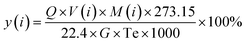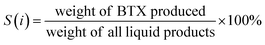Hierarchical ZSM-5 nanosheets for production of light olefins and aromatics by catalytic cracking of oleic acid†
Haoyu
Liu
a,
Wenbo
Luo
a,
Ke
Wang
a,
Yanlin
Wang
a and
Hong
Yuan
 *abc
*abc
aSchool of Chemistry and Chemical Engineering, North Minzu University, Yinchuan, 750021, China. E-mail: 2507486817@qq.com; 444281833@qq.com; 2442488336@qq.com; 2962794253@qq.com; yuanhong@nun.edu.cn
bKey Laboratory for Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University, Yinchuan, 750021, China
cNingxia Key Laboratory of Solar Chemical Conversion Technology, North Minzu University, Yinchuan, 750021, China
First published on 12th November 2024
Abstract
The bolaform surfactant C6H13-N+(CH3)2-C6H12-N+(CH3)2-C6H12-O-C6H4-C6H4-O-C6H12-N+(CH3)2-C6H12-N+(CH3)2-C6H13 (BCPH-6-6-6) was synthesized and used to guide the synthesis of hierarchical ZSM-5 nanosheets (HZN) for use as zeolite catalysts. The number of acidic sites and the Al distribution in the zeolite pores were varied by changing the amounts of sodium sulfate and Al in the initial gel. The X-ray diffraction, Fourier transform infrared spectroscopy, scanning electronic microscopy, NH3 temperature programmed desorption, 27Al magic angle spinning nuclear magnetic resonance spectroscopy and N2 adsorption/desorption were used to characterize these materials. These zeolites each had a well-developed hierarchical system and specific surface areas as high as 631 m2 g−1, indicating that the BCPH-6-6-6 formed lamellar micelles based on π–π stacking. Interconnected hierarchical pore structures were retained after the templating agent was removed. The HZN sample exhibited a 90° rotational intergrowth structure and retained a large number of lamellae. The same material had a high concentration of acidic sites and contained Al in a tetrahedral coordination framework. The catalytic cracking of oleic acid using this zeolite gave light olefin yields up to 52.2% at 500 °C, exceeding the performance of conventional ZSM-5 (38.9%), and the catalyst remained active for up to 60 h. At 450 °C, the BTX selectivity of 6.72% obtained with this material also exceeded that from the conventional ZSM-5. These results were attributed to the connected hierarchical pores of the HZN, which promoted diffusion and provided higher carbon resistance.
1 Introduction
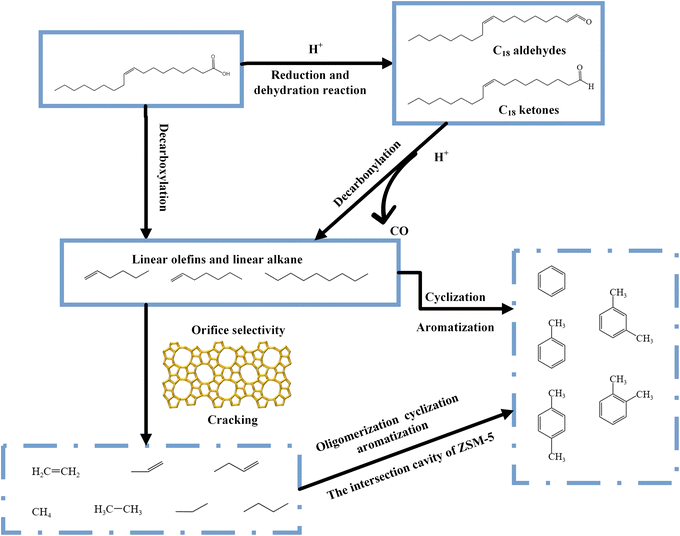
Light olefins such as ethylene, propylene and butene are often used to produce polymers or more complex organic compounds that are important in the petrochemical industry.1 Light olefins are traditionally produced by the steam cracking or catalytic cracking of naphtha, light diesel and other petroleum products.2 The synthesis of aromatics in the petrochemical industry also takes place via the catalytic reforming and cracking of petroleum products.3 However, limited petroleum reserves could at some point greatly curtail the production of light olefins and aromatic compounds and so there is a need to identify alternatives. Biomass has the advantages of being renewable, of generating less pollution and of being widely distributed, and so has become a research focus. In recent years, the production of light olefins and aromatic hydrocarbons via the catalytic cracking of oleic acid, cellulose, bio-methanol, bio-ethanol, fatty acids and triglycerides has been reported.4–8 Oleic acid is of particular interest because this compound is an 18 carbon carboxylic acid with a double bond and is readily cracked to generate light olefins, which in turn can be used to form aromatics through cyclization and aromatization. An additional route to aromatics has also been reported, based on the dehydrocyclization of alkenes with six or more carbons.9The Mobil Fifth (MFI) type zeolite has strong acid sites contained within uniform micropores and so is capable of size- and shape-selective catalysis.10 ZSM-5, which is an MFI zeolite with aluminum substituted framework silicon, is widely used to promote the synthesis of light olefins from waste cooking oil or vegetable oil. Dong et al.11 used a modified ZSM-5 zeolite for the catalytic pyrolysis of the lipid-rich microalga Chlorella pyrenoidosa as a means of producing ethylene, propylene and butene, with carbon-based yields of 7.4%, 16.4% and 8.1%, respectively. Basir et al.7 applied Y, ZSM-5, Y-ZSM-5 hybrid and Y/ZSM-5 composite zeolites to the catalytic cracking of palm oil to produce alkanes and aromatic hydrocarbons. The Y zeolite gave the highest yield (42 wt%) whereas the Y-ZSM-5 composite exhibited greater selectivity for aromatic hydrocarbons (57%). Zakaria et al.12 used various metal-impregnated HZSM-5 zeolites to catalyze the conversion of glucose to light olefins. Cu/ZSM-5 was found to give the highest turnover frequency with a 16.3% light olefin yield because this material contained more moderate to strong acid sites. Sun et al.13 used Fe-modified ZSM-5 to catalyze the fast pyrolysis of biomass. At 600 °C, a 15 wt% Fe/ZMS-5 catalyst provided the highest production of monocyclic and polycyclic aromatic hydrocarbons with amounts of 212.2 and 94.5 mg g−1 biomass, respectively. This material also exhibited a selectivity for monocyclic aromatic hydrocarbons (MAHs) of 69.2%. Li et al.14 used La2O3-modified ZSM-5 to catalyze the processing of waste cooking oil and reported a selectivity for light olefins as high as 33.8%.
Research has shown that ZSM-5 provides significant advantages when used to promote the generation of light olefins and aromatic hydrocarbons.15–17 This material comprises SiO4 and AlO4 tetrahedra linked together to form pentasil chains that in turn produce an MFI structure. This structure contains straight channels parallel to the b axis with diameters in the range of 5.3–5.6 Å and sinusoidal channels in the ac plane with sizes in the range of 5.1–5.5 Å. These channels also contain adjustable acidic sites obtained through hydrogenation and changing the molar ratio of Si/Al in the initial gel, which provide unique selectivity for light olefins and aromatic hydrocarbons. However, the standard ZSM-5 zeolite tends to contain small individual micropores along the [100] and [010] directions. These pores greatly affect the accessibility and diffusion of large molecules and suppress the emission of coke generated during the catalytic reaction. In contrast, the straight channels along the b-axis of ZSM-5 promote the migration of reactants and the removal of coke, meaning that catalytic reactions involving ZSM-5 primarily occur in pores along the b-axis. Because of issues related to micropores in ZSM-5, there have been many attempts to introduce mesopores and macropores to prepare hierarchical ZSM-5 zeolites.18–20 In addition, reducing the thickness of the zeolite crystals both decreases the diffusion lengths that raw materials must travel along the b-axis and extends the service life of the catalyst. Consequently, ultrathin zeolite nanosheets could exhibit enhanced diffusion of macromolecular species and so have received increasing attention in recent years.21 In this regard, it should be noted that zeolite crystallization is accompanied by Ostwald ripening that minimizes the surface free energy of crystals, thereby promoting the dissolution of smaller crystals and further growth of larger crystals.22 As the crystal size decreases, this process becomes more significant and inhibits the formation of ultrathin zeolite nanosheets. Research has shown that stabilizing the micelle structure by introducing hydrophobic chains which have strong interactions can promote the formation of ultrathin zeolite nanosheets.23 Ryoo et al.24 developed a well-designed structure-directing agent (SDA) and demonstrated that the incorporation of a hydrophobic tail into this agent prevented the stacking of layers along the b-axis to produce zeolite nanosheets that were only 2 nm thick. The same group synthesized MFI nanosheets using a diquaternary ammonium-type surfactant that produced an interconnected hierarchical system. Hydrophobic interactions between the long carbon chains in the surfactant generated a mesoscale micellar structure that facilitated the formation of additional mesopores, thus producing a zeolite having an increased specific surface area. The abundant active sites on the external surfaces of zeolite nanosheets can promote the catalytic conversion of organic compounds, whereas the thin sheets allow ready diffusion that avoids catalyst deactivation through coke deposition during methanol-to-gasoline conversion. These effects occur because the hydrophobic tails of the template limit the stacking of layers along the b-axis. This effect both reduces the distance over which raw material molecules must diffuse along the b-axis and slows the surface deposition of coke precursors.
Even so, hierarchical zeolite nanosheets tend to collapse after calcination or multiple uses, meaning that methods of maintaining the laminar structure and improving stability are required. Okubo et al.25 used (C3H7)3N+(CH2)N+(C3H7)3 as an SDA to hydrothermally synthesize silicalite-1 with a 90° rotational intergrowth structure. Tsapatis et al.26 reported the one-step hydrothermal synthesis of a pure-silica self-pillared pentasile zeolite using tetrabutylphosphonium-silica and tetrabutylammonium-silica sols. Rishabh et al.27 synthesized a pure-silica self-pillared pentasile zeolite using MEL-and MFI-type zeolites as crystalline seeds. The resulting material exhibited numerous external acid sites and was less prone to layer collapse. As such, this zeolite displayed markedly improved catalytic performance when applied to Friedel–Crafts alkylation and methanol-to-hydrocarbon reactions. Xu et al.28 produced single-crystalline mesostructured zeolite nanosheets using surfactants incorporating aromatic groups and only a single quaternary ammonium head. Molecular mechanics calculations predicted a low binding energy for the self-assembly of this synthesis system.
Quaternary ammonium compounds are often used as zeolite templates because they undergo strong interactions with the inorganic zeolite framework.29,30 The studies cited above have indicated that the use of double quaternary ammonium salts with special configurations as bolaform molecules can tune the morphology of hierarchical ZSM-5 nanosheets. In particular, the positively charged ammonium groups can strongly interact with negatively charged aluminosilicate particles. Additionally, these SDA molecules contain alkyl groups that significantly decrease the surface tension of developing crystals and thus facilitate the formation of smaller crystals.31 Essentially, the hydrophilic portion of the SDA guides the formation of the MFI topology while the hydrophobic section prevents the stacking of layers along the b-axis and forms micelles that cause nanosheets to join based on a 90° rotational intergrowth structure. These effects help to prevent the collapse of the hierarchical zeolite nanosheets.
In the present work, a bolaform amphiphilic surfactant having dual quaternary ammonium head groups was used to guide the synthesis of hierarchical ZSM-5 nanosheets containing multistage pores. These nanosheets exhibited high specific surface areas and mesopore volumes and were found to stack to form an autocolumnar support structure. The mechanism by which this layered morphology was generated was also investigated.
2 Materials and methods
2.1 Materials
4,4′-Dihydroxybiphenyl (97%), 1,6-dibromohexane (98%), N,N,N′,N′-tetramethyl-1,6-hexanediamine (98%), 1-bromohexane (98%) tetraethyl orthosilicate (TEOS, 98%), sodium meta-aluminate (99%), aluminium isopropoxide (AIP, ≥98.5%), tetrapropylammonium hydroxide (TPAOH, 25 wt%), acetonitrile (99.8%), and oleic acid (97%) were supplied by Shanghai Aladdin Biochemical Technology Co., Ltd., China. Methanol (99.9%), sodium hydroxide (≥96%), and potassium hydroxide (99.99% metals basis) were supplied by Shanghai Macklin Biochemical Technology Co., Ltd, China. Ethanol (99.7%), toluene (99.5%), and ether (99.5%) were purchased directly from Shanghai Pharmaceuticals Holding Co., Ltd, China.2.2 Synthesis of hierarchical ZSM-5 nanosheets
The amphiphilic compound used in this work was synthesized using a method previously reported by Xu et al.32 This process is summarized in Scheme 1. In each synthesis, 5 g of 4,4′-dihydroxybiphenyl and 3.0 g of potassium hydroxide were dissolved in 300 mL of anhydrous ethanol under a nitrogen atmosphere, after which 32 g of 1,6-dibromohexane was added and the mixture refluxed at 85 °C for 24 h. After cooling to room temperature, the solid precipitate was removed by filtration and washed twice with anhydrous ethanol then twice with deionized water, after which the material was dried at 60 °C under vacuum for 24 h to obtain the product. Following the above, 6 g of this product and 42 g of N,N,N′,N′-tetramethyl-1,6-hexanediamine were dissolved in 200 mL of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v mixture of acetonitrile and toluene after which the solution was heated at 65 °C for 30 h. The white powder obtained by filtration was washed three times with anhydrous ether and then dried at 60 °C under vacuum for 24 h to obtain the product. A 5 g quantity of this material and 8 g of 1-bromohexane were subsequently dissolved in 150 mL of acetonitrile and then refluxed at 95 °C for 27 h. The resulting white powder was recovered by filtration and washed three times with anhydrous ether then dried at 60 °C under vacuum for 12 h to obtain the final product (BCPh-6-6-6).
2 v/v mixture of acetonitrile and toluene after which the solution was heated at 65 °C for 30 h. The white powder obtained by filtration was washed three times with anhydrous ether and then dried at 60 °C under vacuum for 24 h to obtain the product. A 5 g quantity of this material and 8 g of 1-bromohexane were subsequently dissolved in 150 mL of acetonitrile and then refluxed at 95 °C for 27 h. The resulting white powder was recovered by filtration and washed three times with anhydrous ether then dried at 60 °C under vacuum for 12 h to obtain the final product (BCPh-6-6-6).
Following the above, 1.2 g of BCPh-6-6-6, 0.2 g of sodium hydroxide, 0.033 g of sodium metaaluminate and 0.43 g of sodium sulfate were dissolved in 14.4 g of deionized water and stirred for 1 h, after which 4.16 g of TEOS was added and the solution was stirred at 60 °C for a further 3 h. This mixture was subsequently transferred to a hydrothermal reactor and crystallized at 150 °C for 4 days. The initial molar ratio of the reactants was approximately 1 BCPh-6-6-6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 SiO2
20 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 Na2O
5.5 Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 Al2O3
0.2 Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 ethanol
80 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 H2O. The ethanol in this material was generated via the hydrolysis of TEOS. Following crystallization, the solid was washed three times with deionized water and then dried at 100 °C for 12 h. The resulting white solid was calcined in air at 600 °C for 6 h, giving the product referred to herein as HZN-5.5-50 (5.5 refers to the molar ratio of BCPH-6-6-6/Na2O and 50 refers to the molar ratio of Si/Al). The HZN-5-50 and HZN-6-50 samples were prepared in the same manner and changing the mass of sodium sulfate to 0.36 and 0.50 g, respectively. The HZN-5.5-30, HZN-5.5-40 and HZN-5.5-60 samples were prepared by changing the mass of sodium metaaluminate to 0.055, 0.041 and 0.027 g, respectively. A conventional ZSM-5 (CZSM-5) was prepared as described in the ESI.†
800 H2O. The ethanol in this material was generated via the hydrolysis of TEOS. Following crystallization, the solid was washed three times with deionized water and then dried at 100 °C for 12 h. The resulting white solid was calcined in air at 600 °C for 6 h, giving the product referred to herein as HZN-5.5-50 (5.5 refers to the molar ratio of BCPH-6-6-6/Na2O and 50 refers to the molar ratio of Si/Al). The HZN-5-50 and HZN-6-50 samples were prepared in the same manner and changing the mass of sodium sulfate to 0.36 and 0.50 g, respectively. The HZN-5.5-30, HZN-5.5-40 and HZN-5.5-60 samples were prepared by changing the mass of sodium metaaluminate to 0.055, 0.041 and 0.027 g, respectively. A conventional ZSM-5 (CZSM-5) was prepared as described in the ESI.†
2.3 Characterization of catalysts
X-ray diffraction (XRD) of the sample was performed on a SmartLab XRD (Rigaku, Japan) equipped with a Cu Kα radiation source at 40 kV and a tube current of 40 mA. Fourier transform infrared spectra (FTIR) were obtained using an FTIR-650 spectrometer (Gangdong, China). Scanning electron microscopy (SEM) was conducted on an EVO18 electron microscope (Zeiss, Germany) operating at 3 kV or 5 kV. The sample was dispersed using anhydrous ethanol and sonicated at 40 kHz for 10 minutes. Transmission electron microscopy (TEM) images were acquired using a JEM-F200 instrument (JEOL, Japan) at an accelerating voltage of 200 kV. The BCPh-6-6-6 solution was measured using a UV-2700 visible spectrophotometer (Shimadzu, Japan), using deionized water as a dispersant. Zeolite nanosheets were measured on a Lambda750S visible spectrophotometer (PerkinElmer, China) using barium sulfate as the substrate, with a testing range of 195–600 nm. The specific surface areas and pore size distribution of the materials were determined using an ASAP 2020 HD88 instrument (Micromeritics, USA). The samples (100–150 mg) were pretreated for 10 h at 573.15 K and 300 mmHg. The specific surface area and pore volume data were calculated according to the Brunauer–Emmet–Teller (BET) method while the pore size distribution was determined based on nonlocal density functional theory (DFT). The ammonia temperature-programmed desorption (NH3-TPD) experiments were performed using an Autochem II 2920 chemical adsorption analyser (Micromeritics, USA). The 27Al magic angle spinning nuclear magnetic resonance (NMR) spectra of specimens were obtained using a 400 MHz instrument (Bruker, Germany) with a spin rate of 12 kHz, pulse length of 0.75 μs (π/8) and cycle delay of 2 s. The spent catalysts were assessed using thermogravimetric analysis with an STA 200 instrument (Hitachi, Japan) in the range of 50 to 850 °C with a heating rate of 10 °C min−1 under a gas mixture (70% N2 and 30% O2). The pyridine-adsorbed Fourier transform infrared (Py-IR) spectra of the samples were obtained at 150 or 350 °C using an infrared spectrometer (Bruker 70, Thermo Fisher, USA) with a scanning range of 1700–1000 cm−1, scan number of 32, and a resolution of 4 cm−1.2.4 Catalytic performance
Catalytic cracking trials using oleic acid were performed in a laboratory-scale fixed-bed apparatus. Prior to each trial, a 30 mL min−1 flow of N2 was used to purge any residual air from the equipment, after which a 0.5 g quantity of an HZN-X-X sample was placed in the reaction tube and the tube was wrapped in quartz cotton (XH-SYM, diameter: 5 μm). The reactor temperature was subsequently increased to the desired value at a rate of 8 °C min−1 at which point oleic acid was injected into the fixed bed at a rate of 0.03 mL min−1. The gaseous reaction products (H2, CO, CO2, CH4, C2 to C4 olefins and C2 to C5 alkanes) were quantified using an online GC-7920 gas chromatograph (Tengzhou, Shandong, China). The chromatograph was equipped with HP-Al/KCl (30 m × 0.530 mm × 15 μm, Agilent Technologies, USA), a flame ionization detector and a thermal conductivity detector. The yield, y(i), of each gaseous product was calculated according to the equation:where i is the gas product, Q is the volume of gas (L), V(i) is the percentage volume of a gaseous substance in the gas products (%), G is the feed quantity (kg), Te is the room temperature (K), and M(i) is the per-unit molar mass of gas products (g mol−1).
Benzene, toluene and xylene (BTX) were analyzed using a GC-7920 chromatograph (Tengzhou, Shandong, China) equipped with a KB-WAX capillary column (30 m × 0.32 mm × 0.5 μm), a flame ionization detector and a thermal conductivity detector. In each trial, a 10–20 mg aliquot of the liquid phase products was transferred into a 5 mL volumetric flask after which and the solution was made up to volume by adding methanol. Each sample was then passed through a 0.22 μm organic filter membrane (NAVIGATOR, Tianjin, China) and then stored in a 2 mL vial. The BTX selectivity S(i), of each liquid phase product was calculated according to the equation:
The residual oleic acid concentration was analyzed by gas chromatography (Celian 456 C, Tianmei) using a flame ionization detector and a DB-5 capillary column (30 m × 0.25 mm × 0.25 μm). The column temperature program comprised an initial hold at 100 °C for 3 min followed by a ramp to 250 °C at 25 °C min−1 and a hold at that temperature for 3 min, with a subsequent ramp to 300 °C at 15 °C min−1 and a hold at that final temperature for 3 min. The oleic acid conversion percentage was calculated as:
| Conversion (%) = 100% − oleic acid concentration (%) |
3 Results and discussion
3.1 Crystallinity and ordering
Fig. 1a shows the low-angle XRD patterns obtained from the HZN-X-50 specimens having different Na+ proportions. In each case a peak is evident at 2θ = 1.44°, which is attributed to the first-order reflections of layered MFI nanosheets with a unit cell constant, indicating an ordered periodic interlamellar structure.21 The low-angle XRD patterns of the calcined HZN-X-50 specimens exhibit broad, low intensity peaks, suggesting that many mesopores were maintained after the removal of the surfactant. The same figure also demonstrates that the HZN-5.5-50 produced the most intense first-order reflection and so presumably contained more lamellae. Compared with the other samples, the peaks obtained from HZN-5.5-50 were shifted to higher angles, indicative of smaller d-spacing. Fig. 1b shows peaks at 7.83°, 8.69°, 13.07°, 13.80°, 14.67°, 20.20°, 23.01° and 23.91° that correspond to reflections from the (101), (200), (012), (031), (202), (103), (051) and (033) planes, respectively.33 Compared with the CZSM-5 sample (Fig. S1†), the HZN-X-X specimens all generated wider high-angle XRD peaks as a result of smaller crystal sizes. Most of these peaks in the high angle range are ascribed to h0l reflections of the MFI skeleton, indicating that these samples were highly crystalline in the a–c plane. As the concentration of Na+ was increased, the peaks gradually become sharper and so it appears that an excess of Na+ may promote the formation of larger crystals.34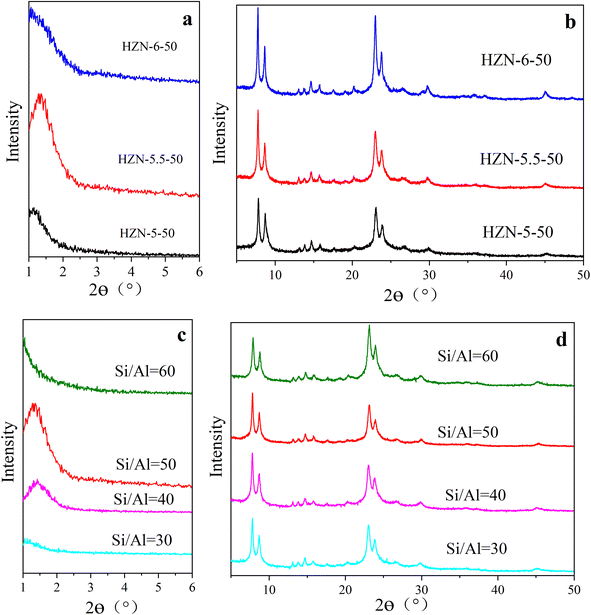 | ||
| Fig. 1 (a) The low-angle XRD and (b) high-angle XRD patterns of the HZN-X-50 specimens, (c) the low-angle XRD and (d) high-angle XRD patterns of the HZN-5.5-X specimens. | ||
Fig. 1c provides the low-angle XRD patterns of HZN-5.5-X specimens having different Si/Al molar ratios. As the Si/Al molar ratio increases, the intensity of the first order reflection first increases and then decreases such that the highest peak intensity appears at a ratio of 50. It is possible that an excess of Al produced a greater number of AlO4− tetrahedra that modified the Al distribution in the material and so inhibited crystallization.35 At a ratio of 60, the first order reflection is not observed, perhaps because of partial collapse of the nanosheets during calcination. Fig. 1d shows the high-angle XRD patterns of HZN-5.5-X specimens having different Si/Al molar ratios. Varying this ratio did not change the positions of the peaks, which were attributed to h0l reflections of the MFI structure. Table S1† summarizes the lattice parameters of the HZN-X-X samples, from which it can be seen that each material had an orthorhombic Pnma crystal system. The a and b lattice constants in each case were similar, indicating that rotational boundaries were commonly formed in the MFI zeolite based on overgrowth of the (h00) and (0k0) faces.36,37 FTIR spectra (Fig. S2†) were obtained from the HZN specimens to further confirm the formation of a ZSM-5 crystal phase.
