Cobalt-doped vanadium nitride composite carbon hollow spheres for enhanced lithium–sulfur battery performance: overcoming sulfur dissolution and the shuttle effect†
Jiangnan
Zhang
a,
Yanshuang
Meng
*ab and
Fuliang
Zhu
 *ab
*ab
aSchool of Materials Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, China. E-mail: chzfl@126.com; mengyanshuang@163.com
bState Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou 730050, China
First published on 21st November 2024
Abstract
This study addresses the challenges of sulfur dissolution and the shuttle effect in the practical application of lithium–sulfur (Li–S) batteries by developing cobalt-doped vanadium nitride composite carbon hollow spheres (CoVN/C-HS). The embedding of CoVN nanoparticles within the carbon hollow spheres creates an efficient charge transport network that significantly reduces electrode interfacial resistance, accelerates charge transfer during charging and discharging, and effectively mitigates polarization, thereby ensuring battery stability under high-rate conditions. Additionally, the strong interaction between CoVN nanoparticles and the carbon hollow sphere matrix enhances the material's adsorption capacity for polysulfides, effectively suppressing their dissolution and shuttle effect, which prolongs battery cycle life. Therefore, the prepared CoVN/C-HS material has demonstrated excellent performance in Li–S battery applications. At a low current density of 0.05C, the battery achieved an initial discharge capacity of up to 1475 mA h g−1, fully demonstrating the efficient utilization of sulfur by the material. Remarkably, even after 100 cycles at 0.2C, the battery retains a capacity of 1067 mA h g−1, showcasing excellent cycle stability. Notably, at a high current density of 2C, the battery achieves an initial capacity of 918.8 mA h g−1 and maintains 662 mA h g−1 after 400 cycles. This success not only presents a novel approach for optimizing Li–S battery performance by meticulously tuning the material structure and composition to concurrently address sulfur dissolution and the shuttle effect but also lays a solid foundation for the large-scale commercialization of this battery type.
1 Introduction
Lithium–sulfur (Li–S) batteries, with their remarkable theoretical specific capacity of 1675 mA h g−1 and the cost-effectiveness of sulfur cathodes, exhibit considerable potential in the pursuit of future high-performance energy storage systems. However, the path to practical application is fraught with challenges, primarily encompassing the dissolution of active sulfur, the shuttle effect, the intrinsic low conductivity of sulfur materials, and pronounced volume changes during charging and discharging cycles, all of which severely limit the further enhancement of Li–S batteries' cycle stability and electrochemical performance.1–5 In light of these issues, significant research efforts have been dedicated to developing novel sulfur host materials, aiming to improve sulfur utilization and optimize the overall battery performance.6Among various candidate materials, carbon-based materials, particularly carbon nanotubes, graphene, and porous carbons, have emerged as popular choices for enhancing the performance of Li–S batteries due to their exceptional conductivity, abundant porous structures, lightweight properties, and superior chemical stability.7–10 The high conductivity of carbon materials effectively elevates the electrical conductivity of sulfur cathodes, mitigates polarization, and thereby enhances the overall battery efficiency. Furthermore, their porous structures, endowed with large specific surface areas and excellent adsorption capabilities, efficiently alleviate the dissolution and loss of sulfur and polysulfides, significantly prolonging the cycle life of batteries. Nonetheless, carbon materials still confront challenges in fully suppressing the polysulfide shuttle effect and reducing the production costs of high-performance materials, necessitating urgent breakthroughs in their structural design and manufacturing processes to propel the large-scale commercialization of Li–S batteries.
In recent years, nitride-composite carbon materials have emerged as promising sulfur cathode materials for Li–S batteries, exhibiting remarkable advantages.11–14 These materials not only inherit the superior conductivity of carbon but also ingeniously harness the abundant surface active sites of nitrides to effectively anchor polysulfide intermediates, thereby significantly inhibiting polysulfide dissolution and migration, vastly enhancing battery cycle stability and rate capability.15 Among them, vanadium nitride (VN) stands out with its conductivity as high as 1.17 × 106 S m−1 at room temperature, making it a leading contender in the field of Li–S batteries.16 VN not only displays catalytic properties akin to precious metals but also possesses robust polysulfide adsorption capabilities and facilitates rapid polysulfide redox reactions.17 Notably, the team of Li18 successfully synthesized a conductive porous VN nanobelt/graphene composite that, leveraging its exceptional anchoring capacity and swift polysulfide conversion kinetics, achieved an initial capacity of 1471 mA h g−1 in Li–S batteries. After 100 cycles at 0.2C, the capacity remained at 1252 mA h g−1, exhibiting a mere 15% decay, underscoring its outstanding performance. In order to further improve the efficiency of VN materials in lithium–sulfur batteries, heteroatom doping is introduced to enhance the catalytic activity.19 Lu Yang20et al. prepared a Co-doped VN composite N-doped carbon material as a sulfur cathode electrode material. This material exhibited excellent performance in lithium–sulfur batteries. At a current density of 0.1C, it achieved 1521 mA h g−1, proving that Co-doped VN materials have high research value in the application of lithium–sulfur batteries. Building upon these foundations, this study innovatively designed a cobalt-doped VN composite carbon hollow sphere material (CoVN/C-HS). This material harnesses the hollow structure of carbon hollow spheres (C-HS) as a container for sulfur cathodes, effectively confining the diffusion of lithium polysulfides through physical confinement. Additionally, the co-doping of cobalt enriches the active sites of VN materials by enhancing interatomic synergy, boosting catalytic activity, accelerating the conversion process of lithium polysulfides, elevating sulfur cathode utilization, and markedly promoting battery reaction kinetics. Experimental results demonstrate that this sulfur-hosting material exhibits a high specific capacity of 1475 mA h g−1 at a current density of 0.05C, maintaining 1067 mA h g−1 after 100 cycles at 0.2C. Even at a high current density of 2C, it achieves an initial capacity of 918.8 mA h g−1 and retains 662 mA h g−1 after 400 cycles, robustly validating its exceptional specific capacity retention and cycle stability.
2 Results and discussion
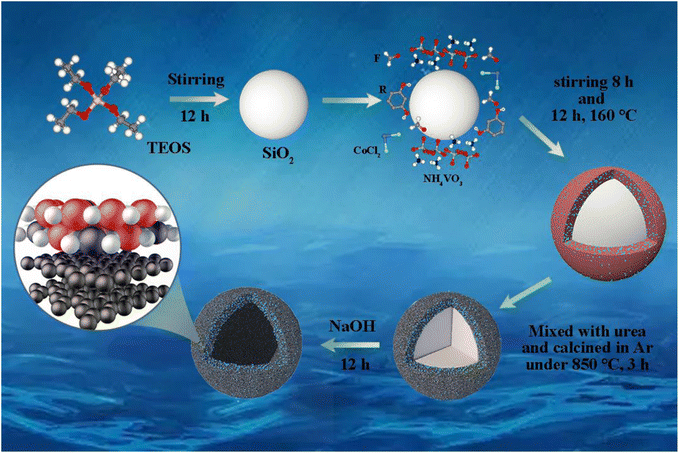
Fig. 1 meticulously outlines the fabrication process of CoVN/C-HS. In this process, SiO2 nanoparticles serve as the rigid template for C-HS (carbon hollow spheres), playing a pivotal role. The carbon source is derived from phenolic resin, synthesized through the precise polycondensation of resorcinol and formaldehyde under alkaline conditions. Critically, during the polycondensation step, the timely addition of NH4VO3 and CoCl2 solutions facilitates the formation of precursors that intimately bind with the phenolic resin. Subsequently, the phenolic resin is blended with urea and subjected to high-temperature carbonization, during which urea pyrolyzes, generating a nitrogen-rich atmosphere that serves as the nitrogen source for vanadium nitride synthesis.21,22 Ultimately, the SiO2 template is effectively removed using NaOH solution, a process that significantly enhances the bonding strength between cobalt-doped vanadium nitride and carbon hollow spheres, further optimizing the dispersion of cobalt-doped vanadium nitride within the carbon matrix. These combined features endow CoVN/C-HS with exceptional performance as a sulfur host material in Li–S batteries, laying a solid foundation for achieving higher specific capacities.Fig. 2 shows the microstructural characteristics and elemental distributions of C-HS and CoVN/C-HS. Fig. 2(a) and (b) exhibit the morphologies of C-HS and CoVN/C-HS, respectively, through scanning electron microscopy (SEM), clearly revealing spherical particles with uniform sizes and well-distributed patterns. Fig. 2(c) utilizes transmission electron microscopy (TEM) to further elucidate the core–shell structure of CoVN/C-HS, with specific annotations for the shell thickness (δ ≈ 12 nm) and particle diameter (d ≈ 330 nm), providing a visual basis for understanding its structural properties. Fig. 2(d) specifically focuses on the composite structure of sulfur-loaded CoVN/C-HS (CoVN/C-HS@S), highlighting the intricacies of the material's internal architecture. Additionally, Fig. 2(e)–(i) employ energy-dispersive X-ray spectroscopy (EDS) elemental mapping to visually display the distributions of vanadium (V), cobalt (Co), carbon (C), sulfur (S), and nitrogen (N) within the CoVN/C-HS@S composite. The uniform distribution of these elements underscores the successful embedding of cobalt-doped vanadium nitride into carbon hollow spheres and the efficient loading of sulfur, yielding an ideal homogeneous composite system. These comprehensive microstructural analyses offer crucial insights and data to support the investigation of the electrochemical properties of the material.
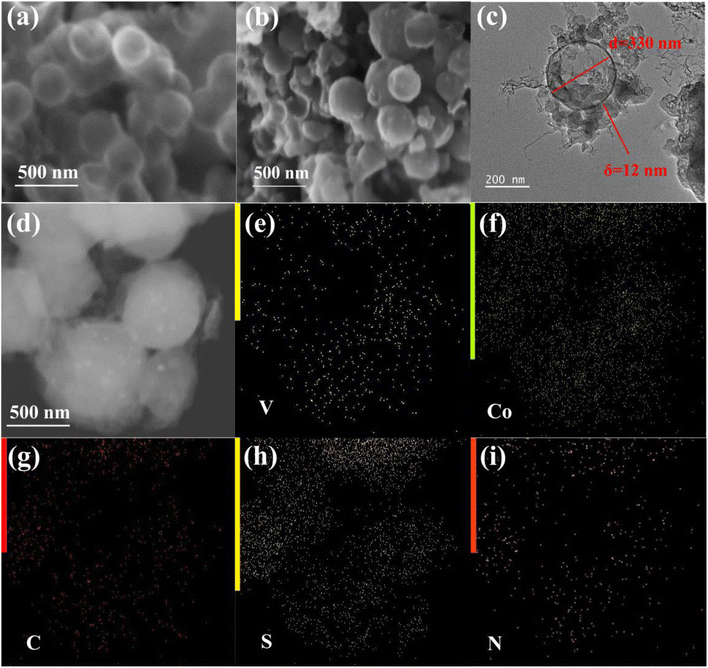 | ||
| Fig. 2 (a) SEM images of C-HS and (b) CoVN/C-HS, (c) TEM images of CoVN/C-HS, (d) TEM images of CoVN/C-HS@S, and (e–i) mapping images of CoVN/C-HS@S showing V, Co, C, S, N. | ||
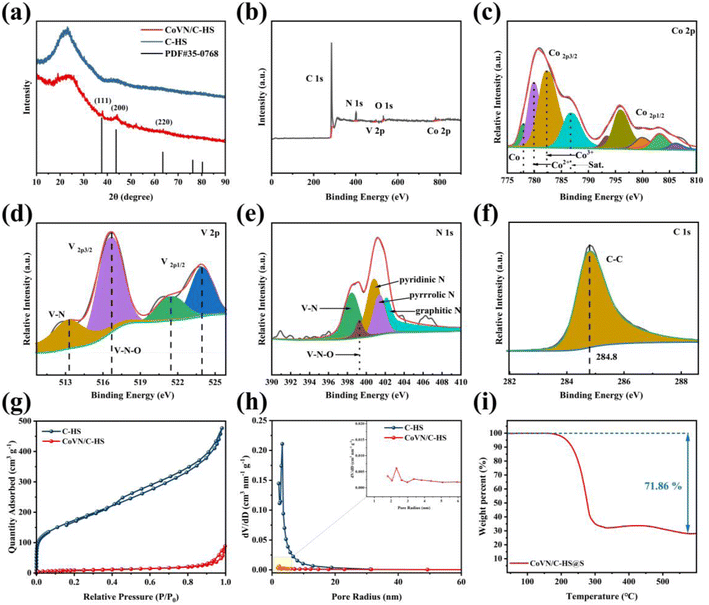
Fig. 3 comprehensively showcases the detailed characterization outcomes of C-HS and CoVN/C-HS utilizing diverse analytical techniques. Specifically, the X-ray diffraction (XRD) patterns in Fig. 3(a) distinctly differentiate the crystallographic phases between C-HS and CoVN/C-HS. Notably, the CoVN/C-HS sample exhibits characteristic peaks at the (111), (200), and (220) planes, which align with the PDF#35-0768 standard, thereby validating the accuracy of its crystalline structure.20,23
Fig. 3(b) employs X-ray photoelectron spectroscopy (XPS) measurements to elucidate the presence of C, N, O, V, and Co elements in CoVN/C-HS. The trace amount of oxygen is likely attributed to minor oxygen contamination during sample preparation and detection. Subsequently, Fig. 3(c)–(f) present the high-resolution XPS spectra of Co 2p, V 2p, N 1s, and C 1s, respectively, providing an in-depth analysis of the chemical states of each element. Fig. 3(c) confirms the existence of multiple valence states of Co, including Co0, Co2+, and Co3+. Fig. 3(d) displays the V 2p spectrum with characteristic peaks of V–N and V–N–O bonds, revealing the nitrided state of vanadium. Fig. 3(e) reveals the presence of various nitrogen species, such as V–N, V–N–O, pyridine, pyrrole, and graphitic nitrogen in the N 1s spectrum, which is attributed to the doping effect of nitrogen during the nitridation of the precursor on the C-HS material.23 Lastly, the dominant peak at 284.8 eV in the C 1s spectrum of Fig. 3(f) corresponds to C–C bonds, further confirming the existence of the carbon matrix. Further analysis of XPS data shows that the atomic ratio of cobalt doping is 37.3%. Collectively, these results verify the successful synthesis of the co-doped vanadium nitride material and its diverse elemental composition.
Fig. 3(g) compares the specific surface areas and porosity characteristics of C-HS and CoVN/C-HS through nitrogen adsorption–desorption isotherms. Notably, the specific surface area of CoVN/C-HS (50.87 m2 g−1) is significantly lower than that of C-HS (624.9 m2 g−1), likely due to the partial occupation of pore spaces within the carbon layers by CoVN during the carbonization process. This is further corroborated by the pore size distribution in Fig. 3(h), which shows that although the specific surface area and pore volume of CoVN/C-HS are reduced, a certain degree of microporosity is retained. Additionally, studies have indicated that the introduction of Co ions during phenol-formaldehyde resin synthesis enhances carbon yield, resulting in a more compact structure upon carbonization and consequently lowering the specific surface area and porosity.24–27 Finally, Fig. 3(i) utilizes thermogravimetric analysis (TGA) to elucidate the thermal stability and sulfur content of the CoVN/C-HS@S composite. The sample experiences significant mass loss at 200 °C, followed by stabilization at 300 °C and cessation of weight loss up to 550 °C. Because CoVN/C-HS was synthesized at a higher calcination temperature, this behavior is attributed to the high volatility of sulfur within the carbon hollow sphere structure, with volatilization partially inhibited by the porous structure. The final sulfur content in the sample is determined to be 71.86%, collectively affirming the success of the synthesis process.
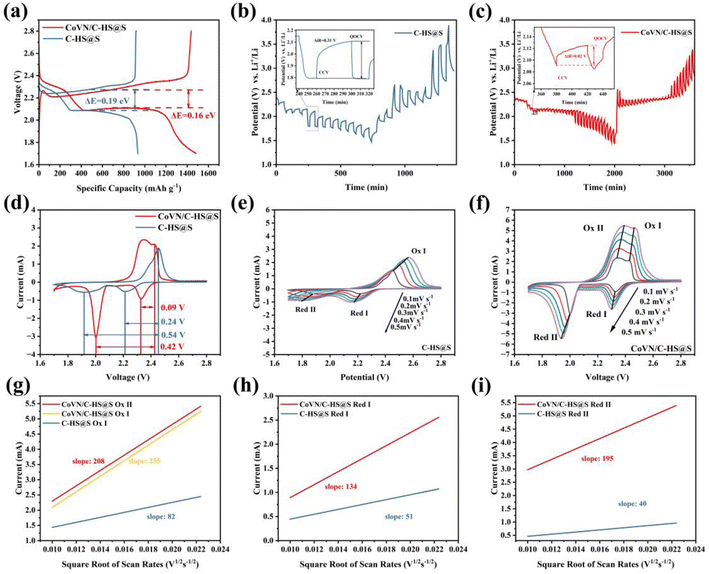
Fig. 4 presents a comprehensive and in-depth analysis of the electrochemical properties of C-HS@S and CoVN/C-HS@S, particularly highlighting the significant advantages of CoVN/C-HS@S in terms of capacity enhancement and potential plateau stability. From the comparison of galvanostatic discharge–charge curves in Fig. 4(a), it is evident that CoVN/C-HS@S exhibits a discharge capacity of 1475 mA h g−1, representing a remarkable increase compared to C-HS@S (910 mA h g−1). This substantial growth can be attributed to the favorable effects of the CoVN nanostructure on the sulfur cathode, which likely enhances electrochemical reaction efficiency and depth by providing additional active sites, and enhancing the adsorption and catalytic conversion of polysulfides. And from Fig. S1,† the possible contribution of CoVN ontology to capacity is excluded. Furthermore, the voltage difference between the charge and discharge platforms of CoVN/C-HS@S is merely 0.16 V, significantly lower than the 0.19 V observed for C-HS@S, indicating that this composite material maintains a more stable voltage platform during charge–discharge cycles. This characteristic is crucial for enhancing battery energy efficiency and cycle stability. The underlying mechanisms may involve the optimization of charge transport pathways by CoVN nanoparticles, reducing interfacial resistance, accelerating charge transfer processes, and effectively suppressing polarization phenomena during charging and discharging. Additionally, CoVN may facilitate the rapid conversion of polysulfides through catalytic effects, thereby mitigating voltage fluctuations caused by intermediate product accumulation.
In Fig. 4(b) and (c), through a meticulous analysis of the equilibrium and overpotentials during the Li2S nucleation process, we unveil pivotal insights into the intrinsic diffusion kinetics and ion transport behavior within the material. The data analysis in the insets reveals that CoVN/C-HS@S exhibits a lower overpotential during the Li2S nucleation stage, indicative of its heightened efficiency in facilitating the conversion of active sulfur cathode species. Firstly, CoVN nanoparticles potentially act as efficacious electrocatalysts, diminishing the activation energy of sulfur cathode reactions and accelerating Li2S nucleation and growth. Secondly, the synergy between CoVN and the carbon matrix creates an efficient electronic transport network, enhancing rapid charge migration within the material. Lastly, CoVN's robust adsorption capability towards polysulfides likely mitigates their dissolution and shuttle in the electrolyte, thereby enhancing sulfur utilization and reducing battery polarization. The internal resistance (ΔRΩ) can be quantified according to the equation ΔRinternal = |ΔVQOCV–CCV|/Iapplied. Here, ΔVQOCV–CCV is the voltage difference between the points of quasi-open-circuit-voltage and closed-circuit-voltage, and Iapplied is the current applied. Analyzing the internal resistance of lithium–sulfur batteries during the nucleation stage can better demonstrate the improvement to the kinetics of the difficult solid–liquid phase transformation. It can better reflect how the main materials address the key issues of lithium–sulfur batteries. CoVN/C-HS@S displays a smaller ΔVQOCV–CCV value, further corroborating its electrochemical and kinetic superiority. This finding not only supports the capacity and potential plateau data presented in Fig. 4(a) but also aligns with previous research,28–30 collectively attesting to the efficacy and feasibility of CoVN composite modification in enhancing Li–S battery performance.
Fig. 4(d) concisely presents the cyclic voltammetry (CV) characteristics of C-HS@S and CoVN/C-HS@S at a scan rate of 0.1 mV s−1. The CV curve provides information about the battery redox reaction, including the voltage at which the reaction occurs, allowing for a better comparison of the role of the two host materials in lithium–sulfur batteries. Both materials distinctly exhibit typical redox peaks, indicative of effective electrochemical activity of sulfur in the sulfur-based composites. Notably, the peaks of CoVN/C-HS@S are more pronounced and sharp, which can be attributed to the incorporation of CoVN nanoparticles, which not only facilitate rapid electron transport as conductive bridges but also optimize the redox reaction pathways of sulfur through their unique surface properties. The significant reduction in the potential separation between the oxidation and reduction peaks directly reflects the lower polarization level of CoVN/C-HS@S. In the lithium–sulfur battery reaction, the mutual conversion between solid phase Li2S2/Li2S and liquid lithium polysulfide occurring in the low voltage stage is the most difficult. Due to the improvement of reaction kinetics in lithium–sulfur batteries by CoVN/C-HS, the battery reaction kinetics are accelerated, resulting in the separation of overlapping oxidation peaks as shown in Fig. 4(d). This observation underscores the positive influence of CoVN on the electrochemical reaction kinetics, mitigating energy losses arising from charge transfer resistance and mass transport limitations. This finding aligns with subsequent galvanostatic charge–discharge tests, collectively validating the material's notable advantage in polarization reduction.31,32
Fig. 4(e) and (f) show the current response characteristics of C-HS@S and CoVN/C-HS@S at various scan rates (0.1, 0.2, 0.3, 0.4, and 0.5 mV s−1), thereby uncovering fundamental differences in their kinetic behaviors and charge transfer mechanisms. Notably, as the scan rate increases, the CoVN/C-HS@S composite retains discernible redox peaks, underscoring its exceptional electrochemical stability and rapid charge transfer kinetics. The CoVN nanoparticles play a pivotal role in this regard, enhancing the overall conductivity of the composite while facilitating interfacial charge transfer, effectively mitigating electrochemical polarization issues at high scan rates. In Fig. 4(g)–(i), a rigorous analysis of the linear relationship between the reaction current and the square root of the scan rate, in conjunction with the classical Randles–Sevcik equation (eqn (1))33–35 enables quantitative calculation of the macroscopic diffusion coefficient of lithium ions (Li+) in C-HS@S and CoVN/C-HS@S during the electrochemical process
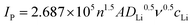 | (1) |
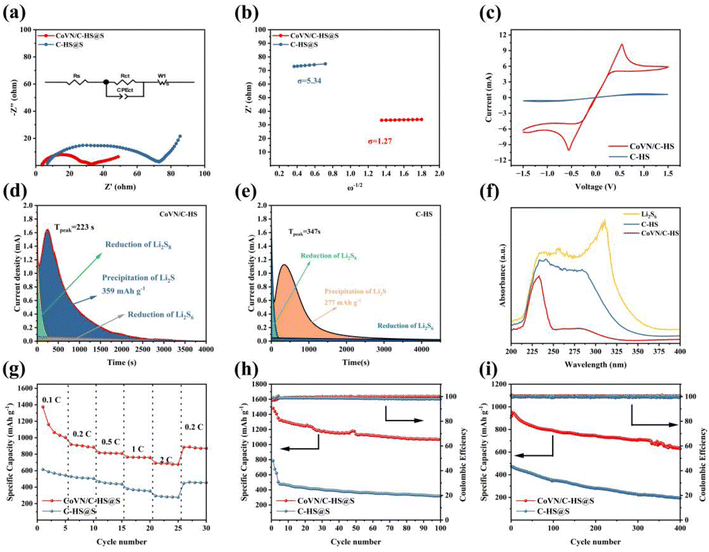
Fig. 5(a) presents the outcomes of electrochemical impedance spectroscopy (EIS), with an inset depicting the equivalent circuit model, offering a visual framework to comprehend the discrepancies in conductive properties of materials. The intercept in the high-frequency region represents the ohmic resistance of the battery. The EIS curve reveals that the CoVN/C-HS@S battery has a smaller intercept and a smaller Rs, proving that CoVN/C-HS improves the conductivity of the sulfur cathode and helps improve battery performance. The diameter of the semicircle in the mid-frequency region is typically associated with the charge transfer resistance (Rct) of the battery. Charge transfer resistance reflects the ease or difficulty of electron transfer from the electrode to the electrolyte during the electrochemical reaction. The Nyquist plot directly reflects the superior conductivity and accelerated electrochemical reaction kinetics of CoVN/C-HS@S, as evidenced by its notably lower charge transfer resistance (Rct) compared to C-HS@S.36 This improvement is attributed to the excellent conductivity of CoVN. Additionally, CoVN acts as an electrocatalyst for the conversion reaction of lithium polysulfides, enhancing the reaction kinetics of the battery and thereby reducing the charge transfer resistance. Furthermore, Fig. 5(b) delves into Warburg impedance through the relationship between Z′ and ω−1/2, a parameter intimately tied to lithium-ion diffusion behavior within materials. Employing eqn (2) and (3), we calculated the lithium-ion diffusion coefficients for both materials.37
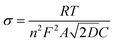 | (2) |
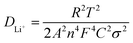 | (3) |
Under the symmetric cell configuration, the cyclic voltammetry (CV) curve analysis presented in Fig. 5(c) explicitly highlights the catalytic superiority of CoVN/C-HS@S in the polysulfide conversion reaction. In symmetrical battery testing, the positive and negative electrodes are the same, which effectively eliminates other influencing factors and allows for a more intuitive evaluation of the interaction between the host material and lithium polysulfide. The C-HS curve showed almost no response, but CoVN/C-HS showed a strong current response. It obviously helps in the conversion reaction of lithium polysulfide. The elevated current density and pronounced redox peaks of this material not only demonstrate its remarkable electrochemical activity but also corroborate its efficacy in catalyzing the polysulfide conversion process. This catalytic effect expedites the rapid conversion of polysulfides, minimizes the loss of active materials, and consequently enhances the overall battery performance.
Fig. 5(d) and (e) delineate the kinetic disparities between the two materials during the reduction of Li2S8, Li2S6 and the precipitation of Li2S through deposition experiments. The earlier onset of peak current (223 s) and the larger nucleation capacity (359 mA h g−1) observed for CoVN/C-HS emphatically attest to its robust catalytic proficiency in polysulfide nucleation reactions. This finding, coupled with the physicochemical analysis of material surfaces, can be attributed to the strong interactions between CoVN and polysulfides, facilitating the rapid progression of nucleation reactions.38–40
The UV-vis absorption spectrum results of the electrolyte, after being left to stand with C-HS and CoVN/C-HS for 12 hours (Fig. 5(f)), further confirm the high adsorption capacity of CoVN/C-HS for polysulfides. The heightened absorbance variation directly reflects its strong adsorption of polysulfide molecules, effectively mitigating the shuttle effect and enhancing battery cycle stability.
Fig. 5(g)–(i) comprehensively exhibit the exceptional performance of CoVN/C-HS@S in terms of rate capability and cycle stability through specific capacity tests and cycle performance evaluations at varying current densities (0.1C, 0.2C, 0.5C, 1C, and 2C). Notably, its ability to maintain high specific capacity and coulombic efficiency even at elevated current densities underscores the pivotal role of CoVN catalysis in ameliorating battery reaction kinetics and augmenting sulfur cathode utilization. Additionally, the potent adsorption mechanism for polysulfides not only alleviates the shuttle effect but also significantly prolongs battery cycle life, offering robust support for the development of high-performance lithium–sulfur batteries.
Fig. 5(g) meticulously compares the specific capacities of two materials across a wide range of current densities (0.1C, 0.2C, 0.5C, 1C, and 2C). The results indicate that the CoVN/C-HS@S composite consistently exhibits significantly higher specific capacities than the reference material, underscoring its exceptional rate capability and implying the presence of efficient electron and ion transport mechanisms within the material. Notably, at high current densities, CoVN/C-HS@S maintains a substantial specific capacity, primarily attributed to its unique structural design and synergistic effects among components, facilitating rapid electrochemical reactions. After undergoing high current charge and discharge, the battery capacity is restored during low current charge and discharge, proving that the CoVN/C-HS@S battery has excellent stability and the battery capacity is reversible. Furthermore, Fig. 5(h) specifically evaluates the cycle stability at 0.2C, revealing that CoVN/C-HS@S retains a high specific capacity close to its initial value (1482 mA h g−1) after numerous charge–discharge cycles. In comparison, its stability surpasses that of C-HS@S, due to CoVN's efficient catalysis of polysulfide conversion and the composite's robust structural stability. This catalysis not only accelerates polysulfide conversion rates but also minimizes the loss of active materials, thereby enhancing battery cycle life. Additionally, Fig. 5(i) showcases the long-term cycling performance of CoVN/C-HS@S under extreme high current density (2C) conditions. Despite this rigorous testing, the material demonstrates remarkable specific capacity retention and coulombic efficiency, outperforming other materials. This phenomenon further attests to CoVN's pivotal role in enhancing battery reaction kinetics, particularly at high current densities, where its catalytic effect is pronounced, effectively overcoming the discharge capacity limitation imposed by the poor conductivity of sulfur cathodes and improving sulfur utilization. Moreover, the robust adsorption capability of the CoVN/C-HS@S cathode towards lithium polysulfides is a crucial factor contributing to its superior performance. This strong adsorption not only effectively mitigates the shuttle effect of lithium polysulfides in the electrolyte, minimizing the loss of active materials, but also reduces self-discharge rates, thereby significantly enhancing battery cycle stability and overall performance. In Table S1,† the CoVN/C-HS host material demonstrates better performance in lithium–sulfur batteries compared with similar materials. However, compared with some cutting-edge work, more research and development is still needed.
3 Conclusion
This study focuses on developing high-performance sulfur anode host materials to enhance the cycle stability and rate capability of Li–S batteries. Significant breakthroughs were achieved through meticulously designed carbon based composites with innovative cobalt-doped vanadium nitride. CoVN/C-HS exhibits superior electrochemical performance due to its excellent conductivity, catalytic properties, and ability to immobilize polysulfide intermediates, thereby mitigating the shuttle effect. The hollow sphere structure offers physical confinement, enhancing sulfur utilization and cycling stability. The study demonstrates remarkable improvements in both specific capacity and cycling stability. Specifically, CoVN/C-HS achieves a specific capacity of 1475 mA h g−1 at 0.05C and retains a capacity of 1067 mA h g−1 after 100 cycles at 0.2C. Additionally, at a high current density of 2C, the material retains a capacity of 662 mA h g−1 after 400 cycles. These findings underscore the potential of CoVN/C-HS as an effective sulfur host material, providing a promising avenue for the development of high-performance Li–S batteries.4 Experimental section
4.1 Preparation of C-HS and CoVN/C-HS
Initially, SiO2 spheres are synthesized via the well-established “Stober” method. Specifically, 0.3 g of SiO2 is ultrasonically dispersed in a mixed solvent of 60 mL deionized water and 30 mL anhydrous ethanol. Subsequently, 0.4 g of CTAB, 1 mL of NH4OH, and 0.0012 g of NH4VO3 are added, followed by stirring for 30 minutes to ensure homogeneity. Thereafter, 0.4 g of resorcinol, 0.6 mL of formaldehyde, and 0.0045 g of CoCl2·6H2O are introduced, and the mixture is stirred at ambient temperature for 12 hours to facilitate the polymerization reaction. The resulting product is collected via centrifugation and thoroughly rinsed with ethanol and deionized water to remove impurities. Subsequently, the precipitate is dried at 80 °C for 12 hours. To obtain the final CoVN/C-HS material, the dried product is ground with twice its mass of urea for 30 minutes and then calcined at 800 °C under an argon atmosphere for 2 hours, yielding a black powder. This powder is subsequently etched in a 4 M NaOH solution at 80 °C for 24 hours to remove unwanted components. The etched product is again collected by centrifugation, washed rigorously with deionized water and ethanol, and dried at 80 °C for 12 hours. For the preparation of C-HS, the procedure is identical except that CoCl2·6H2O and NH4VO3 are omitted, and the carbonization step is performed without the incorporation of urea.4.2 Preparation of C-HS @S and CoVN/C-HS @S
The host materials and sublimed sulfur are thoroughly mixed in a precise 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mass ratio and uniformly ground. This mixture is subsequently transferred into a high-pressure reactor, purged with argon to ensure an inert atmosphere, and hermetically sealed. The reactor is then subjected to heating according to a protocol involving an initial ramp to 155 °C, which is maintained for 12 hours in a blast drying oven to ensure thorough thermal treatment. Following this step, the reactor is transferred to a tube furnace, where it is purged once again with argon and heated to 200 °C, and the temperature is held constant for an additional 30 minutes to complete the desired thermal processing.
3 mass ratio and uniformly ground. This mixture is subsequently transferred into a high-pressure reactor, purged with argon to ensure an inert atmosphere, and hermetically sealed. The reactor is then subjected to heating according to a protocol involving an initial ramp to 155 °C, which is maintained for 12 hours in a blast drying oven to ensure thorough thermal treatment. Following this step, the reactor is transferred to a tube furnace, where it is purged once again with argon and heated to 200 °C, and the temperature is held constant for an additional 30 minutes to complete the desired thermal processing.
4.3 Sample characterization
The XRD analysis was conducted utilizing a Bruker D8 Advance X-ray diffractometer, employing a copper target (λ = 0.15418 nm) at 40 kV and 30 mA, scanning from 10° to 90° at a rate of 10°·min−1, all at ambient temperature (25 °C). For SEM imaging, a JSM-6701F scanning electron microscope was utilized, operating at an accelerated voltage of 10 kV. TEM observations were carried out on a JEM-2010F transmission electron microscope. XPS analysis was performed using a Thermo Scientific K-Alpha XPS system under vacuum conditions, with data corrected to C 1s = 284.80 eV to ascertain the composition and bonding configurations of the prepared sample's constituent elements. The specific surface area and pore size distribution were measured using an ASAP2460, a fully automated analyzer from American Mike Company. Prior to testing, samples were pretreated at 150 °C to mitigate interference from impurities. Furthermore, the sulfur loading of the cathode material was quantitatively analyzed via a DSC-TGA Q500 simultaneous thermal analyzer, operated under an argon atmosphere with a heating rate of 10 °C min−1, spanning from room temperature to 600 °C.4.4 Electrochemical measurements
In accordance with a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the electrode active material, acetylene black, and polyvinylidene fluoride (PVDF) binder were weighed and placed into a grinding bowl for uniform blending. Subsequently, an adequate quantity of N-methyl-2-pyrrolidone (NMP) was added, and the mixture was further ground for 30 minutes to achieve a homogenized electrode slurry. This slurry was then evenly coated onto aluminum foil, which was subsequently dried in a vacuum oven at 80 °C for 12 hours. The dried electrode sheet was then cut into discs, each with a diameter of 12 mm.
1, the electrode active material, acetylene black, and polyvinylidene fluoride (PVDF) binder were weighed and placed into a grinding bowl for uniform blending. Subsequently, an adequate quantity of N-methyl-2-pyrrolidone (NMP) was added, and the mixture was further ground for 30 minutes to achieve a homogenized electrode slurry. This slurry was then evenly coated onto aluminum foil, which was subsequently dried in a vacuum oven at 80 °C for 12 hours. The dried electrode sheet was then cut into discs, each with a diameter of 12 mm.
To evaluate the electrochemical performance of the composite electrode materials, CR2032 coin-cell batteries were assembled, utilizing Celgard 2400 as the separator and 1.0 M LiTFSI solution (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DME and DOL, with a 2.0% LiNO3 additive) as the electrolyte.
1 mixture of DME and DOL, with a 2.0% LiNO3 additive) as the electrolyte.
Charge–discharge tests of the assembled batteries were conducted using a LAND CT2001A battery testing system under a constant current mode, with the voltage window set between 1.7 and 2.8 V. These tests were performed at room temperature.
For deeper analysis, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out using a Shanghai CHI660E electrochemical workstation. During CV testing, the scan potential ranged from 1.7 to 2.8 V, with scan rates varying from 0.1 to 0.5 mV s−1. The EIS test was performed over a frequency range of 0.01 to 100 kHz, with an AC voltage amplitude of 0.005 V. All electrochemical performance tests were conducted at ambient temperature.
Data availability
Data will be made available on request.Author contributions
Yanshuang Meng, and Fuliang Zhu guided the experimental design and led the manuscript preparation and revision work. Jiangnan Zhang did most of the experiments and data analysis. All of the authors have approved the final version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52064035), the Key Research and Development Program of Gansu Province (22YF7GA157), and the Natural Science Foundation of Zhejiang Province (LGG22E020003).References
- N. Nakamura, S. Ahn, T. Momma and T. Osaka, J. Power Sources, 2023, 558, 232566 CrossRef CAS.
- Q. Shao, S. Zhu and J. Chen, Nano Res., 2023, 16, 8097–8138 CrossRef CAS.
- H. Raza, S. Bai, J. Cheng, S. Majumder, H. Zhu, Q. Liu, G. Zheng, X. Li and G. Chen, Electrochem. Energy Rev., 2023, 6, 29 CrossRef CAS.
- J. Xu, S. An, X. Song, Y. Cao, N. Wang, X. Qiu, Y. Zhang, J. Chen, X. Duan and J. Huang, Adv. Mater., 2021, 33, 2105178 CrossRef CAS.
- J. Xu, H. Zhang, F. Yu, Y. Cao, M. Liao, X. Dong and Y. Wang, Angew. Chem., 2022, 134, e202211933 CrossRef.
- S. Tiwari, V. Yadav, A. K. Poonia and D. Pal, J Energy Storage, 2024, 94, 112347 CrossRef.
- Z. Hu, C. Geng, L. Wang, W. Lv and Q.-H. Yang, Adv. Energy Sustainability Res., 2024, 5, 2300148 CrossRef CAS.
- L. Chen, Y. Yuan, R. Orenstein, M. Yanilmaz, J. He, J. Liu, Y. Liu and X. Zhang, Energy Storage Mater., 2023, 60, 102817 CrossRef.
- V. K. Tomer, R. Malik, J. Tjong and M. Sain, Coord. Chem. Rev., 2023, 481, 215055 CrossRef CAS.
- J. Xu, W. Tang, C. Yang, I. Manke, N. Chen, F. Lai, T. Xu, S. An, H. Liu, Z. Zhang, Y. Cao, N. Wang, S. Zhao, D. Niu and R. Chen, ACS Energy Lett., 2021, 6, 3053–3062 CrossRef CAS.
- M. Zhao, H.-J. Peng, B.-Q. Li and J.-Q. Huang, Acc. Chem. Res., 2024, 57, 545–557 CAS.
- Q. Liu, Y. Wu, D. Li, Y.-Q. Peng, X. Liu, B.-Q. Li, J.-Q. Huang and H.-J. Peng, Adv. Mater., 2023, 35, 2209233 CrossRef CAS PubMed.
- N. Mosavati, S. O. Salley and K. Y. S. Ng, J. Power Sources, 2017, 340, 210–216 CrossRef CAS.
- T. Ren, X. Wang, N. Wang, D. Huang, Y. Zhu, P. K. Shen and J. Zhu, J. Mater. Chem. A, 2024, 12, 5307–5318 RSC.
- R. Liu, W. Liu, Y. Bu, W. Yang, C. Wang, C. Priest, Z. Liu, Y. Wang, J. Chen, Y. Wang, J. Cheng, X. Lin, X. Feng, G. Wu, Y. Ma and W. Huang, ACS Nano, 2020, 14, 17308–17320 CrossRef CAS.
- F. Ma, Z. Chen, K. Srinivas, D. Liu, Z. Zhang, Y. Wu, M.-q. Zhu, Q. Wu and Y. Chen, Chem. Eng. J., 2023, 459, 141526 CrossRef CAS.
- X. Li, K. Ding, B. Gao, Q. Li, Y. Li, J. Fu, X. Zhang, P. K. Chu and K. Huo, Nano Energy, 2017, 40, 655–662 CrossRef CAS.
- Z. Sun, J. Zhang, L. Yin, G. Hu, R. Fang, H.-M. Cheng and F. Li, Nat. Commun., 2017, 8, 14627 CrossRef.
- Z. Cheng, Y. Wang, W. Zhang and M. Xu, ACS Appl. Energy Mater., 2020, 3, 4523–4530 CrossRef CAS.
- Y. Lu, M. Zhao, Y. Yang, M. Zhang, N. Zhang, H. Yan, T. Peng, X. Liu and Y. Luo, Nanoscale Horiz., 2022, 7, 543–553 RSC.
- J. Xu, Y. Wang, M. Zhu, R. Wang, H. Jiang, D. Bo, C. Jin and X. Liu, Langmuir, 2024, 40, 9039–9048 CrossRef CAS PubMed.
- W. Ren, L. Xu, L. Zhu, X. Wang, X. Ma and D. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 11642–11651 CrossRef CAS.
- Z. Cheng, Y. Wang, W. Zhang and M. Xu, ACS Appl. Energy Mater., 2021, 4, 6375 CrossRef CAS.
- X. Zhang, X. Chen, Z. Li, P. Guo, Y. Han, Y. Jia and S. Gao, Chem. Res. Appl., 2020, 32, 1409–1414 Search PubMed.
- Z. Lei, Y. X. Yan and W. G. Fei, J. Funct. Polym., 2011, 34, 18–22 Search PubMed.
- X. Dong, X. Liu, P. K. Shen and J. Zhu, Adv. Funct. Mater., 2022, 33, 2210987 CrossRef.
- J. Zhang, M. Xiao, W. Du, J. Feng, Q. Xiang, Y. Meng and F. Zhu, Batter. Supercaps, 2024, 1, e202400310 CrossRef.
- B. Fan, Q. He, Q. Wei, W. Liu, B. Zhou and Y. Zou, Carbon, 2023, 214, 118361 CrossRef CAS.
- S. Hu, M. Yi, H. Wu, T. Wang, X. Ma, X. Liu and J. Zhang, Adv. Funct. Mater., 2022, 32, 2111084 CrossRef CAS.
- Q. Deng, X. Dong, P. K. Shen and J. Zhu, Adv. Sci., 2023, 10, e2207470 CrossRef PubMed.
- X. Huang, Z. Wang, R. Knibbe, B. Luo, S. A. Ahad, D. Sun and L. Wang, Energy Technol., 2019, 7, 1801001 CrossRef.
- F. Liang, Q. Deng, S. Ning, H. He, N. Wang, Y. Zhu and J. Zhu, Adv. Sci., 2024, 11, e2403391 CrossRef.
- D. Li, L. Dai, X. Ren, F. Ji, Q. Sun, Y. Zhang and L. Ci, Energy Environ. Sci., 2021, 14, 424–436 RSC.
- T. L. Nguyen, T. N. Vo, V. D. Phung, K. Ayalew, D. Chun, A. T. Luu, Q. H. Nguyen, K. J. Kim, I. T. Kim and J. Moon, Chem. Eng. J., 2022, 446, 137174 CrossRef CAS.
- A. Ottmann, G. S. Zakharova, B. Ehrstein and R. Klingeler, Electrochim. Acta, 2015, 174, 682–687 CrossRef CAS.
- D. N. Fronczek and W. G. Bessler, J. Power Sources, 2013, 244, 183–188 CrossRef CAS.
- J. Zhang, M. Xiao, T. Liu, Y. Meng, F. Zhu and Z. Fan, Green Chem., 2024, 26, 5546–5555 RSC.
- M. Zhang, W. Chen, L. Xue, Y. Jiao, T. Lei, J. Chu, J. Huang, C. Gong, C. Yan, Y. Yan, Y. Hu, X. Wang and J. Xiong, Adv. Energy Mater., 2020, 10, 1903008 CrossRef CAS.
- Z. Li, Y. Zhou, Y. Wang and Y.-C. Lu, Adv. Energy Mater., 2019, 9, 1802207 CrossRef.
- F. Y. Fan, W. C. Carter and Y.-M. Chiang, Adv. Mater., 2015, 27, 5203–5209 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se01208a |
| This journal is © The Royal Society of Chemistry 2025 |