Synergistic electrochemical properties of conductive additives with 1D–2D carbon networks†
Seon
Lee‡
ab,
Seongjae
Oh‡
ac,
Chae-Lin
Park
ab,
Young-Chul
Song
d,
Hyun
Kim
 ef,
Keon Jung
Kim
g,
Kwang Won
Kim
a,
Seo Won
Song
a,
Joonmyung
Choi
ef,
Keon Jung
Kim
g,
Kwang Won
Kim
a,
Seo Won
Song
a,
Joonmyung
Choi
 h,
Xinghao
Hu
h,
Xinghao
Hu
 i,
Ki Ro
Yoon
i,
Ki Ro
Yoon
 ab,
Youngbok
Lee
ab,
Youngbok
Lee
 *bj and
Shi Hyeong
Kim
*bj and
Shi Hyeong
Kim
 *ak
*ak
aTextile Innovation R&D Department, Korea Institute of Industrial Technology, Ansan, 15588, Republic of Korea. E-mail: shk@kitech.re.kr
bHYU-KITECH Joint Department, Hanyang University, Seoul, 04763, Republic of Korea. E-mail: 5tjdwo@kitech.re.kr
cDepartment of Energy Science, Sungkyunkwan University, Suwon, 16419, Republic of Korea
dChemical Analysis Center, Korea Research Institute of Chemical Technology, Daejeon 34114, Republic of Korea
eAdvanced Materials Division, Korea Research Institute of Chemical Technology, Daejeon 34114, Republic of Korea
fAdvanced Materials and Chemical Engineering, KRICT School, University of Science and Technology, Daejeon, 34114, Republic of Korea
gSemiconductor R&D Center, Samsung Electronics, Hwaseong 18448, Republic of Korea
hSchool of Mechanical Engineering Sungkyunkwan University, Suwon 16419, Republic of Korea
iSchool of Mechanical Engineering, Key Laboratory of Intelligent Flexible Actuation and Control in Universities of Jiangsu Province, Jiangsu University, Zhenjiang 212013, PR China
jDepartment of Energy and Bio Sciences, Hanyang University, Ansan, 15588, Republic of Korea
kDepartment of Advanced Materials Engineering, Chung-Ang University, Anseong 17546, Republic of Korea
First published on 31st March 2025
Abstract
The demand for high energy density lithium-ion batteries (LIBs) has increased due to the miniaturization of portable electronic devices. To enhance the energy density of these batteries, advancements in cathode and anode materials are essential, as their performance is currently limited by low electronic conductivity and poor dispersion efficiency. Therefore, this study proposes a composite conductive additive consisting of carbon nanoscrolls (CNSs) and reduced graphene oxide (rGO) to create a graphene-based cathode network. CNSs prevent rGO aggregation, enhancing slurry dispensability, and the combination of 2D rGO and 1D CNSs forms efficient conductive networks. The composite conductive additive has the potential to increase capacity by up to eight times compared to Ketjenblack and offer better cycle stability than rGO alone. This demonstrates the potential of CNSs and rGO composites to improve the electrochemical properties of conductive materials.
1 Introduction
In recent years, the demand for electric vehicles and energy storage has driven the research of lithium-ion batteries (LIBs) with high energy density.1–3 To improve the electrochemical performance of these batteries, basic electrode components, such as the active materials, conductive additives and binders, need to be developed. The active materials that primarily determine the energy density of cells, the low electronic conductivity and poor dispersion of conductive additives are also crucial factors that limit the energy density and rate capability of the cells.4 Traditionally, granular carbon blacks such as Ketjenblack (KB) or acetylene black have been used as conductive additives. However, their low dispersion quality and point-to-point contact between active materials and carbon materials limit the effective utilization of active materials.Graphene is a two-dimensional (2D) planar conductive material that can form flexible, long-range conductive networks, complementing the traditional point-to-point (short-range) networks produced using zero-dimensional (0D) carbon additives such as carbon black.5–9 Therefore, conductive additives that utilize graphene have attracted considerable attention. Two strategies have been established to use graphene as a conductive additive: modified graphene and composite graphene with carbon-based conductive additives. Some researchers have modified graphene via electrochemical oxidation or by peeling off layers to adjust the particle size and layer thickness. This improves the capacity retention and the charge/discharge cycling stability of the batteries, and small-sized graphene is favourable for use in the cathode.9–11 Other researchers have produced mixed conductive additives consisting of graphene and carbon black, which increases the energy density and cycling stability owing to the excellent mechanical properties.5,11,12 These studies have improved the performance compared to conventional carbon black. However, the planar structure of graphene interferes with diffusion of lithium ions and increases the length of the electron path,11,13 which results in a capacity increase of approximately 10% compared to the excellent electrical properties of graphene. Therefore, in order to express the potential of graphene and lithium-ion batteries, a solution to enhance its electrochemical properties is needed.
In this study, we propose carbon nanoscrolls (CNSs), a scroll-shaped form of graphene, and graphene composite as a conductive additive in a nickel manganese cobalt oxide (NMC) based cathode, which exhibit enhanced electrochemical properties compared to using graphene alone. The inherent structure of the CNSs prevented the aggregation of graphene, which increased the dispersibility of the slurry. Furthermore, when 2D graphene and one-dimensional (1D) CNSs are added to the mixture, long–short-range networks formed, resulting in improved electrochemical performance. Compared to other papers using carbon-based conductive additives, graphene with CNSs showed a capacity increase eight times higher than that of Ketjenblack (a type of carbon black). It also led to 150% capacity improvement and cycle stability compared to only graphene.
2 Experimental
2.1 Materials
rGO powder (size: 7–162 μm, thickness: 2–2.4 nm) was purchased from STANDARD GRAPHENE (rGO-V30-100, STANDARD GRAPHENE, Korea). PVDF powder (Mw ∼ 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Solvay (USA). Nickel manganese cobalt oxide (NMC 811) was provided by POSCO (Korea). NMP (≥99.0%) and lithium hexafluorophosphate (LiPF6, 0.1 M) were purchased from Sigma-Aldrich (USA). Lithium foil was acquired from Thermo Fisher Scientific and Ketjenblack (EC-600JB) was obtained from AkzoNobel.
000) was obtained from Solvay (USA). Nickel manganese cobalt oxide (NMC 811) was provided by POSCO (Korea). NMP (≥99.0%) and lithium hexafluorophosphate (LiPF6, 0.1 M) were purchased from Sigma-Aldrich (USA). Lithium foil was acquired from Thermo Fisher Scientific and Ketjenblack (EC-600JB) was obtained from AkzoNobel.
2.2 Preparation of cathode electrodes
The slurry for all the conductive materials used for the anode was made in the same proportion. The active materials (NMC 811), conductive additive (rGO, rGO/CNSs, Ketjenblack) and binder (PVDF) with a mass ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were mixed in a holder. PVDF was dissolved in 8 wt% NMP for 12 h at 150 °C. An additional 0.6 g of NMP was added for appropriate slurry viscosity. When the weight of the cathode electrode is 0.01024 g, each loading mass of active material is 6.77 mg cm−2.
4 were mixed in a holder. PVDF was dissolved in 8 wt% NMP for 12 h at 150 °C. An additional 0.6 g of NMP was added for appropriate slurry viscosity. When the weight of the cathode electrode is 0.01024 g, each loading mass of active material is 6.77 mg cm−2.
2.3 Instrument and characterization
The morphologies of the rGO/SrGO and rGO particles were determined via SEM (S4700, Hitachi, Japan), TEM (JEM-2100F (FEG)) and AFM (Park NX7, Park SYSTEM). The atomic compositions of rGO/CNSs and rGO powder were obtained by XPS (AXIS SUPRA, KRATOS, United Kingdom). The Raman spectra were obtained using a Raman spectrometer (Nanophoton Ramanforce Raman spectrometer, Nanophoton, Japan) with 532 nm excitation and XRD (Ultima IV, koreaits, Korea). Particle dispersion and size distribution were measured using a DLS (Litesizer DLS 100, Anton Paar, Austria). The specific surface area was determined from nitrogen adsorption–desorption isotherms using a surface area and porosity analyzer (BELSORP MAX X, Microtrac BEL, Japan). The viscosity was measured using a rheometer (MCR 102e, Anton Paar, Austria). EIS was performed using an electrochemical analysis device (Zive SM6, WonA Tech, Republic of Korea) in a coin cell for each conductive additive. The measurements were conducted in the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 0.01 Hz. To evaluate the performance of the coin cell LIBs, galvanostatic discharge and charge measurements were conducted using a multichannel battery testing system (WBCS3000L, Won-Atech, Korea) under ambient conditions.
000 to 0.01 Hz. To evaluate the performance of the coin cell LIBs, galvanostatic discharge and charge measurements were conducted using a multichannel battery testing system (WBCS3000L, Won-Atech, Korea) under ambient conditions.
2.4 Calculation
The loading amount of active material in the electrode was determined using the equation| Loading amount (mg cm−2) = ((total electrode weight (g) − current collector weight (g)) × active material ratio)/(electrode area (cm2)) ×1000 mg g−1 | (1) |
The area of the electrode can be calculated using the formula for the area of a circle
| Electrode area (cm2) = π × (diameter/2)2 = π × (0.5 cm)2 = 0.7854 cm2 | (2) |
The real and imaginary parts of the volumetric capacitance were evaluated as functions of frequency using EIS analysis. The values were calculated using the equation
| C′(f) = (−Z′′(f))/(2πfVdevice|Z(f)|2) | (3) |
| C′′(f)= (−Z′(f))/(2πfVdevice|Z(f)|2) | (4) |
The viscosities at various shear rates were analyzed using the equation
η = τ/(![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) ) | (5) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) (s−1) is the shear rate.
(s−1) is the shear rate.
The size distribution and PDI of the nanoparticles were determined via DLS. The size distribution was extracted from the autocorrelation function, and the PDI was calculated using the equation:
| PDI = σ2/Z2average | (6) |
The diffusion coefficient D (μm2 s−1) was calculated from the DLS data using the equation
| D = (kBT)/(3πηdH) | (7) |
3 Results and discussion
3.1 Fabrication of rGO/CNSs and production process of coin cells
Fig. 1 shows the sonication process used to fabricate the CNSs from graphene and the fabrication process of the LIB coin cells. First, 50 mg of reduced graphene oxide (rGO) powder and 20 mL of ethanol were suspended in a conical tube at a ratio of 0.25 wt% and sonicated with VCX-130 sonication operating at 20% power (4 kHz) for 20 min to evenly disperse the aggregated rGO. Sonication was performed with 1 s operation and 2 s pulses continuously and a 3 mm tip was used (Fig. 1a). The ethanol in the dispersion was evaporated in an oven at 80 °C to attach the rGO to the tube wall (Fig. 1b). Subsequently, 30 mL of deionized (DI) water was added along the wall of the tube and sonicated for 1 h at 70% power (14 kHz) to form the CNSs (Fig. 1c). Some of the rGO was transformed into CNSs, which resulted in a solution containing both 2D rGO and 1D CNSs (Fig. 1d).The rGO/CNS solution was centrifuged to separate it into two layers to determine the ratio of rGO to CNSs. The CNS layer floated on the rGO layer because of its lighter weight than rGO. Hence, the CNS solution could be extracted. Each solution was evaporated and the weight of each component was measured to determine the ratio, which was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.14 Subsequently, a filtration apparatus was used to obtain the rGO/CNS powder. During this process, ethanol was used to prevent the stacking/aggregation of the rGO/CNSs (Fig. 1e). The Brunauer–Emmett–Teller (BET) theory showed that the surface area of the ethanol-treated rGO/CNS powder was 1809.6 cm2 g−1, which was significantly higher than that of the untreated rGO/CNS powder, which was 1165 cm2 g−1 (Fig. S1†). This confirmed that ethanol effectively prevented graphene stacking. The powder was dried in air for 24 h to allow the ethanol to evaporate. The process used to prepare the cathode slurry is shown in Fig. 1f. The slurry components were combined in a holder. Then the mixture was homogenized using a planetary centrifugal mixer for 25 minutes at 2000 rpm for mixing and degassing for 5 minutes at 2300 rpm. The slurry was uniformly cast onto aluminium foil to the wet thickness of 350 μm using a doctor blade (Fig. 1g) and dried under vacuum at 120 °C for 12 h for cell fabrication. The cathode was punched into the required size (diameter = 0 mm) for cell fabrication.
4.14 Subsequently, a filtration apparatus was used to obtain the rGO/CNS powder. During this process, ethanol was used to prevent the stacking/aggregation of the rGO/CNSs (Fig. 1e). The Brunauer–Emmett–Teller (BET) theory showed that the surface area of the ethanol-treated rGO/CNS powder was 1809.6 cm2 g−1, which was significantly higher than that of the untreated rGO/CNS powder, which was 1165 cm2 g−1 (Fig. S1†). This confirmed that ethanol effectively prevented graphene stacking. The powder was dried in air for 24 h to allow the ethanol to evaporate. The process used to prepare the cathode slurry is shown in Fig. 1f. The slurry components were combined in a holder. Then the mixture was homogenized using a planetary centrifugal mixer for 25 minutes at 2000 rpm for mixing and degassing for 5 minutes at 2300 rpm. The slurry was uniformly cast onto aluminium foil to the wet thickness of 350 μm using a doctor blade (Fig. 1g) and dried under vacuum at 120 °C for 12 h for cell fabrication. The cathode was punched into the required size (diameter = 0 mm) for cell fabrication.
As shown in Fig. 1h, a battery was assembled using a 2032 type coin cell in a glove box filled with Ar gas, where the oxygen and water concentrations were less than 1 ppm. The battery consisted of a coin cell lid, spring, spacer, lithium foil, separator, active material, and coin cell base. Microporous polyethylene (diameter = 14 mm) was used as the separator, and 20 mL, 1 M lithium hexafluorophosphate solution (LiPF6; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of dimethyl carbonate and ethylene carbonate) was applied to the top and bottom of the separator as the electrolyte.
1 v/v mixture of dimethyl carbonate and ethylene carbonate) was applied to the top and bottom of the separator as the electrolyte.
3.2 Structural analysis of the rGO/CNS particles
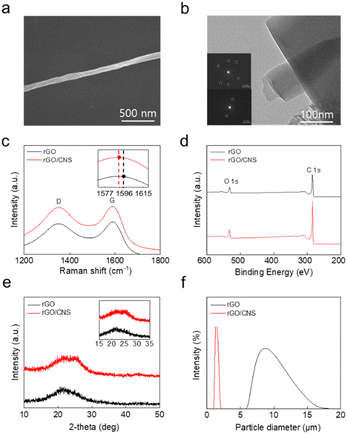
Conductive additives must have high electrical conductivities, chemical stabilities, and specific surface areas to optimize and improve the performance of batteries. Despite their common origin, CNSs and rGO exhibit different chemical and structural characteristics. Therefore, the advantages of CNSs as a conductive additive were determined by investigating the differences between rGO and CNSs.Scanning electron microscopy (SEM), atomic force microscopy (AFM) and transmission electron microscopy (TEM) were used to characterize the morphology of rGO and rGO/CNSs. Particles of the powdered rGO and CNSs were obtained by dispersing them in ethanol at low concentrations. The dispersed solutions were then dropped onto Si wafers or grids and dried in an oven for 24 h. The rGO exists as bulk sheets and had a uniform surface (Fig. S2†). The sonication pressure wave induced temporary bubble cavitation, which involved the sequential nucleation, growth, and collapse of fine bubbles. The sonication energy transferred to the bubbles and rGO particles provides the activation energy required to overcome the energy barrier for the self-formation of CNSs,15,16 and the layer of bulk rGO attached to the wall peeled off and scrolled away to form the CNSs. The CNSs have a cylindrical shape when they were fully scrolled (Fig. 2a), which allowed them to fit between the rGO layers and increased the overall specific surface area. Using the BETcy, the specific surface areas of rGO/CNSs and rGO were determined to be 1809.6 cm2 g−1 and 1373.8 cm2 g−1, respectively. This confirms that the formation of CNSs enhanced the specific surface area of the conductive material (Fig. S3†). Increasing the specific surface area can increase the electrical conductivity because it increases the number of active sites, which allows more electrons to be transferred.
As shown in Fig. 2b, the rGO had a typically planar structure and the selected area electron diffraction (SAED) pattern showed that the carbon atoms were arranged in a hexagonal pattern.17,18 However, structural changes were observed as the rGO was transformed into CNSs. The CNSs consisted of cylindrical scrolls, which formed several layers. Thus, the SAED pattern showed a symmetrical straight line representing the layered cylindrical structure.19 The rGO and the CNS particle sizes were investigated using AFM (Fig. S4†). The length and height of the rGO particles were 1672.45 and 86.02 nm, respectively, whereas those of the CNS particles were 718.7 nm and 457.6 nm, respectively. This indicates that scrolling increased the height and reduced the length of the particles.
In Raman spectroscopy, rGO typically exhibits D- and G-bands. The G-band represents the main mode of graphite and corresponds to the 2D structure of rGO and the sp2 carbon bond. The D-band is observed when the binding of graphene is limited or deformed. In general, the G- and D-band appear at approximately 1550–1630 and 1320–1370 cm−1, respectively. As shown in Fig. 2c the G-band was shifted to a lower value, which indicates that the sp2 domain decreased. Therefore, the intensity ratios of the D- and G-bands (ID/IG) for rGO and rGO/CNSs were 0.94 and 1.02, respectively. ID/IG was slightly higher for rGO/CNSs than for rGO, which indicates that there were more defects on the rGO surface after sonication and suggests that scrolling had occurred.16,20
The carbon and oxygen contents of the rGO and rGO/CNSs were analyzed using X-ray Photoelectron Spectroscopy (XPS) (Fig. 2d). The atomic ratio of carbon in rGO was 88.6%, whereas that in the rGO/CNSs was higher at 90.4%. Furthermore, the atomic ratio of oxygen in the rGO was 10.2%, whereas that in the rGO/CNSs was lower at 8.4%. During the formation process of rGO/CNSs, sonication eliminated some oxygen functional groups, increasing the carbon ratio and decreasing the oxygen ratio.20 Increasing the carbon content can improve the electrical conductivity by increasing electron mobility. Moreover, decreasing the oxygen content can improve the chemical stability of the electrodes by preventing corrosion.
Fig. 2e shows the crystal structure of rGO and rGO/CNSs. The rGO and rGO/CNSs both exhibited diffraction peaks at θ = 20–28°, which indicated the presence of a (0 0 2) plane. The rGO/CNSs exhibited a weak peak, which indicates that the interactions within the crystal were weakened, CNSs contained many defects, and it had a scrolled structure.21 In addition, the diffraction angles of rGO and rGO/CNSs were 21.3° and 24.3°, respectively. The diffraction angle was lower for rGO than for rGO/CNSs, which indicates that the interlayer distance was larger. Therefore, the scrolled structure of the CNSs was denser than that of the rGO.
The particle size distribution and movement in the rGO and rGO/CNS solvents were measured using dynamic light scattering (DLS). The hydrodynamic radius of rGO was 12.99 μm, which was much larger than that of rGO/CNSs, which was 1.922 μm (Fig. 2f). This significant difference in particle size can be attributed to the aggregation of rGO increasing the particle size.22 The percentile distribution was used to analyze the rGO and rGO/CNS particle sizes. For rGO, D10, D50, and D90 were 7.24, 9.57, and 12.89 μm, respectively, and for rGO/CNSs they were 1.17, 1.42, and 1.72 μm, respectively (Fig. S5†). The width of the particle size distribution and the uniformity of particle size were evaluated using the polydispersity index (PDI). A low PDI indicates a narrow particle size distribution and high uniformity, whereas a high PDI indicates a wide particle size distribution and low uniformity. The PDIs of the rGO and rGO/CNSs were 0.59 and 0.24, respectively. This shows that the rGO/CNSs had a narrower size distribution and greater uniformity than the rGO.22 Furthermore, the diffusion coefficient of rGO was measured to be 0.219 μm2 s−1, whereas that of rGO/CNSs was 0.032 μm2 s−1, which was comparatively low and may promote electrochemical reactions. The more uniform particle size of rGO/CNSs suggests that it can form more stable and homogeneous dispersions, which is crucial for maintaining consistent electrical conductivity and structural stability within batteries.23,24 However, the tendency of rGO to aggregate may reduce the uniformity of the conductivity and affect the material stability over time.
3.3 Analysis of physical and electrical properties and network structure of conductive additives
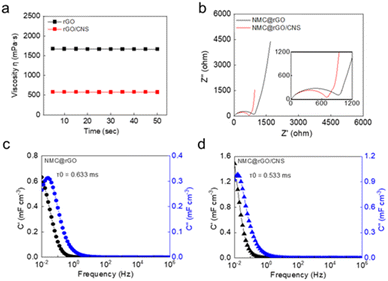
In the slurry, the conductive binder domain (CBD), which consists of a conductive additive and polymeric binder, plays an important role in promoting electron transfer and ion diffusion within the electrode. Percolation is an important mechanism for increasing electron transport, and effective percolation is activated by increased interactions between the PVDF and NMP owing to the disordered arrangement of the carbon in the conductive material.25 Furthermore, the viscosity is closely related to the ratio of ordered to disordered carbon.26 CNSs cause the carbon particles to become disordered, which reduces the viscosity of the slurry, prevents the aggregation of the particles, and allows the particles to move more freely. The viscosities of the rGO and rGO/CNS slurries were 1667 and 576.6 mPa·s, respectively, and in both cases, the viscosity remained constant over time (Fig. 3a). The low viscosity of the rGO/CNS slurry increased the dispersion of particles, which allowed the conductive additive to diffuse widely and allowed the active material to be wrapped efficiently. This improved the formation of conductive networks within the electrode. Therefore, the electrical conductivity may be increased and the internal resistance may be decreased.Electrochemical impedance spectroscopy (EIS) was used to evaluate the formation of electrical networks inside the cathodes depending on the properties of the developed conductive additives (Fig. 3b). The spectra exhibited a partially semicircular shape in the high-frequency region and a linear slope in the low-frequency region. The semicircular shape represents the charge-transfer resistance Rct and the linear slope corresponds to the Warburg impedance, which indicates the diffusion of lithium ions within the electrode. The Nyquist plots show that the charge transfer resistances of the NMC@rGO and NMC@rGO/CNS electrodes were 749 and 700.3 Ω, respectively. This shows that the CNSs actively induced the movement of electrons, which reduced the internal resistance. Furthermore, the reduction in the rGO/CNS slope indicated that the barriers that interfere with ion diffusion were reduced.
The frequency-dependent real C′ and imaginary C′′ capacities of the rGO and rGO/CNS cells were evaluated and the volumetric capability was compared (Fig. 3c and d). The time constant τ0 represents the minimum time required for the device to emit all energy with an efficiency of more than 50%. A lower τ0 value indicates that faster energy dissipation is possible, which is an important indicator for evaluating the fast charge/discharge characteristics of the battery. The τ0 values of the rGO and rGO/CNS cells were 0.633 and 0.533 ms, respectively. This indicates that the rGO/CNS cell has a much faster electron transport capacity than the rGO cell owing to the formation of effective conductive networks.
Bode plots for the two cells are shown in Fig. S6;† when the absolute phase angle is close to 0°, the material mainly exhibits resistance properties, whereas when it is close to 90°, the material mainly exhibits capacitive properties. The rGO/CNS cell showed higher absolute phase angles than the rGO cell in the low-frequency range (10−2 to 10 Hz). This indicates that an electrochemical rGO/CNS bilayer had formed, which can increase the electrochemical stability.
Fig. 4 illustrates a mechanism for comparing the mobility of electrons and ions in the cathode electrode in detail, depending on the form of the conductive additive. In the cathode produced using rGO as the conductive material, aggregated sheets with a hexagonal structure were distributed inconsistently between the active materials (Fig. 4a). This configuration limited the ability of rGO to form efficient electron networks despite its high electronic conductivity. Furthermore, the movement of lithium ions may be limited owing to the aggregated rGO,6 which can disrupt fast and efficient charging/discharging, decrease the battery life, and reduce the stability of the battery. Therefore, to form optimal conductive networks, the transport factors of electrons and ions should be considered. When the CNSs were generated, the peeled rGO and the CNSs are uniformly diffused into the cathode to form a layer that covered a wide area of the active materials (Fig. 4b). The 1D CNSs enabled point-to-line contact and 2D rGO enabled large-area contact. Therefore, the mixture of rGO and CNSs formed 1D–2D networks and established effective electron and lithium-ion transport paths.
 | ||
| Fig. 4 Schematic illustration comparing the mobility of the electrons and ions in cathode electrodes with different conductive additives. (a) NMC@rGO network and (b) NMC@rGO/CNS network. | ||
Additionally, the structural properties of the rGO/CNS composite were investigated in more detail through the SEM image (Fig. S7†). The image on the left shows a high magnification SEM image, where it can be seen that rGO appears in the form of wrinkled graphene sheets, and the CNSs have a more fibrous form. They are in close contact, providing an efficient electron transport network for improving the electrical conductivity of the composite materials.
The image on the right shows the overall distribution and interaction of rGO and CNSs at low magnification. Here, the thin, elongated CNSs show the form of connection between the rGO layers, suggesting that the electron transport path can be formed more smoothly. This network structure serves to maximize the charge transport within the electrode, while at the same time improving the structural stability.
3.4 Electrochemical performance for coin cells of different conductive additives
Coin cell-type LIBs were fabricated using rGO and rGO/CNSs as conductive additives in NMC-based cathode sides to compare their electrochemical properties and confirm the effectiveness of rGO/CNSs for practical applications. KB, the conventional carbon black, was also used for comparison because it is widely used as a commercial conductive additive that can provide a standard for evaluation.25As shown in Fig. 5a, the initial discharge capacities of the three NMC cells were measured at a current rate of 0.1C. A low current density was used to minimize the effects of internal resistance, which increased the accuracy of the measurements of the actual capacities of the batteries. The NMC@rGO/CNS coin cell exhibited the highest discharge capacity of 192.19 mA h g−1, followed by NMC@rGO and NMC@KB coin cells with discharge capacities of 127.75 mA h g−1 and 103.53 mA h g−1, respectively. Therefore, under low current density conditions, the coin cell with CNSs/rGO as the conductive material had a superior capacity compared to those with rGO and KB.
The rate performances of NMC cells are shown in Fig. 5b. The average discharge capacities of the NMC@rGO/CNS cell at 0.2, 0.3, and 0.5C were 168.84, 153.13, and 133.21 mA h g−1, respectively. When it returned to 0.2C, it showed a high retention capacity. This indicates that its capacity and retention rate were significantly higher than those of the other cells. NMC@KB also exhibited good capacity retention at various rates. However, its overall capacity was fairly low. By contrast, NMC@rGO cell exhibited unstable capacity retention. This indicates that the CNSs formed an excellent electrochemical network, which enhanced the stability of the battery during charging and discharging at various rates.
Fig. 5c–e show the charge/discharge curves for the NMC@KB, NMC@rGO and NMC@rGO/CNS cells at rates from 0.2C to 0.5C. When having a capacity of 90 mA h g−1 at 0.3C, the NMC@KB, NMC@rGO, and NMC@rGO/CNS cells had charge/discharge electric potential differences of 0.31, 0.73, and 0.12 V, respectively. A high potential difference indicates that the electrochemical conductivity is low owing to severe polarization. Therefore, the NMC@rGO/CNS cell, which had the lowest potential difference, was highly conductive and required less energy for charging/discharging.
Fig. 5f shows the cycle performance of the coin cells with various conductive additives. After 100 cycles, the NMC@rGO/CNS cell maintained a high capacity of 85.3 mA h g−1, which was superior to the NMC@KB and NMC@rGO cells, which had capacities of 59.7 and 69.6 mA h g−1, respectively. During 100 cycles, the capacity retention rates of the NMC@rGO/CNSs, NMC@KB, and NMC@rGO cells were 90%, 85%, and 85%, respectively, which demonstrates the superior capacity retention rate of the NMC@rGO/CNS cell.
To further understand the structural stability responsible for this performance, we conducted a TEM analysis after cycling (Fig. S8†). As shown in the TEM images, the CNS additive remains effectively attached to the graphene oxide (rGO) surface even after cycling. Although some cathode active materials were separated during pretreatment and sonication, the TEM images clearly confirmed that CNSs, rGO sheets, and active materials remained interconnected. The CNSs maintained their characteristic scroll-like structure, demonstrating structural stability after cycling. Additionally, the graphene sheets formed a wide, interconnected conductive network with CNSs, and dark active material particles were closely bound to both CNSs and rGO. These observations confirm the formation of a robust network that ensures stable electron transport pathways, contributing to the electrode's superior structural and electrochemical stability.
The electrodes using conductive additives with a composite structure exhibited significantly better electrochemical properties than those using conductive additives with a single structure. This shows that the proposed additive is a valuable conductive additive owing to the formation of long–short-range networks.
In this study, a mixed 2D and 1D composite conductive additive based on rGO was used to improve the performance of a battery. To evaluate the potential of rGO-based conductive additives, five related papers of conductive materials based on graphene were compared5,6,10,12,27 (Fig. 6). The battery capacity growth rate at 0.1C was analyzed compared with carbon black, a conventional conductive material that uses the same manufacturing method as the graphene based conductive additives in each paper. Although there were differences in performance depending on the diversity and ratio of materials and manufacturing conditions, the purpose of this study was to understand the potential of graphene-based conductive material which has shown improved results in battery performance compared to carbon black used under the same conditions. As a result, the capacity increase rate of this work increased by 85%, which is greater than that of other reference cells, which was around 10%. We would like to propose this new type of composite conductive additive as an effective solution for improving battery performance.
4 Conclusions
This study investigated the properties of composite conductive additives composed of rGO/CNSs to enhance the electrochemical performance of conductive materials used in batteries. CNSs can improve the specific surface area, dispersibility and electrical conductivity. Therefore, combining 2D rGO and 1D CNSs as a conductive additive forms efficient conductive networks between active materials and carbon composite, outperforming traditional carbon-based materials such as carbon black. Electrochemical analysis of coin cell-type batteries demonstrated that NMC@rGO/CNS cell achieved significantly higher discharge capacity compared to NMC@rGO and NMC@KB cells. It also showed excellent rate performance and cycle stability, highlighting its potential as a conductive additive. The improved performance is attributed to the effective charge transfer path and reduced internal resistance provided by the composite structure. In conclusion, the structurally optimized composite conductive additives provide promising solutions for improving electrochemical properties. They form stable conductive networks, which can overcome the current limitations of conductive materials to maximize battery performance and efficiency.Data availability
Data supporting the findings of this study are available from the corresponding author upon request.Author contributions
S. L. and S. J. O. contributed equally to this study. S. H. K. and Y. L. designed the study. K. J. K., S. L., and S. J. O. designed the experiments and characterization. C. L. P. and H. K. performed AFM and SEM experiments, respectively. Y. C. S. and H. K. characterized the properties of the materials. S. L., K.·W.·K. and K. J. K. contributed to the development of EIS. K. W. K., S.·W.·S., and S. L. analysed the electrochemical performance and produced the coin cells. S. J. O. assisted with rGO/CNS synthesis. S. L., C. L. P., J. C., S.·W. S. and Y.·C. S. wrote the manuscript. J. C., Y. L. and S.·H. K. supervised the study. All authors participated in discussions and reviewed the manuscript. X. Hu contributed to the revision experiments and provided insights for data interpretation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Korea Institute of Industrial Technology [KITECH JE240007] and the Korea Research Institute of Chemical Technology (KRICT) Core Project [KS2521-10], the Korea Research Institute of Chemical Technology (KRICT) [KK2552-10], the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT [NRF-2022R1A2C4001273], the Material Parts Technology Development Program funded by the Ministry of Trade, Industry & Energy (MOTIE) (RS-2024-00469551), the National Natural Science Foundation of China (Grant no. 52105057, 52475059), the Jiangsu Province Natural Science Foundation (No. BK20240155), and the National Research Foundation of Korea (RS-2025-00555988).Notes and references
- G. K. Mishra, M. Gautam, K. Bhawana, J. Ghosh and S. Mitra, High energy density lithium-ion pouch cell with modified high voltage lithium cobalt oxide cathode and graphite anode: prototype stabilization, electrochemical and thermal study, J. Power Sources, 2023, 580, 233395 CAS.
- Z. Guo, Z. Xu, F. Xie, J. Feng and M. Titirici, Strategies for High Energy Density Dual-Ion Batteries Using Carbon-Based Cathodes, Adv. Energy Sustainability Res., 2021, 2(11), 2100074 CrossRef CAS.
- T. Liang, W.-H. Liang, J.-H. Cao and D.-Y. Wu, Enhanced performance of high energy density lithium metal battery with PVDF-HFP/LAGP composite separator, ACS Appl. Energy Mater., 2021, 4(3), 2578–2585 CAS.
- K. Wang, Y. Wu, S. Luo, X. He, J. Wang and K. Jiang, et al., Hybrid super-aligned carbon nanotube/carbon black conductive networks: a strategy to improve both electrical conductivity and capacity for lithium ion batteries, J. Power Sources, 2013, 233, 209–215 CrossRef CAS.
- M. Peddi, S. B. Moodakare, A. K. Budumuru, K. Muthusamy, G. Sundararajan and R. Gopalan, Multilayer Graphene as a Cathode Conductive Additive in Lithium-Ion Pouch Cells: A Correlation of Changes in Electrolyte Uptake and Composition of the Electrode Electrolyte Interface with Enhanced Cycling Stability, ACS Appl. Energy Mater., 2023, 6(6), 3251–3263 CrossRef CAS.
- T. Liu, S. Sun, Z. Zang, X. Li, X. Sun and F. Cao, et al., Effects of graphene with different sizes as conductive additives on the electrochemical performance of a LiFePO4 cathode, RSC Adv., 2017, 7(34), 20882–20887 RSC.
- T. Chi, X. Wang, L. Zeng, Z. Qin, X. Zhou and Z. Liu, Unraveling the effect of conductive additives on Li-ion diffusion using electrochemical impedance spectroscopy: a case study of graphene vs. carbon black, J. Electrochem. Soc., 2023, 170(4), 040515 CrossRef CAS.
- L. Xu, W. Lv, K. Shi, S. Xiao, C. You and Y.-B. He, et al., Holey graphenes as the conductive additives for LiFePO4 batteries with an excellent rate performance, Carbon, 2019, 149, 257–262 CAS.
- J. Wang, Z. Shen and M. Yi, Liquid-exfoliated graphene as highly efficient conductive additives for cathodes in lithium ion batteries, Carbon, 2019, 153, 156–163 Search PubMed.
- T.-H. Hsu and W.-R. Liu, Effects of graphene nanosheets with different lateral sizes as conductive additives on the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode materials for Li ion batteries, Polymers, 2020, 12(5), 1162 Search PubMed.
- D. S. Kim, J. U. Lee, S. H. Kim and J. Y. Hong, Electrochemically exfoliated graphite as a highly efficient conductive additive for an anode in lithium-ion batteries, Battery Energy, 2023, 2(5), 20230012 CAS.
- X. Jiao, A. V. Kirianova, X. Xu, O. O. Kapitanova, V. A. Krivchenko and F. S. Napolskiy, et al., Conductive additives for improving the rate capability of cathode materials in secondary lithium batteries, ACS Appl. Energy Mater., 2023, 6(5), 2855–2862 CrossRef CAS.
- S. Kim, J. Hwang, Y. Jo, C. Park, N. Bansal and R. R. Salunkhe, et al., Minimizing ion/electron pathways through ultrathin conformal holey graphene encapsulation in Li-and Mn-rich layered oxide cathodes for high-performance lithium-ion batteries, J. Mater. Chem. A, 2024, 12(26), 16143–16159 RSC.
- C.-L. Park, S. Ryu, J. Choi, Y.-C. Song, K. J. Kim and S. W. Lee, et al., Wet-spinning of reduced graphene oxide composite fiber by mechanical synergistic effect with graphene scrolling method, Mater. Today Adv., 2024, 22, 100491 CrossRef CAS.
- W. Clower, N. Groden and C. G. Wilson, Graphene nanoscrolls fabricated by ultrasonication of electrochemically exfoliated graphene, Nano-Struct. Nano-Objects, 2017, 12, 77–83 CrossRef CAS.
- C. A. Amadei, I. Y. Stein, G. J. Silverberg, B. L. Wardle and C. D. Vecitis, Fabrication and morphology tuning of graphene oxide nanoscrolls, Nanoscale, 2016, 8(12), 6783–6791 RSC.
- D. Tavar, R. Sharma, M. Ashiq, M. Mudgal and A. Singh, rGO supported cobalt-manganese based nanocomposite with improved electrochemical water oxidation catalysis, J. Mater. Sci., 2023, 58(27), 11270–11285 CAS.
- P. Dash, T. Rout and S. Biswal, Study on the preparation of GO and RGO by chemical and mechanical exfoliation of natural graphite for the aluminum industry, J. Sustain. Metall., 2020, 6(1), 26–33 Search PubMed.
- M. Flygare and K. Svensson, Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour, Mater. Today Commun., 2019, 18, 39–45 CAS.
- A. D. Sontakke and M. K. Purkait, A brief review on graphene oxide nanoscrolls: structure, synthesis, characterization and scope of applications, Chem. Eng. J., 2021, 420, 129914 CAS.
- A. D. Sontakke and M. Purkait, Fabrication of ultrasound-mediated tunable graphene oxide nanoscrolls, Ultrason. Sonochem., 2020, 63, 104976 CAS.
- D. Sahu, G. Kannan and R. Vijayaraghavan, Carbon black particle exhibits size dependent toxicity in human monocytes, Int. J. Inflammation, 2014, 2014(1), 827019 Search PubMed.
- X. Qi, B. Blizanac, A. DuPasquier, M. Oljaca, J. Li and M. Winter, Understanding the influence of conductive carbon additives surface area on the rate performance of LiFePO4 cathodes for lithium ion batteries, Carbon, 2013, 64, 334–340 CrossRef CAS.
- J. H. Choi, C. Lee, S. Park, M. Hwang, T. J. Embleton and K. Ko, et al., Improved electrochemical performance using well-dispersed carbon nanotubes as conductive additive in the Ni-rich positive electrode of lithium-ion batteries, Electrochem. Commun., 2023, 146, 107419 CAS.
- X. Lu, G. J. Lian, J. Parker, R. Ge, M. K. Sadan and R. M. Smith, et al., Effect of carbon blacks on electrical conduction and conductive binder domain of next-generation lithium-ion batteries, J. Power Sources, 2024, 592, 233916 CAS.
- M. Mourshed, S. M. R. Niya, R. Ojha, G. Rosengarten, J. Andrews and B. Shabani, Carbon-based slurry electrodes for energy storage and power supply systems, Energy Storage Mater., 2021, 40, 461–489 Search PubMed.
- C. Teng, R. Zhai, Z. Li, X. Ma, L. Su and C. Chen, et al., Ultrahigh concentration, single-layer of graphene paste as conductive additive for lithium-ion battery, Carbon Trends, 2021, 5, 100104 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta07697d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |