Hydrogen-intercalation PdZn bimetallene for urea electro-synthesis from nitrate and carbon dioxide†
Ziqiang
Wang
 ,
Yanan
Wang
,
Shan
Xu
,
Kai
Deng
,
Hongjie
Yu
,
You
Xu
,
Yanan
Wang
,
Shan
Xu
,
Kai
Deng
,
Hongjie
Yu
,
You
Xu
 ,
Hongjing
Wang
* and
Liang
Wang
,
Hongjing
Wang
* and
Liang
Wang
 *
*
State Key Laboratory Breeding Base of Green-Chemical Synthesis Technology, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, P.R. China. E-mail: hjw@zjut.edu.cn; wangliang@zjut.edu.cn
First published on 13th November 2024
Abstract
Electrochemical co-reduction of carbon dioxide and nitrate is a green technology to replace traditional energy-intensive methods for urea synthesis, and the development of high-performance catalysts is still a great challenge. Here, we propose the incorporation of nonmetallic hydrogen and oxophilic zinc into palladium metallene for the preparation of hydrogen-intercalation PdZn (H-PdZn) bimetallene, serving as an active electrocatalyst for co-reduction of carbon dioxide and nitrate to synthesize urea via the C–N coupling reaction. The H-PdZn bimetallene affords a high urea yield of 314.17 μg h−1 mg−1 and a Faraday efficiency of 24.39%, better than those of PdZn bimetallene (144.25 μg h−1 mg−1 and 16.03%). The strong electronic interaction between the Pd, Zn and H atoms can induce the downshift of the Pd d-band center of H-PdZn bimetallene, which can promote the formation of the key intermediates of *NH2 and *CO, and lower the energy barrier for their C–N coupling to synthesize urea. This work offers a hydrogenation strategy for the construction of advanced PdH-based metallenes towards electrochemical C–N coupling to synthesize urea.
Introduction
Urea (CO(NH2)2) is not only one of the important organic chemicals, but also the most widely used nitrogen fertilizer, which greatly supports global agricultural and social development.1–3 The industrial synthesis of urea usually involves carbon dioxide and ammonia under harsh conditions (NH3 + CO2 → CO(NH2)2, 150–250 bar, and 180–200 °C),4,5 and this process consumes about 80% global production of NH3 obtained from the Haber–Bosch method (N2 + H2 → NH3, 150–350 bar, and 350–550 °C), which requires substantial fossil fuel consumption and results in large CO2 emissions.6–9 To address this problem, there is an urgent need to develop a green and sustainable technology for urea synthesis under mild conditions. Recent reports have demonstrated that electrocatalytic co-reduction of N2 and CO2 has been a promising alternative method for urea production under mild conditions via the C–N coupling reaction.10,11 However, the inherent chemical inertness of N2 and ultralow solubility severely limited the urea yield and Faraday efficiency.10–14 Nitrate has high solubility in water and a relatively low dissociation energy of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (204 kJ mol−1),15–18 which is an ideal nitrogenous reactant with CO2 for C–N coupling to produce urea. However, this method involves a 16-electron reaction process, usually resulting in complex product distribution and unsatisfactory activity and selectivity.11,19,20 Moreover, the competitive hydrogen evolution reaction (HER) is another challenge that inhibits urea electro-synthesis.6 It is highly desired to design highly selective catalysts for urea production.
O bond (204 kJ mol−1),15–18 which is an ideal nitrogenous reactant with CO2 for C–N coupling to produce urea. However, this method involves a 16-electron reaction process, usually resulting in complex product distribution and unsatisfactory activity and selectivity.11,19,20 Moreover, the competitive hydrogen evolution reaction (HER) is another challenge that inhibits urea electro-synthesis.6 It is highly desired to design highly selective catalysts for urea production.
Palladium has been widely demonstrated to be a highly active catalyst for the NO3− reduction reaction (NO3RR) and CO2 reduction reaction (CO2RR).21–23 As such, Pd electrocatalysts have been utilized for co-reduction of NO3− and CO2 to synthesize urea via C–N coupling,24,25 but they usually suffer from unsatisfactory selectivity for the urea product. Designing the morphology and optimizing the composition are considered as two effective strategies for regulating catalytic performance.26–28 Metallene as a novel ultrathin 2D sheet structure has abundant exposed metal active sites, high electrical conductivity and high atomic utilization, attracting intensive attention in electrocatalysis.29,30 Moreover, the combination of Pd with other elements is a promising strategy that not only effectively modifies the electronic structure of Pd, but also reduces the use of precious metals.31,32 Palladium hydride (PdH) has received widespread attention in electrocatalysis due to the unique physicochemical properties, such as nitrogen reduction reaction,33 CO2 reduction,34 formic acid oxidation,35 and methanol oxidation.36 Moreover, it has been reported that the introduction of oxophilic Zn can facilitate the generation of *CO and *NH2 intermediates and facilitate their C–N coupling reaction to synthesize urea because of strong electron transfer from oxophilic Zn to Cu.37 Therefore, it is highly desired to develop hydrogen-intercalated bimetallene for urea electro-synthesis.
In this work, hydrogen-intercalation PdZn (H-PdZn) bimetallene is synthesized by the incorporation of nonmetallic hydrogen and oxophilic zinc into Pd metallene using N,N-dimethylformamide (DMF) as the hydrogen source, which serves as a high-efficiency catalyst for urea electro-synthesis via C–N coupling from CO2 and nitrate. The H-PdZn bimetallene has an ultrathin bent and folded morphology, demonstrating an excellent urea yield (Rurea, 314.17 μg h−1 mg−1) and Faraday efficiency (FE, 24.39%). The ultrathin nanosheet structure and the strong electronic interaction between Pd, Zn and H can provide rich active centers and cause the decrease of the Pd d-band center, which can optimize the adsorption energy of intermediates on active sites, thus enhancing the electrocatalytic performance for urea production. This work offers an attractive approach for the construction of hydrogen-intercalated Pd-based metallene for the electrochemical C–N coupling reaction to synthesize urea.
Experimental
Chemicals and materials
Sodium chloro-palladate (Na2PdCl4), zinc acetylacetonate (Zn(acac)2), potassium hydroxide (KOH), N,N-dimethylformamide (DMF), ethylene glycol (EG), potassium nitrate (KNO3), potassium bicarbonate (KHCO3), potassium sulfate (K2SO4), sodium hydroxide (NaOH), diacetyl monoxime (DAMO), ferric chloride (FeCl3), salicylic acid (C7H6O3), sodium citrate (C6H5Na3O7·2H2O), N-(1-naphthyl) ethylenediamine dihydrochloride (C12H14N2·2HCl), sulfanilamide (C6H8N2O2S), sodium hypochlorite (NaClO), Nafion solution (0.5 wt%), and phosphoric acid (H3PO4, ≥85%) were all purchased from Aladdin Chemicals. Diethylenetriamine (DETA), thiosemicarbazide (TSC) and sodium nitroferricyanide (C5FeN6Na2O) were obtained from Macklin. All chemicals and materials were used as received without further purification.Preparation of H-PdZn bimetallene
All chemicals and reagents were received and used without further purification. PdZn bimetallene was first synthesized via a one-step hydrothermal method according to the literature with slight modifications.26 Typically, 1 g of KOH was dissolved in 6 mL of N,N-dimethylformamide (DMF) and 4 mL of ethylene glycol under ultrasonication for 30 min, followed by adding 300 μL of 0.1 M Na2PdCl4 DMF solution, 150 μL of 0.1 M Zn(acac)2 DMF solution and 5 mL of diethylenetriamine (DETA) to form a homogeneous solution. The solution mixtures were then put into a Teflon-lined reactor and heated at 200 °C for 8 h. After that, PdZn bimetallene was obtained by centrifugation and washing for several cycles. The obtained PdZn bimetallene (8 mg) was dispersed in 20 mL of DMF, which was heated at 160 °C for 16 h. Finally, the H-PdZn bimetallene was collected by centrifugation and washing for several cycles.Electrochemical measurements
All electrochemical measurements were carried out using a CHI660E electrochemical workstation in an H-type cell using a three-electrode system. Catalyst-coated carbon paper (1 × 1 cm2), a Pt sheet and an Ag/AgCl (saturated KCl) electrode were employed as the working electrode, counter electrode and reference electrode, respectively. The obtained electrocatalyst (5 mg) was dispersed in 950 μL of ethanol and 50 μL of Nafion solution to form a catalyst ink under ultrasonication. Then, 100 μL of ink was added dropwise on a piece of carbon paper (1 × 1 cm2) and dried under ambient conditions to form the working electrode with a loading of about 0.5 mg cm−2. Prior to electrochemical experiments, CO2 was continuously bubbled into the cathode chamber to obtain CO2-saturated 0.1 M KNO3. Then, CO2 was continuously flowed into the cathode chamber with stirring at a flow rate of 300 rpm for urea electro-synthesis. For comparison, electrochemical NO3RR tests were conducted in 0.1 M KNO3 without CO2 supply, and electrochemical CO2RR tests were performed in CO2-saturated 0.1 M KHCO3 solution with a constant flow of CO2. All measured potentials were referenced to the reversible hydrogen electrode (RHE). The electrochemical measurements and product detection are described in the ESI file† in detail.Results and discussion
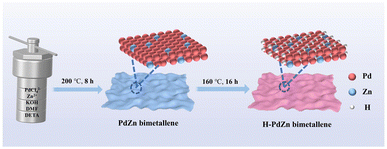
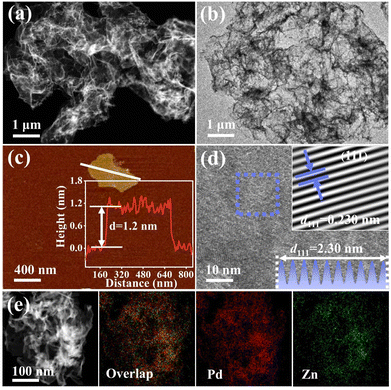
The preparation of H-PdZn bimetallene via a two-step method is depicted in Fig. 1. During the preparation of PdZn bimetallene by a solvothermal reaction, DETA can combine with metal precursors to form chelates, thus resulting in a decreased reduction rate of metal precursors,38 which can allow the selective growth of nanosheets. Furthermore, the DMF is easily decomposed into DMA and CO at elevated temperatures in the presence of KOH,39 which can facilitate facet regulation and induce the formation of ultra-thin nanosheets. After that, active hydrogen atoms can be generated from the decomposition of DMF, which can be easily adsorbed on the surface of the PdZn bimetallene. Subsequently, hydrogen atoms gradually penetrate into the lattices to form H-PdZn bimetallene due to the strong affinity between H and Pd,40,41 resulting in lattice expansion.The morphology of H-PdZn bimetallene is first investigated. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and transmission electron microscopy (TEM) images show that H-PdZn bimetallene has a folded ultrathin nanosheet structure (Fig. 2a and b), which is similar to that of PdZn bimetallene (Fig. S1a and b†). Meanwhile, the transmission electron microscopy (TEM) image and corresponding selected area electron diffraction (SAED) pattern reveal four distinct concentric rings that match well with the (111), (200), (220), and (311) facets of H-PdZn bimetallene (Fig. S2†). The atomic force microscopy (AFM) image of H-PdZn bimetallene exhibits an average thickness of about 1.2 nm (Fig. 2c), which can expose sufficient under-coordinated metal atoms that are favorable for the adsorption and activation of substances in the electrocatalytic process. As shown in Fig. 2d, H-PdZn bimetallene has a clear lattice stripe with a spacing of 0.230 nm, which is larger than that of the (111) plane of PdZn bimetallene (0.222 nm, Fig. S1c†), indicating lattice expansion due to the incorporation of hydrogen atoms into the lattice gap. In addition, element mapping images obviously show the uniform distribution of Pd and Zn in the samples (Fig. 2e), with the similar Pd/Zn atomic ratios of the samples from energy dispersive X-ray spectroscopy (EDS) (Fig. S1d and S3†).
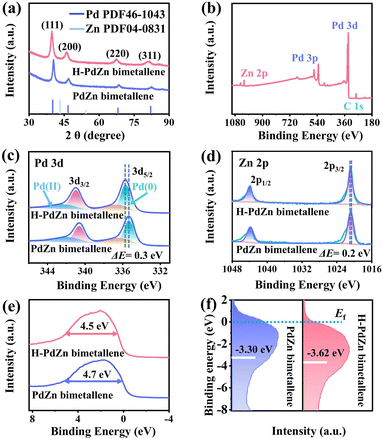
The crystalline structure of the samples is then characterized by X-ray diffraction (XRD). As shown in Fig. 3a, the XRD diffraction peaks of PdZn bimetallene shift to higher angles compared to those of standard face centered cubic (fcc) Pd (JCPDS card 46-1043), which reveals the lattice contraction of the samples because of the introduction of smaller Zn atoms into the Pd lattice, demonstrating the successful formation of PdZn bimetallene. Interestingly, the diffraction peaks of H-PdZn bimetallene are shifted to lower angles compared to those of PdZn bimetallene, suggesting lattice expansion caused by hydrogen incorporation into the Pd lattice gap.33,42 Furthermore, by analyzing the correlation between lattice parameters and the composition of palladium hydride according to Vegard's law, a more precise estimation of the H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Pd + Zn) ratio can be derived, approximating 0.32 (Fig. S4, see the ESI† for details).43 X-ray photoelectron spectroscopy (XPS) is further applied to determine the chemical state of the samples. The existence of Pd and Zn elements in H-PdZn bimetallene is confirmed by the XPS survey spectrum (Fig. 3b). In the high-resolution Pd 3d XPS spectra (Fig. 3c), H-PdZn bimetallene shows two obvious high peaks at 340.96 and 335.69 eV, which are attributed to Pd0 3d3/2 and Pd0 3d5/2, while the other two smaller peaks at 342.37 and 336.53 eV are attributed to Pd2+ due to oxidation in air.44,45Fig. 3d shows the Zn 2p XPS spectra of the samples, in which a pair of peaks for H-PdZn bimetallene are visible at 1021.1 and 1044.3 eV, belonging to the Zn2+ spin–orbit double peaks of Zn 2p3/2 and Zn 2p1/2 due to the high oxophilicity of Zn. Compared to PdZn bimetallene, the Pd 3d and Zn 2p XPS peaks in H-PdZn bimetallene are shifted positively by 0.3 and 0.2 eV, respectively, suggesting stronger electronic interaction between Pd and H than that between Zn and H.46 Furthermore, the XPS valence band spectrum is recorded to analyze the electronic structure of the samples. As shown in Fig. 3e, the peak width at half height of H-PdZn bimetallene (4.5 eV) is smaller than that of PdZn bimetallene (4.7 eV), indicating an increase in the Pd–Pd distance in H-PdZn bimetallene. The shrinkage bandwidth of the valence band spectrum reveals that the incorporation of hydrogen atoms into the metal lattice leads to the formation of metal hydrides.47 Furthermore, the d-band center of Pd is determined from the valence band spectrum (see the ESI Experimental method for details†),48,49 which is calculated to be −3.30 eV for PdZn bimetallene and −3.62 eV for H-PdZn bimetallene (Fig. 3f). Compared to PdZn bimetallene, the d-band center of H-PdZn bimetallene is further away from the Fermi level, which is due to the fact that the state density of H-PdZn bimetallene decreases after the hydrogen intercalation into the PdZn bimetallene. Moreover, the negative shift of the d-band center of H-PdZn bimetallene indicates that hydrogen incorporation can optimize the adsorption energy of intermediates on active sites.50
(Pd + Zn) ratio can be derived, approximating 0.32 (Fig. S4, see the ESI† for details).43 X-ray photoelectron spectroscopy (XPS) is further applied to determine the chemical state of the samples. The existence of Pd and Zn elements in H-PdZn bimetallene is confirmed by the XPS survey spectrum (Fig. 3b). In the high-resolution Pd 3d XPS spectra (Fig. 3c), H-PdZn bimetallene shows two obvious high peaks at 340.96 and 335.69 eV, which are attributed to Pd0 3d3/2 and Pd0 3d5/2, while the other two smaller peaks at 342.37 and 336.53 eV are attributed to Pd2+ due to oxidation in air.44,45Fig. 3d shows the Zn 2p XPS spectra of the samples, in which a pair of peaks for H-PdZn bimetallene are visible at 1021.1 and 1044.3 eV, belonging to the Zn2+ spin–orbit double peaks of Zn 2p3/2 and Zn 2p1/2 due to the high oxophilicity of Zn. Compared to PdZn bimetallene, the Pd 3d and Zn 2p XPS peaks in H-PdZn bimetallene are shifted positively by 0.3 and 0.2 eV, respectively, suggesting stronger electronic interaction between Pd and H than that between Zn and H.46 Furthermore, the XPS valence band spectrum is recorded to analyze the electronic structure of the samples. As shown in Fig. 3e, the peak width at half height of H-PdZn bimetallene (4.5 eV) is smaller than that of PdZn bimetallene (4.7 eV), indicating an increase in the Pd–Pd distance in H-PdZn bimetallene. The shrinkage bandwidth of the valence band spectrum reveals that the incorporation of hydrogen atoms into the metal lattice leads to the formation of metal hydrides.47 Furthermore, the d-band center of Pd is determined from the valence band spectrum (see the ESI Experimental method for details†),48,49 which is calculated to be −3.30 eV for PdZn bimetallene and −3.62 eV for H-PdZn bimetallene (Fig. 3f). Compared to PdZn bimetallene, the d-band center of H-PdZn bimetallene is further away from the Fermi level, which is due to the fact that the state density of H-PdZn bimetallene decreases after the hydrogen intercalation into the PdZn bimetallene. Moreover, the negative shift of the d-band center of H-PdZn bimetallene indicates that hydrogen incorporation can optimize the adsorption energy of intermediates on active sites.50
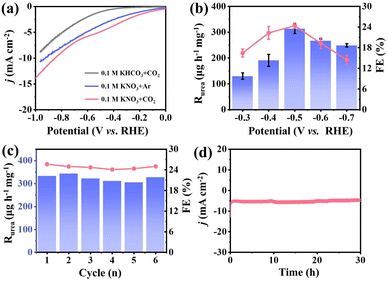
The electrochemical tests of co-reduction of NO3− and CO2 for urea synthesis are carried out in 0.1 M KNO3 electrolyte bubbling with CO2 under ambient conditions (Fig. S5†). The linear sweep voltammetry (LSV) curves of H-PdZn bimetallene in CO2- and Ar-saturated 0.1 M KNO3 electrolytes are displayed in Fig. 4a. In the Ar-saturated electrolyte, the cathodic current density obtained at the given applied potentials could be attributed to the NO3RR and/or HER. Obviously, when CO2 is introduced into the system, H-PdZn bimetallene clearly shows higher current densities at the given potentials from −0.3 to −0.7 V than those in the Ar-saturated electrolyte, which indicates that the catalyst is responsive to CO2 and has the potential for synthesizing urea. Moreover, H-PdZn bimetallene exhibits a lower current density for the CO2 reduction reaction in CO2-saturated 0.1 M KHCO3 solution without KNO3. The increase of current density in the presence of CO2 and NO3− reveals the occurrence of C–N coupling during CO2RR and NO3RR processes for urea synthesis. Besides, we also observe from the LSV curves that the current density of H-PdZn bimetallene is higher than that of PdZn bimetallene (Fig. S6†), revealing its better performance for C–N coupling to synthesize urea. The produced urea is detected by the diacetyl monoxime method according to the standard curve (see the ESI for details, Fig. S7†). Fig. 4b shows the dependence of Rurea and FE on the applied potential in CO2-saturated 0.1 M KNO3 electrolyte for two-hour electrolysis. The H-PdZn bimetallene exhibits the highest Rurea and FE of 314.17 μg h−1 mg−1 and 24.39% at −0.5 V, respectively, which are comparable with those in the literature (Tables S1 and S2†). To further highlight the improvement by inserting H into PdZn bimetallene, the electrochemical performance of PdZn bimetallene was also evaluated under the same conditions (Fig. S8†). In sharp contrast to H-PdZn bimetallene, PdZn bimetallene exhibits poor electrocatalytic performance, with an Rurea and FE of 144.25 μg h−1 mg−1 and 16.03% at −0.5 V, respectively. The enhanced performance of H-PdZn bimetallene can be attributed to the incorporation of interstitial H, which can downshift the Pd d-band center and thus optimize adsorption energy for intermediates. The durability of the H-PdZn bimetallene towards urea electro-synthesis is then investigated. During long-term and cycling tests, H-PdZn bimetallene maintains stable current density and shows slight changes in Rurea and FE, indicating that the H-PdZn bimetallene has good stability in the electrochemical co-reduction of CO2 and NO3− to synthesize urea (Fig. 4c and d). In order to better illustrate the stability of the material, SEM (Fig. S9†) and XRD (Fig. S10†) of H-PdZn bimetallene after electrochemical tests were further performed. The results show that the morphological and structural characteristics of H-PdZn bimetallene essentially remain unchanged after the test, which further emphasizes the good stability of the as-synthesized material.
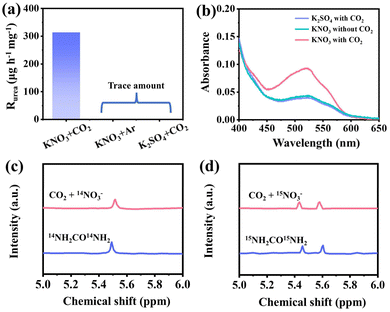
Several comparative experiments are conducted to confirm that the detected urea originated from the electrochemical co-reduction of CO2 and NO3−. Control experiments are conducted in 0.1 M K2SO4 with CO2 and 0.1 M KNO3 without CO2, and a negligible amount of urea is detected for both cases (Fig. 5a and b). The previous results imply that the urea indeed originates from C–N coupling of the CO2RR and NO3RR on the H-PdZn bimetallene. Furthermore, an isotopic labelling experiment is performed using 15NO3− as the nitrogen source. As shown in the 1H NMR spectra (Fig. 5c and d), there is only a single peak of electrolysis solution derived from 14NO3−, while there are double peaks of electrolysis solution derived from 15NO3−, which are located at positions similar to those of the standards 14NH2CO14NH2 and 15NH2CO15NH2, respectively. This result further confirms the formation of urea from the electrochemical co-reduction of CO2 and NO3− by H-PdZn bimetallene. Besides the targeted product of urea, the by-products of NH3, NO2−, CO and H2 are inevitably produced during electrocatalysis. Therefore, the FE of each electrocatalytic product is calculated at different potentials (Fig. S11 and S12†). The NH3 and NO2− concentrations are detected by UV-vis spectroscopy according to the standard curves (see the ESI for details, Fig. S13†) and the gas phase products (CO and H2) are identified by on-line gas chromatography. In addition, the FE of the product is calculated when CO2 is saturated in 0.1 M KHCO3 and in 0.1 M KNO3 in the absence of CO2 supply, indicating that H-PdZn bimetallene shows high performance for the CO2RR and NO3RR (Fig. S14 and S15†). It is clear that there is a significant difference in FE between CO and NH3 in both the single and coupled systems (Fig. S16†), which may be attributed to the electro-synthesis of urea.37
The enhanced electrocatalytic performance of H-PdZn bimetallene for urea synthesis is highly related to the morphology and component. Firstly, CO stripping experiments are carried out to verify the adsorption ability of CO on active sites. As shown in Fig. S17,† the peak potential (1.08 V) of H-PdZn bimetallene is much lower than that of PdZn bimetallene (1.2 V), confirming the low adsorption energy of CO, which is beneficial for the C–N coupling reaction. On the other hand, the bent and ultrathin structure can provide abundant active sites and facilitate channels for the adsorption and activation of intermediates. The electrochemically active surface area (ECSA) serves as an indicator of the quantity of active sites present, which is directly proportional to the value of the capacitance of the electrode.51,52 The ECSA of the catalyst is determined from the double layer capacitance (Cdl), which is derived from the cyclic voltammetry curves at different scan rates (Fig. S18a and b†). The Cdl value of H-PdZn bimetallene (1.2 mF cm−2) is higher than that of PdZn bimetallene (0.7 mF cm−2) (Fig. S18c†), indicating more active sites of H-PdZn bimetallene for urea synthesis due to the strong electronic effect after hydrogen intercalation. Furthermore, the electron transfer kinetics during urea synthesis have been investigated by electrochemical impedance spectroscopy (EIS) using the Nyquist plot. All samples show similar system ohmic resistance (RΩ), while the semicircle radius for H-PdZn bimetallene is smaller than that for PdZn bimetallene (Fig. S18d†), proving the faster charge transfer of H-PdZn bimetallene due to the strong electron transfer among the Pd, Zn and H atoms, which can promote the kinetics of urea synthesis.
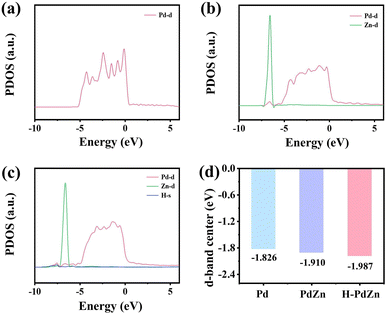
In order to further understand the C–N coupling mechanism of urea electrosynthesis for H-PdZn bimetallene, density functional theory (DFT) calculations are carried out. Firstly, we calculated the projected density of states (PDOS) of the optimized structural models of standard Pd, H-PdZn bimetallene and PdZn bimetallene with an exposed (111) plane (Fig. 6a–c). It can be seen that the main peak of the Zn 3d orbital is located at a more negative position, indicating a relatively electron-rich feature, which can serve as an electron reservoir to supply electrons to Pd and hydrogen.53 Bader charge analysis exhibits that the Zn atoms transfer 6.28 e to Pd (4.98 e) and H (1.30 e) atoms in the H-PdZn bimetallene (Fig. S19†). This result confirms an apparent electron transfer among the Pd, Zn and H atoms of H-PdZn bimetallene, agreeing well with the XPS analysis. After the incorporation of Zn and H atoms, the d orbitals of Pd gradually become wide due to the orbital interaction among Pd, Zn and H, indicating the downshift of the d band center. The Pd d-band centers of Pd (111), PdZn (111), and H-PdZn (111) models are located at about −1.826, −1.910 and −1.987 eV, respectively (Fig. 6d), consistent with the analysis of valence band spectra. This may be attributed to the fact that the electron distribution caused by charge transfer regulates the position of the d-band center, which is conducive to tailoring the adsorption and activation of intermediates.
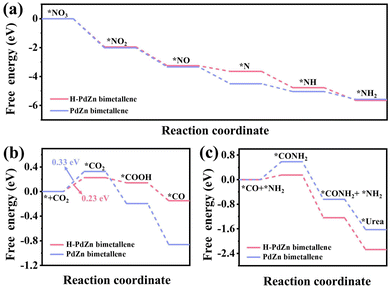
The free energy change (ΔG) values for the NO3RR, CO2RR and C–N coupling to urea processes were further calculated, and the optimized structural models of reaction steps are displayed in Fig. S20 and S21.† As shown in Fig. 7a, H-PdZn bimetallene has more favorable thermodynamics than PdZn bimetallene for the NO3RR, revealing the significant effect of the incorporation of Zn and H. Similarly, for CO2 reduction to CO, it can be seen that the formation of *CO2 to *CO on both H-PdZn bimetallene and PdZn bimetallene is downhill. The potential-determining step (PDS) of the CO2RR on the samples is the formation of *CO2, with a lower energy barrier of H-PdZn bimetallene (0.23 eV) than that of PdZn bimetallene (0.33 eV), indicating the significantly improved CO2RR performance of H-PdZn bimetallene (Fig. 7b). Therefore, the insertion of H into H-PdZn bimetallene can enhance the activity of the co-reduction of nitrate and CO2 to *NH2 and *CO, respectively. Finally, we calculated ΔG for C–N coupling of *NH2 and *CO to form urea (2*NH2 + *CO → *CO(NH2)) on both H-PdZn bimetallene and PdZn bimetallene. The profiles exhibit the smaller uphill process of the reaction step (*NH2 + *CO → *CONH2) of H-PdZn bimetallene compared to that of PdZn bimetallene, with a lower energy barrier on the H-PdZn bimetallene surface compared to PdZn bimetallene (Fig. 7c), indicating promoted C–N coupling to form urea.
Therefore, it can be concluded that the incorporation of Zn and H atoms can promote the formation of key intermediates *CO and *NH2 and reduce the energy barrier for C–N coupling of *CO and *NH2 to form urea.
Conclusions
In conclusion, bent and ultrathin H-PdZn bimetallene is synthesized by inserting H into the as-synthesized PdZn bimetallene, which serves as an active catalyst for electrochemical co-reduction of CO2 and NO3− to urea via C–N coupling. The H-PdZn bimetallene has a superior FE and Rurea of 24.39% and 314.17 μg h−1 mg−1 at −0.5 V, respectively, as well as excellent stability. The enhanced performance of H-PdZn bimetallene is mainly due to its unique 2D morphology and hydrogen insertion, which can provide sufficient active sites and rapid mass/charge transfer and is conducive to the C–N coupling of key *NH2 and *CO intermediates. This study demonstrates the importance of hydrogen intercalation in the enhancement of catalytic performance for electrochemical urea synthesis.Data availability
The data are available from the corresponding author on reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LR24B060001, LQ22B030012 and LQ23B030010) and National Natural Science Foundation of China (No. 22308330, 22372148, 22278369, 21972126, 21978264 and 21905250).References
- C. Lv, C. Lee, L. Zhong, H. Liu, J. Liu, L. Yang, C. Yan, W. Yu, H. H. Hng, Z. Qi, L. Song, S. Li, K. P. Loh, Q. Yan and G. Yu, ACS Nano, 2022, 16, 8213–8222 CrossRef CAS PubMed.
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS.
- J. Peng, X. Wang, Z. Wang, B. Liu, P. Zhang, X. Li and N. Li, Chin. J. Struct. Chem., 2022, 41, 2209094–2209104 CAS.
- Y. Li, S. Zheng, H. Liu, Q. Xiong, H. Yi, H. Yang, Z. Mei, Q. Zhao, Z.-W. Yin and M. Huang, Nat. Commun., 2024, 15, 176 CrossRef CAS.
- X. Huang, Y. Li, S. Xie, Q. Zhao, B. Zhang, Z. Zhang, H. Sheng and J. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202403980 CrossRef CAS PubMed.
- Y. Zhao, Y. Ding, W. Li, C. Liu, Y. Li, Z. Zhao, Y. Shan, F. Li, L. Sun and F. Li, Nat. Commun., 2023, 14, 4491 CrossRef CAS PubMed.
- A. Adalder, S. Paul and U. K. Ghorai, J. Mater. Chem. A, 2023, 11, 10125–10148 RSC.
- C. Chen, S. Li, X. Zhu, S. Bo, K. Cheng, N. He, M. Qiu, C. Xie, D. Song, Y. Liu, W. Chen, Y. Li, Q. Liu, C. Li and S. Wang, Carbon Energy, 2023, 5, e345 CrossRef CAS.
- X. Zhang, Y. Cao, Z. F. Huang, S. Zhang, C. Liu, L. Pan, C. Shi, X. Zhang, Y. Zhou and G. Yang, Carbon Energy, 2023, 5, e266 CrossRef CAS.
- C. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du, T. Liu, D. Yan, C. Xie, Y. Zou, Y. Wang, R. Chen, J. Huo, Y. Li, J. Cheng, H. Su, X. Zhao, W. Cheng, Q. Liu, H. Lin, J. Luo, J. Chen, M. Dong, K. Cheng, C. Li and S. Wang, Nat. Chem., 2020, 12, 717–724 CrossRef CAS PubMed.
- M. Yuan, J. Chen, Y. Bai, Z. Liu, J. Zhang, T. Zhao, Q. Wang, S. Li, H. He and G. Zhang, Angew. Chem., Int. Ed., 2021, 60, 10910–10918 CrossRef CAS PubMed.
- G.-F. Chen, Y. Yuan, H. Jiang, S.-Y. Ren, L.-X. Ding, L. Ma, T. Wu, J. Lu and H. Wang, Nat. Energy, 2020, 5, 605–613 CrossRef CAS.
- H. Xu, Y. Ma, J. Chen, W.-x. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710–2758 RSC.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795 CrossRef.
- Y. Wang, W. Zhou, R. Jia, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2020, 59, 5350–5354 CrossRef CAS.
- Y. Ying, K. Fan, J. Qiao and H. Huang, Electrochem. Energy Rev., 2022, 5, 6 CrossRef CAS.
- Y. Zhang, L. Lu, T. Zhao, J. Zhao, Q. Cai and Z. Chen, J. Mater. Chem. A, 2024, 12, 16704–16715 RSC.
- Y. Wang, A. Xu, Z. Wang, L. Huang, J. Li, F. Li, J. Wicks, M. Luo, D.-H. Nam, C.-S. Tan, Y. Ding, J. Wu, Y. Lum, D. Cao-Thang, D. Sinton, G. Zheng and E. H. Sargent, J. Am. Chem. Soc., 2020, 142, 5702–5708 CrossRef CAS PubMed.
- Y. Wu, H. Lin, Q. Mao, H. Yu, K. Deng, J. Wang, L. Wang, Z. Wang and H. Wang, Small, 2024, 2407679 CrossRef.
- X. Zhang, X. Zhu, S. Bo, C. Chen, M. Qiu, X. Wei, N. He, C. Xie, W. Chen, J. Zheng, P. Chen, S. P. Jiang, Y. Li, Q. Liu and S. Wang, Nat. Commun., 2022, 13, 5337 CrossRef CAS.
- Y. Xu, Y. Wen, T. Ren, Z. Yu, H. Yu, K. Deng, Z. Wang, H. Wang and L. Wang, Chem. Eng. J., 2024, 490, 151519 CrossRef CAS.
- T.-J. Wang, W.-S. Fang, Y.-M. Liu, F.-M. Li, P. Chen and Y. Chen, J. Energy Chem., 2022, 70, 407–413 CrossRef CAS.
- X.-H. Zhao, D.-H. Zhuo, Q.-S. Chen and G.-C. Guo, Chin. J. Struct. Chem., 2021, 40, 376–382 CAS.
- S. Zhang, J. Geng, Z. Zhao, M. Jin, W. Li, Y. Ye, K. Li, G. Wang, Y. Zhang, H. Yin, H. Zhang and H. Zhao, EES Catal., 2023, 1, 45–53 RSC.
- H. Wang, Y. Jiang, S. Li, F. Gou, X. Liu, Y. Jiang, W. Luo, W. Shen, R. He and M. Li, Appl. Catal., B, 2022, 318, 121819 CrossRef CAS.
- H. Zhang, X. Li, Y. Wang, K. Deng, H. Yu, Y. Xu, H. Wang, Z. Wang and L. Wang, Appl. Catal., B, 2023, 338, 123006 CrossRef CAS.
- M. Wu, F. Dong, Y. Yang, X. Cui, X. Liu, Y. Zhu, D. Li, S. Omanovic, S. Sun and G. Zhang, Electrochem. Energy Rev., 2024, 7, 10 CrossRef CAS.
- Y. Sheng, J. Xie, R. Yang, H. Yu, K. Deng, J. Wang, H. Wang, L. Wang and Y. Xu, Angew. Chem., Int. Ed., 2024, 63, e202410442 CrossRef CAS PubMed.
- K. Deng, Z. Lian, W. Wang, J. Yu, H. Yu, Z. Wang, Y. Xu, L. Wang and H. Wang, Small, 2024, 20, 2305000 CrossRef CAS PubMed.
- J. Qin, Z. Yang, F. Xing, L. Zhang, H. Zhang and Z.-S. Wu, Electrochem. Energy Rev., 2023, 6, 9 CrossRef CAS.
- K. Deng, Z. Lian, W. Wang, J. Yu, Q. Mao, H. Yu, Z. Wang, L. Wang and H. Wang, Appl. Catal., B, 2024, 352, 124047 CrossRef CAS.
- Y. Zhou, L. Zhang, Z. Zhu, M. Wang, N. Li, T. Qian, C. Yan and J. Lu, Angew. Chem., Int. Ed., 2024, 63, e202319029 CrossRef CAS PubMed.
- Z. Wang, H. Zhao, J. Liu, D. Zhang, X. Wu, N. Nie, D. Wu, W. Xu, J. Lai and L. Wang, Chem. Eng. J., 2022, 450, 137951 CrossRef CAS.
- C. Ai, T. Vegge and H. A. Hansen, ChemSusChem, 2022, 15, 202200008 CrossRef.
- Y. Shi, R. Schimmenti, S. Zhu, K. Venkatraman, R. Chen, M. Chi, M. Shao, M. Mavrikakis and Y. Xia, J. Am. Chem. Soc., 2022, 144, 2556–2568 CrossRef CAS PubMed.
- Z. Zhao, M. M. Flores Espinosa, J. Zhou, W. Xue, X. Duan, J. Miao and Y. Huang, Nano Res., 2019, 12, 1467–1472 CrossRef CAS.
- N. Meng, X. Ma, C. Wang, Y. Wang, R. Yang, J. Shao, Y. Huang, Y. Xu, B. Zhang and Y. Yu, ACS Nano, 2022, 16, 9095–9104 CrossRef CAS PubMed.
- Q. Yang, L. Shi, B. Yu, J. Xu, C. Wei, Y. Wang and H. Chen, J. Mater. Chem. A, 2019, 7, 18846–18851 RSC.
- L. Shi, Q. Wang, Q. Ren, Q. Yang, D. Zhao, Y. Feng, H. Chen and Y. Wang, Small, 2022, 18, 2103665 CrossRef CAS.
- H.-Y. Sun, Y. Ding, Y.-Q. Yue, Q. Xue, F.-M. Li, J.-X. Jiang, P. Chen and Y. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 13149–13157 CrossRef CAS.
- Z. Zhao, X. Huang, M. Li, G. Wang, C. Lee, E. Zhu, X. Duan and Y. Huang, J. Am. Chem. Soc., 2015, 137, 15672–15675 CrossRef CAS PubMed.
- J. Fan, Z. Feng, Y. Mu, X. Ge, D. Wang, L. Zhang, X. Zhao, W. Zhang, D. J. Singh, J. Ma, L. Zheng, W. Zheng and X. Cui, J. Am. Chem. Soc., 2023, 145, 5710–5717 CrossRef CAS PubMed.
- J. Fan, J. Wu, X. Cui, L. Gu, Q. Zhang, F. Meng, B.-H. Lei, D. J. Singh and W. Zheng, J. Am. Chem. Soc., 2020, 142, 3645–3651 CrossRef CAS PubMed.
- Q. Mao, X. Mu, K. Deng, H. Yu, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, Adv. Funct. Mater., 2023, 33, 2304963 CrossRef CAS.
- Y. M. Liu, B. Q. Miao, H. Y. Yang, X. Ai, T. J. Wang, F. Shi, P. Chen and Y. Chen, Adv. Funct. Mater., 2024, 34, 2402485 CrossRef CAS.
- J. Fan, X. Cui, S. Yu, L. Gu, Q. Zhang, F. Men, Z. Peng, L. Ma, J.-Y. Ma, K. Qi, Q. Bao and W. Zheng, ACS Nano, 2019, 13, 12987–12995 CrossRef CAS.
- W. Xu, G. Fan, J. Chen, J. Li, L. Zhang, S. Zhu, X. Su, F. Cheng and J. Chen, Angew. Chem., Int. Ed., 2020, 59, 3511–3516 CrossRef CAS.
- S. J. Hwang, S.-K. Kim, J.-G. Lee, S.-C. Lee, J. H. Jang, P. Kim, T.-H. Lim, Y.-E. Sung and S. J. Yoo, J. Am. Chem. Soc., 2012, 134, 19508–19511 CrossRef CAS PubMed.
- W. P. Zhou, A. Lewera, R. Larsen, R. I. Masel, P. S. Bagus and A. Wieckowski, J. Phys. Chem. B, 2006, 110, 13393–13398 CrossRef CAS PubMed.
- J. Wellendorff, T. L. Silbaugh, D. Garcia-Pintos, J. K. Norskov, T. Bligaard, F. Studt and C. T. Campbell, Surf. Sci., 2015, 640, 36–44 CrossRef CAS.
- Y. Sheng, R. Yang, K. Shi, H. Yu, K. Deng, Z. Wang, H. Wang, L. Wang and Y. Xu, Chem. Eng. J., 2024, 485, 149769 CrossRef CAS.
- T. Chao, W. Xie, Y. Hu, G. Yu, T. Zhao, C. Chen, Z. Zhang, X. Hong, H. Jin, D. Wang, W. Chen, X. Li, P. Hu and Y. Li, Energy Environ. Sci., 2024, 17, 1397–1406 RSC.
- J. Chen, M. Jiang, F. Zhang, L. Wang and J. Yang, Adv. Mater., 2024, 36, 2401867 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta04802d |
| This journal is © The Royal Society of Chemistry 2025 |