Multicolor solid-state fluorescence and multicolor afterglow carbon dots: preparation, luminescence regulation, and applications
Qiang
Fu†
 *,
Jianye
Zhang†
,
Kailin
Zhang
,
Shouhong
Sun
and
Zhanhua
Dong
*,
Jianye
Zhang†
,
Kailin
Zhang
,
Shouhong
Sun
and
Zhanhua
Dong
College of Engineering, Qufu Normal University, Rizhao, Shandong 276826, People's Republic of China. E-mail: qiang.fu@qfnu.edu.cn
First published on 27th November 2024
Abstract
Carbon dots (CDs), as emerging luminescent materials, have attracted extensive attention from researchers because of their excellent optical properties. However, most reported CDs currently exhibit only a single luminescent color, and research on achieving multicolor solid-state fluorescence (SSF) and multicolor afterglow with CDs is relatively limited. In recent years, increasing research on multicolor SSF and multicolor afterglow CDs has greatly expanded the potential of CDs in various application fields. In this review, we review the research progress on multicolor luminescent CDs from three aspects: synthesis methods, mechanisms and applications. In particular, methods for realizing multicolor luminescence of CDs are summarized in detail, and the multicolor luminescence mechanisms of different realization methods are discussed, which cover multiple aspects, such as element doping, adjustment of the reaction parameters, surface state modulation, CDs with multiple luminescence centers, energy transfer, and matrix assistance. Then, we introduce the applications of multicolor luminescent CDs in the fields of fingerprint identification, optoelectronic devices, sensors, information encryption and advanced anticounterfeiting. Finally, we present and discuss the challenges and developments of multicolor SSF and multicolor afterglow CDs.
1 Introduction
In recent years, carbon dots (CDs), as emerging nanomaterials, have attracted widespread attention because of their unique luminescence properties and excellent chemical stability, showing enormous potential in fields such as bioimaging,1,2 sensors,3,4 and optoelectronic devices,5 anticounterfeiting and information encryption.6,7 Since their discovery, researchers have made significant progress in the synthesis methods, structural properties, luminescence mechanisms, and applications of CDs across various fields.8–11 CDs are carbon materials with diameters typically less than 10 nm, and are usually prepared by the pyrolysis of aromatic hydrocarbons or polymer precursors at high temperatures. The optical performance and stability of CDs in liquids have been extensively studied and improved, and the excellent tunable emission wavelengths and fluorescence quantum yields (QYs) of liquid-state CDs have been remarkably improved.12–14 Achieving fluorescence and afterglow in solid-state CDs has received a great deal of attention because of their limited application in the liquid state, and the study of solid-state CDs faces several challenges. Although there are various methods for the preparation of solid-state CDs, there is still a certain complexity of the problem. Owing to the complexity and variability of the regulation strategy and process flow of CDs, achieving the precise regulation and optimization of fluorescence and afterglow colors is currently one of the key research focuses. Currently, research on methods for the synthesis of multicolored solid CDs, as well as the mechanism of achieving this goal, has made some progress and is emerging as a promising alternative for various applications.15–17To achieve multicolor solid-state fluorescence (SSF) and afterglow, researchers have developed various modulation strategies. These include element doping, adjustment of the reaction parameters, surface state modulation, development of CDs with multiple luminescence centers, energy transfer, and matrix assistance (Fig. 1). Yan et al. used urea and phthalic acid as precursors, and SSF CDs were synthesized via a one-pot solvothermal method.20 When the ratio of phthalic acid to urea decreased, the nitrogen (N) content of the doped CDs in the solvothermal process increased, which further increased the conjugated domain area of the CDs and finally decreased the bandgap of the CDs' highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), resulting in a gradual redshift of the fluorescence emission of the CDs. In addition, phthalimide acts as a carrier for CDs and can inhibit aggregation-caused quenching (ACQ). Zhao et al. prepared solid-state emissive CDs through solvothermal treatment with 4,4-diformyltriphenylamine, terephthalonitrile and acetonitrile in an alkaline solution of ethanol, yielding orange-emissive CDs and yellow-emissive CDs.5 The nonplanar spatial structure of triphenylamine can effectively suppress the fluorescence quenching caused by π–π stacking, leading to efficient SSF emission. The structure of triphenylamine still remains in the resulting CDs, and different triphenylamine-derived luminophores determine the difference in fluorescence emission color between the two types of CDs. By modulating the degree of oxidation on the surface of CDs, Song et al. obtained CDs with SSF colors ranging from white to orange.19 Increasing the amount of HNO3 added increased the number of oxygen atoms on the surface of the CDs, thereby reducing the bandgap and ultimately changing the SSF color of the CDs. Xu et al. used molecular amines with varying numbers of nitrogen atoms and dithiosalicylic acid (DTSA) as precursors to prepare CDs with dual emission centers.26 Different nitrogen contents influence intramolecular charge transfer (ICT). Increasing the nitrogen content increased the ICT efficiency, thereby shifting the fluorescence color of the CDs from green to red. Jin et al. prepared blue fluorescent CDs with spiropyran (SP) groups on their surface and reported that there was effective fluorescence resonance energy transfer (FRET) between the CDs and the SP groups.22 They reported that with prolonged ultraviolet (UV) irradiation, the fluorescence color gradually shifted from blue to red. This change was attributed to the increasing enhancement of the FRET process over time under UV irradiation, thereby promoting red fluorescence emission. In recent years, research on achieving multicolor afterglows for CDs has developed rapidly. Liu et al. used 1,8-naphthalenedicarboximide and AlCl3·6H2O as raw materials to prepare CDs@Al2O3 with luminescence wavelengths ranging from 376 to 619 nm by varying the calcination temperature. Additionally, the afterglow emission mode of the material transitioned from room temperature phosphorescence (RTP) to thermally activated delayed fluorescence (TADF).23 Li et al. developed a new strategy to achieve dynamic phosphorescence from CDs.18 They activated blue phosphorescence color from surface states through surface ionization engineering. Its decay rate is different from that of yellow-green phosphorescence from the carbon core state, thus achieving the dynamic phosphorescence color of the CDs. Shi et al. achieved a time-dependent phosphorescence color (TDPC) from yellow to green by doping a NaCl matrix with Mg2+.25 The introduction of Mg2+ led to an increase in the number of structural defects in the NaCl matrix that could act as exciton trap centers, altering the exciton trapping and transfer paths and achieving yellow phosphorescence. The structural confinement of the NaCl matrix made RTP possible. Song et al. used a facile molecular engineering strategy to obtain efficient blue RTP emission by introducing surface functional groups via a CD-in-hot-urea-bath approach.21 Using the FRET process strategy, blue phosphorescent CDs were used as triplet state donors, and two fluorescent materials (fluorescein sodium salt and Rhodamine B (RhB)) with different singlet states were selected as energy acceptors to obtain green and red emissive CD-based afterglow composites. Li et al. used boric acid (BA) as a matrix to activate the RTP of multicolor (blue, green, yellow-green, and orange) CDs.24 The introduction of boron atoms reduced the energy gap between the singlet and triplet states, and the formed glassy state effectively suppressed nonradiative transitions. Therefore, the RTP of CDs is successfully realized.
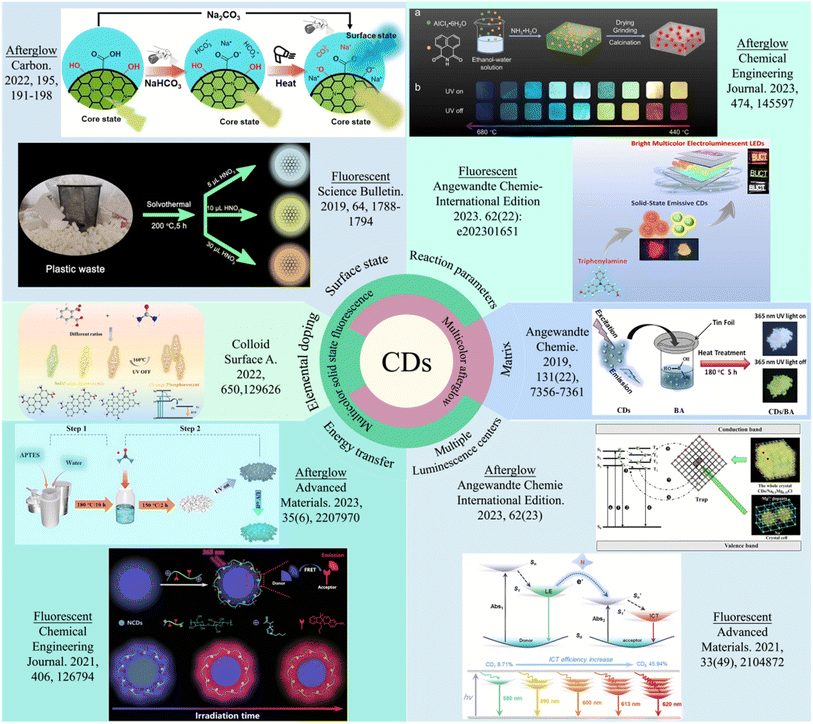 | ||
| Fig. 1 Overview of methods for achieving multicolor SSF and multicolor afterglow in CDs. Reprinted with permission from ref. 18. Copyright 2022 Elsevier Ltd. Reprinted with permission from ref. 19. Copyright 2019 Science China Press. Reprinted with permission from ref. 20. Copyright 2022 Elsevier B.V. Reprinted with permission from ref. 21. Copyright 2022 Wiley-VCH GmbH. Reprinted with permission from ref. 22. Copyright 2021 Elsevier B.V. Reprinted with permission from ref. 23. Copyright 2023 Elsevier B.V. Reprinted with permission from ref. 5. Copyright 2023 Wiley-VCH GmbH. Reprinted with permission from ref. 24. Copyright 2019 John Wiley and Sons Ltd. Reprinted with permission from ref. 25. Copyright 2023 Wiley-VCH GmbH. Reprinted with permission from ref. 26. Copyright 2021 Wiley-VCH GmbH. | ||
In this paper, we review methods for the synthesis of multicolored CDs as well as strategies for modulating the SSF and afterglow color of CDs, including elemental doping, reaction parameters, surface states, multiple emissive centers, and energy transfer. Finally, we propose directions for future research to further develop multicolor SSF and afterglow CDs with superior performance and explore their potential applications in the current field as well as in the wider field.
2 Preparation of multicolor solid-state fluorescent and multicolor afterglow CDs
Currently, the synthesis of multicolored SSF CDs and multicolored afterglow CDs typically involves the aggregation and carbonization of small molecule precursors under specific reaction conditions.27 Therefore, the choice of synthetic method is crucial for the preparation of multicolor SSF CDs and multicolor afterglow CDs. In this section, we divide the synthesis methods into one-step and multistep synthesis routes.2.1 One-step synthesis route
The common one-step synthesis routes currently used to synthesize multicolor SSF CDs and multicolor afterglow CDs include solvothermal, hydrothermal, microwave-assisted, and other synthesis methods, which are described below, and Table 1 lists some examples of multicolored CDs prepared via a one-step synthesis route.| Precursors | Solvent | Method | Temperature and time | Luminescence color | Emission wavelength (nm) | Type | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | Perylene, 1,3-diaminopropane | Sulfuric acid | Solvothermal | 200 °C, 6 h, 8 h, 10 h, 15 h | Yellow, red, NIR | 570, 604, 666, 721 | SSF | 28 |
| 2 | DTSA, o-phenylenediamine (o-PD), m-phenylenediamine (m-PD), p-phenylenediamine (p-PD) | Acetic acid | Solvothermal | 180 °C, 10 h | Blue, green, red | 478, 520, 620 | SSF | 29 |
| 3 | DTSA, ethylamine, ethylenediamine, diethylene triamines, triethylenetetramine, tetraethylenepentamine | Acetic acid | Solvothermal | 180 °C, 10 h | Green to red | 580 to 620 | SSF | 26 |
| 4 | Thiourea, o-PD | Dimethylformamide (DMF)/water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Solvothermal | 120 °C, 210 °C, 10 h | Green to deep red | 525 to 660 | SSF | 2 |
| 5 | Trisodium citrate dihydrate, urea | DMF, dimethylacetamide, diethylformamide | Solvothermal | 160 °C, 4 h | Green, yellow-green, yellow | 537, 530, 513 | SSF | 30 |
| 6 | 3,6,8-Tetra(4-carboxylphenyl)pyrene, ethylenediamine | Solvothermal | Blue to green | 496 to 527 | SSF | 31 | ||
| 7 | Trisodium citrate dihydrate, urea | Microwave | Heat, 5 min | Blue to yellow | 410 to 560 | SSF | 32 | |
| 8 | Phloroglucinol, urea | Microwave | 144 W, 288 W, 464 W | Blue, green, yellow, orange, red | 445 to 643 | SSF | 33 | |
| 9 | Citric acid, L-cysteine | Microwave | Heat, 3 min, 4 min | Blue, yellow, red | 425, 550, 640 | SSF | 34 | |
| 10 | Phloroglucinol dihydrate, BA, ethylenediamine | Microwave | 400 W, 15 min | Yellow, orange | 565, 585 | SSF | 35 | |
| 11 | Maleic acid, (3-aminopropyl) triethoxysilane | MeOH | Hydrothermal | 200 °C, 8 h | Green, yellow, orange-red | 490 to 625 | SSF | 36 |
| 12 | Citric acid, DETA | DMF | Solvothermal | 180 °C, 6 h | Yellow, orange | SSF | 37 | |
| 13 | Urea, m-PD, p-PD, o-PD | Ethanol | Solvothermal | 200 °C, 6 h | Deep blue to red | 431 to 620 | RTP | 38 |
| 14 | 4-(2-Aminoethyl)morpholine, 4,7,10-trioxa-1,13-tridecanediamine | Solvothermal | 170 °C, 7 days | Blue, green | 435 to 525 | RTP, TADF | 39 | |
| 15 | 1,2,4,5-Benzenetetramine, (E)-2-methyl-2-butenedioic acid | Alkaline conditions | Hydrothermal | 180 °C, 8 h | Red, NIR | 600 to 710 | RTP | 40 |
| 16 | Biuret, phosphoric acid | Hydrothermal | 473 K, 353 K, 3 h | Blue to red | 484 to 633 | RTP | 4 | |
| 17 | BA, m-PD, urea, guanidine phosphate | Water | Hydrothermal | 300 °C, 3 h | Violet, deep-blue | RTP | 41 | |
| 18 | Citric acid, NaOH | Deionized water | Microwave | Heat, 3 min | Blue to red | 483 to 635 | RTP | 42 |
| 19 | BA, phosphoric acid, and acrylic acid diethylenetriamine | Deionized water | Heat | 200 °C, 14 h | Green, yellow, orange-red | 538, 574, 605 | RTP | 43 |
| 20 | BA, citric acid | Deionized water | Pyrolysis | 180 °C, 5 h | Green, yellow-green | RTP | 44 | |
| 21 | Arginine, BA | Solid-phase one-step | 220 °C, 240 °C, 260 °C | Green to red | 462 to 623 | RTP | 45 | |
| 22 | BA, levofloxacin | Heat | 220 °C, 3 h | Blue, green | RTP, TADF | 17 | ||
| 23 | ZrCl4, CsCl, o-PD, citric acid | [Bmim]Cl | Heat | Blue to orange-red | RTP, TADF | 46 | ||
| 24 | L-Tyrosine acid, L-glutamic acid, L-aspartic acid, BA, urea | Deionized water | Heat | Cyan to red | RTP | 47 |
The solvothermal method is simple to perform, and many solvents can be used for solvothermal reactions. Common solvents include formamide, DMF, ethanol, acetic acid, ethylene glycol, etc. Different reaction solvents can modulate the structure and surface functional groups of CDs, thus affecting their luminescenceproperties.51 For example, Shen et al. obtained three SSF CDs by embedding them in situ in a trisodium citrate crystalline matrix by the solvothermal method.30 By varying the reaction solvent (DMF, dimethylacetamide, and diethylformamide), the fluorescence colors of the three SSF CDs were green, yellow-green and yellow, with relative photoluminescence quantum yields (PLQYs) as high as 17.6%, 18.7% and 21.6%, respectively. Xu et al. used DTSA as a carbon source, and o-PD, m-PD, and p-PD were subjected to solvothermal reactions in an acetic acid solution to produce CDs with red (o-CD), green (m-CD), and blue (p-CD) SSF emission, respectively.29
The solvothermal method allows the modulation of the SSF and afterglow color of the CDs by adjusting the reaction conditions and selecting different carbon sources. Xu et al. synthesized CDs (named yellow-emitting CDs, red-emitting CDs, deep-red-emitting CDs, and NIR-CDs, respectively) from the visible to near-infrared region via solvent heat treatment by varying the reaction time of perylene and 1,3-diaminopropane at 200 °C through the addition of sulfuric acid as a catalyst and dehydrating agent, controlling the polymerization and deamination carbonization of perylene and 1,3-diaminopropane (Fig. 2A).28 The redshifted fluorescence emission is attributed to a narrowing bandgap caused by an enlarged sp2 carbon crystal cluster from perylene. In addition, the surfaces of the CDs retain many plentiful polymer chains, which can control the interparticle distance, thereby weakening the direct π–π interaction and excessive FRET and achieving self-quenching-resistant SSF characteristics. Yang et al. used citric acid and thiourea as precursors to prepare solid-state luminescent CDs in situ within the confined interlayer of the host matrix LAPONITE® via solvothermal methods.54 By varying the reaction temperature or reaction time, they achieved emissions of cyan, green, yellow-green, yellow, and orange. Liu et al. employed “dots-in-zeolites” to synthesize CD composite materials with zinc-aluminum phosphate (SBT) as the matrix via organic templates 4-(2-aminoethyl)morpholine and 4,7,10-trioxa-1,13-tridecanediamine via a solvothermal method,39 and they are named CDs@SBT-1 and CDs@SBT-2. The CDs synthesized using different organic templates have different structures, leading to different ΔEST values for CDs@SBT-1 and CDs@SBT-2, which are 0.36 and 0.18 eV, respectively, resulting in the two composites exhibiting different RTP and TADF properties. The afterglow emission of CDs@SBT-1 is composed mainly of green RTP and a small amount of blue TADF formed by the reverse intersystem crossing (RISC) process. In contrast, the afterglow emission of CDs@SBT-2 exhibits blue-green RTP from 100 to 225 K. As the temperature increases to 300 K, the blue TADF gradually becomes dominant.
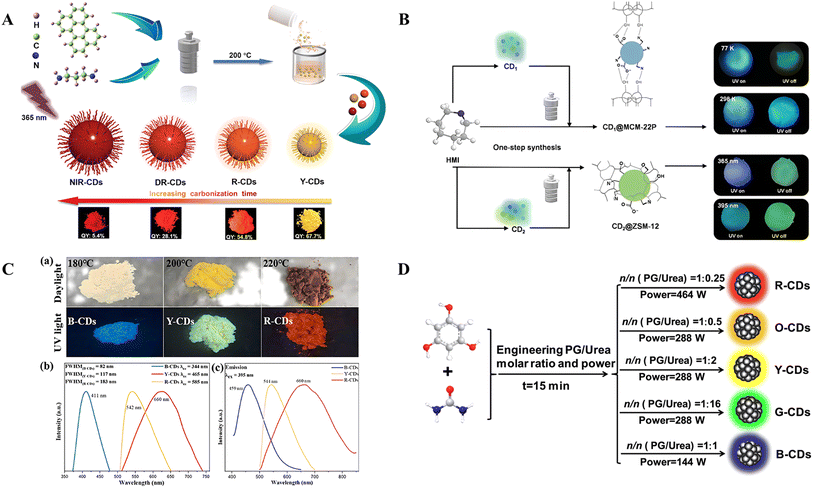 | ||
| Fig. 2 (A) Schematic illustration of the fabrication process of CDs with tunable color from yellow (570 nm) to NIR (721 nm) and optical images of Y-CDs, R-CDs, DR-CDs, and NIR-CDs under 365 nm UV light.28 Copyright 2022 Wiley-VCH GmbH. (B) Synthesis procedure for CD@ZSM-12 and CD@MCM-22.52 Copyright 2023 Tsinghua University Press. (C) (a) Photographs of CDs at different hydrothermal temperatures under daylight (top) and UV light (bottom). (b) Normalized photoluminescence emission spectra of B-CDs, Y-CDs, and R-CDs at their optimal excitation wavelengths.53 Copyright 2024 Elsevier B.V. (D) Synthesis routes of five typical CDs by microwave treatment of PG and urea.33 Copyright 2022 Elsevier Ltd. | ||
The hydrothermal method can control the optical properties of CDs by adjusting the reaction parameters, such as the reaction temperature, pressure and time.52,60 By reasonably selecting the reaction parameters, CDs with different colors can be synthesized.61,62 For example, altering the hydrothermal reaction time changes the interactions between the CDs and the inorganic matrix, thereby altering the luminescence patterns of the CDs and thus affecting their optical properties. Wen et al. synthesized two types of CD composites (CD@ZSM-12 and CD@MCM-22) using hexamethyleneimine as the organic structure-directing agent and carbon source, by varying the reaction time via a one-step hydrothermal method.52 CD@ZSM-12 exhibited an excitation-dependent afterglow, resulting in cyan (365 nm) and green (395 nm) RTPs, whereas CD@MCM-22 exhibited a temperature-dependent afterglow, which, as the temperature increased from 77 to 298 K, changed from green to blue (Fig. 2B). Yao et al. used H3BO3 and arginine as boron and nitrogen dopants, respectively, with tartaric acid as the carbon source, to prepare multicolor SSF CDs by varying the hydrothermal reaction temperature.53 As the reaction temperature increased, the particle size of the CDs increased, and the content of sp2 carbon and carboxyl groups increased, resulting in a redshift of the emission spectrum (Fig. 2C). Furthermore, the synergistic effect of boron and nitrogen doping suppressed ACQ and guaranteed SSF emission.
The preparation of multicolor CDs typically involves introducing different types or concentrations of carbon sources and dopants during the synthesis process to modulate the structure and surface chemical properties of the CDs.67,68 The microwave-assisted method allows the modulation of the SSF and afterglow color of the CDs through the modulation of the ratio of reactants. Wang et al. used benzene-1,3,5-triol and urea as raw materials to prepare blue, green, yellow, orange, and red CDs through a one-step microwave method (Fig. 2D) by adjusting the reactant ratio and microwave power, and these materials exhibited considerable solid-state QYs of 48.2%, 26.0%, 18.5%, 13.7% and 5.7%, respectively.33 Zhao et al. designed a unique microwave-assisted solid-phase synthesis method for preparing tunable fluorescent CD powders with yellow, orange, and red fluorescence (Y-CDs-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, O-CDs-1
8, O-CDs-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, R-CDs-1
2, R-CDs-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by simply adjusting the mass ratio of reactants and utilizing phloroglucinol and butanedioic acid as raw materials.69 As the ratio increased, the conjugated structure of the CDs increased, and the fluorescence redshifted. Ding et al. reported a facile in situ restriction strategy for the microwave-assisted synthesis of full-color room-temperature phosphorescent CDs in NaOH using citric acid as a carbon source.42 A tunable phosphorescence wavelength was achieved by adjusting the mass ratio of citric acid to NaOH. As the NaOH content decreases, the relative amount of CDs in the NaOH matrix increases, resulting in more efficient aggregation of the CDs and reducing the energy difference between the T1 and S0 states of the CDs, which leads to a redshift of the phosphorescence emission.
1) by simply adjusting the mass ratio of reactants and utilizing phloroglucinol and butanedioic acid as raw materials.69 As the ratio increased, the conjugated structure of the CDs increased, and the fluorescence redshifted. Ding et al. reported a facile in situ restriction strategy for the microwave-assisted synthesis of full-color room-temperature phosphorescent CDs in NaOH using citric acid as a carbon source.42 A tunable phosphorescence wavelength was achieved by adjusting the mass ratio of citric acid to NaOH. As the NaOH content decreases, the relative amount of CDs in the NaOH matrix increases, resulting in more efficient aggregation of the CDs and reducing the energy difference between the T1 and S0 states of the CDs, which leads to a redshift of the phosphorescence emission.
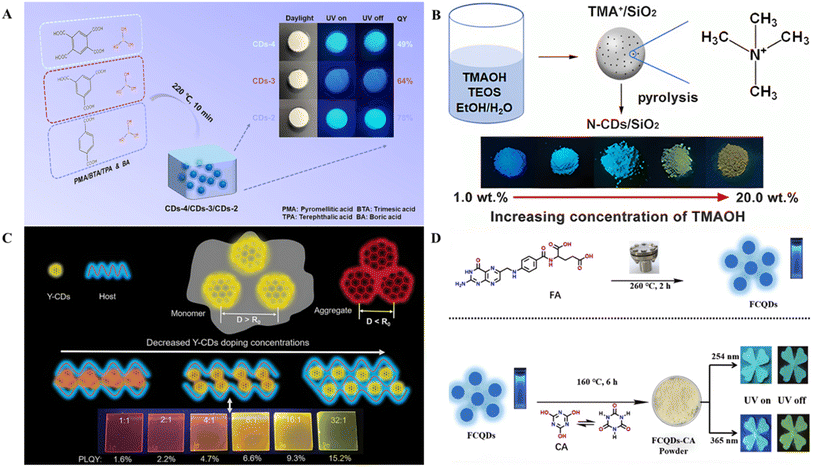 | ||
| Fig. 3 (A) Schematic diagram of the manufacturing process of high color stability blue-to-violet RTP CD composites.72 Copyright 2023 American Chemical Society. (B) Schematic illustration of the preparation of CDs and variation in color of CDs with TMAOH concentration.73 Copyright 2021 American Chemical Society. (C) Schematic diagram of UV irradiation with different ratios of PVK and Y-CDs.74 Copyright 2021 Wiley-VCH GmbH. (D) Synthesis procedure of FCQDs and synthesis procedure of FCQDs–cyanuric acid and RTP and phosphorescence images of FCQDs–cyanuric acid powder with and without UV lamp irradiation (254 and 365 nm, respectively).75 Copyright 2021. The Royal Society of Chemistry and the Chinese Chemical Society. | ||
2.2 Multistep synthesis route
In some cases, employing multistep synthesis routes can effectively achieve multicolor SSF and multicolor afterglow in CDs. Currently, researchers have developed multistep synthesis routes for multicolor SSF CDs and multicolor afterglow CDs (Table 2 lists some of them). The routes generally rely on covalent bonds, hydrogen bonds, ionic bonds, and spatial confinement between the matrix and seed CDs to prevent aggregation quenching and inhibit non-radiative transitions, ensuring the realization of SSF and afterglow.30,39 For example, Chen et al. synthesized CDs with concentration-dependent properties via a hydrothermal method using p-PD and ethylene glycol as precursors.90 The CDs exhibited not only aggregation-dependent emission properties but also antiaggregation-induced irreversible quenching effects, providing a basis for the preparation of SSF materials. They mixed CDs at different concentrations with silica to prepare solid-state CDs@silica composite materials. After the CDs were combined with silica, their optical properties were well preserved, and the emission color of the as-prepared solid-state CDs@silica powders could be tuned from green to red (521–600 nm). Chen et al. used 2,5-diaminobenzenesulfonic acid as the only precursor to synthesize red, green, and blue CDs. CDs with various wavelengths were obtained by changing the solvent.91 R-, G-, and B-CDs were synthesized in DMF, N-methylpyrrolidone, and formamide, respectively. The difference in luminescence between the three colored CDs is determined by their different surface states and doping states. In addition, the SSF emission of red, green and blue CDs was obtained by combining the CD solution with edible lotus root powder. Wang et al. prepared yellow CDs via a solvothermal method.74 Y-CDs were mixed with polyvinyl carbazole (PVK), and luminescent films ranging from yellow-green to red were obtained by controlling the ratio of PVK to Y-CDs. The presence of PVK can inhibit direct contact between Y-CDs. When the distance between Y-CDs is sufficiently small, it can lead to a reduction in the bandgap of the CDs and a redshift in the emission wavelength. Thus, as the Y-CD doping concentration decreases, the distance between the Y-CDs increases, resulting in a blueshift in the emission spectrum (Fig. 3C). Zhu et al. prepared CDs using folic acid as the carbon source through a hydrothermal method and then dispersed the CDs via recrystallization with cyanuric acid as the matrix, which inhibited non-radiative transitions and caused the CDs to exhibit dark blue delayed fluorescence, blue RTP (under 254 nm excitation), and green RTP (under 365 nm excitation).75 (Fig. 3D) Xu et al. prepared five CDs with different fluorescence colors and then dispersed the fluorescent CDs into a metal–organic framework (MOF) to activate RTP.92 The presence of the MOF host provides a rigid environment to inhibit non-radiative transitions, and the metal ions in the MOF can also promote the intersystem crossing (ISC) process. The RTP color of the resulting CD composites can be tuned from blue (478 nm) to red (631 nm).| Seed CDs | Matrix | Multistep preparation method | Temperature and time | Luminescence color | PLQYs/afterglow QY (%) | Type | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | GCDs | Ca(OH)2 | Mix | Room temperature | Green, red | SSF | 79 | |
| 2 | o-CDs, g-CDs, v-CDs | Starch | Stirring | Room temperature, 8 h | Blue, green, pink | 20.7, 12.1, 8.9 | SSF | 80 |
| 3 | R-CDs, Y-CDs | Polyvinyl alcohol (PVA) | Stirring | Room temperature, 6 h | Red, yellow | 47, 36 | SSF | 81 |
| 4 | Y-CDs | PVK | Mix | Room temperature | Yellow-green to red | 1.6, 2.2, 4.7, 6.6, 9.3, 15.2 | SSF | 74 |
| 5 | N-CDs | PVA | Magnetic stirring, dry | 90 °C, 1 h | Blue to yellow | 32.6 | SSF | 82 |
| 48 h | ||||||||
| 6 | G-CDs | B2O3,urea | Heat | 50 °C, 180 °C, 5 h | Yellow, yellow-green | SSF | 83 | |
| 7 | G-CDs | B2O3,urea | Heat | 50 °C, 180 °C, 5 h | Yellow, yellow-green | 61.2 | RTP | 83 |
| 8 | N-CDs | PVA | Ultrasonication, dry | 1 h, 2 h | Green, yellow | RTP | 84 | |
| 9 | CDs | BA | Heat | 180 °C, 5 h | Yellow-green, yellow | 48.4 | RTP | 85 |
| 10 | CDs | BA | Heat | 190 °C, 4 h | Green, cyan, blue | RTP, TADF | 86 | |
| 11 | Cu, N-CDs | BA | Calcination | 180 °C, 5 h | Blue, green | 18.3, 13.8 | RTP, TADF | 87 |
| 12 | CD-I, CDs-VI | B2O3 | Heat | 180 °C, 5 h | Blue, yellow-green, orange | 17.6 | RTP, TADF | 88 |
| 13 | CDs-I, CDs-II, CDs-III, CDs-IV | Urea | Heat | 180 °C, 5 h | Blue, green, yellow, red | 10.18, 12.08, 56.55, 1.86 | RTP, TADF | 16 |
| 14 | F-CDs/3 | BA, urea, polyvinyl pyrrolidone (PVP) | Heat | 180 °C, 5 h | Green to yellow | 515, 510, 535 | RTP | 89 |
| 150 °C, 5 h | ||||||||
| 50 °C, 12 h |
On the basis of the above discussion, we can conclude that the general steps for synthesizing multicolored SSF CDs and multicolored afterglow CDs can be simplified as follows: (1) mix the required raw materials thoroughly or dissolve them in the corresponding solvents; (2) use various heating methods to heat the mixture to make it dehydrated and carbonized; (3) separate and purify them to directly obtain multicolor SSF and multicolor afterglow CDs or seed CDs suitable for multistep synthetic routes; (4) to address the quenching of the fluorescence of CDs prepared in one-step due to ACQ and the disappearance of the afterglow of CDs prepared in one-step caused by internal molecular vibrations, rotations, and external influences such as water and oxygen, a two-step reaction can be performed to introduce a matrix.93 This ensures the realization of SSF and afterglow in CDs, as well as an improvement in their performance. Additionally, the use of a two-step matrix addition method allows the emission wavelength of the CDs to be inherited or emitted at a longer wavelength.
3 Mechanisms for achieving multicolor solid-state fluorescent CDs
In this section, a summary of the multicolor mechanism of reported SSF CDs is provided from both direct and indirect perspectives. The multicolor mechanism of the SSF CDs is summarized in Table 3.| Luminescence mechanism | Fluorescent color | Emission wavelength (nm) | Fluorescence QYs (%) | Applications | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Elemental doping | Yellow, orange | 39, 31.1 | Light emitting diode (LED) | 35 | |
| 2 | Different precursors | Red, green, blue | 12.8, 5.1, 2.1 | White light emitting diode (WLED) | 94 | |
| 3 | Different precursors | Blue, red | 480, 600 | Ink, latent finger-prints | 26 | |
| 4 | Precursor ratio | Green, yellow, orange | 490 to 625 | 34.06, 38.07, 20.37 | LED, CDs/epoxy films | 36 |
| 5 | Precursor ratio | Orange to white | LED | 95 | ||
| 6 | Reaction parameter | Blue to orange | 448 to 585 | 56, 44, 32, 21 | Luminescent solar concentrators | 96 |
| 7 | Reaction parameter | Blue to red | 438 to 633 | 26 to 57 | Full-color LEDs | 97 |
| 8 | Reaction parameter | Blue, green, yellow, orange, red | 445 to 643 | 48.2, 26, 18.5, 13.7, 5.7 | LED | 33 |
| 9 | Solvent regulation | Orange, green, blue | 585, 538, 445 | 13.9, 35.7, 2.6 | Orange, green and blue LED, WLED | 98 |
| 10 | Solvent regulation | Blue, yellow, red | 440, 554, 640 | 64, 57, 51 | B-, Y-, and RCDs/epoxy resin composites, LED, WLED | 99 |
| 11 | Solvent regulation | Green, yellow, red | Ink | 100 | ||
| 12 | Solvent regulation | Blue to green | 18.07, 18.8, 12.6, 10.8, 6.47 | Latent finger-prints | 101 | |
| 13 | Reaction parameter | Blue, yellow, red | 425, 550, 640 | 54.68, 17.93, 2.88 | Multicolor LEDs, WLED | 34 |
| 14 | Surface state | Blue to yellow | 456, 494, 556, 584 | LED, fluorescent films | 102 | |
| 15 | Energy transfer | Blue, purple, pink, red | Complex anti-counterfeiting, and information encryption | 22 |
3.1 Directly achieving multicolor solid-state fluorescent CDs
For the direct realization of the multicolor SSF of CDs, we summarize a variety of strategies, such as elemental doping, changing the reaction parameters and the use of CDs with multiple luminescence centers. Such strategies usually achieve multicolor fluorescence by modulating the energy levels of the CDs.Elemental codoping alters the optical properties of CDs by utilizing the synergistic effect between different dopant atoms. Codoping with elementals can result in unique charge distributions and, owing to the synergistic effect between the elements, can increase the rate of electron transitions, leading to changes in bandgap energies that enhance the fluorescence emission associated with the elemental-doped functional groups.106 Wang et al. prepared yellow- and orange-SSF emissive CDs (y-NB-CDs and o-NB-CDs) via N and B codoping via a facile one-step microwave synthesis method.35 (Fig. 4A) The N-doped CDs showed only weak green emission from the powder, and the B-doped CDs exhibited almost no fluorescence. Interestingly, simultaneously introducing N and B into CDs leads to a bright SSF. Codoping can take advantage of the synergetic coupling effect between heteroatoms, which facilitates the modulation of charge distribution and the enhancement of the optical properties of CDs.107 A higher B content facilitates the formation of hydrogen bonds between B–OH functional groups on the surface of the NB-CDs, further inhibiting ACQ.
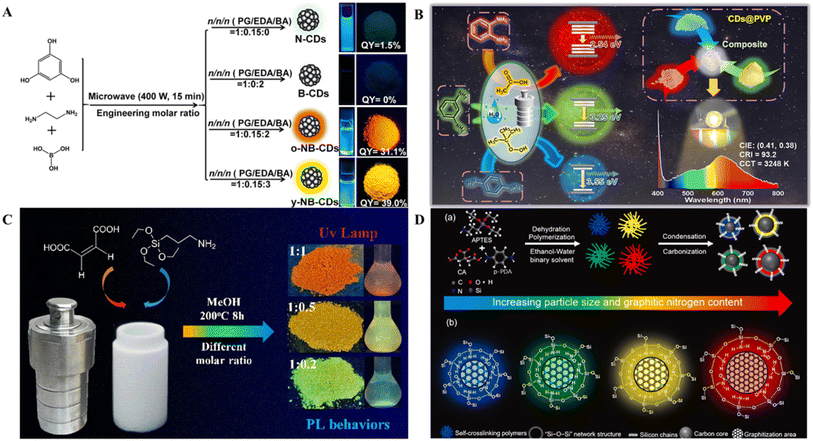 | ||
| Fig. 4 (A) Schematic diagram of the synthesis of CDs and photographs of CDs in solution and powder under UV light and schematic illustration of y-NB-CDs and o-NB-CDs for their structure and luminescence mechanism of highly efficient solid-state emission and dual emission.35 Copyright 2021 American Chemical Society. (B) Figure showing the preparation of R-, G-, and B-CDs@PVP from similar carbon precursors with the same reaction under identical reaction parameters.94 Copyright 2023 American Chemical Society. (C) SiCD composite photographs with different color emissions.36 Copyright 2021 American Chemical Society. (D) Schematic illustration of the four selected SiCDs for their (a) formation mechanism and (b) photoluminescence mechanism.97 Copyright 2022 Elsevier Ltd. | ||
Furthermore, the doping of metal ions (copper (Cu) and manganese (Mn)) can induce changes in the fluorescence of CDs because doping with metal elements can introduce electronic defects or cause new radiative transitions,103 resulting in the development of a multicolor SSF. Latief et al. synthesized carbon quantum dots (CQDs) via a simple and one-step hydrothermal approach with thiourea and citric acid as precursors.108 Owing to the binding of Cu and Mn to the surface passivation groups of the CQDs through oxygen atoms, two emission peaks associated with Cu and Mn ions appeared at 577 and 633 nm, respectively. The emission at 577 nm results from the energy transition of excited electrons in the CQD defect states to the 2 T2 state of copper, whereas the emission at 633 nm is attributed to the energy transition of an excited state of 4 T1 to the ground state of 6 A1 of Mn.109 As a result, multicolor luminescence may be achieved by simply tuning the excitation wavelength.
Regulating the types of raw materials is one method to directly achieve multicolor SSF in CDs. The structural differences in the raw materials can lead to CDs with varying conjugated π-domains and sizes.110,111 This results in a decrease in the bandgap of the CDs as the conjugated structural domain increases, causing a redshift in the emission, which allows tuning of the fluorescent centers originating from the carbon core states.36 In addition, CDs synthesized from different raw materials may also be influenced by surface states; variations in surface oxidation levels and surface defects can affect the energy levels of the CDs, leading to changes in the emission wavelengths. Han et al. synthesized red, green, and blue-emissive CDs (R-, G-, and B-CDs, respectively) from o-, m-, and p-PD via identical reactions.94 (Fig. 4B) Xu et al. used DSTA as the carbon source, acetic acid as the solvent, and melamine, urea, and sulfonamide as the nitrogen sources.112 Red, yellow and green solid-state luminescent CDs with typical aggregation-induced emission (AIE) behavior were synthesized via a simple solvothermal method. The emission behavior of the CDs was regulated by different nitrogen sources so that AIE CDs with different colors could be obtained. With increasing nitrogen doping, the content of pyridine nitrogen gradually increased, and the emission behavior of the CDs gradually redshifted. In addition, as the average particle size of the red, yellow and green solid-state luminescent CDs gradually decreased, the fluorescence emission blueshifted. Furthermore, the disulfide bonds restrict the intramolecular rotation of surface groups around the CDs, thus allowing the CDs to achieve AIE behavior. Cao et al. used 1-butyl-3-methylimidazolium chloride as a solvent and surface modifier and citric acid, pyrocatechol, and o-PD as carbon sources.61 Tunable multicolor luminescent CDs were synthesized in ionic liquids using precursors with different molar ratios, different precursors and varying reaction conditions. As the sp2-conjugated domain increases and the ratio of graphitic nitrogen to pyrrole nitrogen increases, the bandgap decreases, which leads to a redshift of the photoluminescence emission.
By altering the ratio of raw materials, such as the molar ratio, volume ratio, and mass ratio, the multicolor SSF of CDs can be directly achieved. CDs synthesized with different raw material ratios often exhibit varying chemical structures, elemental compositions and sizes.113 By adjusting the raw material ratio, the area of the conjugated π-domains and the size of the CDs can be tuned, resulting in tuning of the bandgap size and opposite changes in the fluorescence emission intensity of the carbon core states and surface states; thus, the SSF color of the CDs can be tuned.95 Yan et al. prepared solid fluorescent CDs in one step via a solvothermal method using maleic acid as the carbon source and 3-aminopropyltriethoxysilane (APTES) as the nitrogen source.36 When the molar ratio of APTES to maleic acid was increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the luminescence center of the Si-doped CDs (Si/CDs) gradually changed from the surface state to the core state, and the fluorescence of the Si/CDs changed from green to yellow to orange (Fig. 4C). When APTES/maleic acid decreased, the amount of doped Si decreased, leading to a decrease in the particle size of the resulting Si/CDs and the formation of a denser aggregate structure. This causes the distance between the luminescent centers of the Si/CDs to decrease, resulting in the formation of new luminescent centers and redshifted emission due to resonance energy transfer. Ding et al. reported the one-step synthesis of AIE CDs with full-color SSF properties via the microwave-assisted pyrolysis of 1,2,4,5-benzylenetetracarboxylic anhydride and o-PD.114 The different surface states that are induced by the increased amount of o-PD are responsible for the observed redshift in the absorption and emission. The increasing number of aromatic structure groups on the CD surfaces leads to different FRET efficiencies of these CDs, resulting in a redshift of the observed photoluminescence from blue to red. Wu et al. prepared CD composites via a solvothermal method using citric acid, urea and APTES as precursors.96 By adjusting the ratios of urea to and APTES to citric acid, the chemical structures of CDs can be affected, such as the sp3 hybrid C from citric acid and APTES, the sp2 hybrid C from citric acid and urea, and the amide bond formed by the carboxyl group of citric acid and amino group of APTES, which affects the optical properties of CDs, and the fluorescence color is adjusted from blue to orange. In addition, CDs can be encapsulated in organosilicon to form a protective shell against self-quenching of the SSF. Changes in the proportion of precursors can affect the amount of elements doped into the CDs, which also enables the regulation of the fluorescent color. Green, red and orange SSF emission was achieved. Using citric acid, p-PD, and APTES as raw materials, Hu et al. used a one-step microwave-assisted route to obtain solid-state silicon-CDs (SiCDs) with full-color emission.97 (Fig. 4D) By adjusting the p-PD amount in the reactions, the particle size and graphitic nitrogen content of the SiCDs were modulated over a wide range, both of which triggered the observed photoluminescence redshift from 438 to 633 nm. The luminescence of the four SiCDs originates from different luminescent centers, which is due to the change in carbon cores regulated by the different mole ratios of p-PD in the reactants. The generated polymer chains and network structure on the surface of SiCDs can prevent the graphitization of nuclei from π–π interactions and subsequently achieve solid-state luminescence.
1, the luminescence center of the Si-doped CDs (Si/CDs) gradually changed from the surface state to the core state, and the fluorescence of the Si/CDs changed from green to yellow to orange (Fig. 4C). When APTES/maleic acid decreased, the amount of doped Si decreased, leading to a decrease in the particle size of the resulting Si/CDs and the formation of a denser aggregate structure. This causes the distance between the luminescent centers of the Si/CDs to decrease, resulting in the formation of new luminescent centers and redshifted emission due to resonance energy transfer. Ding et al. reported the one-step synthesis of AIE CDs with full-color SSF properties via the microwave-assisted pyrolysis of 1,2,4,5-benzylenetetracarboxylic anhydride and o-PD.114 The different surface states that are induced by the increased amount of o-PD are responsible for the observed redshift in the absorption and emission. The increasing number of aromatic structure groups on the CD surfaces leads to different FRET efficiencies of these CDs, resulting in a redshift of the observed photoluminescence from blue to red. Wu et al. prepared CD composites via a solvothermal method using citric acid, urea and APTES as precursors.96 By adjusting the ratios of urea to and APTES to citric acid, the chemical structures of CDs can be affected, such as the sp3 hybrid C from citric acid and APTES, the sp2 hybrid C from citric acid and urea, and the amide bond formed by the carboxyl group of citric acid and amino group of APTES, which affects the optical properties of CDs, and the fluorescence color is adjusted from blue to orange. In addition, CDs can be encapsulated in organosilicon to form a protective shell against self-quenching of the SSF. Changes in the proportion of precursors can affect the amount of elements doped into the CDs, which also enables the regulation of the fluorescent color. Green, red and orange SSF emission was achieved. Using citric acid, p-PD, and APTES as raw materials, Hu et al. used a one-step microwave-assisted route to obtain solid-state silicon-CDs (SiCDs) with full-color emission.97 (Fig. 4D) By adjusting the p-PD amount in the reactions, the particle size and graphitic nitrogen content of the SiCDs were modulated over a wide range, both of which triggered the observed photoluminescence redshift from 438 to 633 nm. The luminescence of the four SiCDs originates from different luminescent centers, which is due to the change in carbon cores regulated by the different mole ratios of p-PD in the reactants. The generated polymer chains and network structure on the surface of SiCDs can prevent the graphitization of nuclei from π–π interactions and subsequently achieve solid-state luminescence.
By controlling the reaction time, reaction temperature, and solvent, the size of the CDs, structure and types of surface functional groups, etc., can be controlled, thereby directly achieving multicolor emission of the SSF CDs.
Different heat treatment temperatures have different effects on the dehydration, polymerization and carbonization processes of CDs.113 Higher reaction temperatures often lead to a greater degree of carbonization in CDs, resulting in larger conjugated π-domains and a reduced number of surface functional groups, thereby altering the optical properties of the CDs. Gao et al. used a one-pot solvothermal method of heating o-PD and thiourea in mixed solvents of DMF and water for the synthesis of CDs.2 By tuning the thermal temperature during solvothermal treatment, the resulting CD solutions displayed tunable fluorescence from green to red under 365 nm UV light irradiation. This is because at low temperature, o-PD and thiourea undergo dehydration and polymerization reactions to form polymeric structures consisting of intertwined polymer chains and crosslinked polymer networks, and subfluorophores or crosslinked polymer chains dominate the green fluorescence. With increasing thermal temperature, the intertwined polymer chains in the CDs further undergo aromatization and carbonization, forming a carbogenic core with an efficient π-conjugated system. Although the temperature is high, many polymer chains or molecules are still not carbonized and remain connected to the carbon core.115 The carbonized core plays a significant role in the redshifted fluorescence of CDs. This causes a portion of the green-emissive CDs formed at 120 °C being converted into with red-emissive CDs, and the rest of the CDs experience insufficient carbonization and excessive oxidation, giving rise to green-emissive CDs with a less carbonized core and a more oxidized surface of the photoluminescence center, which varies from surface subfluorophores to the edge state of the carbon core. The resulting CDs displayed tunable fluorescence from green to red. Controlling the calcination temperature can affect the degree of surface oxidation and the size of the carbon core of the CDs. Zong et al. selected small-pore RHO-type zeolites as a matrix, and the CDs@RHO composite was prepared via in situ hydrothermal synthesis in a reaction gel of Al2O3–P2O5–SiO2–N,N′-dimethylethylenediamine–H2O.60 Furthermore, temperature-controlled calcination (300–500 °C) afforded multicolor fluorescence (yellow, green, and purple) and RTP (orange, yellow, and deep blue) composites. As the temperature increases, the carbon precursors are constantly calcined, resulting in a decrease in the number of surface functional groups, profound carbonization of the carbon cores and a gradual increase in the average particle size of the carbon points. The varied structure of the CDs endows them with varying energy levels, and the fluorescence and RTP emissions of the four samples (CDs-1@RHO, CDs-2@RHO, CDs-3@RHO, and CDs-4@RHO) first redshift and then blueshift (from CDs-2@RHO to CDs-4@RHO) with increasing calcination temperature.
Furthermore, the selection of different types of solvents can alter the morphology, structure, and surface properties of CDs,116,117 thereby tuning the graphite carbon core and surface functional groups and thus affecting the energy band structure of the CDs and the size of the energy gap, ultimately impacting their fluorescence properties. Therefore, during the preparation of CDs, the rational selection of solvent types can directly achieve multicolor SSF from CDs. Bai et al. selected thiourea and p-PD as precursors; three kinds of CDs, emitting red, green, and blue, were successfully synthesized via a one-step hydrothermal reaction with DMF, methanol, and ethanol as the reaction solvents, respectively.118 Using the above solvents during the reaction, the structural size of the synthesized CDs could be adjusted, and amide bonds were introduced into the red CDs to create four structures. From the blue CDs to the green CDs to the red CDs, the increase in structural size and introduction of amide groups cause an increase in the number of sp2 conjugated domains and a decrease in the energy gap between the HOMO and LUMO, which leads to a redshift in the fluorescence wavelengths of the CDs and the acquisition of multicolored CDs (Fig. 5A). An et al. prepared multicolor solid fluorescent CDs via a one-step solvothermal method using citric acid as a carbon source, urea as a nitrogen source and phenylethylamine as a cocarbonization agent.98 The particle sizes and nitrogen contents of the CDs prepared in methanol, ethanol and water tended to decrease. As the particle size of the CDs increases, the energy band structure of the CDs changes, and the energy levels and bandgaps decrease, resulting in a redshift of the emission wavelength of the CDs. In addition, the increase in nitrogen makes the surface state of the CDs uniform, reduces their energy level and bandgap, and causes a redshift of the emission wavelength of the CDs. Furthermore, a phenylethyl structure was introduced on the surface of the CDs through the reaction of the carboxyl group on citric acid with the amino group on phenylethylamine, which inhibited the fluorescence burst caused by the aggregation of CDs and achieved solid fluorescence. An et al. prepared multicolor SSF CDs via a one-step solvothermal method in six amine solvents.119 The formation of multicolor SSF CDs depends on the different alkyl chain substituents, and the aromatic structure at the end of the substituent causes extension of the electron system, which is similar to an increase in π–π conjugation,120 increasing the degree of conjugation of surface luminophores and resulting in a decrease in the bandgap,121 resulting in a redshift of the fluorescence emission (Fig. 5B).
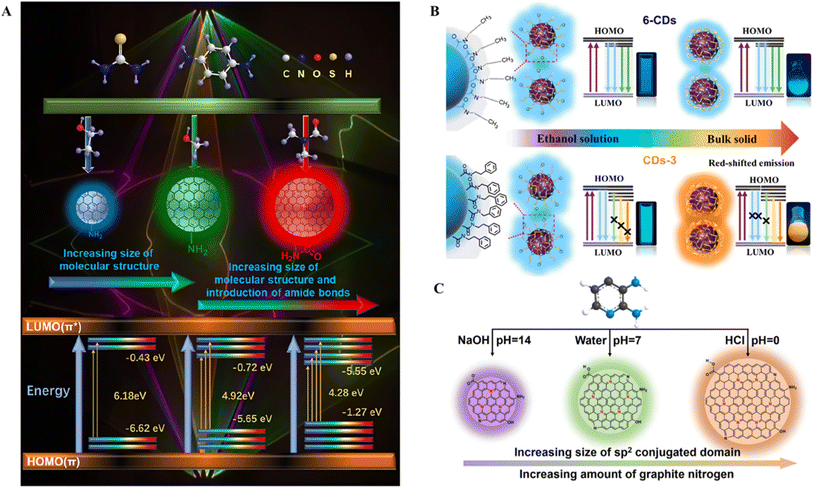 | ||
| Fig. 5 (A) Schematic illustration of multicolor CDs.118 Copyright 2023 Springer Nature Switzerland AG. (B) Schematic diagram of the quenching-resistant SSF mechanism of CDs.119 Copyright 2022 American Chemical Society. (C) Schematic illustration of the emission wavelength controlled by the size of the sp2 conjugated domain and the amount of graphitic nitrogen.80 Copyright 2021 Elsevier B.V. | ||
Changes in solution pH are one of the causes of multicolor emission. The pH of the precursor solution affects the degree of polymerization, crosslinking and carbonation during the reaction process, which ultimately affects the structure of the CDs and the type and content of functional groups on the surface of the CDs and thus the optical properties of the CDs.122 Fu et al. used a microwave-assisted method for the preparation of CDs, and NaOH and KCl were selected to regulate the pH and thus the structure of the CDs.34 They synthesized bright blue-, yellow- and red-emitting SSF multicolor CDs. Dai et al. used 2,3-diaminopyridine as a single precursor to synthesize colorful CDs under different pH conditions.80 By simply regulating the reaction media from alkali to neutral to acid, bright CDs emitting violet, green, and orange fluorescence are prepared. They reported that a lower pH leads to deeper polymerization, resulting in the production of CDs with a larger sp2 conjugated domain and a greater amount of graphitic nitrogen, which have a narrower bandgap and emit a longer wavelength photoluminescence (Fig. 5C).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds produced Em-1 and Em-2 luminescent centers, respectively. An increase in the C
N bonds produced Em-1 and Em-2 luminescent centers, respectively. An increase in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N content generated push and pull electrons in the dual fluorescent groups on the surface of the CDs, which determined the ICT between the dual luminescent centers. With increasing nitrogen content, ICT was induced to shift from Em-1 to Em-2, which achieved luminescence color conversion of the CDs.
N content generated push and pull electrons in the dual fluorescent groups on the surface of the CDs, which determined the ICT between the dual luminescent centers. With increasing nitrogen content, ICT was induced to shift from Em-1 to Em-2, which achieved luminescence color conversion of the CDs.
3.2 Indirectly achieving multicolor solid-state fluorescent CDs
Surface ligand modulation is a feasible strategy for achieving tunable fluorescence colors. By introducing different functional groups or ligand structures on the surface of CDs, the surface chemical properties and band structure of CDs can be modulated.125 Ai et al. used o-PD and sulfuric acid to produce CDs with abundant surface amino groups.127 The CDs were functionalized with salicylaldehyde-type ligands with precise structures, and the CDs were decorated via Schiff base reactions between the amino groups on the surface of the CDs and the aldehyde groups on the aromatic rings of the ligands. By simply varying the structure and functional groups of the aromatic ligand used, a continuous full-color SSF from blue to deep red (with a range of almost 300 nm) can be achieved (Fig. 6A). In the aggregate state, exciton radiation is promoted by intra- and intermolecular hydrogen bonds formed between the ligands and the carbon core, and aromatic ligands successfully conjugate and hybridize with the carbon core to form a new luminous energy level and manipulate the emissive bandgaps. In addition, surface ligands blocked severe aggregation of the graphitized cores. Thus, the ACQ phenomenon caused by direct π–π interactions resulting from carbon core stacking was avoided. Hydrogen bonds formed by the ortho-OH groups on the ligands play a vital role in enhancing aggregation-related emissions.129 Additionally, the optical properties of CDs can be modulated by altering the position of the surface ligands connected to the CDs. Liu et al. prepared seed CDs with abundant surface amino groups.102 The CDs were prepared via Schiff base reactions between the amino groups on the surfaces of the CDs and the aldehyde groups on the aromatic rings of the chlorosalicylaldehyde ligands. By controlling the positions of the ligands' Cl substituents (ortho-, para-, and meta- to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), the emission colors of the CDs could be controlled. The position of Cl affects the electron distribution and energy levels of the molecular orbital of the CDs, influencing the bandgap energy and thus the multicolor emission.
N), the emission colors of the CDs could be controlled. The position of Cl affects the electron distribution and energy levels of the molecular orbital of the CDs, influencing the bandgap energy and thus the multicolor emission.
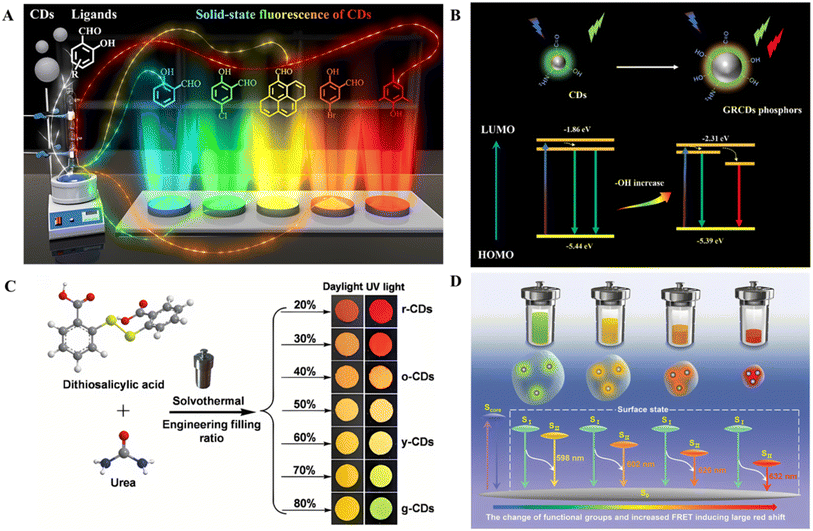 | ||
| Fig. 6 (A) Schematic of SSF from blue to deep red tuning of CDs achieved by surface ligand modulation.127 Copyright 2022 Wiley-VCH GmbH. (B) Possible photoluminescence mechanism of GRCD phosphors.79 Copyright 2021 Elsevier B.V. (C) Synthetic routes of color-tunable AIE CDs by solvothermal treatment of DTSA and urea, and photos of powdered CDs under daylight and 365 nm UV light.128 Copyright 2023 Wiley-VCH GmbH. (D) Schematic mechanism of color-tunable AIE CDs prepared by changing the filling ratio of the autoclave.128 Copyright 2023 Wiley-VCH GmbH. | ||
The presence of alkali also affects the surface state of the CDs and thus modulates their luminescence color. Alkali plays a pivotal role in the surface electron state of CDs. The introduction of alkali enriches the surface electrons of CDs and increases the energy of the HOMO to a higher energy level due to the strong interaction between electrons and the conjugated system and decreases the bandgap, thereby promoting the redshift of CD fluorescence emission.130 Cao et al. synthesized green fluorescent CDs using citric acid and urea as reaction materials in a DMF solution.79 For the first time, dual-emission CD-based phosphors covering the green and red regions were obtained by mixing CDs and Ca(OH)2 at a certain ratio. The addition of Ca(OH)2 introduces many –OH groups with strong electron-donating properties, which leads to an increase in the electron cloud density in the conjugate system, increases the HOMO to a higher energy level, and, as a result, decreases the bandgap (Fig. 6B). Consequently, GRCD phosphors emitting both green and red colors were successfully obtained.
By utilizing the principle of energy transfer, the combination of the intrinsic fluorescence of CDs with the fluorescence generated by FRET can achieve dynamic fluorescence. Jin et al. used citric acid and ethylenediamine as precursors, and one kind of negatively charged CDs, NCDs, was obtained via a hydrothermal process.22 By combining with the positively charged C-Im+-SP, the NCD particles were isolated, inhibiting the ACQ effect. The tunable fluorescence properties can be attributed to the FRET process between the NCDs and C-Im+-SP. Electrostatic attraction ensures the distance conditions required for FRET. Additionally, the emission spectrum of the NCDs, the FRET donor, overlaps with the absorption spectrum of C-Im+-SP, the FRET acceptor, ensuring that the FRET process can occur. Under UV irradiation, the NCDs exhibited blue fluorescence, whereas the red fluorescence originated from the FRET process. With increasing UV irradiation time, the amount of red fluorescence gradually increased, and the amount of blue fluorescence gradually decreased. In addition, by changing the content of the NCDs, the ratio of blue/red fluorescence can be controlled, and dynamic adjustment of the fluorescent color can be achieved.
The FRET efficiency can be influenced by varying the fill ratio of the autoclave to prepare AIE CDs with tunable colors. Wang et al. prepared color-tunable AIE CDs via solvothermal synthesis using DTSA and urea in an acetic acid solution (Fig. 6C).128 The dual-peak-emissive band of the above CDs (Em-1 and Em-2) originates from different photoluminescence centers (green emission and long emission), and FRET occurs between the two emission peaks. The competition of the FRET process with nonradiative transition and radiative transition can cause an increase in the average fluorescence intensity and a redshift of the emission.134 The fluorescence intensity of the donor peak gradually decreases, whereas that of the acceptor peak gradually increases with decreasing filling ratio of the autoclave, and there is a FRET process from green emission to long emission, resulting in the enhancement of red emission from green-emitting CDs to red-emitting CDs. Another reason for the change in the photoluminescence of CDs may be that the foam structures formed under different filling ratios are diverse. When the filling degree is lower, the amount of internal reaction fluid is also lower, and fewer foams are produced. A small amount of foam decreases the distance between CDs, and the FRET efficiency increases, inducing a redshift in the emission wavelength (Fig. 6D).
The switching of fluorescent colors is also possible by controlling the switching of the FRET process. Wang et al. designed and constructed a novel photocontrolled SSF switch based on CDs and diarylethene (DT).135 CDs and DT were prepared according to methods previously reported in the literature.136,137 The photocontrolled fluorescent switching system was dispersed into polymethyl methacrylate to inhibit the fluorescence quenching caused by its aggregation, and SSF was achieved. CDs and DT were used to construct a photocontrolled fluorescence switching system through covalent connections, where the CDs (energy donors) acted as the fluorescence emission unit and DT acted as the switching unit. Upon irradiation with UV light, the open-ring state DT transformed into a closed-ring isomer (energy acceptor), and the FRET channel of the DT–CD fluorescence switching system was opened. With the excitation of 365 nm light, the radiant energy of the CDs was transferred to the closed-ring isomer of DT,138 resulting in quenching of the fluorescence emission at 467 nm, and the color of the fluorescence changed from blue to black. Conversely, the open-ring state DT could be recovered, and the FRET channel of the DT–CD fluorescence switching system was closed upon irradiation with visible light,139 resulting in the system regaining strong emission at 467 nm and blue fluorescence.
4 Mechanisms for achieving multicolor afterglow CDs
In this section, a summary of the multicolor mechanism of reported afterglow CDs is provided from both direct and indirect perspectives. The multicolor mechanism of the afterglow CDs is summarized in Table 4.| Luminescence mechanism | Afterglow color | Emission wavelength (nm) | Afterglow QYs (%) | Applications | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Reaction time | Yellow, green, cyan, blue | 59.41, 11.81, 44.82, 3.38 | Anticounterfeiting, painting | 63 | |
| 2 | Reaction temperature | Purple to red | 376 to 619 | Anticounterfeiting, information encryption | 23 | |
| 3 | Reaction parameter | Green, yellow-green, yellow orange-red | 8.7, 6.3, 3.2, 1.5 | Anticounterfeit patterns | 62 | |
| 4 | Different precursors | Green to yellow | 515 to 535 | Information security | 89 | |
| 5 | Different precursors | Blue, green, yellow | 428 to 546 | Anticounterfeiting and information encryption | 140 | |
| 6 | Reaction parameter | Full-color | 462 to 623 | Multilevel anti-counterfeiting, fingerprint identification | 45 | |
| 7 | Surface state | Yellow-green to blue | 521, 729 | 0.8, 2.6 | Information encryption | 18 |
| 8 | Matrix | Blue, green, orange, red | 476 to 633 | Pattern anti-counterfeiting, information encryption | 141 | |
| 9 | Multiple luminescent center | Blue to green | 424 to 519 | 1.47 | Multilevel information encryption | 142 |
| 10 | Multiple luminescent center | Green, yellow, orange, red, NIR | 530, 555, 585, 625, 645, 750 | 38.2, 20.8, 1.6, 0.51 | Information security | 143 |
| 11 | Multiple luminescent center | Blue, green | 9.6 | Anti-counterfeiting, information encryption | 144 | |
| Multiple luminescent center | ||||||
| 12 | Multiple luminescent center | Blue to red | 484, 522, 560, 617, 633 | 4.08, 0.52, 0.16, 0.21, 0.16 | UV-visible light detection | 4 |
| 13 | Multiple luminescent center | Green to orange-red | 553 to 640 | 3.68, 4.33, 2.16 | Anti-counterfeiting, LED | 145 |
| 14 | Multiple luminescent center | Green, red | 555, 630 | 4.2, 3.2 | Advanced information encryption | 146 |
| 15 | Multiple luminescent center | Green, yellow | 515, 560 | Information encryption | 25 | |
| 16 | Energy transfer | Blue, cyan, green, orange | 425, 477, 506, 598 | Fingerprint recognition | 147 | |
| 17 | Energy transfer | Blue-green, green, yellow, orange, red | 2.82 | Advanced anti-counterfeiting | 148 | |
| 18 | Energy transfer | Blue, green, red | 445, 550, 615 | 50.17 | Information encryption | 21 |
| 19 | Energy transfer | Blue, green, orange, red | 463 to 614 | 4.3, 5.5 | Information multiplexing | 149 |
| 20 | Energy transfer | Green to blue purple | Dynamic anti-counterfeiting | 133 |
4.1 Directly achieving multicolor afterglow CDs
By controlling the reaction time, the size and structure of CDs can be adjusted, affecting the bandgap and energy level structure of the CDs, thus enabling the modulation of afterglow colors. Liu et al. prepared phosphorus- and nitrogen-codoped CDs via fast one-step microwave-assisted heating of 1-[3-(trimethoxy silyl)propyl] urea and phosphoric acid aqueous solutions.63 The afterglow color of the CDs gradually blueshifted from yellow to blue as the microwave reaction time increased. This color change phenomenon can be attributed to the increase in reaction time, increased carbonation of CDs, gradual weakening of the polymer structure, reduction in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group on the surface of the CDs and gradual decrease in the n–π* transition, which gradually weakened the ISC process and caused a blueshift in the afterglow emission.
O functional group on the surface of the CDs and gradual decrease in the n–π* transition, which gradually weakened the ISC process and caused a blueshift in the afterglow emission.
Adjusting the reaction temperature controls the degree of carbonation, the number of surface functional groups, and the degree of conjugation of the CDs to directly achieve afterglow color modulation of the CDs, which enables accurate, low-cost, and simple large-scale synthesis.150 Liu et al. prepared CDs@Al2O3 with afterglow emission ranging from 376 to 619 nm by adjusting the temperature of the one-step calcination method using 1,8-naphthalimide as the precursor and Al2O3 as the matrix.23 As the calcination temperature increases, the number of functional groups on the surface of the CDs and the degree of conjugation decrease, the imide content decreases, and the energy gap increases, resulting in a blueshift of the afterglow emission (Fig. 7A). Furthermore, because the increased crystallinity of the Al2O3 matrix stabilizes the triplet state of the CDs, the RISC between the singlet and triplet states is promoted, resulting in the transformation of the luminescent mode from RTP to TADF. Wang et al. proposed a strategy based on thermally driven amorphous–crystalline phase transitions, where afterglows ranging from green to orange-red were obtained by the thermal annealing of CDs at 200, 260, and 280 °C.62 Thermal annealing causes further cross-linking, dehydration and carbonization of the intertwined polymer chains at the CDs and an increase in the π-conjugated domain, which decreases the bandgap, leading to a redshift in the emission wavelength.
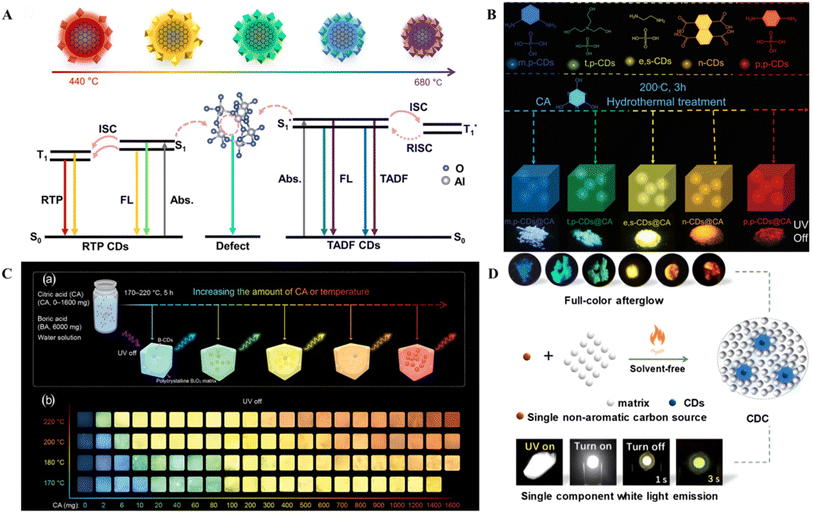 | ||
| Fig. 7 (A) Schematic of the transition model of the multicolor afterglow CDs@Al2O3.23 Copyright 2023 Elsevier B.V. (B) Schematic illustration of the preparation of the CDs and CD-based composite materials.151 Copyright 2022 Wiley-VCH GmbH. (C) Fabrication of B-CD composites. (a) Schematic representation of the fabrication process of B-CD composites with tunable full-color ultralong RTP. (b) Optical images of B-CD composites prepared from citric acid and BA under different reaction conditions (x is the weight of citric acid in milligrams, the weight of BA is 6000 mg, and the reaction temperature is listed on the left) after removing the excitation light under ambient conditions.6 Copyright 2021 The Authors. Advanced Science published by Wiley-VCH GmbH. (D) Synthesis diagram of full-color persistent room-temperature phosphorescent CD complexes and photographs of the device before and after turning off the power.45 Copyright 2022 Wiley-VCH GmbH. | ||
The preparation of multicolored afterglow CDs using different precursors is also a commonly used method, and by choosing different carbon sources as precursors, not only can CDs with different surface structures be produced, but the conjugated structures of different precursors also vary, leading to differences in the degree of conjugation of the resulting CDs,150 and furthermore, the doping atoms of the CDs can also be influenced, thus changing their optical properties. Zheng et al. synthesized CDs with different fluorescent colors via different precursors via a hydrothermal method and prepared CD-based full-color RTP composite materials by constructing rigid hydrogen bonding networks in an aqueous environment with cyanuric acid as the matrix151 (Fig. 7B). CDs with different colors can be produced using different amine precursors. Qi et al. used diethylene-triaminepentakis (methylphosphonic acid), phenylenediamine (either o-PD, m-PD, or p-PD) and BA as precursors and microwave-assisted heating to synthesize CD composites, followed by the removal of BA to obtain matrix-free CDs with wavelength-tunable RTP.67 Owing to the different structures of phenylenediamine, the three types of CDs formed have different particle sizes, surface states, and n–π* transition bandgaps, resulting in yellow, yellow-green, and yellow-white afterglows, respectively. Multicolored CDs can also be prepared using phenolic compounds with different numbers of hydroxyl groups as precursors. Zhang et al. prepared CDs via a facile one-step method using flucytosine and phenolic compounds with different numbers of hydroxyl groups (phenol, resorcinol and phloroglucinol) as precursors.89 As the number of hydroxyl groups increases, the content of C–O–C groups in the CDs progressively increases, and the size of the CDs increases, leading to a gradual increase in the size of the sp2-conjugated domain so that the phosphorescence redshifts and the luminescence color changes to yellow. The use of different precursors affects the type of dopant atoms in CDs and thus the RTP color. Wang et al. prepared CDs with different RTP colors using different precursors (ethylenediamine, diethylenetriamine, and triethylenetetramine) and phosphoric acid heated under vacuum conditions.140 The CDs produced by different precursors were doped with different atoms, and the different dopant atoms affected the energy states of the CDs, followed by the triplet states, leading to different RTP colors.
In addition, during the preparation of CDs, the structure of the CDs and types of doping elements can be influenced by the cooperative interaction of various reaction parameters, thus affecting the afterglow color of the CDs. For example, multicolor afterglow emission from CDs was achieved by controlling the reaction temperature and precursor ratio.152 Ding et al. prepared boron-doped CD (B-CDs) composites with full-color ultralong RTP by pyrolysis of citric acid and BA precursors with various mass ratios at different temperatures.6 With increasing citric acid feeding and pyrolysis temperatures, the average particle size and degree of oxidation of the B-CDs gradually increased, and the RTP color of the CD composites continuously shifted from blue to red (Fig. 7C). The particle size, degree of graphitization and degree of oxidation combined with the luminescent B-CD centers ultimately determined the redshift in the color of the composite RTP. Wang et al. prepared full-color RTP CD composites via a solid-phase one-step method by varying the feed ratio and reaction temperature (Fig. 7D).45 An increase in the feed ratio leads to an increase in the number of heteroatoms, which results in the formation of more covalent and noncovalent bonds between the CDs and the BA matrix, leading to an increase in the degree of spatial conjugation and a decrease in the bandgap. In addition, with increasing temperature, the CDs further cross-linked and carbonized, leading to an increase in graphitization, the formation of larger sp2 conjugated domains, and a higher content of graphitic N, leading to a decrease in the bandgap and a redshift of the emission. The different CD precursors, tunability of the zeolite matrix, and reaction temperature and time also offer the possibility of tunable RTP lifetimes. Yu et al. synthesized CDs@zeolite composites using α-lipoic acid and three kinds of amine analogs with different structures, including ethylenediamine with two –NH2, diethylenetriamine with two –NH2 and one –NH–, and triethanolamine with three –OH and one –(C)3–N as precursors and AlPO-5 (AFI zeotype) with one-dimensional 12-ring channels and SAPO-20 (SOD zeotype) with six rings and sod cages, which were selected as zeolite matrices.153 In addition to regulating the precursors of the CDs, regulating the zeolite matrices as well as the crystallization temperature and time during the synthesis process worked together to obtain nine kinds of CDs@zeolite composites with variable RTP lifetimes (0.38 to 2.1 s). This can be attributed to the high crystallization temperatures and long crystallization times, which favor an increase in the ISC rate (KISC) and a decrease in the nonradiative transition rate (Knr), thus increasing the RTP lifetime. The CD precursors that provide more C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/N–H bonds and a smaller energy gap (ΔEST) increase the KISC, decrease the Knr, and prolong the RTP lifetime. The zeolite matrix provides better confinement for the CDs and enhances the interaction between the CDs and the zeolite matrix, thus decreasing Knr and increasing the RTP lifetime. By taking advantage of the different RTP lifetimes of each CD@zeolite composite, different RTP colors can be observed within 0.38 to 2.1 s of decay.
N/N–H bonds and a smaller energy gap (ΔEST) increase the KISC, decrease the Knr, and prolong the RTP lifetime. The zeolite matrix provides better confinement for the CDs and enhances the interaction between the CDs and the zeolite matrix, thus decreasing Knr and increasing the RTP lifetime. By taking advantage of the different RTP lifetimes of each CD@zeolite composite, different RTP colors can be observed within 0.38 to 2.1 s of decay.
The existence of different luminescent centers at different energy levels leads to different excitation wavelengths corresponding to different emission wavelengths.152 Li et al. prepared boron-doped CDs (B-CDs) from BA and 1,3,5-benzenetricarboxylic acid by heat treatment.144 After UV irradiation at 254 and 365 nm and after switching off, the B-CD powder shows blue and green RTP, respectively, which is attributed to the two different emission centers of the B-CDs. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of CDs is the main source of B-CD RTP emission centers, and the n–π* electronic transition of the C
O bond of CDs is the main source of B-CD RTP emission centers, and the n–π* electronic transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is conducive to the ISC process. Furthermore, the n electron on the O atoms and the vacant p orbital on the B atoms have intramolecular or intermolecular interactions, and the electron-withdrawing boron atom can facilitate the π transition to form the p n–π* conjugated system.154 Thus, confining the n electrons of the oxygen atom to the B–O space is another source of phosphorescence emission centers, resulting in blue and green afterglows. Di et al. prepared carbon dot-based organic long persistent luminescent materials with color-tunable properties via a one-step melting method using urea and isophthalic acid.155 When irradiated with a UV lamp at 275 nm, the CDs emitted a blue color afterglow for up to 60 min. When exposed to UV at 365 nm, the CDs emitted a green color afterglow for 5 min. This was also due to the presence of multiple emission centers in the CDs, and as the excitation wavelength changed, the luminescent centers that dominated the afterglow emission shifted, resulting in a different afterglow color. Cheng et al. reported a universal strategy for preparing CD composites with dual-color phosphorescence emission.156 Various conjugated structures were formed with C
O bond is conducive to the ISC process. Furthermore, the n electron on the O atoms and the vacant p orbital on the B atoms have intramolecular or intermolecular interactions, and the electron-withdrawing boron atom can facilitate the π transition to form the p n–π* conjugated system.154 Thus, confining the n electrons of the oxygen atom to the B–O space is another source of phosphorescence emission centers, resulting in blue and green afterglows. Di et al. prepared carbon dot-based organic long persistent luminescent materials with color-tunable properties via a one-step melting method using urea and isophthalic acid.155 When irradiated with a UV lamp at 275 nm, the CDs emitted a blue color afterglow for up to 60 min. When exposed to UV at 365 nm, the CDs emitted a green color afterglow for 5 min. This was also due to the presence of multiple emission centers in the CDs, and as the excitation wavelength changed, the luminescent centers that dominated the afterglow emission shifted, resulting in a different afterglow color. Cheng et al. reported a universal strategy for preparing CD composites with dual-color phosphorescence emission.156 Various conjugated structures were formed with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups on the surface of the CDs during the heating process, resulting in the formation of multiple luminescent centers. Thus, when the excitation wavelength was changed from 254 to 365 nm, the phosphorescence color changed from cyan to yellow. In addition to the use of various excitation wavelengths to produce multicolored afterglows of CDs, water-triggered strategies for adjusting the color of the afterglow have become possible. Han et al. synthesized TPA-CDs/Si by hydrothermal treatment using terephthalic acid (TPA) as the carbon source and SiO2 as the silicon.142 Si–C covalent bonds were constructed within the CDs by silicon doping, which stabilized the delayed fluorescence at 424 and 467 nm and the RTP at 492 and 519 nm, resulting in blue afterglow emission. In addition, TPA-CDs/Si exhibits a variable water-triggered afterglow color feature from blue to green, which is attributed to the competition between the formation of bridging hydrogen-bonded networks among TPA-CDs/Si nanocomponents connected by water molecules after dissolution of TPA-CDs/Si in water and Si–C covalent bonds, leading to a gradual shift in the emissive center from 424 to 492 and 519 nm. Additionally, TPA-CDs/Si exhibits a reversible stimulus-responsive afterglow effect via the alternating addition of water and heat owing to the formation and breaking of hydrogen-bonded networks in the presence and absence of water, which can be repeated for more than six cycles, indicating good recoverability.
C groups on the surface of the CDs during the heating process, resulting in the formation of multiple luminescent centers. Thus, when the excitation wavelength was changed from 254 to 365 nm, the phosphorescence color changed from cyan to yellow. In addition to the use of various excitation wavelengths to produce multicolored afterglows of CDs, water-triggered strategies for adjusting the color of the afterglow have become possible. Han et al. synthesized TPA-CDs/Si by hydrothermal treatment using terephthalic acid (TPA) as the carbon source and SiO2 as the silicon.142 Si–C covalent bonds were constructed within the CDs by silicon doping, which stabilized the delayed fluorescence at 424 and 467 nm and the RTP at 492 and 519 nm, resulting in blue afterglow emission. In addition, TPA-CDs/Si exhibits a variable water-triggered afterglow color feature from blue to green, which is attributed to the competition between the formation of bridging hydrogen-bonded networks among TPA-CDs/Si nanocomponents connected by water molecules after dissolution of TPA-CDs/Si in water and Si–C covalent bonds, leading to a gradual shift in the emissive center from 424 to 492 and 519 nm. Additionally, TPA-CDs/Si exhibits a reversible stimulus-responsive afterglow effect via the alternating addition of water and heat owing to the formation and breaking of hydrogen-bonded networks in the presence and absence of water, which can be repeated for more than six cycles, indicating good recoverability.
In addition to the above method of presenting different afterglow colors at different excitation wavelengths, multicolored afterglow can also be achieved by using the same excitation wavelength on the basis of the difference in the phosphorescence lifetimes of different luminescence centers. Chen et al. prepared silane-functionalized CDs using 1-[3-(trimethoxysilyl) propyl] urea and sodium citrate as precursors and prepared Si-CDs@SiO2 composites with excitation dependence and time-dependent phosphorescence using SiO2 as a matrix.157 The organosilane functional groups of 1-[3-(trimethoxysilyl)propyl] urea were retained, and the amino-silane functional groups formed various conjugated structures to form emission centers with different energy gaps. Under 365 nm lamp excitation, the two phosphorescent centers are activated simultaneously, and a dynamic phosphorescent color change from yellow to green is achieved because the decay rate of yellow phosphorescence is faster than that of green phosphorescence. The yellow phosphorescence disappears when the silane functional groups on the surface of Si-CDs@SiO2 are reduced with NaBH4, indicating that the yellow phosphorescence originates from the modification of the silane functional groups, whereas the N-related groups remaining after reduction are the main source of the green phosphorescence (Fig. 8A). Tan et al. synthesized CDs with TDPC via a one-pot hydrothermal treatment with levofloxacin.146 During the hydrothermal process, the nitrogen heterocyclic structure of levofloxacin is attached to the CDs, forming the center of the green phosphorescence emission. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O formed on the surface of the CDs, emitting red phosphorescence (Fig. 8B). To verify this, they used NaBH4 to reduce the CDs, the C
O formed on the surface of the CDs, emitting red phosphorescence (Fig. 8B). To verify this, they used NaBH4 to reduce the CDs, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content on the CDs surface was significantly reduced, and the surface was disrupted and accompanied by the disappearance of red phosphorescence. Under 395 nm excitation, both phosphorescent centers are activated simultaneously, while the red phosphorescence decays more rapidly than the green phosphorescence, resulting in a dynamic phosphorescent color that changes over time from orange to green. Further studies have shown that when the excitation wavelength is below 395 nm, green phosphorescence occurs. Above 395 nm, red phosphorescence was observed.
O content on the CDs surface was significantly reduced, and the surface was disrupted and accompanied by the disappearance of red phosphorescence. Under 395 nm excitation, both phosphorescent centers are activated simultaneously, while the red phosphorescence decays more rapidly than the green phosphorescence, resulting in a dynamic phosphorescent color that changes over time from orange to green. Further studies have shown that when the excitation wavelength is below 395 nm, green phosphorescence occurs. Above 395 nm, red phosphorescence was observed.
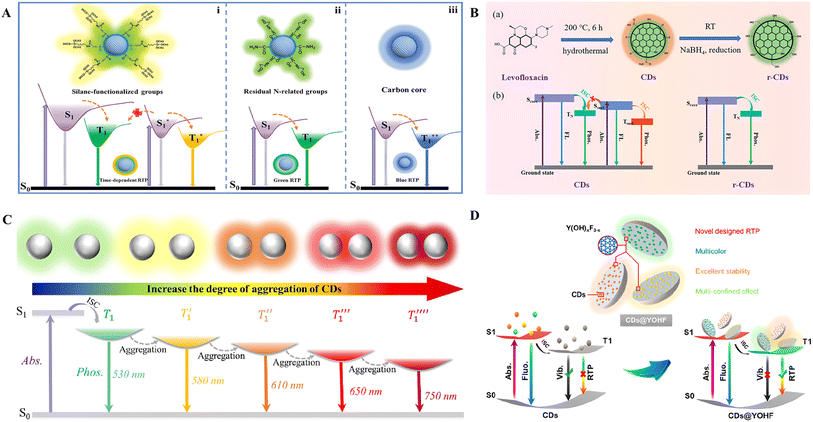 | ||
| Fig. 8 (A) Schematic illustration of RTP color changes with functional groups, and possible phosphorescence emission processes of time-dependent RTP, green RTP, and blue RTP.157 Copyright 2023 Wiley-VCH GmbH. (B) (a) Schematic of the preparation procedure of the CDs and r-CDs. (b) Possible phosphorescence (Phos.) emission processes for the CDs and r-CDs (Abs.: absorption; Score: carbon core singlet state; Ssurf.: surface singlet state; TN.: N-related triplet state; Tsurf.: surface oxide triplet state).146 Copyright 2021 Wiley-VCH GmbH. (C) Possible phosphorescence emission processes and energy level diagram of multicolor phosphorescence CDs@BA composites.143 Copyright 2023 Elsevier B.V. (D) CDs in hydroxy fluorides and schematic illustration of the RTP mechanism of CDs@YOHF.158 Copyright 2022 American Chemical Society. | ||
In addition, luminescent centers other than carbon cores and surface states have been constructed by exploiting the electronic defects of the matrix. The electronic defects possessed by the matrix can capture and store excitons, and upon photoexcitation, these electrons can undergo ISC or RISC, resulting in the formation of RTP and TDPC. Shi et al. synthesized a series of CDs/inorganic nanocomposites via a hydrothermal method.25 They reported that the coexistence of two metal ions in the NaCl matrix triggered TDPC. The doping of dual metal ions (Na+ and Mg2+) can change the RTP color from yellow to green. In contrast, composites doped with only one metal ion (Na+) have only green RTP. The addition of Mg2+ expands the crystal structure of NaCl, forming more electron traps and facilitating the capture, storage and release of excitons. This is due to the combined effect of structural confinement and structural defects in the inorganic salt matrix. The structural restriction stabilizes the triplet exciton, activating the green RTP at the CDs. The structural defect captures and stores the exciton and releases it to the T1 state after the cessation of photoexcitation, generating yellow RTP.
4.2 Indirectly achieving multicolor afterglow CDs
The abundant surface functional groups of CDs can form hydrogen bonds with the matrix. This strong interaction can influence the charge transfer process, enhance the rigidity of the system, and restrict molecular motion, thereby protecting triplet excitons and ensuring the afterglow emission of the fluorescent CDs embedded in the matrix.160,165 Wang et al. synthesized four CDs with different fluorescent colors (blue, green, orange, and red) and dispersed each of them into a polyacrylamide (PAM) matrix to prepare full-color (blue, green, yellow, and red) RTP CDs.141 Intermolecular hydrogen bonds can form between the amide groups in the PAM matrix and the –NH and –OH groups of the CDs and stabilize the triple state of the CDs, facilitating the ISC process and ensuring RTP emission. Similarly, Wang et al. prepared CDs with different fluorescent colors and dispersed them into a PAM matrix to obtain composites with four phosphorescent colors (blue, green, yellow, and red).49 The electron transition at the n–π* position of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond facilitates the ISC process, leading to phosphorescence. In addition, the hydrogen-bonded environment formed by the PAM matrix and the CDs inhibits the nonradiative leaps of triplet excitons, favoring phosphorescence realization. Zhang et al. prepared blue, cyan, and green fluorescent CDs via a one-step hydrothermal carbonization method.166 By dispersing three types of CDs in a sodium alginate matrix, many hydrogen bonds were formed between the CDs and sodium alginate, which inhibited ACQ and non-radiative transitions, resulting in multicolor SFF and multicolor afterglow CDs.
O bond facilitates the ISC process, leading to phosphorescence. In addition, the hydrogen-bonded environment formed by the PAM matrix and the CDs inhibits the nonradiative leaps of triplet excitons, favoring phosphorescence realization. Zhang et al. prepared blue, cyan, and green fluorescent CDs via a one-step hydrothermal carbonization method.166 By dispersing three types of CDs in a sodium alginate matrix, many hydrogen bonds were formed between the CDs and sodium alginate, which inhibited ACQ and non-radiative transitions, resulting in multicolor SFF and multicolor afterglow CDs.
The formation of ionic bonds between the CDs and the matrix results in a relatively stable structure in the matrix due to the stronger attraction of the ionic bonds, which protects the triplet excitons and ensures the realization of afterglow.167 This may also influence the charge transfer process, further affecting the luminescence properties. Li et al. used urea-, HCl- and NaOH-modified seed CDs to obtain color tunable afterglows for u-CDs and u-CDs@NaOH.145 The u-CDs exhibited a weak RTP due to the presence of hydrogen bonding, whereas for the u-CDs@NaOH, due to the addition of NaOH to the u-CDs, ionic bonding formed between NaOH and the carboxyl groups on the surface of the u-CDs, which provided a more stable and rigid structure than hydrogen bonding did, effectively stabilizing the triplet exciton and enhancing the RTP intensity. The phenomenon of tunable afterglow arises from the fact that the introduction of nitrogen/oxygen related groups in the process results in the formation of different luminescent centers. When the excitation wavelength changed from 360 to 450 nm, the phosphorescence color of the CDs changed from green (553 nm) to orange (640 nm). In addition, the addition of HCl increased the degree of carbonation and accelerated the doping of heteroatoms,168 thus promoting the formation of different luminescent centers.
The strength of covalent bonds is much greater than that of hydrogen bonds and ionic bonds, with stability and durability, which significantly enhances the stability of CDs within the matrix, and securely anchors the CDs to the matrix through covalent bonding, effectively inhibiting the non-radiative transitions of triplet excitons and thereby ensuring afterglow emission.161,169 Chen et al. prepared N- and O-doped CDs (N,O-CDs@IP) using insoluble magnesium phosphate as the matrix.78 When the CDs are fixed into the insoluble magnesium phosphate matrix via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O covalent bonds, the rotation and vibration of the CDs and the nonradiative transition of the triplet excitons are suppressed, facilitating the ISC process and enabling the action of the chromogenic functional group C
O covalent bonds, the rotation and vibration of the CDs and the nonradiative transition of the triplet excitons are suppressed, facilitating the ISC process and enabling the action of the chromogenic functional group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. N,O-CDs@IP exhibits an excitation dependence from 254 to 365 nm, and the resulting phosphorescence emission is cyan and green, respectively. Li et al. composited prepared CDs with different contents of BA via heat–melt treatment and obtained phosphorescent CDs with luminescence colors tuned from green to near-infrared based on AIE.143 The CDs and BA were connected by C–B bonds, which effectively suppressed the non-radiative transitions and ensured the realization of phosphorescence. With increasing CD content in BA, the distance between the samples increased, resulting in new aggregation states. As a result, the energy levels continue to split, and multiple triply excited states are formed, leading to a continuous transition of phosphorescence from green to near-infrared (Fig. 8C).
O. N,O-CDs@IP exhibits an excitation dependence from 254 to 365 nm, and the resulting phosphorescence emission is cyan and green, respectively. Li et al. composited prepared CDs with different contents of BA via heat–melt treatment and obtained phosphorescent CDs with luminescence colors tuned from green to near-infrared based on AIE.143 The CDs and BA were connected by C–B bonds, which effectively suppressed the non-radiative transitions and ensured the realization of phosphorescence. With increasing CD content in BA, the distance between the samples increased, resulting in new aggregation states. As a result, the energy levels continue to split, and multiple triply excited states are formed, leading to a continuous transition of phosphorescence from green to near-infrared (Fig. 8C).
In addition, the combined effect of the interactions between the CDs and the matrix, along with the spatial confinement of the matrix, can more effectively inhibit non-radiative transitions and protect triplet excitons in afterglow materials.162,170 Liang et al. developed a universal matrix for multicolor long-wavelength RTP that activates CDs under the same excitation conditions, i.e., Y(OH)xF3−x (YOHF).158 By mixing three classes of CDs (CDs-g, -y, and -o) with different fluorescence colors and a YOHF matrix, CDs@YOHF (green, yellow, and orange) with different RTP colors were prepared via hydrothermal treatment. The presence of the YOHF matrix successfully excited the afterglow of CDs, which was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functional group on the surface of the CDs, and the introduction of electron-withdrawing fluorine atoms reduced the ΔEST, consequently promoting the ISC process and generating triplet excitons. Additionally, the spatial confinement effect of the YOHF matrix and the formation of hydrogen bonds and C–F bonds between CDs and YOHF restrict the intramolecular rotation and vibration of CDs. Therefore, the triplet state was stabilized, which promoted the emission of different colored RTPs (Fig. 8D).
N functional group on the surface of the CDs, and the introduction of electron-withdrawing fluorine atoms reduced the ΔEST, consequently promoting the ISC process and generating triplet excitons. Additionally, the spatial confinement effect of the YOHF matrix and the formation of hydrogen bonds and C–F bonds between CDs and YOHF restrict the intramolecular rotation and vibration of CDs. Therefore, the triplet state was stabilized, which promoted the emission of different colored RTPs (Fig. 8D).
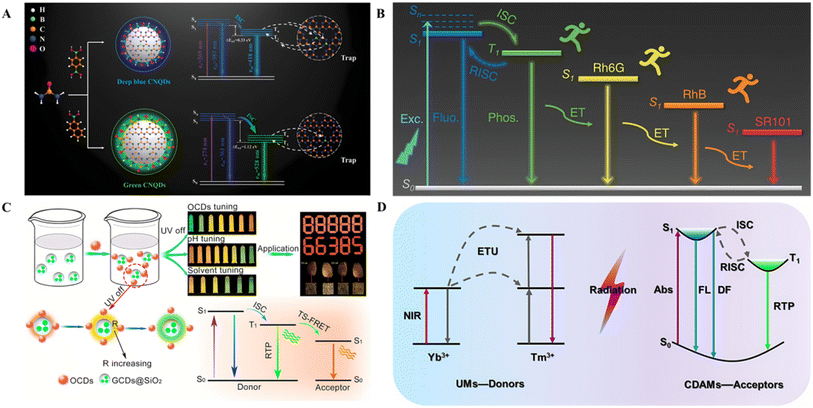 | ||
| Fig. 9 (A) Mechanism of deep blue CNQDs and green CNQDs with ultralong lifetime and wavelength-tunable RTP.172 Copyright 2023 Wiley-VCH GmbH. (B) Proposed possible afterglow mechanism of system 4 in an aqueous environment.173 Copyright 2022 Wiley-VCH GmbH. (C) Schematic illustration of tunable and water-processable room temperature TS-FRET DF successfully obtained from GCDs@SiO2 as the energy donors and OCDs as the energy acceptors with the FRET mechanism.174 Copyright 2022 American Chemical Society. (D) Schematic illustration of activating the multicolor afterglow of the CDAMs under NIR excitation through efficient energy transfer.147 Copyright 2021 Wiley-VCH GmbH. | ||
Fluorescent dyes are important energy receptors. Suitable CDs and fluorescent dyes are selected to construct FRET systems to achieve multicolor afterglow. Mo et al. constructed a cascade FRET system by encapsulating CDs and various fluorescent dyes (rhodamine 6G (Rh6G), RhB, and sulfonamide 101) in SiO2 nanoparticles to achieve multicolor afterglow emission in aqueous solution (Fig. 9B).173 One-, two-, and three-step energy transfers emit yellow (Rh6G), orange (RhB), and red (sulfonamide 101) afterglow, respectively, and the CDs themselves have a green afterglow, thus enabling multicolored afterglow emission from the CDs. Li et al. synthesized RTP CDs using ethanolamine and phosphoric acid as precursors and covalently immobilized the CDs in a SiO2 matrix to produce CDs@SiO2.3 Yellow (553 nm), orange (575 nm), and orange-red (627 nm) afterglows were obtained by using CDs@SiO2 as an energy donor and various organic dyes (Rh6G, RhB, and Nile red) as energy acceptors to fabricate nonradiative energy transfer systems through the surface micelle self-assembly method. Multicolor afterglow emission in aqueous solution was achieved by exploiting the hydrophobicity of the micelles. Sun et al. used citric acid and 1,10-phenanthroline-5-amine (Aphen) as precursors to obtain ACCDs via a one-step hydrothermal method and used PVA as a matrix to produce ACCDs-PVA.180 On the basis of the triplet-to-singlet FRET strategy, afterglow color tuning from yellow to red is achieved via ACCDs-PVA as the energy donor and RhB as the energy acceptor.
In addition, adjusting the ratio between energy acceptors and donors can affect the efficiency of energy transfer, which in turn has an impact on the luminescent color. Jin et al. used cellulose as the raw material to fabricate N-doped cellulose-based CDs with efficient phosphorescence through a solvothermal method.148 N-doped positively charged cellulose-based CDs were used as energy donors, and negatively charged NaOH-treated rhodamine derivatives were used as energy acceptors to construct an energy transfer system in which the afterglow color could be shifted from blue-green to green, yellow, orange and red. The energy transfer process can be effectively facilitated by electrostatic attraction interactions. By increasing the concentration ratio of rhodamine derivatives to CDs, the energy transfer efficiency can be increased, thereby modulating the afterglow color and achieving multicolor afterglow emission.
In energy transfer systems, CDs can act not only as energy acceptors but also as energy donors.150 On this basis, Teng et al. synthesized GCDs with ethanolamine as a precursor in a phosphoric acid solution via a simple microwave method.174,181 Orange-emissive CDs (OCDs) were synthesized via a hydrothermal method using RhB and poly(ethylene glycol) as precursors.182 Silica-coated GCDs (GCDs@SiO2) with green room-temperature phosphorescence were used as energy donors, and OCDs were used as energy acceptors to construct a FRET system (GCDs@SiO2–OCDs). Color-tunable GCDs@SiO2–OCDs with triplet-to-singlet fluorescence resonance energy transfer-induced delayed fluorescence (TS-FRET DF) were obtained in aqueous solution. The TS-FRET DF from the GCDs@SiO2–OCDs can be adjusted from green to orange. As the amount of OCD increases, the efficiency of the TS-FRET DF increases, resulting in a color change from green to orange (Fig. 9C).
The energy transfer of CDs can be divided into radiative energy transfer and non-radiative energy transfer.150 The above content has introduced the method of achieving multicolor afterglow through non-radiative energy transfer, which often requires a sufficiently small distance between the donor and acceptor, as well as an overlap between the emission spectrum of the donor and the absorption spectrum of the acceptor.183,184 In contrast, the radiative energy transfer process only requires spectral overlap and is not limited by distance. Zheng et al. reported an approach based on efficient radiative energy transfer to synthesize NaYF4:Yb,Tm UMs as energy donors and four different types of CD-based room temperature afterglow materials (CDAMs) as acceptors that absorb the light emitted from upconversion materials (Ums).147 Near-infrared light is easily converted into UV or visible light, resulting in multicolor (blue, cyan, green, and orange) afterglow of the CDs (Fig. 9D).150 Efficient energy transfer between the NaYF4:Yb,Tm UMs and CDAMs is possible because of the significant spectral overlap between the UCLs of the UMs and the absorption of the CDAMs and because radiative energy transfer itself is not limited by distance.
5 Applications
Multicolor SSF CDs and multicolor afterglow CDs have advantages such as a wide color gamut and good biocompatibility, demonstrating great potential for applications in anticounterfeiting, information encryption, the photoelectric field, sensing, and biological imaging.5.1 Information encryption and advanced anticounterfeiting
At present, with the development of science and technology, the secure transmission and expression of information is getting more and more attention. Therefore, developing a variety of technologies to ensure information security is becoming increasingly important. Owing to the unique optical properties of CDs, multicolored CDs can be used for fingerprint identification, information encryption and advanced anticounterfeiting.24,185 It provides new technological support in the fields of information security, identification, and information transmission.For fingerprint identification, the characteristic details of fingerprints can be highlighted by applying CDs of different colors to fingerprint acquisition. In addition, the lipid-soluble luminescence property of CDs can clearly show the details of ridges on a variety of substrates, thus improving the accuracy and reliability of identification.186 Owing to the excellent biocompatibility of the CDs, they can be safely used on skin surfaces, ensuring safety and effectiveness.
Currently, the multicolor SSF of CDs has promising applications in fingerprint identification.187,188 Because CDs can emit fluorescence of different colors and have excellent photostability and biocompatibility, they can be used to prepare markers or fluorescent probes, which play a significant role in fingerprint identification. By marking different colored CDs on fingerprints, multiple fluorescence imaging can be achieved, which improves the accuracy and security of fingerprint identification. In addition, the fluorescence properties of CDs can be used to track and record fingerprints over long periods of time, providing a reliable basis for crime investigation and forensic identification. Sekar et al. synthesized CZnO-dots using a gum ghatti carbon precursor. Generation of CZnO-dots/Si by combining CZnO-dots and silica gel.189 The tunable fluorescence property of the CZnO-dot/Si nanopowder aids in displaying the ridges alone, improving fingerprint detection. In addition, the multicolored fluorescence emission of the CZnO-dot/Si nanopowder can be used to qualitatively and quantitatively scrutinize fingerprint details. CZnO-dot/Si nanopowder does not respond to or contaminate the evidence at the crime scene because it is a fluorescence-based imaging probe. Peng et al. synthesized multicolor-emitting CD solutions through simple stratification and extraction.190 Doping the PVP crosslinker into the solutions resulted in red, orange, and yellow powders. The multicolor fluorescence imaging scheme used by Peng et al. can largely inhibit the interference of complex backgrounds, and fingerprint minutiae, such as dots, spurs, terminations and islands, are clearly presented at a higher resolution. Li et al. prepared blue-green fluorescent, yellow and orange-red CD solutions.191 After the CDs were doped as cross-linking agents into g-(2,3-epoxypropoxy) propytrimethoxysilane solutions, orange-, yellow-, and blue-emitting organosilicon-coated powders were fabricated. Compared with the stamp-pad ink commonly used in the office, the ridge patterns (such as the bow, ring and thread) of organosilicon-coated CD powders are clearer and more detailed (Fig. 10A). The collected fingerprints had both high contrast and high sensitivity. With good stability, the fluorescence image is still bright and clear even after several weeks of storage.
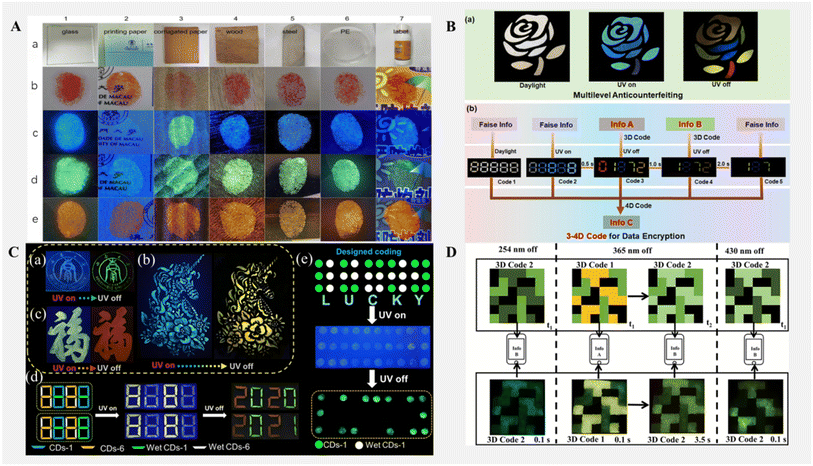 | ||
| Fig. 10 (A) Photographs of CD powder-stained fingerprints on glass sheets, printing paper, corrugated paper, wood, steel, PE (polyethylen), and label, respectively. (a) Various substrates. (b) Stamp-pad ink-stained fingerprint. (c) os-b-CDs, (d) os-y-CDs and (e) os-r-CD stained fingerprints under a UV lamp.191 Copyright 2023 The Royal Society of Chemistry and the Centre National de la Recherche Scientifique. (B) 4D coding applications of the developed CDs@MOF composites. (a) Photographs of the “flower” pattern before and after 365 nm UV irradiation. (b) 4D codes for data encryption.92 Copyright 2022 American Chemical Society. (C) Images of the patterns painted by CDs-1 (a), CDs-3 (b) and CDs-6 (c) with UV light on or off; (d) dual-channel encrypted information (2020, 2021) by CDs-1 and CDs-6; (e) designed code of LUCKY encrypted by CDs-1.192 Copyright 2023 Elsevier B.V. (D) Multilevel phosphorescence colored 3D codes for advanced dynamic information encryption.25 Copyright 2023 Wiley-VCH GmbH. | ||
For information encryption, differently colored CDs can be used to encode information by mixing different colored CDs together to form a fluorescent marker, which can be identified and interpreted only by a specific decoder. This method can effectively protect the security of the information and prevent it from being accessed by unauthorized persons.6 For advanced anticounterfeiting, multicolored afterglow CDs can be added to a product's label or packaging to form a special mark. This type of marker can only be accurately recognized and verified for authenticity via specific testing equipment. Through this method, the inflow of counterfeit products into the market can be effectively prevented, and the rights and interests of consumers can be protected. The emission wavelength of multicolor CDs can vary with factors such as the excitation wavelength,144 water,193 temperature194 and time.195 Therefore, they can be used for information encryption and anticounterfeiting.
Currently, the CDs used for information encryption and advanced anticounterfeiting are mostly based on the excitation dependence of CDs. Xu et al. embedded five fluorescent CDs into MOF to prepare CDs@MOF complexes with adjustable RTP colors ranging from blue to red.92 On this basis, flower patterns were prepared using differently colored CDs@MOF complexes, and multicolored “flowers” were observed after the UV light was switched off. In addition, a new type of code utilizing dynamic color transformation over time via CDs@MOF complexes, called 4D coding, was developed (Fig. 10B). When the UV lamp was turned off, a multicolored 3D code was formed. The code changes further over time. Lu et al. selected four powders with different luminescent properties to achieve information encryption.161 In this case, powder 1 emits red fluorescence, powder 2 emits red phosphorescence after UV irradiation, and powders 3 and 4 emit green light after the UV light is switched off. This approach could transmit specific messages via a safe multichannel approach. If the password number is 243, the three numbers of the password correspond to the message IITTIT. Message encryption is achieved by setting messages to one true and one false. Hu et al. synthesized Si-CDs@B2O3via a simple pyrolysis method.196 Complex patterns of flower capitals and butterflies were made via composites with yellow-orange and red RTP, showing good security capabilities. RTP plates for imaging and information transfer were also prepared, and UV light could be used to write text on the RTP plates, such as “w”, “h”, “u” and “t”. Gan et al. synthesized CDs with multicolored fluorescence and RTP by adjusting the ratio of ethanol.192 CD-1, CD-3 and CD-6 exhibited blue, green and yellow fluorescence and green, yellow and orange phosphorescence, respectively. Two “8888” codes were drawn, with the orange part and the white part coated with CDs-6 and the blue part coated with CDs-3. After the UV light was switched off, the two “8888” signals changed to hidden signals “2020” and “2021”. In addition, 10 sets of vertical spots were used to hide the message, where two sets of spots represented a letter, the pattern that was decoded as LUCKY (Fig. 10C).
CDs with time-dependent characteristics can achieve more sophisticated information encryption and advanced anticounterfeiting. Shi et al. designed a 3D code with switchable phosphorescent colors using CDs/inorganic nanocomposites with TDPC.25 Phosphorescent images composed of dual metal-doped composites and one metal-doped composite represent different codes under different light conditions, and the information in the code can be read via a smartphone (Fig. 10D). Only when the time is correct can correct information be obtained. Zhang et al. synthesized CDs@SiO2 with blue, green, yellow and red colors via the hydrolysis of ethyl orthosilicate on the surface of synthesized fluorescent CDs.197 On the basis of the superior phosphorescence properties of the four CDs@SiO2, digital storage and information encryption were achieved by placing different CD composites in the grooves of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 888. It can encrypt and store information at UV wavelengths in three dimensions in a time dimension limited only by the switching of UV light sources on and off and the presence or absence of filters (Fig. 11A).
888. It can encrypt and store information at UV wavelengths in three dimensions in a time dimension limited only by the switching of UV light sources on and off and the presence or absence of filters (Fig. 11A).
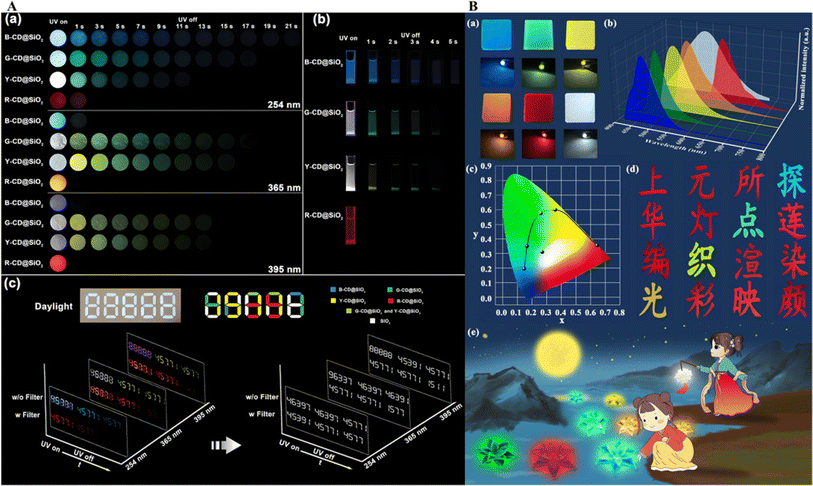 | ||
| Fig. 11 (A) (a) Photographs of four CD@SiO2 composites under different excitations. (b) Photographs of the four aqueous CD@SiO2 dispersions under a UV lamp (254 nm) and after the UV lamp was turned off. (c) 3D information storage and encryption system based on the four composites.197 Copyright 2022 Wiley-VCH GmbH. (B) (a) Photos of square epoxy blocks and LEDs containing blue-, green-, yellow-orange, red- and white-emitting SSF CDs under 365 nm irradiation. (b) Corresponding photoluminescence spectra for the fluorescent epoxy blocks. (c) CIE color coordinates for the fluorescent blocks. (d) A poem describing the Shangyuan Lantern Festival illuminated by blue, green, yellow orange and red SSF CDs. (e) Depiction of three-dimensional seven-color lotus lanterns made by 3D printing technology being set afloat on a river.127 Copyright 2022 Wiley-VCH GmbH. | ||
CDs with dual-mode afterglow emission, featuring both RTP and TADF, can change the luminescent color by adjusting the relative intensity ratio of their dual-mode afterglow emission by adjusting the temperature.194 Currently, this temperature dependence of CDs has been applied in anticounterfeiting and information encryption. Sun et al. reported the use of multicolor anticounterfeiting and encryption via the temperature-responsive conversion characteristics of TADF and RTP of CDs@SiO2.7 CDs@SiO2 exhibited blue fluorescence and green RTP at room temperature. At 120 and 200 °C, CDs@SiO2 is cyan and blue afterglow, respectively. They used CDs@SiO2 to draw the letters “L” and “V” in the pattern “LOVE,” whereas “O” and “E” were drawn with materials that only have blue fluorescence and no afterglow emission. The pattern appears white in sunlight and displays a completely blue “LOVE” under 365 nm UV light. When the UV light is turned off, “L” and “V” are green, whereas “O” and “E” do not exhibit any afterglow color. As the temperature increases to 120 °C, “L” and “V” change to cyan. A further increase in the temperature to 200 °C caused “L” and “V” to appear blue. On the basis of this temperature-dependent characteristic, CDs@SiO2 demonstrates advanced temperature-responsive anticounterfeiting and information encryption technologies.
For some specific CDs, the presence of water has a dual effect on the afterglow emission of the CDs.195 On the one hand, the addition of water breaks the hydrogen bonds between the CDs, leading to the formation of a hydrogen bonding network between the CDs and the matrix, which inhibits the non-radiative transitions and protects the triplet excitons.142 On the other hand, the presence of water and oxygen depletes the triplet excitons in the CDs, causing afterglow quenching. Therefore, after heating to remove free water and oxygen, the CDs can show significant afterglow. Therefore, this property can be utilized for information encryption and dynamic anticounterfeiting. Li et al. prepared boron doped carbon quantum dots (BCQDs) with the above properties and relied on this property for multilevel encryption and anticounterfeiting.133 They used an ethanol solution of BCQDs as ink and printed various pieces of information on filter paper. After the filter paper dried, the information on the filter paper did not appear after UV irradiation because the afterglow of the BCQDs was not activated and did not emit fluorescence. Only after the filter paper has been treated with water and then heated, will the information on the filter paper be fully visible after UV irradiation. Incorrect processing steps would not reveal the information on the filter paper. Additionally, owing to the quenching effect of oxygen on afterglow, the information gradually disappears after 30 min in the air following the cessation of heating, significantly enhancing the security of the information.
5.2 Photoelectric field
Multicolor CDs are excellent luminescent materials, with excellent tunable luminescence properties, light stability and low cost and are suitable for the fabrication of LEDs.198 Its wide range of adjustable emission wavelengths makes it possible to prepare color LEDs,93 and can effectively avoid optical problems such as light leakage and color distortion. Its excellent stability ensures the stability of the photoelectric field and effectively suppresses optical problems such as reduced optical efficiency.LEDs have been widely used in lighting, displays, automotive lamps, backlighting and other fields. Owing to their advantages of high energy efficiency, long life and small size, LEDs have gradually replaced traditional light sources and become mainstream modern lighting and display technology. Han et al. synthesized red, green, and blue-emissive CDs.94 White phosphors were prepared by dispersing three CDs into a PVA matrix at a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Furthermore, WLEDs with a correlated color temperature of 3248 K, an International Commission on Illumination (CIE) coordinate of 0.41 to 0.38 and a high color rendering index of 93.2 were obtained. Zhu et al. prepared white afterglow CDs by means of a synergistic interaction between RTP originating from the carbon core and delayed fluorescence originating from surface C
1.2. Furthermore, WLEDs with a correlated color temperature of 3248 K, an International Commission on Illumination (CIE) coordinate of 0.41 to 0.38 and a high color rendering index of 93.2 were obtained. Zhu et al. prepared white afterglow CDs by means of a synergistic interaction between RTP originating from the carbon core and delayed fluorescence originating from surface C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N.199 The synergistic effect originates from energy transfer by matching the singlet state of the surface group of the CDs with the triplet state of the carbon core. WLEDs were prepared by coating W-CDs on GaN UV (365 nm) chips with luminous efficiencies of up to 31.6 lm W−1 and a decrease of only 12% after 10 h. Han et al. prepared yellow, red and green CDs with excellent photoluminescence properties.200 In this way, monochromatic LEDs were prepared, and the three LEDs operated smoothly at 103.0 V with CIE coordinates of (0.57, 0.42), (0.58, 0.31) and (0.46, 0.49). Wang et al. synthesized CDs with concentration-dependent fluorescence via a one-step microwave method.201 Concentration-dependent CDs with different concentrations were dispersed in N-(3-(trimethoxysilyl) propyl) ethylenediamine for curing to make phosphors and combined with UV chips to make green, yellow, orange and red LEDs, with CIE coordinates of (0.33, 0.38), (0.41, 0.48), (0.49, 0.44) and (0.67, 0.33), respectively, which provides an effective and universal method for the construction of multicolor LEDs. Ai et al. prepared SSF CDs from o-PD and salicylaldehyde.127 A series of color conversion materials for optical displays were prepared using epoxy resin. Composites with CIE coordinates of (0.13, 0.20), (0.15, 0.35), (0.26, 0.58), (0.35, 0.60), and (0.63, 0.36) were obtained, and a variety of monochromatic and white downconverting light-emitting diodes were prepared by coating the CD-containing epoxy on 365 nm chips. The lotus lanterns of the Chinese Shangyuan Lantern Festival as well as poems were also produced (Fig. 11B). Xie et al. synthesized blue- and red-emitting CDs via a hydrothermal method.202 By combining CDs with Mg(OH)2 hexagonal nanosheets in one step, solid-state luminescent B/R-CD@Mg(OH)2 composites were synthesized. Internal quantum efficiency is an important index for evaluating luminescent materials in LED applications. The internal quantum efficiency values of B-CDs@Mg(OH)2 and R-CDs@Mg(OH)2 composites are 42.5% and 31.7%, respectively, indicating their potential applications in LEDs. B/R-CD@Mg(OH)2 composites were coated onto an InGaN NUV-emitting chip (λ = 395 nm) to prepare a plant growth device for LED devices. By adjusting the mass ratio of the two composites, the CIE coordinates of the LEDs ranged from blue (0.185, 0.104) to white (0.352, 0.339) to orange (0.433, 0.375) zones, thus catering to plants with different light needs. By adjusting the current, the CIE coordinates change from (0.254, 0.203) to (0.217, 0.158). In addition to internal quantum efficiency, external quantum efficiency is another key index to evaluate the performance of LEDs. Wang et al. prepared CDs-LEDs doped with different concentrations of Y-CDs into poly(9,9-dioctylfluorene-co-N-(4-(3-methylpropyl))diphenylamine).74 As the doping concentration of Y-CDs increased, the white light emission of CDs-LEDs achieved warm white, pure white and cool white with Lmax and external quantum efficiency reaching 1414–4917 cd m−2 and 0.08–0.87%, respectively, which is one of the best performances of white CD-LEDs.
N.199 The synergistic effect originates from energy transfer by matching the singlet state of the surface group of the CDs with the triplet state of the carbon core. WLEDs were prepared by coating W-CDs on GaN UV (365 nm) chips with luminous efficiencies of up to 31.6 lm W−1 and a decrease of only 12% after 10 h. Han et al. prepared yellow, red and green CDs with excellent photoluminescence properties.200 In this way, monochromatic LEDs were prepared, and the three LEDs operated smoothly at 103.0 V with CIE coordinates of (0.57, 0.42), (0.58, 0.31) and (0.46, 0.49). Wang et al. synthesized CDs with concentration-dependent fluorescence via a one-step microwave method.201 Concentration-dependent CDs with different concentrations were dispersed in N-(3-(trimethoxysilyl) propyl) ethylenediamine for curing to make phosphors and combined with UV chips to make green, yellow, orange and red LEDs, with CIE coordinates of (0.33, 0.38), (0.41, 0.48), (0.49, 0.44) and (0.67, 0.33), respectively, which provides an effective and universal method for the construction of multicolor LEDs. Ai et al. prepared SSF CDs from o-PD and salicylaldehyde.127 A series of color conversion materials for optical displays were prepared using epoxy resin. Composites with CIE coordinates of (0.13, 0.20), (0.15, 0.35), (0.26, 0.58), (0.35, 0.60), and (0.63, 0.36) were obtained, and a variety of monochromatic and white downconverting light-emitting diodes were prepared by coating the CD-containing epoxy on 365 nm chips. The lotus lanterns of the Chinese Shangyuan Lantern Festival as well as poems were also produced (Fig. 11B). Xie et al. synthesized blue- and red-emitting CDs via a hydrothermal method.202 By combining CDs with Mg(OH)2 hexagonal nanosheets in one step, solid-state luminescent B/R-CD@Mg(OH)2 composites were synthesized. Internal quantum efficiency is an important index for evaluating luminescent materials in LED applications. The internal quantum efficiency values of B-CDs@Mg(OH)2 and R-CDs@Mg(OH)2 composites are 42.5% and 31.7%, respectively, indicating their potential applications in LEDs. B/R-CD@Mg(OH)2 composites were coated onto an InGaN NUV-emitting chip (λ = 395 nm) to prepare a plant growth device for LED devices. By adjusting the mass ratio of the two composites, the CIE coordinates of the LEDs ranged from blue (0.185, 0.104) to white (0.352, 0.339) to orange (0.433, 0.375) zones, thus catering to plants with different light needs. By adjusting the current, the CIE coordinates change from (0.254, 0.203) to (0.217, 0.158). In addition to internal quantum efficiency, external quantum efficiency is another key index to evaluate the performance of LEDs. Wang et al. prepared CDs-LEDs doped with different concentrations of Y-CDs into poly(9,9-dioctylfluorene-co-N-(4-(3-methylpropyl))diphenylamine).74 As the doping concentration of Y-CDs increased, the white light emission of CDs-LEDs achieved warm white, pure white and cool white with Lmax and external quantum efficiency reaching 1414–4917 cd m−2 and 0.08–0.87%, respectively, which is one of the best performances of white CD-LEDs.
5.3 Sensing
Multicolor CDs have significant application potential in bioanalysis and environmental monitoring.203,204 They are sensitive to specific ions and excitation wavelengths and use their luminescent color to transform them into variable color signals, providing an efficient and sensitive solution for a wide range of detection scenarios, including ion detection3 and UV detection.205Multicolor luminescent CDs can be used as sensing probes for detecting certain ions. Li et al. applied organosilicon-coated powders for the first time in acetate ion (AcO−) sensing probes.191 Compared with traditional methods, CDs exhibit a wide concentration range (3 × 10−7–3 × 10−3 M) of response to AcO− with a lower fluorescence response threshold (0.3 μM). He et al. used a one-step hydrothermal method to prepare CDs, which were mixed with boron oxide through a heating process to obtain dual-mode afterglow that was applied to visually probe the temperature on the basis of ratio metric sensing.88 When the temperature changed from 20 to 100 °C, the color coordinates could be marked from the orange region (0.3755, 0.4977) to the blue region (0.2028, 0.3105). In addition, the composites exhibit excellent high stability and reversibility in a temperature-dependent manner, providing better conditions for ratio metric temperature sensing and reversible ratio metric detection of temperature. Li et al. designed an energy transfer-based sensing system for the detection of Hg2+ ions.3 The hydrophobic CD composites were used as energy donors, and a rhodamine derivative was used as an energy acceptor. Rhodamine derivatives can be selectively identified as Hg2+ ions. The presence of Hg2+ ions can cause an overlap between the donor emission spectrum and the acceptor absorption spectrum, resulting in a sensing system that appears pink in daylight and orange when UV excitation is switched off. In addition, there is a linear relationship between the intensity of the phosphorescence and the concentration of Hg2+ ions, making the detection of Hg2+ ions more intuitive (Fig. 12A).
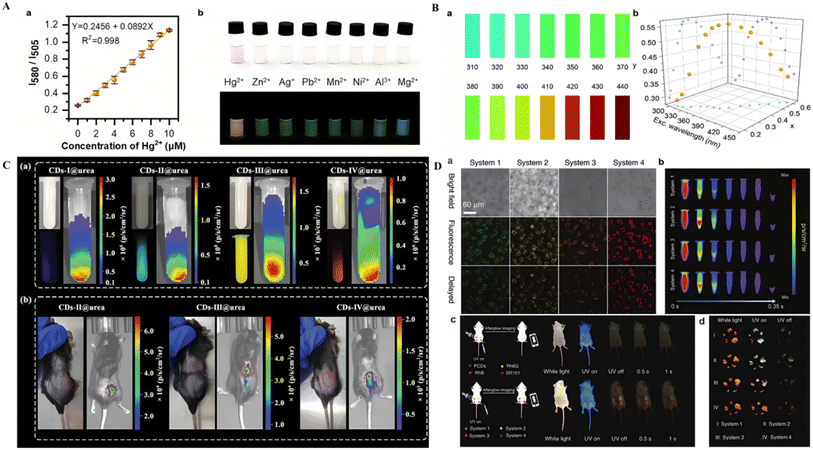 | ||
| Fig. 12 (A) (a) Linear curve of the phosphorescence intensity ratio (I580/I505) versus the Hg2+ ion concentration. (b) Pictures of various metal ion (10 μM) probes in sunlight (top) and after 365 nm excitation was turned off (bottom).3 Copyright 2023 American Chemical Society. (B) (a) Standard color plots at specific wavelengths from 310 to 440 nm were detected with FP-CD powder. (b) Fitting function of the excitation wavelength from 310 to 440 nm with FP-CD emission.4 Copyright 2022 Royal Society of Chemistry. (C) (a) Phosphorescent images of CDs@urea composites using aqueous dispersion. (b) Afterglow images of mice injected subcutaneously with CDs@urea aqueous dispersions.16 Copyright 2024 Wiley-VCH GmbH. (D) (a) Figure validating the potential of afterglow imaging in vitro using HepG2 cells. (b) Schematic of system 1–4 afterglow decay. (c) Afterglow images of control mice injected subcutaneously with fluorescent dyes and treated mice injected subcutaneously with systems 1–4 after the UV lamps were turned on and off. (d) Digital photographs of isolated organs of mice incubated in systems 1–4 under white light, with UV and UV off, respectively.173 Copyright 2022 Wiley-VCH GmbH. | ||
At present, destruction of the ozone layer has led to a gradual increase in the dangers of UV radiation, which are very difficult to observe with the naked eye in daily life, so the detection of UV is very important. Shi et al. achieved UV radiation via the excitation-dependent properties of CDs with RTP emission.4 The one-to-one correspondence between the CIE coordinates of the CDs and the excitation wavelengths was determined, thus enabling the detection of UV wavelengths via the corresponding functional relationship (Fig. 12B). Zhang et al. developed UV detection CDs on the basis of their photochromic properties.205 By mixing CDs with polymethyl methacrylate, they prepared a device that linearly responds to UV. This detection device gradually transitions from an opaque to a transparent state after 10 min of exposure to sunlight, becoming nearly completely transparent within 15 min. This enables effective detection of weak UV light in sunlight.
5.4 Biological imaging
Multicolor CDs exhibit excellent biocompatibility, water solubility, photostability, and a broad tunable wavelength range, making them particularly suitable for biological imaging applications.93,185,206 The tunable wavelength feature allows for the simultaneous analysis of multiple targets. Additionally, long-wavelength emission provides good penetration, enabling observation even at deeper locations, and short-wavelength emission may not be present in organisms.16 Ai et al. prepared four CDs/urea composites (CDs-I@urea (blue), CDs-II@urea (green), CDs-III@urea (yellow), and CDs-IV@urea (red)) with excellent resistance to water quenching properties and photostability.16 These four CDs@urea composites contain only three heavy elements, C, N and O, and are free of metals and heavy atoms, avoiding toxicity issues. Additionally, the biocompatibility of these CDs@urea was tested via a Cell Count Kit-8 experiment on lymphoma U937 cells, ensuring their potential for use in biological imaging. The CDs@urea composites exhibited good dispersion in stroke-physiological saline solution, phosphate buffered saline, bovine serum albumin, and mouse blood. When the dispersed CDs@urea composites in water were injected subcutaneously into the mice, green, yellow, and red afterglows were observed, with intensities of 4.912 × 105, 4.969 × 105, and 1.741 × 106, respectively (Fig. 12C). Blue afterglow from the CDs@urea composite was not observed because of its shorter wavelength or severe bursting. Mo et al. prepared four systems (System 1–4) with multicolor long afterglow (green, yellow, orange, and red) in aqueous solution through cascade FRET.173 They validated the in vitro bioimaging potential of the multicolor long afterglow system using human hepatocellular carcinoma cells (HepG2) for the first time (Fig. 12D). To verify the in vivo bioimaging capability of these systems, they subcutaneously injected systems 1–4 into mice without hair removal and obtained afterglow decay images after exposure to 365 nm UV irradiation. Compared with the fluorescence images of the mice subcutaneously injected with the sulforhodamine 101 fluorescent agent, the afterglow images showed a higher signal-to-noise ratio, enabling the afterglow signals to be observed with a smartphone camera. In addition, they injected systems 1–4 into four different subcutaneous sites of mice as a treated group and used four different fluorescent dyes subcutaneously into another mouse as a control group. Under UV irradiation, the fluorescence signals of both groups were insignificant due to the influence of blue autofluorescence and scattered light. In contrast, the treated group of mice presented distinct green, yellow, orange, and red afterglow signals after excitation stopped (Fig. 12D). In addition, after injecting system 1–4 into the isolated organs of the mice respectively and incubating them for 3 hours, significant afterglow signals were obtained from the connective tissues of the organs, demonstrating the bioimaging capability of systems 1–4 in biological organs (Fig. 12D).6 Summary and outlook
In this review, we have reviewed the synthesis methods, luminescence modulation strategies, and applications of SSF and afterglow luminescent materials. First, the synthesis methods of SSF and afterglow CDs are summarized, mainly including one-step and multistep synthetic routes. Second, it summarizes the luminescence regulation strategies from both direct and indirect realization perspectives, with the main methods including element doping, reaction parameter modulation, surface functionalization, preparation of CDs with multiple luminescent centers, and construction of FRET systems. The detailed elaboration of these luminescence modulation strategies provides relevant assistance for the realization of applications such as fingerprint identification, information encryption and advanced anticounterfeiting, and LEDs. Although much progress has been made in the study of SSF and afterglow CDs, there are still some limitations in certain aspects, and here, we summarize and discuss these challenges.(1) Currently, the SSF and afterglow luminescence mechanisms of CDs are not fully understood. The fluorescence of CDs is generally believed to originate from the carbon core and surface states, whereas the multicolor afterglow emission is typically associated with functional groups and energy gaps related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. However, the structure of CDs is still not well defined; therefore, the understanding of the fluorescence and afterglow luminescence mechanisms of CDs are still somewhat controversial. This requires a systematic understanding of the structural variations of different CDs; therefore, more comprehensive characterization methods as well as advanced calculation methods are needed to fully understand the link between the structure of CDs and the luminescence mechanism.
N. However, the structure of CDs is still not well defined; therefore, the understanding of the fluorescence and afterglow luminescence mechanisms of CDs are still somewhat controversial. This requires a systematic understanding of the structural variations of different CDs; therefore, more comprehensive characterization methods as well as advanced calculation methods are needed to fully understand the link between the structure of CDs and the luminescence mechanism.
(2) Achieving a wider range of emission colors is a key challenge in the preparation of CDs with multicolor emission. Currently, the SSF emission of CDs is mostly blue and yellow, and the afterglow emission is blue and green. This greatly limits the application areas of CDs. Therefore, there is still a need for further development of SSF and afterglow CDs with red and near-infrared emission. The long-wavelength emission of CDs can be achieved through surface modification and the use of precursors with large conjugated structures. Additionally, energy transfer strategies have been widely employed to achieve long-wavelength emission.
(3) The problem of bursting the SSF and afterglow is a key issue affecting the practical application of multicolor CDs. Currently, the most common solution to the SSF and afterglow quenching problem is the use of matrix-dispersed CDs and the formation of hydrogen and covalent bonds between the CDs and the matrix to inhibit the aggregation of the CDs and the nonradiative transitions to ensure the luminescence of the SSF and afterglow. Notably, CDs with AIE properties have been developed to solve the ACQ problem. In addition, the triplet excitons of the CDs tend to quench in water, resulting in the disappearance of afterglow luminescence. The use of matrices with hydrophilic and hydrophobic properties can enable the afterglow luminescence of CDs in water, increasing the feasibility of their implementation in bioimaging applications.
(4) The precise regulation of the SSF and afterglow color of CDs is a problem that urgently needs to be solved. First, the selection of the raw materials, the use of synthetic methods, the ratio between various raw materials and the appropriateness of the reaction conditions during the preparation process all affect the SSF and afterglow color of the CDs. Second, at present, the most important method for preparing SSF and afterglow CDs is to combine CDs with a matrix, and some studies have shown that the use of different matrices will cause the prepared CDs to have different luminescence colors. Therefore, selecting an appropriate matrix to combine with CDs is essential for achieving precise control over the emission color. This requires the exploration of suitable characterization techniques to study and verify the structure of the CDs and the matrix, as well as their interactions, providing a basis for achieving precise control of the emission color.
(5) Enhancing the QYs and lifetimes of SSF and afterglow CDs is one of the challenges we currently face. On the basis of the data in Tables 2–4, the QYs of most CDs are below 50%, and their lifetimes generally range from the nanosecond scale to up to 10 s. Low QYs and short lifetimes significantly impact the practical applications of CDs. Although significant progress has been made in recent years, further optimization of the synthesis and purification processes of CDs for specific application scenarios is needed to improve their overall performance.
Data availability
The datasets generated and/or analyzed during the study are available from the corresponding author upon request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the Youth Talent Program Startup Foundation of Qufu Normal University (No. 602601), and the Natural Science Foundation of Shandong Province (No. ZR2024QB412).References
- J. Xu, Q. Liang, Z. Li, V. Y. Osipov, Y. Lin, B. Ge, Q. Xu, J. Zhu and H. Bi, Rational Synthesis of Solid-State Ultraviolet B Emitting Carbon Dots via Acetic Acid-Promoted Fractions of sp3 Bonding Strategy, Adv. Mater., 2022, 34(17), 2200011, DOI:10.1002/adma.202200011.
- D. Gao, A. Liu, Y. Zhang, Y. Zhu, D. Wei, J. Sun, H. Luo and H. Fan, Temperature triggered high-performance carbon dots with robust solvatochromic effect and self-quenching-resistant deep red solid state fluorescence for specific lipid droplet imaging, Chem. Eng. J., 2021, 415, 128984, DOI:10.1016/j.cej.2021.128984.
- T. Li, X. Li, Y. Zheng, P. Zhu, C. Zhang, K. Zhang and J.-J. Xu, Phosphorescent Carbon Dots as Long-Lived Donors To Develop an Energy Transfer-Based Sensing Platform, Anal. Chem., 2023, 95(4), 2445–2451, DOI:10.1021/acs.analchem.2c04639.
- H. Shi, Z. Niu, H. Wang, W. Ye, K. Xi, X. Huang, H. Wang, Y. Liu, H. Lin and H. Shi, et al., Endowing matrix-free carbon dots with color-tunable ultralong phosphorescence by self-doping, Chem. Sci., 2022, 13(15), 4406–4412, 10.1039/d2sc01167k.
- B. Zhao, H. Ma, H. Jia, M. Zheng, K. Xu, R. Yu, S. Qu and Z. a. Tan, Triphenylamine-Derived Solid-State Emissive Carbon Dots for Multicolor High-Efficiency Electroluminescent Light-Emitting Diodes, Angew. Chem., Int. Ed., 2023, 62(22), e202301651, DOI:10.1002/anie.202301651.
- Y. Ding, X. Wang, M. Tang and H. Qiu, Tailored Fabrication of Carbon Dot Composites with Full-Color Ultralong Room-Temperature Phosphorescence for Multidimensional Encryption, Adv. Sci., 2021, 9(3), 2103833, DOI:10.1002/advs.202103833.
- Y. Sun, J. Liu, X. Pang, X. Zhang, J. Zhuang, H. Zhang, C. Hu, M. Zheng, B. Lei and Y. Liu, Temperature-responsive conversion of thermally activated delayed fluorescence and room-temperature phosphorescence of carbon dots in silica, J. Mater. Chem. C, 2020, 8(17), 5744–5751, 10.1039/d0tc00507j.
- F. Huo, P. G. Karmaker, Y. Liu, B. Zhao and X. Yang, Preparation and Biomedical Applications of Multicolor Carbon Dots: Recent Advances and Future Challenges, Part. Part. Syst. Charact., 2020, 37(4), 1900489, DOI:10.1002/ppsc.201900489.
- F. Du, L.-P. Yang and L.-L. Wang, Synthetic strategies, properties and sensing application of multicolor carbon dots: recent advances and future challenges, J. Mater. Chem. B, 2023, 11(34), 8117–8135, 10.1039/d3tb01329d.
- W. Shi, Q. Han, J. Wu, C. Ji, Y. Zhou, S. Li, L. Gao, R. M. Leblanc and Z. Peng, Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes, Int. J. Mol. Sci., 2022, 23(3), 1456, DOI:10.3390/ijms23031456.
- K. Zuo, W. Liu, X. Liu and X. Liu, Phosphorescence of Carbon Dot: The Intrinsic Mechanism and Recent Progress, Carbon Trends, 2023, 12, 100278, DOI:10.1016/j.cartre.2023.100278.
- Y. Li, C. Liu, H. Sun, M. Chen, D. Hou, Y. Zheng, H. Xie, B. Zhou and X. Lin, Formation and Band Gap Tuning Mechanism of Multicolor Emissive Carbon Dots from m-Hydroxybenzaldehyde, Adv. Sci., 2023, 10(18), 2300543, DOI:10.1002/advs.202300543.
- H. Sun, P. Xia, H. Shao, R. Zhang, C. Lu, S. Xu and C. Wang, Heating-free synthesis of red emissive carbon dots through separated processes of polymerization and carbonization, J. Colloid Interface Sci., 2023, 646, 932–939, DOI:10.1016/j.jcis.2023.05.120.
- B. Wang, J. Yu, L. Sui, S. Zhu, Z. Tang, B. Yang and S. Lu, Rational Design of Multi-Color-Emissive Carbon Dots in a Single Reaction System by Hydrothermal, Adv. Sci., 2020, 8(1), 2001453, DOI:10.1002/advs.202001453.
- W. Sun, Y. Zhang, G. Yin and S. Lu, Afterglow of Dual-Confined Carbon Dots Ranging from Blue to Near-Infrared, Adv. Funct. Mater., 2024, 2402346, DOI:10.1002/adfm.202402346.
- L. Ai, W. Xiang, J. Xiao, H. Liu, J. Yu, L. Zhang, X. Wu, X. Qu and S. Lu, Tailored Fabrication of Full-Color Ultrastable Room-Temperature Phosphorescence Carbon Dots Composites with Unexpected Thermally Activated Delayed Fluorescence, Adv. Mater., 2024, 36(27), 2401220, DOI:10.1002/adma.202401220.
- Y. Liu, D. Cheng, B. Wang, J. Yang, Y. Hao, J. Tan, Q. Li and S. Qu, Carbon Dots-Inked Paper with Single/Two-Photon Excited Dual-Mode Thermochromic Afterglow for Advanced Dynamic Information Encryption, Adv. Mater., 2024, 2403775, DOI:10.1002/adma.202403775.
- Q. Li, S. Meng, Y. Li, D. Cheng, H. Gu, Z. Zhao, Z. Tang, J. Tan and S. Qu, Surface ionization-induced tunable dynamic phosphorescence colors from carbon dots on paper for dynamic multimode encryption, Carbon, 2022, 195, 191–198, DOI:10.1016/j.carbon.2022.03.063.
- H. Song, X. Liu, B. Wang, Z. Tang and S. Lu, High production-yield solid-state carbon dots with tunable photoluminescence for white/multi-color light-emitting diodes, Sci. Bull., 2019, 64(23), 1788–1794, DOI:10.1016/j.scib.2019.10.006.
- F. Yan, C. Yi, Z. Hao, Y. Wang, M. Xu, K. Zhou, F. Shi and J. Xu, Solid-state carbon dots with orange phosphorescence and tunable fluorescence via in-situ growth in phthalimide crystal matrix, Colloids Surf., A, 2022, 650, 129626, DOI:10.1016/j.colsurfa.2022.129626.
- Z. Song, Y. Shang, Q. Lou, J. Zhu, J. Hu, W. Xu, C. Li, X. Chen, K. Liu and C. X. Shan, et al., A Molecular Engineering Strategy for Achieving Blue Phosphorescent Carbon Dots with Outstanding Efficiency above 50, Adv. Mater., 2022, 35(6), 2207970, DOI:10.1002/adma.202207970.
- K. Jin, X. Ji, T. Yang, J. Zhang, W. Tian, J. Yu, X. Zhang, Z. Chen and J. Zhang, Facile access to photo-switchable, dynamic-optical, multi-colored and solid-state materials from carbon dots and cellulose for photo-rewritable paper and advanced anti-counterfeiting, Chem. Eng. J., 2021, 406, 26794, DOI:10.1016/j.cej.2020.126794.
- J. Liu, Y. Luo, Z. Ran, F. Wang, M. Sun, Y. Luo, J. Zhuang, X. Zhang, B. Lei and Y. Liu, et al., Calcination temperature tuning of RTP and TADF with wide range of emission color from carbon dots confined in Al2O3, Chem. Eng. J., 2023, 474, 145597, DOI:10.1016/j.cej.2023.145597.
- W. Li, W. Zhou, Z. Zhou, H. Zhang, X. Zhang, J. Zhuang, Y. Liu, B. Lei and C. Hu, A Universal Strategy for Activating the Multicolor Room-Temperature Afterglow of Carbon Dots in a Boric Acid Matrix, Angew. Chem., Int. Ed., 2019, 58(22), 7278–7283, DOI:10.1002/anie.201814629.
- W. Shi, R. Wang, J. Liu, F. Peng, R. Tian and C. Lu, Time-dependent Phosphorescence Color of Carbon Dots in Binary Salt Matrices through Activations by Structural Confinement and Defects for Dynamic Information Encryption, Angew. Chem., Int. Ed., 2023, 62(23), e202303063, DOI:10.1002/anie.202303063.
- X. Xu, L. Mo, Y. Li, X. Pan, G. Hu, B. Lei, X. Zhang, M. Zheng, J. Zhuang and Y. Liu, et al., Construction of Carbon Dots with Color-Tunable Aggregation-Induced Emission by Nitrogen-Induced Intramolecular Charge Transfer, Adv. Mater., 2021, 33(49), 2104872, DOI:10.1002/adma.202104872.
- T. Zhang, M. Wang, L. Liu, Z. Li and H. Bi, Visible-Light Manipulated Reversible and Ultralong Phosphorescence of Carbon Dots Through Dynamic Cross-Linking, Adv. Funct. Mater., 2024, 2406672, DOI:10.1002/adfm.202406672.
- B. Xu, J. Li, J. Zhang, H. Ning, X. Fang, J. Shen, H. Zhou, T. Jiang, Z. Gao and X. Meng, et al., Solid-State Fluorescent Carbon Dots with Unprecedented Efficiency from Visible to Near-Infrared Region, Adv. Sci., 2022, 10(4), 2205788, DOI:10.1002/advs.202205788.
- X. Xu, L. Mo, W. Li, Y. Li, B. Lei, X. Zhang, J. Zhuang, C. Hu and Y. Liu, Red, green and blue aggregation-induced emissive carbon dots, Chin. Chem. Lett., 2021, 32(12), 3927–3930, DOI:10.1016/j.cclet.2021.05.056.
- C.-L. Shen, J.-H. Zang, Q. Lou, L.-X. Su, Z. Li, Z.-Y. Liu, L. Dong and C.-X. Shan, In-situ embedding of carbon dots in a trisodium citrate crystal matrix for tunable solid-state fluorescence, Carbon, 2018, 136, 359–368, DOI:10.1016/j.carbon.2018.05.015.
- Y. Xu, Y. Li, Y. Meng and H. Li, Mechanofluorochromic carbon dots under grinding stimulation, Nanoscale, 2020, 12(31), 16433–16437, 10.1039/d0nr02964e.
- K. Zhang, Y. Shi, Y. Jia, P. Li, X. Zhang, X. Feng, L. Zhu, Y. Sun, W. Hu and G. Zhao, Tunable dual fluorescence emissions with high photoluminescence quantum yields modulated by Na ion dispersion method for purely solid state N-doped carbon dots, J. Photochem. Photobiol., A, 2020, 397, 112548, DOI:10.1016/j.jphotochem.2020.112548.
- J. Wang, J. Zheng, Y. Yang, X. Liu, J. Qiu and Y. Tian, Tunable full-color solid-state fluorescent carbon dots for light emitting diodes, Carbon, 2022, 190, 22–31, DOI:10.1016/j.carbon.2022.01.001.
- R. Fu, H. Song, X. Liu, Y. Zhang, G. Xiao, B. Zou, G. I. N. Waterhouse and S. Lu, Disulfide Crosslinking-Induced Aggregation: Towards Solid-State Fluorescent Carbon Dots with Vastly Different Emission Colors, Chin. J. Chem., 2023, 41(9), 1007–1014, DOI:10.1002/cjoc.202200736.
- J. Wang, Q. Li, J. Zheng, Y. Yang, X. Liu and B. N. Xu, B-Codoping Induces High-Efficiency Solid-State Fluorescence and Dual Emission of Yellow/Orange Carbon Dots, ACS Sustainable Chem. Eng., 2021, 9(5), 2224–2236, DOI:10.1021/acssuschemeng.0c07992.
- F. Yan, H. Zhang, J. Xu, Y. Wu, Y. Zang and J. Sun, Color Emission Carbon Dots with Quench-ResixAstant Solid-State Fluorescence for Light-Emitting Diodes, ACS Sustainable Chem. Eng., 2021, 9(10), 3901–3908, DOI:10.1021/acssuschemeng.0c09133.
- D. Ozyurt, S. Shafqat, T. T. Pakkanen, R. K. Hocking, A. Mouritz and B. Fox, Aggregation induced emission transformation of liquid and solid-state N-doped graphene quantum dots, Carbon, 2021, 175, 576–584, DOI:10.1016/j.carbon.2021.01.026.
- W. Wang, Q. Chang, L. Li, J.-A. Li, D. Yue and S. Su, Carbon dots with full-color-tunable room-temperature phosphorescence for photo-stimulated responsive application, J. Lumin., 2023, 263, 120017, DOI:10.1016/j.jlumin.2023.120017.
- J. Liu, H. Zhang, N. Wang, Y. Yu, Y. Cui, J. Li and J. Yu, Template-Modulated Afterglow of Carbon Dots in Zeolites: Room-Temperature Phosphorescence and Thermally Activated Delayed Fluorescence, ACS Mater. Lett., 2019, 1(1), 58–63, DOI:10.1021/acsmaterialslett.9b00073.
- C. Zheng, S. Tao, X. Zhao, C. Kang and B. Yang, Crosslink-Enhanced Emission-Dominated Design Strategy for Constructing Self-Protective Carbonized Polymer Dots With Near-Infrared Room-Temperature Phosphorescence, Angew. Chem., Int. Ed., 2024, e202408516, DOI:10.1002/anie.202408516.
- W. Wang, J.-A. Li, S. Ma, Z. Chai, S. Huang, Y. Zhao, S. Wang, Y. Chen, F. Azad and H. Chen, et al., Color-tunable and high-quantum-yield afterglow of carbon dots by covalent fixation, J. Lumin., 2022, 252, 119399, DOI:10.1016/j.jlumin.2022.119399.
- Z. Z. Ding, C. L. Shen, J. F. Han, G. S. Zheng, Q. C. Ni, R. W. Song, K. K. Liu, J. H. Zang, L. Dong and Q. Lou, et al., In Situ Confining Citric Acid-Derived Carbon Dots for Full-Color Room-Temperature Phosphorescence, Small, 2022, 19(31), 2205916, DOI:10.1002/smll.202205916.
- H. Wu, Y. Kang, S. Jiang, K. Wang, L. Qu and C. Yang, Hectogram-Scale Synthesis of Visible Light Excitable Room Temperature Phosphorescence Carbon Dots, Small, 2024, 2402796, DOI:10.1002/smll.202402796.
- Q. Feng, Z. Xie and M. Zheng, Colour-tunable ultralong-lifetime room temperature phosphorescence with external heavy-atom effect in boron-doped carbon dots, Chem. Eng. J., 2021, 420, 127647, DOI:10.1016/j.cej.2020.127647.
- X. Wang, S. Wang, Y. Huang, L. Huang, J. Sun and Z. Lin, Full-color Persistent Room-temperature Phosphorescence from Carbon Dot Composites Based on a Single Nonaromatic Carbon Source, Chem.–Asian J., 2022, 18(2), e202201027, DOI:10.1002/asia.202201027.
- M. Cao, X. Zhao and X. Gong, Ionic Liquid-Assisted One-Step Large-Scale Synthesis of Cs2ZrCl6:CDs Composites with Tunable Multicolor Afterglow Emission, Adv. Funct. Mater., 2024, 2408333, DOI:10.1002/adfm.202408333.
- Y. Huang and P. Li, Visible-light-excited high efficiency multicolor room temperature phosphorescence of carbon dots in boric acid matrix, Chem. Eng. J., 2024, 480, 148157, DOI:10.1016/j.cej.2023.148157.
- J. Wang, S. Zhang, Y. Li, C. Wu, W. Zhang, H. Zhang, Z. Xie and S. Zhou, Ultra-Broadband Random Laser and White-Light Emissive Carbon Dots/Crystal In-Situ Hybrids, Small, 2022, 18(41), 2203152, DOI:10.1002/smll.202203152.
- H. Wang, B. Shi, H. Yu, S. Yang, G. Nie, S. Wang and W. Chen, Visible light-excited full-color phosphorescent material realized by carbon dots dispersed in polyacrylamide and applied to anti-counterfeiting, Mater. Today Adv., 2023, 20, 100429, DOI:10.1016/j.mtadv.2023.100429.
- F. Yan, Y. Jiang, X. Sun, J. Wei, L. Chen and Y. Zhang, Multicolor carbon dots with concentration-tunable fluorescence and solvent-affected aggregation states for white light-emitting diodes, Nano Res., 2019, 13(1), 52–60, DOI:10.1007/s12274-019-2569-3.
- Y. Liu, H. Yang, C. Ma, S. Luo, M. Xu, Z. Wu, W. Li and S. Liu, Luminescent Transparent Wood Based on Lignin-Derived Carbon Dots as a Building Material for Dual-Channel, Real-Time, and Visual Detection of Formaldehyde Gas, ACS Appl. Mater. Interfaces, 2020, 12(32), 36628–36638, DOI:10.1021/acsami.0c10240.
- J. Wen, Z. Zeng, B. Wang, J. Hong, Y. Chen, J. Zhang, J. Li and J. Jiang, Modulating hydrothermal condition to achieve carbon dots-zeolite composites with multicolor afterglow, Nano Res., 2023, 16(5), 7761–7769, DOI:10.1007/s12274-023-5410-y.
- X. Yao, Y. Ma, X. Miao, S. Li, Y. Du, Y. Cheng, Y. Yang, X. Du, H. Deng and H. Geng, et al., Gram-scale synthesis and optical properties of novel tunable solid-state fluorescent carbon dots with self-quenching-resistance for full-carbon-based w-LEDs, J. Alloys Compd., 2024, 1006, 176200, DOI:10.1016/j.jallcom.2024.176200.
- J. Yang, Y. Liu, J. Wang, S. Wang, X. Zhou and H. Li, Visual multiple color emission of solid-state carbon dots, J. Mater. Chem. C, 2019, 7(25), 7806–7811, 10.1039/c9tc01638d.
- S. Moradi, K. Sadrjavadi, N. Farhadian, L. Hosseinzadeh and M. Shahlaei, Easy synthesis, characterization and cell cytotoxicity of green nano carbon dots using hydrothermal carbonization of Gum Tragacanth and chitosan bio-polymers for bioimaging, J. Mol. Liq., 2018, 259, 284–290, DOI:10.1016/j.molliq.2018.03.054.
- T. N. Jebakumar Immanuel Edison, R. Atchudan, N. Karthik, D. Xiong and Y. R. Lee, Facile hydrothermal synthesis of nitrogen rich blue fluorescent carbon dots for cell bio-imaging of Candida albicans, Process Biochem., 2020, 88, 113–119, DOI:10.1016/j.procbio.2019.10.003.
- Z. M. S. H. Khan, S. Saifi, Shumaila, Z. Aslam, S. A. Khan and M. Zulfequar, A facile one step hydrothermal synthesis of carbon quantum dots for label -free fluorescence sensing approach to detect picric acid in aqueous solution, J. Photochem. Photobiol., 2020, 388, 112201, DOI:10.1016/j.jphotochem.2019.112201.
- M. Sabet and K. Mahdavi, Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollution, Appl. Surf. Sci., 2019, 463, 283–291, DOI:10.1016/j.apsusc.2018.08.223.
- N. Akiya and P. E. Savage, Roles of Water for Chemical Reactions in High-Temperature Water, Chem. Rev., 2002, 102(8), 2725–2750, DOI:10.1021/cr000668w.
- S. Zong, B. Wang, X. Yin, W. Ma, J. Zhang and J. Li, Calcination-controlled fabrication of carbon dots@zeolite composites with multicolor fluorescence and phosphorescence, Nano Res., 2022, 15(10), 9454–9460, DOI:10.1007/s12274-022-4558-1.
- M. Cao, X. Zhao and X. Gong, Ionic Liquid-Assisted Fast Synthesis of Carbon Dots with Strong Fluorescence and Their Tunable Multicolor Emission, Small, 2022, 18(11), 2106683, DOI:10.1002/smll.202106683.
- Z. Wang, J. Shen, B. Xu, Q. Jiang, S. Ming, L. Yan, Z. Gao, X. Wang, C. Zhu and X. Meng, Thermally Driven Amorphous-Crystalline Phase Transition of Carbonized Polymer Dots for Multicolor Room-Temperature Phosphorescence, Adv. Opt. Mater., 2021, 9(16), 2100421, DOI:10.1002/adom.202100421.
- Y. Liu, C. Zheng and B. Yang, Phosphorus and Nitrogen Codoped Carbonized Polymer Dots with Multicolor Room Temperature Phosphorescence for Anticounterfeiting Painting, Langmuir, 2022, 38(27), 8304–8311, DOI:10.1021/acs.langmuir.2c00738.
- P. Zuo, X. Lu, Z. Sun, Y. Guo and H. He, A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots, Microchim. Acta, 2015, 183(2), 519–542, DOI:10.1007/s00604-015-1705-3.
- Y. Zhang, P. Zhuo, H. Yin, Y. Fan, J. Zhang, X. Liu and Z. Chen, Solid-State Fluorescent Carbon Dots with Aggregation-Induced Yellow Emission for White Light-Emitting Diodes with High Luminous Efficiencies, ACS Appl. Mater. Interfaces, 2019, 11(27), 24395–24403, DOI:10.1021/acsami.9b04600.
- C. Liu, R. Wang, B. Wang, Z. Deng, Y. Jin, Y. Kang and J. Chen, Orange, yellow and blue luminescent carbon dots controlled by surface state for multicolor cellular imaging, light emission and illumination, Microchim. Acta, 2018, 185(12), 539, DOI:10.1007/s00604-018-3072-3.
- H. Qi, X. Cui, H. Zhang, Y. Tong, M. Qian, W. Zhou, S. Ding and H. Qi, Rationally Designed Matrix-Free Carbon Dots with Wavelength-Tunable Room-Temperature Phosphorescence, Chem.–Asian J., 2023, 18(6), e202201284, DOI:10.1002/asia.202201284.
- H. Sun, H. Ji, E. Ju, Y. Guan, J. Ren and X. Qu, Synthesis of Fluorinated and Nonfluorinated Graphene Quantum Dots through a New Top-Down Strategy for Long-Time Cellular Imaging, Chem.–Eur. J., 2015, 21(9), 3791–3797, DOI:10.1002/chem.201406345.
- L. Zhao, D. Zhang, X. Wang, Y. Li, Z. Li, H. Wei, B. Yao, G. Ding and Z. Wang, Large-Scale Synthesis of Tunable Fluorescent Carbon Dots Powder for Light-Emitting Diodes and Fingerprint Identification, Molecules, 2023, 28(15), 5917, DOI:10.3390/molecules28155917.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization, Adv. Mater., 2017, 30(1), 1704740, DOI:10.1002/adma.201704740.
- Z. Gan, L. Liu, L. Wang, G. Luo, C. Mo and C. Chang, Bright, stable, and tunable solid-state luminescence of carbon nanodot organogels, Phys. Chem. Chem. Phys., 2018, 20(26), 18089–18096, 10.1039/c8cp02069h.
- X. Liu, W. Liu, K. Zuo, J. Zheng, M. Wang and X. Liu, High Color Stability Blue-to-Violet Room Temperature Phosphorescent Carbon Dot Composites with Ultralong Lifetime for Information Encryption, ACS Sustainable Chem. Eng., 2023, 11(5), 1809–1819, DOI:10.1021/acssuschemeng.2c06094.
- M. Wang, Y. Han, Z. Guo, Z. Huang and W. Yang, N-Doped Carbon Dots Embedded in Silica Nanoparticles with Multicolor Luminescence for Light-Emitting Devices, ACS Appl. Nano Mater., 2021, 4(12), 13625–13632, DOI:10.1021/acsanm.1c03057.
- Z. Wang, N. Jiang, M. Liu, R. Zhang, F. Huang and D. Chen, Bright Electroluminescent White-Light-Emitting Diodes Based on Carbon Dots with Tunable Correlated Color Temperature Enabled by Aggregation, Small, 2021, 17(52), 2104551, DOI:10.1002/smll.202104551.
- Y. Zhu, J. Bai, Z. Huang, G. Yuan, L. Zhang, X. Wang and L. Ren, A universal strategy for preparing carbon quantum dot-based composites with blue and green afterglow luminescence, Mater. Chem. Front., 2021, 5(23), 8161–8170, 10.1039/d1qm01235e.
- S. Cui, B. Wang, Y. Zan, Z. Shen, S. Liu, W. Fang, X. Yan, Y. Li and L. Chen, Colorful, time-dependent carbon dot-based afterglow with ultralong lifetime, Chem. Eng. J., 2022, 431, 133373, DOI:10.1016/j.cej.2021.133373.
- M. H. Mokti, H. A. Tajuddin, Z. Abdullah, S. Hisham, N. N. Mohd Yusof Chan, A. Idris and S. Maher, Rapid and facile synthesis of high-color purity solid-state carbon dots with high yield using a direct heating method, Appl. Phys. A, 2023, 129(6), 399, DOI:10.1007/s00339-023-06674-2.
- Z. Chen, X. Liang, D. He, M. Hu and L. Wen, The synthesis and application of an excitation-dependent ultra-long lifetime room temperature phosphorescence carbon dot composite, New J. Chem., 2023, 47(27), 12688–12696, 10.1039/d3nj01775c.
- M. Cao, Y. Liu, M. Zhu, J. Xia, T. Xuan, D. Jiang, G. Zhou and H. Li, A novel and highly stable dual-emission carbon dots-based phosphor, J. Alloys Compd., 2021, 873, 159819, DOI:10.1016/j.jallcom.2021.159819.
- R. Dai, X. Chen, N. Ouyang and Y. Hu, A pH-controlled synthetic route to violet, green, and orange fluorescent carbon dots for multicolor light-emitting diodes, Chem. Eng. J., 2022, 431, 134172, DOI:10.1016/j.cej.2021.134172.
- Y. Kang, D. Li, R. Dong, H. Zhang, W. Li, X. Zhang, X. Yang and B. Lei, Multicolor carbon dots assembled polyvinyl alcohol with enhanced emission for white light-emitting diode, J. Lumin., 2022, 251, 119164, DOI:10.1016/j.jlumin.2022.119164.
- G. Guo, T. Li, Y. Wang, H. Hu, H. Xing, S. Tang, S. Gao, X. Leng and D. Chen, Aggregation-induced bimodal excitation of nitrogen-doped carbon dots for ratiometric sensing of new coccine and solid-state multicolor lighting, J. Colloid Interface Sci., 2023, 645, 96–106, DOI:10.1016/j.jcis.2023.04.114.
- Y. Cui, W. Sun, Y. Dong, Y. Jiang, J. Zhang, T. Zhao, J. Zhang, Y. Wang, M. Sun and G. Yin, Tunable wavelength of fluorescence and afterglow in super-bright carbon dot-based composite materials, New J. Chem., 2024, 48(14), 6254–6260, 10.1039/d4nj00146j.
- B. Xu, Y. Jia, H. Ning, Q. Teng, C. Li, X. Fang, J. Li, H. Zhou, X. Meng and Z. Gao, et al., Visible Light-Activated Ultralong-Lived Triplet Excitons of Carbon Dots for White-Light Manipulated Anti-Counterfeiting, Small, 2023, 20(1), 2304958, DOI:10.1002/smll.202304958.
- H.-J. Wang, Q. Wang, Q.-B. Hu, W. Tian, P.-X. Zhang, R.-L. Gou, H.-L. Chen and G.-Y. Wan, A novel visible light excited room temperature afterglow luminescent carbon dots/boric acid composite for information encryption and Fe3+ sensing, Microchem. J., 2024, 201, 110580, DOI:10.1016/j.microc.2024.110580.
- K. Zhang, S. Huang, J. Gao, L. Tang, R. He, M. Li and W. Shen, Controllable Afterglow Emission of Single-Mode to Dual-Mode Carbon Dot Composites through Matrix Ratio Adjustment, ChemPhotoChem, 2024, e202400068, DOI:10.1002/cptc.202400068.
- J. Hu, M. Lei, L. Yan, L. Chen, Y. Yang, J. Zheng, X. Liu and B. Xu, Carbon dot-based afterglow composite with tunable room temperature phosphorescence and thermally activated delayed fluorescence and their anti-counterfeiting and encryption application, Chem. Eng. J., 2024, 489, 151245, DOI:10.1016/j.cej.2024.151245.
- W. He, X. Sun and X. Cao, Construction and Multifunctional Applications of Visible-Light-Excited Multicolor Long Afterglow Carbon Dots/Boron Oxide Composites, ACS Sustainable Chem. Eng., 2021, 9(12), 4477–4486, DOI:10.1021/acssuschemeng.0c08652.
- L. Zhang, M. Wu, Z. Wang, H. Guo, L. Wang and M. Wu, Phosphorescence Tuning of Fluorine, Oxygen-Codoped Carbon Dots by Substrate Engineering, ACS Sustainable Chem. Eng., 2021, 9(48), 16262–16269, DOI:10.1021/acssuschemeng.1c05525.
- M. Chen, L. Guan, H. Ma, Y. Zhang, Y. Chang, F. Wang, Z. Liu and X. Li, Spectral Regulation of Carbon Dots and the Realization of Single-Component Solar-Simulated White Light-Emitting Diodes, ACS Photonics, 2023, 10(8), 2730–2738, DOI:10.1021/acsphotonics.3c00412.
- X. Chen, J. Wu, J. Zhang and Z. Zhang, Synthesis of trichromatic carbon dots from a single precursor by solvent effect and its versatile applications, Arabian J. Chem., 2023, 16(4), 104576, DOI:10.1016/j.arabjc.2023.104576.
- B. Xu, Z. Wang, J. Shen, J. Li, Y. Jia, T. Jiang, Z. Gao, X. Wang and X. Meng, Metal–Organic Framework-Activated Full-Color Room-Temperature Phosphorescent Carbon Dots with a Wide Range of Tunable Lifetimes for 4D Coding Applications, J. Phys. Chem. C, 2022, 126(28), 11701–11708, DOI:10.1021/acs.jpcc.2c03471.
- Q. Fu, K. Lu, S. Sun and Z. Dong, Recent advances in fluorescence and afterglow of CDs in matrices, Nanoscale Horiz., 2024, 9(7), 1072–1098, 10.1039/d4nh00093e.
- Q. Han, W. Xu, J. Deng, X. Zhang, J. Li and Z. Peng, Multicolor Carbon Dots for Warm White Light-Emitting Diodes with a High Color Rendering Index of 93.2, ACS Appl. Nano Mater., 2023, 6(18), 16373–16382, DOI:10.1021/acsanm.3c02520.
- F. Liu, S. Xu, P. Xia, H. Yang, Z. Qian, Y. Jiang, Z. Wang, D. Ban and C. Wang, Anhydride-Terminated Solid-State Carbon Dots with Bright Orange Emission Induced by Weak Excitonic Electronic Coupling, ACS Appl. Mater. Interfaces, 2022, 14(4), 5762–5774, DOI:10.1021/acsami.1c18786.
- Y. Wu, L. Zhao, X. Cao, Y. Zhang, X. Jiang, Z. Sun and Y. Zhan, Bright and multicolor emissive carbon dots/organosilicon composite for highly efficient tandem luminescent solar concentrators, Carbon, 2023, 207, 77–85, DOI:10.1016/j.carbon.2023.02.055.
- G. Hu, Y. Wang, S. Zhang, H. Ding, Z. Zhou, J. Wei, X. Li and H. Xiong, Rational synthesis of silane-functionalized carbon dots with high-efficiency full-color solid-state fluorescence for light emitting diodes, Carbon, 2023, 203, 1–10, DOI:10.1016/j.carbon.2022.11.048.
- Y. An, C. Liu, Y. Li, M. Chen, Y. Zheng, H. Tian, R. Shi, X. He and X. Lin, Preparation of Multicolour Solid Fluorescent Carbon Dots for Light-Emitting Diodes Using Phenylethylamine as a Co-Carbonization Agent, Int. J. Mol. Sci., 2022, 23(19), 104576, DOI:10.3390/ijms231911071.
- Z. Sun, F. Yan, J. Xu, H. Zhang and L. Chen, Solvent-controlled synthesis strategy of multicolor emission carbon dots and its applications in sensing and light-emitting devices, Nano Res., 2021, 15(1), 414–422, DOI:10.1007/s12274-021-3495-8.
- S. Tang, D. Chen, Y. Yang, C. Wang, X. Li, Y. Wang, C. Gu and Z. Cao, Mechanisms behind multicolor tunable Near-Infrared triple emission in graphene quantum dots and ratio fluorescent probe for water detection, J. Colloid Interface Sci., 2022, 617, 182–192, DOI:10.1016/j.jcis.2022.02.116.
- X. Cao, J. Chen, Y. Chen, X. Jiang, W. Fan, H. Ma, Z. Sun and Y. Zhan, Simple synthesis of carbon dots/organosilicon composites with tunable solid-state emission and size for accurate latent fingerprint identification, J. Mater. Chem. C, 2024, 12(1), 187–196, 10.1039/d3tc03570k.
- T. Liu, G. Yin, Z. Song, J. Yu, X. Yong, B. Zhang, L. Ai and S. Lu, Solid-State Luminescence in Self-Assembled Chlorosalicylaldehyde-Modified Carbon Dots, ACS Mater. Lett., 2023, 5(3), 846–853, DOI:10.1021/acsmaterialslett.2c01130.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, The fluorescence mechanism of carbon dots, and methods for tuning their emission color: a review, Microchim. Acta, 2019, 186(8), 583, DOI:10.1007/s00604-019-3688-y.
- E. S. D'Angelis do, C. Barbosa, J. R. Corrêa, G. A. Medeiros, G. Barreto, K. G. Magalhães, A. L. de Oliveira, J. Spencer, M. O. Rodrigues and B. A. D. Neto, Carbon Dots (C-dots) from Cow Manure with Impressive Subcellular Selectivity Tuned by Simple Chemical Modification, Chem.–Eur. J., 2015, 21(13), 5055–5060, DOI:10.1002/chem.201406330.
- X. Teng, C. Ma, C. Ge, M. Yan, J. Yang, Y. Zhang, P. C. Morais and H. Bi, Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and l-lysine for bioimaging, J. Mater. Chem. B, 2014, 2(29), 4631–4639, 10.1039/c4tb00368c.
- J. Wang, Y. Yang and X. Liu, Solid-state fluorescent carbon dots: quenching resistance strategies, high quantum efficiency control, multicolor tuning, and applications, Mater. Adv., 2020, 1(9), 3122–3142, 10.1039/d0ma00632g.
- J. Y. Wei, Q. Lou, J. H. Zang, Z. Y. Liu, Y. L. Ye, C. L. Shen, W. B. Zhao, L. Dong and C. X. Shan, Scalable Synthesis of Green Fluorescent Carbon Dot Powders with Unprecedented Efficiency, Adv. Opt. Mater., 2020, 8(7), 1901938, DOI:10.1002/adom.201901938.
- U. Latief, S. U. Islam and M. S. Khan, Rare-earth free solid-state fluorescent carbon-quantum dots: Multi-color emission and its application as optical dual-mode sensor, J. Alloys Compd., 2023, 941, 168985, DOI:10.1016/j.jallcom.2023.168985.
- Q.-h. Chen, S.-l. Mei, W. Yang, W.-l. Zhang, G.-l. Zhang, J.-t. Zhu and R.-q. Guo, Tunable emission of cadmium-free transition metal (Cu, Mn, Ag) co-doped ZnInS/ZnS core-shell quantum dots, Trans. Nonferrous Metals Soc. China, 2018, 28(8), 1611–1617, DOI:10.1016/s1003-6326(18)64803-4.
- J. Tan, Z. Yi, Y. Ye, X. Ren and Q. Li, Achieving red room temperature afterglow carbon dots in composite matrices through chromophore conjugation degree controlling, J. Lumin., 2020, 223, 117267, DOI:10.1016/j.jlumin.2020.117267.
- S. Y. Song, K. K. Liu, X. Mao, Q. Cao, N. Li, W. B. Zhao, Y. Wang, Y. C. Liang, J. H. Zang and X. Li, et al., Colorful Triplet Excitons in Carbon Nanodots for Time Delay Lighting, Adv. Mater., 2023, 35(21), 2212286, DOI:10.1002/adma.202212286.
- J. Xu, Y. Zhang, X. Guo, H. Zhang, Y. Deng and X. Zhao, Aggregation-induced emission solid-state multicolor fluorescent carbon dots for LEDs and fingerprints applications, J. Lumin., 2023, 256, 119625, DOI:10.1016/j.jlumin.2022.119625.
- J. Li and X. Gong, The Emerging Development of Multicolor Carbon Dots, Small, 2022, 18(51), 2205099, DOI:10.1002/smll.202205099.
- H. Ding, R. Zhao, Z.-H. Zhang, J.-J. Yang, Z. Wang, L.-L. Xiao, X.-H. Li, X.-J. He and H.-M. Xiong, Kilogram-scale synthesis of carbon dots with high-efficiency full-color solid-state fluorescence using an aggregation-induced emission strategy, Chem. Eng. J., 2023, 476, 146405, DOI:10.1016/j.cej.2023.146405.
- S. Lu, L. Sui, J. Liu, S. Zhu, A. Chen, M. Jin and B. Yang, Near-Infrared Photoluminescent Polymer–Carbon Nanodots with Two-Photon Fluorescence, Adv. Mater., 2017, 29(15), 1603443, DOI:10.1002/adma.201603443.
- Z. He, Y. Sun, C. Zhang, J. Zhang, S. Liu, K. Zhang and M. Lan, Recent advances of solvent-engineered carbon dots: A review, Carbon, 2023, 204, 76–93, DOI:10.1016/j.carbon.2022.12.052.
- H. Ding, J. S. Wei, P. Zhang, Z. Y. Zhou, Q. Y. Gao and H. M. Xiong, Solvent-Controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths, Small, 2018, 14(22), 1800612, DOI:10.1002/smll.201800612.
- H. Bai, X. Jin, Z. Cheng, H. Zhou, H. Wang, J. Yu, J. Zuo and W. Chen, Highly efficient regulation strategy of fluorescence emission wavelength via designing the structure of carbon dots, Adv. Compos. Hybrid Mater., 2023, 6(2), 62, DOI:10.1007/s42114-023-00641-4.
- Y. An, C. Liu, M. Chen, X. Yin, D. Hou, Y. Zheng, R. Shi, X. He and X. Lin, Solid-State Carbon Dots with Tunable Fluorescence via Surface Substitution: Effect of Alkyl Moieties on Fluorescence Characteristics, ACS Sustainable Chem. Eng., 2022, 11(1), 23–28, DOI:10.1021/acssuschemeng.2c06131.
- M. Ge, S. Han, Y. Ma, J. Li, S. Liu, Z. Chen and S. Li, Sensitive Mechanofluorochromic Carbon Dot-Based AIEgens: Promising Reporting Components for Self-Sensing Plastics, Adv. Opt. Mater., 2021, 9(22), 2101092, DOI:10.1002/adom.202101092.
- C. Kang, S. Tao, F. Yang and B. Yang, Aggregation and luminescence in carbonized polymer dots, Aggregate, 2022, 3(2), e169, DOI:10.1002/agt2.169.
- Y. Yan, L. Xia and L. Ma, Solvent-controlled synthesis of multicolor photoluminescent carbon dots for bioimaging, RSC Adv., 2019, 9(42), 24057–24065, 10.1039/c9ra04241e.
- J. Shi, Y. Zhou, J. Ning, G. Hu, Q. Zhang, Y. Hou and Y. Zhou, Prepared carbon dots from wheat straw for detection of Cu2+ in cells and zebrafish and room temperature phosphorescent anti-counterfeiting, Spectrochim. Acta, Part A, 2022, 281, 121597, DOI:10.1016/j.saa.2022.121597.
- H. Yang, F. Li, C. Zou, Q. Huang and D. Chen, Sulfur-doped carbon quantum dots and derived 3D carbon nanoflowers are effective visible to near infrared fluorescent probes for hydrogen peroxide, Microchim. Acta, 2017, 184(7), 2055–2062, DOI:10.1007/s00604-017-2181-8.
- S. Kiran and R. D. K. Misra, Mechanism of intracellular detection of glucose through nonenzymatic and boronic acid functionalized carbon dots, J. Biomed. Mater. Res., Part A, 2015, 103(9), 2888–2897, DOI:10.1002/jbm.a.35421.
- Y. Xu, M. Wu, X. Z. Feng, X. B. Yin, X. W. He and Y. K. Zhang, Reduced Carbon Dots versus Oxidized Carbon Dots: Photo- and Electrochemiluminescence Investigations for Selected Applications, Chem.–Eur. J., 2013, 19(20), 6282–6288, DOI:10.1002/chem.201204372.
- L. Ai, Z. Song, M. Nie, J. Yu, F. Liu, H. Song, B. Zhang, G. I. N. Waterhouse and S. Lu, Solid-state Fluorescence from Carbon Dots Widely Tunable from Blue to Deep Red through Surface Ligand Modulation, Angew. Chem., Int. Ed., 2023, 62(12), e202217822, DOI:10.1002/anie.202217822.
- Y. Wang, S. Zhou, S. Pan, X. Sun, J. Zhou and H. Li, Color-Tunable Carbon Dots with Aggregation-Induced Emission Constructed by FRET between Surface Luminescence Centers, Adv. Opt. Mater., 2023, 12(3), 2301486, DOI:10.1002/adom.202301486.
- F. Zhou, P. Gu, Z. Luo, H. K. Bisoyi, Y. Ji, Y. Li, Q. Xu, Q. Li and J. Lu, Unexpected organic hydrate luminogens in the solid state, Nat. Commun., 2021, 12(1), 2339, DOI:10.1038/s41467-021-22685-0.
- B. Yuan, S. Guan, X. Sun, X. Li, H. Zeng, Z. Xie, P. Chen and S. Zhou, Highly Efficient Carbon Dots with Reversibly Switchable Green–Red Emissions for Trichromatic White Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2018, 10(18), 16005–16014, DOI:10.1021/acsami.8b02379.
- Y. Chen, H. Lian, Y. Wei, X. He, Y. Chen, B. Wang, Q. Zeng and J. Lin, Concentration-induced multi-colored emissions in carbon dots: origination from triple fluorescent centers, Nanoscale, 2018, 10(14), 6734–6743, 10.1039/c8nr00204e.
- Y. Zhan, B. Shang, M. Chen and L. Wu, One-Step Synthesis of Silica-Coated Carbon Dots with Controllable Solid-State Fluorescence for White Light-Emitting Diodes, Small, 2019, 15(24), 1901161, DOI:10.1002/smll.201901161.
- L. Yang, Q. Zhang, Y. Huang, C. Luo, Z. Quan, H. Li, S. Sun and Y. Xu, A sequential dual-lock strategy for generation of room-temperature phosphorescence of boron doped carbon dots for dynamic anti-counterfeiting, J. Colloid Interface Sci., 2023, 632, 129–139, DOI:10.1016/j.jcis.2022.11.062.
- J. Shao, S. Zhu, H. Liu, Y. Song, S. Tao and B. Yang, Full-Color Emission Polymer Carbon Dots with Quench-Resistant Solid-State Fluorescence, Adv. Sci., 2017, 4(12), 1700395, DOI:10.1002/advs.201700395.
- X. Wang, S. Liuye, X. Ma, S. Cui and S. Pu, Construction of a solid-state fluorescent switching with carbon dots and diarylethene, Dyes Pigm., 2023, 216, 111318, DOI:10.1016/j.dyepig.2023.111318.
- S. Qu, D. Shen, X. Liu, P. Jing, L. Zhang, W. Ji, H. Zhao, X. Fan and H. Zhang, Highly Luminescent Carbon-Nanoparticle-Based Materials: Factors Influencing Photoluminescence Quantum Yield, Part. Part. Syst. Charact., 2014, 31(11), 1175–1182, DOI:10.1002/ppsc.201400055.
- Z. Yuwen, H. Mei, H. Li and S. Pu, A novel diarylethene probe with high selective recognition of CN- and Mg2+and its application, J. Photochem. Photobiol., A, 2020, 401, 112754, DOI:10.1016/j.jphotochem.2020.112754.
- H. Zhao, H. Kang, C. Fan, G. Liu and S. Pu, A new multi-functional fluorescent mercuric ion sensor based on diarylethene with triazole-linked rhodamine B unit, Tetrahedron, 2020, 76(33), 131393, DOI:10.1016/j.tet.2020.131393.
- P. V. Karpach, A. A. Scherbovich, G. T. Vasilyuk, V. I. Stsiapura, A. O. Ayt, V. A. Barachevsky, A. R. Tuktarov, A. A. Khuzin and S. A. Maskevich, Photoinduced Reversible Modulation of Fluorescence of CdSe/ZnS Quantum Dots in Solutions with Diarylethenes, J. Fluoresc., 2019, 29(6), 1311–1320, DOI:10.1007/s10895-019-02455-4.
- X. Wang, Y. Han, W. Li, J. Li, S. Ren, M. Wang, G. Han, J. Yu, Y. Zhang and H. Zhao, Doped Carbon Dots Enable Highly Efficient Multiple-Color Room Temperature Phosphorescence, Adv. Opt. Mater., 2023, 12(7), 2301962, DOI:10.1002/adom.202301962.
- H. y. Wang, L. Zhou, H. m. Yu, X. d. Tang, C. Xing, G. Nie, H. Akafzade, S. y. Wang and W. Chen, Exploration of Room-Temperature Phosphorescence and New Mechanism on Carbon Dots in a Polyacrylamide Platform and their Applications for Anti-Counterfeiting and Information Encryption, Adv. Opt. Mater., 2022, 10(15), 2200678, DOI:10.1002/adom.202200678.
- Z. Han, P. Li, Y. Deng and H. Li, Reversible and color-variable afterglow luminescence of carbon dots triggered by water for multi-level encryption and decryption, Chem. Eng. J., 2021, 415, 128999, DOI:10.1016/j.cej.2021.128999.
- Q. Li, D. Cheng, H. Gu, D. Yang, Y. Li, S. Meng, Y. Zhao, Z. Tang, Y. Zhang and J. Tan, et al., Aggregation-induced color fine-tunable carbon dot phosphorescence covering from green to near-infrared for advanced information encryption, Chem. Eng. J., 2023, 462, 142339, DOI:10.1016/j.cej.2023.142339.
- T. Li, C. Wu, M. Yang, B. Li, X. Yan, X. Zhu, H. Yu, M. Hu and J. Yang, Long-Lived Color-Tunable Room-Temperature Phosphorescence of Boron-Doped Carbon Dots, Langmuir, 2022, 38(7), 2287–2293, DOI:10.1021/acs.langmuir.1c02973.
- C. Li, X. Zhao, C. Li, J. Hu, J. Zhu, Q. Lou, N. Chen, Z. Song, X. Chen and G. Pan, Efficient color-tunable room temperature phosphorescence through confining carbon dots in ionic crystal, J. Alloys Compd., 2023, 948, 169674, DOI:10.1016/j.jallcom.2023.169674.
- J. Tan, Q. Li, S. Meng, Y. Li, J. Yang, Y. Ye, Z. Tang, S. Qu and X. Ren, Time-Dependent Phosphorescence Colors from Carbon Dots for Advanced Dynamic Information Encryption, Adv. Mater., 2021, 33(16), 2006781, DOI:10.1002/adma.202006781.
- Y. Zheng, H. Wei, P. Liang, X. Xu, X. Zhang, H. Li, C. Zhang, C. Hu, X. Zhang and B. Lei, et al., Near-Infrared-Excited Multicolor Afterglow in Carbon Dots-Based Room-Temperature Afterglow Materials, Angew. Chem., Int. Ed., 2021, 60(41), 22253–22259, DOI:10.1002/anie.202108696.
- K. Jin, X. Ji, J. Zhang, Q. Mi, J. Wu and J. Zhang, Colourful organic afterglow materials with super-wide color gamut and scaled processability from cellulose, Mater. Today Chem., 2022, 26, 101179, DOI:10.1016/j.mtchem.2022.101179.
- X. Yu, K. Liu, B. Wang, H. Zhang, Y. Qi and J. Yu, Time-Dependent Polychrome Stereoscopic Luminescence Triggered by Resonance Energy Transfer between Carbon Dots-in-Zeolite Composites and Fluorescence Quantum Dots, Adv. Mater., 2022, 35(6), 2208735, DOI:10.1002/adma.202208735.
- Z. Ran, J. Liu, J. Zhuang, Y. Liu and C. Hu, Multicolor Afterglow from Carbon Dots: Preparation and Mechanism, Small Methods, 2023, 8(1), 2301013, DOI:10.1002/smtd.202301013.
- Y. Zheng, Q. Zhou, Y. Yang, X. Chen, C. Wang, X. Zheng, L. Gao and C. Yang, Full-Color Long-Lived Room Temperature Phosphorescence in Aqueous Environment, Small, 2022, 18(19), 2201223, DOI:10.1002/smll.202201223.
- Y. Zhang, L. Chen, B. Liu, S. Yu, Y. Yang and X. Liu, Multicolor Afterglow Carbon Dots: Luminescence Regulation, Preparation, and Application, Adv. Funct. Mater., 2024, 34(25), 2315366, DOI:10.1002/adfm.202315366.
- X. Yu, K. Liu, H. Zhang, B. Wang, G. Yang, J. Li and J. Yu, Lifetime-Engineered Phosphorescent Carbon Dots-in-Zeolite Composites for Naked-Eye Visible Multiplexing, CCS Chem., 2021, 3(12), 252–264, DOI:10.31635/ccschem.020.202000639.
- C. Zhang, H. Wang, X. Lan, Y.-e. Shi and Z. Wang, Modulating Emission of Nonconventional Luminophores from Nonemissive to Fluorescence and Room-Temperature Phosphorescence via Dehydration-Induced Through-Space Conjugation, J. Phys. Chem. Lett., 2021, 12(5), 1413–1420, DOI:10.1021/acs.jpclett.0c03614.
- Y. Di, W. Liu, S. Shi, T. Wu, M. Wang and X. Liu, One-step synthesis of color-tunable carbon dots-based organic long persistent luminescence materials, Chem. Eng. J., 2024, 479, 147589, DOI:10.1016/j.cej.2023.147589.
- H. Cheng, S. Chen, M. Li, Y. Lu, H. Chen, X. Fang, H. Qiu, W. Wang, C. Jiang and Y. Zheng, Ultra-stable dual-color phosphorescence Carbon-Dot@Silica material for advanced anti-counterfeiting, Dyes Pigm., 2023, 208, 110827, DOI:10.1016/j.dyepig.2022.110827.
- J. Chen, J. Tan, P. Liang, C. Wu, Z. Hou, K. Shen, B. Lei, C. Hu, X. Zhang and J. Zhuang, et al., Dynamic Room Temperature Phosphorescence of Silane-Functionalized Carbon Dots Confining within Silica for Anti-Counterfeiting Applications, Small, 2023, 20(16), 2306323, DOI:10.1002/smll.202306323.
- P. Liang, Y. Zheng, X. Zhang, H. Wei, X. Xu, X. Yang, H. Lin, C. Hu, X. Zhang and B. Lei, et al., Carbon Dots in Hydroxy Fluorides: Achieving Multicolor Long-Wavelength Room-Temperature Phosphorescence and Excellent Stability via Crystal Confinement, Nano Lett., 2022, 22(13), 5127–5136, DOI:10.1021/acs.nanolett.2c00603.
- S. Zong, J. Zhang, X. Yin, J. Li and S. Qu, Time-Dependent and Excitation-Dependent Afterglow Color Evolution from the Assembly of Dual Carbon Dots in Zeolite, Nano Lett., 2024, 24(6), 1859–1866, DOI:10.1021/acs.nanolett.3c03501.
- Q. Su, L. Gan and X. Yang, Achieving room temperature phosphorescence in aqueous phase through rigidifying the triplet state and information encryption, Appl. Surf. Sci., 2021, 566, 150726, DOI:10.1016/j.apsusc.2021.150726.
- D. Lu, K. Lu, H. T. Wen, Z. Wei, A. Bianco, G. G. Wang and H. Y. Zhang, Synthesis of Visible Light Excitable Carbon Dot Phosphor-Al2O3Hybrids for Anti-Counterfeiting and Information Encryption, Small, 2023, 19(31), 2207046, DOI:10.1002/smll.202207046.
- C. Wang, Y. Chen, Y. Xu, G. Ran, Y. He and Q. Song, Aggregation-Induced Room-Temperature Phosphorescence Obtained from Water-Dispersible Carbon Dot-Based Composite Materials, ACS Appl. Mater. Interfaces, 2020, 12(9), 10791–10800, DOI:10.1021/acsami.9b20500.
- Y. Wang, K. Jiang, J. Du, L. Zheng, Y. Li, Z. Li and H. Lin, Green and Near-Infrared Dual-Mode Afterglow of Carbon Dots and Their Applications for Confidential Information Readout, Nano-Micro Lett., 2021, 13(1), 198, DOI:10.1007/s40820-021-00718-z.
- C. Zheng, S. Tao, Y. Liu, C. Kang and B. Yang, Achieving blue water-dispersed room-temperature phosphorescence of carbonized polymer dots through nano-compositing with mesoporous silica, Chin. Chem. Lett., 2022, 33(9), 4213–4218, DOI:10.1016/j.cclet.2022.03.041.
- F. Peng, R. Wang, X. Wang, W. Shi and C. Lu, Thermally activated delayed fluorescence from confined carbon dots activated by the triple synergy effect for advanced information encryption, J. Mater. Chem. C, 2023, 11(31), 10712–10721, 10.1039/d3tc01291c.
- H. Zhang, L. Sun, X. Guo, J. Xu, X. Zhao and Y. Xia, Multicolor fluorescent/room temperature phosphorescent carbon dot composites for information encryption and anti-counterfeiting, Appl. Surf. Sci., 2023, 613, 155945, DOI:10.1016/j.apsusc.2022.155945.
- Q. Lou, N. Chen, J. Zhu, K. Liu, C. Li, Y. Zhu, W. Xu, X. Chen, Z. Song and C. Liang, et al., Thermally Enhanced and Long Lifetime Red TADF Carbon Dots via Multi-Confinement and Phosphorescence Assisted Energy Transfer, Adv. Mater., 2023, 35(20), 2211858, DOI:10.1002/adma.202211858.
- Y. Wu, E. Xue, B. Tian, K. Zheng, J. Liang and W. Wu, Tunable multimodal printable up-/down-conversion nanomaterials for gradient information encryption, Nanoscale, 2022, 14(19), 7137–7145, 10.1039/D2NR01380K.
- Y. Jie, D. Wang, W. Li, P. Dai, Y. Gao, F. Li, J. Li and J. Fang, Green Room-Temperature Phosphorescent Carbon Dots with a Lifetime of around 1 s, ACS Appl. Opt. Mater., 2023, 1(5), 1026–1032, DOI:10.1021/acsaom.3c00079.
- X. Guo, H. Zhang, L. Fu, K. Jian and X. Zhao, Carbon Dot-Based Room-Temperature Phosphorescent Materials for Information Encryption, ACS Appl. Nano Mater., 2023, 6(17), 15597–15605, DOI:10.1021/acsanm.3c02454.
- H. Ding, X.-H. Li, X.-B. Chen, J.-S. Wei, X.-B. Li and H.-M. Xiong, Surface states of carbon dots and their influences on luminescence, J. Appl. Phys., 2020, 127(23), 231101, DOI:10.1063/1.5143819.
- B. Han, X. Lei, D. Li, Q. Liu, Y. Chen, J. Wang and G. He, Shallow Traps in Carbon Nitride Quantum Dots to Achieve 6.47 s Ultralong Lifetime and Wavelength-Tunable Room Temperature Phosphorescence, Adv. Opt. Mater., 2023, 11(8), 2202293, DOI:10.1002/adom.202202293.
- L. Mo, H. Liu, Z. Liu, X. Xu, B. Lei, J. Zhuang, Y. Liu and C. Hu, Cascade Resonance Energy Transfer for the Construction of Nanoparticles with Multicolor Long Afterglow in Aqueous Solutions for Information Encryption and Bioimaging, Adv. Opt. Mater., 2022, 10(10), 2102666, DOI:10.1002/adom.202102666.
- X. Teng, X. Sun, W. Pan, Z. Song and J. Wang, Carbon Dots Confined in Silica Nanoparticles for Triplet-to-Singlet Föster Resonance Energy-Transfer-Induced Delayed Fluorescence, ACS Appl. Nano Mater., 2022, 5(4), 5168–5175, DOI:10.1021/acsanm.2c00208.
- H. Peng, G. Xie, Y. Cao, L. Zhang, X. Yan, X. Zhang, S. Miao, Y. Tao, H. Li and C. Zheng, et al., On-demand modulating afterglow color of water-soluble polymers through phosphorescence FRET for multicolor security printing, Sci. Adv., 2022, 8(15), eabk2925, DOI:10.1126/sciadv.abk2925.
- E. R. Sauvé, C. M. Tonge and Z. M. Hudson, Aggregation-Induced Energy Transfer in Color-Tunable Multiblock Bottlebrush Nanofibers, J. Am. Chem. Soc., 2019, 141(41), 16422–16431, DOI:10.1021/jacs.9b08133.
- L. Ma, Q. Xu, S. Sun, B. Ding, Z. Huang, X. Ma and H. Tian, A Universal Strategy for Tunable Persistent Luminescent Materials via Radiative Energy Transfer, Angew. Chem., Int. Ed., 2022, 61(8), e202115748, DOI:10.1002/anie.202115748.
- L. Yuan, W. Lin, K. Zheng and S. Zhu, FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications, Acc. Chem. Res., 2013, 46(7), 1462–1473, DOI:10.1021/ar300273v.
- A. Cravcenco, M. Hertzog, C. Ye, M. N. Iqbal, U. Mueller, L. Eriksson and K. Börjesson, Multiplicity conversion based on intramolecular triplet-to-singlet energy transfer, Sci. Adv., 2019, 5(9), eaaw5978, DOI:10.1126/sciadv.aaw5978.
- W. Sun, W. Hu, B. Shi and C. Lü, 1,10-Phenanthroline-5-amine derived N-doped carbon dots for long-lived visible-light-activated room temperature phosphorescence in the matrix and information encryption application, J. Lumin., 2023, 263, 120078, DOI:10.1016/j.jlumin.2023.120078.
- K. Jiang, Y. Wang, X. Gao, C. Cai and H. Lin, Facile, Quick, and Gram-Scale Synthesis of Ultralong-Lifetime Room-Temperature-Phosphorescent Carbon Dots by Microwave Irradiation, Angew. Chem., Int. Ed., 2018, 57(21), 6216–6220, DOI:10.1002/anie.201802441.
- X. Wang, Y. Wang, W. Pan, J. Wang and X. Sun, Carbon-Dot-Based Probe Designed to Detect Intracellular pH in Fungal Cells for Building Its Relationship with Intracellular Polysaccharide, ACS Sustainable Chem. Eng., 2021, 9(10), 3718–3726, DOI:10.1021/acssuschemeng.0c08160.
- Q. Dang, Y. Jiang, J. Wang, J. Wang, Q. Zhang, M. Zhang, S. Luo, Y. Xie, K. Pu and Q. Li, et al., Room-Temperature Phosphorescence Resonance Energy Transfer for Construction of Near-Infrared Afterglow Imaging Agents, Adv. Mater., 2020, 32(52), 2006752, DOI:10.1002/adma.202006752.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents, Chem. Soc. Rev., 2020, 49(15), 5110–5139, 10.1039/c9cs00318e.
- M. Jiang, Y. Sun, M. Chen, H. Ji, Y. Liu, R. Qin, X. Li, H. Gao, R. Zhang and L. Zhang, Multicolor luminescence of carbon Dots: From mechanisms to applications, Chem. Eng. J., 2024, 496, 153761, DOI:10.1016/j.cej.2024.153761.
- Q. Fu, K. Zhang, K. Lu, N. Li, S. Sun and Z. Dong, Recent advances in solid-state fluorescent of red carbon dots: A comprehensive review, J. Alloys Compd., 2024, 971, 172688, DOI:10.1016/j.jallcom.2023.172688.
- Z. Hu, H. Dai, W. Zhou, J. Wei, H. Zhang, Z. Ye, Y. Qiu, Y. Chen, Z. Duan and J. Wang, et al., Corrosion resistant solid-state carbon dots@silicalite-1 composite for latent fingerprints detection, J. Alloys Compd., 2021, 889, 161660, DOI:10.1016/j.jallcom.2021.161660.
- Q. Feng, Z. Xie and M. Zheng, Room temperature phosphorescent carbon dots for latent fingerprints detection and in vivo phosphorescence bioimaging, Sens. Actuators, B, 2022, 351, 130976, DOI:10.1016/j.snb.2021.130976.
- A. Sekar, R. Vadivel, R. G. Munuswamy and R. Yadav, Fluorescence spotting of latent sweat fingerprints with zinc oxide carbon dots embedded in a silica gel nanopowder: a green approach, New J. Chem., 2021, 45(37), 17447–17460, 10.1039/d1nj03901f.
- R. Wang, Z. Huang, L. Ding, F. Yang and D. Peng, Carbon Dot Powders with Cross-Linking-Based Long-Wavelength Emission for Multicolor Imaging of Latent Fingerprints, ACS Appl. Nano Mater., 2022, 5(2), 2214–2221, DOI:10.1021/acsanm.1c03901.
- T. Li, Q. Yu, Z. Du, J. Gao, D. Lu, R. Liang and G. Sun, A preparation strategy for multicolor carbon dots embedded in silicone for latent fingermarks and detection of AcO, New J. Chem., 2023, 47(25), 11903–11911, 10.1039/d3nj01688a.
- L. Gan, Y. Zhu and X. Yang, Multiple-color room-temperature phosphorescence regulated by graphitization and carbonyls, Chem. Eng. J., 2023, 459, 141635, DOI:10.1016/j.cej.2023.141635.
- D. Hong, X.-y. Xiang, Q.-j. Zhou, Y.-L. Zhu, Y.-p. Wu, J. Yang, S.-h. Chen and K.-j. Tan, One-pot synthesis of aggregation-induced multi-emission and solid-state room-temperature-phosphorescence carbon dots, Dyes Pigm., 2023, 217, 111395, DOI:10.1016/j.dyepig.2023.111395.
- J. Gao, X. Wu, X. Jiang, M. Li, R. He and W. Shen, Achieving purple light excitable high-efficiency temperature-responsive dual- and single-mode afterglow in carbon dots, Carbon, 2023, 208, 365–373, DOI:10.1016/j.carbon.2023.03.048.
- L. Yang, Q. Zhang, Y. Ma, H. Li, S. Sun and Y. Xu, Stimulus-responsive room-temperature phosphorescence with tunable color for time-resolved advanced dynamic anti-counterfeiting, Chem. Eng. J., 2024, 490, 151679, DOI:10.1016/j.cej.2024.151679.
- H. Hu, J. Li and X. Gong, Hour-Level Persistent Multicolor Phosphorescence Enabled by Carbon Dot-Based Nanocomposites Through a Multi-Confinement-Based Approach, Small, 2023, 2308457, DOI:10.1002/smll.202308457.
- Y. Zhang, M. Li and S. Lu, Rational Design of Covalent Bond Engineered Encapsulation Structure toward Efficient, Long-Lived Multicolored Phosphorescent Carbon Dots, Small, 2022, 19(31), 2206080, DOI:10.1002/smll.202206080.
- C. Ji, W. Xu, Q. Han, T. Zhao, J. Deng and Z. Peng, Light of carbon: Recent advancements of carbon dots for LEDs, Nano Energy, 2023, 114, 108623, DOI:10.1016/j.nanoen.2023.108623.
- J. Zhu, J. Hu, Q. Hu, X. Zhang, E. V. Ushakova, K. Liu, S. Wang, X. Chen, C. Shan and A. L. Rogach, et al., White Light Afterglow in Carbon Dots Achieved via Synergy between the Room-Temperature Phosphorescence and the Delayed Fluorescence, Small, 2021, 18(1), 2105414, DOI:10.1002/smll.202105415.
- Q. Han, W. Xu, C. Ji, G. Xiong, C. Shi, D. Zhang, W. Shi, Y. Jiang and Z. Peng, Multicolor and Single-Component White Light-Emitting Carbon Dots from a Single Precursor for Light-Emitting Diodes, ACS Appl. Nano Mater., 2022, 5(10), 15914–15924, DOI:10.1021/acsanm.2c04130.
- J. Wang, J. Zheng, P. He, Q. Li, Y. Yang, X. Liu, J. Yan and Y. Zhang, Realization of solid-state red fluorescence and concentration-induced multicolor emission from N, B co-doped carbon dots, Front. Mater. Sci., 2023, 17(2), 230648, DOI:10.1007/s11706-023-0648-6.
- Y. Xie, X. Geng, J. Gao, W. Shi, Z. Zhou, H. Wang, D. Zhang, B. Deng and R. Yu, Synthesis of carbon dots@Mg(OH)2 solid-state composites with blue, red emitting for horticultural application, J. Alloys Compd., 2021, 873, 159663, DOI:10.1016/j.jallcom.2021.159663.
- D. Xu, Q. Lin and H. T. Chang, Recent Advances and Sensing Applications of Carbon Dots, Small Methods, 2019, 4(4), 1900387, DOI:10.1002/smtd.201900387.
- M. Feng, X. Zhang and Y. Huang, Developing oxygen vacancy-rich CuMn2O4/carbon dots dual-function nanozymes via Chan-Lam coupling reaction for the colorimetric/fluorescent determination of D-penicillamine, Biosens. Bioelectron., 2025, 267, 116864, DOI:10.1016/j.bios.2024.116864.
- K. Zhang, Q. Fu, S. Sun, Z. Dong and M. Yue, Photoluminescent Multicolor Carbon Dots for UV Detection and Dynamic Anticounterfeiting, ACS Appl. Mater. Interfaces, 2024, 16(39), 52833–52841, DOI:10.1021/acsami.4c11417.
- H. Chang, Y. Park, K. Kim, C. Han, Y. Yoon, W. Yoo, J. Yoo, D. Lee, H. Han and K. Kim, et al., Room-temperature phosphorescence of defect-engineered silica nanoparticles for high-contrast afterglow bioimaging, Chem. Eng. J., 2024, 493, 152529, DOI:10.1016/j.cej.2024.152529.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |