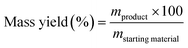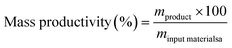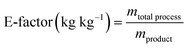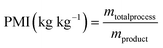Open Access Article
Open Access ArticleSustainable production of raw materials from waste cooking oils†
Alberto
Mannu
*,
Pablo
Almendras Flores
,
Francesco
Briatico Vangosa
 ,
Maria E.
Di Pietro
,
Maria E.
Di Pietro
 and
Andrea
Mele
and
Andrea
Mele
 *
*
Department of Chemistry, Materials and Chemical Engineering “G. Natta”, Politecnico di Milano, PiazzaL. da Vinci 32, 20133 Milano, Italy. E-mail: alberto.mannu@polimi.it
First published on 24th October 2024
Abstract
The current industrial process for recycling Waste Cooking Oils (WCOs) into vegetable lubricants relies on basic decantation and filtration methods, lacking the scientific foundation needed for technical optimization and sustainability. This research addresses these limitations by thoroughly evaluating the technical and environmental impacts of bentonite treatment and water washing techniques. Using a Design of Experiments (DoE) approach coupled with multivariate statistical analysis, key process parameters—temperature, bentonite content, pH, and oil-to-water ratio—were optimized to improve performance and sustainability. Results showed that bentonite had a negligible effect when water treatment was conducted at 75 °C and pH 6 or at 25 °C and pH 2. The efficiency of both recycling methods, as measured by nuclear magnetic resonance spectroscopy and rheological tests, was comparable. However, the green metrics (mass yield, mass productivity, E-factor, and process mass intensity), along with the EcoScale penalty ranking, indicated that water treatment at 75 °C and pH 6 offers the most viable solution. Linear regression of the data acquired through the multivariate analysis driven by the DoE approach provided a mathematical equation which relates the temperature, time, and the oil/water ratio to the equivalent of CO2 eventually produced. This tool provides recycling industries with a practical framework for optimizing process conditions, balancing technical efficiency with minimized environmental impact, a crucial factor for compliance with green certification programs. The results present a scalable, scientifically validated pathway for the WCO recycling industry to enhance both operational performance and sustainability.
Sustainability spotlightIt is well established that Waste Cooking Oils (WCOs) represent a valuable raw material for various industrial applications. Outside of the biodiesel industry, the sustainability associated with their use in fine chemicals production has never been thoroughly assessed. In this context, an operationally simple and low-impact recycling process leading to a raffinate ready for further transformation would facilitate the conversion of the high amount of WCOs produced worldwide. The process presented herein was optimized not only in terms of chemical outcomes, but also with consideration for its impact on sustainability. Green metrics and EcoScale applied to key indicators determined through statistical multivariate analysis allowed for the fine-tuning of the recycling conditions, providing a low-impacting protocol of interest for both industrial and academic purposes. |
1. Introduction
In the current socio-economic context of increasing scarcity of resources, developing new circular economy models for designing new processes, as well as for the re-definition of existing ones, is a crucial target.1–3 This approach has significant potential impact with political support from initiatives like the European Union (EU) Circular Economy Action Plan, 2020,4 or the United States (USA) guidelines “Advancing a Circular Economy to Meet Our Climate, Energy, and Economic Goals” of the White House.5 A key step to achieve circular economy models is using wastes as raw materials.6 Waste Cooking Oils (WCOs) are ideal for circular productions. WCOs are classified in the European Waste Catalogue under the code CER 20 01 25 as municipal wastes (Directive 2006/12/EC). The EU produces 1–2.5 million tons of WCOs annually, and globally, production is between 41 and 52 million tons.7 Looking at the demand of edible oil, its production was estimated in 230 M tons in 2019 (ref. 8) with an increasing rate of 1.5% yearly until 2027.9Chemically, WCOs are comprised of long-chain (C16–C18) triglycerides partially hydrolysed, resulting in varying free fatty acids (FFAs) content.10 EU regulations mandate WCO disposal before advanced decomposition, monitored by total polar compound levels not exceeding 25–27 wt%,11 to minimize the formation of potential toxic compounds like acrylamide.12,13 Early disposal, yields waste with high industrial value. Biodiesel industries convert triglycerides into automotive and aviation fuel via transesterification.14 Triglycerides are used in ecological paintings, asphalt binders, and chain lubricants.15–17 Hydrolysed triglyceride products, like glycerol and FFAs, serve as raw materials for numerous applications. Glycerol, a biodiesel byproduct, finds use in cosmetics,7 animal feed, and chemical processes.18 It's also a precursor for pivotal chemical intermediates like epichlorohydrin.19 FFAs find applications in soap, food, surfactants, and foams.20–22 Glycerol and FFAs also contribute to low-melting mixtures, offering eco-friendly solvents with favourable green metrics.23 Isolating pure glycerol and fatty acids from WCO enhances process sustainability, aligning with circular economy principles.
Among WCO's applications with minimal impacting processing, its use as a lubricant is growing, meeting EU Commission environmental standards.24 The bio-lubricant market, mainly in the EU and USA, is projected to grow by 5.6% in 2024.25 The high value of WCOs may lead to the use of edible vegetable oils for industrial purposes, raising concerns about competition between food and non-food applications.26 In the EU, edible oils can't be industrial ingredients,27 posing challenges when sourcing uncertified WCOs from third countries.28 Regarding this scenario, the attainment of a sustainable and circular approach, viable on an industrial scale, for the facile recycling of WCOs becomes significant.
The chemical processes used for reprocessing and recycling vegetable oils, as well as the physical methods for their purification and large-scale industrial implementation, are well-established. However, when these same processes are examined from the perspective of sustainability, significant and often overlooked gaps in knowledge and practical application remain. This work aims to address these gaps by presenting and analysing the purification, recycling, and industrial utilization of waste vegetable oils as lubricants. Our approach serves as a paradigm for the non-food application of end-of-life food products, offering a novel solution that aligns with sustainability goals while enhancing the value of waste materials.
Preliminary studies showed that water washing under controlled conditions, as well as treatment with clays, are both able to purify the WCOs from many minor organic contaminants, providing a regenerated mixture of triglycerides,29 which can be subjected to many chemical transformations depending on the specific production target.30
Within this framework, the present paper addresses the sustainable production of raw materials from WCOs at different levels. First, the industrial feasibility of the proposed recycling process was considered, exploring the best conditions a recycling company should consider customizing its production. To shed some light on the economical, chemical, and environmental sustainability of different possible scenarios, a Design of Experiments (DoE) approach was used to optimize the process of WCO recycling by water washing followed by bentonite processing. Several green metrics31 were applied as the atom economy, the environmental factor (E-factor), the environmental quotient (EQ), the effective mass yield, and the mass intensity.32,33 The Van Aken EcoScale34 was finally utilized as a tool for selecting the best production process.
2. Materials and methods
2.1. Chemicals
WCOs were provided by a catering company operating in Milan (Italy). About 100 kg of WCOs produced over six months were collected and used for the present study. Hexane, HCl (37% solution), NaOH, CDCl3, and D2O were purchased by Merk Chemicals.Hydrophilic bentonite was purchased by Merk Europe. Samples of milled bentonites were prepared by ball milling procedure for 20 min in a SPEX 8000 (SPEX CertiPrep, USA) shaker mill operating at 875 rpm.
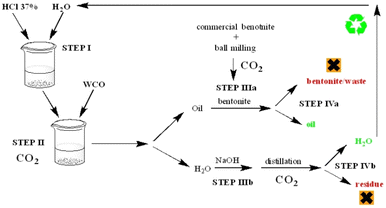
2.2. Recycling protocol
To remove macro contaminants as food residues, WCO was filtered through metallic sieves. Then, 30 g of WCO were added to 12 (40 wt%) or 3 (10 wt%) mL of water (at pH 2 or 6) and the due amount of bentonite (0 g or 0.6 g). Acidic water solutions were prepared by adding 1 mL of HCl 37% to 99 mL of distilled water. The mixture was then stirred at the selected temperature (r.t. or 70 °C). After 2 h, the resulting heterogeneous mixture was cooled down to r.t. and the phases separated with a separatory funnel. The organic phase was dried on Na2SO4, filtered under vacuum, and collected for analysis. The specific conditions of each experiment are reported in the ESI, Table S2.†2.3. Hydrolysis of the recycled oil
To 100 g of recycled oil (0.33 mol), 3.5 equivalents of NaOH (1.16 mol) dissolved in 20 mL of H2O were added. The mixture was stirred at 80 °C during 3 h until a solid was formed. The residual water was removed, and the soap washed 2 times with additional 20 mL (2 × 10 mL) of distilled water. HCl (1.2 mol, 120 mL of HCl 37%) was then added to the solid and the mixture stirred for 2 h at 50 °C. Once all the solid part turned into a yellow oil, the mixture was cooled down to room temperature and the organic phase separated from water in a separatory funnel.2.4. Water recovery
The aqueous phases recovered from the previous described steps were placed into a round bottomed flask and the water distilled under reduced pressure. The residue was then collected for analysis.2.5. NMR spectroscopy
NMR samples were prepared dissolving ca. 25 mg of WCO in 600 μL of CDCl3 and then transferred to 5 mm NMR tubes. NMR measurements were performed with a Bruker Avance III 400 console (9.4 T) equipped with a direct observe PABBO probehead and a variable-temperature unit (1H resonance frequency of 400.13 MHz). 1D 1H spectra were recorded at 302 K with 16 scans using 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 points and a relaxation delay of 1 s, over a spectral width of 20 ppm. Spectra were processed by applying an exponential line broadening (LB = 0.3 Hz), and automatically phased and base-line corrected.
536 points and a relaxation delay of 1 s, over a spectral width of 20 ppm. Spectra were processed by applying an exponential line broadening (LB = 0.3 Hz), and automatically phased and base-line corrected.
2.6. UV-VIS
UV-VIS spectra were acquired with a ONDA SCAN SPECTROPHOTOMETER UV31, in absorbance mode from 1100 to 195 nm, 1.0 step. Few drops of the sample oil dissolved in 1 mL of hexane were placed in a quartz cuvette with 1 cm of path length for analysis.2.7. Rheology
Rheological tests in steady state rotation were performed with an Anton Paar MCR502 modular compact rheometer, equipped with a Peltier Plate and Hood thermal control systems. Test were carried out at −25 °C, 25 °C, 40 °C and 100 °C, in shear rate control. Shear rate ranged from 1 to 100 s−1. At the temperature of −25 °C tests were also carried out in oscillation, at a constant shear strain amplitude of 0.1%, in the linear regime, and at frequencies ranging from 100 rad s−1 to 10 rad s−1. For all experiments a cone and plate (diameter 50 mm, angle 1°, truncation 99 mm) measuring system was adopted.3. Results and discussion
3.1. Waste vegetable oil (WCO) characterization
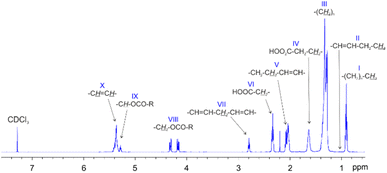
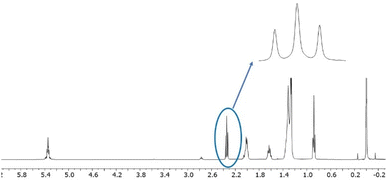
To set a starting point in determining the effectiveness of the recycling process, the chemical profile the WCO was characterized by 1H NMR and UV-VIS spectroscopy (Fig. 1 and 2, Table 1).The 1H NMR spectrum of the starting WCO in CDCl3 is reported in Fig. 1. It is well known that NMR spectroscopy can be used to characterise vegetable oils and several specific techniques, based on 13C, 31P, 1H and other nuclei, were developed so far (Di Pietro 2020). According to Popescu and coworkers,35 the relative distribution between fatty acids (FAs, i.e. linoleic, linolenic, oleic acid) and saturated fatty acids (SFA, as palmitic and/or stearic acid) can be calculated from the 1H NMR spectrum. In Table 1, the chemical composition of the herein considered WCO is reported and compared with other published samples of similar origin and determined with the same methodology.
According to the original methodology of Popescu, the integral corresponding to the signal at 1.02 ppm (II) – assigned to –CH3 of linolenyl groups –is used to calculate the relative percentages of linoleic and linolenic acids. As it is possible to see in Fig. 1, the intensity of signal II is sensibly lower than the signals I and III, representing the amount of –CH3 groups of non-linolenyl type and the (CH2)n of the acyl chains, respectively. Thus, the integral of II is not reliable as it partially overlaps to I and III. For this reason, the linoleic and linolenic acids relative percentages are expressed as a sum.
Looking at Table 1, a consistent degree of variability between different batches of WCOs is observed. All the examples reported in the literature are found to be enriched in oleic acid and contain minor and variable amounts of linoleic and linolenic acids. In the WCO herein considered, the total percentage of linoleic and linolenic acid (respectively containing two and three C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds) reaches 30%, providing a raw material sensitive to oxidation.39 The iodine number (IV), an empirical parameter useful to define the total amount of unsaturation, shows a value of 97 which lays in the middle between classical edible sunflower vegetable oils (around 100–110), and WCOs (80–90).
C bonds) reaches 30%, providing a raw material sensitive to oxidation.39 The iodine number (IV), an empirical parameter useful to define the total amount of unsaturation, shows a value of 97 which lays in the middle between classical edible sunflower vegetable oils (around 100–110), and WCOs (80–90).
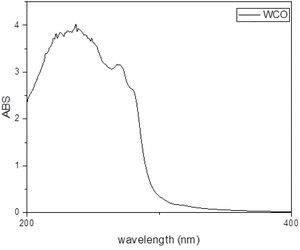
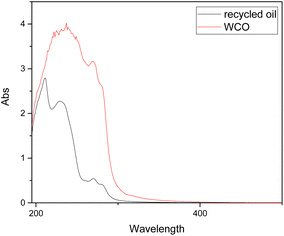
Looking at the UV-VIS spectrum reported in Fig. 2, the typical absorption bands of sunflower oil is observed, according with its wide use in southern Europe. From 400 to 1200 nm no relevant bands are detected, discarding the possibility of the presence of EVO oil (extra virgin olive oil) which exhibits typical polyphenolic signals in that region. Indeed, a small band at 310–320 nm, usually associated with flavonoids or with electron transfer processes in triglycerides (ester moiety) is observed. Also, the bands at 240 nm are symptomatic of linoleic acid and referred to the HOMO–LUMO transition of the conjugated dienes.40 The UV-VIS profile discards the presence in relevant amount of any organic molecule visible with UV-VIS spectroscopy, confirming the assumption that WCOs usually show low degrees of contamination.
3.2. Process designing
After characterizing the raw material, a recycling process involving water washing and treatment with natural clays was undertaken. Previous studies have shown that these two steps are effective in removing metal particles, small organic molecules, and pigments from waste vegetable oils, thereby enhancing numerous physical properties of the recycled material.41–43In fact, both recycling processes (water washing and bentonite processing) were reported as effective alternatives and applied to batches of WCOs. However, what is not clear from the published data is which process performs better, especially considering the possibility of a tandem treatment. While these processes were assessed under different conditions, the overall process has never been fully considered or optimized. It is known that bentonite removes small organic molecules, pigments, and even trace metals from vegetable oils, while water washing under controlled conditions partially removes minor organic chemicals, although this aspect has not been explored in detail. To approach the issue, a full factorial 2k Design of Experiments (DoE) was used to assess the effect of pH, temperature, oil–water ratio (washing step), and clays' amount, on the chemical composition, in terms of oleic acid% and saturated fatty acids%, of the recycled oil (ESI Table S3†).
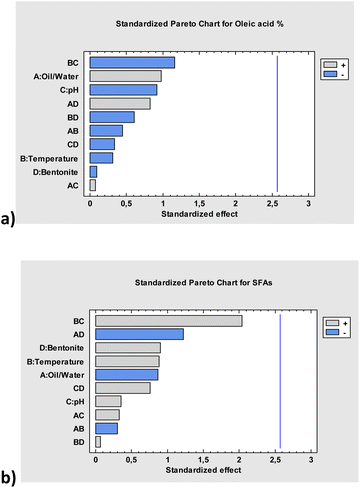
According to the DoE, a total number of 16 experiments were conducted. The ANalysis Of the Variance (ANOVA) revealed small changes in the chemical composition of the treated oil depending on the condition used as expressed by the Pareto charts in Fig. 3.
Pareto charts show that none of the considered parameters reach the blue line which indicates a P-value < 0.05. This threshold is highly relevant as discriminates within parameters which stand out as statistically relevant with an interval of confidence >95%. This means that concerning the chemical composition of the oil, expressed in terms of oleic and saturated fatty acids amount, no relevant variations related to the parameters considered are observed. Thus, it is possible to rule-out decomposition pathways during the recycling, independently of the temperature, pH, presence of bentonite. On this basis, a first approach to the recycling optimization was conducted through Surface Response Analysis (SRA). The two statistical models referred to the oleic acid% and to the SFAs% were combined to generate a desirability function (Mannu 2020b). In our case it is desirable increasing one important parameter in lubricants: the oxidative stability. This means having the higher content of oleic acid with respect to linoleic and linolenic acids (one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond versus two and three C
C bond versus two and three C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively). SRA plot shows how the best compromise can be achieved by varying the parameters (Fig. 4).
C bonds, respectively). SRA plot shows how the best compromise can be achieved by varying the parameters (Fig. 4).
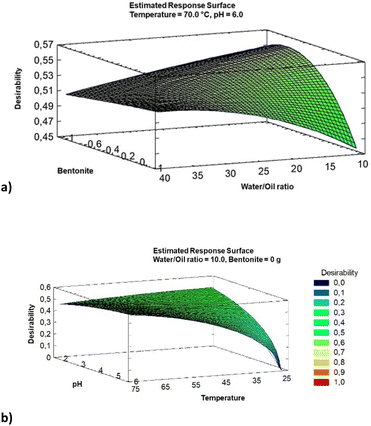
According to Fig. 4a, the best desirability is achieved without bentonite and an oil/water ratio of 10%. Fixing these two parameters (Fig. 4b), a relevant effect of the pH combined with temperature is observed. Thus, it is possible to process the WCO at 25 °C if the pH is lower than 4. Alternatively, WCO can be recycled at 75 °C and pH = 6, providing the same outcome. These two alternative options deserve attention as far as the sustainability of the process is concerned. Naively, higher temperatures are detrimental in terms of energy demand. However, acidic pH reduces the possibility to form emulsions during the washing, allowing us to reduce the energy consumption but introduces environmental issues related to the acid concentration.
The information extracted from the statistical analysis represented in the SRA plots is quite impressive. Purification of WCOs can be achieved solely through water washing (at 75 °C, pH = 6, or at room temperature, pH = 2), avoiding the need for bentonite treatment. Additionally, the amount of water can be kept low, with a water/oil ratio of 10. Under these conditions, the amount of chemicals sensitive to oxidation is reduced, resulting in a highly pure mixture of triglycerides being isolated.
Once resolved the technical issues related to the choice of the best recycling procedures, the sustainability aspect was assessed. At first, the CO2 equivalents (eCO2) related to the main steps involved (Fig. 5) were calculated (Table 2).
| Step | Electric demand (kW) | CO2 (kg) | Comments |
|---|---|---|---|
| Water washing and bentonite processing | 1.26 | 0.470 | Water washing at 25 °C |
| 1.42 | 0.520 | Water washing at 25 °C + bentonite processing | |
| 2.52 | 0.940 | Water washing at 70 °C | |
| 2.68 | 1.000 | Water washing at 70° + bentonite processing | |
| Water recycling | 0.23 | 0.084 | Oil/water = 10% |
| 0.90 | 0.337 | Oil/water = 40% | |
| Pre-treatment of bentonite | 0.03 | 0.010 | For 0.6 g of milled bentonite |
The three most impacting steps on CO2 equivalents production were highlighted. For each of them, the electricity needed was estimated and then transformed in CO2. As conversion factor, an amount of 0.373 kg of CO2 for each kW consumed was considered (Table 2).44
Thus, the data relative to eCO2 were associated to the 16 experiments reported in Table S2† (Table 3).
| Exp. | pH | T (°C) | Water/oil (%) | Bent. (g) | Process (kW) | Milling (kW) | Distillation (kW h) | Total eCO2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 25 | 10 | 0 | 0.47 | 0.00 | 0.084 | 0.554 |
| 2 | 6 | 25 | 40 | 0 | 0.47 | 0.00 | 0.337 | 0.807 |
| 3 | 2 | 25 | 40 | 0 | 0.47 | 0.00 | 0.337 | 0.807 |
| 4 | 6 | 70 | 10 | 0 | 0.52 | 0.00 | 0.084 | 0.607 |
| 5 | 2 | 25 | 40 | 0.6 | 0.94 | 0.01 | 0.337 | 1.287 |
| 6 | 2 | 70 | 10 | 0.6 | 1.00 | 0.01 | 0.084 | 1.094 |
| 7 | 6 | 25 | 40 | 0.6 | 0.94 | 0.01 | 0.337 | 1.287 |
| 8 | 2 | 70 | 40 | 0 | 0.52 | 0.00 | 0.337 | 0.860 |
| 9 | 2 | 70 | 10 | 0 | 0.52 | 0.00 | 0.084 | 0.607 |
| 10 | 6 | 70 | 10 | 0.6 | 1.00 | 0.01 | 0.084 | 1.094 |
| 11 | 6 | 70 | 40 | 0 | 0.52 | 0.00 | 0.337 | 0.860 |
| 12 | 6 | 25 | 10 | 0.6 | 0.94 | 0.01 | 0.084 | 1.034 |
| 13 | 6 | 25 | 10 | 0 | 0.47 | 0.00 | 0.084 | 0.554 |
| 14 | 2 | 70 | 40 | 0.6 | 1.00 | 0.01 | 0.337 | 1.347 |
| 15 | 6 | 70 | 40 | 0.6 | 1.00 | 0.01 | 0.337 | 1.347 |
| 16 | 2 | 25 | 10 | 0.6 | 0.94 | 0.01 | 0.084 | 1.034 |
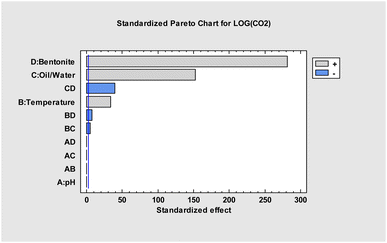
By taking as response the log[eCO2], the model resulted very accurate in explaining the variability in eCO2 generation, with a R2 = 99.995%. The Pareto chart reports the six factors with a p-value < 0.05 (Fig. 6).
It's clear that the use of bentonite and the amount of water have a significant impact on eCO2 equivalents. The interaction between these parameters is particularly noteworthy (see bar CD, Fig. 6). Temperature has a lesser effect, as do the combinations of temperature with bentonite or water amount. The multiple regression model yielded eqn (1), which describes the relationship between estimated eCO2 equivalents and the four parameters under consideration.
| eCO2 = 0.436528 − 4.1431 × 10−18 × pH + 1.25556 × 10−3 × temperature + 8.43333 × 10−3 × oil/water + 0.805833 × bentonite ≈ 0.436528 + 1.25556 × 10−3 × temperature + 8.43333 × 10−3 × oil/water + 0.805833 × bentonite. | (1) |
According to (1), eCO2 equivalents at various temperatures using different amounts of bentonite and water can be estimated. The pH's effect on eCO2 is negligible.
Based on the model's results regarding the variation in chemical composition, minimal electricity consumption was achieved under the same conditions. Employing a low amount of water (10 wt%) for washing can effectively recycle WCOs, potentially eliminating the need for bentonite treatment. The optimal operational pH (either 2 or 6) depends on the chosen temperature, resulting in two possible scenarios that were evaluated in terms of green metrics.
3.4. Sustainability and performance assessment
To design a highly sustainable process of industrial interest, the main recycling steps were evaluated in terms of sustainability. The statistical model for estimating eCO2, as reported above, was complemented by the EcoScale assessment (Section 3.4.1) and several green metrics (Section 3.4.2).The ideal EcoScale score is 100 and it is calculated using the following equation.
| EcoScale = 100 − Σindividual penalty points | (2) |
To estimate the penalty points Koen Van Aken (Van Aken 2006) defined six sets of parameters characteristic of each process. The analytic calculations are reported in the ESI (Table S1†). The results relative to the scenario 1 (pH 2 at room temperature) and scenario 2 (pH 6 at 70 °C) are reported in Table 4.
| Scenario 1 | Penalty | Scenario 2 | Penalty | |
|---|---|---|---|---|
| 1 | Yield: 78.2% | 10.88 | Yield: 78.7% | 10.65 |
| 2 | WCO (10.7 g, 12.2 mmol) | 0 | WCO (10.7 g, 12.1 mmol) | 0 |
| Distilled H2O (1.1 g, 61.1 mmol) | 0 | Distilled H2O (1.1 g, 61.1 mmol) | 0 | |
| HCl (37%), (0.4 mg, 0.01 mmol) | 0 | Na2SO4 (99%, 0.8 g, 5.4 mmol) | 0 | |
| Na2SO4 (99%, 2.4 g, 16.7 mmol) | 0 | |||
| 3 | HCl (T, N) | 5 | None | 0 |
| 4 | Common glassware, stirring | 0 | Common glassware, stirring | 0 |
| 5 | Room temperature, 2 h | 1 | 70 °C, 2 h | 3 |
| 6 | Density-based phase separation | 0 | Density-based phase separation | 0 |
| Filtration of Na2SO4 | 0 | Filtration of Na2SO4 | 0 | |
| EcoScale score | 83.12 | 86.35 |
This method was applied to the two optimal scenarios previously defined. The mass yield was similar in both scenarios, but scenario 2 showed a higher score, as HCl is not used. Given the complications of handling toxic and acidic residues, scenario 2 (pH = 6, T = 70 °C) was selected as the optimal WCO treatment process, despite the extra heating required (see Table 5).
| Metric | Unit | Scenario 1 | Scenario 2 | Ideal value |
|---|---|---|---|---|
| Mass yield | % | 78.23 | 78.70 | 100 |
| Mass productivity | % | 59.22 | 67.19 | 100 |
| E-factor | kg kg−1 | 0.689 | 0.488 | 0 |
| PMI | kg kg−1 | 1.689 | 1.488 | 1 |
| EcoScale | — | 83.12 | 86.35 | 100 |
Mass productivity, a key metric in our analysis, relates the mass of the final product to the mass of all reactants. In our case, it substitutes the concept of Atom Economy as there's no chemical reaction involved. Mass productivity is defined as the mass of refined oil divided by the total mass of raw materials used, including oil, water, and other compounds.
The E factor estimates the total amount of waste generated considering all the process components relative to the final product mass obtained. Higher E-factor means more waste generated.
The Process Mass Intensity (PMI) is defined as the ratio of the total input of materials over the product.
Consistent with the EcoScale results, when calculating these metrics for the two previously defined scenarios, the second one exhibits better values than the first in each studied metric (refer to Table 5). Scenario 2 demonstrates higher values of mass yield and mass productivity, indicating greater mass efficiency towards the product. Additionally, the E-factor and PMI are lower, suggesting reduced waste production during treatment. In this context, considering overall feasibility and sustainability, Scenario 2 is evidently more suitable for industrial application (see Table 5).
3.5. Chemical and physical characterization of the raffinate
Following the optimized process (Scenario 2), a first batch of WCO was recycled. Analysis via1H NMR and UV-VIS confirmed observations made during the optimization study: the main chemical composition of the oil only slightly changes post-recycling. While both 1H NMR spectra exhibit qualitatively the same profile for the WCO before and after treatment (see Fig. 7), quantitative analysis of chemical composition reveals an increase in SFA content post-treatment (from 14.9% to 27.5%) at the expense of the unsaturated portion (oleic + linoleic + linolenic percentage decreases from 85.1% to 72.5%). This change is also reflected in the iodine number, which decreases from 97.0 to 82.4, indicating higher oxidative stability.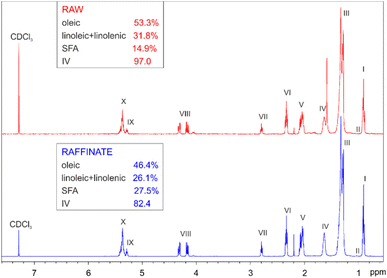 | ||
| Fig. 7 Comparison between the 1H NMR spectra of the raw WCO and the raffinate, including the fatty acid profile. | ||
Comparison of UV-VIS spectra reveal a more resolved portion between 300 and 250 nm. In WCO samples, this region is associated with polyphenols and minor organic compounds, which were removed during the recycling procedure. The band characteristic of the conjugated dienes of linolenic and linoleic acids remains visible and is better resolved at 240 nm (Fig. 8).
3.6 Rheology
Steady state rotational rheometry shows that both WCO and the recycled oil display a Newtonian behaviour (see ESI†) from room to higher temperatures. The viscosities of WCO and recycled oils are very similar and a decrease with temperature according to an Arrhenius dependence, with an energy for the activation of the viscous flow equal to Eatt ≈ 167 J (mol−1 K−1) is observed (Fig. 9).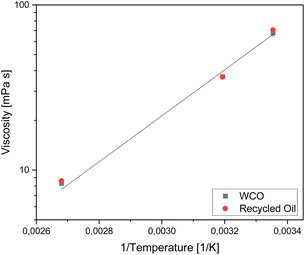 | ||
| Fig. 9 Temperature effect on the shear viscosity of WCO and recycled oil. The straight line represents an Arrhenius fitting (R2 = 0.993). | ||
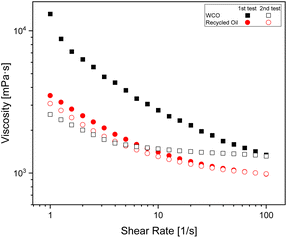
Even if no difference can be observed at room and above room temperatures, at sub-zero temperatures some difference can be highlighted between the untreated and treated sample. Indeed, the viscosity curve in Fig. 10 shows a markedly non-linear, shear thinning behaviour; further WCO and recycled oil show significantly different behaviour when subjected to a shear flow in a flow test performed on an “undisturbed sample”, whereas the differences are very limited –or even neglectable–when the sample is tested a second time.
To investigate this feature and its correlation with the sample structure, oscillatory tests were carried out. The G′ and G′′ curves show a significant difference between WCO and recycled oil before they are subjected to the shear flow (Fig. 11a). Indeed, WCO shows a solid like behavior (G′ > G′′), suggesting the presence of a percolated network for this sample. The shear flow seems to destroy this percolated structure, and the dynamic mechanical analysis performed after flow (Fig. 11b) shows a liquid like behavior (G′ < G′′) comparable for both samples. We speculate that at −15 °C a crystallization phenomenon starts occurring in both WCO and the recycled oil, but in the case of WCO the presence of contaminants either accelerate the crystallization or contributes directly to the formation of the percolated structure. When a shear flow is applied, this weak network breaks, and does not form again before the second dynamic mechanical test, which makes both material liquid-like and comparable.
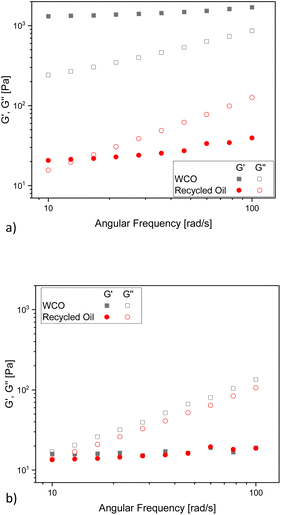 | ||
| Fig. 11 G′ and G′′ dependence on angular frequency for WCO and recycled oil (a) before the application of the shear flow in a flow test and (b) after the application of the shear flow in a flow test. | ||
From rheology measurements, as well as from UV-VIS data, it is evident that the differences within WCO and recycled oil are small and mostly related with the behaviour at low temperature. This agrees with the fact that the recycling procedure, as proposed, just removes minor chemicals and impurities derived by the life cycle of the oil. Further confirmation to this statement came from the 1H NMR analysis of the residue obtained by evaporating the washing waters (ESI, Fig. S2†).
3.7. Hydrolysis of the recycled oil
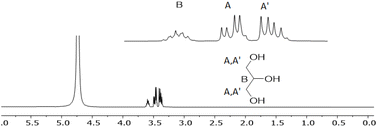
Recycled oil was subjected to alkaline hydrolysis followed by acidification according to Section 2.3 to isolate the corresponding FFAs' mixture. The rate of hydrolysis was monitored by 1H NMR spectroscopy. In fact, the triplets at 2.3 ppm, related to the CH2 in a position to the carbonyl moiety, is diagnostic as its chemical shift is very sensible to the chemical environment (ester of acidic function nearby, Fig. 12).45On the other side, glycerol was recovered in pure form from the water fraction (Fig. 13).
Conclusions
The use of vegetable oils as lubricants is well-known, with recognized technical advantages such as improved lubricating power, higher flash point, and better adhesion compared to synthetic lubricants. While much research has focused on chemically modified lubricants (e.g., esterified or epoxidized oils), our approach was distinct in utilizing raw triglycerides with minimal transformation, offering a more sustainable alternative to chemically modified waste vegetable oils. Although some industries have begun recycling used vegetable oils through basic purification methods, no optimized or scientifically validated processes were established, leaving a critical gap.Our work presents a novel contribution by developing a simple yet rigorously optimized process for obtaining clean triglyceride mixtures from used vegetable oils. By employing multivariate statistical analysis, we identified two potential recycling pathways based on chemical composition, specifically the oleic acid percentage and saturated fatty acids (SFAs) content: one at pH 6 and 75 °C, and another at pH 2 and 25 °C—both without the need for bentonite processing. A statistical model capable of estimating CO2 equivalents (eCO2) generated by these different scenarios provides an innovative decision-making tool to evaluate the sustainability of recycling options.
The recycling process and the analysis of optimal sustainable conditions were further reinforced by spectroscopic characterization of starting materials, intermediates, products, and impurities (using 1H NMR and UV-VIS spectroscopy), as well as rheological analysis (steady-state rotational rheometry). Additionally, for alternative applications requiring pure components, we isolated and characterized pure glycerol, and a mixture of free fatty acids obtained through chemical hydrolysis of waste cooking oil (WCO) processed via the optimized method.
Through extensive experimentation, we optimized key parameters such as pH, temperature, and water usage, eliminating the need for unnecessary filtration, which is traditionally used in industrial processes. Our method is scalable and adaptable, supported by a mathematical model that allows industries to fine-tune process parameters like temperature, pH, and water-to-oil ratio according to specific operational needs. This approach results in the removal of one unit operation, reducing energy consumption and improving the overall energy balance of the process.
This work aligns with several United Nations Sustainable Development Goals (SDGs), including SDG 9 (industry innovation), SDG 10 (reduced inequalities), and SDG 12 (responsible consumption and production). By converting waste into a valuable industrial resource, especially in regions with abundant waste oil supply, our method promotes circular economy development, with relevance for developing countries. We also provide green metrics and a sustainability ranking to further validate the environmental, economic, and safety benefits of this approach.
In conclusion, the analytical and theoretical tools developed in this study offer valuable insights into the sustainability and technical feasibility of potential recycling pathways for WCO recovery. The significance of this research, particularly the DoE (Design of Experiments) approach to sustainability assessment, extends beyond the specific case of WCO recycling and serves as a broader framework for environmental research in general.
Data availability
Data are available upon request from the authors.Author contributions
AM supervision, experimental work, writing and editing, PAF experimental work, writing, MP experimental work, writing and editing, FB experimental work and writing, AnM funding acquisition, supervision, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was funded by the WORLD Project-RISE, a project that has received funding from the European Union's Horizon 2020 research and innovation programme, under the Marie Skłodowska- Curie, Grant Agreement No. 873005.Notes and references
- P. Schroeder, K. Anggraeni and U. Weber, The Relevance of Circular Economy Practices to the Sustainable Development Goals, J. Ind. Ecol., 2018, 77–95 Search PubMed.
- B. Corona, L. Shen, D. Reike, J. Rosales Carreón and E. Worrell, Towards sustainable development through the circular economy - A review and critical assessment on current circularity metrics, Resour., Conserv. Recycl., 2019, 151, 104498 CrossRef.
- J. M. Rodriguez-Anton, L. Rubio-Andrada, M. S. Celemín-Pedroche and M. D. M. Alonso-Almeida, Analysis of the relations between circular economy and sustainable development goals, Int. J. Sustain. Dev. World Ecol., 2019, 26(8), 708–720 CrossRef.
- Circular Economy Action Plan 2020. Available at Available at https://ec.europa.eu/environment/circular-economy/ Search PubMed.
- Guidelines of the White House (Advancing a Circular Economy to Meet Our Climate, Energy, and Economic Goals, white house report, July 5, 2023). Available at https://www.whitehouse.gov/ostp/news-updates/2023/07/05/advancing-a-circular-economy-to-meet-our-climate-energy-and-economic-goals/ Search PubMed.
- J. Cardenas, A. Orjuela, D. L. Sanchez, P. C. Narvaez, B. Katryniok and J. Clark, Pretreatment of used cooking oils for the production of green chemicals: A review, J. Cleaner Prod., 2021, 289, 125129 CrossRef CAS.
- A. Orjuela and J. Clark, Green chemicals from used cooking oils: Trends, challenges, and opportunities, Curr. Opin. Green Sustainable Chem., 2020, 26, 100369 CrossRef PubMed.
- D. Zhou, S. Xiao, X. Xiao and Y. Liu, Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage, Separations, 2023, 10, 49 CrossRef CAS.
- E. Meijaard, T. B. Brooks, K. M. Carlson, E. M. Slade, J. Garcia-Ulloa, D. L. A. Gaveau, J. S. H. Lee, T. Santika, D. Juffe-Bignoli, M. J. Struebig, S. A. Wich, M. Ancrenaz, L. P. Koh, N. Zamira, J. F. Abrams, H. H. T. Prins, C. N. Sendashonga, D. Murdiyarso, P. R. Furumo, N. Macfarlane, R. Hoffmann, M. Persio, A. Descals, Z. Szantoi and D. Sheil, The environmental impacts of palm oil in context, Nat. Plants, 2020, 6, 1418–1426 CrossRef PubMed.
- S. Pyanka, U. Muhammad, S. El-Sayed, R. Margarita, T. Nandini and L. Xiangkai, Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock, Waste Manage., 2021, 136, 219–229 CrossRef PubMed.
- M. C. Dobarganes and G. Marquez-Ruíz, Regulation of used frying fats and validity of quick tests for discarding the fats, Grasas Aceites, 1998, 49, 331–335 CrossRef CAS.
- S. N. S. Ahmad, A. H. A. Tarmizi, R. A. A. Razak, S. Jinap, S. Norliza, R. Sulaiman and M. Sanny, Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets, Foods, 2021, 10, 257 CrossRef CAS PubMed.
- Commission Regulation (EU) 2017/2158 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide, available at https://eur-lex.europa.eu/eli/reg/2017/2158/oj. Accessed on January 15, 2024 Search PubMed.
- L. Nascimento, A. Ribeiro, A. Ferreira, N. Valério, V. Pinheiro, J. Araújo, C. Vilarinho and J. Carvalho, Turning Waste Cooking Oils into Biofuels-Valorization Technologies: A Review, Energies, 2022, 15, 116 CrossRef.
- M. Bartman, S. Balicki and K. A. Wilk, Formulation of Environmentally Safe Graffiti Remover Containing Esterified Plant Oils and Sugar Surfactant, Molecules, 2021, 26, 4706 CrossRef CAS PubMed.
- N. Xu, H. Wang, H. Wang, M. Kazemi and E. Fini, Research progress on resource utilization of waste cooking oil in asphalt materials: A state-of-the-art review, J. Cleaner Prod., 2023, 385, 135427 CrossRef CAS.
- U. Azzena, A. Montenero, M. Carraro, R. Crisafulli, L. De Luca, S. Gaspa, A. Muzzu, L. Nuvoli, R. Polese, L. Pisano, E. Pintus, S. Pintus, A. Girella and C. Milanese, Recovery, Purification, Analysis and Chemical Modification of a Waste Cooking Oil, Waste Biomass Valorization, 2023, 14, 145–157 CrossRef CAS.
- M. T. Mauerwerk, I. V. Zadinelo and F. Meurer, Use of glycerol in fish nutrition: a review, Aquaculture, 2021, 3(2), 853–861 CrossRef.
- G. M. Lari, G. Pastore, C. Mondelli and J. Pérez-Ramírez, Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides, Green Chem., 2018, 20, 148 RSC.
- O. W. Achaw and E. Danso-Boateng. Soaps and Detergents, in Chemical and Process Industries. Springer, Cham, 2021 doi: DOI:10.1007/978-3-030-79139-1_1.
- I. Djuricic and P. C. Calder, Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021, Nutrients, 2021, 13, 2421 CrossRef CAS PubMed.
- A. L. Fameau and A. G. Marangoni, Back to the future: Fatty acids, the green genie to design smart soft materials, J. Am. Oil Chem. Soc., 2022, 99(7), 543–558 CrossRef CAS.
- F. Parveen, A. Madni, V. P. Torchilin, M. Rehman, T. Jamshaid, N. Filipczak, N. Rai, M. M. Khan and M. I. Khan, Investigation of Eutectic Mixtures of Fatty Acids as a Novel Construct for Temperature-Responsive Drug Delivery, Int. J. Nanomed., 2022, 26(17), 2413–2434 CrossRef PubMed.
- S. Lawate. Environmentally friendly hydraulic fluids, in Rudnick L. R., ed. Synthetics, Mineral Oils, and Bio-Based Lubricants. Chemistry and Technology. CRC Press, Philadelphia, 2006, pp. 567–578 Search PubMed.
- S. D. Fernandez-Silva, M. A. Delgado, M. V. Ruiz-Mendez, I. Giraldez and M. García-Morales, Potential valorization of waste cooking oils into sustainable bio-lubricants, Ind. Crops Prod., 2022, 185, 115109 CrossRef CAS.
- W. Li and X. Wang, Bio-lubricants derived from waste cooking oil with improved oxidation stability and low-temperature properties, J. Oleo Sci., 2015, 64, 367–374 CrossRef CAS PubMed.
- J. Ibanez, S. Martel Martín, S. Baldino, C. Prandi and A. Mannu, European Union legislation overview about used vegetable oils recycling: the Spanish and Italian case studies, Processes, 2020, 8(7), 798 CrossRef CAS.
- A. Mannu, M. Poddighe, S. Garroni and L. Malfatti, Application of IR and UV–VIS spectroscopies and multivariate analysis for the classification of waste vegetable oils, Resour., Conserv. Recycl., 2022, 178, 106088 CrossRef.
- A. Mannu, S. Garroni, J. Ibanez Porras and A. Mele, Available technologies and materials for waste cooking oil recycling, Processes, 2020, 8(3), 366 CrossRef CAS.
- A. Mannu, M. Ferro, M. E. Di Pietro and A. Mele, Innovative applications of waste cooking oil as raw material, Sci. Prog., 2019, 102(2), 153–160 CrossRef PubMed.
- R. A. Sheldon, Metrics of Green Chemistry and Sustainability: Past, Present, and Future, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- M. Sajid and J. Płotka-Wasylka, Green analytical chemistry metrics: A review, Talanta, 2022, 238(2), 123046 CrossRef CAS PubMed.
- M. Shi, X. Zheng, N. Zhang, Y. Guo, M. Liu and L. Yin, Overview of sixteen green analytical chemistry metrics for evaluation of the greenness of analytical methods, TrAC, Trends Anal. Chem., 2023, 166, 117211 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters, Beilstein J. Org. Chem., 2006, 2, 1–7 Search PubMed.
- R. Popescu, D. Costinel, O. R. Dinca, A. Marinescu, I. Stefanescu and R. E. Ionete, Discrimination of vegetable oils using NMR spectroscopy and chemometrics, Food Control, 2015, 48, 84–90 CrossRef CAS.
- A. Mannu, M. E. Di Pietro, G. L. Petretto, Z. Taleb, A. Serouri, S. Taleb, A. Sacchetti and A. Mele, Recycling of used vegetable oils by powder adsorption, Waste Manage. Res., 2023, 1–9 Search PubMed.
- A. Mannu, M. Ferro, G. Colombo Dugoni, W. Panzeri, G. L. Petretto, P. Urgeghe and A. Mele, Improving the recycling technology of waste cooking oils: Chemical fingerprint as tool for non-biodiesel application, Waste Manage., 2019, 96, 1–8 CrossRef CAS PubMed.
- A. Mannu, G. Vlahopoulou, P. Urgeghe, M. Ferro, A. Del Caro, A. Taras, S. Garroni, J. P. Rourke, R. Cabizza and G. L. Petretto, Variation of the Chemical Composition of Waste, Cooking Oils upon Bentonite Filtration, Resources, 2019, 8, 108 CrossRef.
- A. Fadda, D. Sanna, E. H. Sakar, S. Gharby, M. Mulas, S. Medda, N. S. Yesilcubuk, A. C. Karac, G. K. Gozukirmizi, M. Lucarini, G. Lombardi-Boccia, Z. Diaconeasa and A. Durazzo, Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils, Sustainability, 2022, 14, 849 CrossRef CAS.
- M. Didham, V. K. Truong, J. Chapman and D. Cozzolino, Sensing the Addition of Vegetable Oils to Olive Oil: The Ability of UV–VIS and MIR Spectroscopy Coupled with Chemometric Analysis, Food Anal. Methods, 2020, 13, 601–607 CrossRef.
- G. Vlahopoulou, G. L. Petretto, S. Garroni, C. Piga and A. Mannu, Variation of density and flash point in acid degummed waste cooking oil, J. Food Process. Preserv., 2018, 42, 13533 CrossRef.
- A. Mannu, G. Vlahopoulou, V. Sireus, G. L. Petretto, G. Mulas and S. Garroni, Bentonite as a Refining Agent in Waste Cooking Oils Recycling: Flash Point, Density and Color Evaluation, Nat. Prod. Commun., 2018, 13(5), 613–616 CrossRef.
- A. Serouri, Z. Taleb, A. Mannu, S. Garroni, N. Senes, S. Taleb, S. Brini and S. K. Abdoun, Variation of Used Vegetable Oils' Composition upon Treatment with Algerian Clays, Recycling, 2021, 6, 6 CrossRef.
- Ember - Yearly Electricity Data 2023. These statistics are updated in continuous, the number used for conversion can vary at any new release of data. Available at https://ember-climate.org/insights/research/global-electricity-review-2023/ Search PubMed.
- M. E. Di Pietro, A. Mannu and A. Mele, A. NMR determination of free fatty acids in vegetable oils, Processes, 2020, 8(4), 410 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00372a |
| This journal is © The Royal Society of Chemistry 2025 |