Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterogeneously catalyzed supramolecular polymerization: essential roles of nucleation and fragmentation-induced autocatalysis in chiral transfer†
Peichen
Shi
,
Ganyu
Chen
*,
Qiang
Chen
,
Huiting
Wu
,
Suixu
Li
,
Xiaoyu
Cao
 *,
Liulin
Yang
*,
Liulin
Yang
 * and
Zhongqun
Tian
* and
Zhongqun
Tian
 *
*
State Key Laboratory of Physical Chemistry of Solid Surface, Key Laboratory of Chemical Biology of Fujian Province, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, P. R. China. E-mail: gychen321@163.com; xcao@xmu.edu.cn; llyang@xmu.edu.cn; zqtian@xmu.edu.cn
First published on 18th February 2025
Abstract
The complexity of multi-component molecular assembly demands precise control strategies to enhance both efficiency and selectivity. Heterogeneous nucleation and the autocatalytic secondary pathway, as key regulatory strategies, have attracted widespread attention for their crucial roles in crystal growth and amyloid protein aggregation. Here, we apply a heterogeneous nucleation strategy to supramolecular polymer systems and report the first direct observation of surface-enrichment-induced primary nucleation and a spontaneous fragmentation-driven autocatalytic secondary process. A heterogeneous nucleating agent promotes primary nucleation, facilitating supramolecular chiral induction. The resulting chiral polymers undergo a catalytic cycle of fragmentation and re-growth at their termini, with the fragments also acting as seeds for nucleation and growth. These pathways play a crucial role in the polymerization process and are essential for chiral transfer and asymmetry amplification, enabling the achievement of maximum enantioselectivity with as little as 0.5% molar equivalent of the heterogeneous nucleating agent. Furthermore, we reveal the existence of an optimal equivalent in their catalytic kinetics, arising from a surface assembly mechanism. In this mechanism, monomers adsorbed on the surface of the heterogeneous nucleating agent assemble with those in solution, rather than through surface diffusion and assembly. This process resembles the surface-catalyzed Eley–Rideal mechanism. Our study highlights the potential of heterogeneous nucleation as an effective strategy for controlling supramolecular polymerization and offers new insights into its underlying mechanism.
Introduction
Supramolecular polymerization, observed in both natural and synthetic systems, involves the polymerization of monomers through non-covalent interactions, exhibiting dynamic and reversible behavior.1–3 While prevalent in biological systems such as actin filaments4 and tubulin,5 achieving the precise control evident in these systems remains challenging in synthetic supramolecular polymers. As a result, researchers are increasingly focusing on kinetically regulating supramolecular polymer systems by shifting from traditional single-step self-assembly to multistep noncovalent synthetic strategies.6–13 Various approaches—such as template utilization,14–16 seed incorporation,17–19 artificial molecular chaperones,20–22 pH23 and temperature modulation,24 and application of external fields such as light,25 electric,26,27 and flow28,29—have been employed to accelerate assembly processes and regulate the product distribution.Nucleation can be significantly accelerated at phase interfaces or when additional particles are introduced into solutions, a phenomenon known as heterogeneous nucleation. In biological systems, heterogeneous nucleation plays a crucial role in protein aggregation. For instance, studies have shown that lipid membranes induce the nucleation and aggregation of Aβ proteins on their surface.30 In artificial supramolecular polymerization systems, researchers have explored chiral templates, such as biomacromolecules like peptides,31 DNA,32–34 and polysaccharides,35,36 to induce chirality in achiral monomers. These chiral templates function similarly to heterogeneous nucleating agents, which are typically regarded as co-assembling with the substrate. However, the potential of heterogeneous nucleation to further enable catalytic cycles and a secondary pathway in supramolecular polymerization remains largely unexplored. These mechanisms, akin to the role of a catalyst, could significantly enhance chiral induction effects with minimal heterogeneous nucleating agents, while allowing chiral inducers to avoid interfering with the assembly and morphology of the final products. Furthermore, the mechanisms of surface assembly in heterogeneous nucleation systems require more comprehensive quantitative analysis.
Herein, we demonstrate the critical role of the fragmentation-induced secondary pathway in promoting chiral transfer and asymmetry amplification in heterogeneously catalyzed supramolecular polymerization (Scheme 1). We report that the polysaccharide carboxymethyl cellulose (CMC) acts as a heterogeneous nucleating agent, effectively catalyzing the supramolecular chiral polymerization of achiral meso-tetraphenylsulfonato porphyrin (TPPS) monomers. CMC promotes the formation of TPPS supramolecular polymers with a predominant p-helical chiral form, achieving maximum enantioselectivity even at molar equivalents as low as 0.5% (calculated based on polymer repeating units relative to TPPS monomers). Real-time super-resolution fluorescence microscopy reveals a surface enrichment-induced primary nucleation and a substantial spontaneous fragmentation-induced secondary pathway, which together account for the high efficiency of CMC in chiral transfer and asymmetry amplification. Structure–function relationship analysis indicates that flexible side chains with carboxyl end groups and high degrees of polymerization enhance the functionality of CMC as an efficient chiral heterogeneous nucleating agent. Furthermore, kinetic analysis reveals an optimal equivalent in the catalytic process, attributed to the assembly of TPPS monomers on the CMC surface with those in solution, rather than through surface diffusion, akin to the Eley–Rideal mechanism. This study reveals the intrinsic catalytic properties of heterogeneous nucleating agents in controlling supramolecular polymerization, with a focus on regulating assembly rates and selecting pathways to enhance chiral transfer and asymmetry amplification.
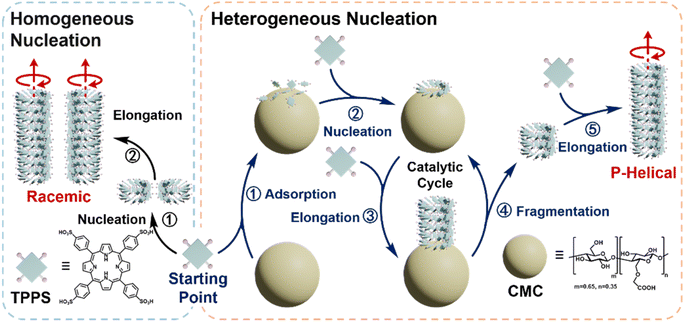 | ||
| Scheme 1 Schematic representation of the competitive pathways of TPPS homogeneous nucleation and heterogeneous nucleation with CMC as the nucleating agent. | ||
Results and discussion
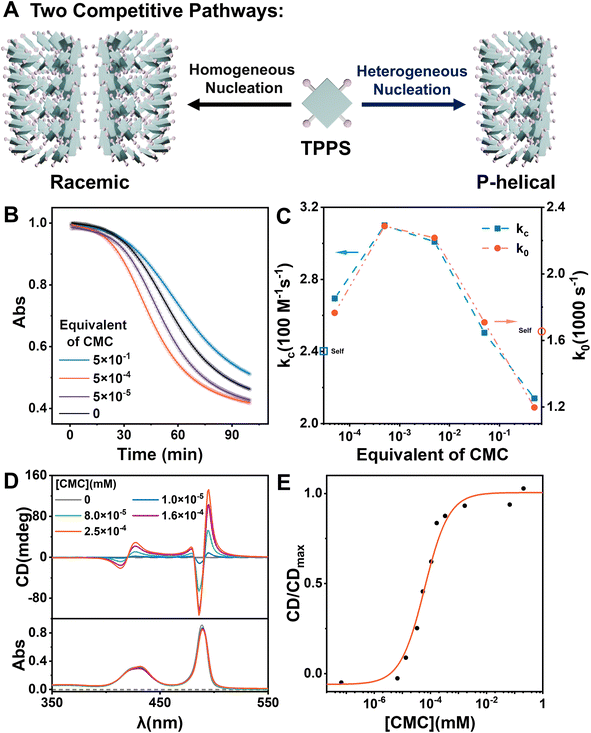
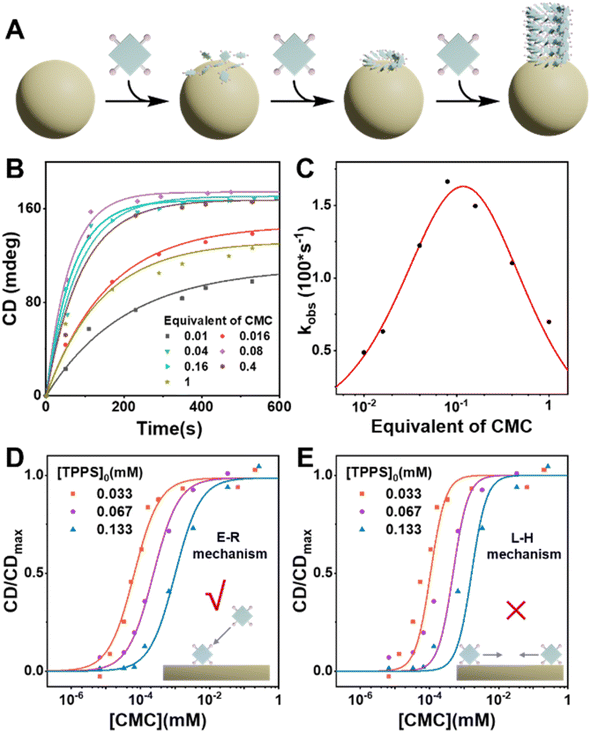
Carboxymethyl cellulose (CMC) was selected as a potential heterogeneous nucleating agent for several reasons: (1) CMC has an approximately spherical morphology in acidic aqueous solution (pH = 1) with a diameter of approximately 50 nm (Fig. S11†), thus providing a substantial specific surface area conducive to the adsorption of TPPS monomers and the induction of nucleation. (2) Under the assembly conditions of TPPS, the carboxyl groups of CMC are protonated, and are anticipated to form double hydrogen bonds with the sulfonic acid groups of TPPS, thus pre-organizing TPPS onto the surface with a high local concentration. (3) CMC is a readily available chiral polymer,37 which could potentially induce the formation of single-handed TPPS supramolecular polymers, consequently enhancing the enantioselectivity of the resulting products.CMC accelerates nucleation and enhances enantioselectivity of TPPS supramolecular polymerization
To investigate the effect of CMC as a heterogeneous nucleating agent, the supramolecular polymerization process of TPPS with varying equivalents of CMC was monitored in real-time. Kinetics data were fitted using a self-similar autocatalytic model38,39 where the initial step (k0) involves the formation of nuclei, alongside a catalytic pathway (kc) mediated by a porphyrin array (Fig. 1B, S2 and Table S1†). The nucleation rate constant (k0) and the catalytic rate constant (kc) were enhanced when the equivalent of CMC (based on repeating units) increased from 0 to 0.05% but subsequently decreased with further increases in equivalent. This indicates that CMC can accelerate nucleation as expected and suggests the presence of an optimal equivalent, with reasons discussed later in the subsequent surface assembly mechanism section (Fig. 5).Self-assembly of TPPS generates racemic supramolecular polymers, while CMC can induce TPPS to form P-type helical supramolecular polymers, achieving pathway selection for TPPS. Therefore, there exists a competitive relationship between CMC-catalyzed enantioselective supramolecular polymerization of TPPS and the racemic self-assembly of TPPS in solution. CD spectroscopy was employed to evaluate the rate ratio of these two pathways. At low CMC equivalents, the homogeneous self-assembly pathway still dominated (Fig. 1A), resulting in a weak CD signal of the assembly product (Fig. 1D and S3†). With increasing CMC concentrations from 0 to approximately 0.5% molar equivalent, the CD signal gradually increased, and reached a plateau (Fig. 1E, S4 and S24 ESI Section 5.2† for the fitting model). At this point, the heterogeneous catalytic pathway predominated, fully suppressing the self-assembly pathway. When the equivalent of CMC was further increased, it did not further enhance the enantioselectivity. Therefore, the threshold of 0.5% molar equivalent (concentration of 0.16 μM calculated by using repeating units) can be referred as the critical saturated equivalent of CMC (Fig. 1E). This indicates the high efficiency of chiral transfer of CMC compared to previously reported polymers.35,40–43
Chiral transfer was amplified through an autocatalytic cycle of fragmentation and growth
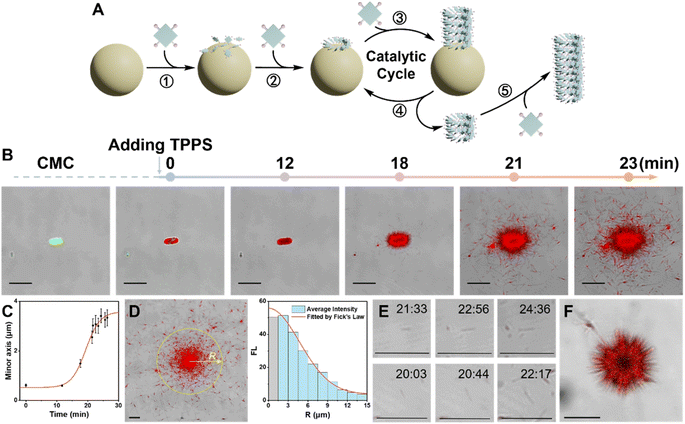
To understand how CMC can efficiently induce the enantioselective supramolecular polymerization of TPPS, the assembly process was monitored using multi-channel confocal fluorescence microscopy in situ (Fig. 2B and Movie S1†). TPPS assemblies emit their own fluorescence (Fig. S9†), while CMC was made visible for observation by labeling it with a fluorescein dye (Fig. S10†).Three stages of CMC-induced TPPS assembly were identified (Fig. 2A and B): (1) rapid adsorption of TPPS onto the CMC aggregate surface. The adsorption reached near saturation within 1 minute after the addition of TPPS. (2) A slow nucleation period, with no significant growth observed at around 12 minutes, indicating the system was in the nucleation stage. (3) A rapid growth process, occurring between 15 and 24 minutes. Quantitative statistical analysis of the thickness of the TPPS layer on the CMC aggregate surface over time revealed a typical nucleation-growth process (Fig. 2C and S5†). During this period, a sea urchin-like assembly structure formed on the CMC surface, indicating radial growth of TPPS on the CMC.
During the growth period, besides the abundant TPPS assemblies attached onto CMC aggregates, numerous discrete TPPS nanowires appeared at 21 and 23 minutes (Fig. 2B). These nanowires predominantly clustered around the CMC aggregates, with fewer found in more distant regions. The fluorescence density of TPPS at various radii around the CMC aggregate was statistically analyzed (Fig. 2D). The results aligned well with Fick's law (Fig. 2D, S6, and ESI Section 3.2† for the Fick's diffusion model), indicating that these TPPS nanowires fragmented from the CMC surface and subsequently diffused into the solution. Furthermore, real-time imaging demonstrated that these fragments actively promoted TPPS growth (Fig. 2E, S6, and Movie S1†), confirming that the fragments act as seeds for nucleation and growth. Additionally, the increasing number of fragments, along with the progressively larger size of the central CMC-TPPS assemblies, suggests that the broken ends of these assemblies, attached to the CMC surface, are undergoing further catalytic cycles of re-growth and fragmentation. Moreover, this phenomenon was not limited to the surface: sea urchin-like TPPS aggregates in solution also exhibited longitudinal extension of TPPS assemblies on the CMC surface, along with numerous nearby TPPS fragments (Fig. 2F, Movies S2 and S3†). These observations further indicate that TPPS undergoes radial growth on the surface of CMC in solution, accompanied by a significant spontaneous fragmentation process.
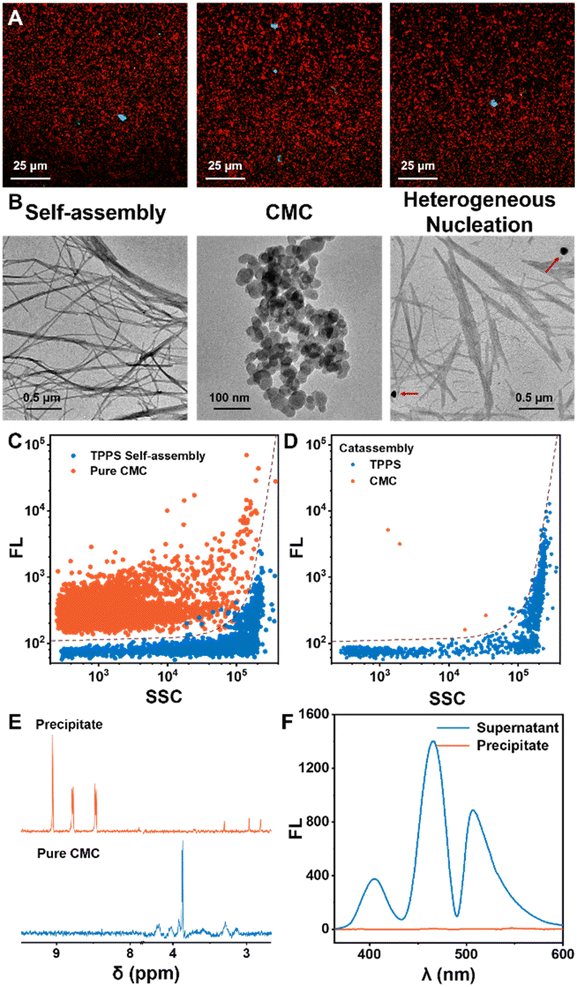
The significant autocatalytic pathways enable chiral inducers to avoid interfering with the assembly and morphology of the final products, unlike classical template co-assembly methods. In situ confocal fluorescence imaging demonstrated the generation of abundant TPPS assemblies in the system, with only a small amount of CMC present, and a notable proportion of TPPS assemblies evidently not bound to CMC (Fig. 3A). TEM results revealed that the morphology of TPPS assemblies induced by CMC resembled nanowires formed by self-assembly, with a diameter of approximately 20 nm (Fig. 3B, S12 and S13†), which further aggregated into bundles. CMC showed a spherical morphology with a diameter of around 50 nm, with no binding observed between the two (Fig. 3B, S11 and S13†).
Moreover, nano-flow cytometry analysis of the final state of the assembly solution, based on fluorescence and scattering, clearly indicated that the predominant TPPS assemblies in the solution were not associated with CMC (Fig. 3C and D). Additionally, the assembly product was isolated by centrifugation, re-dissolved in water (or deuterated water), and characterized using NMR and fluorescence (Fig. 3E, F, S8 and S9†). NMR did not detect characteristic signals of CMC, and fluorescence spectroscopy also did not detect trace amounts of CMC in the TPPS assemblies, further demonstrating that in the final state of assembly, the vast majority of TPPS assemblies do not co-assemble with the nucleating agent CMC.
According to the above observations, it is proposed that the high efficiency of CMC in inducing TPPS supramolecular chiral polymerization can be attributed to two main factors. On the one hand, CMC itself, acting as a heterogeneous nucleating agent, was found to effectively promote TPPS primary nucleation, thereby achieving supramolecular chiral induction. On the other hand, the generated supramolecular chiral polymers undergo spontaneous fragmentation, which leads to two secondary pathways. First, the broken ends of these assemblies, attached to the CMC surface, undergo further catalytic cycles of re-growth and fragmentation. Second, this fragmentation-re-growth catalytic cycle generates a large number of chiral fragments, which then serve as seeds for nucleation and growth. Considering that the proportion of repeating units at the surface relative to the total repeating units of a single CMC particle is approximately 7%, the extent of chiral polymerization achieved on this surface is approximately 0.3% with the addition of a 0.5% equivalent of CMC (see ESI Section 3.5†). Therefore, the fragmentation-induced secondary pathway may make a significant contribution of around 99.7%, resulting in the rapid asymmetry amplification of supramolecular chirality.
Structure–function relationship of chiral induction in heterogeneous nucleation
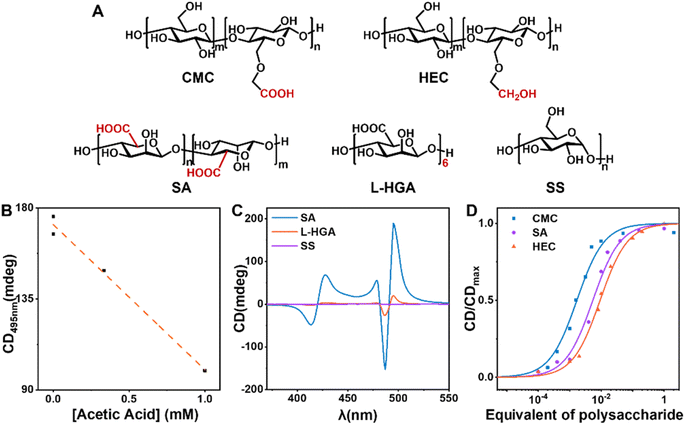
To investigate the active sites and structural characteristics of CMC in efficient chiral induction, a systematic study of the structure–activity relationship was conducted. Using 10% equivalent of soluble starch (SS), which lacks side-chain modification and has a different main chain structure, as a control, the final TPPS assemblies exhibited almost no CD signal (Fig. 4C, S19 and S20†). This indicates that the side chains of CMC serve as key active sites for adsorbing monomers. Hydroxyethyl cellulose (HEC), differing from CMC only in the absence of carbonyl groups in the side chains, showed a critical saturated catalytic equivalent of 4%, much higher than 0.5% of CMC (Fig. 4D, S15 and S16†). This suggests a weaker binding between HEC and TPPS, necessitating a higher concentration to achieve the same induction effect, confirming that the carboxyl group is the key active site of the nucleating agent. To validate the hypothesis that CMC adsorbs TPPS monomers mainly through hydrogen bonding between the carboxyl group of CMC and the sulphonic group of TPPS, acetic acid was introduced as a hydrogen bonding competitor. Acetic acid was supposed to destroy the interactions between CMC and TPPS by forming hydrogen bonds with TPPS competitively, and thereby block the chiral transfer from CMC to TPPS assemblies. A significant reduction in CD intensity (Fig. 4B and S21†) of the TPPS assemblies upon addition of acetic acid was observed, confirming that CMC induces single enantiomer assembly mainly through hydrogen bonding.However, sodium alginate (SA), a water-soluble polysaccharide with carboxyl groups, increased the critical saturated catalytic equivalent to 5% (Fig. 4D, S17 and S18†). This suggests that the flexible side chains in CMC may also play a key role. They may help retain appropriate degrees of freedom for the combined TPPS, allowing TPPS to adjust its conformation and spatial position, thereby facilitating the binding between TPPS towards nucleation.
Furthermore, L-hexaguluronic acid (L-HGA) was used to assess the influence of molecular weight on the efficiency of the nucleating agent. Despite the same equivalent, the induction effect of oligosaccharides was notably weaker than that of polysaccharides CMC (Fig. 4C, S19 and S20†). This underscores that heterogeneous nucleating agents require a certain degree of polymerization, aligning with our initial hypothesis that such agents require a specific surface area to sustain the stable formation of nuclei and initiate the primary nucleation process.
The nucleating agent presents an optimal equivalent, and the heterogeneous nucleation follows the Eley–Rideal mechanism
Kinetic results indicate that there is an optimal equivalent for heterogeneous nucleation, as the assembly rate of TPPS significantly decreased in the presence of high equivalents of the nucleating agent CMC (Fig. 5B, C, S26, and Table S5†). Excess nucleating agent inhibits the assembly process. To investigate the cause of this phenomenon, we compared two common surface catalytic mechanisms: the Langmuir–Hinshelwood (L–H) mechanism,44 where substrates adsorb and diffuse on the surface to form products, and the Eley–Rideal (E–R) mechanism,45 where the catalyst first adsorbs one substrate that reacts with another in solution. We hypothesize that this inhibitory trend arises from the decreasing concentration of free TPPS monomers in solution as CMC concentration increases, which slows the growth rate. This suggests that TPPS monomers adsorbed on the CMC surface assemble with those in solution, resembling the E–R mechanism of surface catalysis (Fig. 5A).Based on this hypothesis, heterogeneous assembly kinetic models based on E–R mechanism were developed (see ESI Section 5.4†), and the relationship between the apparent rate constant and varying equivalents of CMC was fitted using this model. The results indicate a good fit (Fig. S27 and Table S6†), validating that CMC primarily promotes TPPS assembly through the E–R mechanism. Additionally, the curves of enantioselectivity (CD/CDmax) against varying concentrations of CMC at different TPPS concentrations were fitted to kinetic models based on the E–R and L–H mechanisms (Fig. 5D, E, S24, and S25; see ESI Sections 5.2 and 5.3† for the kinetic models for E–R and L–H mechanisms, and original spectra in Fig. S2, S22 and S23†). The results demonstrate that the E–R mechanism model fits the curves well, while the L–H mechanism model performs poorly, further supporting our speculation regarding the E–R mechanism.
Conclusions
This research demonstrates that CMC, as a heterogeneous nucleating agent, exhibits high efficiency in chiral induction, achieving maximum enantiomeric selectivity at low molar equivalents. This high efficiency in inducing TPPS supramolecular chiral polymerization can be attributed to two main factors. First, CMC itself promotes TPPS primary nucleation, facilitating supramolecular chiral induction. Second, the generated supramolecular chiral polymers undergo a catalytic cycle of spontaneous fragmentation and re-growth at their termini, with the resulting fragments also acting as seeds for nucleation and growth. Furthermore, exploring structure–function relationships reveals that key factors—such as the polymerization degree, flexibility of side chains, and the affinity between CMC and TPPS—significantly influence nucleation efficiency, aiding in the rational design of heterogeneous nucleating agents. Kinetic analysis reveals that an optimal equivalent exists in heterogeneous nucleation, following the E–R mechanism. In this process, TPPS monomers adsorbed on the CMC surface assemble with those in solution rather than through surface diffusion.In summary, our study demonstrates that the surface enrichment-induced primary nucleation, along with substantial spontaneous fragmentation-induced autocatalysis, accounts for the high efficiency of the heterogeneous nucleation strategy. Unlike classical template co-assembly strategies, most assemblies remain unbound to the nucleating agent, allowing it to not interfere with the final assembly structure and morphology, akin to the role of a catalyst (or catassembler as proposed by Tian et al.46,47). This work advances the mechanistic understanding of heterogeneous nucleation and highlights its potential as an effective pathway control strategy in supramolecular polymerization systems.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Peichen Shi: data curation, conceptualization, formal analysis, methodology and writing – original draft; Ganyu Chen: data curation, writing – original draft, and review & editing; Qiang Chen, Huiting Wu and Suixu Li: data curation and validation; Xiaoyu Cao, Liulin Yang and Zhongqun Tian: project administration and resources.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank Niangui Cai from Xiaomei Yan's group (Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361001, PR China) for their assistance with the testing of nano-flow cytometry. This work was supported by the National Natural Science Foundation of China (NSFC) (No. 21991130, 21991131, 22372139, and 22250004).References
- M. Wehner and F. Würthner, Nat. Rev. Chem, 2019, 4, 38–53 CrossRef.
- P. K. Hashim, J. Bergueiro, E. W. Meijer and T. Aida, Prog. Polym. Sci., 2020, 105, e101250 CrossRef.
- K. Yano, Y. Itoh, F. Araoka, G. Watanabe, T. Hikima and T. Aida, Science, 2019, 363, 161–165 CrossRef CAS PubMed.
- J. Funk, F. Merino, M. Schaks, K. Rottner, S. Raunser and P. Bieling, Nat. Commun., 2021, 12, 5329 CrossRef CAS PubMed.
- T. Hashimoto, Curr. Opin. Plant Biol., 2013, 16, 698–703 CrossRef CAS PubMed.
- G. Vantomme, G. M. Ter Huurne, C. Kulkarni, H. M. M. Ten Eikelder, A. J. Markvoort, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2019, 141, 18278–18285 CrossRef CAS PubMed.
- N. Sasaki, J. Kikkawa, Y. Ishii, T. Uchihashi, H. Imamura, M. Takeuchi and K. Sugiyasu, Nat. Chem., 2023, 15, 922–929 CrossRef CAS PubMed.
- W. Zhang, W. Jin, T. Fukushima, T. Mori and T. Aida, J. Am. Chem. Soc., 2015, 137, 13792–13795 CrossRef CAS PubMed.
- S. Sarkar, A. Sarkar, A. Som, S. S. Agasti and S. J. George, J. Am. Chem. Soc., 2021, 143, 11777–11787 CrossRef CAS PubMed.
- S. Takahashi, T. Matsumoto, M. J. Hollamby, H. Miyasaka, M. Vacha, H. Sotome and S. Yagai, J. Am. Chem. Soc., 2024, 146, 2089–2101 CrossRef CAS PubMed.
- S. Sarkar, R. Laishram, D. Deb and S. J. George, J. Am. Chem. Soc., 2023, 145, 22009–22018 CrossRef CAS PubMed.
- H. Itabashi, S. Datta, R. Tsukuda, M. J. Hollamby and S. Yagai, Chem. Sci., 2023, 14, 3270–3276 RSC.
- T. Shimada, Y. Watanabe, T. Kajitani, M. Takeuchi, Y. Wakayama and K. Sugiyasu, Chem. Sci., 2023, 14, 822–826 RSC.
- J. Schill, B. Rosier, B. Gumi Audenis, E. Magdalena Estirado, T. F. A. de Greef and L. Brunsveld, Angew. Chem., Int. Ed., 2021, 60, 7612–7616 CrossRef CAS PubMed.
- Y. Zhang, A. Reinhardt, P. Wang, J. Song and Y. Ke, Angew. Chem., Int. Ed., 2020, 59, 8594–8600 CrossRef CAS PubMed.
- B. Liu, M. A. Beatty, C. G. Pappas, K. Liu, J. Ottele and S. Otto, Angew. Chem., Int. Ed., 2021, 60, 13569–13573 CrossRef CAS PubMed.
- W. Wagner, M. Wehner, V. Stepanenko and F. Wuerthner, J. Am. Chem. Soc., 2019, 141, 12044–12054 CrossRef CAS PubMed.
- S. Ogi, K. Sugiyasu, S. Manna, S. Samitsu and M. Takeuchi, Nat. Chem., 2014, 6, 188–195 CrossRef CAS PubMed.
- P. Khanra, A. K. Singh, L. Roy and A. Das, J. Am. Chem. Soc., 2023, 145, 5270–5284 CrossRef CAS PubMed.
- Y. Wang, Y. Sun, P. Shi, M. M. Sartin, X. Lin, P. Zhang, H. Fang, P. Peng, Z. Tian and X. Cao, Chem. Sci., 2019, 10, 8076–8082 RSC.
- W. Li, Y. Zhou, S. He, T. Tong, C. Wang, P. Shi, S. Li, X. Wang, L. Yang, X. Cao and Z. Q. Tian, Aggregate, 2024, 5, e496 CrossRef CAS.
- L. Kleine-Kleffmann, V. Stepanenko, K. Shoyama, M. Wehner and F. Würthner, J. Am. Chem. Soc., 2023, 145, 9144–9151 CrossRef CAS.
- K. L. Morris, L. Chen, J. Raeburn, O. R. Sellick, P. Cotanda, A. Paul, P. C. Griffiths, S. M. King, R. K. O'Reilly, L. C. Serpell and D. J. Adams, Nat. Commun., 2013, 4, 1480 CrossRef PubMed.
- K. V. Rao, M. F. J. Mabesoone, D. Miyajima, A. Nihonyanagi, E. W. Meijer and T. Aida, J. Am. Chem. Soc., 2020, 142, 598–605 CrossRef CAS PubMed.
- E. Moulin, I. A. Nyrkova, N. Giuseppone, A. N. Semenov and E. Buhler, Soft Matter, 2020, 16, 2971–2993 RSC.
- T. K. Ellis, M. Galerne, J. J. t. Armao, A. Osypenko, D. Martel, M. Maaloum, G. Fuks, O. Gavat, E. Moulin and N. Giuseppone, Angew. Chem., Int. Ed., 2018, 57, 15749–15753 CrossRef CAS PubMed.
- S. Selmani, E. Schwartz, J. T. Mulvey, H. Wei, A. Grosvirt-Dramen, W. Gibson, A. I. Hochbaum, J. P. Patterson, R. Ragan and Z. Guan, J. Am. Chem. Soc., 2022, 144, 7844–7851 CrossRef CAS PubMed.
- J. Sun, Y. Li, F. Yan, C. Liu, Y. Sang, F. Tian, Q. Feng, P. Duan, L. Zhang, X. Shi, B. Ding and M. Liu, Nat. Commun., 2018, 9, 2599 CrossRef PubMed.
- S. Matoba, C. Kanzaki, K. Yamashita, T. Kusukawa, G. Fukuhara, T. Okada, T. Narushima, H. Okamoto and M. Numata, J. Am. Chem. Soc., 2021, 143, 8731–8746 CrossRef CAS PubMed.
- J. Habchi, S. Chia, C. Galvagnion, T. C. T. Michaels, M. M. J. Bellaiche, F. S. Ruggeri, M. Sanguanini, I. Idini, J. R. Kumita, E. Sparr, S. Linse, C. M. Dobson, T. P. J. Knowles and M. Vendruscolo, Nat. Chem., 2018, 10, 673–683 CrossRef CAS PubMed.
- D. Kuciauskas, J. Kiskis, G. A. Caputo and V. Gulbinas, J. Phys. Chem. B, 2010, 114, 16029–16035 CrossRef CAS PubMed.
- T. Sugimoto, T. Suzuki, S. Shinkai and K. Sada, J. Am. Chem. Soc., 2007, 129, 270–271 CrossRef CAS PubMed.
- M. Surin, P. G. A. Janssen, R. Lazzaroni, P. Leclère, E. W. Meijer and A. P. H. J. Schenning, Adv. Mater., 2009, 21, 1126–1130 CrossRef CAS.
- P. G. Janssen, A. Ruiz-Carretero, D. Gonzalez-Rodriguez, E. W. Meijer and A. P. Schenning, Angew. Chem., Int. Ed., 2009, 48, 8103–8106 CrossRef CAS PubMed.
- K. Sugikawa, M. Numata, D. Kinoshita, K. Kaneko, K. Sada, A. Asano, S. Seki and S. Shinkai, Org. Biomol. Chem., 2011, 9, 146–153 RSC.
- O.-K. Kim, J. Je, G. Jernigan, L. Buckley and D. Whitten, J. Am. Chem. Soc., 2006, 128, 510–516 CrossRef CAS.
- J. S. Zhao, J. H. Wang, W. B. He, Y. B. Ruan and Y. B. Jiang, Chem.–Eur. J., 2012, 18, 3631–3636 CrossRef CAS.
- R. F. Pasternack, E. J. Gibbs, P. J. Collings, J. C. dePaula, L. C. Turzo and A. Terracina, J. Am. Chem. Soc., 1998, 120, 5873–5878 CrossRef CAS.
- I. G. Occhiuto, M. A. Castriciano, M. Trapani, R. Zagami, A. Romeo, R. F. Pasternack and L. M. Scolaro, Int. J. Mol. Sci., 2020, 21, 4001 CrossRef CAS PubMed.
- L. Zhao, R. Ma, J. Li, Y. Li, Y. An and L. Shi, Biomacromolecules, 2008, 9, 2601–2608 CrossRef CAS PubMed.
- S. L. Thorpe, G. N. Snyder and A. Mammana, Chirality, 2020, 32, 5–16 CrossRef CAS PubMed.
- H. Onouchi, T. Miyagawa, K. Morino and E. Yashima, Angew. Chem., Int. Ed., 2006, 118, 2441–2444 CrossRef.
- I. Occhiuto, G. De Luca, V. Villari, A. Romeo, N. Micali, R. F. Pasternack and L. M. Scolaro, Chem. Commun., 2011, 47, 6045–6047 RSC.
- K. V. Kumar, K. Porkodi and F. Rocha, Catal. Commun., 2008, 9, 82–84 CrossRef CAS.
- R. Prins, Top. Catal., 2018, 61, 714–721 CrossRef CAS.
- Y. Wang, H. X. Lin, L. Chen, S. Y. Ding, Z. C. Lei, D. Y. Liu, X. Y. Cao, H. J. Liang, Y. B. Jiang and Z. Q. Tian, Chem. Soc. Rev., 2014, 43, 399–411 RSC.
- Z. C. Lei, X. Wang, L. Yang, H. Qu, Y. Sun, Y. Yang, W. Li, W. B. Zhang, X. Y. Cao, C. Fan, G. Li, J. Wu and Z. Q. Tian, Chem. Soc. Rev., 2024, 53, 1892–1914 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07894b |
| This journal is © The Royal Society of Chemistry 2025 |