 Open Access Article
Open Access ArticleStudies on poly(butylene succinate) and poly(butylene succinate-co-adipate)-based biodegradable plastics for sustainable flexible packaging and agricultural applications: a comprehensive review
Debarshi
Nath
ab,
Manjusri
Misra
 *ab,
Fadi
Al-Daoud
c and
Amar K.
Mohanty
*ab,
Fadi
Al-Daoud
c and
Amar K.
Mohanty
 *ab
*ab
aSchool of Engineering, University of Guelph, Thornbrough Building, 50 Stone Road East, Guelph, Ontario N1G 2W1, Canada. E-mail: mmisra@uoguelph.ca; mohanty@uoguelph.ca
bBioproducts Discovery and Development Centre, Department of Plant Agriculture, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G 2W1, Canada
cOntario Ministry of Agriculture, Food and Agribusiness, Harrow, Ontario N0R 1G0, Canada
First published on 12th February 2025
Abstract
Due to the increasing use of single-use plastics in daily life, plastic trash is expanding annually, destroying our ecology and producing an unparalleled waste disposal crisis. Bioplastics like poly(butylene succinate) (PBS) and poly(butylene succinate-co-adipate) (PBSA) can substitute certain non-biodegradable polymer materials and can effectively biodegrade under predefined environmental conditions. Both PBS and PBSA were traditionally synthesized from petroleum resources, but in recent years, PBS and PBSA have been reported to be produced from a hybrid of petroleum and renewable resources. PBS and PBSA polymers have good ductility and strength, but their high production costs and limited production volume limit their widespread packaging usage. Therefore, they are usually blended with other polymers and fillers to improve processability, mechanical properties, and biodegradability. Thus, recent polymer processing advances have made these blends/composites an appealing material platform for packaging and agricultural applications with composting compliance. Despite this, few studies have investigated the application of these polymers in real food packaging uses and in agricultural applications, thus highlighting a research gap. Nevertheless, PBS and PBSA-based commercial items are currently on the market, with examples including flexible packaging materials, compostable cutlery, and disposable tableware. Therefore, the purpose of this article is to provide an overview of research trends on PBS and PBSA, including the sustainability of their green synthesis routes using LCA studies, their biodegradability, applications in food packaging and agriculture, and end-of-life considerations. This study aligns with the United Nations' sustainability goal of responsible consumption and production (Sustainable Development Goal 12).
Sustainability spotlightPolybutylene succinate (PBS) and polybutylene succinate-co-adipate (PBSA) are home compostable plastics (at a specific thickness) which are used for flexible applications in food packaging and agricultural sectors. With the global ban on single-use plastics gaining momentum, PBS and PBSA usage is set to increase considerably. This review examines the current state of research on PBS and PBSA synthesis using environmentally friendly methods, emphasizing sustainability assessed by life-cycle assessment studies. Biodegradable blends and composites formed using these polymers using industrial manufacturing techniques, their applications and their end-of-life options post-usage are also discussed. These materials therefore can drive the circular economy forward promoting sustainable resource usage. This study aligns with the Sustainable Development Goal 12 (responsible consumption and production) presented by the United Nations. |
1 Introduction
Packaging is of utmost importance because it safeguards products from different environmental factors such as moisture, light, temperature, mechanical shocks, compressive forces, and vibrations during transportation and storage. Moreover, it is also used to convey information to the consumer, such as nutritional content, expiration date, and tamper identification.1,2 The packaging industry has grown to be one of the biggest industries in the world, not only in the food sector but across various industries as commercializing products without effective packaging solutions would not be feasible. For packaging applications, conventional petroleum-based non-biodegradable polymers such as polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), polystyrene (PS), etc. are widely used because of their wide availability at low prices along with superior tensile and tear strength, flexibility, barrier to moisture and oxygen, heat sealability and so on.3 However, the widespread use of such plastics has an adverse effect on the environment owing to the accumulation of plastic wastes in landfills and oceans and poses an End-of-Life (EoL) disposal challenge worldwide.4,5 To put matters into perspective, 5 trillion plastic bags are generated annually, and 1 million plastic bottles are bought every minute worldwide.6 Almost 36% of the total plastic manufactured is used for production in packaging sectors, which includes bags, containers, bottles, straws, cups, and grocery bags, among others.7 Each year, they produce more than 150 million tonnes of plastic trash that should either be recycled, landfilled, or burned. By the year 2050, the quantity of discarded plastics produced has been predicted to exceed 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 million tonnes.8
000 million tonnes.8
Significant strides have been made in recent years towards the recovery of energy and recycling of plastic wastes; however, the vast majority of these materials are still destined for landfills. In Canada, specific programs are underway to systematically collect and recycle plastic wastes used in agricultural practices. As of 2022, around 149 million units of plastic containers, 392![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units of drums/totes for pesticides and fertilizers, and 12
000 units of drums/totes for pesticides and fertilizers, and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 tonnes of agriculture films/baler twine plastics have been collected for recycling.9 Considering this, planning and implementing environmentally conscious methods to manage such an enormous plastic garbage crisis is of paramount importance. Therefore, the idea of biodegradable packaging materials has garnered popularity over the years, motivating researchers, investors, and concerned institutions to look toward biodegradable plastics that may be sustainable and reduce the harmful impact on the environment.
700 tonnes of agriculture films/baler twine plastics have been collected for recycling.9 Considering this, planning and implementing environmentally conscious methods to manage such an enormous plastic garbage crisis is of paramount importance. Therefore, the idea of biodegradable packaging materials has garnered popularity over the years, motivating researchers, investors, and concerned institutions to look toward biodegradable plastics that may be sustainable and reduce the harmful impact on the environment.
Aliphatic polyesters are a particularly intriguing group of polymers when developing biodegradable polymers for packaging applications because of their biodegradability and good mechanical properties. Aliphatic polyesters, which function as biodegradable structural materials can be categorized into two groups: poly(alkylene dicarboxylate)s, which are synthesised via a polycondensation reaction between diols and dicarboxylic acids, and poly(hydroxyalkanoates), which are polymers of hydroxy acids, HO–R–COOH, as repeating units. Hydroxy acids can again be divided into α-, β-, and ω-hydroxy acids, based on the position of the hydroxyl (OH) group with respect to the carboxyl (COOH) end group. All such structures are depicted in Table 1. Poly(alkylene dicarboxylate) is a class of biodegradable polyesters first pioneered by Showa Highpolymer under the trade name “Bionolle” in 1993. The company developed different grades of Bionolle, including poly(butylene succinate) (PBS) and poly(butylene succinate-co-adipate) (PBSA).10 In 2003, Mitsubishi Chemical Corporation (Japan) began producing PBS branded as GS Pla, that was derived from fossil fuels. In 2015, a bio-based (50% biobased content) PBS commercial production plant was established in Thailand under the brand name BioPBS, in collaboration with PTT Public Company Limited (Thailand).28
| Polymer chemical structure | Examples | Global producers |
|---|---|---|
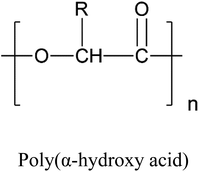
|
• Polyglycolic acid (PGA), R = H | PGA |
| • Polylactic acid (PLA), R = CH3 | • Kureha Corporation11 | |
| • BMG Inc.12 | ||
| PLA | ||
| • NatureWorks LLC13 | ||
| • Total Corbion14 | ||
| • Zhejiang Hisun Biomaterials Co. Ltd15 | ||
| • Futerro16 | ||
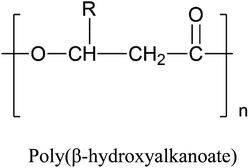
|
• Poly(hydroxybutyrate) (PHB), R = CH3 | • Danimer Scientific17 |
| • Poly(hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), R = CH3, C2H5 | • TianAn Biologic Materials18 | |
| • Yield10 Bioscience (formerly Metabolix, Inc.)19 | ||

|
Poly(ε-caprolactone) (PCL), X = 5 | • Perstorp UK Ltd (sold to Ingevity in 2019)20 |
| • BASF21 | ||

|
• Poly(butylene succinate) (PBS), X = 4, Y = 2 | PBS and PBSA |
| • Poly(butylene succinate-co-butylene adipate) (PBSA), X = 4, Y = 2, 4 | • Mitsubishi Chemical Group22 | |
| • Poly(butylene adipate-co-terephthalate) (PBAT) | • Xinjiang Blue Ridge Tunhe Sci. &Tech. Co., Ltd23 | |
| • Shangdong Life Chemical24,25 | ||
| PBAT | ||
| • Xinjiang Blue Ridge Tunhe Sci. &Tech. Co., Ltd23 | ||
| • BASF26 | ||
| • Jinhui Zhaolong Co. Ltd27 |
PBS and PBSA exhibit properties similar to commonly used packaging materials such as LDPE, HDPE, PP, etc.,29,30 with good elongation properties and a wide processing window, making them suitable for extrusion, cast and blown films, and injection molding.31,32 PBS and PBSA have much lower melting points compared to other bioplastics such as polylactic acid (PLA) and poly(hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). Moreover, properties like high ductility, high heat deflection temperature, and good degradation rate have increased interest in PBS and its copolymers among researchers and industries alike.28,33–35 Though PBS is more rigid and less flexible compared to PBSA, by controlling the molecular weight and copolymerization, the physical characteristics and biodegradation rate can be altered. PBS and its copolymers are biodegradable due to the presence of ester bonds in the soft chains, making them susceptible to hydrolysis.36,37 Presently, for synthesizing PBS and PBSA, more focus is being placed on the green development of their monomer units. They can be commercially synthesized from a variety of renewable resources, including biomass derived from starches, cellulose, and glycerol using fermentation techniques.28 Despite the increase in the popularity of PBS and its copolymers for different applications, its widespread use is hindered because of its high cost. To address this, various strategies are being explored, such as reducing the polymer production cost and adjusting the properties of the polymer to tailor its properties for specific applications. These approaches involve blending, copolymer synthesis, and incorporation of fillers to create composite materials.38 A timeline of the development of PBS and PBSA research is presented in Fig. 1.
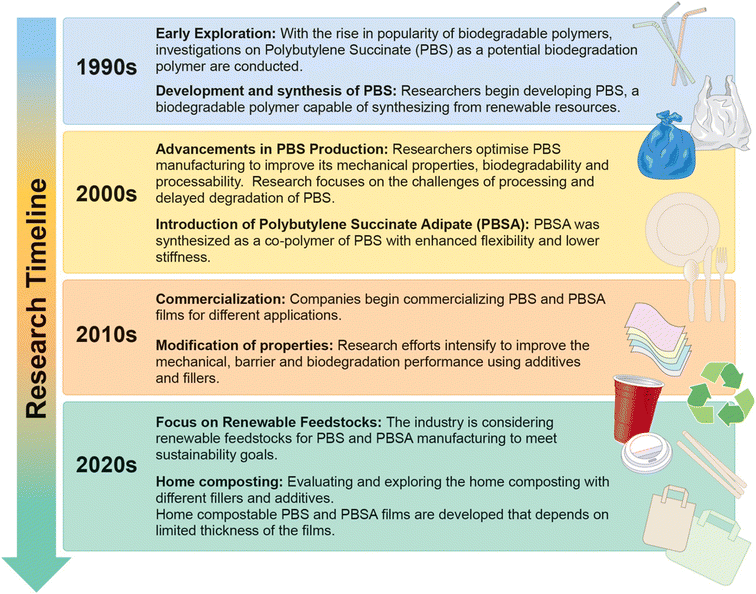 | ||
| Fig. 1 An infographic representation of PBS and PBSA research highlighting key milestones over time. The designer created the figure by incorporating ideas from the authors. | ||
This surge in interest in PBS and PBSA also extends into food packaging applications due to their eco-friendly and user-friendly attributes. Bioplastics used in food packaging are expected to safely enclose the food, protecting it from the external surroundings and preserving its quality. As such, for performing these functions, controlling and altering the polymeric properties with blends and fillers are done extensively.3 Furthermore, PBS and PBSA are often incorporated with nanomaterials or antibacterial ingredients (such as essential oil and plant extracts) to improve their functionality resulting in the shelf-life extension of food products.39–41 These composite materials can interact with the packaged product or release the ingredients into the headspace containing the food product, which can prevent its deterioration.39 For example, molecules like quercetin when incorporated into PBS resulted in the film having superb radical scavenging activity and good antimicrobial activity making them suitable for extending the shelf life of food products.42 Further research is necessary to determine the effect of bioplastics upon contact with food products. By replacing traditional wraps, films, labels, and laminates made of fossil fuels with biodegradable ones, we may make substantial progress in our battle against environmental pollution. Recently, with further advancements in technology, extensive research has been performed on functional polymeric materials for enhancing soil fertility, controlled delivery of agrochemicals and nutrients, and water management, among other applications.43 However, using bioplastics is only the first step towards sustainability; ensuring proper EoL options like recycling and composting facilities is equally important. Composting facilities allow bioplastics to break down naturally under the influence of microorganisms, temperature, etc.44 Effective recycling programs also ensure that bioplastics may be recycled or transformed into other beneficial goods, which preserves resources and minimizes waste. Thus, while the use of bioplastics is a step in the right direction, it is imperative to think about the EoL options for bioplastics that can ultimately reduce packaging waste and lower the carbon footprint. Therefore, it is not surprising to see a growing amount of research being performed around biodegradable food packaging films and their biodegradation behavior. The extent of biodegradation of PBS and PBSA-based blends and composites is studied across different environmental conditions such as soil, compost, and other settings. These studies help understand the biodegradation mechanisms of PBS and PBSA and how to further enhance their biodegradability by the addition of different fibers, polyesters, and additives. Moreover, the ongoing biodegradation studies will help us optimize the composting conditions effectively, which will lead to effective waste management strategies and the creation of environmentally friendly materials.
Extensive research has been conducted on PBS and PBSA-based blends and composites using the injection molding technique35,45–47 and they have been extensively reviewed; this review however specifically focuses on the PBS and PBSA-based films/sheets emphasizing their relevance in flexible packaging and agricultural applications. It addresses the biodegradability case studies and EoL options for these materials which have not been sufficiently summarized. This review offers a perspective on different petroleum and biobased synthesis techniques of PBS and PBSA. Detailed discussions on the production of blends and composite films using PBS and PBSA, as well as their applications in food packaging and controlled-environment agriculture are provided. In a later section, the biodegradability of PBS and PBSA-based blends and composites under industrial, home-composting and other conditions is also presented.
2 PBS and PBSA synthesis
2.1 Synthesis of PBS monomers
PBS is an aliphatic polyester consisting of 1,4-butanediol (BDO) and succinic acid (SA) as its monomer unit that is typically derived from fossil fuels. SA is produced using different methods such as carbonylation of ethylene glycol, oxidation of butane, and maleic anhydride (MA) catalytic hydrogenation.28,48 Similarly, BDO is also synthesized from petrochemical feedstocks by methyl maleate ester hydrogenation, which is derived from MA, reacting acetylene and formaldehyde, isomerizing propylene oxide, and oxidation of butane.28,48 These traditional methods are environmentally damaging and demand a lot of energy.Naturally, to develop sustainable biobased PBS and its co-polymer, the monomeric units should be obtained industrially through an eco-friendly method. Industrial manufacturing of bio-based monomers was made possible by recent advances in fermentation technology. SA is produced in large quantities from a diverse array of fermentable substrates, including corn starch, glycerol, wood hydrolysates, and cane molasses, by using specialized bacterial strains such as recombinant Escherichia coli, Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes, and Mannheimia succiniciproducens to name a few.49,50 The fermentation process used to synthesize SA is considered safe for the environment because it uses carbon dioxide (CO2) to transform the feedstock into the products. Reverdia uses recombinant yeast fermentation technology to produce biobased SA.
Likewise, a biobased method to synthesize BDO includes the fermentation of starch, sugars, glucose, etc., by using microorganisms to yield SA, followed by the catalytic reduction of SA to obtain BDO.51 Typically, BDO is produced via a three-step process: fermenting maize glucose to create benzoic acid, purifying the acid by electrodialysis, and then catalytically reducing the acid to BDO.31 Significant financial resources have been committed to the synthesis of BDO of biological origin. Novamont in 2016 launched the first direct pathway commercial facility for BDO production from renewable raw materials.28 Recently, genetically modified E. coli has been used to directly ferment BDO from xylose, glucose, sucrose, and other biomass-based mixed sugar streams.52 Also, Corynebacterium glutamicum, a genetically modified bacterium, has been tested for improved SA production efficiency.32,53 Early studies focused on microbes using glucose as the energy source, which sparked concerns about SA production's future in light of fossil fuel prices. Over the past decade, low-cost carbon feedstock resources and synthesis routes have been the main focus. Glycerol, a potential alternative carbon source, is a possible option due to its massive production as a byproduct of biofuels.28,54
Different organo-metal-based catalysts used for PBS polycondensation include titanium tetrabutoxide, Sc(NTf2)3, Sc(CF3SO)3, and titanium(IV) isopropoxide phosphate acid.32 Titanium-based catalysts are seen as a sustainable option for synthesizing PBS and PBSA due to their relative abundance in the earth's crust and low supply risk compared to other metal catalysts. Their inherent Lewis acidity and established biocompatibility make the catalysts desirable for sustainable polymerization catalysis. Additionally, they pose fewer challenges in terms of catalyst residue removal, enhancing their suitability for eco-friendly applications.56–58 Titanium-based catalysts offer a broad range of transformations, including α-olefin polymerizations, esterification, and transesterification.57
Titanium tetrabutoxide (TBT) is one of the most effective catalysts for the polycondensation reaction.59 For example, PBS was synthesized using BDO, 1,2-decanediol, and SA in a two-stage esterification and condensation polymerization process with TBT as a catalyst. Other catalysts used in synthesizing PBS include Sn, Zr, Bi, Hf, etc.59,60 Several catalysts such as distannoxane,61 lanthanide triflates,62 and titanium tetraisopropoxide63 were previously used to produce high molecular weight PBS. Han et al.64 introduced an easy method utilizing PBS oligomers with SA and BDO, resulting in the synthesis of a very high molecular weight PBS. Ring-opening polymerization of macrocyclic lactones with a poly(ethylene glycol) macroinitiator results in high molecular weight block copolymer production that cannot be achieved by traditional melt polycondensation of SA and BDO.65 Another technique to obtain high molecular weight PBS involves esterification at a low vacuum and low temperature followed by direct melt polycondensation in a high vacuum.66 Nevertheless, the mechanism behind the role of titanium-based catalysts is challenging due to their uncontrolled and quick exchange reactions with esters, alcohol and carboxylic acids. Additionally, the catalyst readily hydrolyses, resulting in a complex mixture of titanium dioxide, oxo-alkoxides, and oligomeric species.58 Titanium-based catalysts are susceptible to moisture, and water is the byproduct produced during the esterification process. Therefore, it is shown that adding the catalyst during the polycondensation stage increases the catalyst activity, but adding titanium-based catalysts during the esterification process lowers the activity of the catalyst. This is due to the instability of the catalyst during the esterification process in the presence of water (by-product).60,67
Sn-based catalysts are a versatile family of catalysts used in chemical and industrial applications and exist with common oxidation states being +2 and +4. Sn(II) catalysts are employed extensively in the industrial-scale polymerization of polyesters.68 Sn(Oct)2 is a common catalyst used for synthesizing polyesters like PBS and PLA due to its catalytic activity. For polymerizing lactide, Sn(Oct)2 when combined with a primary alcohol, accelerates the polymerization process and creates a stable alkyl ester end group, enhancing the efficiency and stability of the reaction.69 Moreover, it is also U.S. Food and Drug Administration (FDA) approved as a food additive.69 Labruyère et al.70 prepared high molecular weight PBS using Sn(Oct)2 of butyl succinate lactone. This lactone was derived from PBS oligomers using zinc oxide as a catalyst. Lee et al.65 used Sn(Oct)2 as a catalyst to develop high molecular weight PBS with some block co-polymers of PBS in the presence of a macroinitiator polyethylene glycol.
Industrial-scale production of biobased PBS is achieved through fermentation of renewable feedstock (like corn or sugar) by using suitable microorganisms to obtain bio-based SA. SA and BDO are then esterified to form PBS oligomers, followed by a polycondensation reaction to obtain biobased PBS. This process takes place at high temperatures, followed by cooling and purification to obtain the final product (Fig. 2). Expanding upon this foundational approach, more investigation has helped to improve the production of biobased PBS. Velmathi et al.61 synthesized high molecular weight PBS with a residence time of 20 min using microwave irradiation. High molecular weight polymers can also be developed using chain extenders, which cannot be achieved by conventional methods.32 Some of the most common chain extenders used in PBS synthesis are bisoxazoline,71 maleic acid,72 and hexamethylene diisocyanate.73 With a chain extender (hexamethylene diisocyanate), Showa Denko commercially manufactured high-molecular weight PBS (Bionolle) having an Mw of ∼300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 [Fig. 3(a)].38
000 g mol−1 [Fig. 3(a)].38
 | ||
| Fig. 3 (a) Synthesis of high molecular weight PBS using chain extension. Redrawn from ref. 38, MDPI, 2022 (Open Access); (b) synthesis of PBS using the CALB enzyme. Redrawn from ref. 74, MDPI, 2021 (Open Access). | ||
Enzymatic synthesis of PBS has been investigated as a potential substitute for the traditional method that uses toxic metal and metal-oxide catalysts.74Candida antarctica lipase B has recently been explored to synthesize PBS via enzymatic catalysis of BDO and diethyl succinate, and this method has decent yields and prospective industrial uses74,75 [as shown in Fig. 3(b)]. The most critical factor affecting PBS molecular weight is reaction temperature. Generally, the suitable reaction temperature is set below 100 °C for a reaction period of 24 h. Debuissy et al.76 proposed a method where PBS is obtained from BDO and diethyl succinate in the presence of Candida antarctica lipase B (CALB) as a catalyst at 90 °C and 24 h. Enzymatic ring-opening polymerization using CALB as the catalytic agent at temperatures less than 100 °C has also been used to produce cyclic butylene succinate oligomers.77 The results with CALB have shown higher yields via solution polymerization than melt polymerization, with molecular weights ranging from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 73
000 to 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.74,75 Additionally, increasing enzyme concentration enhances the reaction rate; however, excess enzymes have a detrimental effect on the molecular weight.78 The promising outcomes from the studies have demonstrated a greener production technique of the PBS polymer. Unfortunately, difficulties with enzyme leaching and inactivation, along with the use of solvents to prevent polymer precipitation and the low molecular weight of the PBS produced, hindered the development of this approach.79
000 g mol−1.74,75 Additionally, increasing enzyme concentration enhances the reaction rate; however, excess enzymes have a detrimental effect on the molecular weight.78 The promising outcomes from the studies have demonstrated a greener production technique of the PBS polymer. Unfortunately, difficulties with enzyme leaching and inactivation, along with the use of solvents to prevent polymer precipitation and the low molecular weight of the PBS produced, hindered the development of this approach.79
2.2 Synthesis of PBSA monomers
Apart from SA and BDO, adipic acid (AA) is required to be produced in large quantities to meet the demand for PBSA production.80 Commercial AA is generally produced from benzene (a non-renewable petrochemical-based resource), which is its primary feedstock. AA is manufactured through a two-step oxidation process where benzene is first reduced to cyclohexane, which is then oxidized to cyclohexanol and cyclohexanone.81 The mixture is then reacted with excess nitric acid to form AA under the influence of a catalyst (copper or vanadium) [Fig. 4(a)].81 Another method includes butadiene hydrocyanation, following which it is hydroisomerized to adiponitrile, which is then hydrolyzed.83 However, nitrous oxide is the major byproduct of oxidation reactions involving AA synthesis and emits N2, NO, NO2, and N2O into the atmosphere.82 The NOx emissions pose a serious threat to the environment and contribute significantly towards global warming and ozone layer depletion, and as such, several new routes to synthesize AA are being explored. Different petroleum, bio-based feedstocks and oxidants have been assessed in the development of novel NOx-free and more sustainable chemical pathways to AA.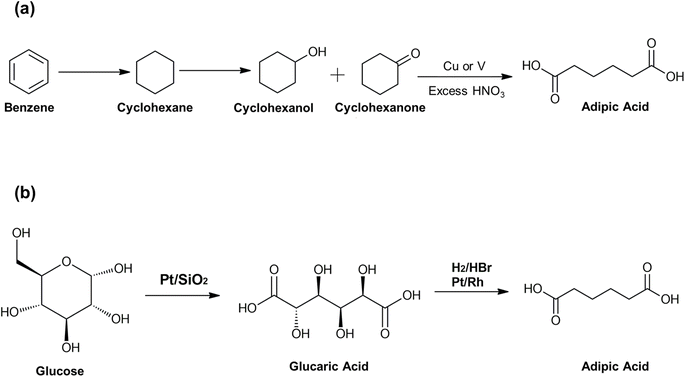 | ||
| Fig. 4 (a) Conventional method for adipic acid (AA) synthesis. Redrawn from ref. 81, MDPI, 2013 (Open Access); (b) a green approach for synthesizing AA. Redrawn from ref. 82, Royal Society of Chemistry, 2021. | ||
One of the sustainable approaches for synthesizing AA includes H2O2 and O2 as alternative oxidants. In recent years, AA has been produced by oxidizing cyclohexane in a one-step oxidation process using H2O2 and peroxy tungstate catalysts.84–86 Several studies have demonstrated higher AA yield with almost 99% purity.85–87 A method using O2 involves a two-step oxidation process in which cyclohexane is initially converted to cyclohexanone/cyclohexanol. The remaining cyclohexane is recycled, whereas the cyclohexanone/cyclohexanol mixture is used to synthesize AA by undergoing a second oxidation step in an acetic acid solvent in the presence of a metal catalyst.84,88 Along with alternative oxidants, phenol, adiponitrile, or butadiene are used to synthesize AA from feedstocks.82 Several routes for the bioproduction of AA are also considered nowadays due to the higher yield of AA by fermentation using engineered or selected microorganisms. These pathways typically generate AA directly from different carbon sources or by biosynthesizing a precursor that is then transformed into AA by conventional chemical catalysis. One approach is the reverse adipate pathway, which produces AA from succinyl CoA and acetyl CoA in Thermobifida fusca.89 An enhanced version of this route in E. coli has been developed using enzymes from many sources, including Clostridium acetobutylicum, Acinetobacter baylyi, Ralstonia eutropha, and Thermococcus gammatolerans.90 Another route for the biosynthesis of AA uses a combination of β-oxidation with ω-oxidation for degrading fatty acids via oxidation. E. coli and Candida tropicalis can be bioengineered to produce AA using glycerol and coconut oil, respectively, as carbon sources using this pathway.82 Another approach includes the synthesis of AA from malonyl-CoA and succinyl CoA intermediates.91
AA can be synthesized by using precursor intermediates like cis,cis-muconic acid, or glucaric acid, whereby a renewable feedstock is transformed chemically into the precursor intermediate through fermentation or another process, and the intermediate is then further processed chemically to become AA.82,91 For instance, glucaric acid is synthesized using a three-step bio-based pathway involving enzymes such as myo-inositol oxygenase synthase, myo-inositol-1-phosphate, and uronate dehydrogenase, where glucose is converted to glucaric acid using E. coli as the host.92 Additionally, biobased AA is produced directly from sugar via glucaric acid using a two-step oxidation–hydrodeoxygenation reaction with Pt/SiO2 and Ru10Pt2 nanoparticles as catalysts [Fig. 4(b)].82,84 For muconic acid synthesis, microorganisms, such as Pseudomonas putida, Klebsiella pneumoniae, and several Sphingobacterium spp., use it as an intermediary in their β-ketoadipate and amino-aromatic biosynthesis pathways.91,93 Various catalysts, such as activated carbon or platinum, can be used to chemically transform muconic acid into AA through hydrogenation.94
 | ||
| Fig. 5 Schematic diagram representing the synthesis of PBSA. Redrawn with permission from ref. 96, Elsevier, 1998. | ||
Titanium-based catalysts are the most effective organometallic catalysts for the transesterification reaction, as shown in the literature, as compared to Zr, Sn, Hf, and Sb-based catalysts.97 Titanium isopropoxide was used as a catalyst for the synthesis of PBSA from SA, BDO, and AA using a two-step procedure of esterification and deglycolization.98 In another study, titanium butoxide was used as a catalyst for the synthesis of PBSA with an average molecular weight (Mw) of 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 from a reaction mixture of dimethyl adipate, dimethyl succinate, and BDO.99 Tserki et al.,100 also used a chain extender, hexamethylene diisocyanate, in their study to obtain high molecular weight PBSA.
400 from a reaction mixture of dimethyl adipate, dimethyl succinate, and BDO.99 Tserki et al.,100 also used a chain extender, hexamethylene diisocyanate, in their study to obtain high molecular weight PBSA.
SnCl2 is a well-known catalyst for the synthesis of aliphatic polyesters, and it is typically used in the production of polyesters of high molecular weight. Compared to other Lewis acids, SnCl2 possesses several desirable qualities like high stability, easy handling, tolerance towards water, and reduced corrosivity.68 Cho et al.101 in their work studied the catalytic activity of SnCl2·H2O in esterification reactions of carboxylic acid with different alcohols. They reported that higher catalyst concentrations and reaction time increased the yield of esters. PBSA can be prepared from the direct polyesterification of its diols and dicarboxylic acid using SnCl2 as a catalyst. In comparing the effect of other catalysts like Na2CO3, p-toluene sulfonic acid, SnCl4, and Sn powder, including SnCl2, it was observed that Na2CO3 and SnCl4 exhibited poor catalysis behavior and a slow reaction rate while SnCl2 was the most effective catalyst.102 Like PBS, biobased PBSA is also synthesized using an enzyme to initiate the reaction. Metal catalysts used in polymer synthesis have been associated with pollution and toxicity problems. Biocatalysts like lipases can help mitigate this problem. Lipases are capable of catalyzing ester bond cleavage in an aqueous medium and can also catalyze ester bond formation in an organic medium. Therefore, by using the enzyme CALB, PBSA can be synthesized by reacting BDO with a dimethyl ester of AA and SA.103
2.3 Disadvantages of bioplastic production and life cycle analysis of biobased PBS and PBSA
The majority of bioplastics have been recognized for their contribution to sustainable living, but we have been looking at them through green-tinted lenses. For instance, brands like Pepsi have been praised for using 100% biobased PET which, while bio-based, is not biodegradable.104 The International Union of Pure and Applied Chemistry (IUPAC) has elucidated that a biobased polymer with attributes comparable to a petroleum-based polymer is not necessarily more environmentally friendly until Life Cycle Analysis (LCA) indicates otherwise.105 Despite the potential of plastics derived from biobased sources, limited research is conducted on different perspectives like acquisition of raw materials, land and water use, optimization procedures, etc.106 For instance, maize or corn are often used for the production of PBS, PBSA, PLA, and other bioplastics and the land allocated to produce the raw materials can cause food insecurity.107 This could lead to a dramatic increase in food prices due to the increased allocation of agricultural land to produce bioplastics and biofuels, disproportionately affecting economically disadvantaged segments of society. LCAs have demonstrated that there is a significant issue with the production of bioplastics vs. food, but according to research from the Institute for Bioplastics and Biocomposites in Hannover, bioplastics require just 0.02% of agricultural land.104 However the production of crops for bioplastics requires the use of chemical fertilizers and pesticides; the chemical processing required to convert biomaterials into bioplastics might also require harmful chemicals.106 Production of crops for bioplastics requires huge amounts of fresh water during cultivation. LCA studies of bio-based plastic production rarely study the water footprint analysis; however, some alarming results indicate that switching to bioplastics for packaging could increase water usage equivalent to nearly one-fifth of the EU's total freshwater withdrawal.108,109 Moreover traditional fossil fuel-based plastic manufacture has a lower impact on the ozone layer than bioplastic production.110Therefore, LCA studies are important to know whether the production process has a lower carbon footprint and is sustainable as claimed by the manufacturer. LCA is an essential method for identifying the potential environmental impacts of bioplastics throughout their entire life cycle. An LCA, also known as a cradle-to-grave study, evaluates the environmental effects of every step in the production process, from the acquisition of raw materials to their disposal.104 LCA studies on PBS and PBSA can tell us whether their production from bioresources is more sustainable than traditional synthesis methods. As mentioned earlier, monomers (SA, BDO, and AA) for PBS and PBSA are nowadays synthesized using biological sources but to ascertain their actual environmental impact, an in-depth LCA study of the production process needs to be carried out.
Bio-SA is a viable industrial substitute for its petroleum counterparts. Moussa et al.111 compared the environmental impact of SA production (biobased and petro-based) using LCA. This LCA's foreground data came from actual production data by an establishment in Louisiana, USA, that produces SA from non-food crop feedstock. Bio-SA exhibited significantly lower global warming potential and non-renewable cumulative energy demand by 385% and 1045%, respectively in comparison to petroleum-based SA. The authors noted that heat generation and electricity use had the highest impact on the environmental and energy efficiency of bio-SA. Gadkari et al.112 studied the LCA of bio-SA developed from bread waste and found that the greenhouse gas (GHG) emissions and non-renewable energy use (NREU) were significantly lower than those of petroleum-based systems. The result indicated that heating oil and steam are the major contributors to NREU and GHG emissions. Bello et al.113 reported that bio-SA from the paper and pulp industry had a lower carbon footprint compared to its fossil alternatives although it had a higher carbon footprint than SA derived from sorghum. LCA of bio-BDO also had a lower impact on the environment compared to fossil-derived BDO. Adom et al.114 in their study found that compared to fossil fuel-based BDO, the cradle-to-grave GHG emissions of bio-BDO made from sugars extracted from maize stover were around 52% lower. The techno-economic analysis of commercial bio-BDO from corn-based dextrose was conducted by Satam et al.115 The procedure was shown to be economically viable having a minimum selling price (MSP) of $1.82 per kg in comparison to the market price of $2.5 per kg.
Evaluating the sustainability of bio-based PBS production requires LCA and techno-economic risk assessment to compare its environmental impact with that of petrochemical-based PBS. The LCA study of PBS production obtained from food waste was conducted by Rajendran et al.116 The cost of BDO and solvent used during PBS purification contributed significantly to the overall economics of the process. The process resulted in a GHG emission equivalent to 5.19 kg CO2-eq. per kg of PBS which is lower than traditional PBS production while achieving an MSP of $3.5 per kg. Ioannidou et al.117 in their study assessed the production of cost-competitive PBS from sugar beet pulp, corn stover, and corn glucose syrup. They reported that acidification potential, human toxicity potential, and eutrophication potential were lower when the above raw materials were used; the MSP for PBS was $1.37 per kg. The techno-economic risk assessment suggests that PBS could be developed from bio-sources at market prices comparable to biaxially oriented polypropylene (BOPP) ($1.4 per kg) and general-purpose polystyrene (GPPS) ($1.72 per kg). Rebolledo-Leiva et al.118 found that wheat straw-based PBS has a lower environmental impact than fossil-based and GPPS. It's worth noting that the majority of the negative effects on the environment from monomer manufacturing occur during the bioconversion or pre-treatment, separation, and post-purification phases.
Sustainable PBSA production requires bio-based SA and BDO, along with an eco-friendly synthesis of AA. Integrating AA with a low carbon footprint is essential to develop PBSA in a sustainable manner. As such, Corona et al.119 developed biobased AA by using lignin only as the fuel and predicted a lower environmental impact for the bio-based route (∼62 to 78% lower) compared to conventional AA synthesis. Another study by Aryapratama et al.120 found that biobased AA had a lower impact on photochemical ozone creation, eutrophication, and global warming, than fossil-based synthesis but had a higher acidification impact. Also, developing biobased AA using acid-catalyzed pretreatment was more sustainable than alkaline (NABH4) pretreatment. Duuren et al.121 reported that CO2 equivalent emissions from AA can be reduced by switching to a biobased method. Currently, there is limited research on the lifecycle analysis of PBSA highlighting the need for more research and focus on studying the effects of PBSA synthesis on the environment. This will provide a comprehensive understanding of PBSA's ecological footprint and help us reduce the carbon footprint associated with bioplastic manufacturing in general by identifying the key contributors to pollution.
3 Developments in PBS and PBSA-based blend and composite films
3.1 Blends of the polyester family in sustainable packaging
The mechanical and barrier performances of a polymer are some of the most important criteria for evaluating the potential commercial viability in flexible packaging applications. With the use of polymer blends, it is possible to combine already-existing components to create new products with improved qualities. Polymer blending allows the mixing of different types of biopolymers to create novel compounds with enhanced mechanical strength, thermal stability, and barrier performance because of the selection of biopolymers with complementary properties and careful optimization of processing conditions.122 Polymers, when blended, can either exhibit miscibility or immiscibility at a molecular level. Melt-blending two miscible polymers forms a homogeneous single-phase material. In such instances, the blend demonstrates properties that fall between those of the individual constituents, like a single glass transition temperature (Tg) value between the Tg of the two original components.123 PBS and PBSA exhibit greater elongation but lower tensile strength and modulus compared to other bioplastics such as PHB, PHBV, PLA, etc. Therefore, the properties of PBS and PBSA can be optimized by blending with other bioplastics to achieve the desired performance.124,125Fig. 6 illustrates the tensile properties of different bioplastics and commodity plastics.28 Although thermodynamically, the majority of the polymers are immiscible and form a two-phase structure when blended. The final blend morphology during processing is determined by a dynamic interplay where competition occurs between the breakdown and coalescence of the constituents because of applied stress. The resultant morphology influences the blend properties, such as rheological and mechanical properties.123 | ||
| Fig. 6 The tensile properties of different biodegradable polyesters and non-biodegradable commodity plastics. Reproduced with permission from ref. 28, Elsevier, 2021. | ||
3.1.1.1 PBS/PBSA and PBAT blends. Aliphatic polyester resins exhibit good stability under typical settings and offer the properties of common polymers. The biodegradable polyester poly(butylene adipate-co-terephthalate) (PBAT) is an excellent polymer choice for PBS blends. It offers toughness and ductility and can be processed using conventional film extrusion techniques to produce films with mechanical properties like LDPE. PBAT has an inferior modulus and stiffness due to its poor crystallinity and random structure, unlike PBS, which is semi-crystalline and less ductile compared to PBAT.30 Therefore, a blend film of PBS and PBAT at 25, 50, and 75 wt% PBS concentration was developed by De Matos Costa et al.30 using compression molding. Due to the stiffer nature of PBS compared to PBAT, the stiffness of the films increased as the PBS percentage went up; also, a dramatic drop in elongation at break (EAB) was seen for films with PBS percentages higher than 25 wt%. However, the gas and moisture barrier properties of neat PBS films decreased with an increase in PBAT content. This is attributed to the higher crystallinity of PBS compared to PBAT. Thus, PBAT mixing can be used to modify the water vapor and gas barrier properties of PBS-based films. A similar trend was reported by Nobile et al.126 for PBS/PBAT films.
3.1.1.2 PBS/PBSA and PHA blends. The crystalline region in the polymers serves as a barrier that prevents the permeation of moisture and oxygen molecules, leading to enhanced barrier performance. The oxygen and moisture permeability of the films are closely related to the crystallinity of the polymer.127 As PHAs such as PHB and PHBV are highly crystalline polymers,128 they can be utilized in packaging materials requiring high barrier performance. Additionally, the rigidity of PHB and PHBV limits their use in flexible packaging applications. Hence, ductile polymers like PBS and PBSA are blended with PHAs to improve the flexibility of the films, blown into films using blown film extrusion while maintaining their high barrier performance. Therefore, Luoma et al.129 developed PBSA and PHB blend films using cast film extrusion. PHB was selected for incorporation in the blend due to its high barrier properties, but PHB has challenges in terms of processing and brittleness.129 The authors found that PHB enhanced the composite film's barrier performance, and flexible PBSA improved the film's processability and ductility. PBSA with 30 wt% PHB exhibited the perfect mixture of tensile properties with an improvement in tensile strength (TS) and modulus by ∼9% and ∼201%, respectively, compared to the neat PBSA film. PHB at 50 wt% reduced the oxygen permeability (OP) by 91% and 82%, respectively, which can be attributed to the increased film crystallinity. Similarly, PBS was used as a polymeric nucleating agent in the polypropylene carbonate (PPC)/PBSA/LOTADER-AX8900 blend film prepared using blown film extrusion to increase the crystallization temperature (Tc) and % crystallinity of the blends.130 The blends solidified at a higher temperature as a result of the higher Tc, which also decreased the plastic deformation of the molecular chain and significantly improved film-blowing stability. Due to this improved crystallinity, the mechanical properties and water vapor barrier properties of the films also increased. Thus, polymers like PHB and PHBV may be blended with PBS and PBSA to promote crystalline nucleation, which also improves mechanical strength, thermal stability, and barrier performance.
3.1.1.3 PBS/PBSA and PLA blends. Blending PBSA or PBS with a more rigid polymer improves bubble stability during film blowing and film mechanical strength. Therefore, a ternary blend of PBS, PBSA, and PLA was developed using blown film extrusion by Puekpoonpoal et al.131 to mitigate the drawback of PBS/PBSA blend films. The authors in this study reported that PBS and PBSA exhibited high miscibility, but the ternary blend introduced an immiscible phase into the matrix. A ternary mix with 10 wt% of PLA was able to achieve a satisfactory combination of stiffness and toughness, making it a promising solution for environmentally friendly flexible packaging applications. Similarly, in another study by Pivsa-Art et al.,132 blending PLA with PBSA led to improved TS, EAB, and impact properties of blown films. Due to the improved properties of PBS and PBSA films when blended with PLA, a significant amount of research has been done to use PBS and PBSA to increase the crystallization, strength, and toughness of PLA. Similarly, Yokohara and co-workers133 in their study reported the immiscibility between PBS and PLA in a compression molded film, but the addition of PBS resulted in accelerated PLA crystallization since PBS droplets serve as crystallization nuclei for PLA. However, they are immiscible; thus, the preparation and inclusion of the compatibilizer have a big impact on their properties.134
The majority of these biodegradable polymers exhibit incompatibility and immiscibility when subjected to melt blending and form a two-phase structure. The phase separation inherent in PLA/PBS mixes is a key reason why their mechanical property improvements are limited. High miscibility improves mechanical, thermal, and barrier qualities compared to immiscible blends. Understanding and controlling the miscibility of polymer blends is essential for tailoring their properties to meet specific application requirements, ranging from packaging materials to biomedical devices. Deng et al.135 evaluated the miscibility of PLA and PBS and found them to be immiscible, which affected the mechanical properties [Fig. 7(A)]. The authors reported that though PLA and PBS are immiscible, the inclusion of PBS may alter the PLA structure in PBS/PLA blends by altering the proportion of amorphous and crystalline phases. Similarly, Ostrowska et al.136 prepared PBS/PLA injection molded composites, which can be later studied for blown film application [Fig. 7(B)]. Improvement in the miscibility between biopolymer blend films can be obtained by adding suitable compatibilizers to make the polymers miscible. This facilitates efficient mixing of the polymers, leading to the development of homogeneous biodegradable films and subsequently improving the performance compared to non-compatibilized blends.
 | ||
| Fig. 7 (A) Optical micrographs of PBS/PLA blends. The poor miscibility of PBS/PLA is evident from the figure with distinct phase separation. Whenever the PBS content reaches 60%, PLA is distributed in a droplet form of different sizes, while PBS is present in a co-continuous phase in the matrix. This demonstrates that there is poor adhesion between the two polymers. Even after poor miscibility, PBS/PLA blending at 40/60 results in a balanced combination of EAB and TS. Reproduced with permission from ref. 135, Elsevier 2015; (B) similarly, PLA/PBS blends have intermediate mechanical characteristics compared to their neat polymer counterparts. This is the main reason behind the blending of a ductile polymer with a rigid one to leverage the distinct characteristics of individual polymers. Reproduced with permission from ref. 136 Wiley 2019. | ||
Reactive extrusion is a simple yet effective process that enhances the interfacial interaction between the two phases in coupled blends. By promoting chemical reactions during melt blending, this technique achieves improved dispersion and a more uniform phase morphology, resulting in enhanced material performance.122,123 The linear structure of most reactive compatibilizers, such as MA, isocyanate, and peroxide groups, allows them to form strong chemical bonds between the blended polymer.140 Polymers grafted with reactive chemicals, like MA, are a type of compatibilizing agent where the grafted materials function as compatibilizers by reacting with one component of the blend through their backbone and with the other component through their grafted reactive group. Therefore, Phetwarotai et al.140 used MA-grafted PLA (MA-g-PLA) and toluene diisocyanate (TDI) as compatibilizers while tricresyl phosphate and triethyl citrate acted as plasticizers to compare the effect of different compatibilizers and plasticizers on the properties of compression molded PLA/PBS blend films. As TDI content increased, the compatibility between PLA and PBS increased while affecting the films toughness. The blending of PLA/PBS with both MA-g-PLA and TDI resulted in an accelerated cold crystallization rate and an increased % crystallinity. The combination of the plasticizer and compatibilizer significantly enhanced the EAB and tensile-impact toughness of PLA/PBS blends relative to pristine PLA. This combination resulted in a transition from brittle to ductile failure mode due to the enhanced miscibility and increased molecular segment mobility between the PBS and PLA phases. Similarly, a blend of PBS and PPC was compatibilized with MA with dicumyl peroxide (DCP) as the initiator. For this study, MA-g-PPC was developed and added to PBS/PPC blends to improve their miscibility which was evident from the increase in mechanical properties of the blends compared to their uncompatibilized counterparts.141 In another study, Ma et al.142 developed PHB/PBS and PHBV/PBS blends using DCP and found that the miscibility between the blends improved which led to significant increment in mechanical performance.
Despite the extensive literature available on the properties of the PLA/PBSA blend with and without compatibilizers, there has been relatively limited exploration of blown films using the same blends. This is significant, considering that blown film extrusion is a highly productive, cost-effective, and widely used process for producing packaging films. Palai et al.143 developed a blown film by blending PBSA with PLA with chain extender epoxy styrene acrylate (ESA) under the trade name of Joncryl for flexible packaging applications. Joncryl, as a chain extender with multiple epoxy groups in its chemical structure has been widely employed to enhance the compatibility and interaction between immiscible blends and composites.144 The PLA/PBSA blend showed reduced TS and modulus compared to the neat PLA film. The authors reported an improved interaction between the polymers due to their interaction with the ESA, resulting in enhanced mechanical properties. Films having 95% PLA/5% PBSA with 3% ESA showed the highest improvement of modulus (26.17%) and TS (29.7%) compared to neat PLA films. This phenomenon may be explained by the interaction of the chain extender, which improves the interfacial adhesion between the matrix and the dispersed phase. Moreover, the oxygen transmission rate (OTR) and water vapor transmission rate (WVTR) of the blend films were also lower than those of the neat PLA film by approximately 60% and 14%, respectively.143
3.2 Role of fillers and fibers in sustainable packaging
The addition of fillers to biopolymer matrices has been identified as a possible method for overcoming the inherent drawbacks of biopolymers and enhancing their capabilities, allowing for a wider range of industrial applications. During the development of composites, organic, inorganic, and metallic particle fillers in the nano- or micro-size range are introduced to reinforce the polymer.150 Filler materials can be used selectively to improve gas barrier qualities, increase stiffness, and decrease material costs.151 The effectiveness of filler-reinforced materials is influenced by several factors, including particle size and shape, particle dispersion, filler structure, surface area, surface reactivity, and matrix-filler bonding properties.150 Different types of fillers used in polymer composite formation include natural fillers and inorganic fillers.Additionally, at lower cellulose loadings, the crystallinity of the films decreased due to the disruption in the polymer molecular chain while the reverse was true at higher cellulose concentration because of the enhanced interaction between the many hydroxyl groups of cellulose and the polymers through hydrogen bonding. Fibers are widely used in the formation of polymer composites; however, they do not mix properly with polyesters, which calls for a compatibilizer to wet the fibers. Moreover, a significant issue in the processing of natural fiber-based composite films is the increase in melt viscosity, which hinders proper fiber dispersion and consequently reduces the overall performance of the films. Su and Wu155 compared the properties of PBSA/bamboo fiber (BF) and acrylic acid-grafted PBSA (AA-g-PBSA)/BF composite sheets prepared using a hot press and found that uniform dispersion of BF within the AA-g-PBSA matrix was achieved. This was due to the formation of ester bonds and the subsequent development of interconnected and branched macromolecules between the carboxyl groups of AA-g-PBSA and the hydroxyl groups in BF. As expected, the composites with AA-g-PBSA had lower melt viscosity due to the development of ester carbonyl groups resulting in the conformational changes in the molecules of BF. However, an alternative approach for enhancing the interaction between the matrix and fibers/fillers can be achieved through their surface modification.156 Ramie fiber was treated with dopamine hydrochloride to induce transcrystallization on its treated surface which considerably enhanced the nucleation ability of dopamine in PBS crystallization.157 Because of this transcrystallization, the interfacial shear strength between the fiber and the PBS increased. Furthermore, the TS of the compression-molded film samples increased by 30%, providing further confirmation that the improvement in interfacial properties was indeed a result of interfacial crystallization. Similarly, treating cellulose-loaded composites with silane coupling agents is a common chemical coupling technique for enhancing the interfacial adhesion of polymers (Fig. 8).158 In a study by Calabia et al.,158 PBS/cotton fiber composites with a silane coupling agent were prepared using compression molding, and its effect was studied. Upon silane treatment of fibers, the TS of the composites increased considerably compared to the untreated composites. Moreover, the thermal stability of the composite films slightly increased upon silane treatment. A weak interaction between the matrix and filler was observed from SEM images, while silane-treated composites exhibit reduced gaps and improved compatibility, indicating enhanced interfacial adhesion.
 | ||
| Fig. 8 (A) Schematic representation of the fabrication of silane-treated PBS/cotton fiber composites; (B) morphology of untreated and silane-treated composites and (C) mechanical properties of PBS/cotton fiber composites with and without silane treatment. Reproduced from ref. 158, MDPI, 2013, Open Access. | ||
While isocyanates are commonly utilized in PBS and PBSA synthesis to enhance miscibility in polymer blends and composites, they pose significant environmental and health risks. Isocyanates are typically derived from amines through reactions with toxic phosgene gas. Moreover, inhaling even trace amounts of isocyanates can be extremely hazardous. Therefore, isocyanates are nowadays prepared by substituting toxic compounds with safer options and by utilizing bio-based precursors.161 Numerous patents like US4749806A and US9950996B2 are also filed which describe the synthesis of non-toxic isocyanates.162 On the other hand, companies like Lemouzy and Delavarde, EVONIK, Vencorex®, Alfa Aesar, Mitsui Chemicals® and Covestro® have started selling isocyanates having high biobased content.161 Detailed study on the synthesis and application of biobased isocyanates can be found elsewhere.161,162
Similarly, PBS and cellulose nanocrystal (CNC) based nanocomposites were prepared by Li et al.163 using compression molding. Thermal analysis revealed that upon the addition of CNCs, the Tg and Tc increased compared to those of pristine PBS. Moreover, an increase in crystallization rate was observed at low CNC levels due to the nucleation effect of CNCs; however, the % crystallinity was unchanged. Tensile tests showed that CNC concentration enhanced TS and modulus but lowered EAB and caused even brittle fractures when CNC concentration increased to 1.0 wt%. However, CNCs tend to congregate together instead of being evenly dispersed in a polymeric matrix. The dispersion of CNCs can be enhanced by the addition of a modifier, such as reactive agents or nonreactive polymers.164 Phthalic anhydride is an example of a reactive agent that can be used to improve the compatibility between the polymer and CNCs. In a study by Zhang and Zhang,164 PBSA was incorporated with CNCs using compression molding at different concentrations to integrate the superior mechanical properties of CNCs into the polymer. Phthalic anhydride (2%) was used as a compatibilizer to enhance PBSA and CNC compatibility. This resulted in enhanced mechanical strength and thermal stability of composites, which was attributed to the better dispersion of CNCs in the PBSA matrix. Moreover, upon the addition of phthalic acid, the surface wettability of the composite film decreased, which suggests the improved hydrophobicity of the films. A similar improvement in compatibility was reported for the PBS and lignin film after the incorporation of MA-grafted lignin into the composites (Fig. 9).165
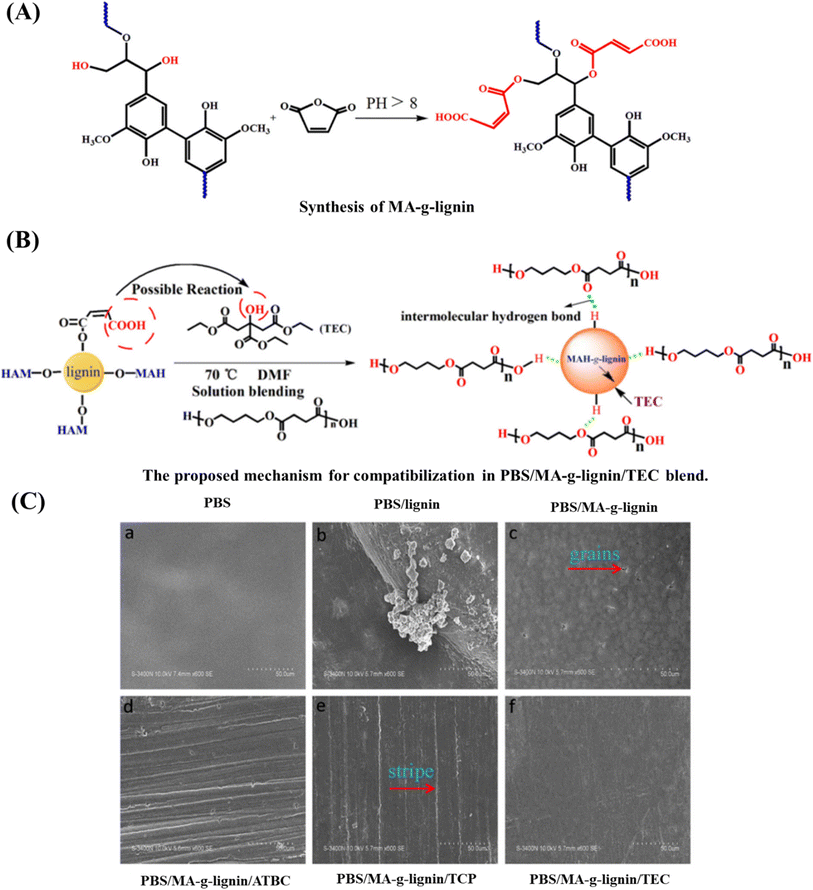 | ||
| Fig. 9 (A) Synthesis of MA-g-lignin; (B) mechanism of compatibilization in PBS/MA-g-lignin/TEC; (C) morphology of blend films. The combination of MA-g-lignin and the plasticizer reduced grain agglomeration and eliminated cracks on the film surface whereas a neat PBS/lignin film exhibited agglomeration of lignin. TEC: triethyl citrate; ATBC: acetyl tributyl citrate; TCP: tricresyl phosphate; DMF: N,N-dimethylformamide. Reproduced with permission from ref. 165, Elsevier 2019. | ||
Another strategy to enhance cellulose nanofiber dispersion in the PBSA matrix is by using the Pickering emulsion process. Kurokawa et al.166 used this technique to fabricate PBSA films incorporated with TEMPO-oxidized cellulose nanofibers (TCN). In this technique, first, through mechanical stirring and ultrasonication, an aqueous suspension of TCN is mixed with a polymer solution. During this process, TCNs were adsorbed on the surface of the droplets of the polymer solution, functioning as a surfactant to stabilize the resultant emulsion. Due to the higher dispersion of TCN, the modulus of the composites increased by ∼120% and prevented the fracture of the nanocomposite films due to stress concentration. The masterbatch technique is another method for improving the dispersion of filler materials in the polymer matrix. Melt processing a highly loaded nanocomposite to the appropriate composition via the masterbatch technique can minimize solvent usage while simultaneously enhancing nanofiller dispersion.167 The masterbatch method is advantageous for processing CNC-based nanocomposites as it facilitates a more uniform distribution of CNCs within the polymer matrices. This homogeneity enhances the processability of the nanocomposites, resulting in improved overall quality and performance of the materials.168 A similar observation was also reported by Platnieks et al.,167 PBS and nano-fibrillated cellulose (NFC) composites were prepared using the masterbatch technique followed by compression molding to improve the dispersion of NFC within the polymer matrix. Due to the masterbatch process, the elastic modulus and storage modulus increased by 1.8 and 2.5 times, respectively attributed to the uniform filler dispersion. SEM analysis corroborated this finding, showing that masterbatch films had a more uniform dispersion of NFC than films made using the solvent-casting method (Fig. 10). Similarly, nano-SiO2 was dispersed onto a cast sheet extruded PBS/PBAT matrix using the masterbatch technique. Due to better dispersion, SiO2 incorporation reduced the WVP and OP by 26 and 8%, respectively. The mechanical properties of the nanocomposites were also enhanced due to the reinforcing effect of SiO2.169
 | ||
| Fig. 10 Morphology of PBS/NFC (a and c) masterbatch and (b and d) solvent cast films. The masterbatch film exhibited a uniform film surface with fewer defects and voids compared to solvent-cast films. Reproduced with permission from ref. 167, Elsevier, 2021. | ||
Co-extruded laminate films with many layers have become more important in the food industry because of their adaptability and ability to maintain food quality. These industrial films generally include three to nine layers, each made up of a different polymer and attached with an adhesive. Their ability to provide high oxygen and water vapor barriers ensures optimal food packaging, safeguarding the product throughout its entire life cycle. These multilayer films have thus emerged as a critical method for preserving food freshness and prolonging shelf life.170 Messin and co-workers171 developed a multilayer film of PBSA incorporated with nanoclay against PLA. The authors reported that the inclusion of nanoclay into the PBSA matrix enhanced the barrier performance of the multilayer films by reducing water vapor diffusivity, WVP, and water vapor solubility. This was attributed to the development of tortuosity in the nanoclay-filled PBSA layer in conjunction with the confinement effects caused by the PLA layer. Table 2 provides a summary of studies conducted on PBS and PBSA-based modified blends and composites.
| Polymer | Filler type | Modifications | Effects on blends and composite performance | Ref. |
|---|---|---|---|---|
| PBS | Lignin | MA-grafted lignin and reactive plasticizer triethyl citrate | • Addition of MA improved the miscibility between lignin and PBS | 165 |
| • The film exhibited good flexibility even after filler addition | ||||
| • Increment in the UV barrier performance of the film while enhancing the transmission of visible light | ||||
| PBS | Inedible wheat flour | PBS-based copolymer [P(BS-co-Pripol)] | • Addition of a co-polymer enhanced the compatibility between PBS and wheat flour | 172 |
| • Composites with 15 and 20 wt% co-polymer had the best balance in mechanical properties with favorable stress and modulus values | ||||
| • The increased copolymer content in the ternary blend decreased the blend disintegration | ||||
| PBS | Starch nanocrystals (SNCs) | L-Lysine diisocyanate (LDI) and N,N-dimethyl-4-aminopyridine modified SNCs | • Modified SNC films have superior tensile and barrier characteristics to untreated or LDI films | 173 |
| • WVTR and OTR of the modified SNC incorporated film were reduced by 52% and 61% respectively, compared to those of neat PBS | ||||
| • Better dispersion of SNCs in the PBS matrix was observed due to the modification | ||||
| • The biocomposite film degraded completely within ∼30 days | ||||
| PBS/PLA | Carbon nanotubes (CNTs) and montmorillonite (MMT) | Poly(butylene succinate-co-lactate) | • CNTs were predominantly in the PBS matrix, while MMT was preferentially localized in PLA domains | 174 |
| • PLA domains changed from spherical to irregular with diffused borders, indicating improved PBS-PLA adhesion | ||||
| • Addition of fillers enhanced the modulus of the composites in the presence of a compatibilizer | ||||
| PBS/starch | Cellulose | Furfural | • The addition of furfural to PBS increased the EAB by up to 16 times compared to that of neat PBS | 175 |
| PBS/PBAT | MA-g-PBAT | • β-Cyclodextrin was used for controlled-release of sweet basil essential oil | 176 | |
| • Increasing PBS in PBAT/PBS blend films increased TS and Young's modulus | ||||
| • The concentration of essential oils in PBAT/PBS films enhanced water vapor and oxygen permeability | ||||
| PBSA/polypropylene carbonate (PPC) | Ethylene–methyl acrylate–glycidyl methacrylate random terpolymer | • Low loadings of the compatibilizer may completely interact with the blend during extrusion, while higher concentrations remain in the blend | 177 | |
| • Compatibilizer increases molecular chain mobility and improves PPC-PBSA compatibility | ||||
| • Compatibilized film exhibited enhanced tensile and barrier performance compared to a neat PPC film | ||||
| PBSA/PLA | Joncryl | • Joncryl resulted in the formation of a long chain branching structures | 143 | |
| • Mechanical properties improved due to the chain extender | ||||
| • The oxygen and moisture barrier performance improved | ||||
| • The addition of PBSA and Joncryl increased the thermal stability of blend films, resulting in the production of heterogeneous nucleation | ||||
| PBSA | Butyl-etherified starch | • Butyl-etherification was proposed to boost starch's compatibility with PBSA | 178 | |
| • The heavily branched amylopectin structures in butyl-etherified starch interact more chemically with the PBSA matrix than linear amylose structures | ||||
| • Increase in starch content reduces the thermal stability | ||||
| • Higher amylopectin content in starch reduced the degree of crystallinity | ||||
| PBSA/PHBV | Dicumyl peroxide (DCP) | • Blown film with 70% PHBV content was developed with the help of DCP | 179 | |
| • Rheological analysis established cross-linkage and the presence of a long branched structure | ||||
| • The films exhibited increased brittleness upon the addition of DCP | ||||
| • Mechanical characteristics were stable over a six-month freezer storage experiment |
 | ||
| Fig. 11 (A) Inhibition of E. coli and S. aureus due to PBS/thymol-based films. E. coli and S. aureus showed inhibition at 10 and 6 wt% thymol content, respectively. Higher concentrations of thymol increased the EAB but reduced the TS and oxygen transmission rate. Thymol acted as a plasticizer for the PBS matrix, reducing the intermolecular forces and making it easier to disentangle. This allowed PBS to elongate more and exhibit less TS. Reproduced with permission from ref. 183 Elsevier, 2015; (B) in PBS/quercetin films, an increase in quercetin concentration increased the antimicrobial activity against E. coli and S. aureus significantly with 0.5 wt% quercetin exhibiting the best antimicrobial performance. Additionally, the TS and EAB decreased at higher quercetin content. This is attributed to the addition of quercetin, which alters the intramolecular bonding. The addition of hydrophobic quercetin disrupts the PBS matrix leading to reduced chain mobility and lower fracture resistance. Reproduced from ref. 42 MDPI, 2021, Open Access. | ||
However, antioxidants and low-molecular-weight antimicrobials in polymer matrices can migrate to the surface from the bulk due to incompatibility between polymer chains. Such instances release active molecules uncontrollably, restricting their utility in the target application. Nanofillers that encapsulate active compounds have been researched extensively as a means of better controlling the release of these substances. The migration of active compounds may be controlled and optimized in these hybrid systems by varying the nature and degree of their interaction with the nanofiller.184,185 This, in turn, significantly improves the shelf life of the packaged product, compensating for the lack of inherent active properties in PBS and PBSA. Therefore, a study by Cicogna et al.184 developed compression-molded PLA/PBS films incorporated with glycyrrhetinic and rosmarinic acid-modified layered double hydroxides (LDHs). LDHs are materials with applications in the capture, storage, and regulated emission of biologically active anions, and because of their versatility, LDHs have been researched in recent years to be developed into functional materials for active packaging. Rosmarinic acid-incorporated LDHs exhibited better antimicrobial properties against Staphylococcus aureus and E. coli. Furthermore, the authors reported a slower migration of active species from the thin layers of composite materials into the hydroalcoholic solvent demonstrating control over the release process. Similarly, silver is widely used as an active agent due to its exceptional antimicrobial properties. However, the quick release of silver from the polymer matrix leads to both a temporary antibacterial effect and an excessive amount of silver in food. Zeolite, an aluminosilicate material in conjunction with silver exhibits antibacterial activity against S. aureus and E. coli.186 Wattanawong and Aht-Ong187 developed antimicrobial films by incorporating silver zeolite with PBS using melt extrusion and solvent casting and found that the active films inhibited the growth of common food-borne pathogens (E. coli and S. aureus) by 99.9%.
Similarly, Mhlabeni and co-workers188 prepared stearic acid-coated LDH (S-LDH) for fabricating PLA/PBSA films. The authors reported that the particles are mainly loaded into the PBSA dispersed phase and 0.5 wt% S-LDH incorporated composites exhibited improved thermal stability and hindrance to the oxygen molecule diffusion rate along with the highest EAB value across all the samples.188Table 3 presents a list of studies on PBS and PBSA-based antimicrobial films.
| Polymer | Active agents used | Inhibited microorganisms | Effects on film performance | Ref. |
|---|---|---|---|---|
| a T m: melting temperature; Tc: crystallization temperature; Tg: glass transition temperature. | ||||
| PBS | Thymol | • Colletotrichum gloeosporioides | • The thermal stability and TS increased upon lignin filler incorporation | 189 |
| • Lasiodiplodia theobromae | • OP and WVP of the film improved | |||
| PBS | Curcumin | • Escherichia coli | • The films exhibited high antioxidant activity | 190 |
| Carvacrol | • Staphylococcus aureus | |||
| • Candida albicans | ||||
| PBS | Zinc oxide | • S. aureus | • Modulus increased upon ZnO incorporation while the tear strength reduced | 191 |
| • E. coli | • The Tm and Tc decreased while the degree of crystallinity increased | |||
| • Zn migrated over a 15 day study period | ||||
| PBS | Thymol | • S. aureus | • PBS was tougher and softer after incorporation of thymol which acted as a plasticizer | 183 |
| • E. coli | • OTR of the film increased due to an increase in the amorphous phase | |||
| • Thymol loading at 10 wt% was effective over a 15 day period | ||||
| PBS/tapioca starch | Biomaster silver (Ag) | • S. aureus | • Ag at 3% loading formed a well-structured PBS film and interacted with the PBS chain | 192 |
| • E. coli | • The crystallinity improved due to the presence of Ag | |||
| • Salmonella typhimurium | • The OP of the blend film reduced upon Ag incorporation | |||
| PBSA/PLA | Thymol | • Aspergillus spp. | • The Tg and Tm decreased for thymol-loaded films | 181 |
| • Penicillium spp | • Films exhibited enhanced flexibility but lower TS and modulus | |||
| • Addition of PBSA and thymol reduced the gas and moisture permeability coefficient | ||||
| PBSA/PHBV | Chitin nanofibrils (CN) | • S. aureus | • Fungal-based chitin had better adhesion compared to chitin from shrimp | 193 |
| PBS | • E. coli | • CN coatings at different concentrations increased the barrier performance | ||
| • Coatings did not affect the biodegradability of the composites | ||||
| PLA, PBAT, and PBS | Eugenol | • S. aureus | • Reduction in the formation of microbial biofilms in food packaging materials | 194 |
| Thymol | • E. coli | |||
| • Bacillus pumilus | ||||
| • Bacillus subtilis | ||||
| • Bacillus tequilensis | ||||
| • Stenotrophomonas maltophilia | ||||
4 Application of PBS and PBSA films for packaging fresh food products
In the area of food packaging, the polymers PBS and PBSA have shown promise as packaging materials. Despite their promise, there is still a dearth of studies examining their use in actual food systems, such as perishable food items like meat, poultry, eggs, and vegetables. Active agent incorporated films are usually used for shelf life extension of packaged food products by limiting microbial growth, reducing the lipid oxidation of food products and obstructing moisture and oxygen passage through the film.195Mohamad et al.196 developed a safe-biopackaging PBSA/PLA-based film by incorporating different antimicrobial agents such as kesum, curry, and thymol to enhance the shelf life of chicken fillets at 4 ± 1 °C storage temperature. The effect of the films on common foodborne pathogens responsible for food spoilage such as E. coli, Aspergillus brasiliensis, and S. aureus was studied. A zone of inhibition against S. aureus was visible in the films incorporated with 10% kesum and 10% thymol. Since Gram-positive bacteria (S. aureus) do not have an outer membrane, hydrophobic active compounds were able to directly penetrate their cell membranes, resulting in a stronger inhibitory impact than Gram-negative bacteria (E. coli). Based on an in vivo direct food contact examination, the chicken fillet wrapped with PBS/15% kesum films had the lowest microbiological count on the sixth day of storage, had a mild odor, and retained its freshness by appearing pink and fleshy in color. However, on the 8th day of storage, films with 15% thymol exhibited better bacterial inhibition than 15% kesum. The samples packaged with films devoid of active ingredients exhibited a potent odor on day 6 with color change of the samples. Protein degradation by microbes reduced carnosine content, which is a small-molecule peptide crucial for meat colour preservation, and this was a major reason for the gradual change in the color of chicken samples. Similarly, double-layered PBS/PBSA films were able to maintain the quality of poultry meat throughout 15 days, similar to polyamide/PE films.197
PBSA-based films can also be designed to increase the shelf life of aquatic products. For example, Yang et al.198 developed PBSA/PLA films incorporated into thymol or carvacrol to impart active properties to the blended films. The addition of essential oils improved the antioxidant properties of the films by a maximum of 262% compared to control films. The phenolic group found in carvacrol and thymol molecules is responsible for the antioxidant properties. Release studies of thymol and carvacrol into salmon samples were conducted and the authors found that the release slows down over time at 4 °C, stabilizing at 48 hours. The authors observed that achieving complete release of active compounds into fatty foods like salmon is challenging, with only partial migration observed. This is likely due to high moisture content which hinders the full release of active agents and causes lower concentrations at equilibrium. TVC (Total Viable Count) is a standard procedure used to assess the microbiological quality of food products, and in this study, the TVC value was reduced for the active films compared to control samples.199 When the active ingredient in the PBSA/PLA films was released at equilibrium onto the salmon samples' surface, the active compound's antibacterial action inhibited bacterial growth. Also, the loading of active agents increased the antioxidant capability of the films and prevented lipid oxidation in the salmon slice. Malondialdehyde which is the end product of lipid oxidation was the lowest in carvacrol incorporated films followed by the thymol films. This is due to the presence of the phenolic groups in thymol and carvacrol, which further assisted in increasing the shelf life of the packaged salmon slices. Therefore, the application of active agents decreased the deterioration and spoilage of the salmon slices, leading to a notable extension of their shelf life by 3–4 days during cold storage.
Breads are typically mold-free after baking, but they become contaminated with mold when exposed to air during chilling, packaging, and storage. The incorporation of organic acids like sorbic acid and propionic acid is a conventional method for preventing the growth of fungi in bread. However, the demand for food products without preservatives is consistently increasing.200 With this in mind, Suwanamornlert et al.181 developed a packaging material for bread. As mentioned earlier, in this study PBSA/PLA films were incorporated with thymol, whose addition improved the barrier properties of the films and led to antifungal properties. Thymol-loaded films, at 3 and 6 wt% concentrations, effectively delayed fungal growth on bread, extending mold-free periods to 7 and 9 days, respectively. In contrast, neat PLA films showed visible mold growth on day 6. On the surface of the bread, the presence of yeast and mold increased significantly when packaged in PLA, and this was significantly reduced when packaged in antifungal packaging films, particularly 6 wt% thymol-loaded PBSA/PLA films. Similar outcomes were noticed after 14 days of storage, with the lowest counts observed in 6% thymol–PBSA/PLA, followed by 3% thymol/PBSA/PLA, and PLA in that order. Moreover, the concentration of CO2 in the packaging headspace was highly correlated with mold and yeast growth. A commercial BOPP had the highest CO2 content, while 6% thymol–PBSA/PLA and 3% thymol/PBSA/PLA had the lowest CO2 content. However, texture analysis indicated that the films had no impact on crumb texture throughout the testing period. The combined effect of the antifungal properties of thymol and the barrier capabilities of PBSA and PLA film inhibited the growth of yeast and mold in bread packaged in antifungal films. This extended the bread's shelf life to at least 9 days compared to 3 days in commercial BOPP films. Varghese et al.201 showed that the shelf life of bananas can be extended using PBS/PBAT-based active films incorporated with TiO2 due to the antimicrobial action of TiO2 (Fig. 12).
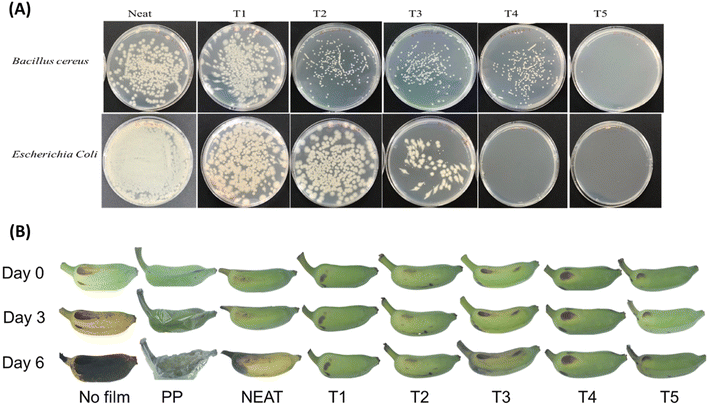 | ||
| Fig. 12 (A) Antimicrobial effect of PBS/PBAT films with different TiO2 concentrations; and (B) comparison of the appearance of bananas in their natural state (no film) to those wrapped in PP and PBAT/PBS films with varying TiO2 levels [neat (0.9%), (T2) 1.8%, (T3) 2.7%, (T4) 3.6%, and (T5) 4.5%]. Reproduced with permission from ref. 201, Elsevier, 2023. | ||
Therefore, the application of PBS and PBSA can be successfully used for the preservation of fresh food products, as demonstrated in the above-mentioned studies. While the existing research presents their ability to enhance the shelf life of food products, limited research has been performed showcasing their use in real food applications. Therefore, the adaptability of these polymers, together with their biodegradability and compostability, makes them viable candidates for environmentally friendly food packaging choices. Table 4 illustrates the studies on the application of PBS and PBSA films in real food systems.
| Polymer | Active agent used | Food sample tested | Observation | Ref. |
|---|---|---|---|---|
| PLA/PBSA | Thymol | Bread | • Shelf life of bread increased to a maximum of 9 days, whereas commercial PP only provided 3 days of shelf life | 181 |
| PLA/PBS | Red grapes (Vitis vinifera L.) | • The shelf life of red grapes increased from 8 to 12 days by postponing the weight loss, hardness loss, and reduction in soluble solid content | 202 | |
| PLA/PBSA | Carvacrol and thymol | Salmon slices | • The films extended the shelf life of the samples by 3 to 4 days under cold storage | 198 |
| PLA/PBS | Alpinia officinarum essential oil | Cooked rice | • The essential oil mainly consisted of compounds like 2-bornanone, eucalyptol, β-ocimene, and geraniol | 203 |
| • Extended the shelf life of cooked rice up to 2 fold by reducing fungal growth | ||||
| PBS and ethylene vinyl alcohol (EVOH) | Geraniol | Bread | • The geraniol migration increased with increasing relative humidity within the container, which corresponded to the bread's moisture release | 204 |
| • The spoilage of bread was delayed by up to 3 weeks while retaining its hardness | ||||
| PLA/PBS | Oregano essential oil | Ready-to-eat lettuce | • The quality and shelf life of lettuce increased up to 8 days of storage when incorporated with 5 and 10% oil | 205 |
| PBS, PLA, Mater-Bi®, and LDPE | — | Rambutan (Nephelium lappaceum) | • In addition to preserving its yellowish-red exterior and fresh green spintern, rambutan stored in PBS bags showed the least amount of weight loss, followed by Mater-Bi® and LDPE | 206 |
| • When kept at 13 °C, the shelf-life following harvest might increase by up to 15 days |
5 Application in controlled-environment agriculture
Controlled Environment Agriculture (CEA) refers to the cultivation of plants and their products, including flowers, fruits, and vegetables, within controlled environment structures (e.g., greenhouses, vertical farms, and growth chambers). Plastics have a wide range of applications in CEA from plastic sheeting for greenhouse covering to ground covering that suppresses weed growth in greenhouses and reflects light up into the crop canopy, to growing media wrapping and agricultural mulch films, which optimize growth conditions for plant growth. Plastics play a crucial role in preserving an optimal microenvironment, providing insulation, regulating humidity, and hindering light penetration.207Polymer sheets and films are widely used in greenhouse coverage, and common polymers include PE, polycarbonate (PC), and polyvinyl chloride (PVC). Greenhouse plastics have high durability, high thickness, and tear strength. Moreover, they need to be UV-stabilized to exhibit durability for up to 3 years since polymers are prone to UV degradation.207 Today, due to the increased waste generation from non-biodegradable plastics, bioplastics can be an eco-friendly alternative to PE and PVC. Bio-based polycarbonates synthesized from renewable feedstock may be utilized in greenhouse cover materials and ground cover materials due to a lower carbon footprint.208 There has been limited to no study on the use of PBS and PBSA in greenhouse and ground covering materials. The main reason behind this could be the high cost and lack of adequate properties of neat PBS and PBSA. These polymers can also be used as multilayer films with PE and PC to enhance the sustainability of the materials without compromising the performance of the sheets. Moreover, PBS and PBSA can be blended with other bioplastics or conventional polymers with or without the presence of compatibilizers to achieve the desired results. In comparison to petroleum-based plastics, bioplastics are more expensive; producing petroleum-based plastics like PVC, PP, and PE costs between 1.59, 0.90, and 1.12 € per kg, respectively, while bioplastics like PLA, PHBV, and PBSA costs 2–5 € per kg making it unsuitable from an economic perspective.209–212
In vertical farming, the use of hydrogels has gained popularity. Hydrogels are superabsorbent polymeric materials that can retain moisture and essential nutrients when used in the soil.213 Hydrogels can absorb water 400-times their dry weight and discharge it gradually to reduce the leaching of herbicides and fertilizers, improving soil quality and reducing irrigation frequencies, making them advantageous for agricultural use.213 Hydrogels can also be used in controlled-release fertilizers and slow-release fertilizers since they can control the release of nutrients that correspond to the plant's nutritional needs. Synthetic-based hydrogels are less hydrophilic and have better mechanical properties compared to bio-based hydrogels. However, due to the biodegradable nature of bio-based hydrogels, along with their non-toxicity and wide availability of materials for bio-based hydrogel synthesis, they are preferred over synthetic hydrogels for use in agriculture. Some research has been performed on PBS-based hydrogels for biomedical applications214 but the application of PBS and PBSA-based hydrogels in agricultural soil is greatly unexplored. Therefore, PBS and PBSA polymers can be used for hydrogel formation, which can act as a growing medium plant in vertical farming. Detailed descriptions of the application of hydrogels in agricultural systems can be found elsewhere.43,213
Urban farming is evolving, and researchers are inventing new techniques to minimize waste and maximize output. For example, film farming can be an alternative to hydrogels in urban farming.213 Film farming requires 90% less water than conventional farming and allows us to grow fruits and vegetables without the need for soil, pesticides, or other toxic chemicals.213,215 A Japanese physicist, Mori, developed a combination of membrane technology and hydrogel, which was introduced to the market as Imec.216 The product is a thin film with nanopores that can support plant growth and prevent microbes and viruses from attacking the plant without the requirement of chemicals. Additionally, this coating is waterproof, minimizing contamination and removing runoff water. This technique allows the plants to be grown on any flat surface, on top of the film that captures and holds the water molecules. Imec has been used in producing cucumber, paprika, tomatoes, lettuce, and melon.213,215 Therefore, film farming can be integrated into vertical farming to improve plant growth and enhance food security. There exists a substantial research gap and immense scope for developing materials for sustainable agriculture from PBS and PBSA. There has been a lack of comprehensive studies, and these polymers show promise in the agricultural domain, especially in controlled agricultural environments. A detailed study on film farming techniques for vertical farming can be found elsewhere.217
Another type of film widely used in greenhouses as well as in open-field agriculture is a mulch film. These films increase crop production when applied to agricultural soils, conserving soil moisture, controlling weed growth, increasing soil temperatures, and safeguarding against pests and weather. The use of conventional PE-based mulch films results in leftover film pieces accumulating in the growing soil, which negatively affects soil productivity and ecology. Recovery of PE mulch films is often difficult because of disintegration and embrittlement caused by weathering, and this also causes accumulation.218 PBS and PBSA can be suitable replacements for PE for agricultural mulch film applications to mitigate these problems. They are particularly important as these films are degraded by mechanical forces, ultraviolet radiation, and weather, and do not cause long-lasting pollution in the soil. This type of mulch film, where the soil is the final destination, also requires less manual labor as it is not required to be extracted at the end of the harvesting season and can later be tilled into the soil.219
Some studies are exploring the use of PBS and PBSA for fabricating eco-friendly mulch films. Ayu et al.220 developed empty fruit brunch fiber reinforced PBS/modified tapioca starch agricultural mulch films using the hot press technique. The films exhibited reduced mechanical properties compared to neat PBS, but the thermal properties remained unchanged. Another study also explored the use of PBSA films for increasing cotton (Gossypium hirsutum L.) yield.221 Bi et al.222 developed PBS films coated with multi-layer fertilizer-infused mulch papers using blade coating, followed by compression molding to study the controlled release of fertilizers. This could be an ideal approach for the controlled release of fertilizers through mulch films to promote plant growth. Similarly, Song et al.223 developed humic acid-enriched PBS mulch films and studied their application in the growth of lettuce. The authors reported that the mulch films could enhance the growth of green vegetables by promoting chlorophyll synthesis, vitamin C, and peroxidase in lettuce and inhibiting malondialdehyde production. Some studies also examined the biodegradation of mulch films to determine the time required for the films to biodegrade effectively. Tamura et al.224 studied the biodegradation of PBSA films in soil collected from cultivated fields in Japan. The biodegradation of PBSA was up to 95% when incubated for 4 weeks; both the esterase activity in soils adhering to the PBSA film and the amount of viable PBSA-degrading fungi increased over time as the film degraded. Another study by Koitabashi et al.225 assessed the fungal ability to biodegrade PBS, PBSA, and commercial biodegradable mulch films. The phylloplane fungal strain B47-9 decomposed 91, 23, and 14 wt% of PBSA, PBS, and commercial biodegradable mulch films on unsterilized soil in 6 days. Therefore, the increased rate of mulch film breakdown was caused by a high distribution of native PBSA/PBS-degrading fungi in the soil.
6 Biodegradation studies on PBS and PBSA
6.1 Under industrial and home composting conditions
Biodegradable plastics biodegrade under specific conditions at the end of their life cycle. Compostable plastics, which are a subset of biodegradable plastics, undergo decomposition in industrial composting facilities. Biodegradable and compostable plastics can be manufactured using either fossil fuels or biological resources.226 Biodegradable plastics are certified following several standards that lay out the precise criteria for composting, such as ISO 14855-2:2018, 17088:2012, EN 13432:2000, ASTM D5338-15, ASTM D6400, and AS 4736.227 One of the most recognized forms of biodegradation is composting, which is defined by standards like ASTM D5338-15 and its equivalent, ISO 14855. The labeling standard requires 90% of the polymer to be disintegrated in 90 days and chemically degraded in 180 days. The product should not affect compost plant development in comparison with biowaste-derived composts not containing that product. ISO 20200 is used to complement composting studies, although it is a disintegration study of the polymeric material rather than biodegradation. ASTM D5929 examines the composting of “organic” materials under aerobic conditions at mesophilic temperatures (25–45 °C), which is another standard that reflects on natural or home composting and has been applied to plastics.44,228 During composting, biodegradable polymers are subjected to a combination of decomposed materials at higher temperatures. Industrial composting is performed at 60 °C, high relative humidity, and in the presence of oxygen. The compost standard is typically intended for industrial or commercial uses, where the environment is optimized or regulated in terms of initial conditions and parameters (such as moisture content and the carbon-to-nitrogen ratio), providing a stable and controlled environment.44 Home composting, is gentler than commercial composting facilities partly because of a lower quantity of biodegradable materials, with temperatures maintained at ∼28 °C. In comparison to commercial composting, biodegradation rates are also lowered at cooler temperatures for home composting; nevertheless, this reduction is highly dependent on the chemical composition of the material as well as the additives used.229Table 5 presents the notable factors responsible for polymer degradation.| Physicochemical conditions | Material properties | Enzymatic effects |
|---|---|---|
| pH value | Molar mass | Microbial diversity |
| Moisture/water content | Size, shape, and surface area | Microbial activity |
| Availability of oxygen | Melting and glass transition temperature | Microbial population density |
| Temperature | Polymer composition | |
| Redox potential | Steric configuration | |
| Availability of nutrients | Thickness | |
| Fillers | ||
| Polymer crystallinity | ||
| Porosity | ||
| Additives |
The above-mentioned standards provide guidance for measuring plastic biodegradability under anaerobic conditions. However, the standards do not mention any specific thickness for the samples but emphasize selecting materials representative of the application. The format and shape of the polymer material are also a significant factor for bioplastic degradation, with thin films degrading faster than thicker films. Certification is granted for bioplastics and bioplastic-based completed products up to a maximum thickness, as measured by a disintegration test. The maximum thickness of a bioplastic during certification is determined by measuring its thinnest section for three-dimensional items like cups or cutlery. The film samples fabricated from the bioplastic are frequently used for testing disintegration.231 Recently, BioPBSA developed by PTT MCC BioChem Co., Ltd (grade: FD92PM) has obtained certification as home compostable with a maximum nominal thickness of 502 μm, as per the OK compost certification scheme. Similarly, biobased PBS (grade: TH803S-Bio) and biobased PBSA (grade: TH802A) from Xinjiang Blue Ridge Tunhe Polyester are also certified home compostable at a maximum thickness of 45 and 68 μm, respectively.
It is reported in the literature that industrial composting is faster than home composting. As such, to confirm this information for blends, the biodegradation of PBS and PBAT blends was compared under both home composting and industrial composting conditions by Nomadolo et al.232 In contrast to home composting conditions, PBS/PBAT blends demonstrated a faster biodegradation rate in industrial composting, which can be attributed to the higher temperature used in industrial composting [Fig. 13(A)]. In another study, the effect of soil compost and natural soil conditions on the biodegradation of PBS composites was studied by Kim et al.235 The authors reported that the % weight loss and mechanical properties of PBS and its biocomposites were higher under compost than under natural soil conditions due to elevated temperature and humid conditions. Moreover, the number of microbial colonies in compost soil plates was higher compared to that in natural soil, which suggests that a higher microbial population resulted in faster biodegradation of biocomposites. A higher reduction in molecular weight of PBS buried in compost soil was observed due to hydrolysis of the aliphatic ester linkage in the PBS main chain. In the case of biocomposites, the biodegradability may be attributed to the chain scission of the C–O–C and C–O bonds of the cellulose main chain in rice husk flour, as well as the degradation of crystalline and amorphous cellulose by bacteria and fungi in the compost soil. Moreover, with an increase in biodegradation, the crystallinity of the samples increased due to the reduction of the amorphous phase in the biocomposites. Similarly, in a study by Liu et al.,236 PBS/jute fiber composite films were tested for biodegradability under a compost-soil burial test. The authors reported that the addition of 10 wt% fiber led to a higher biodegradation rate of 62.5%, which is higher than that of neat PBS films (31.4%) and bulk jute fibers (24.7%). This demonstrates that compounding of PBS and jute fiber promotes the rates of biodegradation.
 | ||
| Fig. 13 (A) The biodegradation behavior of PBAT–PBS and PBAT–PLA compared to cellulose in an (i) industrial and (ii) home composting setting. The biodegradation rate of blends was faster in industrial composting compared to home composting. The mechanism involved here is the change in the hydroxyl and carbonyl groups under composting conditions due to hydrolysis. Reproduced from ref. 232, MDPI 2022, Open Access; (B) optical microscopy images of four strains (Aspergillus versicolor, Penicillium, Bacillus, and Thermopolyspora) isolated from compost. A. versicolor exhibited the fastest growth under composting conditions. Penicillium and Bacillus had a moderate growth rate while Thermopolyspora showed the lowest growth rate. Reproduced with permission from ref. 233, Wiley 2005; (C(i)) biodegradation of PBSA films after incubation for 30 days. The films were co-cultured with A. terreus HC, A. fumigatus L30, and A. oryzae. The upper panel consists of films with attached fungal hyphae on the PBSA surface while the bottom panel illustrates the surface of PBSA after the hyphae have been removed. (ii) Weight loss of the PBSA film after incubating for 0, 14, and 30 days. The weight loss for films exposed to A. terreus strain HC, A. fumigatus strain L30, and A. oryzae RIB40 after 30 days was 47%, 30%, and 27%, respectively. Reproduced from ref. 234, MDPI, 2022, Open Access. | ||
The size and shape of the samples have an important part in the biodegradation rate of PBS in compost. Also, the degradation rate of PBS powder was similar to that of the film, but PBS pellets deteriorated more slowly.237 This was also reported by Zhao et al.,233 where the PBS powder samples had a higher biodegradation rate (compared to granules and films), which is also obvious due to their highly specific surface area. Moreover, the researchers isolated four strains of microbes from the compost, namely Penicillium, Thermopolyspora, Bacillus, and Aspergillus versicolor, and among them, Aspergillus versicolor was the most effective in degrading PBS [Fig. 13(B)]. In a different study, Wu238 used Rhizopus oryzae compost to test the biodegradability of PBSA and sugarcane bagasse (SB), as well as MA-g-PBSA with SB. After 60 days of incubation, morphological examinations showed an extensive breakdown in the structure of the films, and both the PBSA and the MA-g-PHBV/SB composite showed rapid degradation. The biodegradation mechanism involved lysozyme (an enzyme) facilitated by the presence of R. oryzae. The biodegradation rate of MA-g-PBSA/SB was greater than that of pure PBSA but lower than that of PBSA/SB; nevertheless, the MA-g-PBSA/BF (40 wt%) composite exhibited 80% weight loss after 120 days.
In a comparative study on the degradation of PBSA and PHBV under composting conditions, PHBV, as expected, degraded faster than PBSA despite PHBV having higher molecular weight and crystallinity. The primary cause of material loss in both polymers was enzymatic degradation at their surfaces, with a secondary contribution from the hydrolytic chain scission mechanism in their bulk region due to moisture diffusion. Now, with preferential degradation of amorphous areas, mass loss of PHBV increased exponentially, making the PHBV surface porous and rough, exposing more polymer chains, and accelerating enzymatic hydrolysis under composting conditions.239 A similar mechanism might be the reason for the faster biodegradation of PHBV compared to PBS. Like PBS, fungal species belonging to the genus Aspergillus exhibited rapid degradation of PBSA. In a study by Chien et al.234 two fungal strains of Aspergillus fumigatus L30 and Aspergillus terreus HC were extracted from farmland soil and a composting yard; they exhibited a high biodegradation rate for PBSA films even in soil with poor biodegradation ability [Fig. 13(C)]. A bacterial strain, Bacillus pumilus was isolated from soil and compost and was used to compare the biodegradation of different bioplastics such as PBS, PBSA, PBAT, PLA, and poly(ε-caprolactone) (PCL). PBSA had the highest degradation, where B. pumilus effectively degraded the PBSA film, with the adipate units of PBSA being more susceptible to degradation compared to the succinate units. This was followed by PBS (90.2%), and PBS/PCL (50.8%), while PBAT and PLA were minimally degraded by B. pumilus.240
6.2 Soil biodegradation
Plastic wastes are mostly disposed of in soil, and as such it is important to evaluate the effect of this environment on the biodegradation of PBS and PBSA. Essentially, the abundance of microorganisms found under soil conditions makes plastic biodegradation easier than that in other environments like water or air.241 Van der Zee et al.242 tested the disintegration of PHBV, PBS and PLA blends in soil using ASTM G160.243 For this work, the authors blended PBSA with PHBV, PBS and PLA and tested their rate of disintegration. They reported that PHBV can be disintegrated rapidly by adding PBSA. The plastic samples exhibited signs of degradation over time. After 28 days, colored spots began to appear on the surface and within the material. Furthermore, samples that were exposed for 58 days showed several holes, while samples that were retrieved after 112 days of exposure showed even more holes. This is consistent with the gradual reduction in mechanical properties of the blends. The samples with higher PBSA in a PHBV matrix showed higher deterioration. The authors also blended PBS and PBSA and tested the disintegration rate. Unmodified PBS samples in soil began to discolor after 14 days, developed holes by 28 days, and completely disintegrated by 112 days. PBSA degraded even faster, with significant damage within 14 days and substantial disintegration within 28 days. PBS/PBSA blends with higher PBSA content showed faster rates of disintegration. This result supports the idea that PBSA and PBS although similar differ in biodegradability due to the presence of adipate moieties in PBSA, making the former more biodegradable under soil conditions. Blends with higher PBSA content disintegrated quicker, showing that the PBS/PBSA ratio may be adjusted to control the disintegration rate. Interestingly, PBS showed a higher disintegration rate compared to the PHBV material which is certified home compostable and marine biodegradable. Hoshino et al.244 in their soil degradation experiment with PBS, PBSA and other bioplastics reported that the amount of nitrogen is directly correlated with the biodegradation rate.Phua et al.245 investigated the biodegradation of PBS incorporated with montmorillonite under compost soil conditions using the soil burial test. The biodegradation of PBS was significantly slowed down by the presence of montmorillonite because the composite had become more impermeable than neat PBS. Instead, using PBS grafted with MA (MA-g-PBS) results in a nearly complete restoration of the biodegradation ability of PBS, with only minor variations (Fig. 14).
 | ||
| Fig. 14 (A) Mechanism of PBS degradation; (B) SEM micrographs of (i) PBS, (ii) PBS/2% MMT, (iii) PBS/10% MMT and (iv) compatibilized PBS nanocomposites; and (C) weight loss of PBS and its nanocomposites; OMMT: organo-montmorillonite. Reproduced with permission from ref. 245, Elsevier, 2012. | ||
6.3 Other environments
De Falco et al.246 tested the biodegradation of PBSA, PHBV, PCL, and PLA under sandy conditions for 267 days. This experiment was conducted to simulate the sandy beaches which are vulnerable to litter pollution in coastal regions. After the experiment period, PLA was mostly unaffected with minor degradation and only showed physical aging. PHB exhibited the highest degradation rate, with approximately 90% weight loss after 200 days, and clear signs of biological activity on its surface. PBSA degraded more quickly than PCL samples, with 45% weight loss over during the testing period with visible holes and cracks on the surface. PBSA crystallinity increased after degradation which suggests that the degradation starts from the amorphous region. The degradation trend is observed to be as follows: PHB > PBSA > PCL > PLA.While bioplastics are considered biodegradable, their degradation is limited to only aerobic environments. Anaerobic conditions, which is common in nature and in controlled digesters for organic waste, may not support effective bioplastic degradation. Therefore, Jin et al.247 in their study evaluated the degradation of PBS, PBSA, PBAT, PLA, PCL, PPC, PVA, PHB, thermoplastic starch, and cellulose diacetate (CDA) under anaerobic conditions. The authors found that alkaline pretreatment of samples helped accelerate the degradation of all tested bioplastics. Following pretreatment, the mass variation showed that PLA and PBS dissolved slowly over 15 days, but PBSA dissolved rapidly, losing 98.2% of its mass in 24 hours. Except PBAT, pretreatment with a suitable concentration of NaOH may enhance the rates of degradation or minimize the lag phase of degradation of other bioplastic samples. For CDA and PBSA, the biodegradation rate (BD) significantly increased from approximately 4.5% and 30.5% to 85% and 88%, respectively, representing a remarkable 1752% and 190% increase. For PBS, the highest biodegradability rate was observed at 10% after alkaline treatment. The change in microbial community showed that pretreatment had successfully overcome the barriers of strong hydrophobicity and hydrolysis restriction that restrict contact between microbes and bioplastics. This resulted in the notable growth of hydrolytic fermentation bacteria, like Hydrogenispora and Coprothermobacter for PBSA.
7 Circularity of PBS and PBSA
In a perfect circular economy, plastics would be produced from recycled and renewable resources; most plastics, on the other hand, have a linear life cycle.248 Bioplastics like PBS and PBSA can be considered to be within a cyclical framework as follows: using water and sunlight for photosynthesis, plant biomass turns carbon into CO2; the biomass is then harvested and processed into monomers/polymers through extraction, fermentation, chemical, and microbial conversion, and finally into plastic products; these products can either be reused/recycled to increase their useful life and reduce the development of new materials, or biodegraded to recover and recycle CO2 through photosynthesis to form plant-biomass materials.227 To achieve this, environmentally responsible EoL options for PBS and PBSA are required, specifically their biodegradability in particular environments by means of complete bio assimilation or by recyclability. To enable the transition towards fully circular materials, it is important to consider material selection and application design complemented with appropriate EoL management options.227 Biodegradation and chemical and mechanical recycling are very important to minimize or mitigate plastic waste from our ecosystem; reusing plastics after their lifespan also acts as a valuable option.Mechanical recycling is a method of transforming plastic trash into new products without significantly changing the material's chemical structure, with the exception of adding new additives. It can be considered as primary or secondary recycling and is an important part of the circular economy.249 Chemical recycling is performed for materials not suitable for mechanical recycling and falls under tertiary recycling. Techniques such as dissolution and/or precipitation and solvolysis are required to transform plastic waste into pure monomer units or other valuable chemicals.227 Compostable or soil biodegradable materials are preferred for bioplastics, particularly plastics meant for packaging since they contaminate the organic waste stream or are likely to end up in the ecosystem. As mentioned earlier, for bioplastics like PBS and PBSA, it is important to exhibit the conversion of carbon from a polymer to CO2 through microbial activity in specific EoL environments. To completely eliminate the negative effects of microplastics and nanoplastics produced by the partial breakdown of plastics, biodegradability must be combined with full microbial bioassimilation.250,251
Therefore, to achieve sustainability and circularity for all bioplastics important steps such as optimizing resource efficiency and reducing risks, wastes, and pollution should be considered. Moreover, researchers should advise product designers when designing for circularity while taking geographical variations into account. However, the broad range of polymers, additives, and contaminants make recycling more difficult, making the recycling of post-consumer plastic a difficult task.227,252
8 Conclusion and future outlook
PBS and PBSA-based biodegradable blends and composites offer a reliable substitute for petrochemical goods for single-use plastic packaging applications and address environmental concerns. To effectively use PBS and PBSA as a substitute for traditional plastic polymers, it is also necessary to consider their price and availability. As mentioned, PBS and PBSA biopolymers have good mechanical and physical characteristics and can be produced quickly and affordably via melt polycondensation if produced on a large scale. The major properties of these polymers include a good biodegradation rate due to their origin from renewable sources making them adequate for home compostable items. However, they should demonstrate other desirable qualities such as high modulus, high barrier, and cost competitiveness to name a few. As a result, blending and compatibilization techniques can be implemented as a remedy to enhance the features of PBS and PBSA. Additionally, incorporating fillers into the matrix reinforces the polymers, reduces their cost, and improves their biodegradability without compromising their processability. Nevertheless, both blending and composite fabrication approaches offer distinct features compared to the other approaches like increased mechanical performance, reduced cost, sustainability, and application-specific properties. The composite and blend films prepared from PBS and PBSA have been successfully tested for shelf-life extension of meat, fish, fruits, and vegetables by incorporating different antimicrobial nanoparticles and essential oils. Moreover, biodegradable polymers have been used in numerous agricultural applications aiming to substitute traditional materials like PE and PP. Nevertheless, to commercialize the technologies it is imperative that the production capacity of these biobased materials be increased, along with widespread acceptance by industries and consumers alike with help from government policies.The overall finding from biodegradation studies indicated that the biodegradation rate of the PBS and PBSA-based blends was higher than that of the neat polymer while natural filler-based composites had the highest biodegradation rate. Biodegradation of polymers depends on a variety of factors, although temperature, crystallinity, and diverse microorganism populations are considered to be the driving factors. Industrial composting is considered quicker and is used for dealing with compostable films, plastic bags, straws, and bottle-related wastes. It has been observed that the addition of natural fillers from agricultural residues into biomass-derived plastic enhances biodegradability, offering a sustainable EoL approach for waste management. However, the overall sustainability of the product depends mostly on factors like raw material availability, energy consumption, and land and water usage among others. The future success of PBS and PBSA polymers relies on advancements in multiple areas such as
• Mass production, low-cost production, and higher customer requirement can directly affect the final price and affordable bioplastic options. Moreover, biobased synthesis techniques of these polymers are the way forward and different approaches should be explored to manufacture PBS and PBSA with a lower carbon footprint and environmentally sustainable approach.
• The sustainability of the manufacturing techniques can be validated using LCA studies. Sustainable agricultural practices are required for growing raw materials for synthesizing the monomers (like SA, BDO, and AA) required for PBS and PBSA synthesis. Proper EoL scenarios for PBS and PBSA should be formulated by recycling and biodegradation to avoid waste generation.
• The use of certain additives and natural fibers in various ratios and forms is expected to enhance the material's characteristics and functionality. Moreover, new applications of PBS and PBSA in other fields like automotive, biomedical, and water purification will be made possible by further research on their blends and composites.
• Finally, research on PBS and PBSA for practical application in real foods with enhanced antimicrobial capabilities should be performed extensively. This is to assess the safety of these polymers and commercialize them amid an increasing ban on polyolefin-based packaging materials by different governments. Usage of these polymers should be promoted in agricultural applications like mulch films and greenhouse covers.
Abbreviations
| AA | Adipic acid |
| BDO | 1,4-Butanediol |
| BOPP | Biaxially oriented polypropylene |
| CNC | Cellulose nanocrystal |
| DCP | Dicumyl peroxide |
| EAB | Elongation at break |
| EoL | End-of-life |
| GHG | Greenhouse gas |
| LCA | Life cycle assessment |
| MA | Maleic anhydride |
| MDI | Methylenediphenyl diisocyanate |
| MSP | Minimum selling price |
| OP | Oxygen permeability |
| OTR | Oxygen transmission rate |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| PBS | Poly(butylene succinate) |
| PBSA | Poly(butylene succinate-co-adipate) |
| PC | Polycarbonate |
| PCL | Poly(ε-caprolactone) |
| PE | Polyethylene |
| PHB | Polyhydroxybutyrate |
| PHBV | Poly(hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PPC | Polypropylene carbonate |
| PVC | Polyvinyl chloride |
| SA | Succinic acid |
| SEM | Scanning electron microscopy |
| T c | Crystallization temperature |
| TDI | Toluene diisocyanate |
| T g | Glass transition temperature |
| T m | Melting temperature |
| TS | Tensile strength |
| WVP | Water vapor permeability |
| WVTR | Water vapor transmission rate |
Data availability
This is a review article. All the data reported here can be found in the cited papers in the reference section.Author contributions
D. N.: methodology, investigation, data curation, formal analysis, visualization, writing – original draft. M. M.: conceptualization, investigation, methodology, validation, supervision, resources, funding acquisition and administration, writing – review & editing. F. A. D.: investigation, validation, writing – review & editing. A. K. M.: conceptualization, investigation, methodology, validation, supervision, resources, writing – review & editing. All authors contributed to the discussion, reviews, edits and approval of the manuscript for publication.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors would like to acknowledge the financial support from (i) the Ontario Agri-Food Innovation Alliance – Bioeconomy for Industrial Uses Research Program (Project No. 030578, 030671 and 030699); and the Gryphon's Leading to the Accelerated Adoption of Innovative Research (LAAIR) Program (Project No. 030736); (ii) the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chair (CRC) Program (Project No. 460788) and the NSERC, Canada Discovery Grants (Project No. 401716); and (iii) the Ontario Research Fund, Research Excellence Program Round 11 (ORF-RE11) from the Ontario Ministry of Colleges and Universities (Project No. 056106) in carrying out this study. This research also benefited from the facility funding to the Bioproducts Discovery and Development Centre (BDDC) lab by FedDev Ontario; Ontario Ministry of Agriculture, Food, and Agribusiness (OMAFA); Canada Foundation for Innovation (CFI); Federal Post-Secondary Institutions Strategic Investment Fund (SIF); and matching funds from the province of Ontario and numerous University of Guelph's Alumni. The authors would also like to thank our graphic designer, Kimberly Chin, Biomedical Illustration & Design, Toronto, ON, Canada for final designing Fig. 1 and 2 and the Graphical Abstract by incorporating ideas from the authors of this review paper.References
- F. Wu, M. Misra and A. K. Mohanty, Prog. Polym. Sci., 2021, 117, 101395 CrossRef CAS.
- D. Nath, S. R, K. Pal and P. Sarkar, Food Packag. Shelf Life, 2022, 31, 100803 CrossRef CAS.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS.
- A. Valdés, A. C. Mellinas, M. Ramos, M. C. Garrigós and A. Jiménez, Front. Chem., 2014, 2, 6 Search PubMed.
- S. Torres, R. Navia, R. Campbell Murdy, P. Cooke, M. Misra and A. K. Mohanty, ACS Sustain. Chem. Eng., 2015, 3, 614–624 CrossRef CAS.
- United Nations Environment Program, Visual Feature | Beat Plastic Pollution, https://www.unep.org/interactives/beat-plastic-pollution/, accessed 19 August 2023 Search PubMed.
- S. Janaswamy, M. P. Yadav, M. Hoque, S. Bhattarai and S. Ahmed, Ind. Crops Prod., 2022, 179, 114692 CrossRef.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- Annual Reports – Cleanfarms, https://cleanfarms.ca/annual-reports/, accessed 2 December 2023 Search PubMed.
- A. K. Mohanty, M. Misra and G. I. Hinrichsen, Macromol. Mater. Eng., 2000, 276, 1–24 CrossRef.
- Kureha America – Product Groups – PGA, https://kureha.com/product-groups/pga.htm, accessed 29 November 2024 Search PubMed.
- PGA, https://www.bmg-inc.com/en/products/biomaterial/pga, accessed 29 November 2024 Search PubMed.
- NatureWorks | Home, https://www.natureworksllc.com/, accessed 29 November 2024.
- TotalEnergies Corbion, https://www.totalenergies-corbion.com/, accessed 29 November 2024.
- Zhejiang Hisun Biomaterials Co., Ltd, PLA, Plant resources, https://en.hisunplas.com/, accessed 29 November 2024 Search PubMed.
- Futerro PLA leader in the bioplastics market | Futerro, https://www.futerro.com/, accessed 29 November 2024 Search PubMed.
- PHA: Beginning of Life – Danimer Scientific, https://danimerscientific.com/pha-beginning-of-life/, accessed 29 November 2024 Search PubMed.
- TianAn Biopolymer: Nature's Eco-Friendly Solution, https://en.tianan-enmat.com/, accessed 29 November 2024 Search PubMed.
- (PDF) ICI's BIOPOL Cautionary Tales – ScotCHEM – DOKUMEN.TIPS, https://dokumen.tips/documents/icis-biopol-cautionary-tales-scotchem.html?page=1, accessed 3 March 2024 Search PubMed.
- Perstorp completes sale of Capa™, https://www.perstorp.com/en/news_center/news/2019/perstorp_completes_sale_of_capa/, accessed 3 March 2024 Search PubMed.
- Capromer PD1-10, https://products.basf.com/global/en/ci/30468214, accessed 29 November 2024 Search PubMed.
- Biodegradable Polymer ‘BioPBS™’ | Products | Mitsubishi Chemical Corporation, https://www.m-chemical.co.jp/en/products/departments/mcc/sustainable/product/1201025_7964.html, accessed 29 November 2024 Search PubMed.
- Biodegradable Resin PBAT/PBS_Xinjiang Blue Ridge Tunhe Chemical Industry Joint Stock Co., Ltd, https://lanshantunhe.com/en/business/juzhi/chanpin/3038.html, accessed 29 November 2024 Search PubMed.
- Polybutylene Succinate, PBS CAS NO.: 25777-14-4-Lifechemical|1,4 Naphthoquinone|Flame retardant DDP|Polyaluminium Chloride|Anthraquinone|benzoquinone|, https://www.lifechemical.com/content/?179.html, accessed 29 November 2024 Search PubMed.
- Polybutylene Succinate Adipate/PBSA-Lifechemical|1,4 Naphthoquinone|Flame retardant DDP|Polyaluminium Chloride|Anthraquinone|benzoquinone|, https://www.lifechemical.com/content/?203.html, accessed 29 November 2024 Search PubMed.
- Ecoflex® (PBAT), https://plastics-rubber.basf.com/global/en/performance_polymers/products/ecoflex, accessed 29 November 2024 Search PubMed.
- Jin Hui Zhao Long High Tech Co., Ltd, https://www.jinhuizhaolong.com/en/, accessed 29 November 2024.
- O. Platnieks, S. Gaidukovs, V. Kumar Thakur, A. Barkane and S. Beluns, Eur. Polym. J., 2021, 161, 110855 CrossRef CAS.
- L. Liu, J. Yu, L. Cheng and W. Qu, Composites, Part A, 2009, 40, 669–674 CrossRef.
- A. R. de Matos Costa, A. Crocitti, L. H. de Carvalho, S. C. Carroccio, P. Cerruti and G. Santagata, Polymers, 2020, 12, 2317 CrossRef.
- L. Aliotta, M. Seggiani, A. Lazzeri, V. Gigante and P. Cinelli, Polymers, 2022, 14, 844 CrossRef CAS.
- M. Barletta, C. Aversa, M. Ayyoob, A. Gisario, K. Hamad, M. Mehrpouya and H. Vahabi, Prog. Polym. Sci., 2022, 132, 101579 CrossRef CAS.
- E. Pesaranhajiabbas, A. K. Pal, A. Rodriguez-Uribe, A. K. Mohanty and M. Misra, ACS Appl. Polym. Mater., 2022, 4, 5546–5556 CrossRef.
- P. Feijoo, A. K. Mohanty, A. Rodriguez-Uribe, J. Gámez-Pérez, L. Cabedo and M. Misra, Int. J. Biol. Macromol., 2023, 225, 1291–1305 CrossRef CAS PubMed.
- D. C. McNeill, A. K. Pal, A. K. Mohanty and M. Misra, Compos., Part C: Open Access, 2023, 12, 100388 CAS.
- J. Jian, Z. Xiangbin and H. Xianbo, Adv. Ind. Eng. Polym. Res., 2020, 3, 19–26 Search PubMed.
- R. Muthuraj, M. Misra and A. K. Mohanty, J. Appl. Polym. Sci., 2015, 132, 42189 CrossRef.
- M. I. Peñas, R. A. Pérez-Camargo, R. Hernández and A. J. Müller, Polymers, 2022, 14, 1025 CrossRef PubMed.
- R. Ribeiro-Santos, M. Andrade, N. R. de Melo and A. Sanches-Silva, Trends Food Sci. Technol., 2017, 61, 132–140 CrossRef CAS.
- C. v. Garcia, G. H. Shin and J. T. Kim, Trends Food Sci. Technol., 2018, 82, 21–31 CrossRef CAS.
- P. Chakraborty, D. Nath, M. Hoque, P. Sarkar, S. Hati and B. K. Mishra, J. Food Process. Preserv., 2022, 46, e16465 CAS.
- Ł. Łopusiewicz, M. Zdanowicz, S. Macieja, K. Kowalczyk and A. Bartkowiak, Polymers, 2021, 13, 1798 CrossRef PubMed.
- A. Sikder, A. K. Pearce, S. J. Parkinson, R. Napier and R. K. O'Reilly, ACS Appl. Polym. Mater., 2021, 3, 1203–1217 CrossRef CAS.
- K. W. Meereboer, M. Misra and A. K. Mohanty, Green Chem., 2020, 22, 5519–5558 RSC.
- R. Muthuraj, M. Misra and A. K. Mohanty, ACS Sustain. Chem. Eng., 2015, 3, 2767–2776 CrossRef CAS.
- N. Harder, A. Rodriguez-Uribe, M. R. Snowdon, M. Misra and A. K. Mohanty, Materials Advances, 2023, 4, 1502–1514 RSC.
- K. P. Root, A. K. Pal, E. Pesaranhajiabbas, A. K. Mohanty and M. Misra, Compos., Part C: Open Access, 2023, 100358 CAS.
- J. Xu and B. H. Guo, Biotechnol. J., 2010, 5, 1149–1163 CrossRef CAS.
- G. Q. Chen and M. K. Patel, Chem. Rev., 2012, 112, 2082–2099 CrossRef CAS.
- H. Song and S. Y. Lee, Enzyme Microb. Technol., 2006, 39, 352–361 CrossRef CAS.
- M. Crank, M. Patel, F. Marscheider-Weidemann, J. Schleich, B. Huesing and G. Angerer, inis.iaea.org, 2004, vol. 37, 13, NWS-E--2004-111, 37032239, https://inis.iaea.org/Search/search.aspx?orig_q=RN:37032239.
- H. Yim, R. Haselbeck, W. Niu, C. Pujol-Baxley, A. Burgard, J. Boldt, J. Khandurina, J. D. Trawick, R. E. Osterhout, R. Stephen, J. Estadilla, S. Teisan, H. B. Schreyer, S. Andrae, T. H. Yang, S. Y. Lee, M. J. Burk and S. van Dien, Nat. Chem. Biol., 2011,(7), 445–452 CrossRef CAS.
- S. Okino, R. Noburyu, M. Suda, T. Jojima, M. Inui and H. Yukawa, Appl. Microbiol. Biotechnol., 2008, 81, 459–464 CrossRef CAS PubMed.
- T. Zheng, B. Xu, Y. Ji, W. Zhang, F. Xin, W. Dong, P. Wei, J. Ma and M. Jiang, Biotechnol. Biofuels, 2021, 14, 1–10 CrossRef.
- S. A. Rafiqah, A. Khalina, A. S. Harmaen, I. A. Tawakkal, K. Zaman, M. Asim, M. N. Nurrazi and C. H. Lee, Polymers, 2021, 13, 1436 CrossRef CAS PubMed.
- E. Le Roux, Coord. Chem. Rev., 2016, 306, 65–85 CrossRef CAS.
- S. A. Ryken and L. L. Schafer, Acc. Chem. Res., 2015, 48, 2576–2586 CrossRef CAS PubMed.
- L. A. Wolzak, J. I. van der Vlugt, K. J. van den Berg, J. N. H. Reek, M. Tromp and T. J. Korstanje, ChemCatChem, 2020, 12, 5229–5235 CrossRef CAS.
- N. Jacquel, F. Freyermouth, F. Fenouillot, A. Rousseau, J. P. Pascault, P. Fuertes and R. Saint-Loup, J. Polym. Sci., Part A:Polym. Chem., 2011, 49, 5301–5312 CrossRef CAS.
- K. S. Savitha, B. Ravji Paghadar, M. Senthil Kumar and R. L. Jagadish, Polym. Chem., 2022, 13, 3562–3612 RSC.
- S. Velmathi, R. Nagahata, J. I. Sugiyama and K. Takeuchi, Macromol. Rapid Commun., 2005, 26, 1163–1167 CrossRef CAS.
- A. Takasu, Y. Oishi, Y. Iio, Y. Inai and T. Hirabayashi, Macromolecules, 2003, 36, 1772–1774 CrossRef CAS.
- C. H. Chen, J. S. Peng, M. Chen, H. Y. Lu, C. J. Tsai and C. Sen Yang, Colloid Polym. Sci., 2010, 288, 731–738 Search PubMed.
- Y. K. Han, S. R. Kim and J. Kim, Macromol. Res., 2002, 10, 108–114 CrossRef CAS.
- C. W. Lee, K. Masutani and Y. Kimura, Polymer, 2014, 55, 5673–5679 CrossRef CAS.
- M. Ayyoob, S. Lee and Y. J. Kim, J. Polym. Res., 2020, 27, 1–12 Search PubMed.
- A. Fradet and E. Marechal, J. Macromol. Sci., Chem., 1982, 17, 881–891 Search PubMed.
- A. B. Ferreira, A. L. Cardoso and M. J. da Silva, Int. Scholarly Res. Not., 2012, 2012, 142857 Search PubMed.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, J. Polym. Sci., Part A:Polym. Chem., 2018, 56, 1915–1925 CrossRef CAS.
- C. Labruyère, O. Talon, N. Berezina, E. Khousakoun and C. Jérôme, RSC Adv., 2014, 4, 38643–38648 RSC.
- C. Q. Huang, S. Y. Luo, S. Y. Xu, J. B. Zhao, S. L. Jiang and W. T. Yang, J. Appl. Polym. Sci., 2010, 115, 1555–1565 CrossRef CAS.
- H. Jin, D. Kim, B. Lee, M. Kim, I. Lee, H. Lee and J. Yoon, J. Polym. Sci., Part B:Polym. Phys., 2000, 38, 2240–2246 CrossRef CAS.
- L. Zheng, C. Li, W. Huang, X. Huang, D. Zhang, G. Guan, Y. Xiao and D. Wang, Polym. Adv. Technol., 2011, 22, 279–285 CrossRef CAS.
- D. Pospiech, R. Choińska, D. Flugrat, K. Sahre, D. Jehnichen, A. Korwitz, P. Friedel, A. Werner and B. Voit, Processes, 2021,(9), 411 CrossRef CAS.
- L. Ren, Y. Wang, J. Ge, D. Lu and Z. Liu, Macromol. Chem. Phys., 2015, 216, 636–640 CrossRef CAS.
- T. Debuissy, E. Pollet and L. Avérous, Biomacromolecules, 2016, 17, 4054–4063 CrossRef CAS.
- C. Ciulik, M. Safari, A. Martínez de Ilarduya, J. C. Morales-Huerta, A. Iturrospe, A. Arbe, A. J. Müller and S. Muñoz-Guerra, Eur. Polym. J., 2017, 95, 795–808 CrossRef CAS.
- S. Sugihara, K. Toshima and S. Matsumura, Macromol. Rapid Commun., 2006, 27, 203–207 CrossRef CAS.
- M. Gigli, M. Fabbri, N. Lotti, R. Gamberini, B. Rimini and A. Munari, Eur. Polym. J., 2016, 75, 431–460 CrossRef CAS.
- M. S. Nikolic and J. Djonlagic, Polym. Degrad. Stab., 2001, 74, 263–270 CrossRef CAS.
- T. Polen, M. Spelberg and M. Bott, J. Biotechnol., 2013, 167, 75–84 CrossRef CAS.
- J. Rios, J. Lebeau, T. Yang, S. Li and M. D. Lynch, Green Chem., 2021, 23, 3172–3190 RSC.
- M. T. Musser, Ullmann's Encyclopedia of Industrial Chemistry, DOI:10.1002/14356007.A01_269.
- J. C. J. Bart and S. Cavallaro, Ind. Eng. Chem. Res., 2015, 54, 567–576 CrossRef CAS.
- T. Nakajima-Kambe, K. Toyoshima, C. Saito, H. Takaguchi, Y. Akutsu-Shigeno, M. Sato, K. Miyama, N. Nomura and H. Uchiyama, J. Biosci. Bioeng., 2009, 108, 513–516 Search PubMed.
- P. Jin, Z. Zhao, Z. Dai, D. Wei, M. Tang and X. Wang, Catal. Today, 2011, 175, 619–624 CrossRef CAS.
- Y. Wen, X. Wang, H. Wei, B. Li, P. Jin and L. Li, Green Chem., 2012, 14, 2868–2875 RSC.
- US3361806A – Process for oxidizing cyclohexane to adipic acid – Google Patents, https://patents.google.com/patent/US3361806A/en, accessed 14 October 2022 Search PubMed.
- Y. Deng and Y. Mao, J. Appl. Microbiol., 2015, 119, 1057–1063 CrossRef CAS.
- J. le Yu, X. X. Xia, J. J. Zhong and Z. G. Qian, Biotechnol. Bioeng., 2014, 111, 2580–2586 CrossRef.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248–2263 CrossRef CAS PubMed.
- T. S. Moon, S. H. Yoon, A. M. Lanza, J. D. Roy-Mayhew and K. L. Jones Prather, Appl. Environ. Microbiol., 2009, 75, 589–595 CrossRef CAS PubMed.
- H. M. Jung, M. Y. Jung and M. K. Oh, Appl. Microbiol. Biotechnol., 2015, 99, 5217–5225 CrossRef CAS.
- D. R. Vardon, N. A. Rorrer, D. Salvachúa, A. E. Settle, C. W. Johnson, M. J. Menart, N. S. Cleveland, P. N. Ciesielski, K. X. Steirer, J. R. Dorgan and G. T. Beckham, Green Chem., 2016, 18, 3397–3413 RSC.
- T. Debuissy, E. Pollet and L. Avérous, Eur. Polym. J., 2017, 87, 84–98 CrossRef CAS.
- T. Fujimaki, Polym. Degrad. Stab., 1998, 59, 209–214 CrossRef CAS.
- L. P. Ferreira, A. N. Moreira, J. C. Pinto and F. G. de Souza, Polym. Eng. Sci., 2015, 55, 1889–1896 CrossRef CAS.
- B. D. Ahn, S. H. Kim, Y. H. Kim and J. S. Yang, J. Appl. Polym. Sci., 2001, 82, 2808–2826 Search PubMed.
- V. Tserki, P. Matzinos, E. Pavlidou, D. Vachliotis and C. Panayiotou, Polym. Degrad. Stab., 2006, 91, 367–376 CrossRef CAS.
- V. Tserki, P. Matzinos, E. Pavlidou and C. Panayiotou, Polym. Degrad. Stab., 2006, 91, 377–384 CrossRef CAS.
- C. S. Cho, D. T. Kim, H. J. Choi, T. J. Kim and S. C. Shim, Bull. Korean Chem. Soc., 2002, 23, 539–540 Search PubMed.
- C. Zhu, Z. Zhang, Q. Liu, Z. Wang and J. Jin, J. Appl. Polym. Sci., 2003, 90, 982–990 Search PubMed.
- T. Debuissy, E. Pollet and L. Avérous, Eur. Polym. J., 2017, 97, 328–337 CrossRef CAS.
- A. Nandakumar, J. A. Chuah and K. Sudesh, Renewable Sustainable Energy Rev., 2021, 147, 111237 CrossRef CAS.
- M. Vert, Y. Doi, K. H. Hellwich, M. Hess, P. Hodge, P. Kubisa, M. Rinaudo and F. Schué, Pure Appl. Chem., 2012, 84, 377–410 CrossRef CAS.
- S. S. Ali, E. A. Abdelkarim, T. Elsamahy, R. Al-Tohamy, F. Li, M. Kornaros, A. Zuorro, D. Zhu and J. Sun, Environ. Sci. Ecotechnology, 2023, 15, 100254 CrossRef CAS PubMed.
- M. Carus and L. Dammer, Ind. Biotechnol., 2013, 9, 171–176 CrossRef.
- A. Di Bartolo, G. Infurna and N. T. Dintcheva, Polymers, 2021, 13, 1229 CrossRef CAS PubMed.
- J. Brizga, K. Hubacek and K. Feng, One Earth, 2020, 3, 45–53 CrossRef.
- G. Vinci, R. Ruggieri, A. Billi, C. Pagnozzi, M. V. Di Loreto and M. Ruggeri, Sustainability, 2021, 13, 6385 CrossRef CAS.
- H. I. Moussa, A. Elkamel and S. B. Young, J. Cleaner Prod., 2016, 139, 761–769 CrossRef CAS.
- S. Gadkari, D. Kumar, Z. hao Qin, C. S. Ki Lin and V. Kumar, Waste Manage., 2021, 126, 861–871 CrossRef CAS.
- S. Bello, D. Ladakis, S. González-García, G. Feijoo, A. Koutinas and M. T. Moreira, Chem. Eng. J., 2022, 428, 132011 CrossRef CAS.
- F. Adom, J. B. Dunn, J. Han and N. Sather, Environ. Sci. Technol., 2014, 48, 14624–14631 CrossRef CAS.
- C. C. Satam, M. Daub and M. J. Realff, Biofuels, Bioprod. Biorefin., 2019, 13, 1261–1273 CrossRef CAS.
- N. Rajendran and J. Han, Waste Manage., 2023, 156, 168–176 Search PubMed.
- S. M. Ioannidou, D. Ladakis, E. Moutousidi, E. Dheskali, I. K. Kookos, I. Câmara-Salim, M. T. Moreira and A. Koutinas, Sci. Total Environ., 2022, 806, 150594 CrossRef CAS PubMed.
- R. Rebolledo-Leiva, D. Ladakis, S. M. Ioannidou, A. Koutinas, M. T. Moreira and S. González-García, Sustainable Mater. Technol., 2023, 37, e00683 CrossRef CAS.
- A. Corona, M. J. Biddy, D. R. Vardon, M. Birkved, M. Z. Hauschild and G. T. Beckham, Green Chem., 2018, 20, 3857–3866 RSC.
- R. Aryapratama and M. Janssen, J. Cleaner Prod., 2017, 164, 434–443 CrossRef CAS.
- J. B. J. H. Van Duuren, B. Brehmer, A. E. Mars, G. Eggink, V. A. P. M. dos Santos and J. P. M. Sanders, Biotechnol. Bioeng., 2011, 108, 1298–1306 CrossRef CAS.
- R. Muthuraj, M. Misra and A. K. Mohanty, J. Appl. Polym. Sci., 2018, 135, 45726 CrossRef.
- E. Pesaranhajiabbas, M. Misra and A. K. Mohanty, Int. J. Biol. Macromol., 2023, 126231 CrossRef CAS.
- K. Zhang, A. K. Mohanty and M. Misra, ACS Appl. Mater. Interfaces, 2012, 4, 3091–3101 CrossRef CAS.
- L. Henke, N. Zarrinbakhsh, H. J. Endres, M. Misra and A. K. Mohanty, J. Polym. Environ., 2017, 25, 499–509 CrossRef CAS.
- M. R. Nobile, A. Crocitti, M. Malinconico, G. Santagata and P. Cerruti, AIP Conf. Proc., 2018, 1981(1), 020180 CrossRef.
- S. Saengbunkoet, N. Kerddonfag, N. Puekpoonpoal, P. Kumsang, R. Yoksan and P. Jariyasakoolroj, Polymer, 2022, 249, 124859 CrossRef CAS.
- R. G. Hill, Biomaterials, Artificial Organs and Tissue Engineering, 2005, pp. 97–106 Search PubMed.
- E. Luoma, T. Rokkonen, A. Tribot, K. Nättinen and J. Lahtinen, Polym. Renewable Resour., 2022, 2022, 1–19 Search PubMed.
- G. Jiang, H. Wang, L. Yu and H. Li, Polym. Eng. Sci., 2022, 62, 1166–1177 CrossRef CAS.
- N. Puekpoonpoal, S. Phattarateera, N. Kerddonfag and D. Aht-Ong, Polym.-Plast. Technol. Mater., 2021, 60, 1672–1685 CAS.
- W. Pivsa-Art, S. Pavasupree, N. O-Charoen, U. Insuan, P. Jailak and S. Pivsa-Art, Energy Procedia, 2011, 9, 581–588 CrossRef CAS.
- T. Yokohara and M. Yamaguchi, Eur. Polym. J., 2008, 44, 677–685 CrossRef CAS.
- S. Su, R. Kopitzky, S. Tolga and S. Kabasci, Polymers, 2019, 11, 1193 CrossRef PubMed.
- Y. Deng and N. L. Thomas, Eur. Polym. J., 2015, 71, 534–546 Search PubMed.
- J. Ostrowska, W. Sadurski, M. Paluch, P. Tyński and J. Bogusz, Polym. Int., 2019, 68, 1271–1279 CrossRef CAS.
- R. Supthanyakul, N. Kaabbuathong and S. Chirachanchai, Polymer, 2016, 105, 1–9 CrossRef CAS.
- R. Supthanyakul, N. Kaabbuathong and S. Chirachanchai, Polym. Degrad. Stab., 2017, 142, 160–168 Search PubMed.
- B. Tan, S. Bi, K. Emery and M. J. Sobkowicz, Eur. Polym. J., 2017, 86, 162–172 CrossRef CAS.
- W. Phetwarotai, H. Maneechot, E. Kalkornsurapranee and N. Phusunti, Polym. Adv. Technol., 2018, 29, 2121–2133 CrossRef CAS.
- B. A. Calderón, M. S. McCaughey, C. W. Thompson and M. J. Sobkowicz, Ind. Eng. Chem. Res., 2019, 58, 487–495 CrossRef.
- P. Ma, D. G. Hristova-Bogaerds, P. J. Lemstra, Y. Zhang and S. Wang, Macromol. Mater. Eng., 2012, 297, 402–410 CrossRef CAS.
- B. Palai, S. Mohanty and S. K. Nayak, Polym. Test., 2020, 83, 106130 CrossRef CAS.
- A. Surendren, A. K. Pal, A. Rodriguez-Uribe, S. Shankar, L. T. Lim, A. K. Mohanty and M. Misra, Int. J. Biol. Macromol., 2023, 253, 126751 CrossRef CAS PubMed.
- E. Fortunati, D. Puglia, A. Iannoni, A. Terenzi, J. M. Kenny and L. Torre, Materials, 2017, 10, 809 CrossRef PubMed.
- P. Tummala, W. Liu, L. T. Drzal, A. K. Mohanty and M. Misra, Ind. Eng. Chem. Res., 2006, 45, 7491–7496 CrossRef CAS.
- V. Gigante, M. B. Coltelli, A. Vannozzi, L. Panariello, A. Fusco, L. Trombi, G. Donnarumma, S. Danti and A. Lazzeri, Polymers, 2019, 11, 1857 Search PubMed.
- F. M. Fakhouri, L. C. B. Fontes, L. H. Innocentini-Mei and F. P. Collares-Queiroz, Starch/Staerke, 2009, 61, 528–536 CrossRef CAS.
- N. Ratsameetammajak, R. Molloy and R. Somsunan, Plast., Rubber Compos., 2018, 47, 139–146 CrossRef CAS.
- R. K. Thakur and K. K. Singh, J. Braz. Soc. Mech. Sci. Eng., 2021, 43, 1–20 Search PubMed.
- B. E. Itabana, A. K. Pal, A. K. Mohanty and M. Misra, Food Packag. Shelf Life, 2023, 39, 101147 CrossRef CAS.
- D. Nath, A. K. Pal, M. Misra and A. K. Mohanty, Macromol. Mater. Eng., 2023, 2300214 Search PubMed.
- M. Schmid, C. Herbst, K. Müller, A. Stäbler, D. Schlemmer, M. B. Coltelli and A. Lazzeri, Polym.-Plast. Technol. Eng., 2016, 55, 510–517 Search PubMed.
- H. Liu, C. Si, X. He, L. Tang, J. Zheng, Y. Jin, R. Chang, X. Yu, Y. Song and R. Huang, Polymers, 2023, 15, 3000 CrossRef PubMed.
- S. K. Su and C. S. Wu, J. Appl. Polym. Sci., 2011, 119, 1211–1219 CrossRef CAS.
- A. K. Pal, M. Misra and A. K. Mohanty, Int. J. Biol. Macromol., 2023, 229, 1009–1022 CrossRef CAS PubMed.
- M. Zhou, Y. Li, C. He, T. Jin, K. Wang and Q. Fu, Compos. Sci. Technol., 2014, 91, 22–29 CrossRef CAS.
- B. P. Calabia, F. Ninomiya, H. Yagi, A. Oishi, K. Taguchi, M. Kunioka and M. Funabashi, Polymers, 2013, 5, 128–141 CrossRef.
- C. López de Dicastillo, E. Velásquez, A. Rojas, A. Guarda and M. J. Galotto, Compr. Rev. Food Sci. Food Saf., 2020, 19, 1760–1776 CrossRef PubMed.
- J. Xu, P. H. Manepalli, L. Zhu, S. Narayan-Sarathy and S. Alavi, J. Polym. Res., 2019, 26, 1–10 CrossRef CAS.
- A. Delavarde, G. Savin, P. Derkenne, M. Boursier, R. Morales-Cerrada, B. Nottelet, J. Pinaud and S. Caillol, Prog. Polym. Sci., 2024, 101805 Search PubMed.
- J. Niesiobędzka and J. Datta, Green Chem., 2023, 25, 2482–2504 Search PubMed.
- Y. D. Li, Q. Q. Fu, M. Wang and J. B. Zeng, Carbohydr. Polym., 2017, 164, 75–82 CrossRef CAS PubMed.
- X. Zhang and Y. Zhang, Carbohydr. Polym., 2015, 134, 52–59 CrossRef CAS PubMed.
- Y. Zhang, S. Zhou, X. Fang, X. Zhou, J. Wang, F. Bai and S. Peng, Eur. Polym. J., 2019, 116, 265–274 CrossRef CAS.
- N. Kurokawa, K. Matsumoto and A. Hotta, Compos. Sci. Technol., 2022, 223, 109402 CrossRef CAS.
- O. Platnieks, A. Sereda, S. Gaidukovs, V. K. Thakur, A. Barkane, G. Gaidukova, I. Filipova, A. Ogurcovs and V. Fridrihsone, Ind. Crops Prod., 2021, 169, 113669 CrossRef CAS.
- E. Fortunati, J. M. Kenny and L. Torre, Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications, 2019, pp. 87–102 Search PubMed.
- H. Pulikkalparambil, D. Phothisarattana, K. Promhuad and N. Harnkarnsujarit, Food Biosci., 2023, 55, 103023 CrossRef CAS.
- I. S. Bayer, Sustainable Food Packag. Technol., 2021, 395–426 CAS.
- T. Messin, N. Follain, Q. Lozay, A. Guinault, N. Delpouve, J. Soulestin, C. Sollogoub and S. Marais, Nanomaterials, 2020, 10, 2561 Search PubMed.
- M. Soccio, F. Dominici, S. Quattrosoldi, F. Luzi, A. Munari, L. Torre, N. Lotti and D. Puglia, Biomacromolecules, 2020, 21, 3254–3269 CrossRef CAS PubMed.
- J. Saeng-on and D. Aht-Ong, J. Appl. Polym. Sci., 2018, 135, 46836 CrossRef.
- V. Sivanjineyulu, K. Behera, Y. H. Chang and F. C. Chiu, Composites, Part A, 2018, 114, 30–39 CrossRef CAS.
- E. D. Flores, M. Funabashi and M. Kunloka, J. Appl. Polym. Sci., 2009, 112, 3410–3417 CrossRef CAS.
- P. Threepopnatkul, N. Charoendee, N. Wiriyamontree and A. Phodaeng, J. Phys.:Conf. Ser., 2022, 2175, 012023 CrossRef.
- G. Jiang and L. Yu, Macromol. Mater. Eng., 2021, 306, 2000723 CrossRef CAS.
- L. Maubane, S. S. Ray and K. Jalama, Carbohydr. Polym., 2017, 155, 89–100 CrossRef CAS PubMed.
- B. Le Delliou, O. Vitrac, A. Benihya, P. Dole and S. Domenek, Polym. Test., 2023, 124, 108072 Search PubMed.
- D. Nath, R. Santhosh, J. Ahmed and P. Sarkar, J. Food Process Eng., 2022, 45, e14065 CrossRef CAS.
- P. Suwanamornlert, N. Kerddonfag, A. Sane, W. Chinsirikul, W. Zhou and V. Chonhenchob, Food Packag. Shelf Life, 2020, 25, 100515 CrossRef.
- P. Srimalanon, B. Prapagdee, T. Markpin and N. Sombatsompop, Polym. Test., 2018, 67, 331–341 Search PubMed.
- N. Petchwattana and P. Naknaen, Mater. Chem. Phys., 2015, 163, 369–375 Search PubMed.
- F. Cicogna, E. Passaglia, M. Benedettini, W. Oberhauser, R. Ishak, F. Signori and S. Coiai, Molecules, 2023, 28, 347 CrossRef CAS PubMed.
- A. Bahrami, R. Delshadi, E. Assadpour, S. M. Jafari and L. Williams, Adv. Colloid Interface Sci., 2020, 278, 102140 CrossRef CAS PubMed.
- A. Fernández, E. Soriano, P. Hernández-Muñoz and R. Gavara, J. Food Sci., 2010, 75, E186–E193 Search PubMed.
- N. Wattanawong and D. Aht-Ong, Polym. Degrad. Stab., 2021, 183, 109459 Search PubMed.
- T. Mhlabeni, S. Kesavan Pillai and S. S. Ray, J. Appl. Polym. Sci., 2020, 137, 48654 Search PubMed.
- A. J. Basbasan, B. Hararak, C. Winotapun, W. Wanmolee, W. Chinsirikul, P. Leelaphiwat, V. Chonhenchob and K. Boonruang, Polymers, 2023, 15, 989 CrossRef CAS PubMed.
- Ł. Łopusiewicz, S. Macieja, A. Bartkowiak and M. El Fray, Materials, 2021, 14, 7882 Search PubMed.
- N. Petchwattana, S. Covavisaruch, S. Wibooranawong and P. Naknaen, Measurement, 2016, 93, 442–448 Search PubMed.
- N. Aziman, L. K. Kian, M. Jawaid, M. Sanny and S. Alamery, Polymers, 2021, 13, 391 CrossRef CAS PubMed.
- L. Panariello, M. B. Coltelli, A. Hadrich, F. Braca, S. Fiori, A. Haviv, F. Miketa, A. Lazzeri, A. Staebler, V. Gigante and P. Cinelli, Polymers, 2022, 14, 5211 Search PubMed.
- P. Pleva, L. Bartošová, D. Máčalová, L. Zálešáková, J. Sedlaříková and M. Janalíková, Foods, 2022, 11, 2 Search PubMed.
- D. Nath, A. K. Mohanty, A. Mukherjee, S. Kumar and M. Misra, Smart Food Packaging Systems, 2024, pp. 39–72 Search PubMed.
- N. Mohamad, M. M. Mazlan, I. S. M. A. Tawakkal, R. A. Talib, L. K. Kian and M. Jawaid, J. Polym. Environ., 2022, 30, 585–596 Search PubMed.
- S. Vytejčková, L. Vápenka, J. Hradecký, J. Dobiáš, J. Hajšlová, C. Loriot, L. Vannini and J. Poustka, Polym. Test., 2017, 60, 357–364 CrossRef.
- C. Yang, H. Tang, Y. Wang, Y. Liu, J. Wang, W. Shi and L. Li, Food Packag. Shelf Life, 2019, 22, 100393 CrossRef.
- F. Diez-Gonzalez, Encyclopedia of Food Microbiology, 2nd edn, 2014, pp. 630–635 Search PubMed.
- S. Samapundo, F. Devlieghere, A. Vroman and M. Eeckhout, LWT–Food Sci. Technol., 2017, 76, 101–107 CrossRef CAS.
- S. A. Varghese, D. Phothisarattana, A. Srisa, Y. Laorenza, L. Jarupan, N. Bumbudsanpharoke, V. Chonhenchob and N. Harnkarnsujarit, Food Biosci., 2023, 102993 CrossRef CAS.
- W. Hu, F. Gong, Q. Dong, Y. Kang, Y. Zhou, B. Liu and L. Li, J. Food Sci., 2023, 88, 2496–2511 CrossRef CAS PubMed.
- P. Wongphan, P. Nampanya, W. Chakpha, K. Promhuad, Y. Laorenza, P. Leelaphiwat, N. Bumbudsanpharoke, J. Sodsai, J. M. Lorenzo and N. Harnkarnsujarit, Food Packag. Shelf Life, 2023, 37, 101077 CrossRef CAS.
- N. Petchwattana, P. Naknaen, K. Cha-aim, C. Suksri and J. Sanetuntikul, Int. J. Biol. Macromol., 2021, 189, 251–261 CrossRef CAS PubMed.
- M. Llana-Ruíz-Cabello, M. Puerto, S. Pichardo, N. T. Jiménez-Morillo, J. M. Bermúdez, S. Aucejo, A. M. Camean and J. A. González-Pérez, Food Packag. Shelf Life, 2019, 22, 100410 CrossRef.
- N. Leabwan, K. Loylerd, P. Enmak, S. Changprasert, A. Korppiboon and S. Tengrang, in International Symposium on Durian and Other Humid Tropical Fruits 1186, 2015, pp. 177–184 Search PubMed.
- C. Maraveas, Sustainability, 2019, 11, 6129 CrossRef CAS.
- G. Pelletier, C. Maraveas, M. I. Kotzabasaki, I. S. Bayer and T. Bartzanas, AgriEngineering, 2023, 5, 1347–1377 CrossRef.
- A. Rodriguez-Uribe, T. Wang, A. K. Pal, F. Wu, A. K. Mohanty and M. Misra, Compos., Part C: Open Access, 2021, 6, 100201 CAS.
- H. Karan, C. Funk, M. Grabert, M. Oey and B. Hankamer, Trends Plant Sci., 2019, 24, 237–249 CrossRef CAS PubMed.
- M. Seggiani, R. Altieri, P. Cinelli, A. Esposito and A. Lazzeri, J. Polym. Environ., 2021, 29, 392–403 CrossRef CAS.
- J. M. Bressanin, I. L. de M. Sampaio, V. C. Geraldo, B. C. Klein, M. F. Chagas, A. Bonomi, R. M. Filho and O. Cavalett, Sustain. Prod. Consum., 2022, 34, 244–256 CrossRef.
- S. D. Palanivelu, N. A. Z. Armir, A. Zulkifli, A. H. A. Hair, K. M. Salleh, K. Lindsey, M. H. Che-Othman and S. Zakaria, Polymers, 2022, 14, 2590 CrossRef CAS PubMed.
- S. Deepthi, C. V. S. Viha, C. Thitirat, T. Furuike, H. Tamura and R. Jayakumar, Polymers, 2019, 11, 466 CrossRef PubMed.
- Film Farming Revolutionizes How we Grow Fruits and Vegetables, https://www.intelligentliving.co/film-farming-revolutionizes-how-we-grow-fruits-and-vegetables/, accessed 27 October 2023 Search PubMed.
- Y. Mori, React. Funct. Polym., 2013, 73, 936–938 CrossRef CAS.
- Z. Zhang, M. Rod and F. Hosseinian, Sustain. Agric. Res., 2021, 10, 46–53 Search PubMed.
- M. Sander, Environ. Sci. Technol., 2019, 53, 2304–2315 CrossRef CAS PubMed.
- M. A. M. Akhir and M. Mustapha, Polym. Rev., 2022, 62, 890–918 CrossRef CAS.
- R. S. Ayu, A. Khalina, A. S. Harmaen, K. Zaman, N. Mohd Nurrazi, T. Isma and C. H. Lee, Sci. Rep., 2020, 10, 1–7 CrossRef PubMed.
- Z. Wang, Q. Wu, B. Fan, J. Zhang, W. Li, X. Zheng, H. Lin and L. Guo, Soil Tillage Res., 2019, 192, 196–205 CrossRef.
- S. Bi, H. Pan, V. Barinelli, B. Eriksen, S. Ruiz and M. J. Sobkowicz, J. Cleaner Prod., 2021, 294, 126348 CrossRef CAS.
- J. Song, Y. Dou, Y. Niu and N. He, Polym. Test., 2021, 95, 107137 Search PubMed.
- K. Yamamoto-Tamura, S. Hiradate, T. Watanabe, M. Koitabashi, Y. Sameshima-Yamashita, T. Yarimizu and H. Kitamoto, AMB Express, 2015, 5, 1–8 CrossRef CAS PubMed.
- M. Koitabashi, M. T. Noguchi, Y. Sameshima-Yamashita, H. Syuntaro, K. Suzuki, S. Yoshida, T. Watanabe, Y. Shinozaki, S. Tsushima and H. K. Kitamoto, AMB Express, 2012, 2, 1–10 CrossRef PubMed.
- Biobased, biodegradable and compostable plastics – European Commission, https://environment.ec.europa.eu/topics/plastics/biobased-biodegradable-and-compostable-plastics_en#what-are-biobased-biodegradable-and-compostable-plastics, accessed 2 March 2024 Search PubMed.
- A. K. Mohanty, F. Wu, R. Mincheva, M. Hakkarainen, J. M. Raquez, D. F. Mielewski, R. Narayan, A. N. Netravali and M. Misra, Nat. Rev. Methods Primers, 2022, 2, 1–27 CrossRef.
- G. Fredi and A. Dorigato, Adv. Ind. Eng. Polym. Res., 2021, 4, 159–177 CAS.
- R. Mouhoubi, M. Lasschuijt, S. Ramon Carrasco, H. Gojzewski and F. R. Wurm, Waste Manage., 2022, 154, 36–48 CrossRef CAS PubMed.
- S. Kliem, M. Kreutzbruck and C. Bonten, Materials, 2020, 13, 4586 CrossRef CAS PubMed.
- BPI – Certification FAQs, https://bpiworld.org/certification-faq#group-sublicense, accessed 12 October 2023 Search PubMed.
- N. Nomadolo, O. E. Dada, A. Swanepoel, T. Mokhena and S. Muniyasamy, Polymers, 2022, 14, 1894 CrossRef CAS PubMed.
- J. H. Zhao, X. Q. Wang, J. Zeng, G. Yang, F. H. Shi and Q. Yan, J. Appl. Polym. Sci., 2005, 97, 2273–2278 CrossRef CAS.
- H. L. Chien, Y. T. Tsai, W. S. Tseng, J. A. Wu, S. L. Kuo, S. L. Chang, S. J. Huang and C. Te Liu, Polymers, 2022, 14, 1320 CrossRef CAS PubMed.
- H. S. Kim, H. J. Kim, J. W. Lee and I. G. Choi, Polym. Degrad. Stab., 2006, 91, 1117–1127 CrossRef CAS.
- L. Liu, J. Yu, L. Cheng and X. Yang, Polym. Degrad. Stab., 2009, 94, 90–94 CrossRef CAS.
- H. S. Yang, J. S. Yoon and M. N. Kim, Polym. Degrad. Stab., 2005, 87, 131–135 CrossRef CAS.
- C. S. Wu, J. Appl. Polym. Sci., 2011, 121, 427–435 CrossRef CAS.
- M. Salomez, M. George, P. Fabre, F. Touchaleaume, G. Cesar, A. Lajarrige and E. Gastaldi, Polym. Degrad. Stab., 2019, 167, 102–113 CrossRef CAS.
- N. Hayase, H. Yano, E. Kudoh, C. Tsutsumi, K. Ushio, Y. Miyahara, S. Tanaka and K. Nakagawa, J. Biosci. Bioeng., 2004, 97, 131–133 CrossRef CAS PubMed.
- S. M. Emadian, T. T. Onay and B. Demirel, Waste Manage., 2017, 59, 526–536 CrossRef CAS PubMed.
- M. van der Zee, M. Zijlstra, L. J. Kuijpers, M. Hilhorst, K. Molenveld and W. Post, Polym. Test., 2024, 140, 108601 CrossRef CAS.
- G160 Standard Practice for Evaluating Microbial Susceptibility of Nonmetallic Materials By Laboratory Soil Burial, https://www.astm.org/standards/g160, accessed 2 November 2024 Search PubMed.
- A. Hoshino, H. Sawada, M. Yokota, M. Tsuji, K. Fukuda and M. Kimura, Soil Sci. Plant Nutr., 2001, 47, 35–43 CrossRef.
- Y. J. Phua, N. S. Lau, K. Sudesh, W. S. Chow and Z. A. Mohd Ishak, Polym. Degrad. Stab., 2012, 97, 1345–1354 CrossRef CAS.
- F. De Falco, R. Avolio, M. E. Errico, E. Di Pace, M. Avella, M. Cocca and G. Gentile, J. Hazard. Mater., 2021, 416, 126231 CrossRef CAS PubMed.
- Y. Jin, X. Sun, C. Song, F. Cai, G. Liu and C. Chen, Sci. Total Environ., 2023, 873, 162324 CrossRef CAS PubMed.
- J. G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- J. Hopewell, R. Dvorak and E. Kosior, Philos. Trans. R. Soc., B, 2009, 364, 2115–2126 CrossRef CAS PubMed.
- J. Wang, C. Peng, H. Li, P. Zhang and X. Liu, Sci. Total Environ., 2021, 773, 145697 CrossRef CAS PubMed.
- A. C. Albertsson and M. Hakkarainen, Science, 2017, 358, 872–873 CrossRef CAS PubMed.
- B. D. Vogt, K. K. Stokes and S. K. Kumar, ACS Appl. Polym. Mater., 2021, 3, 4325–4346 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |

