Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green hydrogen production for sustainable development: a critical examination of barriers and strategic opportunities
Juan Gabriel
Segovia-Hernández
 a,
Salvador
Hernández
a,
Enrique
Cossío-Vargas
b,
Maricruz
Juarez-García
a and
Eduardo
Sánchez-Ramírez
a
a,
Salvador
Hernández
a,
Enrique
Cossío-Vargas
b,
Maricruz
Juarez-García
a and
Eduardo
Sánchez-Ramírez
a
aUniversidad de Guanajuato, Campus Guanajuato, División de Ciencias Naturales y Exactas, Departamento de Ingeniería Química, Noria Alta s/n, 36050, Guanajuato, Gto, Mexico. E-mail: gsegovia@ugto.mx
bUniversidad Tecnológica del Suroeste de Guanajuato, Carretera Valle-Huanímaro km 1.2, 38400, Valle de Santiago, Guanajuato, Mexico
First published on 27th November 2024
Abstract
As the world endeavors to meet ambitious climate targets and mitigate carbon emissions, green hydrogen stands out as a versatile and scalable solution offering a viable pathway toward sustainable development. Significant advancements in green hydrogen production have been observed in regions demonstrating robust commitments to integrating renewable energy sources, which serve as pioneering models of the feasibility and potential of integrating green hydrogen into existing energy ecosystems. This paper undertakes a comprehensive analysis of the technical challenges hindering the widespread adoption of green hydrogen production, while highlighting the abundant opportunities associated with this transformative technology. The study aims to scrutinize the underlying technologies, methodologies, and structural complexities associated with green hydrogen production to uncover latent opportunities for achieving global decarbonization goals, particularly aligned with the objectives of the 2030 Agenda and the Sustainable Development Goals (SDGs).
Sustainability spotlightGreen hydrogen stands at the forefront of sustainable development, offering a scalable solution to meet global decarbonization targets. This paper explores the technical challenges and opportunities within green hydrogen production, focusing on renewable-powered electrolysis and thermochemical processes that reduce emissions, enhance energy efficiency, and improve energy security. By integrating carbon capture technologies and innovative storage solutions, green hydrogen supports critical SDGs such as affordable clean energy (SDG 7), industrial innovation (SDG 9), and climate action (SDG 13). The study highlights how these technologies can transform energy systems, contributing to the achievement of the 2030 Agenda and fostering a more sustainable, resilient global energy future. |
1. Introduction
Green hydrogen has emerged as a transformative solution poised to revolutionize the global energy landscape, playing a pivotal role in addressing climate and energy challenges while advancing the objectives of the 2030 Agenda for Sustainable Development and the Sustainable Development Goals (SDGs). As the global community accelerates efforts to transition towards a decarbonized energy system, green hydrogen has gained recognition for its potential to bridge gaps in renewable energy integration, reduce greenhouse gas emissions, and drive innovation across multiple sectors. This paper aims to provide a critical review of the underlying technologies, methodologies, and structural complexities associated with green hydrogen production, emphasizing its alignment with global decarbonization goals and its contributions to sustainable development.The adoption of green hydrogen offers substantial environmental and socio-economic benefits. Its production through renewable energy sources enables the storage of surplus renewable energy, addresses intermittency issues in solar and wind power, and ensures a reliable energy supply. Moreover, hydrogen's applications extend beyond power generation, encompassing sectors such as transportation, industry, and heating, thereby positioning it as a versatile energy carrier with the capacity to significantly reduce carbon emissions. Green hydrogen is also instrumental in decarbonizing hard-to-abate sectors, including steel production, chemical manufacturing, and long-haul transportation. In aviation, for instance, advancements in hydrogen-powered technologies, such as fuel cells and hydrogen combustion engines, promise a path toward carbon-neutral air travel, addressing the sustainability challenges of this sector.1
Under the framework of COP28, 198 nations have endorsed the Dubai Agreement, recognizing the urgent need to rapidly and sustainably reduce greenhouse gas emissions in line with 1.5 °C pathways. The agreement highlights several key initiatives:
• Accelerating the phased reduction of coal-based energy consumption.
• Transitioning global energy systems towards net-zero emissions, utilizing low or zero carbon-emitting fuels by mid-century.
• Phasing out fossil fuels from energy systems in an equitable and orderly manner, accelerating efforts in this decade to achieve net-zero emissions by 2050.
• Advancing the development of zero and low-emission technologies, including renewable energy, nuclear power, and carbon capture and storage technologies, particularly in challenging sectors.
• Significantly reducing global emissions of non-CO2 gases, with a specific focus on methane emissions reduction by 2030.
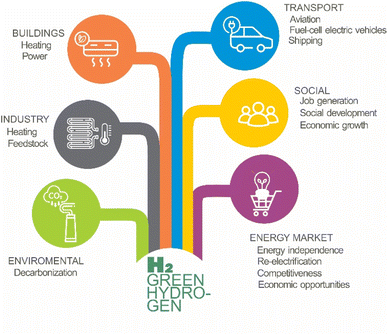
Green hydrogen stands poised as a viable alternative to fulfil the ambitions set forth in the Dubai Agreement, offering a pathway towards a sustainable and low-emission future. Hydrogen, with its high-energy content per unit mass, is a powerful and highly flammable energy carrier. When burned or used in a fuel cell, it releases energy and produces water as a by-product, which represents a significant advantage over conventional fuels. Despite its low solubility in many liquids, including water, the solubility of hydrogen increases with pressure, enhancing its storage potential. Green hydrogen, produced through renewable energy sources, has a wide range of applications2 across various industries, serving as a clean fuel for transportation and a key ingredient in industrial processes (Fig. 1).
In the context of the global energy transition towards sustainable and decarbonized solutions, green hydrogen has emerged as a vital component across the transportation, energy, industry, and heating sectors. Its versatility and potential to significantly reduce carbon emissions position hydrogen as a fundamental element in the future energy matrix. Its role in industrial processes is critical due to its unique properties and the urgent need for cleaner energy alternatives.3
In transportation, hydrogen is utilized in fuel cell electric vehicles (FCEVs), including cars, trucks, and buses. These vehicles generate electricity through a chemical reaction in a fuel cell, emitting only water vapor. Companies like Toyota, Hyundai, and Honda have already launched hydrogen-powered car models, with pilot projects for trucks and buses underway worldwide. The use of hydrogen in transportation is essential for reducing the carbon footprint of this sector, a significant contributor to global greenhouse gas emissions.
Hydrogen also acts as a medium for storing renewable energy. Through electrolysis, surplus solar and wind energy can be converted into hydrogen and later used to generate electricity during periods of high demand or when renewable generation is low. This process helps stabilize the electrical grid and reduces reliance on fossil fuels, addressing the intermittency of renewable energy sources and ensuring a reliable energy supply.4
In the chemical industry, hydrogen is crucial for producing ammonia, used in fertilizers, and in oil refining. It is also used in hydrogenation processes essential for producing chemicals and pharmaceuticals. Transitioning to green hydrogen in these processes can significantly reduce CO2 emissions, making industrial production more sustainable.5
The steel industry is another significant area where hydrogen can make an impact. Traditionally, steel production is carbon-intensive due to the use of coal as a reducing agent. Hydrogen can replace coal in this process, potentially dramatically reducing CO2 emissions. Adopting hydrogen in steel production is vital for decarbonizing this sector and meeting global climate targets.6
In the heating sector, hydrogen can be used in boilers and heating systems for residential, industrial, and commercial applications. Especially in regions where natural gas is widely used for heating, hydrogen can be integrated into existing gas networks with some modifications. Using hydrogen for heating reduces the carbon footprint of this sector and supports the transition to cleaner energy sources.7
Estimating hydrogen demand is challenging, but projections indicate that it will become an indispensable part of the energy system. Various scenarios predict that hydrogen demand will reach approximately 110 million tonnes (Mt) by 2030, with a significant portion produced as clean or low-carbon hydrogen.8–11 Despite variations in projections, a substantial increase in hydrogen demand is expected, potentially doubling by 2050.
The utilization of hydrogen for aviation is an emerging area with significant potential to reduce carbon emissions and enhance sustainability in the aviation sector. Hydrogen can be employed in two primary ways: as a direct fuel in hydrogen combustion engines or through hydrogen fuel cells to power electric propulsion systems. Hydrogen combustion engines can burn hydrogen in modified internal combustion engines or gas turbines, similar to conventional jet fuel, but with adaptations due to its different combustion properties. An example of this is Airbus' ZEROe aircraft concept, which aims to introduce hydrogen-powered commercial aircraft by 2035, including a design with a turbofan engine modified to burn hydrogen.1 On the other hand, hydrogen fuel cells convert hydrogen into electricity through an electrochemical process, powering electric motors to drive the aircraft's propellers. Companies like ZeroAvia and Universal Hydrogen are pioneering in this space. ZeroAvia has successfully conducted test flights of a hydrogen fuel cell-powered six-seater aircraft and is working on scaling up to larger commercial aircraft. Universal hydrogen is developing a modular hydrogen delivery system and partnering with airlines to retrofit existing regional aircraft with hydrogen fuel cell powertrains.2
Hydrogen in aviation offers several benefits. Environmentally, hydrogen combustion produces water vapor, eliminating CO2 emissions, and fuel cells only produce water as a byproduct. Energy efficiency is another advantage, as fuel cells can be more efficient than combustion engines, potentially leading to lower energy consumption for the same amount of thrust. Additionally, electric motors in hydrogen fuel cell systems are quieter than conventional jet engines, reducing noise pollution.3
However, challenges remain. Hydrogen's low energy density by volume compared to traditional jet fuel requires larger storage tanks or high-pressure cryogenic storage, posing technical and logistical issues. Significant investments are needed to develop hydrogen production, storage, and refueling infrastructure at airports. Safety is also a concern, as hydrogen is highly flammable, requiring stringent handling and storage measures.4
Despite these challenges, the future prospects of hydrogen aviation are promising. Advancements in hydrogen production, particularly green hydrogen from renewable energy sources, and improvements in fuel cell technology position hydrogen aviation to play a critical role in achieving carbon-neutral air travel. Companies and governments are investing in research and development, with several pilot projects underway to demonstrate the feasibility and scalability of hydrogen-powered flight. In conclusion, hydrogen for aviation presents a promising path towards more sustainable air travel, with ongoing developments in hydrogen combustion engines and fuel cell technologies paving the way for its future adoption, addressing the challenges of the 2030 agenda.5
The importance of hydrogen in the current industry arises from its unique characteristics and the urgent need for sustainable energy systems. Hydrogen, the most abundant element in the universe, can reduce carbon emissions across various sectors, from transportation to industry and power generation. It can be produced from various domestic sources, improving energy security and resilience. Hydrogen's adaptability to multiple applications, from fuel cells in vehicles to industrial processes and energy storage, supports a comprehensive energy transition. Its production through electrolysis can utilize surplus renewable energy, facilitating the integration of intermittent renewable sources like wind and solar into the energy system, balancing supply and demand, and improving grid stability.12
The rising demand for hydrogen presents numerous opportunities for technological innovation and job creation in sectors related to hydrogen production, storage, distribution, and utilization. The transition to green hydrogen can help countries meet their greenhouse gas emission reduction targets, fostering sustainable economic development.
Technological advances in production, such as high-efficiency electrolysis and methane pyrolysis reactors, and new storage and transportation technologies, including metal hydrides and liquid hydrogen storage solutions, are in development. Expanding hydrogen infrastructure, including production plants, distribution networks, and refueling stations, represents an opportunity for infrastructure investments and job creation. Hydrogen production from renewable sources can benefit regions with abundant renewable resources, leading to balanced regional development and reducing energy import dependence. Integrating green hydrogen into a circular economy, using waste from other industrial processes, and utilizing the oxygen generated as a by-product in industrial and medical applications can further enhance sustainability.13
Despite its immense potential, several technical, economic, and systemic challenges hinder the widespread adoption of green hydrogen. The high costs associated with its production, storage, and distribution remain significant barriers, as does the lack of robust infrastructure and regulatory frameworks. This study underscores the importance of addressing these challenges through advancements in electrolysis efficiency, the development of innovative storage solutions (e.g., metal hydrides, cryogenic storage), and the establishment of policies and incentives that foster hydrogen adoption.
Aligned with the commitments made under the Dubai Agreement at COP28, this study situates green hydrogen as a critical enabler of global decarbonization pathways. The Dubai Agreement outlines urgent measures, including the phased reduction of coal-based energy, the adoption of low-carbon fuels, and the rapid development of zero-emission technologies. Green hydrogen directly supports these measures by offering a clean energy alternative capable of driving emissions reductions across diverse sectors.14
The specific contributions of this review are as follows:
• Comprehensive analysis: the paper critically examines the current state-of-the-art in green hydrogen production technologies, including water electrolysis powered by renewable energy, methane pyrolysis, and other emerging methodologies.
• Identification of opportunities: it highlights latent opportunities in hydrogen's application, including its role in circular economies, regional development, and energy democratization.
• Systemic evaluation: the review explores the systemic intricacies of hydrogen storage and transportation, focusing on scalability and global integration.
• Socio-economic implications: it assesses the broader implications of a hydrogen-based economy, including job creation, industrial transformation, and geopolitical shifts.
• Strategic alignment: the study contextualizes green hydrogen within global frameworks such as the 2030 Agenda and COP28, underscoring its role in achieving long-term sustainability goals.
This review not only presents a holistic exploration of green hydrogen's transformative potential but also identifies pathways to overcome existing barriers, offering actionable insights to accelerate its adoption. By addressing these critical dimensions, this work aims to provide a foundational resource for researchers, policymakers, and industry stakeholders dedicated to advancing green hydrogen as a cornerstone of a sustainable future.
2. Technological landscape of green hydrogen production
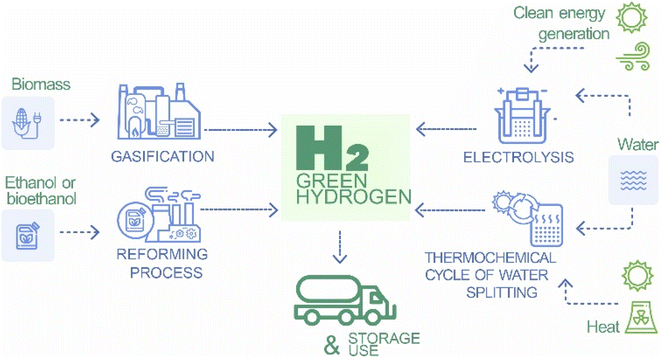
Green hydrogen production encompasses a diverse set of technologies, each method leverages renewable energy sources to contribute to sustainable energy solutions. At the forefront of these technologies is water electrolysis, in this section, various water electrolysis methods, such as alkaline electrolysis, proton exchange membrane (PEM) electrolysis and solid oxide electrolysis, are discussed in depth. Discussions delve into advancements in catalysts, membranes, and system designs with emphasis on the continuous pursuit of greater efficiency and cost-effectiveness.6 The integration of renewable energy, such as solar and wind energy, into the electrolysis process is also discussed (Fig. 2).Another key avenue for green hydrogen production is the biomass gasification, which introduces a novel approach by utilizing biomass as a feedstock. This section explores thermochemical pathways such as steam gasification and pyrolysis, and highlights the potential of biomass-derived green hydrogen as a carbon-neutral alternative, in line with broader sustainability goals.9
High-temperature thermochemical processes, including water splitting and sulfur–iodine cycles, represent a technologically sophisticated aspect of green hydrogen production. This section conducts an in-depth analysis of the efficiency gains and challenges associated with operating at elevated temperatures, offering insights into the potential of these processes in advancing green hydrogen production.10
2.1 Water electrolysis
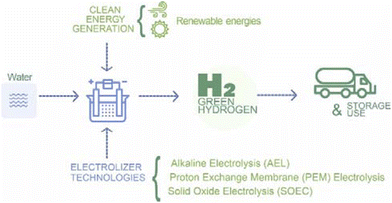
The production of green hydrogen through water electrolysis harnessing electricity generated from renewable sources such as solar and wind, offers a promising approach for hydrogen production.12 The production process begins with the procurement of water, typically in the form of deionized or demineralized water, which is introduced into an electrolyzer (Fig. 3). This device consists of two electrodes submerged in the water and separated by a proton-conducting membrane.13 When electricity is applied across these electrodes, the water undergoes decomposition into hydrogen and oxygen.At the negative electrode (anode), the oxidation reaction of water occurs:
| 2H2O → O2 + 4H+ + 4e− | (1) |
Simultaneously, at the positive electrode (cathode), the reduction of water takes place:
| 4H+ + 4e− → 2H2 | (2) |
The end result is the generation of hydrogen gas (H2) at the cathode and oxygen (O2) at the anode.
Among the diverse technological avenues within water electrolysis, three methods have attracted significant attention: alkaline electrolysis, Proton Exchange Membrane (PEM) electrolysis, and solid oxide electrolysis. All three methods use renewable energy, the mechanisms, advantages and challenges of each are discussed below.
| Cathode: 2H2O + 2e− → H2 + 2OH− | (3) |
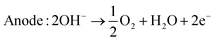 | (4) |
Alkaline electrolysis has demonstrated scalability, making it a favorable choice for large-scale hydrogen production endeavors. Its robust performance and well-established infrastructure contribute to its standing as a reliable technology in the pursuit of sustainable hydrogen generation.16
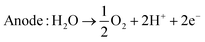 | (5) |
| Cathode: 2H+ + 2e− → H2 | (6) |
PEM electrolysis operates at relatively low temperatures, offering rapid response times and high efficiency. This characteristic makes it particularly advantageous for applications requiring quick start-up and response, such as in decentralized or intermittent renewable energy systems.
The ongoing refinement of PEM electrolysis technology holds promise for enhanced performance, increased durability, and broader deployment across various applications.17 PEM electrolyzers are known for their quick response to changes in electricity input and are suitable for variable renewable energy sources.
| Cathode: H2O + 2e− → H2 + O2− | (7) |
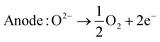 | (8) |
Solid oxide electrolysis is still in the early stages of development,18 it offers notable advantages, including high efficiency because the thermodynamics of the reaction are favored due to the high operating temperature and this process can be thermally integrated with other process streams such as methanol, dimethyl ether and ammonia synthesis,2 making it suitable for industrial processes and applications where waste heat recovery is feasible. However, challenges related to material durability and thermal management are subjects of ongoing research and development efforts.19
The efficiency of the electrolysis process is influenced by factors such as the type of electrolyzer, operating temperature, pressure, and the quality of the electrical input.20 Each type of electrolyzer has specific operating conditions, for example, PEM electrolyzers operate at lower temperatures and are suitable for intermittent renewable energy sources, while solid oxide electrolyzers operate at higher temperatures and may be integrated with high-temperature heat sources. Sophisticated control systems manage the operation of the electrolyzer, ensuring optimal performance and response to varying energy inputs. Integration with the overall hydrogen production system, including energy storage and hydrogen purification, is crucial for system efficiency.21
The current cost of electrolyzers is approximately $1000 to $1500 per kW of capacity. However, this cost can vary depending on the type of electrolyzer, scale of production, and technological advancements. To make green hydrogen viable and competitive with conventional hydrogen production methods such as steam methane reforming, the cost of electrolyzers needs to be reduced to around $200 to $300 per kW. This reduction is crucial to achieve cost parity and widespread adoption of green hydrogen technologies. The efficiency of current electrolyzers typically ranges from 60% to 70%, meaning that 60–70% of the electrical energy input is converted into chemical energy in the form of hydrogen.22
As of recent data, the installed capacity of electrolysis for hydrogen production is estimated to be around 200–300 MW globally. This capacity is expected to grow rapidly with increasing investments and technological advancements. Major projects are being developed, especially in regions with abundant renewable energy resources, such as Europe, North America, and Asia.23 Currently, electrolysis accounts for a small fraction of the global hydrogen production, which is dominated by hydrogen produced from natural gas through steam methane reforming (SMR). Electrolysis is estimated to cover less than 5% of the total hydrogen market. The majority of hydrogen production is still reliant on fossil fuels, contributing to significant CO2 emissions. Regarding electrolysis production, the purity of hydrogen produced through electrolysis is typically very high, often exceeding 99.9%. Proton Exchange Membrane (PEM) electrolyzers can produce hydrogen with purity levels up to 99.999%, making it suitable for most industrial and energy applications without the need for extensive purification.24
The production of green hydrogen through water electrolysis with renewable energy sources is a rapidly evolving field, continuous improvements in efficiency, cost reduction, and expanded application are key areas of research that will further propel the widespread adoption of this technology.25 On an implementation level, certain leading countries, including Germany, Australia, and Japan, have made significant strides in constructing infrastructure for green hydrogen production, establishing pilot projects, and implementing strategies.
The versatility of hydrogen by electrolysis allows its utilization in a wide array of applications, ranging from fueling hydrogen-powered vehicles to industrial processes, making it a versatile resource for decarbonizing multiple sectors.26 The following describes the power generation process to power the electrolyzer with solar and wind-based generation, as well as the key aspects that impact cost, performance, and the overall process of hydrogen production by electrolysis.
The entire process begins with the solar energy harvesting with photovoltaic (PV) panels. Power generation can be grid connected or stand-alone or off-grid where grid connection is not feasible or cost-effective.29 To address intermittency in solar energy production, especially during periods of low sunlight, energy storage solutions like batteries may be integrated30 which add operation cost to the entire production process. Therefore the efficiency and reliability of this process contribute significantly to the overall sustainability and viability of using solar energy for hydrogen production.31
The installed capacity for hydrogen production via PV electrolysis is currently limited but growing. As of recent data, the global installed capacity is estimated to be in the range of tens to hundreds of megawatts. Hydrogen production from PV electrolysis currently covers a small fraction of the global hydrogen market, less than 1%. Hydrogen produced via PV electrolysis is nearly carbon-free, as the primary emissions come from the production and installation of PV panels and electrolyzers. Producing 1 kg of hydrogen through PV electrolysis can prevent the emission of approximately 10–12 kg of CO2 compared to conventional hydrogen production methods.24 The hydrogen produced from PV electrolysis typically has a high purity level, often exceeding 99.99%. This high purity is essential for various applications, particularly for fuel cells and industrial processes such as fuel cells production, or electronics and pharmaceuticals.32
Once the hydrogen is produced, it is necessary a purification step. The hydrogen produced through electrolysis may contain impurities such as water vapor, traces of oxygen, and other gases. Purification processes are employed to remove these impurities and achieve high-purity hydrogen. According to several works, several purification techniques may be applied for hydrogen purification highlighting Pressure Swing Adsorption (PSA), membrane separation, cryogenic eistillation, and combined purification systems.33
Modern wind turbines are equipped with sensors that continuously monitor wind conditions to adjust the turbine's operation to optimize energy capture.37 Many wind farms utilize remote monitoring and control systems that allow operators to oversee multiple turbines from a centralized location.38 Some wind farms incorporate energy storage systems, such as batteries, to store excess electricity during periods of high wind and release it when demand is high or wind conditions are suboptimal. This helps enhance grid stability and enables better utilization of the generated energy.
Once the electricity is generated by the wind turbines is then used in the electrolysis process. The integration of wind-based electrolysis into the energy infrastructure allows for the utilization of excess electricity generated during periods of high wind availability. This helps address the intermittent nature of wind energy production by converting surplus electricity into storable hydrogen, which can be used when energy demand is high or when the wind isn't blowing.39
As of recent data, the installed capacity for hydrogen production via wind-based electrolysis is estimated to be in the range of hundreds of megawatts globally. This includes both pilot projects and a few commercial-scale operations. For instance, some notable projects include the NortH2 project in the Netherlands and the REFHYNE project in Germany. Hydrogen production from wind-based electrolysis currently covers a small fraction of the global hydrogen market, estimated at less than 1%. However, hydrogen production by this means is interesting, if a wind-based electrolysis plant produces 1000 tons of hydrogen annually, it can save 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–12
000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of CO2 emissions per year. Hydrogen produced from wind-based electrolysis typically has a high purity level, often exceeding 99.99%, this high purity is suitable for various applications as mentioned above.23
000 tons of CO2 emissions per year. Hydrogen produced from wind-based electrolysis typically has a high purity level, often exceeding 99.99%, this high purity is suitable for various applications as mentioned above.23
Hydrogen production by electrolysis, encompassing water electrolysis, photovoltaic (PV) electrolysis, and wind-based electrolysis, holds significant potential for advancing sustainable energy systems. However, this potential is accompanied by various challenges and opportunities summarize in Table 1 that need to be addressed to maximize the impact and efficiency of these technologies.
| Category | Challenges | Opportunities | Technological solutions | Actions needed |
|---|---|---|---|---|
| Electrolyzer efficiency | Improve catalysts and electrolytes | Integrate AI for real-time optimization | New catalysts and membrane designs | R&D investment, scaling pilot projects |
| Renewable intermittency | Advanced energy storage | Electrolysis adaptable to renewable energy | Smart grids and AI-based forecasting | Integrate smart grids, regulatory incentives |
| Cost competitiveness | Reduce operating costs | Scale production and automate processes | Automation and process control | Industry-research collaboration, targeted funding |
| Scaling infrastructure | Modular and standardized systems | New business models for H2 | Digital twins, predictive maintenance | Strategic investment, public-private partnerships |
| Material durability | Resist corrosion and degradation | New catalysts and membranes | Advanced materials and coatings | Interdisciplinary collaboration, pilot projects |
2.2 Bioethanol steam reforming
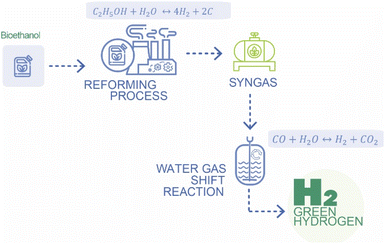
Green hydrogen production through the wet ethanol reforming process stands as a notable and environmentally conscious method within the expansive realm of hydrogen generation and presents a transformative pathway towards sustainable energy.40 This process involves the conversion of ethanol, a renewable and bio-derived alcohol, into hydrogen gas through a sequence of chemical reactions conducted under high-temperature steam reforming conditions11,41 (Fig. 4).Liquid ethanol, sourced from renewable feedstocks like biomass or bioethanol production processes, serves as the primary feedstock for the wet ethanol reforming process. The heart of the process lies within a furnace, a controlled environment where the ethanol undergoes reforming reactions. A catalyst, commonly composed of transition metals such as nickel, plays a pivotal role in facilitating the reactions. The catalyst promotes the breakdown of ethanol into hydrogen and carbon monoxide.
The initial reaction involves the steam reforming of ethanol, where ethanol reacts with water to produce hydrogen and carbon monoxide.
| C2H5OH + H2O ↔ 4H2 + 2CO | (9) |
The subsequent water–gas shift reaction enhances hydrogen production and converts carbon monoxide into additional hydrogen and carbon dioxide.
| CO + H2O ↔ H2 + CO2 | (10) |
The purity of hydrogen produced via ethanol reforming can vary based on the specific process and catalyst used, typically, the hydrogen purity ranges from 98% to 99.9%. The primary byproducts of the process are carbon dioxide (CO2) and trace of other gases. However, the produced hydrogen can be further purified to achieve the desired purity levels for various applications. The integration potential with established ethanol facilities streamlines the adoption of this green hydrogen production method, potentially repurposing existing assets for a dual role in both ethanol and hydrogen production.42 The CO2 released during the reforming process is offset by the CO2 absorbed by the plants during their growth.43 On average, producing 1 kg of hydrogen from ethanol reforming can prevent the emission of approximately 10–12 kg of CO2 compared to conventional SMR.44
Despite its promise, challenges persist in the wet ethanol reforming process, necessitating ongoing research and development efforts. Efficient catalysts are crucial to enhance reaction rates and selectivity, while strategies for managing carbon deposition on the catalyst surface require further exploration. Continued optimization of process parameters and the exploration of innovative catalyst materials are essential for enhancing the overall efficiency and sustainability of this technology. Currently, both decentralized and centralized models of ethanol production coexist, with a tendency towards increasing centralization. However, determining the optimal approach requires a thorough evaluation within the context of a supply chain framework. This assessment should take into account the logistics, economic factors, and environmental impacts of each model to identify which configuration offers the most efficient and sustainable solution for large-scale hydrogen production (Table 2).
| Aspect | Challenge | Opportunity | Technological solutions | Actions needed |
|---|---|---|---|---|
| Process efficiency | Maximize efficiency, heat management issues | Process intensification, thermodynamic optimization | Advanced catalysts, thermal management, innovative reactors | R&D investment, pilot projects, industry collaboration |
| Renewable feedstock integration | Feedstock variability | Standardized sourcing, pre-treatment innovation | Pre-treatment technologies, real-time monitoring, standardization protocols | Collaboration with biofuel industries, guidelines, infrastructure investment |
| Technological integration | Retrofit existing infrastructure | Modular reactor designs, advanced control systems | Modular reactors, advanced control, shared resource optimization | Public-private partnerships, regulatory support, industry collaboration |
| Decentralized applications | Logistics and regulatory hurdles | Innovative storage and distribution | On-site electrolysis, mobile storage, advanced storage solutions | Supportive regulations, infrastructure investment, storage innovation |
The journey towards widespread adoption of green hydrogen through bioethanol wet reforming requires a strategic focus on overcoming challenges while harnessing emerging opportunities. As advancements in catalyst design, process optimization, and renewable feedstock utilization progress, green hydrogen produced via ethanol wet reforming is poised to play a pivotal role in shaping a sustainable and decarbonized energy future.
2.3 Biomass gasification
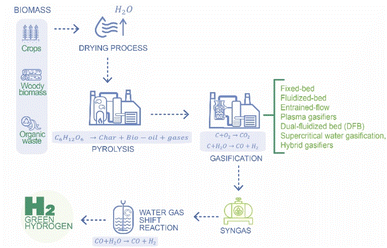
Biomass gasification is a thermochemical process that converts biomass, such as wood, agricultural residues, or organic waste, into a gaseous mixture known as syngas or synthesis gas. This process occurs in a controlled environment with limited oxygen, preventing complete combustion. The resulting syngas is a combination of carbon monoxide (CO), hydrogen (H2), methane (CH4), carbon dioxide (CO2), and other trace gases.45 Biomass gasification offers several advantages, including the utilization of renewable feedstocks, potential carbon neutrality, and the ability to produce a versatile syngas that can be used for heat, power, and hydrogen production (Fig. 5).The International Energy Agency (IEA), the U.S. Department of Energy (DOE), and other similar institutions may provide insights into the current status and trends in hydrogen production from biomass gasification.46 The entire process includes several stages, drying, pyrolysis, gasification, water–gas shift reaction (post-gasification), and tar and soot formation (Fig. 5). The main gasification technologies include fixed-bed gasifiers, fluidized-bed gasifiers, entrained-flow gasifiers, plasma gasifiers, dual-fluidized bed (DFB) gasifiers, supercritical water gasification, and hybrid gasifiers.
Fixed-bed gasifiers are one of the simplest types, where biomass is introduced from the top, and air or oxygen is introduced from either the bottom or the sides. In an updraft gasifier, the syngas exits from the top while char and ash are removed from the bottom, but this design tends to produce a syngas that is rich in tars. The downdraft gasifier, on the other hand, mitigates tar production by allowing syngas to exit from the bottom, which also facilitates the removal of char and ash. Although these designs are relatively simple, they are more suitable for small-scale applications.
Fluidized-bed gasifiers, particularly suited for large-scale operations, offer flexibility and efficiency by introducing biomass into a hot bed of inert material like sand, enabling uniform temperature distribution and effective mixing. Bubbling fluidized-bed (BFB) gasifiers provide this mixing, while circulating fluidized-bed (CFB) gasifiers further enhance efficiency through higher gas velocities. Entrained-flow gasifiers operate at very high temperatures (above 1200 °C) to produce a clean syngas, though they require finely prepared feedstocks. Plasma gasifiers use extreme heat from plasma torches (up to 5000 °C) to break down biomass into clean syngas, making them versatile but energy-intensive. Dual-fluidized bed (DFB) gasifiers separate gasification and combustion into two reactors, allowing for better syngas control and reduced by-products, making them ideal for hydrogen-rich syngas production. Supercritical water gasification, an experimental technology, uses supercritical water conditions to convert wet biomass into hydrogen-rich syngas, but faces challenges with its high operational pressures and temperatures. Finally, hybrid gasifiers combine different technologies to optimize performance, such as integrating fluidized-bed and plasma gasification to enhance syngas quality and reduce tar content.47,48
Drying is a crucial initial step in the biomass gasification process, serving to remove moisture from the feedstock before subsequent stages. The drying stage is important because excessive moisture content can hinder the efficiency of the overall gasification process, since it impedes the efficiency of subsequent thermal processes by absorbing heat and requiring additional energy for vaporization. In order to reduce moisture, conventionally two methods are employed, natural drying and forced-air drying. In natural drying step, the biomass is left in the open air to naturally reduce moisture content through exposure to sunlight and air circulation. This method is cost-effective but weather-dependent. On the other hand, forced-air drying Biomass is subject to forced air using fans or blowers to accelerate the removal of moisture. This method allows for more control over drying conditions. During drying, an important variable is temperature, which is typically in the range from 50 °C to 100 °C, and duration varies based on factors such as biomass type, initial moisture content, and drying method. However, high temperatures must be avoided to prevent thermal degradation of biomass components.49 The choice of drying method, temperature control, and attention to biomass characteristics are all vital aspects in achieving effective moisture reduction. Researchers and engineers continually explore innovations in drying technologies to enhance the overall sustainability and efficiency of biomass gasification systems.50
The second step is the pyrolysis which is a thermochemical process within the biomass gasification sequence, where the dried biomass undergoes thermal decomposition for the generation of valuable products such as solid char, liquid bio-oil, and gases in the absence of oxygen to prevent the formation of combustion products like CO2.
The complex organic compounds in biomass break down into simpler molecules through various chemical reactions. Cellulose, hemicellulose, and lignin are major components of biomass, and their decomposition leads to the formation of volatile compounds. Considering as case base the decomposition of cellulose, the summarized chemical reaction can be expressed as follows:
| C6H12O6 → char + bio-oil + gases | (11) |
The breakdown of cellulose, a complex sugar in biomass, results in the formation of solid char, liquid bio-oil, and gases. As seen in eqn (11), volatile components released during pyrolysis condense to form bio-oil, a complex mixture of oxygenated hydrocarbons. In the same reaction, undesirable tars may form during pyrolysis, and their presence can complicate downstream processes. Strategies for tar removal or reduction are ongoing research areas. Bio-oil composition depends on factors like temperature, heating rate, and biomass type. Also, several gases are produced, including carbon monoxide (CO), hydrogen (H2), methane (CH4), and other volatile organic compounds. These gases contribute to the overall syngas composition in the subsequent gasification stage. Regarding operative conditions, pyrolysis is typically carried out at temperatures ranging from 500 to 800 °C. Higher temperatures favor gas production, while lower temperatures favor bio-oil formation. The rate at which temperature is increased influences product distribution. Rapid heating often leads to more gas production.51
Going forward in the process, it follows gasification that transforms solid biomass into a gaseous mixture known as syngas (synthesis gas). The primary goal of gasification is to convert solid char produced during pyrolysis into a gaseous mixture containing carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and other gases. This mixture is versatile and can be used for various applications, including hydrogen production, power generation, and the synthesis of chemicals. Gasification involves several chemical reactions, primarily between carbon (in the form of solid char) and oxygen or steam. In brief, those chemical reactions can be expressed as follows:
| C + O2 → CO2 | (12) |
| C + H2O → CO + H2 | (13) |
After gasification it may happens Water–Gas Shift Reaction (WGSR) in order to increase hydrogen composition. After the main gasification process, the syngas produced often contains carbon monoxide (CO), which needs to be further processed to increase the hydrogen content and reduce the CO concentration (eqn (14)). The WGS reaction plays a central role in achieving this transformation.53
| CO + H2O → CO + H2 | (14) |
Tar and soot formation during biomass gasification represent challenges that can affect the efficiency and reliability of the process. Tar consists of complex hydrocarbons and other organic compounds that are formed during incomplete combustion and pyrolysis reactions in the gasification process. Tar can condense on the surfaces of equipment, pipes, and heat exchangers, causing fouling and reducing the efficiency of the gasification system. In order to control tar formation, it may be necessary to consider some strategies; tar removal methods include using catalysts, thermal cracking, and filtration.54 Also, integrated gas cleaning systems, such as hot gas filtration and catalytic tar reforming, are employed to minimize tar content. On the other hand, soot consists of fine carbon particles that result from incomplete combustion and pyrolysis reactions during the gasification process. High-temperature conditions and insufficient residence time can contribute to soot formation. Similar to tar formation, some strategies may be considered for soot control such as optimizing gasification conditions, ensuring sufficient residence time, and implementing effective combustion in the gasifier, downstream filtration and particulate removal systems are employed to minimize the presence of soot in the syngas.55
The current global capacity for hydrogen production via biomass gasification is relatively low compared to other methods such as steam methane reforming (SMR) and electrolysis. Most projects are still in the pilot or demonstration phase, with a few commercial-scale plants. The installed capacity is estimated to be in the range of tens of megawatts, with ongoing research and development aimed at scaling up the technology. Hydrogen production from biomass gasification currently covers a very small percentage of the global hydrogen market, less than 1%. The majority of hydrogen is still produced from natural gas through SMR. The CO2 released during gasification is offset by the CO2 absorbed by the plants during their growth. On average, producing 1 kg of hydrogen via biomass gasification can prevent the emission of approximately 10–12 kg of CO2 compared to hydrogen produced from fossil fuels.23
Hydrogen produced from biomass gasification typically has a purity range of 99% to 99.9%, depending on the gas cleanup and purification methods used. Advanced purification techniques, such as pressure swing adsorption (PSA) and membrane separation, can achieve higher purities suitable for various applications. Hydrogen with a purity of 99% is adequate for many industrial processes, including chemical manufacturing and refining.24
In this way, as summary, biomass gasification offers several advantages, including the utilization of renewable feedstocks, potential carbon neutrality, and the ability to produce a versatile syngas that can be used for heat, power, and hydrogen production. However, challenges include feedstock variability, technical complexity, and economic considerations, which researchers and engineers continually address to enhance the efficiency and feasibility of biomass gasification processes. Ongoing research aims to optimize this technology for cleaner and more sustainable energy production. Challenges and opportunities may be expressed as follow (Table 3).
| Aspect | Challenges | Opportunities | Technological solutions | Actions needed |
|---|---|---|---|---|
| Tar and soot formation | Tar and soot affect system reliability | Improved system reliability through advanced mitigation | Advanced filtration, catalytic reforming, plasma-assisted removal | Pilot projects, regulatory standards |
| Feedstock | Feedstock variability and standardization | Maximized resource utilization, reduced landfill use | Flexible gasification, advanced sensors, adaptable reactors | Standardized protocols, adaptive control, biomass databases |
| Economic viability | Cost-effective biomass gasification | Market development through cost-effective solutions | Process optimization, advanced catalysts, cost-effective reactors | Economic assessments, pilot projects, policy support |
| Hydrogen purity | Ensuring high hydrogen purity | Broad compatibility across applications | Advanced purification, membrane separation, adsorption systems | R&D investment, field trials, industry standards |
| Decentralized production | Modular and scalable systems | Localized hydrogen production, reduced logistics costs | Compact gasification systems, localized integration | Decentralized projects, local engagement, supportive regulations |
| Integration with carbon capture | Carbon-negative hydrogen production | Reduced carbon footprint, enhanced sustainability | Advanced CCS technologies, optimized integration | R&D investment, pilot projects, CCS policies |
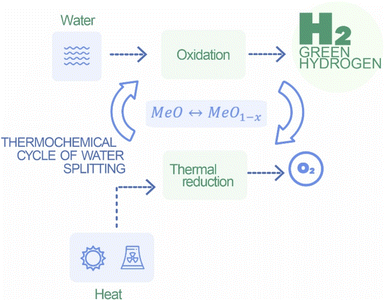
2.4 High-temperature thermochemical processes
High-temperature thermochemical processes are being explored as potential methods for green hydrogen production. These processes involve the use of heat to drive chemical reactions that result in the production of hydrogen (Fig. 6). Many alternatives could be highlighted, sulfur–iodine cycle, hybrid sulfur cycle, cerium oxide-based thermochemical cycle, and ferrite-based thermochemical cycle.56The initial step of this process is decomposition stage.
| H2SO4 → SO2 + H2O + 1/2O2 | (15) |
In the first stage, sulfuric acid is decomposed into sulfur dioxide, water, and oxygen using high-temperature heat. This reaction typically occurs at temperatures above 830 °C. This is why the sulfur–iodine cycle is often integrated with a high-temperature heat source, such as a nuclear reactor or concentrated solar power. This heat source provides the energy required for the thermochemical reactions in the cycle. The second step is Bunsen reaction that could be detailed as follows.
| SO2 + 2I2 → 2HI + H2SO4 | (16) |
| H2SO4 + 2HI → 2H2O + SO2 + I2 | (17) |
The Bunsen reaction in the reactor, where sulfur dioxide reacts with iodine to form hydrogen iodide and sulfuric acid. This stage involves several chemical reactions to regenerate iodine and continue the cycle. The final step is hydrogen production stage where hydrogen iodide is decomposed into hydrogen and iodine, releasing the desired hydrogen product. The detailed equation is:
| 2HI → H2 + I2 | (18) |
It must be considered that the sulfur–iodine cycle is often considered as a high-temperature thermochemical process suitable for integration with nuclear reactors or concentrated solar power. Given the corrosive nature of the chemicals involved and the high temperatures, materials compatibility is a crucial consideration. The construction materials for the reactors and associated components must withstand the harsh conditions over extended periods. The cycle doesn't directly involve water electrolysis, which is an advantage in terms of avoiding high-temperature electrolysis challenges. One of the challenges is the need for materials that can withstand the harsh chemical environment and high temperatures associated with the reactions. The overall efficiency and economic viability of the sulfur–iodine cycle depend on advancements in materials science, reactor design, and heat transfer optimization.
| H2SO4 → SO2 + H2O + 1/2O2 | (19) |
Similar to the sulfur–iodine cycle, the process begins with the thermal decomposition of sulfuric acid at high temperatures to produce sulfur dioxide, water, and oxygen. The second step is the sulfur dioxide depolarization. In this step, sulfur dioxide (SO2) is reacted with hydrogen sulfide (H2S) to produce elemental sulfur (S) and water (H2O). The sulfur dioxide depolarization reaction can be expressed as:
| 2SO2 + 2H2S → 3S + 2H2O | (20) |
The third step is water splitting reaction. Here the elemental sulfur produced in the depolarization reaction is then reacted with water to produce hydrogen sulfide (H2S) and sulfuric acid (H2SO4). The water splitting reaction is given by:
| S + H2O → H2SO4 + H2S | (21) |
The overall reaction for the hybrid sulfur cycle is obtained by combining the sulfuric acid decomposition reaction, the sulfur dioxide depolarization reaction, and the water splitting reaction. By cycling through these reactions, the sulfur compounds are regenerated, and the net result is the decomposition of water into hydrogen and oxygen. The hybrid sulfur cycle has advantages such as the ability to operate at moderate temperatures compared to some other thermochemical cycles, making it suitable for integration with various heat sources, including solar energy and advanced nuclear reactors. However, challenges related to materials, corrosion, and overall system efficiency need to be addressed for practical implementation.57
Initially, in the oxidation stage, cerium oxide (CeO2) is exposed to oxygen at high temperatures. This causes cerium oxide to undergo oxidation, releasing oxygen and absorbing heat. The second stage is hydrogen production. In the hydrogen production stage, cerium oxide, now in the oxidized state, is subjected to high temperatures in the presence of a reducing agent or heat source ΔH. This results in the reduction of cerium oxide, releasing oxygen and producing hydrogen gas. Finally, the oxygen separation and recirculation where the oxygen released in both the oxidation and reduction stages can be separated and recirculated back to the system. This closed-loop process ensures the continuous regeneration of cerium oxide for multiple cycles.58
The cerium oxide-based thermochemical cycle relies on concentrated solar energy or nuclear heat as a high-temperature heat source. This allows for the utilization of abundant and sustainable energy resources. Cerium oxide is chosen for its ability to undergo reversible redox reactions, transitioning between the oxidized (CeO2) and reduced states. This redox reactivity is central to the cyclic nature of the process. The cycle operates in a closed-loop system, with oxygen being recirculated. This feature enhances the overall efficiency of the process by minimizing the need for additional inputs. The absorption and release of heat during the oxidation and reduction stages, respectively, can be exploited for thermal energy storage. This feature contributes to the system's flexibility and ability to operate continuously, even in the absence of sunlight or during periods of low heat availability. Ensuring the durability of materials in the presence of high temperatures and reactive chemicals is a challenge that requires ongoing research. Materials must withstand cyclic oxidation and reduction without significant degradation.
Initially happens the oxidation stage:
| Fe2O3 → 2FeO + 1/2O2 | (22) |
In this stage ferric oxide undergoes oxidation to produce ferrous oxide and release oxygen when exposed to high temperatures. As second stage, the hydrogen is produced according to the next equation.
| 2FeO + H2O → Fe2O3 + H2 | (23) |
Ferrous oxide reacts with water to produce ferric oxide and release hydrogen gas. This reaction is often facilitated by a heat source. Finally, the oxygen separation and recirculation. Oxygen released during the oxidation stage (reaction 16) needs to be separated, and a recirculation system is employed to reintroduce oxygen into the system, ensuring a closed-loop cycle.
Ferrites, particularly iron oxide-based compounds, exhibit reversible redox reactions, allowing for the cyclic nature of the thermochemical cycle. Note that, iron is abundant and relatively inexpensive, making ferrites potentially cost-effective materials for hydrogen production. Similar to other thermochemical cycles, the ferrite-based cycle operates in a closed-loop system, minimizing the need for additional inputs and enhancing overall efficiency.60 The installed capacity for thermochemical hydrogen production is currently limited, primarily existing at the pilot and experimental stage. Large-scale commercial deployment is still in development, and exact figures are difficult to quantify. Given that these technologies are mostly in the pilot or early commercial stages, the market coverage is minimal, representing a small fraction of the global hydrogen market. Hydrogen produced from thermochemical processes typically achieves high purity levels, often exceeding 99%.24 Thus, in general terms, the challenges and opportunities about high-temperature thermochemical processes are described next (Table 4):
| Aspect | Challenges | Opportunities | Technological solutions | Actions needed |
|---|---|---|---|---|
| Materials durability | Corrosive environments, thermal cycling | Advanced alloys, ceramics, coatings | Durable materials, 3D printing for tailored properties | Increased R&D investment |
| Cost of high-temperature reactors | High construction and maintenance costs | Cost-effective designs, scalable reactors | Optimized reactor manufacturing techniques | Policy support, financial incentives |
| Energy efficiency | High energy conversion efficiency | Use of excess heat, thermal storage | Advanced heat exchangers, heat recovery systems | Infrastructure development, pilot projects |
| Reaction kinetics | Slow reaction rates limit hydrogen production | Catalyst development, temperature management | Advanced catalysts, reactor optimization | Collaborative R&D efforts |
| Integration with renewable energy | Intermittency of renewable sources | Flexibility with solar and waste heat | Smart grid technologies, energy storage | Policy support, integration projects |
Ongoing research and development are addressing challenges, leading to innovations in materials, reactor designs, and overall system optimization, all these advancements contribute to the improvement of high-temperature thermochemical processes and continued research, technological innovation, and integration with renewable energy sources will play key roles in unlocking the full potential of these processes.
2.5 H2 standards and purification
Since there are several methods for hydrogen production, there is a need to establish standards for the quality of the hydrogen produced so it can be used in subsequent processes. In 2012, the International Organization for Standardization (ISO) introduced the ISO 14687-2:2012 standard, while the Society of Automotive Engineers (SAE) issued the SAE J2719-201511 standard in 2015. Both standards established uniform criteria for assessing hydrogen (H2) quality in Proton Exchange Membrane Fuel Cells (PEMFCs). Until 2019, China adhered to the GB/T 3634.2-2011 standard, which was focused on industrial H2 applications but lacked specific regulations on certain impurities affecting fuel cell performance.Recognizing this limitation, China introduced the GB/T 37244-2018 standard by the end of 2018, aligning with the ISO 14687-2:2012 and SAE J2719-201511 standards. This standard meticulously regulated the concentration of fourteen impurities, such as water (H2O), total hydrocarbon (HC) (by methane), oxygen (O2), helium (He), nitrogen (N2), argon (Ar), carbon dioxide (CO2), carbon monoxide (CO), total sulfide (by H2S), formaldehyde (HCHO), formic acid (HCOOH), ammonia (NH3), total halide (by halide ions), and maximum particulate matter. In this standard there are three types of hydrogen in terms of purity, pure hydrogen, high pure H2, and ultra-pure H2 with a required purity of 99.99%, 99.999%, and 99.9999% respectively. The remaining impurities shall not exceed 95 ppm, 22 ppm, and 2.3 ppm for pure H2, high pure H2, and ultra-pure H2 respectively.
Advancements in PEMFC technology, including reduced platinum (Pt) usage, thinner electrolyte membranes, higher operating electric current density, and lower humidity, necessitated a reevaluation of previously established impurity limits in H2. Consequently, the ISO technical committee for hydrogen energy, IOS/TC 197, released the ISO 14687:2019 standard in November 2019, consolidating and revising three existing H2 fuel cell-related standards. ISO 14687:2019 requires H2 purities of at least 99.97% with impurities no greater than 714.2 ppm.
Simultaneously, following the ISO 14687:2019 standard, SAE issued the SAE J2719 202003 standard in March 2020, extending the permissible limits for CH4, N2, Ar, and HCHO impurities. ISO 14687:2019 requires H2 purities of at least 99.97% with impurities no greater than 1312 ppm.
Nevertheless, the concentration of impurities is rigorously regulated, influenced by the structure and operational traits of fuel cells. Notably, even minimal carbon monoxide (CO) levels can induce irreversible harm to the efficiency and longevity of fuel cells. The influence of impurities has been reported according to the type of compound. For example, an excess of water (H2O) has the capability to carry water-soluble impurities like Na+ and K+, leading to a reduction in membrane proton conductivity. Additionally, an excess of water can cause corrosion in metal components. Regarding HC, most hydrocarbons (HCs) adsorbed onto the catalyst layer will diminish catalytic efficiency. While methane itself does not contaminate fuel cells, its presence dilutes hydrogen (H2) and impedes overall performance. Carbon dioxide (CO2) exerts a diluting influence on hydrogen (H2). Elevated concentrations of CO2 may undergo a reverse water gas shift reaction, transforming into carbon monoxide (CO), consequently resulting in catalyst poisoning. Carbon monoxide (CO) strongly associates with the active sites of platinum (Pt) catalysts, reducing the effective electrochemical surface area available for the adsorption and oxidation of hydrogen (H2). The attachment of sulfides to the active catalyst sites obstructs the adsorption of hydrogen (H2) on the catalyst surface. These adsorbed sulfides undergo reactions with platinum (Pt) catalysts, resulting in the formation of stable Pt sulfides, causing irreversible degradation of fuel cell performance. Formaldehyde (HCHO) and formic acid (HCOOH) adhere to catalysts, generating carbon monoxide (CO) in the process, ultimately resulting in catalyst poisoning. Ammonium ions (NH4+) have the potential to diminish the proton conductivity of the ionic polymer. Additionally, ammonia (NH3) adsorbed onto the catalyst's surface obstructs the active sites. The electric potential of fuel cells is diminished by the dilution and diffusion of helium (He), argon (Ar), and nitrogen (N2) within hydrogen (H2). Adsorption of halides on the catalyst layer diminishes the catalysts' surface area. Chloride ions are deposited into the fuel cell membrane, creating soluble chlorides and resulting in the dissolution of the platinum (Pt) catalyst. Particulate matter adhering to the active sites of fuel cell catalysts obstructs the adsorption of hydrogen (H2) on the catalyst surface, thereby impeding the filter and causing damage to the overall cell components.60–63
The purification of hydrogen (H2) holds pivotal significance in the entire H2 production-to-utilization chain. The establishment of stable, reliable, and cost-effective H2 sources forms the foundation for the widespread integration of fuel cell vehicles. Consequently, the development of highly efficient and energy efficient H2 purification technologies for fuel cell vehicles plays an integral role in advancing the H2 energy industry.
The optimal functioning of a fuel cell power system is contingent upon the provision of high-quality H2. H2 derived from coal gasification, natural gas reforming, by-product H2, or water electrolysis is collectively termed as crude hydrogen. Without purification in accordance with existing standards, crude hydrogen cannot be directly utilized in fuel cell vehicles. The composition of various crude H2 types may vary according with its source. It is possible to obtain an average composition of 25–35, 70–75, 75–80, 45–60, 70–80, 60–75, 25–35 for coal gasification, natural gas reforming, methanol reforming, coke oven gas, methanol purge gas, synthetic ammonia tail gas, biomass gasification, respectively.63–65
H2 purification methods fall into two main categories: physical methods (including adsorption methods like PSA, temperature swing adsorption, and vacuum adsorption; low-temperature separation methods such as cryogenic distillation and low-temperature adsorption; and membrane separation methods involving inorganic and organic membranes) and chemical methods (encompassing metal hydride separation and catalysis).
The selection of an appropriate H2 purification method is intricately linked to the hydrogen supply mode and gas source. In the case of H2 production through centralized large-scale coal gasification and natural gas reforming with an H2 supply exceeding 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Nm3 h−1, PSA purification is predominantly employed after transformation, desulfurization, and decarbonization. Despite the longstanding presence of PSA technology characterized by low operational costs and a prolonged service life, the H2 produced for fuel cell vehicles through traditional PSA may exhibit a decreased recovery rate and yield due to standard impurity content. Cryogenic distillation, suitable for large-scale production, yields H2 purity between 85% and 99%, falling short of application requirements.66
000 Nm3 h−1, PSA purification is predominantly employed after transformation, desulfurization, and decarbonization. Despite the longstanding presence of PSA technology characterized by low operational costs and a prolonged service life, the H2 produced for fuel cell vehicles through traditional PSA may exhibit a decreased recovery rate and yield due to standard impurity content. Cryogenic distillation, suitable for large-scale production, yields H2 purity between 85% and 99%, falling short of application requirements.66
For H2 production in a centralized by-product mode with an H2 supply ranging from 1000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Nm3 h−1, versatile processes are necessitated to enhance H2 recovery efficiency based on different impurities. In scenarios involving small-scale on-site distributed H2 production with an H2 supply of 1000 Nm3 h−1 or less, and for vehicle H2 supply, traditional PSA separation proves disadvantageous in terms of large floor area, inflexibility, and low adaptability. Consequently, low-temperature adsorption, metal hydride, and metal membrane separations emerge as viable processes based on the types and quantities of impurities. While low-temperature adsorption effectively eliminates multiple impurities, such as sulfide, formaldehyde (HCHO), and formic acid (HCOOH), it entails high energy consumption and is best suited for specific small-scale and cold source applications.67
000 Nm3 h−1, versatile processes are necessitated to enhance H2 recovery efficiency based on different impurities. In scenarios involving small-scale on-site distributed H2 production with an H2 supply of 1000 Nm3 h−1 or less, and for vehicle H2 supply, traditional PSA separation proves disadvantageous in terms of large floor area, inflexibility, and low adaptability. Consequently, low-temperature adsorption, metal hydride, and metal membrane separations emerge as viable processes based on the types and quantities of impurities. While low-temperature adsorption effectively eliminates multiple impurities, such as sulfide, formaldehyde (HCHO), and formic acid (HCOOH), it entails high energy consumption and is best suited for specific small-scale and cold source applications.67
Metal hydride separation and palladium (Pd) membrane separation methods demonstrate reasonable efficacy in separating gas sources with a high content of inert components. However, their inherent disadvantage lies in the reaction of purified materials with impure gas during H2 recovery, leading to a reduction in purification efficiency. Novel membrane technologies like carbon molecular sieve membranes (CMSMs), ionic liquid membranes, and electrochemical H2 pump membranes are currently prominent areas of scientific research, although their industrial-scale implementation remains challenging.68
Thus, hydrogen purification after the aforementioned production processes is crucial to obtain high-purity gas suitable for various industrial and commercial applications (Table 5).69–71
| Production process | Challenges | Opportunities | Technological solutions | Actions needed |
|---|---|---|---|---|
| Water electrolysis | Energy consumption, membrane durability | Improved efficiency, longer membrane lifespan | Ion exchange membranes, molecular sieves, PSA | R&D for energy-efficient technologies, robust membranes |
| Biomass gasification | Diverse impurities, environmental impact | Cleaner syngas, sustainable waste management | Filtration, washing, absorption systems | Advanced filtration systems, sustainable residue management |
| High-temperature thermochemical processes | High temperatures, corrosion, by-product management | Efficient by-product utilization, reduced corrosion | Permeation membranes, corrosion-resistant materials | High-temp resistant membranes, by-product recovery methods |
2.6 Hydrogen transportation
As hydrogen production scales up, the need for an efficient and cost-effective hydrogen transportation infrastructure becomes increasingly urgent. One potential solution that has garnered significant interest is the repurposing of existing natural gas pipelines for hydrogen transport. This approach presents several compelling advantages that justify its consideration as a viable option in the hydrogen economy.72One of the primary justifications for using natural gas pipelines to transport hydrogen is the ability to leverage existing infrastructure. The extensive network of natural gas pipelines already in place provides a ready-made solution for hydrogen transport, potentially avoiding the high capital costs associated with constructing new hydrogen-specific pipelines. Repurposing existing pipelines for hydrogen transport could significantly reduce the time and financial investment required to establish a hydrogen distribution network, thereby accelerating the deployment of hydrogen as a clean energy carrier.
The reuse of natural gas pipelines also offers a strategic advantage in terms of geographic reach. The existing pipeline network spans vast regions, including both urban and rural areas, providing a widespread and accessible means of transporting hydrogen from production sites to end-users. This widespread coverage is particularly beneficial for integrating hydrogen into the energy mix in regions where building new infrastructure would be challenging or cost-prohibitive.73
The technical feasibility of using natural gas pipelines to transport hydrogen has been the subject of extensive research and development. Hydrogen, being a smaller and lighter molecule than methane, exhibits different physical and chemical properties that must be considered when repurposing natural gas pipelines. Key considerations include hydrogen's higher diffusivity, which can lead to increased leakage rates, and its potential to cause embrittlement in certain pipeline materials, particularly high-strength steels.74
Despite these challenges, studies have shown that with appropriate modifications and adaptations, many existing natural gas pipelines can be made suitable for hydrogen transport. These adaptations may include the use of advanced materials and coatings to mitigate embrittlement, as well as enhanced monitoring and maintenance practices to prevent and detect leaks. Additionally, blending hydrogen with natural gas in certain proportions (e.g., up to 20% by volume) has been demonstrated as a feasible interim solution, allowing for the gradual introduction of hydrogen into the natural gas grid while minimizing the need for immediate and extensive infrastructure upgrades.
Ongoing pilot projects and field tests are providing valuable insights into the practical aspects of hydrogen transport via natural gas pipelines. These initiatives are helping to identify best practices for pipeline adaptation and are contributing to the development of industry standards and regulations that will ensure the safe and efficient transport of hydrogen.
The economic viability of using natural gas pipelines for hydrogen transport is another key justification for this approach. By repurposing existing infrastructure, the costs associated with building new hydrogen-specific pipelines can be substantially reduced. This cost savings can play a critical role in making hydrogen more competitive with other energy carriers, particularly in the early stages of market development.75
Moreover, the integration of hydrogen into the existing natural gas grid can help to optimize the utilization of the pipeline network, thereby improving the overall economic efficiency of the energy system. This integration can also create new revenue streams for pipeline operators and provide a pathway for the gradual decarbonization of the natural gas sector.76
Furthermore, the use of natural gas pipelines for hydrogen transport aligns with broader economic and policy objectives related to energy security and diversification. By facilitating the transport of domestically produced hydrogen, this approach can reduce dependence on imported fossil fuels, support the development of local hydrogen industries, and contribute to job creation in the energy sector.
The environmental benefits of using natural gas pipelines to transport hydrogen are closely linked to the broader goals of reducing greenhouse gas emissions and transitioning to a low-carbon energy system. Hydrogen, when produced from renewable sources such as water electrolysis or biogas reforming, has the potential to serve as a zero-emission energy carrier. By utilizing existing natural gas pipelines for hydrogen transport, the carbon footprint associated with building new infrastructure can be minimized, thereby contributing to overall emission reductions.77
In addition to environmental benefits, this approach is also supported by evolving energy policies and regulations that aim to promote the use of hydrogen as a clean energy source. Many governments and regulatory bodies are developing frameworks to support the integration of hydrogen into the natural gas grid, including incentives for pipeline adaptation, safety standards, and targets for hydrogen blending.
The alignment of hydrogen transport via natural gas pipelines with policy goals related to energy transition and decarbonization further strengthens the justification for this approach. As governments and industries work towards achieving net-zero emissions, the repurposing of natural gas pipelines offers a pragmatic and cost-effective pathway for scaling up hydrogen infrastructure in a timely manner.
While the justification for using natural gas pipelines to transport hydrogen is strong, further research and development are needed to fully realize this potential. Areas for future research include the long-term effects of hydrogen on pipeline materials, the development of advanced sensors and monitoring systems for leak detection, and the optimization of hydrogen blending strategies.
Collaboration between industry, academia, and government agencies will be essential to address these challenges and to develop the necessary technical standards and regulatory frameworks. Continued investment in pilot projects and large-scale demonstrations will also be critical for building confidence in the safety and reliability of hydrogen transport via natural gas pipelines.
Thus, the possibility of using natural gas pipelines to transport hydrogen presents a compelling opportunity to accelerate the transition to a hydrogen-based energy system. By leveraging existing infrastructure, addressing technical challenges, and aligning with economic and policy objectives, this approach offers a pragmatic solution for the large-scale distribution of hydrogen, contributing to the broader goals of energy sustainability and decarbonisation (Table 6).72–74,76,78
| Aspect | Challenges | Opportunities | Technological solutions | Actions needed |
|---|---|---|---|---|
| Pipeline adaptation | Material compatibility, embrittlement | Repurposing natural gas pipelines | Advanced coatings, leak detection systems | R&D in materials, industry-academia collaboration |
| Infrastructure investment | High costs of modification | Use existing infrastructure to reduce costs | Reinforced pipelines, hydrogen-compatible storage | Funding incentives, public-private partnerships |
| Regulatory uncertainty | Inconsistent policies | Clear standards can boost investment | Regulatory frameworks and standardization | Establish regulations, international collaboration |
| Geopolitical and public acceptance | Geopolitical tensions, safety concerns | Global hydrogen alignment | Public awareness technologies, safety systems | Diplomatic efforts, public awareness campaigns |
| Environmental challenges | High carbon footprint of grey hydrogen | Green hydrogen aligns with decarbonization | Electrolysis and renewable energy integration | Invest in renewables, support green hydrogen transition |
| Economic growth | Job creation and industry revitalization | New job opportunities in hydrogen sector | Hydrogen infrastructure development | Workforce development, educational initiatives |
| Technology innovation | Material and safety tech advancements | Cross-sector benefits for energy carriers | Monitoring and control technologies | Promote research, cross-sector collaboration |
Thus, addressing the challenges of hydrogen transport through pipeline adaptation, regulatory development, and public engagement is crucial. However, the opportunities for accelerating the hydrogen economy, fostering innovation, creating jobs, and achieving environmental sustainability are vast. Coordinated actions across industry, government, and society are needed to realize these benefits.
3. Technology readiness levels of hydrogen production technologies
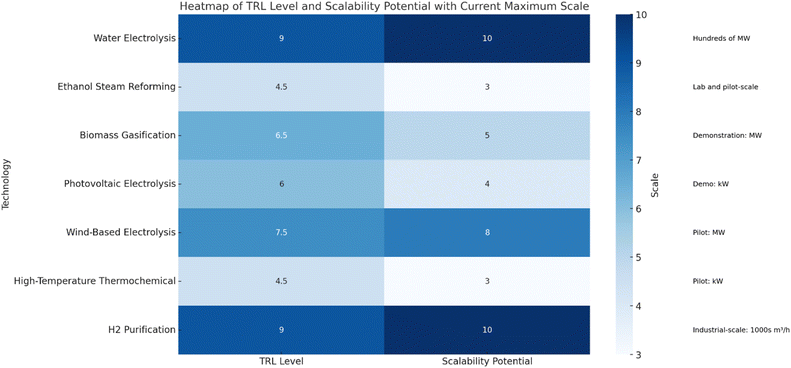
The pursuit of sustainable hydrogen production has led to the advancement of various technologies, each at different stages of maturity. Understanding the Technology Readiness Level (TRL) helps stakeholders recognize which technologies are commercially viable and which need further research (Fig. 7).Water Electrolysis technologies, including Proton Exchange Membrane (PEM), alkaline, and solid oxide electrolyzers, are highly mature and ready for large-scale deployment. Ethanol steam reforming and high-temperature thermochemical processes are in earlier development stages, with ongoing lab and pilot projects. Biomass gasification and photovoltaic (PV) electrolysis are more advanced but remain in the demonstration phase. Wind-based electrolysis is nearing commercial-scale implementation, and hydrogen purification technologies like Pressure Swing Adsorption (PSA) and membrane separation are well-established and widely used.
Overall, this section emphasizes the varying readiness and ongoing development of these hydrogen production methods, highlighting examples of current implementations and future potential.
The development and deployment of hydrogen production technologies are critical for transitioning towards a sustainable energy future. These technologies face a spectrum of challenges and opportunities, each closely tied to their Technology Readiness Levels (TRLs).
4. Policies supporting green hydrogen production technologies
Policies from various organizations supporting the deployment of green hydrogen technologies are multifaceted, aiming to address the technological and economic challenges associated with green hydrogen production (Fig. 8). These policies generally promote research, development, and adoption through funding, incentives, and specific regulatory frameworks. Below is a detailed analysis of key policies implemented by different regions and institutions, focusing on the various production technologies for green hydrogen, and the associated challenges and opportunities for meeting the 2030 agenda.794.1 European Union (EU)
The European Commission, through the European Green Deal, aims to make Europe the first climate-neutral continent by 2050. Green hydrogen is a pivotal element in decarbonizing various industrial sectors, with a particular focus on electrolysis technologies. The EU Hydrogen Strategy outlines plans to install at least 6 GW of renewable hydrogen electrolyzers by 2024 and 40 GW by 2030. This includes both alkaline electrolysis (AEL) and proton exchange membrane (PEM) electrolysis, which are currently the most mature technologies for green hydrogen production.80 Additionally, the innovation fund provides financial support for innovative low-carbon technologies, including solid oxide electrolysis (SOE) projects, which have the potential for higher efficiency but are currently less developed.4.2 United States
In the United States, the Hydrogen and Fuel Cell Technologies Office (HFTO), part of the Department of Energy (DOE), supports R&D in a variety of hydrogen production technologies. The DOE's Hydrogen Program Plan focuses on reducing the cost of hydrogen production from renewable resources to $2 per kg by 2025, emphasizing PEM and AEL electrolysis technologies. The Infrastructure Investment and Jobs Act includes significant funding for clean hydrogen projects, emphasizing the establishment of regional hydrogen hubs and advancing technologies like PEM electrolysis and advanced AEL.81 Additionally, the DOE is exploring next-generation technologies such as high-temperature steam electrolysis (HTSE) using SOE and innovative methods like photocatalytic water splitting.4.3 Japan
Japan, through its basic hydrogen strategy, aims to establish a “hydrogen society” by promoting the use of hydrogen in power generation, transportation, and industrial processes. This strategy includes specific targets for hydrogen production, storage, and infrastructure development, with a strong focus on PEM and AEL electrolysis. Additionally, Japan's Green Innovation Fund allocates around $19 billion over ten years to support innovative technologies, including developments in SOE and photocatalytic water splitting, aiming to enhance the efficiency and reduce the costs of hydrogen production.China has set ambitious goals through its Hydrogen Industry Development Plan (2021–2035), focusing on expanding production capacity and establishing comprehensive hydrogen infrastructure. By 2030, China aims to have one million hydrogen fuel cell vehicles and a robust network of hydrogen refueling stations. The plan emphasizes the development and deployment of AEL and PEM electrolysis technologies, while also investing in advanced research for SOE and novel hydrogen production methods such as biological water splitting using algae or bacteria.82 China's Five-Year Plans emphasize the importance of green hydrogen in achieving carbon neutrality by 2060, supporting R&D and pilot projects for these emerging technologies.
4.4 Australia
Australia's National Hydrogen Strategy aims to position the country as a major global player in hydrogen production and export, leveraging its abundant renewable energy resources. This strategy includes measures to develop hydrogen hubs, enhance regulatory frameworks, and promote international cooperation, with a particular focus on AEL and PEM electrolysis technologies. The Australian Renewable Energy Agency (ARENA) provides grants and funding to support green hydrogen projects, concentrating on reducing production costs and scaling up technologies. Additionally, ARENA is investing in innovative production methods such as solar-to-hydrogen (photovoltaic-driven electrolysis) and exploring the potential of biomass gasification for hydrogen production.4.5 International organizations
The International Energy Agency's (IEA) Hydrogen Technology Collaboration Programme supports international R&D cooperation to advance hydrogen technologies. This program provides comprehensive analysis and recommendations to policymakers on the deployment of various hydrogen production technologies, including AEL, PEM, SOE, and emerging methods such as photocatalytic and biological water splitting. The Hydrogen Council, a global initiative of leading energy, transport, and industry companies, works to accelerate the adoption of hydrogen solutions worldwide, advocating for policies and investments that support the development of a sustainable hydrogen economy834.6 Key policy mechanisms
Collectively, these policies aim to overcome current barriers to green hydrogen deployment, such as high production costs, lack of infrastructure, and market uncertainty. By focusing on advanced electrolysis technologies and emerging production methods, these initiatives drive the transition towards a more sustainable energy system and address the challenges of the 2030 agenda.
5. Green hydrogen and power-to-gas: technological advancements and research contributions
Power-to-Gas (PtG) is an innovative technology that enables the conversion of electrical power, primarily from renewable sources, into hydrogen or synthetic natural gas (SNG) through electrolysis and subsequent methanation processes. This technology serves as a means to store excess energy, thereby enhancing grid stability and contributing to the decarbonization of various sectors. Over the past decade, numerous research groups from prestigious institutions such as ETH Zurich, RWTH Aachen University, Carnegie Mellon University (CMU), and the University of Salamanca (USAL) have significantly contributed to the development and optimization of PtG systems.77ETH Zurich has been at the forefront of PtG research, focusing on the efficiency and integration of electrolysis technologies with renewable energy sources. Their studies have explored the dynamics of coupling electrolysis with fluctuating power supplies, optimizing the operational strategies to maximize hydrogen production efficiency. Additionally, ETH Zurich has investigated the potential of using PtG systems to provide ancillary services to the grid, enhancing overall energy system flexibility.84
RWTH Aachen University has made substantial contributions to the field, particularly in the area of methanation processes. Their research includes the development of advanced catalysts for the Sabatier reaction, which converts hydrogen and carbon dioxide into methane. Aachen's work also extends to the techno-economic analysis of PtG systems, assessing the feasibility and scalability of these technologies for large-scale energy storage and grid balancing applications.75
Carnegie Mellon University (CMU) has focused on the modeling and simulation of PtG systems, providing insights into the optimal design and operation of these technologies. CMU's research has emphasized the integration of PtG with existing natural gas infrastructure, exploring pathways for the utilization of hydrogen-enriched natural gas in various industrial and residential applications. Their work also addresses the life cycle assessment of PtG systems, evaluating their environmental impacts and sustainability metrics.85
The University of Salamanca (USAL) has contributed to the understanding of PtG within the context of smart grids and renewable energy integration. USAL's research includes the development of control algorithms for the dynamic operation of PtG systems, ensuring efficient energy storage and retrieval. Additionally, their studies have investigated the socio-economic implications of PtG deployment, considering factors such as market readiness and policy frameworks.86,87
Collectively, these research efforts have advanced the state-of-the-art in PtG technologies, addressing critical challenges such as efficiency, scalability, and integration with renewable energy sources. The ongoing work by these and other institutions continues to push the boundaries of PtG, making it a viable solution for energy storage and a key enabler of the transition to a sustainable and resilient energy system.
6. Future perspectives for the implementation of green hydrogen production
Within the contextual framework of the United Nations' Agenda 2030, circular economy principles and Industry 4.0, the implementation of green hydrogen production is as promising as it is challenging. This paper discussed the barriers that impede the integration of green hydrogen into the broader sustainability agenda, which go beyond technical complexities and dives into the geopolitical, economic, and socio-environmental dimensions.88 Looking forward, the perspectives outlined here envision a future where green hydrogen becomes a key player in global energy trade.89 This will require an advance in policies, maturing of available technologies as well as proposing new and more competitive alternatives. Achieving the necessary infrastructure deployment on a global scale requires collaboration between nations and industries, overcoming regulatory hurdles and ensuring a coherent global framework. Although economies of scale are anticipated, initial investment costs and ongoing operating expenses remain high, so it is important to develop efficient technologies and processes that minimize energy losses and improve the overall efficiency of production processes. It is crucial to develop a resilient and secure supply chain for critical components such as electrolyzers and catalysts. Dependence on certain regions for raw materials can create vulnerabilities that affect the reliability of green hydrogen production.In the context of the 2030 Agenda and the Sustainable Development Goals, the integration of carbon capture and utilization technologies with green hydrogen production represents a significant advancement in achieving environmental sustainability. By capturing CO2 emissions and utilizing them in downstream processes or converting them into valuable products, such as synthetic fuels or chemicals, green hydrogen production becomes part of a circular economy approach, minimizing environmental impact and enhancing resource efficiency.
Advancements in hydrogen storage technologies are equally crucial. Novel materials for high-density storage and innovative liquid carriers ensure efficient storage, enhancing the availability and flexibility of green hydrogen for diverse applications. These solutions are pivotal in enabling the widespread adoption of green hydrogen across various sectors, supporting sustainable development goals related to clean energy access (SDG 7), industry innovation (SDG 9), and climate action (SDG 13).
Moreover, green hydrogen holds the potential to emerge as a significant energy export commodity, particularly for countries blessed with abundant renewable resources. This not only supports economic growth but also strengthens international partnerships centered on sustainable energy solutions, aligning with SDG 17 (partnerships for the goals).
In the midst of global energy transformation, green hydrogen emerges as a cornerstone, blending advanced technology with geopolitical strategies and the imperative of sustainability. Challenges such as developing scalable infrastructure and optimizing energy efficiency underscore the complexities of transitioning to a hydrogen-based future. Nevertheless, green hydrogen remains steadfast in its promise to decarbonize industries, reshape global trade dynamics, and stimulate economic development.81
The convergence of strategic investments, international collaboration, and forward-thinking policies accelerates progress towards a future where green hydrogen not only surmounts obstacles but also redefines global energy landscapes. Its integration into the energy mix promises resilient, decarbonized, and interconnected systems, paving the way for an environmentally friendly future aligned with the aspirations of the 2030 Agenda.
7. Impact of hydrogen production on the sustainable development goals (SDGs)
Green hydrogen production plays a critical role in advancing the Sustainable Development Goals (SDGs) of the 2030 Agenda for Sustainable Development. The various methods of hydrogen production, such as water electrolysis, ethanol steam reforming, biomass gasification, high-temperature thermochemical processes, and H2 standards and purification, collectively contribute to achieving SDGs by promoting affordable and clean energy, sustainable industrial innovation, and climate action (see Table 2).7.1 SDG 7: affordable and clean energy
Water electrolysis, ethanol steam reforming, biomass gasification and thermochemical processes enhance energy efficiency and reduces energy consumption, making green hydrogen more economically viable and ensuring a sustainable and reliable energy supply through the integration of renewable energy sources.7.2 SDG 9: industry, innovation, and infrastructure
Innovation in water electrolysis, including electrolyzer design and performance, supports sustainable industrial practices and infrastructure development, optimizing energy use and storage through smart grid solutions. Ethanol steam reforming integrates renewable feedstocks and technological advancements, lowering operational costs and improving competitiveness, which supports sustainable industrial practices. Biomass gasification fosters innovation and infrastructure development by promoting sustainable industrialization through the research and development of advanced gasification technologies. High-temperature thermochemical processes drive industrial innovation, infrastructure development, and economic growth, promoting inclusive and sustainable industrialization.7.3 SDG 13: climate action
Technological advancements in water electrolysis reduce the carbon intensity of hydrogen production, supporting global efforts to mitigate climate change by lowering overall carbon emissions and reducing dependency on fossil fuels. Increased efficiency and the use of durable materials in ethanol steam reforming reduce energy consumption and CO2 emissions per unit of hydrogen produced, reinforcing climate mitigation efforts. Biomass gasification, particularly when integrated with carbon capture and storage (CCS) technologies, reduces greenhouse gas emissions, promoting carbon-negative processes and supporting global climate goals. High-temperature thermochemical processes reduce greenhouse gas emissions and fossil fuel dependency, aligning with global climate goals and supporting climate change mitigation and resilience efforts.7.4 SDG 12: responsible consumption and production
Biomass gasification promotes a circular economy by utilizing biomass and waste materials for hydrogen production, converting waste into valuable energy resources, and reducing environmental impact. This method supports responsible consumption and production patterns by integrating sustainable practices into the hydrogen production process.7.5 SDG 15: life on land
Biomass gasification supports the conservation and sustainable use of terrestrial ecosystems by ensuring sustainable sourcing and management of biomass feedstocks. This approach contributes to the sustainable use of land resources and biodiversity conservation.7.6 SDG 17: partnerships for the goals
Achieving the full potential of biomass gasification for hydrogen production requires strong partnerships between governments, the private sector, academia, and civil society. These collaborations enhance the means of implementation and revitalize global partnerships for sustainable development, fostering a cooperative approach to advancing the SDGs.Thus, the strategic implementation of green hydrogen production technologies significantly advances the sustainable development goals. By providing affordable and clean energy, driving sustainable industrial practices, fostering innovation, and supporting climate action, green hydrogen production offers a transformative pathway towards global sustainability and prosperity (Table 7).
| Water electrolysis | Ethanol reforming | Biomass gasification | Thermochemical processes | |
|---|---|---|---|---|
| SDG 7 | ✗ | ✗ | ✗ | ✗ |
| SDG 9 | ✗ | ✗ | ✗ | ✗ |
| SDG 13 | ✗ | ✗ | ✗ | ✗ |
| SDG 12 | ✗ | |||
| SDG 15 | ✗ | |||
| SDG 17 | ✗ |
8. Conclusions
This study has thoroughly explored the implications and strategic opportunities associated with green hydrogen production, focusing on addressing technical challenges and capitalizing on opportunities to achieve the goals of the 2030 Agenda. Through detailed analysis of various green hydrogen production methods, a complex yet promising landscape for integration into the global energy future has been identified.Emerging technologies such as renewable-powered water electrolysis and high-temperature thermochemical processes represent significant advancements toward a more sustainable and resilient energy system. These methods promise not only to reduce carbon emissions and enhance energy efficiency but also offer opportunities for decentralized energy production and strengthened global energy security.
A highlight of our analysis has been the integration of carbon capture and utilization technologies with green hydrogen production, further enhancing environmental benefits by promoting a circular and sustainable approach. CO2 capture and utilization in downstream processes, such as synthetic fuel production or chemical manufacturing, illustrate an innovative approach to maximizing resource efficiency and minimizing environmental impact.
Furthermore, ongoing developments in hydrogen storage technologies, including innovative materials and liquid carriers, are crucial for ensuring the availability and flexibility of green hydrogen across various industrial and commercial applications. These solutions support goals related to clean energy access (SDG 7), industrial innovation (SDG 9), and climate action (SDG 13), while also offering favorable economic prospects by facilitating international trade in sustainable energy.
In conclusion, the strategic implementation of green hydrogen production not only addresses complex technical challenges such as optimizing energy efficiency and scaling up infrastructure but also presents significant opportunities to transform energy systems toward sustainability. By leveraging strategic investments, international collaboration, and progressive policies, we can advance toward a future where green hydrogen not only meets the sustainable development goals of the 2030 Agenda but also catalyzes a transition to more resilient, decarbonized, and interconnected global energy systems.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Hoelzen, D. Silberhorn, T. Zill, B. Bensmann and R. Hanke-Rauschenbach, Hydrogen-Powered Aviation and Its Reliance on Green Hydrogen Infrastructure – Review and Research Gaps, Int. J. Hydrogen Energy, 2022, 47(5), 3108–3130, DOI:10.1016/J.IJHYDENE.2021.10.239.
- H. Lei and B. Khandelwal, Hydrogen Fuel for Aviation, Aviation Fuels, 2021, pp. 237–270, DOI:10.1016/B978-0-12-818314-4.00007-8.
- T. Yusaf, A. S. Faisal Mahamude, K. Kadirgama, D. Ramasamy, K. Farhana, H. A. Dhahad and A. R. Abu Talib, Sustainable Hydrogen Energy in Aviation – A Narrative Review, Int. J. Hydrogen Energy, 2024, 52, 1026–1045, DOI:10.1016/J.IJHYDENE.2023.02.086.
- S. Manigandan, T. R. Praveenkumar, J. Ir Ryu, T. Nath Verma and A. Pugazhendhi, Role of Hydrogen on Aviation Sector: A Review on Hydrogen Storage, Fuel Flexibility, Flame Stability, and Emissions Reduction on Gas Turbines Engines, Fuel, 2023, 352, 129064, DOI:10.1016/J.FUEL.2023.129064.
- J. Huete and P. Pilidis, Parametric Study on Tank Integration for Hydrogen Civil Aviation Propulsion, Int. J. Hydrogen Energy, 2021, 46(74), 37049–37062, DOI:10.1016/J.IJHYDENE.2021.08.194.
- D. S. Falcão, Green Hydrogen Production by Anion Exchange Membrane Water Electrolysis: Status and Future Perspectives, Energies, 2023, 16(2), 943, DOI:10.3390/EN16020943.
- P. Chatterjee, M. S. K. Ambati, A. K. Chakraborty, S. Chakrabortty, S. Biring, S. Ramakrishna, T. K. S. Wong, A. Kumar, R. Lawaniya and G. K. Dalapati, Photovoltaic/Photo-Electrocatalysis Integration for Green Hydrogen: A Review, Energy Convers. Manag., 2022, 261, 115648, DOI:10.1016/J.ENCONMAN.2022.115648.
- H. Rezk, A. G. Olabi, M. A. Abdelkareem, A. Alahmer and E. T. Sayed, Maximizing Green Hydrogen Production from Water Electrocatalysis: Modeling and Optimization, J. Mar. Sci. Eng., 2023, 11(3), 617, DOI:10.3390/JMSE11030617.
- A. H. Martins, A. Rouboa and E. Monteiro, On the Green Hydrogen Production through Gasification Processes: A Techno-Economic Approach, J. Clean. Prod., 2023, 383, 135476, DOI:10.1016/J.JCLEPRO.2022.135476.
- J. E. Lee, I. Shafiq, M. Hussain, S. S. Lam, G. H. Rhee and Y. K. Park, A Review on Integrated Thermochemical Hydrogen Production from Water, Int. J. Hydrogen Energy, 2022, 47(7), 4346–4356, DOI:10.1016/J.IJHYDENE.2021.11.065.
- E. Meloni, M. Martino, G. Iervolino, C. Ruocco, S. Renda, G. Festa and V. Palma, The Route from Green H2 Production through Bioethanol Reforming to CO2 Catalytic Conversion: A Review, Energies, 2022, 15(7), 2383, DOI:10.3390/EN15072383.
- B. S. Zainal, P. J. Ker, H. Mohamed, H. C. Ong, I. M. R. Fattah, S. M. A. Rahman, L. D. Nghiem and T. M. I. Mahlia, Recent Advancement and Assessment of Green Hydrogen Production Technologies, Renew. Sustain. Energy Rev., 2024, 189, 113941, DOI:10.1016/J.RSER.2023.113941.
- G. Squadrito, G. Maggio and A. Nicita, The Green Hydrogen Revolution, Renew. Energy, 2023, 216, 119041, DOI:10.1016/J.RENENE.2023.119041.
- F. Gutiérrez-Martín, L. Amodio and M. Pagano, Hydrogen Production by Water Electrolysis and Off-Grid Solar PV, Int. J. Hydrogen Energy, 2021, 46(57), 29038–29048, DOI:10.1016/J.IJHYDENE.2020.09.098.
- M. David, C. Ocampo-Martínez and R. Sánchez-Peña, Advances in Alkaline Water Electrolyzers: A Review, J. Energy Storage, 2019, 23, 392–403, DOI:10.1016/J.EST.2019.03.001.
- S. W. Sharshir, A. Joseph, M. M. Elsayad, A. A. Tareemi, A. W. Kandeal and M. R. Elkadeem, A Review of Recent Advances in Alkaline Electrolyzer for Green Hydrogen Production: Performance Improvement and Applications, Int. J. Hydrogen Energy, 2024, 49, 458–488, DOI:10.1016/J.IJHYDENE.2023.08.107.
- H. A. Miller, K. Bouzek, J. Hnat, S. Loos, C. I. Bernäcker, T. Weißgärber, L. Röntzsch and J. Meier-Haack, Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions, Sustain. Energy Fuels, 2020, 4(5), 2114–2133, 10.1039/C9SE01240K.
- Q. Hassan, A. Z. Sameen, H. M. Salman and M. Jaszczur, Large-Scale Green Hydrogen Production via Alkaline Water Electrolysis Using Solar and Wind Energy, Int. J. Hydrogen Energy, 2023, 48(88), 34299–34315, DOI:10.1016/J.IJHYDENE.2023.05.126.
- A. Nechache and S. Hody, Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review, Renew. Sustain. Energy Rev., 2021, 149, 111322, DOI:10.1016/J.RSER.2021.111322.
- G. Kakoulaki, I. Kougias, N. Taylor, F. Dolci, J. Moya and A. Jäger-Waldau, Green Hydrogen in Europe – A Regional Assessment: Substituting Existing Production with Electrolysis Powered by Renewables, Energy Convers. Manag., 2021, 228, 113649, DOI:10.1016/J.ENCONMAN.2020.113649.
- A. Grimm, W. A. de Jong and G. J. Kramer, Renewable Hydrogen Production: A Techno-Economic Comparison of Photoelectrochemical Cells and Photovoltaic-Electrolysis, Int. J. Hydrogen Energy, 2020, 45(43), 22545–22555, DOI:10.1016/J.IJHYDENE.2020.06.092.
- A. Boretti, There Are Hydrogen Production Pathways with Better than Green Hydrogen Economic and Environmental Costs, Int. J. Hydrogen Energy, 2021, 46(46), 23988–23995, DOI:10.1016/J.IJHYDENE.2021.04.182.
- H. Canton, International Energy Agency—IEA, The Europa Directory of International Organizations, 2021, pp. 684–686, DOI:10.4324/9781003179900-103.
- Council H., Path to Hydrogen Competitiveness: A Cost Perspective, 2020 Search PubMed.
- S. E. Hosseini and M. A. Wahid, Hydrogen from Solar Energy, a Clean Energy Carrier from a Sustainable Source of Energy, Int. J. Energy Res., 2020, 44(6), 4110–4131, DOI:10.1002/ER.4930.
- G. Kakoulaki, I. Kougias, N. Taylor, F. Dolci, J. Moya and A. Jäger-Waldau, Green Hydrogen in Europe – A Regional Assessment: Substituting Existing Production with Electrolysis Powered by Renewables, Energy Convers. Manag., 2021, 228, 113649, DOI:10.1016/J.ENCONMAN.2020.113649.
- M. A. Khan, I. Al-Shankiti, A. Ziani and H. Idriss, Demonstration of Green Hydrogen Production Using Solar Energy at 28% Efficiency and Evaluation of Its Economic Viability, Sustain. Energy Fuels, 2021, 5(4), 1085–1094, 10.1039/D0SE01761B.
- G. Cheng, E. Luo, Y. Zhao, Y. Yang, B. Chen, Y. Cai, X. Wang and C. Dong, Analysis and Prediction of Green Hydrogen Production Potential by Photovoltaic-Powered Water Electrolysis Using Machine Learning in China, Energy, 2023, 284, 129302, DOI:10.1016/J.ENERGY.2023.129302.
- D. Mazzeo, M. S. Herdem, N. Matera and J. Z. Wen, Green Hydrogen Production: Analysis for Different Single or Combined Large-Scale Photovoltaic and Wind Renewable Systems, Renew. Energy, 2022, 200, 360–378, DOI:10.1016/J.RENENE.2022.09.057.
- L. B. B. Maciel, L. Viola, W. de Queiróz Lamas and J. L. Silveira, Environmental Studies of Green Hydrogen Production by Electrolytic Process: A Comparison of the Use of Electricity from Solar PV, Wind Energy, and Hydroelectric Plants, Int. J. Hydrogen Energy, 2023, 48(93), 36584–36604, DOI:10.1016/J.IJHYDENE.2023.05.334.
- S. Calnan, R. Bagacki, F. Bao, I. Dorbandt, E. Kemppainen, C. Schary, R. Schlatmann, M. Leonardi, S. A. Lombardo, R. G. Milazzo, S. M. S. Privitera, F. Bizzarri, C. Connelli, D. Consoli, C. Gerardi, P. Zani, M. Carmo, S. Haas, M. Lee, M. Mueller, W. Zwaygardt, J. Oscarsson, L. Stolt, M. Edoff, T. Edvinsson and I. B. Pehlivan, Development of Various Photovoltaic-Driven Water Electrolysis Technologies for Green Solar Hydrogen Generation, Sol. RRL, 2022, 6(5), 2100479, DOI:10.1002/SOLR.202100479.
- European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the regions, A hydrogen strategy for a climate-neutral Europe, 2020 Search PubMed.
- R. Bhattacharyya, A. Misra and K. C. Sandeep, Photovoltaic Solar Energy Conversion for Hydrogen Production by Alkaline Water Electrolysis: Conceptual Design and Analysis, Energy Convers. Manag., 2017, 133, 1–13, DOI:10.1016/J.ENCONMAN.2016.11.057.
- R. Ahshan, A. Onen and A. H. Al-Badi, Assessment of Wind-to-Hydrogen (Wind-H2) Generation Prospects in the Sultanate of Oman, Renew. Energy, 2022, 200, 271–282, DOI:10.1016/J.RENENE.2022.09.116.
- A. H. Schrotenboer, A. A. T. Veenstra, M. A. J. uit het Broek and E. Ursavas, A Green Hydrogen Energy System: Optimal Control Strategies for Integrated Hydrogen Storage and Power Generation with Wind Energy, Renew. Sustain. Energy Rev., 2022, 168, 112744, DOI:10.1016/J.RSER.2022.112744.
- A. Kim, H. Kim, C. Choe and H. Lim, Feasibility of Offshore Wind Turbines for Linkage with Onshore Green Hydrogen Demands: A Comparative Economic Analysis, Energy Convers. Manag., 2023, 277, 116662, DOI:10.1016/J.ENCONMAN.2023.116662.
- Y. Zheng, S. You, C. Huang and X. Jin, Model-Based Economic Analysis of off-Grid Wind/Hydrogen Systems, Renew. Sustain. Energy Rev., 2023, 187, 113763, DOI:10.1016/J.RSER.2023.113763.
- M. Nasser and H. Hassan, Thermo-Economic Performance Maps of Green Hydrogen Production via Water Electrolysis Powered by Ranges of Solar and Wind Energies, Sustain. Energy Technol. Assessments, 2023, 60, 103424, DOI:10.1016/J.SETA.2023.103424.
- D. Apostolou and P. Enevoldsen, The Past, Present and Potential of Hydrogen as a Multifunctional Storage Application for Wind Power, Renew. Sustain. Energy Rev., 2019, 112, 917–929, DOI:10.1016/J.RSER.2019.06.049.
- W. H. Chen, S. C. Li, S. Lim, Z. Y. Chen and J. C. Juan, Reaction and Hydrogen Production Phenomena of Ethanol Steam Reforming in a Catalytic Membrane Reactor, Energy, 2021, 220, 119737, DOI:10.1016/J.ENERGY.2020.119737.
- L. Mosca, J. A. Medrano Jimenez, S. A. Wassie, F. Gallucci, E. Palo, M. Colozzi, S. Taraschi and G. Galdieri, Process Design for Green Hydrogen Production, Int. J. Hydrogen Energy, 2020, 45(12), 7266–7277, DOI:10.1016/J.IJHYDENE.2019.08.206.
- Q. Liu, H. Zhou and Z. Jia, Hydrogen Production by Ethanol Reforming on Supported Ni-Cu Catalysts, ACS Omega, 2022, 7(5), 4577–4584, DOI:10.1021/ACSOMEGA.1C06579/ASSET/IMAGES/MEDIUM/AO1C06579_M004.GIF.
- E. Matus, O. Sukhova, I. Ismagilov, M. Kerzhentsev, O. Stonkus and Z. Ismagilov, Hydrogen Production through Autothermal Reforming of Ethanol: Enhancement of Ni Catalyst Performance via Promotion, Energies, 2021, 14(16), 5176, DOI:10.3390/EN14165176.
- M. W. Menezes, D. R. Simmons, S. Winberg, R. Baranwal, P. Hoffman and S. L. Genatowski, U.S. Department of Energy Hydrogen Program Plan Search PubMed.
- S. Valizadeh, H. Hakimian, A. Farooq, B. H. Jeon, W. H. Chen, S. Hoon Lee, S. C. Jung, M. Won Seo and Y. K. Park, Valorization of Biomass through Gasification for Green Hydrogen Generation: A Comprehensive Review, Bioresour. Technol., 2022, 365, 128143, DOI:10.1016/J.BIORTECH.2022.128143.
- A. H. Martins, A. Rouboa and E. Monteiro, On the Green Hydrogen Production through Gasification Processes: A Techno-Economic Approach, J. Clean. Prod., 2023, 383, 135476, DOI:10.1016/J.JCLEPRO.2022.135476.
- Y. Guo, S. Z. Wang, D. H. Xu, Y. M. Gong, H. H. Ma and X. Y. Tang, Review of Catalytic Supercritical Water Gasification for Hydrogen Production from Biomass, Renew. Sustain. Energy Rev., 2010, 14(1), 334–343, DOI:10.1016/J.RSER.2009.08.012.
- A. Molino, S. Chianese and D. Musmarra, Biomass Gasification Technology: The State of the Art Overview, J. Energy Chem., 2016, 25(1), 10–25, DOI:10.1016/J.JECHEM.2015.11.005.
- Y. Kalinci, A. Hepbasli and I. Dincer, Life Cycle Assessment of Hydrogen Production from Biomass Gasification Systems, Int. J. Hydrogen Energy, 2012, 37(19), 14026–14039, DOI:10.1016/J.IJHYDENE.2012.06.015.
- E. E. Ozbas, D. Aksu, A. Ongen, M. A. Aydin and H. K. Ozcan, Hydrogen Production via Biomass Gasification, and Modeling by Supervised Machine Learning Algorithms, Int. J. Hydrogen Energy, 2019, 44(32), 17260–17268, DOI:10.1016/J.IJHYDENE.2019.02.108.
- N. J. Rubinsin, N. A. Karim, S. N. Timmiati, K. L. Lim, W. N. R. W. Isahak and M. Pudukudy, An Overview of the Enhanced Biomass Gasification for Hydrogen Production, Int. J. Hydrogen Energy, 2024, 49, 1139–1164, DOI:10.1016/J.IJHYDENE.2023.09.043.
- C. C. Cormos, Green Hydrogen Production from Decarbonized Biomass Gasification: An Integrated Techno-Economic and Environmental Analysis, Energy, 2023, 270, 126926, DOI:10.1016/J.ENERGY.2023.126926.
- V. G. Nguyen, T. X. Nguyen-Thi, P. Q. Phong Nguyen, V. D. Tran, Ü. Ağbulut, L. H. Nguyen, D. Balasubramanian, W. Tarelko, S. A. Bandh and N. D. Khoa Pham, Recent Advances in Hydrogen Production from Biomass Waste with a Focus on Pyrolysis and Gasification, Int. J. Hydrogen Energy, 2024, 54, 127–160, DOI:10.1016/J.IJHYDENE.2023.05.049.
- B. Cook and C. Hagen, Techno-Economic Analysis of Biomass Gasification for Hydrogen Production in Three US-Based Case Studies, Int. J. Hydrogen Energy, 2024, 49, 202–218, DOI:10.1016/J.IJHYDENE.2023.07.219.
- Ö. Tezer, N. Karabağ, A. Öngen, C. Ö. Çolpan and A. Ayol, Biomass Gasification for Sustainable Energy Production: A Review, Int. J. Hydrogen Energy, 2022, 47(34), 15419–15433, DOI:10.1016/J.IJHYDENE.2022.02.158.
- O. Oruc and I. Dincer, Assessing the Potential of Thermo-Chemical Water Splitting Cycles: A Bridge towards Clean and Sustainable Hydrogen Generation, Fuel, 2021, 286, 119325, DOI:10.1016/J.FUEL.2020.119325.
- S. Abanades, Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy, ChemEngineering, 2019, 3(3), 63, DOI:10.3390/CHEMENGINEERING3030063.
- C. Prieto, P. Cooper, A. I. Fernández and L. F. Cabeza, Review of Technology: Thermochemical Energy Storage for Concentrated Solar Power Plants, Renew. Sustain. Energy Rev., 2016, 60, 909–929, DOI:10.1016/J.RSER.2015.12.364.
- Y. Deng, R. Dewil, L. Appels, S. Li, J. Baeyens, J. Degrève and G. Wang, Thermo-Chemical Water Splitting: Selection of Priority Reversible Redox Reactions by Multi-Attribute Decision Making, Renew. Energy, 2021, 170, 800–810, DOI:10.1016/J.RENENE.2021.02.009.
- K. G. Burra, P. Chandna and A. K. Gupta, Thermochemical Solutions for CO2 Utilization to Fuels and Value-Added Products, Green Energy and Technology, 2021, pp. 59–89, DOI:10.1007/978-981-15-5667-8_4/FIGURES/12.
- Y. Ligen, H. Vrubel and H. Girault, Energy Efficient Hydrogen Drying and Purification for Fuel Cell Vehicles, Int. J. Hydrogen Energy, 2020, 45(18), 10639–10647, DOI:10.1016/J.IJHYDENE.2020.02.035.
- S. Chugh, S. Meenakshi, K. Sonkar, A. Sharma, G. S. Kapur and S. S. V. Ramakumar, Performance Evaluation of PEM Fuel Cell Stack on Hydrogen Produced in the Oil Refinery, Int. J. Hydrogen Energy, 2020, 45(8), 5491–5500, DOI:10.1016/J.IJHYDENE.2019.06.160.
- A. Murugan and A. S. Brown, Review of Purity Analysis Methods for Performing Quality Assurance of Fuel Cell Hydrogen, Int. J. Hydrogen Energy, 2015, 40(11), 4219–4233, DOI:10.1016/J.IJHYDENE.2015.01.041.
- M. Aasadnia, M. Mehrpooya and B. Ghorbani, A Novel Integrated Structure for Hydrogen Purification Using the Cryogenic Method, J. Clean. Prod., 2021, 278, 123872, DOI:10.1016/J.JCLEPRO.2020.123872.
- Z. Du, C. Liu, J. Zhai, X. Guo, Y. Xiong, W. Su and G. He, A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles, Catalysts, 2021, 11(3), 393, DOI:10.3390/CATAL11030393.
- L. Schorer, S. Schmitz and A. Weber, Membrane Based Purification of Hydrogen System (MEMPHYS), Int. J. Hydrogen Energy, 2019, 44(25), 12708–12714, DOI:10.1016/J.IJHYDENE.2019.01.108.
- D. Dunikov, V. Borzenko and S. Malyshenko, Influence of Impurities on Hydrogen Absorption in a Metal Hydride Reactor, Int. J. Hydrogen Energy, 2012, 37(18), 13843–13848, DOI:10.1016/J.IJHYDENE.2012.04.078.
- J. B. S. Hamm, A. Ambrosi, J. G. Griebeler, N. R. Marcilio, I. C. Tessaro and L. D. Pollo, Recent Advances in the Development of Supported Carbon Membranes for Gas Separation, Int. J. Hydrogen Energy, 2017, 42(39), 24830–24845, DOI:10.1016/J.IJHYDENE.2017.08.071.
- S. E. Hosseini and M. A. Wahid, Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development, Renew. Sustain. Energy Rev., 2016, 57, 850–866, DOI:10.1016/J.RSER.2015.12.112.
- G. Di Marcoberardino, D. Vitali, F. Spinelli, M. Binotti and G. Manzolini, Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit, Processes, 2018, 6(3), 19, DOI:10.3390/PR6030019.
- Y. S. Montenegro Camacho, S. Bensaid, G. Piras, M. Antonini and D. Fino, Techno-Economic Analysis of Green Hydrogen Production from Biogas Autothermal Reforming, Clean Technol. Environ. Policy, 2017, 19(5), 1437–1447, DOI:10.1007/S10098-017-1341-1/TABLES/8.
- D. Haeseldonckx and W. D'haeseleer, The Use of the Natural-Gas Pipeline Infrastructure for Hydrogen Transport in a Changing Market Structure, Int. J. Hydrogen Energy, 2007, 32(10–11), 1381–1386, DOI:10.1016/J.IJHYDENE.2006.10.018.
- C. Zhang, Y. Shao, W. Shen, H. Li, Z. Nan, M. Dong, J. Bian and X. Cao, Key Technologies of Pure Hydrogen and Hydrogen-Mixed Natural Gas Pipeline Transportation, ACS Omega, 2023, 8(22), 19212–19222, DOI:10.1021/ACSOMEGA.3C01131/ASSET/IMAGES/LARGE/AO3C01131_0007.JPEG.
- F. Öney, T. N. Vezirolu and Z. Dülger, Evaluation of Pipeline Transportation of Hydrogen and Natural Gas Mixtures, Int. J. Hydrogen Energy, 1994, 19(10), 813–822, DOI:10.1016/0360-3199(94)90198-8.
- M. Rüdisüli, R. Mutschler, S. L. Teske, D. Sidler, D. B. van den Heuvel, L. W. Diamond, K. Orehounig and S. Eggimann, Potential of Renewable Surplus Electricity for Power-to-Gas and Geo-Methanation in Switzerland, Int. J. Hydrogen Energy, 2023, 48(39), 14527–14542, DOI:10.1016/J.IJHYDENE.2022.12.290.
- F. Tabkhi, C. Azzaro-Pantel, L. Pibouleau and S. Domenech, A Mathematical Framework for Modelling and Evaluating Natural Gas Pipeline Networks under Hydrogen Injection, Int. J. Hydrogen Energy, 2008, 33(21), 6222–6231, DOI:10.1016/J.IJHYDENE.2008.07.103.
- R. Qiu, H. Zhang, G. Wang, Y. Liang and J. Yan, Green Hydrogen-Based Energy Storage Service via Power-to-Gas Technologies Integrated with Multi-Energy Microgrid, Appl. Energy, 2023, 350, 121716, DOI:10.1016/J.APENERGY.2023.121716.
- B. C. Erdener, B. Sergi, O. J. Guerra, A. Lazaro Chueca, K. Pambour, C. Brancucci and B. M. Hodge, A Review of Technical and Regulatory Limits for Hydrogen Blending in Natural Gas Pipelines, Int. J. Hydrogen Energy, 2023, 48(14), 5595–5617, DOI:10.1016/J.IJHYDENE.2022.10.254.
- M. Yu, K. Wang and H. Vredenburg, Insights into Low-Carbon Hydrogen Production Methods: Green, Blue and Aqua Hydrogen, Int. J. Hydrogen Energy, 2021, 46(41), 21261–21273, DOI:10.1016/J.IJHYDENE.2021.04.016.
- H. Ishaq, I. Dincer and C. Crawford, A Review on Hydrogen Production and Utilization: Challenges and Opportunities, Int. J. Hydrogen Energy, 2022, 47(62), 26238–26264, DOI:10.1016/J.IJHYDENE.2021.11.149.
- G. Kakoulaki, I. Kougias, N. Taylor, F. Dolci, J. Moya and A. Jäger-Waldau, Green Hydrogen in Europe – A Regional Assessment: Substituting Existing Production with Electrolysis Powered by Renewables, Energy Convers. Manag., 2021, 228, 113649, DOI:10.1016/J.ENCONMAN.2020.113649.
- B. E. Lebrouhi, J. J. Djoupo, B. Lamrani, K. Benabdelaziz and T. Kousksou, Global Hydrogen Development - A Technological and Geopolitical Overview, Int. J. Hydrogen Energy, 2022, 47(11), 7016–7048, DOI:10.1016/J.IJHYDENE.2021.12.076.
- P. J. Megia, A. J. Vizcaino, J. A. Calles and A. Carrero, Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review, Energy Fuel., 2021, 35(20), 16403–16415, DOI:10.1021/ACS.ENERGYFUELS.1C02501/ASSET/IMAGES/LARGE/EF1C02501_0004.JPEG.
- N. Gerloff, Levelized and Environmental Costs of Power-to-Gas Generation in Germany, Int. J. Hydrogen Energy, 2023, 48(49), 18541–18556, DOI:10.1016/J.IJHYDENE.2023.01.347.
- A. Barbaresi, M. Morini and A. Gambarotta, Review on the Status of the Research on Power-to-Gas Experimental Activities, Energies, 2022, 15(16), 5942, DOI:10.3390/EN15165942.
- V. M. Maestre, A. Ortiz and I. Ortiz, Transition to a Low-Carbon Building Stock. Techno-Economic and Spatial Optimization of Renewables hydrogen Strategies in Spain, J. Energy Storage, 2022, 56, 105889, DOI:10.1016/J.EST.2022.105889.
- A. Almena and M. Martín, Chemical Engineering to Aid an Effective Shift Towards Sustainable Low-Carbon Energy Systems: A BECCS Case Study, Contributions of Chemical Engineering to Sustainability, 2024, pp. 359–384, DOI:10.1007/978-3-031-55594-7_12.
- O. Essahili, O. Lakbita, M. Ouafi and O. Moudam, Overview of Advanced Research in Luminescent Solar Concentrators for Green Hydrogen Production, Sol. Energy, 2023, 262, 111859, DOI:10.1016/J.SOLENER.2023.111859.
- V. A. Panchenko, Y. V. Daus, A. A. Kovalev, I. V. Yudaev and Y. V. Litti, Prospects for the Production of Green Hydrogen: Review of Countries with High Potential, Int. J. Hydrogen Energy, 2023, 48(12), 4551–4571, DOI:10.1016/J.IJHYDENE.2022.10.084.
| This journal is © The Royal Society of Chemistry 2025 |