Open Access Article
Open Access ArticleFe3O4@SiO2–BD–DADB-COF is proposed as a novel magnetic covalent organic framework for the determination and extraction of 15 macrolide antibiotics in water and honey†
Hao Zhanga,
Weihao Mabc,
Chunyu Qianga,
Jiayuan Niebc,
Ling Ma*bc,
Yawei Zhang*bc and
Ke Wang *abc
*abc
aCollege of Chemistry and Materials Science, Hebei Normal University, Shijiazhuang 050023, China. E-mail: wkecdc@163.com
bShijiazhuang Center for Disease Control and Prevention, Shijiazhuang 050011, China. E-mail: mamalin001@163.com
cShijiazhuang Technology Innovation Center for Chemical Poison Detection and Risk Early Warning, Shijiazhuang 050011, China. E-mail: 2211291167@qq.com
First published on 17th March 2025
Abstract
In our research, an emerging magnetic covalent organic framework (Fe3O4@SiO2–BD–DADB-COF) was formulated through Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), etc. Several parameters affecting the extraction process were refined. Accordingly, a novel method of determining 15 macrolides (MALs) in honey and water was established through the Fe3O4@SiO2–BD–DADB-COF as a magnetic solid-phase extraction (MSPE) adsorbent and ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). In consequence, the standard curves for the 15 MALs exhibited an exceptional linearity from 0.1 to 200 μg L−1, and the correlation coefficients (R2) varied from 0.9990 to 0.9999. The recoveries fell between 70.01% and 115.56%, with the relative standard deviations (RSDs) being below 9.93% (n = 5). The detection limits reached 0.001–0.075 μg L−1 with the quantification limits being 0.004–0.228 μg L−1. Ultimately, the method was excellently applied to the analysis of MALs in honey and water.
1. Introduction
Macrolides (MALs) are, actually, a category of antibiotics produced by Streptomyces bacteria. MALs are characterized by a big lactone ring (12–16 carbon atoms) attached to sugars via glycosidic bonds, making them effective in combating various infections.1 Gram-positive and Gram-negative bacteria are two common classes of bacteria. Gram-positive bacteria often cause diseases such as skin infections, respiratory tract infections and endocarditis, while Gram-negative bacteria may cause urinary system infections, pneumonia, septicemia and gastrointestinal infections. These bacterial infections, if not treated, can cause serious complications and can even be life-threatening. Fortunately, MALs have been widely used to treat infections caused by Gram-positive and Gram-negative bacteria, providing effective options for clinical treatment.2 Alarmingly, the overuse and inappropriate use of MALs are still common. In addition, humans and animals cannot fully absorb MALs, with 30% to 90% being excreted in their original form through feces and urine. The rate at which MALs are introduced into the environment exceeds their degradation rate.3–5 When MALs enter the environment, they not only affect the microbial population of water bodies but also induce the generation of drug-resistant bacterial strains. Hence, the threat to the environment rises. Correspondingly, MALs are capable of entering the human body via the food chain, resulting in side impacts, like ototoxicity, gastrointestinal issues, nephrotoxicity, and cochlear nerve damage.6,7 According to the severity of the above situation, some organizations and nations have formulated the maximum residue limits (MRLs) for MALs among some foods. For example, Japan has set the MRLs of tylosin and tilmicosin in milk at 50 μg kg−1,8 the European Union has set the MRLs of tilmicosin in sheep meat at 50 μg kg−1.9 As specified by the GB 31650.1-2022, China's National Food Safety Standard, the MRLs for kitasamycin, lincomycin, and tilmicosin from chickens reach 100, 75, and 200 μg kg−1 separately, whereas those for MALs in other foods and water (e.g., honey) are reported seldomly. With respect to food security supervision and risk evaluation, it is crucial to develop the approaches to investigating the MAL residues in food and water.Currently, several methods have been developed for detecting MAL residues. These methods include gas chromatography (GC),10 high-performance liquid chromatography (HPLC),11 capillary electrophoresis (CE),12 and liquid chromatography-tandem mass spectrometry (LC-MS/MS).13,14 As a high-sensitivity and high-selectivity separation and analysis method, LC-MS/MS is supported by scholars and researchers. As a vital factor in analytic method, sample pretreatment matters equally in safeguarding the credibility and precision of experimental data.15,16 To guarantee the precision of LC-MS/MS analyses and overall quality, it is important to value and enhance sample pretreatment technologies.17
The most commonly employed pretreatment techniques for MALs include accelerated solvent extraction (ASE),18 liquid–liquid extraction (LLE),19 solid-phase extraction (SPE),20 QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe)21 and magnetic solid-phase extraction (MSPE).22 Wherein, MSPE is outstanding for a pathbreaking innovation in the realm of separation and enrichment in the 21st century. The most absorbing strength of MSPE becomes the swift and effective analyte extraction through an external magnetic field while highly reducing the pretreatment duration of samples, relative to conventional SPE processes.23 Due to the fact that magnetic materials are gauged by adsorbents and magnetic nanoparticles, developing such novel substances in MSPE becomes a research focus.
The magnetic materials are composed of magnetic nanoparticles and functional adsorbent materials, with the adsorbent typically coated around the surface of the magnetic nanoparticles. Due to their excellent magnetic properties and good dispersion, Fe3O4 nanoparticles are commonly used as the magnetic core for the preparation of magnetic materials.24 Furthermore, the adsorbent materials commonly employed include metal–organic frameworks (MOFs),25 porous organic polymers (POPs),26 molecularly imprinted polymers (MIPs),27 and covalent organic frameworks (COFs).28 COFs are outstanding for a category of crystalline porous material relative to other adsorbent materials and draw remarkable attention of researchers owing to their adjustable pore size, exceptional physicochemical stability, and high specific surface region.29 Wherein, the pore size of COFs substances is capable of being regulated in numerous ways, like choosing discrepant categories of surfactants, integrating pore-expanding agents, etc. (e.g. reaction time, reaction temperature, pH of the reaction solution).30 To sum up, these fascinating properties enable the use of COFs in many fields, encompassing material adsorption,31 gas storage,32 sensors,33 and photochemical and heterogeneous catalysis.34 However, the low density of COFs poses challenges for their separation from solutions, thereby limiting their application in the processing of real samples. The combination of COFs and Fe3O4 perfectly compensates for this shortcoming, resulting in magnetic COFs that not only possess a high adsorption capacity but also facilitate separation, which resolves the challenges associated with COF recycling, thereby increasing the convenience and efficiency of the operational process.24 Although magnetic COF materials for the extraction of antibiotics have been developed, few reports are related to magnetic COF materials for the detection of MALs.35 Currently, the materials for detecting MALs are mainly focused on MOFs and MIPs.7,36 They have good chemical stability, but the synthesis steps are complicated, and the number of MALs is less than 7. Therefore, the development of magnetic COF materials is very important for the detection of multiple MALs simultaneously.
In this study, we designed and synthesized a novel Fe3O4@SiO2–BD–DADB-COF on the basis of the structure of MALs via a simple reaction of Fe3O4@SiO2, 2,2′-bipyridine-5,5′-dicarboxaldehyde, and 1,3,5-tris(4-aminophenyl) benzene. Importantly, the pore volume and size of this Fe3O4@SiO2–BD–DADB-COF were adjusted by the addition of polyethylene–polypropylene glycol (P-123). Subsequently, the synthesized Fe3O4@SiO2–BD–DADB-COF material was further characterized. Actually, our research explored the desorption condition and extraction, encompassing the quantity of adsorbents, the volume of eluent, the extraction time, etc. At last, a novel approach was formulated based on MSPE integrated with UPLC-MS/MS. This was confirmed and utilized to construe 15 MALs in honey and water.
2. Experimental
2.1. Reagents and materials
Tianjin Yongda Chemical Reagent Co., Ltd. (China) provided sodium chloride (NaCl), anhydrous ethanol, ferric chloride hexahydrate (FeCl3·6H2O), ethylene glycol (EG), sodium acetate trihydrate (CH3COONa·3H2O), 1,4-dioxane, and acetic acid (HAc). Xiya Chemical Technology Co. Ltd (Shandong, China) supplied PEG-4000 polyethylene glycol, whereas Tianjin Kaitong Chemical Reagent Co. Ltd (China) provided a 25% (w/w) ammonia solution. Dikma supplied formic acid and tetraethoxysilane (TEOS). Polyethylene–polypropylene glycol (P-123) was purchased from Sigma-Aldrich. In addition to 2,2′-bipyridine-5,5′-dicarboxaldehyde, sodium chloride (NaCl) was offered by Aladdin Chemistry Co., Ltd. (Shanghai, China). Methanol (MeOH) was offered by Thermo Fisher Co., Ltd. (USA) and acetonitrile (ACN) was supplied by Merck (Darmstadt, Germany). Through a Millipore Milli-Q Gradient Water Purification System (USA), acetic acid, acetonitrile, and acetonitrile were of HPLC grade and all other reagents were of analytical grade, with ultra-pure water formulated and utilized throughout the experiment.Ivermectin, tylosin tartrate, selamectin, tilmicosin, eprinomectin, avermectin, kitasamycin, azithromycin, doramectin, emamectin, moximycin tylosin and tylosin were obtained from Dr Ehrenstorfer GmbH. Josamycin, midecamycin and tylvalosin were obtained from MedChemExpress. The chemical structures of the 15 MALs are shown in Table S1.† Table S2† lists the purities and CAS registry numbers of the 15 MALs. Including 1 μg mL−1 of each of the 15 MALs, a blended stock standard solution were prepared in ACN and preserved in darkness at −20 °C.
2.2. Instrumental and analytical conditions
A Phenomenex Kinetex F5 column with a 100 Å pore size, dimensions of 3.0 mm × 100 mm, and a 2.6 particle in diameter, was employed to separate the MALs. With a mobile phase of 0.1% formic acid in water (phase A) and 3.0 mL of acetonitrile (phase B), a flow velocity of 0.3 mL min−1 was achieved. The process of the gradient elution was listed below: (1) 0–2.5 min, 45% B (v/v); (2) 2.5–2.6 min, 45–55% B (v/v); (3) 2.6–7.5 min, 55% B (v/v); (4) 7.5–7.6 min, 55–75% B (v/v); (5) 7.6–10.5 min, 75% B (v/v); (6) 10.5–10.6 min, 75–45% B (v/v); (7) 10.6–14 min, 45% B (v/v). Fig. S1† describes the chromatograms of 15 MALs.Detection and analysis were implemented using a UPLC-MS/MS instrument (Exion TRILPLE QUAD 5500, AB SCIEX, USA) and electrospray ionization (ESI) was applied to the positive ion mode, with the data gathered in multiple reaction monitoring (MRM) pattern. The mass spectrometry details for 15 MALs are provided in Table S3.†
The prepared materials were characterized through Fourier transform infrared (FT-IR) (Thermo Nicolet Co. Ltd., USA), Brunauer–Emmett–Teller (BET) method (Quantachrome Instruments, USA), a SQUID XL-7 vibrating sample magnetometer (Quantum Design, USA), a D8A X-ray diffractometer (Germany), and scanning electron microscopy (SEM) with an S-4800 instrument (Japan), as well as transmission electron microscopy (TEM) with an FEI Tecnai G2 F20 (USA).
2.3. Preparation of Fe3O4@SiO2–BD–DADB-COF
2.4. Sample preparation
In Shijiazhuang, Hebei Province, twenty surface water samples were gathered from discrepant rivers, Hebei Province. A filter membrane of 0.45 μm was employed to filter the total water samples. Then, such samples were preserved for MSPE within a refrigerator at 4 °C.Fifteen honey samples were offered by different supermarkets. Prior to MSPE analysis, 1 g honey sample was delivered to a polypropylene tube of 50 mL in volume. The sample was diluted using 1 mL of ultrapure water. 12 mL acetonitrile was incorporated into the mixture. Next, such solution was sensitive to ultrasonication for 2 min. Subsequently, the tube was centrifuged at 8000 rpm for 5 min. The extract (6 mL) was dehydrated at 35 °C under flowing nitrogen. The residue was lysed in 10 mL ultrapure water again. Afterwards, the mix was vortexed and sonicated. The pH of the mix sample was modulated to 8.
2.5. Procedure of MSPE
Fig. 1 displays the MSPE process. Firstly, 10 mg Fe3O4@SiO2–BD–DADB-COF was added to a 10 mL of sample mixture that was then shaken for 10 min for adsorption. Secondly, an external magnetic field was used to gather the sorbent, with the supernatant abandoned. Thirdly, 8 mL 0.3% ammoniated ACN was supplemented to the sorbent collected, which was then vortexed for 10 min to elute the MALs. Fourthly, the eluent was isolated with a magnet and dehydrated at 35 °C under N2. Ultimately, the residues were lysed through a 1.0 mL mobile phase again and then filtrated via a 0.20 μm membrane. Such residues were injected into the UPLC-MS/MS mechanism for our analysis.3. Results and discussions
3.1. Characterization of the Fe3O4@SiO2–BD–DADB-COF
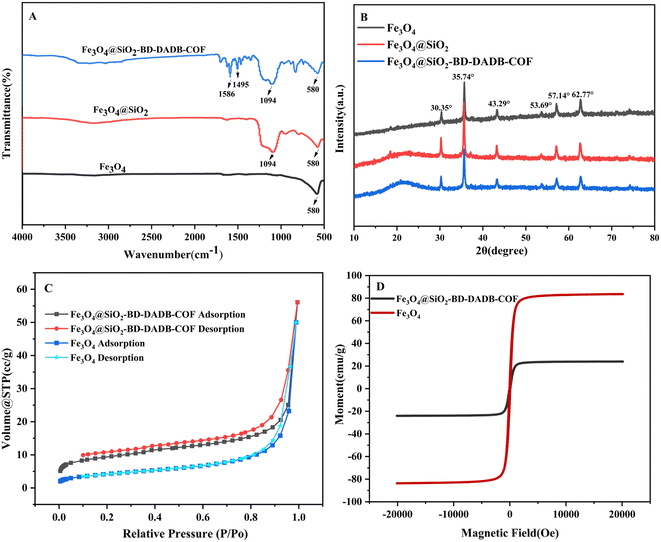
The forms of the prepared Fe3O4@SiO2–BD–DADB-COF and Fe3O4 materials were characterized via TEM and SEM. Fig. 2A exhibits that the Fe3O4 nanoparticles are nearly spherical and well dispersed, whereas the SEM image of Fe3O4@SiO2–BD–DADB-COF (Fig. 2B) reveals a relatively rough surface. Further, as described Fig. 2D, the TEM images exhibits the typical core–shell composition of Fe3O4@SiO2–BD–DADB-COF, implying that the COF was stained on the surface of Fe3O4 nanoparticles. | ||
| Fig. 2 The SEM of (A) Fe3O4, (B) Fe3O4@SiO2–BD–DADB-COF; the TEM of (C) Fe3O4, (D) Fe3O4@SiO2–BD–DADB-COF. | ||
Moreover, energy dispersive X-ray (EDS) analysis was conducted, in an attempt to validate the elemental composition of the Fe3O4@SiO2–BD–DADB-COF material. Fig. S2† shows the observed distributions of C, N, O, Si, and Fe, with contents of 63.91%, 8.94%, 17.35%, 1.94% and 7.86%, separately. Specifically, the atomic content of N was 8.94% in Fe3O4@SiO2–BD–DADB-COF, suggesting the excellent synthesis of the COF layer on the Fe3O4 surface.
Further, Fig. 3A shows the FT-IR spectra of Fe3O4@SiO2–BD–DADB-COF, Fe3O4 and Fe3O4@SiO2. Within the spectra, the absorption peaks at 580 cm−1 and 1094 cm−1 was consistent with the oscillation of the Fe–O–Fe and Si–O–Si bonds, separately. Consequently, it was validated that the smooth encapsulation of silicon shells emerged on the Fe3O4 surface.40 In addition, the two characteristic peaks at 1586 cm−1 and 1495 cm−1 were ascribed to the stretching vibrations of the C–N and C–C ring bonds, respectively.41 Thereby, BD–DADB-COF was grafted on the Fe3O4@SiO2 surface excellently.
What's more, Fig. 3B exhibited that the crystalline structures of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2–BD–DADB-COF were construed by means of XRD patterns. Fe3O4@SiO2–BD–DADB-COF has characteristic peaks at 30.35°, 35.74°, 43.29°, 53.69°, 57.14°, and 62.77°, aligning with the surface of Fe3O4 crystallographic structure (220), (311), (400), (422), (511), and (440), respectively.42 Furthermore, the Fe3O4@SiO2–BD–DADB-COF exhibited inconspicuous broad characteristic peaks at 23.5°, attributing to π–π stacking.43 The data illustrated that Fe3O4@SiO2–BD–DADB-COF became exceedingly crystalline and still kept high crystallinity after staining.
Fig. 3C reveals that the porous structure was evaluated through N2 adsorption determination at 77 K. Fe3O4@SiO2–BD–DADB-COF showed characteristic type IV isotherms. This illustrated its distinctive mesoporous structure.44 In addition, the BET surface of Fe3O4 reached 7.98 m2 g−1 and came short of the 33.71 m2 g−1 surface of Fe3O4@SiO2–BD–DADB-COF. Due to increased specific surface area of Fe3O4@SiO2–BD–DADB-COF, more adsorption sites were thus accessible to MALs. In consequence, it indicated that Fe3O4@SiO2–BD–DADB-COF became appropriate as an adsorbent of the MSPE.
The properties of Fe3O4 and Fe3O4@SiO2–BD–DADB-COF were measured via a vibrating sample magnetometer (VSM). Fig. 3D displays that the saturation magnetization of Fe3O4 reaches 79.12 emu g−1, while Fe3O4@SiO2–BD–DADB-COF presented a lower value of 37.02 emu g−1. This decrease was ascribed to the formation of the core–shell in Fe3O4@SiO2–BD–DADB-COF.45 Despite the decrease in magnetization, Fe3O4@SiO2–BD–DADB-COF still exhibited a magnetic response that was adequate for practical magnetic separation applications. The Fe3O4@SiO2–BD–DADB-COF can be rapidly separated from the solution within 60 seconds via an external magnetic field.46 As depicted in Fig. S3,† a homogeneous dispersion of Fe3O4@SiO2–BD–DADB-COF in water was obtained after ultrasonication, and could be aggregated together by an external magnetic field.
3.2. Condition of MSPE
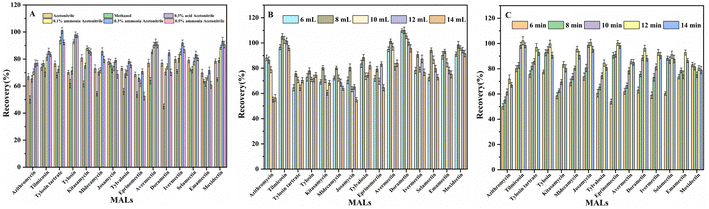
We optimized the adsorbent dosage, the concentration of salts, the pH of samples, eluate type, eluate volume, elution time, and extraction time to improve the MAL extraction efficiency (EE). Such parameters were systemically explored through a single-factor trial.Moreover, the volume of elution solvent (0.3% ammoniated ACN) was analysed. Fig. 5B shows the related results. 8 mL elution solvent was adequate to obtain the excellent desorption efficiencies of the MALs. The recoveries declined mildly with the eluent volume increasing in depth. Therefore, 8 mL was chosen as the elution volume.
Additionally, the desorption time, which was explored within the scope of 6 to 14 min, also impacted the extraction efficiency. The results (Fig. 5C) reveals that the maximal recoveries of MALs were fulfilled at 12 min (71.76–102.13%), no remarkable variations occurred with the prolonged desorption times. As a result, a desorption time of 12 min was chosen for our research.
3.3. Adsorption mechanism
The adsorption systems of Fe3O4@SiO2–BD–DADB-COF towards MALs were investigated in detail. First, the MALs extraction efficiencies of Fe3O4@SiO2 and Fe3O4@SiO2–BD–DADB-COF were compared. Fig. S5† shows that the adsorption of the prepared Fe3O4@SiO2–BD–DADB-COF adsorbent for MALs exceeded that of Fe3O4@SiO2, suggesting that BD–DADB-COF showed outstanding adsorption volume for MALs. Furthermore, The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in MALs could form π–π interactions with the large π-conjugation system in the Fe3O4@SiO2–BD–DADB-COF, thereby contributing to the increase in the adsorption effect.54 The H atoms on the hydroxyl groups in MALs could form hydrogen bonds with the electronegative N atoms in Fe3O4@SiO2–BD–DADB-COF, improving the adsorption efficiency of the material.55
C bonds in MALs could form π–π interactions with the large π-conjugation system in the Fe3O4@SiO2–BD–DADB-COF, thereby contributing to the increase in the adsorption effect.54 The H atoms on the hydroxyl groups in MALs could form hydrogen bonds with the electronegative N atoms in Fe3O4@SiO2–BD–DADB-COF, improving the adsorption efficiency of the material.55
Furthermore, as shown in Fig. S4,† when the pH is 8, the negative charge on the Fe3O4@SiO2–BD–DADB-COF surface led to an increase in the electron cloud density of the material. This further facilitated the formation of hydrogen bonds between the Fe3O4@SiO2–BD–DADB-COF and the H atoms of the hydroxyl groups in MALs. Additionally, through zeta potential analysis, the Fe3O4@SiO2–BD–DADB-COF carries a negative charge under conditions of pH 8. The MALs contain amino structures, These amino groups are prone to protonation. Under neutral to weak alkaline conditions, the positively charged MALs (pKa = 7–9.5) interact with the negatively charged Fe3O4@SiO2–BD–DADB-COF through electrostatic attraction.56 Moreover, the hydrophobicity affected the adsorption efficiency. The hydrophilicity and hydrophobicity were closely related to the magnitude of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value, and the dividing line between hydrophobicity and hydrophilicity was generally considered 1 (greater log
P value, and the dividing line between hydrophobicity and hydrophilicity was generally considered 1 (greater log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values indicated greater hydrophobicity).57 As hydrophobic compounds, MALs are characteristic of a macrocyclic lactone ring (log
P values indicated greater hydrophobicity).57 As hydrophobic compounds, MALs are characteristic of a macrocyclic lactone ring (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P > 1),58,59 with the Fe3O4@SiO2–BD–DADB-COF surface encompassing numerous benzene rings. This contributes to adsorbing the MALs via hydrophobic effects.60 In consequence, the dominant adsorption systems of MALs and Fe3O4@SiO2–BD–DADB-COF were regarded as π–π interactions, hydrophobic interactions, etc.
P > 1),58,59 with the Fe3O4@SiO2–BD–DADB-COF surface encompassing numerous benzene rings. This contributes to adsorbing the MALs via hydrophobic effects.60 In consequence, the dominant adsorption systems of MALs and Fe3O4@SiO2–BD–DADB-COF were regarded as π–π interactions, hydrophobic interactions, etc.
Furthermore, the specific surface area of Fe3O4@SiO2–BD–DADB-COF without the addition of P-123 in the synthesis of magnetic COF materials was 12.46 m2 g−1. However, a larger specific surface area (33.71 m2 g−1) was obtained by adding P-123 in the synthesis, which could offer more contact sites while improving the adsorption volume of the magnetic COF materials. Fig. S6† displays that when the Fe3O4@SiO2–BD–DADB-COF was prepared, adding P-123 as a pore-expanding agent enhanced recoveries of MALs, increasing them from 48.31–94.34% to 78.23–109.32%. The results indicated that the addition of P-123 during the synthesis of Fe3O4@SiO2–BD–DADB-COF improved the material's adsorption efficiency. To deeply investigate the adsorption selectivity of Fe3O4@SiO2–BD–DADB-COF for MALs, three categories of antibiotics, namely, quinolones (QNs), sulfonamides (SAs), and nitroimidazoles (NDZs), were selected for evaluating selectivity. Table S4† exhibits that the adsorption capacity for MALs varied between 82.92 and 109.54%, whereas the adsorption efficiency of all other antibiotics was below 30%. This may primarily be attributed to electrostatic interactions. Based on zeta potential analysis, Fe3O4@SiO2–BD–DADB-COF is negatively charged at pH 8. QNs (pKa = 5.2–6.6), SAs (pKa = 6–7.5), and NDZs (pKa = 3–6) are negatively charged at pH 8, resulting in electrostatic repulsion with the negatively charged Fe3O4@SiO2–BD–DADB-COF, which hinders their adsorption. In contrast, MALs carry a positive charge at pH 8 and interact with the negatively charged Fe3O4@SiO2–BD–DADB-COF through electrostatic interactions, thereby promoting the adsorption of MALs by Fe3O4@SiO2–BD–DADB-COF. The above results further revealed that Fe3O4@SiO2–BD–DADB-COF was capable of showing the selectivity to MALs.
In Fig. 3C, the N2 adsorption–desorption isotherms of Fe3O4@SiO2–BD–DADB-COF exhibited nearly overlapping curves within the relative pressure range of 0.1 < P/P0 < 0.65, accompanied by an upward trend, indicating simultaneous single and multi-layer adsorption in this region. As the relative pressure (P/P0) increased to 0.65–0.95, hysteresis in the desorption isotherm was observed, attributed to capillary condensation. The results demonstrate that the Fe3O4@SiO2–BD–DADB-COF functions through a combination of single-layer and multi-layer adsorption mechanisms.61,62 In addition, the adsorption capacity of the Fe3O4@SiO2–BD–DADB-COF for MALs was investigated by measuring a set of spiked water samples with different concentration, and the capacity of the adsorbent was 678 μg g−1, which displayed good adsorption ability.
3.4. Reusability of the adsorbent
The performance of adsorbents can be evaluated by considering their reusability as a key factor. With adsorption and desorption achieved, the Fe3O4@SiO2–BD–DADB-COF was rinsed using 0.3% ammonia ACN three times, and dehydrated for 4 h in a vacuum at 60 °C, followed by use. Fig. S7† shows that the adsorbent is adopted four times with no prominent decline in the recovery of MALs, which still remained greater than 74.11%. Consequently, it indicated that the Fe3O4@SiO2–BD–DADB-COF was an adsorbent with outstanding reusability.3.5. Matrix effect
Given that the intricate matrices among samples affected the precision of the analysis results, the matrix effects (MEs) were examined. The formula of ME is listed below:63Providing that the matrix effect (ME) came short of 80.0%, it suggested inhibition; conversely, if the ME exceeded 120.0%, it indicated an enhancement effect. When the ME fell within the range of 80.0% to 120.0%, the matrix effect could be considered negligible. Based on the calculation results in Table S5,† the ME values for the 15 MALs varied between 81.10% and 118.89% in water samples and from 81.57% to 115.66% in honey samples. Consequently, the matrix effect was deemed negligible.
3.6. Method validation
| Analytes | Linear range (μg L−1) | Linearity equation | R2 | LOD | LOQ | ||
|---|---|---|---|---|---|---|---|
| Water (μg L−1) | Honey (μg kg−1) | Water (μg L−1) | Honey (μg kg−1) | ||||
| Azithromycin | 0.1–200 | Y = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 856.4X + 27 856.4X + 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654.4494 654.4494 |
0.9990 | 0.011 | 0.008 | 0.037 | 0.025 |
| Tilmicosin | 0.1–200 | Y = 2848.2X + 67.91653 | 0.9995 | 0.038 | 0.025 | 0.114 | 0.076 |
| Tylosin tartrate | 0.1–200 | Y = 561![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50X + 13 50X + 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288.68202 288.68202 |
0.9999 | 0.002 | 0.002 | 0.009 | 0.007 |
| Tylosin | 0.1–200 | Y = 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 568.7X + 3.33625 568.7X + 3.33625 |
0.9991 | 0.011 | 0.012 | 0.037 | 0.036 |
| Kitasamycin | 0.1–200 | Y = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 711.4X + 16 711.4X + 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 478.9553 478.9553 |
0.9990 | 0.010 | 0.002 | 0.029 | 0.007 |
| Midecamycin | 0.1–200 | Y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219 219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57X + 183 57X + 183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 036 036 |
0.9993 | 0.003 | 0.004 | 0.009 | 0.011 |
| Josamycin | 0.1–200 | Y = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331.8X + 64 331.8X + 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300.8 300.8 |
0.9995 | 0.041 | 0.002 | 0.123 | 0.006 |
| Tylvalosin | 0.1–200 | Y = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234.1X + 482.52347 234.1X + 482.52347 |
0.9997 | 0.030 | 0.005 | 0.090 | 0.014 |
| Eprinomectin | 0.1–100 | Y = 8768.3X + 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308.6682 308.6682 |
0.9990 | 0.001 | 0.064 | 0.004 | 0.195 |
| Avermectin | 0.1–200 | Y = 5465.1X + 1863.66417 | 0.9993 | 0.039 | 0.013 | 0.119 | 0.038 |
| Dorametin | 0.1–200 | Y = 4132.8X + 5.73290 | 0.9997 | 0.075 | 0.060 | 0.228 | 0.202 |
| Ivermectin | 0.1–200 | Y = 6069.4X + 5182.20606 | 0.9993 | 0.035 | 0.004 | 0.107 | 0.011 |
| Selamectin | 0.1–200 | Y = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625.2X + 1304.40351 625.2X + 1304.40351 |
0.9999 | 0.029 | 0.036 | 0.087 | 0.109 |
| Emamectin | 0.1–200 | Y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 449 449![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93X + 2.29707 93X + 2.29707 |
0.9991 | 0.014 | 0.030 | 0.042 | 0.092 |
| Moxidectin | 0.1–100 | Y = 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706.9X + 9087.1 706.9X + 9087.1 |
0.9998 | 0.018 | 0.008 | 0.014 | 0.024 |
3.7. Method application in real samples
Our method was employed to construe 15 honey samples and 20 water samples. Azithromycin was explored among two of water samples, showing the concentrations of 0.688 μg L−1 and 0.346 μg L−1. Therefore, despite insufficient regulation for MRLs in water, this deserves attention.3.8. Comparison with other methods
Table 2 summarizes the comparison between the formulated MSPE-UPLC-MS/MS method and other methods to gauge the MALs, including sample matrices, extraction methods, analytical approaches, recoveries, extraction times, and LODs. First, SPE is commonly used pretreatment method for detecting MALs. MSPE eliminates tedious and intricate processes relative to conventional SPE, which simplifies the extraction procedure by separating the sample using an external magnetic field.6,64,65 Comparison with HLB columns, the synthesized Fe3O4@SiO2–BD–DADB-COF demonstrated a shorter extraction time (10 min), more number of MALs (15), and lower detection limits (0.002–0.067 μg kg−1). Second, each previously reported method has focused on the analysis of a single sample type. In contrast, the method developed in this work has been successfully applied to two distinct sample types.56 Furthermore, compared with Ol-Fe3O4 MNPs and Fe3O4@PDA-HPMIPs, the Fe3O4@SiO2–BD–DADB-COF showed significant adsorption capacity and can efficiently adsorb more MALs. Meanwhile, it also exhibits higher recoveries (71.4–105.1%) and lower detection limits (0.001–0.075 μg L−1), showing a high sensitivity of the Fe3O4@SiO2–BD–DADB-COF.56,64 In consequence, our method was highly prone to the enrichment and exceedingly sensitive to measure the trace MALs.| Analysis method | Adsorbent | Matrix (numbers of MALs) | Extraction time (min) | Recovery (%) | LODs | Ref. |
|---|---|---|---|---|---|---|
| MSPE-LC-MS/MS | Ol-Fe3O4 MNPs | Water (4) | 5 | 54–117 | 0.011–0.026 μg L−1 | 64 |
| SPE-LC-MS/MS | Oasis HLB cartridges | Honey (3) | 25 | 80.94–109.26 | 0.4–2 μg kg−1 | 65 |
| SPE-LC-MS/MS | Porous aromatic framework (PAF) | Chicken (6) | 50 | 82.1–101.4 | 0.2–0.5 μg L−1 | 6 |
| MSPE-LC-MS/MS | Fe3O4@PDA–HPMIPs | Honey (7) | 15 | 84.2–117 | 0.003–0.076 μg kg−1 | 56 |
| MSPE-LC-MS/MS | Fe3O4@SiO2–BD–DADB-COF | Water and honey (15) | 10 | 71.4–105.1 | 0.001–0.075 μg L−1 | This work |
| 75.1–101.9 | 0.002–0.067 μg kg−1 |
4. Conclusions
Through the P-123 as a pore-expanding agent, we successfully synthesized a new covalent organic framework material, Fe3O4@SiO2–BD–DADB-COF, which had several advantages, like exceptional magnetism, reusability, and a high specific surface region. A high-throughput analysis technique was formulated, which integrated the adsorbent with UPLC-MS/MS, to synchronously gauge MALs in water and honey samples. Such an approach was characterized by low detection and quantification limits, a simple extraction procedure, and an exceptional linear range. This lays a good groundwork for supervising MALs in both water and honey.Data availability
The data that has been used is confidential.Author contributions
Hao Zhang: conceptualization, methodology, formal analysis, writing – original draft. Weihao Ma: data curation, methodology. Chunyu Qiang: data curation, validation. Jiayuan Nie: methodology, validation. Ling Ma: conceptualization, supervision, funding acquisition. Yawei Zhang: data curation, methodology. Ke Wang: resources, funding acquisition, supervision, data curation, project administration, writing – review & editing. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81903322), the S&T Program of Hebei (223777116D) and the Central Government Guides the Development of Local Science and Technology Project of Hebei Province (246Z7721G).References
- R. Cañadas, R. M. G. Martínez, G. P. González and P. F. Hernando, Polymer, 2022, 249, 124843 CrossRef.
- M. A. García-Mayor, A. Gallego-Picó, R. M. Garcinuño, P. Fernández-Hernando and J. S. Durand-Alegría, Food Chem., 2012, 134, 553–558 CrossRef.
- G. El-Saber Batiha, A. Alqahtani, O. B. Ilesanmi, A. A. Saati, A. El-Mleeh, H. F. Hetta and A. M. Beshbishy, Pharmaceuticals, 2020, 13, 196 CrossRef CAS PubMed.
- A. G. Myers and R. B. Clark, Acc. Chem. Res., 2021, 54, 1635–1645 CrossRef CAS PubMed.
- A. Pani, M. Lauriola, A. Romandini and F. Scaglione, Int. J. Antimicrob. Agents, 2020, 56, 106053 CrossRef CAS PubMed.
- C. Lan, D. Yin, Z. Yang, W. Zhao, Y. Chen, W. Zhang and S. Zhang, J. Anal. Methods Chem., 2019, 2019, 6849457 Search PubMed.
- Y. Zhou, T. Zhou, H. Jin, T. Jing, B. Song, Y. Zhou, S. Mei and Y. I. Lee, Talanta, 2015, 137, 1–10 CrossRef CAS PubMed.
- G. Lu, Q. Chen, Y. Li, Y. Liu, Y. Zhang, Y. Huang and L. Zhu, Food Res. Int., 2021, 147, 110450 CrossRef CAS PubMed.
- W. Zhou, Y. Ling, T. Liu, Y. Zhang, J. Li, H. Li, W. Wu, S. Jiang, F. Feng, F. Yuan and F. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1061–1062, 411–420 CrossRef CAS PubMed.
- Z. Wang, R. C. Beier and J. Shen, TrAC, Trends Anal. Chem., 2017, 92, 42–61 CrossRef CAS.
- M. J. G. de la Huebra, G. Bordin and A. R. Rodríguez, Anal. Chim. Acta, 2004, 517, 53–63 CrossRef.
- A. S. Lorenzetti, A. G. Lista and C. E. Domini, LWT, 2019, 113, 108334 CrossRef CAS.
- J. V. Grutes, R. G. Ferreira, M. U. Pereira, F. S. Candido and B. F. Spisso, J. Environ. Sci. Health, Part B, 2021, 56, 197–211 CrossRef CAS PubMed.
- V. G. Amelin and D. S. Bol'shakov, Moscow Univ. Chem. Bull., 2019, 74, 63–69 CrossRef.
- Q. Zhuang, X. Li, X. Lian, H. Hu, N. Wang, J. Wu, K. Miao, G. Feng and X. Luo, Cryst. Growth Des., 2024, 25, 319–329 CrossRef.
- H. Cheng, R. Chen, Y. Zhan, W. Dong, Q. Chen, Y. Wang, P. Zhou, S. Gao, W. Huang, L. Li and J. Feng, Anal. Chem., 2024, 96, 18555–18563 CrossRef CAS PubMed.
- D. Che, J. Cheng, Z. Ji, S. Zhang, G. Li, Z. Sun and J. You, TrAC, Trends Anal. Chem., 2017, 97, 1–14 CrossRef CAS.
- Y. Tao, G. Yu, D. Chen, Y. Pan, Z. Liu, H. Wei, D. Peng, L. Huang, Y. Wang and Z. Yuan, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 897, 64–71 CrossRef CAS PubMed.
- L. Jank, M. T. Martins, J. B. Arsand, T. M. Campos Motta, R. B. Hoff, F. Barreto and T. M. Pizzolato, Talanta, 2015, 144, 686–695 CrossRef CAS PubMed.
- S. Kheirandish, S. Goudarzi, M. Amirahmadi, S. Ghareghani, H. Ghafari, B. Daraei and M. Ghazi-Khansari, Microchem. J., 2023, 193, 109103 CrossRef CAS.
- B. F. d. M. Pereira, M. U. Pereira, R. G. Ferreira and B. F. Spisso, Food Anal. Methods, 2020, 14, 719–733 CrossRef.
- I. S. Ibarra, J. M. Miranda, J. A. Rodriguez, C. Nebot and A. Cepeda, Food Chem., 2014, 157, 511–517 CrossRef CAS PubMed.
- V. V. Tolmacheva, V. V. Apyari, A. A. Furletov, S. G. Dmitrienko and Y. A. Zolotov, Talanta, 2016, 152, 203–210 CrossRef CAS PubMed.
- L. You, K. Xu, G. Ding, X. Shi, J. Li, S. Wang and J. Wang, J. Mol. Liq., 2020, 320, 114456 CrossRef CAS.
- A. Mohebbi, S. Yaripour, A. S. Alavian, M. M. Daghi and N. Fattahi, J. Food Compos. Anal., 2025, 138, 107006 CrossRef CAS.
- Y. Guo, J. Wang, L. Hao, Q. Wu, C. Wang and Z. Wang, J. Chromatogr. A, 2021, 1649, 462238 CrossRef CAS PubMed.
- D. N. da Silva and A. C. Pereira, Electrochem, 2022, 3, 809–819 CrossRef.
- Y. F. Ma, F. Yuan, X. H. Zhang, Y. L. Zhou and X. X. Zhang, Analyst, 2017, 142, 3212–3218 RSC.
- Q. N. Tran, H. J. Lee and N. Tran, Polymers, 2023, 15, 1279 CrossRef CAS PubMed.
- L. C. Lin, M. Thirumavalavan, Y.-T. Wang and J.-F. Lee, Colloids Surf., A, 2010, 369, 223–231 CrossRef CAS.
- X. Zhong, Z. Ren, Q. Ling and B. Hu, Appl. Surf. Sci., 2022, 597, 153621 CrossRef CAS.
- N. Huang, X. Chen, R. Krishna and D. Jiang, Angew. Chem., Int. Ed. Engl., 2015, 54, 2986–2990 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, H. Xia, Y. Mu and X. Liu, Chem. Commun., 2016, 52, 6613–6616 RSC.
- F. Li, J. L. Kan, B. J. Yao and Y. B. Dong, Angew. Chem., Int. Ed. Engl., 2022, 61, e202115044 CrossRef CAS PubMed.
- Y. Xie, L. Chen, K. Cui, Y. Zeng, X. Luo and X. Deng, Talanta, 2024, 279, 126547 CrossRef CAS PubMed.
- Y. Liu, H. Zhang, D. Xie, H. Lai, Q. Qiu and X. Ma, J. Environ. Chem. Eng., 2022, 10, 108094 CrossRef CAS.
- Y. Zhao, R. Wu, H. Yu, J. Li, L. Liu, S. Wang, X. Chen and T. D. Chan, J. Chromatogr. A, 2020, 1610, 460543 CrossRef CAS PubMed.
- C. Hui, C. Shen, J. Tian, L. Bao, H. Ding, C. Li, Y. Tian, X. Shi and H. J. Gao, Nanoscale, 2011, 3, 701–705 RSC.
- J. Tan, S. Namuangruk, W. Kong, N. Kungwan, J. Guo and C. Wang, Angew. Chem., Int. Ed. Engl., 2016, 55, 13979–13984 CrossRef CAS PubMed.
- Q. B. Fu, H. L. Jiang, L. Q. Qiao, X. Sun, M. L. Wang and R. S. Zhao, J. Chromatogr. A, 2020, 1630, 461534 CrossRef CAS PubMed.
- Z. C. Chen, H. B. Xu, H. Y. Chen, S. C. Zhu, W. F. Huang, Y. He, M. E. Hafez, R. C. Qian and D. W. Li, Anal. Chem., 2022, 94, 14280–14289 CrossRef CAS PubMed.
- S. Bhattacharya, A. Roychowdhury, D. Das and S. Nayar, RSC Adv., 2015, 5, 89488–89497 RSC.
- Q. Sun, B. Aguila, L. D. Earl, C. W. Abney, L. Wojtas, P. K. Thallapally and S. Ma, Adv. Mater., 2018, 30, e1705479 CrossRef PubMed.
- G. Lin, C. Gao, Q. Zheng, Z. Lei, H. Geng, Z. Lin, H. Yang and Z. Cai, Chem. Commun., 2017, 53, 3649–3652 RSC.
- M. Zhang, J. Li, C. Zhang, Z. Wu, Y. Yang, J. Li, F. Fu and Z. Lin, J. Chromatogr. A, 2020, 1615, 460773 CrossRef CAS PubMed.
- Y. Du, X. Yan, Y. Chen, Y. Wu, Q. Qiu, Y. Li and D. Wu, J. Chromatogr. A, 2022, 1675, 463184 CrossRef CAS PubMed.
- Z. Yan, B. Hu, Q. Li, S. Zhang, J. Pang and C. Wu, J. Chromatogr. A, 2019, 1584, 33–41 CrossRef CAS PubMed.
- Z. Ma, L. Fang, L. Liu, B. Hu, S. Wang, S. Yu and X. Wang, Sci. Total Environ., 2023, 901, 166453 CrossRef CAS PubMed.
- J. Kang, S. J. Park, H. C. Park, M. A. Hossain, M. A. Kim, S. W. Son, C. M. Lim, T. W. Kim and B. H. Cho, Appl. Biochem. Biotechnol., 2017, 182, 635–652 CrossRef CAS PubMed.
- X. Guo, H. Tian, F. Yang, S. Fan, J. Zhang, J. Ma, L. Ai and Y. Zhang, Front. Nutr., 2022, 9, 879518 CrossRef PubMed.
- S. Seidi, F. Mohammadi, M. Tajik, M. Baharfar, A. Mohammadi and T. Otoufat, J. Sep. Sci., 2020, 43, 2897–2904 CrossRef CAS PubMed.
- Y. Sun, J. Kuang, Y. Cheng, C. Lin, H. Zhang, M. Zhang, F. Ning and P. Hu, J. Chromatogr. A, 2024, 1713, 464521 Search PubMed.
- J. Chen, G. Mei, X. Zhang, D. Huang, P. He and D. Xu, Foods, 2024, 13, 866 CrossRef CAS PubMed.
- G. Wang, T. Zhou and Y. Lei, RSC Adv., 2020, 10, 11557–11564 RSC.
- M. Li, W. Liu, X. Meng, S. Li, Q. Wang, Y. Guo, Y. Wu, L. Hao, X. Yang, Z. Wang, C. Wang and Q. Wu, Food Chem., 2022, 395, 133596 CrossRef CAS PubMed.
- W. Fan, D. Yang, N. Ding, P. Chen, L. Wang, G. Tao, F. Zheng and S. Ji, Anal. Methods, 2021, 13, 1412–1421 Search PubMed.
- A. Kasperkiewicz and J. Pawliszyn, Food Chem., 2021, 339, 127815 Search PubMed.
- M. Liang, N. Li, H. Zhang, L. Ma and K. Wang, RSC Adv., 2024, 14, 8726–8734 Search PubMed.
- K. D. Lenz, K. E. Klosterman, H. Mukundan and J. Z. Kubicek-Sutherland, Toxins, 2021, 13, 347 CrossRef CAS PubMed.
- Z. Sun, L. Zhao, C. Liu, Y. Zhen and J. Ma, Chem. Eng. J., 2020, 381, 122510 Search PubMed.
- M. Sun, S. Han, H. M. Loussala, J. Feng, C. Li, X. Ji, J. Feng and H. Sun, Microchem. J., 2021, 166, 106263 Search PubMed.
- J. Zhao, H. Cheng, J. Feng, T. Tang and D. Qin, Microchem. J., 2024, 205, 111241 Search PubMed.
- S. Li, C. Zhang, H. X. Tang, Y. Gu, A. J. Guo, K. Wang and K. Q. Lian, J. Food Drug Anal., 2023, 31, 73–84 CrossRef PubMed.
- R. A. Perez, B. Albero, M. Ferriz and J. L. Tadeo, J. Pharm. Biomed. Anal., 2017, 146, 79–85 CrossRef CAS PubMed.
- W. Zheng, J. A. Park, A. M. Abd El-Aty, S. K. Kim, S. H. Cho, J. M. Choi, M. Warda, J. Wang, J. H. Shim and H. C. Shin, Biomed. Chromatogr., 2018, 32, e4145 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00080g |
| This journal is © The Royal Society of Chemistry 2025 |