Open Access Article
Open Access ArticleCNSL-based plasticizers, a promising and sustainable alternative to phthalates, a review
A.
Gartili
ab,
V.
Lapinte
 a,
S.
Caillol
a,
S.
Caillol
 a,
B.
Briou
a,
B.
Briou
 *b and
L.
Jego
b
*b and
L.
Jego
b
aICGM, Univ Montpellier, CNRS, ENSCM, Montpellier, France
bOrpia Innovation, Paris/Montpellier, France. E-mail: b.briou@orpiainnovation.com
First published on 25th October 2024
Abstract
With growing environmental concerns and the depletion of petrochemical resources, biomass-derived chemicals have garnered significant attention. Biomass-derived plasticizers have been widely studied as alternatives to toxic petroleum-based plasticizers. However, the bioressources used for their synthesis, an inedible oil derived from agricultural waste, containing cardanol, cardol and anacardic acid, is attracting new interest. Recent research has focused on cardanol-based plasticizers for various polymers such as PVC, PLA, AC and rubber. Cardanol-based biobased plasticizers offer advantages such as renewability, solvent-resistant extraction and efficient plasticizing performance, making them potentially suitable for partial or total replacement of petroleum-based plasticizers. In this study, we discuss the different types of cardanol-based plasticizers according to their chemical structure, functional groups and applications in polymers. The aim of this study is to increase the interest of researchers in biobased plasticizers based on CNSL derivatives.
Sustainability spotlightThis article focuses on the research and development of efficient bio-based plasticizers from CNSL. Coming from an abundant resource that does not compete with production dedicated to animal and human food, plasticizers derived from CNSL are a relevant and sustainable solution to a problem concerning both environmental and human health. Several polymers are affected: PVC, PLA, cellulose acetate, and rubber. The challenge is twofold. First, for the plasticization of PVC, the vast majority of plasticizers used belong to the phthalate family. These petroleum-based plasticizers are limited or even severely regulated because of their dangerousness: toxicity and endocrine disruption. In addition, plasticizers, over time, migrate to the surface of the polymer and may contaminate the contents. Secondly, the increase in the development of bio-based polymers such as cellulose acetate or PLA is leading to a need for effective, biodegradable, and non-hazardous bio-based additives. Again, at the moment, a large part of the plasticizers used are phthalates. |
Introduction
Our world and today's societies are going through a period of profound transformation, marked by more frequent and intense crises.1,2 Climate change, and the economic, political and ecological disruptions associated with it, are profoundly affecting our collective consciousness and shaping future approaches to development. Consumers, as well as nations and industries, are increasingly attentive to the origin of products, the impact of production processes, and effects on human health and environmental consequences.3,4 There is growing concern about product disposal, recycling possibilities and the pollution they can generate.In response, the field of chemistry is shifting towards bio-based resources as alternatives to fossil ones.5,6 Studies have highlighted the potential toxicity of commonly used products, such as plasticizers,7–10 driving research towards safer, more sustainable alternatives. The goal of sustainability is to harmonize economic, environmental, and social aspects to ensure long-term viability.11,12
Today, components and additives undergo thorough scrutiny and critique, with plasticizers drawing considerable attention.13 Found in diverse daily products ranging from food packaging to children's toys, medical devices, cosmetics, and construction materials, their omnipresence raises concerns. The proximity of plasticizers to our bodies, whether through air, food, or water consumption, makes them potential sources of pollution and hazards, impacting not only humans but also the environment-from flora to fauna-upon which we depend and for which we bear responsibility.14,15
For the past ten years, we have seen the blooming of possible alternatives to current industrial petro-based plasticizer products highlighted by an increase in scientific articles about what are called “biobased plasticizers” within academic research (Fig. 1). There has been a notable surge in research focusing on biobased, non-toxic, and low-migration plasticizers derived from vegetable oils.13,16 These studies aim to provide sustainable alternatives to phthalates, addressing both environmental and health concerns while aligning with the growing emphasis on eco-friendly practices within the plastic industry. Epoxidized linseed oil (ELO) and epoxidized soybean oil (ESBO) have become widely utilized as non-toxic, bio-sourced plasticizers for PVC.17,18 It is crucial to exercise caution and deliberate consideration when it comes to utilizing these renewable resources to prevent any inadvertent impact on food production and expansion land. This approach is essential in order to avert potentially significant social and economic repercussions. Efforts to develop environmentally friendly plasticizers have turned towards utilizing renewable resources derived from agro-industrial residues and non-edible biomass as chemical feedstocks for plasticizer synthesis.19 Recently, attention has shifted to CNSL, a non-edible oil extracted from agro-waste, which is very versatile.20,21
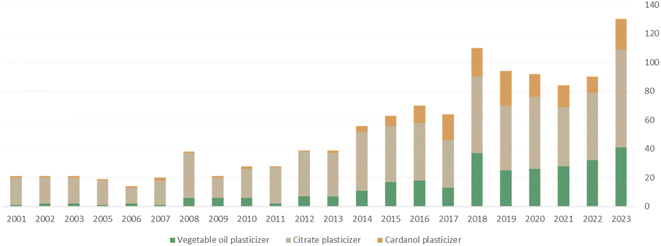 | ||
| Fig. 1 Graphical representation of the number of articles on “vegetable oil, citrate and cardanol plasticizers” per year (Webofknowledge). | ||
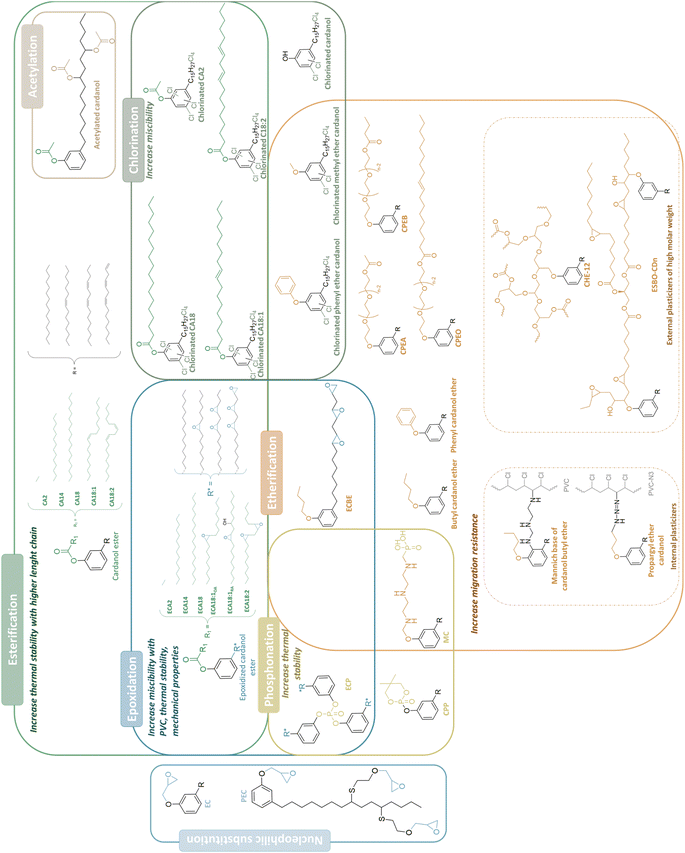
CNSL has been the subject of several reviews focusing on extraction methods, general applications, and more recently, comprehensive examinations regarding its potential as a source of drugs for Alzheimer's disease, and its application in phenolic resins, and biodiesel production.22–27 However, none of these reviews extensively explore its potential applications as a plasticizer. In this review, we aim to examine CNSL as a promising resource for synthesizing biobased plasticizers, while also projecting future perspectives for this raw material in this field. Following a comprehensive introduction to CNSL, we will present various synthesis methods of plasticizers currently derived from this feedstock. Firstly, we will present the synthesis of CNSL-based plasticizers specifically tailored for PVC. Secondly, our attention will shift towards the synthesis of CNSL-based plasticizers for poly(lactic acid) (PLA). Then, we will explore the synthesis of CNSL-based plasticizers for cellulose acetate (CA) and rubber. For all these polymer applications, we will explore the strategies employed, the properties of the synthesized compounds, and the potential plasticizing applications. Finally, we conclude with the promising plasticizing properties and the different applications, and we give an overview of future improvements and potential challenges in the use of these bio-based plasticizers, highlighting avenues for future research and development.
Presentation of cashew nutshell liquid (CNSL)
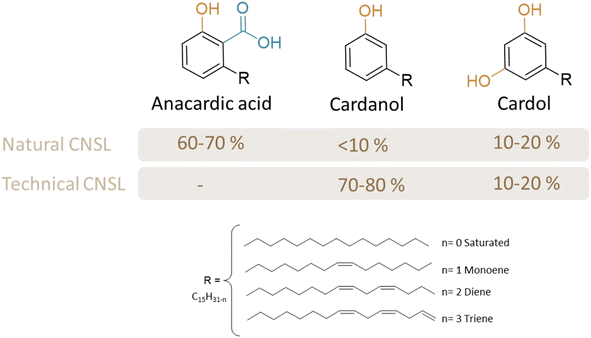
CNSL is a by-product of cashew processing, appearing as a viscous dark reddish-brown to greenish-yellow liquid contained within the porous lattice structure of the cashew nutshell (CNS).22,28 Despite CNSL being an agro-waste product, CNSL stands out as one of the richest natural sources of phenols, comprising four substituted phenols (anacardic acid, cardanol, cardol, and 2-methylcardol).The valorization of CNSL has led to numerous products that compete favorably with those derived from fossil fuels.29 Annual global CNS production is estimated at around 3 million tons per year. It is usually located in tropical climates such as South America (Brazil), Asia (India and Vietnam) and Africa (Ivory Coast, Benin, Burkina Faso, and Tanzania). Various extraction methods, such as screw pressing, solvent extraction, pyrolysis and supercritical CO2 extraction, are used to produce CNSL with the yield ranging up to 30%.30 From these various methods (heated or not) two types of CNSL oil with different compositions can be obtained (Fig. 2).
First, natural CNSL is primarily composed of anacardic acid (60–70%), cardol (10–20%), and cardanol (<10%). Under heat (around 140 °C), anacardic acid tends to decarboxylate into cardanol, resulting in technical CNSL which contains cardanol (70–80%) and cardol (10–20%).28,31 Both types of CNSL also contain methyl cardol (<3%) and traces of urushiol. The formation of residol, a polymeric residue from the polycondensation of CNSL phenolic compounds, may also be observed. Although CNSL is an interesting resource, it should be noted that CNSL, which contains anacardic acid, presents certain issues such as potential skin irritation, allergic reactions, and contact dermatitis, making its safe handling and processing critical. Various CNSL compounds can be separated and purified through precipitation (mostly for anacardic acid), distillation (mostly to separate cardanol from cardol), liquid/liquid extraction, or chromatographic column techniques (to extract cardol, for instance, or to yield other CNSL derivatives in the pure form). It should be noted that these purification methods incur costs, which must be taken into account when designing new molecules intended for the market.
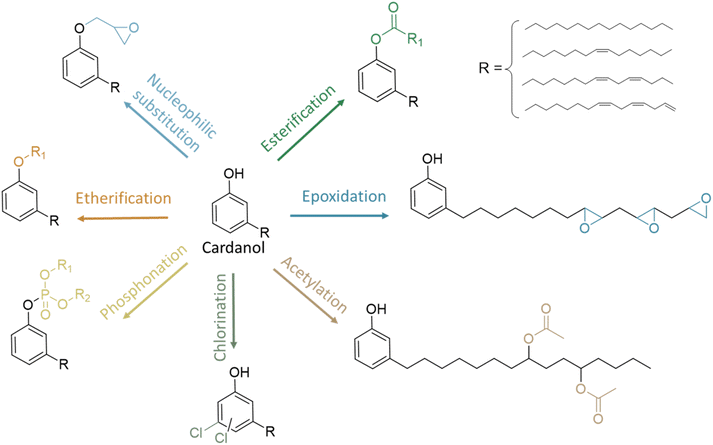
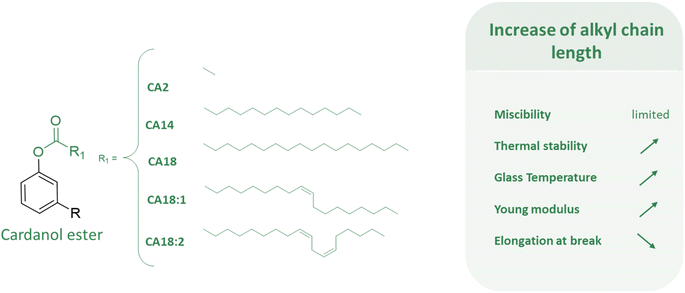
CNSL compounds exhibit diverse alkyl chains, including saturated, monoene, diene, and triene forms. Their chemical structure makes CNSL compounds highly desirable as biobased synthons for chemical synthesis. They consist of natural and non-toxic phenolic compounds that facilitate a wide range of reactions (Fig. 3). The hydroxy functional group of phenol enables various functionalization reactions such as esterification, etherification, or nucleophilic substitution. Furthermore, the hydrophobic alkyl chain in C15, especially when unsaturated, not only imparts a plasticizing effect but also enables various chemical reactions, including epoxidation, hydrogenation, carbonation, and Diels–Alder reactions.32
Although CNSL is a mixture of biobased versatile compounds, it may present toxicity concerns due to the presence of anacardic acid and urushiol, which can cause allergic reactions and dermatitis upon contact. This sensitivity is an important consideration in the use of CNSL and its derivatives, especially in applications where skin contact or exposure is possible. Proper safety measures and handling procedures are essential to mitigate these risks and ensure the safe use of CNSL-based materials.
Cardanol-based plasticizers for poly(vinyl chloride) (PVC)
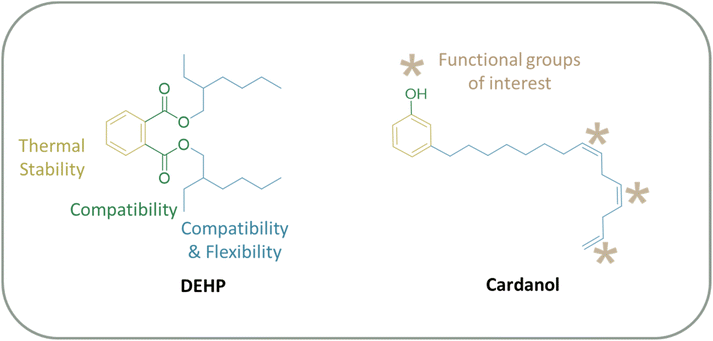
PVC is the third most used polymer, used in various fields such as food packaging, building materials or household appliances, and is indispensable in daily life.33,34 PVC is a brittle plastic that is difficult to process. As essential additives, plasticizers are used (from 20 to 70 phr (parts per hundred parts of resin) i.e. 70 parts of plasticizers for 100 parts of polymers) to improve processability by imparting flexibility when processing.35 Most of the plasticizers currently used in the industry are petroleum phthalates (bis(2-ethylhexyl) phthalate (DEHP) and diisononyl phthalate (DINP)). Phthalates are toxic for the environment and human health, and endocrine disruptors.36 Thus, alternatives such as nontoxic biobased plasticizers are of the utmost interest and have been the subject of many research studies. Vegetable oils such as epoxidized soybean oil or epoxidized linseed oil can be used but are in competition with the agrifood sector.37,38 CNSL is intriguing because it is a non-edible oil and a by-product of the agrifood industry. Moreover, its compounds bear similarities to phthalates, such as the aromatic ring (Fig. 4). It has to be mentioned that most of the literature focuses on cardanol as a pure compound rather than CNSL as a mixture. This may be due to the fact that initial attempts with anacardic acid39 were not successful, and cardanol is easier to obtain than cardol, and is available in larger quantities industrially.Esterification of cardanol
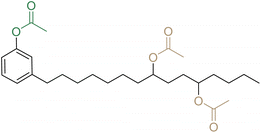
Considering the boom of phthalates in the 1950s and their first regulation in the early 1990s, and the historical implantation of cashew trees, it is not surprising, that one of the first articles available on cardanol as a plasticizer dates back to 1970, from India.39 A biobased-plasticizer from cardanol esterified with acetic acid (C2) has been synthesized to replace DEHP in a PVC formulation. Other similar experiments followed on cardanol acetate (CA2) for plasticizer formulation.20,21,40,41 This has even resulted in a Korean patent in 2017.42 Moreover, similar studies have been conducted on the esterification of cardanol with carboxylic acids with various lengths of the aliphatic chain from C1 to C10 (ref. 42) (only C2 is explicitly detailed in this patent), C14,40 and C18 with 0,39 1 (ref. 21, 39 and 40) or 2 (ref. 39) unsaturated bonds whose effect will be discussed further later. Derivatives of C18 from ricinoleic acid were also investigated in a Chinese patent published in 2021.43
The most used esterification pathway is Steglich esterification with fatty acids and an EDC/DMAP catalyst. Steglich esterification is indeed widely used due to its efficiency. However, from an environmental and economic perspective, these reagents pose significant concerns. EDC and DMAP are associated with toxic by-products and pose challenges in terms of safe disposal and sustainability. EDC, in particular, generates urea by-products, which can complicate purification processes, while DMAP is a toxic and costly reagent, raising questions about its large-scale viability.
Thus, another method using acetic anhydride in bulk was reported44,45 using a zinc perchlorate hexahydrate catalyst45 (Table 1). Although it offers a potentially greener solution, especially in bulk applications, zinc perchlorate is itself not without environmental drawbacks, as perchlorates are known to be highly soluble, persistent in water, and potentially harmful to ecosystems. Thus, while this approach avoids some issues related to EDC/DMAP, it introduces other environmental considerations that require further scrutiny.
| Author | Reactant | Catalyst | Conditions | Purification | Yield |
|---|---|---|---|---|---|
| Neuse et al.46 | — | — | — | — | — |
| Greco et al.20 | Cardanol (1 eq.) | Hexahydrate zinc perchlorate (0.015 eq.) | 50 °C, 2 h, dipyridine | Washed with HCl aqueous solution, dried, and filtered | — |
| Acetic anhydride (3 eq.) | |||||
| Greco et al.45 | Cardanol (1 eq.) | Amberlyst 15, 3% weight | 110 °C, 10 h | — | — |
| Acetic anhydride (1.2 eq.) | |||||
| Greco et al.45 | Cardanol (1 eq.) | Hexahydrate zinc perchlorate (0.015 eq.) | Tr, 24 h | Washed with water to eliminate the traces of hexahydrate zinc perchlorate | — |
| Acetic anhydride (1.5 eq.) | |||||
| Lee et al.44 | Cardanol (1 eq.) | — | 110 °C, 4 h | Distillation from 100 to 150 °C under vacuum | CA2 95% |
| Acetic anhydride (1.2 eq.) | |||||
| Chen et al.47 | Cardanol (1 eq.) | Potassium carbonate (0.6 eq.) | 60 °C, 3 h | Washed with 2% NaHCO3 and distilled water, dried by using anhydrous sodium sulfate and filtered | — |
| Acetic anhydride (1.2 eq.) | |||||
| Briou et al.21 | Cardanol (1 eq.) | EDC/DMAP (2 eq./0.05 eq.) | Tr, 4 h | Washed three times with water, and purified by chromatography on a silica column | CA2 64% |
| Acid (acetic, myristic, and oleic) (1 eq.) | CA14 66% | ||||
| CA18:1 86% | |||||
| Yang et al.55 | Cardanol (1 eq.) | EDC/DMAP (2 eq./0.05 eq.) | Tr, 6 h, N2, dichloromethane | Washed three times with water | CA14 85% |
| Acid (myristic, oleic, and ricinoleic) (1 eq.) | CA18:1OA 84% | ||||
| CA18:1RA 87% | |||||
| Chu et al.48 | Cardanol (1 eq.) | Tetrabutyl titanate (0.05 eq.) | 180 °C, 4.5 h, N2 | Cooled and dissolved in acetone and reprecipitated from water three times, air dried for 36 h and finally dried at 60 °C in a vacuum for 24 h | — |
| Linoleic acid (1 eq.) |
CA2 was tested as a secondary plasticizer of DEHP at different ratios for PVC to increase its low compatibility.46 Increasing CA2 content results in a decrease in Tg. Compared to DEHP (70 phr), the Tg of DEHP-CA2 (40–30 phr) decreased by 18 °C. However, the mechanical properties were almost unchanged and the stiffness was slightly increased. Another study investigates CA2 with another phthalate, DINP, and the enhanced properties of the PVC material.21 DINP-CA2 (35–35 phr) increased the elongation at break and decreased the Young's modulus (340% and 7.6 MPa) compared to DINP (70 phr) (325% and 8.8 MPa). Thus, CA2 could be a good secondary plasticizer for DINP. To go further, another study proposed the use of CA2 with non-toxic biobased edible oil ESBO for PVC, for better compatibility and properties.44 Compared to DEHP, CA2-ESBO (50–5 phr) results in better Young's modulus and tensile stress but lower strain at break values. It was deduced that this combination of biosourced oils could be a promising alternative. Similar studies confirmed the synergistic effect of CA2 with an epoxy plasticizer based on vegetable oils such as sunflower oil, palm oil, castor oil, etc.
To summarize, CA2 provides better thermal stability than phthalate DEHP or biosourced alternatives such as ESBO. For a PVC plasticized material, the Young's modulus and Tg decrease for a higher content of CA2 (114 MPa, 334 K (30 phr), and 5.5 MPa, 245 K (67 phr)). The plasticizing properties are significantly enhanced when used as a secondary plasticizer. Therefore, it shows promising potential as a secondary plasticizer when combined with phthalates or epoxidized bio-sourced oils.
 | ||
| Fig. 5 Study of cardanol esters: influence of the alkyl chain length on plasticized PVC material properties. | ||
It is regrettable that cardanol ester (CA14) based on myristic acid (C14) was not investigated as a primary plasticizer while as secondary plasticizer with DINP (50/50) (70 phr) it shows better thermal stability for DINP-CA14 (247 °C) than CA2 (222 °C), DINP-CA2 (231 °C) and DINP (242 °C).40 However, this reveals that a long aliphatic chain has a negative effect on the properties of the materials. It makes the material more brittle. It decreases the elongation at break and increases the Young's modulus. It has been reported that DINP-CA14 (21.6 MPa) has a higher Young's modulus than DINP-CA2 (5.5 MPa) and DINP (8.8 MPa).40 Despite thermal properties, secondary plasticizer CA14 loses the plasticizing effect compared to CA2.
Cardanol stearate (CA18) was synthesized from stearic acid (C18) and was investigated as a secondary plasticizer for PVC.39 It could also have been interesting to use it as a primary plasticizer. PVC/DEHP-CA18 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) materials were plasticized with different contents of plasticizer (40, 50, 60, and 70 phr). It was noticed that the elongation at break and hardness were really close to those of DEHP. Besides, PVC/CA18 shows lower tensile strength and Young's modulus for contents below 70 phr. Once again, it has to be noted that the effect of the plasticizer on the properties seems to slightly change from 70 phr, and this could be the result of a two phase system in the material.
1) materials were plasticized with different contents of plasticizer (40, 50, 60, and 70 phr). It was noticed that the elongation at break and hardness were really close to those of DEHP. Besides, PVC/CA18 shows lower tensile strength and Young's modulus for contents below 70 phr. Once again, it has to be noted that the effect of the plasticizer on the properties seems to slightly change from 70 phr, and this could be the result of a two phase system in the material.
Nowadays, modelling plasticizers may be a way to predict their plasticizing efficiency.53 For instance, it was predicted that long aliphatic chains of esters decreased plasticizer mobility.53 Long aliphatic chain ester-based plasticizers tend to increase the proportion of non-polar alkyl chains within the molecule, which weakens the bonds with PVC. Consequently, they are both less compatible and less effective for PVC. However, lab experiences with such plasticizers may yield divergent results. From these experimental studies, it is challenging to discern trends in the influence of the chain length on the plasticizing properties of materials due to the lack of comparability among them. Therefore, obtaining more data on this aspect and conducting a more comprehensive evaluation would be valuable.
In conclusion, the use of CA2 as a plasticizer has aroused considerable interest, as it has properties similar to phthalates. When used as a primary plasticizer, CA2 has good mechanical properties, but is less thermally stable than cardanol esters with a longer alkyl chain. However, increasing the length of the alkyl chain could potentially lead to inferior plasticizing properties in plasticized PVC materials. Further research is needed to explore these relationships and optimize the plasticizing effects of cardanol esters in PVC formulations.
The amount of unsaturation of the alkyl chain of the ester implies differences in the mechanical properties of the plasticized PVC.39 PVC materials were plasticized with different contents of the plasticizer (40, 50, 60, and 70 phr) with DEHP-CA18 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The findings of the study reveal that both CA18:1 and CA18:2 decrease the material's hardness compared to CA18:0 and DEHP. Additionally, while saturated chains (CA18:0) increase the stiffness of the material, they also decrease both tensile strength and elongation at break more than higher unsaturation levels (CA18:2), albeit still lower than that due to DEHP. An advantageous characteristic of unsaturated and low-saturated chains is that CA18:0 and CA18:1 decrease the Young's modulus and exhibit similar behaviour.
1). The findings of the study reveal that both CA18:1 and CA18:2 decrease the material's hardness compared to CA18:0 and DEHP. Additionally, while saturated chains (CA18:0) increase the stiffness of the material, they also decrease both tensile strength and elongation at break more than higher unsaturation levels (CA18:2), albeit still lower than that due to DEHP. An advantageous characteristic of unsaturated and low-saturated chains is that CA18:0 and CA18:1 decrease the Young's modulus and exhibit similar behaviour.
However, it is important to note a behavioural shift at 70 phr, where C18:0 to C18:2 increase the elongation at break. Regarding the Young's modulus, C18:2 appears to exhibit a change in behaviour from 70 phr onwards, resulting in a higher Young's modulus compared to DEHP. This shift could potentially stem from a heterogeneous phase system within the material as explained previously. This observed change in behaviour was also noted in another study involving cardanol oleate (CA18:1), synthesized from oleic acid (C18:1).40 In this study, CA18:1 was utilized as a secondary plasticizer, and PVC/DINP-CA18:1 (67 phr) exhibited a higher Young's modulus (8.8 to 30.8 MPa), contrary to the previous study. Additionally, both the hardness and glass transition were increased (shore A from 35 to 60 and Tα from 12 to 46 °C), and the material displayed thermal stability properties quite similar to those of PVC/DINP. Consequently, CA18:1 does not appear to be a suitable candidate for replacing DINP under these conditions. As it stands, it seems difficult to determine the best candidate for a plasticizer based on the amount of unsaturation.
To conclude, esterification is one of the main methods used to introduce an additional spacer to cardanol derivatives, thereby increasing the free volume between PVC chains. Among cardanol derivatives, cardanol acetate CA2 is one of the most investigated compounds. Further research is necessary to clarify the relationships between plasticizing efficiency and alkyl chain length, and to optimize the plasticizing effects of cardanol esters in PVC formulations. It is challenging to draw definitive conclusions as each study operates under unique experimental conditions, including varying parameters such as the plasticizer amount; however, certain trends emerge. While CA2 exhibits good mechanical properties as a primary plasticizer, it demonstrates lower thermal stability compared to longer alkyl chain cardanol esters, and some studies report limitations in PVC compatibility. Elongating the alkyl chain may compromise plasticizing properties when the chain length becomes excessive. Some studies incorporate long alkyl chains with unsaturated bonds, potentially altering the conformation of the molecule. Although notable changes in final material properties have not been observed, the addition of unsaturation may offer intriguing possibilities, given the modifiability of this active site, particularly through methods like epoxidation, which remains an area of active investigation.
Although these synthesized plasticizers show promising properties for partially replacing phthalates as primary or secondary plasticizers, their volatility and chemical resistance are not always thoroughly studied. When such properties are evaluated, the conditions under which tests are conducted often vary, making direct comparisons between studies difficult. Moreover, very few studies assess the toxicity of the synthesized compounds, despite this being a critical parameter alongside plasticizing efficiency. For these plasticizers to be viable replacements for phthalates, it is essential to evaluate their toxicity. Additionally, most papers do not discuss the viscosity or color of these plasticizers. These factors are crucial, as the oils are generally yellow and can be viscous, especially when the alkyl chain length increases, which could limit industrial applications. Therefore, it is necessary to further investigate these parameters to fully assess the potential of these molecules.
Epoxidation of cardanol
The epoxidized plasticizer is of high interest in the plastic industry, rubber industry, and coatings and new polymer materials. For PVC plasticizers, adding epoxy groups is really interesting as it neutralizes the hydrogen chloride released by PVC during thermal degradation, and thus decreases the decomposition of PVC. Moreover, epoxidized plasticizers, such as ESBO or ELO, are generally considered to be low-to-non-toxic options. These plasticizers are extensively used in food packaging and medical equipment materials, leading to an increase in both their production and price over the past few decades.32 Hence, the investigation into the epoxidation of cardanol was undertaken (Table 2). Epoxidation of the unsaturations could be performed with enzymes such as Candida antarctica lipase, with acetic acid and hydrogen peroxide.54 Greco et al. also studied the fact that using an enzymatic pathway for epoxidation results in safer working conditions and less damage to the environment,45 or more recently using Novozyme 435 enzyme,55 but these methods seem to be not widespread. In the most conventional reaction routes, epoxidation requires m-chloroperbenzoic acid (m-CPBA) as the reagent and organic solvent.20 However, such a process may not be suitable at a larger scale as it presents significant challenges in terms of both toxicity and environmental impact. m-CPBA is a hazardous chemical with potential health risks, including irritation and corrosivity. Additionally, the use of organic solvents further compounds environmental concerns due to their volatility and potential to contribute to solvent waste and pollution. Scaling up this process may exacerbate these issues, making it less suitable for large-scale applications. Similarly, as with soybean oil, cardanol can be epoxidized by the in situ generation of peracetic acid from acetic acid and hydrogen peroxide, which is one of the most used methods. However, the primary limitation of epoxidation of unsaturations using peracid catalysis is the opening of the epoxy ring in the final products under acidic conditions, making it challenging to achieve a high conversion yield.55| Author | Reactant | Catalyst | Conditions | Purification | Degree of conversion (%) | Yield (%) |
|---|---|---|---|---|---|---|
| Greco et al.20 | CA2 (1 eq.) | — | 0 °C, 3 h, dichloromethane | Washed with a water solution of Na2CO3, dried and filtered | — | |
| Chloroperbenzoic acid (1.5 eq.) | ||||||
| Greco et al.45 | CA2 (1 eq.) | Lipase B (0.7%) | 50 °C, 24 h | — | 5 | 5 |
| Propionic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.3%) | 60 °C, 7 h | — | 47 | 27 | |
| Acetic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.9%) | 30 °C, 7 h | — | 47 | 29 | |
| Acetic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.9%) | 25 °C, 20 h, dichloromethane | — | 100 | 43 | |
| Acetic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.9%) | 25 °C, 20 h, toluene | — | 92 | 54 | |
| Acetic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.9%) | 50 °C, 20 h, toluene | — | 95 | 68 | |
| Acetic acid (15%) | ||||||
| CA2 (1 eq.) | Sulfuric acid (0.9%) | 25 °C, 20 h | — | 87 | 81 | |
| Acetic acid (15%) | ||||||
| Lee et al.44 | CA2 (1 eq.) | Formic acid | 50 °C, 4 h | Separated using a mixture of ethyl acetate/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and finally washed with water three times. The organic phase was dried using MgSO4 and was filtered 1, v/v) and finally washed with water three times. The organic phase was dried using MgSO4 and was filtered |
99 | 87 |
| Hydrogen peroxide (3.5 eq.) | ||||||
| Chen et al.47 | CA2 (1 eq.) | Formic acid | 65 °C, 3 h | Filtered and washed with 2% NaHCO3 and distilled water | (Epoxy value) 4.21% | — |
| Hydrogen peroxide (3.5 eq.) | APTS | |||||
| Briou et al.21 | CA2, CA14 or CA18:1 (1 eq.) | Formic acid (3 eq.) | 65 °C, 3 h | Extracted by liquid–liquid water–ethyl acetate extraction. The organic phases were washed three times with water and at last dried with MgSO4 | Close to 85% | ECA2 96% |
| Hydrogen peroxide (2 eq./double bond) | Sulfuric acid (0.01 eq.) | ECA14 94% | ||||
| ECA18:1 96% | ||||||
| Yang et al.55 | CA14, CA18:1OA, or CA18:1RA (1 eq.) | Acetic acid (3 eq.) | 65 °C, 6 h | Extracted using ethyl acetate, washed three times with deionized water, and dried over anhydrous MgSO4 | (Epoxy value) ECA14: 3.63% | ECA14:94% |
| Hydrogen peroxide (0.02 eq.) | Sulfuric acid (0.01 eq.) | ECA18:1OA: 4.86% | ECA18:1OA: 94% | |||
| ECA18:1RA: 4.72% | ECA18:1RA: 96% | |||||
| Chu et al.48 | CA18:2 (1 eq.) | Glacial acetic acid (8 eq.) | 50 °C, 4 h | Washed with NaCl saturated solution | (Epoxy value) | — |
| Hydrogen peroxide (0.09 eq.) | Phosphate (0.4 eq.) | 2.21% |
It has been reported that epoxidation of the unsaturated bonds of the cardanol ester enhances its miscibility with PVC (Fig. 6). This is achieved by increasing the overall polarity of the chain, facilitating improved bonding with PVC. Cardanol based plasticizers esterified with C2, C14 and C18 have been epoxidized and studied as plasticizers for PVC.40 Once epoxidized, plasticizers were observed to be more miscible in PVC and could be easily used as the primary plasticizer. It was already observed by Eberhard46 that epoxidized cardanol C18:1 (ECA18:1) was completely compatible with PVC although CA18:1 was partially miscible. It was also confirmed by Greco20 who has performed extensive research in this field, that ECA2 has really good miscibility with PVC compared to CA2, whose miscibility is similar to that of DEHP.
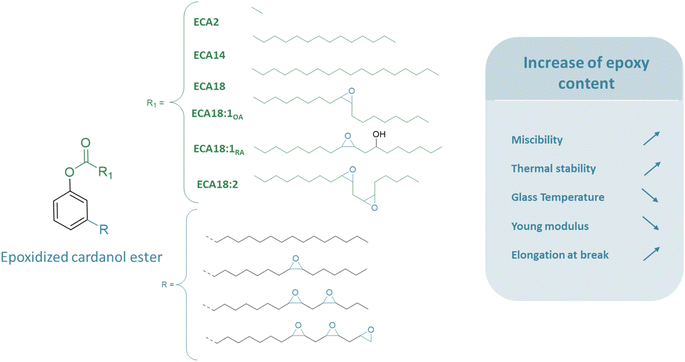 | ||
| Fig. 6 Study of epoxidized cardanol esters: influence of epoxy yield on plasticized PVC material properties. | ||
Adding epoxy groups also influences the thermal stability of the material and drastically limits the dehydrochlorination step. For instance, the weight loss of 5% for PVC/CA2 occurs at 220 °C which increases to 255 °C for PVC/ECA2, which is higher than that of PVC/DINP materials (242 °C). It was already reported by Neuse46 that the PVC/ECA18:1 material was more thermally stable than the PVC/DEHP material, with the degradation temperature increasing from 210 to 283 °C. It was also confirmed by Greco that ECA2 has better thermal stability than DEHP in plasticized PVC materials (54 phr).20 For longer estera with more unsaturated sites, thermal stability was increased too. ECA18:2 was synthesized from linoleic acid and used as secondary plasticizer for PVC (40 phr). It was observed that PVC/ECA18:2 showed higher thermal stability than PVC/DEHP.51 It was proved that thermal stability is increased by high yield of epoxidation,52 and according to aging tests, a higher rate of epoxidation leads to a reduction in leaching of the plasticizer, due to a decrease in the diffusion coefficient of the plasticizer.52
Besides, adding epoxies would lower the glass transition temperature. A Korean patent attests that the Tg of PVC samples plasticized (30 phr) with ECA2 (44 °C) was lower than that of both CA2 (60 °C), and DEHP (59 °C).42 Another study shows that the thermal properties of PVC/ECA18:2 (40 phr) were also enhanced. For instance, for PVC/DEHP Tg decreased from 56 to 37 °C for PVC/ECA18:2 (40 phr).51
In addition to thermal properties, mechanical properties have been improved using epoxidized plasticizers. As the compatibility with PVC was increased by adding polar groups, it resulted in a higher elongation at break. For the C2 based cardanol plasticizer, the elongation at break could be increased from 290 to 330% and the Young's modulus reduced from 5.5 to 4.2 MPa, by the epoxidation of the alkyl chain.56 It was also reported that the elongation at break was increased when using ECA18:1 (ref. 46) and especially ECA18:2 (ref. 51) when compared with DEHP. The strain at break is also increased with epoxidized plasticizers. For PVC samples plasticized (30 phr) with ECA2 (550%) strain at break was higher than that of both CA2 (527%) and DEHP (504%).44 In addition, it was proved that PVC/ECA2 with a high epoxidation rate on the C15 alkyl chain of the cardanol, has mechanical properties similar to those of commercial plasticizers such as fully acetylated glycerol monoesters of 12-hydroxystearic acid or ESBO, and could replace them.20 Other studies report that adding epoxides decrease the Young's modulus and increase the flexibility. For PVC samples plasticized (30 phr) with ECA2 (95 MPa), the Young's modulus was lower than that of PVC/CA2 (114 MPa) and PVC/DEHP (147 MPa).44 For longer esters, it was earlier observed that PVC materials plasticized with ECA18:1 were more flexible compared with PVC/DEHP and PVC/CA18:1 materials. It was noticed that PVC/ECA18:1 has a lower Young's modulus than PVC/DEHP (from 3.4 to 2.9 MPa).46 The same phenomenon was observed with other phthalates and various esters. It was reported that all the films plasticized by epoxidized cardanol ester (ECA2, ECA14, and ECA18:1) are more flexible than the PVC/DINP film, with a Young's modulus ranging from 4 to 6.7 MPa compared to 8.8 MPa.40 Comparing the different cardanol esters with different lengths of alkyl chains (ECA2, ECA14, and ECA18:1), the increase in the length increases the thermal stability but also increases the Tg (12 to 20 °C). No trend could be determined for the Young's modulus, the elongation at break and the hardness.40
To go deeper, the influence of the yield of epoxidation was investigated on the plasticizing properties of the material. It was reported that the Young's modulus for a high yield of epoxidation of ECA2 (81%) was similar to that of DEHP, and was lower than that of other biobased alternatives such as ESBO,57 making ECA2 a potential competitor for ESBO.
Furthermore, as for cardanol esters, epoxy esters of cardanol ECA2 have been studied as a secondary plasticizer. Mixing ECA2 and ESBO improves the affinity of ESBO to PVC, as adding small amounts of ESBO (5 phr) to ECA2 improves the thermal stability and mechanical properties, despite insufficient compatibility of ESBO and PVC.44 The research on ECA2 resulted in a Korean patent42 and relates to a plasticizer composition (a natural cardanol-based plasticizer and a vegetable oil-based epoxidized plasticizer) for polymer resins. When mixed with a vegetable oil-based epoxy plasticizer, not only the performance such as tensile strength and elongation, but also physical properties such as heat loss, elongation, and aging resistance of the polymer resin are improved. Adding ECA18:1 (3 phr) to ESBO (30 phr) in order to plasticize PVC increases the elongation at break and the tensile strength and let barely unchanged the Tg. For higher content (5 phr) the same tendency was observed but the Tg was slightly increased. Compared with PVC/ESO-DEHP, at the same content, using the cardanol derived plasticizer increases the Tg.49
Moreover, the non-toxicity of epoxidized cardanol ester ECA2, which is an environmentally friendly plasticizer was proved along with its non-activity as an endocrine disruptor.42 Other tests such as eco-toxicity tests using daphnia, algae, and plants as model organisms and endocrinal disruptor tests (YES/YAS) were carried out on ECA18:1. In comparison to DINP, ECA18:1 causes a global decrease in toxicity, no harm for the environment and no endocrine perturbation effect for human health.21
Thus, epoxidized cardanol esters appear to be a promising way to obtain good alternatives to phthalate plasticizers, enhancing thermal and mechanical properties of the materials, but also for preexisting biosourced alternatives such as ESBO. ECA2 could be used as both a plasticizer and a stabilizer. This would reduce the amount of thermal and UV stabilizers, which are real issues.20
Although some researchers have investigated the toxicity of synthesized compounds, including their potential endocrine-disrupting effects, this is not always the case. It is crucial to conduct a comprehensive study, as evaluating the plasticizing properties alone is not sufficient. Assessing the toxicity of these molecules is essential. Similarly, their volatility and chemical resistance need thorough evaluation. Additionally, it would be beneficial to examine the stability of these products and study the toxicity of any by-products, such as epoxies opened into diols or compounds resulting from ester hydrolysis. Moreover, providing a description of the color and viscosity of these plasticizers is important, as these parameters significantly influence their industrial applicability. Indeed, compared to phthalates, cardanol derivatives are yellowish and tend to turn browner over time, which could pose a challenge to their industrial use.40,46 Understanding these characteristics is crucial if these molecules are to be effectively used as replacements for phthalates on an industrial scale.
Other design methods of cardanol-based plasticizers: acetylation and etherification
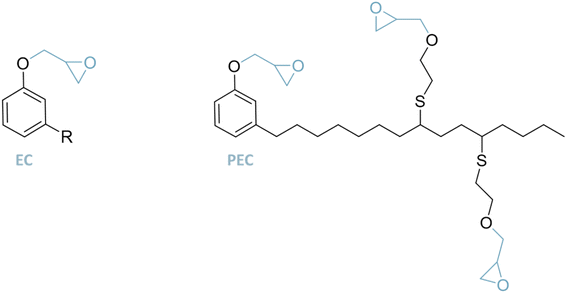
Cardanol glycidyl ether. In 2017, a cardanol-based epoxy plasticizer (PEC) was investigated for PVC using thiolene click chemistry and epichlorohydrin58 (Fig. 8). Although thiol-ene click chemistry is known for its efficiency, the use of epichlorohydrin—a critical reagent in this process—raises substantial environmental and health concerns. Epichlorohydrin is classified as a hazardous substance due to its potential carcinogenicity and persistence in the environment. Its handling necessitates stringent safety measures to mitigate risks associated with exposure and proper waste disposal. Addressing these concerns is crucial to ensure the safety and sustainability of the synthesis process.
In this study it was shown that PEC has favorable plasticizing and stabilizing effects on PVC. The Tg decreased and the tensile strength increased for a low amount of the plasticizer. It was observed that the volatility, the migration and the solvent extraction characteristics of PVC/PEC are similar to those of PVC/DEHP.
In 2021, glycidyl ether of cardanol (EC) was used as the primary and secondary plasticizer.59 The tensile strength, elongation at break, tensile modulus and impact strength of PVC/EC films were higher than those of PVC/DEHP and PVC/ESBO films. The films prepared with DEHP and EC showed higher tensile strength and elongation at break than DEHP. Hence, EC is described to have some good plasticizer properties. However, it has to be noted that EC results from the reaction of cardanol with epichlorohydrin under basic conditions, and is a highly toxic compound. EC is already on the market and commercially available under the trade mark NC-513 (Cardolite). Its reactivity can be enhanced by adding epoxy groups on its unsaturations. It is widely used in solvent free epoxy systems used in protective, industrial, and floor coatings.50
Phenyl ether. In a study in 1974,60 in addition to esterification modification, etherification was also investigated. Phenyl ether of cardanol has been synthesized and used as a secondary plasticizer with DEHP (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for PVC materials (Fig. 9). For 40 and 50 phr content of the plasticizer, the use of this plasticizer decreases the Young's modulus and hardness compared to DEHP alone. However, for a higher content (from 60 phr), the hardness was higher than that of DEHP alone. Moreover, for all contents of the plasticizer (40, 50, 60, and 70 phr), the elongation at break was lower than that DEHP and they present weaker properties than cardanol acetate (CA) or epoxidized cardanol acetate (ECA). These low plasticizing properties could explain why this type of plasticizer was not more investigated over the years.
1) for PVC materials (Fig. 9). For 40 and 50 phr content of the plasticizer, the use of this plasticizer decreases the Young's modulus and hardness compared to DEHP alone. However, for a higher content (from 60 phr), the hardness was higher than that of DEHP alone. Moreover, for all contents of the plasticizer (40, 50, 60, and 70 phr), the elongation at break was lower than that DEHP and they present weaker properties than cardanol acetate (CA) or epoxidized cardanol acetate (ECA). These low plasticizing properties could explain why this type of plasticizer was not more investigated over the years.
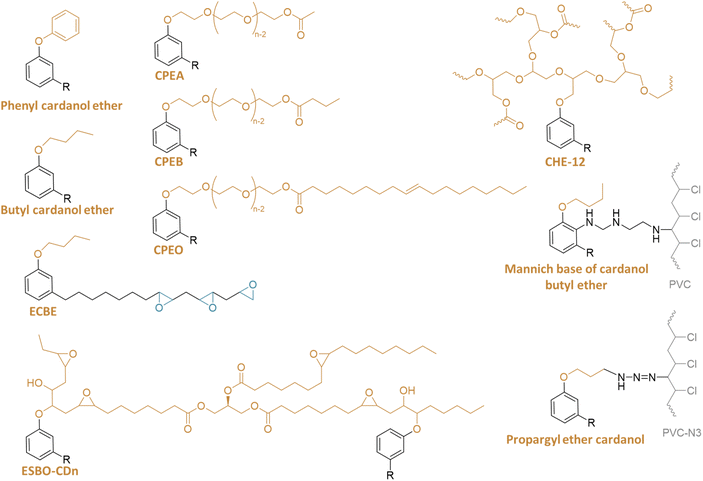
Butyl ethers. Butyl ether of cardanol was also investigated as a primary or secondary plasticizer for PVC (Fig. 9).61,62 It was shown that it significantly improves the flexibility and durability of the plastic. It is also reported to have low toxicity for the environment.
Another study focused on epoxidized butyl ether of cardanol (ECBE).61 Substituting 30 wt% of DEHP with ECBE led to an 18% increase in elongation at break and a 6 °C decrease in Tg. Moreover, increasing ECBE content improved thermal stability, processability, volatility, and exudation resistance of PVC blends. PVC samples with ECBE as the primary or secondary plasticizer showed similar performance to soft PVC plasticized with DEHP.
Long cardanol ether. From a recent study, cardanol-based poly(oxyethylene ether carboxylate)s (CPEC: CPEA, CPEB, and CPO) with high molecular weight (Mn > 3 kg mol−1) were synthesized through cardanol ethoxylation followed by esterification with aliphatic acid63 (Fig. 9). The resulting CPEC addressed the compatibility and molecular weight trade-off, providing highly efficient plasticization and excellent migration resistance in PVC. This was attributed to the abundant oxoethyl units enhancing miscibility and plasticization, along with the high molecular weight improving migration resistance. Overall, PVC plasticized with CPEC exhibited superior mechanical properties, low-temperature resistance, thermal stability, and migration resistance compared to PVC blended with DEHP, suggesting CPEC's potential as a non-toxic alternative to petro-based DEHP.
In 2019,64 a highly branched and nontoxic plasticizer from cardanol hyperbranched ester (CHE-12) was synthesized. It shows better thermal stability, and both better stability and compatibility with PVC than DEHP. It was reported that the Tg decreased from 82 to −8 °C by addition of CHE-12 (60 phr), while the Tg of PVC/DEHP (60 phr) is −1 °C. Besides it was tested and reported to be nontoxic.
To address migration issues in PVC materials, cardanol ether of ESBO with a high molar weight was synthesized65 (Fig. 9). A comparison between PVC/30ESBO/5CD and PVC/35ESBO-CD10 revealed higher tensile properties for the former. However, PVC/35ESBO-CD10 exhibited superior thermal stability and migration resistance. ESBO-CD10 demonstrated the most effective plasticizing and migration resistance properties in the plasticized PVC films. These results suggest that ESBO-CD10 is a promising bio-based PVC plasticizer for various industrial applications.
Another study described the synthesis of a cardanol-based Mannich base, synthesized from cardanol butyl ether and diethylenetriamine, for self-plasticization of PVC67 (Fig. 9). The internal plasticizer makes the molar mass increase from 18.9 to 28.6 kg mol−1. It has to be noted that diethylenetriamine, a key reagent, poses health risks as it is known to be an irritant, necessitating stringent safety measures during handling and processing. Additionally, the environmental impact of using such chemicals must be thoroughly assessed, including their potential release into the environment and their long-term effects. The scalability of this synthesis route from the laboratory to the industrial scale also presents challenges, requiring careful consideration of economic viability and sustainability.
Compared to PVC, it was observed that Tg decreased from 86 to 49 °C, the tensile strength decreased from 0.33 to 18.86 MPa and the elongation at break increased from 180 to 357%, indicating that the Mannich base of cardanol butyl ether had an internal plasticizing effect on PVC. The leaching tests show that the self-plasticized PVC materials exhibited excellent migration resistance compared to PVC/DEHP. This shows that this type of plasticizer is a good internal plasticizer for flexible and non-migration PVC materials.
Phosphorus cardanol derivatives
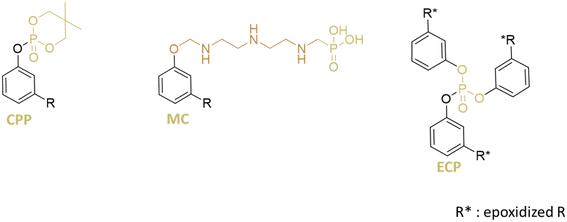
The thermal stability of the plasticizer is of the utmost importance and always needs improvement. Plasticizers should exhibit excellent thermal stability at least up to 170 °C. Otherwise, they will decompose before the end-products are shaped. Therefore, flame retardants are typically incorporated to prevent or slow down ignition by various physical and chemical methods. Inorganic flame retardants are commonly used but are often incompatible with PVC, leading to a deterioration in the mechanical properties. Flame-retardant additives based on phosphonate groups appear to be a promising alternative, and has been introduced into cardanol (Fig. 10).Cardanol phosphoryl plasticizer (CPP) was synthesized and used as a secondary plasticizer for PVC at different rates (10, 20, and 25 phr) with DEHP (40, 30, and 25 phr).68 Increasing the proportion of CPP was found to enhance the thermal stability of the material. Since the weight loss percentage of PVC/DEHP-CPP with a high rate of CPP (25 phr) was just below this limit (0.5%), it was concluded that CPP could be used as a co-plasticizer in PVC with a CPP rate below 25 phr. Thermal properties improved with higher CPP content, resulting in a higher char residue yield, which was reported to slow down the combustion process by preventing the transfer of heat, oxygen, and combustible gas, suggesting that CPP could function as a flame retardant for the PVC material. Additionally, limiting oxygen index (LOI) tests were conducted, showing that PVC/DEHP had a low LOI of 21%, indicating combustibility while PVC/CPP increased to 26%. The presence of organophosphate groups in cardanol contributed to the observed fire-retardant properties, as phosphorus-containing compounds can generate phosphoric acid during combustion. This acid dehydrates into metaphosphoric acid, forming a protective charcoal layer that inhibits oxygen transfer and slows the release of flammable gases, enhancing fire resistance. Furthermore, mechanical properties were examined, showing that increasing the proportion of CPP from 0 to 50% in the plasticizer system led to a rise in tensile modulus and tensile strength of the PVC film while elongation at break slightly decreased. Despite this, the overall properties remained lower than those of PVC/DEHP alone, suggesting that CPP does not significantly reduce the mechanical properties of PVC films compared to inorganic flame retardants.
Other ongoing research studies focus on phosphorus groups on cardanol. Phosphorylated cardanol (MC) was synthesized and utilized as a secondary plasticizer for PVC41 (Fig. 10). It was added to DEHP at various rates (2, 5, 7, and 10 phr) with PVC plasticized using 40 phr of the respective mixture. It has to be noted that calcium stearate and zinc stearate, thermal stabilizers, were also added at 2 phr in the PVC formulation. Substituting a portion of DEHP with modified cardanol (MC) was found to increase thermal stability, tensile strength, and leaching resistance. Tensile strength increased from 17.7 MPa for PVC/DEHP to 25.7 MPa for the PVC/DEHP-MC blend (10 phr of MC), with elongation at break increasing from 256 to 432%. Microstructure analysis revealed that MC addition allowed PVC blends to maintain balanced flexibility and strength properties with excellent migration resistance. Migration tests demonstrated a decrease with increasing MC ratio. The notable thermal stability of MC was attributed to its phosphoric acid, benzene, and nitrogen components, with the observed increase in char content potentially explaining the enhanced thermal stability in PVC blends. Consequently, MC was found to be more effective than DEHP in enhancing the thermal stability of PVC blends.
Other researchers have explored plasticizers derived from cardanol enriched with phosphonate groups: epoxidized cardanol-based plasticizer (ECP).69
The compound is initially synthesized using cardanol and phosphorus oxychloride in chloroform. It has to be noted that phosphorus oxychloride poses challenges due to its corrosive nature and environmental impact. Besides, chloroform, used as a solvent, is also problematic because of its potential health hazards and environmental risks. Following this, the compound undergoes epoxidation with hydrogen peroxide, utilizing quaternary ammonium phosphotungstate as the catalyst. While effective, the catalyst presents toxicity concerns and poses challenges in terms of industrial application and environmental safety. In addition to all this, the synthesis of this compound is relatively complex and could pose a challenge to its industrialization.
ECP was synthesized and employed as the primary plasticizer for PVC at various concentrations (30, 40, 50, and 60 phr). Increasing the proportion of ECP in PVC materials was found to increase elongation at break but decrease tensile strength, along with a reduction in Young's modulus. However, the results did not surpass those obtained with PVC plasticized with DEHP. It is also reported that ECP exhibited good solvent resistance and low volatility. Toxicity testing conducted on rats revealed that the plasticizer was non-toxic for doses below 3 g kg−1.
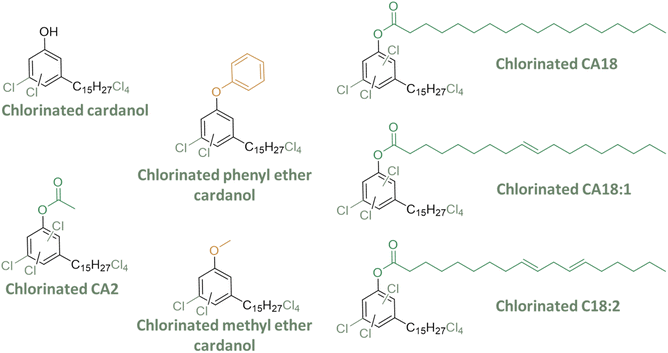
Chlorinated cardanol derivatives
In the 1970s and 1980s, Ghatge et al.39 aimed to enhance the compatibility of the plasticizer with PVC by introducing chlorine atoms to cardanol. Various chlorinated derivatives of cardanol, including chlorinated cardanol, chlorinated acetyl, and methoxy derivatives, were assessed as potential alternatives for DEHP in PVC formulations (Fig. 11).PVC samples were plasticized using both DEHP and synthesized cardanol-based plasticizers, including chlorinated derivatives such as chlorinated cardanol, cardanol acetate, and methyl cardanol.6 They were tested in combination with DEHP at different concentrations (40 and 60 phr). In some cases, the Young's modulus and elongation at break were slightly increased for chlorinated compounds compared to non-chlorinated ones. Among the tested derivatives, chlorinated cardanol methyl ether emerged as a promising PVC plasticizer, exhibiting properties similar to DEHP, especially in terms of thermal properties. Similarly, acetyl derivatives of cardanol were examined for their plasticizing effects on PVC, resulting in PVC materials with comparable thermal and mechanical properties to those plasticized with DEHP. Another study investigated cardanol stearate, cardanol oleate, linoleate, and ethers. When compared with non-chlorinated compounds, it was observed that the hardness, tensile stress, Young's modulus, and elongation at break slightly increased for some of the chlorinated compounds.46 Overall, the addition of chlorinated groups was found to enhance affinity with PVC.
Conclusion
With the rising demand for alternatives to phthalates and edible oils as plasticizers, CNSL presents a promising option. Research efforts have predominantly focused on pure cardanol, excluding cardol and anacardic acid. Experimental conditions vary widely across studies, making it challenging to identify promising compounds. However, etherification, esterification, and epoxidation have emerged as the most studied modification pathways (Fig. 12) (Tables 3–7). Esterification was explored for its PVC compatibility. Epoxidation offers a promising avenue by enhancing compatibility, thermal stability, and mechanical properties. Etherification is commonly employed to obtain higher molecular weight plasticizers or internal plasticizers. The higher molar weight contributes to enhanced chemical migration resistance in the plasticized material. Other modification pathways, such as chlorination and phosphating, were investigated for their potential thermal and fire resistance benefits. Nevertheless, synthesis sustainability and consideration of migration and toxicity are essential for CNSL-derived plasticizers. Indeed, there is a significant lack of data on the toxicological and environmental impact of these cardanol-derived compounds. Despite their promising potential as alternatives to traditional plasticizers, the safety profiles of these materials have not been thoroughly investigated. This highlights a crucial gap in the research, emphasizing the need for comprehensive toxicity evaluations to ensure their safe use in various applications. Besides, the toxicity of decomposition by-products must also be studied. Indeed, the issues of stability and biodegradability are also important. It can be assumed that esters may be easily hydrolyzed, whereas ether bonds are more durable. Moreover, the color and viscosity of plasticizers, though rarely addressed, are important for potential industrialization.| Plasticizer | Structure | Cardanol derived plasticizer content (phr) | Other plasticizers | Other plasticizer contents (phr) | Total amount of plasticizer (phr) | Thermal stabilizer (phr) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (Mpa) | T 5% (°C) | T g (°C) | Hardness A | Hardness D | Volatility chemical resistance | Toxicity | Plasticizing method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esters | ||||||||||||||||||
| CA2 |

|
Primary | 30 | — | — | 30 | — | 527 | 25 | 114 | — | 60 | — | — | Both similar to DEHP | — | Solvent casting | Lee et al.44 |
| CA14 |

|
Secondary | 10 | ESBO | 30 | 40 | — | 184 | 22 | — | 252 | 44 | — | — | Low volatility and chemical migration compared to both DEHP and ESBO | — | Plastisol, pressed | Yang et al.55 |
| CA18:0 |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 150 | 15 | 12 | — | — | 76 (A or D) | — | Both similar to DEHP | — | Plastisol | Ghatge et al.60 |
| CA18:1 OA |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 160 | 16 | 12 | — | — | 78 (A or D) | — | Both similar to DEHP | — | Plastisol | Ghatge et al.60 |
| CA18:1 OA | Secondary | 10 | ESBO | 30 | 40 | — | — | — | — | — | — | — | — | — | — | Plastisol, pressed | Yang et al.55 | |
| CA18:1 RA |

|
Secondary | 10 | ESBO | 30 | 40 | — | — | — | — | — | — | — | — | — | — | Plastisol, pressed | Yang et al.55 |
| CA18:2 |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 180 | 17 | 13 | — | — | 76 (A or D) | — | Both similar to DEHP | — | Plastisol | Ghatge et al.60 |
| Plasticizer | Structure | Cardanol derived plasticizer content (phr) | Other plasticizers | Other plasticizer contents (phr) | Total amount of plasticizer (phr) | Thermal stabilizer (phr) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (MPa) | T 5% (°C) | T g (°C) | Hardness A | Hardness D | Volatility chemical resistance | Toxicity | Plasticizing method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epoxides | ||||||||||||||||||
| ECA2 |

|
Primary | 30 | — | — | 30 | — | 550 | 25 | 95 | — | 44 | — | — | Lower volatility and chemical migration than DEHP | — | Solvent casting | Lee et al.44 |
| ECA14 |

|
Secondary | 10 | ESBO | 30 | 40 | — | 234 | 20 | — | 312 | 30 | — | — | Low volatility and chemical migration lower than DEHP and higher than ESBO | — | Plastisol, pressed | Yang et al.55 |
| ECA18:0 |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 230 | 18 | 14 | — | — | 75 (A or D) | — | Low volatility and similar chemical migration compared to DEHP | — | Plastisol | Ghatge et al.60 |
| ECA18:1 OA |

|
Primary | 43 | — | — | 43 | — | 264 | — | 7 | 268 | — | — | — | — | Non toxic | Plastisol | Briou et al.21 |
| ECA18:1 OA | Secondary | 13 | DEHP | 27 | 40 | 2 | 240 | 19 | 14 | — | — | 70 (A or D) | — | Low volatility and similar chemical migration compared to DEHP | — | Plastisol | Ghatge et al.60 | |
| ECA18:1 OA | Secondary | 10 | ESBO | 30 | 40 | — | 299 | 22 | — | 293 | 48 | — | — | Low volatility and chemical migration compared to both DEHP and ESBO | — | Plastisol, pressed | Yang et al.55 | |
| ECA18:1 RA |

|
Secondary | 10 | ESBO | 30 | 40 | — | 261 | 21 | — | 290 | 44 | — | — | Higher volatility to both DEHP and ESBO and low chemical migration compared to both DEHP and ESBO | — | Plastisol, pressed | Yang et al.55 |
| ECA18:2 |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 250 | 19 | 15 | — | — | 71 (A or D) | — | Low volatility and similar chemical migration compared to DEHP | — | Plastisol | Ghatge et al.60 |
| ECA18:2 | Secondary | 20 | DEHP | 20 | 40 | 2 | 250 | 22 | 129 | 250* | 35* | — | — | Low volatility and chemical migration compared to DEHP | — | Plastisol and molded injection | Chu et al.48 | |
| ECBE |
|
Primary | 40 | — | — | 40 | 2 | 285 | 23 | — | 256 (onset) | 37 | — | — | Low volatility and low to similar chemical migration compared to DEHP | — | Mixed, melted, pressed | Li et al.61 |
| Plasticizer | Structure | Cardanol derived plasticizer content (phr) | Other plasticizers | Other plasticizer contents (phr) | Total amount of plasticizer (phr) | Thermal stabilizer (phr) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (MPa) | T 5% (°C) | T g (°C) | Hardness A | Hardness D | Volatility chemical resistance | Toxicity | Plasticizing method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethers | ||||||||||||||||||
| CPEA |
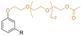
|
Primary | 40 | — | — | 40 | 2 | 675* | 11* | — | 245 | 5 | — | — | Low migration compared to DEHP | — | Solvent casting | Tan et al.63 |
| CPEB |
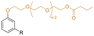
|
Primary | 40 | — | — | 40 | 2 | 590* | 12* | — | 264 | 7 | — | — | Low migration compared to DEHP | — | Solvent casting | Tan et al.63 |
| CPEO |
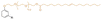
|
Primary | 40 | — | — | 40 | 2 | 525* | 11* | — | 240 | 17 | — | — | Low migration compared to DEHP | — | Solvent casting | Tan et al.63 |
| Phenyl cardanol ether |
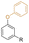
|
Secondary | 13 | DEHP | 27 | 40 | 2 | 200 | 17 | 12 | — | — | 76 (A or D) | — | Both similar to DEHP | — | Plastisol | Ghatge et al.39 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||||
| High molecular weight external plasticizers | ||||||||||||||||||
| ESBO-CD10 |
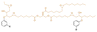
|
Primary | 35 | ESBO (eq. 30 phr) linked to cardanol (eq. 5 phr) | 35 | — | 258 | 22 | — | 316 | 45 | — | — | Low volatility/migration compared to plasticizers mainly containing ESBO | — | Mixed and internally heated | Yang et al.65 | |
| CHE-12 |
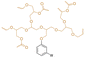
|
Primary | 40 | — | — | 40 | — | 320* | 24* | — | 276 (onset) | 38 | — | — | Low volatility/migration compared to DEHP | Non toxic | Solvent casting | Ma et al.64 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||||
| Internal plasticizers | ||||||||||||||||||
| Mannich base of cardanol butyl ether (CBE) |
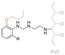
|
Secondary | 40 | DEHP | 75 (PVC) or 50 (PVC-CBE) | 115 | — | 288 | 23 | 121 | 232 (onset) | 59 | — | — | Low migration compared to DEHP | — | Solvent casting | Jia et al.67 |
| Propargyl ethylene cardanol (PEC) |
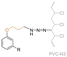
|
Secondary | 50 | DEHP | 75 (PVC) or 50 (PVC-CBE) | 115 | — | — | — | — | 209 (onset) | 59 | — | — | No migration compared to DEHP | — | Solvent casting | Jia et al.66 |
| Plasticizer | Structure | Cardanol derived plasticizer content (phr) | Other plasticizers | Other plasticizer contents (phr) | Total amount of plasticizer (phr) | Thermal stabilizer (phr) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (MPa) | T 5% (°C) | T g (°C) | Hardness A | Hardness D | Volatility chemical resistance | Toxicity | Plasticizing method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleophilic substitution | ||||||||||||||||||
| EC |

|
Primary | 40 | — | — | 40 | 4 | 329 | 21 | 114 | 298 (onset) | 69 | 89 | 43 | Low volatility and chemical migration compared to ESBO and DEHP | — | Mixed, melted, pressed | Satapathy et al.59 |
| PEC |
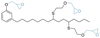
|
Primary | 25 | — | — | 25 | 3 | 9 | 31 | 1382 | — | — | — | — | Higher volatility and chemical migration compared to DEHP | — | Extrusion | Yao et al.58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||||
| Phopshonated | ||||||||||||||||||
| ECP |

|
Primary | 40 | — | — | 40 | — | 325* | 21* | 125* | 258 | 13 | — | — | — | — | Solvent casting | Jia et al.69 |
| CPP |
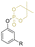
|
Secondary | 25 | DEHP | 25 | 50 | 2 | 196 | 11 | 0,09 | 237 | 8 | — | — | High migration loss compared to DEHP | — | Plastisol, melt mixed, pressed | Hou et al.68 |
| MC |
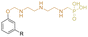
|
Secondary | 10 | DEHP | 30 | 40 | 2 | 432 | 26 | — | 252 | 9 | — | — | Low volatility and chemical migration compared to DEHP | — | Plastisol, pressed | Ali et al.41 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||||
| Acetylated | ||||||||||||||||||
| Acetylated cardanol |
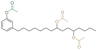
|
Primary | 70 | — | — | 70 | 4 | 445 | 17 | 4 | 255 | — | — | — | — | Compression molded | Neuse et al.46 | |
| Plasticizer | Structure | Cardanol derivedd plasticizer content (phr) | Other plasticizers | Other plasticizer contents (phr) | Total amount of plasticizer (phr) | Thermal stabilizer (phr) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (MPa) | T 5% (°C) | T g (°C) | Hardness A | Hardness D | Volatility chemical resistance | Toxicity | Plasticizing method | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorinated | ||||||||||||||||||
| Cl-cardanol |
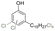
|
Secondary | 13 | DEHP | 27 | 40 | 1 | 118 | 19 | 18 | — | — | 92 (A or D) | — | Lower than DEHP | — | Plastisol | Ghatge et al39. |
| Cl-methyl ether cardanol |
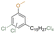
|
Secondary | 13 | DEHP | 27 | 40 | 1 | 200 | 22 | 17 | — | — | 90 (A or D) | — | Lower than DEHP | — | Plastisol | Ghatge et al.39 |
| Cl-phenyl cardanol ether |
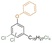
|
Secondary | 13 | DEHP | 27 | 40 | 2 | 300 | 21 | 17 | — | — | 79 (A or D) | — | Lower than DEHP | — | Plastisol | Ghatge et al.39 |
| Cl-CA2 |
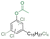
|
Secondary | 13 | DEHP | 27 | 40 | 1 | 210 | 22 | 18 | — | — | 94 (A or D) | — | Lower than DEHP | — | Plastisol | Ghatge et al.39 |
| Cl-CA18:0 |
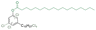
|
Secondary | 13 | DEHP | 27 | 40 | 2 | 210 | 16 | 13 | — | — | 76 (A or D) | — | Similar to DEHP | — | Plastisol | Ghatge et al.39 |
| Cl-CA18:1 OA |
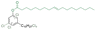
|
Secondary | 13 | DEHP | 27 | 40 | 2 | 220 | 17 | 13 | — | — | 78 (A or D) | — | Similar to DEHP | — | Plastisol | Ghatge et al.39 |
| Cl-CA18:2 |

|
Secondary | 13 | DEHP | 27 | 40 | 2 | 240 | 18 | 14 | — | — | 74 (A or D) | — | Similar to DEHP | — | Plastisol | Ghatge et al.39 |
Thus, these plasticizers must be cost-effective, sustainably synthesized, and exhibit good mechanical, thermal, and migration resistance properties while being non-toxic. Achieving all desired parameters is often challenging, and compromises may need to be made during the process. Therefore, it is essential to continue experimental research in this field to develop solutions that meet the requirements of a plasticizer. Exploring other CNSL compounds to utilize CNSL as a reactant could streamline the synthesis process, leading to reductions in time, energy, and costs. This approach could align with a more sustainable framework.
Cardanol-based plasticizers for poly(lactic acid) (PLA)
CNSL derivatives, and especially, if not exclusively, cardanol derivatives, have been used for the plasticization of other polymers. Among biobased polymers, PLA seems to be one of the most promising and is receiving extensive attention, both in academic and industrial fields for the last 25 years. In addition to having a precursor, lactic acid, from a renewable raw material, the properties and non-hazardousness of PLA allow it to be highly appreciated for applications related to the field of food packaging. Another advantage of PLA is its biodegradability, although this remains to be qualified.70 Indeed, at room temperature, in domestic composting, its degradation can be particularly long and take several months71,72 whereas when composted under specific industrial conditions, its degradation can be completed in less than 30 days.73,74 However, during its implementation, PLA is very often mixed with additives, in particular with plasticizers to gain flexibility and processability. In order not to reduce the sustainability benefits of PLA, the additives used in blends with this polymer must preferably be bio-based, non-toxic and also biodegradable.Having been already proven to be a compatibilizer agent with PLA, notably for PLA-ABS (acrylonitrile butadiene styrene) mixtures,75,76 PLA-PBAT(polybutylene adipate-co-terephthalate)77 or PLA-bamboo,78 cardanol derivatives appear to be relevant candidates for plasticizer synthesis for PLA. Cardaso et al. also showed the influence and interaction of cardanol in the preparation of PLA microparticles, and cardanol then played the role of a stabilizer but also impacted the properties of the material obtained by reducing its Tg, similar to a plasticizing activity.79 Several teams have begun to investigate the use of CNSL derivatives as a plasticizer for PLA with two different strategies.
External plasticizing
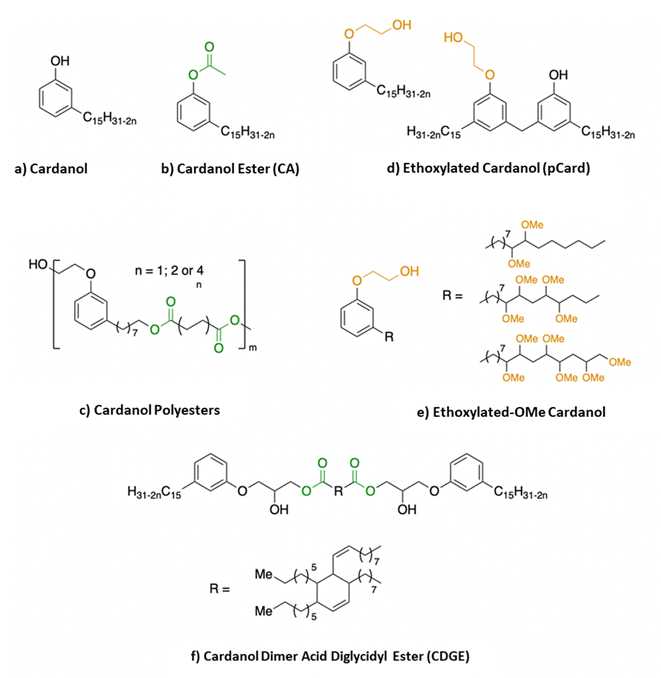
The first way is the use of cardanol or one of its derivatives mixed with the polymer as an external additive. Most of the time, the plasticizer is mixed with PLA using a twin-screw extruder, between 160 and 200 °C. Cardanol alone (Fig. 14a), obtained after distillation of technical CNSL, has been considered by several teams. Kang et al. showed that cardanol, at an amount less than or equal to 25%, mixes well with PLA and that the latter lowers the Tg of the formulated polymer.80 Mele et al. showed that after 28 days, in various media such as salt water, ethanol or acetic acid, PLA plasticized with cardanol did not release plasticizers and that, in view of the properties obtained from the polymer thus formulated, cardanol was a relevant candidate for the development of PLA films for application in food packaging.81 Greco et al. had been interested in cardanol ester (CA) synthesized from the reaction between cardanol and acetic anhydride (Fig. 14b). They first compare it to DEHP,82 a phthalate commonly used with PLA, and then to PEG and cardanol alone.83 Several of their articles focus on the study of the thermal and mechanical properties of plasticized PLA,84–86 demonstrating a real plasticizing effect of this cardanol ester derivative. Vallin et al.87 described the use of a difunctional cardanol with two hydroxy groups, one obtained by ethoxylation of cardanol, and the other at the end of the side chain, reduced to eight carbons instead of fifteen (Fig. 13) after ozonolysis and reduction.88,89 They used this molecule as a co-monomer with dimethyl succinate, adipate or sebacate in the presence of titanium(IV) bromide or CalB Novozym 435, an enzyme. The polyesters obtained have low molecular weights (between 1800 and 4100 g mol−1) (Fig. 14c). Plasticizers were mixed at various rates (2, 5 and 10 wt%) with PLA, and the mixture exhibited a better elongation at break, a slight decrease in Tg (from 58 to 50 °C for 10 wt%) and Young's modulus relatively similar to that of PLA alone. Mixing these two polyesters has allowed, without the need for the addition of a compatibilizing agent, a plasticizing effect on PLA. It should be noted that the plasticizing properties of these polyesters remain relatively low even though they require many synthesis steps beforehand, thus increasing the potential cost of these plasticizers. Hassouma et al. designed a plasticizer from ethoxylated derivatives of cardanol (pCard) (Fig. 14d) by functionalizing the alkyl chain.90 They modified the double bonds of the cardanol side chain into methoxy group by methylation (Fig. 14e). This plasticizer allowed an enhanced ductility of the blend, a better miscibility and compatibility between PLA and the plasticizers compared to the only ethoxylated ones. It should be noted, however, that the synthesis of this plasticizer requires four steps, including one for protection and then one for deprotection. This increase in the number of synthesis steps can have a serious impact on the cost of such a plasticizer and slow down its use and production on an industrial scale.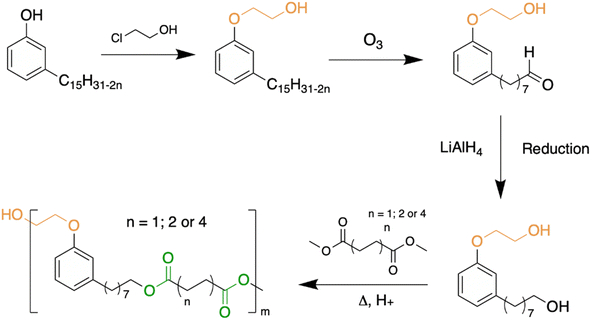 | ||
| Fig. 13 Chemical pathway to obtain a cardanol-ester PLA plasticizer.88,89 | ||
Finally, recently, two patents describe the use of cardanol derivatives for the plasticization of PLA. The first, filed in 2022,91 describes the use of cardanol directly, at about 5–6%, as a plasticizer for the development of biodegradable PLA-PBAT films. The films demonstrate antimicrobial activity as well as hyper-flexibility. The second patent, filed in 2023,92 describes dimer derivatives of cardanol (Fig. 14f) and eugenol synthesized by ring opening etherification of dimer acid diglycidyl ester. The use of this cardanol derivative (CDGE) as a PLA plasticizer allows good flexibility of the material and improves its resistance to the migration of additives formulated with PLA.
Internal plasticizing
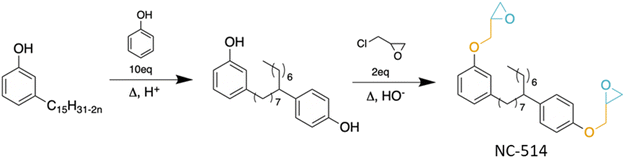
The second method consists of grafting the cardanol derivatives directly onto the PLA in order to provide an internal plasticizing effect to the modified polymer. In a short article, Tang et al. compared PLA plasticized with cardanol at various ratios (between 5 and 25 wt%) to PLA processed with 15 wt% cardanol in the presence of dicumyl peroxide (DCP).93 The PLA made under these conditions shows properties equivalent to those of PLA plasticized with 20 wt% cardanol without DCP and have superior properties to that plasticized with 15 wt% cardanol. According to the authors, this enhancement in properties would result from grafting this 15 wt% cardanol onto PLA, carried out by reactive extrusion in the presence of DCP. However, this remains to be proven by structural characterization methods. It would also be interesting to study the impact on the properties of the polymer resulting in a decrease in the quantity of cardanol “grafted”, with this method, in the presence of DCP. Mihai et al.94 described the use of the Cardolite cardanol derivative NC-514 in PLA reactive extrusion in the presence of ethyltriphenyl phosphorium bromide (ETPB). The grafting was highlighted by structural analyses, including FTIR and SEC. The grafted PLA exhibited a lower Tg, elastic modulus and higher elongation at break, validating the effect of internal plasticization on PLA. It should be noted that the authors mention in the article a “new biobased plasticizer” called NC-514, but it is important to clarify that this compound is only partially bio-based. Indeed, it is produced from cardanol but also from phenol, CMR and petroleum95 and then from epichlorohydrin, and also CMR (Fig. 15).In view of their affinity with PLA, CNSL derivatives, exclusively from cardanol, seem to be relevant, alone or as a precursor for plasticizer synthesis. Studies on PLA application are still very recent (less than 10 years), with results to be confirmed. Finally, even if the bio-based nature of these plasticizers does not need to be proven anymore, but sometimes needs to be qualified, it has to be noted that there is still a lack of additional studies on the toxicity, ecotoxicity and biodegradability of these compounds to really consider them as real viable alternatives for the plasticization of PLA.
Cardanol-based plasticizers for cellulose acetate (CA)
Cellulose is another well used polymer, one of the most abundant biobased polymers on earth with an estimated production of 50–55 × 109 tons per year.96 It is primarily produced by plants and is a principal constituent of plant cell walls. The repeating unit of cellulose comprises three alcohol functional groups which form hydrogen bonds. Consequently, cellulose is a highly crystalline polymer and these interactions confer high mechanical and thermal properties. However, this also makes it difficult to solubilize in solvents and to process. Indeed, its hypothetical melting temperature is higher than its degradation temperature. In 1865, CA was synthesized for the first time by mixing cellulose with acetic anhydride replacing several OH functional groups by acetate groups. CA has quite different properties compared to cellulose depending on the degree of substitution (DS), which refers to the number of hydroxyl (OH) groups replaced by acetyl groups on the repeating unit of cellulose (Fig. 16). In particular, for a degree of substitution of two, the melting temperature of cellulose diacetate (CDA) becomes lower than its degradation temperature. This polymer is also soluble in some common organic solvents such as acetone.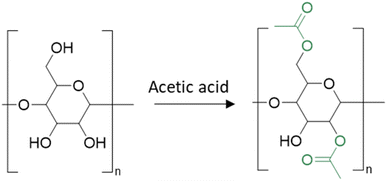 | ||
| Fig. 16 Cellulose into acetate cellulose (the number of acetic units is purely for schematic representation). | ||
Even if cellulose acetate is more processable than cellulose, the high amount of acetyl functional groups and the remaining alcohol functional groups generate strong interactions between macromolecules. It results in high Tg which can be a drawback depending on the application. Adding plasticizers is therefore often needed in order to reduce crystallinity. Iji et al. investigated a thermoplastic polymer of cellulose diacetate plasticized with an internal plasticizer made from cardanol.97 They synthesized hydrogenated cardanoxy acetic acid chloride which was later chemically grafted on CDA through esterification of remaining alcohol functional groups (Fig. 17). Some properties of cellulose diacetate containing modified cardanol were better than those of cellulose diacetate plasticized with an external plasticizer (triethyl citrate) such as lower Tg and water absorption, and higher bending strength.
 | ||
| Fig. 17 Proposed structure of cellulose diacetate bonded with cardanoxy acetic acid (the number of acetate units and hydrogenated cardanol is purely for schematic representation) adapted from ref. 97. | ||
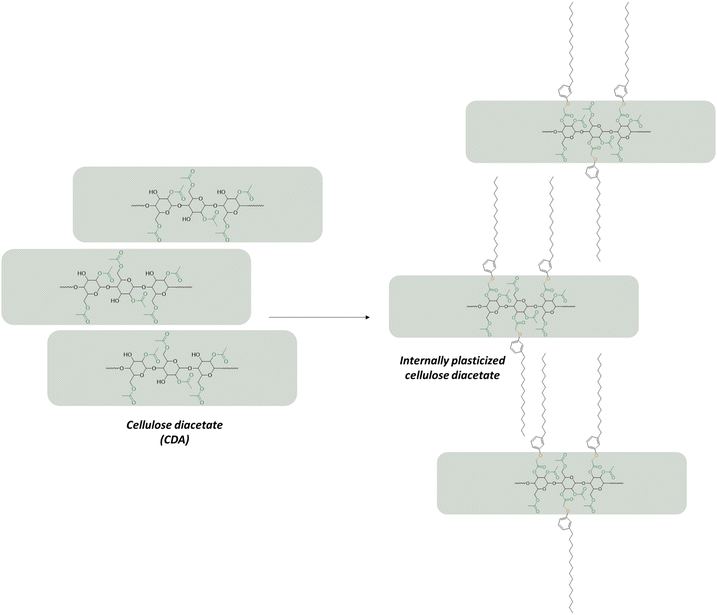
The authors pointed out that the thermoplasticity of cardanol bonded cellulose diacetate was even better than that of cardanol diacetate plasticized with triethyl citrate, a conventional bio-based plasticizer. However, the melt flow rate of two cardanol bonded cellulose diacetates having two different DS are lower than those of two formulations of cellulose diacetate plasticized with the external plasticizer. Similarly, the Tg of the cardanol bonded to cellulose diacetate is higher. They highlighted that the long alkyl chain of cardanol was responsible for high toughness, heat resistance and water resistance while the aromatic ring was responsible for the increasing bending strength, elastic modulus and also water resistance.98
These two papers were recently reviewed by Bonifacio et al.99 The process of grafting cardanol on cellulose diacetate was later improved.100 In the initial process, HCl was formed as a by-product by the reaction of pentadecylphenoxy acetic acid chloride and cellulose diacetate and large amounts of poor solvent were used to isolate the final product. This could be prevented using hexamethylene diisocyanate. This product is able to react with the OH moiety of cardanol leaving one isocyanate functional group which can further react with the remaining OH functional groups of cellulose diacetate (Fig. 18). This procedure has two advantages: no by-product is formed and the unreacted cardanol derivative can coagulate by oligomerization before being filtered. Thus, the plasticized cellulose diacetate can be isolated simply by evaporating the solvent. This procedure greatly reduced the quantity of solvent used.
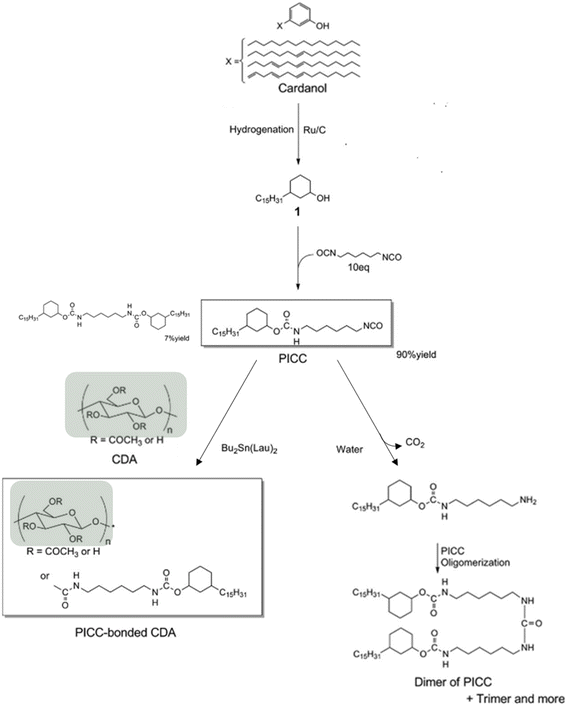 | ||
| Fig. 18 Modifying process of cardanol to PICC and bonding to CDA (adapted from ref. 100). | ||
The product obtained had high strength, hydrophobicity and heat resistance. However, its thermoplasticity was greatly reduced because of strong interactions between modified cardanol units. The melt flow index could be increased while maintaining other characteristics by grafting propionyl groups. These two methods of grafting cardanol derivatives on cellulose diacetate have been described in a patent.101
In a subsequent study, the quantity of solvent used was again greatly decreased by synthesizing plasticized cardanol-bonded cellulose diacetate through a two-step heterogeneous process starting from cellulose instead of cellulose diacetate.102 The reduction of solvent reached about 90% compared to that in the homogeneous process used in ref. 96.
A one-pot heterogeneous process was later developed in which they highlighted the major role of the solvent in the grafting ratio of a long chain (modified cardanol) and short chain (acetyl). It was shown that a good affinity of the solvent with cellulose enhanced the reactivity of the PA (3-pentadecylphenoxy acetyl group) moiety and that the polarity of the solvent had an impact on the reaction ratio of acetyl and PA moieties.103
Despite having good properties such as thermoplasticity, high water and heat resistance and high strength, the impact strength of cardanol-bonded cellulose diacetate remains too low to be used in durable products. Several methods have been employed to improve this parameter: adding small amounts of olefin resins like PE or PP,104 adding small amounts of polyether silicones105 or adding poly(butylene succinate adipate) and glass fiber.106 In a quite different field, cardanol was used as an external plasticizer for CA in order to make protective coatings for seeds against herbicides.107
More recently, a patent concerning cardanol and cardol derivatives has been filed about using these ester derivatives as cellulose acetate plasticizers.108In the examples described in this patent, cellulose acetate with a substitution degree of 2.5 has been formulated by extrusion and shows a lowering of the Tg to around 160–170 °C for many derivatives and to 114 °C with cardol without chemical modification. However, mechanical test results such as the Young's modulus or elongation at fracture are missing to validate the potential of such a plasticizer with a comparable result with citrates or DEP.
In conclusion, over the past decade, only cardanol from CNSL has been studied and presents potential applications in the plasticization of cellulose acetate, in addition to PVC and PLA. Grafting cardanol onto cellulose acetate exhibits favorable properties such as thermoplasticity, high water and heat resistance, and high strength. However, the impact strength of cellulose acetate plasticized with cardanol remains too low for use in durable products. Cardol, a CNSL compound often set aside, seems, in view of the recent results, to be a promising candidate for the plasticization of cellulose acetate, without prior chemical modification. Although these plasticizers are bio-based, it is important to ensure the industrial viability of the synthesis and to study the toxicological and environmental impact of these compounds and their decomposition derivatives. It might also be interesting to study the biodegradability of these molecules, which are used for the plasticization of biodegradable polymers. Furthermore, studies on cellulose acetate by cardanol remain limited, with primarily a research team in Japan focusing on this aspect. This emerging field of application requires more attention to further expand research and understanding.
Cardanol-based plasticizers for rubber
Rubber, natural or synthetic, is a main and central component of tires found in the automotive, transportation or aerospace fields. This material offers unique and highly sought-after mechanical properties in these fields of application. The flexibility of the tires provides the user with a good feeling of safe driving. Depending on the composition of these tires, and the use and the weather conditions during their use, the properties of the tires can vary greatly. As for PVC, in order to maintain good mechanical properties and flexibility, plasticizers compatible with rubber are used in the formulation of these tires. Since the widespread use of petroleum began, petro-based oils, paraffinic and naphthalenic, have been used as plasticizers for tire rubber. Among them, poly(cyclic aromatic hydrocarbon)s (PAH), obtained as an aromatic extract from distillation, are widely used because of the compromise between the good resulting properties of the formulated rubber obtained and the accessibility price of these additives. However, the use of these aromatic petroleum-based oils has demonstrated a certain carcinogenic risk and is a cause of pollution for the environment. In Europe, for example, the amount of PAHs allowed in a tire has been very limited (<3 wt%) since 2010.109 It is therefore necessary and important to find bio-based and non-toxic alternatives to PAHs whose use in tires tends to disappear.Among the vegetable oils available and known to have a plasticizing effect, CNSL derivatives are the only ones having aromatic rings and have therefore, among other things, been investigated as additives and plasticizers for rubber.
Initially, the use of CNSL derivatives in formulation with rubber was studied by Menon et al. in order to improve the self-adhesion strength of natural rubber (NR).110 For this, a phenolic resin prepolymer prepared from phosphorylated CNSL and formaldehyde (PCNSL) is added between 0 and 10 phr with NR (Fig. 19). Subsequently, the same team investigated the influence of the addition of PCNSL and CNSL on vulcanization and crosslinking,111 and on the tensile stress relaxation characteristic at room temperature,112 and their activity as a flame retardant additive113 and as a plasticizer114 on NR.
Subsequently, the use of CNSL as an external plasticizer for different types of rubber was described.115 In 2006, Arayapranee et al.117 studied its formulation with a blend 50/50 NR/EPDM (ethylene propylene diene monomer). NR/EPDM formulated with CNSL shows an increase in thermal stability of the material and better mechanical properties compared to NR/EPDM formulated with paraffin oil. A technical paper by Flanigan et al.117 describes the use of bio-based plasticizers for rubber, including CNSL, which compared to other plasticizers, seems to be among the best candidates. In another article,116 CNSL is used as a plasticizer of carbon black/rubber and is compared to castor oil and paraffin oil. The CNSL shows good plasticizing properties and the best adhesion properties on various substrates among the different plasticizers used in the study.
Cardanol, obtained after distillation of technical CNSL, was investigated by Alexander et al. as an external plasticizer for rubber. It shows good plasticizing properties formulated with silica filled NR118 or carbon black filled NR.119 They demonstrated the versatility and synergistic effect of cardanol when formulated with high abrasion furnace black filled NR. In this formulation, cardanol alone serves as a plasticizer, activator, and antioxidant, yielding even better results than the reference comprising a mixture of conventional additives, each performing one of these distinct roles.121
Recently, in 2022, a polycardanol (PCA) obtained by polymerization by the Friedel–Crafts alkylation reaction was described by Quian et al.122 (Fig. 19). This polymer, obtained by a solvent-free reaction, was formulated with styrene butadiene rubber (SBR) as a plasticizer and lowers Tg. It increases the crosslinking density of the material and also acts as a compatibilizing agent by improving the processability of the SBR and allowing a better dispersion of the silica added to the blend during formulation.
Finally, the strategy of using CNSL derivatives as an internal plasticizer of rubber was also investigated. Wang et al. grafted a cardanol motif directly onto a liquid isoprene rubber (LIR) through a radical route, employing a solvent-free reaction in the presence of AIBN.123 The performance of LIR with and without cardanol was then compared with each other and highlighted a certain plasticizing effect in the presence of grafted cardanol and a better dispersion of silica particles during their formulation. The grafted cardanol here plays both the role of an internal plasticizer and compatibilizer.
Samantarai et al. grafted a prepolymer of phosphorylated cardanol (PCP) on acrylonitrile butadiene rubber (NBR) by a radical route. The PCP-grafted-NBR compared to its counterpart without grafting show a gain in thermal stability, a better processability of NBR, and a decrease in Tg, related to the effect of internal plasticization induced by the cardanol motifs of PCP.95
In conclusion, CNSL derivatives are of increasing interest in the tire industry not only as plasticizers but also for improving the processability and compatibility of rubber with the fillers it is mixed with. CNSL derivatives also contribute to improving the thermal stability of the formulated blends obtained. The use of these molecules as bio-based alternative plasticizers for rubber is still in the beginning stage but seems relevant and attractive in this field for years to come.
Conclusion
In conclusion, the exploration of CNSL derivatives as potential plasticizers has generated significant interest, particularly in industries involving PVC, PLA, cellulose acetate, and rubber. With the increasing demand for alternatives to conventional toxic plasticizers like phthalates or polycyclic aromatic hydrocarbons, CNSL derivatives, such as cardanol or cardol, emerge as a promising solution due to their unique phenolic composition.Experimental studies have investigated various modification pathways for cardanol, including etherification, esterification, and epoxidation, each offering distinct advantages and challenges, particularly in PVC applications. Esterification has demonstrated compatibility with PVC but may compromise mechanical properties, while epoxidation enhances compatibility, thermal stability, and mechanical properties. Etherification is commonly utilized to increase molecular weight and enhance migration resistance. Other pathways, such as chlorination and phosphating, have been explored for potential fire resistance benefits in PVC plasticizing applications. However, toxicity has to be taken into account.
Additionally, cardanol shows potential applications in the plasticization of PLA and cellulose acetate, alongside PVC, representing a relatively recent field compared to PVC applications. While cardanol exhibits promising plasticizer properties for both PLA and cellulose acetate, further research is necessary to validate its effectiveness. Moreover, CNSL, and specifically cardanol, can be utilized in the rubber industry to replace toxic plasticizers like polycyclic aromatic hydrocarbons.
The synthesis sustainability, migration, and toxicity considerations of CNSL-derived plasticizers remain crucial. These plasticizers must be cost-effective, sustainably synthesized, non-toxic, and exhibit favorable mechanical and thermal properties while resisting migration. Achieving these parameters poses challenges, underscoring the need for ongoing research to develop viable solutions. It is worth noting that the vast majority of synthesized plasticizers are derived from cardanol. However, extracting cardanol from CNSL can incur costs. Therefore, exploring other molecules within CNSL, which are not valorized such as cardol or anacardic acid, and potentially utilizing CNSL directly as a starting material could be worthwhile avenues for investigation.
Continued research and development in this field would be appreciated to fully unlock the potential of CNSL-derived plasticizers and ensure their effective integration into industrial applications in the future.
Data availability
The authors confirm that all the data present in this article are available and accessible via the “references” section cited in the last pages of the article in question.Conflicts of interest
There are no conflicts to declare.References
- The World Crisis, in The World Crisis ? and what to Do about it, World Scientific, 2020, pp. 1–5, https://www.worldscientific.com/doi/abs/10.1142/9789811234613_0001 Search PubMed.
- J. Clapp, Concentration and crises: exploring the deep roots of vulnerability in the global industrial food system, J. Peasant Stud., 2023, 50(1), 1–25 CrossRef.
- H. Han, Consumer behavior and environmental sustainability in tourism and hospitality: a review of theories, concepts, and latest research, in Sustainable Consumer Behaviour and the Environment, Routledge, 2021 Search PubMed.
- A. Lubowiecki-Vikuk, A. Dąbrowska and A. Machnik, Responsible consumer and lifestyle: Sustainability insights, Sustainable Prod. Consum., 2021, 25, 91–101 CrossRef PubMed.
- L. Yang, X. C. Wang, M. Dai, B. Chen, Y. Qiao and H. Deng, et al., Shifting from fossil-based economy to bio-based economy: Status quo, challenges, and prospects, Energy, 2021, 228, 120533 CrossRef.
- S. Walker and R. Rothman, Life cycle assessment of bio-based and fossil-based plastic: A review, J. Clean. Prod., 2020, 261, 121158 CrossRef CAS.
- M. A. Kamrin, Phthalate Risks, Phthalate Regulation, and Public Health: A Review, J. Toxicol. Environ. Health, Part B, 2009, 12(2), 157–174 CAS.
- G. Bolívar-Subirats, C. Rivetti, M. Cortina-Puig, C. Barata and S. Lacorte, Occurrence, toxicity and risk assessment of plastic additives in Besos river, Spain, Chemosphere, 2021, 263, 128022 CrossRef PubMed.
- R. Czernych, M. Chraniuk, P. Zagożdżon and L. Wolska, Characterization of estrogenic and androgenic activity of phthalates by the XenoScreen YES/YAS in vitro assay, Environ. Toxicol. Pharmacol., 2017, 53, 95–104 CrossRef CAS PubMed.
- C. G. Basso, A. T. de Araújo-Ramos and A. J. Martino-Andrade, Exposure to phthalates and female reproductive health: A literature review, Reprod. Toxicol., 2022, 109, 61–79 CrossRef CAS PubMed.
- B. J. Brown, M. E. Hanson, D. M. Liverman and R. W. Merideth, Global sustainability: Toward definition, Environ. Manag., 1987, 11(6), 713–719 CrossRef.
- P. Marion, B. Bernela, A. Piccirilli, B. Estrine, N. Patouillard and J. Guilbot, et al., Sustainable chemistry: how to produce better and more from less?, Green Chem., 2017, 19(21), 4973–4989 RSC.
- M. Bocqué, C. Voirin, V. Lapinte, S. Caillol and J. J. Robin, Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(1), 11–33 CrossRef.
- D. F. Cadogan, Plasticizers: A consideration of their impact on health and the environment, J. Vinyl Addit. Technol., 1991, 13(2), 104–108 CrossRef.
- O. Horn, S. Nalli, D. Cooper and J. Nicell, Plasticizer metabolites in the environment, Water Res., 2004, 38(17), 3693–3698 CrossRef CAS PubMed.
- S. Kumar, Recent Developments of Biobased Plasticizers and Their Effect on Mechanical and Thermal Properties of Poly(vinyl chloride): A Review, Ind. Eng. Chem. Res., 2019, 58(27), 11659–11672 CrossRef CAS.
- B. Sun, B. I. Chaudhary, C. Y. Shen, D. Mao, D. M. Yuan and G. C. Dai, et al., Thermal stability of epoxidized soybean oil and its absorption and migration in poly(vinylchloride), Polym. Eng. Sci., 2013, 53(8), 1645–1656 CrossRef CAS.
- M. P. Arrieta, M. D. Samper, M. Jiménez-López, M. Aldas and J. López, Combined effect of linseed oil and gum rosin as natural additives for PVC, Ind. Crops Prod., 2017, 99, 196–204 CrossRef CAS.
- F. Ortega, F. Versino, O. V. López and M. A. García, Biobased composites from agro-industrial wastes and by-products, Emergent Mater., 2022, 5(3), 873–921 CrossRef CAS PubMed.
- A. Greco, D. Brunetti, G. Renna, G. Mele and A. Maffezzoli, Plasticizer for poly(vinyl chloride) from cardanol as a renewable resource material, Polym. Degrad. Stab., 2010, 2169–2174 CrossRef CAS.
- B. Briou, S. Caillol, J. J. Robin and V. Lapinte, Non-endocrine disruptor effect for cardanol based plasticizer, Ind. Crops Prod., 2019, 130, 1–8 CrossRef CAS.
- A. Roy, P. Fajardie, B. Lepoittevin, J. Baudoux, V. Lapinte and S. Caillol, et al., CNSL, a promising building blocks for sustainable molecular design of surfactants: A critical review, Molecules, 2022, 27(4), 1443 CrossRef CAS PubMed.
- M. C. Lubi and E. T. Thachil, Cashew nut shell liquid (CNSL) - a versatile monomer for polymer synthesis, Des. Monomers Polym., 2000, 3(2), 123–153 CrossRef CAS.
- D. Balgude and A. S. Sabnis, CNSL: an environment friendly alternative for the modern coating industry, J. Coat. Technol. Res., 2014, 11(2), 169–183 CrossRef CAS.
- M. Telascrêa, A. L. Leão, M. Z. Ferreira, H. F. F. Pupo, B. M. Cherian and S. Narine, Use of a Cashew Nut Shell Liquid Resin as a Potential Replacement for Phenolic Resins in the Preparation of Panels – A Review, Mol. Cryst. Liq. Cryst., 2014, 604(1), 222–232 CrossRef.
- E. Uliassi, A. S. de Oliveira, L. de Camargo Nascente, L. A. S. Romeiro and M. L. Bolognesi, Cashew Nut Shell Liquid (CNSL) as a Source of Drugs for Alzheimer's Disease, Molecules, 2021, 26(18), 5441 CrossRef CAS PubMed.
- M. L. Adekanbi and T. T. Olugasa, Utilizing cashew nut shell liquid for the sustainable production of biodiesel: A comprehensive review, Clean. Chem. Eng., 2022, 4, 100085 CrossRef.
- D. Lomonaco, G. Mele and S. E. Mazzetto, Cashew Nutshell Liquid (CNSL): From an Agro-industrial Waste to a Sustainable Alternative to Petrochemical Resources, in Cashew Nut Shell Liquid: A Goldfield for Functional Materials, ed. P. Anilkumar, Springer International Publishing, Cham, 2017, pp. 19–38, DOI:10.1007/978-3-319-47455-7_2.
- D. C. Ike, M. U. Ibezim-Ezeani and O. Akaranta, Cashew nutshell liquid and its derivatives in oil field applications: an update, Green Chem. Lett. Rev., 2021, 14(4), 620–633 CrossRef CAS.
- P. Anilkumar, Cashew Nut Shell Liquid: A Goldfield for Functional Materials, Springer, 2017, p. 233 Search PubMed.
- D. K. Viswalingam and D. F. E. Solomon, A Process for Selective Extraction of Cardanol from Cashew Nut Shell Liquid (CNSL) and its Useful applications, 2013, vol. 4.
- P. Jia, H. Xia, K. Tang and Y. Zhou, Plasticizers Derived from Biomass Resources: A Short Review, Polymers, 2018, 10(12), 1303 CrossRef PubMed.
- L. Ciacci, F. Passarini and I. Vassura, The European PVC cycle: In-use stock and flows, Resour. Conserv. Recycl., 2017, 123, 108–116 CrossRef.
- Plastics - the Facts 2022 • Plastics Europe. [cité 22 avr 2024]. Disponible sur: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/.
- L’ingénieur, T. de. Plastifiants. https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/adjuvants-des-plastiques-42138210/plastifiants-a3231/. In.
- T. Schettler, Human exposure to phthalates via consumer products, Int. J. Androl., 2006, 29(1), 134–139 CrossRef CAS PubMed.
- H. Hosney, B. Nadiem, I. Ashour, I. Mustafa and A. El-Shibiny, Epoxidized vegetable oil and bio-based materials as PVC plasticizer, J. Appl. Polym. Sci., 2018, 135(20), 46270 CrossRef.
- J. Parcell, Y. Kojima, A. Roach and W. Cain, Global Edible Vegetable Oil Market Trends, Biomed. J. Sci. Tech. Res., 2018, 2(1), 2282–2291 Search PubMed.
- N. D. Ghatge and S. P. Vernekar, Plasticizer extenders for PVC from cashewnutshell liquid, Eur. Polym. J., 1970, 6(12), 1547–1552 CrossRef CAS.
- B. Briou, Valorisation du cardanol et d’acides et d’aldéhydes lipidiques dans le domaine des matériaux polymères, PhD thesis, Université Montpellier, 2018, [cité 13 avr 2023]. Disponible sur: https://theses.hal.science/tel-02180529 Search PubMed.
- M. Ali, Y. Lu, S. Ahmed, S. Khanal and S. Xu, Effect of Modified Cardanol as Secondary Plasticizer on Thermal and Mechanical Properties of Soft Polyvinyl Chloride, ACS Omega, 2020, 5(28), 17111–17117 CrossRef CAS PubMed.
- Y.-u. Kim, J.-h. Shin, S.-j. Lee, J.-m. Kim, K.-h. Kim, and R.-h. Kim, Plasticizer composition comprising naturally cardanol base plasticizer and vegetable oil base epoxidated, and polymer resin composition comprising the same, KR101981990B1, 2019.
- Z. Caili, Y. Yang and W. Yunxuan, Preparation method of epoxy cardanol ester bio-based plasticizer, product and application thereof, CN113881102A, 2022.
- S. Lee, M. S. Park, J. Shin and Y. W. Kim, Effect of the individual and combined use of cardanol-based plasticizers and epoxidized soybean oil on the properties of PVC, Polym. Degrad. Stab., 2018, 147, 1–11 CrossRef CAS.
- A. Greco, F. Ferrari, R. Del Sole and A. Maffezzoli, Use of cardanol derivatives as plasticizers for PVC, J. Vinyl Addit. Technol., 2018, 24(S1), E62–E70 CAS.
- E. W. Neuse and J. D. Van Schalkwyk, Cardanol derivatives as PVC plasticizers. II. Plasticizer evaluation, J. Appl. Polym. Sci., 1977, 3023–3033 CrossRef CAS.
- J. Chen, Z. Liu, X. Nie, Y. Zhou, J. Jiang and R. Murray, Plasticizers derived from cardanol: synthesis and plasticization properties for polyvinyl chloride (PVC), J. Polym. Res., 2018, 25, 128 CrossRef.
- H. Chu, H. Chu, X. Sun and Y. Zhang, Synthesis of Biomass Based Plasticizer from Linoleic Acid and Cardanol for Preparing Durable PVC Material, J. Polym. Environ., 2022, 30, 3270–3278 CrossRef CAS.
- C. Zhang, Y. Yang and Y. Weng, CN113881102A, 2021.
- L. Puchot, Cardanol : a Bio-Based Building Block for New Sustainable and Functional Materials, PhD thesis, Université de Cergy Pontoise ; Luxembourg Institute of Science and Technology, 2016, https://theses.hal.science/tel-01470742 Search PubMed.
- C. H. ying, L. H. bei, S. X. yan and Z. Y. wang, Synthesis of Biomass Based Plasticizer from Linoleic Acid and Cardanol for Preparing Durable PVC Material, J. Polym. Environ., 2022, 30(8), 3270–3278 CrossRef.
- A. Greco, F. Ferrari and A. Maffezzoli, Effect of the epoxidation yield of a cardanol derivative on the plasticization and durability of soft PVC, Polym. Degrad. Stab., 2016, 134, 220–226 CrossRef CAS.
- D. Li, K. Panchal, N. K. Vasudevan, R. Mafi and L. Xi, Effects of molecular design parameters on plasticizer performance in poly(vinyl chloride): A comprehensive molecular simulation study, Chem. Eng. Sci., 2022, 249, 117334 CrossRef CAS.
- Y. H. Kim, E. S. An, S. Y. Park and B. K. Song, Enzymatic epoxidation and polymerization of cardanol obtained from a renewable resource and curing of epoxide-containing polycardanol, J. Mol. Catal. B: Enzym., 2007, 45(1), 39–44 CrossRef CAS.
- Z. Yang, Y. Zhang, L. Chu, N. Bao and L. Shen, Tailored Acid Carrier Acidity for the Controlled C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C Peracid Epoxidation of Cardanol-Based Materials with Different Polarities, Ind. Eng. Chem. Res., 2023, 62(50), 21644–21653 CrossRef CAS.
C Peracid Epoxidation of Cardanol-Based Materials with Different Polarities, Ind. Eng. Chem. Res., 2023, 62(50), 21644–21653 CrossRef CAS. - A. Greco, F. Ferrari and A. Maffezzoli, UV and thermal stability of soft PVC plasticized with cardanol derivatives, J. Clean. Prod., 2017, 164, 757–764 CrossRef CAS.
- A. Greco, F. Ferrari, R. Velardi, M. Frigione and A. Maffezzoli, Solubility and Durability of Cardanol Derived Plasticizers for Soft PVC, Int. Polym. Process., 2016, 31(5), 577–586 CrossRef CAS.
- L. Yao, Q. Chen, W. Xu, Z. Ye, Z. Shen and M. Chen, Preparation of cardanol based epoxy plasticizer by click chemistry and its action on poly(vinyl chloride), J. Appl. Polym. Sci., 2017, 134(23), 44890–44897, DOI:10.1002/app.44890.
- S. Satapathy and A. Palanisamy, Mechanical and barrier properties of polyvinyl chloride plasticized with dioctyl phthalate, epoxidized soybean oil, and epoxidized cardanol, J. Vinyl Addit. Technol., 2021, 27(3), 599–611 CrossRef CAS.
- N. D. Ghatge and S. V. Vaidya, Plasticizer extenders for PVC. Part I- ester and ethers, Angew. Makromol. Chem., 1975, 43(1), 10, DOI:10.1002/apmc.1975.050430101.
- X. Li, X. Nie, J. Chen and Y. Wang, Preparation of epoxidized cardanol butyl ether as a novel renewable plasticizer and its application for poly(vinyl chloride), Polym. Int., 2017, 66(3), 443–449 CrossRef CAS.
- N. Xiaoan, L. Xiaoying, J. Chen, W. Yigang, J. Huang and L. Ke, A kind of Fructus anacardii phenolic group ether type plasticizer and synthetic method thereof and application, CN106188617A, 2019.
- J. Tan, T. Zhang, F. Wang, N. Huang, M. Yu and L. Wei, et al., One-pot and industrial manufacturing of cardanol-based polyoxyethylene ether carboxylates as efficient and improved migration resistance plasticizers for PVC, J. Clean. Prod., 2022, 375, 133943 CrossRef CAS.
- Y. Ma, F. Song, Y. Hu, Q. Kong, C. Liu and M. A. Rahman, et al., Highly branched and nontoxic plasticizers based on natural cashew shell oil by a facile and sustainable way, J. Clean. Prod., 2020, 252, 119597 CrossRef CAS.
- Y. Yang, C. Zhang, Y. Han, Z. Ma and Y. Weng, Design and Synthesis of Epoxidized Soybean Oil-Branched Cardanol Ethers as Poly(vinyl chloride) Plasticizers, Macromol. Mater. Eng., 2022, 307(11), 2200364 CrossRef CAS.
- P. Jia, M. Zhang, L. Hu, R. Wang, C. Sun and Y. Zhou, Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism, Polymers, 2017, 9(11), 621 CrossRef PubMed.
- P. Jia, L. Hu, Q. Shang, R. Wang, M. Zhang and Y. Zhou, Self-Plasticization of PVC Materials via Chemical Modification of Mannich Base of Cardanol Butyl Ether, ACS Sustainable Chem. Eng., 2017, 5(8), 6665–6673 CrossRef CAS.
- D. Hou, S. Wang, J. Chang, Z. Xu, Q. Zeng and Z. Wang, et al., Cardanol with a Covalently Attached Organophosphate Moiety as a Halogen-Free, Intrinsically Flame-Retardant PVC Bio-Plasticizer, Fibers Polym., 2020, 21(8), 1649–1656 CrossRef CAS.
- P. Jia, M. Zheng, Y. Ma, G. Feng, H. Xia and L. Hu, et al., Clean synthesis of epoxy plasticizer with quaternary ammonium phosphotungstate as catalyst from a byproduct of cashew nut processing, J. Clean. Prod., 2019, 206, 838–849 CrossRef CAS.
- P. McKeown and M. D. Jones, The Chemical Recycling of PLA: A Review, Sustainable Chem., 2020, 1(1), 1–22 CrossRef.
- K. L. G. Ho, A. L. Pometto, P. N. Hinz, A. Gadea-Rivas, J. A. Briceño and A. Rojas, Field Exposure Study of Polylactic Acid (PLA) Plastic Films in the Banana Fields of Costa Rica, J. Polym. Environ., 1999, 7(4), 167–172 CrossRef CAS.
- E. Rudnik and D. Briassoulis, Degradation behaviour of poly(lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing, Ind. Crops Prod., 2011, 33(3), 648–658 CrossRef CAS.
- C. Danyluk, R. Erickson, S. Burrows and R. Auras, Industrial Composting of Poly(Lactic Acid) Bottles, J. Test. Eval., 2010, 38(6), 717–723 CrossRef CAS.
- G. Kale, T. Kijchavengkul, R. Auras, M. Rubino, S. E. Selke and S. P. Singh, Compostability of Bioplastic Packaging Materials: An Overview, Macromol. Biosci., 2007, 7(3), 255–277 CrossRef CAS PubMed.
- A. Rigoussen, P. Verge, J. M. Raquez, Y. Habibi and P. Dubois, In-depth investigation on the effect and role of cardanol in the compatibilization of PLA/ABS immiscible blends by reactive extrusion, Eur. Polym. J., 2017, 93, 272–283 CrossRef CAS.
- A. Rigoussen, J. M. Raquez, P. Dubois and P. Verge, A dual approach to compatibilize PLA/ABS immiscible blends with epoxidized cardanol derivatives, Eur. Polym. J., 2019, 114, 118–126 CrossRef CAS.
- J. M. Farias da Silva and B. G. Soares, Epoxidized cardanol-based prepolymer as promising biobased compatibilizing agent for PLA/PBAT blends, Polym. Test., 2021, 93, 106889 CrossRef CAS.
- X. Song, C. Zhang, Y. Yang, F. Yang and Y. Weng, Cardanol derivatives as compatibilizers for strengthening and toughening polylactic acid/bamboo fiber bio-composites, Polym. Compos., 2023, 44(9), 5675–5688 CrossRef CAS.
- J. J. F. Cardoso, E. Ricci-Júnior, D. Gentili, L. S. Spinelli and E. F. Lucas, Influence of cardanol encapsulated on the properties of poly(lactic acid) microparticles, Quim. Nova, 2018, 41, 273–283 CAS.
- H. Kang, Y. Li, M. Gong, Y. Guo, Z. Guo and Q. Fang, et al., An environmentally sustainable plasticizer toughened polylactide, RSC Adv., 2018, 8(21), 11643–11651 RSC.
- G. Mele, E. Bloise, F. Cosentino, D. Lomonaco, F. Avelino and T. Marcianò, et al., Influence of Cardanol Oil on the Properties of Poly(lactic acid) Films Produced by Melt Extrusion, ACS Omega, 2019, 4(1), 718–726 CrossRef CAS.
- A. Greco and A. Maffezzoli, Cardanol derivatives as innovative bio-plasticizers for poly-(lactic acid), Polym. Degrad. Stab., 2016, 132, 213–219 CrossRef CAS.
- A. Greco and F. Ferrari, Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties, J. Therm. Anal. Calorim., 2021, 146(1), 131–141 CrossRef CAS.
- A. Greco, F. Ferrari and A. Maffezzoli, Mechanical properties of poly(lactid acid) plasticized by cardanol derivatives, Polym. Degrad. Stab., 2019, 159, 199–204 CrossRef CAS.
- A. Greco, F. Ferrari and A. Maffezzoli, Processing of Super Tough Plasticized PLA by Rotational Molding, Adv. Polym. Technol., 2019, 2019, e3835829 CrossRef.
- A. Greco, F. Ferrari and A. Maffezzoli, Thermal analysis of poly(lactic acid) plasticized by cardanol derivatives, J. Therm. Anal. Calorim., 2018, 134(1), 559–565 CrossRef CAS.
- A. Vallin, F. Ferretti, P. Campaner, O. Monticelli and A. Pellis, Environmentally Friendly Synthesis of Cardanol-Based Polyesters and Their Application as Poly(lactic acid) Additives, ACS Sustainable Chem. Eng., 2023, 11(26), 9654–9661 CrossRef CAS.
- F. Dinon, C. Tambe, P. Campaner, A. Natesh and T. Stonis, Oxidized cashew nut shell liquid derivatives and uses thereof, US20190127577A1, 2019, https://patents.google.com/patent/US20190127577A1/en?oq=US+2019%2f0127577+A1.
- Z. Dia and M. J. Chen, Cardanol derivative and method of making the cardanol derivative, WO2000034219A1, 2000, https://patents.google.com/patent/WO2000034219A1/fr?oq=WO+00%2f34219.
- F. Hassouma, I. Mihai, L. Fetzer, T. Fouquet, J. M. Raquez and A. Laachachi, et al., Design of New Cardanol Derivative: Synthesis and Application as Potential Biobased Plasticizer for Poly(lactide), Macromol. Mater. Eng., 2016, 301(10), 1267–1278 CrossRef CAS.
- H. Jia and N. Xiaoyan, High-flexibility and high-antibacterial-property biodegradable polylactic acid material and preparation method thereof, CN115216128A, 2022.
- T. Jihuai, H. Nengkun, X. Min, W. Wang, W. Qi, X. Wang, et al., Natural monophenol grafted dimer acid diglycidyl ester plasticizer, preparation method and application thereof, CN117024274A, 2023.
- H. Tang, L. Zhou, X. Bian, T. Wang, L. Feng and B. Zhang, et al., Highly toughened poly (lactic acid) blends plasticized by cardanol in presence of dicumyl peroxide, Mater. Lett., 2022, 313, 131777 CrossRef CAS.
- I. Mihai, F. Hassouna, T. Fouquet, A. Laachachi, J. M. Raquez and H. Ibn El Ahrach, et al., Reactive plasticization of poly(lactide) with epoxy functionalized cardanol, Polym. Eng. Sci., 2018, 58(S1), E64–E72 CrossRef CAS.
- S. Samantarai, A. Nag, N. Singh, D. Dash, A. Basak and G. B. Nando, et al., Chemical modification of nitrile rubber in the latex stage by functionalizing phosphorylated cardanol prepolymer: A bio-based plasticizer and a renewable resource, J. Elastomers Plast., 2019, 51(2), 99–129 CrossRef CAS.
- K. E. Perepelkin, Renewable Plant Resources and Processed Products in Chemical Fibre Production, Fibre Chem., 2004, 36(3), 161–176 CrossRef CAS.
- M. Iji, S. Moon and S. Tanaka, Hydrophobic, mechanical and thermal characteristics of thermoplastic cellulose diacetate bonded with cardanol from cashew nutshell, Polym. J., 2011, 43(8), 738–741 CrossRef CAS.
- M. Iji, K. Toyama and S. Tanaka, Mechanical and other characteristics of cellulose ester bonded with modified cardanol from cashew nut shells and additional aliphatic and aromatic components, Cellulose, 2013, 20(1), 559–569 CrossRef CAS.
- A. Bonifacio, L. Bonetti, E. Piantanida and L. De Nardo, Plasticizer design strategies enabling advanced applications of cellulose acetate, Eur. Polym. J., 2023, 197, 112360 CrossRef CAS.
- S. Tanaka, H. Honzawa and M. Iji, Development of cardanol-bonded cellulose thermoplastics: High productivity achieved by using isocyanate-modified cardanol, J. Appl. Polym. Sci., 2013, 130(3), 1578–1587 CrossRef CAS.
- M. Iji, S. Moon, S. Tanaka and H. Kai, Cellulose resin and method for producing the same, US8916699B2, 2014, https://patents.google.com/patent/US8916699B2/en.
- K. Toyama, M. Soyama, S. Tanaka and M. Iji, Development of Cardanol-Bonded Cellulose Resin with Nonfood Plant Resources: Low Energy Heterogeneous Synthesis Process, in Green Polymer Chemistry: Biobased Materials and Biocatalysis, ACS Symposium Series, American Chemical Society, 2015, vol. 1192, p. 329–337, DOI:10.1021/bk-2015-1192.ch020.
- S. Tanaka, T. Iwata and M. Iji, Solvent effects on heterogeneous synthesis of cardanol-bonded cellulose thermoplastics, Polymer, 2016, 99, 307–314 CrossRef CAS.
- Y. Kiuchi, M. Soyama, M. Iji, S. Tanaka and K. Toyama, Improvement in impact strength of modified cardanol-bonded cellulose thermoplastic resin by using olefin resins, J. Appl. Polym. Sci., 2014, 131(3), 39829, DOI:10.1002/app.39829.
- M. Soyama, Y. Kiuchi, M. Iji, S. Tanaka and K. Toyama, Improvement in impact strength of modified cardanol-bonded cellulose thermoplastic resin by adding modified silicones, J. Appl. Polym. Sci., 2014, 131(12), 40366, DOI:10.1002/app.40366.
- M. Soyama and M. Iji, Improving mechanical properties of cardanol-bonded cellulose diacetate composites by adding polyester resins and glass fiber, Polym. J., 2017, 49(6), 503–509 CrossRef CAS.
- M. Friuli, P. Nitti, L. Cafuero, A. Prete, M. S. Zafar and M. Madaghiele, et al., Cellulose Acetate and Cardanol Based Seed Coating for Intraspecific Weeding Coupled with Natural Herbicide Spraying, J. Polym. Environ., 2020, 28(11), 2893–2904 CrossRef CAS.
- S. Caillol, V. Lapinte, L. Jégo, B. Briou, A. Gartili. Nouveaux agents plastifiants, leur procédé de préparation et leur utilisation, WO2023187302A1, 2023, https://hal.science/hal-04500210.
- A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks - PMC[Internet]. [cité 28 avr 2024]. Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9571790/.
- A. R. R. Menon, C. K. S. Pillai and G. B. Nando, Self-adhesion of natural rubber modified with phosphorylated cashew nut shell liquid, J. Adhes. Sci. Technol., 1995, 9(4), 443–451 CrossRef CAS.
- A. R. R. Menon, C. K. S. Pillai and G. B. Nando, Thermal degradation characteristics of natural rubber vulcanizates modified with phosphorylated cashew nut shell liquid, Polym. Degrad. Stab., 1996, 52(3), 265–271 CrossRef CAS.
- A. R. R. Menon, Stress-relaxation characteristics of natural rubber modified with phosphorylated cashew nut shell liquid prepolymer, J. Appl. Polym. Sci., 1997, 65(11), 2183–2189 CrossRef CAS.
- A. R. R. Menon, Flame-Retardant Characteristics of Natural Rubber Modified with a Bromo Derivative of Phosphorylated Cashew Nut Shell Liquid, J. Fire Sci., 1997, 15(1), 3–13 CrossRef CAS.
- A. R. R. Menon, C. K. S. Pillai and G. B. Nando, Modification of natural rubber with phosphatic plasticizers: a comparison of phosphorylated cashew nut shell liquid prepolymer with 2-ethyl hexyl diphenyl phosphate, Eur. Polym. J., 1998, 34(7), 923–929 CrossRef CAS.
- W. Zhang, T. Zhang, N. Jiang and T. Zhang, Synthesis of a bio-based internal plasticizer from cardanol and its evaluations, Int. J. Polym. Anal. Charact., 2020, 25(2), 94–104 CrossRef CAS.
- T. R. Kukreja, R. C. Chauhan, S. Chloe and P. P. Kundu, Effect of doses and nature of vegetable oil on carbon black/rubber interactions: Studies on castor oil and other vegetable oil, J. Appl. Polym. Sci., 2003, 87, 1574–1578 CrossRef CAS.
- W. Arayapranee and G. L. Rempel, Effects of cashew nut shell liquid as a plasticizer on cure characteristics, processability, and mechanical properties of 50 : 50 NR/EPDM blends: A comparison with paraffin oil, J. Appl. Polym. Sci., 2007, 106(4), 2696–2702 CrossRef CAS.
- C. Flanigan, L. Beyer, D. Klekamp, D. Rohweder and D. Haakenson, Using Bio-Based Plasticizers, Alternative Rubber, Rubber & Plastic News, 2013, pp. 15–19 Search PubMed.
- M. Alexander, P. Kurian and E. T. Thachil, Effectiveness of Cardanol as Plasticizer for Silica-Filled Natural Rubber, Prog. Rubber Plast. Recycl. Technol., 2007, 23(1), 43–55 CrossRef CAS.
- M. Alexander and E. T. Thachil, A comparative study of cardanol and aromatic oil as plasticizers for carbon-black-filled natural rubber, J. Appl. Polym. Sci., 2006, 102(5), 4835–4841 CrossRef CAS.
- M. Alexander and E. T. Thachil, The Effectiveness of Cardanol as Plasticiser, Activator, and Antioxidant for Natural Rubber Processing, Prog. Rubber Plast. Recycl. Technol., 2010, 26(3), 107–124 CrossRef CAS.
- W. Qian, Y. Gao, P. Wang, X. Lu, Y. Zheng and Q. Chen, Poly-cardanol as plasticizer and compatibilizer on styrene butadiene rubber with improved processability and silica dispersion, J. Appl. Polym. Sci., 2022, 139(28), e52601 CrossRef CAS.
- P. Wang, J. Liu, C. Chi, Y. Zhang, D. Chen and Q. Chen, Solvent-free synthesis, plasticization and compatibilization of cardanol grafted onto liquid isoprene rubber, Compos. Sci. Technol., 2021, 251, 109027 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |