Open Access Article
Open Access ArticleOne-pot acid-catalysed synthesis of bis(1-imidazo[1,5-a]pyridyl)arylmethanes and evaluation of cytotoxic activities†
Tran Quang Hung *a,
Ban Van Phuca,
Ha Nam Dob,
Thu Hue Tranb,
Hai Yen Nguyenb,
Nguyen Truong Phuocc,
Truong Mai Chic,
Dang Van Dob,
Truong T. T. Ngaa,
Hien Nguyend,
Van Tuyen Nguyen
*a,
Ban Van Phuca,
Ha Nam Dob,
Thu Hue Tranb,
Hai Yen Nguyenb,
Nguyen Truong Phuocc,
Truong Mai Chic,
Dang Van Dob,
Truong T. T. Ngaa,
Hien Nguyend,
Van Tuyen Nguyen a and
Tuan Thanh Dang
a and
Tuan Thanh Dang *b
*b
aInstitute of Chemistry, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Hanoi, Vietnam. E-mail: tqhung@ich.vast.vn
bFaculty of Chemistry, VNU-Hanoi University of Science, 19 Le Thanh Tong, Hanoi, Vietnam. E-mail: dangthanhtuan@hus.edu.vn
cHanoi-Amsterdam High School for Gifted, Hoang Minh Giam, Cau Giay, Hanoi, Vietnam
dFaculty of Chemistry, Hanoi National University of Education, 136 Xuan Thuy, Hanoi, Vietnam
First published on 10th February 2025
Abstract
A practical and efficient approach for the acid-catalysed synthesis of bis(1-imidazo[1,5-a]pyridyl)arylmethane (BimPy) derivatives has been described. Interestingly, these BimPys showed potential cytotoxicity against various cancer cell lines (SK-LU-1, MCF-7, HepG2).
Imidazo[1,5-a]pyridines (ImPys) are extremely essential molecules that are receiving a lot of attention because of their unique features.1 Most of them are water soluble and biocompatible, making them easily absorbed by living cells.1 As a result, the ImPy moiety has been exploited as a fundamental component in the development of novel bioactive compounds for a variety of medical purposes.1 ImPy derivatives have been shown in recent pharmaceutical chemistry research to have a variety of important biological activities, including anticancer2 and cardiotonic agents,3 aromatase,4 kinase,5 HIV-protease,6 phosphodiesterase 10A,7 thromboxane A2 synthetase8 and tubulin polymerization inhibitors,9 neurokinin antagonists,10 and agonists11 in several Alzheimer's and inflammatory disease studies (Fig. 1).12
Furthermore, ImPy derivatives have found numerous applications in other fields, including optoelectronic materials,13 confocal microscopy sensors,14 bioimaging,15 and chemotherapeutic drugs for DNA breakage.16 Notably, the ImPy core has been introduced into the structure of carbene ligands, leading to potential applications in the catalysis field.17
Because ImPy derivatives are important in medicinal chemistry,17 organometallics,18 and materials research, several methods for synthesizing them have emerged.1 ImPys could be conventionally synthesized using cyclization processes.1 To date, a number of noncatalytic and catalytic approaches for accessing ImPys have been reported using cyclocondensation.19 A second method to ImPy derivatives via [3 + 2] cycloaddition processes is well known.20 Recently, modern synthetic approaches based on oxidative cyclization and transannulation processes in the presence of transition metal catalysts have provided more effective strategies for preparing ImPys that can tolerate functional groups.21,22
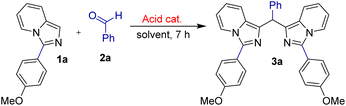
In 2009, Murai et al. published an interesting research on the synthesis of bis(1-imidazo[1,5-a]pyridyl)arylmethanes (BimPy) via iodine-mediated annulation of N-thioacyl-1-(2-pyridyl)-1,2-aminoalcohols.23 These interesting molecules could have applications in pharmaceutical chemistry, catalysis, and materials science. However, N-thioacyl-1-(2-pyridyl)-1,2-aminoalcohols are complicated starting materials that require numerous steps to prepare. In 2023, we presented a facile and efficient iodine-promoted synthesis of BimPy derivatives by tandem cyclization of pyridin-2-ylmethanamines and aldehydes.24 Recently, several studies on the synthesis and exploration of anticancer activities of imidazoheterocycles and other indole-fused N-heterocycles have been developed in our group.25 In continuous progress of our research, herein, we wish to disclose a new approach for the convenient acid-catalysed synthesis of BimPys and evaluation of their cytotoxic activities (Scheme 1).
Initial screening investigations utilizing ImPy 1a and benzaldehyde 2a in the employment of several acid catalysts (10 mol%) were carried out at 50 °C. To confirm the real role of acid catalyst, the reaction was performed in the absence of any acid catalysts, in fact, 3a product was not formed under our condition (entry 1, Table 1). Interestingly, BimPy (3a) was prepared in 98% isolated yield the presence of trifluoroacetic acid (TFA) catalyst (entry 4, Table 1). Then, several organic solvents were examined in order to understand the effect of solvents on the formation of product 3a (entries 3–9, Table 1). However, CHCl3 was determined to be the best solvent for this transformation, producing 3a in highest isolated yield. Further screenings under different temperatures were also investigated (entries 11 and 12). Notably, we did not achieve the target product 3a in better yield.
| Entry | Catalyst (equiv.) | Solvent | Time (h) | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Condition: 1 (0.67 mmol), 2a (0.67 mmol), acid catalyst (10 mol%), solvent (0.3 mL).b Isolated yields. | |||||
| 1 | — | CHCl3 | 7 | 50 | Trace |
| 2 | CH3COOH | CHCl3 | 7 | 50 | 20 |
| 3 | p-TsOH.H2O | 1,4-Dioxane | 7 | 50 | 30 |
| 4 | TFA | CHCl3 | 7 | 50 | 98 |
| 5 | H2SO4 | CHCl3 | 7 | 50 | 96 |
| 6 | TFA | EtOH | 7 | 50 | 69 |
| 7 | TFA | Toluene | 7 | 50 | 90 |
| 8 | TFA | DMSO | 7 | 50 | 72 |
| 9 | TFA | THF | 7 | 50 | 81 |
| 10 | TFA | CHCl3 | 7 | 50 | 75 |
| 11 | TFA | CHCl3 | 7 | 60 | 85 |
| 12 | TFA | CHCl3 | 7 | 40 | 66 |
With the optimal condition in hand, we wanted to investigate the substrates scope of this transformation. In general, the alkylation reaction of ImPy 1a with a series of aldehydes 2a–o under optimal conditions resulted in the desired BimPy products 3a–o in good to excellent yields (Table 2). The coupling reactions of ImPy 1a with halogenated benzaldehydes 2d–j afforded corresponding products 3d–j with excellent yields ranging from 95 to 98%. Interestingly, the reaction of 1a with thiophene-3-carbaldehyde (2l) indole-3-carbaldehyde (2m) resulted in a novel BimPys (3l, 3m) in 98% and 88% yields, respectively. Notably, the reaction of ImPy 1a with challenging aliphatic aldehydes 2n, 2o gave desired BimPys 3n, 3o in lower yields (55% and 60% yields).
To prepare other highly functionalized BimPy derivatives with a variety of substituents, we employed ImPy derivatives 1b–g and benzaldehyde derivatives 2a, 2p, 2q as the starting materials in this synthetic approach. Notably, under optimized condition, eight novel BimPy compounds 3p–x with various substituents were successfully synthesized in excellent yields (82–98%) (Tables 3 and 4).
Relying on previous research on acid-catalysed condensation reactions, we would like to propose a possible mechanism for the formation of BimPys in the presence of TFA (Scheme 2). Firstly, the condensation of ImPy with an aldehyde to form a secondary alcohol (intermediate A) which subsequently attend in an acid-activated dehydration to result in the formation of iminium intermediate B. Then the intermediate B reacts with a second ImPy molecule to give an intermediate C via 1,4-addition reaction. Finally, the BimPy product could be formed by deprotonation process and regenerate TFA catalyst for the next catalytic cycle.
The novel BimPy derivatives were tested for cytotoxicity against three human cancer cell lines SK-LU-1 (non-small cell lung cancer), HepG2 (liver cancer), and MCF-7 (breast cancer). Ellipticine served as a standard sample for the comparison. As shown in Table 5, compounds 3b and 3c (entries 1 and 2) were discovered to be effective cytotoxic agents against human cancer cell lines. Interestingly, compound 3b showed significant toxicity against lung, breast, and liver cell lines. In fact, the high toxicity of compound 3c against the three cell lines (entry 2) was reported in our recent paper.24a As a result, the most promising molecules 3b, 3c will be studied further to determine their ability to impact cell cycle progression and apoptosis in various cells.
| Entry | Compound | SK-LU-1 | MCF-7 | HepG2 |
|---|---|---|---|---|
| 1 | 3b | 5.36 ± 0.30 | 2.58 ± 0.27 | 1.72 ± 0.05 |
| 2 | 3c | 2.49 ± 0.19 | 3.41 ± 0.48 | 4.34 ± 0.67 |
| 3 | 3d | 27.12 ± 2.20 | 23.34 ± 1.40 | 9.19 ± 0.33 |
| 4 | 3j | 50.46 ± 4.20 | 67.91 ± 1.30 | 25.47 ± 0.89 |
| 5 | 3m | 57.37 ± 3.52 | 66.09 ± 4.04 | 47.23 ± 1.71 |
| 6 | 3n | 80.84 ± 4.62 | 87.64 ± 6.44 | 39.33 ± 2.67 |
| 7 | 3q | >100 | >100 | 59.17 ± 4.67 |
| 8 | 3s | >100 | >100 | >100 |
| 9 | Ellipticine | 1.88 ± 0.35 | 1.89 ± 0.29 | 1.46 ± 0.56 |
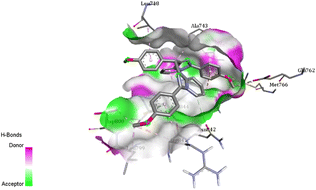
The cytotoxic results in Table 5 revealed that 3b and 3c were the most effective compounds in comparison with other compounds. Indeed, compound 3b (entry 1, Table 5), featuring a 4-methyl group substituent at phenyl ring, exhibited potent cytotoxic activity with IC50 values of 5.36, 2.58, and 1.72 μM against lung, breast, and liver cancer cell lines, respectively. Similarly, compound 3c (entry 2), with a 4-methoxy group substituent at phenyl ring, displayed IC50 values of 2.49, 3.41, and 4.34 μM against the same cell lines, though slightly less effective than compound 3b. However, the halogenated derivatives 3j, 3m (entries 3 and 4) demonstrated low toxicity across all cell lines, suggesting limited activity for these groups. In order to understand the role of functional groups in BimPy structures (3b and 3c), several molecular docking studies have been performed to investigate the interaction between the functional groups and the epidermal growth factor receptor (EGFR), a target kinase previously identified in our group's molecular dynamics (MD) studies.24b Previous studies demonstrated that similar BimPy derivatives exhibited free energy profiles during transitions from bound to unbound states, comparable to those of Erlotinib anticancer drug (PDB: 1M17).24b Encouraged by these findings, docking analyses for 3b and 3c were performed to elucidate their interaction with the EGFR kinase domain. The docking results demonstrated strong binding interactions for both compounds within EGFR's active sites, particularly with residues C797, T790, and L844 (Fig. 2 and 3). Notably, compound 3b showed a notable π–alkyl interaction between its 4-methyl group and residue C797, highlighting the role of the methyl substituent in enhancing binding affinity (Fig. 2). On the other hand, compound 3c formed two π–sulfur interactions with residues M766 and C775, suggesting that sulfur-containing substituents may promote S–S bridge formation, further stabilizing the binding interaction (Fig. 3).
Conclusions
In summary, we present a one-pot convenient method for the TFA-catalysed synthesis of bis(1-imidazo[1,5-a]pyridyl)arylmethane (BimPy) derivatives from simple starting materials such as ImPys and aldehydes. Notably, BimPy derivatives with the tolerance of various substituents can be produced in high yield from imidazo[1,5-a]pyridines (ImPys) and aldehydes in the presence of 10 mol% of TFA catalyst under mild conditions. Interestingly, these BimPy compounds showed some potential applications as anticancer agents against various cancer cell lines (SK-LU-1, MCF-7, HepG2). Notably, compound 3b showed very promising cytotoxic activities with IC50 values of 5.36, 2.58, and 1.72 μM against lung, breast, and liver cancer cell lines, respectively. A molecular docking study was conducted to visualize more information about the interactions between the promising compounds 3b, 3c and the kinase domain of the EGFR. As a result, this discovery has the potential to significantly advance pharmaceutical chemistry and metal-free catalysis into organic synthesis.Experimental
General procedure for preparation of 1,1′-(phenylmethylene)bis(3-(4-methoxyphenyl)imidazo[1,5-a]pyridine) 3a and derivatives
ImPy 1a (150 mg; 0.67 mmol) and benzaldehyde 2a (71 mg; 0.67 mmol) were introduced to a flask fitted with a magnetic stir bar. The flask was added with a 6 μL solution of trifluoroacetic acid (TFA) and 0.3 mL of chloroform. After that, the reaction temperature gradually increased to 50 °C using an oil bath and stirred for 7 hours. After completion, the reaction mixture was cooled to room temperature and quenched with aqueous sodium bicarbonate (NaHCO3, 3 M) solution until the pH reached neutral. The mixture was then extracted using ethyl acetate and water. The organic layer was separated, dried over anhydrous sodium sulfate (Na2SO4), filtered, and the solvent evaporated under low pressure with a rotary evaporator. The resulting brown residue was purified by column chromatography (silica gel, hexane/DCM/ethyl acetate (9/4/2)) to yield 3a (352 mg, 98%) as a bright green solid. 1H NMR (600 MHz, CDCl3) δ 8.1 (dt, J = 7.3, 1.1 Hz, 2H), 7.7–7.6 (m, 4H), 7.5–7.5 (m, 2H), 7.4 (dt, J = 9.3, 1.2 Hz, 2H), 7.3 (t, J = 7.7 Hz, 2H), 7.2–7.2 (m, 1H), 7.0–7.0 (m, 4H), 6.5 (ddd, J = 9.3, 6.3, 1.0 Hz, 2H), 6.4 (ddd, J = 7.4, 6.3, 1.3 Hz, 2H), 6.3 (s, 1H), 3.8 (s, 6H). 13C NMR (151 MHz, CDCl3) δ 159.7, 143.2, 136.7, 133.6, 129.6, 128.9, 128.2, 128.2, 126.2, 123.1, 121.1, 119.7, 117.4, 114.3, 112.7, 55.4, 45.0.Protein and ligand preparation
The X-ray crystal structure coordinates EGFR tyrosine kinase domain (PDB 4ZAU) was retrieved from the RCSB Protein Data Bank26 and prepared with the UCSF ChimeraX.27 During the protein preparation, the AZD9291 was removed, the bond orders were assigned, and hydrogen atoms and formal charges were added to hetero groups. The water molecules in the ligand-binding area were preserved for docking, and all other water molecules 5 Å beyond hetero groups were deleted. The hydrogen bonding network of binding site residues was optimized by selecting the histidine tautomer and by predicting the ionization states. The optimized protein structure was then subjected to all-atom constrained energy minimization using the Optimize Geometry module of Open Babel GUI with MMFF94 force field.28 The prepared domain structure of EGFR tyrosine kinase was used for molecular docking simulations. All chemical structures of the ligands were constructed using MarvinSketch,29 and energy-minimized using the minimization protocol of Avogadro2. Ligand volume was evaluated using the analytical tools included in Avogadro2.Docking protocol
Molecular docking studies were conducted using AutoDock4 software to evaluate the binding affinities of the ligands.30 The default settings in AutoDock4 were applied for grid generation and flexible docking, utilizing the Lamarckian Genetic Algorithm and an empirical free energy scoring function. A binding site radius of 15 Å from the center of the binding cavity was defined, and docking results were clustered with a root mean square deviation (RMSD) of 2.0 Å. For the docking calculations, AutoDock version 4.2 (Scripps Research Institute, San Diego, CA, USA) was used on a Dell personal computer. Grid maps representing the receptor molecule were generated using AutoGrid (Scripps Research Institute), with the grid centered at the ligand-binding pocket (LBP). The grid box was defined with dimensions of x = 80 Å, y = 80 Å, z = 80 Å, and the centroid coordinates were x = −56.673, y = −1.725, z = −23.122. Docking simulations were performed 100 times using the Lamarckian Genetic Algorithm (GA). The run parameters included 100 GA runs, a maximum of 2.5 × 107 energy evaluations, and a maximum of 1.0 × 106 generations. All input structures for proteins and ligands were prepared in the PDBQT format.The free energy change associated with each conformation was calculated as the sum of van der Waals forces, electrostatic interactions, hydrogen bonding events, de-solvation effects, and torsional energetics, as defined by the AutoDock scoring function.31
Data availability
The data supporting this research are available in the ESI.†Author contributions
T. Q. Hung, T. T. Dang wrote this manuscript and conceived this project; B. V. Phuc, T. H. Tran, H. Y. Nguyen, N. T. Phuoc, T. M. Chi performed the experiments and analyzed the data; H. N. Do, H. Nguyen carried out all molecular docking studies; D. V. Do, T. T. T. Nga conducted NMR analysis; V. T. Nguyen edited the manuscript and gave discussions about the mechanism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Vietnam Academy of Science and Technology under Grant Number ĐL0000.04/22-24.Notes and references
- (a) G. Volpi and R. Rabezzana, New J. Chem., 2021, 45, 5737–5743 RSC; (b) M. R. Reddy, C. M. Darapaneni, R. D. Patil and H. Kumari, Org. Biomol. Chem., 2022, 20, 3440–3468 RSC.
- (a) S. Priyanga, T. Khamrang, M. Velusamy, S. Karthi, B. Ashokkumar and R. Mayilmurugan, Dalton Trans., 2019, 48, 1489–1503 RSC; (b) M. Roy, B. V. S. K. Chakravarthi, C. Jayabaskaran, A. A. Karande and A. R. Chakravarty, Dalton Trans., 2011, 40, 4855–4864 RSC.
- D. Davey, P. W. Erhardt, W. C. Lumma, J. Wiggins, M. Sullivan, D. Pang and E. Cantor, J. Med. Chem., 1987, 30, 1337–1342 CrossRef CAS PubMed.
- L. J. Browne, C. Gude, H. Rodriguez, R. E. Steele and A. Bhatnager, J. Med. Chem., 1991, 34, 725–736 CrossRef CAS PubMed.
- G. Hatzivassiliou, J. R. Haling, H. Chen, K. Song, S. Price, R. Heald, J. F. M. Hewitt, M. Zak, A. Peck, C. Orr, M. Merchant, K. P. Hoeflich, J. Chan, S.-M. Luoh, D. J. Anderson, M. J. C. Ludlam, C. Wiesmann, M. Ultsch, L. S. Friedman, S. Malek and M. Belvin, Nature, 2013, 506, 231–236 Search PubMed.
- D. Kim, L. P. Wang, J. J. Hale, C. L. Lynch, R. J. Budhu, M. MacCross, S. G. Mills, L. Malkowitz, S. L. Gould, J. A. DeMartino, M. S. Springer, D. Hazuda, M. Miller, J. Kessler, R. C. Hrin, G. Carver, A. Carella, K. Henry, J. Lineberger, W. A. Schleif and E. A. Emini, Bioorg. Med. Chem. Lett., 2005, 15, 2129–2134 CrossRef CAS PubMed.
- M. S. Malamas, Y. Ni, J. Erdei, H. Stange, R. Schindler, H. J. Lankau, C. Grunwald, K. Y. Fan, K. Parris, B. Langen, U. Egerland, T. Hage, K. L. Marquis, S. Grauer, J. Brennan, R. Navarra, R. Graf, B. L. Harrison, A. Robichaud, T. Kronbach, M. N. Pangalos, N. Hoefgen and N. J. Brandon, J. Med. Chem., 2011, 54, 7621–7638 CrossRef CAS PubMed.
- N. F. Ford, L. J. Browne, T. Campbell, C. Gemenden, R. Goldstein, C. Gude and J. W. F. Wasley, J. Med. Chem., 1985, 28, 164–170 CrossRef CAS PubMed.
- R. Kaur, G. Kaur, R. K. Gill, R. Soni and J. Bariwal, Eur. J. Med. Chem., 2014, 87, 89–124 CrossRef CAS PubMed.
- K. C. Appell, B. E. Babb, R. Goswami, P. L. Hall, L. Kristine B., M. E. Logan, R. Przyklek-Elling, B. E. Tomczuk, B. R. Venepalli and J. M. Yanni, J. Med. Chem., 1991, 34, 1751–1753 CrossRef CAS PubMed.
- R. Nirogi, A. R. Mohammed, A. K. Shinde, N. Bogaraju, S. R. Gagginapalli, S. R. Ravella, L. Kota, G. Bhyrapuneni, N. R. Muddana, V. Benade, R. C. Palacharla, P. Jayarajan, R. Subramanian and V. K. Goyal, Eur. J. Med. Chem., 2015, 103, 289–301 CrossRef CAS PubMed.
- B. P. Fauber, A. Gobbi, K. Robarge, A. Zhou, A. Barnard, J. Cao, Y. Deng, C. Eidenschenk, C. Everett, A. Ganguli, J. Hawkins, A. R. Johnson, H. La, M. Norman, G. Salmon, S. Summerhill, W. Ouyang, W. Tang and H. Wong, Bioorg. Med. Chem. Lett., 2015, 25, 2907–2912 CrossRef CAS PubMed.
- G. Volpi, Asian J. Org. Chem., 2022, 11, e202200171 CrossRef CAS.
- (a) G. Volpi, C. Garino, E. Conterosito, C. Barolo, R. Gobetto and G. Viscardi, Dyes Pigm., 2016, 128, 96–100 CrossRef CAS; (b) G. Volpi, C. Garino, E. Priola, E. Diana, R. Gobetto, R. Buscaino, G. Viscardi and C. Barolo, Dyes Pigm., 2017, 143, 284–290 CrossRef CAS.
- F. Yagishita, C. Nii, Y. Tezuka, A. Tabata, H. Nagamune, N. Uemura, Y. Yoshida, T. Mino, M. Sakamoto and Y. Kawamura, Asian J. Org. Chem., 2018, 7, 1614–1619 CrossRef CAS.
- (a) D. Davey, P. W. Erhardt, W. C. J. Lumma, J. Wiggins, M. Sullivan, D. Pang and E. Cantor, J. Med. Chem., 1987, 30, 1337–1342 CrossRef CAS PubMed; (b) L. J. Browne, C. Gude, H. Rodriguez, R. E. Steele and A. Bhatnager, J. Med. Chem., 1991, 34, 725–736 CrossRef CAS PubMed.
- (a) M. Alcarazo, S. J. Roseblade, A. R. Cowley, R. Fernández, J. M. Brown and J. M. Lassaletta, J. Am. Chem. Soc., 2005, 127, 3290–3291 CrossRef CAS PubMed; (b) S. J. Roseblade, A. Ros, D. Monge, M. Alcarazo, E. Álvarez, J. M. Lassaletta and R. Fernández, Organometallics, 2007, 26, 2570–2578 CrossRef CAS; (c) A. Fürstner, M. Alcarazo, H. Krause and C. W. Lehmann, J. Am. Chem. Soc., 2007, 129, 12676–12677 CrossRef PubMed; (d) M. Nonnenmacher, D. Kunz, F. Rominger and T. Oeser, J. Organomet. Chem., 2007, 692, 2554–2563 CrossRef CAS; (e) D. Schleicher, A. Tronnier, J. Soellner and T. Strassner, Eur. J. Inorg. Chem., 2019, 1956–1965 CrossRef CAS; (f) T. K. Meister, J. W. Kück, K. Riener, A. Pöthig, W. A. Herrmann and F. E. Kühn, J. Catal., 2016, 337, 157–166 CrossRef CAS; (g) A. Schmidt, N. Grover, T. K. Zimmermann, L. Graser and M. Cokoja, J. Catal., 2014, 319, 119–126 CrossRef CAS; (h) F. Yagishita, K. Nomura, S. Shiono, C. Nii, T. Mino, M. Sakamoto and Y. Kawamura, ChemistrySelect, 2016, 1, 4560–4563 CrossRef CAS; (i) A. S. P. Chinna, V. Andrii and R. Graham, Organometallics, 2020, 39, 247–257 CrossRef; (j) F. Yagishita, T. Nagamori, S. Shimokawa, K. Hoshi, Y. Yoshida, Y. Imada and Y. Kawamura, Tetrahedron Lett., 2020, 61, 151782 CrossRef CAS.
- (a) Y. Chen, L. Li, Y. Cao, J. Wu, Q. Gao, Y. Li, H. Hu, W. Liu, Y. Liu, Z. Kang and J. Li, CrystEngComm, 2013, 15, 2675–2681 RSC; (b) Y. Chen, L. Li, Z. Chen, Y. Liu, H. Hu, W. Chen, W. Liu, Y. Li, T. Lei, Y. Cao, Z. Kang, M. Lin and W. Li, Inorg. Chem., 2012, 51, 9705–9713 CrossRef CAS PubMed.
- (a) F. Shibahara, A. Kitagawa, E. Yamaguchi and T. Murai, Org. Lett., 2006, 8, 5621–5624 CrossRef CAS PubMed; (b) G. Pelletier and A. B. Charette, Org. Lett., 2013, 15, 2290–2293 CrossRef CAS PubMed; (c) G. Schafer, M. Ahmetovic and S. Abele, Org. Lett., 2017, 19, 6578–6581 CrossRef CAS PubMed.
- (a) M. Qin, Y. Tian, X. Guo, X. Yuan, X. Yang and B. Chen, Asian J. Org. Chem., 2018, 7, 1591–1594 CrossRef CAS; (b) Y. Li, A. Chao and F. F. Fleming, Chem. Commun., 2016, 52, 2111–2113 RSC; (c) K. Satyam, V. Murugesha and S. Suresh, Org. Biomol. Chem., 2019, 17, 5234–5238 RSC.
- (a) J. R. Huang, Q. R. Zhang, C. H. Qu, X. H. Sun, L. Dong and Y. C. Chen, Org. Lett., 2013, 15, 1878–1881 CrossRef CAS PubMed; (b) Y. Yan, Y. Zhang, Z. Zha and Z. Wang, Org. Lett., 2013, 15, 2274–2277 CrossRef CAS PubMed; (c) D. C. Mohan, S. N. Rao, C. Ravi and S. Adimurthy, Org. Biomol. Chem., 2015, 13, 5602–5607 RSC; (d) J. Sheng, J. Liu, H. Zhao, L. Zhenga and X. Wei, Org. Biomol. Chem., 2018, 16, 5570–5574 RSC; (e) M. Sandeep, P. S. Dushyant, B. Sravani and K. R. Reddy, Eur. J. Org Chem., 2018, 3036–3047 CrossRef CAS.
- (a) M. Regitz, Angew. Chem., Int. Ed. Engl., 1967, 6, 733–749 CrossRef CAS; (b) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862–872 CrossRef CAS PubMed; (c) A. Joshi, D. C. Mohan and S. Adimurthy, Org. Lett., 2016, 18, 464–467 CrossRef CAS PubMed; (d) S. Chuprakov, F. W. Hwang and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 4757–4759 CrossRef CAS PubMed.
- (a) S. Tahara, F. Shibahara, T. Maruyama and T. Murai, Chem. Commun., 2009, 7009–7011 RSC; (b) T. Murai, E. Nagaya, F. Shibahara and T. Maruyama, Org. Biomol. Chem., 2012, 10, 4943–4945 RSC.
- (a) B. V. Phuc, N. T. Nguyen, N. T. H. Van, T. L. Nguyen, V. H. Nguyen, C. M. Tran, H. Nguyen, M. T. Nguyen, T. Q. Hung and T. T. Dang, Chem. Commun., 2023, 59, 1947–1950 RSC; (b) D. T. Truong, K. Ho, H. T. Y. Nhi, V. H. Nguyen, T. T. Dang and M. T. Nguyen, Sci. Rep., 2024, 14, 12218 CrossRef CAS PubMed.
- (a) T. Q. Hung, B. V. Phuc, M. P. Nguyen, T. L. Tran, D. V. Do, H. T. Nguyen, V. T. Nguyen, H. Nguyen and T. T. Dang, RSC Adv., 2024, 14, 29535–29541 RSC; (b) N. T. T. Huyen, B. V. Phuc, T. T. Huyen, T. T. Hong, H. Nguyen, V. H. Nguyen, M. T. Nguyen, T. Q. Hung, C. P. Dinh and T. T. Dang, ChemMedChem, 2024, 19, e202400316 CrossRef CAS PubMed; (c) B. V. Phuc, Q. H. Dinh, N. L. Chi, Q. T. Nguyen, T. T. N. Truong, N. V. Tuyen, H. Nguyen, P. Langer, T. T. Dang and T. Q. Hung, Tetrahedron, 2023, 136, 133360 CrossRef; (d) T. N. Ngoc, O. A. Akrawi, T. T. Dang, A. Villinger and P. Langer, Tetrahedron Lett., 2015, 56, 86–88 CrossRef; (e) N. N. Pham, T. T. Dang, T. N. Ngo, P. Ehlers and P. Langer, Org. Biomol. Chem., 2015, 13, 6047–6058 RSC; (f) N. T. Son, T. A. Nguyen-Tien, M. B. Ponce, P. Ehlers, N. T. Thuan, T. T. Dang and P. Langer, Synlett, 2020, 31, 1308–1312 CrossRef CAS; (g) T. T. Dang, U. Albrecht, K. Gerwien, M. Siebert and P. Langer, J. Org. Chem., 2006, 71, 2293–2301 CrossRef CAS PubMed; (h) N. K. Nguyen, D. H. Nam, B. Van Phuc, V. H. Nguyen, Q. T. Trinh, T. Q. Hung and T. T. Dang, Mol. Catal., 2021, 505, 111462 CrossRef CAS.
- Y. Yosaatmadja, S. Silva, J. M. Dickson, A. V. Patterson, J. B. Smaill, J. U. Flanagan, M. J. McKeage and C. J. Squire, J. Struct. Biol., 2015, 192, 539–544 CrossRef CAS PubMed.
- E. C. Meng, T. D. Goddard, E. F. Pettersen, G. S. Couch, Z. J. Pearson, J. H. Morris and T. E. Ferrin, Protein Sci., 2023, 32, e4792 CrossRef CAS PubMed.
- N. M. O'Boyle, M. Banck, C. A. James, C. Morley, T. Vandermeersch and G. R. Hutchison, J. Cheminf., 2011, 3, 33 Search PubMed.
- A. R. R. P. Almeida and M. J. S. Monte, J. Chem. Thermodyn., 2017, 107, 42–50 CrossRef CAS.
- G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell and A. J. Olson, J. Comput. Chem., 2009, 30, 2785–2791 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08868a |
| This journal is © The Royal Society of Chemistry 2025 |