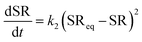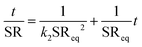DOI:
10.1039/D4TB02354D
(Paper)
J. Mater. Chem. B, 2025,
13, 3602-3617
Development of a novel polyelectrolyte complex nanocomposite of modified chitosan and karaya gum for co-delivery of 5-fluorouracil and curcumin for cancer therapy
Received
20th October 2024
, Accepted 5th February 2025
First published on 10th February 2025
Abstract
Combination chemotherapy is a relatively recent and preferred method for cancer treatment. Sustained delivery of dual drugs can be achieved with a suitable matrix. In the present work, a pH-responsive polyelectrolyte complex (PEC) of trimethylchitosan and carboxymethylkaraya gum containing silver nanoparticles (SNps) has been developed as a matrix material for co-delivery of the drugs, 5-fluorouracil (5-Fu) and curcumin (Cur). The experimental conditions have been optimized for high yield and high swelling of the PEC nanocomposite. 1H-NMR, FT-IR, FE-SEM, P-XRD, HR-TEM, EDS, TGA techniques and zeta potential measurements have been employed in the physico-chemical characterization of the nanocomposite material. The presence of SNps with an average diameter of 16.57 ± 1.25 nm influenced the surface structure and hydrophilicity of the PEC. The swelling study indicated higher swelling at pH 7.4 than at pH 1.2. The two drugs, 5-Fu and Cur, were successfully entrapped and released from the nanocomposite in a sustained manner. Cytotoxicity studies performed with the MCF-10A cell line confirmed the biocompatibility of the nanocomposite and those with the MCF-7 cell line indicated the synergistic effect of the dual drugs in controlling cancer cell growth. The overall study indicates the usefulness of the PEC nanocomposite made from modified polysaccharides, chitosan and karaya gum as a promising material for the development of a dual drug delivery system for cancer treatment.
1. Introduction
Combination drug delivery is the simultaneous administration of two or more active pharmaceutical agents with different therapeutic mechanisms of action. It is more effective compared to conventional therapy, such as the use of a single drug or radiation therapy.1,2 Combination drug delivery in cancer treatment mitigates multidrug resistance and tumor heterogeneity, achieving high synergistic anticancer efficacy and minimizing the toxicity caused by high dosage intake of a single drug.3,4 Among the diverse drug delivery pathways, such as intravenous, intramuscular and oral, oral administration stands out as the most preferred method due to its inherent convenience and non-invasive nature.5 The high stability of the drug and precise dosage through the oral route is achieved through tablet formulation, enhancing therapeutic efficacy. The oral route of administration is associated with less pain, greater convenience, increased patient compliance, decrease in the risk of cross-contamination and needle-stick injuries.6
Polyelectrolyte complexes (PECs) are polymer systems formed by coulombic interaction between two oppositely charged polyelectrolytes.7 The polyelectrolytes utilized for PEC formulations could be of natural or synthetic origin.8 The cytocompatibility properties, pH-sensitivity, swelling ability and biodegradability characteristics make polysaccharide-based PECs promising polymer matrices for biomedical applications.9 Polysaccharide-based PECs can hold a significant volume of water within the space between the polymer chains due to their hydrophilic character. Owing to their pH-sensitive nature stemming from certain functional groups present in them and their ability to swell, some of the PECs are appropriate materials for encapsulation and release of pharmacologically active compounds under desirable conditions.10
Karaya gum (KG), or Indian tragacanth gum, is a biopolymer obtained from Sterculia urens consisting of L-rhamnose, D-galactose and D-glucuronic acid. It is an anionic polysaccharide with significant properties, such as biocompatibility, high water retention capacity, high swelling, low toxicity, and inherent anti-microbial activity. It has been widely used as an emulsifying, thickening, and binding agent in food and healthcare products.11 Chemical modification of KG improves its intrinsic properties, particularly solubility and degradability. Carboxymethylation enhances the hydrophilic nature of the molecule making it a suitable matrix for drug entrapment and release.12 Chitosan (CHN), the deacetylated derivative of chitin, is a natural biocompatible polysaccharide. The amine and hydroxyl groups present in CHN provide an opportunity for chemical modification in order to improve properties, such as pH-responsiveness and solubility.2 Trimethyl chitosan (TMC) is a quaternized derivative of CHN, which shows potential use in various biomedical applications particularly for drug delivery due to its biocompatible and mucoadhesive nature.13 The complex formation between modified KG and modified CHN can result in PECs exhibiting interesting properties.
Metal nanoparticles have attracted the special interest of researchers as multipurpose agents. The synergistic approach which involves the combination of metal nanoparticles and PECs can produce materials with enhanced efficiency over the individual materials in certain applications.14 In this study, the silver nanoparticles (SNps) were selected to enhance the physiochemical properties of the matrix, such as surface area, porous structure, etc., which are intended to enhance the swelling ability of the matrix, resulting in improved drug release behaviour.15
5-Fluorouracil (5-Fu) is a pyrimidine-based chemotherapeutic drug widely used in treating colorectal, breast, and gastrointestinal cancers. Despite its effectiveness, it has severe side effects, which can be overcome by combining it with a low-toxic and effective bioactive compound such as curcumin (Cur).16 Cur possesses anti-cancer, anti-microbial, anti-oxidant, and anti-inflammatory properties. Combining 5-Fu with Cur produces a synergistic effect on cancer cells by promoting apoptosis through modulation of key protein pathways, such as blocking Bcl-2 and inhibiting NF-κB to combat chemoresistance.17 This synergy improves therapeutic efficacy while reducing the toxicity associated with 5-Fu.
A limited number of studies have addressed the combination chemotherapy regimen of 5-Fu (hydrophilic) and Cur (hydrophobic drug). Recently in vitro combinatorial anticancer effects of N,O-carboxymethylchitosan nanoparticles16 and chitosan/reduced graphene oxide nanocomposites18 loaded with 5-Fu and Cur towards colon cancer treatment were studied. Schiff's base cross-linked injectable hydrogels have been used as matrix materials to treat colon cancer using 5-Fu and Cur.19 The injectable shell-crosslinked F127 micelle/hydrogel composites with pH and redox sensitivity were developed as carriers for the co-delivery of 5-Fu and Cur.20
The review of the literature revealed that there are no reports on the use of modified polysaccharide-based PEC nanocomposites for the delivery of 5-Fu and Cur, a combination of hydrophilic and hydrophobic drugs. This study focuses on the development of PECs using TMC/carboxymethyl karaya gum (CMKG) with incorporated SNps for combination delivery of 5-Fu and Cur in the gastrointestinal tract. The developed nanocomposite is characterized by 1H-NMR, FT-IR, FE-SEM, P-XRD, EDS, HR-TEM, and TGA techniques. The surface charges were ascertained by zeta potential (Zp) measurements. The suitability of the PEC-silver nanocomposite as a matrix material for achieving combined drug delivery of 5-Fu and Cur was evaluated by in vitro studies. The biocompatibility of the nanocomposite towards normal cells and toxicity of the dual drug-loaded nanocomposite towards cancer cells were proved by cell viability studies using the MCF-10A and MCF-7 cell lines.
2. Materials and methods
2.1 Materials
Mono-chloroacetic acid (MCA), 5-Fu (99%), CHN (degree of deacetylation being 90%), KG, dimethyl sulphate (DMS), Cur (99%), NaCl, NaOH, and silver nitrate (99%) (AgNO3) were purchased from Sigma-Aldrich (USA); hydrochloric acid, glacial acetic acid, dimethyl sulphoxide (DMSO) (99%), acetone, methanol, potassium dihydrogen phosphate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and disodium hydrogen phosphate were obtained from Merck India Pvt. Ltd. All reagents utilized were of analytical grade.
2.2 Quaternization of CHN
CHN was chemically modified through methylation with DMS, following the previously reported method.21 Approximately 2.0 g of CHN was added to a solution containing 32.0 mL of DMS in 8.0 mL of distilled water. About, 3.2 g of NaOH and 0.96 g of NaCl were added to the above mixture and stirred for 6 h at room temperature. The resulting solution was refrigerated at 5 °C overnight and precipitated in acetone. The product obtained was separated by filtration, washed with methanol and dried at 60 °C for 24 h. It was labelled as TMC.
2.3 Carboxymethylation of KG
Carboxymethylation of KG was carried out using a previously reported method with minor modifications.22 About 0.5 g of KG was dispersed in 40.0 mL of cold NaOH aqueous solution (45% w/v) by maintaining stirring for 60 min. Subsequently, 5.0 mL of MCA solution (75% w/v) was added under constant stirring. The reaction mixture was maintained at 75 °C with continuous stirring for 45 min followed by cooling and it was then suspended in 75% (v/v) aqueous methanol. The resulting precipitate was filtered and washed. The final product was washed three times with 80% (v/v) aqueous methanol and dried at 40 °C for 24 h. It was labelled as CMKG.
2.4 Preparation of PEC
To 10.0 mL of aqueous TMC solution (1% w/v), different volumes of aqueous CMKG (1% w/v) were added in the range of 5.0–50.0 mL to get TMC and CMKG solution in the ratio ranging from 0.04 to 2.0. The resulting precipitate in each case was separated by filtration, washed with water and dried at 40 °C for 24 h. The weight was noted in each case. The product obtained was coded as PEC-1, PEC-2, PEC-3, PEC-4, PEC-5, and PEC-6. The visual appearance of the representative sample PEC-2 is shown in Fig. 1a. The yield (%) in each case was determined using eqn (1):| |  | (1) |
 |
| | Fig. 1 Visual appearance of the prepared samples: (a) PEC-2 and (b) PEC-Ag(6%). | |
2.5 Preparation of the PEC-Ag nanocomposite
To a 50.0 mL solution of TMC and CMKG each with 1% (w/v), known volume (1.0, 3.0 and 5.0 mL) of AgNO3 solution (5.0 mmol L−1) were added and maintained undisturbed under dark conditions till the solution turned brown, confirming the reduction of silver ions from the Ag+ state to the Ag0 state.14,23 The resulting precipitate was filtered, washed with distilled water, and dried at 50 °C. The products obtained by adding 1.0 mL, 3.0 mL, and 5.0 mL of AgNO3 solution were coded as PEC-Ag(2%), PEC-Ag(6%) and PEC-Ag(10%), respectively, and used for further study. The visual appearance of the representative sample PEC-Ag(6%) is shown in Fig. 1b.
2.6 Structural characterization
The 1H NMR spectrum of TMC was recorded in D2O on an NMR spectrometer (JEOL ECX400, USA) with a frequency of 400 MHz. The Fourier transform infrared spectra (FT-IR) of the prepared polymer matrix materials were recorded using a Shimadzu-Prestige-21 spectrophotometer (Japan) from a wavenumber of 4000 to 500 cm−1. The powder X-ray diffractogram (P-XRD), Rigaku Miniflex 600 (Japan), was utilised to record the diffraction peaks with 2θ ranging from 05 to 80° at the recording rate of 2° min−1. Thermogravimetric analysis (TGA) was performed using the SDT Q600 V20.9 (Japan) thermogravimetric analyser by heating the samples from room temperature to 700 °C in a N2 atmosphere. The surface morphological study of the prepared polymer materials was carried out using a field emission scanning electron microscope (FE-SEM) from Carl Zeiss Sigma (Germany), where micrographs were recorded at 5 kV. The elemental composition of the samples was examined using an energy-dispersive X-ray spectrometer, (EDS) by Oxford X-MaxN (USA). A JEOL JEM-2100 (USA) high-resolution transmission electron microscope (HR-TEM) operated at 20 kV was used to record TEM images of the samples. The polydispersity index (PDI), particle size and zeta potential (Zp) of TMC, CMKG, PEC and PEC-Ag(6%) were analyzed using a Malvern Zeta Sizer Nano ZS model Zen3600 (Germany). All readings were recorded at 25 °C with aqueous solutions of the samples used in PEC formation diluted 10 times. The reported values are the average of three readings.
2.7 Degree of carboxymethylation
The procedure for determining the degree of substitution of CMKG was adopted from the previous study.24 About 1.5 g of CMKG was dispersed in 50.0 mL of 2.0 N HCl, and the resulting suspension was stirred for 2 h. During this period, the sodium salt form of CMKG (Na-CMKG) gets converted to the acid form (H-CMKG). The obtained H-CMKG was separated by filtration, subsequently washed with 95% ethanol until it is free from NaCl, as confirmed by the absence of a white precipitate on the addition of AgNO3 solution to the filtrate. The compound is dried at 60 °C for 2 h. Thereafter, 0.5 g of dried H-CMKG was dissolved in 50.0 mL of standard 0.1 N NaOH solution and stirred for 2 h. The excess NaOH was then back-titrated with a 0.1 N HCl solution using phenolphthalein indicator. The degree of substitution (DS) was determined using the following equations:| |  | (2) |
where MA and MB are the molar masses of the anhydroglucose and acetate units, and WA is the mass percentage of the acetyl group (g mol−1) calculated using the equation:| |  | (3) |
where M is the weight of the sample, CA and CB are the concentrations of HCl and NaOH solutions used; and VB is the volume of NaOH initially added (50.0 mL) and VA is the titre value corresponding to the HCl consumed.
2.8 Swelling study
The swelling study was performed by weight measurements25 in media of pH 1.2 (50.0 mL of 0.2 M NaCl and 85.0 mL of 0.2 M HCl are mixed and diluted to 200.0 mL with distilled water) and pH 7.4 (0.238 g of disodium hydrogen phosphate, 0.019 g potassium dihydrogen phosphate and 0.8 g of NaCl dissolved in 100 mL water). A known amount of dry PEC samples were placed in the media maintained at room temperature. The swollen samples were withdrawn from the respective pH buffer media at specific time intervals. The excess water on the surface of the samples was removed by blotting with filter paper, and the weights of the swollen materials were recorded using an analytical electronic balance, Shimadzu AUX120 (Japan) with the accuracy of ±0.1 mg. Measurements were done until the sample attained the equilibrium swelling indicated by the attainment of constant weight. The swelling ratio (SR (g g−1)) was calculated using eqn (4):| |  | (4) |
where W0 and Wt are the weights of the dry PEC material initially taken and the weight of the sample at time ‘t’, respectively.
2.9
In vitro degradation study
The in vitro degradation study26 of PEC and PEC-Ag(6%) was performed in pH 7 (normal), pH 1.2 simulated gastric fluid (SGF) and pH 7.4 simulated intestinal fluid (SIF) at 37 °C by immersing pre-weighed samples in 30.0 mL of respective media. The weight loss of the samples was recorded at definite time intervals for 24 h. The weight loss percentage of the sample was calculated using eqn (5).| | 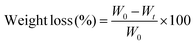 | (5) |
where W0 and Wt are the initial sample weight and weight of the sample at time t, respectively.
2.10 Entrapment efficiency (EE) and drug loading (DL)
The loading of 5-Fu and Cur into PEC and PEC-Ag(6%) was done using the swelling equilibrium method reported earlier27 with minor modifications. To prepare the Cur-loaded polymer matrix, 100 mg of polymer sample was immersed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water mixture containing 0.5 mg mL−1 Cur. The polymer samples were kept in dark conditions for 24 h. The Cur-loaded samples were then taken out and dried. The concentration of the Cur loaded into the polymer matrix was estimated by measuring the absorbance of the Cur solution before and after loading at 429 nm, the λmax of the Cur solution, using a UV-Vis spectrophotometer (Shimadzu UV1900, Japan). For the loading of 5-Fu, Cur-loaded samples were soaked in an aqueous solution of 5-Fu (0.5 mg mL−1). The amount of 5-Fu loaded was quantified from the absorbance measurements of 5-Fu solution before and after loading recorded at 266 nm, the λmax of 5-Fu. The EE (%) and DL (g g−1) were calculated using eqn (6) and (7), respectively.
1 ethanol–water mixture containing 0.5 mg mL−1 Cur. The polymer samples were kept in dark conditions for 24 h. The Cur-loaded samples were then taken out and dried. The concentration of the Cur loaded into the polymer matrix was estimated by measuring the absorbance of the Cur solution before and after loading at 429 nm, the λmax of the Cur solution, using a UV-Vis spectrophotometer (Shimadzu UV1900, Japan). For the loading of 5-Fu, Cur-loaded samples were soaked in an aqueous solution of 5-Fu (0.5 mg mL−1). The amount of 5-Fu loaded was quantified from the absorbance measurements of 5-Fu solution before and after loading recorded at 266 nm, the λmax of 5-Fu. The EE (%) and DL (g g−1) were calculated using eqn (6) and (7), respectively.| |  | (6) |
| |  | (7) |
2.11
In vitro drug release profile
To estimate the extent of 5-Fu and Cur released from the drug loaded samples, the release profile was studied in buffer solution of pH 1.2 as SGF and in buffer solution of pH 7.4 as SIF at 37 °C. The drug release was monitored in the SGF for 4 h and in the SIF for 7 h, considering the fact that the drug loaded sample is retained in the stomach for about 3 h and takes 6 h to pass through the intestinal region where the pH changes from 7.0–7.4, as the nanocomposite is being investigated for drug release by oral administration followed by systemic release.28,29 Dissolution Tester TDT-08LX Electrolab (India) operating with a stirring speed of 100 rpm was employed for the study. About 100 mg of 5-Fu + Cur loaded polymer matrix was placed in the basket containing 900.0 mL of dissolution medium. The aliquots were withdrawn from the pH media at scheduled time intervals and the absorbance was recorded at 266 nm, corresponding to 5-Fu and at 429 nm corresponding to Cur using the UV-Vis spectrophotometer. The quantity of drug released into the medium was determined from the calibration curve made with individual drug solutions of known concentration.20
2.12 Mechanism and kinetics of drug release
The following in vitro drug release kinetic models represented by eqn (8)–(11) were employed to understand the mechanism of release of drugs.30
Zero-order model:
| |  | (8) |
First-order model:
| |  | (9) |
Higuchi square-root model:
| | 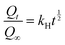 | (10) |
Korsmeyer–Peppas model:
| |  | (11) |
where
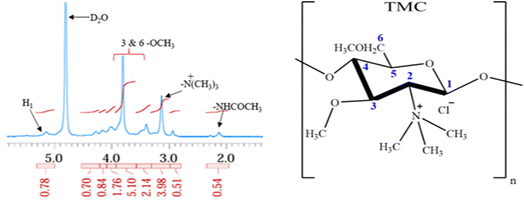
k0,
k1,
kH and
kP are rate constants;
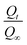
is the fraction of the drug released from the drug-loaded sample at time ‘
t’; and ‘
n’ is the diffusion coefficient. The best-fitting model was decided based on the
R2 values. The diffusion mechanism is indicated as Fickian diffusion (
n ≤ 0.5), non-Fickian diffusion (0.5 <
n ≤ 1), case I transport (
n = 1.0) or super case II transport (
n > 1) based on the value of
n obtained from the Korsmeyer–Peppas model fit.
31
2.13
In vitro cell viability assay
To evaluate the biocompatibility of the matrix material, an in vitro cell viability assay was done against MCF-10A cell lines using the MTT assay method.32 The in vitro cultured cells are incubated at 37 °C for 24 h in an atmosphere of 5% carbon dioxide. In a 96-well culture plate, the 1 × 104 cells per well were distributed to the sample containing 100, 50, 25, 12.5, and 6.25 μg mL−1 of PEC-Ag(6%) and incubated for 24 h. The media was discarded and to each well then, 20 μL of MTT reagent (5 mg mL−1 in phosphate buffer saline) was added and incubated for 4 h. To dissolve the formazan crystal formed in the well, 200 μL of DMSO was added and incubated at 37 °C for 10 min. The absorbance of the cell-cultured plate was measured at 550 nm. The experiments were also performed with the MCF-7 cell lines using 5-Fu, Cur, combined 5-Fu and Cur, the individual drug loaded PEC-Ag(6%) and combined drug loaded PEC-Ag(6%) samples. The cell viability data was used to assess the anti-cancer activity of the component drugs and the matrix:| |  | (12) |
3. Results and discussion
3.1 Formulation of PEC
The addition of CMKG to TMC solution results in varying levels of opalescence of the solution due to partial precipitation of the PEC. The maximum yield of PEC, as indicated by degree of opacity of the solution being minimum due to complete settling of the precipitate formed, was achieved with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of mixing. At this composition, the highest degree of bonding exists between TMC and CMKG, facilitating the formation of insoluble PEC. At higher TMC:CMKG ratios, higher turbidity was observed indicating an increase in the soluble component of PEC. This was also reflected in the decreasing yield of the PEC precipitate with higher ratio. The weight ratio of TMC
1 weight ratio of mixing. At this composition, the highest degree of bonding exists between TMC and CMKG, facilitating the formation of insoluble PEC. At higher TMC:CMKG ratios, higher turbidity was observed indicating an increase in the soluble component of PEC. This was also reflected in the decreasing yield of the PEC precipitate with higher ratio. The weight ratio of TMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMKG at 1
CMKG at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is considered to be optimum for obtaining maximum yield of PEC. The effect of ratio of components on the yield of PEC is shown in Table 1. The schematic representation of the formation of PEC through electrostatic interaction between TMC and CMKG is given in Scheme 1.
1 is considered to be optimum for obtaining maximum yield of PEC. The effect of ratio of components on the yield of PEC is shown in Table 1. The schematic representation of the formation of PEC through electrostatic interaction between TMC and CMKG is given in Scheme 1.
Table 1 Effect of ratio of components on yield of PEC
| Sample code |
TMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMKG (weight ratio) CMKG (weight ratio) |
Volume of 1% TMC (mL) |
Volume of 1% CMKG (mL) |
Yield (%) |
| PEC-1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 |
05 |
58.7 |
| PEC-2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 |
10 |
71.6 |
| PEC-3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
10 |
20 |
64.1 |
| PEC-4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
10 |
30 |
48.3 |
| PEC-5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
10 |
40 |
37.7 |
| PEC-6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
10 |
50 |
27.2 |
 |
| | Scheme 1 Schematic representation of the mechanism of formation of PEC between TMC and CMKG. | |
3.2 Degree of quaternization (DQ) and degree of carboxymethylation
The 1H spectrum of TMC presented in Fig. 2 exhibits the following signals: a singlet at 2.1 ppm corresponding to methyl protons in the N-acetyl groups of partially deacetylated CHN, a singlet at 3.0 ppm attributed to protons in N-dimethyl amino (–N(CH3)2) groups, singlet at 3.2 ppm due to the protons in N-trimethyl amino (–N+(CH3)3) groups and two peaks at 3.4 and 3.8 ppm represent methoxy protons 6(O–CH3) and 3(O–CH3), respectively. The multiple peaks that appear between 3.8–4.4 ppm correspond to the protons linked to carbon atoms 3, 4, 5 and 6 [H3, H4, H5, and H6], and the anomeric proton [H1] appears at 5.4 ppm. The DQ of TMC was calculated using the following equation.33,34| |  | (13) |
 |
| | Fig. 2
1H NMR spectrum of TMC. | |
The concentration of –N+(CH3)3 groups and that of the anomeric proton (H1) is obtained from the integral area under the corresponding peaks at 3.2 and 5.4 ppm, respectively.35 The DQ of TMC was calculated to be 56.7% which is significantly higher than the values achieved in previously reported works.36 The degree of carboxymethylation of KG, determined using the classical acid wash method described in in Section 2.7 was approximately 0.48. This value indicates the fraction of hydroxyl groups that have been substituted by carboxymethyl groups.
3.3 FT-IR analysis
FT-IR spectra of CHN, TMC, KG, CMKG, PEC, PEC-Ag(6%), Cur, 5-Fu, PEC/5-Fu + Cur and PEC-Ag(6%)/5-Fu + Cur are presented in Fig. 3(a–j). In the spectrum of the CHN (Fig. 3a) the broad band present at 3336 cm−1 is related to overlap of –OH and –NH2 stretching vibrations. Absorption bands at 1624 and 1371 cm−1 correspond to symmetric and asymmetric stretching vibrations of amide functionality, and the absorption band at 1013 cm−1 is assigned to C–O–C (ether) stretching vibration. In the spectrum of TMC (Fig. 3b), the absorption band at 1469 cm−1 is attributed to asymmetric angular deformation arising from the C–H bond of –N(CH3)3. The bending vibrations of methyl groups of the N-alkylated moiety, namely, –NH(CH3), –N(CH3)2, and –N+(CH3)3, appear at the absorption band of 1410 cm−1 providing confirmatory evidence for quaternization of CHN.37
 |
| | Fig. 3 FT-IR spectra of (a) CHN, (b) TMC, (c) KG, (d) CMKG, (e) PEC, (f) PEC-Ag(6%), (g) Cur, (h) 5-Fu, (i) PEC/5-Fu + Cur and (j) PEC-Ag(6%)/5-Fu + Cur. | |
The FT-IR spectrum of KG (Fig. 3c) shows a characteristic band at 3322 cm−1 assigned to –OH stretching and a band at 2959 cm−1 corresponding to –CH stretching. The band at 1718 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of acetylated carboxylic acid groups present in KG. Additionally, the carbonyl stretching vibration of carboxylate ions was observed at 1579 (asymmetric) and 1416 cm−1 (symmetric).38 In the FT-IR spectra of CMKG (Fig. 3d), the band at 3408 cm−1 corresponds to –OH stretching, shifted to a higher wavenumber indicating the reduction in the hydrogen bonds due to the substitution of hydroxyl with the carboxymethyl group. The substantial shift of the –OH band from the bonded region into the non-bonded region suggests the involvement of –OH groups in the substitution reaction. Furthermore, the band at 1718 cm−1 is attributed to C
O stretching of acetylated carboxylic acid groups present in KG. Additionally, the carbonyl stretching vibration of carboxylate ions was observed at 1579 (asymmetric) and 1416 cm−1 (symmetric).38 In the FT-IR spectra of CMKG (Fig. 3d), the band at 3408 cm−1 corresponds to –OH stretching, shifted to a higher wavenumber indicating the reduction in the hydrogen bonds due to the substitution of hydroxyl with the carboxymethyl group. The substantial shift of the –OH band from the bonded region into the non-bonded region suggests the involvement of –OH groups in the substitution reaction. Furthermore, the band at 1718 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of partially acetylated carboxylic acid groups observed in KG, which is not seen in CMKG due to alkaline hydrolysis occurring at the ester group.12,22
O of partially acetylated carboxylic acid groups observed in KG, which is not seen in CMKG due to alkaline hydrolysis occurring at the ester group.12,22
The broadening of the –OH band in PEC (Fig. 3e) is attributed to the interaction between the carboxymethyl group of CMKG and the trimethyl group present in TMC of the PEC. Furthermore, the shifting in the carboxymethyl band of CMKG from 1594 to 1576 cm−1 and N-methylated band of TMC from 1410 to 1351 cm−1 confirms the formation of PEC through electrostatic interactions.39,40 The FT-IR spectrum of PEC-Ag(6%) (Fig. 3f) closely resembles that of the parent PEC with slight shift in the bands of hydroxyl/carboxyl ions, indicating the existence of interactions between SNps and the carboxylate ions, which is consistent with other findings in the literature.41,42
The spectrum of Cur (Fig. 3g) exhibits characteristic bands at 3511, 1629, 1504 and 1273 cm−1, corresponding to the stretching vibrations of –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C
O, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –C–O–C– groups, respectively. Additionally, the bands seen at 959 and 811 cm−1 are due to bending of –C–H.43 The FT-IR spectrum of 5-Fu (Fig. 3h) features a band at 1652 cm−1, indicating the overlapping of the stretching vibrations of C
C– and –C–O–C– groups, respectively. Additionally, the bands seen at 959 and 811 cm−1 are due to bending of –C–H.43 The FT-IR spectrum of 5-Fu (Fig. 3h) features a band at 1652 cm−1, indicating the overlapping of the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. The 1426 cm−1 band corresponds to the in-plane bending of C–H while the 1249 cm−1 band represents C–N stretching vibrations. The band at 879 cm−1 corresponds to the bending vibration of C–H bonds of the –CF
C. The 1426 cm−1 band corresponds to the in-plane bending of C–H while the 1249 cm−1 band represents C–N stretching vibrations. The band at 879 cm−1 corresponds to the bending vibration of C–H bonds of the –CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– group, whereas the out of plane bending vibrations of these bonds appear at 815 cm−1.44 The presence of the characteristic bands of 5-Fu and Cur observed in the FT-IR spectra of the drug-loaded PEC sample (Fig. 3i) and of the nanocomposite PEC-Ag(6%) (Fig. 3j) confirms the successful incorporation of 5-Fu + Cur into the polymer matrices.
CH– group, whereas the out of plane bending vibrations of these bonds appear at 815 cm−1.44 The presence of the characteristic bands of 5-Fu and Cur observed in the FT-IR spectra of the drug-loaded PEC sample (Fig. 3i) and of the nanocomposite PEC-Ag(6%) (Fig. 3j) confirms the successful incorporation of 5-Fu + Cur into the polymer matrices.
3.4 Zp, particle size and PDI
The surface charges of TMC, CMKG, PEC and PEC-Ag(6%) were studied using Zp measurements and the corresponding data are presented in Table 2. It is seen that TMC is positively charged, which is due to quaternization of the amine nitrogen of CHN. The sample of CMKG is negatively charged due to the presence of carboxymethyl groups in KG.45,46 Upon the addition of CMKG to TMC, the positive charge on TMC is neutralized by the negative charges on CMKG as indicated by the low value of Zp of PEC, which is a clear evidence for the presence of electrostatic interactions between TMC and CMKG leading to the formation of PEC.47 The slight positive Zp of the PEC indicates residual positive charges on the PEC on neutralization. The decrease in Zp value of PEC-Ag(6%), in comparison with the PEC is due to possible interactions of PEC with SNps. The particle size of TMC and CMKG is found to be much higher than that of PEC due to the colloidal nature of these molecules arising from positive and negative charges respectively on them, which gets reduced during charge neutralization resulting in agrregation.47–49 But, on formation of the nanocomposite, the particle size increases again confirming the influence of Ag on the size of the PEC-Ag(6%) nanocomposite. The PDI values for the systems provide insights into their size distribution.50 TMC exhibited a relatively high PDI, reflecting a broad size distribution due to heterogeneous molecular weightwhereas, the CMKG exhibited significantly lower PDI due to its relatively homogeneous size. The PEC demonstrated the highest PDI, suggesting significant size heterogeneity arising from the complexation process and possible aggregation near charge-neutral conditions.51 Incorporating SNps into the complex reduced the PDI, indicating enhanced size distribution uniformity.
Table 2 Zp, particle size and PDI of the polymer matrices
| Sample code |
Zp (mV) |
Particle size (nm) |
PDI |
| TMC |
67.6 ± 6.12 |
1274 ± 26.4 |
0.65 ± 0.05 |
| CMKG |
−58.1 ± 1.4 |
1611 ± 59.3 |
0.35 ± 0.03 |
| PEC |
9.7 ± 9.72 |
575 ± 34.7 |
0.84 ± 0.04 |
| PEC-Ag(6%) |
5.2 ± 0.67 |
712 ± 46.1 |
0.61 ± 0.02 |
3.5 FE-SEM/EDS and HR-TEM analyses
FE-SEM, EDS and HR-TEM were used to characterize the structural features and elemental composition of the PEC as the drug release characteristics of the matrix are closely related to its morphology. The surface of PEC as depicted in Fig. 4a appears uneven with cavities and wrinkles, which are attributed to the incomplete collapse of the polymer matrix during drying. Diverse folds and aggregates are found due to the presence of bulky carboxymethyl and trimethyl groups in PEC.52 However, the surface of the nanocomposite (Fig. 4b) appears plane compared to PEC (Fig. 4a) and lustrous SNps embedded uniformly in the PEC matrix are seen. The surfaces PEC and the nanocomposite when viewed with higher magnification (Fig. 4c and d) indicate the presence of pores in the latter and similar observations have been reported earlier.53 The presence of SNps in the PEC nanocomposite was confirmed from the appearance of a peak at 2.5 eV in the EDS spectrum of PEC-Ag(6%) corresponding to SNps (Fig. 4e). The HR-TEM image of PEC-Ag(6%) (Fig. 4g) illustrated the average particle size of SNps to be about 16.57 ± 1.25 nm. The spherical-shaped SNps appear to be aggregated, due to the interaction between SNps and the carboxymethyl groups during the reduction process.21
 |
| | Fig. 4 FE-SEM images of (a) PEC (10 k×), (b) PEC-Ag(6%) (10 k×), (c) PEC (25 k×) and (d) PEC-Ag(6%) (25 k×); (e) EDS spectra of PEC-Ag(6%); (f) HR-TEM image of PEC-Ag(6%); (g) particle size distribution curve of PEC-Ag(6%). | |
3.6 P-XRD
The changes in the structure of PEC on the formation of the nanocomposite were studied by P-XRD techniques. The P-XRD pattern observed by PEC and PEC-Ag(6%) is shown in Fig. 5. The PEC (Fig. 5a) shows an amorphous structure as revealed by a broad diffraction peak at 2θ = 23.8° with a shoulder at 42° (Fig. 5a). The destruction of hydrogen bonds due to intermolecular interactions between cationic TMC and anionic CMKG makes the PEC amorphous.54 The diffraction peak of PEC-Ag(6%) (Fig. 5b) showed sharp peaks at 2θ = 32.2, 45.68, 57.6 and 76.01° in addition to the broad band of PEC, featuring (111), (200), (220) and (311) planes, representing the face-centered cubic lattice structure of Ag0 in the nanocomposite.23,55
 |
| | Fig. 5 P-XRD pattern of (a) PEC and (b) PEC-Ag(6%). | |
3.7 TGA analysis
TGA thermograms of PEC and PEC-Ag(6%) are shown in Fig. 6. The PEC system (Fig. 6a) shows three steps of weight loss on heating from room temperature to 700 °C. The weight loss of 15% occurs on heating from room temperature to 200 °C, which is due to the loss of moisture contained in the sample. The second stage of weight loss appearing between 200–300 °C is characteristic of a polysaccharide structure. Around 35% loss is observed during this stage due to polysaccharide chain degradation and loss of low molecular weight products. Subsequently, 20% weight loss is observed on further heating to 500 °C beyond which no change was observed. This gradual weight loss could be due to the loss of functional groups introduced during the modification process, which are involved in electrostatic bonding in the PEC structure. The residual mass of 30% remaining at 700 °C is attributed to the formation of a stable carbon-rich char in an inert atmosphere.56 A slight change in the degradation pattern was observed for PEC-Ag(6%) (Fig. 6b), where the first stage indicated higher moisture content, accounting to 20% weight loss. The second stage, pertaining to the polysaccharide component, occurring from 200 to 300 °C, showed 30% weight loss. The presence of SNps affected the third stage of degradation accounting for 15% weight loss due to the degradation of the polysaccharide functional groups, which interacted with SNps. The residual mass is 5% higher than the PEC sample attributed to the presence of SNps.15,57
 |
| | Fig. 6 TGA thermograms of (a) PEC and (b) PEC-Ag(6%). | |
3.8 Swelling study
The swelling behaviours of the PEC and the corresponding nanocomposite were examined at two different pH conditions and the findings were analysed to provide insights into the water transport mechanism and water retention capacity of the material.58 This understanding is necessary for the potential application of the materials, especially in drug delivery.
3.8.1 Effect of pH on swelling.
Contact time-dependent swelling data were collected for 12 h at room temperature under two different pH conditions and are presented in Fig. 7a and b. The swelling was higher at pH 7.4 compared to pH 1.2 with the nanocomposite showing higher swelling at both pHs when compared to the PEC, the order of swelling being PEC < PEC-Ag(2%) < PEC-Ag(10%) < PEC-Ag(6%). The increase in swelling is due to enhanced polymer relaxation induced by the interaction of the polymer functional groups with buffer media via hydrogen bonding. Both the rate and extent of water diffusion into the polymer are affected by the diffusion of water molecules into the matrix driven by concentration gradients and swelling regulated by relaxation. At pH 7.4, the –COOH groups of the component polyelectrolytes which are not involved in the complexation tend to ionize to form COO−. This leads to the opening up of polymer chains due to the repulsion of charges resulting in polymer relaxation and in turn in higher swelling. Furthermore, by adding SNps into the PEC matrix, there is an increase in swelling at both pHs, which is attributed to the increased hydrophilicity of the PEC material on the addition of SNps.59
 |
| | Fig. 7 Swelling data of PEC and the PEC nanocomposite (a) at pH 7.4 and (b) at pH 1.2. (c) Fit the of second-order kinetic model at pH 7.4, (d) fit of the second-order kinetic model at pH 1.2, (e) fit of the diffusion model at pH 7.4 and (f) fit of the diffusion model at pH 1.2. | |
3.8.2 Effect of concentration of SNps on swelling.
The addition of SNps into PECs results in higher swelling of the PEC material as the osmotic pressure generated by SNps is counterbalanced by the diffusion of water molecules into the nanocomposite.60 Among the three nanocomposite samples, the highest swelling is observed in the sample with 6% SNps content (Fig. 7a and b). On going from PEC-Ag(6%) to PEC-Ag(10%), there is a decrease in the SR as the SNps act as chelating agents making the PEC rigid. Decrease in swelling with increase in polymer rigidity has been a common observation.61 Based on the swelling data, the samples PEC and PEC-Ag(6%) have been considered for furthur studies.
3.9 Kinetics of swelling
In drug delivery studies, the mechanism of water diffusion into PEC can be correlated with the mechanism of drug release. The higher swelling observed initially might result in burst release and gradual swelling may lead to sustained release.62 The swelling data in the present study were analysed using first-order and second-order kinetic63 models represented by eqn (14) and (15), respectively.| |  | (14) |
| | 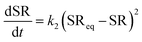 | (15) |
where k1 and k1 are first and second-order rate constants, respectively; SR and SReq are swelling ratios observed at any given time ‘t’ and at equilibrium, respectively.
On integrating eqn (14) and (15) with the limits: at SR = 0, t = 0 and SR = SReq, t = ∞, eqn (16) and (17) are obtained.
| |  | (16) |
| | 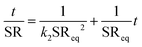 | (17) |
As per the above equations, plots of ln(SR
eq/(SR
eq − SR))
vs. ‘
t’ and
t/SR
vs. ‘
t’ were made and the model that best fits the swelling data was selected based on the
R2 values. The experimental equilibrium SR values along with theoretical calculations are also presented. The
R2 values presented in
Table 3 show that the best fit is obtained for the second-order kinetic model under both pH conditions. Furthermore, the experimental SR
eq values and the values calculated based on the second order model were found to be in close agreement with each other further confirming the fit of the second order kinetic model for swelling. The second-order kinetic model considers swelling to be proportional to the area within the internal specific boundary of the polymer matrix that contains reactive sites, which have not yet interacted with the swelling medium but will eventually undergo hydration.
38
Table 3 Swelling parameters for PEC and PEC-Ag(6%) under different pH conditions
| pH |
Swelling parameter |
PEC |
PEC-Ag(6%) |
| 1.2 |
First-order parameters |
R
2
|
0.746 |
0.798 |
|
k
1 (h−1) |
3.12 |
4.3 |
| Second-order parameters |
R
2
|
0.986 |
0.996 |
|
k
2 × 10−2 ((g g−1)h−1) |
6.10 |
7.3 |
| Diffusion parameters |
R
2
|
0.987 |
0.936 |
|
N
|
0.648 |
0.652 |
| 7.4 |
First-order parameters |
R
2
|
0.894 |
0.901 |
|
k
1 (h−1) |
2.14 |
2.97 |
| Second-order parameters |
R
2
|
0.996 |
0.994 |
|
k
2 × 10−2 ((g g−1)h−1) |
1.93 |
1.95 |
| Diffusion parameters |
R
2
|
0.999 |
0.965 |
|
n
|
0.671 |
0.613 |
3.9.1 Diffusion models.
Knowledge about the water diffusion mechanism is necessary for the feasibility of the selected application of the polymer matrix, which is given by eqn (18).where f, k, and n are the fraction of water absorbed, the rate constant of diffusion, and the diffusion coefficient respectively. The degree of water absorption is proportional to the square root of time for Fickian-type diffusion and n ≤ 0.5 for non-Fickian-type diffusion where absorption is proportional to time ‘t’ and the n value lies between 0.5 and 1.0.31 The data plotted are presented as per eqn (18) for PEC and PEC-Ag(6%) under 2 different pH conditions as shown in Fig. 7e and f. The values of the various swelling parameters calculated based on the plot are listed in Table 3. According to the swelling data, the values of ‘n’ obtained in all cases are in the range 0.5 ≤ 1.0, indicating non-Fickian diffusion. It further suggests that the rate of diffusion of the swelling media into the polymer matrix is comparable with the rate of relaxation of the polymer chain segments.
3.10
In vitro degradation study
The in vitro degradability of polymer matrix materials under three different pH conditions is assessed from weight loss measurements. The PEC exhibited a greater weight loss compared to the PEC nanocomposite at all three pH conditions studied. This implies that the presence of SNps enhances the structural stability of the PEC.14 At pH 1.2, PEC and PEC-Ag(6%) exhibit a greater weight loss attributed to the destabilization of the PEC structure, disruption of hydrogen bonding, and acid-catalyzed hydrolysis of glycosidic linkages, resulting in significant structural breakdown.64,65 The rate of degradation appears to be higher, up to about 100 h compared to the later period. On the other hand, under neutral and slightly alkaline pH conditions, the two materials demonstrated a lower and a more sustained degradation profile, as shown in Fig. 8.66 Although the materials did not fully degrade within the anticipated gastrointestinal transit time, the findings indicate their potential to continue degrading beyond this phase, minimizing environmental concerns. Nevertheless, the degradation behavior and long-term stability must be carefully addressed to overcome challenges in the prolonged use during practical applications and in the commercialization of the nanocomposite.
 |
| | Fig. 8
In vitro degradation study. | |
3.11 Entrapment efficiency and drug loading
Cur, being a hydrophobic molecule, has limited solubility in water. As the preparation of PEC and the PEC nanocomposite is achieved in aqueous medium, significant entrapment of Cur is difficult during the PEC formation. To overcome this challenge, entrapment by the swelling method, achieved using a mixture of solvents, ethanol and water is adopted, favouring the solubility and entrapment of Cur. Entrapment of hydrophilic, water-soluble drug 5-Fu was achieved by the swelling method in an aqueous medium. Based on the absorbance measurements, the DL values for PEC and PEC-Ag(6%) were found to be 0.025 and 0.046 g g−1, respectively, corresponding to an entrapment efficiency of 41.53 and 73.0% for 5-Fu. On the other hand, DL for PEC and PEC-Ag(6%) observed for Cur was 0.029 g g−1 and 0.051 g g−1 leading to an entrapment efficiency of 47.38 and 81.86%, respectively. The higher DL and EE observed in PEC-Ag(6%) for both drugs are attributed to the higher surface area of PEC-Ag(6%) attributed to the presence of SNps.67 Trimethylation of CHN enhanced the hydrophobic character of the polymer matrix, which enhances the absorption of Cur through hydrophobic interactions.67 The involvement of electronegative atoms like N,O, and F of 5-Fu with amino and hydroxyl groups of PEC through hydrogen bonding interactions appears to be responsible for higher entrapment of 5-Fu.68 Regardless of the limited efficiency of the designed material to load the drugs, the ratio and amounts of the incorporated drugs are still significant. The dose-dependent toxicity of 5-Fu is reduced by compensating some of its content with Cur, a non-toxic anticancer drug. This could improve the biocompatibility of the drug-loaded system and overall therapeutic outcomes.69,70
3.12
In vitro drug release profile
The drug release profile is a significant feature for deciding the usage of the polymer matrices in drug delivery applications. As the material can be retained in the stomach for a maximum 3 h and spends relatively more time, about 6 h, in the intestinal tract,29 the release profiles of the two entrapped drugs from the polymer matrices have been studied, in the present work, for 4 h in pH 1.2 medium and for 24 h in pH 7.4 medium.15,71Fig. 9a indicates 95.20% release of 5-Fu from the PEC nanocomposite and 77.76% from the PEC matrix at pH 7.4, which is much higher compared to the extent of release from these matrices at pH 1.2. The data was recorded for the initial 4 h only. The weak hydrogen bonding interactions between 5-Fu and the polymer matrix, combined with a higher solubility of 5-Fu in water, facilitate the diffusion of 5-Fu into the surrounding medium, leading to increased release at pH 7.4. The lower swelling of the matrices at pH 1.2 and reduced solubility of 5-Fu in pH 1.2 lowers the extent of release to 39.29 and 27.27% in PEC-Ag(6%) and PEC, respectively. The corresponding plots for the release of Cur at two different pH conditions are shown in Fig. 9b. At pH 7.4 the PEC nanocomposite exhibits the highest release of about 83.77%, whereas the parent PEC exhibited much lower release (52.26%), and the difference in the maximum release observed for the nanocomposite and the PEC was significant in the case of Cur compared to 5-Fu. A similar trend is observed for the release of Cur at pH 1.2, it being 52.85 and 21.32% for the nanocomposite and the PEC respectively. In general, the release of Cur and 5-Fu from the polymer matrices occurs in a sustained manner. At both pH conditions, the drug release from the nanocomposite was higher than from the PEC sample. The addition of SNps improved the drug release efficiency of the matrix due to an increase in the surface area for drug diffusion.72,73 Also, the drug release data of the polymer matrices are in good agreement with the swelling data, with higher swelling being observed at pH 7.4.74 In earlier studies, similar observation of cumulative release of 56% of 5-Fu and 37% Cur during 24 h has been reported at pH 7.4 from chemically crosslinked xylan-β-cyclodextrin hydrogel.27
 |
| | Fig. 9 Cumulative release of drugs from PEC & PEC-Ag(6%): (a) 5-Fu & (b) Cur. | |
The designed nanocomposite helps to improve the solubility of Cur, which otherwise is practically insoluble in aqueous conditions, resulting in low absorption and bioavailability. In addition, sustained release of 5-Fu could be achieved from the nanocomposite reducing the dosing frequency and accompanying toxic effects of frequent dosing. The existing oral formulations, tablets, capsules and liquid preparations, could not remarkably address the above issues. Thus, the cumulative drug release study, in the present case, shows promising results, which contribute to the progression of the field of combination drug delivery.
3.12.1 Drug release kinetics.
The drug release kinetic data obtained for the two polymer systems at pH 7.4 and 1.2 were fitted into various kinetic models, as shown in Fig. 10(a–c). The goodness of the fit is decided based on the R2 values presented in Table 4. The data for release of 5-Fu and Cur are best fitted to the first-order kinetic model. This suggests release of drugs to be proportional to the concentration of drugs incorporated into the polymer matrices.75 The fit of data to the Higuchi square root model suggests that the release is governed by the diffusion of the medium into the polymer matrix leading to the dissolution of drugs.15 The ‘n’ value obtained from the Korsmeyer–Peppas model is helpful in understanding the mechanism of diffusion. At pH 7.4, the release of 5-Fu appears to follow the non-Fickian diffusion, which implies that diffusion of 5-Fu is related to the concentration of the entrapped drug. Release of Cur, on the other hand, follows Fickian diffusion (anomalous transport), as the polymer chain relaxation and the drug diffusion occur simultaneously with comparable rates.76
 |
| | Fig. 10 The fit of drug release data to kinetic models at pH 7.4: (a) first order, (b) Higuchi and (c) Korsmeyer–Peppas model. | |
Table 4 The kinetic and diffusion parameters for the release of 5-Fu and Cur from the polymer matrices
| Sample code |
pH |
Drug |
Zero order (R2) |
First order (R2) |
Higuchi (R2) |
Korsmeyer–Peppas (R2) |
n
|
| PEC |
1.2 |
5-Fu |
0.87 |
0.83 |
0.91 |
0.48 |
0.58 |
| Cur |
0.64 |
0.73 |
0.86 |
0.77 |
0.43 |
| 7.4 |
5-Fu |
0.97 |
0.99 |
0.99 |
0.99 |
0.57 |
| Cur |
0.99 |
0.99 |
0.98 |
0.98 |
0.34 |
| PEC-Ag (6%) |
1.2 |
5-Fu |
0.88 |
0.95 |
0.97 |
0.97 |
0.62 |
| Cur |
0.96 |
0.94 |
0.91 |
0.93 |
0.35 |
| 7.4 |
5-Fu |
0.99 |
0.96 |
0.99 |
0.99 |
0.56 |
| Cur |
0.99 |
0.98 |
0.99 |
0.98 |
0.27 |
3.13
In vitro cytotoxicity study
Cytotoxicity restricts the clinical use of polymer matrices in drug delivery applications. The evaluation of cytotoxicity of PEC-Ag(6%) was carried out using an MTT assay against MCF-10A normal cell lines at various concentrations of PEC-Ag(6%) ranging from 6.25 to 100 μg mL−1. As shown in Fig. 11a, PEC-Ag(6%), in all concentrations, has not resulted in obvious cell death in the MCF-10A cell line after incubation for 24 h. The minimum cell viability was determined to be 94.42% at the highest tested concentration. These findings demonstrate that the designed nanocomposite is biocompatible and suitable to be utilized for the co-delivery of anticancer drugs. A cell viability study was also carried out to determine the synergetic effects of free drugs (5-Fu, Cur, and 5-Fu + Cur) and drug-loaded samples (PEC-Ag(6%)/5-Fu, PEC-Ag(6%)/Cur and PEC-Ag(6%)/5-Fu + Cur) on the MCF-7 cancer cell line for 24 h and the results are depicted in Fig. 11b. After 24 h of treatment of cells with samples, the cell viability significantly decreased with increasing concentration. The results indicate that in the combined form, the drugs exhibit lower cell viability compared to their individual forms. Also, the free drugs in individual forms exhibit higher cytotoxicity compared to their corresponding drug loaded samples. The observed results are attributed to differences in the cellular uptake pathways of free drugs and drug-loaded materials. Free drugs permeate the cells via passive diffusion across the cell membrane, allowing for rapid intracellular distribution. In contrast, drug-loaded materials are internalized through the endocytosis pathway.77 The IC50 value was lowest for PEC-Ag(6%)/5-Fu + Cur compared to all other formulations. This demonstrates the synergistic effect of 5-Fu and Cur. Thus, the combination drug delivery strategy reduces the toxic effects of 5-Fu and improves treatment efficacy by fine-tuning the concentrations needed to achieve the desired results. Combining 5-Fu and Cur in a polymer nanocomposite shows potential, as it enables lower drug dosages and alleviates concerns about toxicity.
 |
| | Fig. 11 % cell viability vs. concentration on (a) MCF-10A and (b) MCF-7 cell lines. | |
4. Conclusions
The PEC nanocomposite made of CMKG and TMC with SNps incorporated within the matrix was found to be highly suitable for oral delivery of dual drugs, namely 5-Fu and Cur. The FT-IR, FE-SEM, P-XRD, HR-TEM, EDS, TGA and Zp data provided valuable insights into the material's morphological, structural, and thermal characteristics. The polymer nanocomposite exhibited pH-responsiveness in swelling and drug release. The drug release was higher at pH 7.4 compared to pH 1.2. The presence of SNps influenced the swelling and drug release behaviour of the matrix by enhancing the hydrophilicity and surface area of the material. The drug release pattern observed through the in vitro release study demonstrated that at pH 1.2, Cur is released to a higher extent than 5-Fu from the nanocomposite matrix whereas at pH 7.4, the reverse trend is observed. The results demonstrated the potential use of the polymer nanocomposite to release 5-Fu and Cur in a sustained manner over 24 h. The cytotoxicity of the material assessed through an in vitro study was low, indicating the material to be biocompatible. The developed nanocomposite, PEC-Ag(6%)/5-Fu + Cur, has been proven to be highly suitable for the co-delivery of 5-Fu and Cur which can significantly impact the treatment of cancer. This research establishes a strong basis for further investigation and advancement of PEC nanocomposites as effective co-drug delivery systems in cancer treatment, significantly contributing to the field of nanomedicine.
Author contributions
Rakshitha: methodology, investigation, data curation and writing the original manuscript; Manohar M: data curation related to in vitro drug release study; Rompicherla. Narayana Charyulu: visualized and reviewed the data related to in vitro drug release study. Vishalakshi B.: conceptualization, formal analysis and supervision, review and editing.
Data availability
Data will be made available on request.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors gratefully acknowledge STIC INDIA, CUSAT for providing HR-TEM analysis and DST-PURSE, Mangalore University for providing characterization facilities.
References
- R. Singh, A. Prasad, B. Kumar, S. Kumari, R. K. Sahu and S. T. Hedau, ChemistrySelect, 2022, 7, e202201288 CrossRef CAS.
- M. Rahman, F. Chowdhury, K. Uddin, K. S. Ahmed, H. Hossain, P. Jain, H. M. Reza, K. Lee and S. M. Sharker, Int. J. Biol. Macromol., 2023, 241, 124701 CrossRef CAS PubMed.
- A. Okem, C. Henstra, M. Lambert and R. Hayeshi, Med. Drug Discovery, 2023, 17, 100147 CrossRef CAS.
- T. Z. Khan, S. M. Newaj, A. Rahman, R. Tabassum, K. N. Tasnim, H. M. Reza, M. S. Reza, S. Hong and S. M. Sharker, Mater. Adv., 2023, 4, 5175–5183 RSC.
- Y. Hu, S. Hu, S. Zhang, S. Dong, J. Hu, L. Kang and X. Yang, Sci. Reports, 2021, 11, 1–14 Search PubMed.
- B. Brianna and S. H. Lee, Med. Oncol., 2023, 40, 1–13 CrossRef PubMed.
- S. E. Herrera, M. L. Agazzi, E. Apuzzo, M. L. Cortez, W. A. Marmisollé, M. Tagliazucchi and O. Azzaroni, Soft Matter, 2023, 19, 2013–2041 RSC.
- D. Wu, L. Zhu, Y. Li, X. Zhang, S. Xu, G. Yang and T. Delair, Carbohydr. Polym., 2020, 238, 116126 CrossRef CAS PubMed.
- D. Yang, L. Gong, Q. Li, B. Fan, C. Ma and Y. C. He, Int. J. Biol. Macromol., 2023, 227, 524–534 CrossRef CAS PubMed.
- S. Manoj Lalwani, C. I. Eneh and J. L. Lutkenhaus, Phys. Chem. Chem. Phys., 2020, 22, 24157–24177 RSC.
- N. Prasad, N. Thombare, S. C. Sharma and S. Kumar, Polym. Bull., 2023, 80, 3425–3447 CrossRef CAS.
- M. Dhua, S. Maiti and K. K. Sen, Int. J. Biol. Macromol., 2020, 164, 1889–1897 CrossRef CAS PubMed.
- S. Manna, A. Seth, P. Gupta, G. Nandi, R. Dutta, S. Jana and S. Jana, ACS Biomater. Sci. Eng., 2023, 9, 2181–2202 CrossRef CAS PubMed.
- D. H. Hanna, M. H. El-Mazaly and R. R. Mohamed, Int. J. Biol. Macromol., 2023, 231, 123364 CrossRef CAS PubMed.
- A. Luanda, M. Manohar, R. N. Charyulu and V. Badalamoole, Int. J. Biol. Macromol., 2024, 268, 131783 CrossRef CAS PubMed.
- T. Xu, P. Guo, C. Pi, Y. He, H. Yang, Y. Hou, X. Feng, Q. Jiang, Y. Wei and L. Zhao, J. Cancer, 2020, 11, 3955 CrossRef CAS PubMed.
- A. Anitha, N. Deepa, K. P. Chennazhi, V. K. Lakshmanan and R. Jayakumar, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 2730–2743 CrossRef CAS PubMed.
- S. Dhanavel, T. A. Revathy, T. Sivaranjani, K. Sivakumar, P. Palani, V. Narayanan and A. Stephen, Polym. Bull., 2020, 77, 213–233 CrossRef CAS.
- H. Sadeghi-Abandansari, S. Pakian, M. R. Nabid, M. Ebrahimi and A. Rezalotfi, Eur. Polym. J., 2021, 157, 110646 CrossRef CAS.
- N. Gao, S. Lü, C. Gao, X. Wang, X. Xu, X. Bai, C. Feng and M. Liu, Chem. Eng. J., 2016, 287, 20–29 CrossRef CAS.
- M. H. Abu Elella, E. S. Goda, H. M. Abdallah, M. M. Abdel-Aziz and H. Gamal, Carbohydr. Polym., 2023, 303, 120443 Search PubMed.
- B. R. Gangapuram, R. Bandi, R. Dadigala, G. M. Kotu and V. Guttena, J. Clust. Sci., 2017, 28, 2873–2890 CrossRef CAS.
- M. H. Abu Elella, A. E. Shalan, M. W. Sabaa and R. R. Mohamed, RSC Adv., 2022, 12, 1095–1104 RSC.
- H. Gong, M. Liu, J. Chen, F. Han, C. Gao and B. Zhang, Carbohydr. Polym., 2012, 88, 1015–1022 CrossRef CAS.
- S. Khan and N. Anwar, Carbohydr. Polym., 2021, 257, 117617 CrossRef CAS PubMed.
- S. Rahmani, A. Olad and Z. Rahmani, Polym. Bull., 2023, 80, 4117–4138 CrossRef CAS.
- P. Gami, D. Kundu, S. D. K. Seera and T. Banerjee, Int. J. Biol. Macromol., 2020, 158, 18–31 CrossRef CAS PubMed.
- J. Singh and P. Nayak, J. Polym. Sci., 2023, 61, 2828–2850 CrossRef CAS.
- D. M. Mudie, G. L. Amidon and G. E. Amidon, Mol. Pharm., 2010, 7, 1388–1405 CrossRef CAS PubMed.
- P. V. S. Rudhrabatla, R. Jalababu, K. S. V. Krishna Rao and K. V. N. Suresh Reddy, J. Drug Delivery Sci. Technol., 2019, 51, 438–453 CrossRef.
- T. S. Anirudhan, P. L. Divya and J. Nima, Chem. Eng. J., 2016, 284, 1259–1269 CrossRef CAS.
- S. M. Sharker, S. M. Kim, S. H. Kim, I. In, H. Lee and S. Y. Park, J. Mater. Chem. B, 2015, 3, 5833–5841 RSC.
- D. de Britto and O. B. G. Assis, Carbohydr. Polym., 2007, 69, 305–310 CrossRef CAS.
- T. Xu, M. Xin, M. Li, H. Huang and S. Zhou, Carbohydr. Polym., 2010, 81, 931–936 CrossRef CAS.
- W. Sajomsang, P. Gonil and S. Saesoo, Eur. Polym. J., 2009, 45, 2319–2328 CrossRef CAS.
- Y. Jin, D. Zhou, H. Y. Yang, X. Zhu, X. R. Wang, Z. R. Zhang and Y. Huang, Pharm. Dev. Technol., 2012, 17, 719–729 CrossRef CAS PubMed.
- K. B. Rufato, P. R. Souza, A. C. de Oliveira, S. B. R. Berton, R. M. Sabino, E. C. Muniz, K. C. Popat, E. Radovanovic, M. J. Kipper and A. F. Martins, Int. J. Biol. Macromol., 2021, 183, 727–742 CrossRef CAS PubMed.
- B. K. Preetha and B. Vishalakshi, Int. J. Biol. Macromol., 2020, 154, 739–750 CrossRef CAS PubMed.
- M. H. Abu Elella, D. H. Hanna, R. R. Mohamed and M. W. Sabaa, Polym. Bull., 2022, 79, 2501–2522 CrossRef CAS.
- D. C. M. Ferreira, S. O. Ferreira, E. S. de Alvarenga, N. de F. F. Soares, J. S. dos R. Coimbra and E. B. de Oliveira, Carbohydr. Polym. Technol. Appl, 2022, 3, 100197 CAS.
- H. Ghasemzadeh, S. Afraz, M. Moradi and S. Hassanpour, Int. J. Biol. Macromol., 2021, 179, 532–541 CrossRef CAS PubMed.
- R. Kaur, D. Goyal and S. Agnihotri, Carbohydr. Polym., 2021, 262, 117906 CrossRef CAS PubMed.
- A. B. Suresh, M. R. Rajeev and T. S. Anirudhan, J. Environ. Chem. Eng., 2023, 11, 109862 CrossRef CAS.
- L. Huang, W. Sui, Y. Wang and Q. Jiao, Carbohydr. Polym., 2010, 80, 168–173 CrossRef CAS.
- M. Shu, S. Long, Y. Huang, D. Li, H. Li and X. Li, Soft Matter, 2019, 15, 7686–7694 RSC.
- C. V. Pardeshi and V. S. Belgamwar, Int. J. Biol. Macromol., 2016, 82, 933–944 CrossRef CAS PubMed.
- C. Tan, J. Xie, X. Zhang, J. Cai and S. Xia, Food Hydrocolloids, 2016, 57, 236–245 CrossRef CAS.
- S. M. Newaj, T. B. Kashem, J. Ferdous, I. Jahan, H. Rawshan, N. J. Prionty, R. Rakib, M. A. Sadman, F. Bin Faruk, H. M. Reza and S. M. Sharker, ACS Appl. Bio Mater., 2024, 7, 6313–6324 CrossRef CAS PubMed.
- N. P. Birch and J. D. Schiffman, Langmuir, 2014, 30, 3441–3447 CrossRef CAS PubMed.
- K. N. Tasnim, A. Rahman, S. M. Newaj, O. Mahmud, S. Monira, T. Z. Khan, H. M. Reza, M. Shin and S. M. Sharker, ACS Appl. Bio Mater., 2024, 7, 3190–3201 CrossRef CAS PubMed.
- T. Delair, Eur. J. Pharm. Biopharm., 2011, 78, 10–18 CrossRef CAS PubMed.
- M. H. Abu Elella, R. R. Mohamed, M. M. Abdel-Aziz and M. W. Sabaa, Int. J. Biol. Macromol., 2018, 111, 706–716 CrossRef CAS PubMed.
- I. Gholamali, M. Asnaashariisfahani and E. Alipour, Regen. Eng. Transl. Med., 2020, 6, 138–153 CrossRef CAS.
- N. Barroso, O. Guaresti, L. Pérez-Álvarez, L. Ruiz-Rubio, N. Gabilondo and J. L. Vilas-Vilela, Eur. Polym. J., 2019, 120, 109268 CrossRef CAS.
- Z. Shen, G. Han, X. Wang, J. Luo and R. Sun, J. Mater. Chem. B, 2017, 5, 1155–1158 RSC.
- M. P. M. Da Costa, I. L. De Mello Ferreira and M. T. De Macedo Cruz, Carbohydr. Polym., 2016, 146, 123–130 CrossRef CAS PubMed.
- A. K. Kodoth, V. M. Ghate, S. A. Lewis and V. Badalamoole, Int. J. Biol. Macromol., 2018, 115, 418–430 CrossRef CAS PubMed.
- H. Daud, A. Ghani, D. N. Iqbal, N. Ahmad, S. Nazir, M. J. Muhammad, E. A. Hussain, A. Nazir and M. Iqbal, Arab. J. Chem., 2021, 14, 103111 CrossRef CAS.
- A. M. Omer, M. S. Ahmed, G. M. El-Subruiti, R. E. Khalifa and A. S. Eltaweil, Pharmaceutics, 2021, 13, 338 CrossRef CAS PubMed.
- M. W. El-Maadawy, R. R. Mohamed and D. H. Hanna, OpenNano, 2022, 7, 100050 CrossRef CAS.
- P. Le Thi, Y. Lee, T. T. Hoang Thi, K. M. Park and K. D. Park, Mater. Sci. Eng. C, 2018, 92, 52–60 CrossRef PubMed.
- J. Zhao, S. Li, Y. Zhao and Z. Peng, Polymer Bull., 2020, 77, 4401–4416 CrossRef CAS.
- A. Luanda, M. Mahadev, R. N. Charyulu and V. Badalamoole, Int. J. Biol. Macromol., 2024, 282, 137097 CrossRef CAS PubMed.
- R. A. McBath and D. A. Shipp, Polym. Chem., 2010, 1, 860–865 RSC.
- M. Brito-Arias, Synth. Charact. Glycosides, 2022, 459–475 Search PubMed.
- S. O. Solomevich, E. I. Dmitruk, P. M. Bychkovsky, D. A. Salamevich, S. V. Kuchuk and T. L. Yurkshtovich, Int. J. Biol. Macromol., 2021, 169, 500–512 CrossRef CAS PubMed.
- A. F. Martins, P. V. A. Bueno, E. A. M. S. Almeida, F. H. A. Rodrigues, A. F. Rubira and E. C. Muniz, Int. J. Biol. Macromol., 2013, 57, 174–184 CrossRef CAS PubMed.
- G. Li, S. Song, T. Zhang, M. Qi and J. Liu, Int. J. Biol. Macromol., 2013, 62, 203–210 CrossRef CAS PubMed.
- U. Hess, S. Shahabi, L. Treccani, P. Streckbein, C. Heiss and K. Rezwan, Mater. Sci. Eng., C, 2017, 77, 427–435 CrossRef CAS PubMed.
- M. Khafaji, M. Zamani, M. Vossoughi and A. I. Zad, Int. J. Nanomedicine, 2019, 14, 8769–8786 CrossRef CAS PubMed.
- C. E. Iurciuc-Tincu, L. I. Atanase, L. Ochiuz, C. Jérôme, V. Sol, P. Martin and M. Popa, Int. J. Biol. Macromol., 2020, 147, 629–642 CrossRef CAS PubMed.
- P. R. S. Reddy, K. M. Rao, K. S. V. K. Rao, Y. Shchipunov and C. S. Ha, Macromol. Res., 2014, 22, 832–842 CrossRef CAS.
- R. R. Palem, G. Shimoga, K. S. V. K. Rao, S. H. Lee and T. J. Kang, J. Polym. Res., 2020, 27, 1–20 CrossRef.
- S. Eswaramma, N. S. Reddy and K. S. V. K. Rao, Int. J. Biol. Macromol., 2017, 103, 1162–1172 CrossRef CAS PubMed.
- K. Tai, M. Rappolt, X. He, Y. Wei, S. Zhu, J. Zhang, L. Mao, Y. Gao and F. Yuan, Food Chem., 2019, 293, 92–102 CrossRef CAS PubMed.
- T. S. Anirudhan, B. S. Bini and V. Manjusha, J. Mol. Liq., 2021, 340, 116852 CrossRef CAS.
- P. Guo, C. Pi, S. Zhao, S. Fu, H. Yang, X. Zheng, X. Zhang, L. Zhao and Y. Wei, Expert Opin. Drug Delivery, 2020, 17, 1473–1484 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a
*a




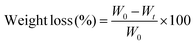
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water mixture containing 0.5 mg mL−1 Cur. The polymer samples were kept in dark conditions for 24 h. The Cur-loaded samples were then taken out and dried. The concentration of the Cur loaded into the polymer matrix was estimated by measuring the absorbance of the Cur solution before and after loading at 429 nm, the λmax of the Cur solution, using a UV-Vis spectrophotometer (Shimadzu UV1900, Japan). For the loading of 5-Fu, Cur-loaded samples were soaked in an aqueous solution of 5-Fu (0.5 mg mL−1). The amount of 5-Fu loaded was quantified from the absorbance measurements of 5-Fu solution before and after loading recorded at 266 nm, the λmax of 5-Fu. The EE (%) and DL (g g−1) were calculated using eqn (6) and (7), respectively.
1 ethanol–water mixture containing 0.5 mg mL−1 Cur. The polymer samples were kept in dark conditions for 24 h. The Cur-loaded samples were then taken out and dried. The concentration of the Cur loaded into the polymer matrix was estimated by measuring the absorbance of the Cur solution before and after loading at 429 nm, the λmax of the Cur solution, using a UV-Vis spectrophotometer (Shimadzu UV1900, Japan). For the loading of 5-Fu, Cur-loaded samples were soaked in an aqueous solution of 5-Fu (0.5 mg mL−1). The amount of 5-Fu loaded was quantified from the absorbance measurements of 5-Fu solution before and after loading recorded at 266 nm, the λmax of 5-Fu. The EE (%) and DL (g g−1) were calculated using eqn (6) and (7), respectively.



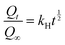

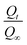 is the fraction of the drug released from the drug-loaded sample at time ‘t’; and ‘n’ is the diffusion coefficient. The best-fitting model was decided based on the R2 values. The diffusion mechanism is indicated as Fickian diffusion (n ≤ 0.5), non-Fickian diffusion (0.5 < n ≤ 1), case I transport (n = 1.0) or super case II transport (n > 1) based on the value of n obtained from the Korsmeyer–Peppas model fit.31
is the fraction of the drug released from the drug-loaded sample at time ‘t’; and ‘n’ is the diffusion coefficient. The best-fitting model was decided based on the R2 values. The diffusion mechanism is indicated as Fickian diffusion (n ≤ 0.5), non-Fickian diffusion (0.5 < n ≤ 1), case I transport (n = 1.0) or super case II transport (n > 1) based on the value of n obtained from the Korsmeyer–Peppas model fit.31

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of mixing. At this composition, the highest degree of bonding exists between TMC and CMKG, facilitating the formation of insoluble PEC. At higher TMC:CMKG ratios, higher turbidity was observed indicating an increase in the soluble component of PEC. This was also reflected in the decreasing yield of the PEC precipitate with higher ratio. The weight ratio of TMC
1 weight ratio of mixing. At this composition, the highest degree of bonding exists between TMC and CMKG, facilitating the formation of insoluble PEC. At higher TMC:CMKG ratios, higher turbidity was observed indicating an increase in the soluble component of PEC. This was also reflected in the decreasing yield of the PEC precipitate with higher ratio. The weight ratio of TMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMKG at 1
CMKG at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is considered to be optimum for obtaining maximum yield of PEC. The effect of ratio of components on the yield of PEC is shown in Table 1. The schematic representation of the formation of PEC through electrostatic interaction between TMC and CMKG is given in Scheme 1.
1 is considered to be optimum for obtaining maximum yield of PEC. The effect of ratio of components on the yield of PEC is shown in Table 1. The schematic representation of the formation of PEC through electrostatic interaction between TMC and CMKG is given in Scheme 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMKG (weight ratio)
CMKG (weight ratio)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of acetylated carboxylic acid groups present in KG. Additionally, the carbonyl stretching vibration of carboxylate ions was observed at 1579 (asymmetric) and 1416 cm−1 (symmetric).38 In the FT-IR spectra of CMKG (Fig. 3d), the band at 3408 cm−1 corresponds to –OH stretching, shifted to a higher wavenumber indicating the reduction in the hydrogen bonds due to the substitution of hydroxyl with the carboxymethyl group. The substantial shift of the –OH band from the bonded region into the non-bonded region suggests the involvement of –OH groups in the substitution reaction. Furthermore, the band at 1718 cm−1 is attributed to C
O stretching of acetylated carboxylic acid groups present in KG. Additionally, the carbonyl stretching vibration of carboxylate ions was observed at 1579 (asymmetric) and 1416 cm−1 (symmetric).38 In the FT-IR spectra of CMKG (Fig. 3d), the band at 3408 cm−1 corresponds to –OH stretching, shifted to a higher wavenumber indicating the reduction in the hydrogen bonds due to the substitution of hydroxyl with the carboxymethyl group. The substantial shift of the –OH band from the bonded region into the non-bonded region suggests the involvement of –OH groups in the substitution reaction. Furthermore, the band at 1718 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of partially acetylated carboxylic acid groups observed in KG, which is not seen in CMKG due to alkaline hydrolysis occurring at the ester group.12,22
O of partially acetylated carboxylic acid groups observed in KG, which is not seen in CMKG due to alkaline hydrolysis occurring at the ester group.12,22![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C
O, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –C–O–C– groups, respectively. Additionally, the bands seen at 959 and 811 cm−1 are due to bending of –C–H.43 The FT-IR spectrum of 5-Fu (Fig. 3h) features a band at 1652 cm−1, indicating the overlapping of the stretching vibrations of C
C– and –C–O–C– groups, respectively. Additionally, the bands seen at 959 and 811 cm−1 are due to bending of –C–H.43 The FT-IR spectrum of 5-Fu (Fig. 3h) features a band at 1652 cm−1, indicating the overlapping of the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. The 1426 cm−1 band corresponds to the in-plane bending of C–H while the 1249 cm−1 band represents C–N stretching vibrations. The band at 879 cm−1 corresponds to the bending vibration of C–H bonds of the –CF
C. The 1426 cm−1 band corresponds to the in-plane bending of C–H while the 1249 cm−1 band represents C–N stretching vibrations. The band at 879 cm−1 corresponds to the bending vibration of C–H bonds of the –CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– group, whereas the out of plane bending vibrations of these bonds appear at 815 cm−1.44 The presence of the characteristic bands of 5-Fu and Cur observed in the FT-IR spectra of the drug-loaded PEC sample (Fig. 3i) and of the nanocomposite PEC-Ag(6%) (Fig. 3j) confirms the successful incorporation of 5-Fu + Cur into the polymer matrices.
CH– group, whereas the out of plane bending vibrations of these bonds appear at 815 cm−1.44 The presence of the characteristic bands of 5-Fu and Cur observed in the FT-IR spectra of the drug-loaded PEC sample (Fig. 3i) and of the nanocomposite PEC-Ag(6%) (Fig. 3j) confirms the successful incorporation of 5-Fu + Cur into the polymer matrices.