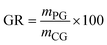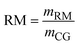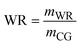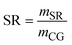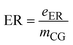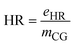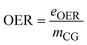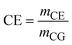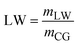Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Techno-economic assessment of biodiesel-derived crude glycerol purification processes†
Yash
Bansod
 a,
Kamran
Ghasemzadeh
a,
Kamran
Ghasemzadeh
 ac and
Carmine
D'Agostino
ac and
Carmine
D'Agostino
 *ab
*ab
aDepartment of Chemical Engineering, University of Manchester, Manchester, M13 9PL, UK. E-mail: carmine.dagostino@manchester.ac.uk
bDipartimento di Ingegneria Civile, Chimica, Ambientale e dei Materiali (DICAM), Alma Mater Studiorum – Università di Bologna, Via Terracini, 28, 40131 Bologna, Italy
cSchool of Engineering, University of Edinburgh, EH9 3JL, UK
First published on 29th January 2025
Abstract
This study presents a comprehensive techno-economic assessment of three glycerol purification processes: Membrane Separation (MBP), Vacuum Distillation (VDP), and Ion Exchange Purification (IEP). The analysis evaluated these processes based on product purity, product recovery, raw material and utility consumption, capital and operating costs, and economic resilience to market fluctuations. IEP achieved the highest glycerol purity (98.75%) and recovery rate (99.00%), followed by VDP (96.91% purity, 94.99% recovery) and MBP (93.92% purity, 86.20% recovery). However, IEP exhibited the highest raw material consumption and liquid waste generation, while VDP demonstrated the most favourable balance between resource utilization and waste production. Economic analysis revealed VDP as the process with the lowest capital cost of 4.44 MUSD and the only profitable process with an annual profit of 0.24 MUSD under the given conditions. Sensitivity analysis, considering variations in raw material prices, utility costs, and product prices, consistently identified VDP as the most economically resilient process. MBP and IEP remained unprofitable across most scenarios, with IEP showing extreme sensitivity to raw material price fluctuations. This assessment provides crucial insights for decision-making in the growing biodiesel industry, emphasizing the need for balancing economic viability with sustainability and adaptability in glycerol purification technologies.
Sustainability spotlightOur work on the techno-economic assessment of biodiesel-derived crude glycerol purification processes addresses a critical aspect for defossilizing chemical industries. As the world transitions away from fossil fuels, the biodiesel industry has emerged as a key player in providing renewable energy sources. However, the sustainability of this industry hinges not only on its primary product but also on the efficient utilization of its by-product, particularly crude glycerol. Crude glycerol, constituting about 10% of biodiesel production, represents a significant opportunity for the chemical industry to reduce its dependence on fossil-based feedstocks. By developing economically viable and environmentally friendly purification processes, we can transform this by-product into a valuable, renewable feedstock for various chemical industries, thereby closing the loop in biodiesel production and contributing to a circular economy. Our study compares three advanced purification technologies – membrane separation, vacuum distillation, and ion exchange purification – providing crucial insights into their technical performance, economic viability, and environmental impact. This comprehensive analysis is essential for decision-makers in the chemical industry as they seek to replace fossil-based raw materials with renewable alternatives. By identifying the most efficient and sustainable purification method, our work contributes to the broader goal of defossilizing chemical industries. It paves the way for increased utilization of bio-based glycerol in various applications, from pharmaceuticals to polymers, thus reducing the industry's carbon footprint and advancing responsible production practices aligned with UN Sustainable Development Goals 12 and 13. |
1. Introduction
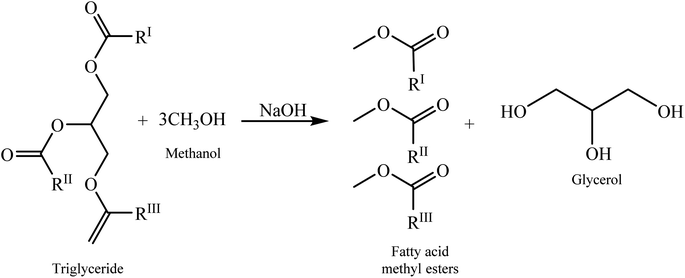
Global population growth is projected to increase energy demand, particularly for transportation fuels. Concurrently, anthropogenic climate change, driven by greenhouse gas emissions from fossil fuel combustion, presents a significant environmental challenge. This process releases carbon sequestered in geological reservoirs into the atmosphere, altering the global carbon cycle. Biofuels have gained prominence as potential sustainable alternatives to conventional fossil fuels, with projections indicating their increasing importance in the coming decades.1 As they are derived from biomass, biofuels theoretically maintain a neutral carbon balance, as the carbon dioxide released during combustion is offset by that absorbed during plant growth. Among biofuels, biodiesel and bioethanol are the most extensively researched. Biodiesel, synthesized from lipid feedstocks, is categorized into three types based on source materials. First-generation biodiesel is derived from edible vegetable oils such as palm and rapeseed, which compete with food production.2,3 Second-generation biodiesel utilizes waste cooking oils and animal fats4 whereas third-generation biodiesel uses on microalgae.5Biodiesel is primarily produced by the transesterification of triglycerides from vegetable oils or animal fats with methanol, usually catalysed by an alkali as shown in Fig. 1. This process yields approximately 10% (w/w) crude glycerol as a by-product.6 Crude glycerol typically contains impurities such as water, inorganic salts (predominantly potassium and sodium compounds), and organic non-glycerol matter (MONG).6 Components included in MONG are free fatty acids (FFAs), fatty acid methyl esters (FAMEs), partial glycerides (mono-, di-, and triglycerides), residual methanol, and saponified fatty acids. The impurity composition of crude glycerol varies significantly depending on the feedstock and production process.7 Second-generation feedstocks, derived from waste, generally result in higher impurity levels in the crude glycerol. The industry trend towards these inexpensive, abundant, and sustainable feedstocks has led to an excess supply of highly impure crude glycerol over the past decade. Currently, this low-value by-product is often incinerated8 or landfilled.9 However, purification processes can enhance its economic value and improve the overall viability of biodiesel production. Furthermore, crude glycerol serves as a potential feedstock for various value-added chemicals, including acrolein, acrylic acid,10 glycerol carbonate, solketal, esters, ethers, 1,2- and 1,3-propanediol, epichlorohydrin, and lactic acid.11,12 These valorisation strategies offer promising opportunities for improving the economics and sustainability of the biodiesel industry.
Glycerol purification to achieve technical-grade levels (>95% w/w) typically involves a series of physicochemical treatments followed by advanced purification techniques.6 The initial physicochemical pre-treatment steps are essential to protect downstream purification processes from potential damage.13 The physicochemical treatment generally comprises several steps as shown in Fig. 2. Saponification converts residual matter organic non-glycerol (MONG) components, such as glycerides and fatty acids, into fatty acid salts (soaps) and glycerol. Acidification then converts the soap bulk to insoluble protonated free fatty acids (FFAs), which form a separate layer. Phase separation results in the formation of two or three distinct layers – an upper FFA layer, a middle glycerol-rich layer, and occasionally, a bottom layer of inorganic salts. The glycerol-rich layer undergoes solvent extraction to remove residual FFAs, followed by neutralization to yield an enriched glycerol fraction. Following these physicochemical processes, the glycerol purity typically reaches 80–85% w/w. To attain technical-grade purity (>95% w/w) for specific applications, advanced purification technologies can be employed such as vacuum distillation, ion exchange treatment, and membrane separation.14 These advanced techniques allow for the removal of remaining impurities and the production of high-purity glycerol suitable for various industrial applications.
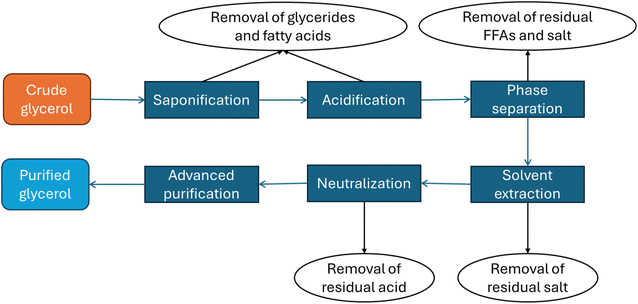 | ||
| Fig. 2 Schematic representation of the physicochemical treatment steps in crude glycerol purification process. | ||
Distillation is typically unsuitable for thermally labile compounds like glycerol, which are susceptible to degradation or polymerization at elevated temperatures. At higher temperatures, glycerol can undergo dehydration to form acrolein or oxidation to produce compounds such as dihydroxyacetone and glyceraldehyde.15 To mitigate these issues, vacuum distillation is employed, reducing the operating temperature and minimizing undesirable side reactions.16 Vacuum distillation is a well-established technology applicable across various scales of continuous operation, from small to large. This process requires minimal pretreatment and can achieve high purity levels in the final glycerol product.17 However, it is important to note that the distillation of crude glycerol is an energy-intensive process. In a study by Yong et al.,18 the purification of crude glycerol via simple vacuum distillation at 120–126 °C yielded approximately 141 g of glycerol per kg of glycerol residue, corresponding to a 14% yield. The resulting product achieved a purity of 96.6% glycerol.
Ion-exchange resins are primarily employed for the removal of low concentrations of salts from aqueous solutions.19 In this process, cations and anions present in the crude glycerol solution are exchanged with ionic species in the resin, resulting in the formation of water. The ion-exchange process is characterized by its low energy intensity and the ability to regenerate the resins, making it potentially cost-effective. However, the economic viability of this method is compromised when the salt content of the glycerol solution exceeds 10 wt%, due to the increased chemical regeneration costs associated with higher salt concentrations.19 Abdul Raman et al.20 demonstrated the efficacy of ion-exchange as a purification method for crude glycerol. In their study, they employed a pre-treatment step involving acidification followed by ion-exchange resin treatment. This process successfully increased the glycerol purity from an initial 35.6 wt% to 98.20 wt%. The researchers utilized a cation exchange H+ resin (Amberlyst 15) and determined the optimal operating conditions to be 40 g of resin, a flow rate of 15 mL min−1, and 60% solvent concentration.
Membrane technology represents an emerging approach in crude glycerol purification, offering several advantages over conventional methods.21 These systems exhibit lower energy requirements compared to vacuum distillation and can effectively handle high salt concentrations, unlike ion exchange processes.22 The reduced energy consumption and lower capital expenditure make membrane separation an attractive option for small and medium-sized plants.23 However, membrane fouling remains a significant challenge, potentially reducing the effective filtration area and compromising performance.24 To mitigate this issue, similar to ion exchange process, a pre-treatment step is typically required to minimize the matter organic non-glycerol (MONG) content in the feed stream. Chol et al.13 investigated membrane separation as a secondary purification step following physico-chemical treatment. Their study employed ceramic membranes composed of ZrO2–TiO2 with a TiO2 support, featuring a molecular weight cut-off (MWCO). The filtration was conducted in a crossflow, semi-continuous mode. The researchers also performed a techno-economic analysis of the process, reporting a unit cost for crude glycerol purification of 50.85 USD per kg and a corresponding revenue of 80.36 USD per kg.
In a separate study, Attarbachi et al.25 employed a series of physicochemical steps combined with activated carbon adsorption to achieve 85% glycerol purity with up to 71% recovery. Their techno-economic analysis indicated a production cost of €19.2 per tonne for the purified glycerol. Arora et al.26 conducted a techno-economic study on membrane purification, concluding that the processes were economically viable when the selling price of purified glycerol was $1.98 per kg or higher. Despite significant research on crude glycerol purification, there remains a gap in comparative techno-economic assessments of different purification technologies. The present work aims to address this gap by evaluating and comparing three distinct routes for crude glycerol purification. This study encompasses comprehensive process simulation, including mass and energy flow analyses, equipment sizing, capital investment estimation, operating cost analysis, and market pricing potential. The primary objective of this research work is to determine the economic viability of crude glycerol purification processes.
2. Methodology
2.1. Process design
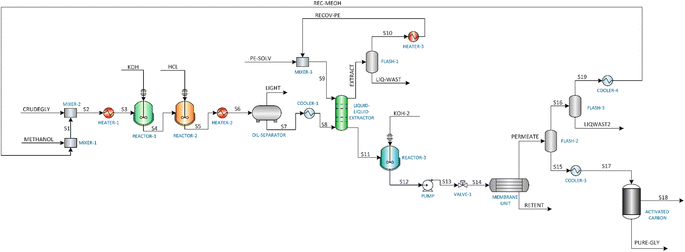
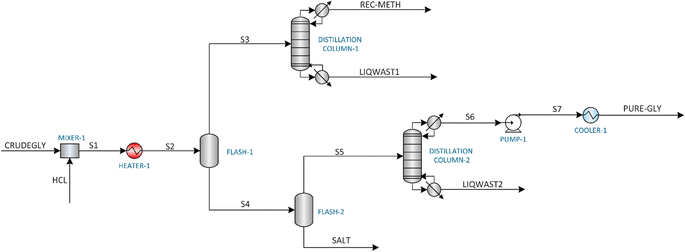
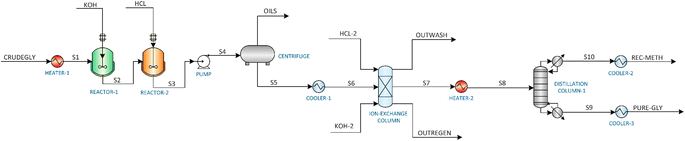
This study investigates three distinct glycerol purification processes: (1) Membrane Purification (MBP) process, (2) Vacuum Distillation Purification (VDP) process, and (3) Ion Exchange Purification (IEP) process. The process simulations were carried out using Aspen Plus V12.1. Given the polar nature of the components involved in the glycerol purification process, the non-random two-liquid (NRTL) model was selected in Aspen Plus to accurately simulate the system. This thermodynamic model was combined with the UNIFAC-LL method to estimate the missing coefficients of the components. For certain unit operations not readily available in Aspen Plus, such as the oil separator, decanting centrifuge, and ion exchange column, the SuperPro Designer software was employed. The results from these SuperPro Designer units were then integrated into the Aspen Plus simulation using a ‘separator2’ unit, which acted as a black box. The integration of Aspen Plus and SuperPro Designer enabled a comprehensive modelling approach, leveraging the strengths of both software platforms to simulate the glycerol purification processes accurately and effectively. The three purification processes were simulated assuming continuous operation of 7920 hours per year, with the target purification capacity of the plant to be approximately 1000 kg h−1. The composition of crude glycerol used in this study is as follows: glycerol (40 wt%), methanol (30 wt%), methyl oleate (10 wt%), oleic acid (13.3 wt%), water (5 wt%) and KOH (1.8 wt%).2.2. Assumptions
The main assumptions made to compute the mass and energy balances are outlined here. Component data and properties were obtained from databases of the mentioned tools, with appropriate substitutions or estimations made for unavailable data. Equipment sizing was performed using established engineering principles and correlations, with complex units estimated based on flow rate ratios and available data. Initial equipment costs were adapted to the current size and updated using the chemical engineering cost index for accurate economic analysis.2.3. Economic analysis
The total capital expenditure (CAPEX) was calculated using the factorial method. The capital and operating costs, along with their calculation methods, are provided in the Tables S1 and S2 of the ESI.† Equipment and installation costs are based on 2010 prices, adjusted for inflation using the Chemical Engineering Plant Cost Index (CEPCI) and a location factor of 1.21 for the United Kingdom. Details on equipment sizing are given in Tables S5–S7 of the ESI.† Based on the UK market, the prices of raw materials, utilities, and the product (purified glycerol) are given Table 1.| Value | References | |
|---|---|---|
| Raw material | ||
| Crude glycerol | −165 USD per tonne | 25 |
| Methanol | 380 USD per tonne | 27 |
| Pentane | 1630 USD per tonne | 28 |
| HCl | 138 USD per tonne | 29 |
| KOH | 1220 USD per tonne | 30 |
| Activated carbon | 500 USD per tonne | 31 |
| Purified glycerol | 900 USD per tonne | 32 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Utilities | ||
| Power electricity | 0.2 USD per kW h | 33 |
| Cooling water | 0.1 USD per tonne | 33 |
| LP steam | 6 USD per tonne | 33 |
| MP steam | 8 USD per tonne | 33 |
| HP steam | 10 USD per tonne | 33 |
2.4. Key performance indicators
The key performance indicators (KPIs) used to compare the glycerol purification processes include the purity of the final product, energy consumption (electricity and heat), and environmental impact. These factors enable a comprehensive technical and environmental assessment across the examined processes, facilitating a fair comparative evaluation of their performance.3. Process description
3.1. Membrane purification (MBP) process
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio from the literature for removing the brown colour and the final impurities in the treated glycerol.36
10 ratio from the literature for removing the brown colour and the final impurities in the treated glycerol.36
3.2. Vacuum distillation purification (VDP) process
3.3. Ion exchange purification (IEP) process
4. Results and discussions
4.1. Technical comparison
Table 2 shows the comparison of the final glycerol purity achieved by each process which reveals significant differences in their purification efficacy. The ion exchange purification process demonstrated superior performance, achieving a final glycerol purity of 98.7%. This high purity level was attributable to the selective nature of ion exchange resins, which can effectively remove ionic impurities from the crude glycerol as well as the usage of centrifuge in the process. The industrial standard vacuum distillation process followed with a purity of 96.91%, leveraging the differences in boiling points between glycerol and its impurities to achieve separation. The membrane separation process (MBP) yielded the lowest purity among the three at 93.9%, which, while still substantial, indicates limitations in the membrane's selectivity. Analysing the glycerol recovery rates provides insight into the process efficiency and potential economic viability. IEP again shows a greater performance with 99.0% recovery rate, suggesting least glycerol loss during purification. VDP with a 94.99% recovery rate, shows a slight reduction in recovery compared to IEP which was be due to glycerol entrainment in the top stream of FLASH-1 and bottom product of the DISTILLATION COLUMN-2. MBP showed the lowest recovery at 86.20% and most of this loss was caused by unit operations performed by LIQUID–LIQUID EXTRACTOR and FLASH-2.| Process | Crude glycerol flow rate (kg h−1) | Purified glycerol flow rate (kg h−1) | Final glycerol purity (%) | Glycerol recovery (%) |
|---|---|---|---|---|
| MBP | 1000.00 | 367.12 | 93.92 | 86.20 |
| VDP | 1000.00 | 392.08 | 96.91 | 94.99 |
| IEP | 1000.00 | 401.03 | 98.75 | 99.00 |
The disparity in both purity and recovery rates among these processes highlights the importance of considering both the factors while choosing a process. IEP's superior performance in both metrics suggests it may be the most promising for industrial applications where high purity and minimal product loss are crucial. However, this technical assessment should be balanced against other factors using KPIs. Fig. 6 shows significant differences in the resource consumption and waste generation of the three crude glycerol purification processes. IEP showed substantially higher raw material consumption (RM = 81.74) compared to MBP (RM = 0.49) and VDP (RM = 0.12). This stark difference suggested that IEP required significant amounts of chemicals needed for regeneration of cationic and anionic resins (5% HCl solution and 6% KOH solution), which could impact its economic viability and sustainability. Due to the least amount of pretreatment required in the VDP process, it had the lowest raw material needs followed by MBP. Only MBP process required solvent because the liquid–liquid extraction unit operation, while VDP and IEP showed no solvent needs. This unique requirement for MBP introduced additional costs as solvent recovery and associated potential emissions. When comparing liquid waste generated by the processes, IEP produces significantly more liquid waste (LW = 82.42) compared to MBP (LW = 2.42) and VDP (LW = 1.77). This substantial difference in waste generation for IEP was attributed to the waste generated after regeneration and washing of ion exchange resins, which required large volumes of regenerant solutions due to high amount of salt in the feed. The lower waste generation from MBP and VDP suggests they may be more environmentally favourable in terms of waste management.
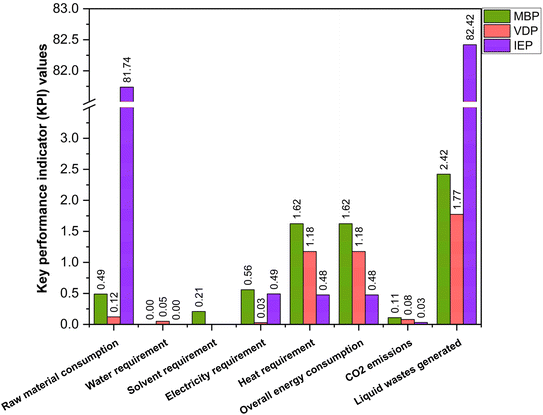 | ||
| Fig. 6 Key performance indicators (per kg of purified glycerol) for the three crude glycerol purification processes. | ||
Fig. 7 shows the hot and cold utility requirement of the three glycerol purification processes and exhibit distinct patterns across various energy forms. MBP had the highest total steam consumption (MP steam: 534.24 kW and LP steam: 61.74 kW), and most of this substantial steam requirement was coming from HEATER-2 which was used to heat the saponified and acidified crude glycerol mixture before flowing it to the oil separator. VDP relied heavily on HP steam (368.73 kW) and LP steam (92.36 kW), totaling 461.09 kW which were mainly needed by the reboilers and flash drums. IEP shows the lowest total steam consumption (190.78 kW) as most of the operations were operating at lower temperatures compared to vacuum distillation or membrane processes. MBP exhibits the highest cooling demand (881.36 kW), and this substantial cooling requirement was because of the COOLER-1 which reduced the temperature of the stream coming from the oil separator before flowing to the liquid–liquid extractor. VDP also shows substantial cooling requirement (417.90 kW), which were required by condensers operating in the distillation columns. IEP demonstrates the lowest cooling requirement (284.57 kW) requirements, mainly for temperature reduction of pretreated crude glycerol before flowing it to the ion exchange column and condenser of the DISTILLATION COLUMN-1 used to separate methanol and glycerol. Electricity usage, which is mainly done by pumps, was relatively low across all processes, with MBP consuming the most (0.206 kW), followed closely by IEP (0.197 kW), and VDP using significantly less (0.012 kW). This analysis reveals that MBP is the most energy-intensive process, consuming approximately 1.68 times more energy than VDP and 3.11 times more than IEP.
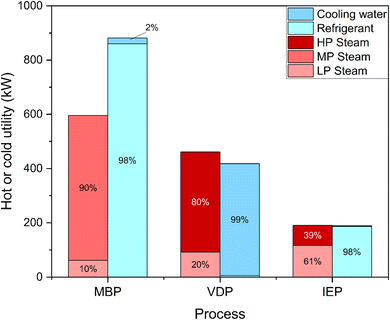 | ||
| Fig. 7 Hot and cold utility requirements for the three crude glycerol purification processes in the form of HP steam, MP steam, LP steam, refrigerant and cooling water. | ||
When comparing CO2 emissions corresponding to heaters and reboilers, MBP shows the highest CO2 emissions (0.11), followed by VDP (0.08) and IEP (0.03). Greater energy demands MBP and VDP reflect on their higher CO2 emissions. In conclusion, VDP appeared to be the most efficient in terms of raw material and solvent usage, with moderate water requirements and CO2 emissions. MBP showed low water needs but required solvents and had the highest CO2 emissions. IEP, while having the lowest CO2 emissions, was characterized by extremely high raw material consumption and liquid waste generation.
4.2. Economic assessment
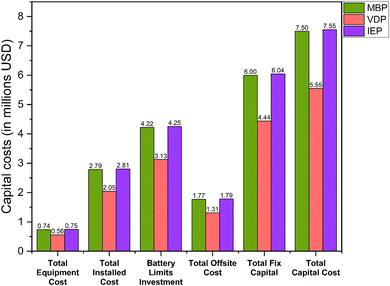
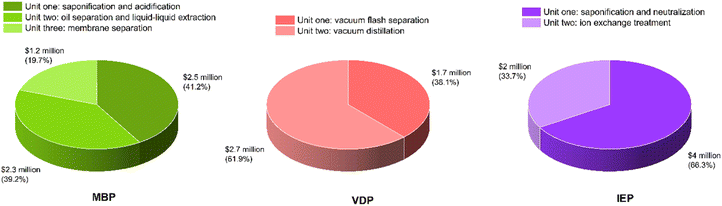
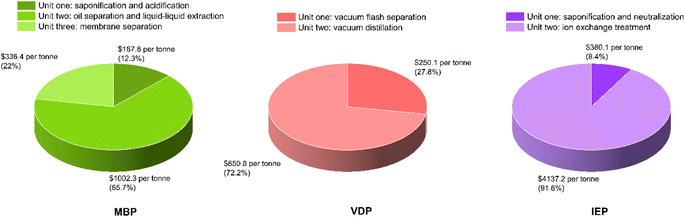
Fig. 8 shows the capital expenditure (CAPEX) analysis for the three glycerol purification processes (MBP, VDP and IEP). IEP showed the highest equipment cost (0.75 MUSD), closely followed by MBP (0.74 MUSD), while VDP had the lowest (0.56 MUSD). The similarity between IEP and MBP costs came from the similar equipment needed for the pretreatment of crude glycerol. Moreover, VDP's lower equipment cost was expected as there is little pretreatment requirement and hence, equipment is not needed. The total fixed capital (TFC), which represented the sum of installed costs, battery limits investment, and offsite costs, shows IEP as the most capital-intensive (6.04 MUSD), closely followed by MBP (6.00 MUSD), with VDP significantly lower (4.44 MUSD). VDP stood out as significantly less capital-intensive across all categories, requiring approximately 26% less total capital investment compared to the other two processes. These capital cost considerations must be balanced against operational expenses to determine the most economically viable process for glycerol purification.Fig. 9 shows the unit-wise capital cost analysis for the three glycerol purification processes where distinct cost distributions for each technology can be seen. MBP demonstrated a relatively balanced allocation of capital across its three units. The saponification and acidification unit required the highest investment at 2.47 MUSD, which was due to the need for reactor vessels for the pretreatment of FAMEs and soaps. The oil separation and liquid–liquid extraction unit followed closely at 2.35 MUSD, indicating substantial investment for this separation equipment. Interestingly, the membrane separation unit itself, despite being the core technology, had the lowest capital cost at 1.18 MUSD. VDP showed a clear emphasis on the vacuum distillation unit, which accounted for 2.75 MUSD, or about 62% of the total capital expenditure which aligned with the complexity and energy-intensive nature of vacuum distillation processes. The vacuum flash separation unit, while still substantial at 1.69 MUSD, required less capital, was serving as an initial separation step. IEP demonstrated the highest single-unit capital cost among all processes, with the saponification, neutralization and centrifuge unit requiring 4.00 MUSD. The ion exchange treatment unit, while less capital-intensive at 2.04 MUSD, still represented a significant investment, due to the cost of ion exchange column systems.
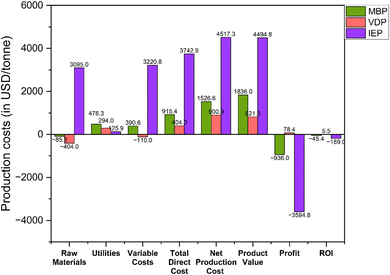
Fig. 10 shows the production costs analysis associated with the three glycerol purification processes. Raw materials costs showed a striking disparity among the three processes. IEP demonstrated an extraordinarily high raw material cost at 3094.96 USD per tonne compared to MDP and VDP, which was mainly due to huge amount of regeneration chemicals required for ion exchange resins. Argent Energy spends around 165 GBP per tonne to dispose the waste crude glycerol feedstock, hence, the cost of this raw material was considered negative while performing calculations.25 Utilities costs were the highest for MBP (476.26 USD per tonne), followed by VDP (294.01 USD per tonne), and lowest for IEP (125.89 USD per tonne). This aligned with the earlier energy consumption data, where MBP showed higher energy requirements, particularly in refrigeration and steam usage. Profits calculations showed that only VDP is profitable with the profit value of 78.38 USD per tonne. Both MBP and IEP show significant losses, with IEP demonstrating the largest loss (−3594.75 USD per tonne), followed by MBP (−936.01 USD per tonne). Producing the highest-purity product and having the highest recovery process, IEP's extremely high raw material and production costs rendered it economically unfeasible under these conditions. MBP, while less loss-making than IEP, still fails to achieve profitability.
Fig. 11 shows the unit-wise operating expenditure analysis for the three glycerol purification processes and provides crucial insights into the operational costs associated with each unit of these processes. For the MBP process, unit one, involving saponification and acidification, had the lowest operating expenditure at 187.84 USD per tonne suggesting that the initial steps of physicochemical treatment of crude glycerol was relatively cost-effective. Unit two, which included oil separation and liquid–liquid extraction, showed the highest operational cost at 1002.34 USD per tonne which was due to usage of solvents and energy requirements by HEATER-1 and COOLER-1. In the VDP process, out of the two units, the vacuum distillation unit (unit two) showed a significantly higher operating cost (650.80 USD per tonne) as expected which was due to the energy-intensive nature of vacuum distillation, including steam generation, cooling water circulation, and maintaining vacuum conditions. The two units in the IEP process demonstrated the most striking cost disparity. The saponification and neutralization unit (unit one) operated at a relatively moderate cost of 380.09 USD per tonne, comparable to some units in the other processes. However, the ion exchange treatment unit (unit two) exhibited an exceptionally high operating cost of 4137.19 USD per tonne. This enormous expense was attributed to the regeneration chemicals required for the ion exchange process. Overall, the unit wise operating expenditures across the processes can show valuable information to provide direction to put efforts for the cost reduction strategies. For instance, efforts to reduce costs in IEP should primarily focus on the ion exchange unit, possibly by exploring more cost-effective resins or optimizing regeneration cycles. In MBP, improving the efficiency of the oil separation and liquid–liquid extraction step could yield significant cost savings.
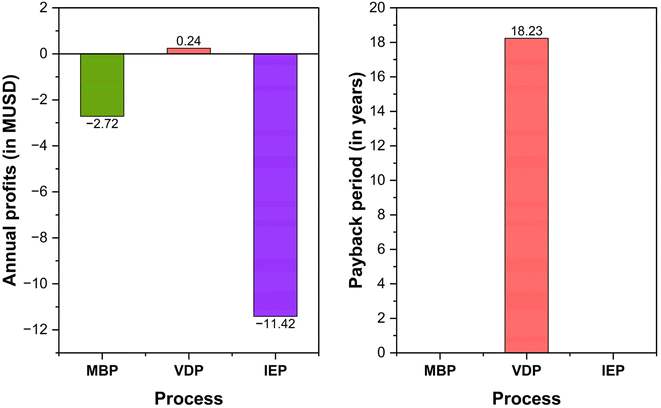
Fig. 12 shows the economic viability of the three glycerol purification processes by analysing their annual profits and payback periods. VDP is the only process showing positive annual profits and a positive payback period of 18.23 years. MBP is operating at a loss of 2.72 MUSD annually whereas IEP shows the worst economic performance with an annual loss of 11.42 MUSD. However, the long payback period of VDP suggests that it may still not be an attractive investment unless there are prospects for improving profitability or reducing initial costs. These findings showed the importance of cost reduction strategies, and potentially exploring hybrid technologies (combination of the three) to glycerol purification to improve the economic outlook of these processes.
4.3. Sensitivity analysis
The techno-economic assessment of glycerol purification processes required a comprehensive understanding of how various economic factors influence their viability. To achieve this, a sensitivity analysis was conducted by changing the three key parameters: raw material prices (−75% to 75%), utility costs (−75% to 75%), and product (purified glycerol) prices (−75% to 75%). These parameters were selected due to their significant impact on operational costs and thereby affecting the profitability. By examining how changes in these parameters affect the annual profits and payback periods of three processes, valuable insights into their economic resilience and opportunities for optimization can be obtained. Fig. 13 illustrates the impact of changes in utilities prices on the annual profits and payback periods for three glycerol purification processes.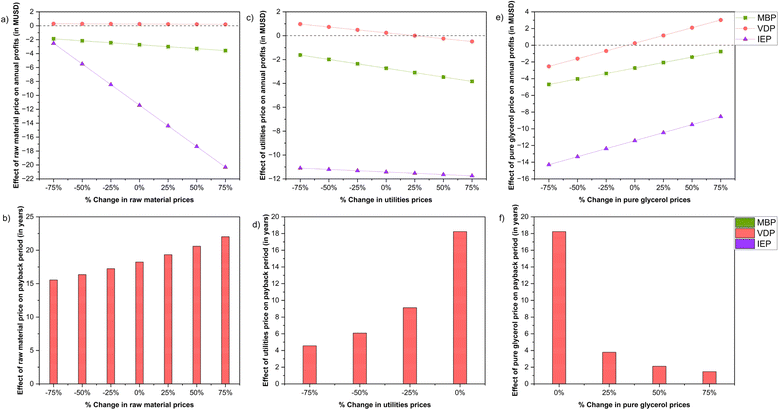 | ||
| Fig. 13 Sensitivity analysis of the effect of change in raw material, utilities and product prices for the three crude glycerol purification processes. | ||
Annual profits remained negative across all raw material price changes for the MBP and IEP process. As raw material prices increase, annual losses grew, reaching 3.57 MUSD for MBP and staggering 20.31 MUSD loss for IEP at a 75% price increase. Even with a 75% decrease in raw material prices, MBP and IEP still had a considerable loss. VDP demonstrated the most stable profitability among the three processes, remaining profitable across all raw material price fluctuations which was expected as this process used the lowest amount of raw materials. Profits range from 0.29 MUSD at a 75% decrease in raw material prices to 0.20 MUSD at a 75% increase. The payback period for VDP increases as raw material prices rise, spanning from 15.56 years at the lowest price point to 22.03 years at the highest (see Fig. 13(a) and (b))
Similar to the change in raw material prices scenarios, the annual profits remained negative across all utility price scenarios. As utility prices increase, the losses increased for MBP, which indicated that MBP is significantly sensitive to utility costs, yet even substantial reductions in these costs are insufficient to bring the process into profitability. IEP exhibited the least sensitivity to utility price changes among the three processes due to less usage of utilities in this process. VDP demonstrated the most favourable response to utility price changes as relatively higher amount of utilities were used in the process. At lower utility prices, VDP showed positive annual profits, peaking at 0.97 MUSD when prices decrease by 75%. The process remains profitable up to a 25% increase in utility prices, breaking even at this point. The payback period for VDP is notably sensitive to utility prices, ranging from 4.56 years at a 75% price decrease to 18.24 years at baseline prices. Overall, for VDP, efforts to reduce utility consumption or negotiate lower utility rates could significantly improve its economic viability (see Fig. 13(c) and (d)).
In the case of increase in product (purified glycerol) prices, there's a clear trend of improving financial performance for all the three processes but the MBP and IEP still remain at loss even after a 75% increase in product price. VDP demonstrated the most favourable response to product price changes. At a 75% decrease in product price, VDP shows a loss of 2.54 MUSD, but it becomes profitable at the baseline price (0.24 MUSD profit). As product prices increase, VDP's profitability improves significantly, reaching 3.02 MUSD at a 75% price increase. The payback period for VDP is only provided for price increases, starting at 18.24 years for the baseline price and improving to 1.47 years at a 75% price increase (see Fig. 13(e) and (f)) The analysis done here suggested across all scenarios examined, VDP consistently emerges as the most economically viable option, demonstrating profitability in favourable conditions.
5. Future perspectives
5.1. Process intensification and optimization opportunities
Process intensification and optimization can be used for improving the efficiency and economic viability of crude glycerol purification processes. For membrane purification, advanced membrane configurations, such as cascade arrangements, could enhance separation efficiency while reducing energy consumption. The implementation of novel membrane materials with enhanced fouling resistance could significantly extend operational lifetime and reduce maintenance requirements.41 Additionally, closed-loop solvent recovery systems could significantly reduce fresh solvent requirements which was implemented in MBP for methanol solvent in the current work. These combined improvements could potentially reduce the current energy requirement and operating costs of the MBP process.In VDP, process intensification would mostly include the heat integration of the process that could minimize heating and cooling utility requirements typically through pinch analysis and heat exchanger networks. Moreover, the integration of renewable energy sources, particularly for heating requirements, could further reduce both operating costs and environmental impact. For instance, solar thermal systems could provide low-temperature heating,42 while biomass-derived steam could support distillation operations.43 After consideration of these enhancements the already favourable economics of the VDP process would improve. For ion exchange purification, process intensification could focus on developing advanced ion exchange materials with higher capacity and selectivity. Such improvements would reduce the frequency of regeneration cycles and decrease chemical consumption, addressing the main economic challenge of the IEP process. The development of continuous ion exchange systems could eliminate the current batch-wise regeneration requirements, leading to more efficient operation.44 Furthermore, optimized regeneration strategies based on real-time monitoring of resin capacity and feed composition could reduce chemical consumption and waste generation. These combined process intensification and optimization strategies could significantly impact the technical and economic performance metrics presented in this study.
5.2. Extended operations and maintenance requirements
For accurate economic assessment of crude glycerol purification processes, understanding long-term operational behaviour and maintenance requirements of each process is important. Starting with membrane purification, extended operations lead to progressive membrane fouling, necessitating regular cleaning cycles every 2–3 months. Each cleaning cycle requires approximately 24–48 hours of downtime and incurs costs related to cleaning chemicals and labour. Complete membrane replacement is typically required every 2–20 years depending on the materials, representing approximately 15–20% of the initial membrane capital cost.45 Vacuum distillation systems are generally more robust and have long-term performance but require periodic maintenance to maintain vacuum integrity and heat exchanger efficiency. Major maintenance operations are typically scheduled every 5 years, with annual maintenance costs representing approximately 4–5% of capital cost. Change-over operations for cleaning and maintenance require 36–48 hours of downtime, during which the entire system must be cooled, cleaned, and restarted.46 This downtime can be factored into annual production planning and economic calculations for an accurate economic assessment. Ion exchange systems face the most frequent operational interruptions due to the need for regular resin regeneration. The regeneration frequency depends on feed impurity levels and desired product purity, but typically occurs every 12–24 hours. While individual regeneration cycles are relatively short, the cumulative downtime significantly impacts annual production capacity. Resin replacement is necessary every 3–4 years, with replacement costs approximately 25–30% of the initial resin cost.44 These operational considerations particularly affect the IEP process economics.6. Conclusions
This study carried out the techno-economic assessment of three glycerol purification processes – membrane separation (MBP), vacuum distillation (VDP), and ion exchange purification (IEP). In terms of product purity and process efficiency, IEP demonstrated the highest glycerol purity (98.75%) and recovery rate (99.00%), followed by VDP (96.91% purity, 94.99% recovery) and MBP (93.92% purity, 86.20% recovery). These results highlighted IEP's superior performance in producing high-quality glycerol, albeit at a higher economic cost. The analysis of resource utilization and waste generation showed marked differences among the processes. IEP exhibited the highest raw material consumption (RM: 81.74) and liquid waste generation (82.42), significantly exceeding MBP (LW: 0.49 and 2.42 respectively) and VDP (LW: 0.12 and 1.77 respectively). However, VDP showed the highest water requirement compared to IEP and MBP. MBP was the only process requiring solvent (SR: 0.21 units), adding to its operating cost. In terms of CO2 emissions related to heat requirements, MBP had the highest emissions (CE: 0.11), followed by VDP (CE: 0.08) and IEP (CE: 0.03).Regarding economic aspects, VDP had the lowest fixed capital cost (4.44 MUSD), compared to MBP (6.00 MUSD) and IEP (6.04 MUSD). The operating costs analysis revealed VDP as the only profitable process (0.24 MUSD per year) under current conditions with, while both MBP and IEP operated at a loss. IEP showed exceptionally high operating costs, primarily due to its substantial raw material requirements needed by ion exchange column.
The sensitivity analysis, examining the impact of changes in raw material prices, utility costs, and product prices, consistently identified VDP as the most economically resilient process. It demonstrated the ability to maintain profitability under various market conditions and showed the most favourable response to positive price changes. MBP and IEP remained unprofitable across most scenarios, with IEP showing extreme sensitivity to raw material price fluctuations.
Despite these findings, several technological gaps and areas requiring further research were identified. For MBP, developing more durable membranes not requiring initial physicochemical treatment to improve separation efficiency and reduce fouling can make the process profitable as then it would require the extra raw materials, utilities or equipment. Across all processes, exploring hybrid systems that combine the strengths of multiple purification methods could lead to more robust and versatile solutions. Future advancements should focus on improving energy efficiency, reducing operational costs, enhancing product quality, and minimizing environmental impact. As the biodiesel industry evolves, the ideal glycerol purification process will need to balance economic viability with sustainability, scalability, and adaptability to changing market conditions. This study provides a foundation for future research and development efforts in glycerol purification, contributing to the ongoing advancement of the biorefinery concept and the broader goal of sustainable industrial processes.
Abbreviations
| MBP | Membrane purification process |
| VDP | Vacuum distillation purification process |
| IEP | Ion exchange purification process |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| HCl | Hydrochloric acid |
| H2SO4 | Sulfuric acid |
| KOH | Potassium hydroxide |
| KCl | Potassium chloride |
| RF | Reflux ratio |
| NaOH | Sodium hydroxide |
| HP, MP, and LP | High pressure, medium pressure, and low pressure |
| AC | Activated carbon |
| ASPEN | Advanced system for process engineering |
| NRTL | Non-random two-liquid |
| UNIFAC-LL | Universal quasi-chemical functional group activity coefficients for liquid–liquid equilibrium |
| GR | Glycerol recovery |
| RM | Raw material consumption |
| WR | Water requirement |
| SR | Solvent requirement |
| ER | Electricity requirement |
| HR | Heat requirement |
| OER | Overall energy consumption |
| CE | CO2 emissions |
| LW | Liquid wastes generated |
| GP | Final glycerol purity |
Symbols
| m CG | Mass flow rate of crude glycerol (kg h−1) |
| m PG | Mass flow rate of purified glycerol (kg h−1) |
| m RM | Mass flowrate of raw materials (kg h−1) |
| m WR | Mass flowrate of water required (kg h−1) |
| m SR | Mass flowrate of solvent (kg h−1) |
| e ER | Electricity consumption in the process (kW) |
| e HR | Energy consumption in the form of heat (kW) |
| e OER | Overall energy consumption in the process (kW) |
| m CE | Mass flowrate of CO2 generated from utilities (kg h−1) |
| m LW | Mass flowrate of liquid wastes generated (kg h−1) |
| mfPG | Mass fraction of glycerol in purified glycerol stream |
Data availability
The data that supports the findings of this study are available within the article and the ESI.†Author contributions
Yash Bansod: conceptualization, data curation, formal analysis, writing – original draft. Kamran Ghasemzadeh: data curation, formal analysis, writing – review & editing. Carmine D'Agostino: funding acquisition, investigation, supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge the EPSRC grant no. EP/V026089/1 for funding this work.References
- OECD/FAO Agricultural Outlook 2024-2033, OECD Publishing, 2024, DOI:10.1787/4c5d2cfb-en.
- H. W. Tan, A. R. Abdul Aziz and M. K. Aroua, Renewable Sustainable Energy Rev., 2013, 27, 118–127 CrossRef CAS
.
- S. P. Singh and D. Singh, Renewable Sustainable Energy Rev., 2010, 14, 200–216 Search PubMed
.
- X. Lang, A. K. Dalai, N. N. Bakhshi, M. J. Reaney and P. B. Hertz, Bioresour. Technol., 2001, 80, 53–62 CAS
.
- N. Moazami, R. Ranjbar, A. Ashori, M. Tangestani and A. S. Nejad, Biomass Bioenergy, 2011, 35, 1935–1939 Search PubMed
.
- T. Attarbachi, M. D. Kingsley and V. Spallina, Fuel, 2023, 340, 127485 Search PubMed
.
- Y. Bansod, B. Crabbe, L. Forster, K. Ghasemzadeh and C. D'Agostino, J. Cleaner Prod., 2024, 437, 140485 Search PubMed
.
- P. Anand and R. K. Saxena, New Biotechnol., 2012, 29, 199–205 CAS
.
- F. D. Pitt, A. M. Domingos and A. A. C. Barros, S. Afr. J. Chem. Eng., 2019, 29, 42–51 Search PubMed
.
- Y. Bansod, P. Pawanipagar, K. Ghasemzadeh and C. D'Agostino, Green Chem., 2024, 26, 9840–9858 CAS
.
- U. C. Abubakar, Y. Bansod, L. Forster, V. Spallina and C. D'Agostino, React. Chem. Eng., 2023, 8, 1819–1838 RSC
.
- A. Sandid, V. Spallina and J. Esteban, Fuel Process. Technol., 2024, 253, 108008 CrossRef CAS
.
- C. G. Chol, R. Dhabhai, A. K. Dalai and M. Reaney, Fuel Process. Technol., 2018, 178, 78–87 Search PubMed
.
- M. S. Ardi, M. K. Aroua and N. A. Hashim, Renewable Sustainable Energy Rev., 2015, 42, 1164–1173 CrossRef CAS
.
-
T. Surrod and C. Pattamaprom, in The Second TSME International Conference on Mechanical Engineering, Krabi, Thailand, 2011 Search PubMed
.
-
R. D. Noble and P. A. Terry, Principles of Chemical Separations with Environmental Applications, Cambridge University Press, Cambridge, 2004 Search PubMed
.
-
J. Van Gerpen, B. Shanks, R. Pruszko, D. Clements and G. Knothe, Biodiesel Analytical Methods: August 2002–January 2004, National Renewable Energy Lab. (NREL), Golden, CO (United States), 2004 Search PubMed
.
- K. C. Yong, T. L. Ooi, K. Dzulkefly, W. M. Z. Wan Yunus and A. H. Hazimah, J. Oil Palm Res., 2001, 13, 39–44 CAS
.
- M. Carmona, A. Lech, A. de Lucas, A. Perez and J. F. Rodriguez, J. Chem. Technol. Biotechnol., 2009, 84, 1130–1135 CrossRef CAS
.
- A. A. Abdul Raman, H. W. Tan and A. Buthiyappan, Front. Chem., 2019, 7, 439904 Search PubMed
.
- R. Govindaraju, S. S. Chen, L. P. Wang, H. M. Chang and M. Pasawan, Curr. Pollut. Rep., 2021, 7, 128–145 CrossRef CAS
.
- N. Sdrula, Desalination, 2010, 250, 1070–1072 Search PubMed
.
- M. Jafari, V. Spallina and K. Ghasemzadeh, Energy Convers. Manage., 2024, 315, 118796 CrossRef CAS
.
- M. A. Indok Nurul Hasyimah, A. W. Mohammad and M. Markom, Ind. Eng. Chem. Res., 2011, 50, 7520–7526 Search PubMed
.
- T. Attarbachi, M. Kingsley and V. Spallina, Ind. Eng. Chem. Res., 2024, 63, 4905–4917 CrossRef CAS PubMed
.
- P. Arora, C. Baroi and A. K. Dalai, J. Basic Appl. Eng. Res., 2015, 2, 60–63 Search PubMed
.
- Methanex Methanol Price Sheet, https://www.methanex.com/wp-content/uploads/Mx-Price-Sheet-April-25-2024.pdf, accessed 2 September 2024 Search PubMed.
- Pentane Prices News Monitor Market Analysis & Demand, https://www.chemanalyst.com/Pricing-data/isopentane-1524, accessed 2 September 2024 Search PubMed.
- Hydrochloric Acid Prices, Monitor, Market Analysis & Demand, https://www.chemanalyst.com/Pricing-data/hydrochloric-acid-61, accessed 2 September 2024 Search PubMed.
- Caustic Potash Prices, Monitor, Market Analysis & Demand, https://www.chemanalyst.com/Pricing-data/caustic-potash-1212, accessed 2 September 2024 Search PubMed.
- S. T. Tibor and C. A. Grande, Clean. Eng. Technol., 2022, 7, 100443 Search PubMed
.
- Glycerine Price Trend and Forecast, accessed 2 September, 2024, https://www.chemanalyst.com/Pricing-data/glycerine-1168.
-
D. Brennan, Process Industry Economics: Principles, Concepts and Applications, Elsevier, 2020 Search PubMed
.
- M. Hunsom, P. Saila, P. Chaiyakam and W. Kositnan, Int. J. Renew. Energy Res., 2013, 3, 364–371 Search PubMed
.
- M. S. Ardi, M. K. Aroua and N. A. Hashim, Renewable Sustainable Energy Rev., 2015, 42, 1164–1173 CrossRef CAS
.
- R. Dhabhai, E. Ahmadifeijani, A. K. Dalai and M. Reaney, Sep. Purif. Technol., 2016, 168, 101–106 Search PubMed
.
- M. Oliveira, A. Ramos, E. Monteiro and A. Rouboa, Sustainability, 2022, 14, 1747 CrossRef CAS
.
- W. Isahak, J. M. Jahim, M. Ismail, N. F. Nasir, M. M. Ba-Abbad and M. A. Yarmo, J. Eng. Sci. Technol., 2016, 11, 1056–1072 Search PubMed
.
-
A. Tiwari, H. K. Patra and A. P. F. Turner, Advanced Bioelectronic Materials, John Wiley & Sons, 2015 Search PubMed
.
- Rohm & Haas, Ion Exchange for Dummies, https://www.lenntech.com/Data-sheets/Ion-Exchange-for-Dummies-RH.pdf, accessed 11 September 2024 Search PubMed.
- S. E. Demirel, J. Li and M. M. F. Hasan, Ind. Eng. Chem. Res., 2021, 60, 7197–7217 CrossRef CAS
.
- M. I. Ismail, N. A. Yunus and H. Hashim, Renewable Sustainable Energy Rev., 2021, 147, 111192 CrossRef
.
- M. N. A. S. Ivan, S. Saha, A. M. Saleque, S. Ahmed, A. K. Thakur, G. Bai, Z. Miao, R. Saidur and Y. H. Tsang, Nano Energy, 2024, 120, 109176 CrossRef CAS
.
-
J. P. Chen, L. Yang, W.-J. Ng, L. K. Wang and S.-L. Thong, Advanced Physicochemical Treatment Processes, 2006, pp. 261–292 Search PubMed
.
- M. B. Asif and Z. Zhang, Chem. Eng. J., 2021, 418, 129481 CAS
.
- A. A. Kiss, J. Chem. Technol. Biotechnol., 2014, 89, 479–498 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00599f |
| This journal is © The Royal Society of Chemistry 2025 |