Open Access Article
Open Access ArticleUnveiling the physicochemical, photocatalytic, antibacterial and antioxidant properties of MWCNT-modified Ag2O/CuO/ZnO nanocomposites
Amjad Latif Lonea,
Sadiq Ur Rehman*a,
Sirajul Haq *a,
Nadia Shahzad
*a,
Nadia Shahzad b,
Mohammad Khalid Al-Sadoonc,
Muhammad Imran Shahzad
b,
Mohammad Khalid Al-Sadoonc,
Muhammad Imran Shahzad d,
Jamoliddin Razzokovefg,
Shafia Shujaata and
Abdus Samadh
d,
Jamoliddin Razzokovefg,
Shafia Shujaata and
Abdus Samadh
aDepartment of Chemistry, University of Azad Jammu and Kashmir, Muzaffarabad 13100, Pakistan. E-mail: srkhattak@hotmail.com; cii_raj@yahoo.com
bUS–Pakistan Centre for Advanced Studies in Energy, National University of Science and Technology (NUST), 44000 Islamabad, Pakistan
cDepartment of Zoology, College of Science, King Saud University, P. O. Box 2455, Riyadh, 11451, Saudi Arabia
dNanosciences and Technology Department (NS & TD), National Center for Physics (NCP), 44000 Islamabad, Pakistan
eInstitute of Fundamental and Applied Research, National Research University TIIAME, Kori Niyoziy 39, 100000 Tashkent, Uzbekistan
fAlfraganus University, Yukori Karakamish street 2a, 100190 Tashkent, Uzbekistan
gDepartment of Biotechnology, Tashkent State Technical University, Universitet 2, Tashkent 100095, Uzbekistan
hSchool of Material Science and Engineering, Nanjing Tech University, P. R China
First published on 16th January 2025
Abstract
Water pollution, oxidative stress and the emergence of multidrug-resistant bacterial strains are significant global threats that require urgent attention to protect human health. Nanocomposites that combine multiple metal oxides with carbon-based materials have garnered significant attention due to their synergistic physicochemical properties and versatile applications in both environmental and biomedical fields. In this context, the present study was aimed at synthesizing a ternary metal-oxide nanocomposite consisting of silver oxide, copper oxide, and zinc oxide (ACZ-NC), along with a multi-walled carbon nanotubes modified ternary metal-oxide nanocomposite (MWCNTs@ACZ-NC). The properties of the synthesized nanomaterials were characterized using Fourier transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), and energy dispersive X-ray (EDX) spectroscopy. The results obtained suggested the successful synthesis of both samples, as evidenced by the morphological changes observed in the SEM images, a transmittance band at 748.02 cm−1 in the FT-IR spectrum, and a diffraction peak at 44.39° in the XRD pattern. The band gap energies were determined via diffuse reflectance spectroscopy (DRS), with a redshift observed in the absorbance edge upon the incorporation of MWCNTs. The synthesized samples were tested as photocatalysts for the degradation of rhodamine 6G (Rh-6G), with the highest degradation efficiency (99.61%) achieved by MWCNTs@ACZ-NC. Additionally, the materials were evaluated for their biological activity as antibacterial and antioxidant agents. The MWCNTs@ACZ-NC exhibited the highest antioxidant potential with an IC50 value of 59.22 μg mL−1. However, the incorporation of MWCNTs resulted in a decrease in antibacterial activity, which may be attributed to the blocking of binding sites.
1. Introduction
Over the past few years, concerns about the impacts of environmental pollution on public health have intensified, particularly regarding water pollution caused by industrialization and unregulated human activities. A large number of people worldwide lack access to safe drinking water due to pollutants, such as heavy metals, dyes, and chemicals contaminating water bodies.1,2 Organic dyes, such as Rh-6G, which are used in various applications, significantly contribute to water pollution and pose risks to human health, including allergic reactions. While numerous methods, such as biological, chemical, and physical processes, exist to remove organic dyes from wastewater, these approaches can be costly and inefficient, often generating substantial sludge. The photocatalytic process, first introduced in 1911, offers a more effective, cost-efficient, and environmentally friendly alternative for treating water pollution.Antioxidants are compounds that inhibit oxidation reactions by scavenging free radicals and converting them into stable, non-reactive molecules.3 The imbalance between the excessive formation of oxidants and the ability of antioxidant defense mechanisms to scavenge them results in a pathophysiological condition known as oxidative stress. In this condition, the levels of reactive nitrogen species (such as peroxynitrite) as well as reactive oxygen species (including hydroxyl radicals, atomic oxygen, and superoxide anions) increase.4 When RNS and ROS are produced in excess, they can damage DNA, proteins, and cellular and subcellular membranes by interacting with them, impairing their normal functions. This can lead to various diseases, such as aging,5 neurodegenerative disorders,6 cardiovascular diseases, diabetes, asthma, pulmonary conditions7 and cancer.8 To counteract the effects of oxidative stress, both synthetic and natural antioxidants are used. Besides natural antioxidants, there is a precise need to develop effective synthetic antioxidants that not only overcome the adverse activity of oxidants but also help in retaining the normal functions of the damaged cells. Among nanomaterials, metal-oxide nanoparticles (MO-NPs) are very reactive particles because of the unpaired valence shell electrons present on their surfaces.9 Therefore, clinical applications of these NPs have more advantages over the conventional therapy treatments, which have numerous side effects and problems of reduced efficiency at target sites. MO-NPs have been investigated for many biological applications such as antimicrobial, antioxidant, drug delivery, bioimaging and biosensing.10
Copper oxide (CuO) is one of the important transition metal oxides having a small energy gap (nearly 2.0 eV). It exhibits good electrochemical, mechanical, and biological applications at the nanoscale.11 Many researchers worked on the synthesis of CuO NPs and studied their antioxidant, antibacterial and photocatalytic activities.12–14 Silver oxide (Ag2O) NPs have gained interest due to their versatility in the field of medical science, like in drug delivery, as oxidation inhibitors, antimicrobial agents, carcinostatic substances, and inflammation reducers.15 Studies of Ag2O-NPs have shown that they exhibit great potential against the oxidants and other inflammatory substances; therefore, they have been investigated as antioxidant and antibacterial agents by many researchers.16–18 Zinc oxide (ZnO) nanocrystals have gained particular interest due to their 3.37 eV band gap and their ability to act as n-type semiconductors.19 Zn is an essential element in the body; although it is required in minute quantities, it plays an important role in enzymatic activities during the synthesis of nucleic acid and proteins. ZnO is a non-toxic and environmentally friendly material that actively participates in combating cancerous and inflammatory cells.20 Photocatalytic, antibacterial and antioxidant potentials of ZnO-NPs have been explored by many researchers in the recent past.21–23 Carbon nanotubes (CNTs) are a major class of nanostructured organic compounds. They are one-dimensional materials having a high aspect ratio.24 CNTs fall into two categories: single-walled CNTs as well as multi-walled CNTs. CNTs have drawn attraction in last decade in the fields of biology and pharmaceutics; hence, the modified CNTs have now diverse applications in nucleic acid sequencers, biocatalysts and biological processes.25 This research explores the potential of functionalized CNTs to either function as standalone materials with desirable properties or to enhance the properties of materials when integrated into composite structures.24,26–28 Chemical interactions between CNTs and free radicals (particularly hydroxyl radicals) have been extensively studied; however, the exact underlying mechanism remains unexplored. There are two proposed mechanisms for the reaction between CNTs and free radicals. One mechanism is the formation of a radical adduct, while another process involves electron transfer during the reaction.29 Di and trimetallic nanocomposites of ZnO, CuO and Ag2O were studied for different potent applications, which showed better ability than the conventional materials.30–34
Different groups of researchers have worked on the nanocomposites of MWCNTs with metals (mono or di or trimetallic) for the biological applications.35–37 Nazal et al. (2018) prepared a composite of copper, molybdenum, and nickel trioxides supported by MWCNTs for electrolytic applications in methanol oxidation reactions.38 Noushin et al. (2021) synthesized Ag–CuO–ZnO@MWCNT nanocomposites by a green synthesis method. These nanocomposites were employed as heterogeneous catalysts for the preparation of substituted dihydropyrroloazepines.39 Roodbari et al. (2023) reported the hydrothermal synthesis of a trioxide-based composite comprising cobalt, copper, and manganese, with rGO-MWCNTs serving as supporting materials. The composite was developed for methanol fuel cell applications.40 Bekmezci et al. (2024) developed Pt-based bi- and trimetallic nanoparticles modified with MWCNTs using an arc discharge process. These materials were applied in the sensing of uric acid.41
After a critical review of literature, it has been found that the data on MWCNT-modified trimetal oxide nanocomposites are very limited, and most of the nanocomposites are used as sensors and catalysts for organic modifications. None of the previously reported nanocomposites were used as photocatalysts, antibacterial agents and antioxidant agents. The novelty of this research lies in reporting MWCNT-Modified Ag2O/CuO/ZnO Nanocomposites for the first time using a sol–gel method, and the multifunctional nature of the nanocomposite as a photocatalyst, antibacterial agent and antioxidant agent is yet to be investigated.
Focusing on the high efficacy of nano hybrid materials and the wide gap in the literature, this research work was designed to synthesize ACZ-NC and MWCNTs@ACZ-NC by the sol–gel method. To study the physiochemical characteristics, EDX (mapping), SEM, FTIR spectroscopy, XRD, and DRS approaches were employed. Under solar light, in order to degrade Rh-6G, the fabricated samples were employed as photocatalysts and the effects of initial concentration, pH, catalyst concentration, and multiple uses of the catalyst were studied. Moreover, the antioxidant potential of both samples was evaluated against ABTS free radicals and the percentage activity was compared to that of ascorbic acid.
2. Materials and methods
2.1 Reagents
In this study, all the chemicals used were of analytical grade. Copper(II) nitrate pentahydrate (Cu(NO3)2·5H2O), zinc(II) nitrate hexahydrate (Zn(NO3)2·5H2O), silver(I) nitrate (AgNO3), rhodamine 6G (C28H31N2O3Cl), sodium hydroxide (NaOH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (C18H18N4O6S4) commonly known as ABTS, and methanol (CH3OH) were bought from Sigma-Aldrich and used without further modification. The MWCNTs were bought from Sigma-Aldrich and functionalized with carboxylic groups. The working solution was prepared using deionized water (DIW). Glassware was washed thoroughly with DIW and then rinsed with acetone. Afterward, the dehydration of glassware, used for the synthesis of NCs, was made in an oven at 100 °C.2.2 Synthesis of ACZ-NCs
In 10 mL of DIW, 4.55 g of Zn(NO3)2·5H2O, 3.15 g of AgNO3, and 3.66 g of Zn(NO3)2·5H2O were individually mixed under constant stirring for 30 min. Aqueous solutions of Zn, Cu, and Ag were blended with energetic stirring in a beaker and thermally treated at 60 °C for 30 min. Then, a 1 M NaOH solution was introduced to adjust the pH to 10 for 3 h, and the prepared material was constantly heated as well as stirred at 60 °C. Following a 24 h cooling period at room temperature, the mixture was clarified and given three DIW washes. After drying at 100 °C for 8 h in an electric oven, the final solid was calcined in a muffle furnace at 350 °C for 4 h.2.3 Synthesis of MWCNTs@ACZ-NC
For the synthesis of MWCNTs@ACZ-NC, 4.55 g of Zn(NO3)2·5H2O, 3.15 g of AgNO3, and 3.66 g of Zn(NO3)2·5H2O were each dissolved separately with constant stirring for 30 min in 20 mL of DIW. The mixture that resulted by vigorously mixing these solutions in a beaker and heating at 60 °C for 30 min was marked as S1. After ultrasonic distribution in 40 mL, 0.154 g of –COOH-functionalized MWCNTs was introduced to S1. Then, 0.5 M NaOH solution was added dropwise to lower the pH down to 10. After heating to 80 °C and stirring for 5 h, the reaction mixture was allowed to age at ambient temperature for 24 h. After filtering and four rounds of rinsing with hot distilled water, the mixture was dried at 100 °C in an oven. After that, the obtained solid product was tightly sealed in a sample vial after calcination for 4 h in a muffle furnace at 350 °C.2.4 Instrumentation
To characterize thoroughly, ACZ-NC and MWCNTs@ACZ-NC were subjected to a series of physicochemical investigations. The Debye–Scherrer equation was used to quantify the crystallite size, while XRD using a Philips X'Pert diffractometer was performed to characterize the crystalline structure. A JEOL JSM-5600LV from Tokyo (Japan) was deployed to investigate the surface morphology as well as microstructure. EDX spectroscopy was performed using an INCA-200 system from the UK to analyze the elemental composition. By using a Nicolet 560 FT-IR spectrometer, the surface functional groups between 4000 and 400 cm−1 were assessed. The DRS spectra were recorded using a DRS model lambda 950, and Tauc plot was used to determine the band gap energy.2.5 Photocatalytic assay
The photocatalytic activity of all the synthesized samples, consisting of ACZ-NC, MWCNTs@ACZ-NC, and the sample without a catalyst, was assessed in the decomposition of Rh-6G dye. First, distilled water was used to prepare Rh-6G's standard solution, at a concentration of 15 ppm. Then, 20 mg of formulated samples were added to a reaction vessel containing 100 mL of this solution. For 30 min, the mixture was blended in darkness in order to achieve sorption equilibrium. A double-beam UV-vis spectrophotometer was used to systematically assess the sample after it had been exposed to simulated solar radiation for a predefined amount of time. The variations in the absorbance maxima were monitored over time.2.6 Antibacterial assay
The antibacterial efficacy of ACZ-CS and MWCNT@ACZ-CS was evaluated against S. aureus and E. coli using the agar well diffusion protocol. Bacterial cultures were inoculated onto nutrient agar plates, and wells were bored to accommodate the test substances. The synthesized samples, i.e. ACZ-NC and MWCNTs@ACZ-NC, were dispersed in distilled water (1 mg in 10 mL) and poured into the wells, and the plates were incubated at 37 °C for 24 h. The diameter of the clear zone of inhibition surrounding each well was measured to determine the extent of bacterial growth inhibition. Larger zones of inhibition indicate greater antibacterial activity. Control wells containing sterile distilled water were included for comparison. The results were analyzed to compare the antibacterial efficacy of ACZ-NC and CNTs@ACZ-NC against the control groups.2.7 Antioxidant assay
To assess the antioxidant activity, the ABTS radical cation was initially produced through mixing 5 mM potassium per sulfate with 14 mM ABTS in a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (w/w) and 1
8 (w/w) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) proportion in the absence of light for 16 h. The solution's absorbance was then assessed at a wavelength of 734 nm. By ultrasonically dispersing 5, 25, 50, 75, 100, 200 and 400 μg of the synthesized nanostructures in 1 mL solution for 30 min at normal temperature, stock suspensions of nanostructures were created. Then, a mixture containing 0.15 mL of ABTS˙+ solution as well as 0.2 mL of produced nanostructure suspension was subjected to UV analysis after aging for 30 min. At 734 nm, the absorbance of this mixture was measured. For computing percent ABTS free radical inhibition activity, eqn (1) was used where Ai stands for sample's absorbance, while Ao refers to control's absorbance. The average of duplicate results is reported herein, and for the statistical analysis, Microsoft excel 2013 version was used where a t-test was applied:
1 (v/v) proportion in the absence of light for 16 h. The solution's absorbance was then assessed at a wavelength of 734 nm. By ultrasonically dispersing 5, 25, 50, 75, 100, 200 and 400 μg of the synthesized nanostructures in 1 mL solution for 30 min at normal temperature, stock suspensions of nanostructures were created. Then, a mixture containing 0.15 mL of ABTS˙+ solution as well as 0.2 mL of produced nanostructure suspension was subjected to UV analysis after aging for 30 min. At 734 nm, the absorbance of this mixture was measured. For computing percent ABTS free radical inhibition activity, eqn (1) was used where Ai stands for sample's absorbance, while Ao refers to control's absorbance. The average of duplicate results is reported herein, and for the statistical analysis, Microsoft excel 2013 version was used where a t-test was applied:
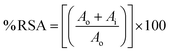 | (1) |
3. Results and discussion
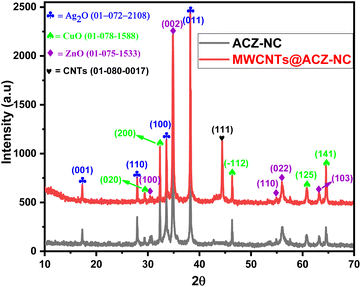
3.1 XRD analysis
Fig. 1 displays the XRD patterns of ACZ-NC as well as MWCNT-ACZ, which exhibit Bragg's reflections corresponding to Ag2O, CuO, ZnO, and CNTs. The diffraction peak at 44.39 resulted from the diffraction of X-rays from the (111) hkl plane of the carbon, confirming the existence of CNTs in the sample, corresponding to JCPDS card no. 01-080-0017. The diffraction peaks for Ag2O were observed at 17.31, 27.93, 33.59 and 38.24, resulting from the efficient overlap of X-rays diffracted from the (001), (110), (100) and (011) hkl planes, respectively. This confirmed the hexagonal geometry of Ag2O, which had a lattice number of 164 and a symmetry group of P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The lengths of two coordinates, i.e., a and b were equal, each measuring 3.0720 Å, while c measured 4.9410 Å. Interfacial angles (α as well as β) were 90° and γ was 120°. These data matched well with that reported on JCPDS card no. 01-072-2108. It was found that Ag2O had a particle size of 14.25 nm with crystal deformation of 0.074 percent. Diffraction peaks along the hkl values, i.e. 29.43(020), 32.35(200), 46.25(–112), 60.83(125), and 64.54(141), corresponded to JCPDS card no. 01-078-1588. These peaks confirmed the orthorhombic configuration of the CuO crystallite with space number of 38 and a space group of Bmm2. Three-dimensional sizes (a = 5.3510, b = 6.0200 and c = 9.3400) were different in length with all the interfacial angles of 90°. With a lattice strain of 0.089 percent, the crystallite size of CuO was determined to be 14.02 nm. Diffraction peaks at 2-theta positions along with the respective hkl planes were 30.38(100), 34.87(002), 54.93(110), 55.59(022), and 63.20(103), corresponding to a hexangular ZnO crystallite with a symmetry group of P63mc and a space number of 186 (JCPDS card: 01-075-1538). The dimensions of two coordinates (a and b) were 3.3520, while that of the third (c) was equal to 5.2260 Å. Additionally, α and β were 90.00° and γ was 120°. The crystallite size of ZnO was found to be 60.3 nm, having a crystal deformation of 0.192 percent.
m. The lengths of two coordinates, i.e., a and b were equal, each measuring 3.0720 Å, while c measured 4.9410 Å. Interfacial angles (α as well as β) were 90° and γ was 120°. These data matched well with that reported on JCPDS card no. 01-072-2108. It was found that Ag2O had a particle size of 14.25 nm with crystal deformation of 0.074 percent. Diffraction peaks along the hkl values, i.e. 29.43(020), 32.35(200), 46.25(–112), 60.83(125), and 64.54(141), corresponded to JCPDS card no. 01-078-1588. These peaks confirmed the orthorhombic configuration of the CuO crystallite with space number of 38 and a space group of Bmm2. Three-dimensional sizes (a = 5.3510, b = 6.0200 and c = 9.3400) were different in length with all the interfacial angles of 90°. With a lattice strain of 0.089 percent, the crystallite size of CuO was determined to be 14.02 nm. Diffraction peaks at 2-theta positions along with the respective hkl planes were 30.38(100), 34.87(002), 54.93(110), 55.59(022), and 63.20(103), corresponding to a hexangular ZnO crystallite with a symmetry group of P63mc and a space number of 186 (JCPDS card: 01-075-1538). The dimensions of two coordinates (a and b) were 3.3520, while that of the third (c) was equal to 5.2260 Å. Additionally, α and β were 90.00° and γ was 120°. The crystallite size of ZnO was found to be 60.3 nm, having a crystal deformation of 0.192 percent.
3.2 SEM analysis
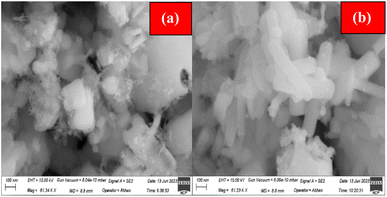
The structural analysis of ACZ-NC and MWCNTs@ACZ-NC was performed, and Fig. 2(a) and (b) demonstrates the obtained FESEM micrographs. The SEM image (Fig. 2(a)) depicts the morphological variations of ACZ-NC, where both the shape and the size of particles were non-uniform. Both individual particles and larger aggregates were clearly seen in the image; the individual particles exhibited spherical, nearly spherical, slightly elongated fiber-like structures, and polyhedral morphologies. All these particles were non-uniform that led to the formation of several defects in the sample. Cavity formation might also be due to the evaporation of solvents and the removal of volatile by-products during the drying process. These cavities were probably a result of gas release or phase separation during the gelation and drying stages. Some individual granules were also observed on the surface of larger aggregates. Individual particles in the sample had wide size distribution ranging between 15 nm and 110 nm, whereas the size of the larger aggregates was over 160 nm. Fig. 2(b) illustrates the SEM image of MWCNTs@ACZ-NC, where changes were observed in the morphology of ACZ-NC due to the incorporation of MWCNTs in their structure. MWCNTs, long cylindrical carbon structures with unique properties, significantly influence the morphology of the resulting composite. The presence of MWCNTs introduced a distinct elongated morphology as tube-like structures were apparent. Upon close observation, the ACZ-NC particles were visible on the surface of elongated tube-like structures; however, their distribution was not uniform. The number of cavities got reduced as MWCNTs filled in voids present in the original nanocomposite. The elongated tube-like structures were regularly distributed and their surfaces appeared smooth and non-porous, possibly due to the synthetic sol–gel method, which produces materials with smooth and non-porous surfaces through uniform deposition and controlled particle growth.3.3 EDX analysis
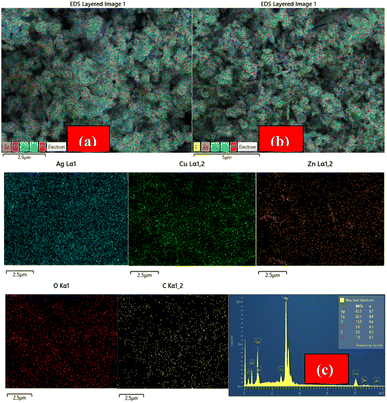
Fig. 3((a) as well as (b)) displays the EDX mapping results of ACZ-NC and MWCNT-ACZ, which confirmed the presence of carbon from the CNTs and the metallic components, including silver (Ag), copper (Cu), zinc (Zn), and oxygen (O), corresponding to the Ag2O, CuO, and ZnO phases. No significant impurities were detected, indicating the high purity of the synthesized nanocomposite. The elemental mapping showed that silver (Ag), copper (Cu), zinc (Zn), and oxygen (O) were uniformly distributed across the CNT matrix, indicating a well-integrated nanocomposite structure. This homogeneous distribution suggested good interaction between CNTs and MO-NPs. No significant areas of agglomeration were observed, highlighting the efficiency of the synthesis method in achieving a well-dispersed nanocomposite. The uniform distribution of elements observed in the EDX mapping aligned well with the XRD results, confirming the formation of distinct Ag2O, CuO, and ZnO phases. The lack of significant agglomeration was further supported by the SEM images, which showed uniformly dispersed nanoparticles.The EDX spectra of MWCNT@ACZ-NC displayed in Fig. 3(c) show that the peak at 0.5 keV indicates oxygen's inclusion in samples, most likely related oxides of Ag, Zn, as well as Cu. The EDX spectra of MWCNT@ACZ-NC displayed in Fig. 3(c) show that the peak at 0.5 keV indicates oxygen's inclusion in samples, most likely related oxides of Ag, Zn, as well as Cu. Ag's peaks became visible at 3.08 keV, for Cu at 0.9 and 8.05 keV, and for Zn at 1.05, 8.7, and 9.6 keV, validating samples' intended elemental composition. Additionally, a peak at 2.62 keV indicated Cl's existence. A peak for C centered at 0.21 keV corresponded to the Kα peak of carbon in EDX analysis. This peak confirmed the incorporation of MWCNTs into the nanocomposite. This peak emerged due to the characteristic X-ray emission of carbon atoms when they were excited by the incident electron beam.
3.4 FT-IR analysis
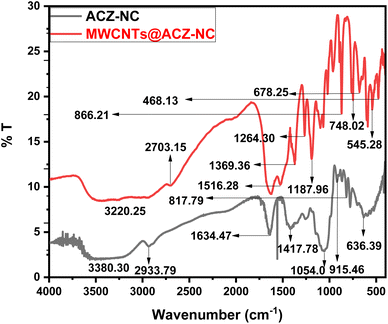
In Fig. 4, the FT-IR spectra display peaks at distinct wavenumbers (cm−1), indicating points where IR radiation transfer occurred. OH absorption as well as H–O–H's stretching collision were identified as causes of two broad bands with centers at 3380.30 and 3220.25 cm−1.42 The band at 2933.79 cm−1 was ascribed to the C–H stretching modes of MWCNTs.43 A slight band resulting from the C–H stretching vibration was noticed at 2703.15 cm−1.44 A notably intense band detected at 1634.47 cm−1 was associated with H–O–H coordinated H2O molecule's in-plane bending.45 A faint band at 1516.28 cm−1 was designated to MWCNTs' C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C expanding mode.46 Another mild band at 1417.78 cm−1 was accredited to the M–OH vibrations.47 Owing to the out-of-plane O–H bending vibrations, a fairly sharp band at 1369.36 cm−1 was identified.46 A second band was detected at 1264.30 cm−1, and it was determined to be a C–O stretching bond.48 C–H in flexural vibration created a band at 1187.96 cm−1.49 The M–OH (Ag, Cu, or Zn) vibrations were assigned to the band at 1054.10 cm−1.50 The O–M–O vibrations of Ag, Cu, and Zn were recognized as band's source at 915.46 cm−1.51 M–O–M (Ag, Cu, or Zn) vibrations were represented by the band at 866.21 cm−1.52 A band at 817.79 cm−1 was influenced by M–O–M vibrations.53 The C–O–M bond was the prime cause of band at 748.02 cm−1.54 The Cu–O elongation mode caused the appearance of band at 678.25 cm−1.55 The AgO bending vibrations gave rise to the band at 636.39 cm−1.56 The band at 545.28 cm−1 was produced by ZnO bending vibrations.57 However, ZnO stretching vibrations caused the band at 468.13 cm−1.58 The incorporation of MWCNTs into the Ag2O/CuO/ZnO nanocomposite significantly altered its FT-IR spectra by introducing new bands, shifting existing bands, changing intensities, broadening bands, causing the disappearance of minor bands, and potentially forming new functional groups. The introduction of MWCNTs led to the appearance of new absorption bands. These bands were attributed to the vibrational modes of the carbon nanotubes, such as C
C expanding mode.46 Another mild band at 1417.78 cm−1 was accredited to the M–OH vibrations.47 Owing to the out-of-plane O–H bending vibrations, a fairly sharp band at 1369.36 cm−1 was identified.46 A second band was detected at 1264.30 cm−1, and it was determined to be a C–O stretching bond.48 C–H in flexural vibration created a band at 1187.96 cm−1.49 The M–OH (Ag, Cu, or Zn) vibrations were assigned to the band at 1054.10 cm−1.50 The O–M–O vibrations of Ag, Cu, and Zn were recognized as band's source at 915.46 cm−1.51 M–O–M (Ag, Cu, or Zn) vibrations were represented by the band at 866.21 cm−1.52 A band at 817.79 cm−1 was influenced by M–O–M vibrations.53 The C–O–M bond was the prime cause of band at 748.02 cm−1.54 The Cu–O elongation mode caused the appearance of band at 678.25 cm−1.55 The AgO bending vibrations gave rise to the band at 636.39 cm−1.56 The band at 545.28 cm−1 was produced by ZnO bending vibrations.57 However, ZnO stretching vibrations caused the band at 468.13 cm−1.58 The incorporation of MWCNTs into the Ag2O/CuO/ZnO nanocomposite significantly altered its FT-IR spectra by introducing new bands, shifting existing bands, changing intensities, broadening bands, causing the disappearance of minor bands, and potentially forming new functional groups. The introduction of MWCNTs led to the appearance of new absorption bands. These bands were attributed to the vibrational modes of the carbon nanotubes, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C elongation and C–H flexing. The incorporation of MWCNTs caused shifts in the existing absorption bands of the Ag2O/CuO/ZnO nanocomposite. This happened due to interactions between the MWCNTs and the metal oxides, which altered the local electronic environment and, consequently, the vibrational frequencies. The intensity of certain absorption bands changed. For example, the peaks corresponding to metal–oxygen stretching vibrations (e.g., Zn–O, Cu–O, and Ag–O) experienced changes in their intensities due to the physical or chemical interactions with the MWCNTs. Enhanced dispersion and improved interfacial bonding led to more prominent bands or changes in the relative intensities. Moreover, the incorporation of MWCNTs enhanced the polarizability of composites due to the presence of π-electrons in carbon nanotubes. This increased polarizability also led to stronger interactions with the infrared radiation, resulting in enhanced peak intensities in the FT-IR spectra. Some minor bands either disappeared or became less noticeable in the FT-IR spectra as they were overshadowed by the more prominent absorption features of the MWCNTs or due to changes in the vibrational modes of the composite. The incorporation of MWCNTs also induced structural changes in the nanocomposite, such as changes in crystallinity or the formation of new phases which also made some peaks more prominent in the FT-IR spectra.
C elongation and C–H flexing. The incorporation of MWCNTs caused shifts in the existing absorption bands of the Ag2O/CuO/ZnO nanocomposite. This happened due to interactions between the MWCNTs and the metal oxides, which altered the local electronic environment and, consequently, the vibrational frequencies. The intensity of certain absorption bands changed. For example, the peaks corresponding to metal–oxygen stretching vibrations (e.g., Zn–O, Cu–O, and Ag–O) experienced changes in their intensities due to the physical or chemical interactions with the MWCNTs. Enhanced dispersion and improved interfacial bonding led to more prominent bands or changes in the relative intensities. Moreover, the incorporation of MWCNTs enhanced the polarizability of composites due to the presence of π-electrons in carbon nanotubes. This increased polarizability also led to stronger interactions with the infrared radiation, resulting in enhanced peak intensities in the FT-IR spectra. Some minor bands either disappeared or became less noticeable in the FT-IR spectra as they were overshadowed by the more prominent absorption features of the MWCNTs or due to changes in the vibrational modes of the composite. The incorporation of MWCNTs also induced structural changes in the nanocomposite, such as changes in crystallinity or the formation of new phases which also made some peaks more prominent in the FT-IR spectra.
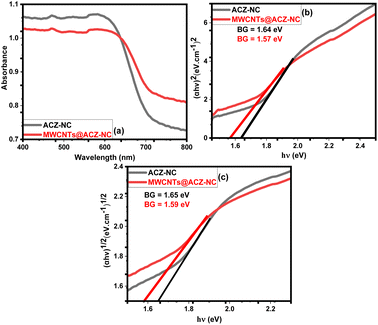
3.5 DRS analysis
The optical characteristics of ACZ-NC as well as MWCNTs@ACZ-NC were investigated using DRS, and Fig. 5(a)–(c) displays the obtained findings. For both samples, absorbance edges appeared at 720.56 nm and 765.70 nm, respectively. The results depicted that both samples exhibited peak absorption in the visible spectrum. Moreover, a redshift was observed in the absorbance edge with the incorporation of CNTs, indicating that the sample absorbed more visible light, extending to a broader spectrum. Band gap energies for both samples were measured using Tauc's plots derived from the respective equations, i.e. eqn (2) and (3) for direct and indirect transitions. The determined energy gaps are given in Fig. 5(b) and (c), showing a slight decrease in the band gap energies, which indicated that the electronic structure of ACZ-NC was altered by the incorporation of MWCNTs. The decrease in band gap energies might be due to the interaction between the electronic structure and conductive π-electron networks of the MWCNTs.| αhν = A(hν − Eg)2 | (2) |
| αhν = A(hν − Eg)1/2 | (3) |
3.6 Photocatalytic activity
The photocatalytic efficacy of ACZ-NC as well as MWCNTs@ACZ-NC, synthesized through a modified sol–gel method, was assessed using Rh-6G dye as the test substrate. The Rh-6G dye was also degraded without any catalyst in order to assess its self-photolytic capability. The assessment was conducted in an outdoor setting from 11 a.m. to 3 p.m. under solar light radiation between June 3 and June 14, 2024. Light brown color's progressive decline over time indicated reaction mixture's discoloration. At 526 nm, a sharp initial decline in absorbance maxima was observed following UV-visible measurement using a double-beam spectrophotometer, which suggested that at that specific wavelength, the chromophore that caused light absorption had degraded.This decrease was pronounced in samples containing catalysts, while it was negligible in the sample without any catalyst. The decay curve shown in Fig. 6 demonstrates a progressive decline in peak absorbance over time. A prominent as well as rapid reduction in maximum absorption occurred during initial 10 min, particularly in the MWCNT-modified sample. However, it was minimal in the sample without any catalyst. Afterward, the reduction rate slowed down in all samples. The sample modified with MWCNTs exhibited the greatest reduction in absorbance maximum.
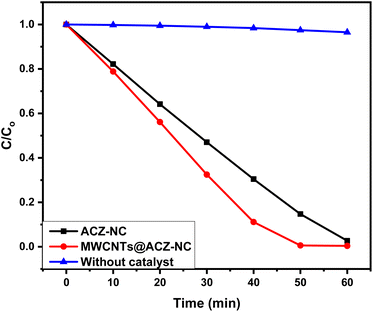 | ||
| Fig. 6 Degradation profile of Rh-6G in the presence of solar light and the synthesized nanocomposites. | ||
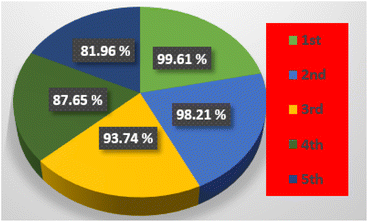
Rh-6G's percentage degradation was determined using eqn (4). Fig. 7 illustrates the results for all samples where the sample containing ACZ-NC showed a degradation of 97.31%, while the MWCNTs@ACZ-NC exhibited 99.61% degradation of Rh-6G. In contrast, the sample without a catalyst showed only 3.52% degradation. These results emphasized the relative performance of each sample to decompose Rh-6G under specified research conditions. The findings showed that among all samples, the MWCNTs@ACZ-NC exhibited the highest photocatalytic efficiency, whereas the sample without any catalyst showed poor catalytic activity.
| % Degradation = Co − Ce/Co × 100 | (4) |
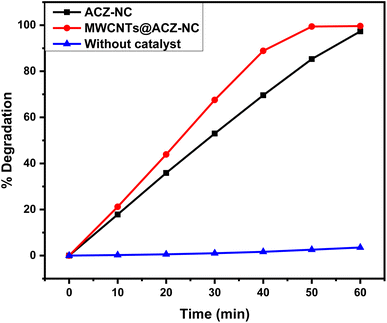 | ||
| Fig. 7 Percentage degradation of Rh-6G in the presence of solar light and the synthesized nanocomposites. | ||
The degradation rate constants determined using eqn (5) for photocatalytic reactions conducted with ACZ-NC, MWCNTs@ACZ-NC, and without a catalyst were 0.05368, 0.10012, and 5.90528 per min, respectively (Fig. 8). These figures simplify Rh-6G breakdown in a specified environment by providing insights into the photocatalytic activity ratio of each sample. A higher rate constant signifies enhanced decomposition rate, implying enhanced photocatalytic activity in fabricated samples. The outcomes indicated that MWCNTs@ACZ-NC showed the fastest rate constant, i.e., 0.10012 per min, among the examined samples. This suggested that MWCNT@ACZ-NC could potentially be more efficient at promoting the target compound's breakdown.
| ln(C/Ct) = −kt | (5) |
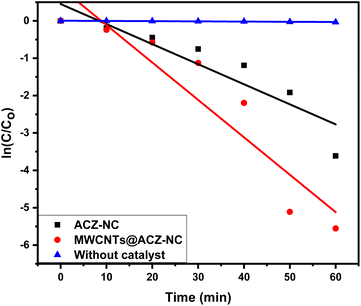 | ||
| Fig. 8 Degradation rate constant of the photocatalytic reaction carried out in the presence of synthesized nanocomposites. | ||
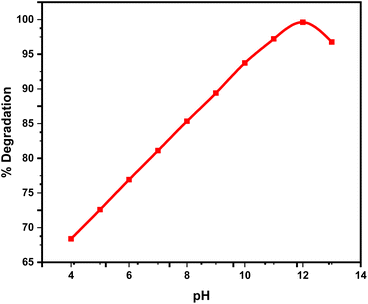
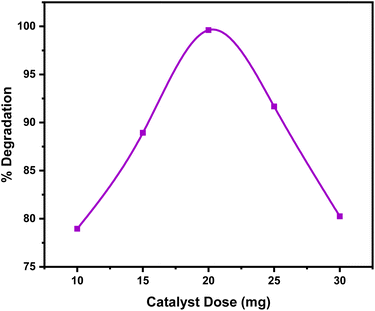
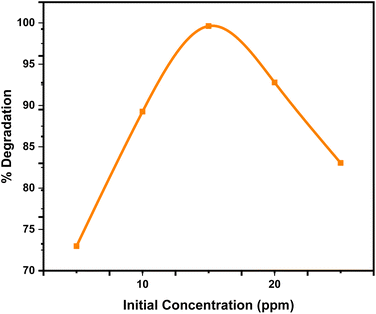
3.7 Factors affecting the photocatalytic reaction
At elevated amounts, the reactive centers of MWCNTs@ACZ-NC became fully occupied and saturated, resulting in reduced degradation. The reduced hydroxyl ion production and, as a result, reduced photocatalytic efficiency can result from the elevated concentrations interfering with light interaction on the catalyst surface. Additionally, the formation of breakdown products that compete with Rh-6G for restricted catalytic sites on the NC surface, along with reactive intermediates formation, could contribute to this decline.
3.8 Antibacterial activity
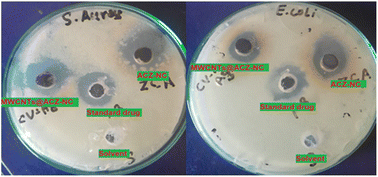
The antibacterial activity of the ACZ-NC and MWCNTs against the selected bacterial strain was determined by an agar well diffusion method, and the obtained results are shown in Fig. 13. The data show (Table 1) that the activity of the sample increases with the increase in the concentration of the samples. The enhanced activity at a higher concentration is due to the large number of particles that interact with the bacterial surface. It is also been observed that the activity of the ACZ-NC is higher than that of the MWCNTs@ACZ-NC, which might be due to the incorporation of CNTs that occupy/cover binding sites responsible for direct interaction with the bacterial cell, which led to a weak interaction between the bacterial surface and the ACZ-NC. The incorporation of CNTs also altered the surface morphology, surface charge and chemical functionality of the ACZ-NC, which made the surface less compatible with the bacterial outer membrane and this reduced interaction led to a lower antibacterial efficacy of the MWCNTs@ACZ-NC. Moreover, the incorporation of MWCNTs led to an increase in particle size, as observed in the SEM analysis. This increase in size may hinder the ability of the particles to effectively penetrate bacterial cells.| Bacterial species | Samples | |||
|---|---|---|---|---|
| ACZ-NC | MWCNTs@ACZ-NC | Standard drug | Solvent | |
| S. aureus | 22.5 | 20 | 19.5 | 00 |
| E. coli | 18.3 | 1.6 | 19 | 00 |
3.9 Antioxidant activity
By testing against the ABTS˙+ solution and compared to that of ascorbic acid as a control, the antioxidant activities of ACZ-NC as well as MWCNTs@ACZ-NC were evaluated, with the findings presented in Table 2. To evaluate the percentage radical scavenging activity, ascorbic acid and the synthesized samples were exposed to a solution containing ABTS˙+ at different doses of 5, 25, 50, 100, 200 and 400 μg mL−1. The antioxidant activity rose as the concentration of both samples increased, suggesting that to neutralize the ABTS˙+ radical cation, a greater amount of antioxidants were needed.67,68 With the increase in concentration, the percentage radical scavenging activity increased. ACZ-NC displayed scavenging activity spanning from 26.076% to 91.336% at different concentrations while the scavenging activity of MWCNTs@ACZ-NC ranged from 27.144% to 95.924%. The antioxidant capacity of both NCs may be explained by the negatively charged NCs' electron-donating surface, which may be in charge of containing the ABTS free radical species.68,69 As it was able to combine more reactive oxygen species, the antioxidant activity was stronger.70 ABTS free radical searching activity was observed to increase when the NP portion was increased, that may be attributed to an increased number of NPs that could engage with ABTS free radicals.71 The antioxidant concentration at which fifty percent of ABTS˙+ radical cation can be neutralized is known as the IC50 value. The IC50 value for ACZ-NC was calculated to be 80.65 μg mL−1. For MWCNTs@ACZ-NC, the IC50 value was 59.22 μg mL−1, while 49.79 μg mL−1 was the IC50 value of vitamin C. This indicates that MWCNTs@ACZ-NC exhibited a greater activity than that of the unmodified ACZ-NC, but was less active than that of ascorbic acid. Standard deviation indicates the spread of antioxidant activity values around the mean and helps assess consistency. A smaller standard deviation suggests high precision and consistency in the results. A larger standard deviation indicates greater variability, suggesting less consistency in the antioxidant activity measurements. The values were found to be 1.63, 1.64, and 1.75 for ACZ-NC, MWCNTs@ACZ-NC, and ascorbic acid, respectively, indicating that ACZ-NC demonstrated the highest precision and consistency in its results. The variance, which is square of the standard deviation, represents the mean square error from the mean and measures the spread of antioxidant activity values. Higher variance suggests greater differences in antioxidant activity between samples or experimental conditions. The variance values were 2.65, 2.71, and 3.09 for ACZ-NC, MWCNTs@ACZ-NC, and ascorbic acid, respectively. These results indicated that ACZ-NC exhibited the least variation in antioxidant activity across samples or conditions. The correlation between dose and % RSA was determined to be 0.110, 0.119, and 0.102 for ACZ-NC, MWCNTs@ACZ-NC, and ascorbic acid, respectively. This suggested that ascorbic acid showed the strongest relationship, where higher doses were not associated with increased % RSA, while higher doses of MWCNTs@ACZ-NC were linked to increased % RSA. ACZ-NC leveraged electron donation, redox cycling, SPR effects, and metal–ligand coordination to neutralize free radicals and reduce oxidative stress while addition of MWCNTs enhanced electron transfer, increased active sites, and stabilized reactive species, making the composite more effective in antioxidant applications.| Samples | Concentration (μg mL−1) | % RSA | IC50 (μg mL−1) | Variance (S2) | Standard deviation (S) | Correlation between dose and % RSA |
|---|---|---|---|---|---|---|
| ACZ-NC | 5 | 26.076 | 80.65 | 2.65 | 1.63 | 0.110 |
| 25 | 34.188 | |||||
| 50 | 48.228 | |||||
| 100 | 65.916 | |||||
| 200 | 81.004 | |||||
| 400 | 91.336 | |||||
| MWCNTs@ACZ-NC | 5 | 27.144 | 59.22 | 2.71 | 1.64 | 0.119 |
| 25 | 36.432 | |||||
| 50 | 53.796 | |||||
| 100 | 71.124 | |||||
| 200 | 84.612 | |||||
| 400 | 95.924 | |||||
| Ascorbic acid | 5 | 21.92 | 92.74 | 3.09 | 1.75 | 0.102 |
| 25 | 33.58 | |||||
| 50 | 47.956 | |||||
| 100 | 57.396 | |||||
| 200 | 83.976 | |||||
| 400 | 94.068 |
4. Conclusions
In this study, ACZ-NC and MWCNTs@ACZ-NC were prepared by the sol–gel method, which allows for better control over size and morphology, leading to the synthesis of uniform samples, as evident from the EDS mapping results. This method is more efficient, faster, and simpler than the traditional approaches, making it a superior strategy for preparing these nanocomposites. The physicochemical analysis revealed that the crystallinity and particle size were significantly affected by the interaction between MWCNTs and the trimetal oxide NC. A slight decrease in the band gap energy of MWCNTs@ACZ-NC was also observed. The incorporation of MWCNTs enhanced the photocatalytic efficiency of the nanocomposite due to increased conductivity and improved electron–hole separation. The optimization process indicated that the pH, catalyst dose, and the initial concentration of Rh-6G significantly influenced the photocatalytic process. A similar improvement was observed in the antioxidant activity of MWCNTs@ACZ-NC, attributed to the increased number of binding sites and enhanced electron transfer, which stabilized free radicals. However, a slight decrease in the antibacterial potential of MWCNTs@ACZ-NC was noted, possibly due to the increase in particle size, which may have hindered the penetration of the material into bacterial cells.Data availability
All relevant experimental details and raw data, including characterization results (XRD, SEM, FTIR, DRS and EDX mapping) and measurements of photocatalytic, antibacterial, and antioxidant activity, have been archived and are available upon request. Due to the large volume of data associated with the calculation results in this study and the limitations of our institution's data-sharing policy, it is not feasible to upload all the data to public networks. However, we have disclosed the relevant calculation script files involved in the research process. The data supporting the findings of this study are available from the corresponding author upon reasonable request. Any additional information regarding the materials and methods used in this study can also be provided upon request to support further inquiries and reproducibility of the results.Author contributions
Amjad Latif Lone – formal analysis and writing – original draft; Sadiq Ur Rehman – supervision, project administration; Sirajul Haq, Nadia Shahzad and Muhammad Imran Shahzad – conceptualization, methodology and project administration; Shafia Shujaat – visualization, and writing – original draft; Mohammad Khalid Al-Sadoon – software, data curation and funding acquisition; Jamoliddin Razzokov and Abdus Samad – validation and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R410), King Saud University, Riyadh, Saudi Arabia.References
- S. Haq, W. Rehman and M. Waseem, J. Inorg. Organomet. Polym. Mater., 2018, 29, 651–658 CrossRef.
- S. Haq, W. Rehman and M. Rehman, J. Inorg. Organomet. Polym. Mater., 2020, 30, 1197–1205 CrossRef CAS.
- V. Lobo, A. Patil, A. Phatak and N. Chandra, Pharmacogn. Rev., 2010, 4, 118–126 CrossRef CAS PubMed.
- P. Gupta, S. P. Authimoolam, J. Z. Hilt and T. D. Dziubla, Acta Biomater., 2015, 27, 194–204 CrossRef CAS PubMed.
- J. Lee, N. Koo and D. B. Min, Compr. Rev. Food Sci. Food Saf., 2004, 3, 21–33 CrossRef CAS PubMed.
- J. A. Klein and S. L. Ackerman, J. Clin. Invest., 2003, 111, 785–793 CrossRef CAS PubMed.
- D. Malakar, H. N. Malik, D. Kumar, S. Saini, V. Sharma, S. Fatima, K. K. Bajwa and S. Kumar, Advances in Animal Genomics, 2020, vol. 4, pp. 33–48 Search PubMed.
- G. Waris and H. Ahsan, J. Carcinog., 2006, 5, 1–8 CrossRef PubMed.
- V. Herynek, P. Matou, M. Moskvin, V. Hunto, M. Slouf and D. Hor, Colloids Surf., B, 2021, 111824, DOI:10.1016/j.colsurfb.2021.111824.
- M. B. Kulkarni and S. Goel, Energy Policy, 2006, 34(5), 1984–1991 Search PubMed.
- N. Verma and N. Kumar, ACS Biomater. Sci. Eng., 2019, 5, 1170–1188 CrossRef CAS PubMed.
- F. Ijaz, S. Shahid, S. A. Khan, W. Ahmad and S. Zaman, Trop. J. Pharm. Res., 2017, 16, 743–753 CrossRef CAS.
- J. Aien, A. A. Khan, S. Haq, A. R. Khan, K. Elmnasri, M. Ben Ali, M. S. AL-Harbi, M. I. Alghonaim, S. A. Alsalamah, A. A. Qurtam, F. Boufahja, A. Hedfi and M. Dellali, Crystals, 2023, 330, DOI:10.3390/cryst13020330.
- S. Ghazal, N. Khandannasab, H. Ali, Z. Sabouri, A. Rangrazi and M. Darroudi, Ceram. Int., 2021, 47, 27165–27176 CrossRef CAS.
- M. Shahzad Shirazi, M. Moridi Farimani, A. Foroumadi, K. Ghanemi, M. Benaglia and P. Makvandi, Sci. Rep., 2022, 12, 1–15 CrossRef PubMed.
- H. Yousaf, A. Mehmood, K. S. Ahmad and M. Raffi, Mater. Sci. Eng., C, 2020, 112, 110901 CrossRef CAS PubMed.
- A. U. Khan, A. U. Khan, B. Li, M. H. Mahnashi, B. A. Alyami, Y. S. Alqahtani, K. Tahir, S. Khan and S. Nazir, Photodiagn. Photodyn. Ther., 2020, 101814, DOI:10.1016/j.pdpdt.2020.101814.
- G. Maheshwaran, A. Nivedhitha Bharathi, M. Malai Selvi, M. Krishna Kumar, R. Mohan Kumar and S. Sudhahar, J. Environ. Chem. Eng., 2020, 8, 104137 CrossRef CAS.
- S. B. Ghaffari, M. H. Sarrafzadeh, Z. Fakhroueian and M. R. Khorramizadeh, Mater. Sci. Eng., C, 2019, 103, 109827 CrossRef CAS PubMed.
- E. Albiter, A. S. Merlano, E. Rojas, J. M. Barrera-andrade, Á. Salazar and M. A. Valenzuela, J. Compos. Sci., 2021, 5(1), 1–40 Search PubMed.
- R. Vinayagam, R. Selvaraj, P. Arivalagan and T. Varadavenkatesan, J. Photochem. Photobiol., B, 2020, 203, 111760 CrossRef CAS PubMed.
- T. A. Singh, A. Sharma, N. Tejwan, N. Ghosh, J. Das and P. C. Sil, Adv. Colloid Interface Sci., 2021, 295, 102495 CrossRef CAS PubMed.
- J. Vera, W. Herrera, E. Hermosilla, M. Díaz, J. Parada, A. B. Seabra, G. Tortella, H. Pesenti, G. Ciudad and O. Rubilar, Antioxidants, 2023, 784, DOI:10.3390/antiox12040784.
- J. Zhang, H. Zhang, S. Wang and M. Liu, Polym. Degrad. Stab., 2017, 144, 93–99 CrossRef CAS.
- G. Cirillo, S. Hampel, R. Klingeler, F. Puoci, F. Iemma, M. Curcio, O. I. Parisi, U. G. Spizzirri, N. Picci, A. Leonhardt, M. Ritschel and B. Büchner, J. Pharm. Pharmacol., 2011, 63, 179–188 CrossRef CAS PubMed.
- R. M. Lucente-Schultz, V. C. Moore, A. D. Leonard, B. K. Price, D. V. Kosynkin, M. Lu, R. Partha, J. L. Conyers and J. M. Tour, J. Am. Chem. Soc., 2009, 131, 3934–3941 CrossRef CAS PubMed.
- K. Rajavel, R. Gomathi, S. Manian and R. T. Rajendra Kumar, J. Taibah Univ. Med. Sci., 2016, 11, 469–477 Search PubMed.
- X. Shi, B. Jiang, J. Wang and Y. Yang, Carbon, 2012, 50, 1005–1013 CrossRef CAS.
- X. Xu, Y. You, X. Liu, D. Wei, Y. Guan and A. Zheng, Carbon, 2021, 177, 189–198 CrossRef CAS.
- R. S. Shinde, R. A. More, V. A. Adole, P. B. Koli, T. B. Pawar, B. S. Jagdale, B. S. Desale and Y. P. Sarnikar, Curr. Res. Green Sustainable Chem., 2021, 4, 100138 CrossRef CAS.
- M. Barwant, Y. Ugale, S. Ghotekar, P. Basnet, S. Pansambal, A. Murthy, T.-D. Pham, M. Bilal, R. Oza and V. Karande, Plant-Mediated Biological Synthesis of Ag-Ago-Ag2O Nanocomposites Using Leaf Extracts of Solanum Elaeagnifolium for Antioxidant, Anticancer, and DNA Cleavage Activities, Research Square, 2021, preprint, DOI:10.21203/rs.3.rs-973781/v1.
- F. Ur Rehman, R. Mahmood, M. Ben Ali, A. Hedfi, M. Almalki, A. Mezni, W. Rehman, S. Haq and H. Afsar, Materials, 2021, 14, 1–13 Search PubMed.
- E. El-Mohsnawy, A. El-Shaer, F. El-Gharabawy, E. E. El-Hawary and A. E. R. R. El-Shanshoury, Braz. J. Microbiol., 2023, 54, 2807–2815 CrossRef CAS PubMed.
- S. Hameed, A. T. Khalil, M. Ali, M. Numan, S. Khamlich, Z. K. Shinwari and M. Maaza, Nanomedicine, 2019, 14, 655–673 CrossRef CAS PubMed.
- J. Zhang, H. Zhang, S. Wang and M. Liu, Polym. Degrad. Stab., 2017, 144, 93–99 CrossRef CAS.
- G. Cirillo, S. Hampel, R. Klingeler, O. Ilaria, U. Gianfranco and N. Picci, JPP, 2011, 179–188 CrossRef CAS PubMed.
- D. M. Stanković, M. Ognjanović, M. Fabián, V. V. Avdin, D. D. Manojlović, S. V. Đurić and B. B. Petković, Surf. Interfaces, 2021, 25, 101211 CrossRef.
- M. K. Nazal, O. S. Olakunle, A. Al-Ahmed, B. Merzougui, A. Abualkibash, A. Sultan, A. Bin Yousaf and S. J. Zaidi, J. Electroanal. Chem., 2018, 823, 98–105 CrossRef CAS.
- A. Noushin, A. Varasteh-Moradi, S. Z. Sayyed-Alangi and Z. Hossaini, Appl. Organomet. Chem., 2021, 35, e6295 CrossRef CAS.
- N. Jamshidi Roodbari, S. R. Hosseini and A. Omrani, Energy Fuels, 2023, 37, 5489–5498 CrossRef CAS.
- M. Bekmezci, N. Y. Ertas, M. Akin, I. Isik and F. Sen, Next Res., 2024, 100081 Search PubMed.
- S. A. Moon, B. K. Salunke, B. Alkotaini, E. Sathiyamoorthi and B. S. Kim, IET Nanobiotechnol., 2015, 9, 220–225 CrossRef PubMed.
- M. Bahgat, A. A. Farghali, W. M. A. El Rouby and M. H. Khedr, J. Anal. Appl. Pyrolysis, 2011, 92, 307–313 CrossRef CAS.
- A. Pozefsky and N. D. Coggeshall, Anal. Chem., 1951, 23(11), 1611–1619 CrossRef CAS.
- A. Ragunathan, R. Krishnan and B. Ameen, J. Chem. Res., 2015, 39, 622–626 CrossRef CAS.
- S. Her and C. Lai, Materials, 2013, 2274–2284 CrossRef PubMed.
- A. Sinha, V. Nand, B. Raj and S. Kumar, J. Hazard. Mater., 2011, 192, 620–627 CrossRef CAS PubMed.
- A. Bayu, D. Nandiyanto, R. Ragadhita and M. Fiandini, IJoST, 2023, 8, 113–126 Search PubMed.
- H. C. A. Murthy, C. H. Prakash and B. Abebe, Current Research in Science and Technology, 2020, vol. 4, pp. 113–131 Search PubMed.
- S. Haq, S. Dildar, M. Ben Ali, A. Mezni, A. Hed and M. I. Shahzad, Mater. Res. Express, 2021, 8(5), 055006 CrossRef CAS.
- S. Haq, F. Abbasi, M. Ben Ali, A. Hed, A. Mezni and W. Rehman, Mater. Res. Express, 2021, 8(7), 075009 CrossRef CAS.
- A. K. Abbas, S. K. Abass and A. M. Bashi, MSE, 2019, 571, 012067 CAS.
- A. Iqbal, A. Haq, G. Antonio, S. Ali, R. Naqvi, P. Westerhoff and S. Garcia-segura, Catalysts, 2021, 11(7), 806 CrossRef CAS.
- T. Oh, Bull. Korean Chem. Soc., 2009, 30, 0–3 CAS.
- A. Manjunath, M. Irfan and K. P. Anushree, Adv. Mater. Phys. Chem., 2016, 263–273 CrossRef CAS.
- B. El-ghmari, H. Farah and A. Ech-chahad, Bull. Chem. React. Eng. Catal., 2021, 16, 651–660 CrossRef.
- M. B. Ali, K. Elmnasri, S. Haq, S. Shujaat, M. Hfaiedh and F. B. Abdallah, Dig. J. Nanomater. Biostruct., 2023, 18, 1577–1585 Search PubMed.
- P. L. Meena, K. Poswal, A. K. Surela and J. K. Saini, Water Sci. Technol., 2021, 84, 2615–2634 CrossRef CAS PubMed.
- N. O. Photocatalysis, O. Al-madanat, B. N. Nunes, Y. Alsalka, A. Hakki, M. Curti, A. O. T. Patrocinio and D. W. Bahnemann, Catalysts, 2021, 1514 Search PubMed.
- V. Polliotto, S. Livraghi and E. Giamello, Res. Chem. Intermed., 2018, 44, 3905–3921 CrossRef CAS.
- V. D. Pakhale and P. R. Gogate, Arabian J. Sci. Eng., 2021, 6473–6484, DOI:10.1007/s13369-020-05074-5.
- D. Mahale, S. P. Hinge and B. S. Banerjee, Desalin. Water Treat., 2016, 18275–18285 Search PubMed.
- U. G. Akpan and B. H. Hameed, J. Hazard. Mater., 2009, 170, 520–529 CrossRef CAS PubMed.
- D. M. Nzilu, E. S. Madivoli, D. S. Makhanu, S. I. Wanakai, G. K. Kiprono and P. G. Kareru, Sci. Rep., 2023, 13, 14030 CrossRef CAS PubMed.
- S. Haq, H. Afsar, I. U. Din, P. Ahmad, M. U. Khandaker, H. Osman, S. Alamri, M. I. Shahzad, N. Shahzad, W. Rehman and M. Waseem, Catalysts, 2021, 11, 1–15 Search PubMed.
- J. Ren, Y. Z. Wu, Y. Dai, D. W. Sha, M. Chen, J. J. Wang, J. M. Pan, H. Tang, X. N. Cheng and X. H. Yan, Mater. Technol., 2017, 32, 574–583 CrossRef CAS.
- B. Siripireddy and B. K. Mandal, Adv. Powder Technol., 2017, 28, 785–797 CrossRef CAS.
- M. A. El-Bindary, M. G. El-Desouky and A. A. El-Bindary, Appl. Organomet. Chem., 2022, 36, 1–16 Search PubMed.
- M. Afifi, O. A. Almaghrabi and N. M. Kadasa, BioMed Res. Int., 2015, 1–6, DOI:10.1155/2015/153573.
- A. Hamid, S. Haq, S. Ur Rehman, K. Akhter, W. Rehman, M. Waseem, S. Ud Din, Z.-u. Abdin, M. Hafeez, A. Khan and A. Shah, Chem. Pap., 2021, 75, 4189–4198 CrossRef CAS.
- S. A. Bhat, F. Zafar, A. H. Mondal, A. Kareem, A. U. Mirza, S. Khan, A. Mohammad, Q. M. R. Haq and N. Nishat, J. Iran. Chem. Soc., 2020, 17, 215–227 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |