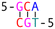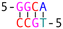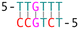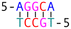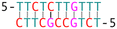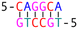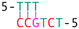DNA crossover flexibilities upon discrete spacers revealed by single-molecule FRET†
Xueqiao
Li‡
a,
Libang
Wang‡
b,
Wenna
Wu
a,
Huajie
Liu
 c,
Chunhua
Xu
*b and
Tao
Zhang
c,
Chunhua
Xu
*b and
Tao
Zhang
 *a
*a
aDepartment of Applied Chemistry, School of Chemistry and Chemical Engineering, Yantai University, Yantai 264006, China. E-mail: tao.zhang@ytu.edu.cn
bBeijing National Laboratory for Condensed Matter Physics and Laboratory of Soft Matter and Biological Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China. E-mail: xch@iphy.ac.cn
cSchool of Chemical Science and Engineering, Shanghai Research Institute for Intelligent Autonomous Systems, Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education, Tongji University, Shanghai, 200092, China
First published on 25th November 2024
Abstract
In this study, we utilized the origami technique to integrate various types of spacers into the double-stranded crossover and examined their flexibilities using single-molecule fluorescence resonance energy transfer (smFRET). We discovered that for the traditional Holliday Junction connection with zero-base spacers, the inter-structural angle measures 58.7 degrees, which aligns well with previous crystallographic research. When introducing non-complementary double-stranded spacers as a free leash, we observed that longer spacers resulted in a more relaxed connection. In contrast, when using complementary segments, the two origami structures rotated as the number of base pairs increased, reflecting the structural characteristics of the B-duplex. Our findings indicate that a stable intramolecular duplex requires a minimum of 5 base pairs. Overall, our results highlight the potential for re-engineering crossovers and designing materials that can change volume with shrink-swell capabilities, as well as applications in torque sensing using short DNA duplexes.
Introduction
The Holliday junction (HJ) serves as a key intermediate in processes such as meiosis, the repair of double-strand breaks, and gene transfer between different strains and species.1,2 Structurally, it consists of a branched DNA molecule featuring four single-stranded DNA segments that intertwine to create four double-helical arms around a central cross-connection. HJs are also very dynamic; the branch point can move on its own or be driven by enzymes, allowing the heteroduplex to lengthen or shorten. At any given branch point, HJs can switch between two different conformations. For example, in the presence of divalent metal ions like magnesium, the Holliday junction typically takes on a right-handed antiparallel configuration, achieving a balance between interhelical stacking and electrostatic repulsion.3–7 From another point of view, the transition from an open-X planar shape to a parallel X configuration connects two rigid DNA duplexes.Due to their helical structure, HJ positions can determine the cross-sectional angle at which new double helices attach. When multiple HJs are introduced between adjacent DNA duplexes, these strands can be packed together to build custom-shaped DNA nanostructures. This field of DNA nanotechnology, pioneered by Ned Seeman,8–11 has shown the potential of DNA as a programmable polymer for nanoconstruction.12 Various DNA nanostructures, including triangles, squares,13 cylinders,14 barrels,15 and hollow frameworks,16 have been created for templated assembly and applications in biophysics and biomedicine.17,18 Beyond serving as static structural motifs, the crossover connections also function as flexible joints for many DNA-based dynamic actuators.19–22 For example, DNA substructures can reconfigure around a single-stranded pivot point to adopt different conformations. However, these spacers are often designed with estimated lengths, leaving their actual flexibility unknown. Additionally, single-molecule manipulation has produced a force-torque phase diagram indicating that naturally stiff double-helical B-DNA exhibits linear torsional elasticity.23,24 By using a specific length of DNA duplex spacer between two rigid supporters, DNA torque devices can be developed to measure desired forces, particularly in biophysical interactions when gravitational effects are minimal. Using the DNA origami technique,25 we incorporated various types of spacers between two crossover connected DNA substructures and examined the dynamics of the crossover. With one DNA substructure fixed on a substrate as a stator and the other one designated as a flexible rotor, we found that, regardless of spacer type – whether complementary or non-complementary DNA strands – the rotor tended to maintain a stable inter-structural angle rather than exhibiting high levels of dynamism.
Results & discussion
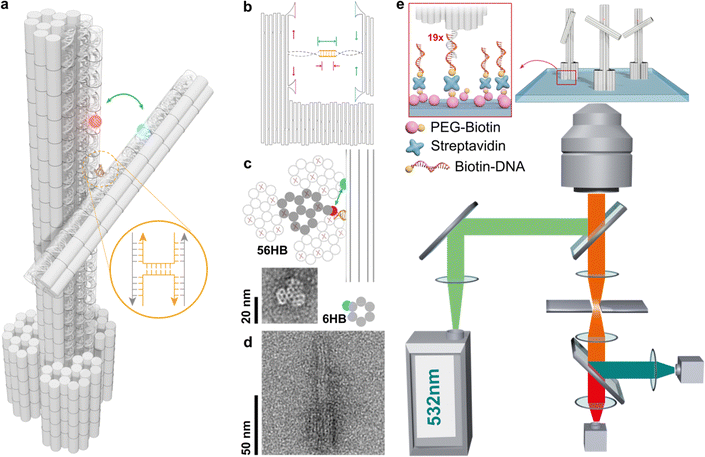
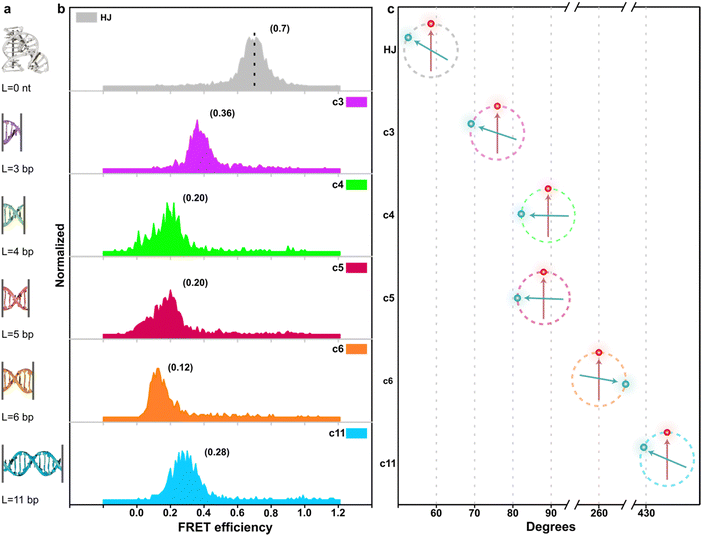
The structural design of the stator and rotor utilizes the DNA origami technique (see Fig. 1a and Fig. S1–S3, ESI†). This involves shaping a circular single-stranded phage DNA (scaffold DNA) and securing it with complementary staple strands. The crossover connections are made through short segments of the scaffold DNA, which are linked to the stator and rotor via T junctions. The length of the double-stranded spacer, whether self-complementary or non-complementary, can be modified by adjusting the position of the scaffold DNA relative to nearby reservoir loops (Fig. 1b). In a single reaction using one scaffold DNA, various objects with different types of spacers can be created using distinct subsets of staple strands. After purification through agarose gel electrophoresis, transmission electron microscopy revealed the cross-sectional honeycomb arrangement of the pedestal section of the 56-helix bundle (56HB) (Fig. 1c and Notes S1, S2 and Fig. S4, S5, ESI†). The well-formed rotor, consisting of a six-helix bundle (6HB), is connected to the upper 14HB of the stator in an X-shaped configuration. However, due to the air-dried negative staining of the specimens, the statistical TEM images showed a wide range of crossing or parallel arrangements rather than the intended angle distribution. Alternatively, cryo-electron microscopy (cryoEM) presents a complementary methodology for elucidating angle distributions. In particular, the sample preparation technique utilized in cryoEM, such as plunge freezing in liquid ethane,26 effectively reduces drying effects and allows single particle analysis. In this work, we rather employ a single-molecule fluorescence microscopy technique.The efficiency of energy transfer between donor and acceptor fluorophores, which is related to their distances apart, can be accurately measured using real-time single-molecule FRET (smFRET). The FRET distance obtained can then be converted into dihedral angles of two DNA bundles. For this study, we labeled cyanine 3 (Cy3) and cyanine 5 (Cy5) on the rotor and stator, respectively, which are positioned 16 and 14 base pairs away from the crossover. The stators were fixed in place by hybridizing extended sticky ends to biotinylated single-stranded DNA (ssDNA), which were attached to a glass slide through a biotin/avidin/biotin complex (as shown in Fig. 1e). The height of the stator is approximately 100 nm, while the rotor measures about 50 nm, with the crossover connection designed to be 73 nm from the bottom of the stator. By vertically aligning the stators, the impact of surface charges and steric effects was reduced, enabling a more unrestricted examination of the crossover dynamics.
Using an objective-based total-internal-reflection fluorescence microscope at room temperature, the samples were excited with a 532 nm laser, and the fluorescence signals were collected and separated using a 630 nm dichroic mirror, then detected by an EMCCD camera (see Note S3, ESI†). FRET efficiencies were calculated using the formula IA/(ID + IA), where IA and ID represent the fluorescence intensities of the donor and acceptor, respectively. In the absence of additional DNA bases in the crossover region, the 6HB rotor is connected to the 14HB stator through a natural Holliday junction, and the angle formed by the 6HB/14HB configuration reflects the natural anti-parallel stacked-X structure, with a measured FRET efficiency of 0.7. Using a Förster radius of R0 = 5.8 nm, the calculated angle using circular functions is 58.7°. This result is consistent with previous studies on Holliday junction configurations, including gel mobility, fluorescence resonance energy transfer, atomic force microscopy, and crystallography research (Table 1).27–29
Subsequently, we introduced various types of spacers (Table 1) in the crossover region and examined the crossover dynamics using smFRET. Initially, we utilized Mfold30 to generate a secondary structure map of p8634, which revealed multiple intramolecular duplex regions. A segment of 11 base pairs (bp) were identified for creating a series of complementary duplex spacers between 14HB and 6HB. In order to prevent the necessity of a completely new structure for each spacer, we kept some extra nucleotides in a nearby scaffold reservoir. This allows for the convenient modification of spacer lengths—whether increasing or decreasing—ensuring that all other staples within the structure remain unchanged. Although negatively-stained TEM images did not capture the subtle structural variations (Fig. S6, ESI†), the FRET efficiencies initially showed a decline followed by an increase as the length of the duplex spacer increased from 3 bp to 11 bp. In B-form DNA, the rotation angle is approximately 36 degrees per bp. For spacer lengths of 3 to 5 bp, the 6HB approaches an angle perpendicular to the 14HB, which does not accurately reflect the true rotation of B-DNA, indicating that these lengths are too short to form a stable duplex, even within an intramolecular context. At a spacer length of 6 bp, the calculated angles indicated that the substructure 6HB rotates counterclockwise to 260 degrees. An 11 bp spacer, which corresponds to one complete turn of B-DNA, resets the angle to the expected standard HJ configuration, but the FRET efficiency significantly decreased due to the increased distance between the rotor and stator (Fig. 2).
The phenomenon whereby complementary spacers yield a stable FRET signal is anticipated due to the formation of well-structured secondary structures within connected DNA bundles. In contrast, non-complementary spacers, which consist of dual linkers formed from two single-stranded DNA (ssDNA) molecules, should exhibit significant flexibility and a wide range of FRET efficiencies (Fig. 3). However, measurements of structures incorporating non-complementary spacers consistently reveal a sharp and stable peak, indicating an inflexible conformation, wherein the relative distances between the two dyes remain constant. DNA origami nanostructures, which are on the nanometer scale, are expected to undergo continuous small and random fluctuations, particularly in the case of dimeric structures connected by flexible linkers. Nevertheless, fluorescence measurements indicate that the FRET efficiency for a non-complementary 3-base spacer is 0.37, which surpasses that of designs utilizing complementary spacers. For non-complementary spacers exceeding 3 bases in length, the FRET efficiency gradually declines, a trend that contrasts with the behavior observed in complementary spacers. The inherent elasticity of ssDNA leads to contraction, thereby reducing its entropy. But direct calculations to ascertain the end-to-end distance are complex, as the persistence length of ssDNA does not accurately reflect the actual interspacing of the two bundles. Employing a modified freely-jointed chain (mFJC) model, the force-extension characteristics of non-complementary spacers can generate pulling forces of approximately 5 pN, which are doubled when two ssDNA tethers are involved.31–33 This contractile force maintains the rotor within a proximate radius, yet renders the end-to-end distance of ssDNA indeterminate. Furthermore, the entropic curling effect does not adequately compensate for the elongated spacers, resulting in a decreased FRET efficiency of 0.16 for 11-base tethers. Importantly, the FRET efficiency of the non-complementary 3-base spacer is greater than that of the asymmetric 3&6 base spacer, thereby demonstrating that two ssDNA strands of equal length can produce greater contraction forces than those generated by an asymmetric spacer design.
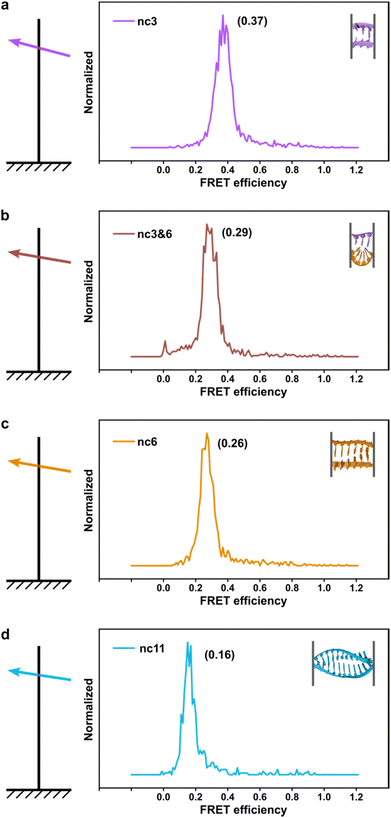 | ||
| Fig. 3 The fluorescence-resonance energy transfer (FRET) efficiencies of rotor and stator connected via non-complementary spacers. From a to d, it showed longer spacers leading to lower FRET signals. | ||
We believe that surface potential is another unmeasured factor at play. When ions condense on charged DNA origami structures, the coulombic repulsion between counterions can create alternating layers of positive and negative charges. As two bundles come within 10 nm of each other, we hypothesize that they adjust their configurations to complement one another, leading to a balance of repulsive and attractive forces that results in an interlocked pattern, which in our case manifests as a stable FRET signal. This aligns with previous findings that two origami blocks connected by an 8-nucleotide spacer tend to adopt a right-handed configuration.34 In the shape-complementary origami assembly, the bridging of multivalent ions also facilitates the formation of gigadalton superstructures35via short-range nucleobase stacking interactions. Furthermore, the attraction between like charges in the absence of multivalent counterions has been supported by counterion condensation theory.36
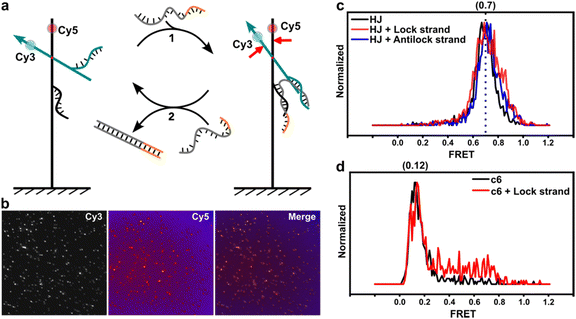
Moreover, we labeled two single-stranded DNAs (ssDNAs) as docking strands on opposite sides of the dye molecules to investigate potential sensing capabilities. The lock strand contains segments that can hybridize with the two corresponding docking strands (see Fig. 4a and b). This interaction brings the two substructures closer together, thereby increasing FRET efficiency. Initially, we examined a design with HJ connections, which demonstrated that the lock strand could enhance FRET efficiencies, with the full width at half maximum (FWHM) increasing from 0.15 to 0.25 (Fig. 4c). Antilock strands can displace the lock strand, returning the system to its previous relaxed state. We explored this effect using designs with complementary spacers. Compared to the relaxed state of the c6 spacer, Fig. 4d indicates that the lock strands partially activated FRET efficiencies to about 0.75, although the overall signal range was still dominated by lower efficiencies. Selected traces (Fig. S7, ESI†) revealed that binding of the lock strand induced blinking states between high and low FRET efficiencies, but the overall FRET profile suggests that the hybridization of the lock strand is influenced by the torque of the duplex DNA, particularly in shorter lengths.37,38
Conclusions
In summary, crossover connections are crucial for constructing both static DNA nanostructures and dynamic configurations. Using the DNA origami technique, we have “redesigned” these crossover connections by introducing various types of spacers between two origami-based structures, 6HB and 14HB. The spacers are formed through two single-stranded scaffold DNAs, ensuring high assembly efficiency. By concealing the scaffold reservoir within a nearby loop, different segments of the scaffold sequences can be shifted into the spacer region to achieve the desired design. Single-molecule FRET analysis revealed that the Holliday junction linking the 14HB and 6HB maintained an expected inter-structural angle of 58.7 degrees. For complementary spacers, we determined that a minimum of 6 bases is necessary for a stable intramolecular duplex, while an 11 base pair spacer can rotate the 6HB nearly a full circle. In contrast, non-complementary spacers act as entropic springs, with longer non-complementary spacers resulting in greater spacing and reduced FRET efficiency. Additionally, the binding of lock strands can improve the FRET efficiency, indicating the potential to create short DNA duplexes that function as torque devices. Moreover, rather than adding extra sequences at a single crossover, replacing each crossover connection with contractile dynamic units can adjust the inter-duplex spacing, leading to structures capable of volume changes through shrinkage and swelling. We believe that these innovations could have significant applications in drug delivery, biomedicine, and nanophotonics.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by the Taishan scholars’ program of Shandong province (tsqn201909083), China. H. L. thanks the National Science Foundation of China (22272119), the Science and Technology Committee of Shanghai Municipality (2022-4-ZD-03), the Shanghai Pilot Program for Basic Research, and the Fundamental Research Funds for the Central Universities. We thank Prof. Dr Ming Li for helpful discussions and insightful suggestions.References
- D. M. J. Lilley, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 9513–9515 CrossRef CAS PubMed.
- D. M. J. Lilley, Q. Rev. Biophys., 2000, 33, 109–159 CrossRef CAS PubMed.
- D. R. Duckett, A. I. H. Murchie, S. Diekmann, E. Von Kitzing, B. Kemper and D. M. J. Lilley, Cell, 1988, 55, 79–89 CrossRef CAS PubMed.
- S. A. McKinney, A.-C. Déclais, D. M. J. Lilley and T. Ha, Nat. Struct. Biol., 2003, 10, 93–97 CrossRef CAS.
- S. Hohng, R. Zhou, M. K. Nahas, J. Yu, K. Schulten, D. M. J. Lilley and T. Ha, Science, 2007, 318, 279–283 CrossRef CAS PubMed.
- P. C. Nickels, B. Wünsch, P. Holzmeister, W. Bae, L. M. Kneer, D. Grohmann, P. Tinnefeld and T. Liedl, Science, 2016, 354, 305–307 CrossRef CAS.
- A. Megalathan, K. M. Wijesinghe and S. Dhakal, ACS Sens., 2021, 6, 1367–1374 CrossRef CAS PubMed.
- N. R. Kallenbach, R.-I. Ma and N. C. Seeman, Nature, 1983, 305, 829–831 CrossRef CAS.
- A. Kimball, Q. Guo, M. Lu, R. P. Cunningham, N. R. Kallenbach, N. C. Seeman and T. D. Tullius, J. Biol. Chem., 1990, 265, 6544–6547 CrossRef CAS.
- S. Zhang, T. J. Fu and N. C. Seeman, Biochemistry, 1993, 32, 8062–8067 CrossRef CAS.
- T.-J. Fu, Y.-C. Tse-Dinh and N. C. Seeman, J. Mol. Biol., 1994, 236, 91–105 CrossRef CAS.
- Y. Hu and C. M. Niemeyer, Adv. Mater., 2019, 31, 1806294 CrossRef PubMed.
- P. W. K. Rothemund, Nature, 2006, 440, 297–302 CrossRef CAS PubMed.
- S. M. Douglas, H. Dietz, T. Liedl, B. Högberg, F. Graf and W. M. Shih, Nature, 2009, 459, 414–418 CrossRef CAS.
- S. F. J. Wickham, A. Auer, J. Min, N. Ponnuswamy, J. B. Woehrstein, F. Schueder, M. T. Strauss, J. Schnitzbauer, B. Nathwani, Z. Zhao, S. D. Perrault, J. Hahn, S. Lee, M. M. Bastings, S. W. Helmig, A. L. Kodal, P. Yin, R. Jungmann and W. M. Shih, Nat. Commun., 2020, 11, 5768 CrossRef CAS PubMed.
- H. Jun, X. Wang, M. F. Parsons, W. P. Bricker, T. John, S. Li, S. Jackson, W. Chiu and M. Bathe, Nucleic Acids Res., 2021, 49, 10265–10274 CrossRef CAS PubMed.
- D. J. Kauert, J. Madariaga-Marcos, M. Rutkauskas, A. Wulfken, I. Songailiene, T. Sinkunas, V. Siksnys and R. Seidel, Nat. Struct. Mol. Biol., 2023, 30, 1040–1047 CrossRef CAS.
- S. C. M. Reinhardt, L. A. Masullo, I. Baudrexel, P. R. Steen, R. Kowalewski, A. S. Eklund, S. Strauss, E. M. Unterauer, T. Schlichthaerle, M. T. Strauss, C. Klein and R. Jungmann, Nature, 2023, 617, 711–716 CrossRef CAS.
- E. M. Willner, Y. Kamada, Y. Suzuki, T. Emura, K. Hidaka, H. Dietz, H. Sugiyama and M. Endo, Angew. Chem., Int. Ed., 2017, 56, 15324–15328 CrossRef CAS PubMed.
- E. Kopperger, J. List, S. Madhira, F. Rothfischer, D. C. Lamb and F. C. Simmel, Science, 2018, 359, 296–301 CrossRef CAS.
- H. Ijäs, I. Hakaste, B. Shen, M. A. Kostiainen and V. Linko, ACS Nano, 2019, 13, 5959–5967 CrossRef.
- M. Vogt, M. Langecker, M. Gouder, E. Kopperger, F. Rothfischer, F. C. Simmel and J. List, Nat. Phys., 2023, 19, 741–751 Search PubMed.
- Z. Bryant, M. D. Stone, J. Gore, S. B. Smith, N. R. Cozzarelli and C. Bustamante, Nature, 2003, 424, 338–341 CrossRef CAS PubMed.
- F. C. Oberstrass, L. E. Fernandes and Z. Bryant, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6106–6111 CrossRef CAS.
- K. F. Wagenbauer, F. A. S. Engelhardt, E. Stahl, V. K. Hechtl, P. Stömmer, F. Seebacher, L. Meregalli, P. Ketterer, T. Gerling and H. Dietz, ChemBioChem, 2017, 18, 1873–1885 CrossRef CAS PubMed.
- T. G. Martin, T. A. M. Bharat, A. C. Joerger, X. Bai, F. Praetorius, A. R. Fersht, H. Dietz and S. H. W. Scheres, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E7456–E7463 CAS.
- M. Coll, M. Ortiz-Lombardía, A. González, R. Eritja, J. Aymamí and F. Azorín, Nat. Struct. Biol., 1999, 6, 913–917 CrossRef.
- C. Mao, W. Sun and N. C. Seeman, J. Am. Chem. Soc., 1999, 121, 5437–5443 CrossRef CAS.
- C. R. Simmons, T. MacCulloch, M. Krepl, M. Matthies, A. Buchberger, I. Crawford, J. Šponer, P. Šulc, N. Stephanopoulos and H. Yan, Nat. Commun., 2022, 13, 3112 CrossRef CAS.
- M. Zuker, Nucleic Acids Res., 2003, 31, 3406–3415 CrossRef CAS PubMed.
- S. B. Smith, Y. Cui and C. Bustamante, Science, 1996, 271, 795–799 CrossRef CAS.
- C. Bustamante, Z. Bryant and S. B. Smith, Nature, 2003, 421, 423–427 CrossRef.
- F. N. Gür, S. Kempter, F. Schueder, C. Sikeler, M. J. Urban, R. Jungmann, P. C. Nickels and T. Liedl, Adv. Mater., 2021, 33, 2101986 CrossRef.
- A. Kuzyk, R. Schreiber, H. Zhang, A. O. Govorov, T. Liedl and N. Liu, Nat. Mater., 2014, 13, 862–866 CrossRef CAS PubMed.
- K. F. Wagenbauer, C. Sigl and H. Dietz, Nature, 2017, 552, 78–83 CrossRef CAS.
- G. S. Manning, Eur. Phys. J. E: Soft Matter Biol. Phys., 2011, 34, 132 CrossRef PubMed.
- H. E. Gaub, M. Rief and H. Clausen-Schaumann, Nat. Struct. Biol., 1999, 6, 346–349 CrossRef.
- M. T. Woodside, W. M. Behnke-Parks, K. Larizadeh, K. Travers, D. Herschlag and S. M. Block, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6190–6195 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm01028k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |