 2b) also verified the greater microbial density of the ACC cathode inserted in the first alum layer in the CW-C3 reactor than in that inserted in the sand layer in the CW-C2 reactor. Moreover, the important role of the cathode and anode ACC electrodes in the CWs-C3 reactor was notably the growth of biofilms, which was significantly greater in CWs-C3 than in CWs-C2, indicating a variety of bacterial cells, compositions of polymeric substances and cell growth over ACC electrodes.25
The trends in the TP and total nitrogen (NH4+ and NO3−) removal efficiencies were consistent with the trends in the COD removal efficiency. As is well known and verified in many studies,26 P removal from wastewater in CWs is strongly influenced by the substrates used, as alum sludge has a high adsorption capacity for P removal. Additionally, as shown in Fig. 2c, in the CWs-C3 reactors, P removal was greater (77%) than that in the CWs-C2 reactors (65%) at higher applied potentials (here, 1 V vs. Ag/AgCl). Notably, the applied lower potential in this work (1 V vs. Ag/AgCl) is lower than that of water electrolysis (theoretically 1.23 V vs. Ag/AgCl), so O2 generation at the cathode of CWs is neglected. P removal at higher potentials can be considered to release more Al3+ or Fe3+ from alum sludge, which could form FePO4 or AlPO4 to remove P effectively.27 Moreover, increasing the release of dissolved Al3+ or Fe3+ ions and further combining with OH− in water or ACC surfaces (Fig. S2a†) and consequently forming AlOOH or FeOOH is another reason for the increased P removal efficiency.28 This was further verified by obtaining FT-IR spectra of alum sludge from the CWs-C2 and CWs-C3 reactors (Fig. S2b†).
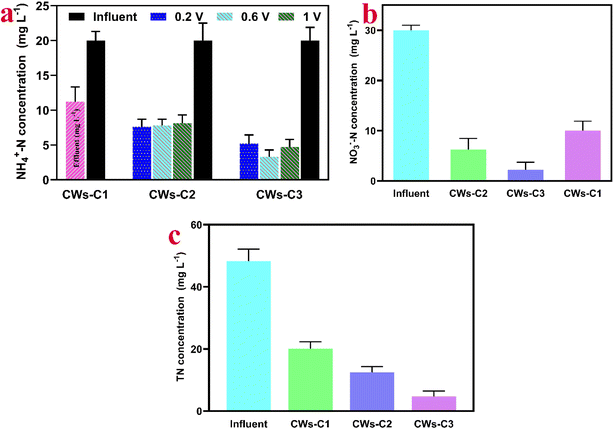
The NH4+–N removal rates of the CWs-C3 reactor were higher (83%) than those of the CWs-C1 (43.8%) and CWs-C2 (61%) reactors at the optimum application potential of 0.6 V vs. Ag/AgCl. NH4+–N can be removed via adsorption to the abundant negatively charged functional groups of biochar and microbial assimilation by nitrifying bacteria.29 Compared with other applied potentials, CWs-C3 enhanced the removal of NH4+–N at 0.6 V vs. Ag/AgCl because a higher applied potential could disturb the charge balance in biochar-doped electrodes and microbial attachments and positively charged NH4+–N.30 Thus, a medium applied potential (here, 0.6 V vs. Ag/AgCl) suggested that both physical and/or chemical adsorption and microbial assimilation caused by rapid growth and reproduction of nitrifying bacteria can effectively contribute to higher removal rates of NH4+–N. The predominant physical or/and chemical adsorption of NH4+–N in the CW-C1 reactor results in lower removal rates of NH4+–N (43.8%). As the growth of nitrifiers over the ACC electrode in CWs-C2 is lower than that over CWs-C3, the potential applied over the ACC electrode could decrease the negative charge and cation exchange capacity of biochar,31 as shown in Fig. 3a. It is believed that the insufficient supply of organic carbon in the shortest period of time of wastewater flow in the biochar ACC cathode inserted in the CWs-C2 reactor compared with that in the CWs-C3 reactor could limit the completion of nitrification, resulting in lower removal rates of NH4+–N in CWs-C2. The abundance of Proteobacteria, which included various groups of nitrifier microorganisms, was greater in CWs-C3 than in CWs-C2 (Fig. 6 and 7). The sufficient retention time and organic matter in the first layer of alum sludge used as the cathode ACC electrode of the CW-C3 reactor, which effectively accumulates and facilitates microbial growth and biofilm formation, can accelerate nitrification and significantly increase NH4+–N removal in the CW-C3 reactor.
The complete nitrification and further accumulation of appropriate NO3–N in the anode section of the CW-C3 reactor as an electron acceptor caused higher removal rates of NO3–N and total nitrogen (TN) in the CW-C3 reactor than in the CW-C1 and CW-C2 reactors (Fig. 3b and c).
3.2. Effects of the C/N ratio
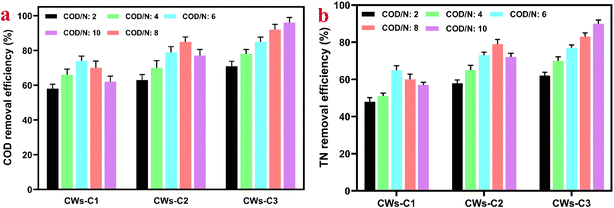
The average removal rates of COD in CW reactors are significantly influenced by different C/N ratios, as shown in Fig. 4a. For the CWs-C1 reactor, the average removal rates of COD slightly increased with increasing C/N ratios from 2 to 6. Notably, the CW-C1 reactor benefited enormously from the application of the alum sludge layer as a substrate to CWs because of the adsorption of organic matter and further accumulation of more microbial density, resulting in higher COD removal rates. The COD removal rates significantly change at C/N ratios greater than 6, suggesting that the high accumulation of organic matter could lead to a high F/M ratio and further decrease the activity of microorganisms.32Inserting the ACC electrode and applying potential into the CWs-C2 and CWs-C3 reactors could be considered reasons for both the higher average COD removal rates and the higher C/N ratios (Fig. 4a). The high porosity of biochar-doped ACC electrodes and the formation and growth of microorganisms massively and variously promoted the removal rates of COD at high C/N ratios. However, higher COD removal rates (96% vs. 85%) and higher C/N ratios (10 vs. 8) were observed in CWs-C3 than in CWs-C2, suggesting that the advantages of inserting an ACC cathode in the first layer of alum sludge in CWs-C3 reactors than in the sand layer of CWs-C2 could be related to the advantages of inserting an ACC cathode.
Notably, a balance should exist between the C/N ratio and the removal rate of NH4+–N and NO3–N. As reported earlier,33 at lower C/N ratios, a lack of enough organic matter sources as electron donors' results in incomplete growth and reproduction of nitrifying bacteria. However, the microbial community and diversity were the lowest, particularly the relative abundance of Proteobacteria, due to the fast growth and dominance of heterotrophic bacteria at relatively high C/N ratios. Fig. 4b clearly shows that the best removal efficiency of TN (NH4+–N and NO3−N) in the CW-C1 system (58%) was achieved at a C/N ratio of 6. However, higher C/N ratios were observed for the CWs-C2 (74%) and CWs-C3 (89%) reactors (C/N ratios of 8/1 and 10/1, respectively), suggesting that the inclusion of biochar-doped ACC electrodes with potential and increased abundance of nitrifying and denitrifying bacteria in the cathode and anode parts of CWs, respectively, could be possible reasons for the increased C/N ratios in the influent flow of wastewater in this study compared with those in other studies.34 Equally importantly, the Fe2+ released from alum sludge layers can act as an electron donor that can induce autotrophic denitrifying bacteria for nitrate reduction.
3.3. Microbial community in the operated reactors
Different configurations of alum sludge substrates and biochar-doped ACC electrodes with different application potentials were used in CW reactors, and their effects on the enhancement of organic matter and nutrient removal efficiency from synthetic wastewater were assessed. As mentioned earlier, the highest diversity and richness of the microbial community were observed in the CW-C2 and CW-C3 reactors at potentials of 0.6 and 1 V vs. Ag/AgCl, respectively, for the highest removal rates of COD; 0.6 V vs. Ag/AgCl for the NH4+–N and NO3–N removal efficiencies for both reactors; and C/N ratios of 8 and 10 for both reactors (Table 3). According to the microbial community composition shown in Fig. 5, the relative abundance of Proteobacteria in the cathode parts of CWs-C3 (20.23–24.36%) was greater than that in the CWs-C2 reactor (16.89–19.87%), suggesting that the use of alum sludge substrate as the cathode parts and the simultaneous application of potential could increase the variety of microorganisms with nitrification ability belonging to this group. Among the other groups, Bacteroidetes, which included nitrite bacteria, nitrate bacteria, and denitrifying bacteria, represented the highest proportion in the CWs-C3 reactor. Moreover, the presence of Chloroflexi at higher abundances in CWs-C3 than in CWs-C2 significantly increased the removal rates of COD and TN in the CWs-C3 reactor because of their ability to utilize organic matter for photosynthesis and NH4+–N and organic N for growth as nitrogen sources.35| Reactors | ACE | Chao 1 | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| CWs-C1 (cathode) | 1559.18 | 1571.27 | 1.76 | 0.051 | 0.99 |
| CWs-C1 (anode) | 1589.96 | 1593.49 | 1.82 | 0.026 | 0.99 |
| CWs-C2 (cathode) | 1885.01 | 1888.17 | 1.97 | 0.028 | 0.99 |
| CWs-C2 (anode) | 1662.70 | 1695.86 | 1.74 | 0.023 | 0.99 |
| CWs-C3 (cathode) | 2137.98 | 2144.18 | 2.11 | 0.028 | 0.99 |
| CWs-C3 (anode) | 1872.67 | 1880.25 | 1.82 | 0.044 | 0.99 |
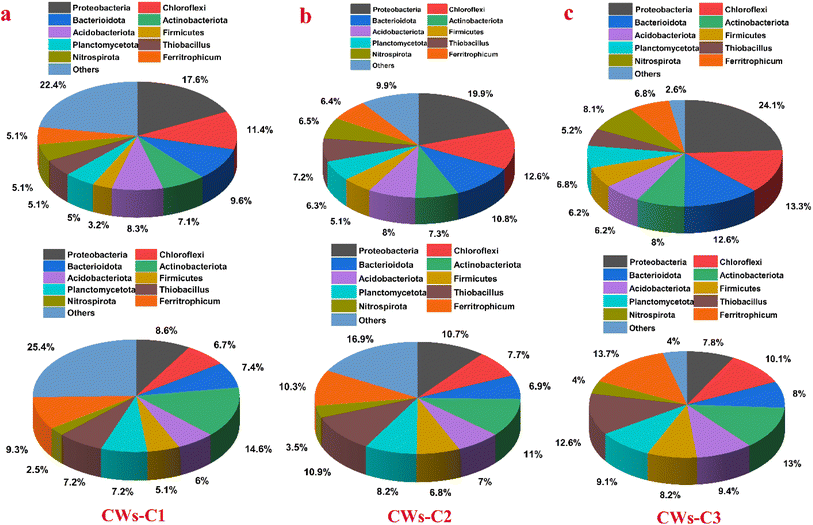 | ||
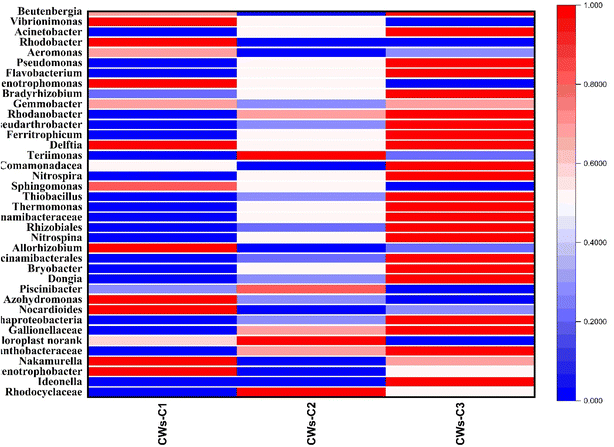
| Fig. 5 Microbial community analysis of reactors at the phylum levels (Note: in all reactors, the top plots represent the cathode parts and bottom plots represent the Anode parts of CWs reactors.). | ||
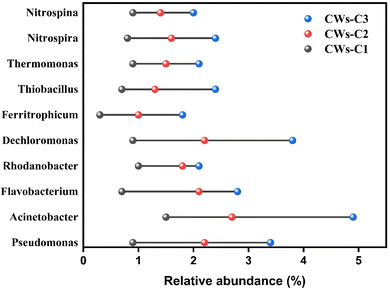
The dominant genera of the microbial community are also illustrated in Fig. 6, which shows that the genera belonging to N and Fe included alum sludge, and electrochemically active bacteria (EAB) were significantly different between the CW-C2 and CW-C3 systems. The dominant genera were Saccharimonadales, norank, Acinetobacter, Pseudomonas, Rhodobacter, and Desulfobacterota. The relative abundance of genera related to nitrification and denitrification was predominant in the CW-C3 reactor, indicating that the use of two alum sludge layers as substrates and the application of electrical potential strongly promoted the removal of N and organic matter by changing the structure of N transformation and the organic matter of degrading bacteria. The main nitrifier bacteria (Nitrospira and Nitrospina) were found to be more abundant in the cathode ACC electrode of CWs-C3 than in the cathode region of CWs-C2 (Fig. 7). The alum sludge substrate and its advantages mentioned earlier and the use of Fe2+-containing alum as an electron donor may increase the metabolism and activity of microorganisms.36 Owing to relatively appropriate nitrification in the cathode of CWs-C3, more denitrifying bacteria were present in the anode region of CWs-C3 than in the CW-C2 reactor. Therefore, the greater opportunity to reduce NO3–N in the anode region of the CWs-C3 reactor could effectively increase the TN removal rate compared with the TN removal rates in the CWs-C2 reactor.
In addition, the microbial community related to Fe(II) autotrophic denitrifying bacteria, such as Ferritrophicum and Thiobacillus, was more abundant and diverse in the anode region of the CW-C3 reactor, which significantly reduced NO3–N, and further TN removal was effectively enhanced in the CW-C3 reactor. The increased abundance of Pseudomonas in the anode region of the CWs-C3 reactor indicates appropriate P removal rates in which applying potential had a moderate effect on P-accumulating bacteria. The presence of EABs in the anode region highlights the important role of interspecies species in enhancing organic matter and nutrient removal in CWs-C2 and CWs-C3 reactors.
3.4. N and P removal mechanisms in CWs-C3
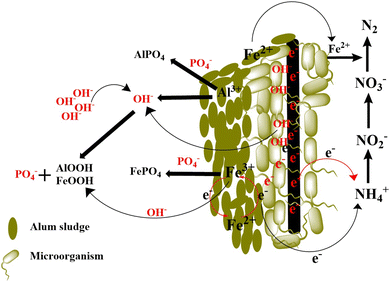
When a potential of 1 V vs. Ag/AgCl was applied to alum sludge containing a biochar-doped ACC electrode, the Fe and Al ions released from alum sludge formed FePO4 and AlPO4 in situ because of interactions with phosphate in wastewater, which promoted high percentages of P removal in the CW-C3 reactor. The greater intensity of bond adsorption at 1030 cm−1 in CWs-C3 corresponds to Fe–O–P vibrations (Fig. S2†). The intensity of the O–H stretching vibration at 3465 cm−1 and O–H bending vibration at 1620 cm−1 and the absorption band near 820 cm−1 correspond to Fe–OH or Al–OH bonds, indicating that the released Al and Fe ions could combine with OH– near the biochar-doped ACC electrode, generate AlOOH or FeOOH and consequently interact with phosphorous in wastewater.In addition, the high contribution of Fe autotrophic denitrifying bacteria in the CWs-C3 reactor, such as Ferritrophicum and Thiobacillus, suggested that Fe2+ as an electron donor and Fe3+ as an electron acceptor due to significant redox cycles can play important roles in the denitrification process. A proposed schematic of the simultaneous mechanism of TN and TP removal in CWs-C3 by focusing on the application of alum sludge as a substrate and inserting an ACC electrode as an inducer and stimulator of initial mechanisms and microbial growth is shown in Fig. 8.
In conclusion, the placing alum sludge substrate in CWs-C3 plays a crucial role in the initial breakdown, transformation of organic matters, effective nitrification and subsequently denitrification. So, the degradation of organic matter and the conversion of ammonium to nitrate and nitrogen gas can be enhanced, significantly contributing the reduction of COD and TN. The redox reactions and enhanced microbial activity induced by placing electrodes within the CWs-C3 can lead to the degradation of organic matters, transformation of nitrogen compounds, and the adsorption and precipitation of phosphorus compounds. Moreover, the alum sludge as substrate provides ideal adaption place for microorganisms and also a robust adsorptive capacity for phosphorus, leading to significant TP removal. The adsorption process captures residual pollutants that may not be fully degraded by biological or electrochemical mechanisms, thereby enhancing the overall removal efficiency of COD, TN, and TP. To sum up, the alum sludge-driven electro-phytoremediation approach integrates biological action, electrochemical effects, and adsorption to provide a comprehensive and efficient solution for nutrient removal. Each mechanism contributes uniquely to the removal of COD, TN, and TP, resulting in a sustainable and high-performance CWs systems. Further optimization and research are essential to maximize the potential of these integrated processes and address the complexities of wastewater treatment.
4. Conclusion
Simultaneously, significant mitigation of organic matter and nutrient-rich wastewater was carried out in CWs designed with alum sludge substrate, and potential was applied to biochar-doped ACC electrodes. The results demonstrated that alum sludge as a substrate and the application of potential could change the microbial diversity and density toward completing nitrification and denitrification and highly biodegraded organic matter. Owing to the iron- and aluminum-containing alum sludge, TP can be mitigated predominantly by the formation of Fe/AlPO4 or Al/FeOOH–P interactions. Moreover, the released Fe ions can act as electron donors and serve as Fe-driven autotrophic denitrifiers, consequently increasing the TN removal efficiency. To conclude, appropriately designing and utilizing waste material as a substrate in CWs and applying a driving stimulator to promote microbial growth and microbial diversity can be promising strategies for simultaneously promoting organic matter and nutrients and utilizing CWs on a large scale and in practical applications. The findings obtained in our study can be conducive to the application of CWs for wastewater treatment, with all the benefits mentioned above.Data availability
Data for this article are available at Origin 2024b [https://www.originlab.com/2024b]. Data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial and technical support provided by the Baqiyatallah University of Medical Sciences. This research was approved by the Ethics Committee of Baqiyatallah University of Medical Sciences (IR.BMSU.REC.1402.064) and project number # 401000559.References
- (a) H. Guven, M. Ersahin, H. Ozgun, I. Ozturk and I. Koyuncu, J. Environ. Manage., 2023, 329, 117130 CrossRef CAS PubMed; (b) M. Faisal, K. M. Muttaqi, D. Sutanto, A. Q. Al-Shetwi, P. J. Ker and M. Hannan, Renew. Sustain. Energy Rev., 2023, 181, 113324 CrossRef CAS; (c) J. Arámbula, S. Mohammadi, A. Mahdaviarab, D. Sanaei, R. P. Patil and H. Sharifan, New J. Chem., 2023, 47, 22235–22245 RSC.
- M. H. Dehghani, D. Sanaei, I. Ali and A. Bhatnagar, J. Mol. Liq., 2016, 215, 671–679 CrossRef CAS.
- (a) M. Sarmadi, M. Foroughi, H. Najafi Saleh, D. Sanaei, A. A. Zarei, M. Ghahrchi and E. Bazrafshan, Environ. Sci. Pollut. Res., 2020, 27, 34823–34839 CrossRef CAS PubMed; (b) W. Piao, Y. Kim, H. Kim, M. Kim and C. Kim, J. Clean. Prod., 2016, 113, 325–337 CrossRef CAS; (c) S. M. Rahman, M. J. Eckelman, A. Onnis-Hayden and A. Z. Gu, Environ. Sci. Technol., 2016, 50, 3020–3030 CrossRef CAS PubMed; (d) R. Boiocchi and G. Bertanza, Water Sci. Technol., 2022, 85, 1673–1687 CrossRef CAS PubMed.
- (a) G. Rodriguez-Garcia, N. Frison, J. Vázquez-Padín, A. Hospido, J. Garrido, F. Fatone, D. Bolzonella, M. Moreira and G. Feijoo, Sci. Total Environ., 2014, 490, 871–879 CrossRef CAS PubMed; (b) T. Sampedro, L. Gómez-Coma, I. Ortiz and R. Ibañez, Sci. Total Environ., 2024, 906, 167154 CrossRef CAS PubMed.
- (a) M. Yang, M. Peng, D. Wu, H. Feng, Y. Wang, Y. Lv, F. Sun, S. Sharma, Y. Che and K. Yang, Resour. Conserv. Recycl., 2023, 190, 106794 CrossRef CAS; (b) Z. Liu, Z. Xu, X. Zhu, L. Yin, Z. Yin, X. Li and W. Zheng, Sci. Total Environ., 2023, 169356 Search PubMed; (c) V. Alevizos, I. Georgousis and A. Kapodistria, Pollutants, 2023, 3, 521–543 CrossRef.
- (a) F. García-Ávila, A. Avilés-Añazco, R. Cabello-Torres, A. Guanuchi-Quito, M. Cadme-Galabay, H. Gutiérrez-Ortega, R. Alvarez-Ochoa and C. Zhindón-Arévalo, Case Studies in Chemical and Environmental Engineering 2023, vol. 7, p. 100307 Search PubMed; (b) Z. Tao, Z. Jing, M. Tao, Y. Kong, L. Guan and Q. Jia, Bioresour. Technol., 2023, 380, 129075 CrossRef CAS PubMed.
- M. M. Waly, T. Ahmed, Z. Abunada, S. B. Mickovski and C. Thomson, Land, 2022, 11, 1388 CrossRef.
- F. Younas, N. K. Niazi, I. Bibi, M. Afzal, K. Hussain, M. Shahid, Z. Aslam, S. Bashir, M. M. Hussain and J. Bundschuh, J. Hazard. Mater., 2022, 422, 126926 CrossRef CAS PubMed.
- (a) J. K. Tang, M. N. H. Jusoh and H. Jusoh, Trop. Aquat. Soil Pollut., 2023, 3, 76–87 CrossRef; (b) H. Wu, R. Wang, P. Yan, S. Wu, Z. Chen, Y. Zhao, C. Cheng, Z. Hu, L. Zhuang and Z. Guo, Nat. Rev. Earth Environ., 2023, 4, 218–234 CrossRef CAS.
- (a) V. Carrillo, G. Gómez and G. Vidal, Ecol. Eng., 2022, 182, 106690 CrossRef; (b) L. Gong, X. Zhao and G. Zhu, Sustainability, 2022, 14, 8819 CrossRef CAS; (c) K. Kill, L. Grinberga, J. Koskiaho, Ü. Mander, O. Wahlroos, D. Lauva, J. Pärn and K. Kasak, Ecol. Eng., 2022, 180, 106664 CrossRef; (d) Y. Liu, L. Feng, Y. Liu and L. Zhang, Environ. Sci. Pollut. Res., 2023, 30, 23035–23046 CrossRef CAS PubMed; (e) J. Li, B. Zheng, X. Chen, Z. Li, Q. Xia, H. Wang, Y. Yang, Y. Zhou and H. Yang, Water, 2021, 13, 476 CrossRef CAS.
- V. Patyal, D. Jaspal and K. Khare, Bioresour. Technol. Rep., 2024, 26, 101870 CrossRef CAS.
- (a) A. Biswas and S. Chakraborty, Sci. Total Environ., 2024, 912, 168809 CrossRef CAS PubMed; (b) D. Kumari and K. Dutta, J. Environ. Chem. Eng., 2024, 12, 113119 CrossRef CAS; (c) Y. Kong, M. Tao, X. Lu, C. Cheng and Z. Jing, Desalination Water Treat., 2024, 318, 100358 CrossRef.
- M. Tao, Y. Kong, S. Cao, Z. Jing, L. Guan, Q. Jia and Y.-Y. Li, Chem. Eng. J., 2024, 487, 150753 CrossRef CAS.
- D. Sanaei, M. Sarmadi, M. H. Dehghani, H. Sharifan, P. G. Ribeiro, L. R. Guilherme and S. Rahimi, Environ. Sci.: Processes Impacts, 2023, 25, 2110–2124 RSC.
- C. H. Ward, K. Balshaw-Biddle and C. L. Oubre, Modular Remediation Testing Systems, CRC Press, 1999 Search PubMed.
- Epa US, Method 3050B: acid digestion of sediments, sludges, and soils, Environ. Prot. Agency, 1996, vol. 2, pp. 3–5 Search PubMed.
- Y. Yang, Y. Zhao, A. Babatunde, L. Wang, Y. Ren and Y. Han, Sep. Purif. Technol., 2006, 51, 193–200 CrossRef CAS.
- (a) T. Hülsen, E. M. Sander, P. D. Jensen and D. J. Batstone, Water Res., 2020, 181, 115909 CrossRef PubMed; (b) A. P. H. Association, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, 1926 Search PubMed; (c) D. Sanaei, M. Massoudinejad, M. S. Javed, S. M. Zarandi, A. Rezaee, H. Sharifan and M. Imran, J. Mater. Chem. C, 2022, 10, 1421–1435 RSC; (d) A. H. Mahvi, M. Sarmadi, D. Sanaei and H. Abdolmaleki, Desalin. Water Treat., 2020, 200, 205–216 CrossRef CAS.
- (a) N. Vyas, R. Sammons, O. Addison, H. Dehghani and A. Walmsley, Sci. Rep., 2016, 6, 32694 CrossRef CAS PubMed; (b) A. Saavedra, D. C. Martínez-Casillas, J. R. Collet-Lacoste and E. Cortón, J. Appl. Microbiol., 2023, 134, lxad140 CrossRef PubMed.
- J. A. Modestra, C. N. Reddy, K. V. Krishna, B. Min and S. V. Mohan, Renew. Energy, 2020, 149, 424–434 CrossRef CAS.
- (a) A. Mirshafiee and A. Rezaee, J. Water Proc. Eng., 2021, 44, 102420 CrossRef; (b) S. Erazo and L. M. Agudelo-Escobar, Processes, 2023, 11, 373 CrossRef CAS.
- C. I. Torres, R. Krajmalnik-Brown, P. Parameswaran, A. K. Marcus, G. Wanger, Y. A. Gorby and B. E. Rittmann, Environ. Sci. Technol., 2009, 43, 9519–9524 CrossRef CAS PubMed.
- (a) A. Omoike, Water Res., 1999, 33, 3617–3627 CrossRef CAS; (b) W. Zhao, H. Xie, J. Li, L. Zhang and Y. Zhao, Processes, 2021, 9, 612 CrossRef CAS; (c) M. B. G. de Almeida, A. M. D. de Jesus, A. S. Pereira and F. A. Fiore, J. Clean. Prod., 2024, 468, 142975 CrossRef.
- H. A. Nabwey and M. A. Tony, Processes, 2023, 11, 2836 CrossRef CAS.
- S. Kumar, A. T. Nguyen, S. Goswami, J. Ferracane and D. Koley, Sensor. Actuator. B Chem., 2023, 376, 133034 CrossRef CAS PubMed.
- (a) D. Georgantas and H. Grigoropoulou, Water Sci. Technol., 2005, 52, 525–532 CrossRef CAS PubMed; (b) N. Maqbool, Z. Khan and A. Asghar, Desalination Water Treat., 2016, 57, 13246–13254 CrossRef CAS; (c) A. Babatunde, Y. Zhao and X. Zhao, Bioresour. Technol., 2010, 101, 6576–6579 CrossRef CAS PubMed; (d) M. Pająk, Int. J. Environ. Sci. Technol., 2023, 20, 10953–10972 CrossRef; (e) A. Chen, L. Lv, R. Hu, X. Wei, J. Guan and X. Meng, Sci. Total Environ., 2023, 867, 161530 CrossRef CAS PubMed; (f) Q. Ping, Z. Zhang, W. Guo, L. Wang and Y. Li, Sci. Total Environ., 2024, 913, 169641 CrossRef CAS PubMed.
- (a) Y. Lei, Z. Zhan, M. Saakes, R. D. van der Weijden and C. J. Buisman, Water Res., 2021, 199, 117199 CrossRef CAS PubMed; (b) X. Ge, X. Cao, X. Song, Y. Wang, Z. Si, Y. Zhao, W. Wang and A. A. Tesfahunegn, Bioresour. Technol., 2020, 296, 122350 CrossRef CAS PubMed; (c) A. Mielcarek, K. Ł. Bryszewski, J. Rodziewicz, K. Kłobukowska and W. Janczukowicz, Energies, 2024, 17, 1352 CrossRef CAS.
- A. Shalaby, E. Nassef, A. Mubark and M. Hussein, Am. J. Environ. Sci., 2014, 1, 90–98 Search PubMed.
- (a) T. Abedi and A. Mojiri, Environ. Technol. Innovat., 2019, 16, 100472 CrossRef; (b) L. Feng, Z. Gao, T. Hu, S. He, Y. Liu, J. Jiang, Q. Zhao and L. Wei, Chem. Eng. J., 2023, 144772 CrossRef CAS; (c) L. Cheng, X. Gong, B. Wang, Z. Liu, H. Liang and D. Gao, ACS ES&T Water, 2024, 4, 1834–1843 Search PubMed; (d) H. Wang, Q. Chen, R. Liu, H. Xia and Y. Zhang, J. Water Proc. Eng., 2023, 55, 104170 CrossRef.
- S. Farhangi-Abriz and K. Ghassemi-Golezani, Chemosphere, 2023, 313, 137365 CrossRef CAS PubMed.
- D. V. Cuong, P.-C. Wu, N.-L. Liu and C.-H. Hou, Sep. Purif. Technol., 2020, 242, 116813 CrossRef CAS.
- M. Zhou, J. Cao, Y. Lu, L. Zhu, C. Li, Y. Wang, L. Hao, J. Luo and H. Ren, Sci. Total Environ., 2022, 847, 157569 CrossRef CAS PubMed.
- (a) W. Lu, Y. Zhang, Q. Wang, Y. Wei, Y. Bu and B. Ma, Bioresour. Technol., 2021, 340, 125661 CrossRef CAS PubMed; (b) K. Hu, W. Li, Y. Wang, B. Wang, H. Mu, S. Ren, K. Zeng, H. Zhu, J. Liang and J. Xiao, J. Water Proc. Eng., 2023, 53, 103673 CrossRef; (c) D. Li, W. Li, D. Zhang, K. Zhang, L. Lv and G. Zhang, Bioresour. Technol., 2023, 367, 128254 CrossRef CAS PubMed.
- (a) D. Yao, N. Dai, X. Hu, C. Cheng, H. Xie, Z. Hu, S. Liang and J. Zhang, Water Res., 2023, 243, 120277 CrossRef CAS PubMed; (b) X. Bai, J. Li and S. Chang, Water, 2023, 15, 4272 CrossRef CAS; (c) C. Xiang, Y. Du, W. Han, B. Guan, H. Liu, Y. An, Y. Liu, H. Jiang, J. Chang and Y. Ge, Environ. Sci. Pollut. Res., 2024, 31, 12036–12051 CrossRef CAS PubMed.
- P. Zhang, Y. Peng, J. Lu, J. Li, H. Chen and L. Xiao, Chemosphere, 2018, 211, 25–33 CrossRef CAS PubMed.
- (a) H. Chen, X. Zhao, Y. Cheng, M. Jiang, X. Li and G. Xue, Environ. Sci. Technol., 2018, 52, 1404–1412 CrossRef CAS PubMed; (b) B. Zhu, R. Yuan, S. Wang, H. Chen, B. Zhou, Z. Cui and C. Zhang, J. Water Proc. Eng., 2024, 59, 104952 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08021a |
| This journal is © The Royal Society of Chemistry 2025 |