3.2 Textural properties
The SEM images provided in Fig. 2 demonstrate that each of the HZN specimens had a zeolite nanosheet structure whereas the CZSM-5 crystals had a typical coffin-shaped morphology (Fig. S3†). HZN-5.5-50 and HZN-5-50 exhibit an unusual boundary structure based on the 90° rotation of adjacent faces. The TEM images in Fig. 3a and d confirm that the nanosheets in these two samples grew in both directions to form the 90° rotational boundaries. Moreover, complete lattice stripes can be observed in these images, indicating that BCPh-6-6-6 promoted the formation of ordered lattice structures. It should also be noted that only HZN-5.5-50 and HZN-5-50 showed the 90° rotation of crystals at a significant level and both had house-of-cards morphologies. The framework connectivity in these materials is thought to have been responsible for the partial retention of a lamellar meso-structure.38 A greater number of 90° rotational boundaries also appears to have been associated with a higher number of mesopores in the calcined materials, and these mesopores are evident in the TEM images. The broad, weak peaks in the low-angle XRD patterns of HZN-5.5-50 and HZN-5-50 provide further evidence for this type of structure. Fig. 3b shows several thick nanosheets that cross over one another in HZN-5-50, which had reduced interlamellar spacing. These nanosheets evidently grew perpendicularly on a single large crystal, similar to the intergrowth phenomenon often observed in traditional ZSM-5.22,37 However, this 90° rotational intergrowth structure was almost completely absent in the HZN-6-50 sample (Fig. 2e and f). Instead, this material exhibited a petal-like morphology resulting from significant collapse of the nanosheets during calcination. The TEM images indicate the presence of multiple zeolite nanosheets stacked along the b-axis direction in the calcined samples. This effect may have occurred as crystallographically ordered MFI layers connected with one another via Si–O–Si bonds during calcination while maintaining their original morphology.32 These results indicate that an optimal Na+ proportion favors the formation of a house-of-cards structure in which crystals are joined with 90° rotation. This structure tends to produce macropores because of the self-pillared structures between nanosheets.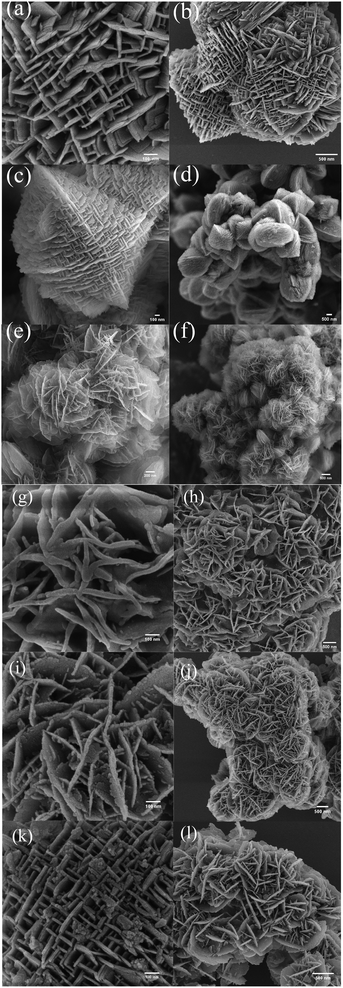 | ||
| Fig. 2 The SEM images of (a, b) HZN-5-50, (c, d) HZN-5.5-50, (e, f) HZN-6-50, (g, h) HZN-5.5-30, (i, j) HZN-5.5-40 and (k, l) HZN-5.5-60. | ||
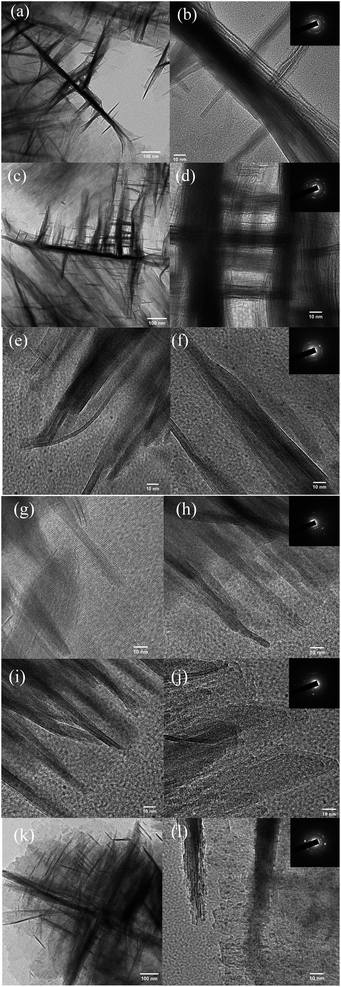 | ||
| Fig. 3 TEM images of (a, b) HZN-5-50, (c, d) HZN-5.5-50, (e, f) HZN-6-50, (g, h) HZN-5.5-30, (i, j) HZN-5.5-40 and (k, l) HZN-5.5-60. | ||
Both SEM and TEM images of HZN-5.5-X specimens with different Si/Al molar ratios are provided in Fig. 2g–l and 3g–l. Nanosheet structures can be observed in each case, confirming that BCPH6-6-6 effectively promoted the formation of such structures. Fig. 2g and h show a large number of nanosheets with a petal-like morphology in the HZN-5.5-30 sample. The number of these nanosheets decreased with increases in the Si/Al molar ratio as the 90° rotational intergrowth structure gradually became dominant. Both the HZN-5.5-30 and HZN-5.5-40 specimens exhibit small particles of debris attached to the nanosheets. It is possible that the zeolite nanosheets did not form a 90° rotational intergrowth structure because there was an excess of the Al source. This ultimately led to the collapse of the zeolite nanosheets to form fragments during calcination. The corresponding TEM images (Fig. 3g–j) indicate that some of the zeolite nanosheets were attached, meaning that the petal-like morphology was more likely to collapse. The TEM image of HZN-5.5-60 confirms a 90° rotational intergrowth structure. In this specimen, the perpendicular crystal plates having a boundary relationship shared a common c axis and the (100) faces were overgrown on the (010) faces.39 The insets to this figure present the selected area electron diffraction data acquired from these samples. These results confirm that the materials comprised polycrystalline MFI zeolites, which may be due to the sequence of the significant exposure of a–c planes.24 It is also possible that the formation of MFI/MEL twins produced polycrystalline MFI zeolite in these intergrowth domains.26 Some ultrathin crossed nanosheets are evident (Fig. 2k), suggesting that the dominant house-of-cards morphology could have two structures with different levels of symmetry and that these morphologies could intergrow. It has been reported that structures having higher symmetry in the 90° rotational intergrowths system can connect structures with lower symmetry to produce rotational boundaries at the connection points.28 However, among the hierarchical zeolites, MFI has lower symmetry (Pnma) while MEL has higher symmetry (I4m2), meaning that the latter would be expected to connect the MFI regions. This phenomenon would produce crystal boundaries at the rotational boundaries.
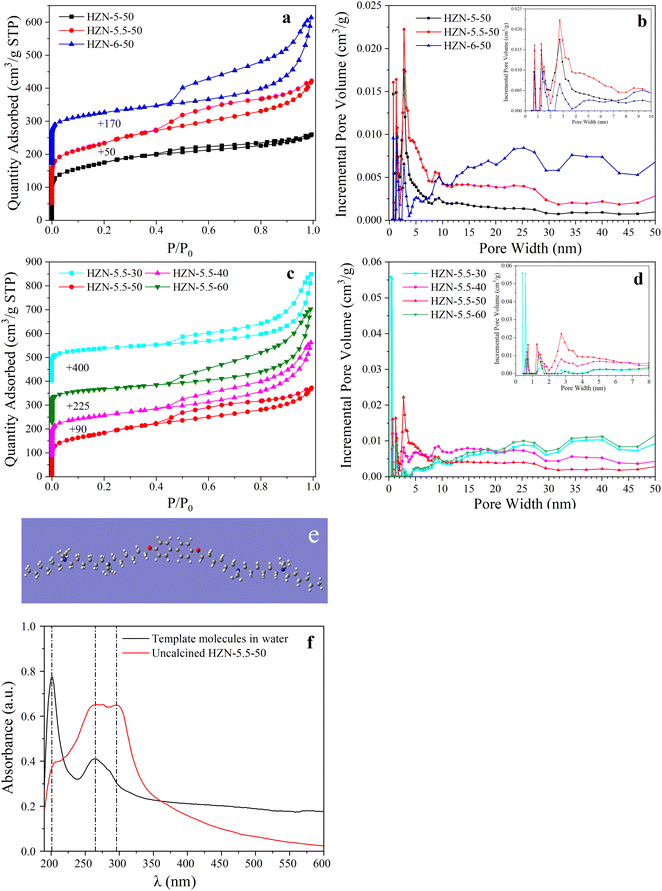
Fig. 4a shows the N2 adsorption–desorption isotherms obtained from the HZN-X-50 specimens having different Na+ proportions. Each of the samples produced a typical type IV isotherm. In the low relative pressure region (P/P0 = 0–0.1), high uptake steps are apparent, indicating that micropores were present because of the formation of a microporous MFI structure.29 In the medium relative pressure region (P/P0 = 0.3–0.8), all samples exhibit a higher uptake based on the presence of numerous uniformly distributed mesopores after calcination. The capillary condensation loops establish the presence of mesopores formed by the collapse of the lamellar structure near the joints. In addition, the hysteresis loop in the range of P/P0 = 0.5–0.7 suggests the presence of interconnected pores formed by the aggregation of card-like sheets. The pore size distributions of three HZN-X-50 samples are displayed in Fig. 4b. Each of the samples produced a small, narrow peak centered at 0.5 nm, characteristic of pores in an MFI structure.40 In the mesopore range, a broad peak was obtained at 2.74 nm in each case, with the HZN-5.5-50 producing the highest peak. The dimensions of the mesopores in these materials were approximately equal to the scale of a single lamellar micelle. Molecular geometry optimization calculations using Gauss View (Fig. 4e) showed that the distance between the two quaternary ammonium groups in the surfactant was approximately 2.65 nm and therefore similar to the characteristic mesopore size of the HZN zeolites (2.74 nm). The hydrophobicity of the template molecule was assessed by studying the stereoregularity of the BCPh6-6-6 using UV-visible absorption spectroscopy(Fig. 4f). In a water solution, the BCPh6-6-6 molecule produced only one peak related to benzene rings (201 nm) and one related to biphenyl groups (264 nm).41 The material in the uncalcined crystalline solid state generated peaks at 295 and 264 nm because the biphenyl groups formed the π–π stacking in a very close and regular manner, which confirmed that such interactions occurred between template agents.30 These results show that, during the synthesis of the zeolite, the template formed lamellar micelles and π–π stacking between biphenyl groups also occurred.
Fig. 4c and d show that HZN-5.5-X specimens generate a type IV isotherm, indicating that both micropores and mesopores were present.42 At a Si/Al molar ratio of 30, the quantity adsorbed increased dramatically in the high relative pressure region (P/P0 = 0.8–1). These data indicate an inhomogeneous pore structure resulting from particle stacking. The pore size distributions showed that the samples contained both micropores (<2 nm) and mesopores (>5 nm). This structure may have been a consequence of the addition of an excess of NaAlO2 to the initial gel such that more Al debris particles were deposited on the external surfaces to block mesopores. The SEM images in Fig. 2g and h also show some debris attached to the nanosheets.43 An excess of NaAlO2 may have disrupted the continuous MFI structure, leading to the formation of a displacement boundary. These effects could generate linear O defects to produce a partially interrupted structure, and in the linear O defects half the Si–O bonds were in registry with the matrix of bonds and half were undercoordinated.22,36 These defects may have led to the partial fragmentation of the nanosheet layers and to the collapse of mesopores to form larger pores following removal of the surfactant. As shown in Table 1, the BET surface areas associated with micropores in the samples gradually decreased as the Si/Al molar ratio was increased from 30 to 50, whereas the BET surface area associated with mesopores slowly increased. Presumably, decreasing the amount of the Al source reduced the number of nanocrystals clogging the mesopores such that the mesopore surface area was primarily associated with slit-shaped pores (2.74 nm) formed by BCPH6-6-6. However, the sample with the Si/Al molar ratio of 60 had an increased micropore surface area and a decreased mesopore surface area. These results are attributed to the spontaneous formation of small precursor nanoparticles at high Si concentrations. These nanoparticles remained nearly constant in size during the initial phase of hydrothermal growth and were able to block some of the pores.44 Small grains can be seen in the TEM image in Fig. 3l, confirming the presence of such nanoparticles. Almost none of the 90° rotational intergrowth structure was found in the HZN-5.5-60 sample (Fig. 2l), as a consequence of the removal of the templating agent during calcination with concurrent collapse of part of the lamellae to form macropores (Fig. 4d). Table 1 shows the hierarchy factor (HF) of each sample, with HZN-5.5-50 possessing the highest HF (0.21) among all the samples, meaning that the external specific surface area was enhanced without severely decreasing the micropore volume.45 For CZSM-5, HF was higher than those for all the other catalysts except for HZN-5.5-50 because TPAOH guided mesopore formation less than BCPH-6-6-6; thus, CZSM-5 retained a higher micropore volume than HZN-5.5-50.
| S BET | S micro | S ext | V total | V micro | V meso | V meso/Vmicro | Average pore size (nm) | HF | |
|---|---|---|---|---|---|---|---|---|---|
| a S BET is the BET surface area. Smicro is the micropore volume calculated by the t-plot method. Sext = SBET − Smicro. Vtotal is obtained from the amount adsorbed. Vmeso is calculated using the t-plot method. Vmeso = Vtotal − Vmicro. Average pore size from N2 adsorption isotherms. Adsorption–desorption curves and pore size distribution of CZSM-5 are derived from Fig. S5. HF (hierarchy factor) = (Vmicro/Vtotal) × (Sext/SBET). | |||||||||
| HZN-5-50 | 580 | 179 | 401 | 0.40 | 0.10 | 0.30 | 3.00 | 2.77 | 0.17 |
| HZN-5.5-50 | 631 | 155 | 476 | 0.58 | 0.16 | 0.42 | 2.63 | 3.66 | 0.21 |
| HZN-6-50 | 509 | 220 | 289 | 0.69 | 0.16 | 0.53 | 3.31 | 5.39 | 0.13 |
| HZN-5.5-30 | 443 | 236 | 207 | 0.69 | 0.12 | 0.57 | 4.75 | 6.28 | 0.08 |
| HZN-5.5-40 | 552 | 215 | 337 | 0.73 | 0.11 | 0.62 | 5.64 | 5.30 | 0.09 |
| HZN-5.5-60 | 460 | 233 | 227 | 0.74 | 0.12 | 0.62 | 5.17 | 6.42 | 0.08 |
| CZSM-5 | 308 | 210 | 98 | 0.18 | 0.11 | 0.07 | 0.64 | 2.32 | 0.19 |
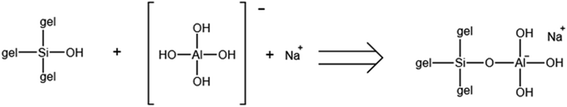
Based on the above results, a mechanism for the formation of the hierarchical ZSM-5 nanosheets was devised (Scheme 2). In this process, NaOH provides OH− and Na+ and so acts as an alkali source and a template. The Al and Si sources in the initial gel form SiO4 and AlO4− tetrahedra based on hydrolysis reactions. In the early stage of crystallization, some of the negatively charged aluminosilicate precursors combine with Na+ to form an aluminosilicate polymer.46 Simultaneously, the BCPH6-6-6 combines with the aluminosilicate to both reduce the surface energy and provide reactive species such as Si–OH and Al–OH. Following this, the strong electrostatic attraction associated with the hydrophilic portions of the template molecules promotes crystal nucleation and growth near these parts. This effect also leads to stacking of the crystals. In addition, the ammonium groups of BCPH6-6-6 decrease the surface tension at the interface, which may also promote crystal stacking.47 As the crystal nuclei continue to grow by consuming Si–OH and Al–OH groups, the hydrophobic portions of the template molecules prevent crystal stacking along the b-axis by forming strong π–π interactions. Si–OH groups were likely responsible for the cross-linking of neighboring crystals via the formation of Si–O–Si bonds based on condensation reactions between Si–OH and –OH groups.48 The BCPH6-6-6 molecules attach to the crystal surfaces to prevent these reactions as a consequence of the π–π stacking interaction between the hydrophobic portions, thus forming hierarchical ZSM-5 nanosheets. Fig. S4† demonstrates that each template molecule was attached to the surface of an isolated nanosheet. Because the BCPH6-6-6 molecules had both hydrophilic and hydrophobic parts, mesopores were retained after calcination. The formation of an interconnected hierarchical MFI structure was therefore guided by the hydrophilic portions of the template molecules.
These analyses confirmed the existence of numerous mesopores in the HZN samples, indicating that the use of BCPH6-6-6 provided a more uniform mesoporous texture. Table 1 shows that both HZN-5-50 and HZN-5.5-50 had large mesopore areas, smaller mesopore volumes and average pore sizes of 2.77 and 3.65 nm, respectively. From these data it is apparent that the mesopores likely originated from the template. However, the Smeso value of HZN-6-50 was only 289 m2 g−1, perhaps because of the partial collapse of the mesopores after removing the template.
3.3 Acidic properties and aluminum state
The acidity values of the HZN-X-50 specimens having different Na+ proportions were determined by NH3-TPD (Fig. 5a). In these plots, the NH3 desorption peaks in the ranges of 100–200 °C and 300–500 °C correspond to weakly and strongly acidic sites, respectively.49 With increase in the Na+ concentration, the density of weakly acidic sites gradually decreased whereas the number of strongly acidic sites gradually increased and the total quantity of acidic sites gradually decreased (Table 2). These results are attributed to the ready condensation of preformed Si oligomers with Al(OH)4− in association with Na+ coordination to form an aluminosilicate polymer in the basic medium. At higher Si/Al molar ratios, all the Al-bearing species were incorporated into this polymer and the preformed Si oligomers condensed to form an aluminosilicate polymer. The Na+ ions acted as counterbalancing charges to promote the formation of this polymer (Scheme 3). The Na+ ions derived from the aluminate or hydroxide were primarily bonded with negatively charged Al centers in the resulting complex and so preferentially interacted with Al centers in the gel.50 On this basis, a moderate concentration of Na+ ions promoted the incorporation of Al into the MFI structure. The classic Brønsted strongly acidic site was generated by the interaction between the proton and [AlO4]− tetrahedron derived from the framework Al atoms in the MFI structure.51 At the same Si/Al molar ratio, more Al atoms entered the MFI structure, increasing the number of strongly acidic sites and decreasing the number of weakly acidic sites derived from extra-framework Al atoms.52 The 27Al-MAS-NMR spectra acquired from the HZN-X-50 specimens are shown in Fig. 5b and exhibit peaks at 55 and 0 ppm with the former being more intense. These peaks are attributed to framework Al with tetrahedral coordination (AlF) or extra-framework Al with octahedral coordination (AlFE).53 The formation of framework and extra-framework Al suggests that Brønsted and Lewis acidic sites were produced in the zeolite. The larger peak at 54 ppm also indicates that more of the Al was present in [AlO4]− groups having a similar structure to that of [SiO4], and so an Al–OH framework was generated that provided Brønsted acid sites. In addition, the formation of a small amount of extra-framework Al demonstrates that each of the HZN-X-50 samples was highly crystalline.54 The relative areas of the peaks at 54 and 0 ppm in the spectra of the HZN-X-50 samples are summarized in Table 3. The relative area of the 0 ppm peak decreased from 6.8% to 3.8% with increase in the Na+ concentration and so it is evident that Al was more easily embedded in the MFI structure with increases in the amount of Na+. The relative area of the AlFE peak was also inversely correlated with the density of weakly acidic sites in the material, in agreement with the NH3-TPD results. The full width at half maximum (FWHM) values of the tetrahedral Al peaks generated by the HZN-X-50 samples were in the range of 6.7–8.3 ppm and so were similar to those obtained from highly crystalline calcined ZSM-5 zeolite specimens. A gradual increase in the FWHM with increase in the Na+ concentration indicates an increase in the proportion of amorphous silica–alumina in the HZN-X-50 samples because these regions did not have crystallographically imposed restrictions.55 This observation clearly shows that the introduction of mesopores into the ZSM-5 zeolite had a significant effect by slightly decreasing the uniformity of the Al environment. This effect can likely be attributed to the less-restricted surface environment of the material.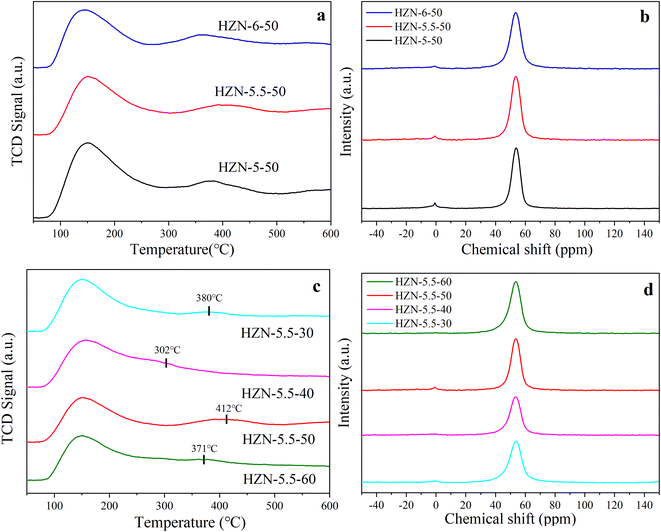 | ||
| Fig. 5 (a) NH3-TPD curves of HZN-X-50, (b) 27Al-MAS-NMR spectroscopy of HZN-X-50, (c) NH3-TPD curves of HZN-5.5-X, (d) 27Al-MAS-NMR spectroscopy of HZN-5.5-X. | ||
| Weak acidity (mmol g−1) | Strong acidity (mmol g−1) | Total acidity (mmol g−1) | WA/SA | 150 °C B/L | 350 °C B/L | |
|---|---|---|---|---|---|---|
| HZN-5-50 | 0.57 | 0.07 | 0.64 | 8.14 | 0.08 | 0.16 |
| HZN-5.5-50 | 0.49 | 0.09 | 0.58 | 5.44 | 0.55 | 1.44 |
| HZN-6-50 | 0.47 | 0.08 | 0.55 | 5.88 | 0.48 | 1.66 |
| HZN-5.5-30 | 0.65 | 0.06 | 0.71 | 10.83 | 0.25 | 0.54 |
| HZN-5.5-40 | 0.66 | 0.07 | 0.73 | 9.42 | 0.18 | 0.41 |
| HZN-5.5-60 | 0.42 | 0.05 | 0.47 | 8.40 | 0.22 | 0.41 |
| CZSM-5 | 0.46 | 0.09 | 0.55 | 5.11 |
| AlFE | AlF | FWHM (ppm) | 45–50 ppm | 50–57 ppm | 57–65 ppm | |
|---|---|---|---|---|---|---|
| HZN-5.5-30 | 2.2% (−0.14 ppm) | 97.8% (53.64 ppm) | 9.3 | 28.7% | 58.4% | 12.9% |
| HZN-5.5-40 | 2.5% (−0.64 ppm) | 97.5% (53.38 ppm) | 8.6 | 26.1% | 62.0% | 11.9% |
| HZN-5.5-50 | 5.6% (−0.89 ppm) | 94.4% (53.67 ppm) | 7.5 | 13.1% | 78.3% | 8.6% |
| HZN-5.5-60 | 0.3% (−0.03 ppm) | 99.7% (53.63 ppm) | 8.7 | 29.7% | 60.7% | 9.6% |
| HZN-5-50 | 6.8% (−0.77 ppm) | 93.2% (53.87 ppm) | 6.7 | 17.2% | 71.8% | 11.0% |
| HZN-6-50 | 3.8% (−0.89 ppm) | 96.2% (53.5 ppm) | 8.3 | 22.8% | 66.3% | 10.9% |
Fig. 5c shows the NH3-TPD results obtained from HZN-5.5-50 samples with different Si/Al molar ratios. It is apparent that variations in this ratio had different effects on the amounts of weakly and strongly acidic sites in HZN-5.5-X. In particular, the number of weakly acidic sites increased with decrease in the Si/Al molar ratio. In contrast, the amount of strongly acidic sites first increased and then decreased, with a maximum value of 0.09 mmol g−1 at a ratio of 50. Fig. 5c shows the peak temperatures for strongly acidic sites in HZN-5.5 prepared at different Si/Al molar ratios. Among all the catalysts, HZN-5.5-40 and -50 possessed the lowest and highest peak temperatures (302 and 412 °C, respectively). Compared with HZN-5.5-50, HZN-5.5-40 possessed a higher Vmeso/Vmicro ratio, which is inconducive to the formation of Al-atom-related strongly acidic sites typically in the MFI structure possessing 10 MR pores.56 The 27Al-MAS-NMR spectrum of the corresponding sample in Fig. 5d demonstrates that the area of the AlF peak decreased and then increased with increase in the ratio and had a maximum value at a ratio of 60 (99.7%). These data indicate that AlO4− tetrahedra were more likely to be present in the framework at higher Si/Al molar ratios. This effect may have occurred because the hydrophilic parts of the BCPH6-6-6 molecules limited the arrangement of AlO4− tetrahedra in the MFI structure. The large benzene rings in the hydrophobic sections of the surfactant molecules (5.8 Å for one benzene ring) tend to block the zeolite pores during the synthesis of the material. As such, only one ammonium group may enter each pore to direct the synthesis of the MFI structure while the other remains at the pore mouth on the external surface.23 As a result, the aluminosilicate polymer formed near the hydrophobic portion is less likely to enter the MFI structure during the primary growth of nanoparticles. The hydrophobic tails prevent continuous growth of the lamellae along the b-axis direction. These tails also prevent Al from being embedded in the MFI structure, which in turn generates AlOx based on extra-framework Al combining with O atoms and free Na+ ions. These effects may provide a smaller number of weak Lewis acid sites.57 Therefore, at lower Si/Al molar ratios, more weakly acidic AlOx sites were formed. The amount of aluminosilicate polymer generated in the vicinity of the ammonium groups was increased as the Si/Al ratio was elevated, leading to an increase in the number of strongly acidic sites. The highest number of strongly acidic sites was produced at a Si/Al molar ratio of 50 because only some of the ammonium groups were able to direct the formation of the MFI structure.
At lower Si/Al molar ratios, more of the Al-rich debris was formed. This debris was selectively extracted and showed little acidity.43 The generation of Si–OH groups from the unsaturated SiO2 network at the external surfaces of the zeolite nanosheets may also have affected the acidity of weakly acidic sites. The strong Brønsted acid sites were formed by the connection of Al atoms to Si via so-called bridging hydroxyl groups such that negative charges were balanced by protons. The Al formed bridging hydroxyl groups by replacing Si in the zeolite skeleton such that stronger Brønsted acid sites were formed. In the case of zeolite crystals with a perfect structure, meaning complete crystallinity without any negative effects of a high Al concentration, each Al atom will represent a Brønsted acid site.58 The data in Table 3 indicate that the FWHM values first increased and then decreased with increase in the Si/Al molar ratio with a minimum at a ratio of 50. These data demonstrate that HZN-5.5-50 was highly crystalline. The FWHM values of the HZN-5.5-X samples were higher than those of the highly crystalline ZSM-5 zeolite (the FWHM values 6.8–6.9 ppm), possibly because the rotational boundaries in the HZN-5.5-50 caused these tetrahedra to have a greater degree of distortion.59
The location of the Al sites was a key factor that determined the chemical characteristics of the zeolite because the AlF sites served as active sites by acting as proton or electron donors and forming metal-oxo complexes. The backbone of conventional ZSM-5 contains 12 non-equivalent T sites, each of which is occupied by eight Si or Al atoms in the crystal cell. The broad peak between 30 and 80 ppm in the 27Al MAS NMR spectra was deconvoluted based on a mixed Gaussian–Lorentzian process (Fig. 6), which estimated the Al distribution in the individual lattice T sites. This deconvolution indicated peaks in the ranges of 45–50 and 50–57 ppm corresponding to Al located at T1 + T2 + T3 and T9 sites in the MFI structure, respectively. In addition, the peaks in the range of 57–65 ppm corresponded to Al atoms located at T4, T10, T8 and T12 sites in the MFI structure, as estimated by the recent DFT calculation.60Table 3 provides the proportional peak areas, which suggest that more AlF sites (that is, at T9 and T1 + T2 + T3 sites) were located in intersection cavities within the ZSM-5. In contrast, only a small portion of the AlF sites (the T4, T10, T8, and T12 sites) were incorporated in the straight and/or sinusoidal channels.53,61
 | ||
| Fig. 6 Deconvolution of the 27Al MAS NMR spectra of (a) HZN-5.5-30, (b) HZN-5.5-40, (c) HZN-5.5-50, (d) HZN-5.5-60, (e) HZN-5-50 and (f) HZN-6-50. | ||
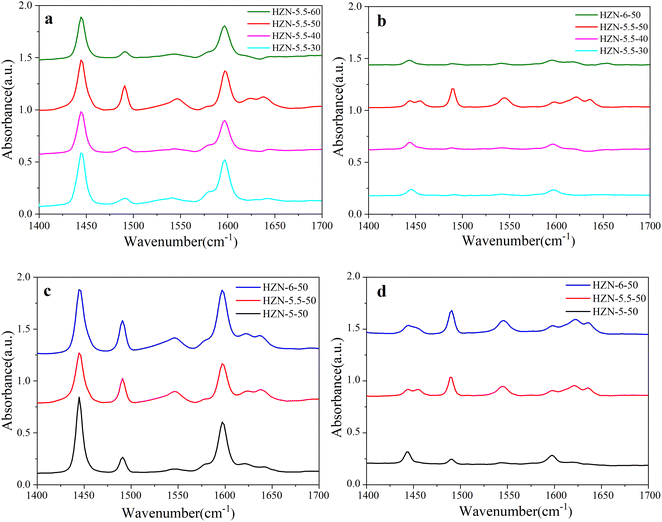
The Py-IR spectra of the HZN samples are provided in Fig. 7. Three peaks can be seen at 1637, 1623 and 1547 cm−1, corresponding to protonated pyridine (Scheme 4).58 The Brønsted acids typically generate a peak at 1547 cm−1. In the present work, a peak also appeared at 1450 cm−1, characteristic of L acid sites (Scheme 4).62Fig. 7a and b present the Py-IR spectra acquired at 150 and 350 °C from the HZN-5.5-X series of samples, respectively. At 150 °C, each specimen generated a strong peak at 1450 cm−1, indicating the presence of a large number of L acid sites. However, the intensity of this peak decreased drastically at 350 °C, demonstrating that the L acid sites were predominantly weakly acidic.63,64Table 2 summarizes the B/L ratios determined for the materials at various desorption temperatures. These data confirm that, with the exception of the HZN-5.5-50, the B/L ratios were all less than 1, and so these hierarchical ZSM-5 nanosheets had more L acid sites. This may have occurred because a loss of continuity along the b-axis direction led to more L acid defects. These sites are associated with NMR-invisible tri-coordinated framework Al and exoskeletal Al.65 White et al.66 characterized HZSM-5 samples having different Si/Al ratios using 27Al MAS NMR and found that 50% to 60% of the aluminum atoms existed as Brønsted acid sites with the others distributed among destroyed framework sites or non-framework sites.
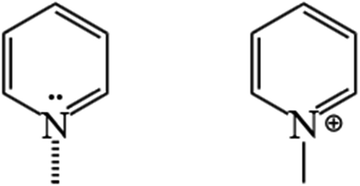 | ||
| Scheme 4 Pyridine adsorption on the constituent unit of active sites in the ZSM-5 zeolite: PyL (left) and PyH+ (right). | ||
The Py-IR spectra acquired at 150 and 350 °C from the HZN-X-50 specimens are presented in Fig. 7c and d. At 150 °C, these materials produced peaks at 1547 and 1450 cm−1, indicating the presence of both B and L acid sites. At the same temperature (Table 2), the B/L ratios of these samples were all less than 1 whereas the ratios of the HZN-5.5-50 and HZN-6-50 at 350 °C were greater than 1 and the relative area associated with AlF sites was larger than the value determined for HZN-5-50 (Fig. 5). These data suggest that the weakly acidic sites were predominantly L acids. The strong Brønsted acid sites may have been related to [AlO4]− tetrahedra. At 350 °C, the B/L ratio gradually increased with increase in the Na+ ion concentration, indicating that the formation of [AlO4]− tetrahedra was favored at higher Na+ concentrations. In addition, the B/L ratio of HZN-5.5-50 differed significantly from those of the other HZN-5.5-X at 350 °C (Table 2). This occurred because of the effects of the geometric structure of the hierarchical ZSM-5 nanosheets and the distortion and dislocation of the grain boundaries in the pore structure in the framework of this zeolite.67
The Al environments in an MFI structure include but are not limited to terminal Al–OH, classic Brønsted acid sites based on Si–(OH)–Al (inside the channels) and external less well defined Brønsted acid sites at which each Al also has a terminal hydroxyl group. The formation mechanism for tri-coordinated framework Al remains unclear. However, the present characterization data allow a theory to be presented. In this proposed process, crystal growth initially consumes the silicate species in the reaction solution. When silicate has been used up to a certain extent, continuous dissolution of the aluminosilicate polymer begins to take place. On this basis, the Al-rich rim of the zeolite readily forms, which is more likely to generate a tri-coordinated Al framework during calcination.68 The calcination of zeolite nanosheets typically results in partial breaking or hydrolysis of the framework Al–O bonds to produce tri-coordinated framework Al and framework silanol groups.65 Compared with the CZSM-5 zeolite, the HZN specimens showed more displacement and rotational boundaries that blocked bond formation in the MFI structure. It is apparent that defects were generated inside the zeolite crystals and more –OH groups were formed on the outer surfaces during calcination. These –OH may have produced a greater quantity of hydroxyl group (FTA-OH) and two hydroxyl groups (FTA-2OH) in conjunction with the tri-coordinated framework Al.69,70
4 Catalyst performance
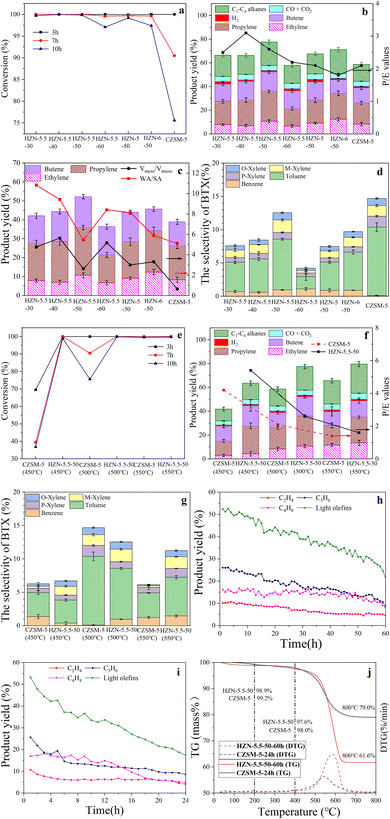
Fig. 8a shows the oleic acid conversion values obtained using the different catalysts. The highest conversion (>99%) was observed for all catalysts at 3 h. With increasing reaction time, HZN-catalyzed oleic acid conversion remained above 95%, while CZSM-5-catalyzed oleic acid conversion decreased from 100 to 75.6% at 3 and 10 h, respectively, because the interconnected hierarchical pores of the HZN promoted the diffusion of the coke precursor from the crystalline interior to the catalyst's surface.19 The surface coke deposition did not deactivate the catalyst unless the pore entrances were completely covered. However, in CZSM-5, coke was deposited mainly in micropores, easily deactivating the catalyst and decreasing the oleic acid conversion rate. Increasing the reaction time to 10 h slightly decreased the oleic acid conversion rates of the HZN-series catalysts, whereas the oleic acid conversion rate of CZSM-5 drastically decreased. Hence, the HZN catalysts possessed more stability. Li et al.15 applied mesoporous ZSM-5 to the catalytic cracking of oleic acid for the production of light olefins and reported >99% conversion at 500 °C with a lower value of 92% for HZSM-5. Fig. 8b summarizes the distribution of gaseous products for the various catalysts. The highest yield of light olefins was obtained with the HZN-5.5-50 (52.2%) and this value was approximately 13.3% higher than that generated using the CZSM-5 (38.9%). The HZN-5.5-50 had a self-pillared structure that retained the majority of the mesopores and these mesopores were connected via straight pore channels in an MFI structure. Thus, this material had an interconnected hierarchical pore structure that inhibited unwanted secondary reactions of hydrocarbons and light olefins by shortening the diffusion pathlengths. This zeolite had a total B/L ratio of 0.55 and a B/L ratio of 1.44 associated with strongly acidic sites. As such, the strongly acidic sites were mainly Brønsted acids that helped crack long-chain hydrocarbons to produce short-chain hydrocarbons.71 The Lewis acid sites in such catalysts play an important role in the catalytic transformation of hydrocarbons by promoting hydrogen transfer and thus can contribute to the deoxygenation of oleic acid.65 The primary products generated using the HZN-X-50 catalysts were propylene and butene, with propylene/ethylene mass-based ratios (P/E) in excess of 1. The P/E ratio was found to decrease with increase in the microporous specific surface area, with a minimum value of 1.8 in the case of HZN-6-50. The P/E ratio for CZSM-5 was lower than those obtained using the HZN-X-50 specimens. This difference was attributed to the dominance of micropores in CZSM-5 whereas the mesopores in HZN-X-50 inhibited the secondary decomposition of C3H6 and C4H8 so that less C2H4 was formed.1The effects of the pore structure and weak acid/strong acid (WA/SA) ratio on the yield of light olefins and the conversion of the oleic acid are summarized in Fig. 8c. Among the HZN specimens, HZN-5.5-50 provided the highest light olefin yield of 52.2%. This value was higher than those obtained from samples having the same Si/Al ratio by approximately 7%. This superior performance may be attributed to the low Vmeso/Vmicro ratio of HZN-5.5-50. In particular, the MFI structure of this material had ten-membered rings that provided sufficient active sites and promoted monomolecular reactions during the catalytic cracking process. The lower Vmeso/Vmicro ratio of this zeolite suggests a higher proportion of micropore volume available for the formation of light olefins. The light olefin yield obtained from CZSM-5 was only 38.9% and so was lower than those generated by the HZN-X-50 specimens. Zhao et al.4 applied mesoporous ZSM-5 catalysts to the cracking of oleic acid to produce light olefins and obtained the highest selectivity for light olefins of 38.1% at 550 °C. The selectivity observed using the conventional ZSM-5 was only 31.3%, presumably because the relatively small pore sizes in the latter led to rapid deactivation of the catalyst. Ishihara et al.6 used a hierarchical zeolite containing mesoporous silica–alumina to catalyze the cracking of soybean oil. The results showed that the gasoline fraction, the olefin/paraffin ratio obtained from the mesoporous zeolite (1.40), was higher than that obtained using conventional ZSM-5 (0.42). The introduction of mesopores evidently inhibited unwanted secondary reactions of hydrocarbons and light olefins. As shown in Fig. 8c, HZN-5.5-30 and -40 produced 42.1% and 44.4% yields of light olefins, which were almost identical to the yield of light olefins produced using CZSM-5. Moreover, HZN-5.5-60 produced a 36.4% yield of light olefins, which was lower than the yield of light olefins produced using CZSM-5, because these zeolites possessed lower HFs. In the hierarchical ZSM-5 nanosheets, the enhanced molecular transport was more efficient than that in the purely microporous counterpart because the former is mostly attributed to the shortened diffusion path through the micropores in the auxiliary mesopore network. However, the introduction of excess interconnected hierarchical pores was disadvantageous because it reduced the micropore volume and decreased the catalytic efficiency.72 The effect of the WA/SA ratio on the light olefin yield is summarized in Fig. 8c. For the series of HZN catalysts, the yields of light olefins increased with decrease in the WA/SA ratio, suggesting that weakly acidic sites favored the formation of light olefins. As shown in Scheme 5, the acidic sites provided by the catalyst interacted with the lone electron pairs of O atoms in the oleic acid which then underwent decarboxylation to form long-chain aldehydes and ketones. Subsequent decarbonylation of the ketone and aldehyde groups generated long-chain hydrocarbons and CO. These long-chain hydrocarbons were selectively cleaved in the MFI structure to form light olefins and short-chain alkanes. In this case, an excess of strongly acidic sites will lead to the over-catalyzed formation of aromatic hydrocarbons or carbon accumulation. Furthermore, the (010) crystalline surfaces of the ZSM-5 nanosheets were more exposed than those of conventional ZSM-5, thus promoting the adsorption of intermediates such as CH3O* and CHOO*.73 The presence of more AlF sites in the intersection cavities of HZN-5.5-50 (Table 3) can possibly be ascribed to the effect of varying Na+ concentrations on the Al distribution in the catalyst. These AlF sites likely enhanced the cycling of the conventional hydrocarbon pool species, resulting in more light olefins.53 Similarly, Di et al.74 showed that the relative molar ratios of an organic structure-directing agent (N,N,N-trimethyl-1-admantylammonium cations, TMAda+) and Na+ ions in the crystallization medium determined the cation/anion charge densities, which in turn affected the distribution of Al in SSZ-13 catalysts. Zhao et al.8 reported that BEA catalysts exhibited higher catalytic stability and propylene selectivity because a greater number of Al atoms at T9 sites promoted olefin cycling.
Fig. 8d shows the selectivity of BTX produced with the different catalysts. The selectivity of BTX 14.7% for CZSM-5 was higher than those from any of the HZN catalysts, possibly because of differences in lattice defects and the hierarchical pore structure. The weakly acidic sites in the HZN specimens resulted from poor adsorption of non-skeletal Al and NH3 on lattice defects or terminal S–OH and Al–OH, implying the presence of lattice defects in the samples. However, the cyclization and aromatization of olefins to produce aromatics was inhibited because the weak acid sites did not promote the hydrogen transfer reaction.17 The aromatization of light olefins was more likely to occur at the smaller intersection cavities. The HZN samples had fewer of these cavities because of the short b-axis lengths in these materials, meaning that more light olefins were discharged from the pores rather than undergoing aromatization.71 HZN-5.5-50 generated mostly light aromatics, possibly because this zeolite produced a higher yield of propylene (25.2%), which was more readily transformed into such compounds.75 Moreover, for HZN-5.5-50, B/L > 1 at 350 °C (Table 2), meaning that HZN-5.5-50 possessed more Brønsted acidic sites than the other HZN-series zeolite catalysts. It has been reported that protons from strong Brønsted acid sites undergo stronger interactions with propylene, leading to a higher rate of formation of secondary carbenium ions, which are easily activated.3 These protonated intermediates would be expected to produce more light aromatic hydrocarbons at the active sites associated with channel intersections. However, among all the HZN-series zeolites, HZN-5.5-50 possessed the highest HF (0.21), meaning that of all the catalysts, HZN-5.5-50 retained the most intersection cavities for producing more light aromatic hydrocarbons.
Fig. 8e summarizes the oleic acid conversion results for the HZN-5.5-50 and CZSM-5 samples at different temperatures. With increasing reaction time, HZN-catalyzed oleic acid conversion remained above 95%, while CZSM-5-catalyzed oleic acid conversion (39.4%) substantially decreased at 450 °C, and CZSM-5 was substantially deactivated at 7 h because the deposition of polycyclic aromatic or coke precursors in pores and at channel intersections externally blocked catalytically active sites.76 With increasing reaction temperature, the rate of the CZSM-5-catalyzed conversion from oleic acid to light olefins also increased; for example, at 550 °C, CZSM-5-catalyzed conversion from oleic acid to light olefins remained above 95% after 10 h because at high temperatures, the selectivity of the polycyclic aromatic conversion decreased, and fewer coke precursors accumulated in pores and channels, somewhat improving CZSM-5-catalyzed oleic acid conversion.76,77 Konno et al.78 employed nanoscale ZSM-5 catalysts to catalyze the cracking of naphtha at 500, 600 and 650 °C and showed that it was easier to generate short-chain products at high temperatures. It has also been established that oleic acid undergoes rapid reaction in the temperature range of 450–550 °C. Zhao et al.4 used hierarchical ZSM-5 catalysts at 500 °C and found conversions exceeding 90% in all cases. Li et al.15 used mesoporous ZSM-5 to promote the cracking of oleic acid to obtain light olefins and found that the conversion increased from approximately 77% to 100% upon raising the temperature from 380 to 550 °C for all samples.
The effect of temperature on the distribution of gaseous products over the range of 450–550 °C is presented in Fig. 8f. The maximum yield of light olefins was obtained at 500 °C using HZN-5.5-50 (52.2%) and 550 °C using CZSM-5 (39.8%). These results are attributed to the hierarchical pore structure of HZN-5.5-50, which promoted the olefin-based cycle. As such, the Haag–Dessau mechanism was dominant and side-reactions were avoided so that more light olefins were generated at lower temperatures.79,80 The light olefin yield from the HZN-5.5-50 sample decreased as the reaction temperature was increased from 500 to 550 °C. This change is ascribed to the enhanced importance of the hydrogen transfer reaction at elevated temperatures. This reaction reduced the yield of lower molecular weight olefins by converting some olefins to the corresponding paraffins.81 In the case of the CZSM-5 sample, the yield of light olefins increased with increase in temperature, indicating that a higher reaction temperature in conjunction with a microporous structure favored the decomposition of oleic acid to form short-chain hydrocarbons. During the catalytic process, both catalytic and thermal cracking occurred simultaneously although the latter was more sensitive to changes in temperature because it involved radical chain reactions. Thus, increasing the temperature increased the yield of thermally cracked products, resulting in an increase in the proportion of C1–C4 alkanes at higher temperatures.82 The P/E ratios for HZN-5.5-50 and CZSM-5 both decreased with increase in temperature because propylene formation involved a carbonium ion mechanism. As noted, thermal cracking was associated with a free radical mechanism such that higher temperatures facilitated the formation of ethylene.11,31 At a given temperature, the P/E ratio for HZN-5.5-50 was higher than that for CZSM-5, indicating that a carbonium ion mechanism was dominant in the case that HZN-5.5-50 was employed. It is possible that the hierarchical pore structure of this material inhibited the secondary cracking of light olefins, leading to the formation of more propylene and butene.20
Fig. 8g shows the selectivity of BTX obtained at different temperatures. Both HZN-5.5-50 and CZSM-5 provided the highest light aromatics yield at 500 °C, and the BTX selectivity of HZN-5.5-50 was higher than that of CZSM-5 at 450 °C and 550 °C. This was attributed to the very different physical properties of HZN-5.5-50 and CZSM-5. At low temperatures (450 °C), oleic acid cracked, forming more long-chain alkane olefins (C6 and C6+). Because HZN-5.5-50 possessed a higher HF than CZSM-5, the former also retained more interfacial cavities, which promoted the direct cyclization and aromatization of long-chain alkanes and olefins to BTX. At high temperatures (550 °C), the interconnected hierarchical pore structures of HZN-5.5-50 promoted further oligomerization, cyclization, and aromatization of light olefins, generating light aromatic hydrocarbons. The cleavage of oleic acid to form light olefins and aromatics can proceed via two pathways (Scheme 5). In pathway I, a proton provided by the catalyst interacts with the lone pair electrons of an O atom in an oleic acid molecule to induce decarboxylation and generate a long-chain aldehyde or ketene. Following this, a C–C bond situated at the β position relative to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is cleaved and the ketone or aldehyde group undergoes decarbonylation reactions to form a long-chain hydrocarbon and CO. The products of such reactions then undergo cleavage at acidic sites on the zeolite to form light olefins and short-chain alkanes, following which oligomerization, cyclization and aromatization reactions generate light aromatic hydrocarbons.83 In pathway II, the C
C bond is cleaved and the ketone or aldehyde group undergoes decarbonylation reactions to form a long-chain hydrocarbon and CO. The products of such reactions then undergo cleavage at acidic sites on the zeolite to form light olefins and short-chain alkanes, following which oligomerization, cyclization and aromatization reactions generate light aromatic hydrocarbons.83 In pathway II, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –COOH groups in the oleic acid molecules are activated by the acidic sites of the catalyst, leading to C–C bond breaking at positions β to the C
C and –COOH groups in the oleic acid molecules are activated by the acidic sites of the catalyst, leading to C–C bond breaking at positions β to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and α to the –COOH. These reactions form long-chain alkanes, C6 and C6+ olefins and CO2. The olefins subsequently generate light aromatics via dehydrocyclization and aromatization processes or produce light olefins based on the selectivity of the MFI structure.9,14
C bonds and α to the –COOH. These reactions form long-chain alkanes, C6 and C6+ olefins and CO2. The olefins subsequently generate light aromatics via dehydrocyclization and aromatization processes or produce light olefins based on the selectivity of the MFI structure.9,14
Fig. 8h summarizes the performance of HZN-5.5-50 at 500 °C over a 60 h reaction. The yield of light olefins continued to decrease as the reaction time increased, from 52.4% at 1 h to 21.7% at 60 h, although overall the decline was slow. The propylene yield decreased significantly and was the main reason for the decrease in the light olefin yield. This may have been a result of the deposition of coke in the mesopores of the zeolite. The reaction time had a lesser effect on the yield of ethylene, which decreased from 10.2% to 4.7%, possibly because this product was less likely to participate in oligomerization cracking and so was less affected by coke.84 The data for CZSM-5 at 500 °C over a time span of 24 h are provided in Fig. 8i. The yield of light olefins significantly decreased over this reaction time, from 53.3% to 17.4%. CZSM-5 contained primarily micropores and so there was resistance to the diffusion of macromolecules and it was difficult for coke precursors to be discharged from the specimen.
Fig. 8j shows the TGA and DTG curves generated for HZN-5.5-50 and CZSM-5 after continuous catalytic reactions for 60 and 24 h, respectively. The mass loss in these plots below 100 °C is attributed to the evaporation of physically adsorbed water from the catalyst while that in the range of 100–200 °C is related to the loss of water in the zeolite framework. The mass loss in the range of 200–400 °C is ascribed to the removal of hydrocarbons and aromatics present in the catalyst pores, often referred to as so-called soft coke. The mass loss throughout the range of 400–800 °C is attributed to the loss of hard coke compounds having high C/H ratios, which can lead to deactivation.82,85 HZN-5.5-50 showed a higher mass loss associated with coke (38.4%) compared with the CZSM-5 (21%). The mass losses of the HZN-5.5-50 and CZSM-5 were only 2.4% and 2.0%, respectively, below 400 °C, indicating that more of the mass loss originated from hard coke. The DTG results exhibit sharp peaks at 580 and 540 °C for HZN-5.5-50 and CZSM-5, respectively, establishing that the coke in the former was more condensed. However, after 60 h, the light olefin yield of the HZN-5.5-50 sample was still higher than that of CZSM-5 (Fig. 8h and i). Moreover, the HZN-5.5-50 reaction time (60 h) is more than twice as long as that of CZSM-5 (24 h). However, the HZN-5.5-50 coke deposition was only 1.84 times higher than that of CZSM-5. The average hourly coke production of HZN-5.5-50 (0.62 mass%/h) was lower than that of CZSM-5 (0.84 mass%/h), indicating that the nanosheet layer was more prone to discharging products and reducing coke accumulation than the CZSM-5 structure. These data confirm that the HZN-5.5-50 sample was more resistant to the effects of carbon deposits. Compared with CZSM-5, HZN-5.5-50 contained more coke because the mesopores in this material facilitated the diffusion of coke precursors to the exterior, leading to the formation of more coke on the external surfaces. However, this coke had a lower effect on the active sites of the zeolite.19 Therefore, even though HZN-5.5-50 had more coke, this material exhibited a longer lifetime and higher catalytic activity. CZSM-5 possessed strongly acidic sites and a large number of micropores and so led to rapid cracking and hydrogen transfer reactions on the catalyst surface, resulting in the formation of hard coke and a reduction in catalytic performance.
5 Conclusion
A template incorporating a biphenyl moiety as the hydrophobic portion and dual ammonium groups as the hydrophilic parts was used to guide the synthesis of hierarchical ZSM-5 nanosheets. This zeolite exhibited a large BET surface area and improved accessibility to acidic sites compared with CZSM-5. The distribution of Al in the zeolite skeleton was modified by changing the concentration of Na+ in the initial gel. The concentration of Al in intersection cavities could be tuned to produce more effective active sites for the production of light olefins and aromatics. When HZN-5.5-50 was used to catalyze the cracking of oleic acid a higher yield of light olefins and greater lifetime were observed relative to the values for CZSM-5. The interconnected hierarchical pore structure of HZN-5.5-50 was well suited to the generation of light aromatics at relatively low temperatures (450 °C) as a consequence of the synergistic action of Brønsted and Lewis acid sites.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Haoyu Liu, Wenbo Luo, Ke Wang and Yanlin Wang. The first draft of the manuscript was written by Haoyu Liu, and Hong Yuan commented on previous versions of the manuscript and supervised the entire work. All authors read and approved the final manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was supported by the Ningxia National Natural Science Foundation of China (2024AAC03185), the National Natural Science Foundation of China (21962001), the Ningxia Key Research and Development Project of China (2023BDE03009), the North Minzu University Graduate Innovation Project of China (YCX24131), the Innovative Team for Transforming Waste Cooking Oil into Clean Energy and High Value-Added Chemicals of China, and the Ningxia Low-Grade Resource High Value Utilization and Environmental Chemical Integration Technology Innovation Team Project of China.References
- S. Zhang, M. Yang, J. Shao, H. Yang, K. Zeng, Y. Chen, J. Luo, F. A. Agblevor and H. Chen, Sci. Total Environ., 2018, 628, 350–357 CrossRef.
- N. Rahimi and R. Karimzadeh, Appl. Catal., A, 2011, 398, 1–17 CrossRef CAS.
- E. A. Uslamin, H. Saito, N. Kosinov, E. Pidko, Y. Sekine and E. J. M. Hensen, Catal. Sci. Technol., 2020, 10, 2774–2785 RSC.
- T. Zhao, F. Li, H. Yu, S. Ding, Z. Li, X. Huang, X. Li, X. Wei, Z. Wang and H. Lin, Appl. Catal., A, 2019, 575, 101–110 CrossRef CAS.
- Y. Zheng, F. Wang, X. Yang, Y. Huang, C. Liu, Z. Zheng and J. Gu, J. Anal. Appl. Pyrolysis, 2017, 126, 169–179 CrossRef CAS.
- A. Ishihara, D. Kawaraya, T. Sonthisawate, K. Kimura, T. Hashimoto and H. Nasu, J. Mol. Catal. A: Chem., 2015, 396, 310–318 CrossRef CAS.
- N. M. Basir, N. A. M. Jamil and H. Hamdan, Nanomater. Nanotechnol., 2021, 11, 184798042098153 CrossRef.
- X. Zhao, L. Wang, J. Li, S. Xu, W. Zhang, Y. Wei, X. Guo, P. Tian and Z. Liu, Catal. Sci. Technol., 2017, 7, 5882–5892 RSC.
- A. Ishihara, D. Kawaraya, T. Sonthisawate, H. Nasu and T. Hashimoto, Fuel Process. Technol., 2017, 161, 8–16 CrossRef CAS.
- M. A. Alabdullah, T. Shoinkhorova, A. Dikhtiarenko, S. Ould-Chikh, A. Rodriguez-Gomez, S. Chung and J. Gascon, Catal. Sci. Technol., 2022, 12(18), 5657–5670 RSC.
- X. Dong, Z. Chen, S. Xue, J. Zhang, J. Zhou, Y. Liu, Y. Xu and Z. Liu, RSC Adv., 2013, 3, 25780 RSC.
- Z. Y. Zakaria, J. Linnekoski and N. A. S. Amin, Chem. Eng. J., 2012, 207, 803–813 CrossRef.
- L. Sun, Z. Wang, L. Chen, S. Yang, X. Xie, B. Zhao, H. Si, J. Li and D. Hua, Int. J. Energy Res., 2021, 45, 6032–6040 CrossRef CAS.
- F. Li, S. Ding, Z. Wang, Z. Li, L. Li, C. Gao, Z. Zhong, H. Lin and C. Chen, Energy Fuels, 2018, 32, 5910–5922 CrossRef CAS.
- Z. Li, F. Li, T. Zhao, H. Yu, S. Ding, W. He, C. Song, Y. Zhang and H. Lin, Catal. Sci. Technol., 2020, 10, 6618–6627 RSC.
- H. Wu, A. Duan, Z. Zhao, T. Li, R. Prins and X. Zhou, J. Catal., 2014, 317, 303–317 CrossRef CAS.
- X. Liu, Y. Wu, J. Zhang, Y. Zhang, X. Li, H. Xia and F. Wang, ACS Omega, 2022, 7, 18953–18968 CrossRef CAS.
- Y. Zhang, X. Shen, Z. Gong, L. Han, H. Sun and S. Che, Chem.–Eur. J., 2019, 25, 738–742 CrossRef CAS.
- T. Fu, R. Qi, W. Wan, J. Shao, J. Wen and Z. Li, ChemCatChem, 2017, 9, 4212–4224 CrossRef CAS.
- Y. Lei, W. Yang, J. Fan, X. Pu, X. Han, X. Qin, M. Ma, Z. Huang, T. Wang, K. Zhu, Y. Xu and J. Liu, Chem. Eng. J., 2024, 482, 148705 CrossRef.
- L. Emdadi, Y. Wu, G. Zhu, C. Chang, W. Fan, T. Pham, R. F. Lobo and D. Liu, Chem. Mater., 2014, 26, 1345–1355 CrossRef CAS.
- J. Lu, M. B. J. Roeffaers, E. Bartholomeeusen, B. F. Sels and D. Schryvers, Microsc. Microanal., 2014, 20, 42–49 CrossRef CAS PubMed.
- W. Park, D. Yu, K. Na, K. E. Jelfs, B. Slater, Y. Sakamoto and R. Ryoo, Chem. Mater., 2011, 23, 5131–5137 CrossRef CAS.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef CAS.
- W. Chaikittisilp, Y. Suzuki, R. R. Mukti, T. Suzuki, K. Sugita, K. Itabashi, A. Shimojima and T. Okubo, Angew. Chem., Int. Ed., 2013, 18, 3355–3359 CrossRef PubMed.
- X. Zhang, D. Liu, D. Xu, S. Asahina, K. A. Cychosz, K. V. Agrawal, Y. A. Wahedi and M. Tsapatsis, Science, 2012, 336, 1684–1687 CrossRef CAS.
- R. Jain, A. Chawla, N. Linares, J. García Martínez and J. D. Rimer, Adv. Mater., 2021, 27, 2100897 CrossRef PubMed.
- D. Xu, G. R. Swindlehurst, H. Wu, D. H. Olson, X. Zhang and M. Tsapatsis, Adv. Funct. Mater., 2014, 102, 201–208 CrossRef.
- Y. Wang, J. Ma, F. Ren, J. Du and R. Li, Microporous Mesoporous Mater., 2017, 240, 22–30 CrossRef CAS.
- D. Xu, Z. Jing, F. Cao, H. Sun and S. Che, Chem. Mater., 2014, 26, 4612–4619 CrossRef CAS.
- W. He, F. Li, Y. Gu, X. Wang, H. Gu, H. Fu and Z. Li, ACS Omega, 2022, 7, 40520–40531 CrossRef CAS PubMed.
- D. Xu, Y. Ma, Z. Jing, L. Han, B. Singh, J. Feng, X. Shen and S. Che, Nat. Commun., 2014, 5, 4262 CrossRef CAS.
- N. P. Radhika, R. Selvin, R. Kakkar and L. S. Roselin, J. Nanosci. Nanotechnol., 2018, 18, 5404–5413 CrossRef CAS.
- K. Kim, L. C. Reimnitz, S. H. Cho, J. Noh, Z. Dong, S. L. Gibbs and D. J. Milliron, Chem. Mater., 2020, 32, 9347–9354 CrossRef CAS.
- M. Alonso-Doncel, A. Peral, M. Shamzhy, J. Čejka, R. Sanz and D. P. Serrano, Catal. Today, 2020, 345, 27–38 CrossRef CAS.
- N. A. Ramsahye and B. Slater, Chem. Commun., 2005, 4, 442–444 Search PubMed.
- L. Karwacki, E. Stavitski, M. H. F. Kox, J. Kornatowski and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2007, 118, 7228–7231 CrossRef.
- X. Shen, W. Mao, Y. Ma, H. Peng, D. Xu, P. Wu and S. Che, Chem.–Eur. J., 2018, 18, 8615–8623 CrossRef PubMed.
- J. Lu, E. Bartholomeeusen, B. F. Sels and D. Schryvers, J. Microsc., 2017, 265, 27–33 CrossRef CAS.
- Z. Zhou, X. Wang, R. Yu, R. Jiang, Y. Gao, X. Chen and H. Hou, Adv. Powder Technol., 2023, 34, 103930 CrossRef CAS.
- Z. Chen, Y. Ma and J. Yao, Thin Solid Films, 2001, 384, 160–165 CrossRef CAS.
- C. García-Sancho, J. A. Cecilia, A. Moreno-Ruiz, J. M. Mérida-Robles, J. Santamaría-González, R. Moreno-Tost and P. Maireles-Torres, Appl. Catal., B, 2015, 179, 139–149 CrossRef.
- H. Vu, M. Schneider, U. Bentrup, T. Dang, B. M. Q. Phan, D. A. Nguyen, U. Armbruster and A. Martin, Ind. Eng. Chem. Res., 2015, 54, 1773–1782 CrossRef CAS.
- S. Kumar, Z. Wang, R. L. Penn and M. Tsapatsis, J. Am. Chem. Soc., 2008, 130, 17284–17286 CrossRef CAS.
- J. Pérez-Ramírez, D. Verboekend, A. Bonilla and S. Abelló, Adv. Funct. Mater., 2009, 18, 3972–3979 CrossRef.
- Y. Quan, S. Li, S. Wang, Z. Li, M. Dong, Z. Qin, G. Chen, Z. Wei, W. Fan and J. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 14899–14910 CrossRef CAS PubMed.
- K. Wang, G. Karlsson and M. Almgren, J. Phys. Chem. B, 1999, 103, 9237–9246 CrossRef CAS.
- K. Aoki and S. Mann, J. Mater. Chem., 2005, 15, 111 RSC.
- S. Lawson, K. Baamran, K. Newport, E. Garcia, G. Jacobs, F. Rezaei and A. A. Rownaghi, ACS Catal., 2022, 12, 14264–14279 CrossRef CAS.
- Z. Gabelica, J. B. Nagy, P. Bodart, N. Dewaele and A. Nastro, Zeolites, 1987, 7, 67–72 CrossRef CAS.
- D. P. Serrano, R. A. García, G. Vicente, M. Linares, D. Procházková and J. Čejka, J. Catal., 2011, 279, 366–380 CrossRef CAS.
- X. H. Vu and U. Armbruster, Adv. Mater. Sci. Eng., 2019, 6, 1–7 Search PubMed.
- V. Pashkova, S. Sklenak, P. Klein, M. Urbanova and J. Dědeček, Chem.–Eur. J., 2016, 69, 3937–3941 CrossRef PubMed.
- J. Dědeček, S. Sklenak, C. Li, B. Wichterlová, V. Gábová, J. Brus, M. Sierka and J. Sauer, J. Phys. Chem. C, 2009, 113, 1447–1458 CrossRef.
- T. Jiang, F. Wang, X. Zhang, Y. Zhai, G. Lv, Y. Shen and Y. Wu, Microporous Mesoporous Mater., 2021, 321, 111112 CrossRef CAS.
- G. Song, D. Xue, J. Xue and F. Li, Microporous Mesoporous Mater., 2017, 248, 192–203 CrossRef CAS.
- H. Hernando, A. M. Hernández-Giménez, C. Ochoa-Hernández, P. C. A. Bruijnincx, K. Houben, M. Baldus, P. Pizarro and D. P. Serrano, Green Chem., 2018, 20, 3499–3511 RSC.
- B.-T. Lønstad Bleken, L. Mino, F. Giordanino, P. Beato, S. Svelle, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2013, 32, 13363 RSC.
- M. Mar Alonso-Doncel, C. Ochoa-Hernández, G. Gómez-Pozuelo, A. Oliveira, J. González-Aguilar, Á. Peral, R. Sanz and D. P. Serrano, J. Energy Chem., 2023, 80, 77–88 CrossRef.
- L. Li, Y. Chen, S. Xu, J. Li, M. Dong, Z. Liu, H. Jiao, J. Wang and W. Fan, J. Catal., 2016, 344, 242–251 CrossRef CAS.
- S. Kim, G. Park, M. H. Woo, G. Kwak and S. K. Kim, ACS Catal., 2019, 9, 2880–2892 CrossRef CAS.
- J. Hao, D. Cheng, F. Chen and X. Zhan, Microporous Mesoporous Mater., 2021, 310, 110647 CrossRef CAS.
- Y. Jin, L. Zong, X. Wang and H. Wei, ACS Omega, 2022, 7, 26289–26297 CrossRef CAS PubMed.
- K. Chen, X. Wu, J. Zhao, H. Zhao, A. Li, Q. Zhang, T. Xia and B. Shen, J. Catal., 2022, 413, 735–750 CrossRef CAS.
- S. Xin, Q. Wang, J. Xu, Y. Chu, P. Wang, N. Feng, G. Qi and F. Deng, Chem. Sci., 2019, 43, 10159–10169 RSC.
- M. Abdolrahmani, K. Chen and J. L. White, J. Phys. Chem. C, 2018, 122, 15520–15528 CrossRef CAS.
- J. Li, S. Liu, H. Zhang, E. Lü, P. Ren and J. Ren, Chin. J. Catal., 2016, 37, 30–315 CAS.
- N. Danilina, F. Krumeich, S. A. Castelanelli and J. A. Bokhoven, J. Phys. Chem. C, 2010, 114, 6640–6645 CrossRef CAS.
- Z. Xue, J. Ma, W. Hao, X. Bai, Y. Kang, J. Liu and R. Li, J. Mater. Chem., 2011, 22, 2532–2538 RSC.
- M. Hu, C. Wang, Y. Chu, Q. Wang, S. Li, J. Xu and F. Deng, Angew. Chem., Int. Ed., 2022, 61, 202207400 CrossRef.
- L. Zhao, P. Xiao, Y. Wang, Y. Lu, T. M. Karim, H. Gies and T. Yokoi, ACS Appl. Mater. Interfaces, 2024, 16, 17701–17714 CrossRef CAS.
- C. Jiao, Q. Jiao, W. Zhang, Z. Ji, J. Zheng, W. Dai, Y. Wang, M. Pan and R. Li, J. Mater. Sci., 2024, 59, 16486–16500 CrossRef CAS.
- H. Tian, H. He, P. Gao, X. Guo, X. Tang, Y. Chang, F. Zha and H. Chen, Appl. Surf. Sci., 2023, 608, 155158 CrossRef CAS.
- J. R. Di Iorio and R. Gounder, Chem. Mater., 2016, 28, 2236–2247 CrossRef CAS.
- P. S. Butolia, X. Xi, J. G. M. Winkelman, M. C. A. Stuart, M. Akker, A. Heeres, H. J. Heeres and J. Xie, Chem.-Ing.-Tech., 2022, 94, 1845–1852 CrossRef CAS.
- J. T. C. Wennmacher, S. Mahmoudi, P. Rzepka, S. Sik Lee, T. Gruene, V. Paunović and J. A. van Bokhoven, Angew. Chem., 2022, 134, e202205413 CrossRef.
- C. Liu, J. Su, S. Liu, H. Zhou, X. Yuan, Y. Ye and Z. Xie, ACS Catal., 2020, 10, 15227–15237 CrossRef CAS.
- H. Konno, T. Tago, Y. Nakasaka, R. Ohnaka, J. Nishimura and T. Masuda, Microporous Mesoporous Mater., 2013, 175, 25–33 CrossRef CAS.
- C. Chizallet, C. Bouchy, K. Larmier and G. Pirngruber, Chem. Rev., 2023, 123, 6107–6196 CrossRef CAS PubMed.
- K. Ma, S. Zhao, M. Dou, X. Ma and C. Dai, ACS Catal., 2024, 14, 594–607 CrossRef CAS.
- H. Liu, Q. Deng, Q. Pan, S. Gu, T. Qiao and H. Gao, J. Mol. Catal. A: Chem., 2004, 215, 195–199 CrossRef.
- L. Dai, N. Zhou, Y. Lv, K. Cobb, P. Chen, Y. Wang, Y. Liu and Y. Cheng, Sci. Total Environ., 2022, 847, 157658 CrossRef CAS.
- K. Wang, J. Zhang, S. Fan, X. Peng, N. Tsubaki and T. Zhao, New J. Chem., 2021, 45, 4860–4866 RSC.
- E. Epelde, M. Ibañez, A. T. Aguayo, A. G. Gayubo, J. Bilbao and P. Castaño, Microporous Mesoporous Mater., 2014, 195, 284–293 CrossRef CAS.
- Z. Zhang, H. Chen, H. Pan, D. Duan, Y. Zhang and D. Long, Appl. Catal., B, 2023, 324, 122226 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available:Preparation of conventional ZSM-5; low-angle XRD pattern of CZSM-5; the SEM images of CZSM-5 samples; the TEM images of uncalcined HZN-5.5-50 series samples; adsorption–desorption curves and pore size distributions of CZSM-5; the lattice parameters of HZN-X-X. See DOI: https://doi.org/10.1039/d4se01167h |
| This journal is © The Royal Society of Chemistry 2025 |