 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A planar pentacoordinate oxygen in the experimentally observed [Be5O6]2− dianion†
Rui
Sun
 ab,
Yang
Yang
a,
Xin
Wu
b,
Hua-Jin
Zhai
ab,
Yang
Yang
a,
Xin
Wu
b,
Hua-Jin
Zhai
 a,
Caixia
Yuan
a,
Caixia
Yuan
 *a and
Yan-Bo
Wu
*a and
Yan-Bo
Wu
 *a
*a
aKey Laboratory of Chemical Biology and Molecular Engineering, Ministry of Education, Key Laboratory of Materials for Energy Storage and Conversion of Shanxi Province, Institute of Molecular Science, Shanxi University, 92 Wucheng Road, Taiyuan, Shanxi 030006, People's Republic of China. E-mail: wyb@sxu.edu.cn; cxyuan@sxu.edu.cn
bBasic Sciences Department, Shanxi Agricultural University, 1 South Mingxian Road, Taigu, Shanxi 030801, People's Republic of China
First published on 16th May 2025
Abstract
Small multiply charged anions (SMCAs) are exceptionally challenging to generate in gas-phase experiments due to the spontaneous detachment of excess electrons. The [Be5O6]2− dianion, first produced in 2006 via electrospray ionization and initially proposed by a concurrent computational study to adopt a linear O–Be-alternating structure, stands as a rare experimentally observed SMCA. In this study, by applying our recently developed electron-compensation strategy, we designed a starlike D5h [O©Be5O5]2− cluster featuring a planar pentacoordinate oxygen (ppO), which intriguingly shares the molecular formula [Be5O6]2−. Remarkably, this ppO isomer is not only 55.8 kcal mol−1 more stable than the previously reported linear isomer but also represents the global energy minimum on the [Be5O6]2− potential energy surface. By adhering to the principles of the electron-compensation strategy, all Be atoms in the ppO isomer are electronically compensated and geometrically shielded by peripheral O atoms, resulting in a well-defined electronic structure. This is evidenced by a positive first vertical detachment energy of 2.44 eV, which effectively prevents the spontaneous loss of excess electrons. Thus, our work serendipitously uncovered and elaborately rationalized an experimentally unprecedented ppO within the previously generated SMCA [Be5O6]2−, marking a significant milestone in the field.
Introduction
Clusters featuring non-classical bonding are typically generated and observed in the gas phase. In general, only the thermodynamically most stable isomer, known as the global energy minimum (GEM), survives the gas-phase annealing process and is subsequently detected through combined spectroscopy. In contrast, although significantly smaller amounts of local energy minima (LEMs) may persist, their spectroscopic signals are often overshadowed by those of the GEMs. For instance, nearly all clusters featuring non-classical planar hyper-coordination have been confirmed to be GEMs. Notable examples include [CB4]+,1,2 [CAl4]−,3 [CAl4Na]−,4 [CAl3X]−/0 (X = Si, Ge),5,6 [CAl4H]−,7 C2Al4,8 and [C5Al5]− (ref. 9) with planar tetracoordinate carbon, [NAl4]−/0 (ref. 10 and 11) with a planar tetracoordinate nitrogen, [Al4X]−/0,12 [Al3X2]− (X = Si, Ge),13 and [Cu3Si3]− (ref. 14) with a planar tetracoordinate silicon or germanium, as well as [CoB8]−/0, [RuB9]−, [TaB10]−, and [NbB10]− with planar octa-, nona-, and decacoordinate transition metals.15,16Consequently, GEMs are highly favored in computational predictions of clusters with non-classical bonding due to their significantly greater compatibility with gas-phase experiments compared to LEMs. This preference is particularly evident in the design of clusters featuring non-classical planar hyper-coordination. Over the past two decades, hundreds of GEMs have been computationally predicted in this field,17–23 most of which exhibit planar pentacoordinate configurations involving H and typical second-row non-metals (ppX, where X = H, C, N, F, etc.).23–35 However, no cluster with a ppX has been experimentally observed to date. Furthermore, stable planar pentacoordinate oxygen (ppO) remains entirely unexplored, even among computationally predicted GEMs.
From the examples above, it is also evident that most experimentally observed clusters are monoanions, likely because they are characterized using photoelectron detachment spectroscopy, which favors monoanions. To the best of our knowledge, no small multiply charged anion (SMCA) with non-classical planar hyper-coordination has been reported, as SMCAs are exceptionally challenging to generate in gas-phase experiments due to the spontaneous detachment of excess electrons. Combined with the experimental gap concerning ppX, a significant breakthrough would be the observation of ppX in SMCAs.
In this work, we report the design of a starlike D5h [O©Be5O5]2− cluster (1a in Fig. 1) featuring a planar pentacoordinate oxygen (ppO). By applying our recently developed electron-compensation strategy,36 this dianion exhibits a positive first vertical detachment energy of 2.44 eV, indicating the avoidance of spontaneous electron detachment. Notably, a literature survey revealed that the corresponding [Be5O6]2− dianion was generated in 2006 via electrospray ionization,37 but a concurrent computational study38 incorrectly proposed a linear O–Be-alternating structure (0 in Fig. 1). Our ppO isomer is not only 55.8 kcal mol−1 more stable than such a linear isomer but also represents the GEM on the [Be5O6]2− potential energy surface. Thus, we have identified a ppO within an experimentally observed SMCA, marking the first experimental observation of a cluster with a ppX and the first SMCA with planar hyper-coordination.
Results and discussion
Design of [O©Be5O5]2−
The design of 1a originated from our systematic effort to create a beryllium-based starlike structure featuring a ppO, which coincidentally resulted in the molecular formula [Be5O6]2−. Specifically, we began by designing [X©BenOn] model structures (see Scheme 1) using our recently proposed “electron-compensation” strategy. By following this approach, all Be atoms in the model structures are electronically compensated and geometrically shielded by peripheral O atoms, resulting in stable BenOn skeletons. Though these skeletons lack valence electrons for centripetal bonding, the Be atoms on such skeletons are ready to accept the donation from the central atom, that is, they are electronically suited to accommodate a central atom (X) with a fully filled valence shell, such as F−, O2−, N3−, Ne, and others. During the evaluation of the compatibility of these candidate atoms with BenOn skeletons for n = 4–6, we discovered that an oxygen atom could perfectly fit the Be5O5 skeleton at a molecular charge of −2.00 |e|, yielding a starlike dianion with D5h symmetry, [O©Be5O5]2− (1a). At the B2PLYP39-D3 (ref. 40) (BJ)41/aug-cc-pVTZ level of theory, 1a was confirmed as an energy minimum, with the lowest vibrational frequency (νmin) at 114 cm−1. The distances between the central O atom and each peripheral Be atom are uniformly 1.765 Å, only 0.030 Å longer than the sum (1.735 Å) of the average radii of Be (1.061 Å) and O (0.674 Å) atoms in tetrahedrally bonded crystals.42 Thus, the central O atom can be considered to be coordinated by five Be atoms, confirming the formation of a non-classical ppO. | ||
| Scheme 1 The X©BenOn (n = 4–6) model structures for designing the clusters with planar hypercoordinations. | ||
Stability consideration
Next, we compared the thermodynamic stability of 1a with the previously reported linear isomer 0. We reoptimized the structure of 0 at the B2PLYP-D3(BJ)/aug-cc-pVTZ level and refined the energies of 0 and 1a using CCSD(T)/aug-cc-pVTZ single-point calculations based on the optimized geometries [abbreviated as CCSD(T)//B2PLYP-D3(BJ)]. Remarkably, 1a is 55.8 kcal mol−1 lower in energy than 0, indicating that 1a should be significantly more likely to be generated and characterized in the gas phase experiment. To further assess the experimental viability of 1a, we extensively explored the singlet and triplet potential energy surfaces of [Be5O6]2− using the stochastic search algorithm43,44 implemented in the GXYZ 3.0 program.45 The low-lying isomers are shown in Fig. 1. As illustrated, 1a was confirmed to be the exclusive GEM. In addition to 1a, we identified three new isomers (1b, 1c, and 1d) that are lower in energy than 0 by 17.0, 10.2, and 0.2 kcal mol−1, respectively. Structurally, 1b adopts a D3h geometry with two axial beryllium atoms linked by three O–Be–O bridges, 1c features a Cs structure with a BeO group attached to a pyramidal Be4O5 moiety, and 1d consists of a Be3O3 ring with a linear Be2O3 tail. Note that the T1 diagnostic values of CCSD(T) calculations for these [Be5O6]2− isomers range from 0.016 to 0.018, lower than the threshold of 0.020, so the results from such single-reference calculations should be reliable. Given that 1a is at least 38.8 kcal mol−1 lower in energy than its isomers, it should be the only experimentally observable isomer, dominating the [Be5O6]2− dianion generated in electrospray ionization experiments. Therefore, we focus on 1a in the subsequent analysis.To evaluate the dynamic stability of 1a,46–49 we performed Born–Oppenheimer molecular dynamics (BOMD) simulations. The structural evolution was monitored using the root-mean-square deviation (RMSD) of atomic positions. As shown in Fig. 2, the RMSD plots for simulations at 298 K, 500 K, and 1000 K exhibit no irreversible upward jumps, and the fluctuations remain small, with variation ranges of 0.02–0.19 Å, 0.03–0.24 Å, and 0.04–0.34 Å, respectively. The average RMSD values are 0.07 Å, 0.10 Å, and 0.15 Å, indicating that 1a is dynamically rigid against isomerization and dissociation at these temperatures.
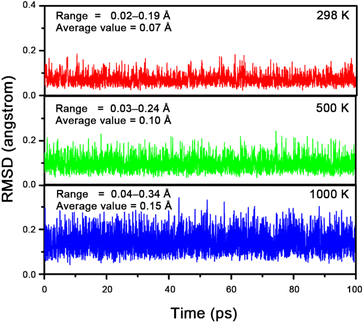 | ||
| Fig. 2 RMSD (in Å) versus simulation time (in ps) for the BOMD simulations (at 298, 500 and 1000 K) of 1a at the PBE/DZVP level. | ||
Electronic structure analyses
The electronic stability of 1a was assessed by examining its vertical detachment energy (VDE) using the outer valence Green's function (OVGF) method at the OVGF/aug-cc-pVTZ level.50 The positive first VDE of 2.44 eV indicates that electron detachment from 1a is endothermic, explaining its stable existence in electrospray ionization experiments. Additionally, we observed a large HOMO–LUMO gap of 4.76 eV at the B2PLYP-D3(BJ)/aug-cc-pVTZ level, suggesting that electron excitation from occupied to Rydberg orbitals is relatively difficult. Together, the positive VDE and large HOMO–LUMO gap confirm the electronic robustness of 1a.To understand the stability of 1a, we analyzed its electronic structure using adaptive natural density partitioning (AdNDP)51,52 to identify characteristic n-center two-electron (nc-2e) bonds. As shown in Fig. 3, among the 24 valence electrons (including two negative charges) in 1a, there are five 1c–2e O lone pairs (occupation numbers, ONs = 1.99 |e|, orbital A), ten 2c–2e Be–O σ bonds (ONs = 1.99 |e|, orbital B), five 3c–2e Be–O–Be π bonds (ONs = 2.00 |e|, orbital C), and four lone pairs on the central ppO (ONs = 1.91–1.97 |e|, orbitals D–G). Notably, the formation of five 3c–2e Be–O–Be π bonds not only reinforces the Be5O5 skeleton but also compensates for the electron deficiency of the five Be atoms through O → Be π-backdonation, aligning with our electron-compensation strategy and stabilizing the starlike structure. Simultaneously, the central ppO satisfies the octet rule (with eight valence lone pair electrons). We also considered an alternative AdNDP scheme with three 6c–2e σ bonds (orbitals E′–G′). Although the ONs for E′–G′ (1.99 |e|) are slightly higher than those for E–G (1.91–1.92 |e|), the differences of 0.07–0.08 |e| are negligible, as they are distributed across five Be atoms (each contributing only 0.014–0.016 |e|). Thus, the AdNDP analysis confirms that the central ppO in 1a is a dianion.
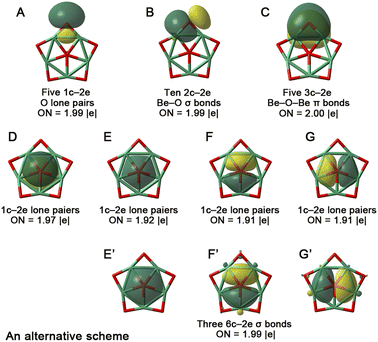 | ||
| Fig. 3 AdNDP view of chemical bonding in 1a. ON denotes the occupation number, which should be close to 2.00 |e| for doubly occupied orbitals. | ||
To further confirm this, the natural bond orbital (NBO)53 analysis was performed for 1a at the B2PLYP-D3(BJ)/aug-cc-pVTZ level. Consistent with the AdNDP analysis, the ppO possesses the electron configuration of 2 s1.912px1.94py1.942pz1.99, suggesting a dianion. The natural charge on ppO (−1.80 |e|), while that on each Be atom is +1.71 |e|, suggesting strong electrostatic characteristics for ppO–Be interactions. Meanwhile, the Wiberg bond index for each ppO–Be bond is 0.08, indicating negligible covalent interactions.
To further investigate the nature of interactions between the central ppO and the peripheral Be5O5 skeleton, energy decomposition analysis with natural orbitals for chemical valence (EDA-NOCV)54,55 was performed at the B3LYP-D3(BJ)/TZ2P level. Table S1† presents the results of different fragmentation schemes. The scheme involving a singlet O2− and a singlet neutral Be5O5 fragment yielded the lowest orbital interaction energy, consistent with the AdNDP analysis, and was therefore selected. As shown in Table 1, the total attractive energy (ΔEattr, −913.6 kcal mol−1) and the Pauli repulsion (ΔEPauli, 475.3 kcal mol−1) result in an interaction energy (ΔEint) of −441.3 kcal mol−1. Within ΔEattr, electrostatic interactions (ΔEelstat, −656.7 kcal mol−1) account for 71.9%, while orbital interactions (ΔEorb, −256.9 kcal mol−1) contribute only 28.1%, indicating that the interaction between the central O2− and the peripheral Be5O5 skeleton is predominantly electrostatic. Furthermore, 79.6% of the total ΔEorb arises from terms exhibiting donation or backdonation characteristics (ΔEorb(1)–ΔEorb(3) and ΔEorb(5)), whereas ΔEorb(4), which reflects electron-sharing bond characteristics, contributes only 5.3% of ΔEorb. This further underscores the dianionic nature of the central ppO.
| Energy terms | Assignments | Interaction energies for O2− + Be5O5 |
|---|---|---|
| a a and b are the percentage contributions to ΔEattr and ΔEorb, respectively. | ||
| ΔEint | −441.3 | |
| ΔEPauli | 475.3 | |
| ΔEattr | −913.6 | |
| ΔEelstat | −656.7 (71.9%)a | |
| ΔEorb | −256.9 (28.1%)a | |
| ΔEorb(1) | L ← O (s) σ backdonation | −102.7 (40.0%)b |
| ΔEorb(2) | L ← O (px) σ backdonation | −43.4 (16.9%)b |
| ΔEorb(3) | L ← O (py) σ backdonation | −43.3 (16.9%)b |
| ΔEorb(4) | L–O (pz) electron-sharing bonds | −13.6 (5.3%)b |
| ΔEorb(5) | L → O (px/py) σ donation | −15.1 (5.8%)b |
| ΔErest | −38.7 (15.1%)b | |
Conclusions
In summary, our targeted design of the starlike [O©Be5O5]2− cluster, featuring a planar pentacoordinate oxygen (ppO), coincidentally shares the molecular formula [Be5O6]2− with a small multiply charged anion generated in 2006 via electrospray ionization experiments. Through extensive exploration of the [Be5O6]2− potential energy surface, we revealed that this ppO isomer is not only 55.8 kcal mol−1 more stable than the previously proposed linear O–Be-alternating isomer but also represents the global energy minimum. This strongly suggests that nearly all the experimentally generated [Be5O6]2− clusters adopt the starlike ppO geometry. The experimental persistence of this dianion is attributed to the mitigation of electron deficiency of beryllium atoms through O → Be π-backdonation, as well as its unique electronic structure, which requires the two additional electrons (corresponding to the molecular charges) for the central ppO to satisfy the octet rule. Moreover, the VDE of 2.44 eV corresponds to an endothermic electron detachment for 1a. This energetically feasible transition implies that 1a is a promising candidate for observation via experimental photoelectron spectra. These findings not only uncover but also rationalize the experimental observation of an unprecedented ppO within a small multiply charged anion [Be5O6]2−, marking a significant breakthrough in the field.Computational
The singlet and triplet [Be5O6]2− potential energy surfaces were explored using the stochastic search algorithm.43,44 Randomly generated structures were initially optimized at the B3LYP/6-31G(d) level and then the ten lowest-energy isomers were re-calculated at the B2PLYP39-D3 (ref. 40) (BJ)41/aug-cc-pVTZ level. The energies of the eight lowest isomers were further refined at the CCSD(T)/aug-cc-pVTZ level. The relative energies of the isomers were compared at the CCSD(T)/aug-cc-pVTZ level, incorporating B2PLYP-D3(BJ)/aug-cc-pVTZ zero-point energy corrections [abbreviated as CCSD(T)//B2PLYP-D3(BJ)]. The geometries of low-lying isomers are also re-optimized at the PBE0-D3/aug-cc-pVTZ level, which are essentially not different from those obtained at the B2PLYP-D3(BJ)/aug-cc-pVTZ level. The structures optimized at the B2PLYP-D3(BJ)/aug-cc-pVTZ level are shown in the text, while the optimized geometries (in Cartesian coordinates) are given in the ESI. Born–Oppenheimer molecular dynamic (BOMD) simulations46–49 were carried out to assess dynamic stability at the PBE/DZVP level. Vertical detachment energies (VDEs) were calculated using the outer valence Green's function (OVGF) procedure at the OVGF/aug-cc-pVTZ level.50 To better understand the chemical bonding, natural bond orbital (NBO)53 analysis and adaptive nature density partitioning (AdNDP) analysis51 were performed at the B2PLYP-D3(BJ)/aug-cc-pVTZ and B3LYP/6-31G* levels, respectively. The AdNDP analysis was done using the AdNDP program,52 while the energy decomposition analysis with natural orbitals for chemical valence (EDA-NOCV)54,55 was further conducted at the B3LYP-D3(BJ)/TZ2P level using the ADF 2022 program package.56 The stochastic search algorithm was implemented using the GXYZ 3.0 program,45 the CCSD(T) calculations were carried out using the MolPro 2012.1 package,57 and all other calculations were performed using the Gaussian 16 package.58Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the NSFC (Grant No. 22073058), the Natural Science Foundation of Shanxi Province (Grant No. 202303021211017 and 202403021222073) and the HPC of Shanxi University.Notes and references
- Z. H. Cui, M. Contreras, Y. H. Ding and G. Merino, J. Am. Chem. Soc., 2011, 133, 13228–13231 CrossRef CAS.
- S. Becker and H. J. Dietze, Int. J. Mass Spectrom. Ion Processes, 1988, 82, 287–298 CrossRef CAS.
- X. Li, L. S. Wang, A. I. Boldyrev and J. Simons, J. Am. Chem. Soc., 1999, 121, 6033–6038 CrossRef CAS.
- X. Li, H. F. Zhang, L. S. Wang, G. D. Geske and A. I. Boldyrev, Angew. Chem., Int. Ed., 2000, 112, 3776–3778 CrossRef.
- L. S. Wang, A. I. Boldyrev, X. Li and J. Simons, J. Am. Chem. Soc., 2000, 122, 7681–7687 CrossRef CAS.
- X. Li, H. J. Zhai and L. S. Wang, Chem. Phys. Lett., 2002, 357, 415–419 CrossRef CAS.
- J. Xu, X. Zhang, S. Yu, Y. H. Ding and K. H. Bowen, J. Phys. Chem. Lett., 2017, 8, 2263–2267 CrossRef CAS PubMed.
- F. Dong, S. Heinbuch, Y. Xie, J. J. Rocca and E. R. Bernstein, Phys. Chem. Chem. Phys., 2010, 12, 2569–2581 RSC.
- C. J. Zhang, P. Wang, X. L. Xu, H. G. Xu and W. J. Zheng, Phys. Chem. Chem. Phys., 2021, 23, 1967–1975 RSC.
- S. K. Nayak, B. K. Rao, P. Jena, X. Li and L. S. Wang, Chem. Phys. Lett., 1999, 301, 379–384 CrossRef CAS.
- B. B. Averkiev, A. I. Boldyrev, X. Li and L. S. Wang, J. Chem. Phys., 2006, 125, 124305 CrossRef PubMed.
- A. I. Boldyrev, X. Li and L. S. Wang, Angew. Chem., Int. Ed., 2000, 39, 3307–3310 CrossRef CAS.
- X. Li, L. S. Wang, N. A. Cannon and A. I. Boldyrev, J. Chem. Phys., 2002, 116, 1330–1338 CrossRef CAS.
- X. H. Yin, H. L. Zeng, X. B. Liu, X. L. Xu, H. G. Xu, G. Merino, W. J. Zheng and Z. H. Cui, Angew. Chem., Int. Ed., 2025, 64, e202415789 CrossRef CAS.
- C. Romanescu, T. R. Galeev, W. L. Li, A. I. Boldyrev and L. S. Wang, Angew. Chem., Int. Ed., 2011, 50, 9334–9337 CrossRef CAS PubMed.
- T. R. Galeev, C. Romanescu, W. L. Li, L. S. Wang and A. I. Boldyrev, Angew. Chem., Int. Ed., 2012, 124, 2143–2147 CrossRef.
- R. Keese, Chem. Rev., 2006, 106, 4787–4808 CrossRef CAS PubMed , and references therein.
- L. M. Yang, E. Ganz, Z. Chen, Z. X. Wang and P. v. R. Schleyer, Angew. Chem., Int. Ed., 2015, 54, 9468–9501 CrossRef CAS , and references therein.
- P. Nag and S. R. Vennapusa, Chemistry, 2022, 4, 1723–1756 CrossRef , and references therein.
- L. Leyva-Parra, L. Diego, O. Yañez, D. Inostroza, J. Barroso, A. Vásquez-Espinal, G. Merino and W. Tiznado, Angew. Chem., Int. Ed., 2021, 60, 8700–8704 CrossRef CAS.
- X. B. Liu, W. Tiznado, L. J. Cui, J. Barroso, L. Leyva-Parra, L. H. Miao, H. Y. Zhang, S. Pan, G. Merino and Z. H. Cui, J. Am. Chem. Soc., 2024, 146, 16689–16697 CrossRef CAS PubMed.
- G. Castillo-Toraya, F. Ortíz-Chi, J. Barroso, M. Orozco-Ic, L. Leyva-Parra and G. Merino, Angew. Chem., Int. Ed., 2025, 64, e202500292 CAS.
- V. Vassilev-Galindo, S. Pan, K. J. Donald and G. Merino, Nat. Rev. Chem., 2018, 2, 0114 CrossRef CAS , and references therein.
- K. Sarmah, A. J. Kalita, S. K. Purkayastha and A. K. Guha, Angew. Chem., Int. Ed., 2024, 63, e202318741 CrossRef CAS.
- A. J. Kalita, S. S. Rohman, C. Kashyap, S. S. Ullah, I. Baruah and A. K. Guha, Inorg. Chem., 2020, 59, 17880–17883 CrossRef CAS PubMed.
- M. H. Wang, X. Dong, Z. H. Cui, M. Orozco-Ic, Y. H. Ding, J. Barroso and G. Merino, Chem. Commun., 2020, 56, 13772–13775 RSC.
- L. J. Cui, L. H. Miao, M. Orozco-Ic, L. Li, S. Pan, G. Merino and Z. H. Cui, Angew. Chem., Int. Ed., 2025, 64, e202416057 CrossRef CAS.
- L. X. Bai, Y. X. Jin, M. Orozco-Ic, G. Merino and J. C. Guo, Chem. Commun., 2024, 60, 14996–14999 RSC.
- R. Sun, B. Jin, J. Zhao, X. Wu and C. Yuan, ChemPhysChem, 2025, 26, e202400882 CrossRef CAS.
- J. H. Bian, B. Jin, X. F. Zhao, R. Sun, C. Yuan, C. Y. Zhou and Y. B. Wu, RSC Adv., 2021, 11, 15841–15846 RSC.
- X. F. Zhao, J. H. Bian, F. Huang, C. Yuan, Q. Wang, P. Liu, D. B. Li, X. T. Wang and Y. B. Wu, RSC Adv., 2018, 8, 36521–36526 RSC.
- R. Sun, X. F. Zhao, B. Jin, B. Huo, J. H. Bian, X. L. Guan, C. Yuan and Y. B. Wu, Phys. Chem. Chem. Phys., 2020, 22, 17062–17067 RSC.
- R. Sun, B. Jin, B. Huo, C. Yuan, H. J. Zhai and Y. B. Wu, Chem. Commun., 2022, 58, 2552–2555 RSC.
- M. H. Wang, X. Dong, Y. H. Ding and Z. H Cui, Chem. Commun., 2020, 56, 7285–7288 RSC.
- L. X. Bai, C. Y. Gao, J. C. Guo and S. D. Li, Molecules, 2024, 29, 3831 CrossRef CAS.
- B. Jin, C. Yuan, G. Lu and Y. B. Wu, Chem. Commun., 2022, 58, 13095–13098 RSC.
- K. Franzreb and P. Williams, Chem. Phys. Lett., 2006, 419, 379–384 CrossRef CAS.
- A. Dreuw, Chem. Phys. Lett., 2006, 419, 385–389 CrossRef CAS.
- S. Grimme, J. Chem. Phys., 2006, 124, 034108 CrossRef PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- P. Pyykkö, J. Phys. Chem. A, 2015, 119, 2326–2337 CrossRef.
- M. Saunders, J. Comput. Chem., 2004, 25, 621–626 CrossRef CAS.
- P. P. Bera, K. W. Sattelmeyer, M. Saunders, H. F. Schaefer and P. v. R. Schleyer, J. Phys. Chem. A, 2006, 110, 4287–4290 CrossRef CAS.
- H. G. Lu and Y. B. Wu, in GXYZ 3.0, Shanxi University, Taiyuan, 2021 Search PubMed.
- The BOMD simulations were performed at the PBE47/DZVP48 level using CP2K package49.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. VandeVondele and J. Hutter, J. Chem. Phys., 2007, 127, 114105 CrossRef PubMed.
- T. D. Kühne and J. Hutter, J. Chem. Phys., 2020, 152, 194103 CrossRef PubMed.
- J. V. Ortiz, V. G. Zakrzewski and O. Dolgounircheva. Conceptual Perspectives in Quantum Chemistry, Kluwer Academic, 1997 Search PubMed.
- D. Y. Zubarev and A. I. Boldyrev, Phys. Chem. Chem. Phys., 2008, 10, 5207–5217 RSC.
- The AdNDP program was downloaded freely at http://ion.chem.usu.edu/%7Eboldyrev/adndp.php .
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- A. Michalak, M. Mitoraj and T. Ziegler, J. Phys. Chem. A, 2008, 112, 1933–1939 CrossRef CAS.
- M. P. Mitoraj, A. Michalak and T. A. Ziegler, J. Chem. Theory Comput., 2009, 5, 962–975 CrossRef CAS PubMed.
- G. Te Velde, et al , J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- H. J. Werner, et al, in MolPro 2012.1, University College Cardiff Consultants Limited, Cardiff U.K., 2012 Search PubMed.
- M. J. Frisch, et al, in Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, U.S.A., 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The EDA results of the Be5O62− cluster obtained using different charged fragments at the B3LYP-D3(BJ)/TZ2P level, the shapes of deformation densities (Δρ) for EDA-NOCV analysis of the Be5O62− cluster, and Cartesian coordinates for the structures reported in this work. See DOI: https://doi.org/10.1039/d5sc02361k |
| This journal is © The Royal Society of Chemistry 2025 |

