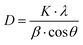Fuel production capacity and DFT analysis of cation modified perovskites for enhanced thermochemical CO2 dissociation
Received
8th November 2024
, Accepted 6th February 2025
First published on 7th February 2025
Abstract
Solar thermochemical redox splitting of CO2 using perovskite oxygen carriers in two-step cycles is a promising method for sustainable fuel production. In this study, a series of 23 potential perovskite candidates for CO production are designed, synthesized, and tested under the same experimental conditions. The material stability and the lattice structure are validated using Goldschmidt's tolerance factor and powder X-ray diffraction. For the reduction step, the high proportion of divalent cations (Sr2+/Ba2+/Ca2+) in the A site promotes oxygen transfer, and the maximum oxygen yield reaches 386 μmol g−1 (δ = 0.164) for Gd0.6Ca0.4MnO3. DFT calculation results indicate that the multi-cationic doping in La0.5Sr0.2Ba0.15Ca0.15MnO3 shows a smaller energy barrier for oxygen transfer compared with the single A-site doping in La0.5Sr0.5MnO3, with an oxygen vacancy formation energy of 2.91 eV per (O atom), and it offers the most favorable CO yields of 225 and 227 μmol g−1 in two consecutive cycles. The designed La0.25Gd0.25Sr0.25Ca0.25MnO3 further decreases the oxygen vacancy formation energy to 2.57 eV per (O atom). Based on the reaction rate analysis, the presence of B-site doping cations, such as in La0.6Sr0.4Mn0.75Zr0.25O3 and La0.5Sr0.5Mn0.8Ce0.2O3, increases the maximum oxidation rate, and the A-site multi doping of perovskites allows maintaining high CO production rates during the oxidation process. This work leverages tunable perovskite redox properties for enhanced CO production performance through DFT and thermochemical performance analysis, providing feasible guidance to promote CO2 splitting by an active cation doping strategy.
Sustainability spotlight
New sustainable and alternative energy carriers are required to limit CO2 emissions and climate change resulting from the intensive use of fossil fuels and their combustion. This study addresses the sustainable production of solar fuels from two-step CO2 splitting cycles using solid oxide intermediates. This thermochemical pathway offers a thermodynamically favourable route for solar energy harvesting and decarbonized fuel production, as it converts the entire solar spectrum to supply the required high-temperature process heat, without using expensive catalysts or intermediate electricity production. Solar recycling/conversion of captured CO2 to synthetic fuels is also an alternative to underground CO2 sequestration. Accordingly, this can contribute to achieving the global net zero carbon emissions and sustainable development goals (particularly SDGs 7, 12 & 13).
|
1 Introduction
The direct conversion of solar energy into fuels by thermochemical methods achieves the utilization of the full solar spectrum, with no greenhouse gas generation and high energy storability in the form of solar chemical fuels.1 Among the most attractive solar fuel production methods, the two-step redox cycles for splitting CO2 or H2O have been widely studied recently with relatively moderate reaction temperatures and high theoretical solar-to-fuel production efficiency up to 68%.2,3 Temperatures typically in the range of 1400–1500 °C for the reduction step and experimental solar-to-fuel efficiencies in the range of 4–10% were achieved.4–7 First, the metal oxides (MOx) are reduced to the oxygen deficient state (MOx−δ) using concentrated solar energy as the process heat source. Then, the reduced oxides absorb the oxygen from CO2 or H2O at a relatively lower oxidation temperature. The redox reactions are presented below:8,9| |  | (1) |
| | | MOx−δ + δH2O/CO2 → MOx + δCO/H2 | (2) |
Ceria (CeO2) and perovskite-based oxides (ABO3 type) are promising oxygen carriers for thermochemical redox reactions.10,11 The rapid fuel production kinetics and multi-cycle stability of ceria make it an attractive option in the past several years.12 However, the low capacity for oxygen non-stoichiometry of reduced ceria limits the maximum fuel production yield.13 To improve the fuel production potential of redox materials, ABO3 type perovskites have been widely studied recently because of their strong oxygen transfer capacity and the large number of available formulations.14,15 Typically, LaMnO3-based perovskites present excellent fuel production performance with an effective cation doping.16–24 Nair et al. found that La0.5Sr0.5MnO3 perovskite synthesized by the Pechini method yielded an optimum CO production and Sr2+ was the best A-site cation dopant.25 Subsequent studies investigated the CO production of Ba/Sr/Co/Fe/Ni/Ce substituted LaMnO3 perovskites, and the results proved that LaxSr1−xMnO3 provided excellent fuel production capacity, while La0.6Sr0.4(CeyMn1−y)O3 showed enhanced CO2 splitting performance.26,27 Through thermodynamic analysis, Carrillo et al. reported that La0.6Sr0.4CrO3−δ has a lower CO2 splitting Gibbs free energy compared with La0.6Sr0.4MnO3−δ, and the synthesized La0.6Sr0.4Cr0.85Mn0.15O3 showed a fast CO production rate of 1.5 mL min−1 g−1.20 Experimentally, the promising perovskites such as CeTi2O6, La0.6Sr0.4Mn0.6Al0.4O3 and BaMn0.75Ce0.25O3 with B site substitution of Mn by Al or Ce showed a rapid oxidation kinetic rate and displayed large reaction entropies.28–32 Although previous studies have proposed numerous high-performance perovskites, the different experimental operation conditions make it difficult to compare the fuel production results.33 In this context, we performed the thermogravimetry analyses of 23 potential perovskites for comparison of their redox activity in the context of two-step CO2 splitting. This is one of the first studies that analyze the fuel production performance of a large number of potential perovskites with different doping strategies under the same experimental conditions. The materials were synthesized by the Pechini sol–gel method and characterized by X-ray diffraction.
The perovskite's oxygen transfer capacity determines the maximum fuel yield and the reaction kinetic rate limits the solar-to-fuel conversion efficiency. Normally, perovskites with excellent oxygen transfer capacity show a high reduction extent (oxygen release), but at the expense of a thermodynamic limitation during oxidation, thus requiring a large temperature swing between redox steps or large oxidant excess to overcome the thermodynamic barrier. The oxidation step can further be hindered by a low kinetic rate due to limitations of the solid–gas surface reaction, thereby limiting the rate of fuel production.28 The oxidation is an exothermic reaction. Thermodynamically, a low reaction temperature is beneficial for H2O or CO2 splitting. However, the kinetic rate decreases at low reaction temperatures. Thus, both thermodynamic and kinetic limitations occur in the oxidation process. The fuel production kinetics in the oxidation process is important because rapid fuel production is needed to decrease the required energy input and increase the fuel production efficiency. To improve the fuel production performance, perovskites with single-cation doping, double-cation doping (cations doped in both A and B sites), and multi-cation doping (more than two cations doped in the A or B site) were synthesized in this work. Actually, some A-site high doping perovskites have been studied with excellent CO production performance.34,35 Therefore, several potential multi-cation doped perovskites were considered to compare the CO yields with single/double cation doped perovskites. We chose the lanthanide elements La, Gd, and Sm as the A-site base elements, and Mn as the B-site base element. As shown in Fig. 1, the divalent cations Sr2+, Ba2+ and Ca2+ are doped in the A site to promote oxygen transfer, and the high valence cations Zr4+, Cr3+, Fe3+, Al3+ and Ce3+ are doped in the B site to adjust the reaction kinetic rate during oxidation. The selected perovskites were synthesized primarily because these formulations were not considered before. For the A site cation doping, the divalent cations (Sr2+, Ba2+, and Ca2+) and the lanthanide elements (Gd3+ and Sm3+) were used to substitute La3+ for better oxygen transfer capacity. Substituting divalent Ca2+, Sr2+, and Ba2+ cations for La3+, Pr3+, Sm3+ or Gd3+ (A site) aims to create several effects leading to structural changes/deviations, cation deficiency, oxygen non-stoichiometry, improved catalytic oxidation activity, and controlled transition between Mn3+ and Mn4+. The substitution of divalent cations causes the valence of the Mn ion to increase above 3, which favors the cation reduction from a high valence state to Mn3+. Similarly, B-site substitution has also beneficial effects. Experimentally, previous studies suggested that B-site cation doping (Al3+, Zr4+, Ce3+, Fe3+, and Cr3+) of LaMnO3 perovskites led to rapid oxidation kinetic rates. Basically, cationic doping of perovskites in the A or B site was shown to be beneficial for the generation of oxygen vacancies within the perovskites. For multi-doped perovskites, the increment of the element number can increase the molar configurational entropy of the perovskites (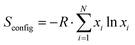 , where R is the universal gas constant and xi is the molar fraction of the element i).36 The high entropy value makes the oxidation process easier to occur because of the low value of reaction Gibbs free energy. The material stability is predicted using the calculated Goldschmidt's tolerance factor and further validated using X-ray diffraction analysis results. Through the thermogravimetric analyses, the CO yields of perovskites are determined and the first principles DFT study of oxygen vacancy formation energy demonstrates the beneficial effects of multi-cation doping on oxygen transfer. Apart from the CO yields, the evolved gas production rates of synthesized perovskites are also obtained in this work. The study analyzes the experimental production performance of the 23 potential perovskites in terms of fuel yields and gas production rates, which supports the search for novel high-performance perovskites for thermochemical CO2 splitting.
, where R is the universal gas constant and xi is the molar fraction of the element i).36 The high entropy value makes the oxidation process easier to occur because of the low value of reaction Gibbs free energy. The material stability is predicted using the calculated Goldschmidt's tolerance factor and further validated using X-ray diffraction analysis results. Through the thermogravimetric analyses, the CO yields of perovskites are determined and the first principles DFT study of oxygen vacancy formation energy demonstrates the beneficial effects of multi-cation doping on oxygen transfer. Apart from the CO yields, the evolved gas production rates of synthesized perovskites are also obtained in this work. The study analyzes the experimental production performance of the 23 potential perovskites in terms of fuel yields and gas production rates, which supports the search for novel high-performance perovskites for thermochemical CO2 splitting.
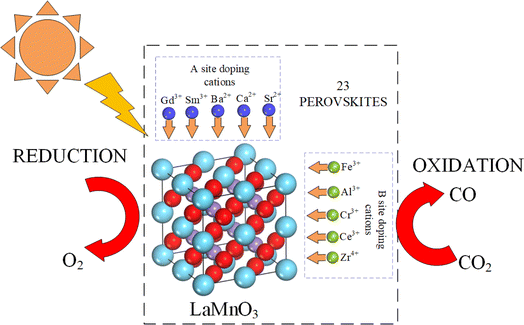 |
| | Fig. 1 Schematic diagram of tunable CO production of perovskites by doping cations. | |
2 Materials and methods
2.1 Materials synthesis
The doped perovskites were synthesized by the Pechini sol–gel method.37 The main raw salt precursors were supplied by the Alfa Aesar company, which include Gd(NO3)3·6H2O (99.9%), La(NO3)3·6H2O (99.9%), Sm(NO3)3·6H2O (99.9%), Sr(NO3)2 (99%), Ca(NO3)2·4H2O (98%), Mn(NO3)·4H2O (97.5%), Fe(NO)3·9H2O (98%), Al(NO3)3·9H2O (99%), Ce(NO3)3·6H2O (99%), ZrN2O7·6H2O (99.5%), Cr(NO3)3·6H2O (99%), citric acid C6H8O7 (99.5%) and ethylene glycol C2H6O2 (99%). First, the nitrates were added into the beaker based on the designed molar proportion. Then, citric acid was mixed with the nitrates in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dissolving in deionized water. After the nitrate solution was stirred and heated to 70 °C, glycol was added to the solution. Then, the solution heating continued to 85 °C with stirring at this temperature to form the gel. The formed gel was placed into a drying oven at 120 °C for 3 h. A two-step calcination process was performed to avoid the loss of Mn.38 First, the powders were calcined at 800 °C for 90 min followed by cooling to room temperature. Then, the powders were heated to 1400 °C for 6 h (3 h ramp up and 3 h dwell) and cooled to form a stable powder structure.
1, dissolving in deionized water. After the nitrate solution was stirred and heated to 70 °C, glycol was added to the solution. Then, the solution heating continued to 85 °C with stirring at this temperature to form the gel. The formed gel was placed into a drying oven at 120 °C for 3 h. A two-step calcination process was performed to avoid the loss of Mn.38 First, the powders were calcined at 800 °C for 90 min followed by cooling to room temperature. Then, the powders were heated to 1400 °C for 6 h (3 h ramp up and 3 h dwell) and cooled to form a stable powder structure.
2.2 Experimental methods
To determine the crystal structures and the lattice parameters of the synthesized perovskite powders, the powder X-ray diffraction (PXRD) patterns were obtained at room temperature with an X'Pert Pro PANalytical diffractometer with Cu Kα radiation (λ = 0.15418 nm). The diffraction angles (2θ) were measured in the range of 10–90° with a step size of 0.017°. The crystal and lattice parameters were analyzed by cell refinement using Jade 6.0 software.
The gas production yields and redox performance of the synthesized perovskites in the two-step thermochemical redox reactions were obtained by thermogravimetry analysis (TGA) using a thermal analyzer SETARAM Setsys Evolution. The processed powders with a weight of about 100 mg were placed in a platinum crucible. The reduction temperature was set at 1400 °C with a heating rate of 20 K min−1. After holding the samples at 1400 °C for 45 min, the reduced perovskites were cooled to the oxidation temperature of 1050 °C, holding for 1 h. In the reduction step, high purity argon (99.999% purity, 2 ppm O2) was used with a flow rate of 20 N mL min−1 (normal conditions). During the oxidation step, CO2 (with 99.995% purity) was injected at a flow rate of 10 N mL min−1 with a CO2 molar fraction of 50% in argon. Two consecutive cycles were carried out to provide an overview of the material's redox performance and capacity for CO2 splitting, and a comparison among the considered formulations in terms of fuel yields and gas production rates. The main aim of this work was to unravel the effect of cation modified perovskites on performance in the fuel production process, which helps for the further selection of effective cations for CO2 splitting. Therefore, various cations incorporated into perovskites were tested under the same experimental conditions. For subsequent cycles after the second cycle, it is generally admitted that the fuel production capacity does not evolve significantly compared to the initial two cycles because the morphological and structural changes generally occur in the first cycle. Thus, the performance of the second cycle is representative of the material's redox activity.
The sample mass variations (loss during reduction due to O2 release and gain during oxidation due to O atom replenishment from CO2 gas to produce CO) were measured continuously to determine the O2 and CO production yields (i.e., the amounts produced per unit mass of perovskite).39
The oxygen yield (mol g−1) can be obtained using eqn (3):37
| | 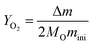 | (3) |
where Δ
m is the perovskite's mass change during the reduction process (g),
MO is the molar mass of the atomic oxygen (16 g mol
−1), and
mini is the initial mass of the perovskites (
g).
To determine the reduction extent, the oxygen non-stoichiometry (δ) is calculated using eqn (4):
| | 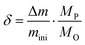 | (4) |
where
MP is the molar mass of the perovskites. The CO yields during oxidation are calculated using the following equation:
| |  | (5) |
In the redox reactions, the O
2 and CO production rates (mol g
−1 s
−1) can be calculated using the following equations:
| |  | (6) |
| |  | (7) |
where
rm (g s
−1) is the rate of sample mass variation obtained from the first order derivative of the mass change.
3 Results and discussion
3.1 Materials characterization
The stability of ABO3 type perovskite structures can be predicted using Goldschmidt's tolerance factor, which reflects the relationship between the average A–O and B–O distances. For the ideal cubic perovskite structure, the tolerance value (t) is 1. The perovskite phase will be distorted to a tetrahedral or hexagonal phase when t > 1, and orthorhombic or rhombohedral phases will form when t < 0.9.40 The original equation is presented below :41,42| |  | (8) |
where rA, rB and rO are the ionic radii of cations A, B and O, respectively. The ionic radii of cations are obtained from Shannon.43 For the ABO3 type perovskites, the 12-fold coordinated A site is filled with low valence metal cations and the 6-fold coordinated B site is occupied by the high valence metal cations. When the A or B site is doped with more than one cation, we adopt the equivalent ionic radius method to get the average cation radii of the A and B site elements.44,45| | 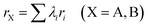 | (9) |
where λi is the molar proportion of the element i and ri represents the ionic radius of i. For example, the tolerance factor of La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 is calculated using eqn (10).| |  | (10) |
The tolerance factor calculation results of the target perovskites are shown in Table 1, ranging from 0.838 to 1.009. The results from a previous study show that perovskites can remain in stable phases in the t range of 0.813 < t < 1.107.46 Therefore, all of the designed materials are predicted to form stable perovskite structures, which are consistent with the X-ray diffraction results in Table 1. The GdMnO3 and SmMnO3 based perovskites present an orthorhombic structure, and the LaMnO3 based perovskites present a cubic structure. Goldschmidt's tolerance factor is useful in preliminary design and can help to assess the perovskite's stability, supporting the effective synthesis and experimental performance studies of the designed materials.
Table 1 Tolerance factors and XRD refinement parameters of perovskites
|
|
Goldschmidt tolerance factor |
Lattice parameters |
Crystallite size (nm) |
|
a (Å) |
b (Å) |
c (Å) |
|
Single A-site cation doping
|
| GdMnO3 |
0.897 |
5.324 |
5.792 |
7.468 |
86.3 |
| Gd0.75La0.25MnO3-Before |
0.92 |
5.376 |
5.738 |
7.535 |
66.1 |
| Gd0.75La0.25MnO3-After |
5.384 |
5.813 |
7.526 |
65.1 |
| Gd0.9Ca0.1MnO3-B |
0.905 |
5.383 |
5.830 |
7.519 |
63.1 |
| Gd0.9Ca0.1MnO3-A |
5.375 |
5.832 |
7.522 |
63.6 |
| Gd0.75Ca0.25MnO3-B |
0.918 |
5.498 |
5.890 |
7.536 |
39.3 |
| Gd0.75Ca0.25MnO3-A |
5.510 |
5.913 |
7.569 |
31.1 |
| Gd0.6Ca0.4MnO3-B |
0.93 |
5.458 |
5.860 |
7.481 |
38.4 |
| Gd0.6Ca0.4MnO3-A |
5.485 |
5.874 |
7.558 |
26.5 |
| Gd0.75Sr0.25MnO3-B |
0.927 |
5.431 |
5.828 |
7.430 |
44.2 |
| Gd0.75Sr0.25MnO3-A |
5.442 |
5.843 |
7.449 |
41.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
Multiple cation doping
|
| Gd0.75Ca0.25Mn0.75Fe0.25O3 |
0.92 |
5.449 |
5.846 |
7.482 |
34.5 |
| Gd0.6Ca0.4Mn0.75Cr0.25O3-B |
0.838 |
5.418 |
5.818 |
7.430 |
48 |
| Gd0.6Ca0.4Mn0.75Cr0.25O3-A |
5.444 |
5.845 |
7.467 |
42.3 |
| La0.6Sr0.4Mn0.5Fe0.25Al0.25O3-B |
0.948 |
3.875 |
3.875 |
3.875 |
47.9 |
| La0.6Sr0.4Mn0.5Fe0.25Al0.25O3-A |
3.881 |
3.881 |
3.881 |
64.5 |
| La0.6Sr0.4Mn0.75Ce0.25O3-B |
1.009 |
3.869 |
3.869 |
3.869 |
40.8 |
| La0.6Sr0.4Mn0.75Ce0.25O3-A |
3.871 |
3.871 |
3.871 |
35.6 |
| La0.6Sr0.4Mn0.75Zr0.25O3-B |
0.982 |
3.901 |
3.901 |
3.901 |
36.5 |
| La0.6Sr0.4Mn0.75Zr0.25O3-A |
3.904 |
3.904 |
3.904 |
25.5 |
| La0.3Gd0.3Sr0.4MnO3-B |
0.954 |
3.908 |
3.908 |
3.908 |
52.2 |
| La0.3Gd0.3Sr0.4MnO3-A |
3.893 |
3.893 |
3.893 |
65.1 |
| La0.25Gd0.25Sr0.25Ca0.25MnO3-B |
0.955 |
3.908 |
3.908 |
3.908 |
37.9 |
| La0.25Gd0.25Sr0.25Ca0.25MnO3-A |
3.880 |
3.880 |
3.880 |
50.6 |
| Sm0.5Sr0.5Mn0.8Ce0.2O3-B |
0.94 |
5.417 |
7.641 |
5.446 |
36.6 |
| Sm0.5Sr0.5Mn0.8Ce0.2O3-A |
5.419 |
7.714 |
5.441 |
52.9 |
| La0.5Sr0.5Mn0.8Ce0.2O3-B |
0.96 |
5.461 |
5.461 |
5.461 |
38.87 |
| La0.5Sr0.5Mn0.8Ce0.2O3-A |
5.468 |
5.468 |
5.468 |
41.4 |
| La0.5Sr0.2Ba0.15Ca0.15MnO3-B |
1 |
3.869 |
3.869 |
3.869 |
26.8 |
| La0.5Sr0.2Ba0.15Ca0.15MnO3-A |
3.875 |
3.875 |
3.875 |
35.4 |
| La0.5Sr0.2Ba0.15Ca0.15Mn0.7Ce0.15Zr0.15O3-B |
0.964 |
3.876 |
3.876 |
3.876 |
33.8 |
| La0.5Sr0.2Ba0.15Ca0.15Mn0.7Ce0.15Zr0.15O3-A |
3.884 |
3.884 |
3.884 |
27.88 |
| Y1/6La1/6Sm1/6Sr1/6Ba1/6Ca1/6Mn0.7Ce0.15Al0.15O3-B |
0.956 |
5.505 |
5.505 |
5.505 |
45.8 |
| Y1/6La1/6Sm1/6Sr1/6Ba1/6Ca1/6Mn0.7Ce0.15Al0.15O3-A |
5.477 |
5.477 |
5.477 |
66.6 |
The crystallite sizes and lattice parameters are presented in Table 1. Crystallite sizes of synthesized perovskites are calculated using the Scherrer equation:47
| | 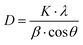 | (11) |
where
D is the mean crystallite size (nm),
β is the full width at half maximum (FWHM) intensity,
λ is the X-ray wavelength,
θ is the Bragg angle, and
K is a dimensionless shape factor, which is assumed to be 0.89.
Fig. 2 shows the change of the crystallite sizes before (black symbols) and after (red symbols) the cycling experiments. For most of the single and double cation doped perovskites, the crystallite sizes decrease after the reaction, indicating that the materials are not significantly sintered after the experiment. In contrast, the multi-cation doped perovskites show an obvious increment in the crystallite size.
 |
| | Fig. 2 Crystallite sizes and Goldschmidt tolerance factors of perovskites. | |
The X-ray diffraction patterns of fresh and cycled materials are presented in Fig. 3. The synthesized perovskites are fitted with the related ICSD (inorganic crystal structure database) patterns. For the single cation doped materials, the replacement of Gd3+ (1.107 Å) with the larger ionic radius elements Ca2+ (1.34 Å), La3+ (1.36 Å), and Sr2+ (1.44 Å) causes the shift of the main Bragg peak (112) to lower angles, indicating that the cations are successfully incorporated into the parent perovskite. Fig. 3(b) shows that the high Ce4+ and Zr4+ doping proportions have generated side peaks of SrZrO3 and CeO2. The same situation occurs in Gd0.75Ca0.25Mn0.75Fe0.25O3, which is extremely sintered after the reaction. It is noted that cation doping in the B site may decrease the purity of target perovskites and generate byproducts. All of the multiple A site doped perovskites remain in the form of pure phases after the experiments, revealing that cation doping in the A site does not affect the material's phase purity.
 |
| | Fig. 3 X-ray powder diffraction patterns of perovskites: (a) single cation doping, (b) double cation doping, (c) multiple cation doping before and after the thermochemical cycles between 1050 °C and 1400 °C. | |
3.2 Thermochemical reaction performance
A summary of the obtained thermochemical reaction yields of synthesized perovskites is presented in Table 2. The overall fuel production performance with O2/CO yields and conversion ratios are reported. The results help to assess the material's reduction capacity and the CO production performance in two successive cycles, and the CO/O2 conversion ratio is determined based on the experimental results.
Table 2 Thermochemical reaction yields of 23 perovskites in two consecutive cycles
|
|
First cycle |
Second cycle |
| O2 (μmol g−1) |
CO (μmol g−1) |
n(CO)/n(O2) |
O2 (μmol g−1) |
CO (μmol g−1) |
n(CO)/n(O2) |
| Gd0.75La0.25MnO3 |
78 |
24 |
0.31 |
22 |
25 |
1.13 |
| Gd0.75Ca0.25MnO3 |
191 |
144 |
0.75 |
104 |
152 |
1.46 |
| Gd0.75Sr0.25MnO3 |
285 |
158 |
0.554 |
97 |
166 |
1.71 |
| Gd0.75Ca0.25Mn0.75Fe0.25O3 |
307 |
119 |
0.387 |
92 |
136 |
1.48 |
| Gd0.75Ca0.25Mn0.75Cr0.25O3 |
318 |
108 |
0.339 |
99 |
109 |
1.1 |
| Gd0.9Ca0.1MnO3 |
100 |
59 |
0.59 |
48 |
63 |
1.3 |
| Gd0.6Ca0.4MnO3 |
386 |
145 |
0.375 |
113 |
156 |
1.38 |
| La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 |
257 |
230 |
0.894 |
121 |
211 |
1.74 |
| La0.25Gd0.25Sr0.25Ca0.25MnO3 |
368 |
184 |
0.5 |
105 |
190 |
1.81 |
| Sm0.75Ca0.25MnO3 |
140 |
112 |
0.8 |
73 |
128 |
1.75 |
| La0.6Sr0.4Mn0.75Zr0.25O3 |
112 |
92 |
0.821 |
52 |
98 |
1.88 |
| La0.6Sr0.4Mn0.75Ce0.25O3 |
126 |
178 |
1.412 |
95 |
178 |
1.87 |
| Pr0.5Sr0.5MnO3 |
268 |
153 |
0.57 |
90 |
160 |
1.77 |
| Sm0.5Sr0.5MnO3 |
373 |
168 |
0.45 |
101 |
175 |
1.73 |
| La0.5Sr0.5MnO3 |
232 |
190 |
0.82 |
104 |
195 |
1.87 |
| Sm0.6Ca0.4Mn0.8Al0.2O3 |
206 |
213 |
1.03 |
180 |
212 |
1.17 |
| Sm0.75Sr0.25MnO3 |
117 |
177 |
1.51 |
95 |
180 |
1.89 |
| La0.3Gd0.3Sr0.4MnO3 |
225 |
182 |
0.8 |
103 |
183 |
1.77 |
| La0.5Sr0.2Ba0.15Ca0.15MnO3 |
271 |
225 |
0.83 |
113 |
227 |
2 |
| La0.5Sr0.5Mn0.8Ce0.2O3 |
205 |
205 |
1 |
110 |
204 |
1.85 |
| Sm0.5Sr0.5Mn0.8Ce0.2O3 |
292 |
164 |
0.56 |
97 |
169 |
1.74 |
| La0.5Sr0.2Ba0.15Ca0.15Mn0.7Ce0.15Zr0.15O3 |
118 |
162 |
1.37 |
88 |
161 |
1.82 |
| Y1/6La1/6Sm1/6Sr1/6Ba1/6Ca1/6Mn0.7Ce0.15Al0.15O3 |
319 |
127 |
0.39 |
91 |
143 |
1.57 |
3.2.1 Reduction capacity.
The synthesized perovskites need to produce sufficient oxygen vacancies to increase maximum CO yields during oxidation. As shown in Fig. 4, TGA of materials was used to determine the mass variation of (a) single doping, (b) double doping, and (c) multiple cation doping of perovskites during two successive reduction–reoxidation cycles. The mass loss in the first reduction step represents the oxygen transfer capacity. Overall, the perovskites Gd0.6Ca0.4MnO3, Sm0.5Sr0.5MnO3 and La0.25Gd0.25Sr0.25Ca0.25MnO3 exhibit the largest mass loss, reaching 1.2%, whereas Gd0.75La0.25MnO3 shows the lowest reduction extent, with a mass loss of only 0.23%. These results reveal that doping of divalent cations in the A site (Sr2+, Ca2+, and Ba2+) is beneficial for the generation of oxygen vacancies within the perovskites. Besides, the trivalent cations in the A site also influence the oxygen transfer, with the reduction capacity from strong to weak being in the following order: Gd3+ > Sm3+ > Pr3+ > La3+. Compared with the single doping of perovskites in Fig. 4(a), the mass losses during reduction of Cr3+/Fe3+ doped Gd0.75Ca0.25Mn0.75Cr0.25O3/Gd0.75Ca0.25Mn0.75Fe0.25O3 and Ce3+ doped Sm0.5Sr0.5Mn0.8Ce0.2O3/La0.5Sr0.5Mn0.75Ce0.25O3 decrease with the existence of B site doping cations. For multi-cation doped perovskites, the mass loss of La0.5Sr0.2Ba0.15Ca0.15MnO3 is 0.5% larger than that of La0.5Sr0.2Ba0.15Ca0.15Mn0.7Ce0.15Zr0.15O3. The above results indicate the unfavorable effect of B site doping cations on oxygen production. Overall, the presence of Gd3+ and the high doping proportion of divalent cations are beneficial for generating more oxygen in the reduction step.
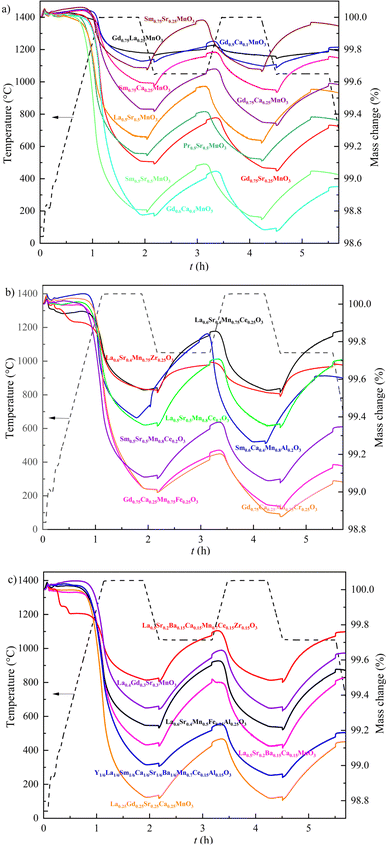 |
| | Fig. 4 TGA mass changes of (a) single doping, (b) double doping, and (c) multi-doping of perovskites during two successive thermochemical cycles between 1050 °C and 1400 °C. | |
Fig. 5 shows the oxygen yields of perovskites in two successive redox cycles by plotting both the oxygen yield and δ values for each material to highlight the reduction capacity. Fig. 5(a and b) represent the oxygen production yields of perovskites in the first reduction step, and Fig. 5(c and d) show the oxygen production yields in the second reduction step. For the first reduction step, the maximum oxygen yield reaches 386 μmol g−1 (δ = 0.164) from Gd0.6Ca0.4MnO3, but the oxygen yield decreases to 113 μmol g−1 in the second cycle. The main reason is that the reaction kinetic rate limits the oxygen vacancy generation in the second cycle. The maximum oxygen non-stoichiometry value of Gd0.6Ca0.4MnO3 is over 10 times larger than the value of CeO2 (δ = 0.016).48 An almost linear relationship can be obtained between the oxygen yield (μmol g−1) and δ in Fig. 5. However, the amount of oxygen produced per unit mass of oxide also relies on the molecular weight of the material, which explains why the values are not directly proportional. Sm0.6Ca0.4Mn0.8Al0.2O3 shows the largest oxygen yield at 180 μmol g−1 in the second reduction step, with a δ value of 0.07. The high ratio of Ca2+ incorporation leads to the best reduction performance in the successive cycles compared with other materials. The cation doping in the B site decreases the oxygen transfer capacity in the first cycle. However, doping of B site cations (Al3+, Fe3+, and Ce3+) presents high oxygen yields in the second redox cycle. Owing to the refill of more oxygen vacancies in the first oxidation step, B-site doped perovskites have improved oxygen production capacity in the successive second reduction step.
 |
| | Fig. 5 Oxygen production yields of perovskites in two successive cycles: (a and b) first cycle for perovskites with low and high reduction extents, and (c and d) second cycle for perovskites with low and high reduction extents. | |
For the A site multi-doping Mn-based perovskites, the reduction extent can be calculated by the variation in the Mn ion valence. As shown in Fig. 6, the decrease in Mn ion valence for Gd0.6Ca0.4MnO3 is larger than that for other perovskites, reaching a value of 0.33. With the same initial Mn ion valence, the reduction capacity of high doping La0.25Gd0.25Sr0.25Ca0.25MnO3 is better than that of La0.5Sr0.2Ba0.15Ca0.15MnO3. For low cation doping proportions, the Sr2+ incorporation shows better reduction performance than the Ca2+ substituted GdMnO3. It is observed that the Mn ion valence of single cation doped perovskites tends to reach a similar final value (below 3.1 in any case), whereas the A site high doping of perovskites cannot reach such a low Mn ion valence state.
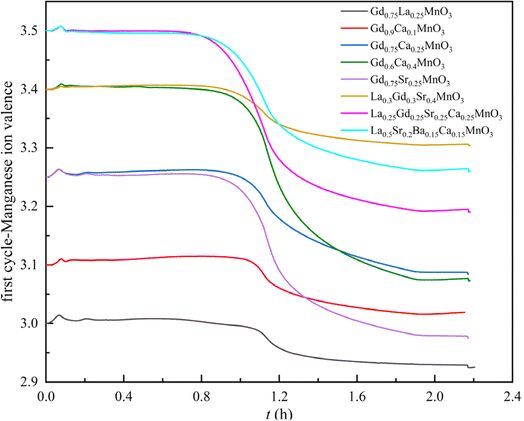 |
| | Fig. 6 Mn ion valence variation of A-site doped Mn-based perovskites for the reduction step of the first cycle. | |
With the same A-site doping proportion of divalent cations, La0.5Sr0.5MnO3, La0.5Sr0.2Ba0.15Ca0.15MnO3 and La0.25Gd0.25Sr0.25Ca0.25MnO3 show different oxygen production capacities. The removal of oxygen from perovskites in the reduction process needs to overcome the energy barrier, which is noted as the oxygen vacancy formation energy ΔEV (eV per (O atom)). In this work, the ΔEV values were predicted using the Cambridge serial total energy package (CASTEP) of Material Studio software through the density functional theory (DFT) calculation, which is based on the periodic boundary conditions and plane-wave expansion of the wave function.49,50 The calculation equation is presented below:51
| |  | (12) |
where
Evac and
E are the DFT total energies of the cubic perovskite cells with and without an oxygen vacancy (eV), and
EO2 is the energy of oxygen molecules (eV). The calculations are based on density functional theory with functions of generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE), using the ultrasoft pseudopotentials.
52,53 The supercells of 2 × 2 × 2 are established with 40 atoms, and 2 × 2 × 2
k point meshes are generated using the Monkhorst–Pack scheme. The setting parameters of analyses include: a cutoff energy of 650 eV, self-consistent field (SCF) of 1.0 × 10
−8 eV per atom, maximum force of 0.01 eV per atom, maximum displacement of 5 × 10
−4 Å and energy convergence criteria of 2.0 × 10
−5 eV per atom. The calculation result of oxygen vacancy formation energy is first verified using existing LaMnO
3 data (3.927 eV per O atom), obtained by Emery
et al.14 The obtained value in our simulation is 3.897 eV per (O atom), with an energy difference of 0.03 eV per (O atom). Then, the divalent cation substitution of La
3+ in the A site of the LaMnO
3 supercell in the model and the oxygen defected structure of supercells are presented in
Fig. 7. It is often considered that the oxygen vacancy formation energy should be above 2.5 eV per (O atom) to overcome the energy barrier for the oxidation step. From the calculation results of the established CASTEP program, doping of the divalent cation Sr
2+ causes an obvious decrease of the required energy for oxygen vacancy formation. Indeed, the oxygen vacancy formation energy decreases from 3.897 eV per (O atom) to 3.07 eV per (O atom). With the presence of multi-doping cations (Sr
2+, Ba
2+, and Ca
2+) in the A site, the reduction energy band further decreases to 2.91 eV per (O atom) for oxygen transfer. Although the global proportion of divalent cation doping is the same, the existence of multi-doping cations can reduce the energy barrier for oxygen transfer. In addition, the incorporation of Gd
3+ leads to a further decrease in the oxygen vacancy formation energy to 2.57 eV per (O atom). The A-site high cation doping thus results in a significant decrease in the energy barriers for oxygen transfer, which supports the experimentally measured oxygen yield results.
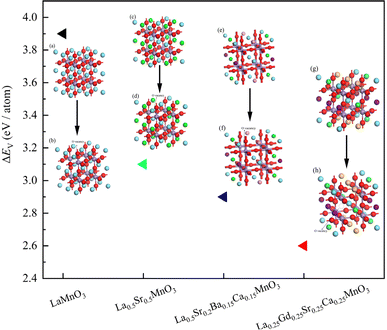 |
| | Fig. 7 Oxygen vacancy formation energy of the perovskites and variations with doping cations: (a) LaMnO3, (b) oxygen deficient LaMnO3, (c) La0.5Sr0.5MnO3, (d) oxygen deficient La0.5Sr0.5MnO3, (e) La0.5Sr0.2Ba0.15Ca0.15MnO3, (f) oxygen deficient La0.5Sr0.2Ba0.15Ca0.15MnO3, (g) La0.25Gd0.25Sr0.25Ca0.25MnO3, and (h) oxygen deficient La0.25Gd0.25Sr0.25Ca0.25MnO3. Blue atoms represent La, light purple atoms represent Mn, red atoms represent O, green atoms represent Sr, pink atoms represent Ba, deep purple atoms represent Ca, and yellow atoms represent Gd. | |
3.2.2 CO production performance.
The CO yields of the synthesized 23 perovskites are presented in Fig. 8. The black symbols represent the CO production results of the first cycle and the red symbols represent the CO yields of the second cycle. La0.5Sr0.2Ba0.15Ca0.15MnO3 exhibits the best CO production performance in two consecutive thermochemical cycles, with the CO yields of 225 and 227 μmol g−1 respectively. Under the same thermochemical cycling conditions of this study, ceria is able to produce up to about 100 μmolCO g−1 after a reduction step at 1400 °C and oxidation with 50% CO2/Ar at 1050 °C.37,54 The highest CO yield is obtained by La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 (230 μmol g−1), but the CO yield decreases slightly to 211 μmol g−1 in the second cycle. All of the synthesized perovskites show overall good stability, as evidenced by the CO production performance remaining stable in multiple cycles. Regarding the single cation doped perovskites, the presence of the Sr2+ cation results in higher CO yields than with the Ca2+ doped perovskites, and the most favorable single cation doped perovskite for CO2 splitting is La0.5Sr0.5MnO3. To improve the CO production performance, a strategy is to incorporate B site cations into the single cation doped perovskites. The doping of Al3+ and Ce3+ in the B site enhances CO production performance, whereas the doping of Fe3+, Zr3+ and Cr3+ in the B site does not improve the CO yields. The Ce3+ incorporation into La0.5Sr0.5MnO3 shows an obvious increase in the CO yield. On the basis of the individual A/B site cation doping effects, the multiple cation doped perovskites are designed with the aim to increase CO yields. Accordingly, two high-performance La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 and La0.5Sr0.2Ba0.15Ca0.15MnO3 perovskites are identified in this work. In contrast, the other considered high doping cations in both A and B sites do not show competitive CO yields.
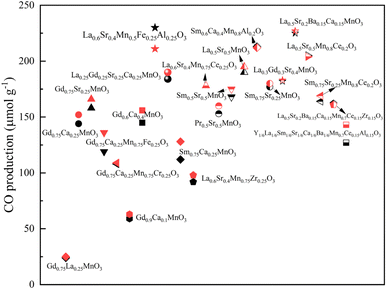 |
| | Fig. 8 CO production yields of 23 single, double, and multiple cation doped perovskites (black symbols represent the results of the first cycle and red symbols represent the results of the second cycle). | |
3.2.3 CO/O2 molar ratio.
The CO/O2 ratios achieved from redox cycling of synthesized perovskites are presented in Fig. 9. It compares the CO/O2 ratio for each group of materials, which allows identification of the materials showing the highest conversion ratios among the considered formulations because this ratio is an important indicator of the reaction extent. The complete redox reactions lead to a conversion ratio of 2. It is observed that the CO/O2 conversion ratio of all the materials exhibits an obvious increase in the second cycle. This is because the oxygen vacancies generated in the first cycle are not refilled completely, which benefits the CO2 splitting in the second cycle. Regarding the single cation doped perovskites, Sm0.75Sr0.25MnO3 shows the best CO/O2 conversion performance, with the maximum conversion ratios of 1.51 and 1.89, respectively, while Gd0.75La0.25MnO3 shows the lowest conversion ratio. The single cation doped perovskites with the incorporation of Sr2+ exhibit better CO/O2 conversion than those with Ca2+ and La3+. For the double cation doped perovskites, Sm0.6Ca0.4Mn0.8Al0.2O3 has the highest CO/O2 conversion ratio in the first cycle, although the ratio of the second cycle is low. In addition, the Fe3+ and Ce3+ doped perovskites show a good CO/O2 conversion ratio in the second cycle. Gd0.75Ca0.25Mn0.75Cr0.25O3 has the lowest conversion ratio compared with other double cation doped perovskites. It is noted that the CO/O2 conversion ratios of all the high cation doped perovskites are over 1.5 in the second cycle.
 |
| | Fig. 9 CO/O2 conversion ratios of (a) single doping, (b) double doping, and (c) multi doping of perovskites during two consecutive thermochemical cycles. | |
3.3 Gas production rate analysis
The reaction kinetics for both cycle steps of the synthesized perovskites is essential for improving the process efficiency. The results of the gas production rate calculation are presented in Fig. 10, and the peak reaction rates are shown in Table 3. All the materials are divided into three groups based on the doping strategies. Overall, Gd0.6Ca0.4MnO3 shows the most rapid oxygen production rate in the first cycle, reaching a maximum value of 0.375 μmol g−1 s−1, and La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 has the highest oxygen production rate of 0.14 μmol g−1 s−1 in the second reduction step. It is found that the oxygen production rates are improved with the existence of active B-site doping cations such as Ce3+, Fe3+, and Al3+. Compared with the single cation doped perovskites, the double doping and high doping of perovskites present better CO production rate performance in two consecutive cycles. The presence of Zr4+ allows for rapid CO production, but the CO rates decrease more rapidly in the oxidation step. The rate evolution tendency indicates that the promising perovskites should have both a high maximum CO production rate along with sustained high CO rates during the oxidation process. The A-site high doping of La0.5Sr0.2Ba0.15Ca0.15MnO3 perovskite shows sustainable high CO production rates in two successive redox cycles, indicating high CO yields, but the maximum CO production rate is lower than the B-site doped La0.6Sr0.4Mn0.75Zr0.25O3 and La0.6Sr0.4Mn0.75Ce0.25O3 perovskites. The Ce3+ incorporated perovskites, such as La0.6Sr0.4Mn0.75Ce0.25O3 and La0.5Sr0.5Mn0.8Ce0.2O3, present high maximum CO production rates and sustainable high CO rates. In addition, the Fe3+ and Al3+ doped La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 shows excellent CO production kinetics in two cycles. The results suggest that the selection of active B site cations is important to increase the maximum CO production rate, and some perovskites with A site high doping show sustainable high CO production rates in multiple cycles.
 |
| | Fig. 10 Dynamic evolution of gas production rates of (a and b) single doping, (c and d) double doping, and (e and f) multi doping of perovskites in two consecutive cycles. | |
Table 3 Peak reaction rates of perovskites in two consecutive cycles
|
|
Peak O2 production rates (μmol g−1 s−1) |
Peak CO production rates (μmol g−1 s−1) |
| 1st cycle |
2nd cycle |
1st cycle |
2nd cycle |
| Gd0.75La0.25MnO3 |
0.1 |
0.02 |
0.01 |
0.03 |
| Gd0.75Ca0.25MnO3 |
0.17 |
0.1 |
0.08 |
0.08 |
| Gd0.75Sr0.25MnO3 |
0.32 |
0.08 |
0.07 |
0.07 |
| Gd0.9Ca0.1MnO3 |
0.13 |
0.05 |
0.04 |
0.05 |
| Gd0.6Ca0.4MnO3 |
0.37 |
0.08 |
0.06 |
0.07 |
| Gd0.75Ca0.25Mn0.75Fe0.25O3 |
0.33 |
0.07 |
0.06 |
0.07 |
| Gd0.75Ca0.25Mn0.75Cr0.25O3 |
0.33 |
0.06 |
0.04 |
0.04 |
| La0.5Sr0.5MnO3 |
0.203 |
0.103 |
0.096 |
0.1 |
| La0.6Sr0.4Mn0.75Zr0.25O3 |
0.11 |
0.05 |
0.11 |
0.15 |
| La0.6Sr0.4Mn0.75Ce0.25O3 |
0.1 |
0.09 |
0.11 |
0.11 |
| La0.5Sr0.5Mn0.8Ce0.2O3 |
0.18 |
0.12 |
0.1 |
0.1 |
| Pr0.5Sr0.5MnO3 |
0.217 |
0.07 |
0.06 |
0.068 |
| Sm0.75Ca0.25MnO3 |
0.111 |
0.095 |
0.061 |
0.113 |
| Sm0.75Ca0.25Mn0.8Al0.2O3 |
0.278 |
0.406 |
0.231 |
0.096 |
| Sm0.5Sr0.5MnO3 |
0.29 |
0.08 |
0.068 |
0.074 |
| Sm0.5Sr0.5Mn0.8Ce0.2O3 |
0.24 |
0.09 |
0.07 |
0.07 |
| Sm0.75Sr0.25MnO3 |
0.148 |
0.14 |
0.097 |
0.136 |
| La0.3Gd0.3Sr0.4MnO3 |
0.21 |
0.1 |
0.08 |
0.08 |
| La0.6Sr0.4Mn0.5Fe0.25Al0.25O3 |
0.2 |
0.14 |
0.14 |
0.13 |
| La0.25Gd0.25Sr0.25Ca0.25MnO3 |
0.31 |
0.08 |
0.07 |
0.07 |
| La0.5Sr0.2Ba0.15Ca0.15MnO3 |
0.23 |
0.1 |
0.1 |
0.1 |
| La0.5Sr0.2Ba0.15Ca0.15Mn0.7Ce0.15Al0.15O3 |
0.11 |
0.09 |
0.1 |
0.11 |
| Y1/6La1/6Sm1/6Sr1/6Ba1/6Ca1/6Mn0.7Ce0.15Al0.15O3 |
0.23 |
0.09 |
0.05 |
0.07 |
4 Conclusion
In summary, the fuel production performance of 23 potential perovskites was investigated in this work through both thermodynamic (DFT) and experimental TGA analyses. The incorporation of various cations into the perovskites was considered to improve gas production yields. The single cation doped, double cation doped, and multiple cation doped perovskites were synthesized by the Pechini sol–gel method and characterized by X-ray diffraction. All of the A-site doped perovskites remained in the form of a pure phase after the reactions, while the formulations with B-site cation incorporation, such as in La0.5Sr0.5Mn0.8Ce0.2O3, may generate side products. The high proportion of divalent cations enhanced the oxygen transfer and the Gd3+ containing perovskite Gd0.6Ca0.4MnO3 had an excellent oxygen yield of 386 μmol g−1. According to the DFT calculation results, the A-site high doping of La0.5Sr0.2Ba0.15Ca0.15MnO3 and La0.25Gd0.25Sr0.25Ca0.25MnO3 showed a lower energy barrier for oxygen transfer than La0.5Sr0.5MnO3, with the oxygen vacancy formation energy decreasing from 3.07 eV per (O atom) (for La0.5Sr0.5MnO3) to 2.91 eV per (O atom) and 2.57 eV per (O atom), respectively. The most favorable perovskite for CO production is La0.5Sr0.2Ba0.15Ca0.15MnO3, with CO yields of 225 and 227 μmol g−1 in two consecutive cycles, indicating that the high cation doping perovskites have the capacity to achieve significant fuel production. It is noted that the CO/O2 conversion ratios of all the high doping perovskites are over 1.5 in the second cycle. The kinetic analysis reveals that the selection of active B site cations is important to increase the maximum CO production rate, and A site high doping perovskites sustained high CO production rates during the CO2 dissociation process. This work focused on the A and/or B site cation doping effects on the fuel production capacity of different perovskites. Future work should focus on the stability during multiple cycles and fuel yield optimization of these perovskites.
Data availability
The data presented in this study are available in this article.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The one-year research stay of J. Cong at CNRS-PROMES was supported by the Chinese Academy of Sciences. The authors acknowledge the materials characterization platform of PROMES for providing the XRD analysis equipment.
References
- R. J. Carrillo and J. R. Scheffe, Advances and Trends in Redox Materials for Solar Thermochemical Fuel Production, Sol. Energy, 2017, 156, 3–20, DOI:10.1016/j.solener.2017.05.032.
- D. Marxer, P. Furler, M. Takacs and A. Steinfeld, Solar Thermochemical Splitting of CO2 into Separate Streams of CO and O2 with High Selectivity, Stability, Conversion, and Efficiency, Energy Environ. Sci., 2017, 10, 1142–1149, 10.1039/c6ee03776c.
- M. Romero and A. Steinfeld, Concentrating Solar Thermal Power and Thermochemical Fuels, Energy Environ. Sci., 2012, 5, 9234–9245, 10.1039/c2ee21275g.
- A. Le Gal, M. Drobek, A. Julbe and S. Abanades, Improving solar fuel production performance from H2O and CO2 thermochemical dissociation using custom-made reticulated porous ceria, Mater. Today Sustain., 2023, 24, 100542, DOI:10.1016/j.mtsust.2023.100542.
- A. Haeussler, S. Abanades, A. Julbe, J. Jouannaux, M. Drobek, A. Ayral and B. Cartoixa, Remarkable Performance of Microstructured Ceria Foams for Thermochemical Splitting of H2O and CO2 in a Novel High–Temperature Solar Reactor, Chem. Eng. Res. Des., 2020, 156, 311–323, DOI:10.1016/j.cherd.2020.02.008.
- S. Zoller, E. Koepf, D. Nizamian, M. Stephan, A. Patané, P. Haueter, M. Romero, J. González-Aguilar, D. Lieftink and E. de Wit,
et al., A Solar Tower Fuel Plant for the Thermochemical Production of Kerosene from H2O and CO2, Joule, 2022, 6, 1606–1616 CrossRef CAS PubMed.
- B. Bulfin, J. Vieten, C. Agrafiotis, M. Roeb and C. Sattler, Applications and Limitations of Two Step Metal Oxide Thermochemical Redox Cycles; A Review, J. Mater. Chem. A, 2017, 5, 18951–18966 RSC.
- S. Abanades, Redox Cycles. Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review, Energies, 2022, 15, 7061, DOI:10.3390/en15197061.
- S. Abanades, A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications, Materials, 2023, 16, 3582, DOI:10.3390/ma16093582.
- C. L. Muhich, S. Blaser, M. C. Hoes and A. Steinfeld, Comparing the Solar-to-Fuel Energy Conversion Efficiency of Ceria and Perovskite Based Thermochemical Redox Cycles for Splitting H2O and CO2, Int. J. Hydrogen Energy, 2018, 43, 18814–18831, DOI:10.1016/j.ijhydene.2018.08.137.
- A. Haeussler, S. Abanades, J. Jouannaux, M. Drobek, A. Ayral and A. Julbe, Recent Progress on Ceria Doping and Shaping Strategies for Solar Thermochemical Water and CO2 Splitting Cycles, AIMS Mater. Sci., 2019, 6, 657–684, DOI:10.3934/MATERSCI.2019.5.657.
- R. R. Bhosale, G. Takalkar, P. Sutar, A. Kumar, F. AlMomani and M. Khraisheh, A Decade of Ceria Based Solar Thermochemical H2O/CO2 Splitting Cycle, Int. J. Hydrogen Energy, 2019, 44, 34–60, DOI:10.1016/j.ijhydene.2018.04.080.
- M. Kubicek, A. H. Bork and J. L. M. Rupp, Perovskite Oxides-a Review on a Versatile Material Class for Solar-to-Fuel Conversion Processes, J. Mater. Chem. A, 2017, 5, 11983–12000 RSC.
- A. A. Emery, J. E. Saal, S. Kirklin, V. I. Hegde and C. Wolverton, High-Throughput Computational Screening of Perovskites for Thermochemical Water Splitting Applications, Chem. Mater., 2016, 28, 5621–5634, DOI:10.1021/acs.chemmater.6b01182.
- M. Ezbiri, M. Takacs, D. Theiler, R. Michalsky and A. Steinfeld, Tunable Thermodynamic Activity of LaxSr1−xMnyAl1−yO3−δ (0 ≤ x ≤ 1, 0 ≤ y ≤ 1) Perovskites for Solar Thermochemical Fuel Synthesis, J. Mater. Chem. A, 2017, 5, 4172–4182, 10.1039/c6ta06644e.
- N. Gokon, K. Hara, Y. Sugiyama, S. Bellan, T. Kodama and C. Hyun-seok, Thermochemical TwoStep Water Splitting Cycle Using Perovskite Oxides Based on LaSrMnO3 Redox System for Solar H2 Production, Thermochim. Acta, 2019, 680, 178374, DOI:10.1016/j.tca.2019.178374.
- L. Wang, M. Al-Mamun, Y. L. Zhong, L. Jiang, P. Liu, Y. Wang, H. G. Yang and H. Zhao, Ca2+ and Ga3+ Doped LaMnO3 Perovskite as a Highly Efficient and Stable Catalyst for Two-Step Thermochemical Water Splitting, Sustainable Energy Fuels, 2017, 1, 1013–1017 RSC.
- A. Haeussler, S. Abanades, J. Jouannaux and A. Julbe, Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review, Catalysts, 2018, 8, 611, DOI:10.3390/catal8120611.
- C. K. Yang, Y. Yamazaki, A. Aydin and S. M. Haile, Thermodynamic and Kinetic Assessments of Strontium-Doped Lanthanum Manganite Perovskites for Two-Step Thermochemical Water Splitting, J. Mater. Chem. A, 2014, 2, 13612–13623, 10.1039/c4ta02694b.
- A. J. Carrillo, A. H. Bork, T. Moser, E. Sediva, Z. D. Hood and J. L. M. Rupp, Modifying La0.6Sr0.4MnO3 Perovskites with Cr Incorporation for Fast Isothermal CO2-Splitting Kinetics in 30 Solar-Driven Thermochemical Cycles, Adv. Energy Mater., 2019, 9, 1803886 CrossRef.
- A. H. Bork, E. Povoden-Karadeniz and J. L. M. Rupp, Modeling Thermochemical Solar-to-Fuel Conversion: CALPHAD for Thermodynamic Assessment Studies of Perovskites, Exemplified for (La,Sr)MnO3, Adv. Energy Mater., 2016, 7, 1601086, DOI:10.1002/aenm.201601086.
- A. Haeussler, A. Julbe and S. Abanades, Investigation of Reactive Perovskite Materials for Solar Fuel Production via Two-Step Redox Cycles: Thermochemical Activity, Thermodynamic Properties and Reduction Kinetics, Mater. Chem. Phys., 2022, 276, 125358, DOI:10.1016/j.matchemphys.2021.125358.
- A. Le Gal, A. Julbe and S. Abanades, Thermochemical Activity of Single- and Dual-Phase Oxide Compounds Based on Ceria, Ferrites, and Perovskites for Two-Step Synthetic Fuel Production, Molecules, 2023, 28, 4327, DOI:10.3390/molecules28114327.
- M. M. Nair and S. Abanades, Cation Synergy in Sr and Al Substituted LaMnO3 during Solar Thermochemical CO2 Splitting, Energy Adv., 2023, 2, 137–147, 10.1039/D2YA00309K.
- M. M. Nair and S. Abanades, Insights into the Redox Performance of Non–Stoichiometric Lanthanum Manganite Perovskites for Solar Thermochemical CO2 Splitting, ChemistrySelect, 2016, 1, 4449–4457, DOI:10.1002/slct.201601171.
- M. M. Nair and S. Abanades, Experimental Screening of Perovskite Oxides as Efficient Redox Materials for Solar Thermochemical CO2 Conversion, Sustainable Energy Fuels, 2018, 2, 843–854, 10.1039/C7SE00516D.
- J. M. Naik, C. Ritter, B. Bulfin, A. Steinfeld, R. Erni and G. R. Patzke, Reversible Phase Transformations in Novel Ce-Substituted Perovskite Oxide Composites for Solar Thermochemical Redox Splitting of CO2, Adv. Energy Mater., 2021, 11, 2003532 CrossRef CAS.
- D. R. Barcellos, M. Sanders, J. Tong, A. H. McDaniel and R. O'Hayre, BaCe0.25Mn0.75O3-δ - A Promising Perovskite-Type Oxide for Solar Thermochemical Hydrogen Production, Energy Environ. Sci., 2018, 11, 3256–3265, 10.1039/c8ee01989d.
- A. H. McDaniel, E. C. Miller, D. Arifin, A. Ambrosini, E. N. Coker, R. O'Hayre, W. C. Chueh and J. Tong, Sr- and Mn-Doped LaAlO3-δ for Solar Thermochemical H2 and CO Production, Energy Environ. Sci., 2013, 6, 2424–2428, 10.1039/c3ee41372a.
- C. Liu, J. Park, H. A. De Santiago, B. Xu, W. Li, D. Zhang, L. Zhou, Y. Qi, J. Luo and X. Liu, Perovskite Oxide Materials for Solar Thermochemical Hydrogen Production from Water Splitting through Chemical Looping, ACS Catal., 2024, 14, 14974–15013 CrossRef CAS PubMed.
- G. Sai Gautam, E. B. Stechel and E. A. Carter, Exploring Ca-Ce-M-O (M = 3d Transition Metal) Oxide Perovskites for Solar Thermochemical Applications, Chem. Mater., 2020, 32, 9964–9982 CrossRef CAS.
- S. S. Naghavi, J. He and C. Wolverton, CeTi2O6 - A
Promising Oxide for Solar Thermochemical Hydrogen Production, ACS Appl. Mater. Interfaces, 2020, 12, 21521–21527 CrossRef CAS.
- A. Bayon, A. de la Calle, K. K. Ghose, A. Page and R. McNaughton, Experimental, Computational and Thermodynamic Studies in Perovskites Metal Oxides for Thermochemical Fuel Production: A Review, Int. J. Hydrogen Energy, 2020, 45, 12653–12679, DOI:10.1016/j.ijhydene.2020.02.126.
- C. Liu, D. Zhang, W. Li, J. Trindell, K. A. King and R. Sean, Bishop Manganese-Based A-Site High-Entropy Perovskite Oxide for Solar Thermochemical Hydrogen Production, J. Mater. Chem. A, 2024, 12, 3910–3922 RSC.
- Q. Wang, Y. Xuan, K. Gao, C. Sun, Y. Gao, J. Liu, S. Chang and X. Liu, High-Entropy Perovskite Oxides for Direct Solar-Driven Thermochemical CO2 Splitting, Ceram. Int., 2024, 50, 1564–1573, DOI:10.1016/j.ceramint.2023.10.248.
- A. Sarkar, Q. Wang, A. Schiele, M. R. Chellali, S. S. Bhattacharya, D. Wang, T. Brezesinski, H. Hahn, L. Velasco and B. Breitung, High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties, Adv. Mater., 2019, 31, 1806236, DOI:10.1002/adma.201806236.
- A. Le Gal, S. Abanades and G. Flamant, CO2 and H2O Splitting for Thermochemical Production of Solar Fuels Using Nonstoichiometric Ceria and Ceria/Zirconia Solid Solutions, Energy Fuels, 2011, 25, 4836–4845, DOI:10.1021/ef200972r.31.
- R. D. Barcellos, M. D. Sanders, J. Tong, A. H. McDaniel and R. P. O'Hayre, BaCe0.25Mn0.75O3-δ - Promising Perovskite-Type Oxide for Solar Thermochemical Hydrogen Production, Energy Environ. Sci., 2018, 11, 3256–3265, 10.1039/c8ee01989d.
- J. Jouannaux, A. Haeussler, M. Drobek, A. Ayral, S. Abanades and A. Julbe, Lanthanum Manganite Perovskite Ceramic Powders for CO2 Splitting: Influence of Pechini Synthesis Parameters on Sinterability and Reactivity, Ceram. Int., 2019, 45, 15636–15648, DOI:10.1016/j.ceramint.2019.05.075.
- M. Rini, R. Tobey, N. Dean, J. Itatani, Y. Tomioka, Y. Tokura, R. W. Schoenlein and A. Cavalleri, Control of the Electronic Phase of a Manganite by Mode-Selective Vibrational Excitation, Nature, 2007, 449, 72–74, DOI:10.1038/nature06119.
- Z. Li, M. Yang, J. S. Park, S. H. Wei, J. J. Berry and K. Zhu, Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys, Chem. Mater., 2016, 28, 284–292 CrossRef CAS.
- S. A. Heuer, R. Schierholz, E. V. Alekseev, L. Peters, D. N. Mueller, T. Duchoň, V. Vibhu, H. Tempel, L. G. J. De Haart and H. Kungl,
et al., Oxygen Nonstoichiometry and Valence State of Manganese in La1-XCaxMnO3+δ, ACS Omega, 2021, 6, 9638–9652 CrossRef CAS.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef.
- T. Maiti, R. Banerjee, S. Chatterjee, M. Ranjan, T. Bhattacharya, S. Mukherjee, S. S. Jana and A. Dwivedi, High-Entropy Perovskites: An Emergent Class of Oxide Thermoelectrics with Ultralow Thermal Conductivity, ACS Sustain. Chem. Eng., 2020, 8, 17022–17032 CrossRef.
- J. Vieten, B. Bulfin, P. Huck, M. Horton, D. Guban, L. Zhu, Y. Lu, K. A. Persson, M. Roeb and C. Sattler, Materials Design of Perovskite Solid Solutions for Thermochemical Applications, Energy Environ. Sci., 2019, 12, 1369–1384, 10.1039/c9ee00085b.
- S. Ašmontas and M. Mujahid, Recent Progress in Perovskite Tandem Solar Cells, Nanomaterials, 2023, 13, 1886, DOI:10.3390/nano13121886.
- S. A. Hassanzadeh-Tabrizi, Precise Calculation of Crystallite Size of Nanomaterials: A Review, J. Alloys Compd., 2023, 968, 171914 CrossRef CAS.
- R. J. Panlener, R. N. Blumenthal and J. E. Garnier Medley, A Thermodynamic study of nonstoichiometric cerium dioxide, J. Phys. Chem. Solids, 1975, 36, 1213–1222 CrossRef CAS.
- V. Milman, B. Winkler, J. A. White, C. J. Pickard, M. C. Payne, E. V. Akhmatskaya and R. H. Nobes, Electronic Structure, Properties, and Phase Stability of Inorganic Crystals: A Pseudopotential Plane-Wave Study, Int. J. Quantum Chem., 2000, 77, 895–910 CrossRef CAS.
- C. C. Pan, Y. H. Chen, N. Wu, M. L. Zhang, L. H. Yuan and C. R. Zhang, First-Principle Study of O Vacancy on LaNiO3 (001) Surface, Int. J. Hydrogen Energy, 2016, 41, 15756–15763, DOI:10.1016/j.ijhydene.2016.04.143.
- A. A. Emery, J. E. Saal, S. Kirklin, V. I. Hegde and C. Wolverton, High-Throughput Computational Screening of Perovskites for Thermochemical Water Splitting Applications, Chem. Mater., 2016, 28, 5621–5634, DOI:10.1021/acs.chemmater.6b01182.
- D. Vanderbilt, Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism, Phys. Rev. B, 1990, 41, 7892–7895 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Abanades, A. Haeussler and A. Julbe, Synthesis and Thermochemical Redox Cycling of Porous Ceria Microspheres for Renewable Fuels Production from Solar-Aided Water-Splitting and CO2 Utilization, Appl. Phys. Lett., 2021, 119, 023902, DOI:10.1063/5.0055282.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article abc,
Eric
Beche
abc,
Eric
Beche
 a and
Stéphane
Abanades
a and
Stéphane
Abanades
 *a
*a

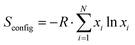 , where R is the universal gas constant and xi is the molar fraction of the element i).36 The high entropy value makes the oxidation process easier to occur because of the low value of reaction Gibbs free energy. The material stability is predicted using the calculated Goldschmidt's tolerance factor and further validated using X-ray diffraction analysis results. Through the thermogravimetric analyses, the CO yields of perovskites are determined and the first principles DFT study of oxygen vacancy formation energy demonstrates the beneficial effects of multi-cation doping on oxygen transfer. Apart from the CO yields, the evolved gas production rates of synthesized perovskites are also obtained in this work. The study analyzes the experimental production performance of the 23 potential perovskites in terms of fuel yields and gas production rates, which supports the search for novel high-performance perovskites for thermochemical CO2 splitting.
, where R is the universal gas constant and xi is the molar fraction of the element i).36 The high entropy value makes the oxidation process easier to occur because of the low value of reaction Gibbs free energy. The material stability is predicted using the calculated Goldschmidt's tolerance factor and further validated using X-ray diffraction analysis results. Through the thermogravimetric analyses, the CO yields of perovskites are determined and the first principles DFT study of oxygen vacancy formation energy demonstrates the beneficial effects of multi-cation doping on oxygen transfer. Apart from the CO yields, the evolved gas production rates of synthesized perovskites are also obtained in this work. The study analyzes the experimental production performance of the 23 potential perovskites in terms of fuel yields and gas production rates, which supports the search for novel high-performance perovskites for thermochemical CO2 splitting.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, dissolving in deionized water. After the nitrate solution was stirred and heated to 70 °C, glycol was added to the solution. Then, the solution heating continued to 85 °C with stirring at this temperature to form the gel. The formed gel was placed into a drying oven at 120 °C for 3 h. A two-step calcination process was performed to avoid the loss of Mn.38 First, the powders were calcined at 800 °C for 90 min followed by cooling to room temperature. Then, the powders were heated to 1400 °C for 6 h (3 h ramp up and 3 h dwell) and cooled to form a stable powder structure.
1, dissolving in deionized water. After the nitrate solution was stirred and heated to 70 °C, glycol was added to the solution. Then, the solution heating continued to 85 °C with stirring at this temperature to form the gel. The formed gel was placed into a drying oven at 120 °C for 3 h. A two-step calcination process was performed to avoid the loss of Mn.38 First, the powders were calcined at 800 °C for 90 min followed by cooling to room temperature. Then, the powders were heated to 1400 °C for 6 h (3 h ramp up and 3 h dwell) and cooled to form a stable powder structure.
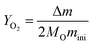
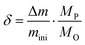




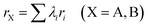

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)