Open Access Article
Open Access ArticleEmerging contaminants in the Mediterranean Sea endangering Lebanon's Palm Islands Natural Reserve†
Bilal Nehmeh‡
a,
Fatima Haydous‡a,
Hiba Aliab,
Adonis Hdaifia,
Bayan Abdlwahaba,
Mariam Bou Orma,
Zohrab Abrahamian ac and
Elias Akoury
ac and
Elias Akoury *a
*a
aDepartment of Physical Sciences, School of Arts and Sciences, Lebanese American University, Beirut 1102-2801, Lebanon. E-mail: elias.akoury@lau.edu.lb; Tel: +961 1 786456, ext. 3950
bUniversité Lille Nord de France, USTL, Cite Scientifique, 59652 Villeneuve d'Ascq Cedex, France
cUS-Middle East Partnership Initiative (MEPI) Tomorrow's Leaders Program, USA
First published on 22nd January 2025
Abstract
The Mediterranean Sea is an intercontinental marine environment renowned for its biodiversity and ecological significance. However, it is also one of the most polluted seas globally with significant levels of microplastics and heavy metals among other emerging contaminants. In Lebanon, inadequate waste management infrastructure and unregulated industrial discharges have exacerbated water quality deterioration by introducing these complex contaminants into surface and seawater. The Palm Islands Natural Reserve in Lebanon is a UNESCO-designated marine protected area and home to endangered species. However, the reserve faces significant threats from pollution, including heavy metals and microplastics, exacerbated by nearby Tripoli's escalating contamination. Plasticisers, particularly phthalates, are recognized for their hormone-disrupting effects, and heavy metals like cadmium, lead, and arsenic pose severe eco-toxicological risks. This study investigates the levels of heavy metals and phthalates in water and sediments from the Palm Islands. Samples were collected from different locations within the reserve, and heavy metals and phthalates were detected, including chromium (13.58 to 19.28 μg L−1), arsenic (2.05 to 5.04 μg L−1), cadmium (1.27 to 3.04 μg L−1), and lead (0.92 to 2.88 μg L−1). Cadmium levels exceeded the permissible limits set by environmental regulatory bodies, highlighting an urgent pollution problem. Phthalates, including DEP and DEHP, were also detected in concentrations of 7.12–10.25 μg L−1 for DEP and 38.47–56.12 μg L−1 for DEHP raising concerns over their potential eco-toxicological impact on marine species. Our research underscores the need for comprehensive environmental monitoring, better waste management infrastructure, and stricter regulatory measures to address pollution in Lebanon's coastal ecosystems.
Introduction
Marine ecosystems, especially those near coastal regions, are increasingly threatened by pollution from various anthropogenic activities.1–3 Among the pollutants, emerging contaminants such as heavy metals and phthalates pose serious risks to marine biodiversity, public health, and environmental sustainability. Heavy metals are persistent, bioaccumulative, and toxic,4,5 while phthalates, widely used as plasticizers, have been identified as endocrine disruptors.6,7 The release of these contaminants from industrial, agricultural, and urban sources leads to their accumulation in marine environments, making coastal areas particularly vulnerable.The Palm Islands Natural Reserve, located off the coast of Tripoli, Lebanon, is a marine protected area covering 4.2 km2. It was officially designated as a protected zone in 1992 under the Barcelona Convention and recognized by UNESCO for its ecological significance.8,9 The reserve comprises three islands and is home to a rich variety of flora and fauna, including endangered species such as monk seals, sea turtles, and the painted lady butterfly.10,11 Furthermore, the reserve provides a crucial habitat for migrating birds, unique marine species, and medicinal plants, underscoring its ecological significance in the eastern Mediterranean. However, despite its protected status, the Palm Islands face growing threats from rising pollution levels in the surrounding waters. The natural reserve is located approximately six nautical miles north of the Tripoli shoreline, an area recently identified for its alarming levels of environmental contaminants, including heavy metals, solid waste, and toxic chemical pollutants, with heavy metals and microplastics being of particular concern.12 Plasticisers are organic chemicals, added during plastic production, that accumulate in marine environments and release phthalates.13 These are hormone-disrupting chemicals that interfere with the production of the male sex hormone, reduce female fertility, and increase birth defects.14–16 A study by Deudero et al. (2015) detected the presence of microplastics in the gastrointestinal tracts of various fish species in the western Mediterranean henceforth indicating widespread contamination.17 A similarly, study revealed substantial microplastic in marine environment underscoring the urgent need for mitigation efforts.18 Notably, elevated levels of heavy metals in sediments were detected along the Lebanese coast19 thus highlighting the significant pollution burden on marine ecosystems. Despite the efforts, these contaminants are poorly examined in marine environment and thus conceal our ability to fully understand their eco-toxicological impact.
A lack of waste treatment infrastructure combined with uncontrolled industrial and household discharges in Lebanon is leading to the progressive deterioration of surface and sea water quality through elevated organic, inorganic, and complex contaminant environmental loading. To mitigate this problem, a range of issues must be addressed that go beyond treatment of traditional point- and nonpoint-source pollution parameters. Given the diversity and interconnectivity of pollutants present in Lebanon's water sources and the massive use of plastics and chemical plasticizers; there is a substantial need for a holistic approach to tackle this issue. The absence of local research on the impact of plastic pollution means, nationwide figures are not available. As per a 2015 study conducted by Cózar et al., it is estimated that the Mediterranean Sea has 1000 to 3000 tons of plastic floating on its surface.20 Microplastic contamination has increased significantly in recent years because of the lack of oversight and poor governance by regulatory agencies,21,22 which resulted in the spread and acceleration of pollution of the water, sediment, and air compartments. Additionally, identification and analysis of heavy metals, phthalates, and their derivatives in the Mediterranean region and specifically in Lebanon, remains largely unexplored. Therefore, this study is of tremendous significance to assess these emerging contaminants and their toxic effects in water and sediments of a marine protected area. In this study, we investigate the presence of heavy metals, phthalates, and their derivatives in water and sediments of the Palm Islands Natural Reserve to highlight the toxicological effects of these emerging contaminants on marine ecosystem.
This study addresses these research gaps by investigating the levels of heavy metals and phthalates in surface water and sediments within the Palm Island Natural Reserve. The research provides a comparative analysis of these environmental compartments, contributing to an improved understanding of pollutant dynamics in coastal ecosystems. By focusing on seasonal variations and proximity to pollution sources, this work offers valuable insights into the ecological risks posed by emerging contaminants. Additionally, the study highlights the need for implementing effective pollution control measures and further research into the long-term environmental implications of contaminant accumulation.
Materials and methods
Reagents and sample preparation
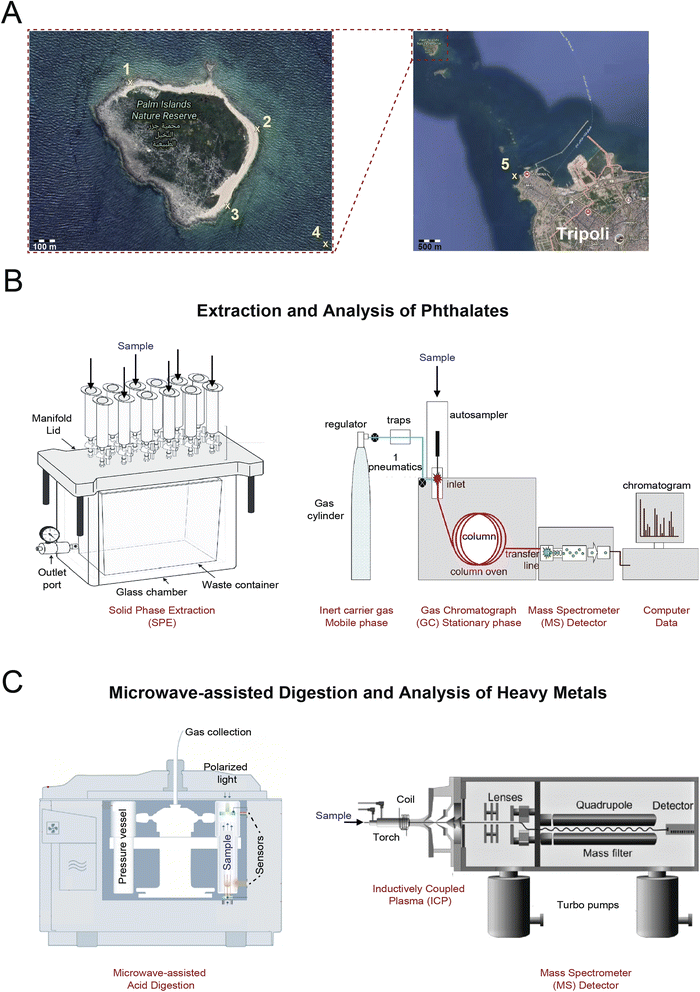
Reagents used in this research were of high purity and analytical grade: ethyl acetate C4H8O2 and dichloromethane CH2Cl2 (Fischer Scientific, Schwerte, Germany); 30% hydrogen peroxide H2O2 solution (Sigma Aldrich, Germany), 69% nitric acid HNO3 (BDH Laboratory Supplies, England), 37% hydrochloric acid HCl (AnalaR Normapur, France). Standards consisted of the heavy metals As, Cd, Cr, and Pb (Merck, Darmstadt, Germany) and the phthalates dimethyl phthalate (DMP – C10H10O4), diethyl phthalate (DEP – C12H14O4), diallyl phthalate (DAP – C14H14O4), diisobutyl phthalate (DiBP – C16H22O4), di-n-butyl phthalate (DBP – C16H22O4), dipentyl phthalate (DPeP – C18H26O4), di-hexylphthalate (DHxP – C20H30O4), benzyl butyl phthalate (BBP – C19H20O4), dicyclohexyl phthalate (DHCP – C20H26O4), di(2-ethylhexyl) phthalate (DEHP – C24H38O4), di(2-propylheptyl) phthalate (DPHP – C28H46O4), di(n-octyl) phthalate (DnOP – C24H38O4), and dibenzyl phthalate (DBzP – C22H18O4) (Santa Cruz Biotechnology, Heidelberg, Germany). Reference materials for salt water and wastewater were acquired to validate the accuracy of the method (ERM-CA403 and ERM-CA713, Institute for Reference Materials and Measurements, Geel, Belgium). All solutions were prepared with analytical reagent grade chemicals and ultrapure water (Millipore S.A., St Quentin-en-Yvelines, France, 18 MΩ cm, 25 °C, total organic carbon content <2 ppb). Samples of seawater and sediments were collected in April 2020 along the palm island using acid-washed plastic bottles and were stored at a temperature of 4 °C. Seawater and sediment samples were collected in triplicates during the month of April 2020 along Palm Island using acid-washed plastic bottles for heavy metal analysis and glass bottles for phthalate analysis. Samples were stored at 4 °C to maintain their integrity. This sampling period was intentionally selected to coincide with seasonal variations in marine conditions and times of increased pollutant discharge, ensuring the collection of representative data aligned with the study's objectives. The sampling program was meticulously designed to gather data from five strategically selected locations, chosen based on their proximity to pollution sources and ecological significance as shown on the map in Fig. 1A. Water samples were collected at a depth of 10 cm from the surface, while sediment samples were obtained from a depth of 20 cm using grab samplers. Grab sampling was employed for its ability to provide a snapshot of environmental conditions at specific moments, facilitating precise analysis of heavy metals and phthalates. The program accounted for spatial and temporal variations through repeated sampling and incorporated stringent quality control measures, such as blank and duplicate samples, to ensure accuracy and consistency across all sites.Macro parameters and total organic carbon
Seawater samples were collected from different locations of the Palm Island Natural Reserve (locations 1–3). Samples were collected from the surface water near the natural reserve (location 4) and location 5 was Tripoli's seashore, the closest major city to the islands. All samples were stored at 4 °C until analysis. Macro parameters of the samples were measured (conductivity, pH, dissolved oxygen species (DOS) and turbidity). The total organic carbon (TOC) of the water samples was measured using a TOC-VCPN instrument (Shimadzu, Kyoto, Japan) and acquired calibration curves for Total Carbon (TC) and Inorganic Carbon (IC) are reported in ESI Fig. 1.†Solid phase extraction for phthalates
Solid phase extraction (SPE) was performed from an adopted method for the extraction of phthalates from the water samples.23 The setup consisted of a CHROMABOND SPE vacuum manifold (Macherey-Nagel, Düren, Germany) with 24 WELCHROM C18E 6 mL, 500 mg, glass cartridges (Welch Materials, Connecticut, United States) as depicted in (Fig. 1B). The cartridges were cleaned three times with 5 mL acetone and 5 mL dichloromethane and conditioned with 5 mL ethyl acetate, 5 mL acetone, and 5 mL ultrapure water prior to sample extraction. Following the percolation step, the cartridge was washed with 5 mL of ultrapure water and dried under vacuum to remove any remaining salt. Phthalates were then eluted with ethyl acetate (2 × 3 mL) into pre-cleaned vials and ethyl acetate was completely evaporated. Prior to GC-MS measurements, 1 mL of ethyl acetate was added to the final samples.GC-MS analysis
Gas Chromatography Mass Spectrometry (GC MS) Thermo Scientific Trace 1310 gas chromatograph coupled to Thermo Scientific TSQ 9000 triple quadrupole mass spectrometer manifold (Massachusetts, USA) operated with electron impact ionization was used to determine phthalates' concentrations (Fig. 1B). Samples (1 μL) were injected automatically in splitless mode at 280 °C on a TG5 silica MS column (30 m × 0.25 mm; 0.25 μm) with the GC oven operating at 40 °C for 1 min, 70 °C at 10 °C min−1 for 2 min, 225 °C at 60 °C min−1 for 3 min, and 280 °C at 8 °C min−1 for 4 min. The carrier gas helium 99.9995% was used at a flow rate of 1 mL min−1. Data collection and analysis were performed using the Xcalibur software.Sample digestion for heavy metal analysis
Heavy metals were extracted from the seawater samples using acid digestion EPA method 3015A 22.5 mL of water sample was combined with 2 mL of concentrated HNO3 and 0.5 mL of concentrated HCl in a reaction vessel (Fig. 1C). Samples were digested at (1) 850 W at 170 °C for 10 min with a ramp of 15 °C min−1 and (2) 850 W at 170 °C for 22 min with Multiwave ECO microwave digestion system (Anton Paar GmbH, Graz, Austria) equipped with 16 pressure-activated-venting vessels (Rotor 24HVT50, Anton Paar GmbH, Graz, Austria) (Table 1). Samples were then transferred into 50 mL falcon tubes, diluted 5 times with 3% HNO3 in ultrapure deionized water and were stored at 4 °C until ICP-MS analysis. Similarly, sediment samples collected from the same locations in the Palm Islands (Fig. 1A) were digested as follows: 0.5 g of each sediment sample was transferred to the microwave tubes and two methods were applied. 2.5 mL of HNO3 and 7 mL of HCl were added to the sediment samples and the microwave program ran for 10 min to reach 165 °C; then the samples were kept at this temperature for 10 additional min. The tubes were placed in the microwave for 8 min to reach a temperature of 175 °C.| Microwave oven digestion | |||||
|---|---|---|---|---|---|
| Step | Power (W) | Pressure (MPa) | Temperature (°C) | Ramp time (min) | Hold time (min) |
| 1 | 850 | 2 | 170 | 10 | 22 |
| 2 | 850 | 2 | 22 | 10 | 1 |
| Inductively coupled plasma mass spectrometer | |
|---|---|
| Operating conditions | |
| Spectrometer | Thermo Scientific, iCAP RQ, ASX-280, autosampler ICP-MS |
| Nebulizer | Borosilicate glass concentric with 0.4 mL min−1 |
| Spray chamber | 2.70 °C, quartz cyclonic |
| Cell geometry | Octopole |
| Sampling cone | Nickel, 1.1 mm diameter orifice |
| Skimmer cone | Nickel, 0.75 mm diameter orifice |
| RF power | 400–1600 W |
| Reflected power | <10 W |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Standard mode | |
| Plasma gas flow | 15 L min−1 |
| Nebulizer gas flow | 1.03 L min−1 |
| Auxiliary gas flow | 0.81 L min−1 |
| Expansion stage | 2.01 mbar |
| Intermediate stage | 10−4 mbar |
| Analyzer stage | 10−6 mbar |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| He mode (collision cell mode) | |
| He gas flow | 4.0 mL min−1 |
| Octopole bias (CCT bias) | −21 V |
| Quadrupole bias (pole bias) | −18 V |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Acquisition parameters | |
| Field | Virtual hyperbolic |
| Frequency | 2 MHz |
| Mass range | 2–290 a.m.u |
| Dwell time | 0.04 s |
| Number of sweeps | 5 |
| Number of replicates | 3 |
| Total acquisition time | 220 s |
ICP-MS analysis
Elemental analysis was performed using inductively coupled mass spectrometer (ICP-MS) iCAP Q/iCAP RQ ICP-MS (Thermo Fisher Scientific Inc., Bremen, Germany) operating with argon gas of spectral purity (99.9995%) and tuned using iCAP Q/RQ TUNE aqueous multi-element standard solution in 2% HNO3 + 0.5% HCl solution (Thermo Scientific, Bremen, Germany) before each experiment (Fig. 1C). To maximize ion signals and minimize interference effects due to high oxide levels, various parameters were optimized with the tuning solution (1 mg L−1): torch position, ion lenses, gas output, resolution axis (10% of peak height) and background (<20 shots) (Table 1). Samples were analyzed twice, and heavy metal content were measured in triplicate to report average values. Quantification of heavy metals was carried out using external calibration curves from standard solutions prepared in 3% nitric acid and plotted using several concentrations (ESI Fig. 2†). Isotopic ratios in digested samples were examined to ensure the absence of polyatomic interferences during the full quantitative mode analysis. Certified reference materials were used to ensure method accuracy. The limit of detection (LOD) for each element was determined through blank determination assays, equivalent to three times the standard deviation of 20 blank replicates. The limit of quantification (LOQ) was calculated as twice the LOD for each element. Precision for heavy metal analysis via ICP-MS was validated through spiking methodology at various concentrations within permissible limits for each matrix, with recovery percentages calculated for each metal.Results and discussion
The water samples were collected from five locations (Fig. 1A) within the Palm Islands Natural Reserve (1 to 4 directly from the island shore, location 5 at 500 meters away). Each sample was analyzed for macro parameters, heavy metals, and phthalates. The GPS coordinates for each sampling location are provided along with the respective results for various parameters in Table 2. All samples showed slightly alkaline pH ranging between 8.32 and 8.41; representative conductivity values between 5.53 and 6.12 mS m−1; and dissolved oxygen (DO) levels (7.1–8.1 mg L−1) indicative of stable marine conditions. The measurement of the total carbon (TC) consists of the sum of the total organic carbon TOC (the carbon covalently bonded in organic molecules) and the inorganic carbon IC (carbonates, bicarbonates, and dissolved CO2 in the samples). TOC involves the purgeable organic carbon POC (removed from the samples by gas purging) and the non-purgeable organic carbon NPOC (not removed by gas purging). TC-IC measurements were conducted and TOC was determined by subtracting IC from TC. Additionally, NPOC measurements were acquired directly by acidifying the sample and bubbling it with the sparging gas to eliminate IC. The TC calibration curve was formed using standard solutions of potassium hydrogen phthalate with concentrations in the range of 0 to 100 ppm. For the IC calibration curve, 10 standard solutions with concentrations ranging between 0 and 100 ppm were prepared from a mixture of sodium hydrogen carbonate and sodium carbonate (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
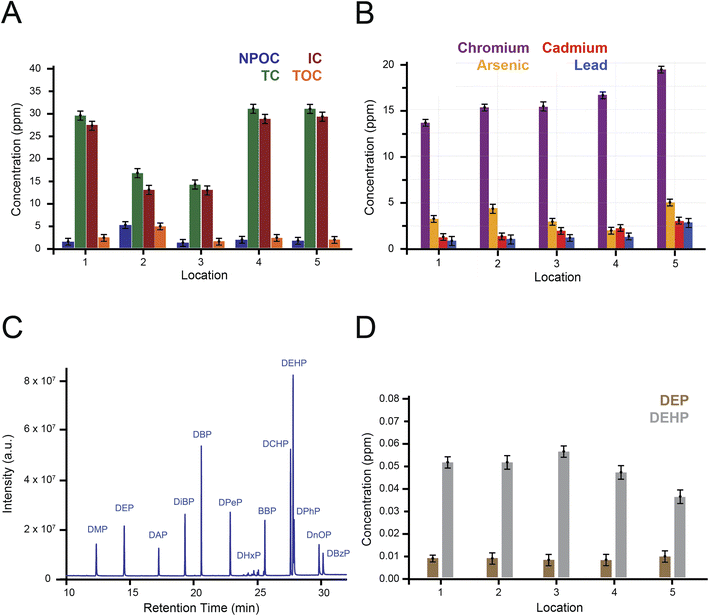
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio). Both calibration curves are represented in ESI Fig. 1.† The results are presented in Fig. 2A and Table 2. NPOC levels vary significantly, with the highest value at location 2 (5.19 ± 0.09 mg L−1), thus indicating higher organic matter content at this site while the other locations have relatively lower and more consistent values.
1 mass ratio). Both calibration curves are represented in ESI Fig. 1.† The results are presented in Fig. 2A and Table 2. NPOC levels vary significantly, with the highest value at location 2 (5.19 ± 0.09 mg L−1), thus indicating higher organic matter content at this site while the other locations have relatively lower and more consistent values.
| Locations of water samples | ||||||
|---|---|---|---|---|---|---|
| Parameters | 1 | 2 | 3 | 4 | 5 | Global concentration averages |
| GPS coordinates | 34°29′45′′N | 34°29′40′′N | 34°29′31′′N | 34°29′28′′N | 34°27′19′′N | |
| 35°46′18′′E | 35°46′35′′E | 35°46′30′′E | 35°46′45′′E | 35°48′35′′E | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Macro parameters | ||||||
| pH | 8.39 | 8.32 | 8.41 | 8.34 | 8.32 | 7.5–8.5 (WHO)24 |
| Conductivity (S m−1) | 5.54 | 5.62 | 5.53 | 5.91 | 6.12 | 0.005–0.05 S m−1 (natural water)25 |
| Dissolved oxygen (DO) | 7.4 | 7.3 | 7.5 | 7.2 | 8.1 | 6–8 mg L−1 (potable water)26 |
| Average NPOC (mg L−1) | 1.38 ± 0.06 | 5.19 ± 0.09 | 0.91 ± 0.06 | 1.76 ± 0.09 | 1.31 ± 0.12 | <2 mg L−1 (oligotrophic), 4–6 (polluted)27 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Heavy metals (μg L−1) | ||||||
| Chromium | 13.58 ± 0.26 | 15.22 ± 0.36 | 15.31 ± 0.34 | 16.57 ± 0.73 | 19.28 ± 0.22 | 0.5–50 μg L−1 (EPA)28 |
| Arsenic | 3.27 ± 0.11 | 4.41 ± 0.79 | 2.98 ± 0.37 | 2.05 ± 0.25 | 5.04 ± 0.49 | Limit 10 μg L−1 (WHO)29 |
| Cadmium | 1.27 ± 0.11 | 1.41 ± 0.24 | 1.98 ± 0.33 | 2.25 ± 0.15 | 3.04 ± 0.47 | 3–5 μg L−1 (EPA)30 |
| Lead | 0.92 ± 0.71 | 1.11 ± 0.79 | 1.25 ± 0.44 | 1.32 ± 0.25 | 2.88 ± 0.49 | 5–10 μg L−1 (EPA)31 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Phthalates (μg L−1) | ||||||
| DEP | 9.36 ± 2.31 | 9.11 ± 1.12 | 7.12 ± 2.16 | 8.32 ± 1.87 | 10.25 ± 1.91 | <10 μg L−1 (ref. 32) |
| DEHP | 51.24 ± 1.03 | 51.61 ± 1.41 | 56.12 ± 2.68 | 46.93 ± 0.91 | 38.47 ± 2.41 | <50 μg L−1 (ref. 32) |
| Locations of sediment samples | ||||
|---|---|---|---|---|
| Parameters | 1 | 2 | 3 | 4 |
| Heavy metals (mg kg−1) | ||||
| Chromium | 8.27 ± 0.34 | 11.69 ± 0.44 | 25.71 ± 0.48 | 14.42 ± 0.35 |
| Arsenic | 3.11 ± 0.91 | 4.25 ± 0.89 | 8.67 ± 0.34 | 8.01 ± 0.28 |
| Cadmium | 3.61 ± 0.48 | 3.17 ± 0.41 | 4.52 ± 0.33 | 4.81 ± 0.55 |
| Lead | 1.15 ± 0.42 | 1.21 ± 0.89 | 3.08 ± 0.33 | 1.85 ± 0.25 |
Next, we accessed the levels of heavy metals in the collected water and sediment samples using ICP-MS after preparing calibration curves from different concentrations of standard solutions (ESI Fig. 2†). To evaluate the method and its reproducibility, reference materials for seawater and wastewater were analyzed and the attributed recovery percentages were in the range of 81.67 and 116.75% (ESI Table 1†). Fig. 2B and Table 2 report the findings of the analysis where levels of chromium (range from 13.58 to 19.28 μg L−1) arsenic (range from 1.98 to 5.04 μg L−1), cadmium (range from 1.27 to 3.04 μg L−1) and lead (range from 0.92 to 2.88 μg L−1) were detected. According to the U.S. Environmental Protection Agency (EPA) recommendations for heavy metals levels in seawater, the threshold effect levels for chromium (50 μg L−1),33 arsenic (36 μg L−1),34 cadmium (0.78 μg L−1)30 and lead (8.1 μg L−1)33 are reported as guidelines for maintaining healthy marine environment. Similarly, we reported the levels of heavy metals found in the collected sediments and their distribution: chromium (range from 7.76 to 18.71 mg kg−1), arsenic (range from 3.11 to 8.67 mg kg−1), cadmium (range from 3.11 to 4.5 mg kg−1), and lead (range from 1.01 to 3.04 mg kg−1). Importantly, the allowed levels of heavy metals in marine sediments are regulated to protect marine ecosystems and human health. According to National Oceanic and Atmospheric Administration (NOAA) guidelines for heavy metals, the threshold effect levels for chromium (52.3 mg kg−1), arsenic (7.24 mg kg−1), cadmium (0.68 mg kg−1) and lead (35.8 mg kg−1) are set to prevent harmful levels for the accumulation of these metals in marine sediments and jeopardize marine ecosystems and human health.35 All metals met regulatory limits set for seawater and sediments except for cadmium which exceeded the expected levels reported by EPA and NOAA (Table 3).
| pH | Conductivity (S m−1) | Dissolved oxygen (DO) | Average NPOC | Chromium | Arsenic | Cadmium | Lead | DEP | DEHP | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.000 | |||||||||
| Conductivity (S m−1) | −0.697 | 1.000 | ||||||||
| Dissolved oxygen (DO) | −0.221 | 0.600 | 1.000 | |||||||
| Average NPOC | −0.571 | −0.184 | −0.371 | 1.000 | ||||||
| Chromium | −0.619 | 0.934 | 0.731 | −0.177 | 1.000 | |||||
| Arsenic | −0.512 | 0.311 | 0.716 | 0.347 | 0.441 | 1.000 | ||||
| Cadmium | −0.389 | 0.888 | 0.735 | −0.438 | 0.959 | 0.262 | 1.000 | |||
| Lead | −0.503 | 0.863 | 0.913 | −0.275 | 0.941 | 0.614 | 0.913 | 1.000 | ||
| DEP | −0.649 | 0.547 | 0.537 | 0.190 | 0.404 | 0.707 | 0.248 | 0.555 | 1.000 | |
| DEHP | 0.703 | −0.951 | −0.684 | 0.150 | −0.850 | −0.476 | −0.780 | −0.869 | −0.769 | 1.000 |
We then used 13 phthalate standards to optimize the solid-phase extraction (SPE) procedure by considering factors such as solvent type, sample volume, and sorbent mass. The gas chromatogram of the phthalate standard mixture displayed distinct retention times for each phthalate, with no peak overlaps (Fig. 2C). Retention times and m/z ratios of the phthalates are listed in ESI Table 2.† The recovery percentages of phthalates from the optimized SPE-GC-MS method ranged between 83 and 95% with ethyl acetate as solvent. After SPE extraction of phthalates from water samples obtained from the Palm Island, identification of phthalates in water samples was confirmed by GC-MS, where the two phthalates DEP and DEHP were detected in all samples with concentrations between 7.12 and 10.25 μg L−1 for DEP and between 38.47 and 56.12 μg L−1 for DEHP (Fig. 2D and Table 2). ESI Fig. 3† represents calibration curves with concentration-dependent variation in the intensity of the chromatogram peaks of the 13 investigated phthalates.
The comparison of the two emerging contaminants between surface water and sediments in the study area reveals significant differences in concentration levels, underscoring the distinct roles of these two environmental compartments in pollutant distribution and accumulation. In surface water, heavy metal concentrations, including chromium (13.58–19.28 μg L−1), arsenic (2.05–5.04 μg L−1), cadmium (1.27–3.04 μg L−1), and lead (0.92–2.88 μg L−1), generally fall within regulatory limits (e.g., EPA and WHO guidelines). These values reflect the dynamic nature of water, where pollutants are diluted and dispersed due to hydrodynamics. In contrast, sediments exhibit significantly higher concentrations of heavy metals, with chromium ranging from 8.27–25.71 mg kg−1, arsenic from 3.11–8.67 mg kg−1, cadmium from 3.17–4.81 mg kg−1, and lead from 1.15–3.08 mg kg−1. This indicates that sediments act as long-term reservoirs, binding metals to particulate matter and accumulating pollutants over time, suggesting persistent contamination and potential historical pollution sources. Similarly, phthalates, such as DEP (7.12–10.25 μg L−1) and DEHP (38.47–56.12 μg L−1), were detected in surface water at levels near or slightly exceeding global standards (<10 μg L−1 for DEP and <50 μg L−1 for DEHP), suggesting active contamination from industrial discharges and plastic waste. While phthalates were not measured in sediments, the accumulation patterns of heavy metals suggest that hydrophobic contaminants like phthalates likely follow similar trends, emphasizing the need for further analysis of their sedimentary concentrations to better understand long-term environmental risks. These findings highlight the importance of monitoring both surface water and sediments to comprehensively assess pollution dynamics and guide effective management strategies.
Next, we analyzed the correlation coefficients between the various physicochemical parameters, heavy metals, and phthalates measured in water samples. Positive correlations indicate a direct relationship between variables, while negative correlations suggest an inverse relationship, providing insights into potential shared sources, environmental dynamics, and contamination patterns. The analysis of correlations among water quality parameters reveals significant interactions between various pollutants and water samples, providing insights into their behavior across the sampled sites. For instance, higher conductivity is associated with increased chromium concentration, suggesting that both may originate from shared sources or environmental conditions, such as industrial discharges or mineral-rich runoff. Elevated lead levels are found alongside higher dissolved oxygen concentrations, indicating site-specific chemical dynamics that might influence metal solubility or mobility in oxygen-rich environments. Also, the very high correlation between cadmium and chromium implies that these heavy metals likely stem from similar sources, such contamination events. Equally important, higher levels of DEHP are detected in water with lower conductivity, indicating that organic and inorganic pollutants may originate from different sources or environmental conditions. This inverse relationship suggests that areas with higher DEHP contamination tend to have lower lead levels and vice versa, possibly reflecting distinct contamination profiles at the sites. On the other hand, a negative correlation between pH and conductivity suggests that water samples with higher pH values tend to have lower ionic strength or salinity, reflecting differences in the underlying water chemistry. Arsenic levels, for instance, show a moderate correlation with DEP suggesting potential shared sources of pollution. The strong correlation between lead and chromium suggests similar sources or processes driving their presence, reinforcing the idea of shared contamination pathways. Higher levels of DEHP correspond with lower dissolved oxygen concentrations, indicating that organic pollutants may impact oxygen dynamics, potentially through increased microbial activity or chemical oxygen demand. Finally, the strong correlations among chromium, cadmium, and lead indicate that these metals are likely introduced from common sources, such as industrial pollution or agricultural runoff. While DEHP and DEP show negative correlations with heavy metals and conductivity, suggesting that different contamination sources or environmental conditions influence the distribution of organic and inorganic pollutants. The negative relationship between pH and conductivity reflects variations in the water's chemical composition across sites. The correlation between conductivity and heavy metals further suggests that contamination affects the ionic balance of the water. These correlations provide a clearer understanding of the complex interactions among pollutants and water chemistry across the sampling sites. They highlight the importance of considering both organic and inorganic contamination sources to develop effective monitoring and remediation strategies.
Thus the findings of this study indicate significant levels of heavy metals and phthalates in the water and sediments of the Palm Islands Natural Reserve, emphasizing the severe pollution issues in this region. The slightly alkaline pH and stable marine conditions observed in the macro parameters of the water samples are consistent with typical marine environments; however, the elevated levels of contaminants highlight ongoing pollution challenges. Meticulously, the presence of heavy metals such as chromium, arsenic, cadmium, and lead in both water and sediment samples raises serious environmental concerns. Cadmium levels in particular exceeded the permissible limits set by both the EPA and NOAA, indicating a potential risk to marine life and human health. For instance, heavy metals like mercury and lead can bioaccumulate in marine organisms leading to neurological damage and reproductive failure while phthalates cause developmental abnormalities. For humans, consuming contaminated seafood can result in mercury poisoning, which affects the nervous system, causing cognitive and motor impairments. Long-term exposure to phthalates through the food chain trigger hormone imbalances, fertility issues, and an increased risk of cancers.
The detection of DEP and DEHP in all water samples confirms the pervasive nature of plastic pollution and its derivatives in the marine environment. The presence of these phthalates poses additional risks to the biodiversity of the Palm Islands Natural Reserve. Notably, the differences observed among the sampling stations in our study can be attributed to several environmental and anthropogenic factors. These variations reflect the influence of both natural and human-induced processes, which vary depending on the specific characteristics of each location. For instance, urban runoff from industrial activities, sewage discharge, and municipal waste carry a wide range of pollutants, including heavy metals and phthalates, into coastal waters and sediments on the shores of Tripoli and reaching the protected palm islands. Agricultural runoff from nearby farmland adds pesticides, fertilizers, and veterinary antibiotics to the marine environment. In addition, maritime activities, including shipping and fishing, release oil spills, antifouling agents, microplastics, and waste discharge. Solid waste dumping along the coast further exacerbates pollution with plastics and chemical leachates. Additionally, coastal tourism at the preserved islands contributes sunscreen residues, microplastics, and personal care product residues. Together, these sources underline the multifaceted nature of contamination in the study area, necessitating targeted monitoring and mitigation strategies.
Sea currents play a significant role in the dispersion of these contaminants that reach the reserve. Stations located downstream of pollutant sources are more likely to exhibit higher contaminant levels. Additionally, hydrodynamic conditions such as tides, waves, and currents can influence the distribution of both heavy metals and phthalates by either dispersing or concentrating them in certain areas. For example, areas with slower-moving waters may experience greater accumulation of contaminants in sediments.
The toxicological effects of heavy metals and phthalates on marine organisms are well-documented, including disruptions to reproductive systems, growth inhibition, and increased mortality rates. The high levels of cadmium and phthalates detected in this study suggest that the marine ecosystem around the Palm Islands is under significant stress, which could have cascading effects on the broader biodiversity of the region. The endangered species and unique flora and fauna of the reserve are particularly vulnerable to such pollutants, potentially leading to long-term ecological imbalances. The interconnected nature of the pollutants underscores the need for an inclusive strategy to tackle the pollution crisis. This involves not only enhancing waste management infrastructure and enforcing stricter regulations on industrial discharges but also conducting continuous monitoring and research to track the levels and effects of emerging contaminants. Public awareness and community engagement are also crucial in driving collective action towards preserving the marine environment.
To further integrate existing literature and place our findings in a broader context, we compared the observed heavy metal and phthalate contamination levels in our study to similar studies from other regions, which have also reported concerning levels of these contaminants in both water and sediment. For example, studies conducted in coastal areas of the Mediterranean Sea and other heavily industrialized regions such as the Gulf of Mexico and the East China Sea has consistently found elevated levels of cadmium and phthalates, suggesting widespread anthropogenic impact on marine ecosystems.36–38 In comparison to these studies, the cadmium and phthalate concentrations in our sampling stations also exceed regulatory guidelines, which emphasizes the global scale of this environmental issue and the critical need for stronger measures to control industrial discharge and plastic pollution. The environmental impact of various pollutants and heavy metals across different settings underscores the urgent need for comprehensive monitoring and remediation efforts. Concurrently, the bioaccumulation of heavy metals in animal species highlights the pervasive nature of environmental contamination and potentially affecting entire ecosystems.39 Moreover, the identification of toxic metals in food products,40 including recent findings of heavy metal contamination in thyme products,41 underscores the importance of stringent food safety measures. Equally important, studies investigating cadmium uptake in native plants provide crucial insights into the mechanisms of metal absorption in terrestrial environments,42 emphasizing the necessity for proactive measures to mitigate environmental pollution and safeguard human health. In addition to heavy metals and microplastics, the presence of other emerging contaminants in water bodies and sediments, notably Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs), poses significant environmental and public health concerns.43 These studies collectively underscore the need for vigilant environmental monitoring and food safety measures to mitigate potential health hazards.
Plastic pollution as well as domestic waste pollution crisis has gained such proportions that it is chocking Lebanon as well as significantly polluting the Mediterranean Sea. Mountains of garbage ends up in the sea since the overflowing trash has already grown to enormous proportions in the second largest city in Lebanon, Tripoli, through land reclamation and disposing it off in the sea. The city is engulfed with dissatisfaction with garbage disposal, inappropriate industrial waste treatment, alarming levels of surface and sea water pollution. More importantly, the implications of this study extend beyond Tripoli to the entire country of Lebanon and the broader MENA region, thus highlighting the need for regional cooperation and comprehensive policies to address marine pollution. Finally, the pollution crisis not only threatens the ecological health of the Mediterranean but also has significant implications for tourism in Lebanon and the MENA region. The natural beauty of the Palm Islands Natural Reserve and other coastal areas in Lebanon are key attractions for tourists. However, the deteriorating water quality and environmental degradation can deter visitors, impacting the tourism industry which is vital for the economy. Addressing these environmental issues is crucial for sustaining tourism and preserving the natural heritage that attracts visitors to Lebanon and the region.44
Conclusion
This study provides critical insights into the pollution dynamics of the Palm Islands Natural Reserve, revealing significant contamination by heavy metals and phthalates in both water and sediment samples. Elevated levels of heavy metals, particularly chromium, arsenic, cadmium, and lead, were found in sediments, indicating persistent accumulation, with cadmium exceeding regulatory limits and posing risks to marine life and human health. Phthalates, including DEP and DEHP, were detected in all water samples, highlighting the widespread issue of plastic pollution and its potential long-term impact on biodiversity. These findings reflect ongoing pollution challenges driven by industrial discharges, agricultural runoff, maritime activities, and waste dumping, underscoring the need for comprehensive monitoring and targeted mitigation strategies. Correlation analysis revealed complex interactions between pollutants, emphasizing shared sources and environmental dynamics shaping contamination patterns. The study highlights the urgent need for stronger pollution controls, enhanced waste management, and ongoing monitoring, particularly given the Palm Islands' ecological significance as a habitat for endangered species. It calls for regional cooperation to combat marine pollution, with broader implications for the Mediterranean and MENA regions, and stresses the importance of preserving Lebanon's vital tourism sector. The research contributes valuable data for environmental monitoring and highlights the necessity of collective action to protect marine ecosystems, safeguard public health, and mitigate the risks posed by these emerging contaminants.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
E. Akoury: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; roles/writing – original draft; and writing – review & editing. B. Nehmeh and F. Haydous: data curation; formal analysis; investigation; and writing – review & editing. H. Ali, A. Hdaifi, Z. Abrahamian, B. Abdlwahab, M. Bou Orm: data curation; formal analysis; and writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
E. Akoury was funded by the US-Middle East Partnership Initiative (MEPI) Tomorrow's Leaders Program. This study is made possible by the generous support of the American people through the United States Department of State. The contents are the responsibility of E. Akoury and do not necessarily reflect the views of the Department of State or the United States Government.References
- I. Kurtul, The critical need for long-term biomonitoring: The case study of a major river system in Anatolia, Environ. Monit. Assess., 2024, 196, 1145 CrossRef PubMed.
- R. Bargagli and E. Rota, Microplastic Interactions and Possible Combined Biological Effects in Antarctic Marine Ecosystems, Animals, 2023, 13(1), 162 CrossRef PubMed.
- A. A. Horton and D. K. A. Barnes, Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems, Sci. Total Environ., 2020, 738, 140349 CrossRef CAS PubMed.
- K. Jomova, S. Y. Alomar, E. Nepovimova, K. Kuca and M. Valko, Heavy metals: toxicity and human health effects, Arch. Toxicol., 2024, 99, 153–209 CrossRef PubMed.
- M. Saleem, D. Pierce, Y. Wang, D. A. Sens, S. Somji and S. H. Garrett, Heavy Metal(oid)s Contamination and Potential Ecological Risk Assessment in Agricultural Soils, J. Xenobiot., 2024, 14, 634–650 CrossRef CAS PubMed.
- M. Kasper-Sonnenberg, C. Palmke, S. Wrobel, T. Bruning, A. Murawski, P. Apel, T. Weber, M. Kolossa-Gehring and H. M. Koch, Plasticizer exposure in Germany from 1988 to 2022: Human biomonitoring data of 20 plasticizers from the German Environmental Specimen Bank, Environ. Int., 2024, 195, 109190 CrossRef.
- A. Maia and M. A. Vieira-Coelho, The impact of exposure to phthalates in thyroid function of children and adolescents: a systematic review, Eur. J. Pediatr., 2024, 184, 111 CrossRef.
- United Nations Environment Programme (UNEP), The Mediterranean Environment and the Comprehensive Report on the Mediterranean and Its Pollution, United Nations Environment Programme, Nairobi, Kenya, 1992, https://www.unep.org/nairobi-convention Search PubMed.
- C. Breen, C. El Safadi, H. Huigens, S. Tews, K. Westley, G. Andreou, R. O. Vazquez, J. Nikolaus and L. Blue, Integrating cultural and natural heritage approaches to Marine Protected Areas in the MENA region, Mar. Pol., 2021, 132, 104676 CrossRef.
- H. Zahreddine, C. Clubbe, R. Baalbaki, A. Ghalayini and S. N. Talhouk, Status of native species in threatened Mediterranean habitats: the case of Pancratium maritimum L. (sea daffodil) in Lebanon, Biol. Conserv., 2004, 120, 11–18 CrossRef.
- M. Kasparek, The Mediterranean Coast of Lebanon: Habitat for Endangered Fauna and Flora: Results of a Coastal Survey in 2004, 2004 Search PubMed.
- N. Jisr, G. Younes, K. El Omari, M. Hamze, C. Sukhn and M. H. El-Dakdouki, Levels of heavy metals, total petroleum hydrocarbons, and microbial load in commercially valuable fish from the marine area of Tripoli, Lebanon, Environ. Monit. Assess., 2020, 192, 705 CrossRef CAS PubMed.
- P. J. Landrigan, H. Raps, M. Cropper, C. Bald, M. Brunner, E. M. Canonizado, D. Charles, T. C. Chiles, M. J. Donohue, J. Enck, P. Fenichel, L. E. Fleming, C. Ferrier-Pages, R. Fordham, A. Gozt, C. Griffin, M. E. Hahn, B. Haryanto, R. Hixson, H. Ianelli, B. D. James, P. Kumar, A. Laborde, K. L. Law, K. Martin, J. Mu, Y. Mulders, A. Mustapha, J. Niu, S. Pahl, Y. Park, M. L. Pedrotti, J. A. Pitt, M. Ruchirawat, B. J. Seewoo, M. Spring, J. J. Stegeman, W. Suk, C. Symeonides, H. Takada, R. C. Thompson, A. Vicini, Z. Wang, E. Whitman, D. Wirth, M. Wolff, A. K. Yousuf and S. Dunlop, The Minderoo-Monaco Commission on Plastics and Human Health, Annals of Global Health, 2023, 89, 23 CrossRef PubMed.
- J. Lu, J. Wu, J. Wu, C. Zhang and Y. Luo, Adsorption and Desorption of Steroid Hormones by Microplastics in Seawater, Bull. Environ. Contam. Toxicol., 2021, 107, 730–735 CrossRef CAS PubMed.
- M. Wang, Y. Wu, G. Li, Y. Xiong, Y. Zhang and M. Zhang, The hidden threat: Unraveling the impact of microplastics on reproductive health, Sci. Total Environ., 2024, 935, 173177 CrossRef CAS PubMed.
- E. Jewett, G. Arnott, L. Connolly, N. Vasudevan and E. Kevei, Microplastics and Their Impact on Reproduction-Can we Learn From the C. elegans Model?, Frontiers in Toxicology, 2022, 4, 748912 CrossRef PubMed.
- S. Deudero and C. Alomar, Mediterranean marine biodiversity under threat: Reviewing influence of marine litter on species, Mar. Pollut. Bull., 2015, 98, 58–68 CrossRef CAS PubMed.
- M. Kazour, S. Terki, K. Rabhi, S. Jemaa, G. Khalaf and R. Amara, Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill, Mar. Pollut. Bull., 2019, 146, 608–618 CrossRef CAS PubMed.
- D. Merhaby, B. Ouddane, S. Net and J. Halwani, Assessment of trace metals contamination in surficial sediments along Lebanese Coastal Zone, Mar. Pollut. Bull., 2018, 133, 881–890 CrossRef CAS.
- A. Cózar, Plastic accumulation in the Mediterranean Sea, PLoS One, 2015, 10, e0121762 CrossRef.
- M. J. Stapleton and F. I. Hai, Microplastics as an emerging contaminant of concern to our environment: a brief overview of the sources and implications, Bioengineered, 2023, 14, 2244754 CrossRef PubMed.
- P. Pastorino and D. Barcelo, Microplastics and their environmental effects, Environ. Toxicol. Pharmacol., 2023, 104, 104324 CrossRef CAS PubMed.
- A. Paluselli, Y. Aminot, F. Galgani, S. Net and R. Sempere, Occurrence of phthalate acid esters (PAEs) in the northwestern Mediterranean Sea and the Rhone River, Prog. Oceanogr., 2018, 163, 221–231 CrossRef.
- W. H. Organization, Guidelines for Drinking-Water Quality, 4th edn, 2017 Search PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, 23rd edn, 2017 Search PubMed.
- EPA, Dissolved Oxygen and Biochemical Oxygen Demand, Environmental Protection Agency, 2003 Search PubMed.
- X. Wu, S. Spencer, S. Gushgari-Doyle, M. O. Yee, J. Voriskova, Y. Li, E. J. Alm and R. Chakraborty, Culturing of “Unculturable” Subsurface Microbes: Natural Organic Carbon Source Fuels the Growth of Diverse and Distinct Bacteria From Groundwater, Front. Microbiol., 2020, 11, 610001 CrossRef PubMed.
- EPA, Drinking Water Health Advisory for Chromium, Environmental Protection Agency, 2016 Search PubMed.
- WHO, Arsenic in Drinking Water, World Health Organization, 2018 Search PubMed.
- U.S. Environmental Protection Agency, Ambient Water Quality Criteria for Cadmium, EPA 440/5-80-025, 1980 Search PubMed.
- CDC, Lead in Drinking Water, Centers for Disease Control and Prevention, 2019 Search PubMed.
- E. C. Agency, Bis(2-ethylhexyl) Phthalate (DEHP) – Substance Information, 2021 Search PubMed.
- U.S. Environmental Protection Agency, Quality Criteria for Water 1986 (“Gold Book”), EPA 440/5-86-001, 1986 Search PubMed.
- U.S. Environmental Protection Agency, National Recommended Water Quality Criteria: 2002, EPA-822-R-02-047, 2002 Search PubMed.
- M. F. Buchman, NOAA Screening Quick Reference Tables, NOAA OR&R Report 08-1, Office of Response and Restoration Division, National Oceanic and Atmospheric Administration, Seattle, WA, 2008 Search PubMed.
- P. A. R. Ardila, R. Alonso, J. J. D. Valsero, R. M. García, F. Cabrera, E. L. Cosío and S. D. Laforet, Assessment of heavy metal pollution in marine sediments from southwest of Mallorca island, Spain, Environ. Sci. Pollut. Res. Int., 2023, 30, 16852–16866 CrossRef CAS PubMed.
- Y. Cao, J. Li, R. Wu, H. Lin, J.-Y. Lao, Y. Ruan, K. Zhang, J. Wu, K. M. Y. Leung and P. K. S. Lam, Phthalate esters in seawater and sediment of the northern South China Sea: Occurrence, distribution, and ecological risks, Sci. Total Environ., 2022, 811, 151412 CrossRef CAS PubMed.
- L. Mi, Z. Xie, Z. Zhao, M. Zhong, W. Mi, R. Ebinghaus and J. Tang, Occurrence and spatial distribution of phthalate esters in sediments of the Bohai and Yellow seas, Sci. Total Environ., 2019, 653, 792–800 CrossRef CAS.
- J. Al-Alam, M. Millet, M. Harb, E. Akoury, S. Tokajian and M. Wazne, Field evaluation of metal bioaccumulation in the gastropod Helix aspersa at agricultural and industrial sites in Lebanon, Environ. Monit. Assess., 2022, 195, 197 CrossRef PubMed.
- E. Akoury, N. Mansour, G. A. Reda, H. Dimassi, L. Karam, N. Alwan and H. F. Hassan, Toxic metals in packed rice: Effects of size, type, origin, packing season, and storage duration, J. Food Compos. Anal., 2023, 115, 104920 CrossRef CAS.
- E. Akoury, C. Baroud, S. El Kantar, H. Hassan and L. Karam, Determination of heavy metals contamination in thyme products by inductively coupled plasma mass spectrometry, Toxicol Rep, 2022, 9, 1962–1967 CrossRef CAS PubMed.
- E. Akoury, S. El Kantar, H. Abdallah, D. Al Timani and Z. Daher, Evaluation of cadmium uptake and consumption of parsley in Lebanese diet, Int. J. Environ. Sci. Technol., 2023, 20, 6079–6090 CrossRef CAS.
- J. Al-Alam, M. Millet, D. Khoury, A. Rodrigues, E. Akoury, S. Tokajian and M. Wazne, Biomonitoring of PAHs and PCBs in industrial, suburban, and rural areas using snails as sentinel organisms, Environ. Sci. Pollut. Res. Int., 2024, 31, 4970–4984 CrossRef CAS PubMed.
- C. Steiner, From heritage to hyper-reality? Tourism destination development in the Middle East between Petra and the Palm, J. Tourism Cult. Change, 2010, 8, 240–253 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra09017a |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |