Open Access Article
Open Access ArticleInter-cluster-linker-absence-enabled sub-Ångstrom pore modulation in a metal–organic framework for multi-scenario CO2 capture†
Jia-Wen
Wang
,
Shu-Cong
Fan
,
Wenyu
Yuan
,
Ying
Wang
 and
Quan-Guo
Zhai
and
Quan-Guo
Zhai
 *
*
Key Laboratory of Applied Surface and Colloid Chemistry (MOE), Key Laboratory of Macromolecular Science of Shaanxi Province, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an, Shaanxi 710062, China. E-mail: zhaiqg@snnu.edu.cn
First published on 14th May 2025
Abstract
Ultrafine aperture control of carbon capture adsorbents is first and foremost important but inscrutable. Herein, an inter-cluster-linker-absence-enabled sub-Ångstrom pore modulation strategy is proposed through the efficient transitivity of coordination bonds in a metal–organic framework (MOF). The feasibility of this strategy is well-demonstrated in SNNU-98-M materials composed of directly connected [M8(TAZ)9] (M = Cd or Cu, TAZ = tetrazolate) triangular prism clusters. The removal of inter-cluster linkers effectively transfers the difference in coordination bond length (approximately 2.3 Å for Cd(II)–N and approximately 2.1 Å for Cu(II)–N) to the size of secondary building blocks (approximately 6.5 × 6.5 × 6.7 Å3 for [Cd8(TAZ)9] and approximately 6.2 × 6.2 × 6.3 Å3 for [Cu8(TAZ)9]), and to the final MOF pore (approximately 5.5 Å for SNNU-98-Cd and approximately 5.1 Å for SNNU-98-Cu). Rational and hyperfine pore control together with optimized Lewis basic N sites endow SNNU-98-M with benchmark multi-scenario CO2 capture performance varying from binary flue gas (CO2/N2) to ternary biogas (CO2/CH4/N2) and even to quinary coal gas (CO2/CH4/N2/CO/H2) mixtures by a one-step process. SNNU-98-Cu is an ideal carbon capture material for practical applications due to its low-cost raw materials, easy scalablity in synthesis, ultra-high stability, and top-level selective adsorption ability as well as multi-scenario adaptability.
Introduction
With the atmospheric CO2 concentration projected to exceed 500 ppm by 2050 due to anthropogenic emissions, the removal of greenhouse gases from dilute emissions has been identified as one of the seven chemical separations that will change the world.1–5 Currently, CO2 removal scenarios can be mainly divided into pre-combustion capture (biogas CO2/CH4/N2 and coal gas CO2/CH4/N2/CO/H2), post-combustion capture (flue gas CO2/N2), and direct air capture.6–12 The conventional amine-scrubbing method is widely used for CO2 capture, but the absorbent regeneration consumes a large amount of energy and requires additional waste treatment processes.13,14Physical adsorption is recognized as a promising carbon-capture method due to its high efficiency, easy operation, non-corrosiveness, and low energy consumption.15–18 A large variety of adsorbents, including zeolites, porous carbon materials, and porous organic networks have been explored and applied in CO2 removal.19,20 From an application perspective, the construction of highly selective CO2 adsorbents suitable for multi-scenarios such as flue gas, biogas, and coal gas is extremely necessary but remains a challenging task.3 As a new type of adsorbent, a metal–organic framework (MOF) has the ability to rationally regulate thermodynamic and kinetic processes and is considered as an ideal platform for developing the next generation of CO2 capture materials.5,21 Most adsorbents are currently suitable for a single separation process,22 and therefore, there is an urgent need to develop MOFs for multi-scenario carbon capture.
Due to the highly similar physical properties and tiny molecular size difference (usual sub-Ångstrom level, Table S1†) of the involved gas molecules, ultrafine pore regulation is important for the adsorbent design of MOFs to be used as adsorbents in multi-scenario carbon capture. However, sub-Ångstrom pore size regulation in typical MOFs containing metal clusters and inter-cluster organic linkers is not easy, although the iso-network principle and building block approach have achieved remarkable success.23 Other common strategies including organic linker functionalization,24–27 framework penetration,28 and pore space partition29 also seem powerless to solve this problem. In our opinion, the main reason for this situation is the presence of inter-cluster organic linkers. Their minor variations (e.g., addition or removal of a single atom) can induce pore size fluctuations exceeding 1 Å, significantly surpassing the sub-Ångstrom-level structural control. However, the flexibility of inter-cluster linkers may also reduce the variations in coordination bond lengths between different metal centers, resulting in nearly identical pore sizes for isostructural MOFs containing distinct metals (Scheme 1a).
 | ||
| Scheme 1 The inter-cluster-linker-absence-enabled sub-Ångstrom pore modulation strategy proposed in this work. | ||
Based on the above considerations, an inter-cluster-linker-absence-enabled sub-Ångstrom pore modulation strategy is proposed herein (Scheme 1b). The removal of inter-cluster linkers will generate architectures consisting of directly connected clusters,30–32 which favorably combines the advantages of inorganic zeolites and MOFs with extra-high stability. Ultra-fine pore regulation can be achieved simply by replacing the metal nodes, which may successfully transmit the sub-Ångstrom level changes of coordination bond length to the entire pore environment.
The feasibility of this strategy is well-demonstrated in SNNU-98-M MOF materials composed of directly connected [M8(TAZ)9] (M = Cd or Cu, TAZ = tetrazolate) triangular prism clusters. Because the ionic radius of Cu2+ (0.73 Å) is significantly smaller than that of Cd2+ (0.95 Å), the absence of inter-cluster linkers effectively transfers the difference from coordination bond length to the size of secondary building blocks and to the final MOF pore capture size (approximately 5.5 Å for SNNU-98-Cd and approximately 5.1 Å for SNNU-98-Cu). This sub-Ångstrom pore modulation is beneficial for the SNNU-98-Cu adsorbent, and enabled it to set a benchmark for challenging multi-scenario CO2 capture ability with ultrahigh CO2/N2 (flue gas) selectivity (1509.3), top-level separation of ternary CO2/CH4/N2 mixtures (biogas, breakthrough interval time of 9 min g−1 for N2, 18.3 min g−1 for CH4, and 80.9 min g−1 for CO2), and even highly efficient one-step CO2 capture from quinary coal gas (CO2/CH4/N2/CO/H2) mixtures, outperforming all advanced materials. The removal of inter-cluster linkers from SNNU-98-Cu also led to superb acid and alkali resistance stability, which lasted for 7 days in solutions with pH −0.5–13, and for as long as half a year in solutions with pH 1–12, which surpassed almost all highly stable MOF materials. In addition, rapid, efficient, and low-energy consumption reflux synthesis routes endow SNNU-98-Cu adsorbents with great potential for large-scale production and commercialization.
Results and discussion
Typical solvothermal reactions of low-cost metal salts and tetrazole ligands produce isostructural SNNU-98-M metal–organic frameworks33,34 with a formula of [M5(TAZ)9(NO3)·(H2O)n] (M = Cd2+/Cu2+, TAZ = tetrazolate). The production of SNNU-98-Cu can be scaled up by an ultra-fast and efficient reflux synthesis process. Only 30 min of a refluxing reaction of raw materials at 150 °C can produce 10.8 g of SNNU-98-Cu with a high yield of approximately 85% (Fig. 1, S2 and S3†). Powder X-ray diffraction (PXRD) patterns and scanning electron microscopy (SEM) images confirmed the high purity and high crystallinity of SNNU-98-Cd/Cu (Fig. S1–S3 and Table S2†) obtained by two different synthesis methods.Single crystal structural analysis indicates that the framework of SNNU-98-Cd/Cu is formed by the direct connection of triangular prism clusters without inter-cluster organic linkers. Specifically, different metal centers result in a gap of approximately 0.2 Å in the metal–N coordination bond lengths (Cd(II)–N of approximately 2.3 Å and Cu(II)–N of approximately 2.1 Å) (Fig. 1a and b). Eight metals are connected through six μ3-TAZs and three μ4-TAZs to form [M8(TAZ)9] clusters. The difference in metal–N bond lengths rationally resulted in a bulk reduction of the [Cu8(TAZ)9] cluster (approximately 6.2 × 6.2 × 6.3 Å3) compared to the [Cd8(TAZ)9] cluster (approximately 6.5 × 6.5 × 6.7 Å3) (Fig. 1c and d). Each [M8(TAZ)9] cluster connects with six equal adjacent neighbors through vertex (metal) sharing patterns to generate a three-dimensional (3D) structure with typical acs topology.35 Due to the removal of inter-cluster linkers, the lattice of the 3D framework formed by [M8(TAZ)9] cluster stacking contracted due to the substitution of metallic cadmium with copper, and the c/a ratio of the cell size decreased by approximately 1% (Fig. 1e and f). Such ultrafine changes in the coordination bond length ultimately transmit to the one-dimensional hexagonal channel, which resulted in the successful achievement of sub-Ångstrom-level modulation (approximately 5.5 Å for SNNU-98-Cd and approximately 5.1 Å for SNNU-98-Cu) (Fig. 1g and h).
The permanent porosity and sub-Ångstrom pore modulation of the activated SNNU-98-Cd/Cu materials was confirmed by N2 (77 K) and CO2 (195 K) adsorption and desorption isotherms. As shown in Fig. 2a, SNNU-98-Cd exhibits a type I adsorption isotherm, while SNNU-98-Cu adsorbs very little N2 at 77 K. Furthermore, CO2 adsorption experiments at 195 K show their permanent microporosity and reversible type I adsorption isotherms (Fig. 2b), with calculated Brunauer–Emmett–Teller (BET) surface areas of 322 m2 g−1 (for SNNU-98-Cd) and 212 m2 g−1 (for SNNU-98-Cu). The pore size distributions of SNNU-98-Cd/Cu were calculated to be 5.1 Å and 4.8 Å, respectively, further confirming the ultra-fine pore size control, which also highly matched with their single crystal structure. Clearly, the removal of inter-cluster linkers results in successful transmission of the coordination bond length gap and rational achievement of sub-Ångstrom pore regulation.
Thermal and chemical stability has long been a limitation of MOF materials for intended use in industrial applications, especially for multi-scenario CO2 capture. These unique structural features may endow SNNU-98-Cd/Cu with ultra-high stability: (i) soft-base N sites and soft-acidic metal sites can form strong coordination bonds; (ii) tetrazolate anions with a lower pKa value can significantly promote acid resistance; and (iii) the absence of inter-cluster linkers will increase the robustness of the MOF framework. Thermogravimetric analysis (TGA) of the synthesized and methanol-exchanged samples showed that the thermal stability is satisfactory for SNNU-98-Cd/Cu (Fig. S4†).
Furthermore, SNNU-98-Cu was immersed in pH 1–14 and 1–3 M HCl solutions to test its acid–base stability. The PXRD results showed that SNNU-98-Cu remained highly crystalline for up to 7 days in pH −0.5–13 solutions (Fig. 2c). Encouragingly, it remained stable for 6 months (pH 1–12), which is extraordinarily long and surpassed that of nearly all MOF adsorbent materials. The pH stability range and duration of SNNU-98-Cu are better than all reported Cu-MOFs, most well-known highly stable MOFs such as UiO-66,36 ZIF-8,37 and MOF-808,38 and even most of the recognized Zr-MOFs39–41 with a high degree of connectivity (Table S3 and Fig. S5–S7†). In addition, the 195 K CO2 adsorption of SNNU-98-Cu after the acid–base stability test showed that a high degree of crystallinity and intact pore structure was maintained (Fig. 2d and e). The adsorption capacity remained unchanged after 6 months of immersion in pH 12 solution, and the pore size distribution slightly changed, suggesting that the pore structure may have undergone a subtle collapse, but the effect on the overall gas adsorption performance was negligible. SNNU-98-Cu is capable of adapting to the complex environment of the industrial CO2 capture process due to its low-cost raw materials, rapid scale-up reflux synthesis, and ultra-high stability.
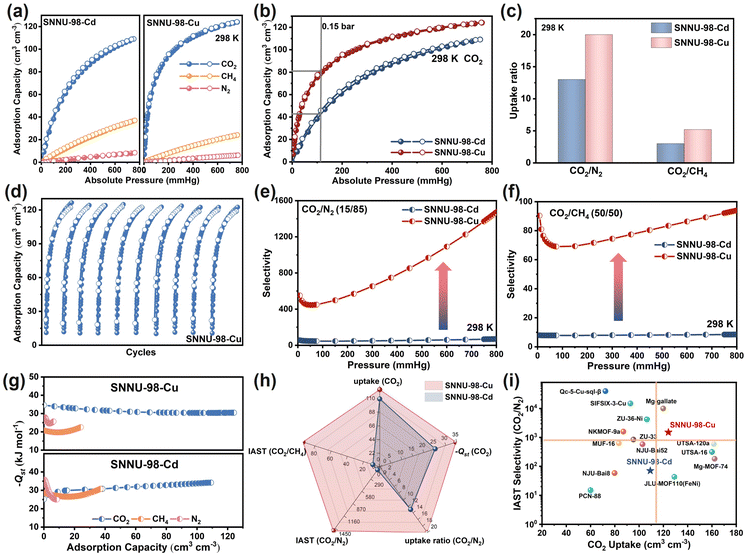
The CO2, CH4, and N2 adsorption of SNNU-98-Cd/Cu was investigated at different temperatures (Fig. S8–S13, Tables S8 and S9†) to systematically evaluate the effect of sub-Ångstrom pore regulation on selective CO2 capture. As shown in Fig. 3a, the CO2 adsorption by SNNU-98-Cu (124.1 cm3 cm−3) was higher than that by SNNU-98-Cd (109.1 cm3 cm−3) at 298 K and 1 bar. In addition, SNNU-98-Cu (80.1 cm3 cm−3, 64.5% at 1 bar) exhibited superior CO2 adsorption as compared to SNNU-98-Cd (42.5 cm3 cm−3, 38.9% at 1 bar) at relatively low pressure (0.15 bar) and at 298 K, which outperformed most of the well-known MOF adsorbents, such as SIFSIX-2-Cu-i42 (58.9 cm3 cm−3), NJU-Bai8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 (38.1 cm3 cm−3), and Qc-5-Cu-sql14 (24.4 cm3 cm−3) (Fig. 3b). The adjustment of the ultra-fine pore size resulted in SNNU-98-Cu with more suitable narrow pores and high-density uncoordinated tetrazolate N sites suitable for rapid mass adsorption of CO2. Conversely, the CH4 and N2 uptakes on SNNU-98-Cd/Cu at 298 K and 1 bar were found to be only 37/24 cm3 cm−3 and 8.4/6.2 cm3 cm−3, respectively, illustrating the molecular-sieving effect of SNNU-98-Cd/Cu. Similarly, the same adsorption characteristics were observed at 273 and 283 K (Fig. S10†).
43 (38.1 cm3 cm−3), and Qc-5-Cu-sql14 (24.4 cm3 cm−3) (Fig. 3b). The adjustment of the ultra-fine pore size resulted in SNNU-98-Cu with more suitable narrow pores and high-density uncoordinated tetrazolate N sites suitable for rapid mass adsorption of CO2. Conversely, the CH4 and N2 uptakes on SNNU-98-Cd/Cu at 298 K and 1 bar were found to be only 37/24 cm3 cm−3 and 8.4/6.2 cm3 cm−3, respectively, illustrating the molecular-sieving effect of SNNU-98-Cd/Cu. Similarly, the same adsorption characteristics were observed at 273 and 283 K (Fig. S10†).
Furthermore, the separation ratios of CO2versus CH4 and N2 of SNNU-98-Cu (5.2/20) were significantly higher than those of SNNU-98-Cd (2.9/13), suggesting that a sub-Ångstrom reduction in pore size favors multi-scene CO2 separation (Fig. 3c). Notably, SNNU-98-Cd/Cu continued to maintain the original adsorption amount after multiple cycles of CO2 adsorption and desorption, indicating good cyclic stability (Fig. 3d and S13†).
The heat of adsorption (Qst) of CO2, CH4, and N2 was further calculated based on the adsorption isotherms at different temperatures to understand the binding affinities between host surface and guest gas molecules (Fig. S14–S17, Tables S4 and S5†). At zero coverage, the −Qst values for CO2, CH4, and N2 are 34.6, 20.8, and 25.9 kJ mol−1 for SNNU-98-Cu and 25.2, 28.6, and 35.7 kJ mol−1 for SNNU-98-Cd, respectively (Fig. 3g). The CO2 adsorption affinity for SNNU-98-Cu was significantly stronger than that for SNNU-98-Cd. The affinity for CO2 was also higher than that for CH4 and N2 in SNNU-98-Cu, which indicated its greater potential for CO2 capture and separation.
Notably, the −Qst value for SNNU-98-Cu was at the maximum at zero loading, whereas the −Qst value for SNNU-98-Cd gradually increased with adsorption, which could be attributed to the smaller pore environment with stronger affinity for CO2 at low pressure, and is consistent with the adsorption performance. In addition, compared with other well-known CO2 separation materials, the CO2 adsorption enthalpy for SNNU-98-Cu is moderate, which is favorable for recycling in practical applications (Table S10†). The CO2 temperature-programmed desorption (CO2-TPD) curves also showed that the CO2 desorption temperature and thermal conductivity detector (TCD) intensity for SNNU-98-Cu (123 °C) were higher than those for SNNU-98-Cd (94 °C), indicating its stronger binding with CO2 (Fig. S18†).
The separation potentials of SNNU-98-Cd/Cu for the CO2/N2, CO2/CH4, and CH4/N2 mixtures were evaluated by ideal adsorbed solution theory (IAST) (Fig. S19–S27, Tables S6 and S7†). For CO2/N2 (15/85, v/v), the selectivity values for SNNU-98-Cd/Cu at 298 K and 1 bar were 70.1 and 1509.3, respectively (Fig. 3e). The ultra-high selectivity value for SNNU-98-Cu exceeds most of the top-level MOF adsorbents, such as ZU-301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (846), MUF-16
10 (846), MUF-16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6,15 (631), NJU-Bai52
6,15 (631), NJU-Bai52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 (581), and UTSA-16
44 (581), and UTSA-16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 (314.7). The IAST selectivity values are 8.5/94.6 for CO2/CH4 (50/50, v/v) and 6.5/5.3 for CH4/N2 (50/50, v/v), respectively, at 298 K and 1 bar (Fig. 3f and S21†). These values further indicate that SNNU-98-Cu has greater potential for separating multi-scenario CO2 (Fig. 3h). In particular, when considering the adsorption and separation selectivity of CO2, the comprehensive capacity of SNNU-98-Cu is superior to that of most benchmark MOF adsorbents, such as ZU-36-Ni,4 NMMOF-9a,3 and ZU-66
45 (314.7). The IAST selectivity values are 8.5/94.6 for CO2/CH4 (50/50, v/v) and 6.5/5.3 for CH4/N2 (50/50, v/v), respectively, at 298 K and 1 bar (Fig. 3f and S21†). These values further indicate that SNNU-98-Cu has greater potential for separating multi-scenario CO2 (Fig. 3h). In particular, when considering the adsorption and separation selectivity of CO2, the comprehensive capacity of SNNU-98-Cu is superior to that of most benchmark MOF adsorbents, such as ZU-36-Ni,4 NMMOF-9a,3 and ZU-66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (Fig. 3i).
8 (Fig. 3i).
To evaluate the feasibility of the actual multi-scenario CO2 separation process, dynamic fixed-bed breakthrough experiments were conducted for the binary gas mixtures, including flue gas (CO2/N2) and CO2/CH4, ternary biogas (CO2/CH4/N2), and quinary coal gas (CO2/CH4/N2/CO/H2) (Fig. 4 and S28–S35†). For CO2/N2 (15/85, v/v) flue gas separation, N2 was first observed from the column due to the lower adsorption capacity and weak affinity with the MOF frameworks, whereas CO2 was not eluted until the breakthrough interval time reached 64.4 min g−1 for SNNU-98-Cd and 82.6 min g−1 for SNNU-98-Cu, with a flow rate of 2 mL min−1 at 298 K (Fig. 4a). Excitingly, CO2 was retained in the column over 109.8 min g−1 and 127.1 min g−1 at 273 K for SNNU-98-Cd and SNNU-98-Cu, respectively (Fig. S28†).
Notably, these breakthrough interval time values are superior to almost all currently used MOF adsorbents under the same conditions, such as UTSA-120a (80 min g−1), JLU-MOF110 (FeNi)2 (49.1 min g−1), and ZU-66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (28 min g−1) and only lower than ZU-301
8 (28 min g−1) and only lower than ZU-301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (175 min g−1) and FJI-H29
10 (175 min g−1) and FJI-H29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (114 min g−1). In addition, the interval time for SNNU-98-Cu was longer than that of SNNU-98-Cd (18.2 min g−1), indicating that the sub-Ångstrom pore modulation is conducive to the separation of flue gas components. Subsequently, the separation effect of SNNU-98-Cu on the CO2/N2 mixed gas remained unchanged in a humid environment (50% and 98% relative humidity (RH)), indicating that it has great potential for industrial applications (Fig. S29†).
7 (114 min g−1). In addition, the interval time for SNNU-98-Cu was longer than that of SNNU-98-Cd (18.2 min g−1), indicating that the sub-Ångstrom pore modulation is conducive to the separation of flue gas components. Subsequently, the separation effect of SNNU-98-Cu on the CO2/N2 mixed gas remained unchanged in a humid environment (50% and 98% relative humidity (RH)), indicating that it has great potential for industrial applications (Fig. S29†).
The cyclic stability of CO2/N2 was tested, which further demonstrated the accuracy and reproducibility of the separation performance of SNNU-98-Cu (Fig. S32†). It was noted that the breakthrough time did not proportionally decrease with increasing flow rate (Fig. 4b), which may be due to the strong interaction between the SNNU-98-Cu adsorbent and CO2 molecules. The high CO2 adsorption at low pressure also indicates that the separation of CO2/N2 (15/85, v/v) in SNNU-98-Cu is mainly dominated by thermodynamics. The superb flue gas separation by SNNU-98-Cu was attributed to a clear thermodynamic effect, whereby CO2 had a higher affinity for the high-density N sites of the pores in a narrower pore environment.
As for the binary biogas separation of CO2/CH4 (50/50, v/v), CH4 was first observed from the column, whereas CO2 was not eluted until the breakthrough interval time reached 29 min g−1 for SNNU-98-Cd and 31 min g−1 for SNNU-98-Cu with a flow rate of 2 mL min−1 at 298 K (Fig. 4c). The interval time for SNNU-98-Cu was nearly the same as that for SNNU-98-Cd, but the outflow time was much earlier (CH4: 12, 27 min g−1, CO2: 43, 56 min g−1). This may be due to the kinetic diffusion effect being greater than the thermodynamic effect for SNNU-98-Cu, resulting in the preferential outflow in SNNU-98-Cu. Furthermore, the CO2/CH4 separation performance was also better when compared to most of the MOF materials. When considering the materials for simultaneous separation of flue gas and biogas, SNNU-98-Cu is nearly superior to all high-performance MOFs, and second only to ZU-301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. 4d and Table S11†). In addition, it also is highly advantageous for the difficult problem of CH4/N2 (10/90, v/v) separation. N2 was first observed from the column, whereas CH4 was not eluted until the breakthrough interval time reached 12.5 min g−1 for SNNU-98-Cd and 27 min g−1 for SNNU-98-Cu (2 mL min−1 at 298 K) (Fig. S31†).
10 (Fig. 4d and Table S11†). In addition, it also is highly advantageous for the difficult problem of CH4/N2 (10/90, v/v) separation. N2 was first observed from the column, whereas CH4 was not eluted until the breakthrough interval time reached 12.5 min g−1 for SNNU-98-Cd and 27 min g−1 for SNNU-98-Cu (2 mL min−1 at 298 K) (Fig. S31†).
Multi-component gas separation plays an important role in adsorption separation technology, but it is still in the initial stage. We evaluated the separation of ternary biogas mixture CO2/CH4/N2 (5/5/90, v/v/v). As shown in Fig. 4e, N2 and CH4 broke through the adsorption column at 12.9 and 22.4 min g−1, respectively, whereas CO2 was not detected at the exit until 47.9 min g−1 for SNNU-98-Cd. Notably, the shrinking of pore size produced a much better performance, with the outflow of N2 detected at 9 min g−1, while the outflow of CH4 and CO2 from the column was 18.3 and 80.9 min g−1, respectively. Such separation of the CO2/CH4/N2 ternary gas mixture in a single process is unprecedented. The separation ability was further promoted as the temperature decreased, and was superior to all adsorbent materials that were used in this separation process (Fig. 4f and Table S12†). Notably, the CH4 breakthrough curve shows a plateau region in the middle stage, which is due to the presence of competing adsorption. Simultaneous adsorption and desorption of CH4 occurs until CO2 adsorption is saturated, and then, the C/C0 rises to 1. In addition, the cyclic stability of this three-component separation is excellent (Fig. 4g). Subsequently, real industrial separation ratios (CO2/CH4/N2: 30/65/5 and 40/55/5, v/v/v) for SNNU-98-Cu were further simulated, which showed that N2 broke through at 6 min g−1, and CH4 at 23 and 29 min g−1, respectively, while CO2 was not detected until 88 and 80 min g−1. This further confirmed that SNNU-98-Cu has great potential for industrial applications (Fig. S34†).
Furthermore, the adsorption capacities were calculated from the breakthrough curves and compared with single-component gas adsorption (Tables S13 and S14†). The results showed that the low-pressure CO2 breakthrough capacity of SNNU-98-Cu was higher than that of SNNU-98-Cd. For CO2/N2 (15/85, v/v) at 298 K, the CO2 adsorption capacity of SNNU-98-Cu/Cd was calculated to be 56.9 and 64.6 cm3 cm−3 compared to 41.2 and 80 cm3 cm−3 for static adsorption, respectively. This is also consistent with GCMC simulation and −Qst values, which further confirms that the separation effect conferred by SNNU-98-Cu is satisfactory.
To further demonstrate the multi-scenario adaptability of SNNU-98-Cd/Cu adsorbents, low calorific value coal gas with a practical composition of N2/CO2/CO/H2O/CH4/H2 (58.4/14.5/9.1/8/5.1/4.8, v/v/v/v/v/v) was selected. Extraordinarily, SNNU-98-Cd/Cu can realize efficient one-step CO2 capture from this five-component gas mixture (Fig. 4h). The N2, CO, CH4, and H2 gas components concurrently broke through the adsorption column at 10 min g−1, whereas CO2 was not detected exiting until 21.5 and 58.9 min g−1 for SNNU-98-Cd and SNNU-98-Cu, respectively. Notably, SNNU-98-Cd/Cu also possessed a high water vapor adsorption capacity (Fig. S36†), which further indicated that these robust MOFs without inter-cluster linkers are suitable for complex industrial separation scenarios and will achieve unprecedented separation results.
Overall, SNNU-98-Cu benefits from an inter-cluster-linker-absence-enabled sub-Ångstrom pore modulation, and its comprehensive CO2 capture ability is much more optimal than that of SNNU-98-Cd (Fig. 4i). The ultra-microporous environments coupled with the high density of bare tetrazolate N sites leads to the strongest interactions with CO2 molecules and achieved efficient multi-scenario separation with thermodynamic and kinetic synergy.
In situ infrared (IR) spectroscopic measurements further probed the interaction of SNNU-Cd/Cu with CO2 and CH4, whereas the N2 molecule has a high degree of symmetry and absorbs IR light very weakly (Fig. 5 and S37–S38†). The results show that the CO2 asymmetric telescopic vibrational peaks (v3 = 2349 cm−1) are redshifted, and the bending vibrational peaks (v2 = 667 cm−1) are split, which proves that CO2 strongly interacts with SNNU-98-Cd/Cu. As shown in Fig. 5a and b, CO2-loaded SNNU-98-Cu (2335–2316 cm−1) is redshifted more than SNNU-98-Cd (2338–2327 cm−1).
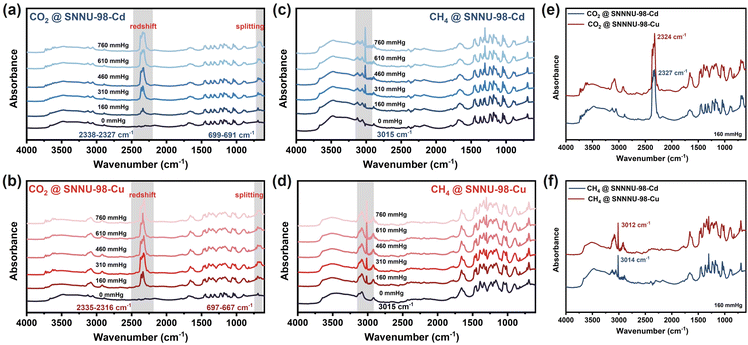 | ||
| Fig. 5 In situ FT-IR spectra for SNNU-98-Cd/Cu: (a and b) for the CO2 adsorption process; (c and d) for the CH4 adsorption process; (e and f) comparison of CO2 and CH4 adsorption. | ||
The positional differences of SNNU-98-Cd/Cu bending vibrational peak splitting were 8 cm−1 and 30 cm−1, respectively, and the vibrational peaks of SNNU-98-Cu at 3000–3250 cm−1 were significantly stronger than those of SNNU-98-Cd, indicating a stronger C–H⋯OCO2 interaction between SNNU-98-Cu and CO2. With the increase in pressure, the characteristic peak of CH4-loaded SNNU-98-Cd/Cu appeared at 3015 cm−1, and it gradually enhanced (Fig. 5c and d). The spectra of CO2 and CH4 at low pressure indicated that the peaks of SNNU-98-Cu are stronger than those of SNNU-98-Cd, which further proved that the affinity of SNNU-98-Cu for CO2 and CH4 was very strong (Fig. 5e and f). All these results not only validate the above adsorption separation performance but also illustrate the power of sub-Ångstrom pore modulation on multi-scenario CO2 capture.
To gain further insight into the host–guest interactions and adsorption behavior at the molecular level, grand canonical Monte Carlo (GCMC) simulations were performed. As shown in Fig. 6, SNNU-98-Cd and SNNU-98-Cu obviously adsorb different amounts of gas molecules in a certain channel, at 298 K and 1 bar. SNNU-98-Cu adsorbed significantly more CO2 molecules than SNNU-98-Cd, and the amount of CO2 exceeded that of CH4 and N2, indicating that the interaction of CO2 was stronger in smaller pores. This is very useful for efficient capture of CO2 from multiple industrial scenarios.
 | ||
| Fig. 6 GCMC-simulated (a and d) CO2, (b and e) CH4, and (c and f) N2 distributions in the channel of SNNU-98-Cd/Cu MOFs. | ||
As shown in Fig. S39,† the binding sites of CO2 mainly interact with high-density bare tetrazolate N sites and hydrogen bond with H, where the interaction with SNNU-98-Cu (N⋯CCO2: 2.7861–3.6808 Å, C–H⋯OCO2: 2.3646–3.108 Å) is significantly stronger than that with SNNU-98-Cd (N⋯CCO2: 3.6063–3.9138 Å, C–H⋯OCO2: 2.8270–3.4154 Å), and thus, it can adsorb additional CO2 molecules. It was confirmed that the reduced pore size at the Ångstrom level enhances the interaction between the framework and CO2. However, CH4 molecules with larger kinetic diameters undergo weaker N⋯H–CCH4 interactions with N atoms in the pores (2.9424–3.8830 Å for SNNU-98-Cd, and 2.5317–3.0746 Å for SNNU-98-Cu). This indicates that CH4 diffuses more slowly in the micropores, and SNNU-98 can efficiently separate CO2 and CH4 by kinetic sieving. In addition, the polarizability and quadrupole moment of N2 are lower than those of CO2, and thus, the interaction with SNNU-98-Cu (H⋯NN2: 2.8799–3.8322 Å) and SNNU-98-Cd (H⋯NN2: 2.5857–3.9138 Å) is weaker, suggesting an excellent separation of CO2 and N2 based on thermodynamic principles. Subsequently, the density distributions of different gas molecules were further calculated, which clearly showed that the interaction between CO2 and the SNNU-98-Cd/Cu adsorbents was stronger than those of CH4 and N2. The CO2 distribution density in SNNU-98-Cu was significantly higher than that of SNNU-98-Cd, especially in the low-pressure region (Fig. S40–S44†). This further demonstrates that the interaction of CO2 in smaller pores is stronger, thereby benchmarking the capture of CO2 in multiple scenarios (flue gas, biogas, and coal gas).
Conclusions
In this work, we propose a rational sub-Ångstrom pore modulation strategy for metal–organic frameworks by the removal of inter-cluster linkers. With the absence of inter-cluster linkers, tiny changes in coordination bond lengths triggered by metal regulation can be efficiently transmitted, enabling ultra-fine pore size control and further significantly promoting multi-scenario CO2 capture performance. Due to the suitable pore environment and high density of bare N sites, SNNU-98-Cu exhibits ultra-high stability (pH −0.5–13), top-level CO2/N2 selectivity (1509.3), and CO2 capture ability superior to nearly all porous adsorbent material, which can efficiently separate CO2 from binary flue gas, ternary biogas, and quinary coal gas mixtures in one step. In situ IR spectra and GCMC calculations clearly indicate that the benchmark CO2 capture performance is due to the coupling effect of thermodynamics and dynamics in the ultra-micropore space. This work introduces a valid strategy realizing hyperfine pore size regulation of MOF adsorbent and prompts the CO2 capture from ‘single-scene passive adaptation’ to ‘multi-scene active response’, which may provide the combination of high efficiency, economy, and sustainability for industrial carbon emission reduction.Data availability
All the associated data are available in the ESI.†Author contributions
Q.-G. Z. and J.-W. W. conceived the idea for this research. J.-W. W. carried out the experiments, analyzed the results, and wrote the manuscript. Q.-G. Z. led the project and edited the manuscript. All authors participated in and contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22471149), the Youth Innovation Team of Shaanxi Universities (2023), and the Fundamental Research Funds for the Central Universities (GK202307009).Notes and references
- L. Zhang, Z. He, Y. Liu, J. You, L. Lin, J. Jia, S. Chen, N. Hua, L.-A. Ma, X. Ye, Y. Liu, C.-X. Chen and Q. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 30394–30401 CrossRef CAS PubMed.
- W. Li, X. Liu, X. Yu, B. Zhang, C. Ji, Z. Shi, L. Zhang and Y. Liu, Inorg. Chem., 2023, 62, 18248–18256 CrossRef CAS PubMed.
- S. Geng, H. Xu, C.-S. Cao, T. Pham, B. Zhao and Z. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202305390 CrossRef CAS PubMed.
- Z. Zhang, Q. Ding, S. B. Peh, D. Zhao, J. Cui, X. Cui and H. Xing, Chem. Commun., 2020, 56, 7726–7729 RSC.
- F. Chen, J. Wang, L. Guo, X. Huang, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, Sep. Purif. Technol., 2022, 292, 121031 CrossRef CAS.
- O. T. Qazvini and S. G. Telfer, ACS Appl. Mater. Interfaces, 2021, 13, 12141–12148 CrossRef CAS PubMed.
- D. Wu, C. Liu, J. Tian, F. Jiang, D. Yuan, Q. Chen and M. Hong, Inorg. Chem., 2020, 59, 13542–13550 CrossRef CAS PubMed.
- L. Yang, X. Cui, Y. Zhang, Q. Wang, Z. Zhang, X. Suo and H. Xing, ACS Sustain. Chem. Eng., 2019, 7, 3138–3144 CrossRef CAS.
- A. Pal, S. Chand, S. M. Elahi and M. C. Das, Dalton Trans., 2017, 46, 15280–15286 RSC.
- C. Yu, Q. Ding, J. Hu, Q. Wang, X. Cui and H. Xing, Chem. Eng. J., 2021, 405, 126937 CrossRef CAS.
- J.-R. Li, J. Yu, W. Lu, L.-B. Sun, J. Sculley, P. B. Balbuena and H.-C. Zhou, Nat. Commun., 2013, 4, 1538 CrossRef PubMed.
- Z. Zhou, T. Ma, H. Zhang, S. Chheda, H. Li, K. Wang, S. Ehrling, R. Giovine, C. Li, A. H. Alawadhi, M. M. Abduljawad, M. O. Alawad, L. Gagliardi, J. Sauer and O. M. Yaghi, Nature, 2024, 635, 96–101 CrossRef CAS PubMed.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228 CrossRef CAS PubMed.
- K.-J. Chen, D. G. Madden, T. Pham, K. A. Forrest, A. Kumar, Q.-Y. Yang, W. Xue, B. Space, J. J. Perry, J.-P. Zhang, X.-M. Chen and M. J. Zaworotko, Angew. Chem., Int. Ed., 2016, 55, 10268–10272 CrossRef CAS PubMed.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Nat. Commun., 2021, 12, 197 CrossRef CAS PubMed.
- Q. Shi, J. Fuel Chem. Technol., 2021, 49, 1531–1539 CrossRef CAS.
- D. Song, S. Zou, Z. Ji, Y. Li, H. Li, Y. Zhou, C. Chen, Q. Chen and M. Wu, Angew. Chem., Int. Ed., 2025, 64, e202423496 CrossRef CAS PubMed.
- Z. Wang, Y. Zhang, E. Lin, S. Geng, M. Wang, J. Liu, Y. Chen, P. Cheng and Z. Zhang, J. Am. Chem. Soc., 2023, 145, 21483–21490 CrossRef CAS PubMed.
- L. F. A. S. Zafanelli, A. Henrique, M. Karimi, A. E. Rodrigues and J. A. C. Silva, Ind. Eng. Chem. Res., 2020, 59, 13724–13734 CrossRef CAS.
- B. Li, S. Wang, Z. Tian, G. Yao, H. Li and L. Chen, Adv. Theory Simul., 2022, 5, 2100378 CrossRef CAS.
- E. Aly, L. F. A. S. Zafanelli, A. Henrique, M. Golini Pires, A. E. Rodrigues, K. Gleichmann and J. A. C. Silva, Ind. Eng. Chem. Res., 2021, 60, 15236–15247 CrossRef CAS.
- E. Wu, X.-W. Gu, D. Liu, X. Zhang, H. Wu, W. Zhou, G. Qian and B. Li, Nat. Commun., 2023, 14, 6146 CrossRef CAS PubMed.
- X. Feng, X. Wang, H. Yan, H. Liu, X. Liu, J. Guan, Y. Lu, W. Fan, Q. Yue and D. Sun, Angew. Chem., Int. Ed., 2024, 63, e202407240 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, Y. Su, X. Liu, P. Zhang, R.-B. Lin, S. Chen, Q. Deng, Z. Zeng, S. Deng and B. Chen, Nat. Commun., 2022, 13, 200 CrossRef CAS PubMed.
- S. Yuan, L. Huang, Z. Huang, D. Sun, J.-S. Qin, L. Feng, J. Li, X. Zou, T. Cagin and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 4732–4738 CrossRef CAS PubMed.
- W. Fan, X. Zhang, Z. Kang, X. Liu and D. Sun, Coord. Chem. Rev., 2021, 440, 213968 CrossRef.
- S. Yuan, Y.-P. Chen, J.-S. Qin, W. Lu, L. Zou, Q. Zhang, X. Wang, X. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 8912–8919 CrossRef CAS PubMed.
- H.-L. Jiang, T. A. Makal and H.-C. Zhou, Coord. Chem. Rev., 2013, 257, 2232–2249 CrossRef CAS.
- Y. Ye, Z. Ma, R.-B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed.
- J.-W. Wang, S.-C. Fan, Z. Li, Q.-Q. Zhang, Y.-F. Zhang, Z.-Y. Wang, W. Yuan, Y. Wang and Q.-G. Zhai, Adv. Funct. Mater., 2024, 35, 2420070 CrossRef.
- S. P. Chan and Y. Zhang, Chem. - Eur. J., 2023, 29, e202301279 CrossRef CAS PubMed.
- Q.-G. Zhai, C.-Z. Lu, S.-M. Chen, X.-J. Xu and W.-B. Yang, Cryst. Growth Des., 2006, 6, 1393–1398 CrossRef CAS.
- Z.-J. Hou, Z.-Y. Liu, N. Liu, E.-C. Yang and X.-J. Zhao, Dalton Trans., 2015, 44, 2223–2233 RSC.
- J.-W. Wang, S.-C. Fan, H.-P. Li, X. Bu, Y.-Y. Xue and Q.-G. Zhai, Angew. Chem., Int. Ed., 2023, 62, e202217839 CrossRef CAS PubMed.
- Z. Chen, P. Li, R. Anderson, X. Wang, X. Zhang, L. Robison, L. R. Redfern, S. Moribe, T. Islamoglu, D. A. Gómez-Gualdrón, T. Yildirim, J. F. Stoddart and O. K. Farha, Science, 2020, 368, 297–303 CrossRef CAS PubMed.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- K. Leus, T. Bogaerts, J. De Decker, H. Depauw, K. Hendrickx, H. Vrielinck, V. Van Speybroeck and P. Van Der Voort, Microporous Mesoporous Mater., 2016, 226, 110–116 CrossRef CAS.
- W. Liang, H. Chevreau, F. Ragon, P. D. Southon, V. K. Peterson and D. M. D'Alessandro, CrystEngComm, 2014, 16, 6530–6533 RSC.
- H. Yang, F. Peng, A. N. Hong, Y. Wang, X. Bu and P. Feng, J. Am. Chem. Soc., 2021, 143, 14470–14474 CrossRef CAS PubMed.
- H.-L. Jiang, D. Feng, K. Wang, Z.-Y. Gu, Z. Wei, Y.-P. Chen and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934–13938 CrossRef CAS PubMed.
- Y. Duan, H. Li, X. Shi, C. Ji, J. Imbrogno and D. Zhao, Ind. Eng. Chem. Res., 2025, 64, 5372–5382 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- L. Du, Z. Lu, K. Zheng, J. Wang, X. Zheng, Y. Pan, X. You and J. Bai, J. Am. Chem. Soc., 2013, 135, 562–565 CrossRef CAS PubMed.
- X. Song, M. Zhang, C. Chen, J. Duan, W. Zhang, Y. Pan and J. Bai, J. Am. Chem. Soc., 2019, 141, 14539–14543 CrossRef CAS PubMed.
- S. Xiang, Y. He, Z. Zhang, H. Wu, W. Zhou, R. Krishna and B. Chen, Nat. Commun., 2012, 3, 954 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2416690. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02144h |
| This journal is © The Royal Society of Chemistry 2025 |