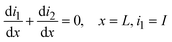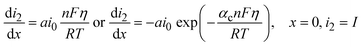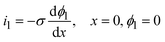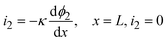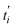Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Separator membranes for aqueous zinc–manganese oxide batteries: a comprehensive review on experimental results and theoretical simulations
T.
Rodrigues-Marinho
ab,
D.
Miranda
bc,
J. C.
Barbosa
d,
R.
Gonçalves
 d,
S.
Lanceros-Méndez
d,
S.
Lanceros-Méndez
 *aef and
C. M.
Costa
*aef and
C. M.
Costa
 *ag
*ag
aPhysics Centre of Minho and Porto Universities (CF-UM-UP), Laboratory of Physics for Materials and Emergent Technologies, LapMET, University of Minho, 4710-057 Braga, Portugal. E-mail: cmscosta@fisica.uminho.pt
b2Ai – School of Technology, IPCA, Barcelos, Portugal
cLASI – Associate Laboratory of Intelligent Systems, Guimarães, Portugal
dCentre of Chemistry, University of Minho, Braga 4710-057, Portugal
eBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940, Leioa, Spain. E-mail: senentxu.lanceros@bcmaterials.net
fIKERBASQUE, Basque Foundation for Science, 48009, Bilbao, Spain
gInstitute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, 4710-053 Braga, Portugal
First published on 30th January 2025
Abstract
Lithium-ion batteries (LIBs) present the highest gravimetric and volumetric energy density, among the different rechargeable battery systems on the market, but still present safety and environmental issues. Thus, batteries based on different chemistries are being explored. Zinc–manganese oxide batteries represent a promising approach since they use components that are more readily available and accessible, especially in light of the scarcity of resources such as lithium. This review focusses on separator materials and the corresponding interface layers for zinc–manganese oxide batteries, from theoretical and experimental points of view, providing an overview of the most recent studies and advancements in the field. The primary obstacles still limiting the widespread application of these batteries are also covered.
1. Introduction
The growing interest in renewable energy generation systems, which need to be associated with convenient storage systems in order to achieve their full potential, has spurred the search for efficient alternatives to traditional lithium-ion battery (LIB) systems.1 The widespread use of these systems, which are estimated to account for around 60% of the total operational electrochemical storage power capacity, particularly at the level of electric mobility, is raising concerns regarding the scarcity of critical materials for LIB manufacturing.2 These materials include lithium, cobalt, and nickel, among others,3 and demand for some of those materials is expected to exceed available reserves in the near future.4 The overexploitation of these materials will create pressure on natural resources, which can lead to increased manufacturing costs, as well as environmental issues.5 In this context, the search for effective alternatives is becoming critical to diversify the range of materials used in energy storage systems, reducing the pressure on each individual one.1In this regard, the development of technologies such as zinc–manganese oxide batteries represents a promising alternative, as they use materials with higher availability and accessibility on earth than lithium, representing lower risks of overexploitation.6 Also, these batteries are relatively simple to produce, which can contribute to reduced manufacturing costs. It is estimated that the cost of raw materials for this type of device ranges between 15 and 30 $ per kW h, a suitable value for energy storage application.7 Furthermore, it has been predicted that with the right processing procedures and materials, these batteries can present performance and capacities comparable to those obtained in traditional LIB technologies, as the energy density of these batteries is 150 W h kg−1, with an estimated capacity loss of 2% per year at room temperature.2
A typical zinc–manganese oxide battery is constituted of a Zn anode and a MnO2 cathode, using a strong basic electrolyte such as potassium hydroxide.2 Also, zinc metal-free anodes8 and a Zn anode decorated with 3D porous-structured Na3V2(PO4)3 (ref. 9) have been used. They were first developed in 1952 and patented in 1960 by Marsal, Kordesch, and Urry.10 This anode/cathode combination is advantageous due to the MnO2 capacity to accommodate the Zn2+ cations, leading to a high theoretical capacity.11 However, there are still several limitations for the wide application of this technology, such as the Zn dendrite growth, the occurrence of side reactions with the electrolyte solution and the collapse of the cathode structure.6 In this regard, improved separator materials and theoretical modeling of zinc–manganese batteries can support overcoming the present limitations. By the theoretical modelling of the materials, architecture and operation of the devices, the main issues affecting battery performance can be identified. This kind of simulation can be a complex topic, as all the components and mechanisms must be accurately described.12 This work reviews the relevant issues and advances in theoretical simulations and separators for zinc–manganese oxide batteries, as materials for electrodes (Zn‖MnO2 (ref. 2, 6 and 13)) and aqueous electrolyte solutions14 have recently been addressed.
2. Aqueous zinc–manganese oxide batteries
In the scope of energetic requirements for human daily activity, energy storage systems are required for the increased mobility and portability of devices and to optimize the use of intermittent renewable energy sources, such as wind and solar energy. At present, lithium-ion batteries (LIBs) and lead–acid batteries dominate the market15 even with the use of toxic lead (lead–acid batteries), large volumetric occupation, environmental issues, and low energy densities (30–50 W h kg−1) of the latter.16 In the case of LIBs, the energy density is improved but the safety problems related to organic electrolytes still persist.17 Environmentally friendly electrolytes with economic viability and high ionic conductivity can be obtained using aqueous secondary batteries.18,19 Using batteries based on aqueous zinc ions (AZIBs) is a promising approach since the materials are of low cost and with low environmental impact fabrication.20 A promising AZIB large-scale energy storage system is based on Zn/MnO2 batteries since zinc presents a high theoretical capacity (820 mA h g−1) with low redox potential (−0.76 V versus the hydrogen electrode),21 low cost, and no toxicity, but with an anion radius larger when compared to the Li ion. Mn-based materials present a theoretical specific capacity of 308 mA h g−1 (for the one-electron redox reaction)22 with non-toxicity and low cost production when compared to other oxide cathodes22 and simple nanostructure production but with low electrical conductivity and instability.23,24 The diversity of crystalline polymorphs in MnO2 arises from the several ways the MnO6 basic units are organized, being classified into three main types: layered structures (δ-MnO2), tunnel structures (α-, β-, γ-, λ-, δ- and R-MnO2) and stacked structures (ε-MnO2). Consequently, the redox of MnO2 can exhibit significant variation based on salt and pH values of the aqueous electrolyte.22Batteries based on Zn/MnO2 as primary cells are considered environmentally friendly and safe since they have been certified by the Environmental Protection Agency (EPA) for landfill disposal25 and they are based on relatively abundant materials in the earth's crust.26,27 For battery proposes, Zn/MnO2 materials need to present reliability for long cycle lifetimes (higher than 4000 cycles) which means 10 to 15 years of battery life for energy storage applications.28 The stability and energy density of aqueous Zn/MnO2 batteries are nonetheless compromised by water splitting in aqueous electrolytes.29,30 To enhance the cycling stability of the Zn/MnO2 battery and reduce the water content, a quasi-solid electrolyte has been implemented by incorporating poly(vinyl alcohol) (PVA) into the aqueous electrolyte.31
At the cathode, the formation of manganite MnOOH occurs from a solid-state proton insertion mechanism from the reduction of MnO2 due to the first electron discharge for voltages between 1.5 and 0.9 V.32 For discharges lower than 0.9 V, the formation of manganese hydroxide (Mn(OH)2) occurs due to a heterogeneous dissolution process from the reduction of MnOOH. The formation of other species such as ZnMn2O4 and Mn3O4 from the reduction of MnOOH at two-electron discharge (Table 1 and Fig. 1) was also observed by Gallaway and Menard.33 The volume expansion of the Mn–O lattice from the reduction of MnO2 leads to the formation of the irreversible, soluble and unstable Mn3+ species.34 The solubilization of the Mn3+ species during the first and second electron discharge is related to the ZnMn2O4 and Mn3O4 formation.35 To obtain a good cycling life of the electrochemical reactions at the cathode, the reduction of the solubilization of the Mn3+ is required by restricting the depth of discharge (DOD)36,37 which leads to a reduction in battery capacity. Although the applications of this restriction by despite this restriction, the capacity failure still occurs due to the formation of ZnMn2O4 and Mn3O4 presenting and insulating and electrochemical irreversible behavior.36,37 Rechargeable Zn/MnO2 batteries demonstrate extended cycle life at reduced DOD between 10% and 20% of the first electron of MnO2 allowing a higher energy density with a cost lower than 100 $ per kW h.37 The increased energy density obtained at higher DOD triggered detrimental phase transformation of MnO2 (ref. 38) and zinc redistribution.39,40 A nearly full two-electron capacity over 6000 cycles, even in the absence of zinc, is obtained using the birnessite type manganese dioxide (δ-MnO2) and Mn(OH)2 with copper and bismuth.41
| Nominal half reactions during discharge | |
|---|---|
| Anode | Zn + 4OH− = Zn(OH)42− + 2e− |
| Cathode | MnO2 + H2O + e− = MnOOH + OH− |
| MnOOH + H2O + e− = Mn(OH)2 + OH− | |
| Overall | Zn + MnO2 + 2H2O + 2OH− → Zn(OH)42− + Mn(OH)2 |
 | ||
| Fig. 1 Illustration of the discharge process of a Zn/MnO2 battery with potassium hydroxide electrolyte. Reproduced from ref. 47, with permission. Copyright 2021, Elsevier. | ||
During the second electron charge/discharge process, the zinc at the anode undergoes a reaction with OH− to produce Zn(OH)42− which precipitates into ZnO.39 The Zn(OH)42− species can be found across the entire cell even on the cathode due to the permeability of the separator layer, resulting in the deposition of an insulator layer of ZnO on the cathode or an irreversible reaction with Mn species to produce ZnMn2O4.42,43 Over several cycles, the accumulation of the ZnMn2O4 species leads to a decrease of Mn atoms participating in the electrochemical reaction which continuously decreases the capacity and increases the cell resistance, diminishing the overall discharged energy and cell potential. Several studies were conducted to reduce the Zn(OH)42− concentration on the cathode side to prevent the Zn/MnO2 battery failure using separators based on polymeric materials (presenting higher permeability to OH− than to Zn(OH)42−),44 anionic exchange based on polystyrene separators,45 and interlayers based on metal oxide materials to enhance the trapping of Zn(OH)42− species.46Table 1 summarizes the redox equations occurring at the cathode and anode and the overall reactions during the discharge process, as illustrated in Fig. 1.
The redox potential of Zn/MnO2 batteries is dependent on the electrode composition and electrolyte concentration, producing an open circuit voltage between 1.4 and 1.7 V.47 Despite the five decades of studies on Zn/MnO2 batteries, the redistribution of the active material on the anode and the limitation of the rechargeable cycles, showing a lifetime between 20 and 30 cycles, are still the limiting factors.47 The company Battery Technologies Inc. (BTI) developed Zn/MnO2 batteries with a capacity retention lower than 50% after 25 cycles with a lifetime of 25 cycles,48 which is poor compared with the rechargeability of LIBs.47
The main characteristics of different battery technologies based on AZIBs,49 Zn/MnO2,50 LIBs51,52 and sodium ion batteries (SIBs)53,54 are summarized in Table 2 for comparison. The energy and power density have been estimated for AZIBs and Zn/MnO2.
| Properties | AZIB | Zn/MnO2 | LIB | SIB |
|---|---|---|---|---|
| Anode | Zn | Zn | Graphite | Hard carbon |
| Cathode | Vanadium-based MFe(CN)6·nH2O | MnO2 | LiCoO2 | NaCoO2 |
| LiNi1−xMxO2 | NaNi1−xMxO2 | |||
| Co3O4 | LiNixMMnyMCozMO2 | NaNixCoyMMnzMO2 | ||
| Electrolyte | Inorganic | Inorganic | Organic | Organic |
| Voltage (V) | 0.6–1.8 | 1.2–2.8 | 3–5 | 2.5–4 |
| Theoretical capacity (mAh g−1) | 65–400 | 100–600 | 80–200 | 80–180 |
| Energy density (W h kg−1) | 100–200 | 70–400 | 150–260 | 120–220 |
| Power density (W kg−1) | 10–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
150–2000 | 40–500 |
| Cost (kW per $) | 450 | <70 | <1530 | 16.8 |
For Zn–MnO2 batteries, the capacity and voltage are limited due to the one-electron redox reaction which can be theoretically increased to 570 mA h g−1 at the two-electron reaction of Mn4+/Mn2+ species.50 The performance of the batteries is diminished due to the instability and lower kinetics of the Zn ions being a drawback for large-scale production. The formation of zinc dendrites at high current, another limitation of these batteries, can be addressed with surface coating, mild acidic aqueous electrolytes or the substitution of the anode current collector.55 The unregulated growth of dendrites poses a substantial risk to the integrity of the anode–electrolyte interface leading to the failure of battery performance. This effect is connected to the local distribution of the electric field, surface texture and the zincophilicity of the substrate.56,57 Efforts have thus been focused on modifying the anode–electrolyte interface and performing electrolyte engineering to suppress dendrite formation using an artificial anode–electrolyte interface to guide the smooth deposition of Zn2+.58–60 Aqueous electrolytes with molecules61,62 and inorganic salts63,64 have also been studied to solve dendrite formation, also supported by theoretical simulations.
2.1. Theoretical model
As mentioned before, theoretical modelling is gaining relevance to predict the best conformations and conditions for optimizing battery performance.A basic electrode configuration consists of a solid metal electrode in contact with an electrolyte. In this setup, the electrochemical reaction occurs solely on the surface of the electrode. However, practical batteries use porous electrodes, implying that active electrode materials are not just at the surface, with the porosity affording a substantial reaction region owing to the electrode's high surface area.65,66 In the Newman model, it is assumed that both the anode and cathode electrodes, as well as the separator, show a porous structure. It is essential to highlight that the electrolyte, in its liquid phase, is distributed within both the electrodes and the separator.65–67 A porous electrode with a random structure can be defined by its porosity, tortuosity, average surface area per unit volume, and the average conductivity of the solid matrix. The separator prevents electron flow, meaning that all current passing through it is ionic. Conversely, the current collector allows only electron flow, making all current at the collector electronic. However, the total current passing through both the separator and the current collector remains constant during operation. Between the separator and the current collector, the conversion between ionic and electronic current occurs at the solid-electrolyte interphase due to the electrochemical reactions.
Chen and Cheh68 advanced on the understanding of alkaline battery discharge behavior by introducing a secondary distribution reaction model. This model considers factors such as the concentration (KOH concentration is 7 M), porosity, and the transference number of species, particularly focusing on their effects on the cathode region. The discharge behavior of the anode was characterized using a simplified mixed-reaction model. The authors further enhanced the model by incorporating a dissolution–precipitation reaction mechanism to elucidate anode discharge behavior, with the assumption that zincate ions were solely present in the anode region.69 The effects of zincate ion transport throughout the entire cell and the temperature variations during discharge were studied in ref. 70 concluding that the temperature effects on alkaline Zn–MnO2 are negligible. The discharge behavior over submicroscopic, microscopic, and macroscopic levels was evaluated in ref. 71 to provide a more precise description of alkaline battery behavior. The relation between the overpotential and the current density, considering the electrochemical kinetic relationships (linear kinetics and Tafel kinetics), can be obtained (eqn (1)) using the Butler–Volmer equation as:
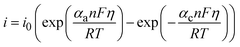 | (1) |
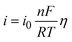 | (2) |
Furthermore, for high overpotential regions, the Tafel kinetics can be used to simplify the Butler–Volmer equation (eqn (3) and (4)) as:
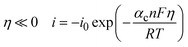 | (3) |
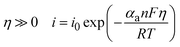 | (4) |
It is further necessary to consider some assumptions to solve this model in one dimension such as during the discharge process the system is at a steady-state and the concentration effects on current density and potential are not considered, as summarized in Table 3. At the current collector and separator, the current is electronic and ionic respectively. It is also assumed that the solid potential and liquid potential are zero at the separator and current collector, respectively.
The principle of mass conservation within the control volume dictates that the accumulation of each species equals (e.g. cation K+, anion OH−, Zn(OH)42−, and water and two salts, i.e., KOH and K2Zn(OH)4) the sum of the net influx of that species into the control volume and the production of that species resulting from electrochemical or chemical reactions. The governing equation for the mass balance of each species (eqn (5)) can be written as
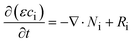 | (5) |
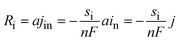 | (6) |
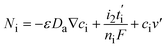 | (7) |
The first term in eqn (7) is related to the diffusion, with Da being the electrolyte effective diffusion coefficient and ∇ci being the concentration gradient of each species on the electrode. The migration phenomena are expressed in the second term 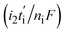 with i2 being the ionic current density and
with i2 being the ionic current density and 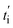 being the transference number (it was assumed constant at battery discharge). The term civ′ describes the convection, with ci being related to the instantaneous concentration of the species. The concentration of binary electrolytes can be expressed (eqn (8)) as:
being the transference number (it was assumed constant at battery discharge). The term civ′ describes the convection, with ci being related to the instantaneous concentration of the species. The concentration of binary electrolytes can be expressed (eqn (8)) as:
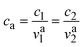 | (8) |
The mass balance in the electrolyte can be obtained (eqn (9)) by substituting eqn (6)–(8) into eqn (5) as
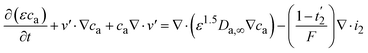 | (9) |
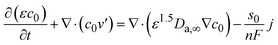 | (10) |
The continuity equation in the battery can be obtained by multiplying the electrolyte molar volume (V−a) and water molar volume (V−0) by eqn (9) and (10), respectively (considering the thermodynamic relationship (caV−a + c0V−0 = 1)) (eqn (11)) as:
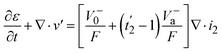 | (11) |
The current density is zero (∇·i1 + ∇·i2 = 0) due to the conservation law considering that the conversion from ionic to electronic current occurs at the active material/electrolyte interface. The conduction in the solid phase and electrolyte is described as eqn (12) and (13) respectively.
| i1 = −σ∇ϕ1 | (12) |
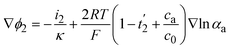 | (13) |
The charge balance can be obtained from Gauss's theorem for an electrode surface area of H × W (eqn (14)) as:
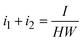 | (14) |
The potential of the electrolyte is related to the ionic current density and chemical potential. Therefore, the overpotential for a Zn/MnO2 battery can be expressed (eqn (15)) as:
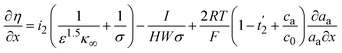 | (15) |
The overall solid balance (eqn (16)) can be obtained from Faraday's law as:
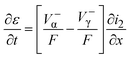 | (16) |
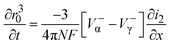 | (17) |
Therefore, the Butler–Volmer equation for the electrochemical reaction rate (eqn (18)) is
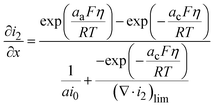 | (18) |
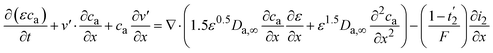 | (19) |
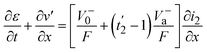 | (20) |
The theoretical modeling of Zn/MnO2 batteries must be complemented with experimental testing in order to validate the data obtained by the simulations. Thus, some of the most relevant studies in the field are described in the following section.
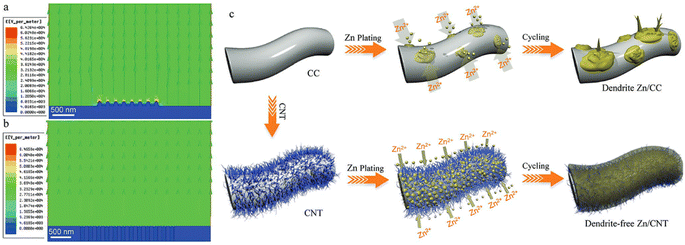 | ||
| Fig. 2 Electric field distributions after Zn nucleation for (a) Zn/carbon cloth and (b) Zn/CNT anodes. (c) Illustration of Zn deposition on both anodes. Reproduced from ref. 72, with permission. Copyright 2019, Wiley. | ||
Li and Sun60 evaluated the electric field distribution and local current density at the anode/electrolyte interface using a vertical graphene carpet on commercial glass fiber Janus separators. Based on Sand's time mode, the initial formation of dendrites can be retarded by reducing the local current density.73,74 The higher surface area of vertical graphene can reduce the current density from 1 to 0.24 mA cm−2 being better when compared to 2D planar graphene (0.38 mA cm−2). The ion concentration field and electric field were theoretically evaluated by Yang and Guo75 to inhibit dendrite formation using an artificial interface layer (hydrogen-substituted graphdiyne (HsGDY)). The electrode with an artificial interface layer presented a uniform concentration field on the Zn interface after 5 and 10 s of diffusion time and a homogeneous electric field, as presented in Fig. 3.
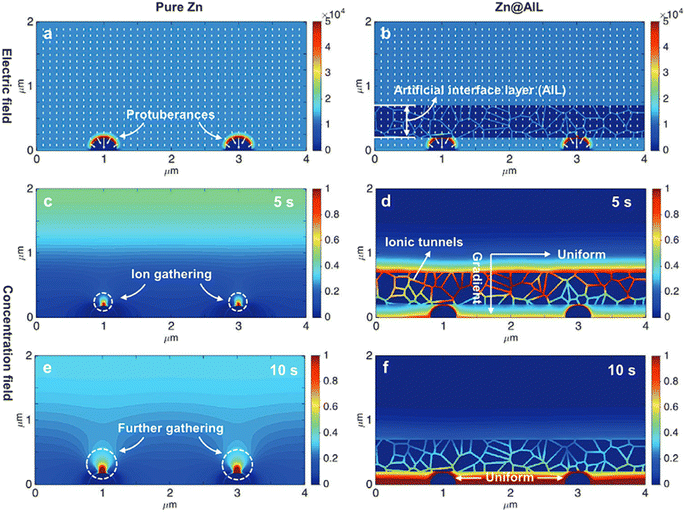 | ||
| Fig. 3 Simulated electric field on (a) Zn electrode and (b) Zn with an artificial interface layer. (c–f) The concentration field of Zn2+ over the two electrodes at diffusion times of 5 s and 10 s. Reproduced from ref. 75, with permission. Copyright 2020, Wiley. | ||
2.2. Materials for separators on zinc–manganese oxide batteries
In rechargeable alkaline Zn/MnO2 batteries, the formation of an inactive zinc manganese spinel phase poisoning the cathode can lead to failure mechanisms mainly at high DOD.46 Preventing the formation of zincate ((Zn(OH)4)42−) anions remains a significant difficulty for ensuring high energy density Zn/MnO2 batteries.32,333D zinc anodes can mitigate dendrite problems as shown in the study by Parker and Chervin76 where monolithic sponge electrodes allow the DOD to be increased at longer cycles. The current density influences the formation of dendrites with the dissolution being enhanced by the decrease of the current density.77 Other approaches to decrease the formation of dendrites are using inorganic78 and polymer coatings.79
The separator is an essential component of the battery systems since it works as an electrolyte contender to mediate the transport of ions and, at the same time, prevent the electron mobility.80,81
Several strategies have been implemented to improve the performance of rechargeable AZIBs by improving the separator properties such as controlling the cathode material dissolution (adsorbing the active material) and suppressing dendrite growth using a homogeneous electric field on the anode surface.82 High mechanical strength and fatigue resistance are required for the separator layer to accumulate repeated cycles and suppress metal dendrite growth. The size and density of the pores are relevant to create a homogeneous ion flux between electrodes to obtain a stable Zn deposition at the anode/separator interface. Furthermore, the separator selectivity for the ion transportation also plays an important role since Zn2+ transport can be improved by the cathode species diffusivity, leading to the contamination of the anode. The separator needs to be inert to oxidation and reduction at the working voltage with no reaction with the active materials.
Typically, commercial primary alkaline Zn/MnO2 batteries use glass fiber separators and nonwoven separators with a paper-like structure, combining layers of cellulose fibers with synthetic fibers based on nylon, rayon or polyvinyl alcohol.83,84 In nonwoven membranes, the electrolyte absorbance can be enhanced using laminated cellophane, allowing OH− consumption to be improved during the discharging process.85
Separator modifications to improve separator–electrolyte and separator–anode interfaces have also been explored.59,89,90 Since the separator is typically an inert component, the chemical and morphological modification using functionalization or surface coating allows battery performance to be improved.91,92 The manipulation of the ionic and electronic conductivity of GF has been extensively studied in zinc ion batteries due to its suitable porosity, low electrical conductivity, and compatibility with aqueous electrolytes.89,93 The short circuit of the battery has been observed for larger and non-uniform pore structures within GF separators due to lower ionic conductivity which induces faster dendrite growth.94,95 Cao and Zhang93 developed Janus separators based on a layer of graphene oxide as an interface between the glass fiber separator and the anode to successfully guide the preferential growth of Zn metal. Moreover, Qin and Liu89 developed a distinct Janus separator by chemical vapor deposition to vertically grow graphene carpets to obtain a more uniform electric field distribution which led to a more uniform Zn deposition.
Song and Ruan96 developed a glass fiber separator with UiO-66 MOFs deposited via a hydrothermal process, showing Zn stripping/plating for 1650 h at 2 mA cm−2 for 1 mA h cm−2. An abundant pore structure and large specific surface area were obtained with a uniform distribution of the electric field. A full cell presented a capacity retention of 85% after 1000 cycles at 1 A g−1.
Yang and Wu97 developed a glass fiber separator coated with a covalent organic framework by the solvothermal growth reaction, presetting polar functional groups and improving electronegativity, which lead to increasing ion diffusion but also dendrite growth. An overpotential of 91.5 mV was achieved for more than 1500 h at a large current density of 10.0 mA cm−2. Capacities higher than 200 mA h g−1 were obtained for a full battery after 700 cycles at 1 A g−1. Cao and Zhang93 used graphene oxide on glass fiber using a vacuum filtration method to develop a separator capable of preferential growth of crystal plane orientation and constant Zn nucleation. A capacity fade over 500 cycles of less than 25% at 0.5 A g−1 was achieved and the full battery exhibits a specific capacity of 126 mA h g−1 at 0.1 A g−1 and high-power density of 20.8 kW kg−1.
Commercial glass fiber was sprayed with Ti3C2Tx ink to produce a Janus separator with 960 μm of thickness capable of inhibiting dendrite growth, showing a stable overpotential of 25.1 mV after 2000 h at 1 mA cm−2 and a capacity retention of 86.7% after 500 cycles at 1 A g−1.98 Lin and Zhou99 developed a glass fiber separator with spray sonicated Ketjen black and zinc oxide nanoparticles to obtain a thickness of 293 μm, capable of homogenizing the electric field at the anode interface, contributing to a uniform Zn growth, stable at 1 mA cm−2 for 2218 h. The full battery presented a specific capacity of ≈100 mA h g−1 after 995 cycles at 0.3 A g−1 (Fig. 4a). The modification of commercial glass fibers using graphite fluoride nanoflakes via vacuum filtration presented high zinc affinity, reducing the by-products and dendrite growth.100 Stable cycles were observed at 1 mA cm−2 and 5 mA cm−2 for 1800 h and 900 h, respectively. A full battery could deliver 1 A g−1 at a capacity retention of 92% after 200 cycles, as presented in Fig. 4b.
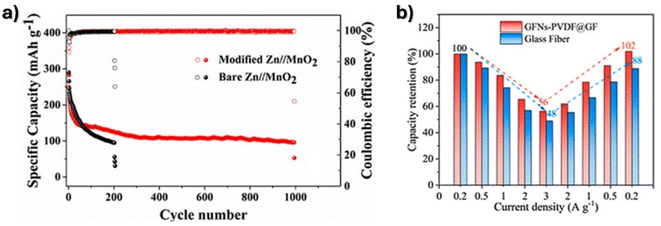 | ||
| Fig. 4 Cycling performance of (a) glass fiber separator with spray sonicated Ketjen black and zinc oxide nanoparticles. Reproduced from ref. 99, with permission. Copyright 2023, Royal Society of Chemistry and (b) capacity retention of commercial glass fibers with graphite fluoride nanoflakes. Reproduced from ref. 100, with permission. Copyright 2023, Elsevier. | ||
Huang and Zhou101 developed a separator based on glass fiber sprayed with Ketjen black on the cathode side, capable of improving the diffusivity and suppressing cathode dissolution. A specific capacity of 194.6 mA h g−1 was obtained at 2 A g−1 after 1500 cycles and a capacity retention of 87% was observed at 5 A g−1 after 6000 cycles.
Graphene sheets with sulfonic cellulose were spin-coated on commercial GF to produce a Janus separator capable of regulating the Zn (002) crystallographic orientation by repelling SO42− and attracting H+ to reduce the side reactions. A capacity retention of 95% after 1900 cycles at 1 A g−1 was obtained for a Zn/CNT/MnO2 cell. An aqueous pouch cell based on Zn/NH4V4O10 presented a capacity of 157 mA h g−1, corresponding to a capacity retention of 87.4% at 2 A g−1 after 260 cycles.102
A prolonged performance up to 500 h was obtained by regulating the Zn deposition and inhibition of dendrite growth using a Sn-coated separator at 10 mA cm−2.59 The discharge capacity after 600 cycles was 200 mA h g−1 at 0.3 A g−1. A dendrite-free Zn anode based on MXene nanoporous NiO heterostructures with an areal capacity of 10 mA h cm−2 and stable cyclability over 500 h was reported in ref. 103.
It has also been demonstrated that metal–organic frameworks (MOFs) as coatings restrict the Zn2+ flux and consequently dendrite formation,97,104 mainly through the combination of inorganic metals and organic ligands showing high resistance to acid and alkaline electrolytes.105–107 The modification of the size of the organic ligands allows ion diffusion channels to be tuned to specific sizes which facilitates Zn2+ ion diffusion.108 MOFs also allow water activity in the electrolyte to be regulated by introducing functional groups capable of producing hydrogen bond networks.109,110
A separator obtained from cellulose fibers on cotton presented a tensile strength and modulus of 29.2 MPa and 4.16 GPa, respectively with a high concentration of hydroxyl groups on a dense and uniform nanoporous structure.91 Cellulose nanofibrils have also been obtained from algae showing a dense and uniform mesopore structure with a size of ≈20 nm.117 The performance of cellulose separators can be improved by grafting sulfonate groups, which enhance the Zn2+ conduction and regulate the cation flux, forming hydrogen bonds with water molecules of the electrolyte and leading to a reduction in the water reactivity.118
Zhou and Chen91 developed a cotton-derived cellulose separator by vacuum filtration with a thickness of 140 μm, a high ionic conductivity of 56.95 mS cm−1 and a tensile strength of 29.2 MPa. This separator presents a network of dense and uniform nanopores with hydroxyl groups, reducing the zinc nucleation overpotential (≈105 mV over 2000 h), which increases the zinc ion transfer number, accelerates zinc deposition and suppresses the side reactions. The zinc plating capacity of Zn//Zn cells reached 1000 mA h cm−2 and 850 mA h cm−2 at 1 mA cm−2 and 2 mA cm−2, respectively. Fig. 5a shows the morphology of this separator applied to a Zn/MnO2 battery, obtaining a capacity retention of 63.6% after 1000 cycles at 1 A g−1. An ultrathin (9 μm) and high-toughness membrane based on bacterial cellulose presenting a tensile strength of 120 MPa showed a chemical affinity to the Zn(100) crystal plane (Fig. 5b), with the hydroxyl groups leading to inhibition of dendrite growth and corrosion suppression and the battery being stable over 4000 h at 0.5 mA cm−2 within 0.1 mA h cm−2.119 The Zn/MnO2 battery presents a specific capacity of 171.7 mA h g−1 after 100 cycles at a 1C rate as presented in Fig. 5c.
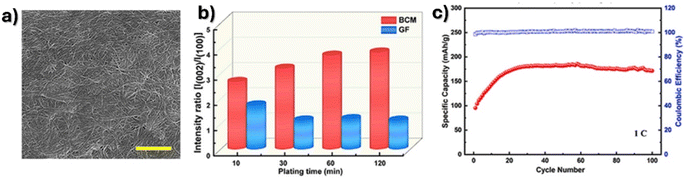 | ||
| Fig. 5 (a) SEM images of a cycled bacterial cellulose based separator; (b) intensity ratio of crystal planes from Zn plating and (c) stability of the battery at 1C. Reproduced from ref. 119, with permission. Copyright 2022, Cell Press. | ||
Solution cast separators based on cellulose nanofibers–ZnO2 composites presented an ionic conductivity of 4.59 mS cm−1, a Zn2+ transfer number of 0.69 and a dielectric constant of 25.115 The separator showed high Zn2+ diffusion while repelling anions, with the uniform Zn deposition leading to inhibition of dendrite growth with an overpotential of 57 mV over 2000 h at 0.5 mA cm−2. A full battery capacity retention of 87.2% was achieved at 2.5 A g−1 over 3000 cycles. Yang and Wu112 modified a conventional filter paper to produce a cellulose-based separator with preferential adsorptions of protons, sulfate, and zinc ions, to inhibit dendrite growth as presented in Fig. 6a. A stable performance was obtained at a current density of 5 mA cm−2 over 2900 h and a full battery capacity retention of 98.4% after 1000 cycles at 1 A g−1 was obtained (Fig. 6b). Furthermore, Liu and Kong111 also developed a separator based on filter paper with strontium titanate coating (Fig. 6c) with an overall thickness of 46 μm, presenting enhanced dielectric properties. A specific capacity higher than 80 mA h g−1 has been obtained for a full battery after 300 cycles at 0.5 A g−1 for a new separator based on a strontium titanate (SrTiO3) coating, which presents high specificity that allows homogenizing the ion flow (Fig. 6d).
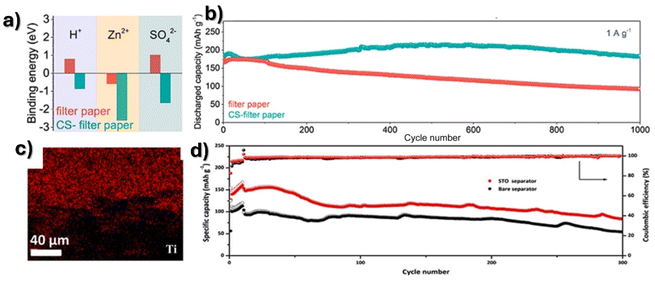 | ||
| Fig. 6 (a) Zinc, proton and sulfate ion adsorption and (b) cycling performance of cellulose-based separators. Reproduced from ref. 112, with permission. Copyright 2022, Elsevier. (c) SEM image of strontium titanate distribution in filter paper separators and (d) the cycling performance at 0.5 A g−1. Reproduced from ref. 111, with permission. Copyright 2023, Elsevier. | ||
A commercial cotton towel was used as a separator for aqueous zinc-ion batteries (AZIBs), presenting good mechanical performance (tensile strength of 6 MPa) and a thickness of 415 μm with high wettability, suppressing side reactions and dendrite growth.120 A lifespan of 700 h at 2 mA cm−2 for 4 mA h cm−2 was achieved, with the full cell presenting a cyclability of 96.9 mA h g−1 after 2400 cycles at 3 A g−1.
Ghosh and Vijayakumar128 developed a Zn/MnO2 cell with a Nafion ionomer separator combined with zinc ion conductive electrolytes to improve the capacity retention up to 75% over 1000 cycles.
A N-butylimidazolium-functionalized polysulfone separator (50 wt% concentration) presented a comparable overall conductivity and hydroxide diffusivity but also lower permeability to zincate when compared to commercial separators.129 This separator was evaluated in a Zn/MnO2 battery, showing an extended lifetime from 21 to 79 cycles for two-electron capacity after falling to 50%.
Also in the scope of polymer-based separators, polyacrylonitrile electrospun fibers with a thickness of 69 μm present a tensile strength of 3.62 MPa with enhanced bending movement and excellent pore connectivity,130 leading to a higher regulation of cation transport due to the uniform ion flux and electrostatic interaction between nitrogen and Zn. Another promising polymer for battery applications is poly(vinylidene fluoride), PVDF, which presents low ionic conductivity and poor hydrophilicity. Liu and Liu87 coated a polydopamine surfactant leading to a uniform Zn deposition on the PVDF nanofiber surface.
Poly(vinylidene fluoride-co-hexafluoropropylene), PVDF-HFP, polymer matrix composites with catalytic ions (manganese sulfate, MnSO4, zinc trifluoromethanesulfonate, Zn(OTf)2, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMI-TFSI]IL) and ionic liquids) facilitate the acceleration of zinc ion deposition and migration kinetics, as well as enhance charge transfer at the electrode/electrolyte interface.131 A Zn/MnO2 battery with a PVDF composite separator with the IL PPCu1C-ZMIL5 showed a specific capacity of 124.6 mA h g−1 at a 1C rate over 1000 cycles (Fig. 7a). The separators developed by Liang and Ma132 based on PVDF/barium titanate composites can efficiently capture and facilitate the Zn2+ transport at the electrolyte–electrode interface and redistributes the ion transport to achieve improved homogeneity at the separator–anode interface. A specific capacity of 108 mA h g−1 at 1 A g−1 after 1800 cycles was achieved presenting a capacity retention improvement of 115% at 0.2 A g−1 after 100 cycles as presented in Fig. 7b.
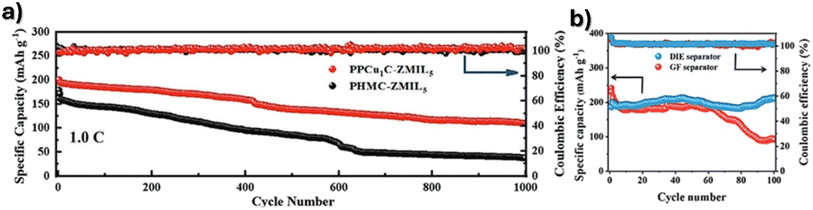 | ||
| Fig. 7 Cycling performance of PVDF composite-based separators with (a) ionic liquid PPCu1C-ZMIL5. Reproduced from ref. 131, with permission. Copyright 2022, Wiley and (b) barium titanate nanoparticles. Reproduced from ref. 132, with permission. Copyright 2022, Wiley. | ||
A stretchable Zn/MnO2 battery with a biocompatible separator based on poly(styrene-isobutylene-styrene) (SIBS) presented an ionic conductivity of 0.05 S m−1 with a specific capacity of 160 mA h g−1 and capacity retention of 75% after 500 charge and discharge cycles.133 The developed battery also presents functionality after 150 cycles when a strain of 100% was applied.
The utilization of nanofibrous polymers with additives allows the formation of size dependent conductive pathways which facilitate the diffusion of hydroxide ions (small size) over the zincate ions (larger size). Huang and Yadav134 developed an ion-selective composite membrane based on graphene oxide/poly(vinyl alcohol) to suppress the zincate ion while the conduction of hydroxyl ions remains constant. The primary and secondary cells showed an area capacity of 20 mA h cm−2 for a near full MnO2 two-electron capacity (617 mA h g−1). The advanced separator reached three times higher cycle life (300) when compared to zincate blocking separators.
A flexible Zn/MnO2 battery was fabricated using a dual-crosslinked Zn-alginate/PAAm hydrogel electrolyte showing a specific capacity of 300.4 mA h g−1 at 0.11 A g−1 with a capacity retention of 82% at 0.88 A g−1 after 500 charge and discharge cycles. The battery also presents capability of supporting high mechanical stresses such as a car run-over for 20 times for 2 days.135
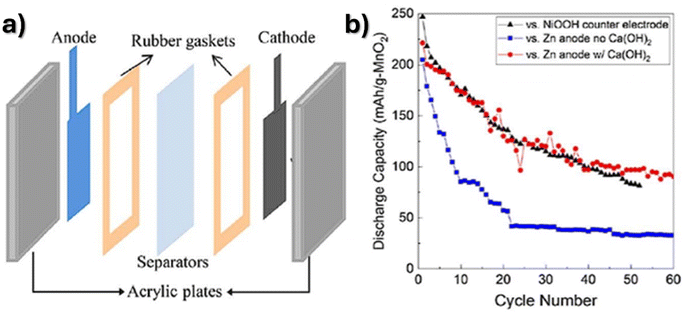 | ||
| Fig. 8 (a) Schematic representation of a Zn/MnO2 battery with a calcium hydroxide sheet between electrodes and (b) discharge capacity over cycling. Reproduced from ref. 46, with permission. Copyright 2017, Elsevier. | ||
Furthermore, printed Zn/MnO2 batteries have also been developed. A printable and flexible Zn/MnO2 battery with a hydrogel separator with a specific capacity of 7.3 mA h cm−2 was reported in ref. 138. A printed planar micro-battery with neutral aqueous electrolyte was reported in ref. 139 based on interdigitated Zn and MnO2 ink patterns. The separator-free battery achieved a capacity of 393 mA h g−1 at 7.5 mA cm−3 and a capacity retention of 83.9% at 5C after 1300 cycles with high mechanical flexibility and capacity stability under several deformations. A capacity retention of 83% over 900 cycles was obtained using a calcium hydroxide layer.42
Typically, the most commonly used separator for this battery system is glass fiber, whose manufacturing process is relatively simple and scalable and, consequently, presents low production costs. These separators are flexible, but they are not mechanically strong enough to withstand pressure and stresses during battery operation. The main disadvantage is the limited chemical compatibility, which must be improved both with the electrolyte and electrodes to avoid degradation and improve ionic conductivity. Separators based on cellulose and other synthetic polymers have some advantages over glass fiber separators, with the main disadvantage being the cost. This issue must be addressed for their future implementation in commercial batteries.
Overall, it is shown that Zn/MnO2 batteries are a potential alternative to lithium-ion batteries for some applications considering the cost and capacity value, but more work is needed to solve dendrite growth and electrode/electrolyte interfaces, among others.
These issues in the separator membrane have been studied using different coatings, but more work is needed, together with the proper engineering of the interface with the electrodes.
For this, it is essential to improve separator materials to obtain high-performance electrochemical energy storage devices.
3. Final remarks and future needs
The key advances in the scope of separator materials and technologies and theoretical simulations for zinc–manganese oxide batteries have been presented. This energy storage system has strong potential for the next generation of high-performance energy storage devices, which aim to replace some critical minerals of lithium-ion batteries, such as lithium, cobalt and nickel, with more available and environmentally friendly materials, including zinc and magnesium.Those batteries will allow a high discharge capacity value to be obtained but present a low voltage, being suitable for specific application areas, such as wearable and related portable devices.
Nevertheless, in order to reach their full potential, electrode interfaces have to be improved and dendrite growth issues have to be solved.
In order to overcome these issues, particular attention must be paid to the separator membrane and electrode/electrolyte interfaces. The most popular separator for this kind of battery is typically made of nonwoven materials, but it also needs to be improved in terms of its mechanical, electrochemical, and thermal characteristics. Utilizing composites based on metal–organic frameworks (MOFs), zeolites, and other ceramics that inhibit dendritic development may allow the mechanical and thermal properties to be improved, combined with coating layers.
In order to investigate synergistic effects and attain flame retardant behavior, wettability, dimensional stability, interface compatibilization, and dendritic growth suppression, innovative ways combining various materials in a separator type are required.
Furthermore, it is necessary to use new types of polymers including UV curing polymers, allowing the interfaces between components to be reduced, obtaining dendrite-free batteries. Additionally, theoretical simulations are essential to go beyond trial-and-error experimental work. In this scope, extensive work on the interfacial electrochemical processes at the separator–electrolyte and separator–anode interfaces is required. These issues such as separator morphology, new electrolyte solutions and their respective compatibility with porous membranes and the use of additive manufacturing techniques for improved battery scalability are necessary in this field.
Separator materials play a significant and critical role in zinc–manganese oxide batteries, where new scientific and technological developments are required in this field to improve the thermal, mechanical, and electrochemical properties in order to obtain high-performance batteries allowing LIBs and their critical materials to be replaced in specific applications, thus contributing to improved performance and sustainability.
Conflicts of interest
There are no conflicts to declare.Abbreviations
List of symbols
Greek symbols
| α a | Anodic transfer coefficient |
| α c | Cathodic transfer coefficient |
| ε | Porosity of electrodes |
| η | Overpotential [V] |
| κ ∞ | Bulk electrolyte conductivity [S cm−1] |
| κ | Electrolyte conductivity [S cm−1] |
| σ | Electrode conductivity [S cm−1] |
| ϕ 1 | Potential of the solid phase [V] |
| ϕ 2 | Potential of electrolyte [V] |
Acknowledgements
The authors thank the Fundação para a Ciência e Tecnologia (FCT) for financial support under the framework of Strategic Funding UIDB/05549/2020, UIDP/05549/2020 Applied Artificial Intelligence Laboratory (2Ai), UIDB/04650/2020, UID/FIS/04650/2020, UID/04650 Physics Centre of Minho and Porto Universities (CF-UM-UP), UID/EEA/04436/2020, LASI-LA/P/0104/2020 and UID/QUI/00686/2020 and under projects, POCI-01-0247-FEDER-046985 and 10.54499/2022.03931.PTDC funded by national funds through FCT and by the ERDF through the COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and project SmartHealth, “NORTE-01-0145-FEDER-000045”, supported by the Northern Portugal Regional Operational Programme (Norte2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF) and NGS-New Generation Storage, C644936001-00000045, supported by IAPMEI (Portugal) with funding from the European Union NextGenerationEU (PRR). The authors also thank the FCT for financial support under UMINHO/BIPD/2024/4 (J. C. B.) and FCT investigator contracts CEECIND/00833/2017 (DOI: 10.54499/CEECIND/00833/2017/CP1458/CT0017) (RG) and 2020.04028.CEECIND (DOI: 10.54499/2020.04028.CEECIND/CP1600/CT0018) (C. M. C.). This study forms part of the Advanced Materials program and was supported by MCIN with funding from the European Union NextGenerationEU (PRTR-C17.I1) and by the Basque Government under the IKUR program.References
- C. M. Costa, J. C. Barbosa, R. Gonçalves, H. Castro, F. J. Del Campo and S. Lanceros-Méndez, Energy Storage Mater., 2021, 37, 433–465 CrossRef.
- M. B. Lim, T. N. Lambert and B. R. Chalamala, Mater. Sci. Eng., R, 2021, 143, 100593 CrossRef.
- T. Prior, P. A. Wager, A. Stamp, R. Widmer and D. Giurco, Sci. Total Environ., 2013, 461–462, 785–791 CrossRef CAS PubMed.
- G. Martin, L. Rentsch, M. Höck and M. Bertau, Energy Storage Mater., 2017, 6, 171–179 CrossRef.
- I. Y. L. Hsieh, M. S. Pan, Y. M. Chiang and W. H. Green, Appl. Energy, 2019, 239, 218–224 CrossRef.
- J. Y. Luan, H. Y. Yuan, J. Liu and C. Zhong, Energy Storage Mater., 2024, 66, 103206 CrossRef.
- G. G. Yadav, D. Turney, J. C. Huang, X. Wei and S. Banerjee, ACS Energy Lett., 2019, 4, 2144–2146 CrossRef CAS.
- J. B. Miao, Y. X. Du, R. T. Li, Z. K. Zhang, N. N. Zhao, L. Dai, L. Wang and Z. X. He, Int. J. Miner., Metall. Mater., 2024, 31, 33–47 CrossRef CAS.
- N. Guo, Z. Peng, W. Huo, Y. Li, S. Liu, L. Kang, X. Wu, L. Dai, L. Wang, S. C. Jun and Z. He, Small, 2023, 19, e2303963 CrossRef PubMed.
- P. Marsal, R. River, K. Kordesch and L. Urry, Dry Cell, US Pat., 2960558, 1960 Search PubMed.
- Y. L. Zhao, Y. H. Zhu and X. B. Zhang, InfoMat, 2020, 2, 237–260 CrossRef CAS.
- D. Miranda, C. M. Costa and S. Lanceros-Mendez, J. Electroanal. Chem., 2015, 739, 97–110 CrossRef CAS.
- C. Li, R. Zhang, H. L. Cui, Y. B. Wang, G. J. Liang and C. Y. Zhi, Trans. Tianjin Univ., 2024, 30, 27–39 CrossRef CAS.
- R. Durena and A. Zukuls, Batteries, 2023, 9, 311 CrossRef CAS.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- A. R. Dehghani-Sanij, E. Tharumalingam, M. B. Dusseault and R. Fraser, Renewable Sustainable Energy Rev., 2019, 104, 192–208 CrossRef.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2009, 22, 587–603 CrossRef.
- D. Chao, W. Zhou, F. Xie, C. Ye, H. Li, M. Jaroniec and S. Z. Qiao, Sci. Adv., 2020, 6, eaba4098 CrossRef CAS PubMed.
- J. H. Huang, Z. W. Guo, Y. Y. Ma, D. Bin, Y. G. Wang and Y. Y. Xia, Small Methods, 2019, 3, 1800272 CrossRef.
- L. E. Blanc, D. Kundu and L. F. Nazar, Joule, 2020, 4, 771–799 CrossRef CAS.
- C. P. Li, X. S. Xie, S. Q. Liang and J. Zhou, Energy Environ. Mater., 2020, 3, 146–159 CrossRef CAS.
- T. Xue and H. J. Fan, J. Energy Chem., 2021, 54, 194–201 CrossRef CAS.
- L. J. Yan, X. M. Zeng, Z. H. Li, X. J. Meng, D. Wei, T. F. Liu, M. Ling, Z. Lin and C. D. Liang, Mater. Today Energy, 2019, 13, 323–330 CrossRef.
- C. Wang, Y. X. Zeng, X. Xiao, S. J. Wu, G. B. Zhong, K. Q. Xu, Z. F. Wei, W. Su and X. H. Lu, J. Energy Chem., 2020, 43, 182–187 CrossRef.
- Part 273—Standards for Universal Waste Management, 40 Cfr, 2017 Search PubMed.
- A. Biswal, B. C. Tripathy, K. Sanjay, T. Subbaiah and M. Minakshi, RSC Adv., 2015, 5, 58255–58283 RSC.
- A. M. Popov, M. O. Kozhnov, T. A. Labutin, S. M. Zaytsev, A. N. Drozdova and N. A. Mityurev, Tech. Phys. Lett., 2013, 39, 81–83 CrossRef CAS.
- I. Gyuk, M. Johnson, J. Vetrano, K. Lynn, W. Parks, R. Handa, L. Kannberg, S. Hearne, K. Waldrip and R. Braccio, Grid Energy Storage, U.S. Department of Energy, 2013 Search PubMed.
- C. Liu, X. Wang, W. Deng, C. Li, J. Chen, M. Xue, R. Li and F. Pan, Angew Chem. Int. Ed. Engl., 2018, 57, 7046–7050 CrossRef CAS PubMed.
- M. H. Alfaruqi, J. Gim, S. Kim, J. Song, J. Jo, S. Kim, V. Mathew and J. Kim, J. Power Sources, 2015, 288, 320–327 CrossRef CAS.
- Y. Zeng, X. Zhang, Y. Meng, M. Yu, J. Yi, Y. Wu, X. Lu and Y. Tong, Adv. Mater., 2017, 29, 1700274 CrossRef PubMed.
- J. W. Gallaway, B. J. Hertzberg, Z. Zhong, M. Croft, D. E. Turney, G. G. Yadav, D. A. Steingart, C. K. Erdonmez and S. Banerjee, J. Power Sources, 2016, 321, 135–142 CrossRef CAS.
- J. W. Gallaway, M. Menard, B. Hertzberg, Z. Zhong, M. Croft, L. A. Sviridov, D. E. Turney, S. Banerjee, D. A. Steingart and C. K. Erdonmez, J. Electrochem. Soc., 2015, 162, A162–A168 CrossRef CAS.
- J. Duay, M. Keny and T. N. Lambert, J. Power Sources, 2018, 395, 430–438 CrossRef CAS.
- D. Im, A. Manthiram and B. Coffey, J. Electrochem. Soc., 2003, 150, A1651–A1659 CrossRef CAS.
- M. Kelly, J. Duay, T. N. Lambert and R. Aidun, J. Electrochem. Soc., 2017, 164, A3684–A3691 CrossRef CAS.
- N. D. Ingale, J. W. Gallaway, M. Nyce, A. Couzis and S. Banerjee, J. Power Sources, 2015, 276, 7–18 CrossRef CAS.
- M. R. Bailey and S. W. Donne, Electrochim. Acta, 2011, 56, 5037–5045 CrossRef CAS.
- D. E. Turney, J. W. Gallaway, G. G. Yadav, R. Ramirez, M. Nyce, S. Banerjee, Y. C. K. Chen-Wiegart, J. Wang, M. J. D'Ambrose, S. Kolhekar, J. C. Huang and X. Wei, Chem. Mater., 2017, 29, 4819–4832 CrossRef CAS.
- X. Wei, D. Desai, G. G. Yadav, D. E. Turney, A. Couzis and S. Banerjee, Electrochim. Acta, 2016, 212, 603–613 CrossRef CAS.
- G. G. Yadav, J. W. Gallaway, D. E. Turney, M. Nyce, J. Huang, X. Wei and S. Banerjee, Nat. Commun., 2017, 8, 14424 CrossRef PubMed.
- G. G. Yadav, X. Wei, J. Huang, J. W. Gallaway, D. E. Turney, M. Nyce, J. Secor and S. Banerjee, J. Mater. Chem. A, 2017, 5, 15845–15854 RSC.
- J. Duay, T. N. Lambert and R. Aidun, Electroanalysis, 2017, 29, 2261–2267 CrossRef CAS.
- H. J. Lee, J. M. Lim, H. W. Kim, S. H. Jeong, S. W. Eom, Y. T. Hong and S. Y. Lee, J. Membr. Sci., 2016, 499, 526–537 CrossRef CAS.
- H. J. Hwang, W. S. Chi, O. Kwon, J. G. Lee, J. H. Kim and Y. G. Shul, ACS Appl. Mater. Interfaces, 2016, 8, 26298–26308 CrossRef CAS PubMed.
- J. C. Huang, G. G. Yadav, J. W. Gallaway, X. Wei, M. Nyce and S. Banerjee, Electrochem. Commun., 2017, 81, 136–140 CrossRef CAS.
- M. B. Lim, T. N. Lambert and B. R. Chalamala, Mater. Sci. Eng., R, 2021, 143, 100593 CrossRef.
- U. Sengupta, S. Megahed, A. Homa and S. Ruth, Proceedings of WESCON 1994 – Idea/Microelectronics, Conference Record, Institute of Electrical and Electronics Engineers Inc., 1994, pp. 249–255 Search PubMed.
- M. Song, H. Tan, D. L. Chao and H. J. Fan, Adv. Funct. Mater., 2018, 28, 1802564 CrossRef.
- D. Chao, W. Zhou, C. Ye, Q. Zhang, Y. Chen, L. Gu, K. Davey and S. Z. Qiao, Angew Chem. Int. Ed. Engl., 2019, 58, 7823–7828 CrossRef CAS PubMed.
- W. Li, B. Song and A. Manthiram, Chem. Soc. Rev., 2017, 46, 3006–3059 RSC.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew Chem. Int. Ed. Engl., 2018, 57, 102–120 CrossRef CAS PubMed.
- C. Vaalma, D. Buchholz, M. Weil and S. Passerini, Nat. Rev. Mater., 2018, 3, 18013 CrossRef.
- Y. M. Li, Y. X. Lu, C. L. Zhao, Y. S. Hu, M. M. Titirici, H. Li, X. J. Huang and L. Q. Chen, Energy Storage Mater., 2017, 7, 130–151 CrossRef.
- Y. Yin, S. Wang, Q. Zhang, Y. Song, N. Chang, Y. Pan, H. Zhang and X. Li, Adv. Mater., 2020, 32, e1906803 CrossRef PubMed.
- Z. Yi, G. Chen, F. Hou, L. Wang and J. Liang, Adv. Energy Mater., 2020, 11, 2003065 CrossRef.
- A. Naveed, T. Rasheed, B. Raza, J. Chen, J. Yang, N. Yanna and J. Wang, Energy Storage Mater., 2022, 44, 206–230 CrossRef.
- Y. Z. Chu, S. Zhang, S. Wu, Z. L. Hu, G. L. Cui and J. Y. Luo, Energy Environ. Sci., 2021, 14, 3609–3620 RSC.
- Z. Hou, Y. Gao, H. Tan and B. Zhang, Nat. Commun., 2021, 12, 3083 CrossRef CAS PubMed.
- C. Li, Z. Sun, T. Yang, L. Yu, N. Wei, Z. Tian, J. Cai, J. Lv, Y. Shao, M. H. Rummeli, J. Sun and Z. Liu, Adv. Mater., 2020, 32, e2003425 CrossRef PubMed.
- P. Sun, L. Ma, W. Zhou, M. Qiu, Z. Wang, D. Chao and W. Mai, Angew Chem. Int. Ed. Engl., 2021, 60, 18247–18255 CrossRef CAS PubMed.
- R. Li, M. C. Li, Y. Chao, J. Q. Guo, G. Xu, B. R. Li, Z. Y. Liu, C. K. Yang and Y. Yu, Energy Storage Mater., 2022, 46, 605–612 CrossRef.
- K. Ouyang, D. Ma, N. Zhao, Y. Wang, M. Yang, H. Mi, L. Sun, C. He and P. Zhang, Adv. Funct. Mater., 2022, 32(7), 2109749 CrossRef CAS.
- Y. T. Xu, J. J. Zhu, J. Z. Feng, Y. Wang, X. X. Wu, P. J. Ma, X. Zhang, G. Z. Wang and X. B. Yan, Energy Storage Mater., 2021, 38, 299–308 CrossRef.
- J. S. Newman and C. W. Tobias, J. Electrochem. Soc., 1962, 109, 1183–1191 CrossRef CAS.
- J. Newman and W. Tiedemann, AIChE J., 2004, 21, 25–41 CrossRef.
- P. M. Gomadam, J. W. Weidner, R. A. Dougal and R. E. White, J. Power Sources, 2002, 110, 267–284 CrossRef CAS.
- J. S. Chen and H. Y. Cheh, J. Electrochem. Soc., 2019, 140, 1205–1213 CrossRef.
- J. S. Chen and H. Y. Cheh, J. Electrochem. Soc., 2019, 140, 1213–1218 CrossRef.
- E. J. Podlaha and H. Y. Cheh, J. Electrochem. Soc., 2019, 141, 15–27 CrossRef.
- T. W. Farrell, C. P. Please, D. L. S. McElwain and D. A. J. Swinkels, J. Electrochem. Soc., 2000, 147, 4034–4044 CrossRef CAS.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Adv. Mater., 2019, 31, e1903675 CrossRef PubMed.
- J. Lee, H. S. Lim, X. Cao, X. Ren, W. J. Kwak, I. A. Rodriguez-Perez, J. G. Zhang, H. Lee and H. T. Kim, ACS Appl. Mater. Interfaces, 2020, 12, 37188–37196 CrossRef CAS PubMed.
- H. L. Qiu, T. Y. Tang, M. Asif, W. Li, T. Zhang and Y. L. Hou, Nano Energy, 2019, 65, 103989 CrossRef CAS.
- Q. Yang, Y. Guo, B. Yan, C. Wang, Z. Liu, Z. Huang, Y. Wang, Y. Li, H. Li, L. Song, J. Fan and C. Zhi, Adv. Mater., 2020, 32, e2001755 CrossRef PubMed.
- J. F. Parker, C. N. Chervin, E. S. Nelson, D. R. Rolison and J. W. Long, Energy Environ. Sci., 2014, 7, 1117–1124 RSC.
- Q. Yang, G. Liang, Y. Guo, Z. Liu, B. Yan, D. Wang, Z. Huang, X. Li, J. Fan and C. Zhi, Adv. Mater., 2019, 31, e1903778 CrossRef PubMed.
- L. T. Kang, M. W. Cui, F. Y. Jiang, Y. F. Gao, H. J. Luo, J. J. Liu, W. Liang and C. Y. Zhi, Adv. Energy Mater., 2018, 8, 1801090 CrossRef.
- Z. M. Zhao, J. W. Zhao, Z. L. Hu, J. D. Li, J. J. Li, Y. J. Zhang, C. Wang and G. L. Cui, Energy Environ. Sci., 2019, 12, 1938–1949 RSC.
- M. F. Lagadec, R. Zahn and V. Wood, Nat. Energy, 2019, 4, 16–25 CrossRef CAS.
- M. S. Gonzalez, Q. Yan, J. Holoubek, Z. Wu, H. Zhou, N. Patterson, V. Petrova, H. Liu and P. Liu, Adv. Mater., 2020, 32, e1906836 CrossRef PubMed.
- J. Q. Huang, W. G. Chong and B. Zhang, J. Power Sources, 2024, 594, 234036 CrossRef CAS.
- Y. Kubo, Y. Hayashi and M. Ueta, Separator Paper for Alkaline Battery and the Alkaline Battery, US Pat., 0014080, 2006 Search PubMed.
- P. Arora and Z. J. Zhang, Chem. Rev., 2004, 104, 4419–4462 CrossRef CAS PubMed.
- P. Kritzer and J. A. Cook, J. Electrochem. Soc., 2007, 154, A481–A494 CrossRef CAS.
- H. Qin, W. Chen, W. Kuang, N. Hu, X. Zhang, H. Weng, H. Tang, D. Huang, J. Xu and H. He, Small, 2023, 19, e2300130 CrossRef PubMed.
- Y. Liu, S. Liu, X. Xie, Z. Li, P. Wang, B. Lu, S. Liang, Y. Tang and J. Zhou, InfoMat, 2022, 5, e12374 CrossRef.
- J. Cao, D. D. Zhang, C. Gu, X. Wang, S. M. Wang, X. Y. Zhang, J. Q. Qin and Z. S. Wu, Adv. Energy Mater., 2021, 11, 2101299 CrossRef CAS.
- Y. Qin, P. Liu, Q. Zhang, Q. Wang, D. Sun, Y. Tang, Y. Ren and H. Wang, Small, 2020, 16, e2003106 CrossRef PubMed.
- J. Li, Y. Chen, J. Guo, F. Wang, H. Liu and Y. Li, Adv. Funct. Mater., 2020, 30, 2004115 CrossRef CAS.
- W. Zhou, M. Chen, Q. Tian, J. Chen, X. Xu and C.-P. Wong, Energy Storage Mater., 2022, 44, 57–65 CrossRef.
- T. C. Liu, J. Hong, J. L. Wang, Y. Xu and Y. Wang, Energy Storage Mater., 2022, 45, 1074–1083 CrossRef.
- J. Cao, D. D. Zhang, X. Y. Zhang, M. Sawangphruk, J. Q. Qin and R. P. Liu, J. Mater. Chem. A, 2020, 8, 9331–9344 RSC.
- Y. C. Liang, Y. Y. Wang, H. W. Mi, L. N. Sun, D. T. Ma, H. W. Li, C. X. He and P. X. Zhang, Chem. Eng. J., 2021, 425, 131862 CrossRef CAS.
- M. Chen, J. Chen, W. Zhou, X. Han, Y. Yao and C. P. Wong, Adv. Mater., 2021, 33, e2007559 CrossRef PubMed.
- Y. Song, P. Ruan, C. Mao, Y. Chang, L. Wang, L. Dai, P. Zhou, B. Lu, J. Zhou and Z. He, Nano-Micro Lett., 2022, 14, 218 CrossRef CAS PubMed.
- D. Yang, X. Wu, L. He, Z. Sun, H. Zhao, M. Wang, Y. Wang and Y. Wei, Nano Lett., 2023, 23, 336–343 CrossRef CAS PubMed.
- J. Zhou, Z. Zhang, W. Jiang, S. J. Hou, K. Yang, Q. Li, L. M. Pan and J. Yang, J. Alloys Compd., 2023, 950, 169836 CrossRef CAS.
- G. Lin, X. Zhou, L. Liu, H. Li, D. Huang, J. Liu, J. Li and Z. Wei, RSC Adv., 2023, 13, 6453–6458 RSC.
- B. H. Wu, P. P. Wang, H. Y. Yang, Y. L. Liang, W. T. Ni, G. B. Xu, X. L. Wei and L. W. Yang, J. Power Sources, 2023, 580, 233323 CrossRef CAS.
- D. Huang, X. L. Zhou, L. M. Liu, H. M. Li, G. Lin, J. Li and Z. H. Wei, Colloids Surf., A, 2023, 663, 130991 CrossRef CAS.
- X. Zhang, J. Li, K. Qi, Y. Yang, D. Liu, T. Wang, S. Liang, B. Lu, Y. Zhu and J. Zhou, Adv. Mater., 2022, 34, e2205175 CrossRef PubMed.
- Y. An, Y. Tian, Q. Man, H. Shen, C. Liu, Y. Qian, S. Xiong, J. Feng and Y. Qian, ACS Nano, 2022, 16, 6755–6770 CrossRef CAS PubMed.
- Z. Wang, L. Dong, W. Huang, H. Jia, Q. Zhao, Y. Wang, B. Fei and F. Pan, Nano-Micro Lett., 2021, 13, 73 CrossRef CAS PubMed.
- C. Wei, L. Tan, Y. Zhang, S. Xiong and J. Feng, ChemPhysMater, 2022, 1, 252–263 CrossRef.
- X. Liu, F. Yang, W. Xu, Y. Zeng, J. He and X. Lu, Adv. Sci., 2020, 7, 2002173 CrossRef CAS PubMed.
- B. Karadeniz, A. J. Howarth, T. Stolar, T. Islamoglu, I. Dejanovic, M. Tireli, M. C. Wasson, S. Y. Moon, O. K. Farha, T. Friscic and K. Uzarevic, ACS Sustain. Chem. Eng., 2018, 6, 15841–15849 CrossRef CAS.
- T. J. Zhao, H. Y. Wu, X. H. Wen, J. Zhang, H. B. Tang, Y. J. Deng, S. J. Liao and X. L. Tian, Coord. Chem. Rev., 2022, 468, 214642 CrossRef CAS.
- W. L. Xin, J. Xiao, J. W. Li, L. Zhang, H. L. Peng, Z. C. Yan and Z. Q. Zhu, Energy Storage Mater., 2023, 56, 76–86 CrossRef.
- Y. Yang, H. M. Hua, Z. H. Lv, W. W. Meng, M. H. Zhang, H. Li, P. X. Lin, J. Yang, G. H. Chen, Y. H. Kang, Z. P. Wen, J. B. Zhao and C. C. Li, ACS Energy Lett., 2023, 8, 1959–1968 CrossRef CAS.
- X. M. Liu, F. G. Kong, Z. R. Wang, M. M. Ren, C. D. Qiao, W. L. Liu, J. S. Yao, C. B. Zhang and H. Zhao, Scr. Mater., 2023, 233, 115520 CrossRef CAS.
- X. P. Yang, W. L. Wu, Y. Z. Liu, Z. R. Lin and X. Q. Sun, Chem. Eng. J., 2022, 450, 137902 CrossRef CAS.
- H. Ma, J. Q. Yu, M. F. Chen, X. Han, J. Z. Chen, B. Liu and S. Q. Shi, Adv. Funct. Mater., 2023, 33, 2307384 CrossRef CAS.
- E. Lizundia, C. M. Costa, R. Alves and S. Lanceros-Méndez, Carbohydr. Polym. Technol. Appl., 2020, 1, 100001 Search PubMed.
- J. Cao, D. D. Zhang, C. Gu, X. Y. Zhang, M. Okhawilai, S. M. Wang, J. T. Han, J. Q. Qin and Y. H. Huang, Nano Energy, 2021, 89, 106322 CrossRef CAS.
- Y. Yang, T. Chen, B. X. Yu, M. K. Zhu, F. B. Meng, W. Shi, M. C. Zhang, Z. C. Qi, K. Y. Zeng and J. M. Xue, Chem. Eng. J., 2022, 433, 134077 CrossRef CAS.
- X. N. Zhang, S. C. Yang, Z. Huang, Z. Zeng, Y. Zhang and Z. H. Wang, Electrochim. Acta, 2022, 430, 141081 CrossRef CAS.
- X. S. Ge, W. H. Zhang, F. C. Song, B. Xie, J. D. Li, J. Z. Wang, X. J. Wang, J. W. Zhao and G. L. Cui, Adv. Funct. Mater., 2022, 32, 2200429 CrossRef CAS.
- Y. Zhang, X. Li, L. S. Fan, Y. Shuai and N. Q. Zhang, Cell Rep. Phys. Sci., 2022, 3, 100824 CrossRef CAS.
- P. H. Cao, H. C. Zhou, X. Y. Zhou, Q. Du, J. J. Tang and J. Yang, ACS Sustainable Chem. Eng., 2022, 10, 8350–8359 CrossRef CAS.
- H. Zhang, M. Y. Zhou, C. E. Lin and B. K. Zhu, RSC Adv., 2015, 5, 89848–89860 RSC.
- M. Guo, J. Xiong, X. Y. Jin, S. J. Lu, Y. F. Zhang, J. Xu and H. S. Fan, J. Membr. Sci., 2023, 675, 121533 CrossRef CAS.
- F. A. Zakil, S. K. Kamarudin and S. Basri, Renewable Sustainable Energy Rev., 2016, 65, 841–852 CrossRef CAS.
- I. Bauer, S. Thieme, J. Brückner, H. Althues and S. Kaskel, J. Power Sources, 2014, 251, 417–422 CrossRef CAS.
- J. T. Wang, P. F. Zhai, T. K. Zhao, M. J. Li, Z. H. Yang, H. Q. Zhang and J. J. Huang, Electrochim. Acta, 2019, 320, 134558 CrossRef CAS.
- M. Ghosh, V. Vijayakumar and S. Kurungot, Energy Technol., 2019, 7, 1900442 CrossRef.
- B. K. Wu, Y. Z. Wu, Z. D. Lu, J. S. Zhang, N. Han, Y. M. Wang, X. M. Li, M. Lin and L. Zeng, J. Mater. Chem. A, 2021, 9, 4734–4743 RSC.
- M. Ghosh, V. Vijayakumar, B. Anothumakkool and S. Kurungot, ACS Sustain. Chem. Eng., 2020, 8, 5040–5049 CrossRef CAS.
- I. V. Kolesnichenko, D. J. Arnot, M. B. Lim, G. G. Yadav, M. Nyce, J. Huang, S. Banerjee and T. N. Lambert, ACS Appl. Mater. Interfaces, 2020, 12, 50406–50417 CrossRef CAS PubMed.
- Y. Fang, X. Xie, B. Zhang, Y. Chai, B. Lu, M. Liu, J. Zhou and S. Liang, Adv. Funct. Mater., 2021, 32, 2109671 CrossRef.
- J. L. Hu, Y. D. Qu, F. W. Shi, J. K. Wang, X. He, S. Q. Liao and L. F. Duan, Adv. Funct. Mater., 2022, 32, 2209463 CrossRef CAS.
- Y. C. Liang, D. T. Ma, N. Zhao, Y. Y. Wang, M. Yang, J. B. Ruan, G. H. Yang, H. W. Mi, C. X. He and P. X. Zhang, Adv. Funct. Mater., 2022, 32, 2112936 CrossRef CAS.
- T. N. Nguyen, B. Iranpour, E. Cheng and J. D. W. Madden, Adv. Energy Mater., 2021, 12, 2103148 CrossRef.
- J. C. Huang, G. Yadav, D. E. Turney, J. S. Cho, M. Nyce, B. R. Wygant, T. N. Lambert and S. Banerjee, ACS Appl. Energy Mater., 2022, 5, 9952–9961 CrossRef CAS.
- Z. X. Liu, D. H. Wang, Z. J. Tang, G. J. Liang, Q. Yang, H. F. Li, L. T. Ma, F. N. Mo and C. Y. Zhi, Energy Storage Mater., 2019, 23, 636–645 CrossRef.
- R. A. Sharma, J. Electrochem. Soc., 2019, 135, 1875–1882 CrossRef.
- Y. M. Wang, J. Electrochem. Soc., 2019, 137, 2800–2803 CrossRef.
- A. Willfahrt, T. Fischer, S. Sahakalkan, R. Martinez, M. Krebes and E. Steiner, Flexible Printed Electron., 2018, 3, 045004 CrossRef CAS.
- X. Wang, S. Zheng, F. Zhou, J. Qin, X. Shi, S. Wang, C. Sun, X. Bao and Z. S. Wu, Natl. Sci. Rev., 2020, 7, 64–72 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |