High-rate rare-earth-based high-entropy Co-free high-Ni cathodes for high-performance lithium-ion batteries†
Yang
Liu
,
Yan
Xin
 ,
Bijiao
He
,
Fang
Zhang
,
Wenbo
Liu
and
Huajun
Tian
,
Bijiao
He
,
Fang
Zhang
,
Wenbo
Liu
and
Huajun
Tian
 *
*
Beijing Laboratory of New Energy Storage Technology, Key Laboratory of Power Station Energy Transfer Conversion, System of Ministry of Education, School of Energy Power and Mechanical Engineering, North China Electric Power University, Beijing, 102206, China. E-mail: Huajun.Tian@ncepu.edu.cn
First published on 25th March 2025
Abstract
In electric transportation and energy storage systems, the development of Co-free Ni-rich layered cathode materials that can maintain high specific discharge capacity and cycling stability under high-rate discharging conditions is critical to the practical application of advanced lithium-ion batteries (LIBs). However, the non-equilibrium delithiation reaction induced by high-rate discharge conditions and the pursuit of low/no cobalt (low cost, low toxicity) leads to hindered interfacial and internal kinetics of Li+ and structural degradation of the cathode. In this work, we have designed a family of rare-earth-based high-entropy Co-free high-Ni cathode materials LiNi0.9Mn0.02Al0.02Mg0.02Ti0.02Si0.02O2 (Lu substituting Si, Ti, Mg, and Al, respectively) for constructing a promising high-rate rare-earth high-entropy Co-free high-Ni layered cathode LiNi0.9Mn0.02Al0.02Mg0.02Ti0.02Lu0.02O2 (HE-Lu). The “entropy stabilization” effect is maximized by optimizing the synergistic relationship between the rare-earth elements and the high-entropy doping elements. The pinning effect of rare-earth elements suppresses the inconsistency of Li+ intercalation under high-rate discharging. High-entropy doping can significantly inhibit the formation of microcracks at high current densities, smooth the H2–H3 detrimental phase transition and induce the formation of an ultra-thin and stable cathode electrolyte interface (CEI) on the cathode. The structure of the LiNi0.9Mn0.02Al0.02Mg0.02Ti0.02Lu0.02O2 cathode remains ultra-stable even under harsh conditions and at a high cut-off voltage (4.7 V). The discharge capacity of the HE-Lu cathode is 218.5 mA h g−1 at 0.2C, and the capacity retention rate is maintained at 84.8% and 81.2% under 1C charging and 5C discharging (1 C/5C) rates even after 1500 cycles (2.7–4.3 V) and 300 cycles (2.7–4.7 V), respectively. The full cell has a high capacity retention of 88.3% even after 1000 cycles. This work provides a promising strategy for designing Co-free, high-Ni cathodes in high-rate lithium-ion batteries.
1 Introduction
High-power fast-discharging lithium-ion batteries (LIBs) are advanced energy storage devices extensively utilized in fields such as smart grid, rail transport and aerospace systems.1–4 The Li storage capacity of cathode materials under high-rate discharging is the key to high-power LIBs.5–8 In high-Ni cathodes LiNixCoyMnzO2 (NCM, x > 0.9) and LiNixCoyAlzO2 (NCA, x > 0.9), the redox couple of Ni2+/4+ and Co3+/4+ can achieve a capacity over 200 mA h g−1 at 4.3 V. In addition, Co and Mn ensure electrical conductivity and structural stability, which are well consistent with conditions of use that require high energy and high power density.9 Cobalt (Co) is a key component for stabilizing cathodes and has been widely used in high-Ni layered cathodes to inhibit Li+/Ni2+ cation mixing and enhance stability.10,11 However, due to the high price and geographic restrictions, Co needs to be removed from high-Ni cathodes.12,13 In addition, Co is even more destructive than Ni due to chemical-mechanical cracking and irreversible oxygen release under high voltages.14 In this context, the development of high-Ni Co-free high-power cathodes has attracted considerable attention.15,16However, in high-Ni Co-free cathodes, increasing the discharge rate will further exacerbate the structural instability, which may lead to microcracks,17 electrolyte decomposition,18,19 cathode surface transition metal dissolution,20,21 and cathode–electrolyte interface (CEI) degradation.22 In addition, long-term fast discharge can induce prolonged delithiation of the cathode material, a phenomenon comparable to the further delithiation process caused by charge/discharge tests at high cut-off voltages.23–25 Charging and discharging at high cut-off voltages lead to severe side reactions between Ni4+ and the electrolyte.26,27 Moreover, Ni4+ reduction at high voltages further aggravates irreversible phase transitions and antisite defects. Therefore, the slow kinetics of Li+ intercalation and poor lattice stability under high-rate discharging need to be addressed simultaneously. Typically, various strategies including transition metal doping and surface coating have been used well to solve the problem of Co-free high-Ni layered cathodes by improving Li+ diffusion kinetics, restraining structural failures and suppressing side reactions, thereby leading to efficient and stable Li+ storage.28–31 However, single-element doping cannot simultaneously solve the problematic crosstalk induced by Co-free and high-Ni.32–34 Surface coating needs to balance the thickness and nature of the protective layer.35–37 The coating film needs to be ultra-thin and stable and have good compatibility with the cathode particle surface. The co-optimization strategy of doping and coating will increase the time and difficulty of the preparation process. Therefore, an advanced Co-free high-Ni layered cathode needs to be designed to overcome structural degradation and severe side reactions under high-rate discharging.
High-entropy oxides (HEOs) are a new class of oxide materials designed based on high-entropy (HE) compositions. Typically, HEOs consist of five or more metal oxides, each with a molar ratio (atomic concentration) between 5% and 35%, forming a solid solution material.38 The flexibility of high entropy design reduces the cathode's dependence on any single key metal source while simultaneously optimizing multiple properties.39,40 In addition, the “entropy stabilization” effect can be maximized to compensate for Co-free high-Ni structural stability. Therefore, it is critical to optimize the balance between high-rate discharge performance and HE design by adjusting the composition and ratio of each element.41 Among the selected high-entropy elements, rare-earth elements (REs), due to their unfilled 4f electron orbitals and high oxophilicity, not only provide structural stability to layered cathodes during Li+ intercalation and deintercalation but also inhibit the reduction of Ni3+ to Ni2+, which can lead to cation mixing during calcination and cycling.42 Therefore, designing a rare-earth high-entropy synergistic strategy to provide crack-free, ultra-stable cathodes without sacrificing capacity, especially under harsh electrochemical conditions, is a critical task. In addition, a full understanding of the effect of high-entropy rare-earth elements on the structure–performance relationship, in particular the stability of cathodes during long cycling under a high-rate discharge, has not been fully explored.
In this work, we purposefully designed a novel lattice engineering strategy for high-rate discharge performance, i.e., through a synergistic strategy of rare-earth doping and high-entropy doping, to construct a collaborative doped cathode LiNi0.9Mn0.02Al0.02Mg0.02Ti0.02Lu0.02O2 (HE-Lu) as a competitive cathode material for advanced LIBs. The spherical secondary particle cathode with a uniform distribution of elements was achieved by an optimized spray drying technique. Further comprehensive physicochemical/electrochemical characterization demonstrated that Ni is expected to be used to improve reversible capacity and charge compensation. Al, Mg, Ti and Lu alleviate the bulk phase structure degradation and accelerate the kinetics of Li+ during deep delithiation. Due to the synergistic effect of rare-earth high-entropy doping, the HE-Lu cathode exhibits better electrochemical Li+ storage performance under a high-rate discharge and high cut-off voltage compared to LiNi0.9Mn0.1O2 (NM90). The HE-Lu cathode exhibits an initial capacity of 218.5 mA h g−1 at 0.2C. After 500 cycles at 1C/15C rate (1C charging and 15C discharging rate), the capacity retention of the HE-Lu cathode is 81.9% in half cells. The capacity retention of HE-Lu is 88.3% (1C/5C) after 1000 cycles in full cells. Overall, this novel lattice engineering strategy effectively eliminates the Co dependence and greatly enhances the stability of HE-Lu cathodes under a high-rate discharging process, which is an important step for the practical application of Co-free high-Ni layered cathodes in the specialty industry and high-power LIB systems.
2 Results and discussion
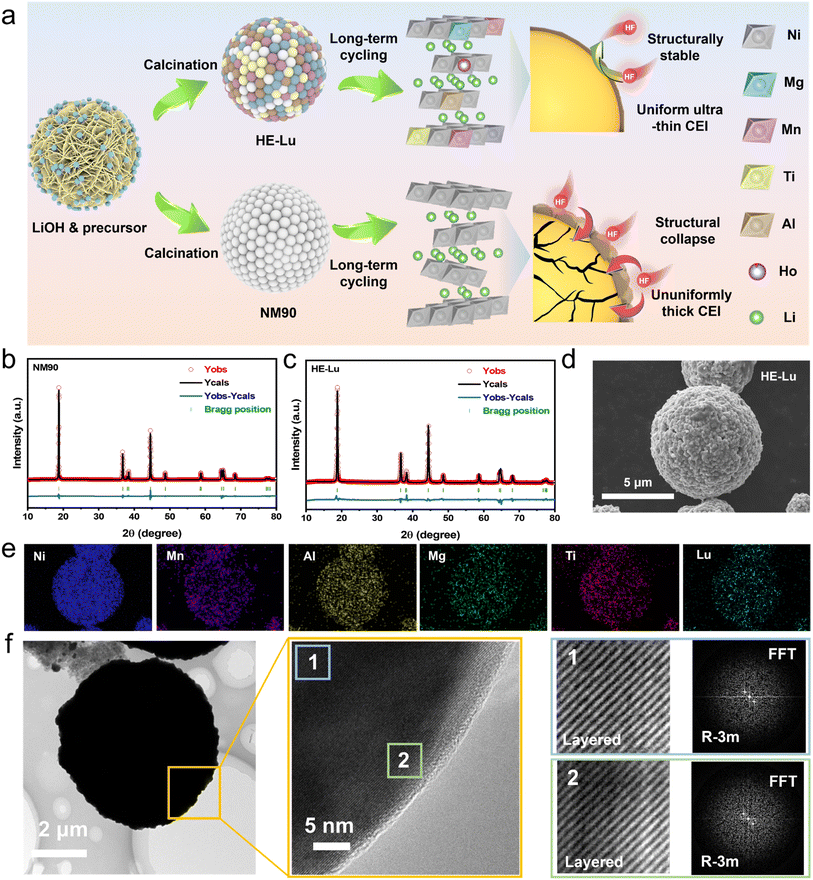
The synthetic process of Ni-rich Co-free NM90 cathode materials and HE-Lu cathode materials is illustrated in Fig. 1a. The process includes the mixing of raw materials and subsequent annealing. The detailed synthesis steps are described in the experimental section in the ESI.† Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) demonstrates that the actual composition does not deviate significantly from the design composition (Table S1†). X-ray diffraction (XRD) was used to analyze the crystal structures of NM90 and HE-Lu (Fig. 1b). The XRD results show that all samples exhibit strong diffraction peaks, which indicate that the materials have high crystallinity. All the characteristic peaks correspond well to the layered hexagonal α-NaFeO2 structure, which is characteristic of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, and no additional peaks are detected in both the pristine and modified samples. Meanwhile, the obvious splitting of the (006)/(102) and (018)/(110) peaks indicates that the samples have highly ordered layered structures.43 This implies that the rare-earth high-entropy synergistic strategy does not cause serious distortions to the structure of the matrix material. To gain insight into crystallographic information, we analyzed the obtained XRD data by Rietveld refinement. All relevant parameters are listed in Table S2.† The lattice parameters of HE-Lu (a = 2.8746 Å, c = 14.2535 Å) are slightly larger than those of NM90 (a = 2.8732 Å, c = 14.2147 Å). This result could be due to a consequence of lattice distortion caused by the diffusion of heterogeneous ions into the bulk phase of NM90 during the high-temperature calcination process.
m space group, and no additional peaks are detected in both the pristine and modified samples. Meanwhile, the obvious splitting of the (006)/(102) and (018)/(110) peaks indicates that the samples have highly ordered layered structures.43 This implies that the rare-earth high-entropy synergistic strategy does not cause serious distortions to the structure of the matrix material. To gain insight into crystallographic information, we analyzed the obtained XRD data by Rietveld refinement. All relevant parameters are listed in Table S2.† The lattice parameters of HE-Lu (a = 2.8746 Å, c = 14.2535 Å) are slightly larger than those of NM90 (a = 2.8732 Å, c = 14.2147 Å). This result could be due to a consequence of lattice distortion caused by the diffusion of heterogeneous ions into the bulk phase of NM90 during the high-temperature calcination process.
The expansion of layer spacing along the c-axis is conducive to the rapid lithiation/delithiation process. In addition, the Li+/Ni2+ antisite defects in HE-Lu (2.39%) are lower than those in NM90 (4.34%). The presence of Lu, which is strongly bonded to oxygen in the cathode material lattice, may result in limited structural changes occurring during Li+ insertion.43 Strengthening of the Lu–O bond limits the reduction of Ni3+ to Ni2+, which may inhibit cation mixing during calcination and charge/discharge. The lower Li+/Ni2+ antisite defects facilitate the stability of the crystal structure during cycling and effectively increase the specific discharge capacity.
The micromorphology of the spray-dried precursors and the rare-earth high-entropy-doped precursors was characterized by scanning electron microscopy (SEM). The precursors for spray drying were secondary particles with a size range of 8–12 μm (Fig. S1†). After the calcination process (see the Experimental section in the ESI†), all spherical precursors were transformed into oxide particles. The primary particles grew to form spherical cathode particles with high tap density (Fig. 1d). In addition, energy dispersive spectroscopy (EDS) mapping was used to further analyze the distribution of elements in the HE-Lu particles, as shown in Fig. 1e. The results showed that the elements Ni, Al, Mn, Ti, Mg and Lu were uniformly distributed in the HE-Lu particles. In particular, the cross-sectional SEM image and the corresponding EDS mapping of HE-Lu show that the high entropy doping elements are uniformly distributed in the bulk and on the surface (Fig. S2†). The modified samples with other elements replaced by Lu also maintain good spherical morphology and elemental distribution (Fig. S3†). The HRTEM image of HE-Lu shows obvious lattice fringes, indicating a well-layered structure. Further characterization of different regions inside and on the surface of cathode particles and the corresponding FFT transformations show that HE-Lu maintains a layered structure belonging to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group both on the surface and inside the bulk phase (Fig. 1f and g).
m space group both on the surface and inside the bulk phase (Fig. 1f and g).
To evaluate the effect of rare-earth high-entropy doping on the ability of NM90 to store Li+ efficiently, the electrochemical performance of the samples after rare-earth and high-entropy doping of NM90 was evaluated using the as-prepared cathodes. The charge–discharge profiles (Fig. 2a) show that HE-Lu delivers an initial discharge capacity of 218.5 mA h g−1 at 0.2C, which is comparable to that of NM90 (208.3 mA h g−1), and the initial coulombic efficiency of HE-Lu reaches 89.3%, which is significantly improved compared with the 81.5% of NM90. This improvement could be attributed to the synergistic effect of rare earth and high entropy, which reduces the Li+/Ni2+ antisite defects and stabilizes the layered structure, thus improving the reversible utilization efficiency of Li+. As shown in Fig. 2b, HE-Lu still provides a reversible discharge capacity of 126.9 mA h g−1 at 15C. When the rate returns to 0.2C, the capacity of HE-Lu is almost completely restored. Fig. 2c and d compares the cyclic voltammetry (CV) curves of the original and modified cathodes. Each curve contains three pairs of redox peaks, which correspond to different phase transitions such as H1–M, M–H2, and H2-H3 occurring during the charging and discharging processes, respectively. It is believed that the difference in the potential between the oxidation and reduction peaks can reflect the degree of Li+ polarization and the reversibility of lithiated and delithiated states during the charge/discharge process. As shown in Fig. 2c and d, the redox peak differences for HE-Lu are Δ1 = 0.171 V, Δ2 = 0.097 V, and Δ3 = 0.078 V. In contrast, the redox peak differences for NM90 are Δ1 = 0.178 V, Δ2 = 0.116 V, and Δ3 = 0.097 V, suggesting that HE-Lu possesses good lithiated/delithiated reversibility and inhibition of the electrode polarization. A comparison of the discharge curves for different cycles at 1C/5C shows that HE-Lu has less polarization even after 1500 cycles. In contrast, NM90 exhibited a large polarization with a capacity decay of 71.5 mA h g−1 after 368 cycles (Fig. 2e and f). By comparing the average potential of charging and discharging at 1C/5C rate, it can be found that the ΔE of HE-Lu is 0.437 V after 1500 cycles, while the ΔE of NM90 is 0.875 V after 368 cycles, which further proves the effects of rare-earth high-entropy doping on the stability of the long-cycle and the inhibition of polarization (Fig. S4†). The capacity retention and discharge specific capacity of HE-Lu and NM90 are 90.6% (155.3 mA h g−1) and 49.7% (69.5 mA h g−1) after 500 cycles at 3C, respectively (Fig. S5b†). As shown in Fig. 2g and i, we tested the long-cycling stability of the pristine and modified cathodes at 1C charging/5C discharging (1/5C) and 1C charging/10C discharging (1/10C) rates, respectively. HE-Lu exhibits extraordinarily long cycling stability under fast discharging. After 1500 cycles at 1/5C and 1/10C rates, the capacity retention of HE-Lu is 84.1% (1/5C) and 74.4% (1/10C). Under the same conditions, the capacity retention rates of NM90 after 200 cycles are 58.8% (1/5C) and 58.1% (1/10C). When cycled even at an ultra-high rate of 15C, HE-Lu was still able to deliver 107.5 mA h g−1 discharge specific capacity after 500 cycles, while the NM90 capacity was almost completely lost (Fig. S6†). This could be attributed to the fact that the ultra-high rate further deepens the polarization of NM90, and the long-term Li-depleted state inside the cathode severely reduces the structural stability of the cathode. The excellent stability of HE-Lu is further demonstrated by the fact that the peaks and intensities of the reduction peaks did not undergo significant shifts and decays during the long-term cycling, whereas the peaks and intensities of the reduction peaks of NM90 underwent significant shifts and decays (Fig. 2h and j). In addition, we have conducted the electrochemical performance tests of HE-Lu cathodes at a low rate to further demonstrate that the rare-earth high-entropy doping optimized HE-Lu could be widely used in a wide range of scenarios. Notably, HE-Lu still has excellent performance at a low rate. The capacity retention of HE-Lu after 100 cycles at 0.5C and 0.2C is 97.6% and 99.2%, respectively, which further demonstrates that the rare-earth high-entropy doping synergistic optimization for the modification of the Co-free high-Ni layered cathode can achieve excellent performance under various operating conditions (Fig. S7†). To quantitatively evaluate the H2–H3 phase transition, ex situ XRD was performed at 0.1C during the first cycle in the voltage range from 3.7 to 4.3 V, and the evolution of peaks (003) and (101) was tracked (Fig. S8†). In the initial stage, as Li+ is extracted from the cathode, the (003) peak gradually shifts to lower angles, indicating an increase in interlayer spacing due to enhanced electrostatic repulsion within the lattice. Subsequently, during the H2–H3 phase transition, the (003) peak abruptly shifts to higher angles, suggesting a significant contraction along the c-axis caused by lattice collapse. Simultaneously, the shift of the (101) peak to higher angles reflects the reduction in the radius of transition metal ions due to oxidation, leading to a gradual contraction along the a- and b-axes. Notably, the peak shifts for HE-Lu are significantly smaller, with 0.277° for the (003) peak and 0.529° for the (101) peak. Rietveld refinement reveals that the c-axis lattice parameter of NM90 undergoes a sudden decrease of 2.41% during the H2–H3 phase transition, whereas the c-axis contraction for HE-Lu is only 1.44%. The H2–H3 phase transition in the HE-Lu cathode is not entirely eliminated. In contrast, Lu doping optimizes the reversibility and uniformity of the phase transition process. This is further supported by the reduction of microcracks observed in post-cycling SEM images of the electrode.
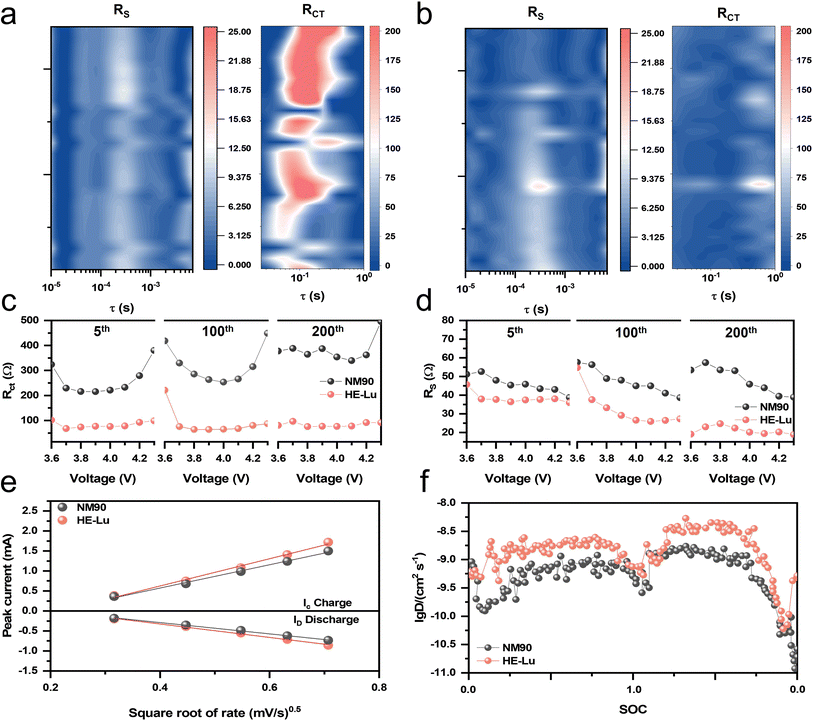
To reveal the stabilization mechanism of rare-earth high-entropy doping during long-term cycling, in situ electrochemical impedance spectroscopy (EIS) analyses were carried out on the pristine and modified cathodes. EIS profiles (Fig. S9†) obtained by the equivalent circuit model (Fig. S9g†) reveal the change in charge transfer resistance (Rct) and cathode interfacial resistance (RCEI) for both NM90 and HE-Lu. The distribution of relaxation times (DRT) was used to de-convolve the electrochemical processes from the EIS data at different SOCs. Typically, charge transfer occurs with a time constant between 10−2 and 100 s, and Li+ transport across the cathode electrolyte interface (CEI) occurs between 10−4 and 10−2 s. As shown in Fig. 3a–d, the Rct and RCEI become larger with an increasing number of cycles at the same voltage. Compared with NM90, the increase in Rct and RCEI of HE-Lu is suppressed and maintains a low value, which proves that the synergistic effect of rare-earth high-entropy doping can effectively suppress the increase of Rct and RCEI during the charge/discharge processes.44 The rapidly growing Rct and RCEI should result from the deposition of Li-insulating by-products as a result of surface-side reactions and continuous microcrack propagation after long cycling.45 It is noteworthy that the lower RCEI of HE-Lu could be due to the combination of ultra-thin, low-impedance CEI and fast Li+ diffusion kinetics. The low Rct and RCEI can further satisfy the requirements of fast Li+ kinetics and interfacial stability during the high-rate discharging process.
To gain insight into the electrochemical reaction kinetics associated with the rare-earth high-entropy strategy, CV tests were performed at different scan rates of 0.1–0.5 mV s−1 (Fig. 3e). The Li+ diffusion process can be reflected by the linear relationship between the square root of the scan rate and the peak current.46 According to the classical Randles–Sevcik equation:
| Ip = 2.69 × 105n3/2AD1/2Cv1/2 |
where MB (g mol−1) and mB (g) correspond to the molecular weight and mass of the electrode materials, respectively. VM (cm3 mol−1) represents the molar volume of electrode materials. S (cm2) is the BET surface area of the cathode material. τ (s) is the pulse time.
The results show that the Li+ diffusion rate is the fastest at a medium SOC, while it is slower at low and high SOCs (Fig. 3f). The high activation energy and the small number of lithium vacancies may be the reason for the slow Li+ diffusion rate at low SOCs. Under high voltages, the H2–H3 phase transition process leads to a sharp contraction of the c-axis of the lattice, which further inhibits the Li+ diffusion. The SBET of the cathode materials obtained by the BET test can accurately reflect the Li+ diffusion coefficient in the GITT test. The SBET values of NM90 and HE-Lu were 0.642 and 0.497 m2 g−1, respectively. The GITT results show that the diffusion coefficient of Li+ is improved after the modification. The large ionic radius of rare-earth elements widens the interlayer spacing, which facilitates the Li+ transport, and the high-entropy doping significantly improves the long-term cycling stability of HE-Lu under a high-rate discharge through the “entropy stabilization” effect. Obviously, the stability and Li+ diffusion rate of the high-Ni layered cathode were significantly improved under the synergistic effect of rare-earth and high-entropy doping.
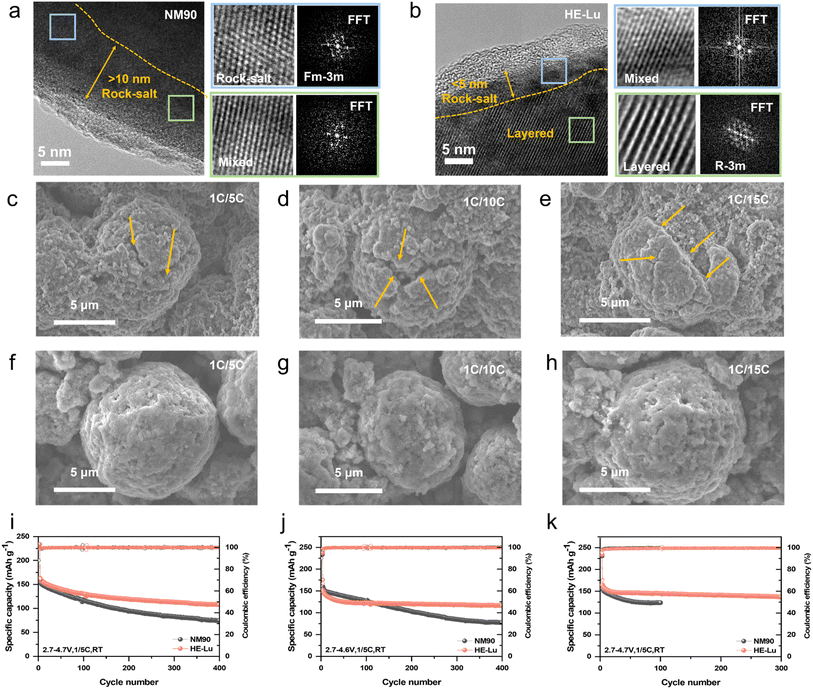
In the deeply delithiated state, the highly valent Ni4+ is reduced to Ni2+ and migrates into the Li layer, leading to a shift of the layered structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) to the rock salt phase (Fm
m) to the rock salt phase (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and impeding of the Li+ migration pathway. This undesired structural degradation will be exacerbated with electrochemical cycling and coupling with interfacial parasitic reactions, ultimately leading to significant polarization and capacity degradation. HRTEM tests were performed to investigate the structural evolution of NM90 and HE-Lu after 200 cycles in the voltage range of 2.7–4.3 V at 1C/5C. As shown in Fig. 4a and S12,† the CEI on the surface of the NM90 cathode has some thicker areas and is not uniformly continuous. This is caused by the inconsistency of lithiation on the particle surface due to long-term high-rate discharge. The hysteresis of lithiation in different regions will further trigger the Li+ concentration gradient, which in turn generates microcracks. In contrast, the uniformly continuous and ∼5 nm CEI in HE-Lu ensures highly reversible Li+ insertion. Furthermore, HRTEM analysis of the near-surface cathode particles shows that a mixture of rock salt and layered phases with a thickness greater than 10 nm can be observed on the NM90 surface. The layer was carefully analyzed using the FFT method, and its typical characteristics of rock salt and mixed phases were determined, as indicated by the blue and green boxed areas. For HE-Lu, the thickness of the observed surface phase transition region is less than 5 nm, which is much thinner than that of NM90 (Fig. 4b). These HRTEM results indicate that the surface structural degradation of HE-Lu is significantly suppressed compared to that of NM90, and the rare-earth high-entropy design in HE-Lu has a strong stabilizing effect on the surface lattice oxygen in the deep delithiated state, which can mitigate the reduction of Ni4+ to Ni2+ and the escape of oxygen, thus suppressing the detrimental surface phase transition.
m) and impeding of the Li+ migration pathway. This undesired structural degradation will be exacerbated with electrochemical cycling and coupling with interfacial parasitic reactions, ultimately leading to significant polarization and capacity degradation. HRTEM tests were performed to investigate the structural evolution of NM90 and HE-Lu after 200 cycles in the voltage range of 2.7–4.3 V at 1C/5C. As shown in Fig. 4a and S12,† the CEI on the surface of the NM90 cathode has some thicker areas and is not uniformly continuous. This is caused by the inconsistency of lithiation on the particle surface due to long-term high-rate discharge. The hysteresis of lithiation in different regions will further trigger the Li+ concentration gradient, which in turn generates microcracks. In contrast, the uniformly continuous and ∼5 nm CEI in HE-Lu ensures highly reversible Li+ insertion. Furthermore, HRTEM analysis of the near-surface cathode particles shows that a mixture of rock salt and layered phases with a thickness greater than 10 nm can be observed on the NM90 surface. The layer was carefully analyzed using the FFT method, and its typical characteristics of rock salt and mixed phases were determined, as indicated by the blue and green boxed areas. For HE-Lu, the thickness of the observed surface phase transition region is less than 5 nm, which is much thinner than that of NM90 (Fig. 4b). These HRTEM results indicate that the surface structural degradation of HE-Lu is significantly suppressed compared to that of NM90, and the rare-earth high-entropy design in HE-Lu has a strong stabilizing effect on the surface lattice oxygen in the deep delithiated state, which can mitigate the reduction of Ni4+ to Ni2+ and the escape of oxygen, thus suppressing the detrimental surface phase transition.
SEM characterization of the cathode material after cycling at different discharge rates is shown in Fig. 4c–h. Different decay characteristics are exhibited at different discharge rates. At 1C/5C rate, tiny cracks appear on the surface of NM90 cathode particles, and with the further increase of the discharge rate, even some particles are broken at 1C/10C rate. It is worth noting that the NM90 particles are almost completely broken at 1C/15C ultra-high discharge rate. The more serious structural degradation under a high-rate discharge is attributed to the large amount of heat generated in the cell by the excessive discharge current, which leads to the oxidative decomposition of the electrolyte and CEI reconstruction, resulting in a serious parasitic reaction and the destruction of the crystal structure of the NM90 cathode. In addition, under high-rate discharge conditions, the decomposition products of the anode migrate to the cathode, so that a large number of nanoparticles can be observed on the surface of the NM90 particles. Due to the low depth of discharge (DOD) of the battery under high-rate discharge conditions, Li+ will not be completely removed from the anode, and the interior of the cathode is in the state of Li depletion for a long time. During this process, the loss of active lithium inside the cathode is the main cause of performance aging. In contrast, as shown in Fig. 4h, HE-Lu maintains good integrity even at an ultra-high discharge rate of 15C, which demonstrates the importance of rare-earth high-entropy doping to improve the stability of Co-free high-Ni layered cathodes.
In addition, high-rate discharges can lead to prolonged delithiation of the cathode material, which can be achieved by increasing the cut-off voltage. Therefore, the electrochemical performance of HE-Lu under more severe operating conditions was further evaluated to demonstrate its commercial viability. We evaluated the cycling stability at high cut-off voltages of 4.5–4.7 V and 1C/5C rate. Under the high-rate discharge and high cut-off voltage, on the one hand, the fast discharge leads to incomplete insertion of Li+ from the anode into the cathode material. On the other hand, the high cut-off voltage leads to further extraction of Li+ from the Li layer inside the cathode. Therefore, the long-term depletion of Li+ in the cathode exerts higher requirements on the stability of the cathode. As shown in Fig. 4i–k, a further improvement of the structural tolerance of rare-earth high-entropy doping at high cut-off voltages and high-rate discharges can be seen by evaluating the long-cycle stability. HE-Lu provides excellent capacity conservation with capacity loss rates of 30.2% (4.5 V) and 23.2% (4.6 V) after 400 cycles, which are considerably lower than the capacity loss rates of 52.2% (4.5 V) and 48.1% (4.6 V) for NM90 after 400 cycles. In addition, the voltage plateau of HE-Lu was simultaneously well-maintained after long-term cycling, whereas the voltage decay of NM90 became increasingly severe, suggesting a relatively fast polarization growth and a large resistance build-up after long cycles (Fig. S13†). Even when the charging cut-off potential was increased to 4.7 V, HE-Lu was able to withstand 300 long cycles with 87.4% capacity retention accompanied by a significant voltage plateau and a moderate polarization gap, which greatly exceeded those of NM90 (78.5%). Notably, the post-cycling cathode particles were characterized by SEM. The results show that the NM90 cathode particles exhibit significant structural degradation at 4.5 V, with cracks on the surface gradually forming a connecting network and expanding to the interior. When the cut-off voltage was increased to 4.6 V, the stability of the cathode structure was further degraded by the structural collapse and various anisotropic volume changes due to deep delithiation. When the cut-off voltage was further increased to 4.7 V, the spherical particle structure of NM90 is lost, which is the result of the combined effect of high cut-off voltage and high-rate discharge. However, in HE-Lu, the spherical structure was well maintained, and only tiny cracks appeared on the particle surface at 4.7 V (Fig. S14†). The spherical structure of HE-Lu was also well preserved at 4.7 V. The above morphology analysis further demonstrates that rare-earth high-entropy doping enhances the stability of the cathode structure even under harsh conditions (Fig. S15 and 16†).
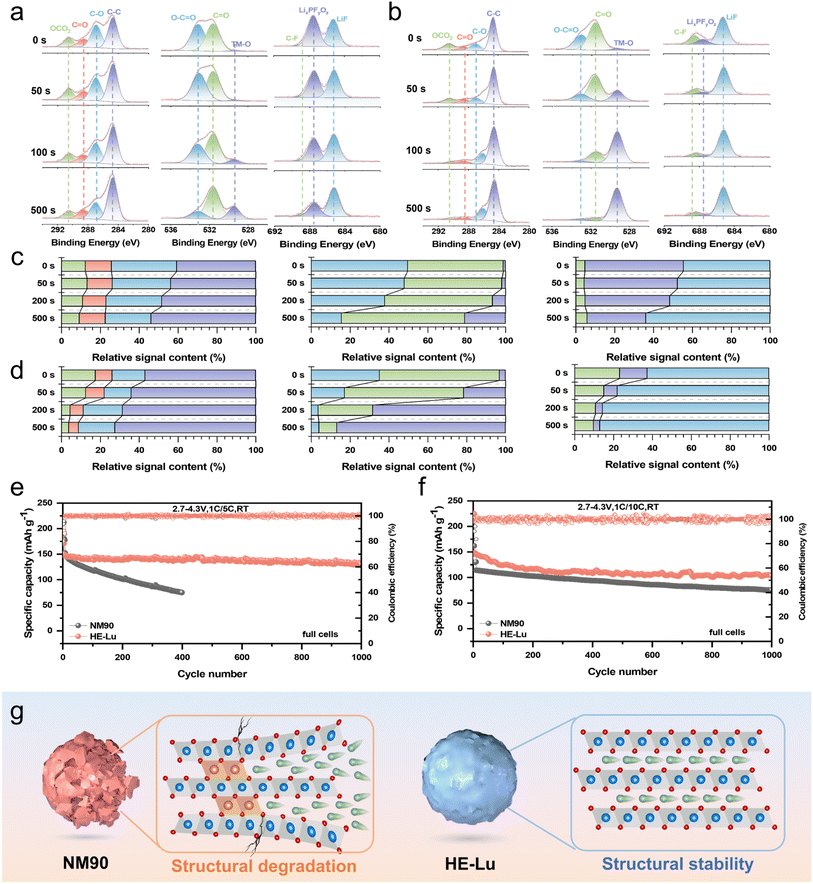
To further investigate the properties of CEI, the dependence of CEI thickness and composition on the etching depth was examined by XPS for NM90 (Fig. 5a and c) and HE-Lu (Fig. 5b and d).49 In the C 1s spectra, electrolyte solvent decomposition can be reflected on C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and OCO2 as the fitted peaks are associated with ROCO2Li, ROLi, and Li2CO3 substances (R = alkyl), respectively. The percentage of fitted peak area indicates that the rare-earth high-entropy doped HE-Lu interfacial side reactions and solute decomposition are significantly reduced. The peak intensity of O–C
O, and OCO2 as the fitted peaks are associated with ROCO2Li, ROLi, and Li2CO3 substances (R = alkyl), respectively. The percentage of fitted peak area indicates that the rare-earth high-entropy doped HE-Lu interfacial side reactions and solute decomposition are significantly reduced. The peak intensity of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O decreases rapidly and disappears almost completely when the etching time reaches 100 s, which proves that HE-Lu has no side reaction deep inside the particles. In contrast, the peak of O–C
O decreases rapidly and disappears almost completely when the etching time reaches 100 s, which proves that HE-Lu has no side reaction deep inside the particles. In contrast, the peak of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can still be detected at 500 s etching time. In addition, the lagged appearance of TM-O peaks of NM90 relative to that of HE-Lu further proves that the thicker and unstable CEI and particle cracks in NM90 lead to severe side reactions from further solute decomposition.50,51 More convincing evidence can also be found in the signal of LixPFyOz in the F 1s spectrum. where the LixPFyOz signal intensity is largely suppressed in HE-Lu compared to that in NM90. Furthermore, even higher intensities of LiF at all etch depths in HE-Lu are believed to be the result of forming an ultra-stable CEI with a higher LiF content, which is of great benefit for prolonged cycling stability. In addition, analysis of the photographs of the Li anode in the NM90//Li cell after 200 cycles at 1C/5C cycling shows that there is a severe morphological change in Li of NM90, which may be caused by a severe side reaction due to the high-rate discharge. In contrast, morphology of the HE-Lu//Li cell on the Li side remains basically unchanged (Fig. S17†).
O can still be detected at 500 s etching time. In addition, the lagged appearance of TM-O peaks of NM90 relative to that of HE-Lu further proves that the thicker and unstable CEI and particle cracks in NM90 lead to severe side reactions from further solute decomposition.50,51 More convincing evidence can also be found in the signal of LixPFyOz in the F 1s spectrum. where the LixPFyOz signal intensity is largely suppressed in HE-Lu compared to that in NM90. Furthermore, even higher intensities of LiF at all etch depths in HE-Lu are believed to be the result of forming an ultra-stable CEI with a higher LiF content, which is of great benefit for prolonged cycling stability. In addition, analysis of the photographs of the Li anode in the NM90//Li cell after 200 cycles at 1C/5C cycling shows that there is a severe morphological change in Li of NM90, which may be caused by a severe side reaction due to the high-rate discharge. In contrast, morphology of the HE-Lu//Li cell on the Li side remains basically unchanged (Fig. S17†).
To further assess the feasibility of our optimized HE-Lu as a high-power LIB cathode material, we assembled graphite//HE-Lu full cells using commercial graphite as the anode material for device-level electrochemical evaluation. Fig. S18† shows the long-term cycling performance of the full cells in the voltage range of 2.7–4.3 V. The capacity retention rates of NM90 and HE-Lu are 28.1% and 82.8% after 500 cycles at 1C, respectively. HE-Lu still delivers a specific capacity of discharge of 131.1 mA h g−1 after 1000 cycles at 3C, with an average capacity decay of 0.0186% per cycle, which demonstrates the excellent electrochemical stability and reversibility of HE-Lu in full cells.
To have a more comprehensive evaluation of the harsh working conditions under real conditions, we evaluated the electrochemical performance under high-rate discharge and in a wide temperature region. It is noteworthy that the capacity retention of HE-Lu after 1000 cycles is 88.3% (1C/5C) and 71.3% (1C/10C), which demonstrates that HE-Lu has excellent capability under a high-rate discharge in full cells (Fig. 5e and f). The charging and discharging curves of different cycles show that HE-Lu not only has less capacity decay but its voltage plateau is also well maintained (Fig. S19†). SEM-EDS mapping of the graphite anode after 200 cycles shows that the graphite anode surface of HE-Lu full cells contains less transition metal. This proves that the rare-earth high-entropy doping suppressed the dissolution of transition metals in the HE-Lu layered cathode (Fig. S20†). In addition, the cycling performance of full cells was tested at 55 and −5 °C at 1C (Fig. S21†). Remarkably, after 200 cycles, the average per cycle capacity degradation of HE-Lu was only 0.131% (55 °C) and 0.092% (−5 °C), which demonstrates excellent adaptability to a wide operating temperature range. Finally, we installed the full cells on a light bulb to test their practical application. HE-Lu has a longer duration and no significant change in brightness after 35 min of continuous illumination (Fig. S22†).
3 Conclusion
In conclusion, we have successfully prepared a high-performance Co-free high-Ni layered cathode HE-Lu via a synergistic strategy of rare-earth high-entropy doping, which enable a high-power LIB with high-rate discharge capability. Electrochemical performance tests and mechanistic analyses indicate that rare-earth doping, in combination with high-entropy doping, plays an important role in promoting charge diffusion kinetics, inducing stable interfacial layers and inhibiting structural degradation, thus rationally explaining the competitive Li+ storage properties of the HE-Lu cathode under high-rate discharging conditions. Specifically, the enhancement of the “entropy stabilization” effect has been achieved by optimizing the substitution of the rare-earth element Lu for Si. The synergistic effect of Lu, Mn, Al, Mg and Ti contributes to the excellent long-term cycling stability of HE-Lu under high-rate discharging. The rare-earth high-entropy doping improves the Li+ diffusion kinetics and reduces the interfacial resistance. The ultra-thin homogeneous CEI further reduces the loss of active Li+ in the cathode and suppresses the phase transition near the surface. As a result, the HE-Lu cathode can maintain the integrity of spherical particles under harsh conditions of high cut-off voltage and high-rate discharging. The initial capacity of the HE-Lu cathode is 218.5 mA h g−1 at 0.2C. The capacity retention of HE-Lu is 88.3% (1C/5C) even after 1000 cycles in full cells. Our innovative modification strategy provides valuable guidance and insights for the development of advanced high-power Co-free high-Ni cathodes for further application in smart energy storage systems and specialized industry applications.Data availability
The data supporting this article have been included as part of the ESI.† The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
H. T. conceived the idea for the project and supervised the project. Y. L., Y. X., and B. H. wrote the manuscript. W. L. helped Y. L. prepare the cathode materials. F. Z. helped Y. L. analyze the cathodes in this work. All the authors agreed upon the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
H. T. offers thanks to support from the Beijing Laboratory of New Energy Storage Technology, North China Electric Power University and the Program of the National Energy Storage Industry-Education Platform, and the Interdisciplinary Innovation Program of North China Electric Power University (No. XM2212315).References
- G.-T. Park, N.-Y. Park, H.-H. Ryu, H. H. Sun, J.-Y. Hwang and Y.-K. Sun, Chem. Soc. Rev., 2024, 53, 11462–11518 RSC
.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS
.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed
.
- H. Yuan, K. Wen, S. Guan, Y. Liang, Y.-H. Wu, S. Liu, D. Yu, L. Li and C.-W. Nan, J. Materiomics, 2024, 10, 643–651 CrossRef
.
- Y.-K. Sun, Z. Chen, H.-J. Noh, D.-J. Lee, H.-G. Jung, Y. Ren, S. Wang, C. S. Yoon, S.-T. Myung and K. Amine, Nat. Mater., 2012, 11, 942–947 CrossRef CAS PubMed
.
- C. Huang, H. Zheng, N. Qin, C. Wang, L. Wang and J. Lu, Acta Phys.-Chim. Sin., 2024, 40, 2308051 CrossRef
.
- F. Zhang, B. He, Y. Xin, T. Zhu, Y. Zhang, S. Wang, W. Li, Y. Yang and H. Tian, Chem. Rev., 2024, 124, 4778–4821 CrossRef CAS PubMed
.
- B. He, F. Zhang, Y. Xin, C. Xu, X. Hu, X. Wu, Y. Yang and H. Tian, Nat. Rev. Chem, 2023, 7, 826–842 Search PubMed
.
- A. Manthiram, Nat. Commun., 2020, 11, 1550 CrossRef CAS PubMed
.
- D. Aurbach, Z. Lu, A. Schechter, Y. Gofer, H. Gizbar, R. Turgeman, Y. Cohen, M. Moshkovich and E. Levi, Nature, 2000, 407, 724–727 CrossRef CAS PubMed
.
- R. J. Clément, Z. Lun and G. Ceder, Energy Environ. Sci., 2020, 13, 345–373 RSC
.
- Y.-K. Hou, C. Li, D. Ren, F. He, K. Zhuang, S. Yin, G. Yuan, Y. Wang, Y. Guo, S. Liu, P. Sun, Z. Zhang, T. Tan, G. Zhu, L. Lu, X. Liu and M. Ouyang, Adv. Sci., 2023, 10, 2306347 CrossRef CAS PubMed
.
- Y. Kim, H. Kim, W. Shin, E. Jo and A. Manthiram, Adv. Energy Mater., 2023, 13, 2204054 CrossRef CAS
.
- G.-T. Park, S.-B. Kim, B. Namkoong, N.-Y. Park, H. Kim, C. S. Yoon and Y.-K. Sun, Mater. Today, 2023, 71, 38–49 CrossRef CAS
.
- T. Liu, L. Yu, J. Liu, J. Lu, X. Bi, A. Dai, M. Li, M. Li, Z. Hu, L. Ma, D. Luo, J. Zheng, T. Wu, Y. Ren, J. Wen, F. Pan and K. Amine, Nat. Energy, 2021, 6, 277–286 CrossRef CAS
.
- G.-T. Park, B. Namkoong, S.-B. Kim, J. Liu, C. S. Yoon and Y.-K. Sun, Nat. Energy, 2022, 7, 946–954 CrossRef CAS
.
- J. Zhang, S. Wang, X. Yang, Y. Liu, Z. Wu, H. Li, S. Indris, H. Ehrenberg and W. Hua, Chem. Eng. J., 2024, 484, 149599 CrossRef CAS
.
- Y. Yang, R. Xu, K. Zhang, S.-J. Lee, L. Mu, P. Liu, C. K. Waters, S. Spence, Z. Xu, C. Wei, D. J. Kautz, Q. Yuan, Y. Dong, Y.-S. Yu, X. Xiao, H.-K. Lee, P. Pianetta, P. Cloetens, J.-S. Lee, K. Zhao, F. Lin and Y. Liu, Adv. Energy Mater., 2019, 9, 1900674 CrossRef
.
- J. Li, N. Sharma, Z. Jiang, Y. Yang, F. Monaco, Z. Xu, D. Hou, D. Ratner, P. Pianetta, P. Cloetens, F. Lin, K. Zhao and Y. Liu, Science, 2022, 376, 517–521 CrossRef CAS PubMed
.
- J. Wandt, A. Freiberg, R. Thomas, Y. Gorlin, A. Siebel, R. Jung, H. A. Gasteiger and M. Tromp, J. Mater. Chem. A, 2016, 4, 18300–18305 RSC
.
- D.-S. Ko, J.-H. Park, S. Park, Y. N. Ham, S. J. Ahn, J.-H. Park, H. N. Han, E. Lee, W. S. Jeon and C. Jung, Nano Energy, 2019, 56, 434–442 CrossRef CAS
.
- T. Weigel, F. Schipper, E. M. Erickson, F. A. Susai, B. Markovsky and D. Aurbach, ACS Energy Lett., 2019, 4, 508–516 CrossRef CAS
.
- E. S. Zsoldos, M. M. E. Cormier, M. Ball, D. Rathore and J. R. Dahn, J. Electrochem., 2023, 170, 070502 CAS
.
- S.-M. Han, G.-T. Park, D.-H. Kim, M.-G. Seo, N.-Y. Park and Y.-K. Sun, ACS Energy Lett., 2024, 9, 5859–5868 CrossRef CAS
.
- X. Chen, Y. Li, Y. Lu, J. Xie, C. Huang, X. Xu, J. Tu, X. Zhao and T. Zhu, J. Materiomics, 2024, 10, 682–693 CrossRef
.
- H. Liu, Z. Xie, W. Qu, E. Dy, S. Niketic, S. Brueckner, K. Tsay, E. Fuller, C. Bock, N. Zaker and G. A. Botton, Small, 2022, 18, 2200627 CrossRef CAS PubMed
.
- X. Liu, G.-L. Xu, V. S. C. Kolluru, C. Zhao, Q. Li, X. Zhou, Y. Liu, L. Yin, Z. Zhuo, A. Daali, J.-J. Fan, W. Liu, Y. Ren, W. Xu, J. Deng, I. Hwang, D. Ren, X. Feng, C. Sun, L. Huang, T. Zhou, M. Du, Z. Chen, S.-G. Sun, M. K. Y. Chan, W. Yang, M. Ouyang and K. Amine, Nat. Energy, 2022, 7, 808–817 CrossRef CAS
.
- C.-G. Shi, C.-H. Shen, X.-X. Peng, C.-X. Luo, L.-F. Shen, W.-J. Sheng, J.-J. Fan, Q. Wang, S.-J. Zhang, B.-B. Xu, J.-J. Xian, Y.-M. Wei, L. Huang, J.-T. Li and S.-G. Sun, Nano Energy, 2019, 65, 104084 CAS
.
- P. Yan, J. Zheng, T. Chen, L. Luo, Y. Jiang, K. Wang, M. Sui, J.-G. Zhang, S. Zhang and C. Wang, Nat. Commun., 2018, 9, 2437 Search PubMed
.
- W. Guo, W. Wei, H. Zhu, Y. Hu, H. Jiang and C. Li, eScience, 2023, 3, 100082 Search PubMed
.
- Y.-H. Luo, Q.-L. Pan, H.-X. Wei, Y.-D. Huang, P.-Y. Li, L.-B. Tang, Z.-Y. Wang, C. Yan, J. Mao, K.-H. Dai, Q. Wu, X.-H. Zhang and J.-C. Zheng, eScience, 2024, 4, 100229 Search PubMed
.
- L. Mu, R. Lin, R. Xu, L. Han, S. Xia, D. Sokaras, J. D. Steiner, T.-C. Weng, D. Nordlund, M. M. Doeff, Y. Liu, K. Zhao, H. L. Xin and F. Lin, Nano Lett., 2018, 18, 3241–3249 CAS
.
- C. Wang, L. Han, R. Zhang, H. Cheng, L. Mu, K. Kisslinger, P. Zou, Y. Ren, P. Cao, F. Lin and H. L. Xin, Matter, 2021, 4, 2013–2026 CrossRef CAS
.
- Z. Wang, H. Zhu, H. Yu, T. Zhang, Y. Hu, H. Jiang and C. Li, Chin. Chem. Lett., 2023, 34, 107718 CrossRef CAS
.
- S. J. Shi, J. P. Tu, Y. Y. Tang, Y. Q. Zhang, X. Y. Liu, X. L. Wang and C. D. Gu, J. Power Sources, 2013, 225, 338–346 CrossRef CAS
.
- X. Zhou, F. Hong, S. Wang, T. Zhao, J. Peng, B. Zhang, W. Fan, W. Xing, M. Zuo, P. Zhang, Y. Zhou, G. Lv, Y. Zhong, W. Hua and W. Xiang, eScience, 2024, 4, 100276 CrossRef
.
- B. Jiang, H. Li, B. Luo, L. Liu, L. Chu, Q. Zhang and M. Li, Chin. Chem. Lett., 2024, 35, 108649 CrossRef CAS
.
- R. Zhang, C. Wang, P. Zou, R. Lin, L. Ma, T. Li, I.-h. Hwang, W. Xu, C. Sun, S. Trask and H. L. Xin, Nat. Energy, 2023, 8, 695–702 CrossRef CAS
.
- X.-H. Meng, T. Lin, H. Mao, J.-L. Shi, H. Sheng, Y.-G. Zou, M. Fan, K. Jiang, R.-J. Xiao, D. Xiao, L. Gu, L.-J. Wan and Y.-G. Guo, J. Am. Chem. Soc., 2022, 144, 11338–11347 CrossRef CAS PubMed
.
- L. Liang, X. Li, M. Su, L. Wang, J. Sun, Y. Liu, L. Hou and C. Yuan, Angew. Chem., Int. Ed., 2023, 62, e202216155 CAS
.
- F. Ding, C. Zhao, D. Xiao, X. Rong, H. Wang, Y. Li, Y. Yang, Y. Lu and Y.-S. Hu, J. Am. Chem. Soc., 2022, 144, 8286–8295 CAS
.
- X. Zhu, H. Yu, L. Cheng, F. Xu, Z. Wang and L.-Z. Fan, J. Materiomics, 2023, 9, 82–89 Search PubMed
.
- X. Qu, H. Huang, T. Wan, L. Hu, Z. Yu, Y. Liu, A. Dou, Y. Zhou, M. Su, X. Peng, H.-H. Wu, T. Wu and D. Chu, Nano Energy, 2022, 91, 106665 CAS
.
- K. Ding, E. J. Begin, S. Yuan, M. Zhong, Y. Wang, Y. Zhang, X. Zeng, J. L. Bao and Y. Wang, Adv. Energy Mater., 2023, 13, 2302443 CAS
.
- Y. Zhou, H. Zhang, Y. Wang, T. Wan, P. Guan, X. Zhou, X. Wang, Y. Chen, H. Shi, A. Dou, M. Su, R. Guo, Y. Liu, L. Dai and D. Chu, ACS Nano, 2023, 17, 20621–20633 CAS
.
- Q. Xu, X. Li, H. M. Kheimeh Sari, W. Li, W. Liu, Y. Hao, J. Qin, B. Cao, W. Xiao, Y. Xu, Y. Wei, L. Kou, Z. Tian, L. Shao, C. Zhang and X. Sun, Nano Energy, 2020, 77, 105034 CAS
.
- J. Li, X. Wang, X. Kong, H. Yang, J. Zeng and J. Zhao, ACS Sustain. Chem. Eng., 2021, 9, 10547–10556 CrossRef CAS
.
- Y. Zhang, Z.-B. Wang, F.-D. Yu, L.-F. Que, M.-J. Wang, Y.-F. Xia, Y. Xue and J. Wu, J. Power Sources, 2017, 358, 1–12 CrossRef CAS
.
- T. R. Tanim, Z. Yang, D. P. Finegan, P. R. Chinnam, Y. Lin, P. J. Weddle, I. Bloom, A. M. Colclasure, E. J. Dufek, J. Wen, Y. Tsai, M. C. Evans, K. Smith, J. M. Allen, C. C. Dickerson, A. H. Quinn, A. R. Dunlop, S. E. Trask and A. N. Jansen, Adv. Energy Mater., 2022, 12, 2103712 CrossRef CAS
.
- X. Cheng, X. Liu, L. Zhao, D. Zhang, J. Biao, Z. Chen, Y. Yuan, M. Liu, Y.-B. He and F. Kang, Adv. Funct. Mater., 2023, 33, 2211171 CrossRef CAS
.
- H. Huang, L. Zhang, H. Tian, J. Yan, J. Tong, X. Liu, H. Zhang, H. Huang, S.-m. Hao, J. Gao, L. Yu, H. Li, J. Qiu and W. Zhou, Adv. Energy Mater., 2023, 13, 2203188 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta00576k |
| This journal is © The Royal Society of Chemistry 2025 |