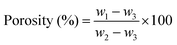Interfacing bioactive glass with silk fibroin: a soft matter approach to tunable mechanics and enhanced biocompatibility
Apipon
Methachittipan†
a,
Ayuth
Vejpongsa†
bcd,
Juthatip
Manissorn
bcj,
Duangruedee
Khwannimit
bcj,
Thanaphum
Wannalobon
 bcj,
Chayanon
Ngambenjawong
e,
Siriporn
Damrongsakkul
df,
Kittikhun
Wangkanont
bcj,
Chayanon
Ngambenjawong
e,
Siriporn
Damrongsakkul
df,
Kittikhun
Wangkanont
 g,
Khaow
Tonsomboon
h,
Chonlatep
Usaku
i and
Peerapat
Thongnuek
g,
Khaow
Tonsomboon
h,
Chonlatep
Usaku
i and
Peerapat
Thongnuek
 *bcj
*bcj
aInternational School of Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand
bBiomedical Engineering Program, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand. E-mail: peerapat.t@chula.ac.th
cBiomedical Engineering Research Center, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand
dCenter of Excellence in Biomaterial Engineering in Medical and Health, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand
eSchool of Biomolecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology (VISTEC), Wangchan Valley, Rayong 21210, Thailand
fDepartment of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand
gDepartment of Biochemistry, Faculty of Science, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand
hNational Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Klong Luang, Pathum Thani 12120, Thailand
iResearch Unit on Sustainable Algal Cultivation and Applications, Bio-Circular-Green-economy Technology & Engineering Center, BCGeTEC, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Phayathai Road, Patumwan, Bangkok 10330, Thailand
jBiomedical Materials and Devices for Revolutionary and Integrative Systems Engineering (BMD-RISE), Faculty of Engineering, Chulalongkorn University, Pathumwan, 10330, Thailand
First published on 28th April 2025
Abstract
Tissue-engineering scaffolds must balance mechanical compatibility with biological performance to support effective tissue regeneration. Bioactive glass (BG), valued for its strength and bone-bonding ability, often suffers from high stiffness, risking stress shielding. To address this limitation, we hybridized BG with silk fibroin (SF), a soft, biocompatible protein, to create (3-glycidyloxypropyl)trimethoxysilane (GPTMS)-crosslinked BG–SF scaffolds with tunable mechanics and enhanced cellular interactions. Fabricated via the sol–gel technique with varying BG-to-SF ratios, the scaffolds demonstrated increased porosity with higher SF content, positioning SF as a natural alternative to chemical porogens. Mechanical testing revealed that incorporating SF reduced BG stiffness, improved flexibility, and enhanced toughness, aligning the scaffold properties with those of native tissues. Fatigue testing confirmed greater durability in SF-enriched scaffolds, while degradation studies highlighted controllable rates conducive to tissue regeneration. Remarkably, as little as 10 wt% SF increased cell survival by 6.5-fold in biocompatibility assays. These findings underscore the synergy between BG and SF, presenting a soft matter strategy for designing scaffolds with customizable properties for tissue-engineering applications.
1. Introduction
Bioactive glass (BG) is a well-established material in bone tissue engineering and orthopaedic applications due to its ability to bond with bone and its structural strength.1,2 Beyond hard tissues, BG has also been explored for applications in soft tissues such as cartilage, blood vessels, cardiac tissues, skin, nerves, laryngeal, and lung tissues.3 However, the high stiffness of BG, characterized by its high Young's modulus, poses a challenge for mechanical compatibility with human tissues, often resulting in stress shielding.4 Addressing this limitation requires strategies to modulate the mechanical properties of BG, often by softening it, for effective integration with biological environments while preserving or enhancing its biocompatibility.Bone exemplifies a natural composite material that combines rigid ceramics and flexible polymers to achieve exceptional mechanical properties. Approximately 70% of bone mass is composed of hydroxyapatite, a calcium phosphate ceramic, while the remaining 30% consists of collagen fibres, a proteinaceous polymer.5 This hierarchical composite design utilizes the rigidity of hydroxyapatite and the energy-dissipating ductility of collagen to enhance bone toughness, flexibility, and fracture resistance. Inspired by this biomimetic synergy, developing BG-based scaffolds with finely tuned mechanical properties and composite-like behaviour offers a promising avenue for tissue-engineering applications.
Strategic combinations of BG with biocompatible polymers in a composite form offer a means to fine-tune BG mechanical properties for tissue engineering purposes. This is attributed to the superior energy dissipation capability of polymeric molecules compared to BG, thereby reducing BG brittleness. Moreover, the inclusion of polymers can lower the stiffness, since polymers deform more readily than glass. The polymer chains act as a matrix within the composite, facilitating greater deformability. There are many reports that combine BG with biopolymers such as chitosan, gelatin, and silk fibroin (SF).6–11 Amongst those biopolymers, SF is of interest in this work. SF is a well-studied polymeric biomaterial recognized for its biocompatibility, mechanical strength, and biodegradability.12,13 Despite these advantages, prior studies involving BG–SF composites have focused primarily on improving the biological response, with limited attention on customising mechanical properties such as stiffness, toughness, and fatigue resistance, which are critical for mimicking native bone properties and other potential applications.10,14–17 The lack of control over these mechanical parameters often results in scaffolds that are either too brittle or too rigid to effectively mimic the dynamic mechanical environment of native tissue. For instance, some BG–SF composites exhibit biocompatibility but fail to provide the flexibility needed to withstand repeated mechanical loads or adequately mimic the natural stiffness gradient of bone.16,17 Similarly, without proper modulation of toughness and fatigue resistance, these materials may not possess the long-term durability required for sustained implantation in load-bearing applications.
Therefore, a significant gap remains in developing BG–SF composites that not only promote a favourable biological response but also closely replicate the mechanical properties of tissues, ensuring both biological functionality and structural integrity in tissue engineering applications. This study thus seeks to elucidate how variation in the organic and inorganic proportion of the scaffold affects the mechanical properties and biocompatibility.
In this study, we hypothesized that integrating SF into BG scaffolds, mimicking the collagen-hydroxyapatite composite structure of bone, could provide a means to tune mechanical properties and enhance biocompatibility. To test this, we fabricated GPTMS-crosslinked BG–SF hybrids with varying BG to SF ratios. By systematically evaluating their stiffness, toughness, fatigue resistance, degradation behaviours, and in vitro biocompatibility, we demonstrated that SF incorporation not only enhanced the mechanical properties but also significantly improved cell viability, even at low concentrations. These findings offer insights into the design of hybrid composite scaffolds that synergize the properties of hard and soft matter, advancing their applicability in tissue-engineering applications.
2. Experimental
2.1 Materials
The silk cocoons, Bombyx mori Nangnoi Srisaket I, were generously provided by the Queen Sirikit Sericulture Center, Nakhon Ratchasima, Thailand. Commercial chemicals and equipment are provided in the methods section.2.2 Silk fibroin solution preparation
The SF solution was prepared according to the previously published procedure.11 In brief, silk cocoons were cleaned by removing the outermost layer and contaminated debris. The cocoons were subsequently degummed twice by boiling for 20 minutes in 20 mM Na2CO3 solution (Ajax Finechem, Australia) and then thoroughly washed with de-ionized (DI) water. The degummed fibres were air-dried. The dry silk fibres were dissolved for 4 hours in 9.3 M lithium bromide (LiBr; Sigma-Aldrich Laborchemikelien, Germany) at 60 °C. The solution was then dialysed (MWCO 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–16
000–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Viskase Company Inc, Japan) for 3 days against DI water. The water was changed regularly during the dialysis. After that, the resulting SF solution was transferred into centrifuge tubes for centrifugation for 20 minutes at 9000 rpm and 4 °C to remove impurities. The SF solution was stored in a 4 °C refrigerator.
000; Viskase Company Inc, Japan) for 3 days against DI water. The water was changed regularly during the dialysis. After that, the resulting SF solution was transferred into centrifuge tubes for centrifugation for 20 minutes at 9000 rpm and 4 °C to remove impurities. The SF solution was stored in a 4 °C refrigerator.
2.3 Bioactive glass–silk fibroin hybrid composite scaffold fabrication
Hybrid composite scaffolds were fabricated according to the previously published procedure.11 The SF solution was diluted to 4% w/v in DI water. The SF solution was mixed with (3-glycidyloxypropyl)trimethoxysilane (GPTMS; Gelest, Inc, USA). The GPTMS was used at 20 wt% of the SF dry weight. The mixture was then incubated for 6 hours at 37 °C. In parallel to the SF–GPTMS mixture, tetraethyl orthosilicate (TEOS; Sigma-Aldrich, China) was hydrolysed in 0.25 N HCl solution (J. T. Baker, NJ, USA). The TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl mole ratio was 1
HCl mole ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12. The solution was stirred for 1.5 hours. Next, CaCl2 (Ajax Finechem, Australia) was added to the TEOS solution with the Si
12. The solution was stirred for 1.5 hours. Next, CaCl2 (Ajax Finechem, Australia) was added to the TEOS solution with the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca mole ratio of 70
Ca mole ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The solution was stirred further for 5 minutes. The prepared SF–GPTMS solution was added to the TEOS sol, and the sol was stirred lightly for 5 minutes. Then, the sol was foamed for approximately 25 minutes using a foaming machine (iMix, Cat. No. 1610-555, Thailand). At this point, no liquid should be visible in the beaker. The foam was then transferred to silicone moulds. The moulds were covered with aluminium foil and left to age for 3 days at 60 °C. After that, they were dried further for one more day. For the control that had no SF addition, the TEOS sol was prepared as mentioned earlier and was foamed with 100 μL Teepol (Sigma-Aldrich, Singapore) as a surfactant. The scaffolds with varied ratios of BG and SF are listed in Table 1.
30. The solution was stirred further for 5 minutes. The prepared SF–GPTMS solution was added to the TEOS sol, and the sol was stirred lightly for 5 minutes. Then, the sol was foamed for approximately 25 minutes using a foaming machine (iMix, Cat. No. 1610-555, Thailand). At this point, no liquid should be visible in the beaker. The foam was then transferred to silicone moulds. The moulds were covered with aluminium foil and left to age for 3 days at 60 °C. After that, they were dried further for one more day. For the control that had no SF addition, the TEOS sol was prepared as mentioned earlier and was foamed with 100 μL Teepol (Sigma-Aldrich, Singapore) as a surfactant. The scaffolds with varied ratios of BG and SF are listed in Table 1.
| Nomenclature | Weight ratio | |
|---|---|---|
| BG | SF | |
| BG100 | 100 | 0 |
| BG95–SF5 | 95 | 5 |
| BG90–SF10 | 90 | 10 |
| BG85–SF15 | 85 | 15 |
| BG80–SF20 | 80 | 20 |
2.4 Morphological study by scanning electron microscopy
The scaffolds were cut into the dimensions of 5 × 5 × 5 mm3. The scaffolds were gold-coated by sputtering prior to the observation using an SEM microscope (JEOL JSM-6610LV, Oxford X-Max 50) with the voltage of 5 kV. The SEM images were given yellow pseudocolour to highlight SF deposition on the BG surfaces.2.5 Porosity measurement
The porosity of the scaffolds was analysed using n-hexane displacement.18 Each scaffold was submerged in n-hexane (Sigma-Aldrich, Singapore) until the scaffold fully absorbed the n-hexane. The scaffold was then retrieved, and the weight of n-hexane remaining after submersion was measured. The experiment was done in triplicate. The porosity was determined using the following equation:where w1 is the initial weight of n-hexane, w2 is the weight of the submerged scaffold and n-hexane, and w3 is the remaining weight of n-hexane after submersion.
2.6 Thermogravimetric analysis
The scaffolds were crushed with a mortar and pestle and then subjected to a thermogravimetric analysis (TGA Model 209 F3 Tarsus, Germany) in a nitrogen atmosphere with a heating rate of 20 °C minute−1. Organic matter, SF, will decompose when a certain temperature is reached. This was done to determine the actual ratio of BG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SF present in the scaffolds compared to their nominal composition. The results were normalized at 150 °C to account for different humidity in the scaffolds.
SF present in the scaffolds compared to their nominal composition. The results were normalized at 150 °C to account for different humidity in the scaffolds.
2.7 Compression test
Compression testing of dry scaffolds (∼15 × 15 × 15 mm3) was performed at room temperature using a universal testing machine (8872, INSTRON Instruments, UK) with the crosshead speed of 0.5 mm minute−1. The elastic moduli were determined from the slope of the initial linear regime of the stress–strain curve. The compressive strength was determined using the maximum stress of the elastic regime before reaching the plateau. The representative stress–strain curves are shown. Each type of scaffold was made of two samples that had been fabricated from different batches of preparation, and they were tested on different days.2.8 Fatigue failure analysis
The scaffolds were cut to the dimension of 10 × 10 × 10 mm3 and tested with a universal testing machine (8872, INSTRON Instruments, UK). The load used was half maximum stress obtained from the static compression test. The number of cycles needed to drastically disfigure or fracture the scaffold was recorded. The test was performed in triplicate.2.9 Degradation test
The scaffolds (5 × 5 × 5 mm3) were submerged in phosphate buffered saline (PBS; Bio Basic Inc., Japan) at 37 °C and pH 7.4. The scaffold degradation was observed by weight for 15 days. The PBS was replaced every 24 hours. On each day, the scaffolds were retrieved, dried, and weighed. The experiment was conducted in triplicate. The remaining weight of a scaffold after a period in PBS was calculated as follows:where Wf is dry weight of the scaffold after incubation, and Wi is dry weight of the scaffold before incubation.
2.10 In vitro biocompatibility test
The in vitro biocompatibility of the scaffolds was assessed using an indirect assay in accordance with ISO10993-5 using MTT tests on NIH3T3 mouse fibroblasts. The scaffolds were sterilised using the ethylene-oxide sterilization facility (Faculty of Medicine, Chulalongkorn University, Thailand) prior to the test. Each scaffold was cut into a cube (5 × 5 × 5 mm3) and washed for 10 minutes in PBS. The washing was done twice. The scaffold was then submerged for 24 hours in serum-free Dulbecco's modified Eagle's medium (DMEM; Hyclone, USA) at 37 °C to harvest the scaffold extract. The extract was subsequently used to replace FBS-supplemented media that had been in the cell culture for 24 hours. The final concentrations of the scaffold extracts were 100%, 50%, 25%, or 12.5% of the serum-free media. After the extract treatment, the cells were grown further for 24 hours, and the MTT assay was performed to examine cell viability.For the MTT assays, the fibroblast cells were grown in DMEM medium for 24 hours prior to the scaffold-extract treatment. The culture medium was replaced with the scaffold extracts. The medium was subsequently replaced with the MTT solution after 24 hours of treatment. After 1 hour, the MTT solution was removed and dimethylsulfoxide (DMSO; Sigma-Aldrich, Singapore) was added. The resulting solution was analysed using a microplate reader at wavelength 570 nm (BioTek Synergy H1, Agilent Technologies, Inc., USA). The in vitro biocompatibility test was performed in triplicate. The cell viability was calculated as follows:
2.11 Statistical analysis
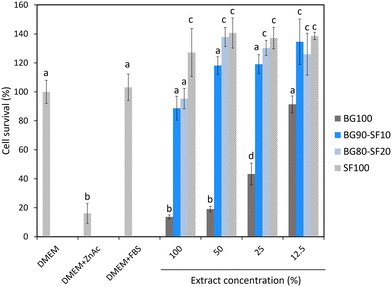
Comparisons of means between groups were performed using the non-parametric Kruskal–Wallis test, followed by pairwise Mann–Whitney U tests with Bonferroni correction. Statistical groupings indicated by lowercase letters (a, b, c, d, etc.) represent comparisons among all scaffold compositions. Different letters denote statistically significant differences at p < 0.05, while samples sharing the same letter are not significantly different. All graphs display mean values with error bars representing standard deviations.3. Results and discussion
3.1 Morphology of BG–SF hybrid composite scaffolds
Electron micrographs of the scaffolds revealed the scaffold morphology and porosity. BG100 had a smooth surface and relatively sharp edges, and small pores could be seen (Fig. 1A). The scaffolds that had been hybridised to SF had protein deposition on the glass surface (Fig. 1B–E). The amount of SF deposition, seen as rough patches, was more apparent when more SF had been introduced in the fabrication. For example, more SF deposition could be seen in BG80–SF20 than BG95–SF5.In addition, the scaffold porosity was greater when there was more SF content (Fig. 1F). This is due to the SF acting as a foaming agent during agitation.19 The porosity was increased from 30.62% in BG100 to 95.03% in BG80–SF20. It was also reported in other literatures that high porosity up to approximately 99% could be observed when SF was used in scaffold fabrication.14,20,21 Our BG100 scaffolds had low porosity probably because a minute volume of Teepol, as a foaming agent, was used. Also, the bubbles created by Teepol in the foam structure were not stable and tended to collapse before ageing into a scaffold. It was reported that scaffolds with higher porosity provide a more favourable environment for cell growth and proliferation.22 Adding SF that can increase the porosity of the hybrid composite scaffolds, rather than using a chemical surfactant, can thus reduce the concern over toxicity. This approach will be beneficial for tissue engineering applications.
While higher porosity is generally beneficial for scaffolds as it provides more space for cell attachment and proliferation,23 other published works showed that pore size, surface area, and pore interconnectivity are also important.24,25 Optimal pore size facilitates nutrient and oxygen diffusion, while interconnected pores enhance cell migration and vascularization. Additionally, a larger surface area increases the availability of attachment points for cells, promoting better cell adhesion and growth. Therefore, achieving a balance between these parameters is essential for designing scaffolds that support tissue regeneration effectively.
It is also important to note that scaffold porosity is not only relevant to biological performance but can significantly influence mechanical behaviour as well. The varying porosity observed across the compositions is expected to affect scaffold stiffness, strength, and deformation characteristics, which will be further examined in Section 3.3.
3.2 Thermogravimetric profiles
Thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) were used to determine the organic and inorganic composition in the hybrid composite scaffolds and the controls. The TGA results were normalized to account for the different moisture trapped in the scaffolds (Fig. 2A). The sudden weight loss at 250–400 °C was due to the decomposition temperature of SF, as evident in the SF100 control.26 When the SF content was increased, the weight loss was greater at this temperature range. As expected, BG100 did not show sudden weight loss. The constant weight loss during 500–800 °C was observed in all scaffold types. A study reported that the temperature rise during TGA is similar to sintering, and the heating can cause additional condensation of the Si–OH groups present in BG and GPTMS into Si–O–Si bonds, with water as a byproduct that evaporates away.27 At 800 °C, the remaining weights of the scaffolds showed the same trend with the amount of the SF used in fabrication. In fact, the more proteinaceous SF used, the smaller weight remained after the analysis. Notably, the remaining weight in the SF100 control might be attributed to the GPTMS crosslinkers, which would condense into an organosilane network. | ||
| Fig. 2 Analysis of inorganic–organic composition. (A) Representative TGA profiles of the scaffolds. (B) Derivatives of the TGA curves in (A). | ||
The DTG confirmed that there was unequal moisture in different scaffolds even when all specimens had been kept in a desiccator, and the extent to which the decomposition occurred depended on the amount of SF in the scaffolds. The DTG peaks around 50–150 °C, representative of water evaporation, increased with the BG content in the hybrid composite scaffolds. The peak was highest in the BG95–SF5 and lowest in the SF100 scaffolds. This is due to the hygroscopicity of the calcium silicate.28 However, we found that the BG100 did not absorb as much moisture as BG95–SF5 did. This could probably be explained by the much lower porosity of the BG100. The peaks at 250–400 °C showed SF thermal decomposition. The peak areas were greater in the scaffolds with more SF content. Noteworthily, the peak shifted to higher decomposition temperatures when there was more BG. The shift could be because the organic SF polypeptides were covalently crosslinked to the inorganic calcium silicate network via GPTMS crosslinkers. Also, we previously showed that the GPTMS crosslinking results in ethanol formation.11 The ethanol byproduct could induce the crystalline β-sheet formation in SF.29 The more crystalline structures in the hybrid composite scaffolds might increase the decomposition temperature, similar to other published works.30,31
3.3 Stress–strain profiles
The stress–strain profiles from the unconfined compression of the dry scaffolds revealed that the scaffolds could be distinguished into 3 groups according to Lorna J. Gibson and Michael F. Ashby:32 solid ceramic (BG100), elastic-brittle foams (BG95SF5 and BG90SF10) and elastomeric foams (BG85SF15, BG80SF20, and SF100) (Fig. 3). This classification was based on the characteristic shapes of the stress–strain curves as described in Gibson and Ashby's model for cellular solids.32 Elastic-brittle foams exhibit stress plateaus with stepwise drops due to micro-failures, while elastomeric foams show smooth, gradual stress–strain transitions indicating energy dissipation through bending and ductile deformation. The BG100 scaffold had an ultimate strength of 915.52 kPa and its strain at fracture was 0.082. The BG100 was also very stiff with the modulus of 10.75 MPa. These mechanical properties are unlike bone or other tissues in humans that are much more flexible. | ||
| Fig. 3 Representative stress–strain responses in unconfined compression tests. (A) Stress–strain profiles of the dry scaffolds. (B) Stress–strain profiles during the strain of 0–0.25 from (A). | ||
Adding SF dramatically reduced the stiffness and changed the mechanical profiles of the hybrid composite scaffolds. BG95–SF5 and BF90–SF10 had the ultimate strength of 70.43 and 39.88 kPa, respectively. Their stiffnesses were reduced to 684.44 and 398.15 kPa much closer to the range of biological tissues. The stress–strain profiles of these two scaffolds can be divided into 3 parts: elastic regime, plateau, and densification. In the plateau, the scaffolds repetitively exhibited small drops in stress, which can be explained by successive micro-failures and breakages of the small struts of the pore wall.33
The elastomeric foams including BG85SF15, BG80SF20, and SF100 had the ultimate strength of 34.48, 21.99, and 1.65 kPa, respectively. The stress–strain curves of this group smoothened out when compared to that of the elastic-brittle group. This suggests that the SF helps mitigate the strut failures and instead improves structure integrity throughout the compression, although being able to withstand smaller compressive load. Hence, adding SF to the hybrid composite scaffolds will make BG more mechanically comparable to biological tissues, and will improve the practicability for handling by physicians.
Furthermore, we analysed the scaffold toughness from the areas under the stress–strain curves. In agreement with composite theory, we found that the hybrid composite scaffolds had toughness greater than that of the BG100 scaffolds (Table 2). This can be explained using the composite theory. SF, being flexible and ductile polymer chains, can act as a toughening agent within the brittle BG matrix.34 When cracks propagate in the ceramic, the presence of SF can hinder their advancement by absorbing energy and redistributing stress. This mechanism is known as crack deflection, where the flexible polymer fibres deflect the crack path, preventing catastrophic failure. Additionally, SF can bridge across cracks, effectively holding them together and preventing further propagation. This mechanism, known as crack bridging, helps maintain the structural integrity of the composite material even in the presence of flaws or damage. Moreover, the interaction between SF and BG at the interface can lead to toughening mechanisms such as frictional sliding or interlocking. These interactions effectively dissipate energy and prevent crack propagation, further enhancing the toughness of the composite. It is worth noting that the toughness reduced when the SF fraction exceeded 10% by weight. This can be explained by the fact that polymers generally have lower stiffness and strength compared to ceramics. As the polymer fraction increases, the overall stiffness and strength of the composite decrease. While this may enhance the material's ductility and deformation capacity, it can also make the composite more susceptible to deformation and failure under applied loads, leading to lower toughness.
| Scaffolds | Young's modulus (kPa) | Ultimate strength (kPa) | Toughness (kJ m−3) |
|---|---|---|---|
| BG100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 747.56 747.56 |
912.61 | 42.42 |
| BG95–SF5 | 684.44 | 70.43 | 112.65 |
| BG90–SF10 | 398.15 | 39.88 | 179.41 |
| BG85–SF15 | 67.61 | 34.48 | 41.692 |
| BG80–SF20 | 81.84 | 21.99 | 52.62 |
| SF100 | 16.80 | 1.65 | 32.73 |
In the case of our pristine BG, it exhibited higher stiffness and strength and was more prone to brittle fracture, especially in porous forms. It was established that defects in ceramics and glasses such as pores or cracks can act as stress concentrators.35,36 The lack of ductility in these materials can result in rapid crack propagation and catastrophic failure under applied stress.
The mechanical behaviour observed can also be attributed, in part, to the porosity differences among the scaffold groups. As previously noted, increasing SF content led to higher porosity due to its foaming ability. Porosity is a critical factor influencing the mechanical response of scaffolds: higher porosity generally reduces stiffness and strength due to decreased solid fraction and increased void space.37 This aligns with our observation that scaffolds with more SF, and thus higher porosity, transitioned from stiff and brittle to more compliant and foam-like behaviours. The elastic-brittle scaffolds exhibited intermediate porosity and mechanical properties, whereas the elastomeric foams, with the highest porosity, displayed the most ductile and energy-absorbing behaviour.
While mechanical testing in the dry state is a common first-step characterization for comparing scaffold formulations, it does not fully replicate physiological conditions where the scaffolds would be hydrated. Upon hydration, SF, being a hydrophilic biopolymer, is expected to swell, potentially increasing the overall porosity and altering the load-bearing capabilities of the hybrid composite scaffolds. Swelling may lead to a decrease in stiffness and strength due to plasticization of the SF matrix. However, GPTMS-mediated crosslinking is anticipated to moderate excessive swelling by stabilizing the SF structure, as previously demonstrated in our earlier work.11 Additionally, the ceramic phase (BG) remains relatively unaffected by hydration, helping preserve a degree of mechanical rigidity. Future studies may include wet-state mechanical and swelling assessments to better approximate in vivo conditions.
3.4 Fatigue of the hybrid composite scaffolds
The scaffolds were subjected to cyclic compressive loads equal to half of the maximum stress experienced in the unconfined compression. The scaffolds are considered fractured when the load cell has moved more than 50% of its original height. The numbers of cycles used to fracture BG100, BG95–SF5, BG90–SF10, BG85–SF15 and BG80–SF20 was 240, 3303, 6230, 8046, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and 13
500, and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543 cycles, respectively (Fig. 4). After these numbers of cycles, the scaffolds were crushed into a dense pellet with crumbling pieces scattered around, with the exception of SF100. In general, stiff materials store energy and disperse it throughout the structure causing micro-damage, whereas compliant materials bend in response to the forces to mitigate the damages suffered.38,39 Therefore, including SF into the scaffolds will improve the long-term use of the scaffolds because biological tissues surrounding the scaffolds typically exert cyclic loads. In fact, several research groups documented the effects of cyclic physiological loading on the accumulation of microcracks.40–43 To further improve the fatigue life of bone scaffolds, the scaffold microstructure such as strut thickness and alignment may need further customization.44
543 cycles, respectively (Fig. 4). After these numbers of cycles, the scaffolds were crushed into a dense pellet with crumbling pieces scattered around, with the exception of SF100. In general, stiff materials store energy and disperse it throughout the structure causing micro-damage, whereas compliant materials bend in response to the forces to mitigate the damages suffered.38,39 Therefore, including SF into the scaffolds will improve the long-term use of the scaffolds because biological tissues surrounding the scaffolds typically exert cyclic loads. In fact, several research groups documented the effects of cyclic physiological loading on the accumulation of microcracks.40–43 To further improve the fatigue life of bone scaffolds, the scaffold microstructure such as strut thickness and alignment may need further customization.44
3.5 Degradation of the scaffolds
Scaffolds need to have a degradation rate that matches with the rate of specific tissue repair. The scaffolds exhibited different rates of degradation when submerged in PBS solution, meaning that the degradation rate could be customized (Fig. 5). The weight loss was marginal even when submerged for 15 days, marking the success of GPTMS crosslinking. We observed that the rate of degradation was in correlation with the SF content. In fact, the degradation rates were 0.10, 0.41, 0.46, 0.57, and 0.58 percent of the original weight per day for BG100, BG95–SF5, BG90–SF10, BG85–SF15, and BG80–SF20, respectively. This allows us to adjust the scaffold degradation rate using SF.SF dissolution and uncatalyzed hydrolysis may be a cause of the scaffold degradation. Another factor that affected the degradation rates could be the porosity, as the pores provide more surface area for dissolution and might affect the exposure of the scaffold matrix to degradation agents in the solution.
In other reports, BG scaffolds degrade slowly. It may take 15 days to degrade approximately 5% of their original weight.45,46 One strategy to increase the degradation rate is to incorporate boron into the silicate network.47 However, the boron derived from degradation caused cytotoxicity in vitro.48 This suggests that adjusting the BG scaffold degradation rate by hybridizing BG with a biological polymer such as SF can be a more biocompatible strategy.
Apart from being able to customize the degradation of the scaffolds, SF degradation was shown in other works to promote tissue repair such as osteogenesis and angiogenesis.11,13,49 The SF degradation products were also shown to modulate the transition from pro-inflammatory (M1) to anti-inflammatory (M2) macrophages.12 The M1-to-M2 transition would help resolve the inflammation, known to be an early stage of tissue repair, and proceed to the stages of tissue regeneration and remodelling.
3.6 In vitro biocompatibility
Biocompatibility is important for implantable scaffolds. We found that the biocompatibility of BG could be improved by combining with SF. In fact, BG100 scaffolds caused cytotoxicity to an extent similar to the zinc acetate control even when we washed the scaffolds thoroughly in PBS (Fig. 6). It was possible that residual Teepol used during foaming might be trapped in the porous structures of the scaffolds. The cytotoxicity of BG depended on the scaffold–extract concentration. For hybrid composite scaffolds, the cytotoxicity was diminished, as the cell survival was similar to or greater than that of the DMEM or DMEM with FBS control. The hybrid composite scaffolds were more biocompatible than the BG100 scaffolds possibly because SF supported cell growth and Teepol was not used during foaming.13 In the case of hybrid composite scaffolds, SF acted as a surfactant that stabilized the foam formation. This property of SF allowed us to avoid the use of Teepol that caused cell death. When comparing the 100% extracts of BG90–SF10 and BG100, we also found that only a small amount of SF (10%) could enhance the cell survival by 6.5 times. We believe that degradation products of SF, which are smaller peptides and amino acids, could interact with cells and might positively affect cell metabolism.NIH3T3 fibroblasts were used in this study as representative mesenchymal cells that are present in various tissues and organs. Given the aim of this study to develop a tunable scaffold system adaptable to different tissue types, fibroblasts provide a useful general model to assess cytocompatibility across potential applications. Moreover, NIH3T3 cells are known for their sensitivity to cytotoxic agents and are widely employed in standard ISO 10993-5 cytotoxicity tests. While not specific to bone tissue, they offer a reliable initial indication of scaffold biosafety. Future studies may expand upon these findings by employing direct cell-seeding and testing with bone-relevant cells such as osteoblasts or mesenchymal stem cells.
4. Conclusions
This study demonstrates that incorporating SF into BG scaffolds using GPTMS as a crosslinker provides a versatile approach to modulate their mechanical properties and compressive load response. The use of GPTMS as a crosslinker in the current study was based on our previously reported work,11 where FTIR analysis confirmed the formation of siloxane and organosilane bonds between BG and SF during the sol–gel process. While direct spectroscopic analysis was not repeated here, similar processing conditions and mechanical improvements support the successful formation of the hybrid network.The BG100 scaffolds exhibited high strength but lacked compliance, mirroring the properties of solid glass rather than biological tissues. In contrast, hybrid composite scaffolds such as BG95SF5 and BG90SF10 behaved like elastic-brittle foams, while BG85SF15 and BG80SF20 resembled elastomeric foams, achieving mechanical characteristics closer to native tissues. The addition of SF enabled precise tuning of porosity, stiffness, fatigue resistance, and degradation rate, all in a fraction-dependent manner, while significantly enhancing the biocompatibility of the scaffolds.
These findings underscore the potential of BG–SF hybrid composite scaffolds as a soft matter-inspired platform for designing biomaterials that bridge the mechanical and biological requirements of tissue engineering. However, further investigations are required to fully realize their clinical potential. Specifically, in vitro assessments should be complemented by in vivo studies to account for the dynamic and complex biological environments that influence scaffold performance. Future work should also explore the effects of pore size and interconnectivity on cellular behaviours and examine long-term degradation and mechanical stability in animal models. By addressing these aspects, BG–SF hybrids could be advanced as highly customizable and clinically relevant biomaterials for tissue regeneration.
Author contributions
AP – formal analysis, funding acquisition, resources, writing – original draft, AV – investigation, methodology, validation, writing – original draft, JM – investigation, methodology, validation, writing – review & editing, DK – investigation, methodology, TW – investigation, methodology, CN – writing – review & editing, SD – funding acquisition, supervision, writing – review & editing, KW – project administration, writing – review & editing, KT – writing – review & editing, CU – writing – review & editing, PT – conceptualization, formal analysis, funding acquisition, project administration, resources, supervision, validation, visualization, writing – original draft, writing – review & editing.Data availability
Data for this article are available at the Science Data Bank at https://doi.org/10.57760/sciencedb.19763.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Assoc. Prof. Sorada Kanokpanont, and Assoc. Prof. Juthamas Ratanavaraporn for sharing materials and tools required in this work. We are also grateful for their discussion. The project was supported by A New Researcher Grants sponsored by the Ministry of Science & Technology managed by the National Science and Technology Development Agency (JRA-CO-2563-13126-TH). The work was also supported by course budgets from the International School of Engineering, Faculty of Engineering, Chulalongkorn University. P. Thongnuek is supported by The Asahi Glass Foundation (RES_66_111_2100_011) and the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation (B38G670008). This project is funded by the National Research Council of Thailand (NRCT) and Chulalongkorn University (N42A670600). This research is also funded by the Thailand Science Research and Innovation Fund Chulalongkorn University (BCG_FF_68_294_2100_040). K. Tonsomboon and P. Thongnuek have received funding support from the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation (Grant number B05F640156). P. Thongnuek heartfully thanks Pimsen Thongnuek and Tubtim Chueabunmee for their support during the manuscript preparation.References
- J. R. Jones, Acta Biomater., 2013, 9, 4457–4486 CrossRef CAS PubMed.
- D. Greenspan, Biomed., Ther. Clin. Appl. Bioact. Glasses, 2019, 5, 178–184 Search PubMed.
- V. Miguez-Pacheco, L. L. Hench and A. R. Boccaccini, Acta Biomater., 2015, 13, 1–15 CrossRef CAS.
- O. Mahony, O. Tsigkou, C. Ionescu, C. Minelli, L. Ling, R. Hanly, M. E. Smith, M. M. Stevens and J. R. Jones, Adv. Funct. Mater., 2010, 20, 3835–3845 CrossRef CAS.
- J. Watkins, in Comprehensive Biomedical Physics, ed. A. B. T.-C. B. P. Brahme, Elsevier, Oxford, 2014, pp. 1–37 Search PubMed.
- M. Peter, N. S. Binulal, S. V. Nair, N. Selvamurugan, H. Tamura and R. Jayakumar, Chem. Eng. J., 2010, 158, 353–361 CrossRef CAS.
- K. Maji, S. Dasgupta, K. Pramanik and A. Bissoyi, Int. J. Biomater., 2016, 9825659 Search PubMed.
- J. Lao, X. Dieudonné, F. Fayon, V. Montouillout and E. Jallot, J. Mater. Chem. B, 2016, 4, 2486–2497 RSC.
- D. Nadeem, M. Kiamehr, X. Yang and B. Su, Mater. Sci. Eng., C, 2013, 33, 2669–2678 CrossRef CAS PubMed.
- H. Zhu, N. Liu, X. Feng and J. Chen, Mater. Sci. Eng., C, 2012, 32, 822–829 CrossRef CAS.
- J. Manissorn, P. Wattanachai, K. Tonsomboon, P. Bumroongsakulsawat, S. Damrongsakkul and P. Thongnuek, Mater. Today Commun., 2020, 101730 Search PubMed.
- J. Sapudom, M. Kongsema, A. Methachittipan, S. Damrongsakkul, S. Kanokpanont, J. C. M. Teo, M. Khongkow, K. Tonsomboon and P. Thongnuek, J. Mater. Chem. B, 2023, 11, 3607–3616 RSC.
- T. Watchararot, W. Prasongchean and P. Thongnuek, R. Soc. Open Sci., 2021, 8, 201618 CrossRef CAS PubMed.
- M. R. Bidgoli, I. Alemzadeh, E. Tamjid, M. Khafaji and M. Vossoughi, Mater. Sci. Eng., C, 2019, 103, 109688 CrossRef CAS.
- D. Li, G. Jiao, W. Zhang, X. Chen, R. Ning and C. Du, RSC Adv., 2016, 6, 19887–19896 RSC.
- X. Du, D. Wei, L. Huang, M. Zhu, Y. Zhang and Y. Zhu, Mater. Sci. Eng., C, 2019, 103, 109731 CrossRef CAS.
- R. B. Phetnin, S. Suksaweang, C. Giannasi, A. T. Brini, S. Niada, S. Srisuwan and S. T. Rattanachan, Int. J. Appl. Ceram. Technol., 2020, 17, 2779–2791 CrossRef CAS.
- U.-J. Kim, J. Park, H. J. Kim, M. Wada and D. L. Kaplan, Biomaterials, 2005, 26, 2775–2785 CrossRef CAS PubMed.
- R. Maxwell, M. C. Costache, A. Giarrosso, C. Bosques and S. Amin, Int. J. Cosmet. Sci., 2021, 43, 68–77 CrossRef CAS PubMed.
- Y. Hu, F. Zhang, W. Zhong, Y. Liu, Q. He, M. Yang, H. Chen, X. Xu, K. Bian, J. Xu, J. Li, Y. Shen and H. Zhang, J. Mater. Chem. B, 2019, 7, 7525–7539 RSC.
- L.-M. Yu, T. Liu, Y.-L. Ma, F. Zhang, Y.-C. Huang and Z.-H. Fan, Front. Bioeng. Biotechnol., 2020, 8, 578988 CrossRef.
- D. W. Hutmacher, T. B. F. Woodfield and P. D. Dalton, in Tissue Engineering, ed. C. A. Van Blitterswijk and J. de Boer, Academic Press, Oxford, 2nd edn, 2014, pp. 311–346 Search PubMed.
- J. Jiao, Q. Hong, D. Zhang, M. Wang, H. Tang, J. Yang, X. Qu and B. Yue, Front. Bioeng. Biotechnol., 2023, 11, 1117954 CrossRef.
- N. Abbasi, S. Hamlet, R. M. Love and N.-T. Nguyen, J. Sci. Adv. Mater. Devices, 2020, 5, 1–9 CrossRef.
- J. Manissorn, P. Wattanachai, K. Tonsomboon, P. Bumroongsakulsawat, S. Damrongsakkul and P. Thongnuek, Mater. Today Commun., 2020, 101730 Search PubMed.
- K. Kaewprasit, A. Promboon, S. Kanokpanont and S. Damrongsakkul, J. Biomed. Mater. Res., Part B, 2014, 102, 1639–1647 CrossRef.
- S. Lin, C. Ionescu, K. J. Pike, M. E. Smith and J. R. Jones, J. Mater. Chem., 2009, 19, 1276–1282 RSC.
- E. Kamseu, Z. N. M. Ngouloure, L. K. Tiogning-Djiogue, F. Andreola, B. Nait-Ali, S. Rossignol and C. Leonelli, J. Build. Eng., 2023, 67, 106020 CrossRef.
- K. Kaewprasit, T. Kobayashi and S. Damrongsakkul, Int. J. Biol. Macromol., 2018, 118, 1726–1735 CrossRef CAS PubMed.
- X. Hu, D. Kaplan and P. Cebe, Macromolecules, 2006, 39, 6161–6170 CrossRef CAS.
- P. Cebe, X. Hu, D. L. Kaplan, E. Zhuravlev, A. Wurm, D. Arbeiter and C. Schick, Sci. Rep., 2013, 3, 1130 CrossRef PubMed.
- L. J. Gibson and M. F. Ashby, Cellular Solids: Structure and Properties, Cambridge University Press, Cambridge, 2nd edn, 1997 Search PubMed.
- L. Řehořek, Z. Chlup, D. Meng, D. M. Yunos, A. R. Boccaccini and I. Dlouhý, Ceram. Int., 2013, 39, 8015–8020 CrossRef.
- R. Bai, Q. Sun, Y. He, L. Peng, Y. Zhang, L. Zhang, W. Lu, J. Deng, Z. Zhuang, T. Yu and Y. Wei, Front. Bioeng. Biotechnol., 2022, 10, 840372 CrossRef.
- E. Martin, D. Leguillon, O. Sevecek and R. Bermejo, Eng. Fract. Mech., 2018, 201, 167–175 CrossRef.
- T. Chen, B. Gong and C. Tang, Materials, 2023, 16, 5508 CrossRef CAS.
- I. J. Gibson and M. F. Ashby, Proc. R. Soc. London, Ser. A, 1997, 382, 43–59 Search PubMed.
- S. Suresh, Fatigue of Materials, Cambridge University Press, Cambridge, 2nd edn, 1998 Search PubMed.
- D. R. H. Jones and M. F. Ashby, Fatigue Failure, in Engineering Materials 1, ed. D. R. H. Jones and M. F. Ashby, Butterworth-Heinemann, 5th edn, 2019, ch. 18, pp. 283–299 Search PubMed.
- C. A. Pattin, W. E. Caler and D. R. Carter, J. Biomech., 1996, 29, 69–79 CrossRef CAS.
- S. Dendorfer, H. J. Maier, D. Taylor and J. Hammer, J. Biomech., 2008, 41, 636–641 CrossRef CAS.
- F. M. Lambers, A. R. Bouman, C. M. Rimnac and C. J. Hernandez, PLoS One, 2013, 8, e83662 CrossRef.
- M. G. Goff, F. M. Lambers, T. M. Nguyen, J. Sung, C. M. Rimnac and C. J. Hernandez, Bone, 2015, 79, 8–14 CrossRef CAS PubMed.
- A. Torres, A. Trikanad, C. Aubin, F. Lambers, M. Luna, C. Rimnac, P. Zavattieri and C. Hernandez, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 201905814 Search PubMed.
- M. N. Rahaman, D. E. Day, B. Sonny Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- A. M. Deliormanlı, Ceram. Int., 2013, 39, 8087–8095 CrossRef.
- Q. Fu, M. N. Rahaman, H. Fu and X. Liu, J. Biomed. Mater. Res., Part A, 2010, 95A, 164–171 CrossRef CAS PubMed.
- R. F. Brown, M. N. Rahaman, A. B. Dwilewicz, W. Huang, D. E. Day, Y. Li and B. S. Bal, J. Biomed. Mater. Res., Part A, 2009, 88A, 392–400 CrossRef CAS.
- S.-H. Park, E. S. Gil, H. Shi, H. J. Kim, K. Lee and D. L. Kaplan, Biomaterials, 2010, 31, 6162–6172 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |