Recent advances in non-noble metal catalysts toward N-ethylcarbazole hydrogen storage
Hansai
Wu†
,
Junming
Zhang†
,
Zicong
Wang
,
Xianglong
Kong
,
Gaofu
Li
,
Ying
Zhao
*,
Piaoping
Yang
* and
Zhiliang
Liu
 *
*
College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, P. R. China. E-mail: zhying@hrbeu.edu.cn; yangpiaoping@hrbeu.edu.cn; zhiliangliu@hrbeu.edu.cn
First published on 12th November 2024
Abstract
N-Ethylcarbazole (NEC) hydrogen storage has garnered significant attention owing to its superior large-scale safe storage of hydrogen. However, the sluggish hydrogenation kinetics of NEC are extremely dependent on catalysts with superior catalytic activity and high selectivity. Numerous research studies have demonstrated that noble metal catalysts present the best catalytic performance in NEC hydrogen storage, but their high cost impedes widespread commercial applications. In contrast, non-noble metal catalysts effectively address the high-cost issue associated with noble metal catalysts. Furthermore, by optimizing the structural design of the catalysts and increasing the density of active sites, their catalytic performance can be significantly enhanced. This approach achieves a better balance between cost-effectiveness and catalytic efficiency. Therefore, this review first summarizes the synthetic methods and typical structures of NEC hydrogenation non-noble metal catalysts. Subsequently, the recent advances and catalytic mechanisms regarding non-noble metal catalysts for hydrogenation are discussed in detail. Finally, through in-depth analysis of potential problems and active exploration of future prospects, we can better guide the relevant research and application work in the future to continuously reach new heights.
1. Introduction
As fossil fuel sources decrease and environmental pollution becomes more severe, it is urgent to introduce and accelerate the pace of energy structure transformation and vigorously develop a low-carbon economy.1–5 Hydrogen energy is considered to be a sustainable and eco-friendly energy source due to its large reserves, high energy density and zero carbon emissions.6–8 However, the highly flammable and explosive characteristics of hydrogen pose significant challenges for safe and efficient storage and transportation, which remain key barriers to its widespread use.9–11 For many years, scientists have focused on enhancing hydrogen storage technologies to be safer, more efficient, and economically viable. These technologies encompass metal hydride storage,12–14 coordination hydride hydrogen storage,15–17 complex hydride hydrogen storage18–20 and liquid organic hydrogen carrier (LOHC) storage.21–23 Among these various methods, LOHCs feature large hydrogen storage density, high safety and low transportation cost, which is beneficial for large-scale, highly safe, long-duration storage and long-distance transportation of hydrogen energy. LOHCs allow for hydrogen storage in an organic liquid state, effectively addressing the challenges related to limited storage capacity and safety concerns. The reversible catalytic hydrogenation/dehydrogenation reactions enable easy storage and transportation of hydrogen from LOHCs. In addition, during the reversible storage of hydrogen, LOHCs themselves are hardly consumed.24,25 These advantages lead to extensive research into the development of LOHCs and their gradual industrial production.Aromatic compounds without heterocycles, as the earliest studied LOHCs, have been used in primary commercial applications such as toluene26,27 and dibenzyltoluene.28,29 However, an important application bottleneck of traditional aromatic hydrocarbons is the slow kinetics of hydrogenation and dehydrogenation, and it is easy to produce side reactions such as carbon deposition on the catalyst. To address this issue, the structure of LOHCs must be enhanced. Researchers have discovered that the dehydrogenation enthalpy of heteroatoms (such as N, O, S, etc.) incorporated into the aromatic ring of LOHCs decreases in correlation with the bond energy of the C–H bond, resulting in a significant reduction in the dehydrogenation temperature of these hydrogen storage materials.30,31 It has been reported that heterocyclic LOHC compounds exhibiting effective reversible hydrogen storage and release primarily consist of nitrogen heterocycles, including pyridine, carbazole, quinoline, and their derivatives.
Among these LOHCs, N-ethylcarbazole (NEC) stands out as one of the most promising candidates for applications, owing to its high hydrogen storage density of 5.8 wt%, a high boiling point of 348 °C, and a medium dehydrogenation enthalpy of 50.6 kJ mol−1 H2. Notably, NEC is the first proposed LOHC capable of completing hydrogenation/dehydrogenation cycles at temperatures below 200 °C.32–34 Additionally, NEC demonstrates good safety and has relatively small impacts on the environment and humans with a released hydrogen purity of 99.99%. However, the sluggish hydrogen absorption and desorption kinetics of NEC have seriously hindered the development and application of LOHCs.
The development of highly efficient, stable and economical catalysts is crucial for the commercialization of NEC hydrogen storage and transportation technology. At present, extensive research has shown that during the hydrogen absorption process of NEC, the supported noble metal catalysts exhibit superior catalytic performance in the hydrogen absorption reaction of NEC, such as ruthenium-based catalysts35,36 and palladium-based catalysts.37,38 Although noble metal catalysts offer advantages such as strong stability and desirable selectivity, their high cost is not conducive to large-scale applications. Therefore, researchers have turned their attention to non-noble metal catalysts. These non-noble metal catalysts usually include transition metals, such as nickel,39 cobalt,40etc., which are widely studied as catalysts because of their relatively low cost. The slow reaction kinetics, poor stability and low activity are the key reasons restricting further development of non-noble metal catalysts. How to ensure that non-noble metal catalysts have similar catalytic performance to noble metal catalysts while reducing the cost of raw materials has become the direction that researchers are striving for. To address this, by adjusting metal active sites in the catalyst, the interface structure between the metal and the support, and the interaction between the host and the dopant, a series of non-noble metal catalysts with high hydrogenation activity have been synthesized.
In the following sections, a systematic summary of the latest research achievements regarding the non-noble metal hydrogenation catalysts of NEC will be presented (Fig. 1). The focus will be on the development of various typical synthetic methods and structure characteristics. The details of the NEC system are described, including its hydrogen storage mechanism, advanced characterization and catalytic performance of non-noble metal hydrogenation catalysts. Additionally, supplementary viewpoints on the development prospects of non-noble metal hydrogenation catalysts are offered. Future research prospects for these metal catalysts, like synthesis methods, cost-effectiveness and exploration of reaction mechanisms, will also be discussed. It is hoped that this review can promote further research and application of non-noble metal catalysts and boost the development of the hydrogen economy.
2. Synthetic methods for non-noble metal catalysts
To allow non-noble metals to be widely used in NEC hydrogen storage, it is particularly important to design and prepare high-performance catalysts. Recently, numerous novel strategies have been put forward for designing non-noble metal catalysts boasting outstanding activity. This review lists some common methods for the synthesis of non-noble metal catalysts. Table 1 summarizes the preparation methods of non-noble metal catalysts and their advantages and limitations.| Methods | Advantages | Limitations |
|---|---|---|
| Impregnation | Uniform dispersion of active components and controllable structures | Accurate impregnation conditions and operation steps |
| Molten salt-assisted chemical reduction method | Low reaction temperature and effective prevention of material agglomeration | Uncontrollable preparation process |
| Carbothermal reduction | Low cost, simple operation process and no pollution | Existence of the carbon by-product |
2.1 Impregnation method
The method of manufacturing catalysts with impregnation as a key and special step is called the impregnation method, which is also a widely used method in the current catalyst preparation and production.41 The impregnation method is based on the principle that the porous carrier is impregnated with the active components in the form of salt solutions that penetrate the inner surface to form a highly efficient catalyst. These supports can provide an effective surface and a suitable pore structure, enabling the active components to be evenly dispersed on the surface and in the pores, thus improving the utilization rate of the active metal components and the catalytic activity of the catalyst. Therefore, this method selects the appropriate carrier according to different requirements to provide the physical structural characteristics required by the catalyst, such as specific surface area, pore radius, etc.For example, Chen et al. added the precursor of Ni(NO3)2·6H2O to the dispersion solution with SiO2 as the carrier, fully stirred and impregnated, and then calcined to prepare the Ni/SiO2 catalysts.42 As shown in Fig. 2a, the average particle size of nickel in the catalyst is 20.4 ± 8.8 nm, and it is highly dispersed on the surface of the carrier. The silica carrier has a large specific surface area, adjustable pore size and pore volume, and uniform pore channels, so that nickel-based catalysts with small size and uniform dispersion can be easily obtained via a simple impregnation method.
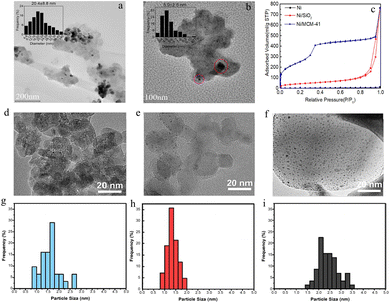 | ||
| Fig. 2 TEM images and particle size distributions of Ni/SiO2 (a) and Ni/MCM-41(b). (c) Adsorption/desorption isotherms of N2 of Ni/MCM-41. Reprinted with permission from ref. 42. Copyright 2022, Elsevier Ltd. TEM images of Ru–Ni/rutile (d), Ru–Ni/anatase (e) and Ru–Ni/P25 (f). The particle size distributions of Ru–Ni/rutile (g), Ru–Ni/anatase (h) and Ru–Ni/P25 (i). Reprinted with permission from ref. 43. Copyright 2019, Elsevier B.V. and Science Press. | ||
Molecular sieves with large pore sizes and pore volumes as well as an ultrahigh surface area are a potential catalyst carrier. Similarly, Chen et al. loaded non-noble metal Ni into mesoporous molecular sieves via the impregnation method to obtain Ni/MCM-41.42 In the TEM image of Ni/MCM-41, two types of Ni particles are observed (Fig. 2b). One is large-size Ni crystals on the external surface of MCM-41. The other is small-size Ni nanorods or nanodots uniformly distributed in the mesopores of MCM-41, and the average particle size of Ni is reduced to 5 nm, which is smaller than that of the synthesized Ni/SiO2. This is because the large specific surface area of the mesoporous molecular sieve MCM-41 of 616.4 m2 g−1 (Fig. 2c) is much higher than that of SiO2 (111.1 m2 g−1). Therefore, various types of supports such as silica, titanium dioxide, molecular sieves, etc. can all be loaded by the impregnation method. Different supports possess distinct physicochemical properties and can meet the requirements of different catalytic reactions. Moreover, these support materials are abundantly available in the market with relatively low prices, which enables the procurement cost of raw materials to be effectively controlled in large-scale production.
Furthermore, TiO2 is also a common catalyst carrier because of its adjustable and controllable metal anchoring sites. Yu et al. synthesized bimetallic catalysts (bimetallic Ru–Ni loaded on three types of TiO2 supports, namely Ru–Ni/rutile, Ru–Ni/anatase and Ru–Ni/P25) using this method.43 In this research, most of the metal species are highly dispersed on the surface of the carrier, and the average metal sizes of Ru–Ni/anatase and Ru–Ni/P25 are as small as 1.2 and 1.4 nm, respectively (Fig. 2d–i). The uniform dispersion of the active components increases their chances of coming into contact with the NEC during the subsequent hydrogenation process. They can participate more effectively in the catalytic reaction, thereby improving the utilization rate of the active components. Therefore, when achieving the same catalytic effect, compared with other preparation methods such as the deposition–precipitation method and the CVD method, the impregnation method requires a relatively smaller amount of active components, which can reduce the cost of the active components and have certain economic advantages in industrial applications.
2.2 Molten salt-assisted chemical reduction method
Due to advantages such as low reaction temperature, short reaction time, and being green and highly efficient, the molten salt-assisted chemical reduction method is often used to prepare metal catalysts.44 In the reaction process of this method, one or several types of low-melting-point metal salts are used as the reaction medium, and the reactants are placed in the molten salt to synthesize materials through a series of chemical and physical transformations. Among them, the selection of the types of molten salts, the amounts of molten salts used, and the ratio of raw materials to salts all need to be reasonably regulated. Specifically, in the presence of reducing agents (e.g. Ca and CaH2), the liquid-phase reaction medium can be used with the help of molten salt medium to expand the dispersion degree of metal nanoparticles. Thus, this method can effectively reduce the reaction sintering temperature and avoid the agglomeration between powders.For example, Yu et al.45 synthesized uniform LaNi5 particles via a molten salt-assisted CaH2 reduction method (Fig. 3a). The TEM results (Fig. 3b and e) reveal that the LaNi5 particles have a unique core–shell structure with a particle size around 100 nm. Compared with other synthesis methods, the molten salt-assisted CaH2 reduction method can obtain smaller LaNi5 particles. The greatest advantage of this method is the addition of excessive KCl. The addition of the KCl molten salt medium can provide a uniform reaction medium at a lower temperature and assist the diffusion reaction between metals. At the same time, the molten salt can also prevent the growth of the generated metal particles and prevent agglomeration. Previously, the main chemical synthesis methods for LaNi5-based hydrogen storage alloys mainly included the coprecipitation method, the gel network method, and the combustion method. Although the coprecipitation method is easy to operate, the difference in the solubility of different substances may lead to uneven precipitation rates, resulting in the inability to obtain uniformly mixed coprecipitates. The gel network method and the combustion method are relatively complex to operate, but the obtained oxide precursors are uniformly mixed, and the final alloy particles are of small size. Therefore, the nano-LaNi5 alloy synthesized by the molten salt-assisted chemical reduction method has a small size, a large specific surface area, an active Ni-rich layer on the surface, and is especially suitable for the catalytic hydrogenation reaction of NEC. This method can effectively control the composition and structure of the alloy, prepare alloy-type catalysts with high purity and high dispersity, reduce the subsequent purification treatment steps, and lower the production cost.
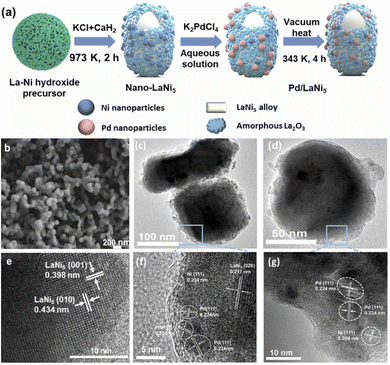 | ||
| Fig. 3 (a) Schematic illustration of the LaNi5 and Pd/LaNi5 synthetic process. (b) SEM image of LaNi5 and (e) HR-TEM image of LaNi5. Reprinted with permission from ref. 45. Copyright 2021, Elsevier Ltd. HRTEM images of Pd/LaNi5 after the galvanic replacement reaction (c and f) and Pd/LaNi5 after vacuum heat treatment (d and g). Reprinted with permission from ref. 46. Copyright 2022, Elsevier B.V. | ||
With the continuous progression of research, the combination of the molten salt method with other techniques can further improve the performance of alloy-type catalysts, providing more possibilities for the scalability of preparing alloy-type catalysts by the molten salt method. Yu et al.46 also proposed a two-step molten salt-assisted chemical reduction method to prepare Pd–LaNi5 (Fig. 3a). Under the effect of galvanic replacement between K2PdCl4 and nano-LaNi5, Pd particles are not only deposited on the surface of the originally smooth LaNi5 particles (Fig. 3c and f) but also will further move to the inner LaNi5 nucleus by vacuum heat treatment, making the surface smooth from being rough (Fig. 3d and g). Furthermore, the mean particle size of Pd–LaNi5 is 100 nm, similar to that of nano-LaNi5 mentioned above. The nano-LaNi5 synthesized by the molten salt method has certain stability in water and oxygen, and the displacement reaction between Pd and Ni can be conveniently carried out in an aqueous solution. Therefore, by using the displacement reaction between Pd and Ni to construct a Pd–Ni composite catalyst, this method is more convenient to operate compared with some complex multi-step synthesis methods that first adopt the hydrothermal method and then conduct heat treatment.
2.3 Carbothermal reduction method
The carbothermal reduction reaction uses a carbon source as a reducing agent to reduce substances (such as metal oxides) at relatively high temperatures.47 The carbothermal reduction can be used to prepare various transition metal nanomaterials. Due to its low raw material cost, it has good application value in industry. Biochar serves as both the carrier and the reducing agent without the need for any additional chemical reducing agents or stabilizers. This method has a wide range of raw material sources, is low in price, and uses mature technology. It is an efficient, stable, and green reduction method for preparing supported nano-metal catalysts.Qin et al.48 used biomass carbon as a catalyst carrier and Ni (NO3)2·6H2O and RuCl3·3H2O as metal sources to prepare Ni–Ru bimetallic catalysts by carbothermal reduction without adding any other reducing agent or stabilizer, named Ni0.5Ru4.5/pg-BC. The thus-prepared Ni0.5Ru4.5/pg-BC exhibits excellent dispersion of the alloy particles, which are evenly scattered on the carrier without any significant agglomeration (as shown in Fig. 4a and b). Besides, the metal particles have a relatively small size, with an average particle dimension of 2.8 nm. The resulting alloy nanoparticles are embedded in the recesses on the surface of the carrier that are formed due to carbon loss during the reduction process, demonstrating extremely high reactivity and stability.
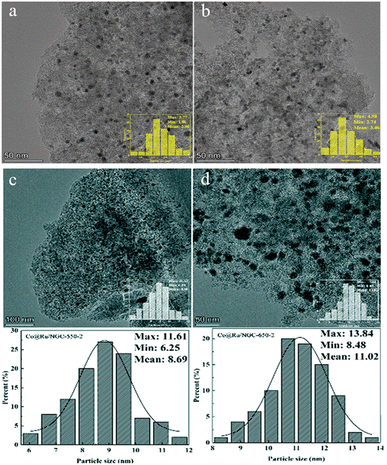 | ||
| Fig. 4 TEM images and particle size distributions of Ni0.5Ru4.5/pg-BC-550-2 (a) and Ni0.5Ru4.5/pg-BC-650-2 (b). Reprinted with permission from ref. 48. Copyright 2021, Elsevier Ltd. TEM images and particle size distributions of Co@Ru/NGC-550-2 (c) and Co@Ru/NGC-650-2 (d). Reprinted with permission from ref. 49. Copyright 2022, The Royal Society of Chemistry. | ||
In recent work, Qin et al.49 also synthesized a Co@Ru core–shell structured bimetallic catalyst supported by N-doped graphitized carbon (NGC) using urea as the nitrogen source through carbothermal reduction, named Co@Ru/NGC. The CoRu bimetallic nanoparticles have a relatively narrow distribution without obvious aggregation. As can be seen from Fig. 4c and d, the shell layer formed by many ruthenium nanoparticles coats cobalt nanoparticles of about 5 nm, and the average particle size of Co@Ru/NGC-550 is 8.69 nm. As the temperature is increased to 650 °C, the metal ions grow in size, resulting in an increase in the average particle diameter to 11.02 nm. Compared with the traditional chemical reduction method, the carbothermal reduction method does not involve the use of a large amount of strongly reducing reagents and flammable and explosive gases. Only by selecting appropriate metal precursors and carbon materials catalysts with high purity and good high activity can be prepared, and its operation process is simpler and safer. In addition, the carbothermal reduction method is applicable to the preparation of catalysts with different metal active components, demonstrating strong applicability of this method.
2.4 Other methods
In the field of catalytic research, extensive and in-depth studies have also been carried out on other methods for synthesizing non-noble metal catalysts such as the chemical reduction method and electrostatic adsorption method, and there have been comprehensive reports.For example, the chemical reduction method,50 which uses reducing agents to convert metal ions into metal atoms and aggregate them to form nano-scale particles, is one of the commonly used methods for preparing metal nanoparticle catalysts. The catalysts prepared by this method have low cost, simple operation conditions and short reaction time. Moreover, the microscopic performances of these catalysts can be regulated by altering the macroscopic preparation conditions. Wu et al.51 prepared the Co–B/Al2O3 precursor using NaBH4 as the reducing agent, and simply ground it with the YH3 precursor prepared by the high-temperature hydrogenation method, and finally obtained the Co–B/Al2O3–YH3 catalyst. As shown in Fig. 5a and b, amorphous spherical Co–B with the size of only 20 nm and highly crystalline YH3 with the size of 50–500 nm are uniformly dispersed in an Al2O3 carrier. The catalyst prepared by the sodium borohydride reduction method has good dispersion and strong stability and can effectively control the agglomeration of Co–B particles.
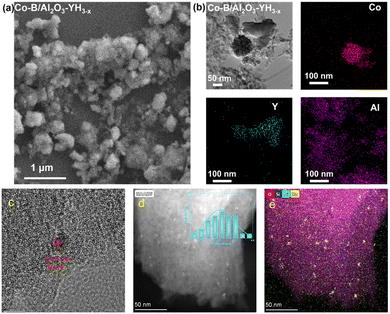 | ||
| Fig. 5 Co–B/Al2O3–YH3 before and after catalytic hydrogenation: (a) TEM image and (b) HRTEM image. Reprinted with permission from ref. 51. Copyright 2020, Chinese Chemical Society. Ru0.7Ni0.3/SBA15: (c) HRTEM image, (d) HAADF-TEM image, and (e) mapping. Reprinted with permission from ref. 52. Copyright 2022, Elsevier Ltd. | ||
The electrostatic adsorption method is a technique that uses electrostatic action to realize the mutual attraction between the adsorbed substance and the adsorbent surface to achieve the purpose of adsorption separation.53 A large number of literature studies indicate that this method will also be applicable to the preparation of highly dispersed small-sized metal nanoparticles on other carrier materials. Wang et al.52 enhanced the interaction between the carrier and the metal precursor by adjusting the acidity and alkalinity and obtained Ru–Ni bimetallic catalysts with similar carriers loaded with metal particles in different proportions (Fig. 5c–e). As a result, the catalyst has a regular mesostructure, and the molecular sieve structure is not destroyed during the electrostatic adsorption process. The RuNi alloy is highly dispersed and accumulates to form intergranular pores. Moreover, the size of the metal nanoparticles is 2.52–3.57 nm, and there is no agglomeration.
3. The structural characteristics of non-noble metal catalysts
In recent years, researchers have investigated various structural characteristics and compositions of non-noble metal catalysts with the aim of improving the performance and reducing the cost. In many reports, non-noble metals mainly exist in the form of being loaded on other carriers. The carriers in these catalysts not only play the role of dispersing and stabilizing metal nanoparticles but also have strong interactions with the metal particles, thereby influencing the activity, selectivity, and stability of the catalysts. Similarly, the size effect of metals has an important impact on the catalytic activity and selectivity of supported metal nanomaterials. How to select the particle size of the catalyst, expose more active sites, and then optimize the design of catalysts with better performance is a major research direction in the field of heterogeneous catalysis at present.54–563.1 The structural features of non-noble metal nanoparticle (NP) catalysts
The activity of the catalyst is closely related to the size of the metal particles. Metal nanoparticle catalysts supported on metal oxides with a high specific surface area or molecular sieves (supported metal NP catalysts) are currently the most widely studied and the most common types of catalysts in chemical production.57 Ding et al.58 conducted research on the electronic structure of Ni nanoparticles supported by SiO2–Al2O3 (Ni/AlSiO). They used silicon dioxide and aluminum oxide as catalyst carriers, enabling Ni atoms to be dispersed in the large pores formed between the stacked layers. Besides, X-ray photoelectron spectroscopy (XPS) revealed Ni0 and Ni2+ states in the Ni/AlSiO catalyst (Fig. 6a). The introduction of Si and Al elements in the catalyst forms a strong charge interaction, reduces the formation of spinel-structured NiAl2O4, promotes the reduction of Ni and prevents particle agglomeration, thus presenting outstanding catalytic performance without adding noble metals.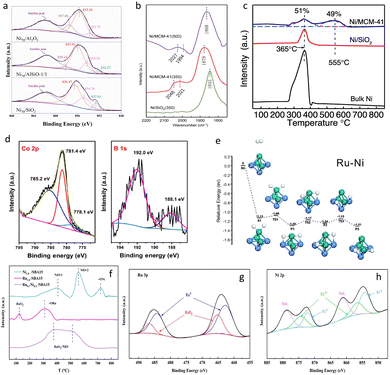 | ||
| Fig. 6 (a) XPS spectra of Ni/AlSiO. Reprinted with permission from ref. 58. Copyright 2023, Elsevier B.V. (b) In situ FT-IR spectra of CO adsorbed on Ni/MCM-41 and (c) H2-TPR profiles of the precursors of Ni/MCM-41. Reprinted with permission from ref. 42. Copyright 2022, Elsevier Ltd. (d) The XPS spectra of Co–B/A2O3–YH3. Reprinted with permission from ref. 51. Copyright 2020, Chinese Chemical Society. (e) Calculated energy diagram of dissociative chemisorption of an H2 molecule and the subsequent migration of the H atoms on Ru–Ni. Reprinted with permission from ref. 59. Copyright 2020, The Royal Society of Chemistry. (f) H2-TPR profiles of the precursors of RuNi/SBA15 and (g and h) the XPS spectra of RuNi/SBA15. Reprinted with permission from ref. 52. Copyright 2022, Elsevier Ltd. | ||
Chen et al.42 studied the electronic structure and particle size of nickel nanoparticles supported on the molecular sieve MCM-41. The shift of the B band to a higher wavenumber and the existence of the L band in the CO-IR spectrum (Fig. 6b) of Ni/MCM-41 (350) indicate that the electron density of Ni particles in Ni/MCM-41 (350) is lower than that of Ni/SiO2 (350). With the increase of the reduction temperature, the electron density of nickel particles on the surface of MCM-41 increases, which is beneficial to improving the selectivity of 12H-NEC. The H2 temperature-programmed reduction (TPR) curve (Fig. 6c) shows that the interaction between Ni and MCM-41 leads to an increase in the reduction temperature of nickel oxide species and prevents the aggregation of nickel particles in the mesopores at high temperatures due to the physical confinement of the mesopores of MCM-41. Therefore, the particle size of Ni/MCM-41 is significantly smaller than that of nickel particles on the surface of the SiO2 carrier. The active component Ni particles formed in the pores are smaller, generating more active centers, which is more conducive to the progress of the structure-sensitive NEC hydrogenation reaction.
Wu et al.51 developed a Co-based non-noble metal catalyst Co–B/Al2O3–YH3 that can simultaneously catalyze the efficient absorption and desorption of hydrogen in NEC. Co–B has amorphous nanoparticles with a size of about 20 nm, but most of them agglomerate into small spheres with a size of 50–200 nm, dispersed on the surface of Al2O3, and have a certain interfacial contact with YH3 to achieve hydrogen transfer. The d-band center of Co is located at −1.17 eV (relative to the Fermi level), and its position is even higher than that of the d-band center of Ni. Therefore, the adsorption ability of Co for NEC and its hydrogen-absorbed products may be too strong, resulting in the low catalytic activity of Co–B/Al2O3–YH3. Through the comparison of XPS spectra of Co 2p3/2 of the Co/Al2O3 and Co–B/Al2O3 catalysts (Fig. 6d), the signal peak of the former is attributed to Co–O, while that of the latter belongs to Co–B–O. This is because both have undergone surface oxidation, but it also indicates that the presence of B causes part of the electrons of Co to transfer to B, thereby shifting the d-band center of the surface Co downward, so that there is suitable adsorption strength for NEC and its hydrogen-absorbed products, and thus Co–B/Al2O3–YH3 exhibits high catalytic activity.
3.2 The structural features of non-noble metal alloy catalysts
Alloy materials have also been widely applied in the field of catalysis. Alloy catalysts can regulate element compositions, sizes, active sites, etc. to reduce the cost of the catalyst and enhance the durability. Compared with monometallic catalysts, the alloy catalysts can reduce the usage amounts of precious metals. For example, by forming alloys with some non-noble metals and noble metals, the catalyst cost can be reduced while maintaining good catalytic performance. In addition, alloy catalysts can change the electronic structure and geometric configuration through the synergistic effect between different metals, thereby improving the catalytic activity.60,61For example, in the single-metal catalyst Ru/TiO2, the Ru0 peak shows a slight positive shift from the standard value, indicating an electron transfer effect between Ru and TiO2. Subsequently, a second metal Ni, is doped into the single-metal catalyst. With the incorporation of Ni, the Ru0 peak shifts further positively. The higher binding energy implies that electrons transfer from the more electronegative Ni to Ru.43 Likewise, the calculation results of the electron density difference (Fig. 6e) also confirm this point.59 During the catalytic process, NEC is more readily adsorbed and activated by the electron-deficient metal Ru, thereby accelerating the reaction progress.
In another study, the RuNi/SBA15 alloy catalyst exhibited a similar trend.45 Hydrogen temperature-programmed reduction (H2-TPR) analysis revealed the formation of alloy species between ruthenium and nickel as well as the interactions between these metals (Fig. 6f). The bimetallic catalyst Ru0.7Ni0.3/SBA15 has a broad reduction peak around 300–600 °C. Compared with the temperature ranges of the monometallic catalysts (Ru and Ni), this reduction peak can probably be attributed to the formation of new alloy species. In addition, the hydrogen spillover effect between adjacent Ru sites and Ni sites leads to the emergence of active H. XPS characterization was further used to explore the electronic interactions of the two metals. Compared with the monometallic catalysts, the binding energy of Ru in the bimetallic catalyst increased, which can be attributed to the electron transfer between Ni and Ru. The electronegativity of Ru is greater than that of Ni, so in the bimetallic system the electron transfer from Ni to Ru will occur spontaneously, resulting in a decrease in the electron cloud density of Ni and an increase in the electron cloud density of Ru (Fig. 6g and h). The electronic interaction between Ru and Ni may enhance the catalytic activity. According to the literature reports, the electron transfer in bimetallic systems is one of the important signs of alloy formation. Moreover, through FT-IR spectroscopy testing, it was found that the –Si–OH peak of Ru0.7Ni0.3/SBA15 was enhanced. This is because the oxygen with enhanced electronegativity can attract hydrogen ions to recover the amount of –Si–OH. The RuNi alloy NPs complex with the surface hydroxyl groups of SBA15, and there is a strong interaction between the metal and the carrier, which improves the stability of the catalyst in the NEC hydrogenation.
4. Non-noble metal catalysts for catalytic NEC storage
Catalysts have a significant influence on the activity and selectivity of hydrogenation reactions. Choosing different catalysts can make the target hydrogenation reaction more active and the target product more selective.62,63 In order to reduce the cost of catalysts, the research focus has shifted to non-precious metal catalysts such as La, Ni and Co, so as to obtain catalysts with comparable performance to precious metals. It is an effective strategy to improve the catalytic performance of non-noble metal catalysts for NEC hydrogenation by adding cocatalysts or selecting suitable carriers. A second metal is also introduced into the catalyst supported by non-noble metals to obtain a catalyst with multiple metals as the active component. This section systematically introduces the hydrogen storage mechanism of NEC and the research results of non-noble metal catalysts. Table 2 summarizes the hydrogenation catalytic performance of some catalysts for NEC.| Catalysts | T (°C) | P (MPa) | Time (h) | Conva (%) | Yieldb (%) | H2 release (wt%) | Cycling performance | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Conversion of LOHCs (complete hydrogenation carriers). b Yield of complete dehydrogenation products. | ||||||||
| 5 wt% Ru/Al2O3 | 170 | 7 | 14 | 100 | — | 5.7 | — | 61 |
| 5.2 wt% Ru/Al2O3 | 160 | 6 | 0.6 | 100 | 98 | 5.6 | — | 64 |
| 1.0 wt% Ni/Al2O3–YH3 | 150 | 3 | 4.8 | 100 | 100 | 5.8 | 100% in 3 cycles | 65 |
| Ni70/AlSiO-1/1 | 150 | 7 | 1.5 | 100 | 100 | 5.8 | — | 58 |
| 5.0 wt% Ni0.5Ru4.5/pg-BC | 130 | 6 | 1.17 | 100 | 99.06 | 99.7% | 97.9% in 10 cycles | 48 |
| Ru0.7Ni0.3/SBA15 | 100 | 5 | 1.33 | 100 | 99.82 | — | 99% in 3 cycles | 52 |
| Ru–Ni/P25 | 150 | 7 | 24 | 100 | 93 | — | — | 43 |
| 5.0 wt% Co@Ru/NGC | 130 | 6 | 1 | 100 | 99.1 | 99.7% | 97.5% in 10 cycles | 49 |
| 10 wt% LaNi5 | 180 | 7 | 4.5 | 100 | 96.8 | 5.5 | 98.6% in 9 cycles | 45 |
| Co–B/Al2O3–YH3 | 180 | 10 | 2 | 100 | 100 | 5.6 | 90% in 3 cycles | 51 |
4.1 NEC hydrogen storage mechanism
Fig. 7a illustrates the complete hydrogenation process of NEC.66,67 It can be clearly observed that there are many hydrogenation intermediates during the NEC hydrogenation process.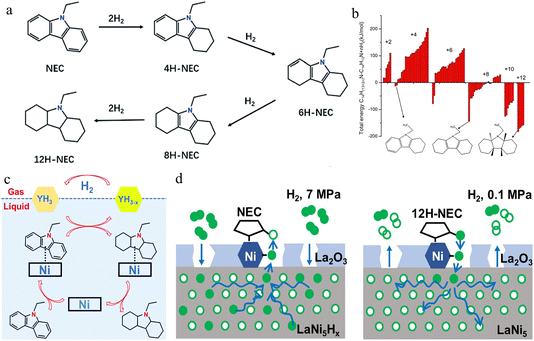 | ||
| Fig. 7 (a) Schematic diagram of the NEC stepwise hydrogenation reaction process. Reprinted with permission from ref. 66. Copyright 2024, Advanced Materials. (b) Relative stability of possible products in NEC hydrogenation as calculated by DFT. Reprinted with permission from ref. 62. Copyright 2010, Elsevier Ltd. (c) The schematic diagram of the synergetic hydrogenation mechanism of NEC on the Ni/Al2O3–YH3 catalyst. Reprinted with permission from ref. 65. Copyright 2019, The Royal Society of Chemistry. (d) Structure diagram of a LaNi5 nanoparticle and the mechanism schematic for NEC hydrogenation and 12H-NEC dehydrogenation (the solid and hollow circles represent occupied and unoccupied hydrogen binding sites, respectively. Pentagons with empty or filled H-binding sites denote NEC or 12H-NEC molecules). Reprinted with permission from ref. 45. Copyright 2021, Elsevier Ltd. | ||
From a theoretical perspective, Eblagon et al.62 concluded using density functional theory (DFT) that there are relatively stable potential hydrogenation products of 4H-NEC, 6H-NEC and 8H-NEC in NEC without solvent68 (as shown in Fig. 7b and c). Meanwhile, they found 8H-NEC to be the most stable among these intermediates. Gas chromatography-mass spectrometry (GC-MS) was used to further study the hydrogenation products of NEC, the result revealing the presence of only 4H-NEC and 8H-NEC intermediates. The 4H-NEC intermediate gradually disappears during hydrogenation, while the accumulation of 8H-NEC is observed.
Mehranfar et al.69 employed DFT calculations to investigate the energy variation during the hydrogenation process of NEC in both gaseous and solvent decalin at 150 °C and 7 MPa H2. Following the same conclusion as Eblagon et al.,62 8H-NEC is the most stable intermediate under both conditions. The activation energy for the conversion from 8H-NEC to 12H-NEC is the highest, serving as the rate-determining step for the entire reaction. So, how to design and improve the hydrogenation efficiency of catalysts and reduce the intermediates produced in the hydrogenation process is the research direction.
4.2 The mechanism of hydrogen transfer mediated by non-noble metal catalysts
The hydrogen absorption process of NEC involves the stepwise addition of H2 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond through multiple intermediates. Therefore, the hydrogenation process can be regarded as a hydrogen transfer process between H2 gas and NEC molecules. Thus, an efficient catalyst should be able to activate the substrate molecules and promote hydrogen transfer simultaneously. During the hydrogenation process of NEC, H2 needs to first dissociate into atomic H on the catalyst surface and then transfer to the H-bonding sites in the activated NEC molecules. Atomic hydrogen is a crucial intermediate in the hydrogen transfer process.
C bond through multiple intermediates. Therefore, the hydrogenation process can be regarded as a hydrogen transfer process between H2 gas and NEC molecules. Thus, an efficient catalyst should be able to activate the substrate molecules and promote hydrogen transfer simultaneously. During the hydrogenation process of NEC, H2 needs to first dissociate into atomic H on the catalyst surface and then transfer to the H-bonding sites in the activated NEC molecules. Atomic hydrogen is a crucial intermediate in the hydrogen transfer process.
Eblagon et al.62 explained the catalytic activities of different metal catalysts based on the position of the d-band center (Ed). The Ed values of the above metals are Pt (2.25) > Pd (1.83) > Ru (1.41) > Ni (1.29). It is difficult to explain the different activities of the non-noble metal Ni and the noble metal Ru solely by the slight differences in the Ed values. The hydrogen absorption of NEC requires the activation of both NEC and H2. Therefore, one possible reason for the low activity of the non-noble metal Ni/Al2O3 catalyst is the competitive adsorption of NEC and H2 on Ni. On traditional catalysts, the H atoms or vacant H-bonding sites on the catalyst surface must be around the activated NEC molecules. Each NEC molecule requires up to 12 H atoms. Consequently, due to the insufficient H atoms around the activated NEC molecules or the vacant H-bonding sites, the hydrogen transfer efficiency is rather low. Wu et al.65 combined a non-stoichiometric YH3 hydride with the non-noble metal catalyst Ni/Al2O3 to obtain Ni/Al2O3–YH3. YH3 is a large reservoir of atomic H and H-bonding sites, with rapid hydrogen absorption and desorption kinetics. Its addition provides an additional pathway for H2 activation and hydrogen transfer. During the hydrogenation process, Ni is used to activate NEC molecules, and YH3 is used to adsorb and dissociate H2. The H in YH3 overflows across the Ni/YH3 interface to the surface of Ni and undergoes a hydrogenation reaction with the NEC adsorbed on the Ni surface (Fig. 7c). Compared with the direct hydrogenation of NEC using H2, the hydrogen transfer from YH3 to NEC is more favorable. When the partially dehydrogenated YH3 absorbs H2, the H2 molecules dissociate into atomic H and reside in the Y lattice. Therefore, the reversible hydrogen absorption and desorption of YH3 continuously dissociates H2 into atomic H and transfers the atomic H from the Y lattice phase to NEC. This synergistic hydrogenation mechanism is illustrated, which includes the adsorption and activation of NEC on Ni, the dissociation of H2 by YH3, the transfer of H from YH3 to NEC, and the regeneration of the Ni surface.
The hydrogen spillover phenomenon generally refers to the process in which H2 is adsorbed and dissociated on the surface of species capable of activating hydrogen, and then the hydrogen adsorbed on its surface is transferred to other chemical species (mostly the carriers of the species that activate hydrogen). The hydrogen spillover phenomenon is of great importance for the catalytic reaction of NEC hydrogenation, opening up a new hydrogen transfer path and improving the catalytic performance of the catalyst. Hydrogen spillover can occur not only between the active metal and the carrier but also between the active metal and the hydrogen storage alloy. Yu et al.45 introduced the hydrogen storage alloy LaNi5 on the basis of the Ni-based catalyst. Ni nanocrystals can adsorb and activate the organic molecules of NEC/12H-NEC, while LaNi5, due to its rapid hydrogen absorption and desorption kinetics and good reversibility of hydrogen absorption and desorption, can provide sufficient dissociation H-binding sites (Fig. 7d). In addition, the LaNi5 nanoparticles have a naturally formed stable Ni/LaNi5 interface, which is conducive to the hydrogen transfer between the activated organic molecules on the Ni nanocrystals and the internal LaNi5. The hydrogen transfer in the hydrogenation–dehydrogenation cycle of NEC/12H-NEC can be subdivided into the following two steps: (1) the hydrogen transfer between the dissociated hydrogen and the NEC/12H-NEC adsorbed on the Ni nanocrystals after activation and (2) the hydrogen transfer between H2 and the dissociated H. LaNi5 has the ability to rapidly absorb and release hydrogen at room temperature, which means that the dissociation of H2 on the LaNi5 surface, the recombination of the dissociated H, and the transfer of hydrogen in the alloy lattice are all very fast. Therefore, the introduction of LaNi5 accelerates the hydrogen transfer in step (2). And the naturally formed stable LaNi5/Ni interface ensures the rapid progress of the hydrogen transfer in step (1). The synergistic effect between LaNi5 and Ni leads to the rapid absorption and release of hydrogen by NEC.
4.3 Monometallic hydrogenation catalysts
Ni-based catalysts have emerged as the primary focus in the research on hydrogenating NEC. The conventional Ni/Al2O3 catalyst exhibits low catalytic activity in the NEC hydrogenation reaction because of the formation of low-reduction NiAl2O4, thereby reducing the reduction of Ni.70,71 To enhance the catalytic performance, Wu et al.65 proposed a strategy for preparing rare earth hydride-transition metal cocatalysts and successfully synthesized a Ni/Al2O3–YH3 catalyst. The specific surface area of the catalyst was slightly smaller than the weighted average of the specific surface areas of Ni/Al2O3 and YH3. Therefore, a new interface is formed between Ni/Al2O3–YH3 during the grinding process. Rare earth hydride YH3 plays the role of a cocatalyst during the hydrogen absorption process. This co-catalytic effect avoids the problem of competitive adsorption between the organic substrate and H2 on the metal surface, significantly enhancing its catalytic activity. It is verified by isotope labeling that the incorporation of YH3 introduces a novel hydrogen transfer pathway and realizes the separation of activation centers (Fig. 8a–d). Hydrogen is dissociated on the surface of YH3 to obtain activated H which is then transferred to the surface of Ni to react with the NEC adsorbed on the surface of Ni. Moreover, under the reaction conditions, the hydrogen lost by YH3 can be quickly replenished from H2, enabling the hydrogen absorption reaction to proceed rapidly, which significantly enhances the catalytic performance. This catalyst can enable the complete hydrogen absorption of NEC at 150 °C, rivaling the currently best-performing Ru/Al2O3 catalyst (Fig. 8e and f). Moreover, the hydrogen absorption capacity of the Ni/Al2O3–YH3 catalyst and its XRD pattern hardly change after four cycles, which strongly prove that the Ni/Al2O3–YH3 catalyst has good structural stability and catalytic stability (Fig. 8g and h). Furthermore, this work discovers that Ni/Al2O3–YH3 exhibits much superior catalytic activity in hydrogen evolution of 12H-NEC compared to Ni/Al2O3 which barely has any catalytic effect. Although its performance is difficult to compare with the existing Pd-based catalysts, this result provides a direction for the future development of bidirectional non-noble metal catalysts.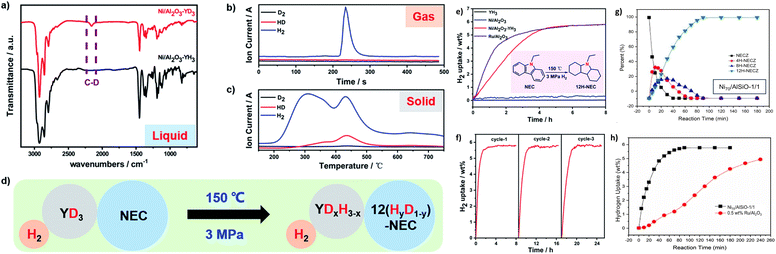 | ||
| Fig. 8 Analysis of the distribution of D after the hydrogen absorption reaction of NEC catalyzed by Ni/Al2O3–YD3: (a) FT-IR diagram of liquid-phase products (with 12H-NEC as a comparison sample). (b) MS diagram of gases in the gas phase. (c) TPD/MS diagram of the catalyst after separation, washing, and drying after use. (d) Schematic diagram of the flow direction of D during the reaction process. (e) Hydrogen absorption of NEC on different catalysts (Ni/Al2O3, Ru/Al2O3, YH3, and Ni/Al2O3–YH3). (f) Catalytic curves of Ni/Al2O3–YH3. Reprinted with permission from ref. 65. Copyright 2019, The Royal Society of Chemistry. (g) Time dependent product distribution for NECZ over Ni70/AlSiO-1/1 and (h) hydrogen absorption curves of Ni70/AlSiO-1/1 and 0.5 wt% Ru/Al2O3 as catalysts. Reprinted with permission from ref. 58. Copyright 2021, Elsevier Ltd. | ||
Similarly, different carriers in the catalyst and their proportions have varying impacts on the size of metal particles, further influencing the catalytic performance. Ding et al.58 selected Al2O3, SiO2 and their mixture as carriers to prepare Ni-based catalysts by a simple coprecipitation method, which can effectively catalyze the hydrogenation of dibenzyltoluene, benzene, NEC and N-propylcarbazole. By adjusting the ratio of silicon to aluminum, the Ni70/AlSiO-1/1 catalyst with the molar ratio of silicon to aluminum of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the carrier has the best catalytic performance for the above-mentioned LOHC hydrogenation, and it is a versatile hydrogenation catalyst to some extent. The Ni70/AlSiO-1/1 catalyst delivers excellent catalytic performance with a hydrogen uptake of 5.8 wt%. As the reaction proceeds, NEC gradually absorbs hydrogen and is finally completely hydrogenated into 12H-NEC, with its conversion rate exceeding 100% (Fig. 8i and j). In contrast, the commercially available 0.5 wt% Ru/Al2O3 catalyst requires 4 h to completely absorb hydrogen under the same conditions. Besides 12H-NEC, the product also contains a large amount of 8H-NEC, and its conversion rate is only 59.5%. In addition, the catalytic efficiency of this catalyst is still maintained at 95% after 5 continuous cycles, which can demonstrate its high catalytic stability. More importantly, this catalyst selects non-noble metal and low-cost carriers, demonstrating superior performance and high universality. Undoubtedly, this catalyst provides a case reference for replacing Ru-based catalysts, which is beneficial for further realizing the large-scale application of organic liquid hydrogen storage.
1 in the carrier has the best catalytic performance for the above-mentioned LOHC hydrogenation, and it is a versatile hydrogenation catalyst to some extent. The Ni70/AlSiO-1/1 catalyst delivers excellent catalytic performance with a hydrogen uptake of 5.8 wt%. As the reaction proceeds, NEC gradually absorbs hydrogen and is finally completely hydrogenated into 12H-NEC, with its conversion rate exceeding 100% (Fig. 8i and j). In contrast, the commercially available 0.5 wt% Ru/Al2O3 catalyst requires 4 h to completely absorb hydrogen under the same conditions. Besides 12H-NEC, the product also contains a large amount of 8H-NEC, and its conversion rate is only 59.5%. In addition, the catalytic efficiency of this catalyst is still maintained at 95% after 5 continuous cycles, which can demonstrate its high catalytic stability. More importantly, this catalyst selects non-noble metal and low-cost carriers, demonstrating superior performance and high universality. Undoubtedly, this catalyst provides a case reference for replacing Ru-based catalysts, which is beneficial for further realizing the large-scale application of organic liquid hydrogen storage.
To date, advancements in high-performance, cost-effective hydrogenation catalysts for NEC compounds as LOHCs primarily revolve around incorporating accelerators like YH3 and SiO2 to enhance conventional carriers, exploring non-noble metal catalysts as substitutes for noble metal catalysts. Furthermore, a combination of these two approaches holds promise for the future development of even more effective hydrogenation catalysts.
4.4 Bimetallic hydrogenation catalysts
Due to the relatively low intrinsic catalytic activity of some non-noble metal-based catalysts, their activation effect on organic substrates is poor, resulting in particularly unsatisfactory catalytic ability for the hydrogenation reaction of NEC. Noble metals are still mainstream in the current research on NEC/12H-NEC catalysts. Noble metals have a relatively strong activation effect on the C–H bonds of organic substrates.72 Therefore, people have been continuously exploring bimetallic hydrogenation catalysts with added noble metal components in non-noble metal catalysts, which has led to remarkable progress in the catalytic hydrogenation of NEC. Multi-metal active components are one of the effective strategies for reducing costs and developing highly active non-noble metal catalysts.Currently, adding the second metal Ru to Ni-based non-noble metal catalysts is the most common RuNi bimetallic catalyst in hydrogen storage applications. Qin et al.48 combined different amounts of Ni with Ru to prepare Ni–Ru NPs/graphitized biomass carbon (pg-BC) catalysts. The experimental results indicate that the Ni–Ru/pg-BC catalyst delivers superior performance with 99.06% 12H-NEC yields and 99.7% hydrogen storage (6 MPa H2 at 130 °C) (Fig. 9a and b). In addition, even when the amount of Ru added was reduced by 10%, the bimetallic catalyst still exhibited excellent catalytic activity. The non-noble metal Ni promoted the electron transfer between the two metals, and the partial graphitization of the support enhanced its conductivity and electron transfer ability.73 Meanwhile, Ni also promoted the activation of H2 to H, generating the hydrogen spillover effect and effectively increasing the active sites of the catalyst.74 After 10 cycles, the catalyst maintains a 100% conversion rate of NEC and a 12H-NEC yield of over 93.47%, significantly outperforming commercial Ru/Al2O3. The catalyst prepared via carbon thermal reduction shows an 11.4% decrease in metal loading after 10 cycles (Fig. 9c–f). Qin et al.48 prepared Ni0.5Ru4.5/pg-BC-NaBH4 catalysts using a conventional reduction method for comparison. After 10 cycles, the metal loading of the catalyst prepared by carbon thermal reduction decreased by 11.4%, while the metal loading of the catalyst prepared by the reduction method decreased by 48.2%. Therefore, carbon thermal reduction allows metal particles to be embedded in the depressions on the support surface formed by carbon loss during reduction, effectively preventing metal particle agglomeration and loss and demonstrating exceptionally high stability.
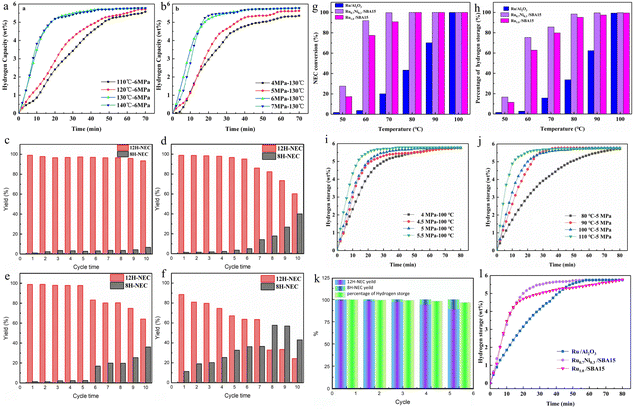 | ||
| Fig. 9 Catalytic curves of Ni0.5Ru4.5/pg-BC under different conditions: (a) different temperatures and (b) different H2 pressures. Recycling experiment histograms of different catalysts: (c) Ru/pg-BC-550-2, (d) commercial Ru/Al2O3, (e) commercial Ru/C and (f) Ru/BC-NaBH4 in NEC. Reprinted with permission from ref. 48. Copyright 2022, Elsevier Ltd. (g and h) Hydrogenation curves depicting the catalytic performance of different catalysts of Ru0.7Ni0.3/SBA15 (5 MPa H2 pressure for 80 min). Catalytic curves of Ru0.7Ni0.3/SBA15 under different conditions: (i) different H2 pressures, (j) different temperatures, (k) reusability test and (l) various catalysts (100 °C and 5 MPa H2). Reprinted with permission from ref. 52. Copyright 2022, Elsevier Ltd. | ||
The strategy of preparing bimetallic catalysts by adding noble metal components to non-noble metal catalysts can further improve the catalytic hydrogenation performance by selecting appropriate carriers. Wang et al.52 used SBA15 molecular sieves as a catalyst carrier and prepared the Ru0.7Ni0.3/SBA15 alloy nano-catalyst with high activity via an electrostatic adsorption method. It also confirms the feasibility of the strategy of adding noble metal components to non-noble metal catalysts to improve the catalytic activity. Under Ru0.7Ni0.3/SBA15 catalysis, NEC can be fully hydrogenated within 0.5 h at 100 °C and 5.5 MPa H2 (Fig. 9g–i). With the increase of H2 pressure, the hydrogen storage rate reaches a peak value of 99.82% under 5 MPa H2 pressure. The influence of temperature on the reaction rate is more significant than that of pressure. The hydrogen storage rates at 90–110 °C all exceed 99.0%. By comparing the NEC conversion rates of different catalysts at different temperatures, it is found that the NEC hydrogenation reaction catalyzed by Ru0.7Ni0.3/SBA15 synthesized by electrostatic adsorption can achieve a 100% NEC conversion rate at 70 °C. Moreover, both the NEC conversion rate and the percentage of hydrogen storage of this catalyst at 60 °C are higher than those of the commercial Ru/Al2O3 at 90 °C (Fig. 9g and h). Repeating the hydrogenation reaction five times under the same conditions, the used catalyst still facilitates rapid hydrogen absorption of NEC, with the hydrogen storage rate remaining above 96% (Fig. 9k). This indicates that the catalyst has excellent catalytic stability. There is a charge transfer between the non-noble metal Ni and the added noble metal Ru, resulting in synergistic hydrogenation catalysis. The alloy nanoparticles are electrostatically adsorbed on the SBA15 surface, forming a robust structure that is uniformly distributed and highly dispersed. It is also noted that this catalyst achieves over 90% hydrogenation conversion efficiency even at the lowest reaction temperature (60 °C), which can effectively reduce the cost and energy consumption (Fig. 9l). During the hydrogenation process, H radicals generated by adsorption on the Ni active sites of the catalyst migrate to the Ru active sites via the support, facilitating hydrogen overflow. This is a crucial step in the entire alloy synergistic catalysis process, and the new hydrogen transfer pathway also reduces the formation of oxides.
As a representative of oxide carriers, TiO2 has an obvious hydrogen overflow effect on its surface. Therefore, the hydrogen overflow effect on its surface is significant, making it highly suitable for leveraging the synergistic effects of bimetallic catalysts. TiO2 has various crystal forms, and the catalytic performance and effects of these different crystal forms as supports for bimetallic catalysts can vary. Yu et al.43 investigated the changes in the catalytic performance of Ru–Ni bimetallic catalysts supported on three different types of TiO2 carriers, namely rutile, anatase, and commercial P25 (a mixture of rutile and anatase in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Fig. 10a and b). Ru–Ni/P25 is a mixture of rutile and anatase. It can form small-sized alloys on the surface of anatase, and due to the presence of rutile, it can avoid excessive growth covering the surface of the active metal. Therefore, it exhibits high activity and selectivity for the hydrogenation of NEC. Under the catalysis of Ru–Ni/P25, NEC can be completely hydrogenated under the conditions of 150 °C and 7 MPa H2. Among them, the catalyst with the highest selectivity for the fully hydrogenated product 12H-NEC is Ru/anatase. After 24 h of reaction, the proportion of the fully hydrogenated product 12H-NEC in the product is as high as 93%, and the TOF reaches 3.4. There are dual activation centers in the Ru–Ni bimetallic catalyst. The presence of the precious metal Ru serves as an activation center for NEC, increasing the hydrogenation activation sites. The non-precious metal Ni serves as an activation center for H2, reducing the competitive adsorption between NEC and dissociated hydrogen, thereby improving the activity and selectivity. In bimetallic catalysts, the hydrogen overflow effect between Ru and Ni allows for rapid transfer of dissociated H on the reducible TiO2 surface, leading to continuous hydrogenation of NEC adsorbed on Ru. Experimental data also show that the effectiveness of the dual-active-center design is related to the crystal form of TiO2, with Ru–Ni/P25 showing significant improvement. Dual active sites are provided by the Ru–Ni bimetallic catalyst, which reduces the competitive adsorption between NEC and dissociated hydrogen. Therefore, while maximizing the advantages of dual active centers, it is also essential to enhance the ability of the second active center to dissociate H2 and improve the efficiency of hydrogen transfer between the two active centers.
4) (Fig. 10a and b). Ru–Ni/P25 is a mixture of rutile and anatase. It can form small-sized alloys on the surface of anatase, and due to the presence of rutile, it can avoid excessive growth covering the surface of the active metal. Therefore, it exhibits high activity and selectivity for the hydrogenation of NEC. Under the catalysis of Ru–Ni/P25, NEC can be completely hydrogenated under the conditions of 150 °C and 7 MPa H2. Among them, the catalyst with the highest selectivity for the fully hydrogenated product 12H-NEC is Ru/anatase. After 24 h of reaction, the proportion of the fully hydrogenated product 12H-NEC in the product is as high as 93%, and the TOF reaches 3.4. There are dual activation centers in the Ru–Ni bimetallic catalyst. The presence of the precious metal Ru serves as an activation center for NEC, increasing the hydrogenation activation sites. The non-precious metal Ni serves as an activation center for H2, reducing the competitive adsorption between NEC and dissociated hydrogen, thereby improving the activity and selectivity. In bimetallic catalysts, the hydrogen overflow effect between Ru and Ni allows for rapid transfer of dissociated H on the reducible TiO2 surface, leading to continuous hydrogenation of NEC adsorbed on Ru. Experimental data also show that the effectiveness of the dual-active-center design is related to the crystal form of TiO2, with Ru–Ni/P25 showing significant improvement. Dual active sites are provided by the Ru–Ni bimetallic catalyst, which reduces the competitive adsorption between NEC and dissociated hydrogen. Therefore, while maximizing the advantages of dual active centers, it is also essential to enhance the ability of the second active center to dissociate H2 and improve the efficiency of hydrogen transfer between the two active centers.
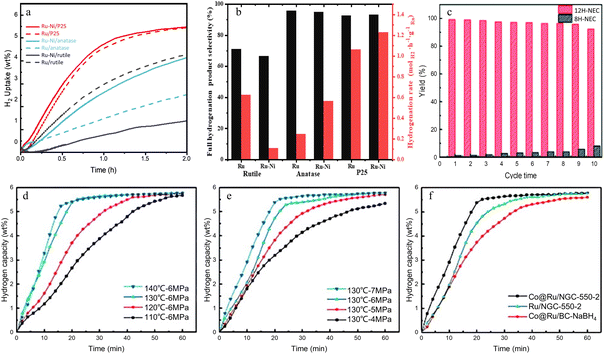 | ||
| Fig. 10 (a) Catalytic curves of various catalysts and (b) catalytic performances of as-prepared catalysts. The left axis shows selectivity towards the full hydrogenation product 12H-NEC; the right axis shows the hydrogenation rate. Reprinted with permission from ref. 43. Copyright 2019, Elsevier B.V. and Science Press. Catalytic curves of Co@Ru/NGC under different conditions: (c) reusability test, (d) different temperatures, (e) different H2 pressures and (f) various catalysts (130 °C and 6 MPa H2). Reprinted with permission from ref. 49. Copyright 2022, The Royal Society of Chemistry. | ||
In addition, adding the second metal Ru to non-noble metal Co-based catalysts is also a common strategy. Based on the above challenges, Qin et al.49 proposed a Co@Ru core–shell bimetallic catalyst with nitrogen-doped graphitized carbon (NGC), which exhibits high activity and superstability, confirming the feasibility of the strategy of adding noble metal components to other non-noble metal catalysts to improve the catalytic activity. This design, with the addition of a small amount of the noble metal Ru, has significantly enhanced the catalytic activity of the original non-noble metal catalyst. With the increase of temperature and hydrogen pressure, the reaction rate of the catalyst increases gradually. Under the conditions of 130 °C, 6 MPa H2, and a catalyst loading of 0.3 wt%, the final hydrogen storage rate is 99.7% and the NEC yield reaches 99.1% (Fig. 10d and e). This is attributed to the successful graphitization of the carbon carrier and the doping of N, which improves the electrical conductivity and electron transfer ability of the composite material, thereby enhancing the catalytic activity of the catalyst, being significantly superior to Co@Ru/BC-NaBH4 synthesized by the chemical reduction method (Fig. 10f). In addition, the presence of Ru increases the activation sites of the catalyst, while Co promotes the activation of H2, resulting in the hydrogen spillover effect, which further promotes the catalytic performance of the catalyst. Furthermore, even after 10 consecutive cycles, the Co@Ru/NGC catalyst maintains an NEC conversion rate of 100% and a hydrogen storage capacity of over 97.5%, which demonstrate its exceptional catalytic stability (Fig. 10c). This study provides a reference for the preparation of core–shell bimetallic catalysts for NEC hydrogenation.
4.5 Other hydrogenation catalysts
Using carriers with specific functional properties can play a pivotal role in achieving highly efficient catalyst hydrogenation. Yu et al.45 synthesized LaNi5 hydrogen storage alloys using a molten salt-assisted chemical reduction method, which enables reversible hydrogen storage for NEC. Catalytic tests reveal that LaNi5 exhibited superior performance, with a hydrogen uptake of 5.5 wt% for NEC within 5.5 h at 180 °C, and a dehydrogenation capacity of 5.5 wt% for 12H-NEC within 4.5 h at 200 °C (Fig. 11a and b). In addition, the LaNi5 also presents good stability under 9 cycles, and the reversible hydrogen storage capacity of hydrogen absorption and desorption cycles can still reach more than 5.5 wt% (Fig. 11c). Thus, LaNi5 is not only a unique reversible catalyst for the NEC/12H-NEC hydrogenation/dehydrogenation reaction but also is the best catalyst for its long-term cycle stability. The catalyst incorporates the hydrogen storage alloy LaNi5 onto a Ni-based catalyst. The La2O3 layer on the alloy surface is not dense, allowing H2 and reactants to permeate through it and interact with the embedded Ni nanocrystals and the underlying LaNi5. The Ni nanocrystals can adsorb and activate NEC/12H-NEC organic molecules. Due to its rapid hydrogen absorption and desorption kinetics and good hydrogen absorption/desorption reversibility, LaNi5 provides ample dissociation hydrogen binding sites. Additionally, the naturally formed stable Ni/LaNi5 interface within LaNi5 nanoparticles facilitates hydrogen transfer between the activated organic molecules on the Ni nanocrystals and the internal LaNi5.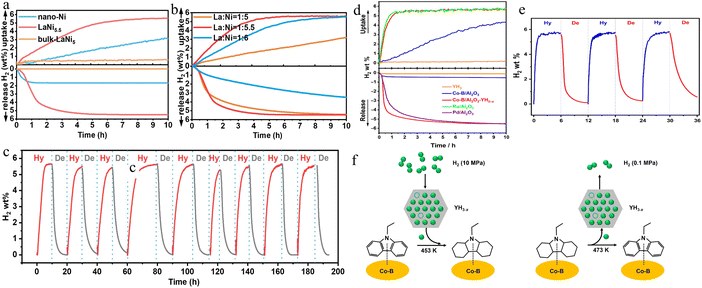 | ||
| Fig. 11 Catalytic curves of LaNi5 under different conditions: (a) various catalysts and (b) LaNi5 with different excessive Ni contents and (c) reusability test. Reprinted with permission from ref. 45. Copyright 2021, Elsevier Ltd. Hydrogen uptake and release catalytic curves of Co–B/YH3 under different conditions: (d) various catalysts and (e) reusability test. (f) The surface hydrogen transfer mechanism of the Co–B/Al2O3–YH3 catalyst for NEC hydrogenation and 12H-NEC dehydrogenation reactions. Reprinted with permission from ref. 51. Copyright 2020, Chinese Chemical Society. | ||
In addition to using carriers with specific functional properties, incorporating promoters is also an effective approach for preparing high performance catalysts. For example, Wu et al.51 used rare earth oxides to enhance the Co–B/Al2O3 catalyst, yielding remarkable outcomes. The synthesized Co–B/Al2O3–YH3 catalyst exhibited outstanding catalytic performance in facilitating the hydrogenation and dehydrogenation processes. Co–B/Al2O3 has some catalytic activity for hydrogen absorption reactions, but its activity is relatively low. Surprisingly, Co–B/Al2O3–YH3, obtained by simply mixing with YH3, exhibits excellent catalytic performance for both hydrogen absorption and desorption reactions. Under the catalysis of Co–B/Al2O3–YH3, the hydrogen absorption reaction of NEC can be completed within 2 h at 180 °C and 10 MPa H2. The corresponding hydrogen desorption reaction can release more than 5.5 wt% hydrogen within 7 h at 200 °C and 0.1 MPa H2 (Fig. 11d and e). This catalyst shows far superior performance for NEC hydrogen absorption reactions compared to non-noble metal catalysts reported in the literature, approaching the performance of noble metal catalysts. In addition, the synergistic catalytic process of rare earth hydride-transition metal is verified again by isotope labeling and kinetic isotope effect. Within the Co–B/Al2O3–YH3 framework, Co–B serves to activate NEC/12H-NEC molecules, while YH3 facilitates the rapid H transfer. Hydrogen transfer kinetics are significantly accelerated by the presence of lattice H and H vacancies in YH3. After simple grinding, Co–B/Al2O3 and YH3 form a new contact interface, where both synergistically catalyze the hydrogen absorption and desorption of NEC. As illustrated in Fig. 11f, activated hydrogen exists within the YH3 lattice, simultaneously transferring some hydrogen to the Co–B surface and reacting with NEC adsorbed on the Co–B surface. The hydrogen lost from YH3 is quickly replenished from H2 and then transferred to the NEC attached to the Co–B surface for hydrogen storage. This shows that rare earth hydrides can not only promote the catalytic hydrogen absorption reaction process but also promote the catalytic hydrogen release reaction process, further expanding the application of metal hydrides in catalytic and liquid organic hydrogen storage systems and providing a direction for the development of cheap and efficient LOHCs.
5. Conclusions
To sum up, this review provides a detailed overview of the latest research advancements in non-noble metal catalysts for NEC hydrogen storage. Research on non-noble metal catalysts for NEC hydrogen storage covers various aspects, from synthesis methods and structural features to microscopic mechanisms and catalytic performances. These studies provide a valuable foundation for further advancing the application of non-precious metal catalysts in hydrogen storage technology.Although significant headway has been achieved in non-noble metal catalysts, there remain several challenges that impede their further progress in LOHCs. Consequently, we present the current issues and future development directions as follows:
(I) Development and enhancement of non-noble metal catalysts
Currently, existing methods for catalyst preparation have not fully addressed how to achieve efficient and precise structural tuning at the nanoscale. Additionally, there are technical challenges in enhancing the overall catalytic performance of catalysts to meet higher reaction rates, better selectivity, and greater stability. The development of new technologies should focus on improving the efficiency and simplicity of the preparation process while also exploring new catalyst design concepts to promote widespread industrial applications and enhance practical benefits.
Non-noble metal catalysts have a high degree of tunability in their electronic and geometric structures. First, various preparation parameters such as temperature, pressure, and reaction time can be finely adjusted to control these structures. Factors related to chemical composition, such as the choice of the primary support and the type and ratio of metal elements, play a crucial role in the catalysts' performance. The particle size and morphology of the catalysts also affect the specific surface area, the number and dispersion of active sites, and mass transfer properties. Furthermore, precise control of the local structure and distribution of active sites, such as the platforms, corners, and steps on the catalyst surface, is essential. Therefore, by finely tuning these factors during the NEC hydrogenation process, non-precious metal catalysts can exhibit exceptionally significant potential performance, greatly enhancing the efficiency and selectivity of the hydrogenation reaction. This is facilitated by innovative synthesis methodologies, including in situ preparation techniques, carbothermal reduction methods, and molten salt-assisted chemical reduction processes. In addition, the synergistic interactions among multiple elements offer a diverse range of adsorption sites, making these metal catalysts particularly suited for complex hydrogenation reactions, such as those involving multiple steps. Nevertheless, significant challenges persist in the development of highly efficient catalysts, precise control of their fine structures, and understanding the structure–property relationships in NEC hydrogenation catalytic processes.
(II) Explore the structure–property relationship from multiple angles
Due to the potential changes in the morphology and electronic structure of active centers during the NEC hydrogenation reaction, accurately determining their true state and function becomes challenging. Additionally, the catalytic reaction often involves multiple intermediates and reaction steps, complicating the comprehensive understanding of the catalytic mechanism. These technical and theoretical obstacles not only hinder a deeper understanding of existing catalysts' performance but also limit the design and development of new catalysts. Therefore, addressing these challenges is crucial for advancing the field further. These issues hamper the development of insights for designing new catalysts, thereby constraining further advancements in this area.
First of all, improvements can be made in terms of in situ characterization techniques. Reactions during the hydrogenation process of catalysts are often very rapid. Faster detectors, optimization of data acquisition systems and algorithms, etc. can be employed to shorten the data acquisition time interval, capture these transient reaction processes and intermediate species more accurately, and thus gain an in-depth understanding of the dynamic change process of the reactions. Second, different in situ techniques can also be combined. For example, gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) can be used to study in detail how the catalyst interacts with the reactants during the reaction process and the structural changes of the active centers on the catalyst surface throughout the reaction process. By real-time monitoring and analysis of the changes that occur during the reaction process, a deeper understanding of the essence of the catalytic reaction can be achieved, providing valuable guidance for the development and application of new catalysts in the future. In addition, in situ techniques can also be combined with ex situ techniques. Before conducting in situ characterization experiments, the initial structure and morphology of the catalyst can be observed using ex situ electron microscopy techniques. Then, during the in situ experiment, the structural changes of the catalyst can be monitored in real time. Finally, the catalyst after the reaction can be characterized using ex situ analysis techniques to verify the results of the in situ experiment, enabling a more comprehensive understanding of the changes in the catalyst throughout the entire hydrogenation process. Finally, high-throughput calculations or density functional theory calculations (DFT) can be utilized to simulate the entire catalytic reaction process at the atomic level, revealing the active sites involved in the reaction, the reaction pathways, as well as the stability and reactivity of the intermediates, which can be used to predict the performance of different catalyst materials, overcome the limitations of experimental techniques, and provide profound insights and directions for the design and optimization of catalysts.
(III) Improvement of catalytic system and exploration of reversible hydrogenation/dehydrogenation catalysts
The choice of the active components of a catalyst has a significant impact on its catalytic performance. Currently, there are relatively few studies on non-noble metal catalysts for catalyzing the dehydrogenation reaction of NEC. The development of catalysts that combine non-noble metals and noble metals can not only significantly reduce the cost of the catalysts but also improve their bifunctional catalytic performance by optimizing the composition of the catalysts. Combining non-noble metals with relatively good catalytic activity (such as Ni, Co, etc.) with metals having a strong hydrogen adsorption capacity (such as Pt, Pd, etc.) can utilize the catalytic activity of the former and the hydrogen adsorption and dissociation capabilities of the latter to jointly promote the hydrogenation reaction of NEC. Combining and adjusting non-noble metal and noble metal catalysts in different proportions can not only significantly reduce the cost of the catalysts but also improve their bifunctional catalytic performance by optimizing the composition of the catalysts.
In addition, an appropriate carrier can not only stabilize the structure and increase the anchoring sites but also enhance the dispersion of the active metals to ensure sufficient contact between the active sites and NEC. Furthermore, carriers with good hydrogen spillover performance, such as reducible carriers, zeolite molecular sieves, carbon materials, etc., can be selected. These carriers can interact with the metals to promote the occurrence of hydrogen spillover and improve the hydrogenation performance of the catalyst. The carrier can also be modified, such as introducing defect sites, acidic sites or alkaline sites, etc., which can increase the interaction between the carrier and the metal and enhance the hydrogen spillover effect. Or some electron promoters, such as alkali metals, alkaline earth metals, etc., can be added to change the electronic structure of the catalyst, improve its hydrogen adsorption and dissociation capabilities, and thus enhance the hydrogen spillover effect and the bimetallic synergistic effect.
Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Author contributions
Hansai Wu: writing – original draft, conceptualization. Junming Zhang: writing – review & editing, writing – original draft. Zicong Wang: writing – review & editing. Xianglong Kong: writing – review & editing. Gaofu Li: writing – review & editing. Ying Zhao: writing – review & editing, supervision. Piaoping Yang: writing – review & editing, supervision. Zhiliang Liu: writing – review & editing, supervision.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 22101065), the Natural Science Foundation of Heilongjiang Province (No. YQ2021B001), the China Postdoctoral Science Foundation (No. 2020M681075 and 2023T160153), and Fundamental Research Funds for the Central Universities.Notes and references
- Y. X. Gong, J. S. Yao, P. Wang, Z. X. Li, H. J. Zhou and C. M. Xu, Chin. J. Chem. Eng., 2022, 43, 282–296 CrossRef CAS.
- P. K. Liu and X. Han, Int. J. Hydrogen Energy, 2022, 47, 9485–9503 CrossRef CAS.
- M. Niermann, A. Beckendorff, M. Kaltschmitt and K. Bonhoff, Int. J. Hydrogen Energy, 2019, 44, 6631–6654 CrossRef CAS.
- A. Z. Arsad, M. A. Hannan, A. Q. Al-Shetwi, M. Mansur, K. M. Muttaqi, Z. Y. Dong and F. Blaabjerg, Int. J. Hydrogen Energy, 2022, 47, 17285–17312 CrossRef CAS.
- F. Razi and I. Dincer, Renewable Sustainable Energy Rev., 2022, 168, 112763 CrossRef CAS.
- I. Staffell, D. Scamman, A. V. Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- Q. M. Sun, N. Wang, Q. Xu and J. H. Yu, Adv. Mater., 2020, 32, 2001818 CrossRef CAS.
- Q. Y. Zhao, X. D. Zhao, Z. Y. Liu, Y. Ge, J. X. Ruan, H. Y. Cai, S. S. Zhang, C. L. Ye, Y. Xiong, W. Chen, G. Meng, Z. L. Liu and J. Zhang, Adv. Mater., 2024, 36(36), 2402971 CrossRef CAS.
- U. Eberle, M. Felderhoff and F. Schüth, Angew. Chem. Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed.
- S. Sharma and S. K. Ghoshal, Renewable Sustainable Energy Rev., 2015, 43, 1151–1158 CrossRef CAS.
- S. S. Zhang, X. D. Zhao, Y. J. Qiu, Y. Xiong, G. Meng, W. Chen, Z. L. Liu and J. Zhang, Nano Lett., 2024, 24, 5165–5173 CrossRef CAS PubMed.
- G. C. Li, M. Niu, R. Z. An, H. Y. Zhang, B. X. Sun and G. L. Li, Inorg. Chem., 2024, 63, 20792–20801 CrossRef CAS PubMed.
- B. Galey, A. Auroux, S. Sabo-Etienne, S. Dhaher, M. Grellier and G. Postole, Int. J. Hydrogen Energy, 2019, 44, 28848–28862 CrossRef CAS.
- K. S. Nivedhitha, T. Beena, N. R. Banapurmath, M. A. Umarfarooq, V. Ramasamy, M. E. M. Soudagar and Ü. Agbulut, Int. J. Hydrogen Energy, 2024, 61, 1259–1273 CrossRef CAS.
- Y. K. Huang, Y. Zheng, J. D. Li, X. Z. Bao, J. P. Guo, J. J. Shen, Y. Guo, Q. Zhang, J. Li, W. Lei and H. Y. Shao, J. Mater. Sci. Technol., 2023, 153, 181–204 CrossRef CAS.
- X. Zhang, Z. H. Ren, X. L. Zhang, M. X. Gao, H. G. Pan and Y. F. Liu, J. Mater. Chem. A, 2019, 7, 4651–4659 RSC.
- O. Zavorotynska, S. Deledda and B. C. Hauback, Int. J. Hydrogen Energy, 2016, 41, 9885–9892 CrossRef CAS.
- M. Baricco, M. Bang, M. Fichtner, B. Hauback, M. Linder, C. Luetto, P. Moretto and M. Sgroi, J. Power Sources, 2017, 342, 853–860 CrossRef CAS.
- S. Kiruthika, P. Sundar and P. Ravindran, J. Solid State Chem., 2023, 321, 12867 CrossRef.
- K. T. Moller, D. Sheppard, D. B. Ravnsbæk, C. E. Buckley, E. Akiba, H. W. Li and T. R. Jensen, Energies, 2017, 10, 1645 CrossRef.
- S. P. Safronov, S. V. V. Vostrikov, A. A. Samarov, P. Wasserscheid, K. Mueller and S. P. Verevkin, Fuel, 2023, 331, 125764 CrossRef CAS.
- B. S. Shin, C. W. Yoon, S. K. Kwak and J. W. Kang, Int. J. Hydrogen Energy, 2018, 43, 12158–12167 CrossRef CAS.
- L. B. Shi, S. T. Qi, J. F. Qu, T. H. Che, C. H. Yi and B. L. Yang, Int. J. Hydrogen Energy, 2019, 44, 5345–5354 CrossRef CAS.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118–18132 CrossRef CAS.
- Y. Dong, M. Yang, L. L. Li, T. Zhu, X. D. Chen and H. S. Cheng, Int. J. Hydrogen Energy, 2019, 44, 4919–4929 CrossRef CAS.
- A. H. Al-ShaikhAli, A. Jedid, D. H. Anjum, L. Cavallo and K. Takanabe, ACS Catal., 2017, 7, 1592–1600 CrossRef CAS.
- C. H. Chan, S. Y. Lee and S. S. Han, Int. J. Hydrogen Energy, 2023, 48, 33590–33598 CrossRef CAS.
- K. Sisáková, N. Podrojková, R. Orinaková and A. Orinak, Energy Fuels, 2021, 35, 7608–7623 CrossRef.
- N. Brückner, K. Obesser, A. Bösmann, D. Teichmann, W. Arlt, J. Dungs and P. Wasserscheid, ChemSusChem, 2014, 7, 229–235 CrossRef.
- G. P. Pez, A. R. Scott, A. C. Cooper and H. Cheng, CA2465555A1, 2004.
- E. Clot, O. Eisenstein and R. H. Crabtree, Chem. Commun., 2007, 22, 2231–2233 RSC.
- H. Liu, J. W. Xue, P. F. Yu, Y. K. Zhang, J. H. Wang and D. F. Che, Fuel, 2023, 331, 125920 CrossRef CAS.
- I. Y. Choi, B. S. Shin, S. K. Kwak, K. S. Kang, C. W. Yoon and J. W. Kang, Int. J. Hydrogen Energy, 2016, 41, 9367–9373 CrossRef CAS.
- S. Kiermaier, D. Lehmann, A. Bösmann and P. Wasserscheid, Int. J. Hydrogen Energy, 2021, 46, 15660–15670 CrossRef CAS.
- K. M. Eblagon, K. Tam, K. M. K. Yu and S. C. E. Tsang, J. Phys. Chem. C, 2012, 116, 7421–7429 CrossRef CAS.
- X. H. Zhao, X. L. Kong, G. F. Li, Y. Zhao, Z. M. Jia, F. He, P. P. Yang, K. Ge, M. L. Zhang and Z. L. Liu, Fuel, 2024, 360, 130605 CrossRef CAS.
- F. Sotoodeh, L. Zhao and K. J. Smith, Appl. Catal., A, 2010, 378, 124 CrossRef CAS.
- C. X. Tang, Z. L. Feng and X. F. Bai, Fuel, 2021, 302, 121186 CrossRef CAS.
- X. F. Ye, Y. An and G. H. Xu, J. Alloys Compd., 2011, 509, 152–156 CrossRef CAS.
- B. Wang, P. Y. Li, Q. Dong, L. Q. Chen, H. Q. Wang, P. L. Han and T. Fang, ACS Appl. Energy Mater., 2023, 6, 1741–1752 CrossRef CAS.
- B. A. T. Mehrabadi, S. Eskandari, U. Khan, R. D. White and J. R. Regalbuto, Adv. Catal., 2017, 61, 1–35 CAS.
- B. Chen, B. W. Hui, Y. T. Dong, Q. Sheng, X. Li, Q. L. Hao and C. J. Liu, Fuel, 2022, 324, 124405 CrossRef CAS.
- H. E. Yu, X. Yang, Y. Wu, Y. R. Guo, S. Li, W. Lin, X. G. Li and J. Zheng, J. Energy Chem., 2020, 40, 188–195 CrossRef.
- Y. Yang, Y. P. Ma, C. C. Lu, S. J. Li and M. Y. Zhu, Green Chem., 2023, 25, 10209–10234 RSC.
- H. G. Yu, X. Yang, X. J. Jiang, Y. M. Wu, S. P. Chen, W. Lin, Y. Wu, L. Xie, X. G. Li and J. Zheng, Nano Energy, 2021, 80, 10547 Search PubMed.
- H. E. Yu, Y. Wu, S. P. Chen, Z. W. Xie, Y. M. Wu, N. Cheng, X. Yang, W. Lin, L. Xie, X. G. Li and J. Zheng, Appl. Catal., B, 2022, 317, 121720 CrossRef CAS.
- Y. F. Shen, J. Mater. Chem. A, 2015, 3, 13114–13188 RSC.
- Y. B. Qin and X. F. Bai, Fuel, 2022, 307, 121921 CrossRef CAS.
- Y. B. Qin and X. F. Bai, Catal. Sci. Technol., 2022, 12, 2829–2836 RSC.
- C. D. De Souza, B. R. Nogueira and M. Rostelato, J. Alloys Compd., 2019, 798, 714–740 CrossRef.
- Y. Wu, CCS Chem., 2022, 4, 3894 Search PubMed.
- Y. D. Wang and X. F. Bai, Fuel, 2023, 331, 125709 CrossRef CAS.
- A. Wong, Q. Lin, S. Griffin, A. Nicholls and J. R. Regalbuto, Science, 2017, 358, 1427–1430 CrossRef CAS PubMed.
- H. R. Liu, S. R. Shi, Z. H. Wang, Y. H. Han and W. Huang, Small, 2022, 18(10), 2103747 CrossRef CAS PubMed.
- X. Gong, S. Y. Guo, Z. Jiang, B. L. Yang and T. Fang, Int. J. Hydrogen Energy, 2021, 46, 33835–33848 CrossRef CAS.
- F. Sotoodeh and K. J. Smith, J. Catal., 2011, 279, 36–47 CrossRef CAS.
- C. Zhu, S. Fu, Q. Shi, D. Du and Y. Lin, Angew. Chem., 2017, 56(45), 13944–13960 CrossRef CAS PubMed.
- Y. H. Ding, Y. Dong, H. S. Zhang, Y. H. Zhao, M. Yang and H. S. Cheng, Int. J. Hydrogen Energy, 2021, 46, 27026–27036 CrossRef CAS.
- C. G. Li, M. Yang, Z. J. Liu, Z. L. Zhang, T. Zhu, X. D. Chen, Y. Dong and H. S. Cheng, Catal. Sci. Technol., 2020, 10, 2268–2276 RSC.
- J. R. Kitchin, J. K. Norskov, M. A. Barteau and J. G. Chen, Phys. Rev. Lett., 2004, 93, 156801 CrossRef CAS.
- Y. Yang, Z. Y. Lun, G. L. Xia, F. C. Zheng, M. N. He and Q. W. Chen, Energy Environ. Sci., 2015, 8, 3563–3571 RSC.
- K. M. Eblagon, D. Rentsch, O. Friedrichs, A. Remhof, A. Zuettel, A. J. Ramirez-Cuesta and S. C. Tsang, Int. J. Hydrogen Energy, 2010, 35, 11609–11621 Search PubMed.
- K. M. Eblagon, K. Tam and S. C. E. Tsang, Energy Environ. Sci., 2012, 5, 8621–8630 Search PubMed.
- W. J. Xue, H. X. Liu, B. B. Zhao, L. X. Ge, S. Yang, M. H. Qiu, J. Li, W. Han and X. Q. Chen, Appl. Catal., B, 2023, 327, 122453 CrossRef CAS.
- Y. Wu, H. G. Yu, Y. R. Guo, Y. X. Zhang, X. J. Jiang, B. X. Sun, K. Fu, J. Chen, Y. Qi, J. Zheng and X. G. Li, J. Mater. Chem. A, 2019, 7, 16677–16684 Search PubMed.
- M. J. Zhou, Y. L. Miao, Y. W. Gu and Y. J. Xie, Adv. Mater., 2024, 2311355 CrossRef CAS.
- J. X. Zhang, F. S. Yang, B. Wang, D. Li, M. Wei, T. Fang and Z. X. Zhang, Materials, 2023, 16, 3735 CrossRef CAS.
- K. M. Eblagon, K. Tam, K. M. K. Yu, S. L. Zhao, X. Q. Gong, H. Y. He, L. Ye, L. C. Wang, A. J. Ramirez-Cuesta and S. C. Tsang, J. Phys. Chem. C, 2010, 114, 9720–9730 CrossRef.
- A. Mehranfar, M. Izadyar and A. A. Esmaeili, Int. J. Hydrogen Energy, 2015, 40, 5797–5806 CrossRef CAS.
- B. W. Hoffer, A. D. V. Langeveld, J. P. Janssens, R. L. C. Bonné, C. M. Lok and J. A. Moulijn, J. Catal., 2000, 192, 432–440 CrossRef CAS.
- L. H. Zhu, Z. Li, K. Q. Du, F. Hao, Y. H. Li, G. R. You and B. H. Chen, RSC Adv., 2013, 3(3), 713–719 Search PubMed.
- C. C. Zhang, L. X. Sun, Y. F. Ouyang, F. Xu, Y. J. Zou, H. L. Chu, K. X. Zhang, B. Li and H. G. Pan, J. Alloys Compd., 2023, 937, 168334 CrossRef CAS.
- S. B. Yoon, G. S. Chai, S. K. Kang, J. S. Yu, K. P. Gierszal and M. Jaroniec, J. Am. Chem. Soc., 2005, 127, 4188–4189 CrossRef CAS PubMed.
- E. Truszkiewicz, K. Kowalczyk, A. Debska, D. Wojda, E. Iwanek, L. Kepinski and B. Mierzwa, Int. J. Hydrogen Energy, 2020, 45, 31985–31999 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |