Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Three-step process for the synthesis of 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives†
Farid M. Sroor *a,
Thierry Terme
*a,
Thierry Terme b,
Patrice Vanelle
b,
Patrice Vanelle b and
Cédric Spitz
b and
Cédric Spitz *b
*b
aOrganometallic and Organometalloid Chemistry Department, National Research Centre, 12622 Cairo, Egypt. E-mail: faridsroor@gmx.de
bAix Marseille Univ., CNRS, ICR, UMR 7273, Equipe Pharmaco-Chimie Radicalaire, Faculté de Pharmacie, 27 Boulevard Jean Moulin – CS 30064 Cedex 05, 13385 Marseille, France. E-mail: cedric.spitz@univ-amu.fr
First published on 3rd March 2025
Abstract
We reported the synthesis of 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives, a class of compounds that has been limitedly investigated in the literature. Our approach streamlines the synthetic process, allowing straightforward access to various substituted 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives. This advancement not only enhances the accessibility of these derivatives for further research but also contributes to the development of potential therapeutic agents targeting various medical conditions.
Introduction
Dibenzazepine derivatives are a significant class of compounds in medicinal chemistry, recognized for their wide range of biological activities and therapeutic potential. The tricyclic dibenzazepine system, characterized by a fused bicyclic skeleton containing a sandwiched azepine ring between two benzene rings, displays a distinctive three-dimensional configuration that enables interactions with a wide range of biological targets.1 Dibenzazepine derivatives synthesis has attracted much attention. Several approaches, including cyclization reactions, ring expansion, and multicomponent reactions, are used to produce structurally different analogs efficiently.2In the same context, the dibenzo[b,f]azepine scaffold-containing compounds are known to possess an abundance of pharmacological features, making their biological activity extremely impressive, including anticancer, anti-inflammatory, antiepileptic, and antidepressant activities (Fig. 1).3 Their capacity to impact neurotransmitter systems, especially those in the central nervous system, has made them useful in creating medications such as novel antiepileptic drugs and mood disorders. Furthermore, certain dibenzo[b,f]azepine derivatives have shown encouraging results in blocking GPR4, suggesting their potential as a therapeutic lead for the treatment of myocardial infarction.4
In particular, a 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivative, eslicarbazepine acetate, was quite recently commercialized as a novel antiepileptic drug indicated for the treatment of partial-onset seizures.5 Eslicarbazepine acetate improves upon its predecessors, carbamazepine, and oxcarbazepine, by being available in a once-daily treatment, interacting with a reduced range of drugs, and causing lower side effects.3e 10,11-Dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives are usually synthesized through the ketone reduction of oxcarbazepine derivatives.6 Despite the critical importance of this class of heterocycles in medicinal chemistry, there is a lack of efficient and straightforward methods for their preparation. Even though the influence of the substituent on the nitrogen was already well-documented, studies on the effect of substituents on both phenyl rings are minimal. In this context, there is a need for a new process for preparing 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives with broad substitution patterns to modulate their biological activity. Indeed, the importance of 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives lies not only in their therapeutic potential but also in their role as scaffolds for exploring novel pharmacophores in the quest for new and effective treatments.
Therefore, we aim to develop a rapid and highly productive synthesis method of phenyl-substituted 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives.
Results and discussion
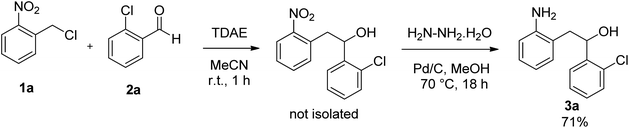
In our endeavor, and in continuation of our previous studies to discover novel biologically active organic compounds,7 we established only a three-step process for the synthesis of novel 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol compounds from commercially available starting materials.Tetrakis(dimethylamino)ethylene (TDAE) is an organic reducing agent, that reacts with halogenated derivatives to generate a carbanion under mild conditions.8 In particular, from o-nitrobenzyl chloride, TDAE was able to generate the corresponding o-nitrobenzyl carbanion, which can react with 2-chlorobenzaldehyde to allow the formation of 1-(2-chlorophenyl)-2-(2-nitrophenyl)ethanol. Then, the nitro group was reduced in the presence of Pd/C and hydrazine hydrate to afford 2-(2-aminophenyl)-1-(2-chlorophenyl)ethanol 3a in 71% overall yield (Scheme 1).
The final and more challenging step was an intramolecular Buchwald–Hartwig coupling to obtain the dibenzazepine scaffold. The reaction conditions optimization for this step was performed on 2-(2-aminophenyl)-1-(2-chlorophenyl)ethanol 3a (Table 1). In the literature, a recent patent9 described exactly this reaction using palladium acetate as palladium source, Xantphos as ligand, K2CO3 as base, and tetrahydrofuran as solvent at 50–60 °C for 2 h. So, we tried these conditions for the intramolecular Buchwald–Hartwig coupling of 2-(2-aminophenyl)-1-(2-chlorophenyl)ethanol 3a. Unfortunately, we obtained the dibenzazepine 4a in only 9% yield (entry 1, Table 1). No significant improvement was observed with degassed THF (entry 2). Increasing the reaction time (entry 3) and the temperature (entry 4) afforded dibenzazepine 4a in low yields. We do not have any satisfying explanation about the impossibility for us to reproduce the high yield (85–95%) described in the patent. The large scale (49.5 g, 0.2 mol of 3a) described in the patent may be more suitable for this reaction. We tried the reaction with a maximum of 5 mmol of 2-(2-aminophenyl)-1-(2-chlorophenyl)ethanol 3a but no improvement was observed. Our purpose to discover novel biologically active organic compounds was not suitable for large scale experiment. So, we performed the optimization reactions in a 0.2 mmol scale. Our studies began with screening palladium sources, ligands, bases, and solvents. Our first results (entries 5−7, Table 1) indicated that the Xphos/NaOt-Bu combination is unsuitable for the reaction. Using Cs2CO3 or K2CO3, poor and similar yields were obtained with a catalytic amount of Pd(OAc)2 and BINAP in toluene at 110 °C (entries 8 and 9). A slightly better yield was achieved using Xantphos as a ligand (entry 10). Performing the reaction under microwave irradiation and increasing the temperature allowed better yields (entries 11−13). So, the best reaction conditions for the intramolecular Buchwald–Hartwig coupling of compound 3a was the use of palladium acetate as palladium source, Xantphos as ligand, K2CO3 as base, and toluene as solvent at 170 °C under microwave irradiation for 8 h (entry 13, Table 1). As the crude NMR of compound 3a was quite clean and to save time and money, we decided not to purify intermediate 3a with column chromatography. Thus, after the two first steps, crude intermediate 3a was directly cyclized by an intramolecular Buchwald–Hartwig coupling using the previously optimized reaction conditions to give dibenzazepine 4a with a 39% overall yield (Table 2, 4a). In comparison, when intermediate 3a was purified, dibenzazepine 4a was isolated with a 37% overall yield (71% for 3a and 52% for 4a). Given this result and to save time and money, we selected the process without intermediate purification to do the scope of the reaction.
| Entry | Pd source (10 mol%) | Ligand (10 mol%) | Base (2 equiv.) | Solvent (C = 0.5 mol L−1) | Temperature (° C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reactions are performed on a 0.2 mmol scale.b Isolated yields.c THF was degassed with N2.d Performed under microwave irradiation. | |||||||
| 1 | Pd(OAc)2 | Xantphos | K2CO3 | THF | 60 | 2 | 9 |
| 2 | Pd(OAc)2 | Xantphos | K2CO3 | THFc | 60 | 2 | 10 |
| 3 | Pd(OAc)2 | Xantphos | K2CO3 | THF | 60 | 16 | 13 |
| 4 | Pd(OAc)2 | Xantphos | K2CO3 | THF | 100 | 48 | 27 |
| 5 | Pd2(dba)3 | XPhos | NaOt-Bu | Toluene | 110 | 16 | <5 |
| 6 | Pd2(dba)3 | XPhos | NaOt-Bu | 1,4-Dioxane | 110 | 16 | <5 |
| 7 | Pd(OAc)2 | XPhos | NaOt-Bu | 1,4-Dioxane | 110 | 16 | <5 |
| 8 | Pd(OAc)2 | BINAP | Cs2CO3 | Toluene | 110 | 16 | 7 |
| 9 | Pd(OAc)2 | BINAP | K2CO3 | Toluene | 110 | 16 | 8 |
| 10 | Pd(OAc)2 | Xantphos | K2CO3 | Toluene | 110 | 16 | 14 |
| 11 | Pd(OAc)2 | Xantphos | K2CO3 | Toluene | 110d | 8 | 16 |
| 12 | Pd(OAc)2 | Xantphos | K2CO3 | Toluene | 150d | 8 | 31 |
| 13 | Pd(OAc)2 | Xantphos | K2CO3 | Toluene | 170d | 8 | 52 |
| a Reaction conditions: (1) 1 (1 mmol), 2 (1.2 mmol), and TDAE (1.2 mmol) in MeCN (4 mL) were stirred at r.t. for 1 h under an inert atmosphere. (2) After evaporation of MeCN, MeOH (5 mL), Pd/C (5%, 46 mg), and hydrazine hydrate (50–60%, 312 μL) were added and the reaction was stirred at 70 °C for 18 h under an inert atmosphere. (3) Intermediate 3, Pd(OAc)2 (22.5 mg, 0.1 mmol, 0.1 equiv.), Xantphos (58 mg, 0.1 mmol, 0.1 equiv.), K2CO3 (276 mg, 2 mmol, 2 equiv.) and anhydrous toluene (2 mL) were stirred under microwave irradiation at 135 °C for 8 h.b Microwave temperature for step (3) was 170 °C.c Microwave temperature for step (3) was 150 °C.d Isolated overall yields of the three steps are given. |
|---|
 |
Starting from di-halogenated aldehydes, dibenzazepines 4b and 4c were obtained in 42 and 31% overall yields (Table 2). Interestingly, the presence of an electron-withdrawing substituent allowed the temperature of the Buchwald–Hartwig coupling to decrease to 135 °C. As the trifluoromethyl group could enhance the biological activity, metabolic stability, lipophilicity, and pharmacokinetic properties of heterocyclic drug molecules,10 diversely trifluoromethyl-substituted dibenzazepines were synthesized in modest to excellent overall yields for the three-step process (Table 2, 4d–4g). An electron-donating methyl group was well-tolerated on both aromatic parts of the dibenzazepine (Table 2, 4h–4j). The structure of product 4i was unequivocally established by X-ray analysis (Fig. 2). Even though the reactivity was lower by introducing a chlorine atom on the left aromatic part of the dibenzazepine, compounds 4k and 4l were afforded in 21 and 20% overall yields (Table 2). Remarkably, the chlorine atom could be replaced by selected substituents, such as aromatic patterns with palladium-catalyzed cross-coupling reactions. This is a great advantage to further explore structure–activity relationship of the 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol scaffold.
Conclusions
In conclusion, we have successfully reported a new, practical, and straightforward approach for synthesizing 10,11-dihydro-5H-dibenzo[b,f]azepin-10-ol derivatives in only three steps. Firstly, using TDAE as a mild reductant allowed the nucleophilic addition reaction of diversely substituted o-nitrobenzyl chlorides to different 2-chlorobenzaldehydes. Then, reduction of the nitro group afforded 2-(2-aminophenyl)-1-(2-chlorophenyl)ethanol intermediates 3. Finally, an intramolecular Buchwald–Hartwig coupling was carried out to obtain the dibenzazepine scaffold with substitution on one or both phenyl rings, contrary to the patent9 which only described the synthesis of dibenzazepine 4a.Data availability
The data supporting this article have been included as part of the ESI.† CCDC-2391789 (https://www.ccdc.cam.ac.uk/mystructures/viewinaccessstructures/39ce8207-9c8c-ef11-96ca-00505695281c) contains the supplementary crystallographic data of compound 4i for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/structures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Farid Sroor greatly appreciates the postdoctoral scholarship from the French embassy in Cairo and Institut Français d'Egypte (IFE) at Aix-Marseille University. This work was supported by the CNRS and Aix-Marseille University. The authors also thank the Spectropole team for their elemental analysis.Notes and references
- (a) M. Erdoğan and A. Daştan, Synthesis of N-substituted dibenzoazepine–pyridazine derivatives as potential neurologically active drugs, Synth. Commun., 2020, 50, 3845–3853 CrossRef; (b) D. B. Kastrinsky, J. Sangodkar, N. Zaware, S. Izadmehr, N. S. Dhawan, G. Narla and M. Ohlmeyer, Reengineered tricyclic anti-cancer agents, Bioorg. Med. Chem., 2015, 23, 6528–6534 CrossRef CAS PubMed; (c) J. M. Munson, L. Fried, S. A. Rowson, M. Y. Bonner, L. Karumbaiah, B. Diaz, S. A. Courtneidge, U. G. Knaus, D. J. Brat, J. L. Arbiser and R. V. Bellamkonda, Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma, Sci. Transl. Med., 2012, 4, 127ra36 Search PubMed; (d) R. Pal, B. Kumar, Md. J. Akhtar and P. A. Chawla, Voltage gated sodium channel inhibitors as anticonvulsant drugs: a systematic review on recent developments and structure activity relationship studies, Bioorg. Chem., 2021, 115, 105230 CrossRef CAS PubMed; (e) P. K. Gillman, Tricyclic antidepressant pharmacology and therapeutic drug interactions updated, Br. J. Pharmacol., 2009, 151, 737–748 CrossRef PubMed; (f) K. Yue, S. Sun, G. Jia, M. Qin, X. Hou, C. J. Chou, C. Huang and X. Li, First-in-Class Hydrazide-Based HDAC6 Selective Inhibitor with Potent Oral Anti-Inflammatory Activity by Attenuating NLRP3 Inflammasome Activation, J. Med. Chem., 2022, 65, 12140–12162 CrossRef CAS PubMed.
- (a) B. P. Das, R. W. Woodard, L. K. Whisenant, W. F. Winecoff and D. W. Boykin, Synthesis of some substituted 10-amino-10,11-dihydro-5H-dibenz[b,f]azepines, J. Med. Chem., 1970, 13, 979–981 CrossRef CAS PubMed; (b) L. J. Kricka and A. Ledwith, Dibenz[b,f]azepines and related ring systems, Chem. Rev., 1974, 74, 101–123 CrossRef CAS; (c) M. Carril, R. SanMartin, F. Churruca, I. Tellitu and E. Dominguez, An Advantageous Route to Oxcarbazepine (Trileptal) Based on Palladium-Catalyzed Arylations Free of Transmetallating Agents, Org. Lett., 2005, 7, 4787–4789 CrossRef CAS PubMed; (d) T. Stopka, L. Marzo, M. Zurro, S. Janich, E.-U. Würthwein, C. G. Daniliuc, J. Aleman and O. García Mancheno, Oxidative C–H Bond Functionalization and Ring Expansion with TMSCHN2: A Copper(I)-Catalyzed Approach to Dibenzoxepines and Dibenzoazepines, Angew. Chem., Int. Ed., 2015, 54, 5049–5053 CrossRef CAS PubMed; (e) H.-J. Song, E. Yoon and J.-N. Heo, Efficient synthesis of dibenzazepine lactams via a sequential Pd-catalyzed amination and aldol condensation reaction, Tetrahedron Lett., 2020, 61, 151536 CrossRef CAS; (f) D. I. H. Maier, B. C. B. Bezuidenhoudt and C. Marais, Strategies in the synthesis of dibenzo[b,f]heteropines, Beilstein J. Org. Chem., 2023, 19, 700–718 CrossRef CAS PubMed; (g) J. Tsoung, J. Panteleev, M. Tesch and M. Lautens, Multicomponent-Multicatalyst Reactions (MC)2R: Efficient Dibenzazepine Synthesis, Org. Lett., 2014, 16, 110–113 CrossRef CAS PubMed.
- (a) M. P. Sadashiva, S. NanjundaSwamy, F. Li, K. A. Manu, M. Sengottuvelan, D. S. Prasanna, N. C. Anilkumar, G. Sethi, K. Sugahara and K. S. Rangappa, Anti-cancer activity of novel dibenzo[b,f]azepine tethered isoxazoline derivatives, BMC Chem. Biol., 2012, 12, 5 CrossRef PubMed; (b) L. Xu, F.-W. Guo, X.-Q. Zhang, T.-Y. Zhou, C.-J. Wang, M.-Y. Wei, Y.-C. Gu, C.-Y. Wang and C.-L. Shao, Discovery, total syntheses and potent anti-inflammatory activity of pyrrolinone-fused benzoazepine alkaloids Asperazepanones A and B from Aspergillus candidus, Commun. Chem., 2022, 5, 80 CrossRef CAS PubMed; (c) M. Berger and M. Gastpar, Trimipramine: a challenge to current concepts on antidepressives, Eur. Arch. Psychiatr. Clin. Neurosci., 1996, 246, 235–239 CrossRef CAS PubMed; (d) S. M. Grant and D. Faulds, Oxcarbazepine: A Review of its Pharmacology and Therapeutic Potential in Epilepsy, Trigeminal Neuralgia and Affective Disorders, Drugs, 1992, 43, 873–888 CrossRef CAS PubMed; (e) G. L. Galiana, A. C. Gauthier and R. H. Mattson, Eslicarbazepine Acetate: A New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures, Drugs R&D, 2017, 17, 329–339 Search PubMed; (f) S. Sinning, M. Musgaard, M. Jensen, K. Severinsen, L. Celik, H. Koldsø, T. Meyer, M. Bols, H. H. Jensen, B. Schiøtt and O. Wiborg, Binding and Orientation of Tricyclic Antidepressants within the Central Substrate Site of the Human Serotonin Transporter, J. Biol. Chem., 2010, 285, 8363–8374 CrossRef CAS PubMed.
- H. Fukuda, S. Ito, K. Watari, C. Mogi, M. Arisawa, F. Okajima, H. Kurose and S. Shuto, Identification of a Potent and Selective GPR4 Antagonist as a Drug Lead for the Treatment of Myocardial Infarction, ACS Med. Chem. Lett., 2016, 7, 493–497 CrossRef CAS PubMed.
- R. P. Singh and J. J. Asconapé, A Review of Eslicarbazepine Acetate for the Adjunctive Treatment of Partial-Onset Epilepsy, J. Cent. Nerv. Syst. Dis., 2011, 3, 179–187 CAS.
- B. Ravinder, S. Rajeshwar Reddy, M. Sridhar, M. Murali Mohan, K. Srinivas, A. Panasa Reddy and R. Bandichhor, An efficient synthesis for eslicarbazepine acetate, oxcarbazepine, and carbamazepine, Tetrahedron Lett., 2013, 54, 2841–2844 CrossRef CAS.
- (a) F. Mathias, Y. Kabri, L. Okdah, C. Di Giorgio, J. M. Rolain, C. Spitz, M. D. Crozet and P. Vanelle, An Efficient One-Pot Catalyzed Synthesis of 2,4-Disubstituted 5-Nitroimidazoles Displaying Antiparasitic and Antibacterial Activities, Molecules, 2017, 22, 1278 CrossRef PubMed; (b) C. Spitz, F. Mathias, S. Péchiné, T. H. D. Doan, J. Innocent, S. Pellissier, C. Di Giorgio, M. D. Crozet, C. Janoir and P. Vanelle, 2,4-Disubstituted 5-Nitroimidazoles Potent against Clostridium difficile, ChemMedChem, 2019, 14, 561–569 CrossRef CAS PubMed; (c) F. M. Sroor, A. Younis, M. Abdelraof and I. A. Abdelhamid, Synthesis, molecular docking and anti-biofilm activity of novel benzo[4,5]imidazo[2,1-a]quinazoline, 4H-chromene, and acridine derivatives as potent anti-candida agents, J. Mol. Struct., 2025, 1331, 141520 CrossRef CAS; (d) M. G. Kamel, F. M. Sroor, M. K. H. Hanafy, K. F. Mahrous and H. M. Hassaneen, Design, synthesis and potent anti-pancreatic cancer activity of new pyrazole derivatives bearing chalcone, thiazole and thiadiazole moieties: gene expression, DNA fragmentation, cell cycle arrest and SAR, RSC Adv., 2024, 14, 26954–26970 RSC; (e) F. M. Sroor, A. F. El-Sayed and K. Mahmoud, Novel 5-Fluorouracil analogues versus perfluorophenyl ureas as potent anti-breast cancer agents: design, robust synthesis, in vitro, molecular docking, pharmacokinetics ADMET analysis and dynamic simulations, Bioorg. Chem., 2024, 153, 107944 CrossRef CAS PubMed.
- C. Spitz, O. Khoumeri, T. Terme and P. Vanelle, Diastereoselective Synthesis of N-tert-Butanesulfinylamines through Addition of p-Nitrobenzyl Chloride to N-tert-Butanesulfinimines Using a TDAE Strategy, Synlett, 2013, 24, 1725–1727 CrossRef CAS.
- D. He, Z. Hu, M. Xu, D. Che and Y. Li, Preparation method of hydroxyl-substituted dibenzazepine compound, CN116514670A, 2023-08-01.
- Y. Liu, Q. Tian, J. Ge, X. Wu, Z. Li and G. Cheng, Recent advances in the synthesis of trifluoromethyl-containing heterocyclic compounds via trifluoromethyl building blocks, Org. Biomol. Chem., 2024, 22, 6246–6276 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, characterization data, and NMR spectra. CCDC 2391789. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra00909j |
| This journal is © The Royal Society of Chemistry 2025 |