Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring new horizons in mid-to-far infrared nonlinear optical crystals: the significant potential of trigonal pyramidal [TeS3]2− functional units†
Bo
Zhang
 abc,
Sheng-Hua
Zhou
*ad,
Bing-Xuan
Li
ab,
Xin-Tao
Wu
abc,
Sheng-Hua
Zhou
*ad,
Bing-Xuan
Li
ab,
Xin-Tao
Wu
 ab,
Hua
Lin
ab,
Hua
Lin
 *ab and
Qi-Long
Zhu
*ab and
Qi-Long
Zhu
 *ab
*ab
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China. E-mail: linhua@fjirsm.ac.cn; qlzhu@fjirsm.ac.cn
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350002, China
cUniversity of the Chinese Academy of Sciences, Beijing 100049, China
dResource Environment & Clean Energy Laboratory, School of Chemistry and Chemical Engineering, Jiangsu University of Technology, Changzhou 213001, China. E-mail: zhoushenghua@jsut.edu.cn
First published on 10th January 2025
Abstract
Traditional tetrahedral-based mid-to-far infrared (MFIR) nonlinear optical (NLO) crystals often face limitations due to the optical anisotropy constraints imposed by their highly symmetric structures. In contrast, the relatively rare trigonal pyramidal [TeS3]2− functional unit characterized by its asymmetric structure and stereochemically active lone pair (SCALP), offers improved optical anisotropy, hyperpolarizability and a broader IR transparency range. Despite its potential, synthetic challenges have hindered the development of MFIR NLO crystals that incorporate this unit, with only one example reported to date. Herein, an innovative MFIR NLO crystal, Cu10Te4S13 has been successfully constructed using the trigonal pyramidal [TeS3]2− units, via a simple high-temperature solid-state method. The novel three-dimensional structure of Cu10Te4S13 is interconnected by butterfly-orchid-like [Cu6Te4S13] anionic clusters and [CuS4] groups, where the former are composed of trigonal pyramidal [TeS3]2− groups and [Cu6S13] hexamers. Cu10Te4S13 exhibits a remarkable second harmonic generation effect, approximately 3.75 times that of AgGaS2 at 2900 nm in the particle size range of 30–45 μm. Additionally, it demonstrates favorable crystal growth habits, producing single crystals with maximum dimensions of about 7 × 3 × 2 mm3. This polished single crystal appears to exhibit complete transparency within the MFIR spectral window ranging from 2.5 to 25 μm, representing the widest IR transmission in all reported NLO chalcogenides. Furthermore, the structure–property relationship is also elucidated through first-principles analysis. This work confirms the potential of the unique trigonal pyramidal [TeS3]2− as a MFIR NLO functional unit, paving the way for the development of unconventional MFIR NLO materials.
Introduction
Mid-to-far infrared (MFIR) nonlinear optical (NLO) crystals are essential for advancements in IR technology, significantly improving the performance, stability, and application range of IR light sources.1 As the demand for IR technology increases across various sectors, including the military, aerospace, environmental monitoring, and communications, the quest for high-performance MFIR NLO crystals has become increasingly paramount.2 Currently, the availability of effective MFIR NLO crystals, particularly those operating beyond 5 μm, is limited. Traditional MFIR NLO crystals, such as chalcopyrite-type AgGaS2,3 AgGaSe2,4 and ZnGeP2,5 face significant challenges due to their inherent limitations. These materials often exhibit intrinsic defects that severely restrict their high-power laser output. Moreover, AgGaS2 and ZnGeP2 demonstrate pronounced multi-phonon absorption at around 9 μm, which prevents them from effectively utilizing the vital “8–14 μm” transparency window.6,7 Given these constraints, the urgent development of novel MFIR NLO candidates with enhanced properties is not only desirable but essential.Traditional tetrahedral-based MFIR NLO crystals often face limitations due to the optical anisotropy constraints imposed by their highly symmetric structures.8–21 To enhance optical anisotropy, it is imperative to explore other MFIR NLO functional units beyond tetrahedral units. Compared to tetrahedral units, asymmetric functional units with stereochemically active lone pairs (SCALPs) have attracted our attention due to their enhanced optical anisotropy and improved hyperpolarizability.22–28 Among these, many triangular pyramidal functional units exhibit broader IR transmission than their tetrahedral counterparts, making them more suitable for practical applications in MFIR NLO crystals.29 Additionally, trigonal pyramids offer greater structural flexibility and design potential than tetrahedral motifs. This design flexibility permits researchers to explore and optimize the overall performance of NLO materials to meet specific requirements.
Currently, NLO crystals based on trigonal pyramidal functional units have been more extensively studied in the ultraviolet-visible (UV-vis) range compared to the MFIR range. Among them, the [TeO3]2− group has been recognized as an excellent UV-vis NLO-active functional unit.30–38 Over the past two decades, extensive research has focused on NLO crystals containing [TeO3]2− units, leading to the discovery of many NLO materials with high optical anisotropy and strong second-harmonic generation (SHG) effects, such as Cd2Nb2Te4O15 (31 × KDP, 0.12 @ 546 nm),35 Mo(H2O)Te2O7 (5.4 × KDP, 0.528 @ 546 nm),36 β-K2TeW3O12 (15 × KDP, 0.196 @ 1064 nm),37 and LiNbTeO5 (17 × KDP, 0.083 @ 632.8 nm).38 The [TeO3]2− unit offers several advantages for constructing NLO materials: (i) its highly asymmetric coordination environments facilitate the formation of non-centrosymmetric (NCS) structures;39 (ii) the uniform arrangement of asymmetric trigonal pyramidal units enhances optical anisotropy, conducive to a large birefringence;40 (iii) SCALPs in Te4+ promote secondary Jahn–Teller distortions, favoring powerful SHG effects.35,41 Nevertheless, the strong absorption of Te–O bonds in the 400–800 cm−1 IR range prevents their application in the MFIR range.42 To overcome this limitation, the oxygen atoms in the [TeO3]2− unit are replaced with sulfur to derive the pyramidal [TeS3]2− units. [TeS3]2− not only retains the trigonal pyramidal configuration with a pair of SCALPs, inheriting the advantages of large optical anisotropy and hyperpolarizability of [TeO3]2− units, but also extends its transmission window to the MFIR range. Consequently, [TeS3]2− presents itself as a promising candidate for MFIR NLO applications, distinct from traditional tetrahedral functional units.
Nonetheless, only one NLO crystal containing the [TeS3]2− unit, CsAg2TeS6,43 has been reported. This limited occurrence is primarily due to synthetic challenges: (i) bond strength: Te–S bonds are relatively weak compared to Te–O bonds. Additionally, the small electronegativity difference between Te and S complicates the formation and stability of the [TeS3]2− units during synthesis. (ii) Chemical properties of Te: as a metalloid, Te displays complex chemical behavior, exhibiting multiple oxidation states with varying reactivities and reaction products. This variability requires precise control of synthesis conditions to ensure that Te remains in the desired oxidation state, further complicating the process of creating compounds with [TeS3]2− units. As a result, research on NLO crystals containing [TeS3]2− units is greatly limited.
Cu+ with a d10 electronic configuration exhibits a large polar displacement, which contributes to strong SHG effects.44 Additionally, Cu+ can adopt multiple coordination modes, namely CuSn (n = 2–4), further enhancing the structural diversity of compounds.45–47 Utilizing [TeS3]2− as the functional units and Cu+ as the cations, we successfully synthesized a novel MFIR NLO crystal, Cu10Te4S13 through a high-temperature solid-state reaction. It exhibits a remarkable SHG effect, measuring 3.75 times that of AgGaS2 at 2900 nm. Furthermore, Cu10Te4S13 demonstrates favorable crystallographic growth habits, producing single crystals with dimensions up to 7 × 3 × 2 mm3. Such polished single crystals display a complete transmission window across 2.5–25 μm, representing the widest IR transmission in all reported NLO chalcogenides. First-principles analysis elucidates the NLO and linear optical properties, providing insights into the structure–property relationship. Our research confirms the promise of the [TeS3]2− unit as an MFIR NLO-active functional unit and presents a novel perspective for designing and exploring MFIR NLO materials beyond traditional chalcopyrite structures.
Results and discussion
Using the high-temperature solid-state method, black metallic lustrous block Cu10Te4S13 single crystals were successfully synthesized. Energy-dispersive X-ray spectroscopy (EDS) elemental analysis revealed the presence of Cu, Te, and S elements in the single crystals (Fig. S1, ESI†), and Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te
Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 9.7
S = 9.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.4, close to the atomic ratio of the molecular formula. Field emission scanning electron microscopy (FESEM) analysis confirmed the uniform distribution of Cu, Te, and S elements (Fig. S2, ESI†).
12.4, close to the atomic ratio of the molecular formula. Field emission scanning electron microscopy (FESEM) analysis confirmed the uniform distribution of Cu, Te, and S elements (Fig. S2, ESI†).
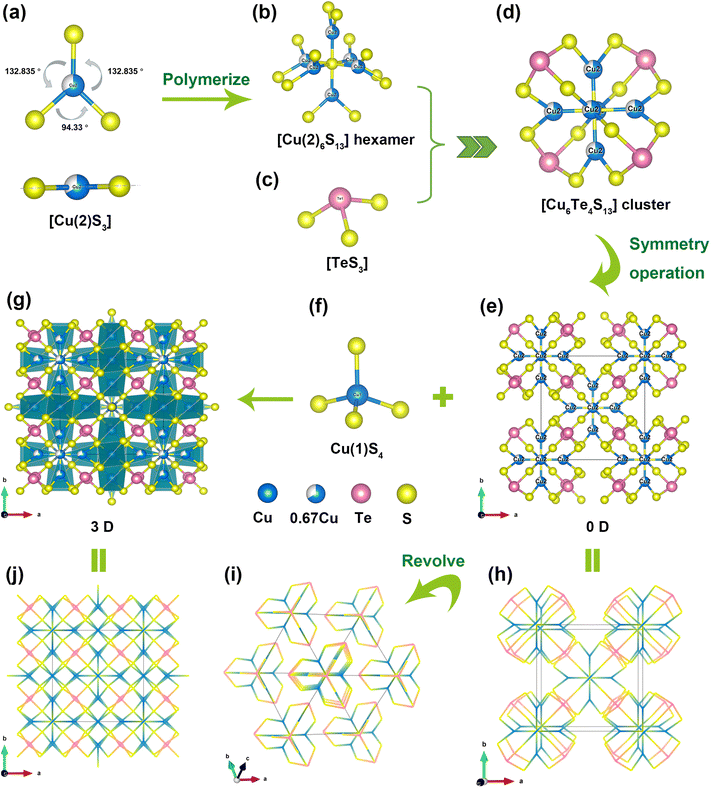
The crystal structure of Cu10Te4S13 was determined by single-crystal X-ray diffraction, with crystallographic data summarized in Table S1.† Cu10Te4S13 crystallizes in the NCS space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m [Pearson code: CI58; Wyckoff sequence: gedca]. Within its asymmetric unit, there are two unique Cu, one unique Te and two unique S atoms (Table S2†). The Cu(2) atoms adopt three-coordinated planar triangular [Cu(2)S3] motifs, where Cu(2)–S bond lengths range from 2.2173(18) to 2.183(2) Å, and S–Cu(2)–S bond angles vary from 94.33(2) to 132.83(6)°. Moreover, the sum of the three bond angles around Cu(2) is 360° (Fig. 1a). The Cu(2) atom is located at the central position of the triangular plane determined by the three S atoms (Fig. 1a), and these results all confirm that the [CuS3] motif is planar triangular. In the structure of Cu10Te4S13, six planar [Cu(2)S3] units share the S(2) atom located at the Wyckoff 2a position to form a unique [Cu(2)6S13] hexamer (Fig. 1b). As depicted in Fig. 1c, Te(IV) atoms exhibit triangular pyramid coordination with three S atoms forming [TeS3] motifs, with Te–S bond lengths of 2.3812 Å. Four [TeS3] units are connected to the hexamer by sharing the S(1) atoms of the [Cu(2)6S13] hexamer, forming a butterfly-orchid-like [Cu6Te4S13]4− zero-dimensional (0D) anion cluster (Fig. 1d). The anionic clusters stack repetitively along the a-, b-, and c-axes by symmetry operation, constituting a [Cu(2)–Te–S] 0D anionic framework, with the centers of the clusters residing at the body-centered and vertex positions within the unit cell (Fig. 1e, h and i).
3m [Pearson code: CI58; Wyckoff sequence: gedca]. Within its asymmetric unit, there are two unique Cu, one unique Te and two unique S atoms (Table S2†). The Cu(2) atoms adopt three-coordinated planar triangular [Cu(2)S3] motifs, where Cu(2)–S bond lengths range from 2.2173(18) to 2.183(2) Å, and S–Cu(2)–S bond angles vary from 94.33(2) to 132.83(6)°. Moreover, the sum of the three bond angles around Cu(2) is 360° (Fig. 1a). The Cu(2) atom is located at the central position of the triangular plane determined by the three S atoms (Fig. 1a), and these results all confirm that the [CuS3] motif is planar triangular. In the structure of Cu10Te4S13, six planar [Cu(2)S3] units share the S(2) atom located at the Wyckoff 2a position to form a unique [Cu(2)6S13] hexamer (Fig. 1b). As depicted in Fig. 1c, Te(IV) atoms exhibit triangular pyramid coordination with three S atoms forming [TeS3] motifs, with Te–S bond lengths of 2.3812 Å. Four [TeS3] units are connected to the hexamer by sharing the S(1) atoms of the [Cu(2)6S13] hexamer, forming a butterfly-orchid-like [Cu6Te4S13]4− zero-dimensional (0D) anion cluster (Fig. 1d). The anionic clusters stack repetitively along the a-, b-, and c-axes by symmetry operation, constituting a [Cu(2)–Te–S] 0D anionic framework, with the centers of the clusters residing at the body-centered and vertex positions within the unit cell (Fig. 1e, h and i).
Two types of S atoms respectively form [S(1)Cu3Te] tetrahedral and [S(2)Cu6] octahedral groups (Fig. S3†). Each Cu(1) atom is coordinated by four S atoms forming a [CuS4] tetrahedral unit (Fig. 1f), with Cu(1)–S bond lengths ranging from 2.3233(7) to 2.3233(8) Å. Notably, the Cu(1) atom located at the Wyckoff 12d position is independently occupied in crystallography, while the Cu(2) atom at the Wyckoff 12e position is partially occupied in crystallography, with a 2/3 occupancy and a 1/3 vacancy occupancy in a single cell. This partial occupancy of Cu is a common feature in many Cu-containing structures, such as CuZnPS4 (ref. 45) and Cu5Zn0.5P2S8.46 The 0D anionic framework is interconnected by the [Cu(1)S4] units with tetrahedral configurations, resulting in the ultimate three-dimensional (3D) structure of Cu10Te4S13 (Fig. 1g and j). In conclusion, Cu10Te4S13 is an interesting and unique 3D structure composed of three completely different configurations of functional units, namely the trigonal pyramid configuration [TeS3], the planar triangle [CuS3] group and the tetrahedral configuration [CuS4] unit, which are interconnected by sharing S atoms.
Additionally, the phenomenon of multiple coordination modes of metal centers is commonly observed in chalcogenides containing d10 metals.48 The crystal structure can be quantitatively evaluated through bond valence sum (BVS) and global instability index (G) calculations. As shown in Table S3,† BVS calculations for Cu(1), Cu(2), Te, S(1) and S(2) yield 1.1432, 1.1789, 3.6129, 1.1968 and 2.5050 respectively, indicating oxidation states of +1, +1, +4, −2 and −2. The calculated BVS values can be used with the expected bond valence (vi) of each ion for G calculation (eqn (1)).
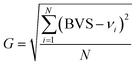 | (1) |
The calculation results of Mulliken population (MP) for bonds and atoms in Cu10Te4S13 and AgGaS2 are shown in Table S5.†52 In Cu10Te4S13, the MP of the Cu(1)–S(1) bond in the [CuS4] building block is 0.38, resulting in an average MP of 0.38 for the [CuS4] motif. In the planar π-conjugated [CuS3] building block, the MPs of the two types of Cu–S bonds are 0.30 and 0.56, with an average MP of 0.39. For AgGaS2, the average MP of the Ag–S bond is 0.29, which is smaller than that of the Cu–S bond in Cu10Te4S13 (0.38–0.39). This confirms that the covalence of the Cu–S bonds in Cu10Te4S13 is greater than that of the Ag–S bonds in AgGaS2, and the overall covalence of the planar π-conjugated [CuS3] motif is greater than that of the tetrahedral [CuS4] motif. Furthermore, the MP value for Te–S bonds in Cu10Te4S13 is 0.32, which is smaller than the 0.42 for Ga–S bonds in AgGaS2, aligning with established trends. Notably, the charge on Te atoms in Cu10Te4S13 is 0.86e, greater than that of Ga atoms in AgGaS2 (0.82e), indicating that Te indeed carries a higher charge than Ga. This finding concurs with bond valence calculations, wherein Te adopts a +4 oxidation state while Ga exhibits a +3 state.
The valence states of each element in Cu10Te4S13 were probed by X-ray photoelectron spectroscopy (XPS). The binding energy (BE) for surface charging was calibrated by taking the C 1s peak of contaminated carbon as a reference at 284.5 eV. The Cu 2p XP spectrum exhibits two main peaks at 932.5 eV and 952.3 eV, respectively, with a splitting (Δ) value of 19.8 eV, corresponding to Cu 2p1/2 and 2p3/2.53 Additionally, besides the two main peaks, there is an extremely weak satellite peak at around 947.6 eV, indicating the presence of Cu+ in the sample (Fig. 2a).54 Furthermore, we confirmed the existence of Cu+ at BE ∼569.7 eV through the Auger Cu LMM spectrum (Fig. 2b), which is in accordance with the previous reports on Cu-containing compounds.55 In the Te 3d XP spectrum, two main peaks were detected (575.4 and 585.8 eV), corresponding to Te4+ 3d5/2 and 3d3/2, confirming that the valence of Te in the sample is +4 (Fig. 2c). As depicted in Fig. 2d, the BEs of S 2p3/2 and S 2p1/2 are 162.4 eV and 163.5 eV, respectively, and the Δ value is 1.1 eV, which is typically attributed to S2−.
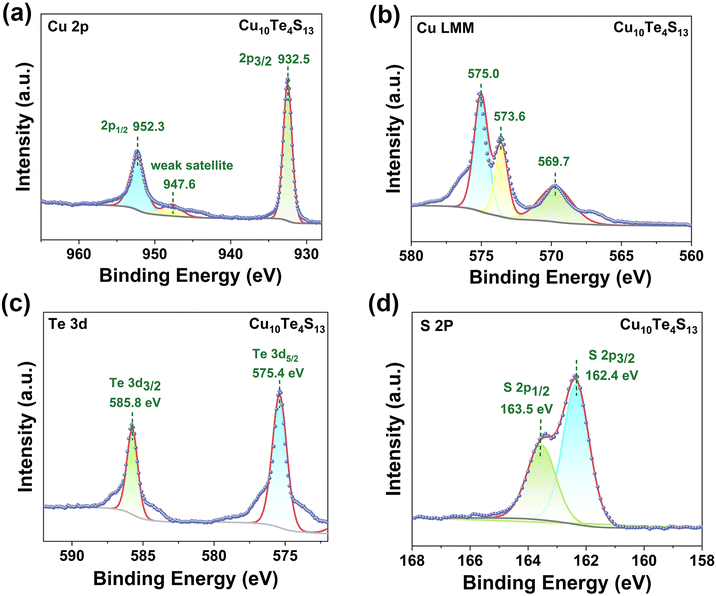 | ||
| Fig. 2 (a) Cu 2p XP spectrum; (b) Cu LMM auger XP spectrum; (c) Te 3d XP spectrum; (d) S 2p XP spectrum of Cu10Te4S13. | ||
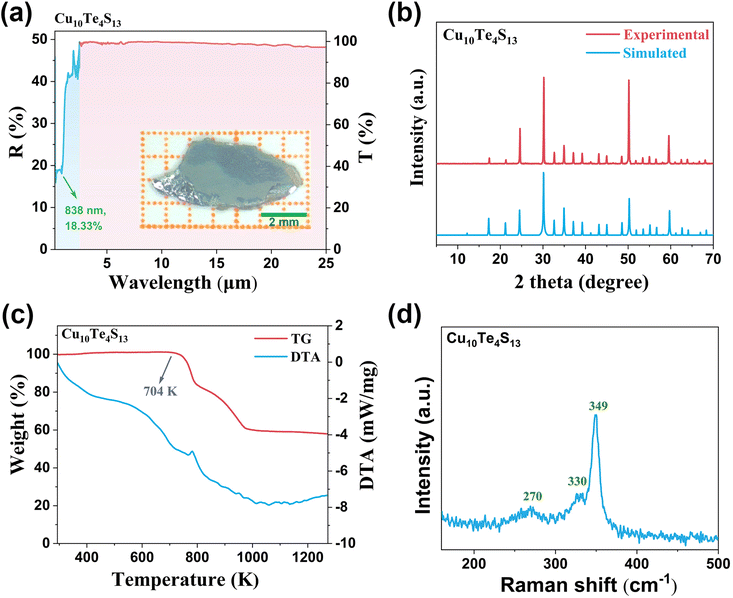
The UV-vis-NIR diffuse reflectance spectrum of Cu10Te4S13 was measured in the range of 200–2500 nm, and its bandgap was inferred to be 1.09 eV (Fig. S4†). As depicted in Fig. 3a, Cu10Te4S13 is transparent from 0.84 to 25 μm, spanning visible and MFIR spectral regions. The IR transmission spectrum of Cu10Te4S13 was tested using a single crystal after polishing. It exhibits complete transmission in the range of 2.5–25 μm, encompassing the vital MFIR atmospheric windows, and can be effectively simulated by laser sources at 1.064, 2.09, and 10.6 μm wavelengths. As shown in Fig. 3b, experimental powder X-ray diffraction (PXRD) patterns of Cu10Te4S13 matched well with calculated results, confirming the purity.
The thermogravimetric-differential thermal analysis (TG-DTA) of Cu10Te4S13 was conducted in a nitrogen atmosphere within the temperature range of 293–1273 K. As shown in Fig. 3c, Cu10Te4S13 can remain stable at 704 K. Such thermal stability is higher than that of some NLO crystals featuring pyramidal motifs, such as Li2TeSe3 (701 K),56 Hg3AsS4Cl (578 K),16 and Hg3AsS4Br (603 K).16
The Raman spectrum of Cu10Te4S13, spanning the range from 500 to 100 cm−1, is presented in Fig. 3d. The [TeS3] units within Cu10Te4S13 exhibit an ideal C3V symmetry, a crystalline feature that manifests in the Raman spectrum as precisely two distinctive vibrational bands. One of these bands is attributed to the νasym mode at 359 cm−1, while the other, positioned at 336 cm−1, corresponds to the νsym mode of the Te–S bond. Additionally, the peak at 270 cm−1 is identified as arising from Cu–S vibrational activity.57
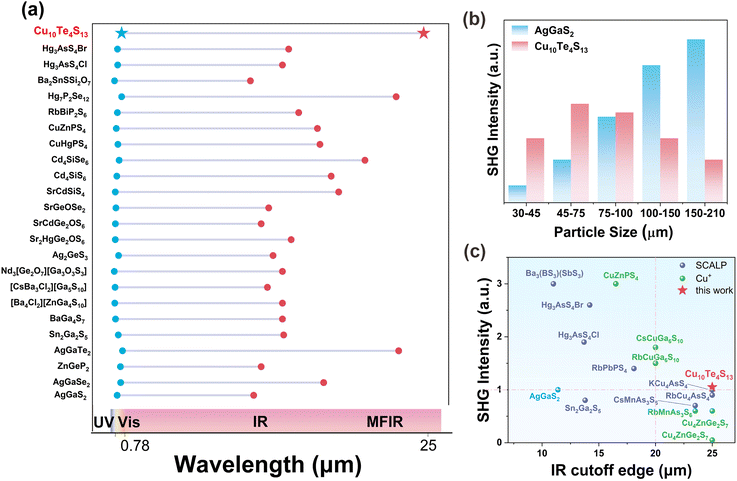
As shown in Table S6† and Fig. 4a, the low-energy end corresponding to the transmission range of Cu10Te4S13 is longer than that of current excellent IR NLO crystals, such as Hg3AsS4Cl (13.7 μm),16 Hg3AsS4Br (14.2 μm),16 AgGaS2 (11.4 μm),58 Ag2GeS3 (12.95 μm),59 Hg7P2Se12 (22.8 μm),7 AgGaTe2 (23 μm),41,60etc. This indicates that Cu10Te4S13 is a promising candidate material for MFIR NLO crystal applications.
Employing the Kurtz–Perry method, the powder SHG intensities of Cu10Te4S13 and AgGaS2 were measured under Q-switched laser irradiation at 2900 nm. As shown in Fig. 4b, Cu10Te4S13 achieves the maximum SHG intensity (3.75 × AgGaS2) in the particle size range of 30–45 μm. Its SHG intensity is greater than that of many NLO crystals characterized by pyramidal motifs, such as Na4SrAs2S8 (0.95 × AgGaS2),61 KAg2AsS4 (1.36 × AgGaS2),62 RbAg2AsS4 (1.34 × AgGaS2),62 and Ag3AsS3 (1.1 × AgGaS2).63 Generally, chalcogenides containing SCALP groups tend to exhibit greater structural anisotropy, leading to higher birefringence (Δn) and enabling phase matching in the infrared range.64 However, despite containing SCALP groups, Cu10Te4S13 cannot achieve phase matching at 2900 nm due to its cubic crystal system (Fig. 4b), which results in an isotropic structure (Δn = 0). In future work, a chemical substitution strategy65 could be considered, where alkali or alkaline earth metals with high electronegativity replace the Cu element. This would break the high symmetry of the structure and potentially enhance Δn, thereby enabling phase matching. A statistical analysis and comparison of the SHG intensities (70–110 μm) and IR cutoff edge of Cu10Te4S13 and some currently reported typical IR NLO crystal materials characterized by triangular pyramidal units were carried out. The results show that Cu10Te4S13 has good balanced properties: a long IR cutoff edge greater than 25 μm and moderate SHG effect of 1.05 × AgGaS2 in the particle size range of 70–100 μm (Fig. 4c and Table S7†). This shows that the novel [TeS3]2− group with a pair of SCALPs is a potential MFIR NLO active motif.
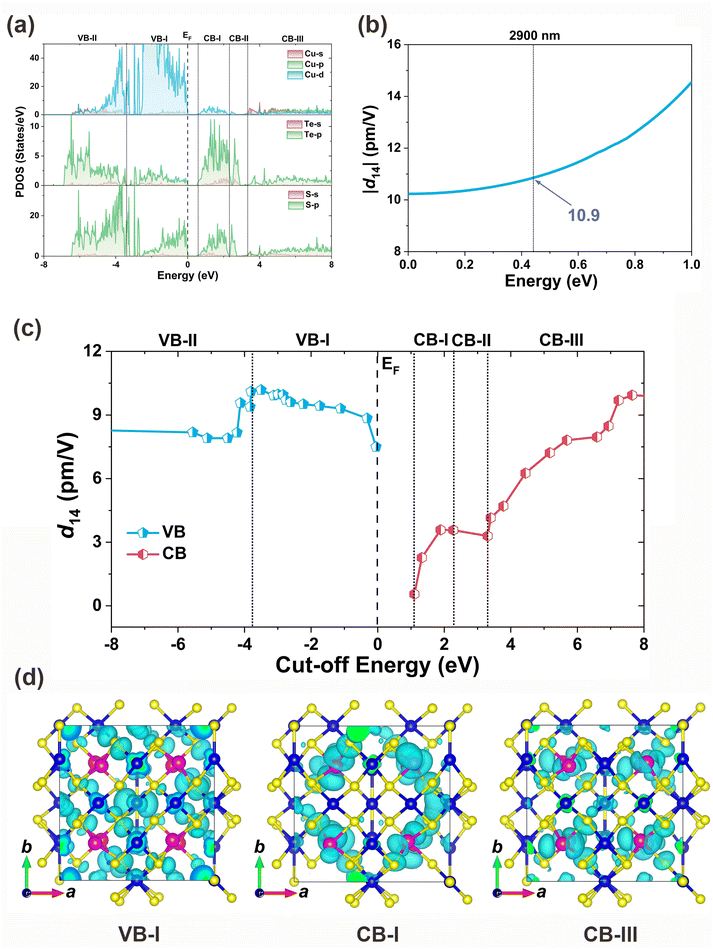
To elucidate the intrinsic structure–property relationship of Cu10Te4S13, we conducted a systematic first-principles computational study.66 Cu10Te4S13 exhibits a direct band gap of 0.82 eV based on PBE calculations. When accounting for the spin–orbit coupling effect of Te4+, the theoretical band gap decreases to 0.67 eV. For a more accurate estimation, HSE calculations were also performed, yielding a theoretical band gap of 1.42 eV (see Fig. S5† for details). Analyzing the partial density of states (PDOS) diagram can reveal the origin of optical properties. In the PDOS diagram of Cu10Te4S13, the top of the valence band (VB) is mainly composed of Cu 3d and S 3p orbitals, while the bottom of the conduction band (CB) is mainly contributed by Te 5p and S 3p orbitals (Fig. 5a). Therefore, the optical properties of Cu10Te4S13 are mainly synergistically determined by the [CuS3], [CuS4], and [TeS3] FBUs. Considering the I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m point group and Kleinman symmetry rules, Cu10Te4S13 has only one independent non-zero tensor (d14). At a wavelength of 2900 nm, the SHG tensor is calculated to be d14 = 10.9 pm V−1 (Fig. 5b), which is approximately 0.8 times more than that of d36 = 13.4 pm V−1 of AgGaS2. It should be noted that the calculated SHG value is smaller than the measured one, and this discrepancy may arise from two main factors. First, the Cu(2) sites in the actual structure are partially occupied, so we used an approximate structure in our calculations, which could lead to deviations. Second, the theoretical model assumes a perfect crystal and does not account for factors such as particle size, whereas in practice, the SHG intensity is influenced by crystal quality and particle size. For example, the SHG intensity for samples with particle sizes in the range of 74–106 μm is closer to the theoretical value. In addition, a cut-off energy dependence analysis of the SHG coefficient d14 is performed to clarify the contribution of orbitals.67 We find that its SHG effect mainly comes from the VB-I, CB-I, and CB-III regions (Fig. 5c). Through the corresponding analysis of PDOS and charge density diagram (Fig. 5d), it is found that its SHG effect is mainly contributed by the Cu 3d, S 3p, and Te 5p orbitals corresponding to the triangular [CuS3] (19.5%), tetrahedral [CuS4] (19.6%), and triangular pyramid [TeS3] (60.9%) units.
3m point group and Kleinman symmetry rules, Cu10Te4S13 has only one independent non-zero tensor (d14). At a wavelength of 2900 nm, the SHG tensor is calculated to be d14 = 10.9 pm V−1 (Fig. 5b), which is approximately 0.8 times more than that of d36 = 13.4 pm V−1 of AgGaS2. It should be noted that the calculated SHG value is smaller than the measured one, and this discrepancy may arise from two main factors. First, the Cu(2) sites in the actual structure are partially occupied, so we used an approximate structure in our calculations, which could lead to deviations. Second, the theoretical model assumes a perfect crystal and does not account for factors such as particle size, whereas in practice, the SHG intensity is influenced by crystal quality and particle size. For example, the SHG intensity for samples with particle sizes in the range of 74–106 μm is closer to the theoretical value. In addition, a cut-off energy dependence analysis of the SHG coefficient d14 is performed to clarify the contribution of orbitals.67 We find that its SHG effect mainly comes from the VB-I, CB-I, and CB-III regions (Fig. 5c). Through the corresponding analysis of PDOS and charge density diagram (Fig. 5d), it is found that its SHG effect is mainly contributed by the Cu 3d, S 3p, and Te 5p orbitals corresponding to the triangular [CuS3] (19.5%), tetrahedral [CuS4] (19.6%), and triangular pyramid [TeS3] (60.9%) units.
Conclusions
In summary, Cu10Te4S13, a novel crystal containing a trigonal pyramidal [TeS3]2− MFIR NLO-active functional unit, has been synthesized for the first time. It exhibits favorable crystal growth habits (7 × 3 × 2 mm3) and remarkable SHG efficiency (3.75 × AgGaS2 at 2900 nm). Moreover, single crystal IR transmission tests confirm that it is completely transparent across the 2.5–25 μm range. These findings indicate that Cu10Te4S13 is a promising MFIR NLO candidate material and confirm for the first time that the [TeS3]2− motif represents a potential MFIR NLO functional unit. The incorporation of the [TeS3]2− functional units, or analogous triangular pyramidal units, into future materials research may well spawn a novel class of MFIR NLO materials, poised for practical utilization, fundamentally distinct from the conventional chalcopyrite-type MFIR materials in terms of their unique structural configurations. In essence, this study opens up exciting avenues for the exploration and development of innovative MFIR NLO materials.Data availability
All supplementary data for the results of this study are available in the article and its ESI file.† Crystallographic data for Cu10Te4S13 have been deposited at the CCDC under 2391396 and can be obtained from https://www.ccdc.cam.ac.uk/structures.Author contributions
Bo Zhang: investigation, formal analysis, writing – original draft. Sheng-Hua Zhou: investigation, methodology, validation. Bing-Xuan Li: investigation, formal analysis. Xin-Tao Wu: conceptualization, writing – review & editing. Hua Lin: supervision, conceptualization, writing – review & editing. Qi-Long Zhu: supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22175175), Natural Science Foundation of Fujian Province (2022L3092 and 2023H0041), Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR118), and the Youth Innovation Promotion Association CAS (2022303). The authors thank Prof. Yong-Fan Zhang at Fuzhou University and Prof. Long-Hua Li at Jiangsu University for helping with the DFT.Notes and references
- (a) F. J. Duarte, Tunable Laser Applications, CRC Press, Boca Raton, FL, 2nd edn, 2008, ch. 2, pp. 9–12 CrossRef; (b) V. Petrov, Prog. Quantum Electron., 2015, 44, 1 CrossRef; (c) V. A. Serebryakov, E. V. Boiko, N. N. Petrishchev and A. V. Yan, J. Opt. Technol., 2010, 77, 6 CrossRef CAS; (d) X. T. Wu and L. Chen, Structure–Property Relationships in Nonlinear Optical Crystals II The IR Region. Structure, Bonding, 2012, 145, 1 Search PubMed; (e) L. Kang, M. L. Zhou, J. Y Yao, Z. S. Lin, Y. C Wu and C. T. Chen, J. Am. Chem. Soc., 2015, 137, 13049 CrossRef CAS PubMed; (f) J.-X. Zhang, P. Feng, M.-Y. Ran, X. T. Wu, H. Lin and Q. L. Zhu, Coord. Chem. Rev., 2024, 502, 215617 CrossRef CAS.
- (a) K. Wu, Y. Yang and L. Gao, Coord. Chem. Rev., 2020, 418, 213380 CrossRef CAS; (b) H. Chen, W.-B. Wei, H. Lin and X.-T. Wu, Coord. Chem. Rev., 2021, 448, 214154 CrossRef CAS; (c) H. Chen, M.-Y. Ran, W.-B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Coord. Chem. Rev., 2022, 470, 214706 CrossRef CAS; (d) X. Liu, Y.-C. Yang, M.-Y. Li, L. Chen and L.-M. Wu, Chem. Soc. Rev., 2023, 52, 8699 RSC; (e) W. Zhou and S.-P. Guo, Acc. Chem. Res., 2024, 57, 648–660 CrossRef CAS.
- A. Harasaki and K. Kato, Jpn. J. Appl. Phys., 1997, 36, 700–703 CrossRef CAS.
- B. Tell and H. M. Kasper, Phys. Rev. B, 1971, 4, 4455–4459 CrossRef.
- G. D. Boyd, T. J. Bridges, C. K. N. Patel and E. Buehler, Appl. Phys. Lett., 1972, 21, 553–555 CrossRef CAS.
- M. Sun, C. Li, J. Shi, M.-H. Lee and J. Yao, Mater. Today Phys., 2023, 36, 101166 CrossRef CAS.
- Y. Chu, H. Wang, Q. Chen, X. Su, Z. Chen, Z. Yang, J. Li and S. Pan, Adv. Funct. Mater., 2023, 34, 2314933 CrossRef.
- (a) H. Lin, L. J. Zhou and L. Chen, Chem. Mater., 2012, 24, 3406–3414 CrossRef CAS; (b) H. Lin, L. Chen, L. J. Zhou and L. M. Wu, J. Am. Chem. Soc., 2013, 135, 12914–12921 CrossRef CAS PubMed.
- R. H. Duan, P. F. Liu, H. Lin, Y. J. Zheng, J. S. Yu, X. T. Wu and L. Chen, J. Mater. Chem. C, 2017, 5, 7067–7074 RSC.
- L. Wang, D. Chu, Z. Yang, J. Li and S. Pan, Chem. Sci., 2024, 15, 6577–6582 RSC.
- M. M. Chen, S. H. Zhou, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Adv. Opt. Mater., 2022, 10, 2102123 CrossRef CAS.
- J. Li, J.-N. Li, L.-Y. Hu, J.-J. Ni, W.-D. Yao, W. Zhou, W. Liu and S.-P. Guo, Inorg. Chem., 2024, 63, 6116–6121 CrossRef CAS.
- P. Feng, S.-H. Zhou, B.-X. Li, J.-X. Zhang, M.-Y. Ran, X.-T. Wu, H. Lin and Q.-L. Zhu, ACS Appl. Mater. Interfaces, 2024, 16, 52682–52691 CrossRef CAS.
- Y. Y. Li, P. F. Liu, H. Lin, M. T. Wang and L. Chen, Inorg. Chem. Front., 2016, 3, 952–958 RSC.
- W. F. Zhou, J. J. Wu, W. L. Liu and S. P. Guo, Coord. Chem. Rev., 2023, 477, 214950 CrossRef CAS.
- H. Lin, H. Chen, Y. J. Zheng, J. S. Yu, X. T. Wu and L. M. Wu, Dalton Trans., 2017, 46, 7714–7721 RSC.
- L. Gao, J. Huang, S. Guo, Z. Yang and S. Pan, Coord. Chem. Rev., 2020, 421, 213379 CrossRef CAS.
- M. Y. Ran, A. Y. Wang, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Coord. Chem. Rev., 2023, 481, 215059 CrossRef CAS.
- F. Liang, L. Kang, Z. Lin, Y. Wu and C. Chen, Coord. Chem. Rev., 2017, 333, 57–70 CrossRef CAS.
- P. Feng, J.-X. Zhang, M.-Y. Ran, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Sci., 2024, 15, 5869–5896 RSC.
- H. Chen, M. Y. Ran, S. H. Zhou, X. T. Wu, H. Lin and Q. L. Zhu, Chin. Chem. Lett., 2023, 34, 107838 CrossRef CAS.
- M. Yan, H.-G. Xue and S.-P. Guo, Cryst. Growth Des., 2021, 21, 698–720 CrossRef CAS.
- B. Zhang, M.-Y. Ran, X.-T. Wu, H. Lin and Q.-L. Zhu, Coord. Chem. Rev., 2024, 517, 216053 CrossRef CAS.
- (a) T. K. Bera, J.-H. Song, A. J. Freeman, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, Angew. Chem., Int. Ed., 2008, 47, 7828–7832 CrossRef CAS; (b) M.-M. Chen, Z. Ma, B.-X. Li, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, J. Mater. Chem. C, 2021, 9, 1156–1163 RSC.
- (a) H. Lin, Y. Y. Li, M. Y. Li, Z. J. Ma, L. M. Wu, X. T. Wu and Q. L. Zhu, J. Mater. Chem. C, 2019, 7, 4638–4643 RSC; (b) Y. Xiao, M. M. Chen, Y. Y. Shen, P. F. Liu, H. Lin and Y. Liu, Inorg. Chem. Front., 2021, 8, 2835–2843 RSC; (c) H.-J. Zhao, H.-D. Yang, P.-F. Liu and H. Lin, Cryst. Growth Des., 2022, 22, 1437–1444 CrossRef CAS; (d) Y. Zhou, L.-T. Jiang, X.-M. Jiang, B.-W. Liu and G.-C. Guo, Chin. Chem. Lett., 2024, 35, 109740 CrossRef; (e) C. Liu, S.-H. Zhou, Y. Xiao, C. Zhang, H. Lin and Y. Liu, J. Mater. Chem. C, 2021, 9, 15407–15414 RSC.
- (a) M. M. Chen, S. H. Zhou, W. B. Wei, B. X. Li, M. Y. Ran, X. T. Wu, H. Lin and Q. L. Zhu, ACS Mater. Lett., 2022, 4, 1264–1269 CrossRef CAS; (b) V. Nguyen, B. Ji, K. Wu, B. Zhang and J. Wang, Chem. Sci., 2022, 13, 2640–2648 RSC.
- (a) M. Y. Li, B. X. Li, H. Lin, Z. J. Ma, L. M. Wu, X. T. Wu and Q. L. Zhu, Chem. Mater., 2019, 31, 6268–6275 CrossRef CAS; (b) X. H. Li, Z. H. Shi, M. Yang, W. Liu and S. P. Guo, Angew. Chem., Int. Ed., 2022, 61, e202115871 CrossRef CAS; (c) C. Zhao, B. Zhang, X. Tian, G. Zhou, J. Xu and K. Wu, Inorg. Chem. Front., 2023, 10, 5726–5733 RSC.
- (a) R. H. Duan, J. S. Yu, H. Lin, Y. J. Zheng, H. J. Zhao, S. X. Huang-Fu, M. A. Khan, L. Chen and L. M. Wu, Dalton Trans., 2016, 45, 12288–12291 RSC; (b) X. Chen, Q. Jing and K. M. Ok, Angew. Chem., Int. Ed., 2020, 59, 20323–20327 CrossRef CAS; (c) A.-Y. Wang, S.-H. Zhou, M.-Y. Ran, B.-X. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, Inorg. Chem. Front., 2024, 11, 3744–3754 RSC; (d) J. Wang, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Adv. Opt. Mater., 2022, 10, 2102673 CrossRef CAS; (e) W. F. Chen, B. W. Liu, S. M. Pei, X. M. Jiang and G. C. Guo, Adv. Sci., 2023, 10, 2207630 CrossRef CAS PubMed.
- F. Xu, X. Xu, B.-X. Li, G. Zhang, C. Zheng, J. Chen and N. Ye, Inorg. Chem. Front., 2024, 11, 2105–2115 RSC.
- W. Liu, Z. Du, B. Ahmed, Y. Zhao, J. Zheng, X. Song, Z. Wang, X. Kuang, J. Luo and S. Zhao, Adv. Opt. Mater., 2024, 12, 2400474 CrossRef CAS.
- X. Dong, H. Huang, L. Huang, Y. Zhou, B. Zhang, H. Zeng, Z. Lin and G. Zou, Angew. Chem., Int. Ed., 2024, 63, e202318976 CrossRef CAS.
- Y. Y. Yang, Y. Guo, B. B. Zhang, T. Wang, Y. G. Chen, X. H. Hao, X. X. Yu and X. M. Zhang, Inorg. Chem., 2022, 61, 1538–1545 CrossRef CAS PubMed.
- B. Zhang, C.-L. Hu, J.-G. Mao and F. Kong, Chem. Sci., 2024, 15, 18549–18556 RSC.
- M. L. Liang, Y. X. Ma, C. L. Hu, F. Kong and J. G. Mao, Chem. Mater., 2020, 32, 9688–9695 CrossRef CAS.
- Q. Wang, X. H. Dong, L. Huang, K. M. Ok, Z. E. Lin and G. H. Zou, Small, 2023, 19, e2302797 CrossRef.
- T. Wu, X. Jiang, K. Duanmu, C. Wu, Z. Lin, Z. Huang, M. G. Humphrey and C. Zhang, Adv. Sci., 2024, 11, 2306670 CrossRef CAS PubMed.
- M. Chen, Z. Liang, Y. Shui, B. Li, X. Jiang, Z. Lin and H. Liu, Inorg. Chem., 2024, 63, 12894–12900 CrossRef CAS PubMed.
- K. C. Chen, C. S. Lin, G. Peng, Y. Chen, H. Z. Huang, E. Z. Chen, Y. X. Min, T. Yan, M. Luo and N. Ye, Chem. Mater., 2022, 34, 399–404 CrossRef CAS.
- (a) P. F. Li, C. L. Hu, J. G. Mao and F. Kong, Coord. Chem. Rev., 2024, 517, 216000 CrossRef CAS; (b) R. L. Tang, M. Yan, W. D. Yao, W. L. Liu and S. P. Guo, Inorg. Chem., 2022, 61, 2333–2339 CrossRef CAS PubMed; (c) Y. P. Gong, Y. X. Ma, S. M. Ying, J. G. Mao and F. Kong, Inorg. Chem., 2019, 58, 11155–11163 CrossRef CAS.
- (a) W. Zeng, Y. Tian, X. Dong, L. Huang, H. Zeng, Z. Lin and G. Zou, Chem. Mater., 2024, 36, 2138–2146 CrossRef CAS; (b) P. F. Li, C. L. Hu, J. G. Mao and F. Kong, Chem. Sci., 2024, 15, 7104–7110 RSC; (c) M. Zhao, W. m. Dong, Y. d. Wu, D. j. Mei, S. g. Wen and T. Doert, J. Alloys Compd., 2021, 865, 158785 CrossRef CAS.
- (a) Y. She, J. Jiao, Z. Wang, J. Chai, J. Song, N. Ye, Z. Hu, Y. Wu and C. Li, Inorg. Chem. Front., 2023, 10, 6557–6565 RSC; (b) Q. Wu, J. F. Zhou, X. M. Liu, X. Jiang, Q. Zhang, Z. Lin and M. J. Xia, Inorg. Chem., 2021, 60, 18512–18520 CrossRef CAS PubMed.
- (a) X. Y. Zhou, X. Mao, P. Zhang, X. H. Dong, L. Huang, L. L. Cao, D. J. Gao and G. H. Zou, Inorg. Chem. Front., 2024, 11, 3221–3228 RSC; (b) B. Zhang, J. H. Wu, C. L. Hu, Y. F. Li, F. Kong and J. G. Mao, Inorg. Chem. Front., 2023, 10, 1328–1337 RSC.
- S. L. Nguyen, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, Inorg. Chem., 2010, 49, 9098–9100 CrossRef CAS PubMed.
- (a) M. Y. Li, Z. J. Ma, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Chem. Mater., 2020, 32, 4331–4339 CrossRef CAS; (b) X. Zhao, C. Lin, C. Wang, H. Tian, P. Dong, T. Yan, B. Li, N. Ye and M. Luo, Scr. Mater., 2024, 242, 115935 CrossRef CAS; (c) M.-Y. Li, C.-Y. Zhao, H.-P. Xu, W. Zhou, W. Liu, J. Zhu and S.-P. Guo, Inorg. Chem., 2024, 63, 18484–18488 CrossRef CAS PubMed.
- Z. Li, S. Zhang, Z. Huang, L.-D. Zhao, E. Uykur, W. Xing, Z. Lin, J. Yao and Y. Wu, Chem. Mater., 2020, 32, 3288–3296 CrossRef CAS.
- B. J. Song, Z. Ma, B. Li, X. T. Wu, H. Lin and Q. L. Zhu, Inorg. Chem., 2021, 60, 4357–4361 CrossRef CAS.
- N. Zhao, D. Lu, J. Xu, K. Wu, H. Yu and H. Zhang, Inorg. Chem. Front., 2024, 11, 4603–4610 RSC.
- (a) H. Lin, H. Chen, Y. J. Zheng, Y. K. Chen, J. S. Yu and L. M. Wu, Chem. Commun., 2017, 53, 2590–2593 RSC; (b) H. Lin, G. J. Tan, J. N. Shen, S. Q. Hao, L. M. Wu, N. Calta, C. Malliakas, S. Wang, C. Uher, C. Wolverton and M. G. Kanatzidis, Angew. Chem., Int. Ed., 2016, 55, 11431–11436 CrossRef CAS PubMed; (c) Z. Li, Y. Yang, Y. Guo, W. Xing, X. Luo, Z. Lin, J. Yao and Y. Wu, Chem. Mater., 2019, 31, 1110–1117 CrossRef CAS; (d) P.-F. Liu, X. Li, J. Li, J. Zhu, Z. Tong, M. Kofu, M. Nirei, J. Xu, W. Yin, F. Wang, T. Liang, L. Xie, Y. Zhang, D. J. Singh, J. Ma, H. Lin, J. Zhang, J. He and B.-T. Wang, Natl. Sci. Rev., 2025, 12, nwae216 Search PubMed.
- I. D. Brown, Z. Kristallogr., 1992, 199, 255–272 CrossRef CAS.
- J. C. Kelly, C. E. Hoyer, S. H. Shin, J. Bin Cho, S. S. Stoyko, A. Bonnoni, A. J. Craig, K. E. Rosello, E. T. O'Hara, J. I. Jang and J. A. Aitken, J. Alloys Compd., 2023, 970, 172437 CrossRef.
- T. Bernert and A. Pfitzner, Z. Kristallogr., 2005, 220, 968–972 CAS.
- I. Mayer, Int. J. Quantum Chem., 1984, 26, 151–154 CrossRef CAS.
- N. Ma, F. Li, J.-G. Li, X. Liu, D.-B. Zhang, Y.-Y. Li, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2021, 143, 18490–18501 CrossRef CAS PubMed.
- (a) K. Huang, K. Tang, M. Wang, Y. Wang, T. Jiang and M. Wu, Adv. Funct. Mater., 2024, 34, 2315324 CrossRef CAS; (b) I. Platzman, R. Brener, H. Haick and R. Tannenbaum, J. Phys. Chem. C, 2008, 112, 1101–1108 CrossRef CAS.
- P. Liu and E. J. M. Hensen, J. Am. Chem. Soc., 2013, 135, 14032–14035 CrossRef CAS.
- C. Preitschaft, M. Zabel and A. Pfitzner, Z. Anorg. Allg. Chem., 2005, 631, 1227–1232 CrossRef CAS.
- A. Pfitzner, Inorg. Chem., 1998, 37, 5164–5167 CrossRef CAS.
- D. N. Nikogosyan, Nonlinear Optical Crystals: A Complete Survey, Springer, Berlin, 2005 Search PubMed.
- H. Chen, M. Y. Ran, S. H. Zhou, X. T. Wu and H. Lin, Adv. Opt. Mater., 2024, 12, 2401100 CrossRef CAS.
- L. Bai, Z. Lin, Z. Wang, C. Chen and M.-H. Lee, J. Chem. Phys., 2004, 120, 8772–8778 CrossRef CAS PubMed.
- Z. Xiong, A. Yao, Z. Li, X. Meng, Q. Wu, G. Deng, L. Kang and M. Xia, Inorg. Chem., 2024, 63, 14816–14820 CrossRef CAS PubMed.
- J. Tang, A. Yao, Q. Wu, C. Tang, J. Wu, L. Kang, M. Xia, W. Yin, B. Kang and J. Deng, Adv. Opt. Mater., 2024, 12, 2401126 CrossRef CAS.
- K. F. Hulme, O. Jones, P. H. Davies and M. V. Hobden, Appl. Phys. Lett., 1967, 10, 133–135 CrossRef CAS.
- (a) C. Zhang, M.-Y. Ran, X. Chen, S.-H. Zhou, H. Lin and Y. Liu, Inorg. Chem. Front., 2023, 10, 3367–3374 RSC; (b) Z. Bai and K. M. Ok, Inorg. Chem. Front., 2023, 10, 1919–1925 RSC; (c) Y.-F. Shi, S.-H. Zhou, P.-F. Liu, X.-T. Wu, H. Lin and Q.-L. Zhu, Inorg. Chem. Front., 2023, 10, 4425–4434 RSC; (d) C. Liu, S.-H. Zhou, C. Zhang, Y.-Y. Shen, X.-Y. Liu, H. Lin and Y. Liu, Inorg. Chem. Front., 2022, 9, 478–484 RSC; (e) C.-L. Hu, Q.-Q. Chen, F. Kong and J.-G. Mao, Inorg. Chem. Front., 2024, 11, 3150–3158 RSC; (f) P.-F. Li, C.-L. Hu, B. Zhang, J.-G. Mao and F. Kong, Chin. Chem. Lett., 2025, 36, 110588 Search PubMed.
- (a) H. Lin, W.-B. Wei, H. Chen, X.-T. Wu and Q.-L. Zhu, Coord. Chem. Rev., 2020, 406, 213150 CrossRef CAS; (b) G. Zou and K. M. Ok, Chem. Sci., 2020, 11, 5404–5409 RSC; (c) M. Y. Ran, A. Y. Wang, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Coord. Chem. Rev., 2023, 481, 215059 CrossRef CAS.
- (a) M. Y. Ran, S. H. Zhou, W. B. Wei, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Small, 2023, 19, 2300248 CrossRef CAS PubMed; (b) A.-Y. Wang, S. H. Zhou, M.-Y. Ran, X. T. Wu, H. Lin and Q. L. Zhu, Chin. Chem. Lett., 2024, 35, 109377 CrossRef CAS; (c) M. Y. Ran, S. H. Zhou, W. B. Wei, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Small, 2024, 20, 2304563 CrossRef CAS PubMed.
- (a) H. D. Yang, M. Y. Ran, S. H. Zhou, X. T. Wu, H. Lin and Q. L. Zhu, Chem. Sci., 2022, 13, 10725 RSC; (b) J.-X. Zhang, Q.-G. Yue, S.-H. Zhou, X.-T. Wu, H. Lin and Q.-L. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202413276 CrossRef CAS PubMed; (c) M. Y. Ran, S. H. Zhou, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Mater. Today Phys., 2024, 44, 101442 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental and theoretical results, and additional tables and figures. CCDC 2391396. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07322c |
| This journal is © The Royal Society of Chemistry 2025 |