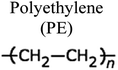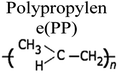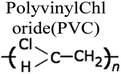Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pyrolysis of waste plastics for alternative fuel: a review of key factors
Haseeb
Yaqoob
 *a,
Hafiz Muhammad
Ali
*ab and
Umair
Khalid
c
*a,
Hafiz Muhammad
Ali
*ab and
Umair
Khalid
c
aMechanical Engineering Department, King Fahd University of Petroleum & Minerals, 31261, Dhahran, Saudi Arabia
bInterdisciplinary Research Center for Sustainable Energy Systems (IRC-SES), King Fahd University of Petroleum & Minerals, 31261, Dhahran, Saudi Arabia
cSABIC MS, Cluster#2, Jubail United Petrochemical Company, Jubail, Saudi Arabia
First published on 10th December 2024
Abstract
Plastic is a threat to the environment since it does not biodegrade, but it also has the potential to become a substantial resource to produce alternative energy sources, creating a multibillion-dollar untapped market. Every year, millions of tons of plastic are produced, resulting in a significant rise in plastic waste, which causes ecological and environmental problems. According to estimates, only around 10% of this waste plastic is now recycled. Plastic waste may be handled in two ways: recycling or converting it into energy. The first alternative, recycling, has several challenges, including the need for labor-intensive processes and concerns about water pollution, which may threaten its long-term sustainability. As a result, the second technique for turning waste plastic into energy has been developed, enhanced, and extensively researched. Pyrolysis is a technique that involves heating plastics at temperatures ranging from 455–700 °C without oxygen. This process yields high-calorific fuel that can be utilized as an alternative fuel. This study explores the thermal and catalytic cracking processes involved in waste plastic pyrolysis, focusing on crucial factors such as temperature, time, feedstock, reactor type, and catalyst that impact results such as oil production, gases, and heat. Furthermore, the study investigates the properties of the liquid oil produced and offers suggestions for enhancing the liquid fuel yield for each kind of plastic.
Sustainability spotlightThis study emphasizes the long-term potential of turning waste plastics into high-calorific oil by pyrolysis, providing a possible avenue for addressing plastic waste's increasing environmental and ecological problems. The study advances energy recovery from waste plastic by improving the pyrolysis process, which includes optimizing aspects such as temperature, time, and catalyst selection. This approach reduces plastic's environmental impact while utilizing an unexplored resource, aligning with worldwide initiatives to promote sustainable waste and energy management. The work promotes numerous UN Sustainable Development Goals, including SDG-7 (affordable and clean energy), SDG-12 (responsible consumption and production), and SDG-13 (climate action). |
1. Introduction
The production of alternative fuels has vital significance in response to growing energy needs and the subsequent impact on fossil fuel reserves and limited natural resources.1,2 This effort includes carefully harnessing non-biodegradable waste materials, thereby realizing their significant energy value.3,4 One important aspect of this need is the pyrolytic conversion of waste plastic, providing an alternate oil product suitable for deployment as an alternative fuel in diesel engines and as a possible source of electrical power generation.5,6 Local governments across the globe confront formidable challenges in effectively managing the growing issue of waste plastic disposal.7 Due to its widespread use in packaging and as a medium for transporting liquids, plastic is essential to the safe and effective movement of these goods. However, the rapid rate of urbanization has made it necessary to move landfills to remote, more difficult-to-reach places, which has resulted in higher transportation logistics costs. Consequently, this voluminous plastic waste's mechanized handling and processing of this voluminous plastic waste have emerged as a pressing imperative. Notably, it is noteworthy that an annual quantity of approximately 280 million metric tons of plastic waste is generated globally,8,9 a fact graphically illustrated in Fig. 1.10,11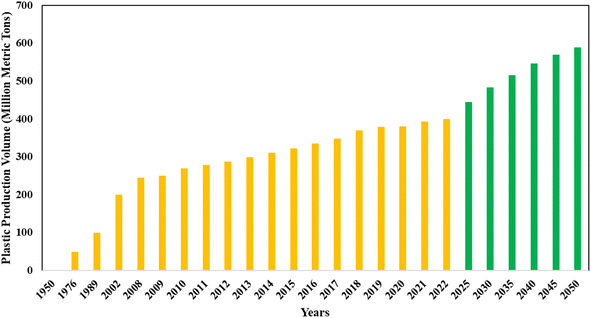 | ||
| Fig. 1 Worldwide variation of plastic waste production in the past few years and future forecast.10,11 | ||
Plastics are complex organic compounds primarily derived from hydrocarbon sources within petroleum-based constituents. Their rapid production can be attributed to their unique characteristics. On an annual basis, a staggering volume of approximately 280 million metric tons of plastic is transported globally, inevitably ending in the waste stream. The exponential growth in Municipal Solid Waste (MSW) generation, reaching an astounding 1.3 billion metric tons annually worldwide, is mainly a consequence of both rapid population expansion and urbanization, coupled with heightened expectations of living standards.12,13 This rising demand for products made of plastic prompts many waste management challenges, including the collection, disposal, and transportation of waste plastic to treatment facilities, exerting substantial environmental and socio-economic burdens.14
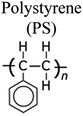
By a broad categorization, plastics encompass a variety of types, notably high-density polyethylene (HDPE), low-density polyethylene (LDPE), polystyrene (PS), polypropylene (PP), polyethylene terephthalate (PET), and polyvinyl chloride (PVC). Their distinct characteristics, applications, and roles in the pyrolysis process are elucidated in Table 1.15–17 Concurrently, the expanding use of plastics has presented an opportunity to harness waste plastic as a source for fuel production, given its inherent hydrocarbon-based composition. Numerous organizations have developed frameworks for managing plastic waste to mitigate its adverse environmental impact. It is estimated that a staggering 600 billion pieces of plastic are produced annually, with a mere 10% being subjected to recycling, while the remainder contributes to waste generation and environmental issues. The various types of plastics and their applications are depicted in Fig. 2.18
 | ||
| Fig. 2 Plastics along with the use.18 | ||
With the advent of more efficient mechanical processes, the traditional landfill approach for managing plastic waste has become increasingly impractical. Due to its non-soluble properties, plastic continues to pose a persistent threat to the environment. In this context, thermal treatments such as pyrolysis, gasification, and waste-to-energy methods emerge as more sustainable and feasible approaches for plastic waste management. From a chemical standpoint, plastic comprises elements including hydrogen, carbon, nitrogen, chlorine, and various other constituents, rendering it highly durable.19
The consistent disposal of plastic in landfills results in significant ecological challenges.20 Additionally, the reusability of plastic necessitates source segregation, rendering it a difficult and resource-intensive process,21 thereby making plastic reuse both problematic and costly. Furthermore, not all types of plastic can be effectively recycled or reused, as these possibilities are contingent upon numerous factors such as design, colour, texture, chemical composition, and more. Coloured or blistered plastics, for instance, are considerably less preferred by consumers. Transparent plastics, however, can be modified through colouring to create new products with greater versatility and appeal to manufacturers.21–23 Traditional recycling methods, primarily involving grinding, manage to reuse only a modest fraction, approximately 15–20%, of the total plastic waste generated.24,25
In recent times, thermal and catalytic pyrolysis and plasma gasification, along with plasma gasification, have gained significant attention from researchers as alternative methods for recycling plastic waste.12,26 These processes involve subjecting plastic waste to high temperatures for a specific duration without oxygen,27 making pyrolysis a particularly versatile approach.
As a tertiary recycling process, pyrolysis employs high-temperature thermal oxidation to convert natural polymers into liquid oil, heat, and gases.16,26,28 Extensive research has been conducted across a range of temperatures, spanning from 300–900 °C; however, the optimal temperature for plastic waste pyrolysis typically falls within the range of 500–550 °C.16 Moreover, pyrolysis of various types of plastic waste has been explored at varying heating rates, such as 4 °C min−1,29 20–25 °C min−1, and 10 °C min−1.30 Additionally, different researchers have employed various residence times for the pyrolysis process, with experiments conducted at durations ranging from 40–70 minutes, 120 minutes, and 45 minutes.9 Mangesh et al.31 identified an investigation gap in plastic combustion and developed a clean fuel made from polypropylene that passes environmental standards. The present investigation was carried out by hydroprocessing post-consumer polypropylene pyrolysis oil with nickel–gold metal implanted on a mordenite support as a catalyst at 70 bar hydrogen pressure and 350 °C.
This review paper provides a comprehensive overview of the production of waste plastic pyrolysis oil (PPO). The primary focus is on plastic pyrolysis oil (covered in Section 2), where the advantages of the pyrolysis process are discussed. Within this section, subheadings delve into the types of pyrolysis processes, specifically thermal and catalytic pyrolysis processes. Furthermore, Section 2.3 explores the various parameters that influence the pyrolysis process, encompassing the impact of temperature, residence time, feedstock, catalyst application, pyrolysis reactor type, and product yield. Finally, Section 3 discusses the reviewed studies, with a concise summary of the reviewed studies and a concise summary in the conclusion, followed by future recommendations.
2. Plastic pyrolysis process
Pyrolysis emerges as a viable strategy for recovering energy from plastic waste, representing an effective method for transforming waste materials into energy-rich liquid and gaseous products.32 Pyrolysis, or thermal breakdown, uses heat to break down long-chain polymer molecules into smaller parts. Many experts contend that pyrolysis can yield a substantial quantity of liquid oil, approximately 79% by weight at around 500 °C.27,33The pyrolysis procedure typically unfolds in four stages: initiation, transition, decomposition, and termination.34 Computational models in computer simulations consider numerous potential reactions during the thermal decomposition of feedstock materials.35 Factors such as temperature, residence time, heating rates, feedstock composition, and the presence of moisture or hazardous substances all play pivotal roles in influencing the outcomes of the pyrolysis process.36
The primary focus of the studies above was to maximize the yield of liquid oils through pyrolysis while establishing the carbon chain composition necessary for producing liquid oil.17,37,38 However, details regarding the density, viscosity, high heating value (HHV), flash point, and cold flow properties (e.g., pour point and solidification point) of liquid pyrolysis fuels derived from blends of various types of plastics, as well as their compatibility with conventional diesel fuel properties, have been rarely disclosed.15,39,40
Most experiments utilised a feedstock comprising 1 kg of waste plastic within the pyrolysis reaction chamber and the blend structure. The reaction chamber was maintained at 455 °C, with a heating rate of 15 degrees Celsius per minute. Across all tests, a consistent maintenance period of 70 minutes was employed, as outlined in the initial experimental setup detailed in Table 2. The selection of these parameters, including the temperature and duration, was guided by Thermogravimetric Analysis (TGA) of the specific plastic materials under controlled conditions, alongside safety considerations. The waste pieces of plastic changed into gaseous organic compounds, which then condensed into liquid oil and collected in a condenser at the bottom of the accumulation tank. To optimize the accumulation of organic vapors in the coolant, the cooling cell's temperature was maintained below 10 °C, with a flow rate of 35 mL min−1.9
| Waste plastic type | Amount taken (kg) | Ratio (%) | Retention time (min) | Temperature (°C) | Heating rate (°C min−1) |
|---|---|---|---|---|---|
| PE | 1 | 100 | 70 | 455 | 10 |
| PP | 1 | 100 | 70 | 455 | 10 |
| PP/PE | 1 | 50/50 | 70 | 455 | 10 |
| PS/PE/PP/PET | 1 | 40/20/20/20 | 70 | 455 | 10 |
The volatile components and solid debris in the feedstock are the primary factors influencing the yield of liquid oil in the pyrolysis process. Higher levels of volatile matter promote the production of liquid oil, while a significant presence of solid debris reduces the liquid oil yield, leading to increased gas production and heat generation.33 The analysis revealed that the volatile matter content for all plastics is substantially higher than that of solid debris, which is relatively low. These characteristics underscore that plastics have the potential to yield a significant amount of liquid oil through the pyrolysis process. Given the compelling results of this analysis of plastics, the subsequent discussion will focus on the process system requirements associated with the pyrolysis process, as these factors have a notable impact on liquid oil production.
In a study by Kumar and Singh,41 a semi-batch reaction chamber was employed to analyze the thermal pyrolysis of HDPE at temperatures ranging from 400 to 550 °C. It was observed that the highest liquid output (80.05 wt%) and gas product (24.65 wt%) were achieved at a temperature of 550 °C, in contrast to wax, which dominated the product composition at higher temperatures between 500 and 550 °C. The resulting light brown oil from the breakdown of plastic waste contained no valuable residue and exhibited a boiling range of 82 to 352 °C. As depicted in Table 3, blending various oil components, such as coal, kerosene, and diesel, with the HDPE pyrolytic oil allows for attaining conventional fuel properties. The HDPE pyrolytic oil boasted a low sulfur content (0.019%), rendering it environmentally safe for use on Earth.
| Types of plastics | Moisture (wt%) | Fixed carbon (wt%) | Volatile (wt%) | Ash content (wt%) | Ref. |
|---|---|---|---|---|---|
| Polyethylene terephthalate (PET) | 0.46 | 6.95 | 92.85 | 0.03 | 30 |
| 0.61 | 13.17 | 86.83 | 0.00 | 22 | |
| High-density polyethylene (HDPE) | 0.00 | 0.02 | 99.75 | 0.17 | 23 |
| 0.00 | 0.03 | 98.57 | 1.40 | 22 | |
| Polyvinyl chloride (PVC) | 0.80 | 6.30 | 93.70 | 0.00 | 33 |
| 0.74 | 5.19 | 94.82 | 0.00 | 22 | |
| Low-density polyethylene (LDPE) | 0.30 | 0.00 | 99.70 | 0.00 | 42 |
| — | — | 99.60 | 0.40 | 43 | |
| Polypropylene (PP) | 0.15 | 1.22 | 95.08 | 3.55 | 44 |
| 0.18 | 0.16 | 97.85 | 1.99 | 22 | |
| Polystyrene (PS) | 0.25 | 0.12 | 99.63 | 0.00 | 45 |
| 0.30 | 0.20 | 99.50 | 0.00 | 42 | |
| Polyethylene (PE) | 0.10 | 0.04 | 98.87 | 0.99 | 44 |
| Nylons | 0.00 | 0.69 | 99.78 | 0.00 | 46 |
| Acrylonitrile butadiene styrene (ABS) | 0.00 | 1.12 | 97.88 | 1.01 | 46 |
2.1. Advantages of pyrolysis
The quality of the liquid oil produced is exceptional due to the multiple operations with no modifications to the process.27 Additionally, the gaseous fuel generated possesses a high calorific value, making it suitable for fulfilling the energy requirements of the pyrolysis plant itself. Pyrolysis is preferred over traditional recycling methods because it offers easier and more flexible handling. Unlike recycling, pyrolysis does not pose a water pollution concern. It is considered a green technology, and the gaseous byproduct of pyrolysis holds a significant calorific value and can be recycled to meet the energy needs of the pyrolysis plant.33 The resulting liquid oil can be utilized in various applications, including furnaces, boilers, generators, and diesel engines, without requiring any modifications.32The pyrolysis process yields various products such as gas, oil/wax, and heat, with their composition and production influenced by factors such as plastic-type, reactor type, and process conditions, particularly reaction temperature and heating rate.47–51 Numerous researchers have also explored the impact of catalysts on enhancing the extraction of crude oil from the process.47,52–57
2.2. Types of pyrolysis
| Plastic | Thermal temp. [°C] | Time [min] | Oil (wax) [%] | Gas [%] | Char [%] | Ref. |
|---|---|---|---|---|---|---|
| PS | 450 | 75 | 80.8 | 13 | 6.2 | 34 |
| PS | 330 | — | 80 | — | — | 58 |
| LDPE | 437–486 | — | 94 | — | — | 59 |
| PP | 378–456 | — | 86 | — | — | 59 |
| PP | 350 | — | 82.6 | — | — | 58 |
| PP | 450 | 30 | 67.48 | 8.85 | 23.67 | 60 |
| HDPE | — | — | 84 | 13 | 3 | 61 |
| HDPE | — | — | — | — | — | 62 |
| HDPE | — | — | — | — | — | 62 |
| HDPE | 430 | — | 75.5 | 20 | 4.5 | 62 |
| HDPE | 450 | — | 82 | 18 | — | 63 |
| HDPE | 450 | — | 80 | — | — | 58 |
| PP | 540 | — | 61 | 31 | 7 | 64 |
| PP | — | — | — | — | — | 65 |
| LDPE | — | — | — | — | — | 65 |
| HDPE | — | — | — | — | — | 65 |
| LDPE | 550 | — | 93.1 | 14.6 | — | 64 |
| HDPE | 550 | — | 84.7 | 16.3 | — | 64 |
| LDPE | 375 | — | 68 | — | 22 | 66 |
| HDPE | 430 | 150 | 72.66 | — | — | 67 |
| LDPE | 450 | 150 | 73.91 | — | — | 67 |
| PP | 400 | 150 | 83.81 | — | — | 67 |
The thermal degradation of polystyrene (PS) was completed under milder conditions compared to the thermal degradation of high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP). This difference arises from the fact that polyethylene (HDPE and LDPE) and PP require significantly higher temperatures for decomposition when compared to PS plastic.44 Polyethylene (PE) is converted without a catalyst into wax rather than liquid oil.45 The liquid oil produced through thermal degradation consists of complex mixtures of heavy oil compounds with long carbon chains. However, due to its lower octane number and solid residues and contaminants such as sulfur, nitrogen, and phosphorus, the quality of the liquid oil obtained was relatively poor.44
| Plastic | Catalyst | Catalytic oil (%) | Temp. (°C) | Gas (%) | Char (%) | Ref. |
|---|---|---|---|---|---|---|
| PS | Natural zeolite | 54 | 450 | 12.8 | 32.8 | 34 |
| Synthetic zeolite | 50 | 450 | 22.6 | 27.4 | ||
| PP | Silica aluminium | 59.57 | 320 | — | — | 58 |
| LDPE | Zeolite | 51 | — | — | — | 59 |
| PS | Silica aluminium | 50 | 290 | — | — | 58 |
| PP | Zeolite | 58 | — | — | — | 59 |
| HDPE | Alumina | 82 | 450 | 15.9 | 2.1 | 62 |
| PP | Kaolin | 69.75 | 450 | 14.01 | 16.24 | 60 |
| HDPE | ZSM-5 | 35 | — | 63.5 | 1.5 | 61 |
| HDPE | Silica aluminium | 48.3 | 350 | — | — | 58 |
| HDPE | FCC | 79.7 | — | 19.4 | 0.9 | 62 |
| HDPE | Silica/NaOH | 81 | 450 | 19 | 0 | 63 |
| HDPE | Mordenite | 78.5 | 450 | 18.5 | 3 | 62 |
| PP | Calcium bentonite | 88.5 | 500 | — | — | 65 |
| PP | Fe-SBA-15 | 73–77 | 540 | 24–21 | 2–0.8 | 64 |
| LDPE | Calcium bentonite | 82 | 500 | — | — | 65 |
| HDPE | Calcium bentonite | 82.5 | 500 | — | — | 65 |
| LDPE | HZSM-5 | 18.3 | 550 | 70.7 | 0.5 | 64 |
| HDPE | HUSY | 41.0 | 550 | 39.5 | 1.9 | 64 |
| LDPE | KAB/kaolin | 84 | 295 | — | <1 | 66 |
| HDPE | 10% dolomite | 80.73 | 430 | — | — | 67 |
| LDPE | 10% dolomite | 83.04 | 450 | — | — | 67 |
| PP | 10% dolomite | 85.2 | 400 | — | — | 67 |
2.3. Factors affecting the pyrolysis process
Variables such as temperature,70 residence time,40 the physical structure of waste plastic pieces,71 catalyst use,72, moisture content29 heating rate73 and molecular composition,74 all play an important role in influencing the outcome of the plastic pyrolysis process.In the thermal cracking of plastics, temperature is a paramount operating parameter in the process, as it governs the breaking reactions of carbon chains. Different plastics have distinct degradation temperature ranges based on their molecular structures. For instance, thermal degradation temperatures of common plastics like PET, LDPE, HDPE, PS, and PP range between 250 and 360 °C, while PVC decompresses at a lower temperature of around 230 °C. The specific operational temperature requirements depend strongly on the desired product composition. If the preference is for gaseous products, higher temperatures exceeding 450 °C are recommended, whereas a lower temperature range of 300–500 °C is suitable for obtaining liquid products.17,77
Apart from temperature, selecting an appropriate reaction chamber configuration is crucial for the economically viable conversion of plastic waste into fuel. Each reactor type has unique advantages and limitations, depending on its intended use. Batch or semi-batch reactors have traditionally been used for plastic decomposition without catalysis, as they allow for controlled conditions. However, these reactors may not be suitable for catalytic pyrolysis due to the tendency of coke formation, which can hinder the contact between the catalyst and plastic pieces, thus reducing the catalytic activity. In contrast, fluidized bed reactors, such as conical spouted-bed reactors (CSBRs), are considered suitable for catalytic plastic decomposition as they allow periodic catalyst replenishment and offer excellent mixing to handle large amounts of feedstock and promote bed circulation compared to bubbling fluidized beds.78
Nevertheless, certain operational challenges are associated with reactor operation, including complex system arrangements, catalyst handling, and the collection of solid and liquid products, which can affect overall efficiency.79
3. Discussion
In summary, feedstock weight and reaction time play significant roles in pyrolysis. Larger feedstock loads can increase the production of gaseous products and affect the distribution of molecular weights in both the liquid and vapor phases. However, this effect is more pronounced at higher temperatures. Incineration is a commonly used waste management method that does not differentiate waste composition, including plastics. Therefore, catalytic pyrolysis of plastic waste is considered a preferable approach. Additionally, the type and flow rate of the fluidizing gas used during pyrolysis also influence the process.The fluidizing gas, often referred to as the carrier gas, serves the sole purpose of transporting vaporized products and does not participate in the actual decomposition of the feedstock. Various gases can be used for fluidization, including nitrogen, helium, argon, ethylene, propylene, and hydrogen.80 After careful consideration, most experts have concluded that nitrogen is the most suitable fluidizing gas for plastic pyrolysis. It is easier and safer to handle compared to highly reactive gases like hydrogen and propylene, which can be more volatile.
At the lowest fluidizing gas flow rate of 300 ml min−1, Lin and Yen81 observed a considerable increase in the rate of deterioration. This is because a lower flow rate provides a longer contact time for the feedstock, allowing for the accumulation of coke precursor compounds (BTX) and increasing the secondary cracking reactions, even though the overall cracking rate is relatively mild.82 The separation between gas and hydrocarbon products becomes more pronounced at the highest flow rate of 910 ml min−1. This discussion underscores the influence of fluidizing gas flow rate on the final product distribution.
Regarding catalytic degradation, thermal technology employs a catalyst to accelerate the desired reactions and enhance hydrocarbon formation, resulting in pyrolysis liquids with properties similar to conventional fuels like gasoline and diesel.
During the heating of waste plastic, three types of catalysts are commonly used: basic zeolites, fluidized cracking catalysts, and silica–alumina catalysts. The utilization of zeolite catalysts during plastic heating has been found to reduce the impurities in the resulting oil, as demonstrated by Miskolczi et al.36 Lee et al.83 reported that the solid residue left after catalytic pyrolysis decreased from 4.5% to 0.9% by weight. In contrast to these catalytic applications, more research has been conducted on silica–alumina catalysts, which are non-destructive. The mole ratio of SiO2/Al2O3 determines the catalytic activity of silica–alumina catalysts. The precise acidity characteristics of the catalyst have a significant impact on the outcome of plastic pyrolysis.
Table 6 provides information on the physical properties of pyrolyzed liquid fuel obtained from plastic. The calculated calorific values of pyrolyzed oil from plastic waste exceed 40 MJ kg−1, which makes it a valuable energy source. The calculations indicate that polystyrene (PS) has a lower calorific value compared to low-density polyethylene (LDPE) and high-density polyethylene (HDPE) because PS contains aromatic rings, which have lower energy content compared to other straight-chain hydrocarbons.87 On the other hand, PVC and PET have lower calorific values of around 30 MJ kg−1 due to the presence of rings in PET and the negative impact of chlorine compounds in PVC.
| Properties | Units | Types of plastics (typical experimental value) | Commercial standard value (ASTM 1979) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PET77 | HDPE23 | PVC77,84 | LDPE85 | PP23 | PS80,86 | Gasoline82 | Diesel82 | ||
| Calorific value | MJ kg−1 | 28.2 | 40.5 | 21.1 | 39.5 | 40.8 | 43.0 | 42.5 | 43.0 |
| API gravity @ 60 °F | — | — | 27.48 | 38.98 | 48.05 | 33.03 | — | 55 | 38 |
| Viscosity | mm2 s−1 | — | 5.06 | 7.05 | 5.56 | 5.02 | 1.8 | 1.18 | 1.9–4.1 |
| Density @ 15 °C | g cm−3 | 0.90 | 0.90 | 0.79 | 0.79 | 0.85 | 0.80 | 0.658 | 0.907 |
| Ash | wt% | — | 0.00 | — | 0.035 | 0.00 | 0.007 | — | 0.02 |
| Octane number MON (min) | — | 86 | — | — | 88 | — | 82–86 | — | |
| Octane number RON (min) | — | 96 | — | — | 98 | 90–98 | 90–94 | — | |
| Pour point | °C | — | −4 | — | — | −10 | −68 | — | 7 |
| Flashpoint | °C | — | 49 | 39 | 40 | 29 | 27 | 43 | 51 |
| Aniline point | °C | — | 56 | — | — | 39 | — | 69 | 78 |
| Diesel index | — | 30 | — | — | 35 | — | — | 39 | |
Table 7 presents ideal heating conditions to enhance liquid oil production in catalytic and thermal pyrolysis under various scenarios. The pyrolysis of plastics is influenced by factors such as the type of reaction chamber, feedstock mass, heating rate, and residence time. Nitrogen gas is commonly used to create an inert atmosphere in all these processes. However, two types of plastics, PET and PVC, yield lower liquid products, which have led to fewer research studies on these materials.
| Plastic-type | Reaction chamber | Procedure parameter | Production (wt%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (atm) | Heating rate (°C min−1) | Duration (min) | Oil | Gas | Solid | |||
| PET | Fixed bed | 510 | — | 12 | — | 23 | 77 | 0 | 77 |
| PET | — | 505 | 1 atm | 6 | — | 39 | 53 | 9 | 12 |
| HDPE | Horizontal steel | 360 | — | 19 | 29 | 81.1 | 16.9 | 2 | 82 |
| HDPE | Semi-batch | 400 | 1 atm | 6 | — | 82 | 15 | 3 | 88 |
| HDPE | Batch | 455 | — | — | 65 | 74 | 6 | 20 | 89 |
| HDPE | Semi-batch | 455 | 1 atm | 28 | — | 92 | 3 | 5 | 89 |
| HDPE | Fluidized bed | 550 | — | — | 59 | 90 | 9 | 1 | 87 |
| HDPE | Batch | 500 | — | 6 | — | 83 | 17 | 0 | 90 |
| HDPE | Fluidized bed | 600 | — | — | 21–26 | 69.1 | 30.9 | 0 | 91 |
| PVC | Fixed bed | 550 | — | 12 | — | 13.1 | 86.9 | 0 | 77 |
| PVC | Vacuum batch | 550 | 2.5 kPa | 9 | — | 11 | 1 | 29 | 92 |
| LDPE | Pressurized batch | 450 | 0.8–4.3 mPa | 12 | 59 | 90 | 9 | 1 | 93 |
| LDPE | Batch | 440 | — | 4 | — | 76 | 8.9 | 8 | 94 |
| LDPE | Batch | 550 | 1 atm | 5 | — | 79.5 | 20 | 0.8 | 27 |
| LDPE | Fixed bed | 520 | — | 11 | 19 | 94 | 6 | 0 | 95 |
| LDPE | Batch | 500 | — | 8 | — | 87 | 13 | 0 | 90 |
| LDPE | Fluidized bed | 580 | 1 atm | — | — | 50 | 25 | 0 | 96 |
| PP | Horizontal steel | 350 | — | 19 | 29 | 68 | 29 | 1.5 | 82 |
| PP | Semi-batch | 420 | 1 atm | 8 | — | 85 | 12 | 3 | 88 |
| PP | Semi-batch | 425 | 1 atm | 20 | — | 93 | 3 | 4 | 97 |
| PP | Semi-batch | 520 | 1 atm | 5 | — | 81 | 18 | 0.25 | 27 |
| PP | Batch | 720 | — | — | — | 49 | 48 | 3 | 37 |
| PS | Semi-batch | 450 | 1 atm | 6 | — | 89 | 5 | 3.5 | 88 |
| PS | Pressurized batch | 450 | 0.25–1.7 MPa | 11 | 59 | 96 | 3 | 0.25 | 93 |
| PS | Batch | 550 | — | — | 149 | 96.5 | 3.5 | 0 | 98 |
| PS | Batch | 579 | — | — | — | 89 | 10 | 1 | 37 |
Pyrolysis of PVC, in particular, results in low liquid oil production and poses environmental hazards due to the release of hydrochloric acid during the process. The pyrolysis oil derived from PVC can degrade the oil's quality and harm the environment.
Studies have shown that the optimal temperature range for increasing liquid oil production in thermal pyrolysis typically falls between 500 and 550 °C. This optimal temperature can be lowered to 300–400 °C using suitable catalysts, resulting in higher oil yields. While it is possible to increase liquid products by providing extreme conditions during the heating of different plastic types, polystyrene (PS) stands out as a unique material in this regard.87
In contrast, polystyrene (PS) is the most favorable plastic for pyrolysis because it yields more liquid oil than other plastics. Furthermore, in thermal pyrolysis, low-density polyethylene (LDPE) yielded the highest liquid oil content at 93.1% by weight, followed by high-density polyethylene (HDPE) at 84.7% by weight, and polypropylene (PP) at 82.12% by weight. However, with the introduction of suitable catalysts, especially fluidized cracking catalysts (FCC), operating at optimal temperatures, the oil yield can be further enhanced to exceed 89% by weight.
Moreover, the potential value of the obtained oil can be estimated based on the total amount of plastic waste available in Saudi Arabia. The Kingdom of Saudi Arabia generates roughly 53 MMT of waste annually, 95% of which is in landfills. From 2020 to the first part of 2021, the Kingdom recycled barely 5% of its total garbage, including plastic, metal, and paper.99 Saudi Arabia's total GHG emissions have increased by 225% since 1990, accounting for 1.47% of world emissions.100
4. Conclusion & future recommendations
Plastic waste can be effectively processed through pyrolysis to produce a valuable alternative fuel source. Pyrolysis of plastic waste results in high-performance oil, offering environmental sustainability and reducing the dependence on fossil fuels while also addressing the issue of plastic waste disposal. Extensive experimental research has been conducted to optimize the pyrolysis process for extracting oil from plastic waste, leading to critical findings summarized below:• Thermal pyrolysis of plastic waste often yields low-quality liquid oil and requires high heating temperatures and extended maintenance times. To address these challenges, catalytic pyrolysis of plastic waste has been introduced.
• Various catalysts have been found to enhance the efficiency of the pyrolysis process by improving the quality of liquid oil and gas products while reducing the required heating temperature and time. Notable catalysts researchers use include ZSM-5, HZSM-5, FCC, Al2O3, Red Mud, and natural zeolite (NZ).
• Natural zeolite is increasingly being utilized as a catalyst in pyrolysis due to its availability and cost-effectiveness.
• Catalyst modifications, such as metal doping (e.g., Co, Ni, Zn, and Mo) on acidic catalysts, have been shown to enhance catalyst activity. Catalysts with larger surface areas and varying acidity levels can influence gas and liquid oil production differently.
Further studies can improve the efficiency of the pyrolysis process for plastic waste, such as exploring the potential of generated liquids and gases with high heating values as alternative energy sources.
• Using the huge surface area of pyrolysis heat for environmental applications such as heavy metal adsorption and pollutant removal from wastewater and air.
• Assessing how heating temperatures, maintenance time, plastic waste composition, and catalyst use affect the pyrolysis process and final product quality.
• Investigating advanced catalyst modifications, including thermal, acidic, and metal doping treatments, to enhance catalytic activity and properties.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
The authors declare no conflict of interest.Nomenclature
| ABS | Acrylonitrile butadiene styrene |
| BTX | Butene toluene xylene |
| C–C | Carbon–carbon |
| Co–Mo/Z | Cobalt–molybdenum/zeolite |
| CSBR | Conical spouted bed reactor |
| Cu–Al2O3 | Copper–aluminum trioxide |
| FCC | Fluidized catalytic cracking |
| HDPE | High-density polyethylene |
| HHV | High heating value |
| HCs | Hydrocarbons |
| HZSM-5 | Hydrogen zeolite Socony mobile-5 |
| LDPE | Low-density polyethylene |
| MPW | Municipal plastic waste |
| MSW | Municipal solid waste |
| NZ | Natural zeolite |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| SA-1 | Silicon aluminium-1 |
| TGA | Thermo-gravimetric analysis |
| ZSM-5 | Zeolite Socony mobile-5 |
Acknowledgements
The authors would like to acknowledge the King Fahd University of Petroleum & Minerals, Saudi Arabia, for support of this study.References
- V. L. Mangesh, T. Perumal, S. Subramanian and S. Padmanabhan, Energy Fuels, 2020, 34, 8824–8836 Search PubMed.
- S. Muhammad Hammad, H. Yaqoob, M. Umer Farooq, H. Muhammad Ali, U. Sajjad, M. Ahmad Jamil and K. Hamid, Energy Convers. Manage.: X, 2024, 24, 100710 CAS.
- H. Yaqoob, H. M. Ali, H. Abbas, O. Abid, M. A. Jamil and T. Ahmed, Clean Technol. Environ. Policy, 2023, 25, 3177–3187 CrossRef CAS.
- H. Yaqoob and H. M. Ali, Arabian J. Sci. Eng., 2024, 49, 11763–11773 CrossRef CAS.
- P. Saravanan, D. Mala, V. Jayaseelan and N. M. Kumar, Energy Sources, Part A, 2019, 1–14 CAS.
- S. Padmanabhan, T. V. Kumar, K. Giridharan, B. Stalin, N. Nagaprasad, L. T. Jule and K. Ramaswamy, Sci. Rep., 2022, 12, 21719 CrossRef CAS PubMed.
- D. Dillikannan, J. Dilipsingh, M. V. De Poures, G. Kaliyaperumal, A. P. Sathiyagnanam, R. K. Babu and N. Mukilarasan, Energy Sources, Part A, 2019, 1–16 Search PubMed.
- W. Sriningsih, M. G. Saerodji, W. Trisunaryanti, T. Triyono, R. Armunanto and I. I. Falah, Procedia Environ. Sci., 2014, 20, 215–224 CrossRef CAS.
- R. Miandad, M. A. Barakat, A. S. Aburiazaiza, M. Rehan, I. M. I. Ismail and A. S. Nizami, Int. Biodeterior. Biodegrad., 2017, 119, 239–252 CrossRef CAS.
- S. R. Department, Annual production of plastics worldwide from 1950 to 2022, https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/, accessed 21 August 2024.
- M. Jaganmohan, Production forecast of thermoplastics worldwide from 2025 to 2050, https://www.statista.com/statistics/664906/plastics-production-volume-forecast-worldwide/, accessed 21 August 2024.
- A. S. Nizami, M. Rehan, O. K. M. Ouda, K. Shahzad, Y. Sadef, T. Iqbal and I. M. I. Ismail, Chem. Eng. Trans., 2015, 45, 337–342 Search PubMed.
- E. Martinot, A. Chaurey, D. Lew, J. R. Moreira and N. Wamukonya, Annu. Rev. Energy Environ., 2002, 27, 309–348 CrossRef.
- A. Liu, F. Ren, W. Y. Lin and J. Y. Wang, Int. J. Sustainable Built Environ., 2015, 4, 165–188 CrossRef.
- R. Miandad, M. A. Barakat, A. S. Aburiazaiza, M. Rehan and A. S. Nizami, Process Saf. Environ. Prot., 2016, 102, 822–838 CrossRef CAS.
- D. Chen, L. Yin, H. Wang and P. He, Waste Manage., 2014, 34, 2466–2486 CrossRef CAS PubMed.
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Energy Convers. Manage., 2016, 115, 308–326 CrossRef CAS.
- G. Gourmelon, Global Plastic Production Rises, Recycling Lags, WorldWatch Inst., 2015, pp. 1–7 Search PubMed.
- A. Brems, J. Baeyens and R. Dewil, Therm. Sci., 2012, 16, 669–685 CrossRef.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- E. Masanet, R. Auer, D. Tsuda, T. Barillot and A. Baynes, IEEE Int. Symp. Electron. Environ., 2002, 5–10 Search PubMed.
- J. M. Heikkinen, J. C. Hordijk, W. De Jong and H. Spliethoff, J. Anal. Appl. Pyrolysis, 2004, 71, 883–900 CrossRef CAS.
- I. Ahmad, M. Ismail Khan, M. Ishaq, H. Khan, K. Gul and W. Ahmad, Polym. Degrad. Stab., 2013, 98, 2512–2519 CrossRef CAS.
- A. Studies, Int. J. Innovation Appl. Stud., 2013, 4, 101–113 Search PubMed.
- M. N. Siddiqui and H. H. Redhwi, Fuel Process. Technol., 2009, 90, 545–552 CrossRef CAS.
- O. K. M. Ouda, S. A. Raza, A. S. Nizami, M. Rehan, R. Al-Waked and N. E. Korres, Renewable Sustainable Energy Rev., 2016, 61, 328–340 CrossRef.
- S. M. Fakhrhoseini and M. Dastanian, J. Chem., 2013, 2013, 7–9 Search PubMed.
- M. Anjum, R. Miandad, M. Waqas, I. Ahmad, Z. Omar, A. Alafif, A. S. Aburiazaiza, M. A. E. Barakat and T. Akhtar, J. Appl. Agric. Biotechnol., 2016, 1, 13–26 Search PubMed.
- I. Velghe, R. Carleer, J. Yperman and S. Schreurs, J. Anal. Appl. Pyrolysis, 2011, 92, 366–375 CrossRef CAS.
- F. Zannikos, S. Kalligeros, G. Anastopoulos and E. Lois, J. Renewable Energy, 2013, 2013, 1–9 CrossRef.
- V. L. Mangesh, P. Tamizhdurai, P. Santhana Krishnan, S. Narayanan, S. Umasankar, S. Padmanabhan and K. Shanthi, J. Cleaner Prod., 2020, 276, 124200 CrossRef CAS.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS.
- F. Abnisa and W. M. A. Wan Daud, Energy Convers. Manage., 2014, 87, 71–85 CrossRef CAS.
- M. Rehan, R. Miandad, M. A. Barakat, I. M. I. Ismail, T. Almeelbi, J. Gardy, A. Hassanpour, M. Z. Khan, A. Demirbas and A. S. Nizami, Int. Biodeterior. Biodegrad., 2017, 119, 162–175 CrossRef CAS.
- J. H. Zhang, Z. B. Wang, H. Zhao, Y. Y. Tian, H. H. Shan and C. H. Yang, Appl. Petrochem. Res., 2015, 5, 255–261 CrossRef CAS PubMed.
- N. Miskolczi, A. Angyal, L. Bartha and I. Valkai, Fuel Process. Technol., 2009, 90, 1032–1040 CrossRef CAS.
- A. Demirbas, J. Anal. Appl. Pyrolysis, 2004, 72, 97–102 CrossRef CAS.
- N. Miskolczi, F. Ateş and N. Borsodi, Bioresour. Technol., 2013, 144, 370–379 CrossRef CAS PubMed.
- S. Lee, K. Yoshida and K. Yoshikawa, Energy Environ. Res., 2015, 5, 18–32 Search PubMed.
- A. López, I. De Marco, B. M. Caballero, M. F. Laresgoiti and A. Adrados, Chem. Eng. J., 2011, 173, 62–71 CrossRef.
- S. Kumar and R. K. Singh, Braz. J. Chem. Eng., 2011, 28, 659–667 CrossRef CAS.
- S. S. Park, D. K. Seo, S. H. Lee, T. U. Yu and J. Hwang, J. Anal. Appl. Pyrolysis, 2012, 97, 29–38 CrossRef CAS.
- A. Aboulkas, K. El harfi and A. El Bouadili, Energy Convers. Manage., 2010, 51, 1363–1369 CrossRef CAS.
- S. H. Jung, M. H. Cho, B. S. Kang and J. S. Kim, Fuel Process. Technol., 2010, 91, 277–284 CrossRef CAS.
- S. Bayu, Environ. Prog., 2011, 30, 925–932 Search PubMed.
- N. Othman, N. E. A. Basri and M. N. M. Yunus, Iccbt, 2008, 169–180 Search PubMed.
- C. Muhammad, J. A. Onwudili and P. T. Williams, Energy Fuels, 2015, 29, 2601–2609 CrossRef CAS.
- G. Manos, A. Garforth and J. Dwyer, Ind. Eng. Chem. Res., 2000, 39, 1198–1202 CrossRef CAS.
- P. T. Williams and E. Slaney, Resour., Conserv. Recycl., 2007, 51, 754–769 CrossRef.
- D. P. Serrano, J. Aguado, J. M. Escola and E. Garagorri, Appl. Catal., B, 2003, 44, 95–105 CrossRef CAS.
- M. Del Remedio Hernández, Á. N. García and A. Marcilla, J. Anal. Appl. Pyrolysis, 2005, 73, 314–322 CrossRef.
- N. Z. Muradov, Energy Fuels, 1998, 12, 41–48 CrossRef CAS.
- F. Ateş, N. Miskolczi and N. Borsodi, Bioresour. Technol., 2013, 133, 443–454 CrossRef PubMed.
- M. Syamsiro, H. Saptoadi, T. Norsujianto, P. Noviasri, S. Cheng, Z. Alimuddin and K. Yoshikawa, Energy Procedia, 2014, 47, 180–188 CrossRef CAS.
- P. Tiwary and C. Guria, J. Polym. Environ., 2010, 18, 298–307 CrossRef CAS.
- S. H. Chung, J. J. Park, S. G. Jeon and D. C. Kim, Korea Inst. Energy Resour., 2003, 71, 305–343 Search PubMed.
- J. F. Mastral, C. Berrueco, M. Gea and J. Ceamanos, Polym. Degrad. Stab., 2006, 91, 3330–3338 CrossRef CAS.
- P. A. Owusu, N. Banadda, A. Zziwa, J. Seay and N. Kiggundu, J. Anal. Appl. Pyrolysis, 2018, 130, 249–255 CrossRef.
- A. F. Anene, S. B. Fredriksen, K. A. Sætre and L. A. Tokheim, Sustainability, 2018, 10, 1–11 CrossRef.
- I. G. Hakeem, F. Aberuagba and U. Musa, Appl. Petrochem. Res., 2018, 8, 203–210 CrossRef CAS.
- Y. Zhao, W. Wang, X. Jing, X. Gong, H. Wen and Y. Deng, J. Anal. Appl. Pyrolysis, 2020, 146, 104755 CrossRef CAS.
- Y. H. Seo, K. H. Lee and D. H. Shin, J. Anal. Appl. Pyrolysis, 2003, 70, 383–398 CrossRef CAS.
- F. Obeid, J. Zeaiter, A. H. Al-Muhtaseb and K. Bouhadir, Energy Convers. Manage., 2014, 85, 1–6 CrossRef CAS.
- R. Navarro, A. Marcilla and M. I. Beltra, Appl. Catal., B, 2009, 86, 78–86 CrossRef.
- A. K. Panda, Int. J. Ind. Chem., 2018, 9, 167–176 CrossRef CAS.
- S. Attique, M. Batool, M. Yaqub, O. Goerke, D. H. Gregory and A. T. Shah, Waste Manage. Res., 2020, 38, 689–695 CrossRef CAS PubMed.
- Y. B. Sonawane, M. R. Shindikar and M. Y. Khaladkar, Nat., Environ. Pollut. Technol., 2017, 16, 879–882 CAS.
- S. Y. Lee, J. H. Yoon, J. R. Kim and D. W. Park, Polym. Degrad. Stab., 2001, 74, 297–305 CrossRef CAS.
- A. López, I. De Marco, B. M. Caballero, M. F. Laresgoiti, A. Adrados and A. Torres, Waste Manage., 2011, 31, 1973–1983 CrossRef PubMed.
- L. Ji, A. Hervier and M. Sablier, 2006, Chemosphere, vol. 65, pp. 1120–1130 Search PubMed.
- J. C. Acomb, C. Wu and P. T. Williams, J. Anal. Appl. Pyrolysis, 2015, 113, 231–238 CrossRef CAS.
- L. C. Lerici, M. S. Renzini and L. B. Pierella, Procedia Mater. Sci., 2015, 8, 297–303 CrossRef CAS.
- B. K. Sharma, B. R. Moser, K. E. Vermillion, K. M. Doll and N. Rajagopalan, Fuel Process. Technol., 2014, 122, 79–90 CrossRef CAS.
- S. Luo, B. Xiao, Z. Hu and S. Liu, Int. J. Hydrogen Energy, 2010, 35, 93–97 CrossRef CAS.
- A. López, I. De Marco, B. M. Caballero, M. F. Laresgoiti, A. Adrados and A. Aranzabal, 2011, Appl. Catal., B, vol. 104, pp. 211–219 Search PubMed.
- M. Sarker and M. M. Rashid, Int. J. Renew. Energy Technol. Res., 2013, 2, 17–28 Search PubMed.
- S. D. A. Sharuddin, F. Abnisa, W. M. A. W. Daud and M. K. Aroua, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 334, 012001 Search PubMed.
- M. Olazar, G. Lopez, M. Amutio, G. Elordi, R. Aguado and J. Bilbao, 2009, J. Anal. Appl. Pyrol., vol. 85, pp. 359–365 Search PubMed.
- R. Desai, H. Ahmed, S. Pawar and B. Balkunde, Waste Plastic Pyrolysis, Vijaypur, 2016 Search PubMed.
- F. Pinto, P. Costa, I. Gulyurtlu and I. Cabrita, 1999, J. Anal. Appl. Pyrolysis, 51, 39–55 Search PubMed.
- Y. Lin and H. Yen, Polym. Degrad. Stab., 2005, 89, 101–108 CrossRef CAS.
- I. Ahmad, M. Ismail Khan, H. Khan, M. Ishaq, R. Tariq, K. Gul and W. Ahmad, Int. J. Green Energy, 2015, 12, 663–671 CrossRef CAS.
- K. Lee, S. Jeon, K. Kim, N. Noh, D. Shin, J. Park, Y. Seo, J. Yee and G. Kim, Korean J. Chem. Eng., 2003, 20, 693–697 CrossRef CAS.
- E. Manickaraja and S. Tamilkolundu, 2014, Semant. Sch., vol. 23, pp. 74–78 Search PubMed.
- S. B. Desai, Int. J. Eng. Res. Gen. Sci., 2015, 3, 590–595 Search PubMed.
- N. Garcı, A. Go, J. Agullo and A. Marcilla, 2007, Appl. Catal., A, vol. 317, pp. 183–194 Search PubMed.
- G. Luo, T. Suto, S. Yasu and K. Kato, Polym. Degrad. Stab., 2000, 70, 97–102 CrossRef CAS.
- K. H. Lee, N. S. Noh, D. H. Shin and Y. Seo, Polym. Degrad. Stab., 2002, 78, 539–544 CrossRef CAS.
- N. Miskolczi, L. Bartha, G. Deák, B. Jóver and D. Kalló, J. Anal. Appl. Pyrolysis, 2004, 72, 235–242 CrossRef CAS.
- R. Navarro, A. Marcilla and M. I. Beltra, 2009, Appl. Catal., B, vol. 86, pp. 78–86 Search PubMed.
- J. A. Conesa, R. Font and A. Marcilla, Energy Fuels, 1997, 0624, 126–136 CrossRef.
- R. Miranda, J. Yang, C. Roy and C. Vasile, Polym. Degrad. Stab., 1999, 64, 127–144 CrossRef CAS.
- J. A. Onwudili, N. Insura and P. T. Williams, 2009, J. Anal. Appl. Pyrolysis, vol. 86, pp. 293–303 Search PubMed.
- A. Wddin, K. Koizumip, K. Murata and Y. Sakata, Polym. Degrad. Stab., 1997, 56, 37–44 CrossRef.
- J. C. Garcı, A. Marcilla and J. Herna, J. Anal. Appl. Pyrolysis, 2006, 76, 254–259 CrossRef.
- P. T. Williams and E. A. Williams, J. Anal. Appl. Pyrolysis, 1999, 51, 107–126 CrossRef CAS.
- M. S. Abbas-Abadi, M. N. Haghighi, H. Yeganeh and A. G. McDonald, J. Anal. Appl. Pyrolysis, 2014, 109, 272–277 CrossRef CAS.
- J. S. Adnan and M. R. Jan, J. Taiwan Inst. Chem. Eng., 2014, 45, 2494–2500 CrossRef CAS.
- S. Hersey, Saudi Arabia - Country Commercial Guide - Waste Management, https://www.trade.gov/country-commercial-guides/%0Asaudi-arabia-waste-management, accessed 21 August 2024.
- A. E. A. Abdulkareem, Saudi Arabia Greenhouse Gas Emissions Have Increased by 225% Since 1990, https://www.climatescorecard.org/2020/12/saudi-arabia-greenhouse-gas-emissions-have-increased-by-225-since-1990/#_ftn1, accessed 21 August 2024.
| This journal is © The Royal Society of Chemistry 2025 |