Nanoparticles based image-guided thermal therapy and temperature feedback
Carlos
Jacinto
 *a,
Wagner F.
Silva
*a,
Wagner F.
Silva
 a,
Joel
Garcia
a,
Joel
Garcia
 *b,
Gelo P.
Zaragosa
*b,
Gelo P.
Zaragosa
 b,
Carlo Nonato D.
Ilem
b,
Carlo Nonato D.
Ilem
 b,
Tasso O.
Sales
a,
Harrisson D. A.
Santos
a,
Blessed Isaac C.
Conde
b,
Tasso O.
Sales
a,
Harrisson D. A.
Santos
a,
Blessed Isaac C.
Conde
 b,
Helliomar Pereira
Barbosa
c,
Sonia
Malik
*de and
Surender Kumar
Sharma
b,
Helliomar Pereira
Barbosa
c,
Sonia
Malik
*de and
Surender Kumar
Sharma
 *fg
*fg
aNano-Photonics and Imaging Group, Institute of Physics, Universidade Federal de Alagoas, 57072-900, Maceió-AL, Brazil. E-mail: cjacinto@fis.ufal.br
bDepartment of Chemistry, De La Salle University, Manila, Philippines. E-mail: joel.garcia@dlsu.edu.ph
cDepartment of Chemistry and Biomolecular Sciences, University of Ottawa, Canada
dPhysiology, Ecology & Environmental Laboratory (P2e), University of Orléans, 45067, France. E-mail: sonia.malik@univ-orleans.fr
eDepartment of Biotechnology, Baba Farid College, Bathinda, 151001, India
fDepartment of Physics, Central University of Punjab, Bathinda 151401, India. E-mail: surender.sharma@cup.edu.in
gDepartment of Physics, Federal University of Maranhão, São Luís, 65080-805, Brazil
First published on 8th November 2024
Abstract
Nanoparticles have emerged as versatile tools in the realm of thermal therapy, offering precise control and feedback mechanisms for targeted treatments. This review explores the intersection of nanotechnology and thermal therapy, focusing on the utilization of nanoparticles for image-guided interventions and temperature monitoring. Starting with an exploration of local temperature dynamics compared to whole-body responses, we delve into the landscape of nanomaterials and their pivotal role in nanomedicine. Various physical stimuli employed in therapy and imaging are scrutinized, laying the foundation for nanothermal therapies and the accompanying challenges. A comprehensive analysis of nanomaterial architecture ensues, delineating the functionalities of magnetic, plasmonic, and luminescent nanomaterials within the context of thermal therapies. Nano-design intricacies, including core–shell structures and monodisperse properties, are dissected for their impact on therapeutic efficacy. Furthermore, considerations in designing in vivo nanomaterials, such as hydrodynamic radii and core sizes at sub-tissue levels, are elucidated. The review then delves into specific modalities of thermally induced therapy, including magnetically induced hyperthermia and luminescent-based thermal treatments. Magnetic hyperthermia treatment is explored alongside its imaging and relaxometric properties, emphasizing the implications of imaging formulations on biotransformation and biodistribution. This review also provides an overview of the magnetic hyperthermia treatment using magnetic nanoparticles to induce localized heat in tissues. Similarly, optical and thermal imaging techniques utilizing luminescent nanomaterials are discussed, highlighting their potential for light-induced thermal therapy and cellular-level temperature monitoring. Finally, the application landscape of diagnosis and photothermal therapy (PTT) is surveyed, encompassing diverse areas such as cancer treatment, drug delivery, antibacterial therapy, and immunotherapy. The utility of nanothermometers in elucidating thermal relaxation dynamics as a diagnostic tool is underscored, alongside discussions on PTT hyperthermia protocols. Moreover, the advancements in nanoparticle magnetic imaging and implications of imaging formulations especially in creating positive MRI contrast agents are also presented. This comprehensive review offers insights into the evolving landscape of nanoparticle-based image-guided thermal therapies, promising advancements in precision medicine and targeted interventions, underscoring the importance of continued research in optimization for the full potential of magnetic hyperthermia to improve its efficacy and clinical translation.
1. Introduction
1.1. Local temperature versus whole body temperature
Temperature is a crucial physical parameter that holds significant importance in various domains, including electronics, engineering, biology, and more. In the context of organisms, temperature is closely linked to the average thermal energy of molecules within the human body, where the typical temperature hovers around 37 °C. Maintaining a normal core body temperature (normothermia) is essential for optimal bodily function, as it helps reduce the occurrence of adverse cardiac events.1 On the contrary, deviations from normal body temperature, whether in the form of excessive heat (hyperthermia) or cold (hypothermia), can lead to tissue damage and metabolic disturbances. It is widely recognized that even slight temperature anomalies can be associated with various diseases and health dysfunctions, such as inflammation and tumors.2 In such cases, local temperature monitoring is an effective early detection method.3–5 There are many important review papers in the literature on this topic.6,7 For example, a schematic diagram illustrating the various effects caused by different thermal treatments or temperature changing effect, as classified by the corresponding operating temperature range, is presented in the review published by Prof. Jaque et al.6Body temperature exhibits significant variation influenced by various physiological and meteorological factors. Although the standard body temperature is commonly considered to be 37 °C, different parts of the body can exhibit slight variations, and daily fluctuations can range from 0.25 to 0.5 °C.8Fig. 1 illustrates the dependence of body temperature on measurement sites, highlighting the temperature differences among body parts. Consequently, various types of thermometers are available, including sublingual, rectal, and axillary, among others. For example, oral measurements can range from 33.2 to 38.1 °C, while rectal measurements typically range from 36.7 to 37.5 °C in humans.9 A comprehensive study on thermometry and the interpretation of body temperature can be found in the work published by W. Chen.10 Furthermore, it is noteworthy that, on average, younger adults tend to have slightly higher body temperatures than healthy elderly individuals.11 Additionally, apart from surface temperature measurements, there is sometimes a need to assess deep body positions such as the brain or heart. In such cases, the temperature tends to remain relatively constant, since temperature changes can significantly impact metabolic reactions and overall bodily function.
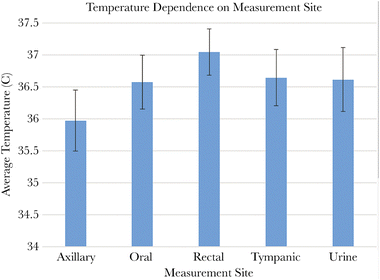 | ||
| Fig. 1 The dependence of body temperature on measurement site. Reproduced from ref. 11 with permission from Oxford University Press, copyright 2019. | ||
1.2. Nanomaterials and nanomedicine
Nanotechnology is a groundbreaking paradigm that has revolutionized our understanding and manufacturing processes in various industrial sectors. Over the past two decades, nanotechnology has profoundly impacted on numerous domains, including transportation, nuclear weapons, detection systems, and telecommunications, among others. Further, the term “nanomedicine”, which is the application of nanotechnologies to medicine, is considered the field where nanotechnologies may noticeably improve medical practice for prevention and therapeutic purposes. However, because nanomedicine is a nascent science, its risks and benefits are only hypothetically assessed at this stage but give us hope. The novelty of nanotechnology demands a new approach to fully understand its broad applications, assess potential benefits, and assess potential risks to human health.Nanomedicine is a generic term to indicate products, processes, and properties at the nano/micro scale. Finding a general definition of nanomedicine is a difficult task because of its hybrid nature and broad range of applications in different fields such as biology, chemistry, mathematics, and bioengineering. Therefore, different definitions exist for nanomedicine. The European Science Foundation on nanomedicine defines nanomedicine as: ‘the science and technology of diagnosing, treating, and preventing disease and traumatic injury, relieving pain, and of preserving and improving human health, using molecular tools and molecular knowledge of the human body’. In fact, most nanomedicine technologies challenge the boundaries between different areas of science such as chemistry, biology, or engineering, because they do not fit into traditional classifications of drugs or medical devices. Conversely, nanomedicine applications demand an accurate understanding of the complex processes that involve different expertise and disciplines. Nanomedicine holds great expectations for scientific advances in regenerative medicine – tissue engineering12–14 – and cancer therapy, enabling techniques that cannot be performed otherwise. The use of nanomedicine has made tremendous advances for the treatment of serious and terminal diseases15,16 Nanomedicine applications are related to novel therapies and diagnostic techniques in three major areas such as molecular imaging agents, drug delivery systems, and biosensors.17–20
Over the past three decades, we have witnessed remarkable advancements in this field, leading to significant progress in our scientific understanding of the mechanisms governing the organization and interaction of matter with biological systems at the nanoscale. Nanomedicine, leveraging the utilization of engineered particles typically ranging in size from 1 to 100 nm, strives to harness the potential of nanotechnology for various biomedical applications, primarily focused on disease treatment, diagnosis, molecular imaging, as well as regenerative medicine, and tissue engineering. From its inception, nanomedicine has often been associated with the utilization of nanoparticles (NPs) in oncology. Notably, the seminal work of Maeda and his colleagues in 1986 established the groundwork for cancer nanomedicine, wherein they validated the phenomenon known as the “EPR” (enhanced permeability and retention) effect for particles up to 400–600 nm.21–23
Current cancer therapies cannot often specifically target therapeutic drug delivery to tumors, posing one of the greatest challenges in cancer treatment. Chemotherapy and radiotherapy serve as the main pillars of conventional cancer therapies for various cancer types. However, despite advances in these approaches, patient survival rates have not surpassed 30%. Moreover, these therapies are associated with significant limitations, such as severe toxic side effects on patients’ health, including damage to the immune system. Additionally, the continuous administration of chemotherapeutic drugs to tumors leads to the development of drug resistance mechanisms in cancer cells, ultimately failing of chemotherapy. Therefore, developing next-generation cancer therapies is imperative to improve patient outcomes and overall health.
1.3. Physical stimulations in therapy and imaging
As discussed above, there have been multiple conventional ways for therapeutics and imaging of diseases, and modern nanotechnological approaches help to investigate unconventional ways for the same purpose. Among many unconventional cancer therapeutic strategies being interrogated in the last decade, therapies responsive to external physical stimuli are of particular interest and can have an advantage over conventional therapies. Scientists have invented novel ways to utilize nanoscale agents that are capable of responding to physical stimuli such as (i) light, (ii) magnetic field (MF), (iii) ultrasound, (iv) radio frequency, and (v) X-ray.Specially designed nanostructures termed hybrid nanomaterials (HNMs) are highly sensitive to physical stimulus and can perform “on-demand” cancer theranostics (therapy + diagnostics). The term “theranostics” symbolizes a new class of treatment options that can provide therapy and diagnostics to patients. Taking advantage of the interesting physical properties of different nanomaterials, especially HNMs, a variety of different physical stimuli theranostics strategies have been exploited as the next generation of cancer therapeutics. For example, theranostics combined with engineering, known as nanotheranostics, integrate nanotechnological and biochemical approaches, presenting promising opportunities for cancer therapy and diagnostics. Physically stimulated nanotheranostics can target and destroy cancer tissues or tumors only in response to specific external stimuli, enhancing treatment specificity and reducing the impact on healthy tissues. Additionally, physically responsive nanomedicine can be combined with other conventional cancer treatments, such as chemotherapy, to achieve synergistic anticancer effects via diverse biochemical and physical mechanisms. The use of externally applied physical stimuli such as magnetic or light signals in the form of physical forces along with administered signaling molecules, i.e., HNMs, could allow for chemotherapeutic drug presentation at the right time and at the right dose; this is the main requisite of cancer therapy.
There are a few novel physically stimulated strategies: (i) to enhance remotely controlled and targeted drug delivery to the tumor; (ii) to induce hyperthermia ablation using different types of magnetic NPs (MNPs), or near-infrared (NIR) light-induced hyperthermia using gold nanorods (GNRs); (iii) to enhance radiotherapy in a clinical setting and with HNMs as theranostic agents particularly magnetic@gold, silica@silver, carbon@gold, or gadolinium; (iii) to promote a photodynamic effect with multifunctional HNMs; and (iv) to permeate the blood–brain barrier (BBB) using MNPs. These are examples of clinical or human trials, but along with them many other nanomaterials or careers have been used.
As far as physical stimulus or responsive therapies are concerned, nanotheranostics may offer unique advantages. Unlike chemotherapy, which applies to the whole body, external physical stimulus-responsive nanotheranostics are often applied locally to the tumor. Physically stimulated nanotheranostics, e.g., light, MF, ultrasound, allow a real monitoring window of the distribution of therapeutic agents. Thus, physically stimulated nanotheranostics applied at the best timing opens effective therapeutic options by combining imaging required to real-time monitor of the tumor when the stimulus-responding nanostructures reach their maximal level in the tumor after its administration. Additionally, nanotheranostics coupled with imaging modalities could also be helpful in monitoring under- or overdosed therapeutic responses (e.g., optical energy, X-ray dose, etc.) under physical stimuli.
1.4. Nanothermal therapies and challenges
In the present scenario, most powerful thermal therapies at the nanoscale are based on magnetic hyperthermia and photothermal therapies. They might be called magnetic-stimulated and photo-stimulated ones. Considering the term nanotheranostics where the MF works as a stimulating agent, two broader categories can occur: (i) non heating MF (<103 Hz) and (ii) heating MF (>104 Hz).Biological objects, such as cells, tissues, and bodies are tightly regulated systems that represent a good example of a heat generating object that operates within narrow limits of a few degrees. These limits are controlled through numerous physiological pathways that regulate the kinetics of heat loss and its production for attaining balance. The loss of balance leads to severe life-threatening conditions, such as hyperthermia and heatstroke. From pain to emotional distress, relatively small temperature fluctuations have large effects on many physiological functions and might be symptoms of serious diseases such as viral and bacterial infection, diabetes, vitamin deficiency, autoimmune disease, and cancer. Yet, local applications of high and low temperatures might have a strong healing effect. Controlled heating or cooling has been known for centuries to provide therapeutic intervention for treating various conditions as it destroys bacteria, viruses, and dysfunctional cells and tissues, all within a targeted region.
Nanothermometry is an emerging field that has garnered attention for its ability to address fundamental questions about temperature regulation in cell biology, enhance our understanding of disease mechanisms, and aid in the thermal treatment strategies for deep tissues.24–28 The search for nanothermometers (NThs) has been driven by primarily two fields: fundamental cell biology to understand cell thermogenesis on a single-cell level, and in vivo imaging to monitor and control clinically important thermal ablation procedures. Being conceptually different, both fields came to rely on the same class of biologically compatible, temperature-sensitive nanosensors, collectively known as molecular NThs (later denoted as NThs), which enable temperature readout remotely and in real time.29–31
The size, sensitivity, and response time of the NThs are critical for both fields. The nanodimension is important to not affect biological functions within the cell, while whenever possible to either evenly distribute within the cells or to localize to a specific subcellular location. Extremely high sensitivity is needed to resolve temperatures of less than 0.1 K, and a rapid response (millisecond) is required to map thermogenesis and detect temperature fluctuations due to fast metabolic processes. For thermal ablation applications, the size of the NThs is critical in preventing toxicity and immune responses, as well as providing fast clearance. Sensitivity is less critical in this application and 1 K is sufficient. Since the thermal ablation procedure takes several minutes, a response time of 1 sec is adequate. Regardless of the application, nanometer size, biocompatibility, rapid equilibration with the environment, and independence from the type of cell or tissue are the key parameters considered in NThs design.
2. Nanomaterials and architecture
2.1. Functional nanomaterials in context of thermal therapies
Temperature is a crucial thermodynamic state that regulates the dynamics of the life cycle of biological cells. Typically, cancer cells have higher temperatures than healthy ones.32 However, in some cases, cancer cells can be cooler than the normal body temperature due to factors such as reduced metabolic activity, diminished tumor blood supply, or necrotic areas within the tumor.33 In this sense, real-time temperature sensing at the cellular level enables pathologies identification while also expanding the frontier of the most cutting-edge temperature measurement techniques.32 One method involves calibrating the optical properties of rare earth luminescent nanomaterials to measure temperature without direct contact.34 These nanomaterials are used in remote thermometers with high spatial and temporal resolution, offering excellent thermal sensitivity and stable spectroscopic properties even under electromagnetic or thermal stress.34 Thermal sensitivity refers to how the thermometric parameter changes in response to temperature variations.32 Additionally, temperature influences various photonic properties, such as luminescence intensity,28,35 lifetime,36 emission color,37 narrow band position,38 and emission bandwidth.39,40Another approach of interest is the Photodynamic Therapy (PDT), which is an alternative non-invasive treatment strategy for cancer. This method involves selecting and applying a specific wavelength of light to a photosensitizer (PS) or photochemotherapeutic material into the targeted tumor. In the presence of molecular oxygen, the excitation wavelength activates the photosensitizer, producing phototoxic species that interfere with the metabolic activity of the tumor and suppress cancer cells.41 PDT is painless, and patients tolerate it well because of its cancer cell selectivity. It consists of a PS molecule that accumulates in tumor cells, a local source of light to excite and activate the PS, and tumor molecular oxygen.
The selection of the appropriate wavelength to activate PSs must consider the higher depth of penetration into the tissues, but whose energy does not damage the biological components. The application of wavelengths in the visible spectrum range (400–700 nm) in in vivo imaging has been a bottleneck due to the low penetration in the biological material. In addition, many endogenous fluorophores in the body absorb in the 200–650 nm range, causing autofluorescence and interfering with the optical image.42 On the other hand, the ultraviolet (UV) wavelength (200–400 nm) can damage biological tissue, therefore, their biomedical applications are restricted. For this reason, the most suitable wavelengths for PDT are located between 550 and 900 nm, known as a “phototherapeutic window”.43 The NIR fluorescence windows, NIR-I (650–950 nm) and NIR-II (1000–1380 nm) windows, known also as biological windows (BW), present a great advantage for biophotonic images due to the low absorption and dispersion of tissues and water, as well as minimal autofluorescence, resulting in penetration of the NIR light into the deeper tissues.41,42 However, some problems or difficulties have recently been presented due to spectral distortions caused by biological tissues,24,26,44 which appear to be greatly minimized in NIR-III (1500–1800 nm).45 There is still the NIR-IV (2100–2300 nm), however it has been ignored mainly due to water absorption and the lack of high sensitivity imaging detector, but it is very important for some studies, for example, tissues with a higher collagen content such as bone or malignant tumors, because collagen is a chromophore with prominent spectral peaks between 2100 and 2300 nm. NIR-IV has also been considered for PTT due to the high absorption of water in this spectral region.46
Photonic materials with nanometric dimensions have allowed the creation of well-located heating techniques using a combination of Photodynamic/Photothermal therapy (PDT/PTT).47 PTT is a mechanism based on hyperthermia induced by the occurrence of light to trigger thermal damage in the diseased cell.47 Temperatures over 42 °C have been shown to make cancer cells more susceptible to the effects of additional therapies such as irradiation and induce some apoptosis, while temperatures over 45 °C can activate direct cell death (i.e., thermoablation).48
In a PTT technique, a temperature detector is necessary to overcome the lack of control of the NIR excitation and to regulate the heat emitted in the neighboring environment, enabling proper therapy targeting.49 A non-invasive technique for remote temperature measurement with high spatial/temporal resolution is needed for this application. The fluorescence signal from lanthanide ions can be used to build a nanoscale non-contact thermometer.50 The main procedure is based on measuring the fluorescence intensity ratio (FIR) between two luminescence bands arising from thermally coupled energy levels.50 Inorganic NPs outperform organic nanomaterials in terms of stability, tunable size, and optical properties, as well as the ease with which they can be surface functionalized to make them more biocompatible in biological applications.41
Gold NPs (AuNPs) can be employed as imaging contrast agents and generate heat under NIR laser irradiation due to surface plasmon resonance effects. This capability enables them to induce hyperthermia in tumor cells, thereby enhancing the effectiveness of PDT treatment. According to Calavia and co-workers,51 AuNPs are biocompatible and typically present low intrinsic toxicity, which is ideal for biological applications. Moreover, the large surface area-to-volume ratio allows their functionalization with PSs. These AuNPs present NIR absorption and act as photothermal agents (PTAs).
Carbon-based nanomaterials, such as carbon quantum dots (CQDs) and graphene quantum dots (GQDs), possess intrinsic electronic, mechanical, and thermal properties that have garnered significant attention for their applications in biomedicine, nanomedicine, and drug delivery platforms.52 With an exceptionally high specific surface area (2630 m2 g−1), excellent thermal conductivity, and favorable biocompatibility, these materials are increasingly recognized for a wide range of applications.53
The results from imaging and therapeutic approaches using hyperthermia are promising. This technique involves the targeted destruction of cancer cells through localized heating within the 41–47 °C range, sparing surrounding healthy tissue. Due to their superior heat-dissipating capabilities compared to traditional therapies, carbon-based materials result in minimal side effects on healthy cells. Furthermore, the high selectivity of this method leads to the rapid annihilation of cancer cells.
Hyperthermia therapy can also enhance the sensitivity of cancerous tissues to radiation or effectively eliminate cancer cells that are resistant to radiation. Notably, hyperthermia has been shown to significantly improve the efficacy of certain anticancer drugs by increasing perfusion in tumor tissues, which raises local oxygen levels and creates optimal conditions for radiation to effectively kill tumor cells.54
Because of their ability to absorb and emit NIR light, NThs based on lanthanide-doped materials and NIR quantum dots (QDs) are among the most promising structures. Because of the high temperature dependence of the relative strength of its two green emission lines, correlated with the effect of thermal equilibrium in the population of excited states (2H11/2; 4S3/2), erbium (Er3+) is the most widely used dopant ion in lanthanide-based NThs.49 QDs have a tunable absorption/emission spectrum from visible to NIR, narrow emission bandwidth, high absorption coefficient, high QY, excellent photostability, and straightforward synthetic technique. Besides, these doped materials have a high response and temperature sensitivity over a wide temperature range (10–400 K).55,56 Nd3+ co-doped CaF2:Y3+ nanomaterials by using the intensity ratio of two thermally coupled Stark components of 4F3/2 → 4I11/2 multiplet with emission wavelengths close to each other (I1041nm/I1062nm) have been reported, yet with a much low relative thermal sensitivity (Sr) of less than 0.2% K−1 at 27 °C.56,57
Besides presenting controllable size and shape during preparation, silica NPs for PDT applications also have high thermal stability and direct functionalization, providing high biocompatibility due to aqueous solubility. Photosensitizers, crucial in PDT, can be encapsulated or covalently attached to the surface of silica NPs.58 The high efficiency of grafted PSs is attained when molecular oxygen and singlet oxygen (1O2) can easily diffuse in and out of the silica NPs.58 The synergy of PS with specific wavelengths results in energy transfer (ET) to oxygen molecules, resulting in the generation of reactive oxygen species (ROS) that trigger apoptotic and necrotic cell death. The resulting ROSs cause irreversible damage to the target tissues/cells.59
Trinidad and co-workers60 investigated applying rat alveolar macrophages loaded with gold nanoshell as the drug delivery system for an in vitro study on head and neck squamous cell carcinoma (HNSCC) using PDT/PTT treatments. Combining the two treatments (irradiation at λ: 670 nm for PDT with aluminum phthalocyanine disulphonate and λ: 810 nm for PTT) led to an increase of 40% cell death.
Ceramic NPs consist of materials that have properties that fall somewhere between metals and non-metals, as follows: metals and metalloid oxides, carbides, sulfides, carbonates, hydroxyapatite, among others.61 They usually have low electrical and thermal conductivity, a high elastic modulus, a high stiffness, and are corrosive-resistant. The nature of bonding between their constituent atoms, a mixture of ionic and covalent bonds, determines their properties.62 Moreover, these ceramic NPs present a controlled release of drugs and easy incorporation of hydrophilic and hydrophobic drugs with high loading capacity. Hosseinzadeh and Khorsandi63 evaluated the biocompatibility of nano-platelet zirconium phosphate (ZrP) as a drug delivery vehicle for methylene blue (MB) to enhance the PDT efficacy in MDA-MB-231 human breast cancer cells. The findings indicated that ZrP-MB nano-hybrids significantly improve PDT effectiveness against human breast cancer cells, most likely via an apoptosis cell death mechanism. Furthermore, no toxicity was observed, suggesting that ZrP NPs could be used as a carrier for cationic photosensitizers for successful PDT.
Ceramic NPs present high loading capability, stability, chemical inertness, heat resistance and ease of conjugation to hydrophilic or hydrophobic drugs, making them ideal for drug delivery, imaging, and photocatalysis applications.
MNPs, primarily iron oxide forms like γ-Fe2O3 (maghemite) and Fe3O4 (magnetite), are significant in nanomedicine due to their superparamagnetic properties. Magnetic fluid hyperthermia uses these MNPs to locally heat and destroy cancer cells when exposed to an AMF. This method can induce cell apoptosis or enhance the efficacy of radiation and chemotherapy. MNPs also enable targeted drug delivery, reducing side effects of traditional treatments. Superparamagnetic NPs are preferred as they don't maintain magnetization after the field is removed. Additionally, MNPs facilitate magnetic targeting and can be used in resonance imaging for confirming drug delivery efficiency.67–74
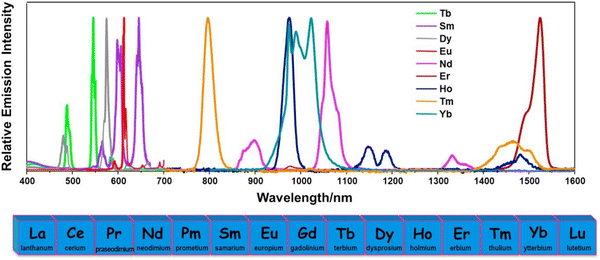 | ||
| Fig. 2 Emission spectra of different Ln3+ ions in solutions. Reproduced from ref. 87 with permission from Elsevier, copyright 2017. | ||
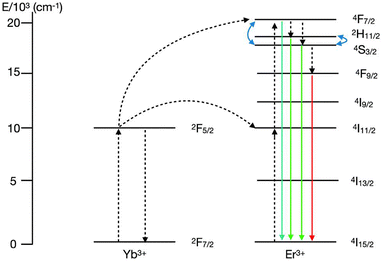
One of the most widely investigated UC NPs are those doped with the Yb3+–Er3+ ions pair due to their highly efficient UC luminescence. In these materials, green and red emissions arising from Er3+ are usually observed when under excitation at 980 nm diode laser, due to narrow bands located at 525 nm (2H11/2 → 4I15/2), 545 nm (4S3/2 → 4I15/2) and 655 nm (4F9/2 → 4I15/2). Because of its two suitable thermally coupled energy levels, 2H11/2 and 4S3/2, with an energy separation of ∼800 cm−1, Er3+ is the most widely used dopant ion in lanthanide-based thermometers.92,93 Yb3+–Er3+ co-doped phosphors could be effectively triggered by both the 980 and 405 nm laser diodes to generate the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions via up- and downconversion mechanisms, respectively.94,95
The UC mechanism involves first excitation of ions from their ground states, 4I15/2(Er3+); and 2F7/2(Yb3+), to their respective excited states, 4I11/2(Er3+) and 2F5/2(Yb3+), under incident ∼980 nm radiation.96 The Er3+ ion is excited from the ground state 4I15/2 to the excited state 4I11/2via energy transfer (ET) from the excited Yb3+ ion, as the excitation states of both Er3+ and Yb3+ ions are near resonant, facilitating ET. At higher energy levels, transitions such as 4I11/2 → 4F7/2 occur through the excited-state absorption (ESA) process. Subsequent non-radioactive relaxations in Er3+ ions populate lower energy levels, specifically 2H11/2 and 4S3/2 (Fig. 3). Furthermore, the narrow-band emissions from Er3+ ions arise from transitions including 2H11/2 → 4I15/2 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 cm−1), 4S3/2 → 4I15/2 (18
047 cm−1), 4S3/2 → 4I15/2 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cm−1), and 4F9/2 → 4I15/2 (15
416 cm−1), and 4F9/2 → 4I15/2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267 cm−1) (Fig. 3).91,96,97 Therefore, it is suitable to recognize that the Yb3+/Er3+ ions pair is one of the most renowned in UC luminescent materials, showcasing the anti-Stokes mechanism effectively.
267 cm−1) (Fig. 3).91,96,97 Therefore, it is suitable to recognize that the Yb3+/Er3+ ions pair is one of the most renowned in UC luminescent materials, showcasing the anti-Stokes mechanism effectively.
Since iron oxide has limitations (oxidation and chemical reactivity) for nanomedicine, appropriate modifications in MNPs are needed to give them stability and biocompatibility. As a result, MNPs coated with specific luminescent probes can provide a thorough understanding of cellular processes at the molecular level by functioning as nanothermometric sensors.99 By stimulating various optical methods, these luminescent MNPs can distinguish tumor cells. This precision is critical for identifying cancer as early as possible and prior to local treatment when the tumor tissue is still small enough.
Swain and co-workers101 reported the preparation of hybrid Fe3O4/BaMoO4:Dy3+ NPs functionalized with β-cyclodextrin (CD) and 3-aminopropyl triethoxysilane (APTES). The authors confirmed the superparamagnetic properties at room temperature as well as that of a drug nano vehicle. Moreover, the photonic features were investigated, indicating these materials are promising candidates for targeted and cellular imaging.
At the same time, a silica-coated SrAl2O4:Eu2+ (SAO) nanoscintillators for X-ray mediated PDT was documented by Chen and co-workers.102 APTES and tetraethyl orthosilicate (TEOS) were used as silane precursors to synthesize nanoscintillators, and the NPs were coated with a solid silica film. The SAO persistent luminescence material can be activated by specific wavelength excitation and generate cytotoxic 1O2via ET. In aqueous solutions, the lifetime of SAO coated NPs can be extended to more than 3 days, which is appropriate for therapeutic purposes. SrAl2O4:Eu2+ exhibits a high persistent luminescence (PeL), where light is emitted for long periods, from minutes to hours, after the excitation, resulting in a glow-in-the-dark phenomenon.103 Besides, SAO is an efficient luminescence material that can convert X-ray photons to visible photons, known as the scintillation phenomenon.104 X-ray absorption spectroscopy methods based on synchrotron radiation (XANES, X-ray absorption near edge structure and EXAFS, extended X-ray absorption fine structure) can be used to explain the task of Eu2+ and the other RE2+,3+,IV ions in the mechanism of PeL.105
By modifying the organic ligand and/or metal center, metal–organic framework (MOF) can be used as an effective nanocarrier for delivering drugs and imaging agents for cancer therapy.106 According to Pei et al.,107 the Gd complexes can be distributed independently in the structure of the nanoporous MOF, resulting in reduced molecular aggregation in the Gd-TCPP MOF nanoforms. Furthermore, the synthesized Gd-TCPP MOF nanosheets exhibited intense image effects and precise ablation of cancer cells in vitro due to photosensitizers as organic ligands. The space limiting effect of MOFs has been shown to increase the Gd relaxation rate, giving the material the potential for an effective MRI-guided PDT. The nanosheets passed the cytotoxic test on KB cancer cells without apparent toxic or side effects. After centrifugation of Gd-TCPP MOF nanosheets in the cell culture medium for 48 h, the nanostructures did not alter significantly. The crystal stability of the Gd-TCPP MOF nanosheets in the cells prevents the release of Gd ions, resulting in low cytotoxicity. Meanwhile, due to their unique periodic porous structure, the Gd-TCPP MOF nanosheets had a high capacity for producing singlet oxygen under specific excitation wavelengths.
2.2. Nano-design and geometry
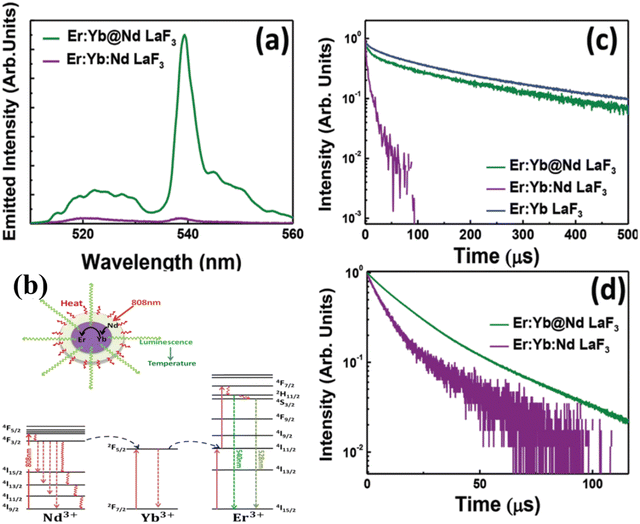 | ||
| Fig. 4 (a) Room temperature upconversion emission spectra generated by the single-core and core@shell LaF3 samples under optical excitation at 808 nm in Nd3+ sensitizer. (b) Schematic representation of the active-core/active shell nanoparticles designed especially for simultaneous 808 nm light-activated heating and thermal sensing. Simplified energy level diagrams of Nd3+, Yb3+ and Er3+ ions for purpose of light induced heat by means of Nd3+ ions at the shell and thermal sensing by the Yb3+ and Er3+ ions at the core. (c) Fluorescence decay curves corresponding to the emission from the 2H11/2:4S3/2 thermally coupled levels of erbium ions after 488 nm optical (Er3+) excitation. (d) Fluorescence decay curves corresponding to the 4F3/2 Nd3+ level as obtained under 808 nm optical excitation. Reproduced from ref. 108 with permission from Royal Society of Chemistry, copyright 2016. | ||
With the same goal of controlling ET and then emissions, Fig. 5 presents the potential use of active-core/active-shell based on Nd3+ and Yb3+ doped NPs.109Fig. 5a–c depicts schematic representations of single-core Nd:Yb LaF3 and spatially separated active-core/active-shell NPs: Yb@Nd LaF3 and Nd@Yb LaF3. The concentrations were both fixed to 10 mol%. Fig. 5d displays room temperature emission spectra for all samples under 790 nm infrared (IR) excitation, which is primarily absorbed by Nd3+ ions as Yb3+ ions have minimal absorption at this wavelength. The spectra reveal characteristic emissions of Nd3+ (around 900, 1060, and 1350) and Yb3+ (970–1030 nm) ions, though with varying relative intensities, especially between single-core and core–shell samples. Fig. 5f illustrate the simplified energy diagram for the Nd:Yb system, outlining the main processes involved. Upon 790 nm of excitation, the Nd3+ ions are excited from the ground state to the 4F5/2 level, followed by multiphonon relaxation to the metastable 4F3/2 state. At this level, energy migration and cross-relaxation among Nd3+ ions could occur, and ET to Yb3+ ions as well, promoting the Yb3+ to the 2F5/2 state. Both ions at its metastable levels produce emissions in the transitions: 4F3/2 →4I9/2 (∼900 nm), 4F3/2 →4I11/2 (∼1060nm), 4F3/2 →4I13/2 (∼1350nm) for Nd3+ and 2F3/2 →2F7/2 (970–1030 nm) for Yb3+. The effect of spatial ions separation in controlling the Nd3+–Yb3+ ET is clearly and better visualized in the fluorescence decay curves for the 4F3/2 level presented in Fig. 5e. The lifetime of the 4F3/2 state for the single-core sample is more of 10 times shorter than that for the core–shell NPs, indicating the strong ET from the Nd3+ to Yb3+ ions. These findings, illustrated in Fig. 4 and 5, underscore the potential of NPs engineering in controlling emissions through precise ET management. This diverse NPs engineering strategy has been used to tailoring nanomaterials, especially multifunctional materials to act as luminescent NThs (LNThs) and nanoheaters.
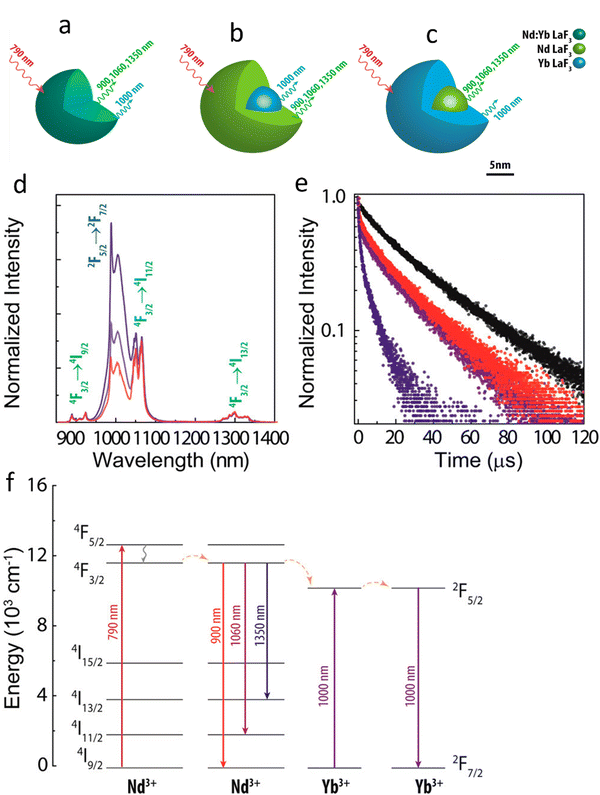 | ||
| Fig. 5 Schematic scaled representation of (a) Nd:Yb LaF3 (single-core), (b) Yb@Nd LaF3 (core–shell), (c) Nd@Yb LaF3 (core–shell) nanoparticles. (d) Room temperature normalized (at 1350 nm) down-conversion emission spectra of single-core Nd:Yb LaF3 (blue) and core–shell Nd@Yb LaF3 (red) and Yb@Nd LaF3 (purple) nanoparticles under 790 nm laser excitation. Transition relatives to Nd3+ and Yb3+ ions are identified in green and blue, respectively. (e) Fluorescence decay curves of the 4F3/2 Nd3+ emitting level of Nd@Yb LaF3 (red), Yb@Nd LaF3 (purple), Nd:Yb LaF3 (blue), and Nd:LaF3 (black) nanoparticles. (f) Simplified energy level diagrams of Nd3+ and Yb3+ emitting ions with excitation at 790 nm, emission from Nd3+ and Yb3+ ions and possible energy transfer. Reproduced from ref. 109 with permission from American Chemical Society, copyright 2016. | ||
Directly in nanomedicine, self-assembly methods are used to make nanoscale coordination polymers (NCPs) from organic bridge ligands and metal ions. They can overcome the drawback of conventional drug delivery systems due to their customizable formulations, shapes, and sizes, inherent biodegradability, and ease of surface adjustment.106 The surfaces of inorganic core materials can be coated with different types of organic materials, for instance: polymers,110 biomolecules,111 or lipids.112
Liu and co-workers113 prepared functional gold NPs (Ce6-AuNR@SiO2-d-CPP) for treating breast cancer MCF-7 cells by PTT/PDT. Gold nanorods were coated by a mesoporous silica layer as the shell for entrapping photosensitizer chlorin e6 (Ce6) and bonding the D-type cell penetrating peptide (d-CPP). MCF-7 cells were cultured and treated with a combination of photothermal and photodynamic evaluations to assess the cytotoxic effects of PTT/PDT. The following irradiation modes were used: 808 nm (2 W) for 10 minutes, followed by 650 nm (50 mW) for 10 minutes. Ce6-AuNR@SiO2-d-CPP exhibited high cytotoxicity and apoptosis-inducing effects in breast cancer cells in vitro, as well as a robust treatment efficacy in breast cancer-bearing mice, when given in combination with PTT and PDT.
The nanoshell thickness is a critical aspect of harvesting and converting energy regarding AuNPs PDT/PTT. Shan and co-workers114 synthesized gold nanorods (GNR/R-SiO2) in shell thickness silica up to 83 with size pores ∼4.8 nm. Using the ultra-thick silica shell, GNR/R-SiO2 exhibited ultra-high thermal stability, retaining the integrity and photothermal effects even after thermal annealing at 800 °C, offering fascinating insights into the application under extreme conditions. The photothermal effects of GNRs coated with R-SiO2 remained perfect after continuous irradiation for twenty times, with no output degradation or shape shift. Furthermore, having a lot of mesopores could help GNRs improve their photothermal conversion performance. The GNRs can convert NIR excitation into heat efficiently. Under 808 nm laser power excitation, the temperature increased from 25 to more than 60 °C in less than 8 min. Besides, the maximum temperature of the samples increased even more with the rise in thermal annealing temperature, reaching 89.7 °C after heat treatment at 800 °C. This is due to the LSPR blue-shifted from 935 nm to 818 nm after annealing at various temperatures, eventually approaching 808 nm, resulting in a higher efficiency in photothermal conversion. These findings suggest that GNRs with ultra-thick silica shells may be used as a stimuli-responsive NIR drug delivery system in cancer therapy.
This is a historic milestone for the large-scale application of NCPs as effective drug delivery systems for cancer treatment. Lin and co-workers115 developed NCPs core–shell carry oxaliplatin in the core and the photosensitizer pyropheophorbide-lipid conjugate (pyrolipid) in the shell (NCP@pyrolipid) for effective chemotherapy and PDT. The three-in-one treatment model greatly aided tumor-specific T cell development and improved T cell penetration into primary and remote tumors. It not only removed the primary tumor, but it also triggered a systemic antitumor immune response by rejecting the distant tumor. Besides, NCP@pyrolipid chemotherapy/PDT effectively induces immunogenicity in the tumor microenvironment and boost anti-PD-L1 therapy. In two bilateral syngeneic mouse models of colorectal cancer, PDT of NCP@pyrolipid in combination with anti-PD-L1 slowed the development of both primary and distant tumors.
A NaYF4:Yb3+,Tm3+ UCNP core was prepared and coated with photo-effecting material TiO2–ZrO2 as a shell to increase NIR-triggered PDT (NaYF4:Yb,Tm@TiO2/ZrO2 core@shell NPs) in a more recent study by Ramírez-García and co-workers.116 To enhance its overall NP PS active targeting inside HER-2 positive in vitro cultured SK-BR-3 human BC cells, the monoclonal antibody Trastuzumab was applied to the UCNPs surface. The NaYF4:Yb,Tm@TiO2/ZrO2–Tras nanocomposite showed 76% cell death in PDT assays at 975 nm power excitation at 400 μg mL−1, while control groups treated with single TiO2 UCNP that lacked ZrO2 attachment showed only 40% cell death. Overall, these findings showed that the combined photocatalytic activity of TiO2–ZrO2 within the final nanocomposite improved PDT treatment outcomes in BC cells by producing higher levels of ROS.
If nanocarriers circulating in normal blood vessels have a diameter of 100–150 nm, they cannot easily leave the capillaries that perfuse tissues like the kidney, lung, and heart. Smaller particles in the 20–100 nm range, on the other hand, can distribute to the bone marrow, spleen, and liver sinusoids and, to some degree, leave the bloodstream through the leaky capillaries of these organs. It's understood that lung alveoli can capture particles as small as a few micrometers in diameter, and the pulmonary capillary barrier's pore size is estimated to be about 35 nanometers. Smaller pores with diameters of 15 nm can be found in the glomerulus in the kidneys and islet tissues in the pancreas. Particles with a diameter of less than 10 nm are filtered by the glomerular capillary wall and are not reabsorbed.120,121 Due to these tissue and capillary pore size ranges, most nanocarriers of 50–200 nm cannot escape from continuous blood capillaries in their intact form.
In the formulation of nanoencapsulated therapeutic agents, variations in NP size should be considered. To achieve optimal clinical results, nanocarrier formulations with a constant and narrow size distribution are needed. Furthermore, particle size and size distribution are critical factors in determining a dosage form's stability during storage and medical use. When it comes to nanosystems, size stability is more important than when it comes to microscale drug delivery systems. This occurs because nanosystems have a much larger real surface area than microscale structures.
Supramolecular interactions, such as hydrogen bonds, van der Waals forces, electrostatic forces, hydrophobic forces, and π–π stacking, are important for drug molecule assembly and the structural stability and integrity of the self-assembly system. To better understand the molecular assembly process, computer-aided molecular structure simulation, and single crystal diffraction can be used to investigate the spatial geometry of drug molecules and possible weak interactions among active molecules.
Theranostic NPs can be designed in several ways to achieve the desired properties. For example, for diagnostic applications, one can use paramagnetic materials such as gadolinium or iron oxide as the MRI contrast-enhancing entities in a nanoparticle. To enable the therapeutic capability of these agents, these particles are loaded with ligands and drugs for targeted therapeutic applications. The incorporation of antioxidants such as cerium oxide122 into the NPs holds promise for sensitizing cancer cells for radiation therapy while protecting normal cells.
Cerium-based NPs are capable of switching from being an antioxidant to a prooxidant when pH changes from neutral to acidic, which may be the case in cancerous tissues. This switching behavior of cerium particles could be used to selectively sensitize cancer cells. Li et al. have demonstrated this switching behavior in the oxidation state of cerium (from +3 to +4) in gadolinium-containing cerium oxide NPs (CeOx:Gd) when ROS hydrogen peroxide is present in the solution, indicating that this nanomaterial could behave as an antioxidant. It should be noted that coating is needed for cerium oxide NPs containing higher Gd content, as it was also observed in the same study that non-coated cerium oxide NPs containing higher gadolinium content induced ROS production from neutrophils.
While most commercially available contrast agents for MRI are gadolinium chelates, some gadolinium-based contrast agents have been reported to be nephrotoxic. Recently, increasing reports on gadolinium deposition in the brain of patients who received Gd-based contrast agents have spurred renewed interest in the safety of gadolinium-based contrast agents. A brief review summarizing the known biological effects of gadolinium-based contrast agents and their potential mechanism for the biological effects is available in the literature.123 To reduce the risk of nephrotoxicity, a method was developed to incorporate gadolinium into biocompatible iron oxide NPs, minimizing overall Gd content used. Shen et al. reported dotted core–shell NPs where gadolinium oxide NPs are introduced on the surface of iron oxide NPs to form dotted core–shell type iron oxide NPs/gadolinium oxide NPs.124 Nonspherical NPs such as core–shell Fe3O4/Gd2O3 nanocubes have also been developed.125 These nanocubes have larger surface-to-volume ratios than spherical particles, a favorable property for increased water accessibility of the paramagnetic gadolinium on the NPs. In these particles, the Fe3O4 is mainly concentrated in the core of these nanocubes, while the Gd2O3 is embedded in the surface of the NPs.
Superparamagnetic iron oxide (SPIO) NPs have been used as platforms for drug delivery, MRI contrast agents, and tumor thermotherapy due to their biocompatibility, biodegradability, and magnetic properties. Nosrati et al. used iron oxide NPs as imaging agents for image-guided delivery of an anticancer drug, paclitaxel (PTX), into the brain using glutathione (GSH) as the blood–brain barrier (BBB) shuttle.126 In their work, iron oxide NPs were coated with PEG, PTX, and GSH (PTX-GSH-ION). The resulting particles have a core diameter of 15.85 nm. The PTX release was monitored using UV-vis spectrophotometry and found to depend on pH, with higher PTX released at pH 5.8 (2% w/v tween 80) than at pH 7.4 (PBS). Finally, the PTX brain delivery was investigated by intravenously injecting PTX-GSH-ION into the cross-tail vein of BALB/c mice at 20 mg Fe per kg dose monitored using MRI. While a significant amount of PTX-GSH-ION remains in the bloodstream, brain scans revealed significant darkening when PTX-GSH-ION was used compared to the control brain 30 min post-injection. Some NPs were detected in other organs such as kidneys, heart, spleen, and liver. Overall, the successful use of GSH as a BBB shuttle peptide is clearly demonstrated in this work. The drug delivery into the brain could be improved by increasing the GSH loading onto the NPs. Decreasing the size of the iron oxide NPs further to take advantage of the T1 effect is also something to consider for future work.
While some of these particles only passively target tumors or macrophages, i.e., through enhanced permeability and retention, introducing biological moieties such as antibodies, peptides, and drugs onto the NP surface enables some nanoprobes to target disease and improve theranostic efficacy. Li et al. have used epidermal growth factor receptor (EFGR) monoclonal antibody (Cetuximab) as the targeting ligand to deliver SPIO nanocomposite to EGFR-overexpressing H460 lung cancer.127 The cytotoxicity of this nanocomposite was evaluated using MTT assay at different iron concentrations (5, 10, 20, 40, 60, and 80 5 μg mL−1). It showed 96% viability of H460 cells at 5 μg mL−1 and decreased to 85% at 5 μg mL−1, indicating no apparent cytotoxicity at tested concentrations in H460 cells.
Another ligand utilized for targeting EGFR is erlotinib, a highly selective small-molecule tyrosine kinase inhibitor. Erlotinib was introduced on the surface of dextran-coated iron oxide NPs to add therapeutic and targeting functionality to the NPs against highly invasive cancer cells while being monitored by MRI.128 The erlotinib-functionalized iron oxide NPs (erlotinib-ION) exhibited cytotoxicity to EGFR-overexpressing CL1-5-F4 (generated from the CL1 human lung adenocarcinoma) in a dose-dependent manner, while no cytotoxic effects were observed in Jurkat cells, which do not express EGFR. This observation confirmed the selectivity of erlotinib to tumor cells that overexpress EGFR. Further, cellular uptake of the NPs in CL1-5-F4 cells was enhanced by a factor of four when the cells were treated with erlotinib-ION compared to those nontreated cells. pH-dependent release of erlotinib from erlotinib-ION was also observed when the NPs were exposed to intracellular endocytotic mimicking fluid (pH = 5, 76.6% of cumulative erlotinib release was observed after 140 min) and extracellular environment mimicking fluid (pH = 7, 60.8%). When these NPs were tested for their in vivo antitumor activity in a tumor xenograft model of CL1-5-F4 cells in BALB/c nude mice, erlotinib-ION exhibited tumor inhibition, while no cytotoxic effects were observed in mice.
Glu-{Cyclo[Arg-Gly-Asp-(D-Phe)-Lys]}2, a dimeric RGD peptide (RGD2) is used for active targeting to integrin αvβ3 positive tumors. The presence of this ligand to the core–shell iron oxide-gadolinium oxide hybrid NPs (FeGd-HN3-RGD2) enabled tumor detection as evidenced by strong MRI signal in tumor region on U-87 MG human glioblastoma-bearing nude mice.124
Folic acid was also used as a ligand for tumor targeting. In one study, folic acid was introduced to Prussian Blue (PB) NPs to have tumor-targeting capability.129 Prior to introducing the ligand to the NPs, the particles were incubated in dopamine to produce polydopamine and subsequently conjugated with biamino polyethylene glycol for physiological stability. The resulting folic acid-coated PB NPs (FA-PB NPs) exhibited excellent stability in water, phosphate buffered saline, and 0.9% NaCl solution. Doxorubicin (DOX) was loaded as a model drug to FA-PB NPs to yield DOX-FA-PB NPs, which showed 45.2% cell viability using CCK-8 assay for HL-7702 cells after 24 h incubation at concentrations up to 200 μg mL−1. However, cell viability decreased to 40.5% when irradiated with NIR, demonstrating the promising potential of these NPs for targeted chemotherapy and PTT. The DOX release depended on pH (17.1%DOX release over 24 h at pH 5.5 vs. 6.6% at pH 7.4) and NIR irradiation (28.75% at pH 5.5). Finally, in vivo MRI data revealed significant tumor degeneration exhibited by tumor-bearing mice that received DOX-FA-PB NPs with NIR irradiation (808 nm laser for 600 s) confined to the tumor site at 6 h post-injection.
Integrating different agents into a single platform is more beneficial for temporal and spatial correlation than using separate agents as biological systems are inherently dynamic. If these agents are introduced separately, the differential biodistribution of these agents will make it challenging to conduct simultaneous monitoring using multiple imaging modalities as each agent may have different biodistribution in vivo, increasing the risk of toxicity and other side effects. A more comprehensive discussion on the development of nanoparticle-based multimodal PET/MRI imaging nanoprobes and their applications in diagnosis and treatments is available in other reviews in the literature.130
Besides using magnetic ferrite NPs in PET/MRI, these NPs have been explored as potential MPI/MRI theranostic agents.131 MPI is a highly sensitive imaging technique but does not use ionizing radiation like nuclear imaging modalities such as PET and CT scans. Because of the ability of MPI to directly detect MFNPs, MPI can be used to predict specific absorption rate dose, ensuring the safety and efficacy of magnetic hyperthermia treatment (MHT). The high-resolution MRI can complement the high-sensitive MPI for biomedical applications such as cancer imaging and hyperthermia therapy.
Du et al.132 reported iron oxide NPs (IOs) functionalized with penta-peptide Cys-Arg-Glu-Lys-Ala (CREKA) for targeting fibrin–fibronectin complexes, which are expressed by breast cancer cells and interstitial cells.132,133 These IOs were prepared using high-temperature thermal decomposition in the presence of acetylacetonate and subsequently exchanged with hydrophilic 3,4-dihydroxyhydrocinnamic acid (DHCA). DLS measurements of IOs revealed a hydrodynamic diameter of 33.1 nm, which remained constant for 24 days at ambient conditions in 0.9% NaCl solution and cell culture medium containing 10% FBS. CREKA was then conjugated to IOs via EDC/NHS-coupling reaction, resulting in 7% loading of the peptide as determined by thermogravimetric analysis. Injection of IOs–CREKA intratumorally on an orthotopic 4T1 breast tumor-bearing mouse model showed higher MPI signal intensity and more uniformly distributed MPI signal in the tumor region relative to MFNPs without CREKA and commercially available Vivotrax (Fig. 6). This observation was validated in T2-weighted MR images where the darkening of the tumor was most uniform for IOs–CREKA compared to IOs only and Vivotrax. Finally, the in vivo antitumor activity of IOs–CREKA was investigated by injecting these particles intratumorally into 4T1 tumor-bearing mice, followed by treatment with AMF every 2 days for a total of three treatments. Tumor growth was completely inhibited for IOs–CREKA, showing the best effect of MHT relative to just IOs and Vivotrax with AMF treatment.
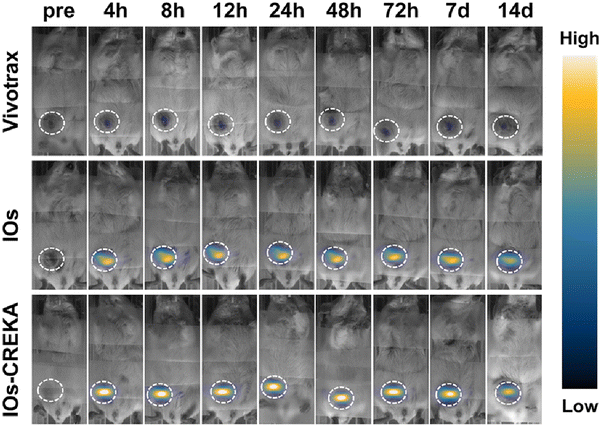 | ||
| Fig. 6 MPI images showing the distribution of Vivotrax, IOs only, IOs–CREKA in 4T1 orthotopic break mouse model taken at different time points. The homogeneity of MPI signals in the tumor region is more prominent for IOs–CREKA than for IOs. Reproduced from ref. 132 with permission from American Chemical Society, copyright 2019. | ||
Integration of agents for photoluminescence (PL), CT, and MRI imaging into one platform for multimodal imaging complement the inherent advantages of these imaging techniques such as high detection sensitivity (PL), unlimited detection depth (MRI and CT), and high resolution (MRI for soft tissues, CT for hard tissues) while addressing the limitations of each modality. However, it is critical to carefully design the NPs incorporating all these agents.
He et al. strategically modulated the thickness of the CT agent NaLuF4-containing interfacial layer to enhance the efficacy of PL, CT, and MRI modalities in β-NaYb0.2/Er0.8F4@NaLuF4@NaGdF4 NPs. The position of the NaLuF4-containing interfacial layer in the β-NaYb0.2/Er0.8F4@NaLuF4@NaGdF4 NPs keeps the luminescent β-NaYb0.2/Er0.8F4 core safe from being quenched while ensuring bulk water accessibility for the paramagnetic Gd in NaGdF4, a key condition in relaxing water protons for MRI applications.134 Changing the thickness of the NaLuF4-containing interfacial layer from 1.0 nm to 10.1 nm resulted in enhanced UC PL (∼13-fold), X-ray attenuation capability for CT due to increased Lu3+ content in the nanoparticle (25% to 84%), and improved relaxivity due to slower tumbling time (0.28 μs to 12.7 μs) at clinically relevant field 1.5 T. While the multimodal properties of these β-NaYb0.2/Er0.8F4@NaLuF4@NaGdF4 NPs were thoroughly investigated, their toxicity has not yet been explored for possible biological applications.
Nanomaterials have a high tendency to aggregate and subsequently settle out from the solution. Consequently, stabilizing them at physiological conditions is critical for their in vivo applications. The stability of NPs is modulated by either electrostatic interaction or steric hindrance brought upon by adsorbed or grafted polymers onto the NPs. Coating the particles with polyionic molecules can modulate surface charge. Variation in the surface charge of the particle changes the electrostatic interaction among NPs and between NPs and their biological targets. Introducing polymer chains such as polyethylene glycol (PEG)127 and oleic acid can provide the necessary stability via sterics, thereby limiting direct contact among particles. NPs with polymers on their surface have reduced blood protein adsorption and lower clearance via the reticuloendothelial system. Soares et al. coated iron oxide NPs with an oleic acid bilayer and tested their use as magnetic hyperthermia agents.124 The resulting NPs have a hydrodynamic diameter of 170 nm and zeta potential of −120 mV at pH 7, indicating high stability of the particle at physiological conditions. However, it should be noted that the heating ability of the iron oxide NPs is reduced when the oleic acid bilayer is present.
3. Magnetically induced thermal therapy
This section provides an overview of magnetic hyperthermia treatment (MHT) using MNPs to induce localized heat in cancerous tissues. Over the years, MHT has progressed into clinical trials for treating various cancers. MNPs offer advantages such as deep tissue penetration and selective targeting of cancer cells while sparing healthy tissues. Despite its effectiveness in inducing apoptosis/necrosis in cancer cells by raising local tumor temperatures, challenges persist in optimizing thermal conversion efficiency and understanding localized heat effects about the tumor microenvironments. Integrating MRI enables real-time temperature monitoring during MHT, ensuring precise control over therapy. We present advancements in nanoparticle magnetic imaging and relaxometric properties, and implications of imaging formulations (especially in creating positive MRI contrast agents), which show promising cancer therapy outcomes, underscoring the importance of continued research in nanoparticle design and optimization for MH's full potential to improve its efficacy and clinical translation.3.1. Magnetic hyperthermia treatment (MHT)
The term magnetic hyperthermia (MH) was first coined long back in 1957 when Gilchrist et al. selectively heated the tumor cells by exploiting MNPs in the presence of a MF.1 With substantial progress in the field of nanotechnology, the introduction of MNPs has further progressed into a well-researched field.2 One of the finest advantages of MNPs facilitated hyperthermia therapeutic modality is the deep tissue penetration and selective killing cancerous cells without affecting the surrounding healthy tissues.3–5,8 It aids in understanding intracellular hyperthermia, as it directly provides therapeutic heating to the cancer cells, which can be improved by bio-conjugating the cell-targeting ligands with MNPs.9 Indeed, the localized and consistent heat leads to superior selectivity and effectiveness of the treatment.Based upon the above benefits, MNPs-mediated magnetic hyperthermia has been translated from the laboratory to clinical trials, and successfully utilized for treating glioblastoma and prostate cancer.10 MagForce (Berlin, Germany) further completed several clinical trials to explore this heat-based therapy for pancreatic cancer. Though the thermal therapy based MNPs have been piloted in clinical trials, further research is required to realize their full potential in cancer based thermal therapy. Particularly, substantial hurdles related to the efficacy of therapeutic modality in cancer must be researched and addressed, as these challenges pose significant obstacles to effective treatment.
Typically, MNPs-based thermal therapy involves raising the tumor local temperature to the range of 43–46 °C, which enhances the physiology of cancer cell and ultimately induces apoptosis or necrosis.5,8,11 To make this therapy clinically practicable for eradicating cancer cells, it is crucial to ensure that sufficient heat is uniformly distributed throughout the tumor mass while leaving the surrounding healthy tissues unaffected. As such, a stringent restriction in applied ac field, H and frequency f, where H × f < 5.1 × 109 A m−1 s−1 for biomedical reasons is imposed.11 With this restriction, the therapeutic efficacy of MNPs-based thermal therapy hereafter depends on their thermal conversion efficiency.
Among the library of MNPs, superparamagnetic iron oxide NPs, also called SPIONS, are broadly explored as thermal therapy agents due to their biocompatibility and biodegradability. Nonetheless, their inadequate thermal energy conversion efficiency, attributed to the degraded magnetic susceptibility, has been exposed as a serious problem in hands-on applications. To address such important concern, major research efforts have been dedicated to the enhancement of magnetic susceptibility so as to achieve considerably enhanced induction heating properties, with familiarized strategies such as controlling the particle size,21–23,122–124,127,135 controlling phase composition,128,129,131,134,136–138 deploying the shape anisotropy,125,128,139–143 and modifying the surface properties.144 Even though, these tactics are ready, there is an ultimate limit to the thermal energy conversion efficiency due to their inherent non-resonance absorption nature under an AC MF. Aside from this well-established macroscopic heating effect, topical works have advocated that a single MNP can increase the local temperature of the molecules either attached to or in the neighborhood of the MNP. This immediate nanoscale heat can standardize both the physiological and biochemical properties of molecules and hence cause functional alterations in organisms.145,146 Also, these effects of localized heat induction might add new vitality to enhancing the antitumor therapeutic efficacy of MNPs mediated magnetic hyperthermia.9,147–149 For instance, under the AC-applied external MF, lysosome-pile-up MNPs would induce lysosomal cell death by increasing the local temperature and improving reactive oxygen species (ROS) production within the lysosomes.150,151 However, the biological effects of localized heat in cellular death and its well-regulated mechanism still necessitate much research and tests. Moreover, due to the limited understanding of MNPs based thermal therapy, progress in this area is slow with inadequate new developments that lead to finite expansion in this field. Also, the therapeutic using MNPs is generally exploited as a combinatorial tactic to improve the sensitivity of traditional cancer treatments such as chemotherapy, radiotherapy, immunotherapy, photothermal, and PDT. Such a combinatorial approach has been swiftly realized as a strategy to improve the tumor treatment efficacy, posing a potential alternative therapeutic modality for cancer treatment.
3.2. Magnetic imaging & relaxometric properties
The efficacy of nanoparticle-based contrast agents for MRI is given by the relaxivity of a contrast agent. This molecular parameter refers to the ability of the agent to enhance MRI contrast by modifying the longitudinal and transverse relaxation of water protons near these contrast agents within the tissues/regions of interest. Several factors that can influence the magnitude of this parameter for nanoparticle-based systems: composition of the particle, size of the metal core, and thickness and nature of the coating material used. Depending on the nature of the material, a nanoparticle can be considered either a positive or a negative MRI contrast agent. Positive contrast agents such as gadolinium-based complexes catalytically increase the longitudinal relaxation of water protons, resulting in an increase in signal intensities in MR images. On the other hand, negative contrast agents such as iron oxide NPs decrease the signal intensities in MR image. One limitation of negative contrast agents is that the signal intensities arising from these agents are similar to that of internal bleeding, metal deposits, calcification, or air-tissue boundaries. Another limitation is the large magnetic moments of these agents that can lead to a susceptibility artifact resulting in unclear images. Consequently, positive contrast agents are preferred for imaging applications.Gadolinium(III) is a positive contrast agent because of its seven unpaired electrons and its ability to shorten longitudinal relaxation time more than the transverse relaxation time of water protons. This lanthanide ion has been incorporated into various NPs to create positive MRI contrast agents. In other NPs, gadolinium is combined with other metals for added functionality. For example, gadolinium-containing cerium oxide NPs, designed as theranostic probes due to their ROS scavenging potential of cerium and diagnostic properties of Gd, have been found to have higher r1 relaxivity than commercially available contrast agents. However, it is not yet clear what contributes to its superior relaxivity and therefore further studies are needed for better understanding of the relaxometric properties for this type of system.
Core–shell Fe3O4/Gd2O3 nanocubes have been found to have higher r1 and r2 relaxivity values than clinically used contrast agents Gd-DTPA, Fe3O4, and Gd2O3 nanocubes. The enhanced longitudinal relaxation rate is likely caused by the lower tumbling rate of these nanocubes compared to gadolinium-containing chelates and the increased local MF intensity caused by the Fe3O4 component. On the other hand, the enhancement of the transverse relaxation rate is likely due to the synergistic contribution by the Gd ions that are close to the Fe3O4.125 The larger effective radius of Fe3O4 in cubic-shaped NPs compared to spherical NPs may also contribute to the superior r2 value of these Fe3O4/Gd2O3 nanocubes.125
The risks associated with some of the gadolinium-based contrast agents and their reduced efficacy at higher field strengths136 have spurred synthetic chemists to develop alternative contrast agents for MRI. This is the motivation for Bawendi and coworkers to develop extremely small iron oxide NPs (3-nm core) with 1-nm ultrathin hydrophilic shells (ES-ION).137 While relatively large iron oxide NPs are known to be negative contrast agents, the reported extremely small iron oxide NPs exhibit properties similar to positive contrast agents. The key is to optimize the r2/r1 ratio while keeping r1 similar to those of clinically approved Gd-based contrast agents. The lower maghemite (Fe2O3) and magnetite (Fe3O4) ratio was found to minimize the T2 effect, a promising step for optimizing r2/r1. To ensure that the particles have higher maghemite content, an oxidation step using trimethylamine N-oxide was performed during the synthesis of the NPs. The synthesized ES-ION exhibited an r2/r1 ratio of 2.0 at a clinical field strength of 1.5 T. This ratio is better than that of clinically approved iron oxide NPs, Ferumoxytol (r2/r1 = 5.9) but lower than Gd-DTPA (r2/r1 = 1.1). T1-weighted MR imaging of mice injected with ES-ION was performed at 7.0 T (Fig. 7), and MR images revealed contrast enhancement in the heart and vena cava immediately after injection of the ES-ION. At the same time, the bladder increased in signal intensity 8 min post-injection. These observations indicate the potential of ES-ION for use in angiography and that these NPs can be effectively cleared through the renal system. Based on these promising results, ES-ION holds promise as an alternative positive contrast agent for MRI. The introduction of ligands on the surface of ES-ION using known bioconjugation methods maybe pursued for targeted imaging in vivo.
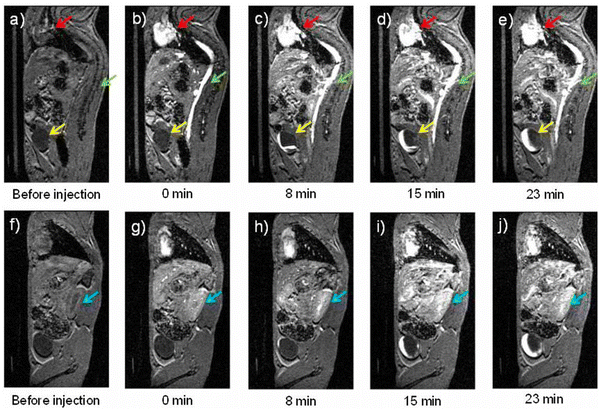 | ||
| Fig. 7 T 1-weighted MR images (a)–(j) of a mouse injected with ES-ION taken at 7 Tesla. Panels A–E correspond to one sagittal slice while panels F–J are another sagittal slice showing the kidney (blue arrow). The heart (red arrow) and vena cava (green arrow) exhibited positive contrast enhancement immediately after injection. The bladder (yellow arrow) and kidney showed contrast enhancement at 8 min post-injection. Reproduced from ref. 137 with permission from PNAS, copyright 2017. | ||
To improve the relaxivity of iron oxide NPs, Martínez-Gonzáles et al.139 evaluated the effect of using liposomes encapsulating iron oxide NPs on the relaxivity of the NPs. Liposomes of different formulations were prepared, testing the influence of varying fatty-acid chain length, presence or absence of cholesterol, and presence or absence of negative charge on phosphatidylserine. The resulting magnetoliposomes have diameters ranging from 220 to 335 nm with a polydispersity index of 0.200 and 0.300. Relaxivity measurements at 7 T provided r1 values of 0.9–9.1 mM−1 s−1 and r2 values of 230–1282 mM−1 s−1, highlighting the significant influence of liposome formulation on the efficacy of these magnetoliposomes as MRI contrast agents. Among the liposome formulations used in their study, hydrophilic 1,2-dimyristoyl-sn-glicerol-3-phosphatidylcholine (DMPC) exhibited the highest r1 and r2 values. It was noted that hydrophilicity enhances water accessibility and is therefore favorable for increasing r1. However, hydrophilic particles tend to cluster and may increase magnetic inhomogeneity resulting in higher r2 compared. While these magnetoliposomes are promising negative contrast agents for MRI, their toxicity and biodistribution should be investigated to further their utility in vivo (Table 1).
| Core | Hydrodynamic Size (nm) | Magnetic field (T) | Relaxivity (mM−1 s−1) | Ref. | |
|---|---|---|---|---|---|
| r 1 | r 2 | ||||
| n.d. – no data. | |||||
| SPIO | 45.7 | 4.7 | n.d. | 97.1 | 127 |
| CeOx:Gdx% (x% = 9–46%Gd) | 3–5 | 1.41 | 7–13.4 | n.d. | 122 |
| FeGd-HN3-RGD2 | 8.5 | 1.50 | 24.0 | 47.6 | 124 |
| core–shell Fe3O4/Gd2O3 nanocubes | 9.2 (edge length) | 1.50 | 45.24 | 186.51 | 125 |
| ES-ION | 4.7 | 1.50 | 4.8 | 5.1 | 137 |
| DOX-FA-PB | 40 | 7 | 0.366 | n.d. | 129 |
| H-DMPC magnetoliposomes | 220–335 | 7 | 9.1 | 1282 | |
| Mn-LDHs | 48 | n.d. | 9.48 (pH 5) | n.d. | 140 |
| Mn-LDHs | 48 | n.d. | 1.16 (pH 7.4) | n.d. | 140 |
| IOs | 33.1 | 7 | n.d. | 241.3 | 131 |
| NaYb0.2/Er0.8F4@NaLuF4@NaGdF4 | 37.7 | 1.5 | 52.9 | n.d. | |
Biogenic Mn(II) was also explored as an alternative to Gd(III)-based contrast agents because of its high paramagnetism (S = 5/2).140 Li et al. used layered double hydroxides (LDH) to chelate manganese(II) ions forming Mn-containing LDHs (Mn-LDHs) NPs. The hydroxides in LDHs are sensitive to acidic environments; consequently, Mn-LDHs NPs could be used as pH sensors and imaging agents. The Mn-LDHs NPs were prepared using the co-precipitation method followed by isomorphic substitution of Mg2+ with Mn2+ to produce Mn-LDHs with a hydrodynamic diameter of 48 nm and polydispersity index of 0.16. The reported r1 showed pH dependence, changing from 1.16 mM−1 s−1 at pH 7.4 to 9.48 mM−1 s−1 at pH 5.0. While Mn-LDHs showed significant change in r1 values when pH was changed from 7.4 to 5.0, further studies should be directed towards measurements of molecular parameters such as number of inner-sphere water molecules and water exchange rates of these Mn-LDHs and investigate the changes of these parameters when pH is varied. Finally, these NPs, when labeled with dsDNA-cy5, exhibited internalization into cancer cells in the mouse melanoma skin cancer cell line (B16F10), as evidenced by the intense red fluorescence around the prenuclear area. This observation is supported by significant enhancement of B16F10 cells in T1-weighted MR images following co-incubation of these cells with Mn-LDHs NPs in culture media.
3.3. Implications of imaging formulations: biotransformation & biodistribution
MRI of iron oxide NPs in vivo is typically carried out in a 3.0 T MR system with a high-resolution animal coil and T2W spin-echo sequence127 (TR/TE = 4000/96.7 ms) with a 256 × 256 matrix size. After imaging, animals are sacrificed. To determine the biodistribution of the contrast agent, iron concentration in the liver, spleen, heart, lung, stomach, intestines, kidneys, blood, and tumor tissues is measured using inductively coupled plasma atomic emission spectroscopy. In the study of SPIO NPs with anti-EGFR,127 a decrease in signal intensities was observed in the lung tumor 30 min postinjection of the NPs on nude rats bearing H460 lung tumors. This observation suggests active targeting of the NPs to the EGFR overexpressed H460 lung cancer xenograft. While this SPIO with anti-EGFR nanoparticle targets lung tumor tissues, its biodistribution patterns are similar to non-targeted SPIO, where the liver and spleen have higher iron concentrations. The clearance mechanism of these NPs is through the reticuloendothelial system and renal/urinary route.Because Gd is implicated in nephrogenic systemic fibrosis, biodistribution of Gd-containing NPs is important. The core–shell FeGd-HN3-RGD2, when injected intravenously on U-87 MG human glioblastoma bearing nude mice at 5.0 mg kg−1 of Gd dosage, exhibited measurable Gd level at 6 h time point for liver, spleen, and tumors.124 The Gd levels become comparable to those without injection at 6 d post-injection, indicating that the NPs can be cleared from the body in part by renal excretion.
Other alternatives to Gd-based contrast agents follow the same imaging protocol when doing in vivo studies. Slight modification of the NPs may be needed prior to introducing the agent into the biological system. For example, Li et al. precoated Mn-LDHs with bovine serum albumin (BSA/Mn-LDHs) to prevent nanoparticle aggregation in vivo. Following intravenous injection of BSA/Mn-LDHs NPs at a dose of 3.4 mg Mn/kg in the melanoma tumor model, the NPs were found to accumulate around the tumor tissue as indicated by enhanced signal intensity in this region 24 hours post-injection. The enhanced signal intensity in the tumor tissue persisted for 72 hours, 30× longer than that of Gd-DTPA (2 hours). The particles were found to be metabolized in the liver and tumor (acidic environment) followed by excretion by the renal system.
4. Luminescent nanomaterials: optical and thermal images and light-induced thermal therapy
Luminescent nanomaterials are at the forefront of advanced materials science, bridging the gap between optical innovation and therapeutic applications. These materials, often engineered to exhibit unique luminescent properties, play a crucial role in fields including imaging, sensing, and therapy. Their ability to emit light under specific conditions makes them invaluable for both diagnostic and treatment purposes, particularly in the realm of medical imaging and light-induced thermal therapies. By harnessing the precision of optical signals, researchers can enhance the accuracy of imaging techniques while simultaneously exploring novel approaches to targeted therapies.In recent years, the integration of luminescent nanomaterials into thermal imaging and therapy has gained significant attention. These materials can be engineered to produce distinct optical signatures that are used to monitor temperature variations and assess tissue conditions in real-time. This capability is particularly transformative for light-induced thermal therapy, where the localized heating of tissues is achieved through light absorption by these nanomaterials. This technique promises to revolutionize treatments for various medical conditions, offering a non-invasive approach to targeting and destroying pathological tissues with high precision. As research continues to advance, the potential applications of luminescent nanomaterials in both diagnostic imaging and therapeutic interventions are poised to expand, offering new avenues for improving patient outcomes.
4.1. Optical and thermal imaging: a multiplexed fluorescence system
Multiplexed fluorescence imaging has gained extensive utilization in health sciences due to its exceptional capacity to allow multichannel (multicolor) observations with high sensitivity and precise spatio-temporal resolution.141–143 Recent efforts have focused on developing fluorescent materials that function within the NIR windows of biological transparency because these windows enable enhanced tissue penetration, thereby facilitating imaging at significant depths.144–153 The application of multiplexed fluorescence techniques for investigating various analytes has proven to be valuable in numerous in vitro diagnostics. However, achieving spectral multiplexing in vivo is often impractical, particularly for fluorescent imaging in deep tissues. This is primarily due to the challenges posed by the absorption and scattering of complex tissue structures across different regions of the electromagnetic spectrum, including skin, muscle, fat, bone, and body fluids. These factors present significant obstacles to effective spectral multiplexing.154,155 Another limitation arises from the prevalent practice of performing multiplexed in vivo fluorescent imaging using a single probe,145,149,150,153 with occasional use of two probes simultaneously. However, in such cases, the quantitative precision of these multiplexed imaging approaches is often inadequately evaluated.147,156Due to the inherent variability of biological composition across different locations, achieving quantitative multiplexing that accounts for this heterogeneity at various wavelengths remains a challenge. Consequently, developing techniques to address these limitations has gained significant interest in recent years.157–159 Among these techniques, time-gated imaging, which is based on materials with long fluorescence lifetimes, has become a particularly relevant approach.160–162 Simultaneously, there has been a growing interest in the scientific community regarding the development of new materials with varying lifetimes to serve as contrast agents for acquiring multiplexed fluorescent images. Given that endogenous fluorophores in biological tissues typically exhibit fluorescence lifetimes in the nanosecond range, efforts have been made to engineer contrast agents with lifetimes ranging from several microseconds to milliseconds.163–167
A promising candidate for multiplexed fluorescence lifetime imaging is the NPs doped with lanthanide ions (Ln-NPs), whose f–f transitions allow the generation of emissions with long lifetimes and sharp peaks.162,167–170 Specifically, these types of NPs are interesting systems because their spectral properties, including luminescence and lifetime, can be altered simply by changing their size. Therefore, Ln-NPs of different sizes are well-suited for multiplexing in the time domain. Additionally, the hydrodynamic size of NPs is a crucial factor for effective biodistribution within biological systems.171,172 Thus, precise control over their size also contributes to efficient incorporation into cells. Furthermore, many Ln-NPs are biocompatible, making them promising contrast agents for multiplexed in vivo imaging based on lifetime.
An example of this capability was demonstrated by Jingke Yao et al.173 They employed NaGdF4:Nd,Yb,Tm of varying sizes (termed in the paper as τNPs) to achieve different fluorescence lifetimes for the Yb3+ emission (2F5/2 → 2F7/2), which can be used in multiplexed fluorescence lifetime imaging (Fig. 8). The crystal size of the τNPs varied from 9 to 42 nm (Fig. 8a–f), but their hydrodynamic diameter remained constant. In this case, the τNPs displayed lifetimes for 2F5/2 Yb3+ level ranging from 760 μs to 1510 μs (Fig. 8h). Among the five types of τNPs synthesized, the authors selected three (with sizes of 9, 30, and 35 nm) to carry out experiments using chicken breast tissue. Two different tissue thicknesses (2 and 5 mm) were used, and the results showed that different lifetimes could still be clearly visualized (Fig. 8i–t). This demonstrates the advantage of operating at the same wavelength in the NIR-II region. Furthermore, in the in vivo experiments using a mouse, two types of τNPs with different measured lifetimes were subcutaneously injected into each animal flank. This resulted in a subtissue lifetime of approximately 0.9 ms on the left flank and 1.4 ms on the right flank of the animal. These results demonstrate the efficacy of the time-gated technique in conjunction with different τNPs for achieving multiplexing in vivo, underscoring the value of working in the time domain.
 | ||
| Fig. 8 Morphological characterization: TEM images (a), (b), (c), (d), and (e) and size distributions (f) of five different sizes of NaGdF4:3% Nd3+, 2% Yb3+, 0,2% Tm3+ NPs. Scale bar: 50 nm. (g) Infrared emission spectra of five different sizes of τNPs modified with polyacrylic acid (PAA), dispersed in phosphate buffered saline (PBS) and excited at 808 nm. (h) Normalized fluorescence decay curves of the emission observed at 980 nm corresponding to the five different sizes of τNPs modified with PAA in PBS and excited at 808 nm. Experiments with chicken breast: (i) optical image, (j) infrared image, (k) merged image of the optical image and infrared image and (l) fluorescence lifetime mapping image of different τNP dispersions in PBS, specifically with 9, 20 and 35 nm core diameters (5 mg mL−1, 5 mg mL−1 and 1 mg mL−1 respectively). (m) Optical image, (n) infrared image, (o) merged image of optical image and infrared image, and (p) fluorescence lifetime mapping image of the same τNPs covered with 2 mm chicken. (q) Optical image, (r) infrared image, (s) merged image between optical image and infrared image, and (t) fluorescence lifetime mapping image of the same τNP dispersions covered with 5 mm chicken. In vivo experiments in a mouse: (u) Infrared image, (v) merge between optical image and infrared image, and (x) fluorescence lifetime mapping image after 50 μL injection of τNP (20 nm and 35 nm) dispersions in PBS (5 mg mL−1 and 1 mg mL−1, respectively). Reproduced from ref. 173. | ||
Another study showcasing the potential for time-domain multiplexing was conducted by Fan Zhang et al.174 They designed a systematic approach based on controlled energy relaying in a core–multi-shell nanostructure. This structure involved an outer layer co-doped with Nd3+ and Yb3+, an intermediate layer doped solely with Yb3+, and an inner layer co-doped with Yb3+ and Er3+ (Fig. 9a), overall forming a hexagonal (β) phase (Fig. 9b). Under 808![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm illumination, these NPs emit luminescence at 1525
nm illumination, these NPs emit luminescence at 1525![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm. The primary advantage of this system is that increasing the thickness of the energy relaying layer extends the average duration from absorption to emission, thereby lengthening the lifetime from 1.25
nm. The primary advantage of this system is that increasing the thickness of the energy relaying layer extends the average duration from absorption to emission, thereby lengthening the lifetime from 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ms to 7.21
ms to 7.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ms (Fig. 9c). On the contrary, when the thickness of the energy relay layer is fixed, increasing Er3+ concentration accelerates the conversion of stored energy into luminescence emission, effectively shortening the lifetime from 2.75
ms (Fig. 9c). On the contrary, when the thickness of the energy relay layer is fixed, increasing Er3+ concentration accelerates the conversion of stored energy into luminescence emission, effectively shortening the lifetime from 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ms to 292
ms to 292![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μs (Fig. 9d).
μs (Fig. 9d).
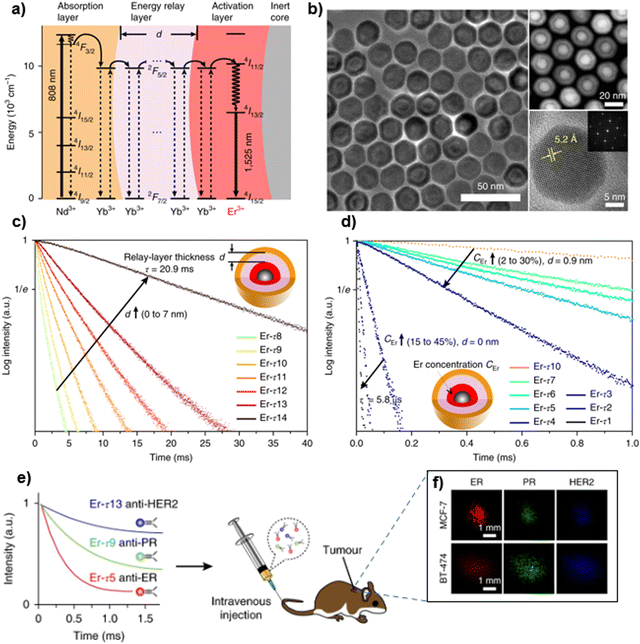 | ||
Fig. 9 (a) Energy level diagram illustrating the luminescence process of the core–multi-shell nanoparticles. (b) Transmission electron microscopy (TEM) image (left), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image (top right), high-resolution (HR) TEM image and the corresponding fast Fourier transform (bottom right) of the Er-τ12 (NaGdF4@NaGdF4:Yb, Er@NaYF4:Yb@NaNdF4:Yb) nanoparticles. (c) Luminescence decay curves were measured at 1525![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm from the as-prepared Er nanoparticles with energy relay shells of increasing thickness d from 0 to 7 nm from the as-prepared Er nanoparticles with energy relay shells of increasing thickness d from 0 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm (identical composition). (d) Luminescence decay curves of the nanoparticles with incremental Er3+ doping concentration CEr from 2% to 30% for d = nm (identical composition). (d) Luminescence decay curves of the nanoparticles with incremental Er3+ doping concentration CEr from 2% to 30% for d = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm and from 15% to 45% for d = nm and from 15% to 45% for d = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm. (e) Schematics illustrating animal experiment procedures: three batches of Er nanoparticles exhibiting distinct lifetimes are conjugated to three antibodies (anti-ER, anti-PR and anti-HER2), respectively, and intravenously injected into the mouse via tail vein. (f) Lifetime-resolved images for the MCF-7 and BT-474 tumors are decomposed into the three lifetime channels, represented by the red, green, and blue monochromatic image sets. Reproduced from ref. 174 with permission from Springer Nature, copyright 2018. nm. (e) Schematics illustrating animal experiment procedures: three batches of Er nanoparticles exhibiting distinct lifetimes are conjugated to three antibodies (anti-ER, anti-PR and anti-HER2), respectively, and intravenously injected into the mouse via tail vein. (f) Lifetime-resolved images for the MCF-7 and BT-474 tumors are decomposed into the three lifetime channels, represented by the red, green, and blue monochromatic image sets. Reproduced from ref. 174 with permission from Springer Nature, copyright 2018. | ||
To investigate its potential application in cancer diagnostics in vivo, the authors used a xenograft tumor model in nude mice, where the mice were injected with MCF-7 or BT-474 breast cancer cells. To achieve multiplexing, they used three distinct populations of Er NPs (Er-τ5, Er-τ9, and Er-τ13), which were conjugated with primary antibodies against three commonly examined biomarkers for breast cancer: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2). These NPs were intravenously injected into a mouse via the tail vein, as schematized in Fig. 9e. Through imaging at multiple time points following nanoparticle injection, the study revealed that the NIR-II luminescence emitted by the tumors of both subtypes remained sufficiently strong to enable differentiation of the positive groups. In contrast, the control groups exhibited non-specific nanoparticle accumulation at the tumor site, which eventually cleared over time. Exploiting this distinction, the researchers were able to quantify the expression levels of the biomarkers in the tumor subtypes. This was achieved by simultaneously resolving the three lifetime components using a pattern recognition algorithm. The results revealed that, although there were variations in overall intensities, the expression patterns of the three biomarkers exhibited clear and statistically significant differences between the two tumor subtypes (Fig. 9f). In resume, they have demonstrated the feasibility of engineering NIR-II lanthanide NPs to establish distinct lifetime channels for multiplexed in vivo imaging. Importantly, this approach overcomes the challenge of wavelength-dependent attenuation caused by various biological substances, ensuring accurate quantification of imaging signals.
4.2. Nanomaterials used as thermometers at cellular level
Accurate temperature measurement at the sub-micron scale holds significance across various domains, including microfluidics,175 microelectronics,176 and the study of living cells.177 In biological applications, especially at the cellular level, it is crucial to employ minimally invasive thermometers. This precaution is essential to prevent disruptions in metabolic processes. Notably, small temperature anomalies are a compelling avenue for detecting tumors and inflammation. This is due to the distinct metabolic processes occurring in these areas compared to those in a healthy part of the body.2–5 Real-time temperature monitoring becomes particularly pertinent in applications such as PTT and optimal anticancer treatments based on hyperthermia. Such monitoring plays a pivotal role in preventing the overheating of healthy tissues, ensuring the efficacy of treatments.178–180A very efficient way to measure the local temperature is using LNThs, which are based on the temperature-dependent changes in the luminescence properties (band-shape, spectral position, intensity, bandwidth polarization, lifetime, etc.).181,182 Previous studies have reported a wide range of nanomaterials as optical temperature probes, including exogenous fluorescent probes,183 nanodiamonds,184 hydrophilic fluorescent nanogel,185 semiconductor quantum dots (QDs), metallic NPs, rare-earth (RE) based NPs, carbon nanotubes186–190 and so on. However, due to some limitations (e.g., stability, toxicity, and accuracy) only a few of these LNThs have been used to measure the temperature of living cells. Many examples of thermometers for thermal sensing and imaging of living cells and biological tissues can be found at ref. 191. Two very complete summaries of luminescence were published separately in 2012 by Jaque et al.182 and Brites et al.,192 and more recently Tingting Bai and Ning Gu published a review about the current developments in cellular thermometers and a picture of their applications in cell biology.191 For biological applications, when operating within NIR windows,144,193 it is possible to excite the nanomaterials and collect their emissions at millimeter to centimeter tissue depths.
Fig. 10 shows a schematic representation of the possible effects on the emission features caused by the increase in temperature increasing and related to the temperature-dependent changes in the luminescence properties: spectral shift, polarization, intensity, lifetime, bandwidth, and band-shape.32,181,182 For example, the temperature can be measured using thermal coupled levels, in which the separation between emitting electronic states (or Stark levels) is maximum 10kBT ≈ 2000 cm−1 (at room temperature). The population of thermally coupled states follows the Boltzmann's distribution law. In this case, the fluorescence intensity ratio (FIR) can be expressed as the following equation:194,195
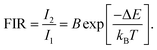 | (1) |
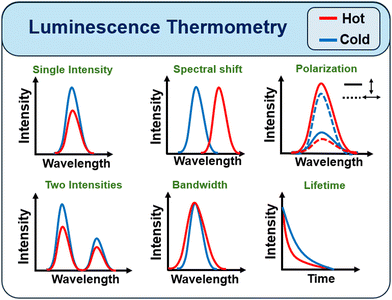 | ||
| Fig. 10 Schematic representation of the possible effects on the phosphors’ emission features caused by temperature increasing. | ||
The performance of LNThs should be quantified, including comparisons with other distinct types of LNThs. A parameter that has been widely used for this purpose is the relative thermal sensitivity (Sr) which measures the relative change of Δ per degree of temperature change (Δ = FLIR in the case of ratiometric LNThs), defined by:
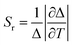 | (2) |
This parameter has been used as a figure of merit to compare different thermometers, regardless of their nature. Another important parameter is the temperature uncertainty (δT), which indicates the smallest temperature variation that can be detected in a given measurement. In a first order of approximation, this parameter is given by:
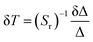 | (3) |
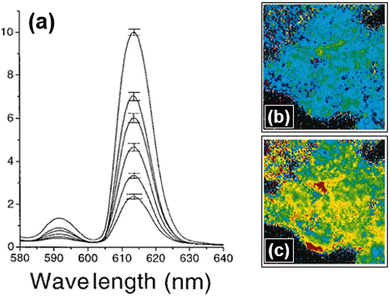 | ||
| Fig. 11 (a) Emission intensity of Eu-TTA showing that emission decreases with rising temperature from 15 °C to 40 °C, at 5 °C intervals. The error bar at the peak of each curve is to indicate that it was performed an average of a steady-state measurement (n = 6). The figures on the left are the thermal imaging of the heat wave evoked in a cluster of Eu-TTA cells (b) before and (c) 9s after the ACh application. In these pseudo-color images, the blue and red colors are associated with colder and warmer areas, respectively. Reproduced from ref. 201 with permission from Elsevier, copyright 1998. | ||
In 2018, L. Labrador-Páez et al.204 published an interesting review about core–shell rare-earth-doped nanostructures in biomedicine, describing the most relevant examples of their applications in imaging, sensing, and therapy. In 2020, Zhou et al.,24 reviewed the advances and challenges for fluorescence nanothermometry, highlighting applications and achievements, discussed the scenarios that may lead to biased sensing, etc., and pointed out directions for improving the field. More recently, in 2021, Ansari et al. published a complete review of thermometric luminescent lanthanide nanocomposites.205 The authors summarized many concepts about the photophysical properties of Ln3+ ions and their use as luminescence optical thermometry. In addition, they discussed the recent progress of the scientific community in boosting the thermometric performances of these systems. Many reasons made LNThs:RE an exciting system for biological applications, and one of them is because luminescence detections are relatively easy to obtain. They have already been used to detect cardiovascular problems in small animals and to access the basic properties of living tissues.27,109,206 In 2024, several short-review papers discussing temperature in nanoscale materials were published. For instance, Li et al.207 explored several factors, such as, lanthanide concentration, particle size, and crystal structure, focusing on optical diagnosis and temperature feedback through photothermal conversion pathways. Puccini et al.208 delved into the challenges and limitations of LNThs, particularly concerning excitation and emission wavelengths, drawing from publications over the past four years. Meanwhile, Wu et al.209 conducted a noteworthy study on lanthanide LNThs with working wavelengths extending beyond 1500 nm, specifically for in vivo cerebrovascular temperature imaging, among other significant contributions.210–212
A LNTh:RE is typically obtained by doping a suitable host with Ln3+ ions. The precise selection of the host is crucial, as it directly influences the intensity of luminescence. For instance, hosts with high phonon energy may be undesirable due to the increased likelihood of non-radiative decays, which diminish luminescent emissions. Thus, achieving optimal luminescence necessitates a judicious pairing of the host and Ln3+ ions, characterized by a well-suited coordination chemistry surrounding Ln3+ ions or crystal field.32,205 A more complete discussion of the host media's impact on the luminescence of RE-doped systems can be found in ref. 213. Mahaling et al. pioneered the development of the first single-core multifunctional NPs doped with RE (NPs:RE) by introducing Yb3+/Tm3+ ions into LiYF4 Nanocrystals in the core. This approach yielded strong emissions across the deep-UV to NIR regions. However, a drawback of this structural design is the potential for undesirable energy-transfer-assisted luminescence quenching due to the coexistence of these two ions in the core. The inaugural RE-doped NPs designed for nanomedicine were introduced by Zijlmans et al.,214 who employed upconverting phosphors for detecting antigens in tissue sections or on cell membranes. Despite being somewhat dated, the 2009 review on Ln3+-doped UC nanocrystals published by Feng Wang and Xiaogang Liu remains a valuable resource for understanding various synthetic approaches, possibilities for chemical tuning of UC properties, and biological applications of these systems.215 Additionally, Lee et al.216 recently presented NPs exhibiting giant nonlinear optical responses via a photon-avalanching process, demonstrating the potential for super-resolution imaging with sub-70-nanometer spatial resolution using a simple scanning confocal microscopy technique, free of computational analysis.
Considering exactly NPs for biological applications, we can categorize the LNThs:RE into two groups: (G1) LNThs:RE that are excited in the IR region but with emission in UV/VIS and (G2) LNThs:RE with excitation and emission in the IR region. An example of G1 we can cite are the UC NPs that, via a two-photon or multiphoton mechanism, convert long wavelength radiation (e.g., IR light) into short wavelength fluorescence, e.g., visible light. The LNThs:RE of G2 (hereafter LNThs:RE-G2) has great potential for biological applications. With a suitable choice of host and Ln3+ ions, these systems can exhibit both excitation and emission bands within the BWs. Although different types of LNThs:RE-G2 have already been reported in the literature on nanothermometry, this subject is still constantly explored because most of them have low thermal sensitivity, such as Nd:YAG and Nd:LaF3.198,217
Illustrating the application of LNThs:RE for thermal imaging, a notable example is the erbium, thulium, and ytterbium core–shell LaF3 NPs, as published by Ximendes et al. in 2017, functioning within the biological windows.29 These NPs exhibit exceptional thermal sensitivity, reaching values as high as 5% °C−1, a notable achievement among RE-doped systems. Under 690 nm excitation, as depicted in Fig. 12a, this system displays emission bands around 1230 and 1470 nm (Tm3+), 1000 nm (Yb3+), and 1550 nm (Er3+). Notably, the core@shell structure is intriguing due to its promotion of spatial separation between the ions, such as Er3+ and Tm3+, thereby mitigating processes like back ET. In their study, the authors successfully employed these NPs as subcutaneous thermal probes with significant optical penetration into tissues. This highly efficient system allowed the acquisition of time-resolved 2D thermal images in a small animal model. Fig. 12b and c present thermal images obtained just before initiating a heating procedure (t = 0 s) and after 700 s, respectively. This groundbreaking work represents the first demonstration of the viability of using LNThs:RE for obtaining accurate in vivo subcutaneous 2D thermal videos.
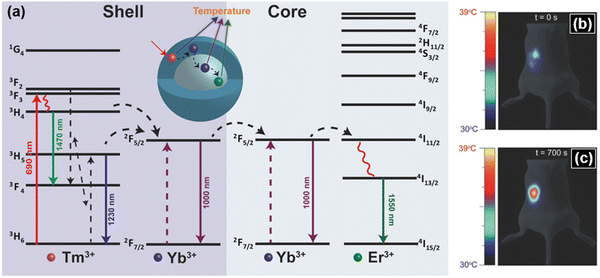 | ||
| Fig. 12 (a) Simplified energy scheme of the Tm3+/Yb3+/Er3+ system showing the excitation at 690 nm and non-radiative decays (curved line), radiative decays (full lines), and possible cross-relaxations and ion–ion energy transfer paths (dashed lines). The inset shows the schematic representation of the Er–Yb@Yb–Tm (core@shell structure) LaF3 NPs. (b) Thermal image obtained just (b) before starting a heating procedure (t = 0 s) and (c) after 700 s. Reproduced from ref. 29 with permission from John Wiley and Sons, copyright 2017. | ||
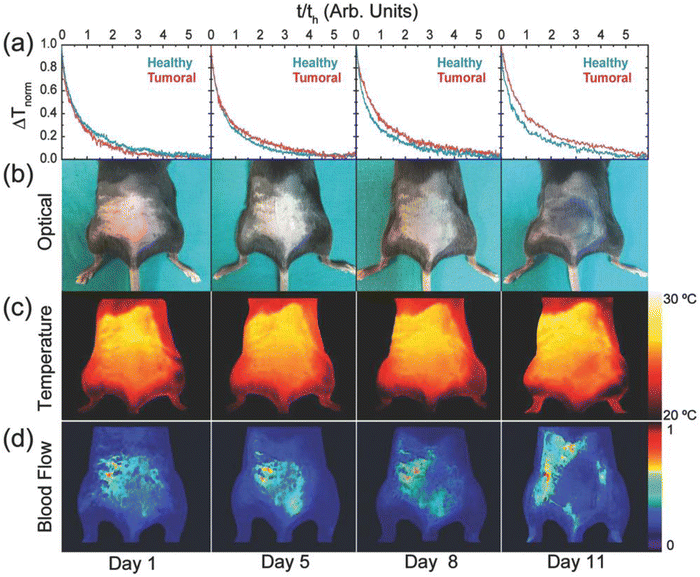 | ||
| Fig. 13 (a) Thermal transient curves at different times after inoculating a mouse with B16 cancer cells. Blue and red curves correspond to healthy and tumoral tissues, respectively. (b) Optical images of a representative mouse after cancer cell inoculation, at different time points. (c) Thermographic images and (d) blood perfusion images corresponding to the mouse shown in (b). Reproduced from ref. 2 with permission from John Wiley and Sons, copyright 2018. | ||
Still using Ag2S NCs, the same research groups225 produced superbright short-wave IR dots for low-dose in vivo imaging by ultrafast photochemistry induced via pulsed intense laser. The increase in brightness was over 80-fold in the quantum yield of the Ag2S NCs and this allowed in vivo imaging with super low excitation intensities (<10 mW cm−2) and extremely low doses (<0.5 mg Kg−1). Fig. 14 (top left) presents the results of the optical transformation of Ag2S dots into superdots, where the shape of the emission spectrum did not change with the irradiation (inset of this figure) and the main result is the fluorescence enhancement over 50 times during the irradiation process. The quantum yield and fluorescence lifetime also presented a super-enhancement after different ultrafast laser irradiation durations, reaching its maximum value or saturation for 50 min. The quantum yield increased by 80-fold at this exposure time of 50 min, while the lifetime enhancement was over 10-fold, but with a similar trend to quantum yield. Fig. 14 (top right) shows the comparison of the in vivo NIR-II imaging for different systems, exactly Ag2S superdots, commercial Ag2S dots, Nd3+ doped LaF3 nanocrystals (LaF3:Nd) and single wall carbon nanotubes (SWNTs). The same volume of NPs (100 μL) and concentration (1.5 mg mL−1) were injected in all cases and excited at the same 808 nm excitation wavelength. The Ag2S superdots presented a super brightness in comparison with the other fluorescence probes used, and this superior brightness enabled the acquisition of fluorescence images with sufficient contrast even using ultralow irradiation power density such as 0.3 mW cm−2, which enables using superlow doses of superdots and also obtaining in vivo imaging with cost-effective excitation sources. The exceptional brightness of Ag2S superdots also allows the acquisition of in vivo dynamic fluorescent imaging (video). Fig. 14 (bottom) shows the NIR-II images obtained at different times after intravenous administration of PEG-coated Ag2S superdots. This implies the feasibility of minimal administration doses for in vivo imaging, offering several advantages. This approach facilitates the development of a cost-effective probe for NIR-II in vivo imaging, minimizing the quantity of material used. This enhances cost-effectiveness and mitigates potential toxicity associated with the probe. Additionally, the Ag2S superdots exhibit a photothermal effect that demands low administration doses and low illumination intensities, further contributing to the overall efficacy of the imaging process.
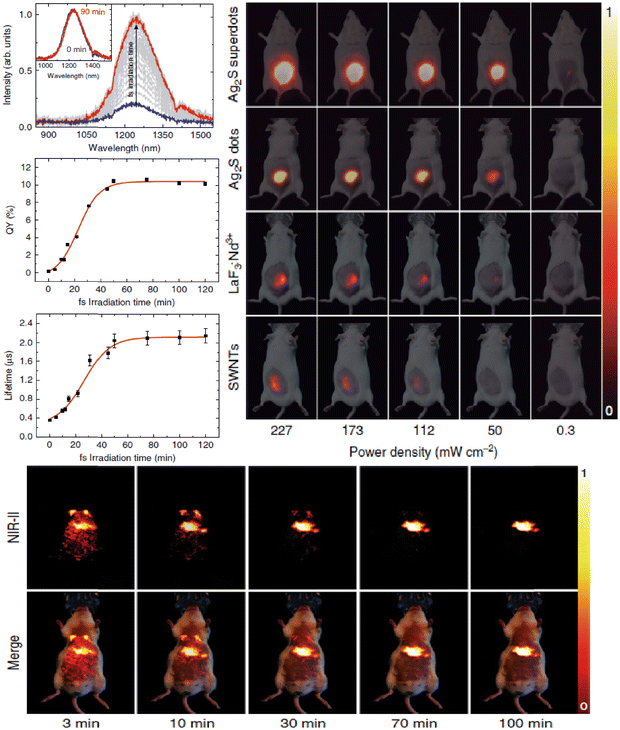 | ||
| Fig. 14 Top left: NIR-II emission spectra generated by a colloidal suspension of dos in CHCl3 during 90 min-long ultrafast laser irradiation. The inset presents the normalized emission spectra of the sample before and after the laser irradiation. Fluorescence quantum yield and fluorescence lifetime of Ag2S dots in CHCl3 after being subjected to ultrafast laser irradiation processes with different durations. Top right: NIR-II fluorescence images of a group of four mice subcutaneously injected with colloidal aqueous dispersions containing Ag2S superdots, commercial Ag2S dots, SWNTs, and LaF3: Nd NPs. Bottom: NIR-II fluorescence images were obtained at different times after the intravenous injection of Ag2S superdots dispersed in PBS (100 μL, 0.15 mg mL−1). Reproduced from ref. 225 with permission from Springer Nature, copyright 2020. | ||
5. Diagnosis and photothermal therapy (PTT)
5.1. Applications of photothermal therapy
PTT is a therapeutic method employing light and PTAs, such as nanomaterials alone or small molecules encapsulated within NPs, to generate localized heat to destroy cells, particularly cancer cells. Generally, NIR light is used due to its enhanced penetration into biological tissues. While PTT's primary application is in cancer treatment,226 it has also demonstrated utility in diverse areas such as drug delivery,227 antibacterial therapy, thermal ablation,228 immunotherapy,229etc. Bellow, we provide a brief overview of some of the PTT applications mentioned here and others:Another very interesting example of PTT was presented by Carrasco et al.,84 who performed intratumoral thermal reading during PTT using multifunctional fluorescent Nd:LaF3 NPs. Nd:LaF3 is a well-known LNTh, and with a high Nd3+ concentration these NPs exhibit multifunctional properties, including functioning as nanoheaters.83 Carrasco and co-authors aimed to conduct PTT while monitoring the temperature in real-time to avoid unnecessary overheating. Fig. 15a shows an optical image of a mouse with two tumors. Solutions of PBS with and without Nd:LaF3 NPs were injected into the tumors on the left and right sides, respectively. The presence of Nd:LaF3 NPs was confirmed in the left tumor detecting the Nd3+ luminescence under 808 nm laser excitation with an intensity of 4 W cm−2 (Fig. 15b), while no luminescence signal was detected in the right tumor. The pumped-induced local heating was monitored by both luminescence (for intratumoral temperature) and a thermographic camera (model FLIR E40) for surface temperature measurement. Fig. 15c exhibits a thermal image of the tumor's surface during laser irradiation. The most noteworthy result was the simultaneous monitoring of intratumoral temperature during PTT, as shown in Fig. 15d. This figure compares the intratumoral temperature (measured via Nd3+ luminescence), the surface temperature (measured with the thermographic camera), and the control temperature of the right tumor (without NPs), which also experienced pump-induced heating due to residual tissue absorption. Notably, after 3 minutes, the intratumoral temperature was approximately 20% higher than the surface temperature, highlighting the importance of intratumoral monitoring to avoid unnecessary overheating. The efficacy of the PTT was further assessed by tracking tumor size over time, as illustrated in Fig. 15e–g. Fig. 15e and f show optical images of the mouse 15 minutes and 20 days after treatment, respectively. The tumor treated with Nd:LaF3 NPs had completely disappeared within 5–6 days (Fig. 15g, red dots), while the control tumor, treated with PBS and laser, continued to grow throughout the treatment period (Fig. 15g, blue dots).
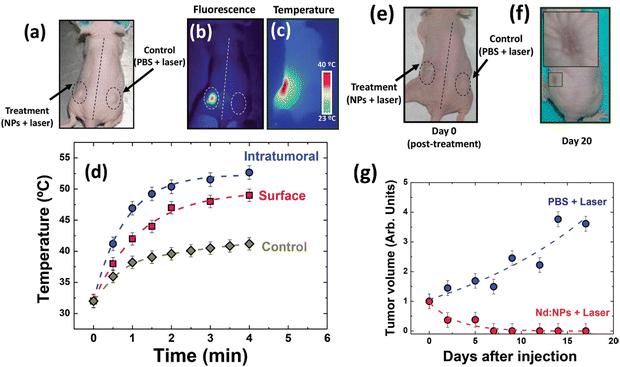 | ||
| Fig. 15 (a) Optical image of a representative mouse with two tumors. A solution of Nd:LaF3 NPs (with high Nd3+ concentration – 25 at%) was injected only in the left-side tumor whereas the right-side one was used as a control. (b) and (c) Infrared fluorescence and thermal images of the same mouse under 808 nm with intensity of 4 W cm−2, respectively. The images show the fluorescent and heating signals differentially emitted by the treated tumor. (d) Time evolution of the temperature at the tumor surface as obtained from the analysis of infrared thermal images (surface) and the intratumoral temperature obtained from the analysis of sub-tissue fluorescence (intratumoral). Time evolution of the surface temperature as a control is also presented (control). Optical images of the same representative mouse are shown (e) 15 min and (f) 20 days after the photo thermal treatment with concentrated Nd:LaF3 NPs and 4 W cm−2 of 808 nm laser irradiation. (g) Volume evolution of both treated and control tumors versus days of treatment. Symbols are experimental data, and dashed lines serve as guides for the eyes. Reproduced from ref. 84 with permission from John Wiley and Sons, copyright 2014. | ||
A recent study by Khalili et al.,233 clearly demonstrates the effect of drug delivery in combination with light-induced heating. The release dynamics of DOX and DTX from DOX/DTX@HCOF@Au-βCD-PEG-FA were studied in buffer solutions at two different pH levels (7.4 and 5.0) to mimic the conditions of blood serum and the acidic environment within cancer cells. Fig. 16a illustrates that the release of drugs is significantly pH-dependent. Over a 30-hour period, only about 8% of DTX was released at pH 7.4, likely due to its low solubility in neutral water and the strong hydrophobic interactions that hinder drug release. Conversely, the release of DOX was also low at this pH level. The minimal leakage of DOX in physiological conditions can be attributed to the stability of the imine bonds and strong π–π interactions and hydrogen bonding between DOX and HCOF (hollow covalent organic frameworks), further protected by the Au NPs on the nanoplatform. When the pH was lowered to 5.0, the release rates increased substantially to 67% for DOX and 46% for DTX. This increase is due to the acidic environment breaking down the host–guest interactions between the drugs and cyclodextrin, facilitating greater drug release. The MTT assay was used to assess the in vitro biocompatibility and cytotoxicity of various formulations. Breast 4T1 cancer cells were treated with pure HCOF@Au-βCD-PEG-FA, DOX@HCOF@Au-βCD-PEG-FA, DTX@HCOF@Au-βCD-PEG-FA, HCOF@Au-βCD-PEG-FA with NIR laser, DTX/DOX@HCOF@Au-βCD-PEG-FA, DTX/DOX@HCOF@Au-βCD-PEG-FA with NIR laser, and a cocktail, all at concentrations of 0, 6, 12, 25, 50, and 100 μg mL−1. Results shown in Fig. 16b indicate that pure HCOF@Au-βCD-PEG-FA displayed minimal cytotoxicity even at high concentrations, suggesting that this carrier is highly biocompatible. Single-drug formulations using HCOF@Au-βCD-PEG-FA had lower toxicity than the dual-drug formulations, demonstrating the enhanced efficacy of the combination delivery system. The cocktail was less effective than DOX/DTX@HCOF@Au-βCD-PEG-FA, possibly due to varied pharmacokinetics of the drugs leading to less effective therapy. The CI50 value of 0.33 for DOX/DTX@HCOF@Au-βCD-PEG-FA, less than 1, signifies a synergistic effect between the two drugs. The combination therapy showed a greater inhibitory effect on cancer cells than individual treatments. The synergistic potential of chemotherapy and PTT was further examined with dual-drug-loaded HCOF under 808 nm laser irradiation (1 W cm−2). The results showed a significant decrease in cancer cell viability under NIR laser treatment, with enhanced growth inhibition compared to drug formulations without laser. Confocal laser scanning microscopy (CLSM) analysis (Fig. 16c) revealed that nearly all cells in the dual-drug plus laser group were dead, in contrast to other groups, emphasizing the superior effectiveness of combination therapy. The internalization of DOX/DTX@HCOF@Au-βCD-PEG-FA was investigated using confocal microscopy. Fig. 16d shows that both DOX (red fluorescence) and DTX (green fluorescence) were present in 4T1 cells after 6 hours. DOX was primarily localized in the cell nuclei, while DTX was detected in the cytoplasm, indicating that the coloaded nanocarrier effectively enters cancer cells and releases the drugs into their respective cellular compartments through endocytosis.
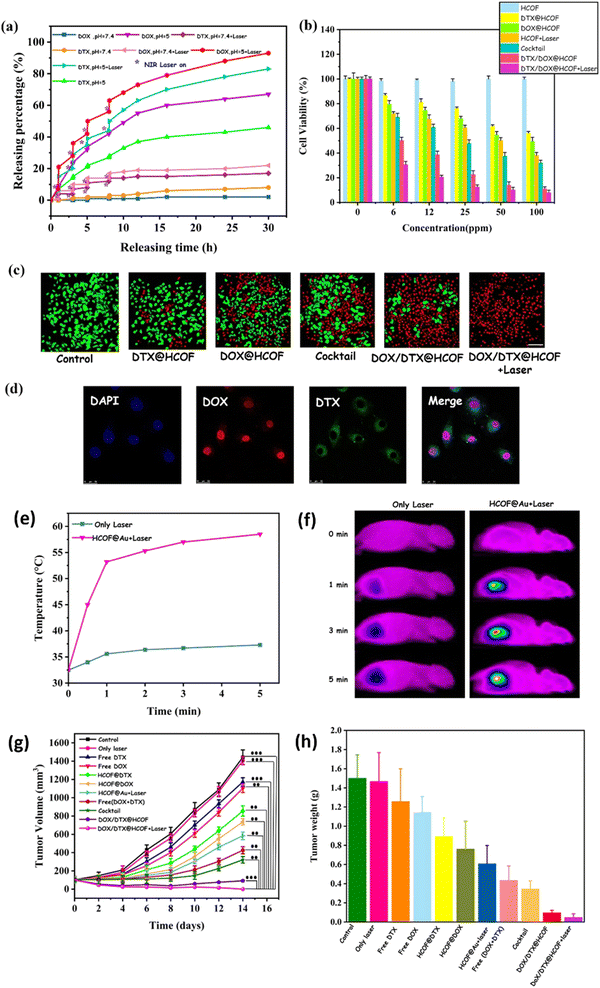 | ||
| Fig. 16 (a) Release profiles of DTX and DOX with and without 808 nm NIR laser irradiation at different pH levels (pH 7.4 and 5.0). (b) Viability of 4T1 cells following treatment with HCOF (hollow covalent organic frameworks), DTX/HCOF, DOX/HCOF, HCOF with laser, cocktail, DOX/DTX@HCOF, and DOX/DTX@HCOF with laser at various concentrations. (c) CLSM images showing live (green) and dead (red) cells after different treatments, stained with calcein AM and propidium iodide (PI), respectively (scale bar = 100 μm). (d) CLSM images of 4T1 cells post 6-hour incubation with DOX/DTX-loaded HCOF@Au-βCD-PEG-FA (all scale bars: 25 μm). (e) and (f) Temperature variations and IR thermal images of tumors from the laser-only and HCOF@Au + laser groups, subjected to 808 nm laser irradiation (1 W cm−2) for 5 minutes. (g) Tumor growth inhibition following different treatments (n = 5, mean ± SD, **P < 0.01, and ***P < 0.001). (h) Hematoxylin and eosin staining of major organs (liver, spleen, kidney, heart, and lung) from healthy and tumor-bearing mice post-treatment (scale bar = 200 μm). Reproduced from ref. 233 with permission from American Chemical Society, copyright 2024. | ||
To assess the in vivo antitumor efficacy, 4T1 tumor-bearing mice were grouped (n = 5 per group) and treated with various regimens, including control, laser-only, free DOX, free DTX, DTX@HCOF@Au-βCD-PEG-FA, DOX@HCOF@Au-βCD-PEG-FA, HCOF@Au-βCD-PEG-FA with laser, free DOX + DTX, cocktail, DOX/DTX@HCOF@Au-βCD-PEG-FA, and DOX/DTX@HCOF@Au-βCD-PEG-FA with laser. NIR laser treatment (808 nm, 1 W cm−2) was applied 12 hours after the last intravenous injection. Tumor temperature increased to 58.5 °C within 5 minutes in the HCOF@Au plus laser group, indicating effective tumor ablation. In contrast, tumors in the control group showed no significant temperature change (Fig. 16e and f). Tumor growth was measured regularly (Fig. 16g), showing that tumors in the control group and laser-only group grew rapidly. Mice treated with DOX and DTX in HCOF@Au-βCD-PEG-FA exhibited superior tumor inhibition compared to those treated with single-drug formulations or cocktails, demonstrating the benefits of combination therapy. The dual-drug-loaded HCOF provided a more controlled pharmacokinetic profile and enhanced drug delivery to tumors, improving therapeutic outcomes over traditional methods. The group receiving both drug-loaded HCOF and laser treatment had the most significant reduction in tumor growth, demonstrating the high efficacy of combining dual-drug chemotherapy with PTT. Tumor weights recorded for each group (Fig. 16h) confirmed that the combination treatment produced the most favorable therapeutic results.
Indeed, MoS2 has been extensively explored for biomedical applications due principally to its excellent photothermal conversion. Zhang et al.,236 introduced a folic acid (FA)-targeted, dual-stimuli responsive MoS2 nanosheet nanoplatform for treating FA-receptor positive breast cancer. The nanocomposites, with an average diameter of approximately 133 nm, demonstrate a high drug loading capacity of 151.4 mg g−1 for doxorubicin (DOX). DOX release was significantly accelerated at pH 5.0 compared to pH 7.4 and further enhanced under NIR laser irradiation, indicating dual responsiveness. Fig. 17a demonstrates that the photothermal effect of FBPMP (FA-BSA-PEI-LA-MoS2-LA-PEG) is concentration-dependent when irradiated at 0.5 W cm−2, highlighting its suitability for PTT and potential for tumor thermal ablation. The optimal drug loading efficiency was achieved with a FBPMP to DOX mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where 1 g of FBPMP could load 151.4 mg of DOX. Fig. 17b illustrates the in vitro release profiles of FBPMP (DOX) in two pH environments (5.0 and 7.4), both with and without laser irradiation. DOX release was significantly faster and more extensive at pH 5.0 compared to pH 7.4, with respectively cumulative releases of 61.3% and 15.0% after 68 hours. This is attributed to the reduced hydrophobic interactions between FBPMP and DOX in acidic conditions, which is beneficial for targeting the acidic tumor microenvironment. Additionally, at a given pH, laser irradiation further enhances DOX release, suggesting that NIR-induced photothermal heating can facilitate drug release and increase the efficacy of cancer cell destruction. The therapeutic performance of FBPMP(DOX) was evaluated using MTT assays on MDA-MB-231 and L929 cells (Fig. 17c and d). The cell viability remained above 80% for FBPMP-treated cells, indicating high biocompatibility even at a concentration of 10 μg mL−1. NIR irradiation alone had no significant effect on cell viability, with both L929 and MDA-MB-231 cells showing similar viabilities (>90%) with or without laser treatment. However, for L929 cells, the FBPMP(DOX) treatment resulted in significantly higher cell viability than free DOX, while MDA-MB-231 cells exhibited slightly lower viability with FBPMP(DOX). For both cell types, combining FBPMP with laser irradiation resulted in lower cell viability compared to FBPMP alone, demonstrating the photothermal effect of the MoS2 nanocomposites. Notably, MDA-MB-231 cells in the combined therapy group showed markedly lower viability than those in either chemotherapy or PTT alone, reflecting the synergistic effect of combined chemo-PTT. The NIR laser not only generates heat for PTT but also accelerates DOX release from FBPMP(DOX) by disrupting π–π stacking interactions, enhancing the overall chemotherapy efficacy. The increased cytotoxicity of FBPMP(DOX) with NIR irradiation against MDA-MB-231 cells compared to L929 cells indicates a potential for selective targeting of cancer cells. These findings confirm the effectiveness and promise of the multifunctional drug delivery system for combined chemo-photothermal cancer therapy.
1, where 1 g of FBPMP could load 151.4 mg of DOX. Fig. 17b illustrates the in vitro release profiles of FBPMP (DOX) in two pH environments (5.0 and 7.4), both with and without laser irradiation. DOX release was significantly faster and more extensive at pH 5.0 compared to pH 7.4, with respectively cumulative releases of 61.3% and 15.0% after 68 hours. This is attributed to the reduced hydrophobic interactions between FBPMP and DOX in acidic conditions, which is beneficial for targeting the acidic tumor microenvironment. Additionally, at a given pH, laser irradiation further enhances DOX release, suggesting that NIR-induced photothermal heating can facilitate drug release and increase the efficacy of cancer cell destruction. The therapeutic performance of FBPMP(DOX) was evaluated using MTT assays on MDA-MB-231 and L929 cells (Fig. 17c and d). The cell viability remained above 80% for FBPMP-treated cells, indicating high biocompatibility even at a concentration of 10 μg mL−1. NIR irradiation alone had no significant effect on cell viability, with both L929 and MDA-MB-231 cells showing similar viabilities (>90%) with or without laser treatment. However, for L929 cells, the FBPMP(DOX) treatment resulted in significantly higher cell viability than free DOX, while MDA-MB-231 cells exhibited slightly lower viability with FBPMP(DOX). For both cell types, combining FBPMP with laser irradiation resulted in lower cell viability compared to FBPMP alone, demonstrating the photothermal effect of the MoS2 nanocomposites. Notably, MDA-MB-231 cells in the combined therapy group showed markedly lower viability than those in either chemotherapy or PTT alone, reflecting the synergistic effect of combined chemo-PTT. The NIR laser not only generates heat for PTT but also accelerates DOX release from FBPMP(DOX) by disrupting π–π stacking interactions, enhancing the overall chemotherapy efficacy. The increased cytotoxicity of FBPMP(DOX) with NIR irradiation against MDA-MB-231 cells compared to L929 cells indicates a potential for selective targeting of cancer cells. These findings confirm the effectiveness and promise of the multifunctional drug delivery system for combined chemo-photothermal cancer therapy.
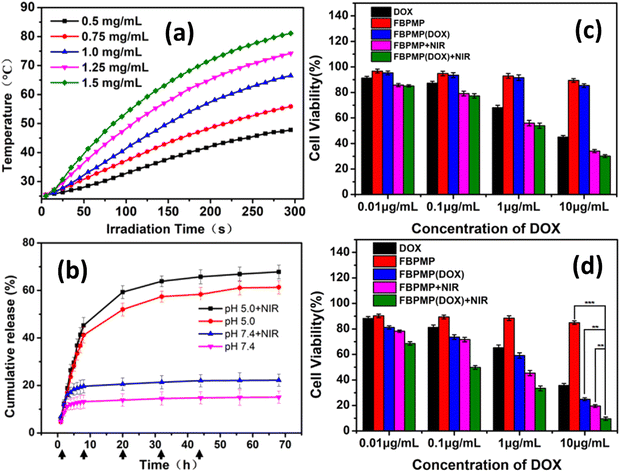 | ||
| Fig. 17 PEI = poly(ethylene imine), PEG = poly(ethylene glycol); DOX = doxorubicin; BSA = bovine serum albumin; LA = alpha-lipoic acid; FA = folic acid; FA-BSA = folic acid-grafted bovine serum albumin; PMP = PEI-LAMoS2-LA-PEG; FBPMP = FA-BSA-PEI-LA-MoS2-LA-PEG. (a) Temperature increase curves for various concentrations of FBPMP (FA-BSA-PEI-LA-MoS2-LA-PEG) under 0.5 W cm−2 laser power; (b) release profiles of DOX from FBPMP(DOX) at different pH levels, with and without 808 nm laser irradiation (0.5 W cm−2; laser irradiation indicated by arrows). Results are shown as mean ± SD (n = 3); (c) viability of L929 cells and (d) MDA-MB-231 cells treated with different concentrations of DOX or DOX-loaded nanocomposites, with or without laser exposure. Data are expressed as mean ± SD (n = 3). Statistical significance is indicated as P < 0.01 (**) and P < 0.001 (***). Reproduced from ref. 236 with permission from Elsevier, copyright 2019. | ||
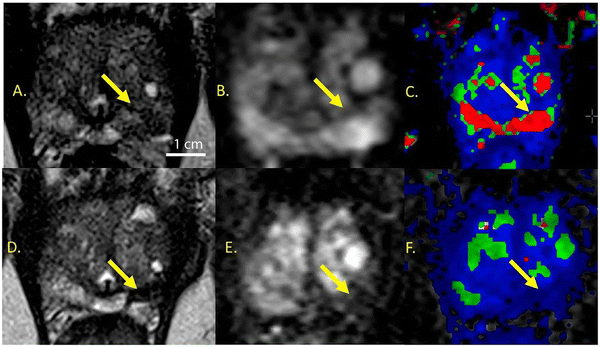 | ||
| Fig. 18 Case study of a 70-year-old man with localized prostate cancer successfully treated using GSN-directed laser excitation and ablation. (A)–(C) Images from pre-treatment and (D)–(F) from 3 months post-treatment. Follow-up biopsy at 3 months showed no cancer presence. (A) Axial T2-weighted MRI image showing a tumor in the left apex with a Gleason score of 3 + 4 on targeted biopsy (arrow). (B) Diffusion-weighted imaging (DWI) at b = 2000 reveals restricted diffusion in the tumor, indicated by hyperintensity compared to the normal peripheral zone. (C) Dynamic contrast-enhanced MRI (DCE-MRI) parametric map (Ktrans/Ve) shows heightened tumor enhancement. (D) Post-treatment axial T2-weighted MRI image displays a contracted ablation zone with marked hypointensity, suggestive of hemorrhagic or necrotic changes. (E) DWI at b = 2000 shows the resolution of restricted diffusion in the treated area (arrow). (F) DCE-MRI parametric map (Ktrans/Ve) indicates the resolution of abnormal enhancement in the treated tumor (arrow) (scale bar: 1 cm). Reproduced from ref. 228 with permission from PNAS, copyright 2019. | ||
Chen et al.242 explored a multifunctional nanoplatform designed for effective PDT, PTT, and chemotherapy, all regulated by a single NIR laser. Typically, these three therapeutic modalities require different stimuli, which can complicate treatment and impact efficacy. To address this, the study utilized a single NIR wavelength (700–1250 nm) known for its deeper tissue penetration, making it a suitable choice for phototherapy. Human serum albumin (HSA), known for its biocompatibility, low toxicity, and abundance, was employed as a carrier due to its ability to encapsulate drugs through hydrophobic interactions. Chen et al.242 developed a reactive oxygen species (ROS)-responsive prodrug, DTD, by linking thioketal (TK) and doxorubicin (DOX). Through a self-assembly method, DTD and IR780 were incorporated into HSA to form DTD/IR780@HSA NPs (Fig. 19a and b). When exposed to 808 nm laser light, IR780, a NIR fluorescent dye, exhibited significant photothermal activity and produced substantial ROS. This ROS not only facilitated PDT but also triggered the breakdown of thioketal linkages, leading to the release of DOX. Thus, the 808 nm laser could concurrently manage PTT, PDT, and chemotherapy for a synergistic therapeutic effect.
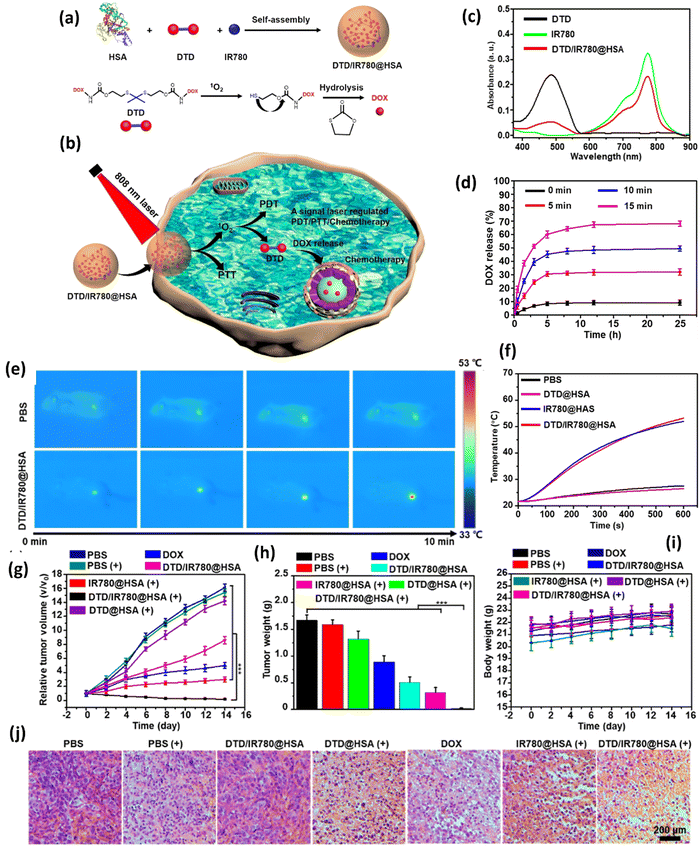 | ||
| Fig. 19 Overview of DTD/IR780@HSA preparation and NIR light-regulated combination therapy: (a) schematic representation of the synthesis of DTD/IR780@HSA. (b) Diagram showing the mechanism of combination therapy regulated by NIR light. (c) UV-vis absorption spectra for DTD, IR780, and DTD/IR780@HSA. (d) Release profiles of DTD/IR780@HSA under various laser irradiation conditions. The symbol (+) denotes laser irradiation, whereas (−) denotes no laser irradiation. (e) Infrared thermal imaging of tumor-bearing mice during laser exposure. (f) Temperature changes at the tumor site during laser irradiation. (g) Changes in tumor volume for different treatment groups. (h) Tumor weights post-treatment. (i) Body weight variations throughout the treatment period. (j) H&E staining results of tumor tissues following different treatments. The scale bar in the final image applies to all similar images. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01, and ***P < 0.001). Reproduced from ref. 242 with permission from American Chemical Society, copyright 2024. | ||
The efficacy of the system was evaluated through in vitro and in vivo experiments. Transmission electron microscopy (TEM) and dynamic light scattering confirmed the uniform spherical shape of the DTD/IR780@HSA NPs, with hydrodynamic diameters of 123 ± 5 nm, 105.2 ± 6 nm, and 106.5 ± 3 nm for DTD/IR780@HSA, IR780@HSA, and DTD@HSA, respectively. The UV-Vis spectra (Fig. 19c) revealed distinct absorption peaks at 504 and 808 nm, corresponding to DTD and IR780, respectively, indicating successful loading of these compounds into the NPs.
The drug release behavior was tested under 808 nm laser irradiation (Fig. 19d). Without laser exposure, DOX release from DTD/IR780@HSA was less than 10%, demonstrating the nanoparticle stability. However, laser irradiation for 5 minutes increased DOX release to approximately 31.2%, which further rose to 70.6% after 15 minutes, showing that the 808 nm laser effectively controlled drug release through ROS generation and photothermal effects.
The photothermal performance of DTD/IR780@HSA was evaluated (Fig. 19f), revealing that DTD@HSA did not exhibit photothermal effects due to the absence of IR780. In contrast, DTD/IR780@HSA and IR780@HSA displayed notable photothermal properties under laser irradiation, with the intensity of photothermal effects modulated by the NIR laser power.
To assess in vivo antitumor efficacy, HeLa cell-bearing mice were treated with various formulations. Following tumor growth to 100 mm3, NPs were administered intratumorally. Thermal imaging revealed a significant temperature rise to 51.3 °C in tumors treated with DTD/IR780@HSA after 10 minutes of laser irradiation (Fig. 19e). Tumor-bearing mice were divided into seven treatment groups: PBS, NIR, DOX, DTD/IR780@HSA, DTD@HSA + laser, IR780@HSA + laser, and DTD/IR780@HSA + laser. Due to the low DOX release rate, DTD/IR780@HSA without laser did not inhibit tumor growth. However, significant tumor growth suppression was observed in the DTD/IR780@HSA + laser group, due to the combined effects of PTT, PDT, and chemotherapy. The efficacy was further supported by tumor weight measurements (Fig. 19h) and H&E staining, which revealed extensive necrosis in tumors treated with DTD/IR780@HSA and laser (Fig. 19j). These results demonstrated that DTD/IR780@HSA effectively inhibited tumor growth through synergistic PTT/PDT/chemotherapy and extended mouse survival without significant weight loss (Fig. 19i). H&E staining of major organs showed no significant differences across treatments, indicating low toxicity and high biocompatibility of DTD/IR780@HSA.
 | ||
| Fig. 20 (a) Schematic depiction of PVP-MPDA@R837 nanoparticles and the mechanism of intrigued antitumor immune responses. (b) Photothermal effect of PVP-MPDA solution (250 μg mL−1) with laser irradiation (808 nm, 0.5 W cm−2) for 20 min and left to cool down then, Inset: Linear time data versus negative natural logarithm of the temperature driving force which is obtained from the cooling stage. The thermal time constant for heat transfer from the system was determined to be τs = 304.69 s. (c) Tumor volumes of B16F10-bearing mice receiving different treatments. Footpad injections of various formulations were performed once every 3 d from 0 d for three times and tumor laser irradiation was applied at 24 h after injection. Reproduced from ref. 243 with permission from Elsevier, copyright 2020. | ||
 | ||
| Fig. 21 (a) Schematic representation of Ag@SiO2@Agseed, Ag@SiO2@AgNPs core–shell nanoparticles use them for photothermal therapy- PTT. (b) Time-dependent photothermal temperature measurements of the Ag@SiO2@Agseed, Ag@SiO2@AgNPs core–shell nanoparticles using halogen light sources. (c) HeLa cells stained with annexin V followed by propidium iodide indicator in the presence of images Ag@SiO2@Agseed, Ag@SiO2@ANps core–shell nanoparticles (light irradiation time 10, 20 min and concentration 10 μg/1 mL, each scale bar indicates 50 μm. Reproduced form ref. 245 with permission from Elsevier, copyright 2019. | ||
Finally, it is important to clarify that our goal here is not to provide an exhaustive exploration of all potential applications of the PTT technique. Rather, we aim to highlight some of the most prominent applications in the literature. Moving forward, we will delve into a well-established application of the PTT technique, focusing specifically on using NThs for diagnostic purposes, particularly in assessing thermal relaxation dynamics.
5.2. Using nanothermometers for thermal relaxation dynamics as a diagnostic tool
Early diagnostic detection is highly sought by physicians looking for techniques to increase the chances of early treatment and monitor the status of the disease after appropriate treatment. As we have mentioned, NPs can act as NThs. For example, we discussed rare-earth doped NPs and silver sulfide NPs, Ag2S, in Section 4.2.3. In both cases, the luminescence parameters can change with high temperature sensitivity. Thus, by monitoring changes in luminescence, we can read the temperature in real time.182,192 On the other hand, the thermal relaxation dynamics associated with a body transitioning from a higher to a lower temperature can provide numerous information about the body.27,246,247 Within a biological tissue or even a single cell, it can provide insight into thermoregulation, chemical events, and, in general, the status of the biological system, such as whether it is healthy or contains a tumor.248,249To address this challenge, Ximendes et al.5 used PbS/CdS/ZnS quantum dots (NIR-QDs) for the first time to detect ischemia by using transient thermometry (TTh). The quantum dots used have the advantage of emitting in the NIR-II, with the emission centered at 1200 nm, making it ideal for biological applications. The TTh technique involves applying a heating pulse to a tissue, causing its temperature to increase from the natural level (considering the surrounding conditions) to a higher one. After the pulse is switched off, the tissue's temperature starts to cool down until it returns to its basal temperature. This dynamic process is monitored using a thermal relaxation curve. The diagnosis is made by examining changes in the biophysical properties of the tissue, which can be inferred from the thermal relaxation curve. In other words, the thermal relaxation curve is strongly dependent on the biophysical properties of the tissue.
Like every physical observation, the thermal relaxation dynamics also have a mathematical description, known as Penne's bioheat equation:250
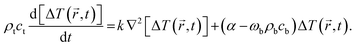 | (4) |
The biophysical properties involved are the specific heat (ct), the mass density (ρt) and the thermal conductivity of tissue k; the local blood perfusion rate (ωb), the mass density (ρb) and specific heat of blood (cb); the local metabolic heat generation rate, blood and tissue temperatures; α is a term associated with the local metabolic activity of the tissue under investigation; and ΔT(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) , t)(=T(
, t)(=T(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) , t) − Tbasal(
, t) − Tbasal(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) )) is a temperature variation from a basal value Tbasal(
)) is a temperature variation from a basal value Tbasal(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) ) to an arbitrary value reached after exposing the tissue to heat.
) to an arbitrary value reached after exposing the tissue to heat.
The authors compared the thermal relaxation dynamics of healthy tissue (used as a control) with tissue exhibiting ischemic dysfunction, as shown in Fig. 22a. It is challenging to control how each property changes, but some of these changes can be predictable depending on the disease. For example, an induced ischemic lesion will likely cause a significant decrease in local blood perfusion in the tissue. Conversely, if surgery is not successful, inflammation can occur. In such cases, the trends in blood perfusion tend to increase significantly.
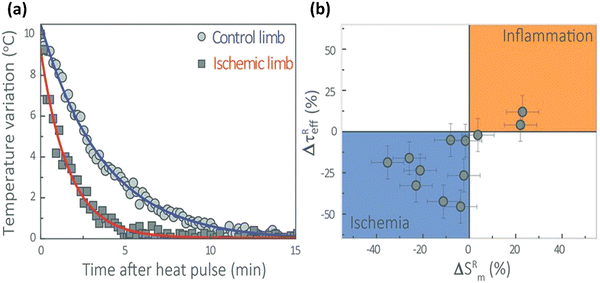 | ||
Fig. 22 (a) Representative thermal transients obtained from an ischemic and healthy leg. The faster thermal dynamics of the ischemic leg is evidenced. (b) Statistical results of ΔτReffversus ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔSRm, taken over 12 mice. Reproduced from ref. 5 with permission from John Wiley and Sons, copyright 2016. ΔSRm, taken over 12 mice. Reproduced from ref. 5 with permission from John Wiley and Sons, copyright 2016. | ||
Fig. 22b highlights the divergence between the characteristic thermal relaxation times (also termed effective relaxation times) of tissues undergoing ischemic and inflammatory episodes, as seen on the graph's vertical axis. Ischemic tissues generally exhibit a negative effective thermal relaxation time (ΔτReff), while inflammatory tissues show a positive result. The horizontal axis provides additional information, representing the relative modification of the distal area of the surae triceps muscles (ΔSRm) for each tissue type. As expected, tissues undergoing inflammation display larger values than those with ischemia, which typically suffer from atrophy and necrosis, thus reducing muscle size.
Another noteworthy study, also conducted by Ximendes et al.,109 used the core/shell engineering of rare-earth-doped luminescent NPs, specifically Nd3+- and Yb3+-doped LaF3 NPs with active-core/active-shell structures (Nd@Yb core/shell LaF3 NPs). Unlike the previously mentioned study using quantum dots5 that relied on temperature-dependent intensity emission to monitor thermal relaxation time, this study used the temperature dependence of the ratio between two-band emissions, a method known as the ratiometric approach. This method has a significant advantage over simple intensity luminescent thermometry as it is not influenced by environmental factors or experimental setup, including detectors, filters, and sample position.251–253Fig. 23a describes the procedure used for monitoring the thermal relaxation curves by observing the intensity ratio Δ = INd/IYb. This ratio was derived from the intensities emitted by the Nd3+ and Yb3+ ions at 1060 nm and 976 nm, respectively. A laser at 808 nm served as the heating source, while another laser at 790 nm, at a low power of 30 mW, was used to excite the NPs injected into the mice's left limb. The thermal relaxation curve is depicted in Fig. 23b–d. This work introduced the innovative approach of studying fundamental tissue properties under in vivo conditions through subcutaneous thermal relaxation, leveraging the luminescence of injected core/shell NPs. The authors managed to determine one of the most crucial properties related to biological applications – the tissue absorption coefficient. This coefficient is influenced by the fat, water, and melanin content within the small animal being studied. This finding opened new possibilities for understanding and analyzing tissue properties.
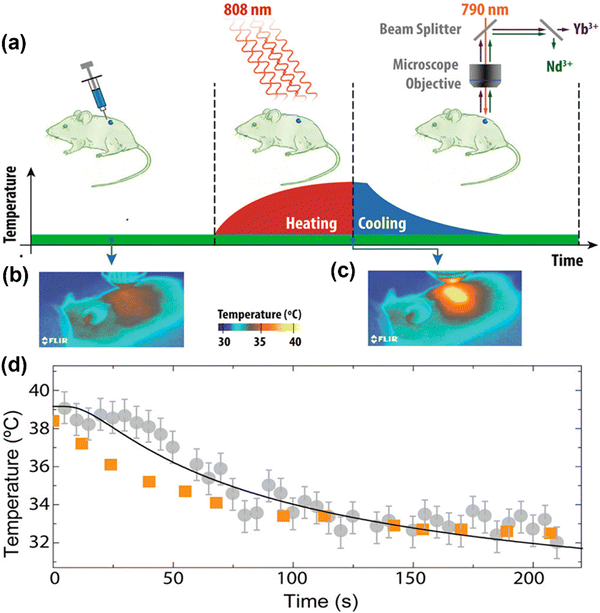 | ||
| Fig. 23 (a) Schematic representation of the subcutaneous thermal relaxation experiments. Thermal infrared images of the CD1 mouse before (b) and at the end (c) of the heating stimulus. (d) Time evolution of the temperatures measured by the subcutaneous luminescent thermometer (gray) and the IR thermal camera (orange). Reproduced from ref. 109 with permission from American Chemical Society, copyright 2016. | ||
In another groundbreaking research effort, designed by the same research groups mentioned before, this time led by Santos et al.,2 the team conducted in vivo experiments on murine model melanoma induced in C75Bl/6 mice. Again, thermal relaxation dynamics was the main tool used to monitor the progression of the disease from its onset to the final stage (where tumor necrosis was evident). The luminescent NPs used in this work were Ag2S NPs. Their remarkable properties and aspects have been discussed previously in Section 3.1.3. As discussed in that section, the changes in thermal relaxation dynamics as the tumor develops allowed for early tumor detection. This detection occurred several days before the tumor could be visually identified by optical inspection (seven days) or even surface temperature readings (six days) using the IR thermography technique. This highlights the potential of thermal relaxation dynamics as a promising early detection method for various types of tumor.
In summary, thermal relaxation dynamics is capable of detecting a tumor at a very early stage and of providing an initial diagnosis by distinguishing two different stages of tumor growth. The first stage, at the onset of the development of the tumor, is known as angiogenesis. This is marked by high metabolic activity and, consequently, a high blood perfusion rate in the tumor volume and periphery. This stage can be easily identified through thermal relaxation dynamics, as this process is highly dependent on the rate of blood perfusion. The second stage, occurring at more advanced stages of tumor development, is defined as necrosis. This stage is characterized by a significant reduction in intratumoral blood perfusion and metabolic activity, changes that are readily detected by thermal relaxation dynamics of the tissues. In conclusion, thermal relaxation dynamics has great potential to be used as a diagnostic tool in the early detection and monitoring of cancerous tumors.
5.3. PTT hyperthermia protocols
In this section, we will provide an overview of PTT hyperthermia protocols, highlighting their key aspects and potential implications. The following characteristics of PTT agents are important to consider: appropriate absorption efficiency, cross-sectional area, excellent photothermal conversion efficiency, long-term photothermal stability, biocompatibility, low toxicity, biosafety, and ease of surface modification.254 First, a crucial aspect to discuss concerns the precise control of the light irradiation parameters, such as wavelength, light intensity, and exposure time. By adjusting these parameters, researchers can achieve localized and controlled heating in the tumor region. NIR light is commonly used because it can penetrate biological tissues without causing significant damage. This was previously addressed in sections of this work that discuss the concept of biological windows.Determining the optimum light intensity and exposure time for PTT, hyperthermia requires consideration of several factors, including the properties of the NPs used, the targeted tissue, and the desired therapeutic outcome. The optimum source intensity for PTT can vary depending on the absorption characteristics of the NPs and the target tissue. It is typically determined by the balance between achieving sufficient heat generation within the target area and avoiding excessive heating that could lead to tissue damage or thermal ablation. In the same way, preclinical studies and experimental evaluations are typically performed to establish the optimum exposure time for PTT. These studies involve subjecting NPs-treated tissues or cells to laser irradiation for varying durations and monitoring the resulting temperature changes and therapeutic outcomes. As an example, we can cite the study titled “Tumor acidity and near-infrared light responsive drug delivery MoS2-based nanoparticles for chemo-photothermal therapy”, in which the authors investigated the photothermal properties of MoS2/PDA-TPP.255 For assessing these properties, MoS2/PDA-TPP NPs and a PBS solution were separately placed in quartz cuvettes. Subsequently, they were irradiated with NIR 808 nm light at a intensity of 0.5 W cm−2 for 10 minutes. The temperature of each solution was measured using a microprobe thermocouple at intervals of 30-second. Furthermore, the researchers examined the photothermal killing effect of MoS2/PDA-TPP on A549 cells. The survival rate of the cells was evaluated after being exposed to irradiation for 0, 5, 10, 15, and 20 minutes under the 808 nm LED cold light lamp with a light intensity of 0.5 W cm−2. This analysis aimed to determine the impact of varying the duration of irradiation on the viability of A549 cells. The values for light intensity and exposure time cited here are only to provide a general idea of how these studies are conducted.
Another important aspect to highlight pertains to the choice of light-absorbing agents. Various agents, including gold NPs, carbon nanotubes, and organic dyes, have been explored for use in PTT.256–258 A key feature of these agents is their ability to absorb and emit in the NIR region, allowing deep tissue penetration while minimizing damage to healthy tissues. Furthermore, for biological applications, these agents must be biocompatible. Size is another crucial parameter, as incorporation into cells requires small dimensions. The optimum size of a nanoparticle is influenced by several factors related to the therapy's principles and practical considerations. Although there is no strict upper limit for NPs size in PTT, smaller NPs are generally favored because of their advantages in achieving efficient heat generation and effective tumor targeting. For example, the smaller size of nanocarriers, typically less than 200 nm, provides several advantages for passive targeting of tumor cells.254 This is achieved through the enhanced permeation and retention (EPR) effect, which exploits tumor vasculature and microenvironment characteristics. The reduced size facilitates a prolonged circulation of the nanocarriers in the bloodstream, allowing them to reach the leaky vasculature of the tumor microenvironment. As a result, the formulation accumulates at the tumor site, enhancing the delivery of photothermal therapeutic agents for effective treatment.
It is important to note that size-dependent considerations exist in PTT. For instance, extremely small NPs (e.g., below 10 nm) may exhibit limited light absorption capacity, potentially compromising their ability to generate sufficient heat. Conversely, very large NPs may encounter challenges related to their clearance from the body, potential aggregation, and limited penetration into tumor tissues. In summary, while there is no strict maximum size limit for NPs used in PTT, smaller NPs within the 10 to 200 nm range are commonly preferred. These smaller NPs offer advantages such as improved tumor targeting, efficient heat generation, and enhanced therapeutic outcomes. By striking a balance between size and functionality, these NPs demonstrate better performance in PTT applications. In addition, surface modification is also a crucial aspect to consider since the accumulation of nanocarriers at the tumor site is further facilitated by active targeting through surface modification. Introducing a specific ligand on the carrier surface allows targeting tumor cells that overexpress specific receptors.259,260
Additionally, imaging techniques, such as fluorescence or photoacoustic imaging, can be employed to visualize the distribution and accumulation of light-absorbing agents in real-time.261,262 This allows selective accumulation in tumor tissues, maximizing the therapeutic efficacy while minimizing off-target effects. For example, Rong Wu et al. successfully developed a MXene-based composite nanoplatform for multiple imaging-guided photothermal hyperthermia of cancer.262 In their work, the photothermal conversion performance of MnOx/Ta4C3 composite nanosheets facilitated contrast-enhanced photoacoustic imaging and significantly suppressed tumor growth through photothermal hyperthermia.
As mentioned in this study, precise monitoring of the temperature distribution during PTT plays a crucial role in achieving optimal heat generation while minimizing harm to healthy tissues. In this regard, non-invasive techniques like magnetic resonance thermometry can be employed for real-time temperature monitoring and control.263 Despite the promising results observed in preclinical studies, the successful translation of PTT hyperthermia protocols into clinical practice requires addressing several challenges. These challenges encompass the standardization of protocols, optimization of light-absorbing agents, comprehensive long-term safety assessments, and the scalability of manufacturing processes. Furthermore, further elucidation of the intricate interplay between tumor biology, immune response, and treatment parameters is necessary to improve treatment outcomes. In addition, before clinical translation, thorough evaluations of safety considerations such as potential phototoxicity, particle stability, and long-term biocompatibility of light-absorbing agents are imperative.
In conclusion, the utilization of hyperthermia protocols via PTT presents a promising avenue for cancer treatment that is targeted and minimally invasive. Ongoing advancements in the field, including the development of improved light-absorbing agents, refined tumor targeting strategies, the exploration of combination therapies, and the implementation of sophisticated treatment monitoring techniques, collectively driving the potential clinical translation of these protocols. However, further research and development efforts are necessary to tackle existing challenges and optimize these protocols, ensuring their safety and efficacy when applied to cancer patients. The continued exploration and refinement of this field holds great potential to improve cancer treatment outcomes in the future.
6. Summary and future directions
This review has highlighted the role, significance, properties, and applications of NPs in thermal therapy, particularly their use in image-guided interventions and temperature monitoring. Employing magnetic NPs for biomedical applications greatly benefits the rapid emergence, improvements, and development of nanotechnology. They show promise as versatile foundations for cancer therapeutic agents when paired with MRI and function as both therapeutic and diagnostic agents for more targeted drug delivery and hyperthermia therapy while providing real-time monitoring of the treatment process and its progress. These technologies are being improved through recent and ongoing studies to bring them closer to clinical implementations. On one hand, NPs with a specific chemical composition and a controlled size distribution can be effectively synthesized by choosing the optimal experimental conditions. On the other hand, modifications can be done, making them highly target-specific and designed to work efficiently for their target applications. These functionalized NPs that offer high selectivity, sensitivity, and possess excellent physio-chemical properties are considered superior not only over traditionally used methods but also in the field of targeted drug delivery and hyperthermia treatment since increasing their specificity might be able to prevent the occurrence of damage brought by high temperatures to healthy surrounding tissues during the treatment process. The use of these is not only limited to therapeutic processes but also in the field of diagnostics. It can be effectively used for magnetic particle imaging for MRI as a contrast agent and to monitor and visualize temperature changes and distribution using thermometry. Although many promising developments and successful studies uplift NPs for cancer theranostics, as described in this paper, more extensive studies on these should still be run as numerous challenges and issues remain unresolved. For instance, clinical trials still need to be more restricted due to toxicity and biocompatibility issues. Another one is to address and consider the different factors that affect the effect of functionalized NPs on hyperthermia and thermometry, such as its composition, size, structure, surface modification, and interactions, as well as the role of external factors, such as the applied magnetic field, excitation/emission wavelengths and host/target environment.Indeed, magnetic NPs show promising potential for hyperthermia therapy and MRI thermometry, but there are still some challenges to be addressed and factors to be considered. Among included are optimizing the design of NPs in terms of choosing the optimal chemical composition, size, morphology, and structure and also including their modification, such as optimal surface coatings and ligands, ensuring safety through biocompatibility since these NPs may induce immune responses or cause toxicity which may further complicate or prevent their target applications, limiting or avoiding non-specific accumulation since accumulation can lead to uneven distribution within the target area potentially affecting the therapeutic process, and effectively targeting and delivering the therapeutics to the target site since non-specificity may lead to unintended heating and damage to healthy tissues. Other factors considered are the limited penetration of the magnetic field, inefficient heat dissipation, unprecise control and non-uniformity of the temperature, and host/environment stimulus since responses to hyperthermia therapy can vary based on factors such as cancer type, its microenvironment, and genetics. Predicting and optimizing patient responses to hyperthermia treatment using functionalized NPs remains a complex challenge, as well as their long-term effects on the body. Clinical validation and pre- and post-therapy assessments are considered significant, before performing any clinical implementations. Therefore, for more NPs to be approved for clinical implementations and widely used in clinical practice, more in vivo experiments and trials on animals should be done, and their long-term effects should be assessed. Other challenges include the reduction of inter-batch variability of the synthesized NPs, ensuring efficient large-scale production, and standardizing its storage and retrieval conditions. Marketed NPs for cancer theranostics must be tested in every batch as produced to ensure no significant batch-to-batch variability. Addressing these challenges requires interdisciplinary collaboration between researchers and clinical professionals, but despite the said challenges, recent and ongoing research is now in progress to overcome these challenges and further develop NP-based hyperthermia therapy as a safe and effective treatment option for cancer patients. Additionally, creating multifunctional NPs that, for instance, can deliver drugs while undergoing hyperthermia treatment or combine imaging with thermometry is of great interest in ongoing and current research studies. This is one of the future directions of creating smart nanomaterials for theranostic purposes. This is in addition to developing multifunctional NPs with optimal properties and features of wide versatility, better bioavailability, biocompatibility, and targeted drug delivery. Due to widespread large-scale production, future improvements in production and storage processes also ensure product quality, safety, and lower-cost production. Such developmental approaches and future perspectives have several benefits and would help us advance the use of theranostics in treating oncogenic diseases.
Author contributions
C. Jacinto: conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing. W. F. Silva: data curation, writing original draft. J. Garcia: conceptualization, methodology, project administration, writing original draft. G. P. Zaragosa: data curation, writing original draft. C. N. D. Ilem: data curation, writing original draft. T. O. Sales: data curation, writing original draft. H. D. A. Santos: data curation, writing original draft. B. I. C. Conde: data curation, writing original draft. H. P. Barbosa: data curation, writing original draft. S. Malik: conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing. S. K. Sharma: conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing. All authors have contributed to original draft writing and revision of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from Brazilian Agencies: FINEP (Financiadora de Estudos e Projetos); CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through the grants: scholarship in Research Productivity 1C under the Nr. 304967/2018-1 (C. J.) and Process Nr. 408179/2023-6 (C. J.). FAPEAL (Fundação de Amparo à Pesquisa do Estado de Alagoas through the grant E:60030.0000000161/2022 (C. J.). The researches of T. O. Sales are supported by a Post-Doctoral Fellowship grant of the PNPD-CAPES project – CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Ensino Superior). S. K. S. is very thankful to UGC-DAE Consortium for Scientific Research, India for providing financial support (CSR-IC-ISUM-54/CRS-337/2020-21/795).References
- S. M. Frank, Perioperative Maintenance of Normothermia Reduces the Incidence of Morbid Cardiac Events, JAMA, 1997, 277, 1127, DOI:10.1001/jama.1997.03540380041029.
- H. D. A. Santos, E. C. Ximendes, M. D. C. Iglesias-de la Cruz, I. Chaves-Coira, B. del Rosal, C. Jacinto, L. Monge, I. Rubia-Rodríguez, D. Ortega, S. Mateos, J. GarcíaSolé, D. Jaque and N. Fernández, In Vivo Early Tumor Detection and Diagnosis by Infrared Luminescence Transient Nanothermometry, Adv. Funct. Mater., 2018, 28, 1803924, DOI:10.1002/adfm.201803924.
- A. M. Stark and S. Way, The use of thermovision in the detection of early breast cancer, Cancer, 1974, 33, 1664–1670, DOI:10.1002/1097-0142(197406)33:6<1664::AID-CNCR2820330629>3.0.CO;2-7.
- D. H. Ortgies, Á. L. García-Villalón, M. Granado, S. Amor, E. M. Rodríguez, H. D. A. Santos, J. Yao, J. Rubio-Retama and D. Jaque, Infrared fluorescence imaging of infarcted hearts with Ag2S nanodots, Nano Res., 2019, 1–9, DOI:10.1007/s12274-019-2280-4.
- E. C. Ximendes, U. Rocha, B. del Rosal, A. Vaquero, F. Sanz-Rodríguez, L. Monge, F. Ren, F. Vetrone, D. Ma, J. García-Solé, C. Jacinto, D. Jaque and N. Fernández, In Vivo Ischemia Detection by Luminescent Nanothermometers, Adv. Healthcare Mater., 2017, 6, 1601195, DOI:10.1002/adhm.201601195.
- D. Jaque, L. Martínez Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martín Rodríguez and J. García Solé, Nanoparticles for photothermal therapies, Nanoscale, 2014, 6, 9494–9530, 10.1039/C4NR00708E.
- D. Jaque, C. Richard, B. Viana, K. Soga, X. Liu and J. G. Solé, Inorganic nanoparticles for optical bioimaging, Adv. Opt. Photonics, 2016, 8, 1–103, DOI:10.1364/AOP.8.000001.
- V. E. Del Bene, Chapter 218 – Temperature, in Clinical Methods: The History, Physical, and Laboratory Examinations, ed. H. Kenneth Walker, W. Dallas Hall and J. Willis Hurst, Butterworths, Boston, 3rd edn, 1990 Search PubMed.
- M. Sund-Levander, C. Forsberg and L. K. Wahren, Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review, Scand. J. Caring Sci., 2002, 16, 122–128, DOI:10.1046/j.1471-6712.2002.00069.x.
- W. Chen, Thermometry and interpretation of body temperature, Biomed. Eng. Lett., 2019, 9, 3–17, DOI:10.1007/s13534-019-00102-2.
- I. I. Geneva, B. Cuzzo, T. Fazili and W. Javaid, Normal Body Temperature: A Systematic Review, Open Forum Infect. Dis., 2019, 6, ofz032, DOI:10.1093/ofid/ofz032.
- K. L. McKinley, M. T. Longaker and S. Naik, Emerging frontiers in regenerative medicine, Science, 1979, 380(2023), 796–798, DOI:10.1126/science.add6492.
- K. Dzobo, N. E. Thomford, D. A. Senthebane, H. Shipanga, A. Rowe, C. Dandara, M. Pillay and K. S. C. M. Motaung, Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine, Stem Cells Int., 2018, 2018, 1–24, DOI:10.1155/2018/2495848.
- A. Bhattacharyya, M. R. Khatun, S. Narmatha, R. Nagarajan and I. Noh, Modulation of 3D Bioprintability in Polysaccharide Bioink by Bioglass Nanoparticles and Multiple Metal Ions for Tissue Engineering, Tissue Eng. Regener. Med., 2024, 21, 261–275, DOI:10.1007/s13770-023-00605-1.
- M. J. Nirmala, U. Kizhuveetil, A. Johnson, G. Balaji, R. Nagarajan and V. Muthuvijayan, Cancer nanomedicine: a review of nano-therapeutics and challenges ahead, RSC Adv., 2023, 13, 8606–8629, 10.1039/D2RA07863E.
- S. R. Dash and C. N. Kundu, Advances in nanomedicine for the treatment of infectious diseases caused by viruses, Biomater. Sci., 2023, 11, 3431–3449, 10.1039/D2BM02066A.
- J. Kim, N. Lee and T. Hyeon, Recent development of nanoparticles for molecular imaging, Philos. Trans. R. Soc., A, 2017, 375, 20170022, DOI:10.1098/rsta.2017.0022.
- O. Afzal, A. S. A. Altamimi, M. S. Nadeem, S. I. Alzarea, W. H. Almalki, A. Tariq, B. Mubeen, B. N. Murtaza, S. Iftikhar, N. Riaz and I. Kazmi, Nanoparticles in Drug Delivery: From History to Therapeutic Applications, Nanomaterials, 2022, 12, 4494, DOI:10.3390/nano12244494.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H.-S. Shin, Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16, 71, DOI:10.1186/s12951-018-0392-8.
- M. Ramesh, R. Janani, C. Deepa and L. Rajeshkumar, Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications, Biosensors, 2022, 13, 40, DOI:10.3390/bios13010040.
- Y. Nakamura, A. Mochida, P. L. Choyke and H. Kobayashi, Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?, Bioconjugate Chem., 2016, 27, 2225–2238, DOI:10.1021/acs.bioconjchem.6b00437.
- F. Yuan, M. Dellian, D. Fukumura, M. Leunig, D. A. Berk, V. P. Torchilin and R. K. Jain, Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size, Cancer Res., 1995, 55, 3752–3756 CAS.
- Y. Matsumura and H. Maeda, A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs, Cancer Res., 1986, 46, 6387–6392 CAS.
- J. Zhou, B. del Rosal, D. Jaque, S. Uchiyama and D. Jin, Advances and challenges for fluorescence nanothermometry, Nat. Methods, 2020, 17, 967–980, DOI:10.1038/s41592-020-0957-y.
- C. D. S. Brites, R. Marin, M. Suta, A. N. Carneiro Neto, E. Ximendes, D. Jaque and L. D. Carlos, Spotlight on Luminescence Thermometry: Basics, Challenges, and Cutting-Edge Applications, Adv. Mater., 2023, 35, 2302749, DOI:10.1002/adma.202302749.
- M. Quintanilla, M. Henriksen-Lacey, C. Renero-Lecuna and L. M. Liz-Marzán, Challenges for optical nanothermometry in biological environments, Chem. Soc. Rev., 2022, 51, 4223–4242, 10.1039/D2CS00069E.
- B. del Rosal, E. Ximendes, U. Rocha and D. Jaque, In Vivo Luminescence Nanothermometry: from Materials to Applications, Adv. Opt. Mater., 2017, 5, 1600508, DOI:10.1002/adom.201600508.
- M. A. R. C. Alencar, G. S. Maciel, C. B. de Araújo and A. Patra, Er3+-doped BaTiO3 nanocrystals for thermometry: Influence of nanoenvironment on the sensitivity of a fluorescence based temperature sensor, Appl. Phys. Lett., 2004, 84, 4753–4755, DOI:10.1063/1.1760882.
- E. C. Ximendes, U. Rocha, T. O. Sales, N. Fernández, F. Sanz-Rodríguez, I. R. Martín, C. Jacinto and D. Jaque, In Vivo Subcutaneous Thermal Video Recording by Supersensitive Infrared Nanothermometers, Adv. Funct. Mater., 2017, 27, 1600508, DOI:10.1002/adfm.201702249.
- E. Carrasco, B. del Rosal, F. Sanz-Rodríguez, Á. J. de la Fuente, P. H. Gonzalez, U. Rocha, K. U. Kumar, C. Jacinto, J. G. Solé and D. Jaque, Intratumoral Thermal Reading During Photo-Thermal Therapy by Multifunctional Fluorescent Nanoparticles, Adv. Funct. Mater., 2015, 25, 615–626, DOI:10.1002/adfm.201403653.
- Y. Shen, H. D. A. Santos, E. C. Ximendes, J. Lifante, A. Sanz-Portilla, L. Monge, N. Fernández, I. Chaves-Coira, C. Jacinto, C. D. S. Brites, L. D. Carlos, A. Benayas, M. C. Iglesias-de la Cruz and D. Jaque, Ag2S Nanoheaters with Multiparameter Sensing for Reliable Thermal Feedback during In Vivo Tumor Therapy, Adv. Funct. Mater., 2020, 30, 2002730, DOI:10.1002/adfm.202002730.
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry, Adv. Opt. Mater., 2019, 7, 1801239, DOI:10.1002/adom.201801239.
- J. P. Knapp, J. E. Kakish, B. W. Bridle and D. J. Speicher, Tumor Temperature: Friend or Foe of Virus-Based Cancer Immunotherapy, Biomedicines, 2022, 10, 2024, DOI:10.3390/biomedicines10082024.
- R. V. Perrella and P. C. de Sousa Filho, High-sensitivity dual UV/NIR-excited luminescence thermometry by rare earth vanadate nanoparticles, Dalton Trans., 2020, 49, 911–922, 10.1039/C9DT04308J.
- Y. Tian, Y. Tian, P. Huang, L. Wang, Q. Shi and C. Cui, Effect of Yb3+ concentration on upconversion luminescence and temperature sensing behavior in Yb3+/Er3+ co-doped YNbO4 nanoparticles prepared via molten salt route, Chem. Eng. J., 2016, 297, 26–34, DOI:10.1016/j.cej.2016.03.149.
- J. R. Shakirova, N. N. Shevchenko, V. A. Baigildin, P. S. Chelushkin, A. F. Khlebnikov, O. A. Tomashenko, A. I. Solomatina, G. L. Starova and S. P. Tunik, Eu-Based Phosphorescence Lifetime Polymer Nanothermometer: A Nanoemulsion Polymerization Approach to Eliminate Quenching of Eu Emission in Aqueous Media, ACS Appl. Polym. Mater., 2020, 2, 537–547, DOI:10.1021/acsapm.9b00952.
- Q. Wang, Z. Mu, S. Zhang, Q. Du, Y. Qian, D. Zhu and F. Wu, Bi3+ and Sm3+ co-doped La2MgGeO6: A novel color-temperature indicator based on different heat quenching behavior from different luminescent centers, J. Lumin., 2019, 206, 462–468, DOI:10.1016/j.jlumin.2018.10.112.
- D. K. Amarasinghe and F. A. Rabuffetti, Bandshift Luminescence Thermometry Using Mn4+:Na4 Mg(WO4)3 Phosphors, Chem. Mater., 2019, 31, 10197–10204, DOI:10.1021/acs.chemmater.9b03886.
- L. Zhou, P. Du, W. Li and L. Luo, Lithium ion doping triggered splendid quantum efficiency and thermal stability in Li2SrSiO4:xEu2+ phosphors for optical thermometry and high luminous efficiency white-LED, New J. Chem., 2019, 43, 16445–16453, 10.1039/C9NJ04102H.
- Q. Wang, M. Liao, Q. Lin, M. Xiong, Z. Mu and F. Wu, A review on fluorescence intensity ratio thermometer based on rare-earth and transition metal ions doped inorganic luminescent materials, J. Alloys Compd., 2021, 850, 156744, DOI:10.1016/j.jallcom.2020.156744.
- H. Montaseri, C. A. Kruger and H. Abrahamse, Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer, Pharmaceutics, 2021, 13, 296, DOI:10.3390/pharmaceutics13030296.
- J. Son, G. Yi, J. Yoo, C. Park, H. Koo and H. S. Choi, Light-responsive nanomedicine for biophotonic imaging and targeted therapy, Adv. Drug Delivery Rev., 2019, 138, 133–147, DOI:10.1016/j.addr.2018.10.002.
- X. Yin, L.-L. Luo, H. Li, X.-Y. Lai, X. Wang and Y.-T. Liu, Theoretical insights on type I/II photoreactions of potential Zn(II) polypyridyl photosensitizers for two-photon photodynamic therapy, Spectrochim. Acta, Part A, 2020, 242, 118771, DOI:10.1016/j.saa.2020.118771.
- Y. Shen, J. Lifante, N. Fernández, D. Jaque and E. Ximendes, In Vivo Spectral Distortions of Infrared Luminescent Nanothermometers Compromise Their Reliability, ACS Nano, 2020, 14, 4122–4133, DOI:10.1021/acsnano.9b08824.
- A. C. C. Soares, T. O. Sales, E. C. Ximendes, D. Jaque and C. Jacinto, Lanthanide doped nanoparticles for reliable and precise luminescence nanothermometry in the third biological window, Nanoscale Adv., 2023, 5, 3664–3670, 10.1039/D2NA00941B.
- E. D. Onal and K. Guven, Plasmonic Photothermal Therapy in Third and Fourth Biological Windows, J. Phys. Chem. C, 2017, 121, 684–690, DOI:10.1021/acs.jpcc.6b10060.
- B. Gong, Y. Shen, H. Li, X. Li, X. Huan, J. Zhou, Y. Chen, J. Wu and W. Li, Thermo-responsive polymer encapsulated gold nanorods for single continuous wave laser-induced photodynamic/photothermal tumour therapy, J. Nanobiotechnol., 2021, 19, 41, DOI:10.1186/s12951-020-00754-8.
- A. S. Thakor and S. S. Gambhir, Nanooncology: The future of cancer diagnosis and therapy, Ca-Cancer J. Clin., 2013, 63, 395–418, DOI:10.3322/caac.21199.
- K. Nigoghossian, S. Ouellet, J. Plain, Y. Messaddeq, D. Boudreau and S. J. L. Ribeiro, Upconversion nanoparticle-decorated gold nanoshells for near-infrared induced heating and thermometry, J. Mater. Chem. B, 2017, 5, 7109–7117, 10.1039/C7TB01621B.
- K. Nigoghossian, Y. Messaddeq, D. Boudreau and S. J. L. Ribeiro, UV and Temperature-Sensing Based on NaGdF4:Yb3+:Er3+@SiO2–Eu(tta)3, ACS Omega, 2017, 2, 2065–2071, DOI:10.1021/acsomega.7b00056.
- P. García Calavia, G. Bruce, L. Pérez-García and D. A. Russell, Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer, Photochem. Photobiol. Sci., 2018, 17, 1534–1552, 10.1039/c8pp00271a.
- M. Sajjadi, M. Nasrollahzadeh, B. Jaleh, G. J. Soufi and S. Iravani, Carbon-based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects, J. Drug Targeting, 2021, 29, 716–741, DOI:10.1080/1061186X.2021.1886301.
- S. Augustine, J. Singh, M. Srivastava, M. Sharma, A. Das and B. D. Malhotra, Recent advances in carbon based nanosystems for cancer theranostics, Biomater. Sci., 2017, 5, 901–952, 10.1039/C7BM00008A.
- B. Saifullah, A. Maitra, A. Chrzastek, B. Naeemullah, S. Fakurazi, S. Bhakta and M. Hussein, Nano-Formulation of Ethambutol with Multifunctional Graphene Oxide and Magnetic Nanoparticles Retains Its Anti-Tubercular Activity with Prospects of Improving Chemotherapeutic Efficacy, Molecules, 2017, 22, 1697, DOI:10.3390/molecules22101697.
- H. Zhao, A. Vomiero and F. Rosei, Tailoring the Heterostructure of Colloidal Quantum Dots for Ratiometric Optical Nanothermometry, Small, 2020, 16, 2000804, DOI:10.1002/smll.202000804.
- S. Yu, J. Xu, X. Shang, W. Zheng, P. Huang, R. Li, D. Tu and X. Chen, A Dual-Excitation Decoding Strategy Based on NIR Hybrid Nanocomposites for High-Accuracy Thermal Sensing, Adv. Sci., 2020, 7, 1–11, DOI:10.1002/advs.202001589.
- M. Quintanilla, Y. Zhang and L. M. Liz-Marzán, Subtissue Plasmonic Heating Monitored with CaF2:Nd3+,Y3+ Nanothermometers in the Second Biological Window, Chem. Mater., 2018, 30, 2819–2828, DOI:10.1021/acs.chemmater.8b00806.
- L. Colombeau, S. Acherar, F. Baros, P. Arnoux, A. M. Gazzali, K. Zaghdoudi, M. Toussaint, R. Vanderesse and C. Frochot, Inorganic Nanoparticles for Photodynamic Therapy, in Light-Responsive Nanostructured Systems for Applications in Nanomedicine, ed. S. Sortino, Springer, Cham, 2016, pp. 113–134 DOI:10.1007/978-3-319-22942-3_4.
- K. Shanmugapriya and H. W. Kang, Engineering pharmaceutical nanocarriers for photodynamic therapy on wound healing: Review, Mater. Sci. Eng., C, 2019, 105, 110110, DOI:10.1016/j.msec.2019.110110.
- A. J. Trinidad, S. J. Hong, Q. Peng, S. J. Madsen and H. Hirschberg, Combined concurrent photodynamic and gold nanoshell loaded macrophage-mediated photothermal therapies: An in vitro study on squamous cell head and neck carcinoma, Lasers Surg. Med., 2014, 46, 310–318, DOI:10.1002/lsm.22235.
- A. Malakar, S. R. Kanel, C. Ray, D. D. Snow and M. N. Nadagouda, Nanomaterials in the environment, human exposure pathway, and health effects: A review, Sci. Total Environ., 2021, 759, 143470, DOI:10.1016/j.scitotenv.2020.143470.
- S. Thomas, B. S. P. Harshita, P. Mishra and S. Talegaonkar, Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery, Curr. Pharm. Des., 2015, 21, 6165–6188, DOI:10.2174/1381612821666151027153246.
- R. Hosseinzadeh and K. Khorsandi, Photodynamic effect of Zirconium phosphate biocompatible nano-bilayers containing methylene blue on cancer and normal cells, Sci. Rep., 2019, 9, 14899, DOI:10.1038/s41598-019-51359-7.
- A. Bhardwaj, N. Jain and K. Parekh, Investigating the effect of outer layer of magnetic particles on cervical cancer cells HeLa by magnetic fluid hyperthermia, Cancer Nanotechnol., 2021, 12, 7, DOI:10.1186/s12645-021-00076-w.
- A. Hervault and N. T. K. Thanh, Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer, Nanoscale, 2014, 6, 11553–11573, 10.1039/C4NR03482A.
- D. Chang, M. Lim, J. A. C. M. Goos, R. Qiao, Y. Y. Ng, F. M. Mansfeld, M. Jackson, T. P. Davis and M. Kavallaris, Biologically Targeted Magnetic Hyperthermia: Potential and Limitations, Front. Pharmacol., 2018, 9, 831, DOI:10.3389/fphar.2018.00831.
- A. Hervault and N. T. K. Thanh, Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer, Nanoscale, 2014, 6, 11553–11573, 10.1039/C4NR03482A.
- B. McCarthy, R. Singh and N. Levi-Polyachenko, Oxaliplatin-resistant colorectal cancer models for nanoparticle hyperthermia, Int. J. Hyperthermia, 2021, 38, 152–164, DOI:10.1080/02656736.2021.1876253.
- T. J. Carter, G. Agliardi, F. Lin, M. Ellis, C. Jones, M. Robson, A. Richard-Londt, P. Southern, M. Lythgoe, M. Zaw Thin, V. Ryzhov, R. T. M. de Rosales, C. Gruettner, M. R. A. Abdollah, R. B. Pedley, Q. A. Pankhurst, T. L. Kalber, S. Brandner, S. Quezada, P. Mulholland, M. Shevtsov and K. Chester, Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation, Small, 2021, 17, 2005241, DOI:10.1002/smll.202005241.
- J. G. Ovejero, F. Spizzo, M. P. Morales and L. Del Bianco, Mixing iron oxide nanoparticles with different shape and size for tunable magneto-heating performance, Nanoscale, 2021, 13, 5714–5729, 10.1039/D0NR09121A.
- M. Ramazanov, A. Karimova and H. Shirinova, Magnetism for Drug Delivery, MRI and Hyperthermia Applications: a Review, Biointerface Res. Appl. Chem., 2020, 11, 8654–8668, DOI:10.33263/BRIAC112.86548668.
- F. Fabris, J. Lohr, E. Lima, A. A. de Almeida, H. E. Troiani, L. M. Rodríguez, M. Vásquez Mansilla, M. H. Aguirre, G. F. Goya, D. Rinaldi, A. Ghirri, D. Peddis, D. Fiorani, R. D. Zysler, E. De Biasi and E. L. Winkler, Adjusting the Néel relaxation time of Fe3O4/ZnxCo1−xFe2O4 core/shell nanoparticles for optimal heat generation in magnetic hyperthermia, Nanotechnology, 2021, 32, 065703, DOI:10.1088/1361-6528/abc386.
- Z. Hedayatnasab, F. Abnisa and W. M. A. W. Daud, Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application, Mater. Des., 2017, 123, 174–196, DOI:10.1016/j.matdes.2017.03.036.
- M. Suciu, C. M. Ionescu, A. Ciorita, S. C. Tripon, D. Nica, H. Al-Salami and L. Barbu-Tudoran, Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements, Beilstein J. Nanotechnol., 2020, 11, 1092–1109, DOI:10.3762/bjnano.11.94.
- A. Espinosa, G. R. Castro, J. Reguera, C. Castellano, J. Castillo, J. Camarero, C. Wilhelm, M. A. García and Á. Muñoz-Noval, Photoactivated Nanoscale Temperature Gradient Detection Using X-ray Absorption Spectroscopy as a Direct Nanothermometry Method, Nano Lett., 2021, 21, 769–777, DOI:10.1021/acs.nanolett.0c04477.
- Z. Zhang, H. Wang, Z. Chen, X. Wang, J. Choo and L. Chen, Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications, Biosens. Bioelectron., 2018, 114, 52–65, DOI:10.1016/j.bios.2018.05.015.
- E. D. Martínez, A. Prado, M. González, S. Anguiano, L. Tosi, L. S. Alarcón and H. Pastoriza, Integrating photoluminescent nanomaterials with photonic nanostructures, J. Lumin., 2021, 233, 117870, DOI:10.1016/j.jlumin.2020.117870.
- J. Son, G. Yi, J. Yoo, C. Park, H. Koo and H. S. Choi, Light-responsive nanomedicine for biophotonic imaging and targeted therapy, Adv. Drug Delivery Rev., 2019, 138, 133–147, DOI:10.1016/j.addr.2018.10.002.
- A. S. Thakor and S. S. Gambhir, Nanooncology: The future of cancer diagnosis and therapy, Ca-Cancer J. Clin., 2013, 63, 395–418, DOI:10.3322/caac.21199.
- M. D’Acunto, P. Cioni, E. Gabellieri and G. Presciuttini, Exploiting gold nanoparticles for diagnosis and cancer treatments, Nanotechnology, 2021, 32, 192001, DOI:10.1088/1361-6528/abe1ed.
- D. Monga, S. Soni, H. Tyagi and R. A. Taylor, Optimization of tumor ablation volume for nanoparticle-mediated thermal therapy, Int. J. Therm. Sci., 2020, 157, 106515, DOI:10.1016/j.ijthermalsci.2020.106515.
- C. Yang, K. Bromma, C. Di Ciano-Oliveira, G. Zafarana, M. van Prooijen and D. B. Chithrani, Gold nanoparticle mediated combined cancer therapy, Cancer Nanotechnol., 2018, 9, 4, DOI:10.1186/s12645-018-0039-3.
- U. Rocha, K. Upendra Kumar, C. Jacinto, J. Ramiro, A. J. Caamano, J. Garcia Sole and D. Jaque, Nd3+ doped LaF3 nanoparticles as self-monitored photo-thermal agents, Appl. Phys. Lett., 2014, 104, 053703, DOI:10.1063/1.4862968.
- E. Carrasco, B. Del Rosal, F. Sanz-Rodríguez, Á. J. De La Fuente, P. H. Gonzalez, U. Rocha, K. U. Kumar, C. Jacinto, J. G. Solé and D. Jaque, Intratumoral thermal reading during photo-thermal therapy by multifunctional fluorescent nanoparticles, Adv. Funct. Mater., 2015, 25, 615–626, DOI:10.1002/adfm.201403653.
- C. Xu and K. Pu, Second near-infrared photothermal materials for combinational nanotheranostics, Chem. Soc. Rev., 2021, 50, 1111–1137, 10.1039/D0CS00664E.
- X. Ge, R. Wei and L. Sun, Lanthanide nanoparticles with efficient near-infrared-II emission for biological applications, J. Mater. Chem. B, 2020, 8, 10257–10270, 10.1039/D0TB01745K.
- I. Martinić, S. V. Eliseeva and S. Petoud, Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials, J. Lumin., 2017, 189, 19–43, DOI:10.1016/j.jlumin.2016.09.058.
- A. Dwivedi, D. Kumar and S. B. Rai, Monochromatic NIR UC emission in Tm3+/Yb3+ co-doped GdVO4 phosphor: the effect of the Bi3+ ion concentration and pump power of a diode laser, Opt. Lett., 2018, 43, 5785, DOI:10.1364/OL.43.005785.
- M. Runowski, P. Woźny, I. R. Martín, V. Lavín and S. Lis, Praseodymium doped YF3:Pr3+ nanoparticles as optical thermometer based on luminescence intensity ratio (LIR) – Studies in visible and NIR range, J. Lumin., 2019, 214, 116571, DOI:10.1016/j.jlumin.2019.116571.
- D. Pominova, V. Proydakova, I. Romanishkin, A. Ryabova, S. Kuznetsov, O. Uvarov, P. Fedorov and V. Loschenov, Temperature Sensing in the Short-Wave Infrared Spectral Region Using Core-Shell NaGdF4:Yb3+, Ho3+, Er3+@NaYF4 Nanothermometers, Nanomaterials, 2020, 10, 1992, DOI:10.3390/nano10101992.
- S. K. Maurya, M. Mohan, R. Poddar, D. Senapati, S. Singh, A. Roy, A. K. Munirathnappa, J. C. G. E. da Silva and K. Kumar, Synthesis of NaGdF4:Er3+/Yb3+ Upconversion Particles as Exogenous Contrast Agent for Swept-Source Optical Coherence Tomography: In Vitro Animal Tissue Imaging, J. Phys. Chem. C, 2020, 124, 18366–18378, DOI:10.1021/acs.jpcc.0c05786.
- F. Vetrone, R. Naccache, A. Zamarrón, A. J. De La Fuente, F. Sanz-Rodríguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Sole and J. A. Capobianco, Temperature sensing using fluorescent nanothermometers, ACS Nano, 2010, 4, 3254–3258, DOI:10.1021/nn100244a.
- P. V. Dos Santos, M. T. De Araujo, A. S. Gouveia-Neto, J. A. M. Neto and A. S. B. Sombra, Optical thermometry through infrared excited upconversion fluorescence emission in Er3+ and Er3+-Yb3+-doped chalcogenide glasses, IEEE J. Quantum Electron., 1999, 35, 395–399, DOI:10.1109/3.748846.
- L. Li, F. Qin, Y. Zheng and Z. Zhang, Comparative study on the up- and down-conversion optical ratiometric thermometry in one phosphor, Mater. Res. Express, 2019, 6, 106203, DOI:10.1088/2053-1591/ab3ecd.
- P. Singh, N. Jain, A. K. Tiwari, S. Shukla, V. Baranwal, J. Singh and A. C. Pandey, Near-infrared light-mediated Er3+ and Yb3+ co-doped CaTi4O9 for optical temperature sensing behavior, J. Lumin., 2021, 233, 117737, DOI:10.1016/j.jlumin.2020.117737.
- S. K. Maurya, R. Kushawaha, S. P. Tiwari, A. Kumar, K. Kumar and J. C. G. E. da Silva, Thermal decomposition mediated Er3+/Yb3+ codoped NaGdF4 upconversion phosphor for optical thermometry, Mater. Res. Express, 2019, 6, 086211, DOI:10.1088/2053-1591/ab20b4.
- S. K. Maurya, J. C. G. Esteves da Silva, M. Mohan, R. Poddar and K. Kumar, Assessment of colloidal NaGdF4:Er3+/Yb3+ upconversion phosphor as contrast enhancer for optical coherence tomography, J. Alloys Compd., 2021, 865, 158737, DOI:10.1016/j.jallcom.2021.158737.
- S. H. Mir, L. A. Nagahara, T. Thundat, P. Mokarian-Tabari, H. Furukawa and A. Khosla, Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies, J. Electrochem. Soc., 2018, 165, B3137–B3156, DOI:10.1149/2.0191808jes.
- K. C. Barick, A. Sharma, N. G. Shetake, R. S. Ningthoujam, R. K. Vatsa, P. D. Babu, B. N. Pandey and P. A. Hassan, Covalent bridging of surface functionalized Fe3O4 and YPO4:Eu nanostructures for simultaneous imaging and therapy, Dalton Trans., 2015, 44, 14686–14696, 10.1039/C5DT01522G.
- E. Alphandéry, Light-Interacting iron-based nanomaterials for localized cancer detection and treatment, Acta Biomater., 2021, 124, 50–71, DOI:10.1016/j.actbio.2021.01.028.
- S. K. Swain, A. Sahoo, S. K. Swain, S. K. Tripathy and G. Phaomei, Synthesis of a novel β-cyclodextrin-functionalized Fe3O4/BaMoO4:Dy3+ magnetic luminescent hybrid nanomaterial and its application as a drug carrier, Dalton Trans., 2020, 49, 14605–14612, 10.1039/D0DT02314K.
- W. Park, H. Shin, B. Choi, W.-K. Rhim, K. Na and D. Keun Han, Advanced hybrid nanomaterials for biomedical applications, Prog. Mater. Sci., 2020, 114, 100686, DOI:10.1016/j.pmatsci.2020.100686.
- D. L. Fritzen, L. Giordano, L. C. V. Rodrigues and J. H. S. K. Monteiro, Opportunities for Persistent Luminescent Nanoparticles in Luminescence Imaging of Biological Systems and Photodynamic Therapy, Nanomaterials, 2020, 10, 2015, DOI:10.3390/nano10102015.
- D. Nakauchi, G. Okada, M. Koshimizu and T. Yanagida, Storage luminescence and scintillation properties of Eu-doped SrAl2O4 crystals, J. Lumin., 2016, 176, 342–346, DOI:10.1016/j.jlumin.2016.04.008.
- M. Lastusaari, H. F. Brito, S. Carlson, J. Hölsä, T. Laamanen, L. C. V. Rodrigues and E. Welter, Valences of dopants in Eu2+ persistent luminescence materials, Phys. Scr., 2014, 89, 044004, DOI:10.1088/0031-8949/89/04/044004.
- J. Zhou, L. Rao, G. Yu, T. R. Cook, X. Chen and F. Huang, Supramolecular cancer nanotheranostics, Chem. Soc. Rev., 2021, 50, 2839–2891, 10.1039/D0CS00011F.
- Y. Zhao, Y. Kuang, M. Liu, J. Wang and R. Pei, Synthesis of Metal–Organic Framework Nanosheets with High Relaxation Rate and Singlet Oxygen Yield, Chem. Mater., 2018, 30, 7511–7520, DOI:10.1021/acs.chemmater.8b02467.
- E. C. Ximendes, U. Rocha, C. Jacinto, K. U. Kumar, D. Bravo, F. J. López, E. M. Rodríguez, J. García-Solé and D. Jaque, Self-monitored photothermal nanoparticles based on core-shell engineering, Nanoscale, 2016, 8, 3057–3066, 10.1039/c5nr08904b.
- E. C. Ximendes, W. Q. Santos, U. Rocha, U. K. Kagola, F. Sanz-Rodríguez, N. Fernández, A. D. S. Gouveia-Neto, D. Bravo, A. M. Domingo, B. Del Rosal, C. D. S. Brites, L. D. Carlos, D. Jaque and C. Jacinto, Unveiling in Vivo Subcutaneous Thermal Dynamics by Infrared Luminescent Nanothermometers, Nano Lett., 2016, 16, 1695–1703, DOI:10.1021/acs.nanolett.5b04611.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv. Drug Delivery Rev., 2016, 99, 28–51, DOI:10.1016/j.addr.2015.09.012.
- Y. Lv, Y. Yuan, N. Hu, N. Jin, D. Xu, R. Wu, H. Shen, O. Chen and L. S. Li, Thick-Shell CdSe/ZnS/CdZnS/ZnS Core/Shell Quantum Dots for Quantitative Immunoassays, ACS Appl. Nano Mater., 2021, 4, 2855–2865, DOI:10.1021/acsanm.0c03483.
- J.-H. Lee, Y. Shin, W. Lee, K. Whang, D. Kim, L. P. Lee, J.-W. Choi and T. Kang, General and programmable synthesis of hybrid liposome/metal nanoparticles, Sci. Adv., 2016, 2, e1601838, DOI:10.1126/sciadv.1601838.
- L. Liu, H.-J. Xie, L.-M. Mu, R. Liu, Z.-B. Su, Y.-N. Cui, Y. Xie and W.-L. Lu, Functional chlorin gold nanorods enable to treat breast cancer by photothermal/photodynamic therapy, Int. J. Nanomed., 2018, 13, 8119–8135, DOI:10.2147/IJN.S186974.
- C. Shan, Y. Huang, J. Wei, M. Chen and L. Wu, Ultra-high thermally stable gold nanorods/radial mesoporous silica and their application in enhanced chemo-photothermal therapy, RSC Adv., 2021, 11, 10416–10424, 10.1039/D1RA00213A.
- C. He, X. Duan, N. Guo, C. Chan, C. Poon, R. R. Weichselbaum and W. Lin, Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy, Nat. Commun., 2016, 7, 12499, DOI:10.1038/ncomms12499.
- G. Ramírez-García, E. De la Rosa, T. López-Luke, S. S. Panikar and P. Salas, Controlling trapping states on selective theranostic core@shell (NaYF4:Yb,Tm@TiO2-ZrO2) nanocomplexes for enhanced NIR-activated photodynamic therapy against breast cancer cells, Dalton Trans., 2019, 48, 9962–9973, 10.1039/C9DT00482C.
- N. Zhao, L. Yan, X. Zhao, X. Chen, A. Li, D. Zheng, X. Zhou, X. Dai and F.-J. Xu, Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications, Chem. Rev., 2019, 119, 1666–1762, DOI:10.1021/acs.chemrev.8b00401.
- L. Huang, S. Zhao, F. Fang, T. Xu, M. Lan and J. Zhang, Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy, Biomaterials, 2021, 268, 120557, DOI:10.1016/j.biomaterials.2020.120557.
- K. Ulbrich, K. Holá, V. Šubr, A. Bakandritsos, J. Tuček and R. Zbořil, Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies, Chem. Rev., 2016, 116, 5338–5431, DOI:10.1021/acs.chemrev.5b00589.
- M. Danaei, M. Dehghankhold, S. Ataei, F. Hasanzadeh Davarani, R. Javanmard, A. Dokhani, S. Khorasani and M. Mozafari, Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems, Pharmaceutics, 2018, 10, 57, DOI:10.3390/pharmaceutics10020057.
- N. Bertrand and J.-C. Leroux, The journey of a drug-carrier in the body: An anatomo-physiological perspective, J. Controlled Release, 2012, 161, 152–163, DOI:10.1016/j.jconrel.2011.09.098.
- P. Eriksson, A. A. Tal, A. Skallberg, C. Brommesson, Z. Hu, R. D. Boyd, W. Olovsson, N. Fairley, I. A. Abrikosov, X. Zhang and K. Uvdal, Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement, Sci. Rep., 2018, 8, 6999, DOI:10.1038/s41598-018-25390-z.
- J. Garcia, S. Z. Liu and A. Y. Louie, Biological effects of MRI contrast agents: gadolinium retention, potential mechanisms and a role for phosphorus, Philos. Trans. R. Soc., A, 2017, 375, 20170180, DOI:10.1098/rsta.2017.0180.
- Z. Shen, J. Song, Z. Zhou, B. C. Yung, M. A. Aronova, Y. Li, Y. Dai, W. Fan, Y. Liu, Z. Li, H. Ruan, R. D. Leapman, L. Lin, G. Niu, X. Chen and A. Wu, Dotted Core–Shell Nanoparticles for T1-Weighted MRI of Tumors, Adv. Mater., 2018, 30, 1803163, DOI:10.1002/adma.201803163.
- F. Li, D. Zhi, Y. Luo, J. Zhang, X. Nan, Y. Zhang, W. Zhou, B. Qiu, L. Wen and G. Liang, Core/shell Fe3O4/Gd2O3 nanocubes as T1–T2 dual modal MRI contrast agents, Nanoscale, 2016, 8, 12826–12833, 10.1039/C6NR02620F.
- H. Nosrati, M. Tarantash, S. Bochani, J. Charmi, Z. Bagheri, M. Fridoni, M. A. Abdollahifar, S. Davaran, H. Danafar and H. Kheiri Manjili, Glutathione (GSH) Peptide Conjugated Magnetic Nanoparticles As Blood-Brain Barrier Shuttle for MRI-Monitored Brain Delivery of Paclitaxel, ACS Biomater. Sci. Eng., 2019, 5, 1677–1685, DOI:10.1021/acsbiomaterials.8b01420.
- Z. Wang, R. Qiao, N. Tang, Z. Lu, H. Wang, Z. Zhang, X. Xue, Z. Huang, S. Zhang, G. Zhang and Y. Li, Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer, Biomaterials, 2017, 127, 25–35, DOI:10.1016/j.biomaterials.2017.02.037.
- A. A. A. Ali, F.-T. Hsu, C.-L. Hsieh, C.-Y. Shiau, C.-H. Chiang, Z.-H. Wei, C.-Y. Chen and H.-S. Huang, Erlotinib-Conjugated Iron Oxide Nanoparticles as a Smart Cancer-Targeted Theranostic Probe for MRI, Sci. Rep., 2016, 6, 36650, DOI:10.1038/srep36650.
- X. Lin, Y. Cao, J. Li, D. Zheng, S. Lan, Y. Xue, F. Yu, M. Wu and X. Zhu, Folic acid-modified Prussian blue/polydopamine nanoparticles as an MRI agent for use in targeted chemo/photothermal therapy, Biomater. Sci., 2019, 7, 2996–3006, 10.1039/C9BM00276F.
- J. Garcia, T. Tang and A. Y. Louie, Nanoparticle-based multimodal PET/MRI probes, Nanomedicine, 2015, 10, 1343–1359, DOI:10.2217/nnm.14.224.
- Y. Du, X. Liu, Q. Liang, X.-J. Liang and J. Tian, Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy, Nano Lett., 2019, 19, 3618–3626, DOI:10.1021/acs.nanolett.9b00630.
- Y. Du, X. Liu, Q. Liang, X.-J. Liang and J. Tian, Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy, Nano Lett., 2019, 19, 3618–3626, DOI:10.1021/acs.nanolett.9b00630.
- A. A. A. Ali, F.-T. Hsu, C.-L. Hsieh, C.-Y. Shiau, C.-H. Chiang, Z.-H. Wei, C.-Y. Chen and H.-S. Huang, Erlotinib-Conjugated Iron Oxide Nanoparticles as a Smart Cancer-Targeted Theranostic Probe for MRI, Sci. Rep., 2016, 6, 36650, DOI:10.1038/srep36650.
- S. He, N. J. J. Johnson, V. A. Nguyen Huu, E. Cory, Y. Huang, R. L. Sah, J. V. Jokerst and A. Almutairi, Simultaneous Enhancement of Photoluminescence, MRI Relaxivity, and CT Contrast by Tuning the Interfacial Layer of Lanthanide Heteroepitaxial Nanoparticles, Nano Lett., 2017, 17, 4873–4880, DOI:10.1021/acs.nanolett.7b01753.
- H. Nosrati, M. Tarantash, S. Bochani, J. Charmi, Z. Bagheri, M. Fridoni, M.-A. Abdollahifar, S. Davaran, H. Danafar and H. Kheiri Manjili, Glutathione (GSH) Peptide Conjugated Magnetic Nanoparticles As Blood–Brain Barrier Shuttle for MRI-Monitored Brain Delivery of Paclitaxel, ACS Biomater. Sci. Eng., 2019, 5, 1677–1685, DOI:10.1021/acsbiomaterials.8b01420.
- J. Garcia, J. Neelavalli, E. M. Haacke and M. J. Allen, EuII-containing cryptates as contrast agents for ultra-high field strength magnetic resonance imaging, Chem. Commun., 2011, 47, 12858, 10.1039/c1cc15219j.
- H. Wei, O. T. Bruns, M. G. Kaul, E. C. Hansen, M. Barch, A. Wiśniowska, O. Chen, Y. Chen, N. Li, S. Okada, J. M. Cordero, M. Heine, C. T. Farrar, D. M. Montana, G. Adam, H. Ittrich, A. Jasanoff, P. Nielsen and M. G. Bawendi, Exceedingly small iron oxide nanoparticles as positive MRI contrast agents, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 2325–2330, DOI:10.1073/pnas.1620145114.
- P. I. P. Soares, C. A. T. Laia, A. Carvalho, L. C. J. Pereira, J. T. Coutinho, I. M. M. Ferreira, C. M. M. Novo and J. P. Borges, Iron oxide nanoparticles stabilized with a bilayer of oleic acid for magnetic hyperthermia and MRI applications, Appl. Surf. Sci., 2016, 383, 240–247, DOI:10.1016/j.apsusc.2016.04.181.
- R. Martínez-González, J. Estelrich and M. Busquets, Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI, Int. J. Mol. Sci., 2016, 17, 1209, DOI:10.3390/ijms17081209.
- B. Li, Z. Gu, N. Kurniawan, W. Chen and Z. P. Xu, Manganese-Based Layered Double Hydroxide Nanoparticles as a T1-MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity, Adv. Mater., 2017, 29, 1700373, DOI:10.1002/adma.201700373.
- M. L. James and S. S. Gambhir, A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications, Physiol. Rev., 2012, 92, 897–965, DOI:10.1152/physrev.00049.2010.
- V. Ntziachristos, Going deeper than microscopy: the optical imaging frontier in biology, Nat. Methods, 2010, 7, 603–614, DOI:10.1038/nmeth.1483.
- S. H. Yun and S. J. J. Kwok, Light in diagnosis, therapy and surgery, Nat. Biomed. Eng., 2017, 1, 0008, DOI:10.1038/s41551-016-0008.
- A. M. Smith, M. C. Mancini and S. Nie, Second window for in vivo imaging, Nat. Nanotechnol., 2009, 4, 710–711, DOI:10.1038/nnano.2009.326.
- G. Hong, S. Diao, J. Chang, A. L. Antaris, C. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo and H. Dai, Through-skull fluorescence imaging of the brain in a new near-infrared window, Nat. Photonics, 2014, 8, 723–730, DOI:10.1038/nphoton.2014.166.
- D. Ghosh, A. F. Bagley, Y. J. Na, M. J. Birrer, S. N. Bhatia and A. M. Belcher, Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 13948–13953, DOI:10.1073/pnas.1400821111.
- O. T. Bruns, T. S. Bischof, D. K. Harris, D. Franke, Y. Shi, L. Riedemann, A. Bartelt, F. B. Jaworski, J. A. Carr, C. J. Rowlands, M. W. B. Wilson, O. Chen, H. Wei, G. W. Hwang, D. M. Montana, I. Coropceanu, O. B. Achorn, J. Kloepper, J. Heeren, P. T. C. So, D. Fukumura, K. F. Jensen, R. K. Jain and M. G. Bawendi, Next-generation in vivo optical imaging with short-wave infrared quantum dots, Nat. Biomed. Eng., 2017, 1, 0056, DOI:10.1038/s41551-017-0056.
- W. Shao, G. Chen, A. Kuzmin, H. L. Kutscher, A. Pliss, T. Y. Ohulchanskyy and P. N. Prasad, Tunable Narrow Band Emissions from Dye-Sensitized Core/Shell/Shell Nanocrystals in the Second Near-Infrared Biological Window, J. Am. Chem. Soc., 2016, 138, 16192–16195, DOI:10.1021/jacs.6b08973.
- G. Chen, F. Tian, Y. Zhang, Y. Zhang, C. Li and Q. Wang, Tracking of Transplanted Human Mesenchymal Stem Cells in Living Mice using Near-Infrared Ag2S Quantum Dots, Adv. Funct. Mater., 2014, 24, 2481–2488, DOI:10.1002/adfm.201303263.
- X. Dang, L. Gu, J. Qi, S. Correa, G. Zhang, A. M. Belcher and P. T. Hammond, Layer-by-layer assembled fluorescent probes in the second near-infrared window for systemic delivery and detection of ovarian cancer, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 5179–5184, DOI:10.1073/pnas.1521175113.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, A small-molecule dye for NIR-II imaging, Nat. Mater., 2016, 15, 235–242, DOI:10.1038/nmat4476.
- M. H. Wong, J. P. Giraldo, S.-Y. Kwak, V. B. Koman, R. Sinclair, T. T. S. Lew, G. Bisker, P. Liu and M. S. Strano, Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics, Nat. Mater., 2017, 16, 264–272, DOI:10.1038/nmat4771.
- Y. Sun, M. Ding, X. Zeng, Y. Xiao, H. Wu, H. Zhou, B. Ding, C. Qu, W. Hou, A. Er-bu, Y. Zhang, Z. Cheng and X. Hong, Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery, Chem. Sci., 2017, 8, 3489–3493, 10.1039/C7SC00251C.
- S. L. Jacques, Optical properties of biological tissues: a review, Phys. Med. Biol., 2013, 58, R37–R61, DOI:10.1088/0031-9155/58/11/R37.
- A. N. Bashkatov, E. A. Genina, V. I. Kochubey and V. V. Tuchin, Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm, J. Phys. D: Appl. Phys., 2005, 38, 2543–2555, DOI:10.1088/0022-3727/38/15/004.
nm, J. Phys. D: Appl. Phys., 2005, 38, 2543–2555, DOI:10.1088/0022-3727/38/15/004. - D. J. Naczynski, M. C. Tan, M. Zevon, B. Wall, J. Kohl, A. Kulesa, S. Chen, C. M. Roth, R. E. Riman and P. V. Moghe, Rare-earth-doped biological composites as in vivo shortwave infrared reporters, Nat. Commun., 2013, 4, 2199, DOI:10.1038/ncomms3199.
- J. Lifante, Y. Shen, E. Ximendes, E. Martín Rodríguez and D. H. Ortgies, The role of tissue fluorescence in vivo optical bioimaging, J. Appl. Phys., 2020, 128, 171101, DOI:10.1063/5.0021854.
- B. del Rosal and A. Benayas, Strategies to Overcome Autofluorescence in Nanoprobe-Driven In Vivo Fluorescence Imaging, Small Methods, 2018, 2, 1800075, DOI:10.1002/smtd.201800075.
- D. H. Ortgies and E. Martín Rodríguez, Near Infrared-Emitting Bioprobes for Low-Autofluorescence Imaging Techniques, Near Infrared-Emitting Nanoparticles for Biomedical Applications, Springer International Publishing, Cham, 2020, pp. 199–229 DOI:10.1007/978-3-030-32036-2_9.
- Y. Gu, Z. Guo, W. Yuan, M. Kong, Y. Liu, Y. Liu, Y. Gao, W. Feng, F. Wang, J. Zhou, D. Jin and F. Li, High-sensitivity imaging of time-domain near-infrared light transducer, Nat. Photonics, 2019, 13, 525–531, DOI:10.1038/s41566-019-0437-z.
- M. Tan, B. del Rosal, Y. Zhang, E. Martín Rodríguez, J. Hu, Z. Zhou, R. Fan, D. H. Ortgies, N. Fernández, I. Chaves-Coira, Á. Núñez, D. Jaque and G. Chen, Rare-earth-doped fluoride nanoparticles with engineered long luminescence lifetime for time-gated in vivo optical imaging in the second biological window, Nanoscale, 2018, 10, 17771–17780, 10.1039/C8NR02382D.
- B. del Rosal, D. H. Ortgies, N. Fernández, F. Sanz-Rodríguez, D. Jaque and E. M. Rodríguez, Overcoming Autofluorescence: Long-Lifetime Infrared Nanoparticles for Time-Gated In Vivo Imaging, Adv. Mater., 2016, 28, 10188–10193, DOI:10.1002/adma.201603583.
- M. Y. Berezin and S. Achilefu, Fluorescence Lifetime Measurements and Biological Imaging, Chem. Rev., 2010, 110, 2641–2684, DOI:10.1021/cr900343z.
- J.-H. Park, L. Gu, G. von Maltzahn, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Biodegradable luminescent porous silicon nanoparticles for in vivo applications, Nat. Mater., 2009, 8, 331–336, DOI:10.1038/nmat2398.
- K. Y. Zhang, Q. Yu, H. Wei, S. Liu, Q. Zhao and W. Huang, Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing, Chem. Rev., 2018, 118, 1770–1839, DOI:10.1021/acs.chemrev.7b00425.
- L. Gu, D. J. Hall, Z. Qin, E. Anglin, J. Joo, D. J. Mooney, S. B. Howell and M. J. Sailor, In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles, Nat. Commun., 2013, 4, 2326, DOI:10.1038/ncomms3326.
- W. Yang and S.-L. Chen, Time-gated fluorescence imaging: Advances in technology and biological applications, J. Innovative Opt. Health Sci., 2020, 13, 2030006, DOI:10.1142/S1793545820300062.
- X. Zheng, X. Zhu, Y. Lu, J. Zhao, W. Feng, G. Jia, F. Wang, F. Li and D. Jin, High-Contrast Visualization of Upconversion Luminescence in Mice Using Time-Gating Approach, Anal. Chem., 2016, 88, 3449–3454, DOI:10.1021/acs.analchem.5b04626.
- L. Cai, Y. Huang, P. Sun, W. Zheng, S. Zhou, P. Huang, J. Wei, D. Tu, X. Chen and Z. Liang, Accurate detection of β-hCG in women's serum and cervical secretions for predicting early pregnancy viability based on time-resolved luminescent lanthanide nanoprobes, Nanoscale, 2020, 12, 6729–6735, 10.1039/C9NR10973K.
- Y. Lu, J. Lu, J. Zhao, J. Cusido, F. M. Raymo, J. Yuan, S. Yang, R. C. Leif, Y. Huo, J. A. Piper, J. Paul Robinson, E. M. Goldys and D. Jin, On-the-fly decoding luminescence lifetimes in the microsecond region for lanthanide-encoded suspension arrays, Nat. Commun., 2014, 5, 3741, DOI:10.1038/ncomms4741.
- L. Balogh, S. S. Nigavekar, B. M. Nair, W. Lesniak, C. Zhang, L. Y. Sung, M. S. T. Kariapper, A. El-Jawahri, M. Llanes, B. Bolton, F. Mamou, W. Tan, A. Hutson, L. Minc and M. K. Khan, Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models, Nanomedicine, 2007, 3, 281–296, DOI:10.1016/j.nano.2007.09.001.
- F. Alexis, E. Pridgen, L. K. Molnar and O. C. Farokhzad, Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles, Mol. Pharmaceutics, 2008, 5, 505–515, DOI:10.1021/mp800051m.
- J. Yao, G. López-Peña, J. Lifante, M. C. Iglesias-de la Cruz, R. Marin, E. Martín Rodríguez, D. Jaque and D. H. Ortgies, (INVITED)Adjustable near-infrared fluorescence lifetime emission of biocompatible rare-earth-doped nanoparticles for in vivo multiplexing, Opt. Mater.: X, 2023, 17, 100225, DOI:10.1016/j.omx.2022.100225.
- Y. Fan, P. Wang, Y. Lu, R. Wang, L. Zhou, X. Zheng, X. Li, J. A. Piper and F. Zhang, Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging, Nat. Nanotechnol., 2018, 13, 941–946, DOI:10.1038/s41565-018-0221-0.
- C. Gosse, C. Bergaud and P. Löw, Molecular Probes for Thermometry in Microfluidic Devices, 2009, pp. 301–341 DOI:10.1007/978-3-642-04258-4_10.
- L. Aigouy, G. Tessier, M. Mortier and B. Charlot, Scanning thermal imaging of microelectronic circuits with a fluorescent nanoprobe, Appl. Phys. Lett., 2005, 87, 184105, DOI:10.1063/1.2123384.
- J. M. Yang, H. Yang and L. Lin, Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells, ACS Nano, 2011, 5, 5067–5071, DOI:10.1021/nn201142f.
- B. Del Rosal, A. Pérez-Delgado, M. Misiak, A. Bednarkiewicz, A. S. Vanetsev, Y. Orlovskii, D. J. Jovanovic, M. D. Dramicanin, U. Rocha, K. Upendra Kumar, C. Jacinto, E. Navarro, E. Martin Rodríguez, M. Pedroni, A. Speghini, G. A. Hirata, I. R. Martín and D. Jaque, Neodymium-doped nanoparticles for infrared fluorescence bioimaging: The role of the host, J. Appl. Phys., 2015, 118, 143104, DOI:10.1063/1.4932669.
- P. Cortelletti, C. Facciotti, I. X. Cantarelli, P. Canton, M. Quintanilla, F. Vetrone, A. Speghini and M. Pedroni, Nd3+ activated CaF2 NPs as colloidal nanothermometers in the biological window, Opt. Mater., 2017, 68, 29–34, DOI:10.1016/j.optmat.2016.11.019.
- M. Dewhirst, B. L. Viglianti, M. Lora-Michiels, P. J. Hoopes and M. A. Hanson, in Thermal dose requirement for tissue effect: experimental and clinical findings, ed. T. P. Ryan, 2003, p. 37 DOI:10.1117/12.476637.
- R. Liang, R. Tian, W. Shi, Z. Liu, D. Yan, M. Wei, D. G. Evans and X. Duan, A temperature sensor based on CdTe quantum dots–layered double hydroxide ultrathin films via layer-by-layer assembly, Chem. Commun., 2013, 49, 969–971, 10.1039/C2CC37553B.
- D. Jaque and F. Vetrone, Luminescence nanothermometry, Nanoscale, 2012, 4, 4301–4326, 10.1039/c2nr30764b.
- C. F. Chapman, Y. Liu, G. J. Sonek and B. J. Tromberg, The use of exogenous fluorescent probes for temperature measurements in single living cells, Photochem. Photobiol., 1995, 62, 416–425, DOI:10.1111/j.1751-1097.1995.tb02362.x.
- N. Mohan, C.-S. Chen, H.-H. Hsieh, Y.-C. Wu and H.-C. Chang, In Vivo Imaging and Toxicity Assessments of Fluorescent Nanodiamonds in Caenorhabditis elegans, Nano Lett., 2010, 10, 3692–3699, DOI:10.1021/nl1021909.
- C. Gota, K. Okabe, T. Funatsu, Y. Harada and S. Uchiyama, Hydrophilic Fluorescent Nanogel Thermometer for Intracellular Thermometry, J. Am. Chem. Soc., 2009, 131, 2766–2767, DOI:10.1021/ja807714j.
- G. W. Walker, V. C. Sundar, C. M. Rudzinski, A. W. Wun, M. G. Bawendi and D. G. Nocera, Quantum-dot optical temperature probes, Appl. Phys. Lett., 2003, 83, 3555–3557, DOI:10.1063/1.1620686.
- L. M. Maestro, P. Haro-González, B. del Rosal, J. Ramiro, A. J. Caamaño, E. Carrasco, A. Juarranz, F. Sanz-Rodríguez, J. G. Solé and D. Jaque, Heating efficiency of multi-walled carbon nanotubes in the first and second biological windows, Nanoscale, 2013, 5, 7882, 10.1039/c3nr01398g.
- A. Carattino, M. Caldarola and M. Orrit, Gold Nanoparticles as Absolute Nanothermometers, Nano Lett., 2018, 18, 874–880, DOI:10.1021/acs.nanolett.7b04145.
- L. Marciniak, K. Prorok and A. Bednarkiewicz, Size dependent sensitivity of Yb3+, Er3+ up-converting luminescent nano-thermometers, J. Mater. Chem. C, 2017, 5, 7890–7897, 10.1039/C7TC02322G.
- D. Valerini, A. Cretí, M. Lomascolo, L. Manna, R. Cingolani and M. Anni, Temperature dependence of the photoluminescence properties of colloidal CdSe/ZnS core/shell quantum dots embedded in a polystyrene matrix, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 235409, DOI:10.1103/PhysRevB.71.235409.
- T. Bai and N. Gu, Micro/Nanoscale Thermometry for Cellular Thermal Sensing, Small, 2016, 12, 4590–4610, DOI:10.1002/smll.201600665.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Thermometry at the nanoscale, Nanoscale, 2012, 4, 4799, 10.1039/c2nr30663h.
- R. Weissleder, A clearer vision for in vivo imaging, Nat. Biotechnol., 2001, 19, 316–317, DOI:10.1038/86684.
- J. Cao, F. Hu, L. Chen, H. Guo, C. Duan and M. Yin, Optical thermometry based on up-conversion luminescence behavior of Er3+-doped KYb2F7 nano-crystals in bulk glass ceramics, J. Alloys Compd., 2017, 693, 326–331, DOI:10.1016/j.jallcom.2016.09.163.
- S. A. Wade, S. F. Collins and G. W. Baxter, Fluorescence intensity ratio technique for optical fiber point temperature sensing, J. Appl. Phys., 2003, 94, 4743–4756, DOI:10.1063/1.1606526.
- H. Suo, X. Zhao, Z. Zhang, Y. Wang, J. Sun, M. Jin and C. Guo, Rational Design of Ratiometric Luminescence Thermometry Based on Thermally Coupled Levels for Bioapplications, Laser Photonics Rev., 2021, 15, 1–25, DOI:10.1002/lpor.202000319.
- F. Vetrone, R. Naccache, A. Zamarrón, A. J. De La Fuente, F. Sanz-Rodríguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Sole and J. A. Capobianco, Temperature sensing using fluorescent nanothermometers, ACS Nano, 2010, 4, 3254–3258, DOI:10.1021/nn100244a.
- U. Rocha, C. Jacinto, W. Ferreira Silva, I. Guedes, A. Benayas, L. Martinez Maestro, M. Acosta Elias, E. Bovero, F. C. J. M. van Veggel, J. A. Garcia Sole and D. Jaque, Subtissue Thermal Sensing Based on Neodymium-Doped LaF3 Nanoparticles, ACS Nano, 2013, 7, 1188–1199, DOI:10.1021/nn304373q.
- A. F. Pereira, K. U. Kumar, W. F. Silva, W. Q. Santos, D. Jaque and C. Jacinto, Yb3+/Tm3+ co-doped NaNbO3 nanocrystals as three-photon-excited luminescent nanothermometers, Sens. Actuators, B, 2015, 213, 65–71, DOI:10.1016/j.snb.2015.01.136.
- C. D. S. Brites, A. Millán and L. D. Carlos, Lanthanides in Luminescent Thermometry, 2016, pp. 339–427 DOI:10.1016/bs.hpcre.2016.03.005.
- O. Zohar, M. Ikeda, H. Shinagawa, H. Inoue, H. Nakamura, D. Elbaum, D. L. Alkon and T. Yoshioka, Thermal imaging of receptor-activated heart production in single cells, Biophys. J., 1998, 74, 82–89, DOI:10.1016/S0006-3495(98)77769-0.
- A. Bednarkiewicz, J. Drabik, K. Trejgis, D. Jaque, E. Ximendes and L. Marciniak, Luminescence based temperature bio-imaging: Status, challenges, and perspectives, Appl. Phys. Rev., 2021, 8, 011317, DOI:10.1063/5.0030295.
- A. S. Laia, D. A. Hora, M. V. dos, S. Rezende, Y. Xing, J. J. Rodrigues, G. S. Maciel and M. A. R. C. Alencar, Nd3+-doped LiBaPO4 phosphors for optical temperature sensing within the first biological window: A new strategy to increase the sensitivity, Chem. Eng. J., 2020, 399, 125742, DOI:10.1016/j.cej.2020.125742.
- L. Labrador-Páez, E. C. Ximendes, P. Rodríguez-Sevilla, D. H. Ortgies, U. Rocha, C. Jacinto, E. Martín Rodríguez, P. Haro-González and D. Jaque, Core–shell rare-earth-doped nanostructures in biomedicine, Nanoscale, 2018, 10, 12935–12956, 10.1039/C8NR02307G.
- A. A. Ansari, A. K. Parchur, M. K. Nazeeruddin and M. M. Tavakoli, Luminescent lanthanide nanocomposites in thermometry: Chemistry of dopant ions and host matrices, Coord. Chem. Rev., 2021, 444, 214040, DOI:10.1016/j.ccr.2021.214040.
- E. C. Ximendes, A. F. Pereira, U. Rocha, W. F. Silva, D. Jaque and C. Jacinto, Thulium doped LaF3 for nanothermometry operating over 1000 nm, Nanoscale, 2019, 11, 8864–8869, 10.1039/C9NR00082H.
- Z. Li, J. Gong, S. Lu, X. Li, X. Gu, J. Xu, J. U. Khan, D. Jin and X. Chen, Photothermal lanthanide nanomaterials: From fundamentals to theranostic applications, BMEMat, 2024, 2, e12088, DOI:10.1002/bmm2.12088.
- A. Puccini, N. Liu and E. Hemmer, Lanthanide-based nanomaterials for temperature sensing in the near-infrared spectral region: illuminating progress and challenges, Nanoscale, 2024, 16, 10975–10993, 10.1039/D4NR00307A.
- Y. Wu, F. Li, Y. Wu, H. Wang, L. Gu, J. Zhang, Y. Qi, L. Meng, N. Kong, Y. Chai, Q. Hu, Z. Xing, W. Ren, F. Li and X. Zhu, Lanthanide luminescence nanothermometer with working wavelength beyond 1500
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm for cerebrovascular temperature imaging in vivo, Nat. Commun., 2024, 15, 2341, DOI:10.1038/s41467-024-46727-5.
nm for cerebrovascular temperature imaging in vivo, Nat. Commun., 2024, 15, 2341, DOI:10.1038/s41467-024-46727-5. - M. Wang, A. Skripka, Y. Zhang, T. Cheng, M. Ng, S. Y. Wong, Y. Zhao, X. Sun, X. Li, K. K. Bhakoo, A. Y. Chang, F. Rosei and F. Vetrone, Theranostic Nanocapsules: Heating, Imaging, and Luminescence Nanothermometry, Chem. Mater., 2024, 36, 3285–3295, DOI:10.1021/acs.chemmater.3c03216.
- L. Marciniak, W. M. Piotrowski, M. Szymczak, M. Drozd, V. Kinzhybalo and M. Back, Customizing thermometry: Optimizing the operating temperature range of phase transition-based ratiometric luminescence thermometers, Chem. Eng. J., 2024, 487, 150363, DOI:10.1016/j.cej.2024.150363.
- P. Martínez-Merino, M. A. Hernández-Rodríguez, J. C. Piñero, C. D. S. Brites, R. Alcántara and J. Navas, Morphology does not matter: WSe2 luminescence nanothermometry unravelled, Nanoscale, 2024, 16, 8470–8478, 10.1039/D4NR00014E.
- Y. Hasegawa, Y. Wada and S. Yanagida, Strategies for the design of luminescent lanthanide(III) complexes and their photonic applications, J. Photochem. Photobiol., C, 2004, 5, 183–202, DOI:10.1016/j.jphotochemrev.2004.10.003.
- H. J. M. A. A. Zijlmans, J. Bonnet, J. Burton, K. Kardos, T. Vail, R. S. Niedbala and H. J. Tanke, Detection of Cell and Tissue Surface Antigens Using Up-Converting Phosphors: A New Reporter Technology, Anal. Biochem., 1999, 267, 30–36, DOI:10.1006/abio.1998.2965.
- F. Wang and X. Liu, Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals, Chem. Soc. Rev., 2009, 38, 976, 10.1039/b809132n.
- C. Lee, E. Z. Xu, Y. Liu, A. Teitelboim, K. Yao, A. Fernandez-Bravo, A. M. Kotulska, S. H. Nam, Y. D. Suh, A. Bednarkiewicz, B. E. Cohen, E. M. Chan and P. J. Schuck, Giant nonlinear optical responses from photon-avalanching nanoparticles, Nature, 2021, 589, 230–235, DOI:10.1038/s41586-020-03092-9.
- A. Benayas, B. Del Rosal, A. Pérez-Delgado, K. Santacruz-Gómez, D. Jaque, G. A. Hirata and F. Vetrone, Nd:YAG Near-Infrared Luminescent Nanothermometers, Adv. Opt. Mater., 2015, 3, 687–694, DOI:10.1002/adom.201400484.
- T. Yang, Y. Tang, L. Liu, X. Lv, Q. Wang, H. Ke, Y. Deng, H. Yang, X. Yang, G. Liu, Y. Zhao and H. Chen, Size-Dependent Ag2S Nanodots for Second Near-Infrared Fluorescence/Photoacoustics Imaging and Simultaneous Photothermal Therapy, ACS Nano, 2017, 11, 1848–1857, DOI:10.1021/acsnano.6b07866.
- S. Li, L. Xu, M. Sun, X. Wu, L. Liu, H. Kuang and C. Xu, Hybrid Nanoparticle Pyramids for Intracellular Dual MicroRNAs Biosensing and Bioimaging, Adv. Mater., 2017, 29, 1606086, DOI:10.1002/adma.201606086.
- X. Zhang, M. Liu, H. Liu and S. Zhang, Low-toxic Ag2S quantum dots for photoelectrochemical detection glucose and cancer cells, Biosens. Bioelectron., 2014, 56, 307–312, DOI:10.1016/j.bios.2014.01.033.
- J. Amaya Suárez, J. J. Plata, A. M. Márquez and J. Fernández Sanz, Ag2S Quantum Dot-Sensitized Solar Cells by First Principles: The Effect of Capping Ligands and Linkers, J. Phys. Chem. A, 2017, 121, 7290–7296, DOI:10.1021/acs.jpca.7b07731.
- M. Jagadeeswararao, A. Swarnkar, G. B. Markad and A. Nag, Defect-Mediated Electron–Hole Separation in Colloidal Ag2S–AgInS2 Hetero Dimer Nanocrystals Tailoring Luminescence and Solar Cell Properties, J. Phys. Chem. C, 2016, 120, 19461–19469, DOI:10.1021/acs.jpcc.6b06394.
- D. Ruiz, M. Mizrahi, H. D. A. Santos, D. Jaque, C. M. S. Jones, J. Marqués-Hueso, C. Jacinto, F. G. Requejo, A. Torres-Pardo, J. M. González-Calbet and B. H. Juárez, Synthesis and characterization of Ag2S and Ag2S/Ag2 (S,Se) NIR nanocrystals, Nanoscale, 2019, 11, 9194–9200, 10.1039/C9NR02087J.
- J. Zhang, G. Hao, C. Yao, J. Yu, J. Wang, W. Yang, C. Hu and B. Zhang, Albumin-Mediated Biomineralization of Paramagnetic NIR Ag2S QDs for Tiny Tumor Bimodal Targeted Imaging in Vivo, ACS Appl. Mater. Interfaces, 2016, 8, 16612–16621, DOI:10.1021/acsami.6b04738.
- H. D. A. Santos, I. Zabala Gutierrez, Y. Shen, J. Lifante, E. Ximendes, M. Laurenti, D. Mendez-Gonzalez, S. Melle, O. G. Calderon, E. Lopez Cabarcos, N. Fernandez, I. Chaves-Coira, D. Lucena-Agell, L. Monge, M. D. Mackenzie, J. Marques-Hueso, C. M. S. Jones, C. Jacinto, B. del Rosal, A. K. Kar, J. Rubio-Retama and D. Jaque, Ultrafast photochemistry produces superbright short-wave infrared dots for low-dose in vivo imaging, Nat. Commun., 2020, 11, 2933, DOI:10.1038/s41467-020-16333-2.
- S. G. Alamdari, M. Amini, N. Jalilzadeh, B. Baradaran, R. Mohammadzadeh, A. Mokhtarzadeh and F. Oroojalian, Recent advances in nanoparticle-based photothermal therapy for breast cancer, J. Controlled Release, 2022, 349, 269–303, DOI:10.1016/j.jconrel.2022.06.050.
- X. Yao, X. Niu, K. Ma, P. Huang, J. Grothe, S. Kaskel and Y. Zhu, Graphene Quantum Dots-Capped Magnetic Mesoporous Silica Nanoparticles as a Multifunctional Platform for Controlled Drug Delivery, Magnetic Hyperthermia, and Photothermal Therapy, Small, 2017, 13, 1602225, DOI:10.1002/smll.201602225.
- A. R. Rastinehad, H. Anastos, E. Wajswol, J. S. Winoker, J. P. Sfakianos, S. K. Doppalapudi, M. R. Carrick, C. J. Knauer, B. Taouli, S. C. Lewis, A. K. Tewari, J. A. Schwartz, S. E. Canfield, A. K. George, J. L. West and N. J. Halas, Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18590–18596, DOI:10.1073/pnas.1906929116.
- X. Huang, Y. Lu, M. Guo, S. Du and N. Han, Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view, Theranostics, 2021, 11, 7546–7569, DOI:10.7150/thno.56482.
- B. Liu, W. Cao, G. Qiao, S. Yao, S. Pan, L. Wang, C. Yue, L. Ma, Y. Liu and D. Cui, Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer, Acta Biomater., 2019, 99, 307–319, DOI:10.1016/j.actbio.2019.08.046.
- T. Sun, J. Han, S. Liu, X. Wang, Z. Y. Wang and Z. Xie, Tailor-Made Semiconducting Polymers for Second Near-Infrared Photothermal Therapy of Orthotopic Liver Cancer, ACS Nano, 2019, 13, 7345–7354, DOI:10.1021/acsnano.9b03910.
- C. Bonamy, S. Pesnel, M. Ben Haddada, O. Gorgette, C. Schmitt, A.-L. Morel and N. Sauvonnet, Impact of Green Gold Nanoparticle Coating on Internalization, Trafficking, and Efficiency for Photothermal Therapy of Skin Cancer, ACS Omega, 2023, 8, 4092–4105, DOI:10.1021/acsomega.2c07054.
- N. Rahmani Khalili, A. Banitalebi Dehkordi, A. Amiri, G. Mohammadi Ziarani, A. Badiei and P. Cool, Tailored Covalent Organic Framework Platform: From Multistimuli, Targeted Dual Drug Delivery by Architecturally Engineering to Enhance Photothermal Tumor Therapy, ACS Appl. Mater. Interfaces, 2024, 16, 28245–28262, DOI:10.1021/acsami.4c05989.
- Y. Chen, Y. Gao, Y. Chen, L. Liu, A. Mo and Q. Peng, Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment, J. Controlled Release, 2020, 328, 251–262, DOI:10.1016/j.jconrel.2020.08.055.
- X. Zhang, J. Wu, G. R. Williams, S. Niu, Q. Qian and L.-M. Zhu, Functionalized MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy, Colloids Surf., B, 2019, 173, 101–108, DOI:10.1016/j.colsurfb.2018.09.048.
- X. Zhang, J. Wu, G. R. Williams, S. Niu, Q. Qian and L.-M. Zhu, Functionalized MoS2-nanosheets for targeted drug delivery and chemo-photothermal therapy, Colloids Surf., B, 2019, 173, 101–108, DOI:10.1016/j.colsurfb.2018.09.048.
- S. D. Quinn and W. M. Gedroyc, Thermal ablative treatment of uterine fibroids, Int. J. Hyperthermia, 2015, 31, 272–279, DOI:10.3109/02656736.2015.1010608.
- S. Ren, X. Cheng, M. Chen, C. Liu, P. Zhao, W. Huang, J. He, Z. Zhou and L. Miao, Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC, ACS Appl. Mater. Interfaces, 2017, 9, 31509–31518, DOI:10.1021/acsami.7b09522.
- H. Kim, M. Yang, N. Kwon, M. Cho, J. Han, R. Wang, S. Qi, H. Li, V. Nguyen, X. Li, H. Cheng and J. Yoon, Recent progress on photodynamic therapy and photothermal therapy, Bull. Korean Chem. Soc., 2023, 44, 236–255, DOI:10.1002/bkcs.12655.
- D. Zhi, T. Yang, J. O’Hagan, S. Zhang and R. F. Donnelly, Photothermal therapy, J. Controlled Release, 2020, 325, 52–71, DOI:10.1016/j.jconrel.2020.06.032.
- X. Li, X. Li, S. Park, S. Wu, Y. Guo, K. T. Nam, N. Kwon, J. Yoon and Q. Hu, Photodynamic and Photothermal therapy via human serum albumin delivery, Coord. Chem. Rev., 2024, 520, 216142, DOI:10.1016/j.ccr.2024.216142.
- Y. Chen, Z. Lu and D. Wang, Multifunctional Nanoplatform for Single NIR Laser-Regulated Efficient PDT/PTT/Chemotherapy, Biomacromolecules, 2024, 25, 1038–1046, DOI:10.1021/acs.biomac.3c01100.
- L. Wang, Y. He, T. He, G. Liu, C. Lin, K. Li, L. Lu and K. Cai, Lymph node-targeted immune-activation mediated by imiquimod-loaded mesoporous polydopamine based-nanocarriers, Biomaterials, 2020, 255, 120208, DOI:10.1016/j.biomaterials.2020.120208.
- J. Wang, C. Yao, B. Shen, X. Zhu, Y. Li, L. Shi, Y. Zhang, J. Liu, Y. Wang and L. Sun, Upconversion-Magnetic Carbon Sphere for Near Infrared Light-Triggered Bioimaging and Photothermal Therapy, Theranostics, 2019, 9, 608–619, DOI:10.7150/thno.27952.
- K. Manivannan, C.-C. Cheng, R. Anbazhagan, H.-C. Tsai and J.-K. Chen, Fabrication of silver seeds and nanoparticle on core-shell Ag@SiO2 nanohybrids for combined photothermal therapy and bioimaging, J. Colloid Interface Sci., 2019, 537, 604–614, DOI:10.1016/j.jcis.2018.11.051.
- M. P. Çetingül and C. Herman, A heat transfer model of skin tissue for the detection of lesions: sensitivity analysis, Phys. Med. Biol., 2010, 55, 5933–5951, DOI:10.1088/0031-9155/55/19/020.
- A. J. Welch and M. J. C. Gemert, Optical-Thermal Response of Laser-Irradiated Tissue, Springer, Netherlands, Dordrecht, 2011 DOI:10.1007/978-90-481-8831-4.
- R. K. Jain, Temperature distributions in normal and neoplastic tissues during normothermia and hyperthermia, Ann. N. Y. Acad. Sci., 1980, 335, 48–66, DOI:10.1111/j.1749-6632.1980.tb50736.x.
- W. H. Newman and P. P. Lele, A Transient Heating Technique for the Measurement of Thermal Properties of Perfused Biological Tissue, J. Biomech. Eng., 1985, 107, 219–227, DOI:10.1115/1.3138546.
- H. H. Pennes, Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm, J. Appl. Physiol., 1948, 1, 93–122, DOI:10.1152/jappl.1948.1.2.93.
- H. D. A. Santos, S. M. V. Novais and C. Jacinto, Optimizing the Nd:YF3 phosphor by impurities control in the synthesis procedure, J. Lumin., 2018, 201, 156–162, DOI:10.1016/j.jlumin.2018.04.051.
- K. Trejgis, K. Ledwa, A. Bednarkiewicz and L. Marciniak, A single-band ratiometric luminescent thermometer based on tetrafluorides operating entirely in the infrared region, Nanoscale Adv., 2022, 4, 437–446, 10.1039/D1NA00727K.
- K. Kniec, A. Kochanowska, L. Li, M. Suta and L. Marciniak, A ratiometric and lifetime-based luminescent thermometer exploiting the Co3+ luminescence in CaAl2O4:Co3+ and CaAl2O4:Co3+,Nd3+, J. Mater. Chem. C, 2022, 10, 9278–9286, 10.1039/D2TC00952H.
- A. V. P. Kumar, S. K. Dubey, S. Tiwari, A. Puri, S. Hejmady, B. Gorain and P. Kesharwani, Recent advances in nanoparticles mediated photothermal therapy induced tumor regression, Int. J. Pharm., 2021, 606, 120848, DOI:10.1016/j.ijpharm.2021.120848.
- W. Zhang, M. Ding, H. Zhang, H. Shang and A. Zhang, Tumor acidity and near-infrared light responsive drug delivery MoS2-based nanoparticles for chemo-photothermal therapy, Photodiagn. Photodyn. Ther., 2022, 38, 102716, DOI:10.1016/j.pdpdt.2022.102716.
- L. Cheng, W. He, H. Gong, C. Wang, Q. Chen, Z. Cheng and Z. Liu, PEGylated Micelle Nanoparticles Encapsulating a Non-Fluorescent Near-Infrared Organic Dye as a Safe and Highly-Effective Photothermal Agent for In Vivo Cancer Therapy, Adv. Funct. Mater., 2013, 23, 5893–5902, DOI:10.1002/adfm.201301045.
- Z. sobhani, M. A. Behnam, F. Emami, A. Dehghanian and I. Jamhiri, Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes, Int. J. Nanomed., 2017, 12, 4509–4517, DOI:10.2147/IJN.S134661.
- M. A. Dheyab, A. A. Aziz, P. M. Khaniabadi, M. S. Jameel, N. Oladzadabbasabadi, A. A. Rahman, F. S. Braim and B. Mehrdel, Gold nanoparticles-based photothermal therapy for breast cancer, Photodiagn. Photodyn. Ther., 2023, 42, 103312, DOI:10.1016/j.pdpdt.2023.103312.
- S. Hejmady, R. Pradhan, A. Alexander, M. Agrawal, G. Singhvi, B. Gorain, S. Tiwari, P. Kesharwani and S. K. Dubey, Recent advances in targeted nanomedicine as promising antitumor therapeutics, Drug Discovery Today, 2020, 25, 2227–2244, DOI:10.1016/j.drudis.2020.09.031.
- H. Choudhury, B. Gorain, M. Pandey, R. K. Khurana and P. Kesharwani, Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting, Int. J. Pharm., 2019, 565, 509–522, DOI:10.1016/j.ijpharm.2019.05.042.
- J. Stabile, D. Najafali, Y. Cheema, C. T. Inglut, B. J. Liang, S. Vaja, A. J. Sorrin and H.-C. Huang, Engineering gold nanoparticles for photothermal therapy, surgery, and imaging, Nanoparticles for Biomedical Applications, Elsevier, 2020, pp. 175–193 DOI:10.1016/B978-0-12-816662-8.00012-6.
- C. Dai, Y. Chen, X. Jing, L. Xiang, D. Yang, H. Lin, Z. Liu, X. Han and R. Wu, Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation, ACS Nano, 2017, 11, 12696–12712, DOI:10.1021/acsnano.7b07241.
- H. Odéen and D. L. Parker, Magnetic resonance thermometry and its biological applications – Physical principles and practical considerations, Prog. Nucl. Magn. Reson. Spectrosc., 2019, 110, 34–61, DOI:10.1016/j.pnmrs.2019.01.003.
| This journal is © The Royal Society of Chemistry 2025 |