Advancing paper-based sensors with MXenes and MOFs: exploring cutting-edge innovations
Sepehr
Larijani
 a,
Atefeh
Zarepour
b,
Arezoo
Khosravi
c,
Siavash
Iravani
a,
Atefeh
Zarepour
b,
Arezoo
Khosravi
c,
Siavash
Iravani
 *d,
Mahnaz
Eskandari
*a and
Ali
Zarrabi
*d,
Mahnaz
Eskandari
*a and
Ali
Zarrabi
 *ef
*ef
aDepartment of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran. E-mail: eskandarim@aut.ac.ir
bDepartment of Research Analytics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600077, India
cDepartment of Genetics and Bioengineering, Faculty of Engineering and Natural Sciences, Istanbul Okan University, Istanbul 34959, Turkey
dIndependent Researcher, W. Nazar ST, Boostan Ave, Isfahan, Iran. E-mail: siavashira@gmail.com
eDepartment of Biomedical Engineering, Faculty of Engineering and Natural Sciences, Istinye University, Istanbul 34396, Turkey. E-mail: alizarrabi@gmail.com
fGraduate School of Biotechnology and Bioengineering, Yuan Ze University, Taoyuan 320315, Taiwan
First published on 12th November 2024
Abstract
MXenes and metal–organic frameworks (MOFs) are emerging as promising materials for integration into paper-based sensors (PSs), offering unique properties that can enhance sensor performance in various applications. MXenes, with their high conductivity and large surface area, and MOFs, known for their tunable porosity and chemical functionalities, provide distinct advantages to PSs. By leveraging the exceptional properties of MXenes and MOFs, researchers can develop PSs with improved sensitivity, selectivity, and stability, paving the way for advanced sensing platforms with diverse capabilities in environmental monitoring, healthcare diagnostics, and beyond. However, challenges still exist for incorporating MXenes and MOFs into PSs, including sensitivity, stability, interference, and scalability. Addressing these challenges is crucial for optimizing sensor performance and reliability. Herein, recent developments pertaining to the applications of MXenes and MOFs in PSs are discussed, focusing on challenges and future perspectives. By examining the unique properties of these materials, exploring innovative sensor designs, and discussing potential solutions to current challenges, this review seeks to pave the way for the development of next-generation PSs with enhanced sensitivity, selectivity, and reliability.
1 Introduction
Paper-based sensors (PSs) have emerged as a cost-effective, portable, and versatile solution for various sensing applications. Leveraging the simplicity and ubiquity of paper substrates, these sensors offer advantages such as low cost, portability, ease of use, biodegradability, and versatility in detecting a wide range of analytes.1,2 However, challenges like sensitivity limitations, stability concerns, and interference issues pose hurdles that need to be addressed for optimal sensor performance and reliability.3 Looking ahead, the future of PSs has promising prospects for enhanced sensitivity, multiplexed detection capabilities, integration with internet of things (IoT) platforms, point-of-care diagnostics, and environmental monitoring applications. Continued research efforts aim to improve sensor performance, expand their utility in complex sample analysis, enable real-time data monitoring, provide rapid healthcare testing solutions, and enhance on-site detection of pollutants.4–6One notable innovation is the development of flexible and wearable PSs, enabling real-time monitoring of various analytes directly on the skin or clothing. These wearable sensors offer non-invasive, convenient, and continuous monitoring capabilities for applications in healthcare, sports performance tracking, and environmental sensing.7,8 Another significant innovation is the integration of microfluidics into PSs, allowing for precise sample handling, controlled fluid flow, and multiplexed detection of multiple analytes on a single platform. This advancement has expanded the utility of PSs in point-of-care diagnostics, food safety testing, and environmental monitoring, providing rapid and cost-effective solutions for complex analytical challenges.9 Additionally, the incorporation of smartphone-based readout systems and cloud connectivity has enhanced data collection, analysis, and sharing capabilities of PSs. By leveraging smartphone cameras, apps, and cloud services, users can access real-time sensor data, perform on-the-spot analysis, and share results remotely, enhancing the accessibility and usability of PSs in various settings. These innovations continue to drive the evolution of PSs, offering versatile, portable, and user-friendly sensing solutions for a wide range of applications.10
MXenes are a family of two-dimensional (2D) nanomaterials characterized by their layered structure and composition of transition metal carbides or nitrides. The general chemical formula for MXenes can be represented as Mn+1XnTx, where M represents an early transition metal (such as titanium, tantalum, or niobium), X is carbon and/or nitrogen, T denotes surface terminations (such as hydroxyl, oxygen, or fluorine), and n corresponds to the number of carbon/nitrogen layers in the MXene structure that varies between 1 and 4.11 The layered structure of MXenes consists of transition metal layers interleaved with functionalized X-containing layers, resulting in a unique structure that imparts exceptional properties to these materials. The presence of terminations on the surface of MXenes influences their physicochemical properties, such as conductivity, surface area, and interlayer spacing, making them highly tunable for various applications in sensor technology. By selectively etching the A layer from MAX phases (precursor materials for MXenes), researchers can obtain MXene nanosheets with tailored properties suited for specific sensor applications. The versatile structure and chemical composition of MXenes allow for customization to meet the requirements of different sensing platforms, highlighting their potential as key components in the development of advanced sensing technologies.12–15 One key application of MXenes in PSs is their ability to improve the conductivity and surface area of the sensing platform.16 These properties enable efficient electron transfer and enhanced adsorption of analytes, leading to increased sensor sensitivity and detection capabilities. By functionalizing paper substrates with MXenes, researchers can create sensors that exhibit superior performance in detecting various target molecules, making them valuable tools for environmental monitoring, healthcare diagnostics, and food safety applications. Moreover, MXenes play a crucial role in enhancing the stability and durability of PSs. Their robust nature and resistance to environmental factors make them ideal candidates for improving sensor reliability over prolonged periods. By incorporating MXenes into sensor designs, researchers can overcome challenges related to stability and ensure consistent performance under varying conditions, thus expanding the practicality and utility of paper-based sensing platforms.17
MOFs are a class of porous materials composed of metal ions or clusters connected by organic ligands, forming crystalline structures with high surface areas and tunable properties. The properties and structure of MOFs contribute to their versatility and suitability for various applications, including in sensor technology.18–20 One key property of MOFs is their exceptional porosity, which arises from the arrangement of metal ions and organic linkers in a three-dimensional network. This porous structure provides MOFs with a large surface area, enabling efficient adsorption of gases, liquids, or analytes. The tunable nature of MOFs allows researchers to modify the pore sizes and chemical functionalities, tailoring them for specific applications in gas storage, separation, catalysis, and sensing.21 Additionally, the modular design of MOFs, with metal clusters serving as nodes and organic linkers as struts, offers flexibility in controlling the physical and chemical properties of the material. The choice of metal ions, ligands, and synthesis conditions allows for the precise tuning of MOF properties such as pore size, surface area, thermal stability, and guest molecule interactions.22 This versatility in structure and composition makes MOFs highly adaptable for designing sensors with customized functionalities and performance characteristics. When integrated into PSs, MOFs provide a range of advantages that enhance sensor performance. The high surface area of MOFs allows for efficient analyte adsorption, leading to enhanced sensitivity and selectivity in detection.23 By functionalizing paper substrates with MOFs, researchers can design sensors capable of capturing target molecules with high affinity, enabling precise and reliable analysis in various fields, from environmental monitoring to healthcare diagnostics. Furthermore, the tunable nature of MOFs allows for the design of sensors tailored to specific analytes of interest. By selecting appropriate metal ions and organic linkers, researchers can customize the properties of MOFs to selectively bind to target molecules, improving the sensor's specificity and accuracy. This versatility in MOF design opens up a wide range of possibilities for developing PSs with tailored capabilities for diverse sensing applications.24,25
Both MXenes and MOFs contribute unique properties that can be leveraged in the design of PSs. MXenes are recognized for their excellent electrical conductivity, mechanical stability, and ability to facilitate electron transfer, making them ideal candidates for the development of sensitive and rapid detection methods. On the other hand, MOFs possess high surface areas and tunable porosity, which enable them to capture and selectively adsorb target analytes, thereby improving the sensitivity and specificity of sensors. This review aims to explore the potential and challenges of using MXenes and MOFs in PSs. By examining the integration of advanced materials like MXenes and MOFs into paper substrates, researchers seek to enhance sensor performance, sensitivity, and selectivity for various applications. The review will address challenges such as material compatibility, scalability, stability, and integration dynamics that impact the successful deployment of MXene- and MOF-based PSs. Additionally, it will discuss future perspectives, focusing on advancing sensor technology through improved sensitivity, multiplexed detection capabilities, IoT integration, point-of-care diagnostics, and environmental monitoring applications. By shedding light on the opportunities and obstacles associated with MXene- and MOF-enhanced PSs, this review aims to guide further research and innovation in the field, leading to the development of next-generation sensing platforms with enhanced capabilities and functionalities.
2 Design and preparation of PSs
2.1 Design of PSs
The PS design is crucial for its intended application, leading to the creation of both 2D and 3D designs. These designs incorporate hydrophilic channels and hydrophobic barriers within specific regions of the paper.26LFAs are frequently based on antibodies to ensure significant binding specificity, commonly seen in home pregnancy tests and Covid-19 tests. Unlike DSAs, LFAs are more intricate devices, consisting of porous membranes with distinct components like a sample pad, conjugate pad, test line, control line, and absorbent pad.4 Conducting a test with LFAs involves adding the sample solution to the sample pad, allowing it to diffuse through the membrane and interact precisely with a labeled conjugate antibody and a capture antibody. When the sample contains the antigen being targeted, a colored band appears on the strip due to the specific affinities between the labeled antibody and the capture antibody. Recent advancements in LFAs have integrated enzymes, DNA, and nanoparticles to enhance their detection limits, as reported by Li et al.28 These improvements have expanded the capabilities of LFAs, allowing for more sensitive and accurate detection of various substances. LFAs have found diverse applications in detecting pollutants, toxins, pathogens, and diseases, including the crucial role they play in Covid-19 detection. The adaptability and versatility of LFAs have made them indispensable tools in various fields, contributing significantly to rapid and reliable diagnostic processes.4,29 Notably, LFAs are characterized by their specificity, affordability, ease of use, and independence from additional instruments.30 However, they are limited to providing yes or no detection (qualitative analysis) due to their specificity for detecting a single analyte, resulting in lower detection limits compared to established clinical methods like ELISA. To address the limitations of LFAs and DSAs, current developments in PSs aim to build upon the strengths of both methods. These advancements focus on improving the sensors to enable quantitative, sensitive, and multi-analyte assays. The objective is to create devices that are not only simple to operate and cost-effective but also capable of providing more comprehensive and accurate results. The integration of enhanced quantitative capabilities, sensitivity, and the ability to perform multi-analyte assays in PSs holds great promise for various applications.4,31 By overcoming the drawbacks of traditional LFAs and DSAs, these innovative sensors are poised to help develop diagnostic processes, offering efficient and reliable solutions for a wide range of analytical needs. The continuous evolution of PSs underscores the importance of ongoing research and development efforts in this field.
2.2 Physical patterning
Paper cutting stands out as one of the oldest and simplest methods for channel fabrication on paper, whether done manually with tools like knives or scissors or through advanced techniques like laser cutting. To reinforce the paper channels and ensure stability, materials like glass, plastic, or tape are often used for backing.36,37 A recent development in channel fabrication involves a parafilm-based cutting technique, where a parafilm is fused with paper through hot pressing to create infused and laminated paper layers. Laser cutting is then utilized to etch channels onto the laminated paper, allowing for the creation of hydrophilic channels by binding plain paper within the etched areas.38 In the plotting technique, hydrophobic inks are used to create barriers on paper using pens filled with polymer inks, correction pens, or permanent markers. This method requires optimization of ink viscosity and plotter head pressure for effective penetration into the paper thickness.39 While the plotting technique is cost-effective, it may be time-consuming and limited in pattern resolution, making it suitable for small-batch device fabrication. To improve throughput, desktop pen plotters with multiple pen holders or digital craft plotters with drawing pens can be utilized.40 Various manual pen techniques, including acrylate pens and 3D pens, provide additional options for rapid prototyping of raw devices, showcasing the versatility and adaptability of paper-based fabrication methods.412.3 Chemical patterning
Chemical patterning of PSs encompasses a wide array of techniques, including wax printing, inkjet printing, flexographic printing, screen printing, laser printing, photolithography, plasma treatment, chemical vapor deposition (CVD), and ink stamping.4 Each method offers unique advantages and challenges for creating precise patterns and functional elements on paper surfaces. Wax printing is a commonly used method due to its simplicity, rapidity, and cost-effectiveness. Molten wax is printed on paper surfaces using solid ink printers, allowing the wax to penetrate the paper thickness upon heating. Strategies to enhance printing resolution include utilizing hot lamination presses for dual-sided wax printing and employing sodium periodate solutions for pattern miniaturization.42,43 In inkjet printing, a solvent is used in place of ink to create hydrophobic barriers on paper. By printing hydrophobic polymers or inks directly onto the paper surface, channels and patterns can be formed, facilitating the fabrication of complete PSs in a single process.44,45 Flexographic printing involves sequentially integrating print units to apply hydrophobic inks onto paper, offering benefits for mass production of PSs in a roll-to-roll manner. This technique allows for the printing of reagents during a single printing process, enhancing efficiency and scalability.46 Screen printing utilizes designed screens to press hydrophobic inks or polymers onto paper, creating defined patterns for sensor applications. While versatile in accommodating various hydrophobic inks, the need for new screens for each design pattern limits its suitability for rapid prototyping with varying designs.47In laser printing, toner is applied to a paper surface which is heated to melt the toner, allowing it to spread through the paper thickness. This technique, although requiring longer baking times and higher temperatures, offers resistance to certain solvents and surfactants. Laser printing is a cost-effective method commonly available in laboratories equipped with laser printers and ovens.4 Photolithography, known for its precision and high resolution, involves applying a negative photoresist to paper, exposing it to UV light through a photomask, and developing the paper to remove the unexposed photoresist. While effective for precise patterning, photolithography requires specialized equipment like high UV-light sources and photoresists, making it more suitable for labs with existing facilities.4 Plasma treatment can be used to create hydrophobic patterns on PSs by selectively removing hydrophobic layers with plasma exposure through a mask. This process involves the use of chemical solutions like octadecyl trichlorosilane and alkyl ketene dimer, along with equipment such as plasma cleaners and handheld corona generators.48 CVD enables the creation of hydrophobic paper patterns using a physical mask. By depositing hydrophobic monomers like dichloro-[2,2]-paracyclophane on exposed paper regions through polymerization, CVD offers precise control over pattern formation. Additionally, polyvinyl tape can act as a mask to create defined patterns on paper, further enhancing the versatility of CVD in fabricating PSs.49 Stamping techniques offer a straightforward and cost-effective method for patterning PSs.50 These techniques involve using a stamp to apply hydrophobic ink onto paper, with variations in ink composition leading to different application methods. Stamping techniques can be classified into two main categories based on the type of ink used: room temperature stamping with inks that cure or dry upon application, and heated stamping with molten polymers or waxes that solidify upon cooling. The primary advantages of stamping techniques include their quick application, low cost, and minimal resource requirements. However, drawbacks include the necessity of creating a new stamp for each unique pattern, as well as potential issues with precision and resolution compared to other patterning techniques. Optimization of the stamping procedure and ink selection is crucial to ensure the creation of clear and precise hydrophobic patterns on paper without blurring. One example of a stamping technique is the use of a polydimethylsiloxane (PDMS)-based rubber stamp or indelible ink to imprint patterns on paper. By utilizing these stamps with hydrophobic ink, researchers can efficiently create well-defined patterns on paper surfaces for various sensor applications. These stamping methods provide a practical and accessible approach for patterning PSs, offering a balance between simplicity, cost-effectiveness, and pattern clarity.50,51 Overall, plasma treatment, CVD, laser printing, ink stamping, and photolithography offer distinct advantages and applications in the chemical patterning of PSs. These diverse methods contribute to the advancement of sensor fabrication, enabling precise control over channel design and functionality for various analytical needs.
3 MXenes and MOFs in PSs: properties and advantages
The interesting features of paper including flexibility, cheapness, porous structure, and high surface-to-volume ratio introduce it as a suitable platform for sensing and biosensing applications. Indeed, PSs have the capability of detecting different types of analytes (like biomarkers, DNA, drugs, metals, and gases) using various methods such as optical and electrochemical methods.52 These types of sensors are composed of cellulose as the main compound that is functionalized with other compounds to improve its sensitivity and selectivity, and among the newest types of them are MXenes and MOFs.3.1 MXenes for enhancing sensing capabilities
MXenes exhibit interesting features like electrical conductivity, optical and mechanical properties, thermo-sensitive nature, stability, large surface area, and biocompatibility that make them one of the ideal candidates for sensor applications.53 These properties are tunable by altering their chemical composition, the value of n, and their functional groups.54 MXenes offer a range of unique electrochemical and surface properties, enhancing their utility in sensors by improving charge transfer rates, minimizing background noise, and strengthening interactions with biomolecules. The 2D structure and high conductivity of MXenes, like Ti3C2Tx, facilitate swift charge transfer, which is crucial in electrochemical sensing. This occurs because MXenes have a layered architecture that supports rapid ion and electron mobility, critical for applications requiring fast electrochemical responses. Their surface can also be functionalized with groups like hydroxyls and oxygen, increasing interaction with aqueous environments and further improving charge transport efficiency across the sensor's interface, leading to faster response times and higher sensitivity.55 Conventional 2D materials like graphene and MoS2 require intricate synthesis methods and conditions, which often result in low hydrophilicity and pose challenges for effective functionalization. In contrast, MXenes possess not only the beneficial attributes of traditional 2D materials—such as exceptional conductivity, favorable mechanical characteristics, and substantial specific surface area—but also exhibit significant hydrophilicity, adjustable layer spacing, and a variety of surface functional groups. Their unique surface chemical properties facilitate easy functionalization and ensure good biocompatibility.56 The structure and surface properties of MXenes, combined with their inherent hydrophilicity and large surface area, lead to better signal transduction and less interference from noise. For instance, the lamellar structure of Ti3C2Tx MXenes allows them to interact closely with the analyte, which enhances signal strength and minimizes background noise. This quality is particularly beneficial in optical and electrochemical sensors, where clear, consistent signals are essential for detecting low-concentration biomarkers.57 Moreover, MXenes can be engineered to improve their interaction with specific biomolecules, making them exceptionally useful in biomarker detection. Functional groups on MXene surfaces (like –OH, –F, and –O) can serve as binding sites for antibodies, aptamers, or other biomolecules, forming strong, stable complexes with biomarkers. This stability supports long-term, repeatable sensing and allows MXenes to be customized for different biomarkers, increasing sensitivity and specificity across various biosensing applications.58 The surface functional groups could also affect mechanical features of MXenes and making them suitable for specific types of sensors. Indeed, MXenes with –O– terminations display higher stiffness levels compared with those having –OH or –F groups. This variation in stiffness is largely due to differences in lattice constants: typically, OH or F-terminated MXenes feature larger lattice parameters compared to their O-terminated counterparts. Additionally, surface-functionalized MXenes tend to be more flexible than those without functional groups. Moreover, in functionalized MXenes, a reduction in the value of n generally leads to an increase in the hardness and strength of the Mn+1XnTx structure. The unique combination of MXenes' mechanical robustness, flexibility, metal-like conductivity, and advanced surface characteristics renders them effective as transducing materials for a variety of sensor types, including optical, electrochemical, and gas sensors. MXenes' broad optoelectronic properties—including high optical absorption, varied work functions, and adaptable electronic band structures—make them versatile for applications in optoelectronic devices. These features enable MXenes to efficiently manage charge production and transport, allowing these devices to detect molecular interactions and biological reactions with high sensitivity, significantly enhancing the precision and functionality of sensing technologies.58 These properties make MXenes highly versatile and powerful in developing sensitive, robust, and high-performance sensors for medical diagnostics and environmental monitoring applications. Their multifaceted benefits underscore the growing interest in furthering MXene-based sensing research and development in advanced sensing platforms.Since their discovery, MXenes have been utilized in various types of sensors such as electrochemical sensors, gas sensors, strain/stress sensors, optical sensors, etc. to detect different types of analytes like disease biomarkers and environmental pollutants.59 They could be used in the structure of PSs to enhance their sensing ability. For example, Wang et al., synthesized a paper-based electrochemical sensor with the ability of detecting cardiac troponin I (cTnI), a biomarker of acute myocardial infarction (AMI), via modifying the working electrode's surface with aminosilane functionalized MXene (f-Ti3C2) that could improve the conductivity of the sensor and provide a widespread surface for the immobilization of anti-cTnl antibody. The results of electrochemical impedance spectroscopy (EIS) showed a notable decrease in the impedance of the surface after modification with MXenes, which resulted in a higher electrical conductivity of the surface and an enhanced electron transfer between the surface and sample. Furthermore, the modified electrode exhibited a higher sensitivity compared to other previously studied platforms, with the linear range between 5 and 100 ng mL−1 and detection limit of about 0.58 ng mL−1. It also showed high reproducibility and excellent selectivity for the detection of cTnI target analyte in a mixture of non-specific proteins.60
Tissue paper is one of the materials used as a substrate for paper-based sensing due to its good absorption capacity and gas permeability.52 Shu et al. developed a paper-based pressure sensor using MXene/tissue paper as the pressure-sensing layer and screen-printed silver paste to transmit the produced signal. Tissue paper was modified by adding an aqueous dispersion of Ti3C2Tx to the paper. Infiltration of the MXene dispersion, followed by a drying step at 70 °C for 20 minutes, resulted in a conductive sensitive layer which was used as a pressure sensor. The fabricated sensor exhibited fast response and recovery time (30 ms) in a wide linear range (0–100 kPa), with a limit of detection of about 0.89 Pa (that confirmed its high sensitivity).61
The hydrophilic nature of MXenes and the presence of functional groups such as hydroxyl in their structure enables them to be well-dispersed in water. As a result, MXene molecules are more likely to evenly cover the dipped paper in this method.62 For instance, Ma et al. used the dip-drying method to fabricate pressure sensors. In this method, the paper substrate, which consisted of filter and A4 papers, was immersed in MXene solutions with different concentrations. Then the papers were dried in a vacuum to obtain normal MXene (P-MXene) coated paper, or in an air-blast oven to obtain oxidized MXene (O-MXene) coated paper (Fig. 1A). Oxidation of MXenes is achieved by heating in air which results in the transformation of Ti3C2Tx into TiO2 nanocrystals. It was also observed that these two modification methods affect the sensitivity of the sensor so that the sensor with an O-MXene coating had a sensitivity of about 28.43 kPa−1 in the range of 0–1.9 kPa while the sensor with P-MXene had a sensitivity of 1.33 kPa−1 in the same range (Fig. 1B). Furthermore, it was shown that the degree of oxidation is a factor that can be used to alter the sensitivity and response of the sensor, with higher degrees of oxidation leading to lower sensitivity and response.63
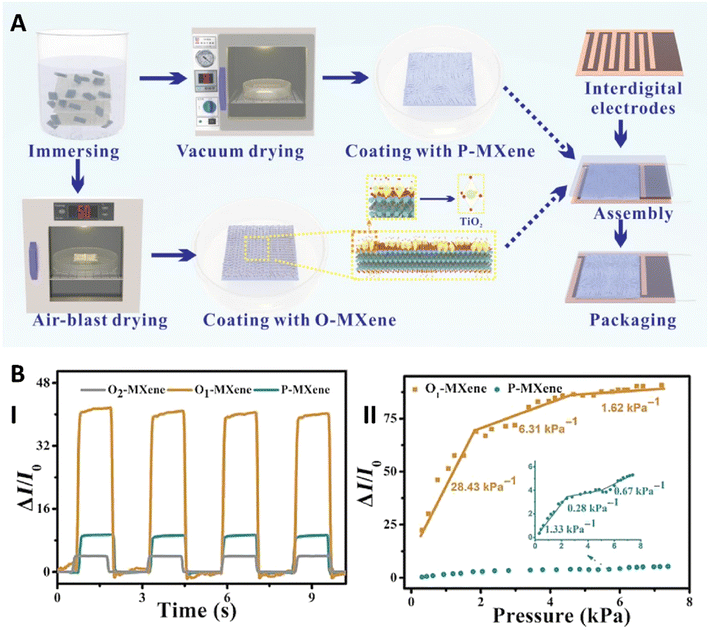 | ||
| Fig. 1 (A) Production of pristine MXene (P-MXene) and oxidized MXene (O-MXene) coatings for a PS using vacuum drying and air-blast drying, respectively and (B) the effect of modification on the sensitivity of the sensor, with higher sensitivity observed in partially oxidized MXene (O1-MXene) (I) and a decrease in the sensor response on increasing the degree of oxidation (O2-MXene) (II). Reprinted from ref. 63 under the terms of the Creative Commons CC BY License. Copyright 2022, Wiley. | ||
MXenes are widely studied as a new emerging sensory material with the ability of improving the sensitivity and selectivity of sensors.64 They possess high surface area and different functional groups that provide a good potency for immobilizing various biological compounds such as enzymes,65,66 DNA nanostructures,67 proteins, and antibodies.68,69 Therefore, application of MXenes in the structure of sensors could enhance their sensitivity and selectivity. For instance, Suk-in et al.70 designed a dual electrochemical/colorimetric sensor on a microfluidic paper-based platform to detect carbaryl that contained silver nanoparticle/MXene as the working electrode with enhanced surface area and conductivity. It was observed that using MXenes in this sensor resulted in a higher sensitivity for detection due to the enhancement of electron transfer between the solution and electrode. This MXene-modified electrode showed a sensitivity of about 0.4667 μA μM−1 cm−1 and limit of detection (LOD) of 0.01 μM, and could detect low amounts of carbaryl.
Wang et al.71 developed a triboelectric nanosensor (TENS) based on MXene/bacterial cellulose paper to detect Cu2+, Zn2+, and Cr3+. To fabricate the sensitive layer of this sensor, MXene and bacterial cellulose (BC) solutions were mixed in different ratios. After vacuum filtering this mixture, MXene/BC composite PSs were obtained. It was found that due to the presence of defective sites on the surface of MXenes, the sensor was able to detect heavy metal ions as small as 1 μM within a high linearity range of 10–300 μM.
In sensors, especially biosensors, signals resulting from a chemical or biological reaction should be transformed into electrical or optical signals. Compared to other types of 2D materials, MXenes have ideal features such as excellent electrical conductivity, hydrophilic functional groups, and tunable band gaps. Furthermore, MXenes are less prone to agglomeration and produce stable colloids.72 These advantages of MXenes make them an ideal platform to obtain high-output signals with less noise compared to other 2D materials. They were used in the structure of a paper based wearable sensor for the detection of glucose and lactate of sweat, simultaneously. It was a highly integrated sensing paper with a 3-dimensional structure in which Ti3C2Tx single layer MXene was coated on the screen-printing carbon working electrode (SPCE) and functionalized with methylene blue that improved the electrochemical performance of the sensor through facilitating electron transfer. Then, glucose oxidase (GOx) and lactate oxidase (LOx) were immobilized on the surface of this electrode that were used for the detection of glucose and lactate, respectively. It was a flexible low-cost sensor that showed high sensitivity for the detection of lactate and glucose (0.49 μA mM−1 and 2.4 nA μM−1, respectively).73
Bu et al. reported a paper-based strain sensor with a Ti3C2Tx hydrophobic coating to detect strain, which worked based on the change in the resistance of the sensor after bending at certain strains. When the sensor was bent, tension was applied to the outer layer of the sensor that caused the formation of micro-cracks on the outer surface of the sensor. Generation and propagation of these micro-cracks resulted in the destruction of the MXene conductive network. On the other hand, bending caused compression stress on the inner surface of the paper, resulting in the closure of these micro-cracks and forming a better conductive network. Overall, the effect of micro-crack closure due to compression surpassed their formation because of the tension on the outer surface and led to a decrease in the resistance by applying different strains up to 0.8%. The gauge factor of this sensor, which is the ratio of the resistance change to applied strain (GF = (ΔR/R0)/ε), represented the sensitivity of this sensor. This sensor showed a stable GF of 17.4 for strains in the range of 0–0.6% and dropped afterwards and reached 3.9 at 0.8%.74
3.2 MOFs for enhancing surface performance
MOFs are one of the other groups of 2-dimensional nanomaterials with unique physicochemical properties such as high porosity, tunable structure, surface functionalizing ability, large surface area, and good adsorption capability, which make them a suitable replacement for conventional sensory platforms. Owing to these properties, MOF-based sensors have been widely used for detecting heavy metals,75 toxic gases,76,77 and biological molecules.78 They could serve as an ideal modification for PSs to improve the properties of these sensors. They could be functionalized with different recognition elements, leading to increased sensitivity and selectivity. Moreover, these materials could act as signal transducers that enable the detection of analytes using various methods such as colorimetry, fluorescent imaging, and electrochemistry.79Drop casting is another method to modify paper substrates with MOFs. In this method, MOFs are incorporated on paper by adding drops of a MOF-containing mixture to the surface of the paper. For instance, Ortiz-Gómez et al. developed a microfluidic paper-based colorimetric sensor to detect glucose and used Fe–MIL-101 MOF to mimic the action of horseradish peroxidase (HRP) in the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2. In this sensor, MOF was used to catalyze the production of OH˙ radicals from H2O2 and the oxidation of TMB to produce a blue color in the presence of different concentrations of glucose. The value of saturation increased on increasing the glucose concentration and a linear relationship was established between the reciprocal of the saturation of the blue color and concentration of glucose up to 150 μM, with a LOD of 2.5 μM.82
In another study, Lu et al.83 synthesized a nickel-MOF/gold nanoparticle (Ni-MOF/AuNPs) composite to improve the performance of an electrochemical paper-based biosensor with cellulose paper. In this sensor, first, the paper was covered with polyvinyl alcohol and carbon nanotubes (PVA/CNT) by vacuum filtration of their mixture. Furthermore, this paper was modified by using the Ni-MOF/AuNP composite by the drop casting method in which 5 μL of the composite was dropped on the prepared PVA/CNT paper to obtain a flexible paper-based electrode modified with a MOF. The obtained electrode was further modified with single-stranded DNA probes that were able to hybridize with the target HIV DNA and produce a double-stranded DNA. To generate an electrical signal, the sensor was immersed in 20 μM MB solution to produce currents. Using modified Ni-MOF/AuNPs increases the current by 48% compared to the unmodified electrode. The incorporation of MOFs enhanced the adsorption of ssDNA probes on the electrode. Besides, the presence of conjugated π electrons in the structure of the MOF linker (terephthalic acid) and an increased surface area of MOFs resulted in more binding sites for ssDNA probes and MB and amplified the produced current.
Utilizing MOFs in PSs can lead to an improved interaction of the sensor with the target analyte, which can enhance the specificity of the sensor. For example, Vaid et al. used a mixed cobalt/molybdenum MOF (Co/Mo-MOF) to detect arsenic(V) (As(V)) metal ions. It was observed that the analyte only interacted with Co–Mo MOF, which resulted in a color change from purple to blue, while other metal ions did not cause the color change. This was due to the interaction of Co/Mo MOF with As(V) and the formation of a blue [AsMo12O40]7− complex. This sensor showed a LOD value of 0.04 ppb for As(V) metal ions, which was improved compared to that of other materials such as gold nanoparticles.85
In another study, Leelasree et al. aimed to combine the high water absorption capacity of MOFs and high electrical conductivity of MoS2 particles by incorporating them on cellulose paper coated MoS2 and MOF layers. After growing MoS2 nanoparticles on cellulose paper, a suspension of HKUST-1 MOFs in ethanol was added dropwise to the substrate. The resulting composite was used to detect different breathing patterns to study sleep apnea disorders in which the MOF layer of the sensor was able to absorb a large amount of water present in exhaled breath due to its large pore volume and diameter. After maximum water absorption occurred, water molecules were transported to the MoS2 layer, where electron transfer from water molecules to the MoS2 layer led to an increase in the recorded current.86
Another way by which MOFs take part in enhancing detection is by eliminating the need for enzymes in the system. For example, G. C. Ilacas et al.87 encapsulated glucose oxidase (GOx) enzyme in an Fe-centered porphyrinic Zr–PCN-222 MOF (GOx@MOF). The presence of the MOF mimicked the presence of heme-peroxidase which resulted in a yellow color with the reaction of glucose with GOx. This GOx@MOF was used to determine the concentration of glucose using colorimetry methods. Two different sensors were prepared using this MOF, a lateral flow assay (LFA) and a well-based platform and the increase in the yellow color intensity with increasing concentration of glucose was recorded. The former platform showed a LOD of 0.5 mM and the linear range of 0–2.5 mM while the LOD for the latter was 0.25 mM.
Xia et al. embedded a core–shell gold nanorod/quantum dot (GNR–QD) structure in a NU-901 MOF to detect the amount of benzaldehyde (BA) in human breath. The incorporation of GNR–QDs in the NU-901 framework enabled the diffusion of BA into its porous structure which reduced the speed of BA flow in the structure and increased its reaction time with GNR–QDs, resulting in the breakage of the bond between GNR and QDs and increased fluorescent and SERS signals.88
MOFs can also be used as a coating to preserve the biorecognition of PSs. For instance, Jiang et al.89 developed a plasmonic paper-based biosensor with AuNPs and ZIF-8 to detect Zika virus (ZIKV), in which ZIF-8 acted as a protective layer to preserve the recognition capabilities in the absence of proper refrigeration. It was observed that the ZIF-8-protected sensors retained 89% and 78% of their recognition capability at room temperature and 60 °C, respectively, while unprotected sensors lost their sensing capabilities at both temperatures.
Natural enzymes such as GOx possess some drawbacks which limit their application in biosensors. Some of these disadvantages include high cost, strict storage conditions, and the limited stability of enzymes. To overcome these limitations, a class of materials known as nanoenzymes has been developed. Nanoenzymes are nanomaterials that show enzyme-like properties and provide high stability and easier sample preparation.90 In the case of PSs, Wei et al. produced an electrochemical paper-based biosensor for the detection of glucose which used cobalt MOF (Co-MOF) on carbon cloth (Co-MOF/CC) as a nanoenzyme. The catalytic properties of Co-MOF caused a drop in the charge-transfer resistance between the electrode and the sample from 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω to 4000 Ω. This indicates that the presence of Co-MOF can facilitate the electrochemical reaction without the need for an enzyme and act as a nanoenzyme. This sensor exhibited a linear response in the range of 0.8–16 mM and an LOD of 0.15 mM.91
000 Ω to 4000 Ω. This indicates that the presence of Co-MOF can facilitate the electrochemical reaction without the need for an enzyme and act as a nanoenzyme. This sensor exhibited a linear response in the range of 0.8–16 mM and an LOD of 0.15 mM.91
Dual-emission ratiometric fluorescence is an alternative way of detecting analytes using two fluorescent emissions instead of one. In this method, two fluorescent signals are generated by the presence of the analyte, and the ratio of the produced signals is used to quantify the amount of the analyte in a sample, which is a more accurate way of quantification than measuring the absolute intensity of a single emission. Chi et al. developed a PS and modified it with UiO-66–NH2 MOFs with rhodamine B (RhB) encapsulated in it to detect arginine. In the presence of arginine, the intensity of the UiO-66–NH2 MOFs was increased, while no change was seen in the intensity of RhB emission (that was used as the reference emission). Here, the ratio of the fluorescence intensity of the MOF (IMOF) to the fluorescence intensity of RhB (IRhB) can be used to indicate the presence of the analyte since in a solution containing arginine, the IMOF/IRhB increased 30 times compared to that of the blank MOF@RhB. The LOD of the PSs with UiO-66–NH2@RhB was calculated to be 6.33 μM.95
Some MOFs have also been reported to have phosphorescence properties. For example, Chen et al.96 used iridium(III)–Zn(II) MOFs (Ir–Zn MOFs) as phosphorescence luminophores to find glucose in human serum. Using Ir–Zn MOFs resulted in a LOD of 0.05 mM and a response time of 0.12 seconds. It was observed that the presence of Ir–Zn MOFs significantly reduced the limit of detection and response time compared to previous studies which used rhodamine97 or bisazobiphenyl.98 Lawati et al.99 investigated the effect of cobalt-imidazole MOFs (CoMOF) on the chemiluminescence (CL) emission caused by the luminol–H2O2 reaction. It was found that the presence of CoMOFs significantly increased the CL emission due to its catalytic activity in creating hydroxyl radicals (OH˙) and excited double oxygen (O2) which is the source of the CL emission. This sensor was used to find different phenolic compounds by eliminating the OH˙ radicals in the system and quenching the CL emission. Also, direct synthesis of CoMOFs on the paper resulted in a lower relative standard deviation (% RSD) than adding synthesized CoMOFs to the PS. Utilizing gallic acid (GA) as a sample of a polyphenol compound led to high sensitivity with a LOD of 0.12 μg mL−1 in the range from 0.5 to 50 μg mL−1.
An electrochemical biosensor was reported by Wang et al. for the detection of miRNA-155, in which the incorporation of gold nanoparticles functionalized with Cu-MOFs resulted in an amplified electrical signal. The Cu-MOF functionalized gold nanoparticles were added to the working electrode of the sensor by depositing 10 μL of their solution and incubating at 4 °C for 120 minutes. Target measurements were performed in 2 mL of a 0.1 M PBS solution which contained 240 μL of 5 mM glucose. The role of Cu-MOF was to catalyze the glucose oxidation to gluconolactone and amplify the produced electrical signal. Using Cu-MOF as the catalyst resulted in an 18.15 μA increase in the recorded current. This signal was further amplified when Cu-MOFs were modified with gold nanoparticles and the recorded current was increased by 24.95 μA.100
Zhu et al. synthesized conductive MOFs by the hydrothermal reaction of nickel acetic acid tetrahydrate (Ni(CH3COO)2·4H2O) and triphenylene-2,3,6,7,10,11-hexaol (HHTP) on carbon paper at a temperature of 85 °C for 30 minutes to create carbon paper modified with conductive MOFs (CP-CMOF). The MOFs were densely grown on the fibers of the paper and covered the smooth surface of the fibers with rod-like MOFs. After the in situ growth of MOFs on carbon paper, further modification was carried out by the electrodeposition of gold nanoparticles on CP-CMOF. The resulting paper composite (CP-CMOF@Au) was studied as a sensing platform to detect diazepam using the surface enhanced Raman scattering (SERS) method. In this sensor, carbon paper provided a flexible substrate. When carbon paper was exposed to the rhodamine 6G (R6G) probe molecule, it did not show any notable Raman characteristic peaks. However, the increase in the conductivity due to the presence of conductive MOFs, followed by the plasmonic effects of the deposited gold nanoparticles, resulted in a sensor with superior Raman performance. This was proven by calculating the efficacy factor (EF) for the composite sensor, which is a factor used for assessing the Raman enhancement of the sensor. Here, the EF was calculated to be 4.6 × 106, which indicated superior Raman signals compared to bare carbon paper. Finally, the ability of this sensor to detect diazepam was evaluated for diazepam concentrations ranging from 0.1 ng mL−1 to 1 mg mL−1. The linear detection range of diazepam was from 0.001 to 10 μg mL−1 and the LOD was determined to be 0.64 ng mL−1.101 Other types of MOFs grown on paper have also been reported such as Fe-MOFs with 2-amino-1,4-benzene dicarboxylic acid (2-NH2–BDC) linkers102 and HKUST-1 (Cu) MOFs.103
Jiang et al. also modified carbon paper with MOFs and gold nanoparticles to develop an electrochemical sensor to detect glyphosate. To fabricate this sensor, gold nanoparticles were deposited on the surface of carbon paper. Then, a dispersion of Cu-TTCP MOFs in water and Nafion were drop-cast on the modified paper. In this composite electrode, the presence of AuNPs significantly improved the electrical conductivity. It was seen that the Cu-TTCP/CP composite had an impedance of 7000 Ω while the impedance of the Cu-TTCP/AuNPs/CP composite was only 25 Ω which indicated that the effective incorporation of gold nanoparticles greatly improved the electron transfer of this composite with increasing electroactive area. Furthermore, cyclic voltammetry evaluations confirmed that the Cu-TTCP/AuNPs/CP composite had higher electrical conductivity than AuNPs/CP which proved that the simultaneous incorporation of MOF and gold nanoparticles had a synergic effect on increasing the electrical conductivity and electron transfer of the developed composite.104
4 Applications
4.1 MXene-based paper sensors
Since their discovery, MXenes have been widely investigated for biomedical applications, especially in the field of biosensors. MXene-based biosensors are used to detect different analytes like cancer biomarkers, small molecules such as glucose and hydrogen peroxide (H2O2),105 and heavy metal ions and toxic gases.106 Some of the MXene based sensors are described in the following parts.Zhou et al. fabricated a core–shell structure of Ti3C2Tx (core) and poly(1,5-diaminonaphthalene-croconaine) (PDAC) polymer (shell) by the in situ polymerization of croconic acid and 1,5-diaminonaphthalene to develop a composite which can provide both stability and conductivity for sensors to detect ammonia (Fig. 2A). It was a highly sensitive and repeatable biosensor with a low detection limit (50 ppb), long-term stability (for at least 40 days), and good selectivity. The polymeric shell presented in the structure of this compound acted as the protective material for the core part to eliminate its oxidation, from one side, and facilitated NO3 adsorption, from the other side, that enhanced the sensitivity of this compound. The composite prepared with a mass ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of Ti3C2Tx, croconic acid, and 1,5-diaminonaphthalene showed the best responsiveness and selectivity to NH3 concentrations ranging from 500 ppb to 10 ppm. Different concentrations of NH3 increased in the electrical resistance of the composite (Fig. 2B). Furthermore, a PS was fabricated by drawing this composite on cellulose paper using a pencil and was able to detect different concentrations of NH3 in flat, rolled or folded states (Fig. 2C).110
5 of Ti3C2Tx, croconic acid, and 1,5-diaminonaphthalene showed the best responsiveness and selectivity to NH3 concentrations ranging from 500 ppb to 10 ppm. Different concentrations of NH3 increased in the electrical resistance of the composite (Fig. 2B). Furthermore, a PS was fabricated by drawing this composite on cellulose paper using a pencil and was able to detect different concentrations of NH3 in flat, rolled or folded states (Fig. 2C).110
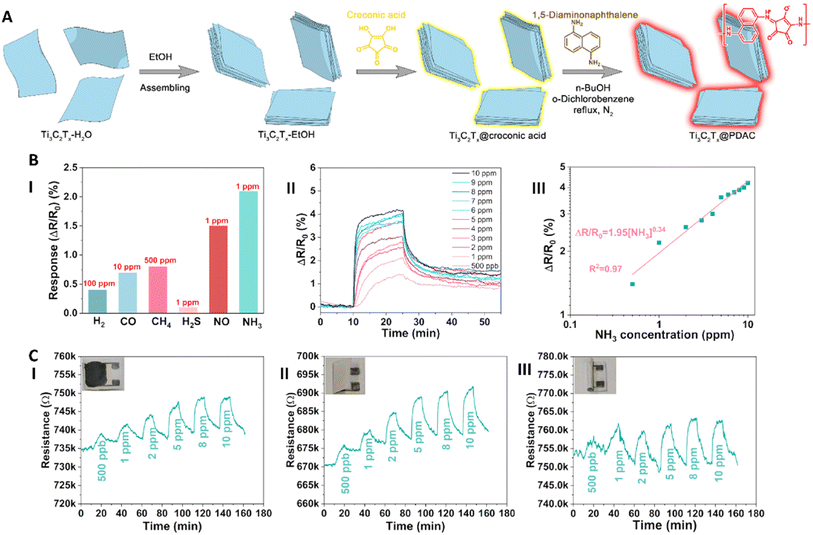 | ||
| Fig. 2 (A) The process of modifying MXenes using PDAC; first, MXene flakes are assembled into multilayers by dispersing them in ethanol. Then PDAC was grafted on the surface of MXenes by a condensation reaction between croconic acid and 1,5-diaminonaphthalene; (B) the response of the modified MXenes in the presence of different gases (I), and different concentrations of NH3 (II). The double log plot of the response versus concentration (III); and (C) fabrication of PSs using inks fabricated from this composite in (I) flat, (II) folded and (III) rolled states. Reprinted from ref. 110 under a Creative Commons Attribution 3.0 Unported License. Copyright 2023, Royal Society of Chemistry. | ||
Zhu et al. benefited from the high conductivity of MXenes for the rapid in situ detection of H2 gas via synthesizing a flexible sensor using Ti3C2Tx MXene and palladium colloidal nanoclusters (Ti3C2Tx@Pd CNC). The presence of Pd CNCs in the structure of this sensor provided the capability of interacting with H2 gas and transferring electrons to the MXene. The cluster shape of this compound provided a high surface area as well as the potential for further functionalization. In here, the concentration of loaded Pd CNCs was important so that at low concentration of this compound the detection was poor due to low absorption of H2 gas, and high amounts of it led to aggregation of clusters. The mechanism of action of this sensor is based on the interaction between H2 gas and the Pd CNCs that led to producing PdHxvia gaining electron from the MXene part. Releasing the H2 gas from this surface occurred in a reverse method which led to recovery of resistance ability of MXenes. It was a rapid and highly sensitive sensor that could detect H2 gas within 32 ± 7 s and could be recovered within 161 ± 23 s. Besides, it showed stable detection during multiple cycles and different bending magnitudes so that just about 21–25% reduction was observed in its sensitivity. The selectivity of the fabricated Ti3C2Tx@Pd CNC sensor was also confirmed in the presence of other gases in the microenvironment of the sensor that confirmed its high selectivity towards the detection of H2 gas.111
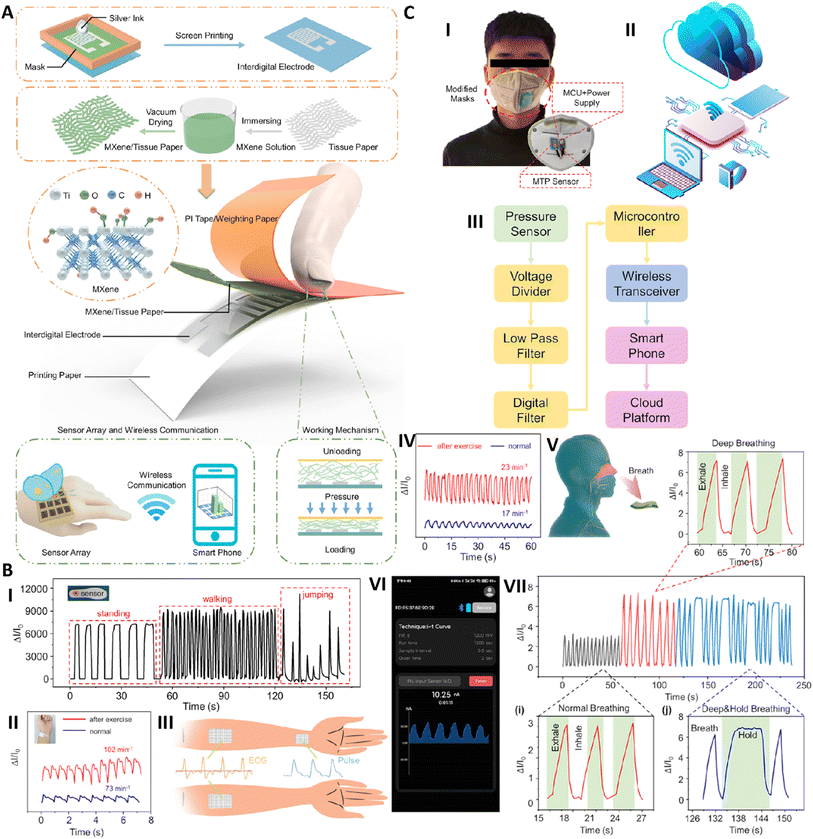 | ||
| Fig. 3 (A) Fabrication and application of MXene based wearable sensors. (B) Utilizing an MTP pressure sensor in real samples for the detection of (I) different motions (walking, jumping, and standing) and (II) pulse signal of the wrist. (III) Utilizing the MTP pressure sensor as an ECG electrode. (C) Application of the MTP pressure sensor in wireless detection of respiratory depression. (I) Fabricating a smart mask via conjugation of the MTP pressure sensor into the structure of the mask. Remote monitoring of the fabricated respiratory sensor: schematic image (II) and block diagram (III). (IV) Change in the current of the respiratory sensor before and after exercise. (V) Schematic image of exhaled air detection. (VI) Wireless monitoring of breath signals. (VII) Different patterns of breath signals. Reprinted with permission from ref. 16. Copyright 2021, American Chemical Society. | ||
Yu et al.113 designed a wearable flexible electrode using MXenes and MXenes modified with gold nanoparticles (AuNPs@MXene). It was seen the bare MXene showed greater electrical conductivity than AuNPs@MXene and the conductivity of MXenes dropped on increasing the concentration of AuNPs. Different types of papers, including Xuan paper, watercolor paper, photocopy paper, drawing paper and bamboo fiber paper, were investigated for electrical conductivity. It was observed that Xuan paper showed the best conductivity of 0.3467 S cm−1 compared to other papers after three dip coatings in MXenes. The ability of this sensor was investigated to detect the electromyography (EMG) signal of the biceps brachii muscle when lifting 0, 5, 10 and 15 kg dumbbells. The EMG signals obtained with MXene papers had lower noise with a more stable baseline and higher root-square-mean (RMS) values and sensitivity compared to a commercial Ag/AgCl electrode. This sensor also showed good breathability in comparison to other similar sensors that introduced it for application as a comfortable skin patch electrode with the ability of being used for a long time.
Liu et al. developed a flexible and degradable wearable sensor utilizing the assembly of two layers, the sensing layer that was fabricated using Ti3C2Tx MXene ink printed on a conductive printing paper (MPP) and MXene printed interdigitated electrodes (MPIE). The fabricated assembly was then placed between two layers of polyethylene terephthalate (PET) films to improve its durability (Fig. 4). It was an eco-friendly, cheap, and flexible biosensor that could be easily degraded in water without leaving any electronic waste. The mechanism of action of this biosensor was based on the distances between the two layers; indeed, at first these two layers had a normal contact state, which was decreased by applying pressure which resulted in more contact between MXene sheets. This led to the creation of conductive paths that reduced the sensor resistance and increased the recorded current. As more pressure was applied, more conductive paths were created and the current was increased further until reaching its limit at which no more space was available between MXene based layers. It was a highly sensitive and stable pressure sensor with the ability of detecting pressure in a wide range (up to 1200 kPa) that could be used for a long time. The pressure sensing of this sensor was used to determine various human motions such as single or double-clicking on a mouse, knocking or tapping a finger, the vibration of vocal cords, and breathing, all of which confirmed the high sensitivity of the fabricated sensor.114
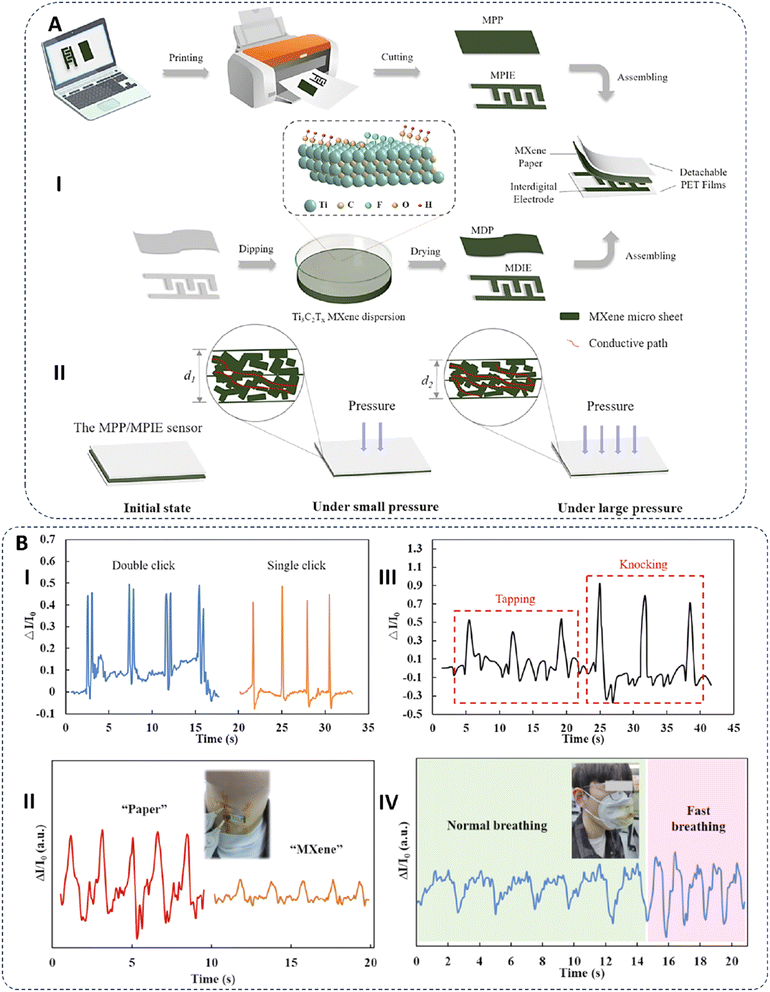 | ||
| Fig. 4 (A) Schematic image related to the fabrication (I) and mechanism of action (II) of the MXene-based printed wearable biosensor. (B) The response of the prepared sensor in detecting human motions such as (I) single or double clicks on a computer mouse, (II) making different sounds such as saying the words “paper” and “MXene”, (III) tapping or knocking with a finger, and (IV) normal and fast breathing. Reprinted with permission from ref. 114. Copyright 2023, American Chemical Society. | ||
One of the interesting fields of application of thermal sensors is their usage as thermal alarm systems in different environments that could prevent serious problems like forest fires. Utilizing deep eutectic solvents (DESs) with high thermal stability and ideal conductivity as printing ink made it possible to achieve flexible and portable printing of thermal alarm systems for use as thermal sensors. Accordingly, Wang et al. fabricated a type of thermal sensor via utilizing MXene Ti2C3Tx and DES (containing FeCl3 and ethylene glycol as a printing ink) that was printed on A4 paper. In this sensor, MXenes were used due to their photothermal conversion properties under irradiation with near-infrared (NIR) light, and DES was applied due to its ability to change its conductivity with changing temperature. A NIR laser with a wavelength of 808 nm was irradiated onto different amounts of MXene, and it was shown that the amounts of MXene sheets, types of DES and soft substrates could affect the responsiveness of the fabricated sensor, among which utilizing 0.1 grams of MXene, FeCl3, and A4 paper showed the best response where the temperature could even reach about 200 °C.116
4.2 MOF-based paper sensors
MOFs have garnered significant attention for their application in PSs due to their unique structural properties, such as high surface area, tunable porosity, and versatile chemistry. MOFs can be integrated with cellulose fibers in paper to create sensors that are highly sensitive and selective for detecting various analytes, including gases, heavy metals, and biological molecules. The use of MOFs in PSs offers a cost-effective, portable, and environmentally friendly approach for real-time monitoring in diverse fields such as environmental monitoring, food safety, and healthcare diagnostics.Photoelectrochemical (PEC) sensors are a type of electrochemical sensors in which a light source causes stimulation in a photoactive material which in turn, causes electron transfer from the analyte to the electrodes that produces currents.119 Yan et al. used zinc porphyrin MOFs (2D-TTCP(Zn)) with multi-walled carbon nanotubes (MWCNTs) as photoactive materials for fabricating a paper-based wearable PEC sensor for the detection of vitamin C in sweat using the light of a smartphone (Fig. 5). It was seen that incorporation of MWCNTs with 2D-TTCP(Zn) significantly improved the generated current by 8.42 and 8.13 times in air and O2, respectively. Moreover, the combined use of these two materials led to enhancement in the detected current compared to bare TTCP(Zn) and MWCNTs. This sensor showed high sensitivity and good selectivity for vitamin C with a LOD of about 3.61 μM with a wide range of detection (10–1100 μM). Utilizing a smartphone light led to electron transfer from 2D-TCPP(Zn) to the MWCNTs that further required O2 molecules and converted them into the O2˙−. The lost electrons of the MXene part were then replaced by the electrons produced via oxidation of vitamin C. It showed long-term stability for about 16 days and after utilizing for different number of cycles. Furthermore, it was used for fabricating a wearable sensor via fabricating a patch composed of five layers; it had a transparent polyurethane (PU) film on top and bottom part which incorporated the PS, a ring-shaped PU film, and the sweat-absorbing non-woven fabric. Sweat could reach the electrode via creating some pores on the PU film and was then evaporated from the other part. Here, the presence of a ring-shaped PU film prevented wetting of the electrode by sweat. The fabricated patch showed rapid diffusion and stable response at acidic pH and on increasing the temperature. Applying the fabricated patch on a volunteer confirmed its effectiveness in detection of vitamin C present in sweat using a smartphone.120
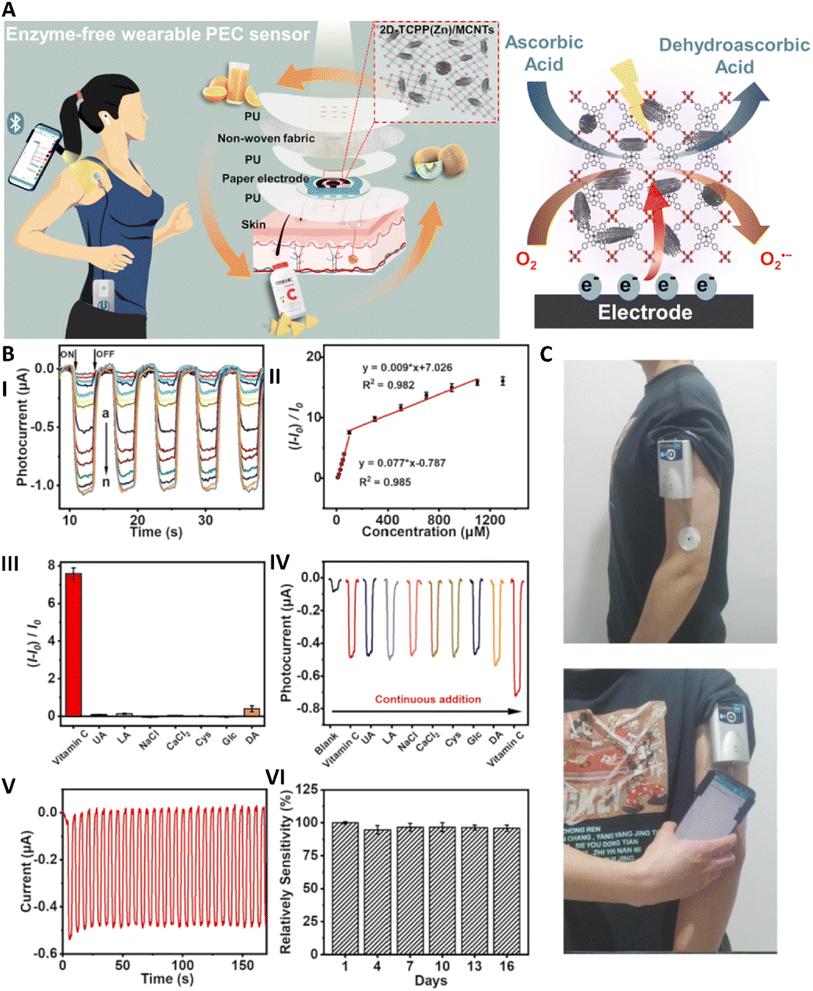 | ||
| Fig. 5 (A) Schematic image related to the application of a wearable PS composed of 2D-TTCP(Zn) MOFs used for the detection of vitamin C in sweat. (B) The photocurrent response (I) and calibration curve (II) related to the application of the PEC sensor for the detection of different concentrations of vitamin C. Detection of selectivity (III) and anti-interference (IV) abilities of the fabricated sensor towards vitamin C and in the presence of other materials. Determining the photocurrent (V) and storage (VI) stability of the sensor. (C) Application of the fabricated sensor for real-time monitoring of vitamin C on the body. Reprinted with permission from ref. 120. Copyright 2023, Elsevier. | ||
Kou et al.121 developed a colorimetric PS with ZIF-8 MOFs as nanoreactors to encapsulate single or multiple enzymes. In their study, they encapsulate GOx and horseradish peroxidase (HRP) to sense glucose. The presence of GOx and HRP initiated an enzymatic cascade in which glucose was oxidized to gluconic acid and H2O2. Subsequently, the produced H2O2 reacted with HRP in the presence of 3,30,5,50-tetramethylbenzidine (TMB) and produced a blue color, which was analyzed with a smartphone camera and a designed software. The saturation of the produced blue color was recorded for different glucose concentrations and showed a linear relationship for the concentrations ranging between 100 and 3000 μM and the LOD of 250 μM. Also, entrapping the enzymes in the MOF enhanced the stability of the enzymes and allowed the long-term storage of the sensor. It was observed that the enzymes significantly lost their activity after 28 days in free solution at room temperature and only lost about 20% of their activity when entrapped within the MOF.
Lawati and Hassanzadeh122 developed a dual-function colorimetric/fluorometric PS using CoMOFs as a catalyst to detect glucose. In this sensor, the oxidation of glucose resulted in the production of H2O2, which subsequently reacted with OPD in the presence of CoMOFs. The results showed that using CoMOFs as the catalyst led to a LOD of 16.3 μM and 3.2 μM for colorimetry and fluorescence detection respectively which was stated to be lower than that of other types of MOFs. The validation of this sensor was also performed by taking blood samples from healthy and diabetic patients. It was proven that the sensor had high accuracy for the detection of glucose in blood samples.
A paper-based electrochemiluminescence sensor was designed for the detection of glycated hemoglobin (HbA1c), which is a biomarker for diabetes. It was composed of two parts; a signal amplifier, composed of an antibody-labeled nanocomposite of zirconium metal–organic framework/Fe3O4 (trimethyl chitosan)/gold nanocluster (Zr-MOF/Fe3O4(TMC)/AuNCs), and a sensing platform, containing the paper-based Au electrode modified with reduced graphene oxide/aminophenylboronic acid (rGO/APBA). The signal amplifying feature of Zr-MOF/Fe3O4(TMC)/AuNCs was resulted from two different strategies; one of them was related to the highly positive charge of the TMC-functionalized Fe3O4 nanoparticles that enhanced the capability of interaction of the Zr-MOF/Fe3O4(TMC) part with the negatively charged BSA-capped AuNCs. The second one was the Zr-MOF/Fe3O4(TMC) compound that exhibited co-reaction accelerator properties in the presence of tri ethylene amine (TEA), as the co-reactant, and enhanced the production of TEA free radicals (TEA˙) from TEA resulting in the fabrication of Zr-MOF/Fe3O4(TMC)/AuNCs* with exceptional ECL properties. To assess the performance of this sensor, they prepared different concentrations of HbA1c with plasma, from 0–20%. The fabricated sensor showed a linear range of 2–18%, which was higher compared to that of other biosensors without the MOF in their label structure, and a LOD of 0.072%. The reproducibility of this sensor was also assessed for the HbA1c sample with concentrations of 3%, 10% and 16% by four measurements in both intra-assay and inter-assay ways. The intra-assay measurements showed a coefficient of variation (CV) of 4.1, 2.6 and 3.1%, respectively, and the CV of inter-assay measurements were calculated to be 3.8, 2.8 and 1.8%, respectively, that indicated adequate reproducibility for this sensor.123
Hassanzadeh et al. fabricated a chemiluminescence PS for the detection of phenolic compounds via modifying the sensor with cobalt-MOF (CoMOF) containing rhodamine B (RhoB) (R@CoMOF). Indeed, the paper base was first fabricated using wax printing, and then R@CoMOF was incorporated into it through dropping a suspension of R@CoMOF and NaOH on the paper, followed by a 15 minute drying. Entrapping RhB inside the pores of CoMOFs resulted in an amplified CL emission compared to that with RhB alone or with free Co2+ ions. The presence of phenolic compounds caused the quenching of this emission and a decrease in its intensity that was used as an indicator of the presence of phenolic compounds. The initial tests were performed on gallic acid that showed a linear relationship between the reduction of the CL emission and the concentration of gallic acid ranging from 2 to 300 ng mL−1 with a LOD of about 0.98 ng mL−1.127
The limitations of the microfluidic paper-based device (μPAD) like low sensitivity and limited volume that could be used in this type of sensor make it important to use them with other materials that could overcome these challenges. In this context, MOFs with high stability and high surface area could be a good candidate to form composites with paper sensors and produce new sensors with improved features. In this context, Martínez-Pérez-Cejuela et al. used ZIF-8 MOFs to improve the sensitivity of a μPAD for the detection of phenolic compounds in different fruits. It was composed of two layers, one of them had a MOF and Folin–Ciocalteu (FC) reagent and the other one had Na2CO3 that provided alkaline conditions. The results showed that the presence of ZIF-8 MOFs in the structure of the sensor increased the sensitivity of the sensor by 50% compared to the sample without MOFs. The developed sensor showed a linear response in the range of 1.2–30 mg L−1 and a LOD and limit of quantification of about 0.36 mg L−1 and 1.2 mg L−1, respectively, for gallic acid reference solution. It also showed color stability for about 6 h and storage stability for at least 2 weeks via incubating the sensor inside a freezer without the need to use vacuum conditions.128
Dey et al. synthesized a nanocomposite paper using copper MOFs with 1,3,5-benzenetricarboxylic acid (BTC) linkers (Cu(BTC) MOFs) and carbon nanofibers (CNFs) as an electrochemical sensor to detect the 4-nitrophenol toxin. The working electrode of this sensor was composed of this nanocomposite stuck to a glass carbon electrode (GCE). The presence of 4-nitrophenol in a solution causes a redox reaction on the surface of Cu(BTC)MOF@CNF/GCE that reduces 4-nitrophenol to 4-hydroxyaminophenol and generates a current. Different concentrations of 4-nitrophenol were investigated and the sensor showed a linear response in the range of 5–400 μM with a LOD of 0.0871 μM.132
Lanthanide MOFs (Ln-MOFs) are a group of MOF materials that have stable and tunable luminescence emissions. Depending on the type of lanthanide used in the synthesis of these MOFs, they can emit various wavelengths of light upon excitation. For instance, under excitation with UV light, europium MOFs (Eu-MOFs) emit red light, while terbium MOFs (Tb-MOFs) emit green light.133 Ln-MOFs are being investigated in luminescence PSs. Zhang et al. reported a paper microsensor for determining triazophos using Tb-MOFs and a filter paper. The suspension of Tb-MOFs displayed a green fluorescent emission when excited with 254 nm UV radiation. In the presence of triazophos, the fluorescent emission of Tb-MOFs was reduced in the presence of increasing amounts of analyte. The fabricated sensor showed linearity in concentrations ranging from 3 to 800 ng mL−1 with a LOD of 0.12 ng mL−1. This sensor also showed specificity towards triazophos in a mixture of multiple pesticides. Compared to triazophos, other samples did not show notable changes in the fluorescent emission, which shows that this sensor had specificity towards triazophos.134
Table 1 summarizes some of the other studies which used PSs with MXene and MOF modifications and the effects of these modifications on the performance of these sensors.
| Sensor type | Modification | Analyte | LOD | Linear range | Results of MXene or MOF addition | Ref. |
|---|---|---|---|---|---|---|
| Electrochemical | MXene/AuNPs | Fumonisin B1 | 21 fg mL−1 | 50–100 fg mL−1 | • Increased electroactive surface | 135 |
| • Increased electrical conductivity | ||||||
| MXene/ZIF-8 MOF | Apigenin | 2.6 nM | 0.01 to 10 μM | • Ultralow detection limit | 136 | |
| • High sensitivity, linear range and reproducibility | ||||||
| AuNPs/Cu-TCPP(Fe) | Lactate | 0.91 pM | 0.013 nM to 100 mM | • Cu-TCPP(Fe) MOFs increased the smoothness and surface area of the electrode | 137 | |
| Co-MOF/AuNPs/carbon paper electrode (CPE) | Pb2+ and Cd2+ ions | 0.07 ng mL−1 (Pb2+) | 0.5–20 ng mL−1 (Pb2+) | • 20.8% increase in the electroactive surface of CPE | 138 | |
| 0.011 ng mL−1 (Cd2+) | 0.75–35 ng mL−1 (Cd2+) | |||||
| SERS | MXene/AuNPs | Glucose | 0.39 μM | 1–50 μM | • Quenching fluorescence interference | 139 |
| • Amplification of the Raman signal | ||||||
| Wearable pressure sensor | MXene/bacterial cellulose (BC) | — | 0.57 Pa | 0.002–30 kPa | • Hydrophobic modification of the paper using hydroxyl moieties of MXenes | 140 |
| • A waterproof sensor was obtained using this modification | ||||||
| MXene/bamboo microfibril (BF) | — | (Not specified) | 0–2.5 kPa | • Flexible sensor | 141 | |
| • Enhanced mechanical properties; tensile strength = 49.46 ± 2.25 MPa | ||||||
| Carbon black/MXene/silicon rubber/fiber | — | (Not specified) | 0.1–1700 kPa | • Highly-sensitive sensor (2.18 kPa−1 for pressures under 15 kPa) | 142 | |
| Piezoelectric pressure sensor | MXene/PVA/copper electrode/polyethylene terephthalate (PET) film | — | 6 Pa | 65.3 Pa to 294 kPa | • Ultrahigh-sensitive, easy-to-produce, low-cost sensor with high durability (more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
143 |
| Colorimetry | Cu–PyC MOF | Cr4+ ion | 0.051 μM | 0.5–50 μM | • Cu-PyC MOFs catalyzed the oxidation of TMB by reducing Cr4+ to Cr3+ and producing a detectable blue color | 144 |
| Luminescence | Eu-MOF | Fe3+ ion | 1 μM | 0–3 mM | • Emission of detectable red light using UV light from Eu-MOFs | 145 |
| 0.012CD/Eu0.05Tb0.95BTC/PVA | Carbamazepine | 1.7 nM (in water) | (Not specified) | • Eu3+ and Tb3+ MOF nodes emitted yellow light upon excitation at 365 nm | 146 | |
| 2.8 nM (in artificial urine) | • Concentration variation of carbamazepine quenched the emission of MOFs | |||||
| Fluorescence | Eu-MOF | Tryptophan | 0.8 μM | 0–350 μM | • Quenching of Eu-MOF fluorescence by varying tryptophan concentrations | 147 |
| Eu-MOF | Folic acid | 0.12 mM | 1–9 mM | • Eu-MOF fluorescence was quenched using folic acid | 148 | |
| Eu-MOF | Sulfamethazine (SMZ) | 1.1 μM | 0–50 μM | • Fluorescence of Eu-MOF showed a 97% quenching efficiency towards SMZ | 149 | |
| Luminescence | Cu2+@UiO-66 MOF | L-Histidine | 7 nM | 0.01–40 μM | • The luminescence of UiO-66 MOF was quenched in the presence of Cu2+ and L-histidine | 150 |
| Chemiluminescence | UiO-66 MOF | Acetone | 0.03 ppm | 0.1–8 ppm | • More efficient adsorption of acetone on the sensor | 151 |
| • Reducing the LOD from 3.5 ppm to 0.03 ppm | ||||||
| Bioluminescence | ZIF-8 MOF | Adenosine triphosphate (ATP) | 0.4 μM | — | • Improvement of the sensitivity and stability | 152 |
| • Capability of use in real samples |
5 Challenges
Challenges in PSs present significant hurdles that researchers must address to optimize sensor performance and reliability.1,153 One key challenge is the sensitivity of PSs. Achieving high sensitivity levels to detect low concentrations of analytes reliably is essential for various applications, such as environmental monitoring and healthcare diagnostics. Researchers face the task of enhancing signal amplification techniques, improving detection limits, and minimizing background noise to boost the sensor's sensitivity and ensure accurate detection of target analytes. Another critical challenge lies in the selectivity of PSs. Ensuring that sensors can differentiate between target analytes and potential interferents in complex sample matrices is crucial for reliable and specific detection. Researchers must develop sensor designs that incorporate selective recognition elements, such as specific receptors or functionalized surfaces, to enhance the sensor's selectivity and reduce the likelihood of false positives or cross-reactivity. Improving selectivity is essential for enhancing the accuracy and reliability of sensor measurements in real-world applications. Furthermore, scalability poses a significant challenge in the development of PSs. Achieving consistent sensor performance across multiple sensor platforms and production batches can be challenging due to variations in material properties, fabrication processes, and environmental conditions. Standardizing sensor fabrication methods, optimizing material characterization protocols, and implementing quality control measures are essential to ensure uniform sensor performance and reliability on a larger scale. Addressing scalability challenges is vital for the widespread adoption and practical implementation of PSs in various industries and applications.154Challenges in incorporating MXenes into PSs present significant hurdles that need to be addressed for optimal sensor performance and reliability.107 One of the primary challenges is related to the sensitivity of MXene-based sensors. While MXenes offer high conductivity, achieving high sensitivity levels in PSs can be challenging due to limitations in signal amplification and detection thresholds. Researchers must develop strategies to enhance the signal-to-noise ratio and improve the detection limits of MXene-based sensors to ensure accurate and reliable detection of target analytes. Another critical challenge is the stability of MXene-based PSs. Maintaining the stability and shelf-life of sensors, especially under harsh environmental conditions, is essential for long-term reliability. MXenes are susceptible to oxidation and degradation, which can impact sensor performance over time. Researchers need to explore protective coatings, encapsulation techniques, or alternative MXene compositions to enhance the stability of PSs and ensure consistent performance under varying conditions. Interference is another challenge that researchers face when developing MXene-based PSs. Paper substrates and environmental samples may introduce background interference or matrix effects that affect the accuracy and specificity of sensor measurements. Addressing these interference issues requires the development of robust sensor designs that can differentiate between target analytes and potential interferents, ensuring reliable and selective detection in complex sample matrices. Furthermore, the integration of advanced materials like MXenes into PSs poses scalability challenges. Achieving a uniform and reproducible sensor performance across multiple sensor platforms can be difficult due to variations in material properties, synthesis methods, and sensor fabrication processes. Researchers must optimize sensor production workflows, standardize material characterization protocols, and establish quality control measures to ensure consistent sensor performance and reliability on a larger scale.
On the other hand, challenges associated with integrating MOFs into PSs present hurdles that need to be addressed to maximize sensor performance and reliability.79 One significant challenge is the stability of MOF-based PSs. MOFs are susceptible to degradation under certain environmental conditions, such as humidity, temperature variations, and exposure to air, which can compromise sensor performance over time. Researchers need to develop strategies to enhance the stability of MOFs in PSs through protective coatings, encapsulation techniques, or alternative synthesis approaches to ensure long-term sensor reliability. Another critical challenge is the scalability of MOF-based PSs. Achieving consistent sensor performance across multiple sensor platforms can be difficult due to variations in MOF properties, synthesis methods, and sensor fabrication processes. Standardizing material characterization protocols, optimizing sensor production workflows, and implementing quality control measures are essential to ensure uniform sensor performance and reliability on a larger scale. Addressing scalability challenges is crucial for the widespread adoption of MOF-based PSs in practical sensing applications. Interference is another challenge that researchers face when developing MOF-based PSs. Background interference or matrix effects from paper substrates and sample matrices can affect sensor specificity and accuracy, leading to false readings or reduced detection performance. Mitigating interference issues requires the design of robust sensor architectures that can effectively differentiate between target analytes and potential interferents, ensuring accurate and selective detection in complex sample matrices. Furthermore, the sensitivity of MOF-based PSs poses a challenge that researchers must overcome. Enhancing the sensitivity levels of MOF sensors to detect low concentrations of analytes reliably is essential for their practical utility in various sensing applications. Improving signal amplification strategies, optimizing sensor designs, and exploring novel detection mechanisms are critical steps in advancing the sensitivity of MOF-based PSs and unlocking their full potential for sensitive and selective detection in real-world scenarios.
6 Future perspectives
Future perspectives and innovations in PSs hold immense potential for advancing sensor technology and expanding their applications across diverse fields. One key future direction is the development of self-powered PSs that harness energy from the surrounding environment or the analyte itself to enable autonomous operation. By integrating energy harvesting components or self-sustaining power sources into PSs, researchers can enhance their portability, longevity, and versatility for long-term monitoring applications in remote or resource-limited settings. Another exciting innovation on the horizon is the incorporation of artificial intelligence (AI) algorithms and machine learning techniques into PSs to enable real-time data analysis, pattern recognition, and predictive modeling. By leveraging AI capabilities, PSs can adapt to changing environmental conditions, optimize sensor performance, and provide actionable insights for decision-making in fields such as healthcare, environmental monitoring, and industrial process control. Furthermore, the additional explorations ought to be conducted on bioinspired designs and biomimetic sensor architectures in PSs. Drawing inspiration from nature, researchers can develop sensors that mimic biological sensing mechanisms, such as olfaction or vision, to enhance sensitivity, selectivity, and robustness. By incorporating biomimetic principles into sensor design, PSs can achieve higher levels of performance, reliability, and adaptability to complex analytical challenges. Additionally, advancements in nanotechnology, nanomaterials, and printable electronics are poised to help develop the fabrication and functionality of PSs.The future of MXenes in PSs holds immense promise for advancing sensor technology and addressing critical needs in various fields. One key aspect of future developments lies in enhancing the sensitivity of MXene-based PSs. Continued research efforts aim to optimize sensor designs, signal amplification strategies, and detection mechanisms to achieve ultra-sensitive detection capabilities for a wide range of analytes. By pushing the limits of sensitivity, researchers can unlock new opportunities for detecting trace levels of molecules in complex samples with unprecedented precision and accuracy. Another future perspective involves the integration of MXenes into multiplexed sensing platforms. By incorporating multiple MXene-based sensors within a single device, researchers can enable simultaneous detection of multiple analytes, expanding the utility of PSs for comprehensive analysis in diverse applications. Multiplexed sensing capabilities offer the potential for rapid, cost-effective, and high-throughput screening of complex samples, making MXene-enhanced PSs valuable tools for point-of-care diagnostics, environmental monitoring, and personalized healthcare applications. Furthermore, future advancements in MXene-based PSs will focus on enhancing their selectivity and specificity. By tailoring the surface chemistry of MXenes and functionalizing paper substrates with specific receptors or ligands, researchers can develop sensors that exhibit high selectivity towards target analytes while minimizing interference from background compounds. Improved selectivity is crucial for reducing false positives, enhancing the reliability of sensor measurements, and enabling accurate detection in real-world scenarios. Additionally, future perspectives include the integration of MXene-based PSs with emerging technologies such as IoT platforms and wearable devices. By connecting PSs to wireless networks, cloud-based data storage systems, and mobile applications, researchers can enable real-time monitoring, remote data access, and on-demand analytics for sensor data. This connectivity opens up new possibilities for deploying MXene-based PSs in smart environments, enabling continuous monitoring, rapid response capabilities, and data-driven insights for various applications.
The future of MOFs in PSs holds great promise for advancing sensor technology and opening up new possibilities for diverse applications. One key aspect of future developments involves enhancing the sensitivity of MOF-based PSs. Continued research efforts aim to optimize sensor designs, signal amplification techniques, and detection mechanisms to achieve ultra-sensitive detection capabilities for a wide range of analytes. By pushing the boundaries of sensitivity, researchers can unlock new opportunities for detecting trace levels of molecules with unparalleled precision and accuracy in various fields. Another future perspective lies in the customization and functionalization of MOFs in PSs to enhance their selectivity and specificity. By tailoring the chemical functionalities of MOFs and designing specific receptor sites within the structure, researchers can develop sensors with high selectivity towards target analytes while minimizing interference from background compounds. Improved selectivity is crucial for reducing false positives, increasing the reliability of sensor measurements, and enabling accurate detection in complex sample matrices. Furthermore, future advancements in MOF-based PSs will focus on addressing scalability challenges to enable widespread adoption and practical implementation. Standardizing material synthesis processes, optimizing sensor fabrication techniques, and implementing quality control measures are essential steps to ensure consistent sensor performance across multiple platforms. By enhancing scalability, researchers can accelerate the integration of MOFs into PSs for real-world sensing applications in environmental monitoring, healthcare diagnostics, and beyond. Additionally, future perspectives include exploring the integration of MOF-based PSs with emerging technologies such as IoT platforms and smart devices. By connecting PSs to wireless networks, cloud-based data storage systems, and mobile applications, researchers can enable remote monitoring, real-time data analysis, and seamless integration with IoT ecosystems. This connectivity opens up new avenues for deploying MOF-based PSs in smart environments, enabling continuous monitoring, data-driven insights, and personalized sensing solutions for various applications.
7 Conclusion
The integration of MXenes and MOFs into PSs represents a significant advancement in sensor technology, offering unique properties and opportunities for enhancing sensor performance in various applications. Both MXenes and MOFs provide distinct advantages to PSs, such as high sensitivity, selectivity, and stability, which have the potential to help develop sensing platforms across different fields. Despite their promising capabilities, challenges persist, including issues related to sensitivity, stability, interference, and scalability. Overcoming these challenges through innovative research and development efforts is crucial for harnessing the full potential of MXene- and MOF-based PSs for practical applications.Looking towards the future, exciting prospects abound for MXenes and MOFs in PSs. Continued research endeavors aim to enhance sensitivity levels, improve selectivity, address scalability challenges, and integrate these advanced materials with emerging technologies for enhanced sensor performance. By pushing the boundaries of sensor design, signal amplification strategies, and connectivity with IoT platforms, researchers can unlock new opportunities for deploying MXene- and MOF-based PSs in smart environments, point-of-care diagnostics, environmental monitoring, and beyond. Through collaborative efforts and interdisciplinary approaches, the field of PSs is poised for significant advancements, paving the way for the development of next-generation sensing platforms with enhanced capabilities, reliability, and versatility to meet the evolving needs of diverse industries and applications.
Abbreviations
| AuNPs | Gold nanoparticles |
| BC | Bacterial cellulose |
| CL | Chemiluminescence |
| CNTs | Carbon nanotubes |
| GOx | Glucose oxidase |
| HRP | Horseradish peroxidase |
| I | Current |
| LOD | Limit of detection |
| MB | Methylene blue |
| MOFs | Metal–organic frameworks |
| PSs | Paper-based sensors |
| PVA | Polyvinyl alcohol |
| R | Resistance |
| RhB | Rhodamine B |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
Data availability
No data were used for the research described in the article.Author contributions
Sepehr Larijani: writing – review & editing; Atefeh Zarepour: writing – review & editing; Arezoo Khosravi: visualization, writing – review & editing; Siavash Iravani: supervision, conceptualization, writing – review & editing; Mahnaz Eskandari: writing – review & editing; Ali Zarrabi: supervision, writing – review & editing.Conflicts of interest
The author(s) declare no conflict of interest.References
- S. Malik, J. Singh, K. Saini, V. Chaudhary, A. Umar, A. A. Ibrahim, S. Akbar and S. Baskoutas, Paper-based sensors: affordable, versatile, and emerging analyte detection platforms, Anal. Methods, 2024, 16, 2777–2809 RSC.
- O. Chailapakul, W. Siangproh, S. Jampasa, S. Chaiyo, P. Teengam, A. Yakoh and C. Pinyorospathum, Paper-based sensors for the application of biological compound detection, in Comprehensive Analytical Chemistry, 2020, vol. 89, pp. 31–62 Search PubMed.
- B. Suslu, F. Ali and I. K. Jennions, Understanding the Role of Sensor Optimisation in Complex Systems, Sensors, 2023, 23, 7819 CrossRef PubMed.
- B. Kuswandi, M. A. Hidayat and E. Noviana, Paper-based sensors for rapid important biomarkers detection, Biosens. Bioelectron.: X, 2022, 12, 100246 CAS.
- S. Kumar, J. B. Kaushal and H. P. Lee, Sustainable Sensing with Paper Microfluidics: Applications in Health, Environment, and Food Safety, Biosensors, 2024, 14, 300 CrossRef CAS PubMed.
- S.-H. Choi, J.-S. Lee, W.-J. Choi, J.-W. Seo and S.-J. Choi, Nanomaterials for IoT Sensing Platforms and Point-of-Care Applications in South Korea, Sensors, 2022, 22, 610 CrossRef.
- A. Erdem, E. Eksin, H. Senturk, E. Yildiz and M. Maral, Recent developments in wearable biosensors for healthcare and biomedical applications, TrAC, Trends Anal. Chem., 2024, 171, 117510 CrossRef CAS.
- G. Vulpe, G. Liu, S. Oakley, D. Pletsas, G. Yang, R. Dutra, O. Guy, Y. Liu, M. Waldron, J. Neary, A. A. Mohan and S. Sharma, Wearable technology for one health: charting the course of dermal biosensing, Biosens. Bioelectron.: X, 2024, 19, 100500 CAS.
- M. B. Kulkarni, N. H. Ayachit, T. M. Aminabhavi and B. W. Pogue, Recent advances in microfluidics-based paper analytical devices (μPADs) for biochemical sensors: from fabrication to detection techniques, Biochem. Eng. J., 2023, 198, 109027 CrossRef CAS.
- S. Arumugam, D. A. M. Colburn and S. K. Sia, Biosensors for Personal Mobile Health: A System Architecture Perspective, Adv. Mater. Technol., 2020, 5, 1900720 CrossRef CAS.
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Fundamentals of MXene synthesis, Nat. Synth., 2022, 1, 601–614 CrossRef CAS.
- Q. Meng, C. Yang, X. Tai, K. Cheng, P. Li, H. Li, X. Liu and S. Liu, Recent advances in MXenes and their composites for wearable sensors, J. Phys.: Condens. Matter, 2022, 34, 453001 CrossRef CAS.
- K. Grabowski, S. Srivatsa, A. Vashisth, L. Mishnaevsky Jr and T. Uhl, Recent advances in MXene-based sensors for Structural Health Monitoring applications: A review, Measurement, 2022, 189, 110575 CrossRef.
- S. Hajian, D. Maddipatla, B. B. Narakathu and M. Z. Atashbar, MXene-based flexible sensors: a review, Front. Sens., 2022, 3, 1006749 CrossRef.
- D.-J. Yao, Z. Tang, L. Zhang, Z.-G. Liu, Q.-J. Sun, S.-C. Hu, Q.-X. Liu, X.-G. Tang and J. Ouyang, A highly sensitive, foldable and wearable pressure sensor based on MXene-coated airlaid paper for electronic skin, J. Mater. Chem. C, 2021, 9, 12642–12649 RSC.
- L. Yang, H. Wang, W. Yuan, Y. Li, P. Gao, N. Tiwari, X. Chen, Z. Wang, G. Niu and H. Cheng, Wearable pressure sensors based on MXene/tissue papers for wireless human health monitoring, ACS Appl. Mater. Interfaces, 2021, 13, 60531–60543 CrossRef CAS.
- W. Niamsi, N. Larpant, P. K. Kalambate, V. Primpray, C. Karuwan, N. Rodthongkum and W. Laiwattanapaisal, Paper-based screen-printed ionic-liquid/graphene electrode integrated with prussian blue/MXene nanocomposites enabled electrochemical detection for glucose sensing, Biosensors, 2022, 12, 852 CrossRef CAS.
- M. B. Alahri, R. Arshadizadeh, M. Raeisi, M. Khatami, M. S. Sajadi, W. K. Abdelbasset, R. Akhmadeev and S. Iravani, Theranostic applications of metal–organic frameworks (MOFs)-based materials in brain disorders: recent advances and challenges, Inorg. Chem. Commun., 2021, 134, 108997 CrossRef.
- A. Aldhaher, N. Rabiee and S. Iravani, Exploring the synergistic potential of MXene-MOF hybrid composites: a perspective on synthesis, properties, and applications, Hybrid Advances, 2024, 5, 100131 CrossRef.
- A. Bigham, N. Islami, A. Khosravi, A. Zarepour, S. Iravani and A. Zarrabi, MOFs and MOF-Based Composites as Next-Generation Materials for Wound Healing and Dressings, Small, 2024, 20, 2311903 CrossRef CAS PubMed.
- X. Liu, L. Zhang and J. Wang, Design strategies for MOF-derived porous functional materials: preserving surfaces and nurturing pores, J. Materiomics, 2021, 7, 440–459 CrossRef.
- Y. An, X. Lv, W. Jiang, L. Wang, Y. Shi, X. Hang and H. Pang, The stability of MOFs in aqueous solutions—research progress and prospects, Green Chem. Eng., 2024, 5, 187–204 CrossRef.
- H. Meskher, S. B. Belhaouari and F. Sharifianjazi, Mini review about metal organic framework (MOF)-based wearable sensors: challenges and prospects, Heliyon, 2023, 9, e21621 CrossRef CAS.
- D. Li, A. Yadav, H. Zhou, K. Roy, P. Thanasekaran and C. Lee, Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review, Global Chall., 2024, 8, 2300244 CrossRef.
- W. Chen and C. Wu, Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine, Dalton Trans., 2018, 47, 2114–2133 RSC.
- L. M. Fu and Y.-N. Wang, Detection methods and applications of microfluidic paper-based analytical devices, TrAC, Trends Anal. Chem., 2018, 107, 196–211 CrossRef CAS.
- H. Tai, Z. Duan, Y. Wang, S. Wang and Y. Jiang, Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review, ACS Appl. Mater. Interfaces, 2020, 12, 31037–31053 CrossRef CAS.
- H.-Y. Li, W.-N. Jia, X.-Y. Li, L. Zhang, C. Liu and J. Wu, Advances in detection of infectious agents by aptamer-based technologies, Emerg. Microbes Infect., 2020, 9, 1671–1681 CrossRef CAS.
- M. A. El-Mokhtar, S. G. Elgendy, A. S. Eldin, E. A. Hassan, A. A. A. Hasan, M. R. A. Hameed, D. Sayed and E. H. Salama, Hepatitis C Virus Affects Tuberculosis-Specific T Cells in HIV-Negative Patients, Viruses, 2020, 12, 101 CrossRef CAS PubMed.
- L. Ochola, P. Ogongo, S. Mungai, J. Gitaka and S. Suliman, Performance Evaluation of Lateral Flow Assays for Coronavirus Disease-19 Serology, Clin. Lab. Med., 2022, 42, 31–56 CrossRef.
- D. Evans, K. I. Papadimitriou, N. Vasilakis, P. Pantelidis, P. Kelleher, H. Morgan and T. Prodromakis, A Novel Microfluidic Point-of-Care Biosensor System on Printed Circuit Board for Cytokine Detection, Sensors, 2018, 18, 4011 CrossRef PubMed.
- W. Suntornsuk and L. Suntornsuk, Recent applications of paper-based point-of-care devices for biomarker detection, Electrophoresis, 2020, 41, 287–305 CrossRef CAS PubMed.
- R. A. G. de Oliveira, F. Camargo, N. C. Pesquero and R. C. Faria, A simple method to produce 2D and 3D microfluidic paper-based analytical devices for clinical analysis, Anal. Chim. Acta, 2017, 957, 40–46 Search PubMed.
- C. Srisomwat, P. Teengam, N. Chuaypen, P. Tangkijvanich, T. Vilaivan and O. Chailapakul, Pop-up paper electrochemical device for label-free hepatitis B virus DNA detection, Sens. Actuators, B, 2020, 316, 128077 CrossRef CAS.
- C. A. Chen, H. Yuan, C. W. Chen, Y. S. Chien, W. H. Sheng and C. F. Chen, An electricity- and instrument-free infectious disease sensor based on a 3D origami paper-based analytical device, Lab Chip, 2021, 21, 1908–1915 Search PubMed.
- C. Carrell, A. Kava, M. Nguyen, R. Menger, Z. Munshi, Z. Call, M. Nussbaum and C. Henry, Beyond the lateral flow assay: a review of paper-based microfluidics, Microelectron. Eng., 2019, 206, 45–54 Search PubMed.
- E. Noviana, T. Ozer, C. S. Carrell, J. S. Link, C. McMahon, I. Jang and C. S. Henry, Microfluidic Paper-Based Analytical Devices: From Design to Applications, Chem. Rev., 2021, 121, 11835–11885 CrossRef CAS.
- Y. S. Kim, Y. Yang and C. S. Henry, Laminated and infused Parafilm® – paper for paper-based analytical devices, Sens. Actuators, B, 2018, 255, 3654–3661 Search PubMed.
- R. Amin, F. Ghaderinezhad, C. Bridge, M. Temirel, S. Jones, P. Toloueinia and S. Tasoglu, Pushing the Limits of Spatial Assay Resolution for Paper-Based Microfluidics Using Low-Cost and High-Throughput Pen Plotter Approach, Micromachines, 2020, 11, 611 Search PubMed.
- F. Ghaderinezhad, R. Amin, M. Temirel, B. Yenilmez, A. Wentworth and S. Tasoglu, High-throughput rapid-prototyping of low-cost paper-based microfluidics, Sci. Rep., 2017, 7, 1–9 CrossRef CAS.
- V. N. Ataide, L. F. Mendes, L. I. L. M. Gama, W. R. De Araujo and T. R. L. C. Paixaõ, High-throughput rapid-prototyping of low-cost paper-based microfluidics, Anal. Methods, 2020, 12, 1030–1054 RSC.
- A. Nilghaz, X. Liu, L. Ma, Q. Huang and X. Lu, Development of fabric-based microfluidic devices by wax printing, Cellulose, 2019, 26, 3589–3599 CrossRef.
- S. Kasetsirikul, M. J. A. Shiddiky and N. T. Nguyen, Challenges and perspectives in the development of paper-based lateral flow assays, Microfluid. Nanofluid., 2020, 24, 1–18 CrossRef.
- R. Tortorich, H. Shamkhalichenar and J.-W. Choi, Inkjet-Printed and Paper-Based Electrochemical Sensors, Appl. Sci., 2018, 8, 288 CrossRef.
- N. N. Le, H. C. T. Phan, H. K. Tran, D. M. T. Dang and C. M. Dang, Fabrication of paper-based microfluidic channels by electrohydrodynamic inkjet printing technology for analytical biochemistry applications, Int. J. Nanotechnol., 2020, 17(7–10), 673–688 CrossRef CAS.
- E. Rosqvist, E. Niemelä, J. Frisk, H. Öblom, R. Koppolu, H. Abdelkader, D. Soto Véliz, M. Mennillo, A. P. Venu, P. Ihalainen, M. Aubert, N. Sandler, C. E. Wilén, M. Toivakka, J. E. Eriksson, R. Österbacka and J. Peltonen, A low-cost paper-based platform for fast and reliable screening of cellular interactions with materials, J. Mater. Chem. B, 2020, 8, 1146–1156 Search PubMed.
- D. Capoferri, R. Álvarez-Diduk, M. Del Carlo, D. Compagnone and A. Merkoçi, Electrochromic Molecular Imprinting Sensor for Visual and Smartphone-Based Detections, Anal. Chem., 2018, 90, 5850–5856 Search PubMed.
- Y. Jiang, Z. Hao, Q. He and H. Chen, A simple method for fabrication of microfluidic paper-based analytical devices and on-device fluid control with a portable corona generator, RSC Adv., 2016, 6, 2888–2894 RSC.
- T. Lam, J. P. Devadhasan, R. Howse and J. Kim, A Chemically Patterned Microfluidic Paper-based Analytical Device (C-μPAD) for Point-of-Care Diagnostics, Sci. Rep., 2017, 7, 1188 Search PubMed.
- Y. Guan and B. Sun, Detection and extraction of heavy metal ions using paper-based analytical devices fabricated via atom stamp printing, Microsyst. Nanoeng., 2020, 6, 14 Search PubMed.
- L. A. Santana-Jiménez, A. Márquez-Lucero, V. Osuna, I. Estrada-Moreno and R. B. Dominguez, Naked-Eye Detection of Glucose in Saliva with Bienzymatic Paper-Based Sensor, Sensors, 2018, 18, 1071 Search PubMed.
- E. W. Nery and L. T. Kubota, Sensing approaches on paper-based devices: a review, Anal. Bioanal. Chem., 2013, 405, 7573–7595 CrossRef CAS.
- D. H. Ho, Y. Y. Choi, S. B. Jo, J. M. Myoung and J. H. Cho, Sensing with MXenes: progress and prospects, Adv. Mater., 2021, 33, 2005846 CrossRef CAS.
- L. Wu, X. Yuan, Y. Tang, S. Wageh, O. A. Al-Hartomy, A. G. Al-Sehemi, J. Yang, Y. Xiang, H. Zhang and Y. Qin, MXene sensors based on optical and electrical sensing signals: from biological, chemical, and physical sensing to emerging intelligent and bionic devices, PhotoniX, 2023, 4, 15 CrossRef.
- Y. Pei, X. Zhang, Z. Hui, J. Zhou, X. Huang, G. Sun and W. Huang, Ti3C2TX MXene for sensing applications: recent progress, design principles, and future perspectives, ACS Nano, 2021, 15, 3996–4017 CrossRef CAS.
- Y. Z. Zhang, Y. Wang, Q. Jiang, J. K. El-Demellawi, H. Kim and H. N. Alshareef, MXene printing and patterned coating for device applications, Adv. Mater., 2020, 32, 1908486 CrossRef CAS.
- B. Zazoum, A. Bachri and J. Nayfeh, Functional 2D MXene inks for wearable electronics, Materials, 2021, 14, 6603 CrossRef CAS.
- H. Wu, Y. Xie, Y. Ma, B. Zhang, B. Xia, P. Zhang, W. Qian, D. He, X. Zhang and B. W. Li, Aqueous MXene/Xanthan Gum Hybrid Inks for Screen-Printing Electromagnetic Shielding, Joule Heater, and Piezoresistive Sensor, Small, 2022, 18, 2107087 Search PubMed.
- T. Zhang, Y. Zhao, Q. Long, X. Zhu, L. He, Z. Li, X. Qian, X. He, J. Li and C. Lv, Graphene/MXene/cellulose cellulosic paper-based flexible bifunctional sensors utilizing molecular bridge strategy with tunable piezoresistive effect for temperature-pressure sensing, Chem. Eng. J., 2024, 497, 154972 CrossRef CAS.
- L. Wang, Y. Han, H. Wang, Y. Han, J. Liu, G. Lu and H. Yu, A MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I, J. Semicond., 2021, 42, 092601 CrossRef CAS.
- J. Shu, L. Gao, Y. Li, P. Wang, X. Deng, X. Yan, K. Qu and L. Li, MXene/tissue paper composites for wearable pressure sensors and thermotherapy electronics, Thin Solid Films, 2022, 743, 139054 CrossRef CAS.
- C. Lin, S. Luo, F. Meng, B. Xu, T. Long, Y. Zhao, H. Hu, L. Zheng, K. Liao and J. Liu, MXene/air-laid paper composite sensors for both tensile and torsional deformations detection, Compos. Commun., 2021, 25, 100768 CrossRef.
- Y. Ma, Y. Cheng, J. Wang, S. Fu, M. Zhou, Y. Yang, B. Li, X. Zhang and C. W. Nan, Flexible and highly-sensitive pressure sensor based on controllably oxidized MXene, InfoMat, 2022, 4, e12328 CrossRef CAS.
- A. Sinha, H. Zhao, Y. Huang, X. Lu, J. Chen and R. Jain, MXene: an emerging material for sensing and biosensing, TrAC, Trends Anal. Chem., 2018, 105, 424–435 CrossRef CAS.
- R. Rakhi, P. Nayak, C. Xia and H. N. Alshareef, Novel amperometric glucose biosensor based on MXene nanocomposite, Sci. Rep., 2016, 6, 36422 Search PubMed.
- J. Liu, X. Jiang, R. Zhang, Y. Zhang, L. Wu, W. Lu, J. Li, Y. Li and H. Zhang, MXene-enabled electrochemical microfluidic biosensor: applications toward multicomponent continuous monitoring in whole blood, Adv. Funct. Mater., 2019, 29, 1807326 CrossRef.
- H. Wang, H. Li, Y. Huang, M. Xiong, F. Wang and C. Li, A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes, Biosens. Bioelectron., 2019, 142, 111531 CrossRef CAS PubMed.
- H. Liu, C. Duan, C. Yang, W. Shen, F. Wang and Z. Zhu, A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2, Sens. Actuators, B, 2015, 218, 60–66 CrossRef CAS.
- Q. Wu, N. Li, Y. Wang, Y. Xu, S. Wei, J. Wu, G. Jia, X. Fang, F. Chen and X. Cui, A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection, Biosens. Bioelectron., 2019, 144, 111697 CrossRef CAS.
- N. Suk-in, K. Thongpim, W. Phamonpon, J. Yukird, S. Ummartyotin and N. Rodthongkum, Dual Colorimetric/Electrochemical Sensor of Carbaryl in Fruits on Microfluidic Paper-Based Analytical Device Connected with Smartphone Readout, J. Food Compos. Anal., 2024, 133, 106445 CrossRef CAS.
- E. Wang, Y. Cao, Y. Bai, Y. Gai, Y. Shan, Q. Li, T. Jiang, H. Feng and Z. Li, A triboelectric nanosensor based on ultra-thin MXene composite paper for heavy metal ion detection, J. Micromech. Microeng., 2022, 32, 044003 CrossRef CAS.
- X. Li, Y. Lu and Q. Liu, Electrochemical and optical biosensors based on multifunctional MXene nanoplatforms: progress and prospects, Talanta, 2021, 235, 122726 CrossRef CAS PubMed.
- M. Li, L. Wang, R. Liu, J. Li, Q. Zhang, G. Shi, Y. Li, C. Hou and H. Wang, A highly integrated sensing paper for wearable electrochemical sweat analysis, Biosens. Bioelectron., 2021, 174, 112828 CrossRef CAS.
- Y. Bu, T. Shen, W. Yang, S. Yang, Y. Zhao, H. Liu, Y. Zheng, C. Liu and C. Shen, Ultrasensitive strain sensor based on superhydrophobic microcracked conductive Ti3C2Tx MXene/paper for human-motion monitoring and E-skin, Sci. Bull., 2021, 66, 1849–1857 CrossRef CAS.
- S. K. Panda, S. Mishra and A. K. Singh, Recent progress in the development of MOF-based optical sensors for Fe3+, Dalton Trans., 2021, 50, 7139–7155 RSC.
- H. Sohrabi, S. Ghasemzadeh, Z. Ghoreishi, M. R. Majidi, Y. Yoon, N. Dizge and A. Khataee, Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: a review of current status and future prospects, Mater. Chem. Phys., 2023, 299, 127512 CrossRef CAS.
- L. J. Small, M. E. Schindelholz and T. M. Nenoff, Hold on tight: MOF-based irreversible gas sensors, Ind. Eng. Chem. Res., 2021, 60, 7998–8006 CrossRef CAS.
- J. Yang and Y. W. Yang, Metal–organic frameworks for biomedical applications, Small, 2020, 16, 1906846 CrossRef CAS.
- J. Huang, J. Pan, Y. Song, Q. Lin, Y. Xu, Z. Dai and S.-Y. Liu, MOF-functionalized paper-based biosensors: fabrications, mechanisms and applications, TrAC, Trends Anal. Chem., 2024, 117619 CrossRef CAS.
- L. Zhong, J. Qian, N. Wang, S. Komarneni and W. Hu, Metal–organic frameworks on versatile substrates, J. Mater. Chem. A, 2023, 11, 20423–20458 RSC.
- P. Goel, S. Singh, H. Kaur, S. Mishra and A. Deep, Low-cost inkjet printing of metal–organic frameworks patterns on different substrates and their applications in ammonia sensing, Sens. Actuators, B, 2021, 329, 129157 CrossRef CAS.
- I. Ortiz-Gómez, A. Salinas-Castillo, A. G. García, J. A. Álvarez-Bermejo, I. de Orbe-Payá, A. Rodríguez-Diéguez and L. F. Capitán-Vallvey, Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic, Microchim. Acta, 2018, 185, 1–8 CrossRef.
- Q. Lu, T. Su, Z. Shang, D. Jin, Y. Shu, Q. Xu and X. Hu, Flexible paper-based Ni-MOF composite/AuNPs/CNTs film electrode for HIV DNA detection, Biosens. Bioelectron., 2021, 184, 113229 CrossRef CAS.
- X. Yang, Y. Deng, H. Yang, Y. Liao, X. Cheng, Y. Zou, L. Wu and Y. Deng, Functionalization of mesoporous semiconductor metal oxides for gas sensing: recent advances and emerging challenges, Adv. Sci., 2023, 10, 2204810 CrossRef CAS PubMed.
- K. Vaid, J. Dhiman, S. Kumar, K.-H. Kim and V. Kumar, Mixed metal (cobalt/molybdenum) based metal-organic frameworks for highly sensitive and specific sensing of arsenic (V): spectroscopic versus paper-based approaches, Chem. Eng. J., 2021, 426, 131243 CrossRef CAS.
- T. Leelasree, V. Selamneni, T. Akshaya, P. Sahatiya and H. Aggarwal, MOF based flexible, low-cost chemiresistive device as a respiration sensor for sleep apnea diagnosis, J. Mater. Chem. B, 2020, 8, 10182–10189 RSC.
- G. C. Ilacas, A. Basa, K. J. Nelms, J. D. Sosa, Y. Liu and F. A. Gomez, Paper-based microfluidic devices for glucose assays employing a metal-organic framework (MOF), Anal. Chim. Acta, 2019, 1055, 74–80 CrossRef CAS PubMed.
- Z. Xia, D. Li and W. Deng, Identification and detection of volatile aldehydes as lung cancer biomarkers by vapor generation combined with paper-based thin-film microextraction, Anal. Chem., 2021, 93, 4924–4931 CrossRef CAS.
- Q. Jiang, Y. J. Chandar, S. Cao, E. D. Kharasch, S. Singamaneni and J. J. Morrissey, Rapid, point-of-care, paper-based plasmonic biosensor for Zika virus diagnosis, Adv. Biosyst., 2017, 1, 1700096 CrossRef.
- L. Alvarado-Ramírez, M. Rostro-Alanis, J. Rodríguez-Rodríguez, J. E. Sosa-Hernández, E. M. Melchor-Martínez, H. M. Iqbal and R. Parra-Saldívar, Enzyme (single and multiple) and nanozyme biosensors: recent developments and their novel applications in the water-food-health nexus, Biosensors, 2021, 11, 410 CrossRef PubMed.
- X. Wei, J. Guo, H. Lian, X. Sun and B. Liu, Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics, Sens. Actuators, B, 2021, 329, 129205 CrossRef CAS.
- B. Feng, Z. Wang, Y. Feng, P. Li, Y. Zhu, Y. Deng, L. Wu, Q. Yue and J. Wei, Single-Atom Au-Functionalized Mesoporous SnO2 Nanospheres for Ultrasensitive Detection of Listeria monocytogenes Biomarker at Low Temperatures, ACS Nano, 2024, 18, 22888–22900 CrossRef CAS PubMed.
- Y. Chen, Y. Li, B. Feng, Y. Wu, Y. Zhu and J. Wei, Self-templated synthesis of mesoporous Au-ZnO nanospheres for seafood freshness detection, Sens. Actuators, B, 2022, 360, 131662 CrossRef CAS.
- S. Liu, C. Lai, X. Liu, B. Li, C. Zhang, L. Qin, D. Huang, H. Yi, M. Zhang and L. Li, Metal-organic frameworks and their derivatives as signal amplification elements for electrochemical sensing, Coord. Chem. Rev., 2020, 424, 213520 CrossRef CAS.
- J. Chi, Y. Song and L. Feng, A ratiometric fluorescent paper sensor based on dye-embedded MOF for high-sensitive detection of arginine, Biosens. Bioelectron., 2023, 241, 115666 CrossRef CAS.
- Y. A. Chen, F. J. Tsai, Y. T. Zeng, J. C. Wang, C. P. Hong, P. H. Huang, H. L. Chuang, S. Y. Lin, C. T. Chan and Y. C. Ko, Fast and Effective Turn-on Paper-based Phosphorescence Biosensor for Detection of Glucose in Serum, J. Chin. Chem. Soc., 2016, 63, 424–431 CrossRef CAS.
- J. Yu, L. Ge, J. Huang, S. Wang and S. Ge, Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid, Lab Chip, 2011, 11, 1286–1291 RSC.
- H. Liu, X. Li and R. M. Crooks, based SlipPAD for high-throughput chemical sensing, Anal. Chem., 2013, 85, 4263–4267 CrossRef CAS PubMed.
- H. A. Al Lawati, J. Hassanzadeh, N. Bagheri and I. Al Lawati, On paper synthesis of metal-organic framework as a chemiluminescence enhancer for estimating the total phenolic content of food samples using a smartphone readout, Talanta, 2021, 234, 122648 CrossRef CAS.
- H. Wang, Y. Jian, Q. Kong, H. Liu, F. Lan, L. Liang, S. Ge and J. Yu, Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks, Sens. Actuators, B, 2018, 257, 561–569 CrossRef CAS.
- C. Zhu, C. Ren, W. Jiang, D. Liu, Y. Huang, W. Wang, K. Chang, L. Zhu and Q. Wang, A versatile SERS platform based on conductive MOF-enforced carbon paper for rapidly and sensitively monitoring diazepam in aquatic products, Food Chem., 2024, 435, 137608 CrossRef CAS.
- J. Hassanzadeh, H. A. Al Lawati and N. Bagheri, On paper synthesis of multifunctional CeO2 nanoparticles@ Fe-MOF composite as a multi-enzyme cascade platform for multiplex colorimetric detection of glucose, fructose, sucrose, and maltose, Biosens. Bioelectron., 2022, 207, 114184 CrossRef CAS PubMed.
- W. Xiu, P. Zhao, Y. Pan, X. Wang, L. Zhang, S. Ge and J. Yu, Flexible SERS strip based on HKUST-1 (cu)/biomimetic antibodies composite multilayer for trace determination of ethephon, Anal. Chim. Acta, 2023, 1253, 341097 CrossRef CAS.
- R. Jiang, Y.-H. Pang, Q.-Y. Yang, C.-Q. Wan and X.-F. Shen, Copper porphyrin metal-organic framework modified carbon paper for electrochemical sensing of glyphosate, Sens. Actuators, B, 2022, 358, 131492 CrossRef CAS.
- J. Huang, Z. Li, Y. Mao and Z. Li, Progress and biomedical applications of MXenes, Nano Sel., 2021, 2, 1480–1508 CrossRef CAS.
- T. Rasheed, MXenes as emerging two-dimensional analytical modalities for potential recognition of hazardous environmental contaminants, Mater. Today Chem., 2022, 24, 100859 CrossRef CAS.
- M. Mathew and C. S. Rout, Electrochemical biosensors based on Ti3C2Tx MXene: future perspectives for on-site analysis, Curr. Opin. Electrochem., 2021, 30, 100782 CrossRef CAS.
- Q. Xia, Y. Fan, S. Li, A. Zhou, N. Shinde and R. S. Mane, MXene-based chemical gas sensors: recent developments and challenges, Diamond Relat. Mater., 2023, 131, 109557 CrossRef CAS.
- W. Quan, J. Shi, H. Luo, C. Fan, W. Lv, X. Chen, M. Zeng, J. Yang, N. Hu and Y. Su, Fully flexible MXene-based gas sensor on paper for highly sensitive room-temperature nitrogen dioxide detection, ACS Sens., 2023, 8, 103–113 CrossRef CAS PubMed.
- J. Zhou, S. H. H. Shokouh, L. Cui, T. Järvinen, O. Pitkänen, Z.-P. Lv and K. Kordas, An ultra-sensitive NH3 gas sensor enabled by an ion-in-conjugated polycroconaine/Ti3C2Tx core–shell composite, Nanoscale Horiz., 2023, 8, 794–802 RSC.
- Z. Zhu, C. Liu, F. Jiang, J. Liu, X. Ma, P. Liu, J. Xu, L. Wang and R. Huang, Flexible and lightweight Ti3C2Tx MXene@ Pd colloidal nanoclusters paper film as novel H2 sensor, J. Hazard Mater., 2020, 399, 123054 CrossRef CAS PubMed.
- C. Ma, M. G. Ma, C. Si, X. X. Ji and P. Wan, Flexible MXene-based composites for wearable devices, Adv. Funct. Mater., 2021, 31, 2009524 CrossRef CAS.
- S.-C. Yu, T.-Y. Huang and T.-E. Lin, MXene Nanosheets-Decorated Paper as a Green Electronics Material for Biosensing, ACS Meas. Sci. Au, 2024, 4(1), 81–91 CrossRef CAS.
- H. Liu, Q. Zhang, N. Yang, X. Jiang, F. Wang, X. Yan, X. Zhang, Y. Zhao and T. Cheng, Ti3C2Tx MXene Paper-Based Wearable and Degradable Pressure Sensor for Human Motion Detection and Encrypted Information Transmission, ACS Appl. Mater. Interfaces, 2023, 15, 44554–44562 CrossRef CAS PubMed.
- L. Zhang, Y. Huang, H. Dong, R. Xu and S. Jiang, Flame-retardant shape memory polyurethane/MXene paper and the application for early fire alarm sensor, Composites, Part B, 2021, 223, 109149 CrossRef CAS.
- Y. Wang, N. Sun, H. Cheng, S. Zhou, X. Ouyang, X. Zhang and N. Ma, Highly Sensitive Flexible Thermal Sensors Based on a Kind of MXene/DES Inks, ACS Appl. Electron. Mater., 2023, 5, 1252–1261 CrossRef CAS.
- J. E. d. S. Souza, G. P. d. Oliveira, J. Y. Alexandre, J. G. Neto, M. B. Sales, P. G. d. S. Junior, A. L. d. Oliveira, M. C. d. Souza and J. C. d. Santos, A comprehensive review on the use of metal–organic frameworks (MOFs) coupled with enzymes as biosensors, Electrochem, 2022, 3, 89–113 CrossRef CAS.
- Y. Yang, W. Ji, Y. Yin, N. Wang, W. Wu, W. Zhang, S. Pei, T. Liu, C. Tao and B. Zheng, Catalytic modification of porous two-dimensional Ni-MOFs on portable electrochemical paper-based sensors for glucose and hydrogen peroxide detection, Biosensors, 2023, 13, 508 CrossRef CAS PubMed.
- X. Ma, J. Kang, Y. Wu, C. Pang, S. Li, J. Li, Y. Xiong, J. Luo, M. Wang and Z. Xu, Recent advances in metal/covalent organic framework-based materials for photoelectrochemical sensing applications, TrAC, Trends Anal. Chem., 2022, 157, 116793 CrossRef CAS.
- T. Yan, G. Zhang, K. Yu, H. Chai, M. Tian, L. Qu, H. Dong and X. Zhang, Smartphone light-driven zinc porphyrinic MOF nanosheets-based enzyme-free wearable photoelectrochemical sensor for continuous sweat vitamin C detection, Chem. Eng. J., 2023, 455, 140779 CrossRef CAS.
- X. Kou, L. Tong, Y. Shen, W. Zhu, L. Yin, S. Huang, F. Zhu, G. Chen and G. Ouyang, Smartphone-assisted robust enzymes@ MOFs-based paper biosensor for point-of-care detection, Biosens. Bioelectron., 2020, 156, 112095 CrossRef CAS PubMed.
- H. A. Al Lawati and J. Hassanzadeh, Dual-function 2D cobalt metal-organic framework embedded on paper as a point-of-care diagnostic device: application for the quantification of glucose, Anal. Chim. Acta, 2020, 1139, 15–26 CrossRef CAS.
- A. Ahmadi, S. M. Khoshfetrat, S. Kabiri, P. S. Dorraji, B. Larijani and K. Omidfar, Electrochemiluminescence paper-based screen-printed electrode for HbA1c detection using two-dimensional zirconium metal-organic framework/Fe3O4 nanosheet composites decorated with Au nanoclusters, Microchim. Acta, 2021, 188, 296 CrossRef CAS.
- W. Cheng, X. Tang, Y. Zhang, D. Wu and W. Yang, Applications of metal-organic framework (MOF)-based sensors for food safety: enhancing mechanisms and recent advances, Trends Food Sci. Technol., 2021, 112, 268–282 CrossRef CAS.
- W. Wu, Y. Li, P. Song, Q. Xu, N. Long, P. Li, L. Zhou, B. Fu, J. Wang and W. Kong, Metal-organic framework (MOF)-based sensors for exogenous contaminants in food: mechanisms, advances, and prospects, Trends Food Sci. Technol., 2023, 138, 238–271 CrossRef CAS.
- A. Hitabatuma, P. Wang, X. Su and M. Ma, Metal-organic frameworks-based sensors for food safety, Foods, 2022, 11, 382 CrossRef CAS.
- J. Hassanzadeh, H. A. Al Lawati and I. Al Lawati, Metal–organic framework loaded by rhodamine B as a novel chemiluminescence system for the paper-based analytical devices and its application for total phenolic content determination in food samples, Anal. Chem., 2019, 91, 10631–10639 CrossRef CAS PubMed.
- H. Martínez-Pérez-Cejuela, R. B. Mesquita, E. Simó-Alfonso, J. Herrero-Martínez and A. O. Rangel, Combining microfluidic paper-based platform and metal–organic frameworks in a single device for phenolic content assessment in fruits, Microchim. Acta, 2023, 190, 126 CrossRef.
- G. L. Yang, X. L. Jiang, H. Xu and B. Zhao, Applications of MOFs as luminescent sensors for environmental pollutants, Small, 2021, 17, 2005327 CrossRef CAS.
- Y. Shen, A. Tissot and C. Serre, Recent progress on MOF-based optical sensors for VOC sensing, Chem. Sci., 2022, 13, 13978–14007 RSC.
- S. H. Al-Jaf, S. S. M. Ameen and K. M. Omer, A novel ratiometric design of microfluidic paper-based analytical device for the simultaneous detection of Cu2+ and Fe3+ in drinking water using a fluorescent MOF@ tetracycline nanocomposite, Lab Chip, 2024, 24, 2306–2316 RSC.
- B. Dey, G. Sarkhel and A. Choudhury, Facile synthesis of copper MOF/carbon nanofiber nanocomposite paper for electrochemical detection of toxic 4-nitrophenol, J. Macromol. Sci., Part A: Pure Appl. Chem., 2023, 60, 150–160 CrossRef CAS.
- X. Wang, Y. Jiang, A. Tissot and C. Serre, Luminescent sensing platforms based on lanthanide metal-organic frameworks: current strategies and perspectives, Coord. Chem. Rev., 2023, 497, 215454 CrossRef CAS.
- L. Zhang, G. Liang, P. Han, Z. Zhang and Q. Liu, A convenient lanthanide metal–organic framework based visual fluorescence paper microsensor and logic device for triazophos, J. Sci.: Adv. Mater. Devices, 2022, 7, 100502 CAS.
- X. Zhang, Z. Li, L. Hong, X. Wang and J. Cao, Tetrahedral DNA Nanostructure-Engineered Paper-Based Electrochemical Aptasensor for Fumonisin B1 Detection Coupled with Au@ Pt Nanocrystals as an Amplification Label, J. Agric. Food Chem., 2023, 71, 19121–19128 CrossRef CAS.
- W. Pang, Y. Gao, X. Chang, G. Du, Z. Hu, T. Hu and X. Ma, Highly Sensitive Detection of Apigenin Using a Paper-Based Sensor with ZIF-8@ MXene, J. Inorg. Organomet. Polym. Mater., 2024, 1–9 Search PubMed.
- G. Ji, W. Zhu, X. Jia, S. Ji, D. Han, Z. Gao, H. Liu, Y. Wang and T. Han, AuNP/Cu-TCPP (Fe) metal–organic framework nanofilm: a paper-based electrochemical sensor for non-invasive detection of lactate in sweat, Nanoscale, 2023, 15, 5023–5035 RSC.
- Y.-H. Pang, Q.-Y. Yang, R. Jiang, Y.-Y. Wang and X.-F. Shen, A stack-up electrochemical device based on metal-organic framework modified carbon paper for ultra-trace lead and cadmium ions detection, Food Chem., 2023, 398, 133822 CrossRef CAS PubMed.
- X. Cui, J. Li, Y. Li, M. Liu, J. Qiao, D. Wang, H. Cao, W. He, Y. Feng and Z. Yang, Detection of glucose in diabetic tears by using gold nanoparticles and MXene composite surface-enhanced Raman scattering substrates, Spectrochim. Acta, Part A, 2022, 266, 120432 CrossRef CAS.
- J. Yang, C. Wang, L. Liu, H. Zhang and J. Ma, Water-tolerant MXene epidermal sensors with high sensitivity and reliability for healthcare monitoring, ACS Appl. Mater. Interfaces, 2022, 14, 21253–21262 CrossRef CAS PubMed.
- W.-B. Zhu, F.-L. Yi, P. Huang, H. Zhang, Z.-H. Tang, Y.-Q. Fu, Y.-Y. Wang, J. Huang, G.-H. Dong and Y.-Q. Li, Flexible but robust Ti3C2Tx MXene/bamboo microfibril composite paper for high-performance wearable electronics, J. Mater. Chem. A, 2021, 9, 26758–26766 RSC.
- X. Guo, W. Hong, B. Hu, T. Zhang, C. Jin, X. Yao, H. Li, Z. Yan, Z. Jiao and M. Wang, Human touch sensation-inspired, ultrawide-sensing-range, and high-robustness flexible piezoresistive sensor based on CB/MXene/SR/fiber nanocomposites for wearable electronics, Compos. Struct., 2023, 321, 117329 CrossRef CAS.
- R. Qin, X. Li, M. Hu, G. Shan, R. Seeram and M. Yin, Preparation of high-performance MXene/PVA-based flexible pressure sensors with adjustable sensitivity and sensing range, Sens. Actuators, A, 2022, 338, 113458 CrossRef CAS.
- S. Kulandaivel, W.-C. Lo, C.-H. Lin and Y.-C. Yeh, Cu-PyC MOF with oxidoreductase-like catalytic activity boosting colorimetric detection of Cr (VI) on paper, Anal. Chim. Acta, 2022, 1227, 340335 CrossRef CAS.
- Y. Yu, D. Pan, S. Qiu, L. Ren, S. Huang, R. Liu, L. Wang and H. Wang, Polyphenylene sulfide paper-based sensor modified by Eu-MOF for efficient detection of Fe3+, React. Funct. Polym., 2021, 165, 104954 CrossRef CAS.
- Y. Li, M. Sun, Y. Yang, H. Meng, Q. Wang, C. Li and G. Li, Luminescence-colour-changing sensing toward neurological drug carbamazepine in water and biofluids based on white light-emitting CD/Ln-MOF/PVA test papers, J. Mater. Chem. C, 2021, 9, 8683–8693 RSC.
- Q.-R. Liu, B. Liu, M.-M. Qiu, W.-N. Miao and L. Xu, A Europium MOF-based turn-off fluorescent sensor for tryptophan detection in human serum, urine and lake water, J. Solid State Chem., 2022, 311, 123138 CrossRef CAS.
- K. F. Kayani and K. M. Omer, A red luminescent europium metal organic framework (Eu-MOF) integrated with a paper strip using smartphone visual detection for determination of folic acid in pharmaceutical formulations, New J. Chem., 2022, 46, 8152–8161 RSC.
- J. Yan, J. Zhang, M. Zhang and G. Shi, Lanthanide metal-organic framework as a paper strip sensor for visual detection of sulfonamide with smartphone-based point-of-care platform, Talanta, 2022, 237, 122920 CrossRef CAS.
- A. F. Kateshali, F. Moghzi, J. Soleimannejad and J. Janczak, Bacterial Cellulose–Based MOF Hybrid as a Sensitive Switch Off–On Luminescent Sensor for the Selective Recognition of l-Histidine, Inorg. Chem., 2024, 63, 3560–3571 CrossRef PubMed.
- C. Lv, Y. Hou, Y. Guo, X. Ma, Y. Zhang, Y. Liu, Y. Jin, B. Li and W. Liu, A metal–organic framework loaded paper-based chemiluminescence sensor for trace acetone detection in exhaled breath, Anal. Methods, 2022, 14, 4514–4522 RSC.
- H. Martínez-Pérez-Cejuela, M. M. Calabretta, V. Bocci, M. D'Elia and E. Michelini, Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection, Biosensors, 2023, 13, 451 CrossRef.
- M. Sher, R. Zhuang, U. Demirci and W. Asghar, Paper-based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms, Expert Rev. Mol. Diagn., 2017, 17, 351–366 CrossRef CAS PubMed.
- S. R. Benjamin, F. de Lima, V. A. do Nascimento, G. M. de Andrade and R. B. Oriá, Advancement in Paper-Based Electrochemical Biosensing and Emerging Diagnostic Methods, Biosensors, 2023, 13, 689 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |
