Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-catalysed asymmetric cascade transformations of 4-alken-2-ynyl carbonates to construct complex frameworks†
Ze-Liang
He
a,
Li
Li
a,
Zhi-Chao
Chen
*a,
Wei
Du
 a and
Ying-Chun
Chen
a and
Ying-Chun
Chen
 *ab
*ab
aKey Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry and Sichuan Province, Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu 610041, China. E-mail: chenzhichao@scu.edu.cn; ycchen@scu.edu.cn; Fax: +86 28 85502609
bCollege of Pharmacy, Third Military Medical University, Shapingba, Chongqing 400038, China
First published on 27th December 2024
Abstract
As a class of readily available and multifunctional building blocks, the chemistry of 4-alken-2-ynyl carbonates remains to be explored. Presented herein is a palladium-catalysed cascade transformative reaction between 4-alken-2-ynyl carbonates and ortho-functionalised activated alkenes. Achiral 1,1-bisalkyl-4-alken-2-ynyl carbonates undergo highly regioselective propargylic substitution with ortho-hydroxyphenyl-tethered activated alkenes, and an auto-tandem vinylogous addition, unusual central-carbon Tsuji–Trost alkylation, protonation and β-H elimination process is followed to furnish fused and spirocyclic frameworks with high structural complexity. Even kinetic transformations with racemic 1-monoalkylated 4-alken-2-ynyl carbonates can be accomplished in the assemblies with ortho-aminophenyl-tethered activated alkenes to afford the analogous alkaloid architectures. This palladium-catalysed auto-tandem protocol exhibits excellent chemo-, regio-, stereoselectivity and reaction efficacy, and substantial functionality compatibility is also observed.
Introduction
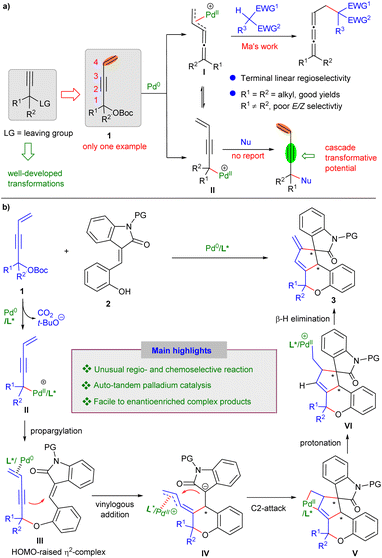
Owing to their ready availability and versatile reactivity, propargylic substrates have emerged as valuable reagents in organic synthesis.1 Upon the oxidative addition of propargylic alcohol derivatives with transition metals, the formed allenyl or propargyl metal species can undergo a variety of transformations, such as allenylation,2 1,3-dienylation,3 propargylation4 and others,5 to afford structurally diverse products, even enantioselectively. In addition, pre-installation of a pendent alkene moiety into propargylic skeleton, as in 4-alken-2-ynyl carbonates 1, would further enrich the transformative potential, since more reactive sites might be envisaged upon activation by transition metals. In this regard, Ma recently revealed that vinylidene-π-allyl palladium intermediates I, which were generated in situ from 1,1-bisalkyl-substituted 4-alken-2-ynyl carbonates 1 and Pd0, could undergo Tsuji−Trost-type reaction with stabilized carbon-centred nucleophiles to furnish achiral 1,2,3-butatrienes with exclusive linear selectivity.6 Unfortunately, the reactivity of racemic propargylic substrates (R1 ≠ R2) was not well investigated in this work, and poor E/Z selectivity was observed for the sole example. In addition, switching the regioselectivity of nucleophilic substitution to the more sterically hindered C1 position via potential species II is a formidable challenge, but would provide valuable opportunities in latent reaction design owing to the versatile features of the enyne products (Scheme 1a).7 Taking advantage of the multiple catalytic roles of palladium,8 here, we would like to present an unprecedented cascade transformative reaction between 4-alken-2-ynyl carbonates 1 and ortho-functionalised activated alkenes, such as 3-olefinic oxindoles 2. As outlined in Scheme 1b, the oxidative addition of Pd0 to carbonates 1 would generate propargylic palladium complexes II. In sharp contrast to Ma's work, exclusive C1-regioselective substitution with O-centered nucleophiles was observed to deliver multifunctional propargylated intermediates III. By employing our recently developed π-Lewis base catalysis,9 Pd0 further enhanced the nucleophilicity of the alkyne moiety by forming η2-complexes with increased highest occupied molecular orbital (HOMO) energy levels, thus facilitating intramolecular vinylogous addition to the 3-olefinic oxindole motif to produce ene-π-allyl-Pd intermediates IV. Intriguingly, an unusual nucleophilic attack on the central carbon of π-allylpalladium-type species occurred to afford palladacyclobutanes V.10 Subsequent protonation and β-H elimination delivered fused and spirocyclic architectures 3 with high molecular complexity.3,11 It is noteworthy that highly regio-, chemo- and enantioselective assemblies were achieved in this palladium-based auto-tandem catalysis.12Results and discussion
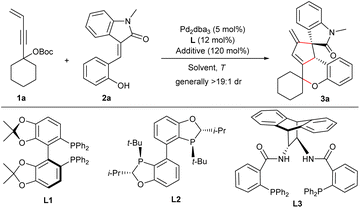
Reaction optimisation
The reaction of 4-alken-2-ynyl carbonate 1a and (E)-3-(2-hydroxybenzylidene)oxindole 2a was initially examined in MeCN at 80 °C. The reaction proceeded smoothly in the presence of Pd(PPh3)4, and product 3a was obtained straightforwardly in a good yield through the proposed pathway as virtually a single diastereomer (Table 1, entry 1). Next, we turned our attention to the enantioselective synthesis of 3a. After a brief survey of various chiral ligands, we quickly found that bisphosphine ligands exhibited good catalytic performance for the current transformations.13 Fair enantioselectivity was observed for ligands L1 and L2 in combination with Pd2dba3 (entries 2 and 3), and ligand L3 having an anthracene diamine backbone substantially boosted both the efficiency and enantiocontrol (entry 4). Lowering the temperature enhanced the enantioselectivity, although a longer time was required to achieve better conversions (entry 5). A solvent survey suggested enantiocontrol was slightly improved in polar solvents (entries 6–8), and a higher yield was obtained in diluted DMA (entry 9). Then, several additives were screened,13 and KHCO3 was found to be beneficial to the yield (entry 10). A satisfactory yield with an excellent ee value was finally obtained by employing 1.5 equivalents of carbonate 1a at 45 °C (entry 11), whereas the yield was reduced significantly when using 2.5 mol% of Pd2dba3 (entry 12).| Entry | L | Solvent | T (°C) | t (h) | Additive | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|---|
a Unless noted otherwise, reactions were performed with carbonate 1a (0.1 mmol), alkene 2a (0.12 mmol), Pd2dba3 (5 mol%), ligand L (12 mol%) and additive (1.2 equiv.) in degassed solvent (1.0 mL) under Ar.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase, and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr was generally obtained through 1H NMR analysis.
d With Pd(PPh3)4 (5 mol%).
e In DMA (2.0 mL).
f With 1a (0.15 mmol) and 2a (0.1 mmol).
g With Pd2dba3 (2.5 mol%) and L3 (6 mol%). 1 dr was generally obtained through 1H NMR analysis.
d With Pd(PPh3)4 (5 mol%).
e In DMA (2.0 mL).
f With 1a (0.15 mmol) and 2a (0.1 mmol).
g With Pd2dba3 (2.5 mol%) and L3 (6 mol%).
|
|||||||
| 1d | — | MeCN | 80 | 36 | — | 78 | — |
| 2 | L1 | MeCN | 80 | 24 | — | 56 | 29 |
| 3 | L2 | MeCN | 80 | 24 | — | 33 | 58 |
| 4 | L3 | MeCN | 80 | 24 | — | 63 | 74 |
| 5 | L3 | MeCN | 50 | 48 | — | 63 | 85 |
| 6 | L3 | NMP | 50 | 48 | — | 58 | 87 |
| 7 | L3 | DMF | 50 | 48 | — | 62 | 85 |
| 8 | L3 | DMA | 50 | 48 | — | 57 | 88 |
| 9e | L3 | DMA | 50 | 48 | — | 60 | 88 |
| 10e | L3 | DMA | 50 | 48 | KHCO3 | 73 | 89 |
| 11e,f | L3 | DMA | 45 | 60 | KHCO3 | 72 | 91 |
| 12e,f,g | L3 | DMA | 45 | 60 | KHCO3 | 48 | 91 |
Substrate scope and limitations
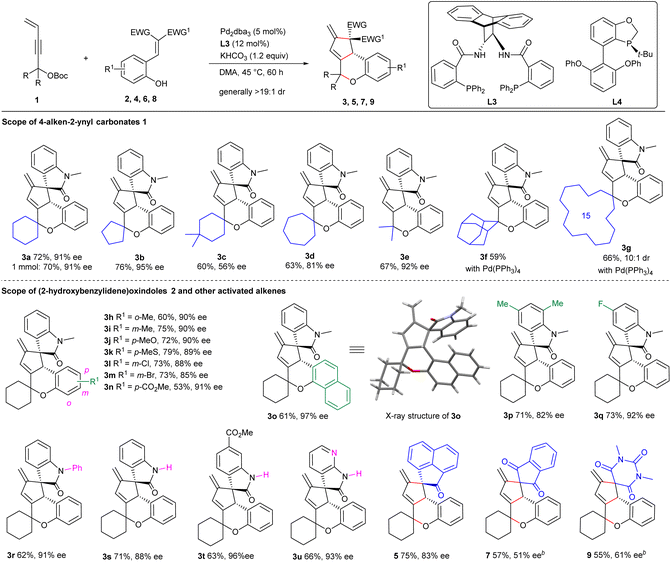
Under the optimised conditions, the scope of the 3-vinyl propargylic carbonates 1 was first explored in the reactions with (E)-3-(2-hydroxybenzylidene) oxindole 2a under the catalysis of Pd2dba3/L3 with KHCO3 as an additive. As summarised in Scheme 2, an array of carbonates 1 derived from different cyclic ketones reacted smoothly with 2a, generally affording the corresponding products 3a–3d in moderate yields with remarkable diastereo- and enantioselectivity, even in a 1.0 mmol scale reaction (product 3a), whereas a moderate ee value was observed for product 3c bearing a 4,4-dimethylcyclohexane moiety. Pleasingly, product 3e having gem-dimethyl groups was furnished with comparably good results. Unfortunately, no apparent conversion was observed when using propargylic carbonates bearing an admantyl or cyclopentadecyl group, while racemic 3f and 3g could be obtained in moderate yields catalysed by Pd(PPh3)4. Next, the scope of functionalized alkenes 2 was evaluated (Scheme 2). Substrates with a broad range of electron-donating and -withdrawing groups at various positions of the phenyl or oxindole unit were well tolerated, generally affording the desired products 3h–3q in moderate yields with high levels of enantioselectivity. Notably, similarly good data were obtained for (7-aza)oxindoles 2 having an N-phenyl or even a free NH group (products 3r–3u).In addition to 3-olefinic oxindoles 2, activated alkenes derived from other skeletons were also applicable. As outlined in Scheme 2, benzylideneacenaphthenone 4 was successfully assembled with carbonate 1a to deliver 5 in good yield and enantioselectivity under the standard conditions. Moreover, both activated alkenes 6 and 8 condensed from 1,3-indandione and barbituric acid with salicylaldehyde, respectively, proved to be reliable counterparts in the reactions with 1a under the catalysis of Pd2dba3/L4, albeit with moderate enantiocontrol (products 7 and 9). These results not only showcased the robustness of the current catalytic strategy, but also enriched the structural diversity of the frameworks constructed.
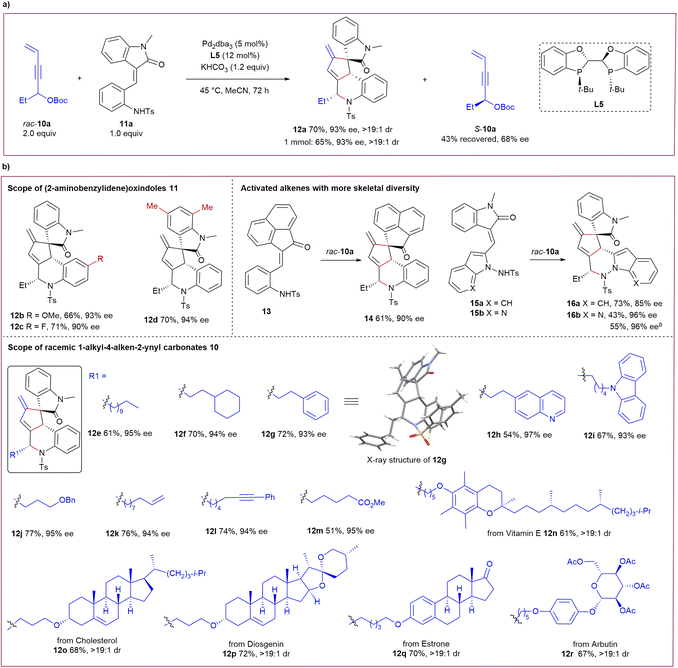
The successful cascade transformations of achiral 1,1-bisalkyl-substituted 4-alken-2-ynyl carbonates 1 inspired us to investigate the potential application of more challenging racemic propargylic carbonates. As illustrated in Scheme 3a, gratifyingly, 1-ethyl carbonate rac-10a (2 equiv.) could be efficiently utilised in similar asymmetric cascade transformations with (2-aminobenzylidene) oxindole 11a catalysed by Pd2dba3 and ligand L5, furnishing an analogous alkaloid architecture 12a in a moderate yield with excellent stereoselectivity, even on a larger scale. In addition, simultaneous kinetic resolution for recovered rac-10a was observed, albeit with moderate enantioselectivity.14
The substrate scope for this type of kinetic transformation is substantial. As summarised in Scheme 3b, good yields and high enantioselectivity were uniformly obtained for activated alkenes 11 with different substituents on the aryl unit under the standard catalytic conditions (products 12b–12d). Activated alkene 13 and newly designed 15 smoothly participated in the reactions with rac-10a to produce complex frameworks 14 and 16a–16b with high efficiency. Importantly, a spectrum of racemic propargylic carbonates 10 with diverse 1-alkyl substitutions, including those bearing various functionalities, were compatible in the reactions with activated alkene 11a, yielding products 12e–12m with high enantiocontrol. This method also provides an efficient tool for the late-stage modification of bioactive molecules. A broad range of chiral drugs (or their fragments) containing a 3-vinyl motif were competent in this process, which led to the complex products 12n–12r in moderate yields with excellent diastereoselectivity.
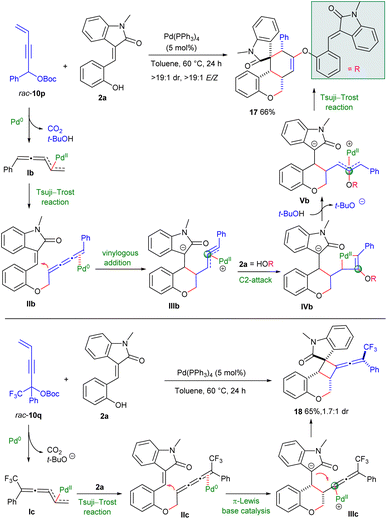
Of particular note, 1-phenyl-substituted carbonate rac-10p underwent O-allylic alkylation with (E)-3-(2-hydroxybenzylidene) oxindole 2a with distinct terminal regioselectivity under the catalysis of Pd(PPh3)4,6 probably resulting from the generation of thermally more stable 1-phenyl allenyl-π-allyl species Ib. The formed 1,2,3-butatriene intermediate IIb could be similarly HOMO-activated by Pd(0) via a π-Lewis base pattern, thus facilitating intramolecular vinylogous addition to the 3-olefinic oxindole motif. Interestingly, the resultant η3-propargylpalladium species IIIb was C2-attacked by another molecule of 2a, delivering palladacyclobutene intermediate IVb. Subsequent protonation and intramolecular Tsuji–Trost reaction of Vb would furnish spirocyclic product 17 with high diastereo- and E/Z-selectivity (Scheme 4).5 Moreover, tertiary propargylic carbonate rac-10q was found to undergo similar Tsuji–Trost reaction/vinylogous addition with 2a efficiently through intermediates Ic and IIc, respectively, whereas the formed η1-allenylpalladium moiety of IIIc was intramolecularly captured by enolate to provide intriguing cyclobutane-fused chromanone 18 having a tetrasubstituted exocyclic allene motif in a moderate yield with fair diastereoselectivity.2g–i Although the asymmetric variants were not applicable at the current stage, these explorations clearly demonstrated the versatile reactivity of 4-alken-2-ynyl carbonates and significantly enriched the product diversity.
Synthetic transformations
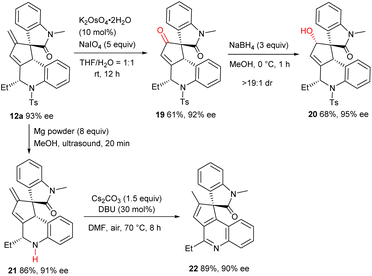
Further transformations successfully exemplified the synthetic utility of the multifunctional products. As depicted in Scheme 5, osmium-catalysed oxidative cleavage of the terminal olefin moiety of 12a afforded ketone 19 in a moderate yield, which was further reduced to alcohol 20 with exclusive diastereoselectivity. Additionally, the N-Ts group was efficiently removed to deliver amine 21, and fused quinoline 22 was obtained in high yield via a base-promoted isomerization/oxidative aromatization process. It should be noted that the spirooxindole-fused polycycles and their derivatives are core subunits of many bioactive natural products and drug candidates.15Conclusions
In summary, we successfully developed a cascade transformative reaction between 4-alken-2-ynyl carbonates and ortho-functionalized activated alkenes under auto-tandem palladium catalysis. This process showed high regio-, chemo-, and stereoselectivity through integrating classical palladium-mediated nucleophilic substitution with our newly uncovered π-Lewis base catalysis into a one-pot fashion. Both achiral 1,1-bisalkyl- and racemic 1-alkyl-4-alken-2-ynyl carbonates with broad substitution patterns underwent C1-selective propargylation to afford enyne intermediates, and fused and spirocyclic frameworks with high structural complexity were finally furnished enantioselectively upon tandem palladium catalysis, which proceeded via a cascade vinylogous addition, unusual central carbon Tsuji–Trost alkylation, protonation and β-H elimination process. In contrast, 1-aryl-substituted 4-alken-2-ynyl carbonates favoured terminal substitution to generate 1,2,3-butatriene intermediates, and regiodivergent transformations gave distinct polycyclic architectures, albeit in a racemic pattern. This work exhibits the versatile reaction potential of multifunctional 4-alken-2-ynyl carbonates, which offer a platform to construct structurally complex compounds via auto-tandem catalysis. More results will be reported in due course.Data availability
The data that support the findings of this study are available in the ESI† or on request from the corresponding author.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from NSFC (21931006 and 21921002). We thank Dr Meng Yang from College of Chemistry, Sichuan University, for X-ray diffraction analysis.Notes and references
- For selected reviews, see: (a) C. Q. O'Broin and P. J. Guiry, J. Org. Chem., 2020, 85, 10321–10333 CrossRef PubMed; (b) Y. Nishibayashi, Chem. Lett., 2021, 50, 1282–1288 CrossRef CAS; (c) M. Dai, L. Song and L.-A. Chen, Sci. China:Chem., 2024, 67, 1384–1396 CrossRef CAS.
- For selected examples, see: (a) S. Teng, Z. Jiao, Y. R. Chi and J. S. Zhou, Angew. Chem., Int. Ed., 2020, 59, 2246–2250 CrossRef CAS PubMed; (b) S. Teng, Y. R. Chi and J. S. Zhou, Angew. Chem., Int. Ed., 2021, 60, 4491–4495 CrossRef CAS PubMed; (c) Y. Jin, H. Wen, F. Yang, D. Ding and C. Wang, ACS Catal., 2021, 11, 13355–13362 CrossRef CAS; (d) X. Xu, M. Wang, L. Peng and C. Guo, J. Am. Chem. Soc., 2022, 144, 21022–21029 CrossRef CAS PubMed; (e) D. Posevins, A. Bermejo-López and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2021, 60, 22178–22183 CrossRef CAS PubMed; (f) H. Wu, Z. Zheng, K. Zhang, J. Kajanus, M. J. Johansson, A. Córdova and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2023, 62, e202314512 CrossRef CAS PubMed; (g) H. Wang, H. Qian, J. Zhang and S. Ma, J. Am. Chem. Soc., 2022, 144, 12619–12626 CrossRef CAS PubMed; (h) H. Wang, H. Luo, Z.-M. Zhang, W.-F. Zheng, Y. Yin, H. Qian, J. Zhang and S. Ma, J. Am. Chem. Soc., 2020, 142, 9763–9771 CrossRef CAS PubMed; (i) W.-F. Zheng, W. Zhang, C. Huang, P. Wu, H. Qian, L. Wang, Y.-L. Guo and S. Ma, Nat. Catal., 2019, 2, 997–1005 CrossRef CAS.
- For selected examples, see: (a) T. M. Locascio and J. A. Tunge, Chem.–Eur. J., 2016, 22, 18140–18146 CrossRef CAS PubMed; (b) C. Q. O'Broin and P. J. Guiry, Org. Lett., 2020, 22, 879–883 CrossRef PubMed; (c) M. Dai, Z. Sun and L.-A. Chen, Angew. Chem., Int. Ed., 2022, 61, e202203835 CrossRef CAS PubMed; (d) J. Xu, Z. Ge, K. Ding and X. Wang, JACS Au, 2023, 3, 2862–2872 CrossRef CAS PubMed; (e) X.-Q. Chu, R. Cheng and C.-J. Li, ACS Catal., 2024, 14, 574–584 CrossRef CAS.
- For selected examples, see: (a) M. J. Ardolino and J. P. Morken, J. Am. Chem. Soc., 2012, 134, 8770–8773 CrossRef CAS PubMed; (b) K. Nakajima, M. Shibata and Y. Nishibayashi, J. Am. Chem. Soc., 2015, 137, 2472–2475 CrossRef CAS PubMed; (c) R.-Q. Wang, C. Shen, X. Cheng, X.-Q. Dong and C.-J. Wang, Chem. Commun., 2022, 58, 8552–8555 RSC; (d) J. Zhang, X. Chang, X. Xu, H. Wang, L. Peng and C. Guo, Nat. Commun., 2022, 13, 7049 CrossRef CAS PubMed; (e) G.-Y. Han, P.-F. Su, Q.-Q. Pan, X.-Y. Liu and X.-Z. Shu, Nat. Catal., 2024, 7, 12–20 CrossRef CAS; (f) Q. Hu, B. Wei, M. Wang, M. Liu, X.-W. Chen, C.-K. Ran, G. Wang, Z. Chen, H. Li, J. Song, D.-G. Yu and C. Guo, J. Am. Chem. Soc., 2024, 146, 14864–14874 CrossRef CAS PubMed.
- For selected examples, see: (a) T. D. Montgomery and V. H. Rawal, Org. Lett., 2016, 18, 740–743 CrossRef CAS PubMed; (b) L. Ding and S.-L. You, Org. Lett., 2018, 20, 6206–6210 CrossRef CAS PubMed; (c) N. Chauhan, S. Pradhan and M. K. Ghorai, J. Org. Chem., 2019, 84, 1757–1765 CrossRef CAS PubMed; (d) M. Abe, M. Kawamoto, A. Mizukami, T. Kimachi and K. Inamoto, J. Org. Chem., 2024, 89, 10037–10046 Search PubMed.
- (a) C. Li, Z. Zhou, Y. Li, Y. Guo and S. Ma, Chem. Commun., 2023, 59, 3727–3730 RSC; (b) Z.-L. Wang and R. Zhu, J. Am. Chem. Soc., 2024, 146, 19377–19385 Search PubMed.
- (a) K. Kant, C. K. Patel, R. Reetu, Y. A. Teli, P. Naik, S. Some, C. K. Hazra, N. Aljaar, A. K. Atta and C. C. Malakar, Synthesis, 2025, 57, 39–70 Search PubMed; (b) L. Fu, S. Greßies, P. Chen and G. Liu, Chin. J. Chem., 2020, 38, 91–100 CrossRef CAS.
- (a) L. F. Tietze, H. Ila and H. P. Bell, Chem. Rev., 2004, 104, 3453–3516 CrossRef CAS PubMed; (b) S. Sain, S. Jain, M. Srivastava, R. Vishwakarma and J. Dwivedi, Curr. Org. Synth., 2019, 16, 1105–1142 CrossRef CAS PubMed.
- For a review, see: (a) Z.-C. Chen, Q. Ouyang, W. Du and Y.-C. Chen, J. Am. Chem. Soc., 2024, 146, 6422–6437 CrossRef CAS PubMed ; for selected examples, see:; (b) Q. He, L. Zhu, Z.-H. Yang, B. Zhu, Q. Ouyang, W. Du and Y.-C. Chen, J. Am. Chem. Soc., 2021, 143, 17989–17994 CrossRef CAS PubMed; (c) Z. Zhao, J.-L. Zheng, W. Du and Y.-C. Chen, Chem Catal., 2022, 2, 3174–3184 CrossRef CAS.
- For selected examples, see: (a) H. M. R. Hoffmann, A. R. Otte, A. Wilde, S. Menzer and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1995, 34, 100–102 CrossRef CAS; (b) R. Shintani, S. Park and T. Hayashi, J. Am. Chem. Soc., 2007, 129, 14866–14867 CrossRef CAS PubMed; (c) R. Shintani, T. Tsuji, S. Park and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 7508–7513 CrossRef CAS PubMed; (d) S. Duan, B. Cheng, X. Duan, B. Bao, Y. Li and H. Zhai, Org. Lett., 2018, 20, 1417–1420 CrossRef CAS PubMed; (e) B. M. Trost, Z. Jiao, Y. Liu, C. Min and C.-I. J. Hung, J. Am. Chem. Soc., 2020, 142, 18628–18636 CrossRef CAS PubMed; (f) L. Yang, Z. Tao, H.-D. Xu, M.-H. Shen and H. Chu, Org. Lett., 2024, 26, 5782–5787 CrossRef CAS PubMed.
- For preliminary mechanism investigation, see the ESI†.
- (a) D. E. Fogg and E. N. dos Santos, Coord. Chem. Rev., 2004, 248, 2365–2379 CrossRef CAS; (b) N. Shindoh, Y. Takemoto and K. Takasu, Chem.–Eur. J., 2009, 15, 12168–12179 CrossRef CAS PubMed.
- For more details, see the ESI†.
- For kinetic transformations of racemic propargylic derivatives via palladium catalysis, see ref. 2g−i.
- For selected examples, see: (a) M. Banerjee, H. Liu, O. Wiest, J. Zartman and B. L. Ashfeld, ChemMedChem, 2022, 17, e202100512 CrossRef PubMed; (b) T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao and J. Kobayashi, J. Org. Chem., 2005, 70, 9430–9435 CrossRef CAS PubMed; (c) C.-B. Cui, H. Kakeya and H. Osada, J. Antibiot., 1996, 49, 832–835 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data for new compounds, NMR and HRMS spectra, and HPLC chromatograms, CIF files of enantiopure 3o, 12g and racemic 17, 18, 20 (CIF). CCDC 2381131–2381135. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07823c |
| This journal is © The Royal Society of Chemistry 2025 |