Ordered crown-ether 2D framework based loose nanofiltration membranes for improved separation and stability†
Jae Jun
Kim
a,
Huiran
Seo
a,
Jinseok
Kim
a,
Mun Hyeon
Kim
a,
Jinwook
Park
a,
Hyunkee
Hong
a,
Hee Joong
Kim
 *b and
Jong-Chan
Lee
*b and
Jong-Chan
Lee
 *a
*a
aDepartment of Chemical and Biological Engineering and Institute of Chemical Processes, Seoul National University, 1 Gwanak-ro, Seoul, 08826, Republic of Korea. E-mail: jongchan@snu.ac.kr
bDepartment of Polymer Science and Engineering & Program in Environmental and Polymer Engineering, Inha University, Incheon, 22212, Republic of Korea. E-mail: heejoong@inha.ac.kr
First published on 11th November 2024
Abstract
Loose nanofiltration (LNF) membranes, known for high water permeability and dye/salt selectivity, have been widely used. However, LNF membranes fabricated through layer-by-layer (LbL) assembly lack structural stability and uniformity. Herein, we employed a 2D crown-ether framework (C2O) for LbL assembly due to its physical stability, uniform pores, and chemical robustness. The LbL membrane exhibited a high water permeance of 39 L m−2 h−1 bar−1, excellent dye rejection of 99.8% for methyl blue (MB) and congo red (CR), and 99.7% for eriochrome black T (EBT), as well as superior dye/salt selectivity (EBT/NaCl selectivity of 295). Furthermore, the membrane showed remarkable stability under varying pH conditions and throughout prolonged operation.
Purification of wastewater is a crucial process in industrial facilities, essential for safeguarding environmental health and ensuring regulatory compliance.1,2 Organic pollutants, such as bisphenol A, phthalates, polycyclic aromatic hydrocarbons, and dyes are known to be carcinogenic and endocrine disruptors, posing significant risks to both the environment and human health.3,4 Among various wastewater purification techniques including adsorption, coagulation, and biological degradation, the pressure-driven membrane filtration process is considered to be the most efficient given the simple operation, relatively small floor space, and high productivity.5,6 The membrane filtration processes are categorized based on their selectivity: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). NF membranes have been widely used in wastewater treatment due to their moderate operating pressure and relatively high rejection of small organic pollutants.
Conventional NF membranes are characterized by (i) small pore diameter (ca. <2 nm), (ii) reasonable rejection of divalent ions, and (iii) molecular weight cut-off (MWCO) < 500 Da, specifically categorizing them as tight NF membranes.7 Despite high rejection of tight NF membranes for multivalent ions and small organic solutes, they suffer from low water productivity and a narrow application window due to the small pore size and high concentration polarization.8 Currently, loose nanofiltration (LNF) membranes, characterized by relatively high water permeability and effective organic pollutant/salt fractionation, have received significant attention as they are more suitable for wastewater treatment and/or resource recovery involving relatively large molecules.9,10 These LNF membranes offer a promising alternative, addressing the limitations of tight NF membranes by providing higher water flux and higher selectivity against organic pollutants and inorganic salts.11
Several methods, such as interfacial polymerization, surface grafting, dip-coating, and layer-by-layer (LbL) assembly, have been employed to fabricate NF membranes.12,13 Among these, LbL assembly is a promising method for preparing LNF membranes because it allows for precise control of membrane parameters (i.e., thickness, composition, functionality, and architecture).14 In general, the LbL assembly process involves the sequential adsorption of alternating layers of oppositely charged materials, typically polyelectrolytes, onto a substrate. For example, Joseph et al. reported various polyelectrolyte multilayer membranes, which showed excellent separation performance.15 However, most polyelectrolytes are not stable under filtration conditions, resulting in the degradation of membrane performance.16 Charged nanofillers such as graphene oxide (GO),17,18 carbon nanotubes (CNTs),19 covalent organic frameworks (COFs),20,21 and metal–organic frameworks (MOFs)22 have been included to stabilize polyelectrolyte multilayers. These nanocomposite LbL membranes exhibited improved separation performance and mechanical strength.23 However, most of them contain defective structures (i.e., non-uniform pores), require multiple synthetic/purification steps, and are unstable under harsh conditions, making them difficult to apply in practice. Therefore, employing nanomaterials that are scalable, stable, possess uniform pores, and can be easily functionalized in the LbL process is a critical challenge.
In this context, we identified an ordered crown-ether 2D framework, designated as C2O, which primarily consists of carbon and oxygen in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, as an attractive structural material for LbL assembly in the fabrication of LNF membranes. C2O is relatively inexpensive, scalable, physicochemically stable, possesses numerous functionalizable edges (i.e., hydroxyl and halide groups), and has a uniform pore size distribution (pore size ∼ 0.88 nm),24 making it a promising alternative to conventional 2D nanofillers. In this study, LNF membranes were prepared via LbL assembly of polycation-grafted C2O and polyanion in a controlled manner. The resultant LbL membranes demonstrated excellent water permeability and rejection of model organic pollutants, with the added benefit of long-term chemical stability, attributed to the unique characteristics of C2O structures. We concluded that C2O-based LbL membranes have significant potential for future advanced LNF applications.
1 molar ratio, as an attractive structural material for LbL assembly in the fabrication of LNF membranes. C2O is relatively inexpensive, scalable, physicochemically stable, possesses numerous functionalizable edges (i.e., hydroxyl and halide groups), and has a uniform pore size distribution (pore size ∼ 0.88 nm),24 making it a promising alternative to conventional 2D nanofillers. In this study, LNF membranes were prepared via LbL assembly of polycation-grafted C2O and polyanion in a controlled manner. The resultant LbL membranes demonstrated excellent water permeability and rejection of model organic pollutants, with the added benefit of long-term chemical stability, attributed to the unique characteristics of C2O structures. We concluded that C2O-based LbL membranes have significant potential for future advanced LNF applications.
A 2D covalent organic framework with uniform crown-ether holes (C2O) was prepared via a simple condensation reaction, as reported previously (see Fig. S1† for structural characterization).24 The hydroxyl groups at the edges were functionalized using 2-bromo-2-methylpropionyl bromide, followed by atom transfer radical polymerization (ATRP) of quaternized 2-(dimethylamino)ethyl methacrylate (QDM) (Fig. 1a). The characteristic functional groups were investigated by FT-IR (Fig. 1b). Bromination of C2O resulted in the appearance of new peaks at 1760 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 610 cm−1 (C–Br stretching). Subsequent graft polymerization produced peaks at 2978 cm−1 (C–H stretching), 1722 cm−1 (C
O stretching) and 610 cm−1 (C–Br stretching). Subsequent graft polymerization produced peaks at 2978 cm−1 (C–H stretching), 1722 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), and 1135 cm−1 (C–O stretching), indicating the successful graft polymerization of PQDM at the edges of C2O. This is further supported by the characteristic peaks of the quaternized ammonium group at 1478 cm−1 (C–N+ stretching) and 963 cm−1 (C–N+ stretching).25 For all C2O, C2O–Br, and C2O–PQDM samples, TEM images clearly show that the framework structures remained intact and were not degraded or destroyed upon functionalization (Fig. 1c and S4†). Furthermore, the lattice distance increased after bromination and subsequent polymerization, which is attributed to the intercalation of functional groups. This observation is consistent with AFM images (Fig. 1d). Additional analytic data shown in the ESI (Fig. S5 and S6 and Tables S1–S3†) confirmed the successful functionalization and graft polymerization. Specifically, TGA data suggested that the weight fraction of the cationic polymer is approximately 77 wt%. This aligns with the atomic compositions (82 wt%) calculated from the XPS and EA (Tables S1 and S2†). The positive zeta potential (34 mV) of C2O–PQDM further indicated the successful grafting of a cationic polymer (i.e., PQDM).
O stretching), and 1135 cm−1 (C–O stretching), indicating the successful graft polymerization of PQDM at the edges of C2O. This is further supported by the characteristic peaks of the quaternized ammonium group at 1478 cm−1 (C–N+ stretching) and 963 cm−1 (C–N+ stretching).25 For all C2O, C2O–Br, and C2O–PQDM samples, TEM images clearly show that the framework structures remained intact and were not degraded or destroyed upon functionalization (Fig. 1c and S4†). Furthermore, the lattice distance increased after bromination and subsequent polymerization, which is attributed to the intercalation of functional groups. This observation is consistent with AFM images (Fig. 1d). Additional analytic data shown in the ESI (Fig. S5 and S6 and Tables S1–S3†) confirmed the successful functionalization and graft polymerization. Specifically, TGA data suggested that the weight fraction of the cationic polymer is approximately 77 wt%. This aligns with the atomic compositions (82 wt%) calculated from the XPS and EA (Tables S1 and S2†). The positive zeta potential (34 mV) of C2O–PQDM further indicated the successful grafting of a cationic polymer (i.e., PQDM).
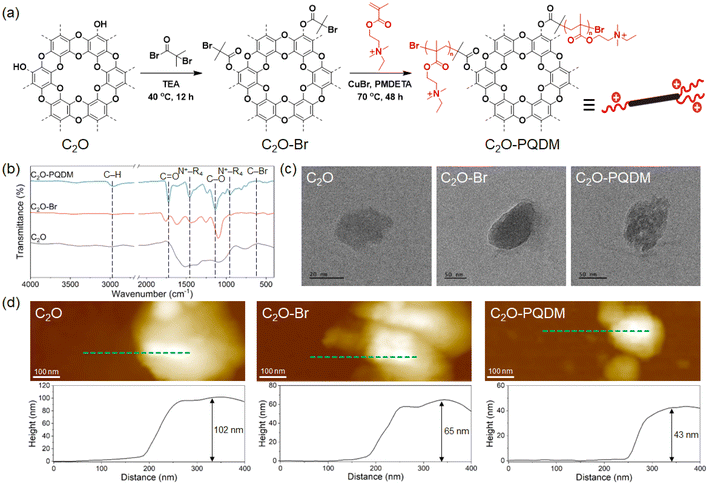 | ||
| Fig. 1 (a) Synthetic routes for C2O–Br and C2O–PQDM. (b) FT-IR spectra, (c) TEM images, (d) AFM images and height profiles of C2O, C2O–Br, and C2O–PQDM. | ||
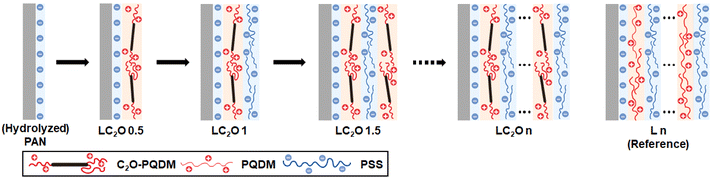
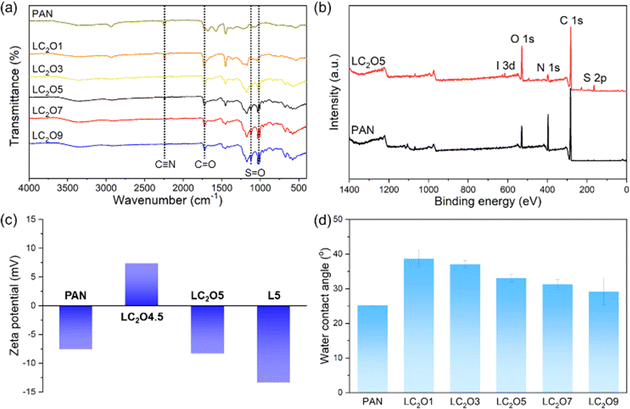
A polyacrylonitrile (PAN) ultrafiltration membrane was employed as a support layer for the preparation of an LNF membrane by the layer-by-layer method. C2O–PQDM was employed as the cationic component, with poly(styrene sulfonate) (PSS) serving as the counterpart (Fig. 2). LC2O# indicates the C2O–PQDM/PSS LbL membrane with # layers; for example, LC2O5 membrane consists of five pairs of C2O–PQDM/PSS layers. Note that the LbL membrane prepared by only polyelectrolytes – PQDM and PSS – without C2O was named L#. Fig. 3a shows FT-IR spectra for PAN and C2O-based LbL membranes. The absorption peak at 2230 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching) decreased with increasing the number of layers, suggesting successful LbL progress. This is consistent with an increase in peak intensity observed at 1717 cm−1 (C
N stretching) decreased with increasing the number of layers, suggesting successful LbL progress. This is consistent with an increase in peak intensity observed at 1717 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) from C2O–PQDM, as well as at 1040 cm−1 and 989 cm−1 (S
O stretching) from C2O–PQDM, as well as at 1040 cm−1 and 989 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching) and 1121 cm−1 (S
O symmetric stretching) and 1121 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching) from PSS. The membrane surface was further investigated by XPS analysis. Peaks corresponding to iodine (0.24%) and sulfur (2.25%) content appeared due to the PQDM of the cationic layer and PSS of the anionic layer, respectively (Fig. 3b and S7†).
O asymmetric stretching) from PSS. The membrane surface was further investigated by XPS analysis. Peaks corresponding to iodine (0.24%) and sulfur (2.25%) content appeared due to the PQDM of the cationic layer and PSS of the anionic layer, respectively (Fig. 3b and S7†).
Zeta potential analysis was used to identify surface charge characteristics during LbL assembly (Fig. 3c). The zeta potential value of the PAN membrane was −7.57 mV because of the hydrolyzed structure (e.g., –COOH). The LC2O4.5 membrane exhibited positive charge (7.38 mV) because its surface was covered by the C2O–PQDM layer. In a similar fashion, negative surface charges were observed for LC2O5 and L5 membranes due to the surface PSS layer. These results demonstrate that the LbL assembly is driven by electrostatic interaction between C2O–PQDM and PSS, and indicate that the surface charge is controllable. The surface hydrophilicity is another important characteristic to consider for filtration membranes (Fig. 3d). The PAN membrane exhibited a low contact angle value (25°), possibly due to its hydrolyzed and porous structure. In contrast, the LC2O1 membrane displayed an increased contact angle (38°) due to the blocking of the porous PAN substrate by the deposition of the C2O–PQDM/PSS layer. An increase in the number of layers resulted in a decrease in contact angle, attributed to the hydrophilic polyelectrolytes on the membrane surface and increased surface roughness. AFM and SEM data demonstrated that an increase in the number of layers led to an increase in roughness and thickness, due to the complex electrostatic C2O–PQDM/PSS structures (Fig. S8–S11 and Table S4†).
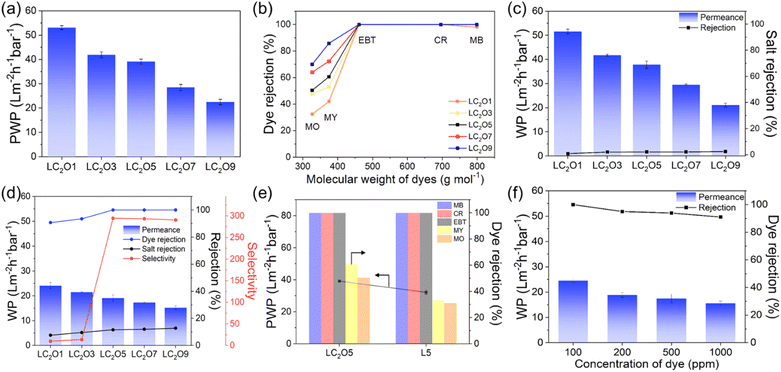
Fig. 4a and b show pure water permeance and dye rejection of LbL membranes, respectively, where five types of dyes with different molar masses were employed as model pollutants: methyl blue (MB), congo red (CR), eriochrome black T (EBT), metanil yellow (MY), and methyl orange (MO) (see Table S5† for dye information). As expected, thicker membranes exhibited lower water permeability and higher dye rejection due to the increased resistance to permeance. For model pollutants with molar masses larger than 460 Da, all LbL membranes exhibited high dye rejection exceeding 99%, indicating that a few C2O–PQDM/PSS layers provide reasonable selectivity (see Fig. S12†). However, complete rejection was not observed for small dyes such as MY and MO. Given that the rejection of the NF membrane is determined by a complex combination of the Donnan effect and size sieving,26 the negatively charged surface likely enhanced the Donnan effect while providing size sieving selectivity through the uniformly porous C2O structure.
Fig. 4c and S13† show the separation performance of LbL membranes during the filtration of NaCl, Na2SO4, and MgSO4 solutions. Since dye wastewater contains a certain amount of salt in actual industrial processes, saline solutions were selected as additional model solutions. Generally, the salt rejection for NaCl and MgSO4 was lower than that of Na2SO4, possibly due to smaller hydrodynamic volume and Donnan effect. Also, thicker membranes exhibited lower water permeability and higher salt rejection, consistent with dye wastewater filtration results. In contrast to high dye rejection, salt rejection remained quite low, attributed to the smaller hydrodynamic volume compared to dyes. These results demonstrate the possibility of selective dye separation from saline wastewater.
To test the possibility, an additional filtration test was conducted with a mixed solution of EBT (100 ppm) and NaCl (1000 ppm), as shown in Fig. 4d. The membranes exhibited high rejection of EBT and low rejection of NaCl, resulting in significant EBT/NaCl selectivity; LC2O5 showed the highest selectivity (∼295), concluding that the 5-layered structure is the optimal design for achieving efficient dye/salt separation (Table S6†). When filtration tests were performed using a mixed solution of NaCl and other dyes, the membrane exhibited moderate selectivity retaining high water permeability (>16 L−1 m−2 h−1 bar−1) for all tested solutions, further demonstrating the membrane's ability to selectively separate organic dyes from salts (Fig. S14†). Note that a slight decrease in dye rejection compared to the single-dye filtration test is possibly due to enhanced electrostatic shielding, reducing the electrostatic repulsion between the dye and membrane surface.27,28
The separation performance of 5-layer LbL membranes, i.e., LC2O5 and L5, was evaluated to demonstrate the role of the C2O structure (Fig. 4e). While both LC2O5 and L5 membranes have comparable surface charge and thickness (Fig. 3c and S9†), the LC2O5 membrane showed higher water permeability and higher dye rejection. This possibly suggests the critical role of C2O in the LbL structure; the crown-ether holes (D ∼ 0.88 nm) of C2O are precisely sized to allow only water molecules to pass through while blocking larger dye molecules (Davg ∼ 1.8 nm). To further validate the results, the separation performance of 9-layer LbL membranes (i.e., LC2O9 and L9) was additionally conducted. As shown in Fig. S15,† the LC2O9 membrane exhibited both higher water permeability and higher dye rejection compared to the L9 membrane, consistent with the trend observed in Fig. 4e for the 5-layer membranes. It is worth noting that the separation performance of the LC2O5 membrane is aligned with other state-of-the-art membranes currently reported (Table S7†). The separation performance of the LC2O5 membrane with various MB, CR, and Na2SO4 concentrations was further investigated (Fig. 4f and S16†). As the concentration of MB and CR increased from 100 ppm to 1000 ppm and the Na2SO4 concentration rose from 1000 ppm to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm, both the permeability and rejection consistently decreased, which is likely attributed to concentration polarization and increasing osmotic pressure.29 Nevertheless, the LC2O5 membrane exhibited a high MB rejection (>91%) even under extremely concentrated conditions (1000 ppm), indicating its potential for practical applications.
000 ppm, both the permeability and rejection consistently decreased, which is likely attributed to concentration polarization and increasing osmotic pressure.29 Nevertheless, the LC2O5 membrane exhibited a high MB rejection (>91%) even under extremely concentrated conditions (1000 ppm), indicating its potential for practical applications.
In industrial practices, membranes are exposed to harsh conditions such as acidic and alkaline environments, therefore, membrane stability is another important factor to consider. As shown in Fig. 5a and b, the LC2O5 membrane maintained higher MB dye rejection compared to the L5 membrane at a wide pH range, demonstrating that the LC2O5 membrane exhibits excellent chemical stability in both acidic and alkaline environments. A slight decrease in water permeability under acidic conditions is probably due to the blockage of pores by the aggregated protonated MB.30 In contrast, the L5 membrane exhibited a significant decrease in water permeability and dye rejection under harsh pH conditions. This is probably due to the simultaneous swelling and coagulation of polyelectrolytes, and this result indicates that the LbL assembly composed of only polymeric electrolytes is not stable under highly acidic or alkaline conditions.31 Therefore, the C2O structure can provide structural stability as well as improved selectivity.
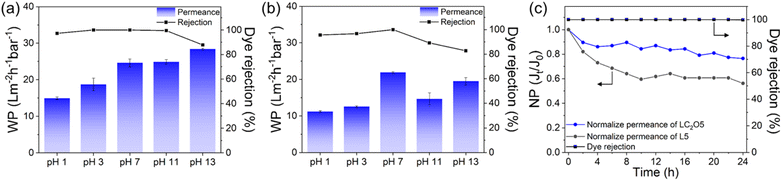 | ||
| Fig. 5 Water permeance (WP) and dye rejection measured using MB solution at various pH values: (a) LC2O5, (b) L5, (c) long-term performance of LC2O5 and L5 membranes using MB solution. | ||
Long-term stability is another crucial property for real-world applications. A feed solution containing MB was filtered through 5-layer LbL membranes for 24 hours (Fig. 5c). Both membranes exhibited consistently high MB rejection (>99%) and a decline in water permeability, possibly due to pore blocking by dye molecules and/or membrane compression. The permeability of the LC2O5 membrane exhibited a smaller decrease compared to that of the L5 membrane, presumably due to (i) the high mechanical strength of the C2O structure, which withstands the operating pressure, (ii) uniformly porous C2O structure, which is less likely to be blocked by dye molecules.
Conclusions
In conclusion, LNF membranes were successfully prepared via the layer-by-layer assembly of cationic polymer grafted C2O and an anionic polymer counterpart. Membrane performance could be tuned by adjusting the number of layers, and the optimized membrane, LC2O5, exhibited excellent water permeance of 39 L m−2 h−1 bar−1 with high dye rejection – 99.8% for methyl blue (MB) and congo red (CR), and 99.7% for eriochrome black T (EBT), along with superior dye/salt selectivity (EBT/NaCl selectivity of 295). Furthermore, the LC2O5 membrane demonstrated high stability under various pH conditions and during long-term operation. These high performances are attributed to the uniform crown-ether holes and structural stability of C2O. This study highlights the eco-friendly, easy, and simple fabrication of loose nanofiltration membranes that exhibit outstanding dye removal, making them suitable for wastewater treatment applications.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Jae Jun Kim: conceptualization, validation, formal analysis, investigation, data curation, visualization, writing – original draft. Huiran Seo: methodology, investigation. Jinseok Kim: formal analysis, investigation. Mun Hyeon Kim: investigation. Jinwook Park: resources. Hyunkee Hong: validation. Hee Joong Kim: conceptualization, investigation, supervision, writing – review & editing. Jong-Chan Lee: conceptualization, supervision, project administration, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (NRF-2020R1A2C2008114 and NRF-2018R1A5A1024127), the Materials/Parts Technology Development Program funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea) (project number: 00432777), and Inha University Research Grant (69932-1).Notes and references
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- S. Miller, H. Shemer and R. Semiat, Desalination, 2015, 366, 2–8 CrossRef CAS.
- E. R. Kabir, M. S. Rahman and I. Rahman, Environ. Toxicol. Pharmacol., 2015, 40, 241–258 CrossRef CAS PubMed.
- L. He, F. Michailidou, H. L. Gahlon and W. Zeng, Chem. Res. Toxicol., 2022, 35, 901–915 Search PubMed.
- A. Saravanan, P. Senthil Kumar, S. Jeevanantham, S. Karishma, B. Tajsabreen, P. R. Yaashikaa and B. Reshma, Chemosphere, 2021, 280, 130595 CrossRef CAS PubMed.
- B. Van Der Bruggen, C. Vandecasteele, T. Van Gestel, W. Doyen and R. Leysen, Environ. Prog., 2003, 22, 46–56 CrossRef CAS.
- Y. Chen, F. Liu, Y. Wang, H. Lin and L. Han, J. Membr. Sci., 2017, 537, 407–415 CrossRef CAS.
- D. J. Miller, D. R. Dreyer, C. W. Bielawski, D. R. Paul and B. D. Freeman, Angew. Chem., Int. Ed., 2017, 56, 4662–4711 CrossRef CAS.
- S. Guo, X. Chen, Y. Wan, S. Feng and J. Luo, ACS Appl. Mater. Interfaces, 2020, 12, 13327–13337 CrossRef CAS PubMed.
- Y. Liang and S. Lin, Environ. Sci. Technol., 2021, 55, 738–748 CrossRef CAS.
- S. Guo, Y. Wan, X. Chen and J. Luo, Chem. Eng. J., 2021, 409, 127376 CrossRef CAS.
- Z.-Y. Ma, Y.-R. Xue, H.-C. Yang, J. Wu and Z.-K. Xu, Macromolecules, 2022, 55, 3363–3383 CrossRef CAS.
- S. Zhang, L. Shen, H. Deng, Q. Liu, X. You, J. Yuan, Z. Jiang and S. Zhang, Adv. Mater., 2022, 34, 2108457 CrossRef CAS.
- G.-R. Xu, S.-H. Wang, H.-L. Zhao, S.-B. Wu, J.-M. Xu, L. Li and X.-Y. Liu, J. Membr. Sci., 2015, 493, 428–443 CrossRef CAS.
- N. Joseph, P. Ahmadiannamini, R. Hoogenboom and I. F. J. Vankelecom, Polym. Chem., 2014, 5, 1817–1831 RSC.
- L. Y. Ng, A. W. Mohammad, C. Y. Ng, C. P. Leo and R. Rohani, Desalination, 2014, 351, 19–26 CrossRef CAS.
- T. Lee, S. H. Min, M. Gu, Y. K. Jung, W. Lee, J. U. Lee, D. G. Seong and B.-S. Kim, Chem. Mater., 2015, 27, 3785–3796 CrossRef CAS.
- S. Rajesh and A. B. Bose, ACS Appl. Mater. Interfaces, 2019, 11, 27706–27716 CrossRef CAS PubMed.
- H. Paloniemi, M. Lukkarinen, T. Ääritalo, S. Areva, J. Leiro, M. Heinonen, K. Haapakka and J. Lukkari, Langmuir, 2006, 22, 74–83 CrossRef CAS PubMed.
- X. Zhao, J. Sun, X. Cheng, Q. Qiu, G. Ma, C. Jiang and J. Pan, ACS Appl. Mater. Interfaces, 2023, 15, 53924–53934 CrossRef CAS PubMed.
- S. Zhao, N. Di, R. Lei, J. Wang and Z. Wang, J. Membr. Sci., 2023, 672, 121424 CrossRef CAS.
- Y. Deng, Y. Wu, G. Chen, X. Zheng, M. Dai and C. Peng, Chem. Eng. J., 2021, 405, 127004 CrossRef CAS.
- J. Yang, Z. Li, Z. Wang, S. Yuan, Y. Li, W. Zhao and X. Zhang, Adv. Mater. Technol., 2021, 6, 2000862 CrossRef CAS.
- J. Kim, S. Kim, J. Park, S. Kang, D. J. Seo, N. Park, S. Lee, J. J. Kim, W. B. Lee, J. Park and J.-C. Lee, J. Am. Chem. Soc., 2024, 146, 4532–4541 CrossRef CAS PubMed.
- D. Jeong, D. G. Hong, J. Yook, C. Y. Koong, S. Kim, K.-H. Kim, K. Sohn and J.-C. Lee, RSC Adv., 2021, 11, 25305–25313 RSC.
- P.-Y. Pontalier, A. Ismail and M. Ghoul, Sep. Purif. Technol., 1997, 12, 175–181 CrossRef CAS.
- M. Jiang, K. Ye, J. Deng, J. Lin, W. Ye, S. Zhao and B. Van der Bruggen, Environ. Sci. Technol., 2018, 52, 10698–10708 CrossRef CAS.
- J. Lin, W. Ye, M.-C. Baltaru, Y. P. Tang, N. J. Bernstein, P. Gao, S. Balta, M. Vlad, A. Volodin, A. Sotto, P. Luis, A. L. Zydney and B. Van der Bruggen, J. Membr. Sci., 2016, 514, 217–228 CrossRef CAS.
- L. Shen, P. Li and T. Zhang, J. Appl. Polym. Sci., 2019, 136, 47438 CrossRef.
- Y. Qin, L. Wang, C. Zhao, D. Chen, Y. Ma and W. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 16690–16698 CrossRef CAS.
- C. Naas, U. Scheler and U. Lappan, Macromolecules, 2021, 54, 1043–1051 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06349j |
| This journal is © The Royal Society of Chemistry 2025 |