Open Access Article
Open Access ArticleMetallic nanomaterials in Parkinson's disease: a transformative approach for early detection and targeted therapy
Amira
Mansour†
,
Mariam Hossam
Eldin†
and
Ibrahim M.
El-Sherbiny
 *
*
Nanomedicine Laboratories, Center for Materials Science, Zewail City of Science and Technology, 6th October City, 12578 Giza, Egypt. E-mail: ielsherbiny@zewailcity.edu.eg
First published on 17th February 2025
Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by substantial loss of dopaminergic neurons in the substantia nigra, leading to both motor and non-motor symptoms that significantly impact quality of life. The prevalence of PD is expected to increase with the aging population, affecting millions globally. Current detection techniques, including clinical assays and neuroimaging, lack the sensitivity and specificity to sense PD in its earliest stages. Despite extensive research, there is no cure for PD, and available treatments primarily focus on symptomatic relief rather than halting disease progression. Conventional treatments, such as levodopa and dopamine agonists, provide limited and often temporary relief, with long-term use associated with significant side effects and diminished efficacy. Nanotechnology, particularly the use of metallic-based nanomaterials (MNMs), offers a promising approach to overcome these limitations. MNMs, due to their unique physicochemical properties, can be engineered to target specific cellular and molecular mechanisms involved in PD. These MNMs can improve drug delivery, enhance imaging and biosensing techniques, and provide neuroprotective effects. For example, gold and silver nanoparticles have shown potential in crossing the blood–brain barrier, providing real-time imaging for early diagnosis and delivering therapeutic agents directly to the affected neurons. This review aims to reveal the current advancements in the use of MNMs for the detection and treatment of PD. It will provide a comprehensive overview of the limitations of conventional detection techniques and therapies, followed by a detailed discussion on how nanotechnology can address these challenges. The review will also highlight recent preclinical research and examine the potential toxicity of MNMs. By emphasizing the potential of MNMs, this review article aims to underscore the transformative impact of nanotechnology in revolutionizing the detection and treatment of PD.
1. Introduction
Parkinson's disease (PD) is a public debilitating progressive neurodegenerative disorder primarily affecting the elderly. It ranks second in prevalence after Alzheimer's disease. The prevalence of PD has increased over the past 40 years compared to previous periods, reaching about 9% among the elderly population over 60 years, with a higher incidence in males. This gender-related occurrence is thought to be due to the potential neuroprotective effects of estrogen.1,2 According to the WHO, the population affected by PD is expected to rise to about 2 million by 2030.3,4 Although the etiology of PD is not yet fully understood, it is attributed to both non-genetic factors, such as dietary imbalances and pesticides, and genetic mutations related to α-syn and dopamine, which are more prevalent in patients under 40 years of age.3,5 To achieve a definitive diagnosis and efficient therapy, the pathophysiology of PD should be elucidated. While the PD etiology remains unclear, ongoing research aims to provide a coherent and substantial mechanistic explanation.The dopaminergic neurons of the substantia nigra (SN) provide dopamine to the striatum. In PD, the substantia nigra pars compacta (SNpc) neurons, which are responsible for motor functions, are disrupted, leading to a depletion of extracellular dopamine in the synapse. This results in striatal malfunction and induces motor disability.6–9 Other coherently pertinent factors that exacerbate the PD ailment include altered mitochondrial function, triggered cerebral inflammatory response, exacerbated ROS generation and activation of microglia.10–12
Remarkably, the aggregation of α-synuclein (α-syn) and the formation of Lewy bodies within the dopaminergic neurons of the substantia nigra are some of the main hallmarks. The α-syn protein is composed of 140 amino acids divided into 3 regions; N-terminal, amyloid-binding central domain and C-terminal. The first region is responsible for the protein's aggregation where the phosphorylation of serine-129 within the last region abrogates this event.13–15 The aggregation of α-syn contributes to the dissipation of dopaminergic neurons within the SNpc leading to dopamine deficiency.16,17
The PD development and progression are mostly explained by the Braak hypothesis that divides the disease into six stages, where the symptoms start with olfactory disorders and end with cognitive impairment.18,19 PD symptoms can be classified as motor and non-motor symptoms as well (Fig. 1). Neuromotor disability is mainly observed as tremors at rest and bradykinesia. The latter class is heterogeneous and includes cognitive disorders in addition to the fast movement of the eye and some non-PD-specific symptoms. All these symptoms culminate in declined life quality and can occasionally embarrass the patients.20–23 The focus of this review is on the inciting development of metallic nanomaterial (MNM)-based detection techniques, therapeutics and theranostics. Furthermore, toxicity, biocompatibility, challenges, and future outlook are delineated.
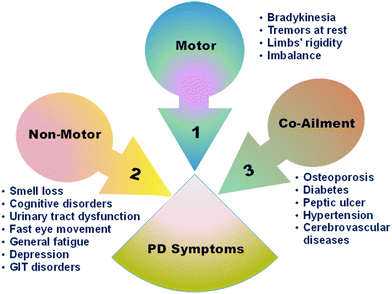 | ||
| Fig. 1 Summary of the symptoms of PD.20,24,25 | ||
2. Detection of PD
PD clinical diagnosis remains a challenge as it is misleading in most cases due to the overlap of its symptoms with other neurodegenerative disorders. Intriguingly, the accumulation of misfolded α-syn protein clumps and the development of Lewy bodies in various parts of the brain are evident to be the primary pathogenic hallmarks of PD. These α-syn aggregates, as well as DA, levodopa (LD), and miRNAs, form essential biomarkers for the early identification and control of this disease.26–29 Current diagnostic platforms involve enzyme-linked immunosorbent assay (ELISA), fluorescence and electrochemical immunoassays, high-performance liquid chromatography (HPLC), real-time polymerase chain reaction (rt-PCR) and neurobiosensors.26,30,31 ELISA is the most widely used method for the detection of markers such as PS65-Ub, indicating any mitochondrial dysfunction, and α-syn, especially in CSF because its levels are higher than those in biofluids.32 Other methods, such as electrochemiluminescence and immunomagnetic reduction are becoming more common and undergo more developments to detect extremely low levels of biomarkers.33 These conventional techniques are used to selectively detect and quantify the levels of DA and α-syn in body fluids such as blood and urine.34 However, they suffer from several limitations, as they can be complex, expensive, time-consuming, and inaccurate, providing false results, and requiring result interpretation.34,35Molecular imaging techniques are also implemented in PD diagnosis; they include magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), positron emission tomography (PET), transcranial ultrasound imaging and their advancements. They provide quantitative information about structural changes in the brain according to their modalities and computer algorithms, and utilize tracers and contrast agents to bind to specific targets and markers.36–38 Nevertheless, they are still expensive and labor-intensive techniques, and this limits their accessibility for PD patients.
2.1. Artificial intelligence (AI)-based PD detection
With the urgent need for development and advancements of PD differential diagnosis techniques, scientists have implemented and integrated artificial intelligence (AI) and machine learning (ML) in this process. They have employed data analytics to accurately diagnose PD in the early stages and it is showing promising results. AI holds a great potential for the automation of diagnosis and accessibility in remote areas with high demands.39,40 They mainly depend on using different models and algorithms to process inputs according to well-established libraries. These inputs can be medical history, genetic information, symptoms or PET images that need expertise to be analyzed. Such inputs are provided to AI systems possibly through wearable devices (WDs), so that they can detect patterns and anomalies indicating PD incidences with remarkable precision. Even breath patterns, as shown in Fig. 2, were proved to have a link with PD, where a study was conducted on nocturnal breath signals to predict the disease.41 The developed model achieved specificity and sensitivity of 82.83% and 86.23%, respectively using wireless signals. While ML uses algorithms to enable computers to process data without being programmed, deep learning (DL) exploits more sophisticated multi-layered neural networks for more complex data analysis and accurate predictions.39 This allowed expanding the sample size, reaching remote places, early decision making, and optimizing more algorithms to outperform human expertise. Moreover, WDs emerged as a promising tool that allows the non-invasive detection and continuous monitoring of PD. They have advanced sensors that capture motion patterns and physiological signals in real time, and collect data to analyze motor functions indicative of PD. The design of these devices was integrated with AI to aid in data interpretation and highlight any deviation from normal motor functions.42 AI also facilitates the continuous improvement of WDs and adaptation through learning from any new inputs for further enhancement of the accuracy.43 Besides, different types of sensors such as inertial, acoustic, optical, electrical, and force sensors were exploited in WDs. Once the patient uses the device, it begins retrieving and collecting specific data to be processed and analyzed using different AI and DL or ML models and algorithms.44 However, this technology suffers from several drawbacks; ML models implemented in the studies lack full description of findings or techniques. Moreover, the number and kind of subjects and their medical history are not accurately reported and assessed, while DL models are limited due to difficulty in handling their datasets.45,46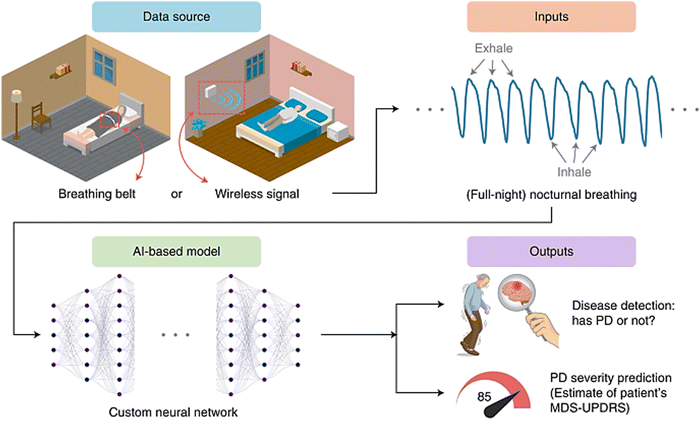 | ||
| Fig. 2 Scheme of AI-based diagnosis process for PD. Breathing patterns are collected through signals or wearable devices and processed using AI models. Based on the analysis, PD is predicted if present, and its severity is determined according to the movement disorder society unified Parkinson's disease rating scale (MDS-UPDRS) questionnaire. Reproduced from ref. 41. | ||
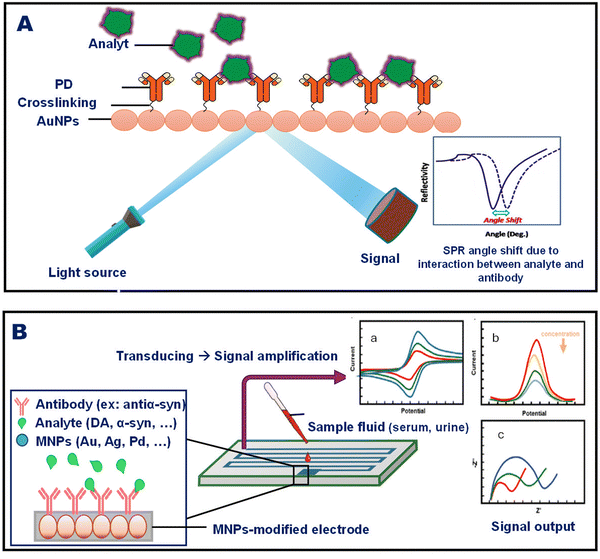
2.2. MNM-based biosensors for the detection of PD
Nanotechnology holds great potential to overcome the aforementioned challenges and confer revolutionary advances in developing diagnostic assays to enable the early detection of this incurable neurological disorder. Although the mechanism of nanomaterials in biosensing remains unclear, they are currently being explored for their ability to offer high sensitivity, specificity and low limit of detection (LOD).47 Metal nanoparticles (MNPs) such as gold and silver nanoparticles (NPs) exhibit unique tunable electronic and optical properties, which make them applicable for the imaging of PD. They can interact with incident light and form characteristic absorption signals that shift depending on their sizes and shapes.48 Moreover, they can be functionalized with specific chemical moieties to be able to cross the blood–brain barrier (BBB) and act as contrast agents or bind to any desired biomarker and quantify it with high sensitivity.49–51 Different metals can be used to develop diagnostic assays for the detection of PD depending on their properties and the type of sensors.52,53 For instance, a study was conducted to develop an immunosensor based on electrochemical impedance spectroscopy. They used an electrode system modified with palladium nanoparticles (PdNPs) to enhance the signals and conductivity. This PdNP-plated electrode was functionalized with α-syn antibodies to quantify the levels of α-syn and epinephrine in clinical serum samples. It showed linear response in phosphate buffer and serum samples with LOD values of 0.13 μg mL−1 and 1.3 μg mL−1, respectively.54 Based on that, research has recently focused mainly on exploiting MNMs to enhance the sensitivity and performance of different types of biosensors. Biosensors can be categorized into immunosensors, DNA- and enzyme-based biosensors, and piezoelectric and thermal biosensors.35,55 This review focuses mainly on different MNMs such as zinc, platinum, gold, silver, cerium, and iron in the fabrication of biosensors for the detection and imaging of PD.Another research work was performed using activated charcoal modified with AuNPs to detect the levels of LD in synthetic serum, urine, and river water. This nanocomposite was deposited on a glassy carbon substrate to fabricate an electrode that measures the electrochemical response of LD by square-wave adsorptive anodic stripping voltammetry. Various factors were also studied according to the Doehlert experimental matrix to assess their impact on the electrode performance. The results indicated the linear detection of LD and LOD of 50 nmol L−1–10 μmol L−1 and 8.2 nmol L−1, respectively.34
Several studies were also conducted using the same integrated nanocomposite with different carbon allotropes to construct an electrochemical immunosensor. The reason is that it proved to have high surface area and conductivity, unique electronic properties, and biocompatibility. For example, recent work was conducted using multi-walled carbon nanotubes with AuNP-doped indium tin oxide electrodes. This electrode was developed to detect levels of DJ-1 protein as a biomarker of PD in cerebrospinal fluids and saliva. The analysis of the biosensor efficiency was performed by electrochemical impedance spectroscopy (EIS), single-frequency impedance, and cyclic voltammetry (CV), and it showed the detection range and LOD of 4.7–4700 fg mL−1 and 0.5 fg ml−1, respectively.59,60
Carbon nanotubes were also used in another work as single-walled (SWCNTs) to fabricate interdigitated electrodes functionalized chemically with anti-α-syn-conjugated Au nanourchin.56 This electrode was designed to detect the levels of α-syn, and the results were confirmed by ELISA technique. Due to the integration of Au nanomaterials into electrodes, higher sensitivity and selectivity were achieved because they can detect low currents. Therefore, the LOD was enhanced from 1 pM using the bare SWCNT electrode to 1 fM after applying conjugated gold nanourchin.56
A different study was conducted using labelled gold nanobipyramids (GNBPs) to construct a lab-on-a-chip diagnostic system based on surface-enhanced Raman scattering (SERS). This nanostructure was anisotropic, which offered sharp tips and edges, and consequently, enriched the plasmonic hot points. In addition, it was functionalized with hairpin DNA and Raman reporter moieties which caused GNBPs to aggregate if the analyte was present. This platform enabled the detection of altered expression of miR-221 and miR-214 as biomarkers of PD through amplified SERS signals due to aggregation.61
SERS technique was also implemented in another study conducted on AuNPs. However, this study aimed to image DA in retinal tissues and live cells. AuNPs were labelled with modified thiol molecules due to their high affinity to the Au surface where DA-positive samples result in the aggregation of functionalized AuNPs. The positive response was detected by the Raman scattering signals due to formed plasmonic hot spots, and it is speculated that this technique can be further applied in the detection of DA in live cells in PD patients.62 Furthermore, the biodistribution of administered gold nanoclusters (AuNCls) was studied as a function of the route of administration in mice as the animal model. Intravenous, intraperitoneal, intranasal, and intragastric routes were assessed, and it was found that AuNCs were mostly located in the brain in the case of intraperitoneal administration. This implies that they had the ability to cross the BBB, and therefore, can be further investigated to use such nanoparticles in the imaging of PD upon irradiation along with treatment.63 Besides, Adam et al. focused on developing a AuNP-based interdigitated tetraelectrode to detect α-syn in the fibril formation process. This electrochemical biosensor was investigated using a cyclic and differential pulse voltammeter, and the results showed a linear range of 1 aM–1 pM and an LOD of 100 aM.64 All these biosensing models will enable the early detection and monitoring of PD once they are well developed and pass the stages of the clinical trials.
The same technique was implemented in another work to detect the levels of DA in situ using AgNPs where the label-free SERS measurements were conducted with a laser of wavelength 488 nm. The results displayed the reproducibility of SERS spectrum when using citrate-reduced AgNPs upon binding to DA through surface adsorption.67 Besides, Rouhani et al. worked on developing a biosensing electrode made of AgNPs with GO as a nanocomposite to detect LD in serum and urine samples. The performance of the modified electrode was measured through CV, and it showed enhanced oxidation–reduction peaks. Different parameters such as concentration of GO, pH, and accumulation time were optimized, and the results showed that this accurate assay achieved an LOD of 0.76 nM and a linear range of 0.003–10 μM of LD.68
Another study employed PEGylated SPIONs conjugated to a W20 antibody as the MRI probe to detect amyloid oligomers in PD transgenic mice. The results proved the BBB penetration of these small SPIONs and showed high magnetic resonance relaxation and significant contrast in T2-weighted image. Therefore, this system was able to detect amyloids at the early stages because of its high sensitivity and biocompatibility.72 Additionally, magnetic IONs were exploited in the biosensing of different biomarkers of PD due to the ease of their functionalization and their high sensitivity. For instance, Yang et al. developed a sensitive immunosensor depending on immunomagnetic reduction (IMR) of magnetic IONs labelled with anti-α-syn. The IMR signals in plasma samples were detected using a magnetosusceptometer, and the results showed that they achieved an LOD and range of α-syn concentration of 0.3 fg mL−1 and 0.1–100 pg mL−1, respectively.73,74 Another work was done using anti-α-syn-functionalized ION hybrids with GO to construct Mg-based micromotors for the detection of α-syn in whole-blood samples. This sensitive electrochemical biosensor enabled the effective capturing of the biomarker in 100 s and its concentration was inversely proportional to the signal amplification capability of the developed biosensor.75 Besides, Zhang et al. investigated ION-based interdigitated electrode for the identification of α-syn through using both the aptamer and the antibody to sandwich the analyte. They enhanced the performance of this biosensor by modifying it with AuNPs where the current changed as a response to binding to the target in a linear manner. The results recorded an LOD of 10 aM and a linear range of 10–107 aM with R2 = 0.9729, thereby enabling the sensitive and accurate detection of α-syn (Fig. 4).76Table 1 summarizes the main studies of MNM-based biosensors of PD.
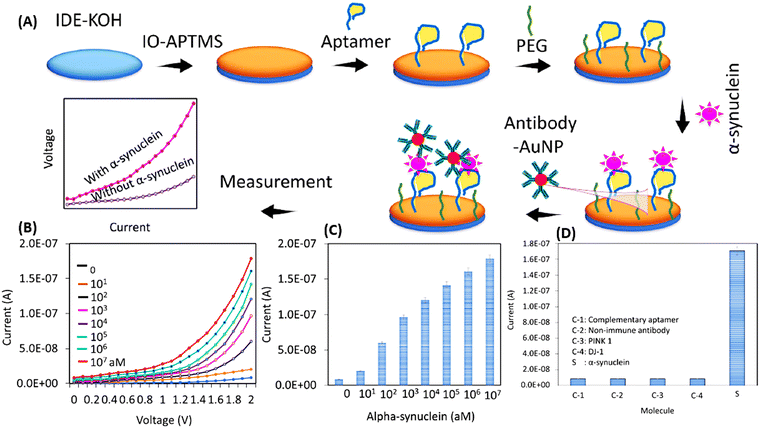 | ||
| Fig. 4 (A) Scheme of the biosensor construction. Iron oxide (IO)-modified aptamers and polyethylene glycol (PEG) were immobilized on the surface of interdigitated di-electrode (IDE), where α-syn is sandwiched between the anti-α-syn aptamer and antibody-AuNPs (B) and (C) quantitative detection of α-syn at various concentrations and (D) selective detection of α-syn in the presence of other analytes. Reproduced from ref. 76. | ||
| Target | Targeting ligand | Sample type | Detection method | Nanomaterial | Linear range | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| α-syn | Rabbit Ab138501 mAb | Serum | Label-free SPR | Iron oxide (Fe3O4) NPs | 0.01–100 pg mL−1 | 5.6 fg mL−1 | 30 |
| α-syn | Specific antibodies | Synthetic cerebrospinal fluid | Electrochemical impedance spectroscopy | Palladium (Pd) NPs | 1.5–15 μg mL−1 | 0.13 μg mL−1 | 54 |
| Epinephrine | — | 0.75–100 μmol L−1 | 0.051 μmol L−1 | ||||
| DA | — | Live human nerve cells | Enzyme-less electrochemical biosensor (CV) | NiAl layered double hydroxides nanosheets integrated with GO | 0.1–97 μM | 2 nM | 77 |
| α-syn | Anti-α-syn | Diluted human sera | Label-free electrochemical immunoassay | Cysteamine-functionalized fluorine-doped tin oxide NPs | 10–1000 ng mL−1 | 1.13 ng mL−1 | 78 |
| Anti-α-syn | Human plasma | Electrochemical immunoassay | Gold nanostars-decorated zinc oxide (ZnO) nanowires | 0.5–10 pg mL−1 | 0.08 pg mL−1 | 79 | |
| Monoclonal antibody | Plasma and serum | Immunomagnetic reduction assay | Magnetic Fe3O4 NPs | ND | In plasma: 3.60 ± 2.53 pg mL−1 | 80 | |
| In serum: 0.03 ± 0.04 pg mL−1 | |||||||
| Thiolated aptamer | Diluted serum | Electrochemiluminescence (ECL) | AuNPs@Metal organic frameworks (MOFs) composite | 2.43 fM–0.486 pM | 0.42 fM | 81 | |
| Carboxylated aptamer | 1.39 fM–0.243 pM | 0.38 fM |
These MN-based electroanalytical techniques have advantages over conventional ones as they require a small volume of sample and exhibit high sensitivity and specificity. They also allow multiplexing where one electrochemical biosensor can be functionalized with various ligands for the detection of multiple analytes simultaneously, and this, as a return, enhances the accuracy of the result. As for the imaging techniques, contrast agents such as IONs enabled higher resolution in MRI and easy penetrability due to their ultra-small sizes, and they can detect any minor changes in the brain structure. However, using MNPs may encounter some limitations where they can be expensive to manufacture, complex according to the design, and difficult in standardization because of the low reproducibility and high reactivity of the nanoparticles. These hurdles require more advanced research and optimization in implementing nanotechnology in diagnostic techniques to overcome such limitations and enhance the nanoparticle applicability.
3. Treatment of PD
BBB is a semi-permeable membrane that surrounds the central nervous system including the brain. It confers selective permeability that constrains the passage of pathogens and toxins but passively transport the essential nutrients for survival and homeostasis.82 On the other hand, this semi-permeability constrains the treatment as only 1% of the therapeutic doses reaches the brain. However, increasing the doses is stumbled by the severity of the adverse effects.16,83 PD is irremediable and the available therapy is mainly palliative for both the motor and non-motor symptoms. Therefore, the patients’ plight is ameliorated through the easiness of the symptoms by increasing the intraneuronal dopamine (DA) or enhancing DA receptors.20 Due to the noted DA deficiency in the PD patient's brains, the efforts lead to the development of a DA precursor, levodopa (L-3,4-dihydroxyphenylalanine, known as L-dopa). L-Dopa is characterized by its ability to penetrate the BBB and get converted into DA when absorbed by the nerve cells. Hitherto, the DA precursor, L-dopa, is the indispensable choice for the treatment and it exerts its effect through replenishing the deficiency. L-Dopa can be undesirably converted to DA in the peripheral nerves; therefore, carbidopa (peripheral dopa-carboxylase inhibitor) is co-administered with L-dopa.84 However, DA does not exert efficient remedial effect on the non-motor symptoms as hallucination and can culminate dopaminergic “type A” adverse effects.85 There are some drug classes that were proven to have anti-PD effects as monoamine oxidase-B (MAO-B) inhibitors and catechol-O-methyltransferase (COMT) inhibitors. However, they may also induce the dopaminergic “type A” adverse effects.85 Different treatment mechanisms are illustrated in Fig. 5.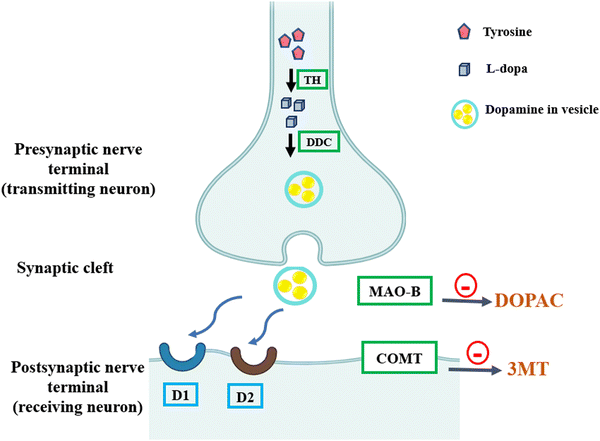 | ||
| Fig. 5 Classes of anti-PD drugs affecting the DA (dopamine) synapse. In the presynaptic nerve terminal, tyrosine is converted to L-dopa by tyrosine hydroxylase (TH) and then to DA by L-dopa-decarboxylase (DDC). Monoamine oxidase-B (MAO-B) inhibitors prevent DA breakdown, and catechol-O-methyltransferase (COMT) inhibitors prevent the peripheral degradation of L-dopa. D1 and D2 are DA agonists, specifically non-ergot agonists. DOPAC, dihydroxyphenylacetic acid; 3MT, 3 methoxy-tyramine. Recreated from ref. 86. | ||
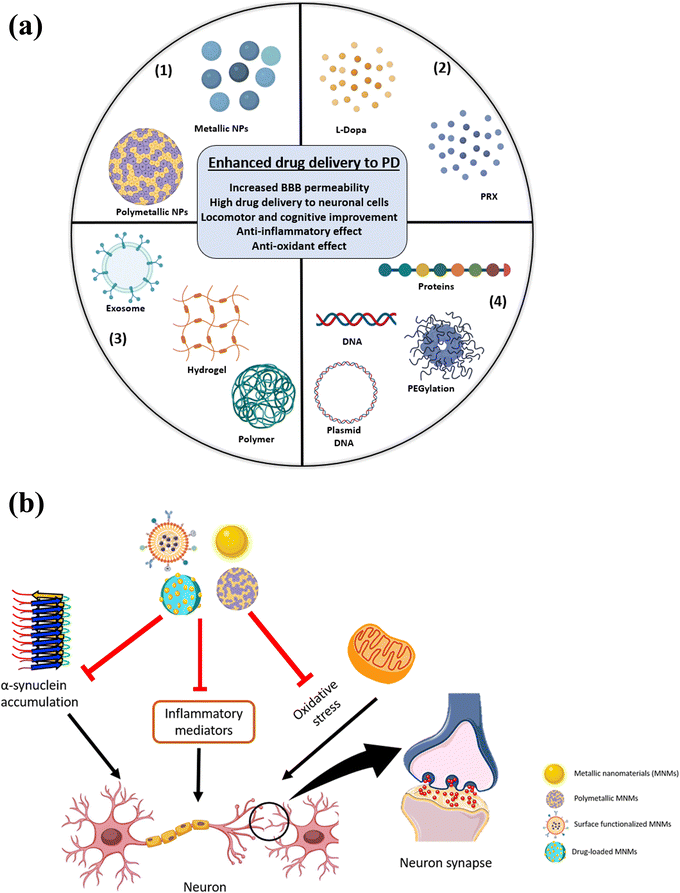
The resistance-dependent debilitating ability of the neurons to maintain the DA in addition to the limited plasma half-life of L-dopa aggravates the PD motor stability. For instance, the DA level fluctuates causing on/off cycles of akinesia and dyskinesia. Therefore, non-ergot DA agonists such as ropinirole and pramipexole are currently used as long-acting treatments available for PD.21,86 In order to sequester the limitations of PD, the application of nanotechnology and the development of nano-based therapeutics emerged as efficient and probable definitive treatment. Nanomaterials can penetrate the BBB, interact with the cells, encapsulate and deliver drugs to obtain desirable drug release rate while dissipating off-targeting of the drugs.87,88 Intriguingly, many studies have shown the ability of the MNMs to overcome this permeability obstacle and effortlessly penetrate the BBB.89 Consequently, the MNMs' effect is potentiated to play a crucial role in the development of central nervous system (CNS) novel treatments (Fig. 6). Other classes of nanostructures are described in elsewhere in the literature as liposomes,90 nanoemulsions91 and polymeric nanoparticles.92
3.1. MNMs-based treatment of PD
Naturally derived AuNPs were found to be promising for effective treatment of PD,101,102 and can ameliorate the induced neurotoxicity through exerting protective effects from destructive oxidation, inflammation and cell death.103 For instance, AuNPs derived from Hibiscus sabdariffa have been reported to have remedial effects on PD by preventing the aggregation of α-lactalbumin.101 AuNPs (30–50 nm) derived from Cinnamomum verum neutralized the oxidative activity and neuromotor dysfunction in the MPTP-induced PD model in addition to mitigated tumor necrosis factor-α (TNF-α), Interleukin-1β (IL-β) and Interleukin-6 (IL-6) levels and normalized TLR/NF-κB signal.104 Hu et al. adopted gene drug delivery for the treatment of PD using a nanocomposite based on chitosan and AuNPs loaded with plasmid DNA and nerve growth factor (CTS@GNP-pDNA-NGF) (Fig. 7). The NGF facilitated the cellular endocytosis into the PC12 cells in the in vitro model. Moreover, the MPP+-based apoptosis was inhibited by the nanocomposite as proven by confocal microscopy due to the suppressed overexpression of α-syn. These results resembled those obtained from the Western blot analysis, which proved that CTS@GNP-pDNA-NGF deteriorated the expression level of α-syn. Additionally, in the PD in vivo model induced in C57bl/6 mice by MPTP, CTS@GNP-pDNA-NGF nanocomposite improved the body weight, healed the substantia nigra density, crossed the BBB and were cleared from the body through the spleen after exerting its therapeutic effect.105
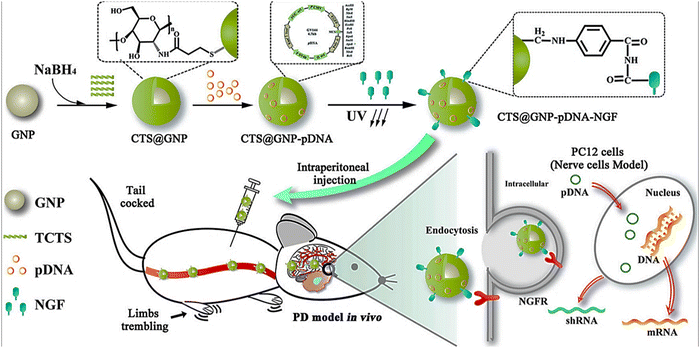 | ||
| Fig. 7 Schematic of the preparation of the nanocomposite, coated with pDNA (plasmid DNA) and nerve growth factor (NGF). The NFG-driven endocytosis into PC12 cells resulted in enhanced cellular and dopaminergic neuron proliferation. In vivo models of PD showed BBB permeability and inhibition of α-syn overexpression. Adapted from ref. 105 with permission from Elsevier, copyright 2018. | ||
AuNPs were exploited as neuronal drug delivery cargoes for the most efficient therapies of PD. Nanoflowers of multibranched AuNPs were prepared and used as transposing carriers of L-DOPA to penetrate the BBB.106 A novel AuNP-based platform for the cerebral drug delivery of L-DOPA and DA was developed. The AuNP surface was functionalized by three different amantadine derivatives due to their ability to bypass the BBB and their biocompatibility.87,107,108 The surface-functionalized AuNPs were compared to polyethylene glycol-coated AuNPs (PEG-AuNPs). L-DOPA or DA were attracted to the AuNPs by several potential surface interactions. The developed systems were investigated in the presence of bovine serum albumin (BSA) as it is the most abundant protein in the body that may form a protein corona hindering the drug's release. Peptidoglycan monomer-AuNPs (PGM-AuNPs) was proved to be the most successful drug delivery tool as it had the highest drug cargo especially from the DA. Additionally, the impact of the BSA on pharmacokinetics and pharmacodynamics was almost diminished.87 However, neither in vitro assessments nor in vivo studies were performed to further reveal the anti-PD activity and efficiency. AuNPs were reported to be incorporated with other therapeutic agents to exploit the beneficial characters of the AuNPs. For example, it acted as the conductive component in the self-healing hydrogel that exerted anti-inflammatory effects.109 Another hydrogel was also prepared using AuNP conductive properties to design an injectable implant in the brain for PD treatment.110 Additionally, AuNPs acted as a drug carrier through electrostatic interactions,87 as NIR-responsive agents,111 gene carriers and112 for CT imaging.113 Diverse applications of AuNPs for the treatment of PD are listed in Table 2.
| Nanoformulation | Size (nm) | Zeta potential (mV) | Biological assessment | Efficacy outcome | Mechanism of action | Ref. |
|---|---|---|---|---|---|---|
| Electroconductive hydrogel using dialdehyde polyurethane as nano-crosslinker, AuNPs (CDAH) | 15 | −26.2 ± 2.7 | In vitro cytotoxicity by neural stem cells and in vivo by oxidopamine (6-OHDA)-induced rat model | CDAH2 hydrogel was found to be biocompatible by quantifying M2/M1 macrophages. The ratio of the CDAH2 group was double the control one. In the in vivo model, there was significant functional recovery | The hydrogel neutralized the ROS production, decrease inflammation of neurons and delay dopaminergic neurons degradation in SNpc, striatum glial fibrillary acidic protein (GFAP+) astrocytes were limited | 109 |
| AuNPs-pDNA loaded into liposomes, NGF and docosahexaenoic acid linked to the liposomes surface as targeting ligands to develop AuNPs(pDNA)-Lipo-NGF-DHA | 20–40 nm of the AuNPs, increased to 200 nm | −15 | Cell viability test of SH-SY5Y cells and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in vivo model | AuNPs-(pDNA)-Lipo-NGF-DHA significantly enhanced the cell viability relative to the control but not a significant difference compared to AuNPs(pDNA), in PD disease model, TH was increased, improved bradykinesia, improvement in the cognitive impairment and motor dysfunction | AuNPs (pDNA)-Lipo-NGF-DHA conferred protection of the dopaminergic neurons by elevating the TH and constrain the overexpression of α-syn, opposed long-term potentiation (LTP) inhibition | 112 |
| AuNCs functionalized with dihydrolipoic acid (DHLA-AuNCs) | 1.87 ± 0.14 | — | Cytotoxicity and microglial protein-inflammatory response on BV2 cells, cytotoxicity, neuronal differentiation and axonal growth assay on N2a cells, ex vivo organotypic brain slice model | Inhibited proinflammation in BV2 cell, improved neurogenesis in N2a cells and in ex vivo brain slice stroke model | Stimulated polarization near to the M2-like phenotype in addition to enhanced anti-oxidant effect and decreased NF-κB signal, prohibited apoptosis that led to enhanced cell viability, limited astrogliosis | 94 |
| Hybrid nanoassembly of AuNPs decorated with thiolated amphiphilic β-cyclodextrin incorporated with DA; AuNPs@SC16SH/DA | 150–300 nm, size of AuNPs was 20–30 nm | — | Estimation the rate of DA release after the additions of stimulants by CV | Release of 70% of the DA amount through half an hour as the AuNPs@SC16SH/DA acted as stimuli responsive nanocarrier | Thiolated-compounds dependent redox responsive drug delivery system for the DA, β-cyclodextrin acted as carrier for the DA by forming a complex with it, AuNPs allowed the monitoring of the DA release intracellularly followed the penetration of the BBB | 114 |
| AuNPs coated with brain-targeting exosomes functionalized with rabies viral glycoprotein (RVG) | AuNPs: 48 ± 1.5 and AuNPs/exosomes: 105 ± 10.1 | About-7 | In vitro binding efficiency using bEnd.3 mouse brain endothelial cell line, C6 rat glioma cells, or HeLa cervical cancer cells, in vitro BBB model using astrocyte cells and bEnd.3 cells and murine model and in vivo assay to determine the accumulation of the AuNPs/exosomes in the brain | bEnd.3 and C6 displayed the greatest binding affinity towards the AuNPs/exosomes than the HeLa cell so the nanoparticles have brain-specific affinity, the AuNPs/exosome crossed the BBB with a significant increase relative to the AuNPs coated with unmodified exosomes, the in vivo model demonstrated intense fluorescence from the brains treated with RVG-exosomes-coated AuNPs | Enhanced BBB permeability of the AuNPs after the coating with brain targeting RVG-protein ligated to exosomes | 115 |
| L-DOPA-functionalized multi-branched nanoflower-like gold nanoparticles (L-DOPA-AuNF) | 90 nm | −34.6 ± 1 | In vitro test of the L-DOPA-AuNF accumulation in the hCMEC/D3 brain endothelial cells, penetration of L-DOPA-AuNF across the brain endothelial monolayers, internalization of L-DOPA-AuNF into brain macrophages | High intracellular accumulation in the brain endothelial cells in the cytoplasm and the peri-nuclear space, penetration and internalization of L-DOPA-AuNF into the brain microglia without inflammation | Energy-dependent cellular internalization of L-DOPA-AuNF into brain endothelial monolayers and brain microglia due to L-DOPA functionalization and may be due to receptor-independent mechanism | 106 and 116 |
| NIR-responsive PLGA microspheres loaded with pramipexole (PRX) and hollow gold nanospheres (HGNS) (PRX/HGNS MS) | HGNS was 40 nm, PRX/HGNS MS was 24 μm | — | Cytotoxicity assessment using C2C12 myotubes, RAW macrophages, PC12 model neurons and 3T3 fibroblasts, in vivo model including pharmacokinetics, pharmacodynamics and immunohistochemistry | HGNS and PRX/HGNS MS did not exert significant cytotoxicity were found biocompatible, the release rate of PRX from PRX/HGNS MS significantly increased compared to PXR from PXR MS, NIR-exposed mice treated with PRX/HGNS MS exhibited faster improvement relative to non-NIR exposed mice, significant decrease of TH in the striatum | Significant post-treatment elevation if DA, homovanilic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC), protective effect of the neurons conferred by PRX/HGNS MS and NIR-treated mice | 111 |
| Self-healing composite hydrogel of O-carboxymethyl chitosan (CMC)OTA@Au (oxidized tannic acid modified gold nanocrosslinker) (COA) | 27.79 ± 2.89 nm | −45.6 ± 2.7 | In vitro antioxidant test by neural stem cells (NSCs) and anti-inflammatory test by J774A.1 murine macrophages, in vivo PD model followed by behavioral and electrophysiological assessment, immunofluorescent and immunohistochemical assays | NSCs proliferation was enhanced in the presence of COA, upregulation of GFAP, β-tubulin and MAP2, about 90% of the inflamed cells were healed after 12 h of COA treatment, inflammatory proteins expression was not expressed in COA-treated macrophages, significant increase in the counterclockwise rotation period, improved forelimbs contact, low spikes count in the projection neurons discharge behavior | The developed COA has efficient self-healing ability and injectability, can enhance the cellular proliferation and subsequent differentiation, can be developed as implant injected to the brain | 110 |
The green synthesis of AgNPs for the treatment of PD123–126 and exerting anti-oxidant effects have been reported previously.127,128 The plant extract of Mucuna pruriens was found to have reduction capability that was exploited to produce AgNPs and simultaneously, containing high L-dopa amounts.129 Sardjono et al. reported the preparation of AgNPs (36.5 nm) by extraction from seeds and performed catalepsy assessment to investigate the efficiency of the prepared NPs in the in vivo model. Gradually increasing doses starting from 5 to 25 mg kg−1 were tested on 3 months old male mice. Groups of mice treated with 5, 10 and 15 mg kg−1 of AgNPs significantly improved the catalepsy symptoms relative to the control and pure extract-treated groups, where the dose of 5 mg kg−1-treated group was remarkably the most efficient.124 Therefore, in order to circumvent the potential toxicity of AgNPs, green synthesis, coating with biocompatible polymers or decorating the surface with neural-cell-specific targeting ligands are all potential pathways that should be adopted.130
The second approach is underpinned on the application of an external magnetic field. For example, dextran-coated IONPs can ameliorate the remedial effect of human mesenchymal stem cells (hMSCs). Both rotational and motor behaviors were significantly improved relative to the control. This improvement was attributed to the enhanced migration of hMSCs towards the damaged DA neurons and their subsequent differentiation to resemble the DA-neurons.137 To enhance the selectivity of the SPIONs towards PD, the binding affinity of streptavidin (SA) and biotin was exploited. The developed SA/PEI-SPIONs were tested using a biotin-treated PC12 cell as an in vitro model. The surface-modified SPIONs demonstrated greater binding of the PC12 cell membrane, as observed by TEM.138
Selenium NPs (Se NPs) were proven to exert neuroprotective effects, and hence, were reviewed as potential therapy for CNS diseases such as PD.142 For instance, the effect of the Se NPs is attributed to the crucial role of Se to allow the normal function of several peroxidase enzymes143 and inhibit inflammatory mediators such as TNF-α, Nf-κB and PEG2144 to exert its anti-oxidant and anti-inflammatory effects, respectively. Derivatives of aminothiazole were synthesized and used for the development of Se NPs pursuing fortified enhancement of the neurological functions. The molecular docking studies revealed blocking of the hMAO isoforms (A and B). Simultaneously, the IC50 value of the NPs was only 0.033 μM, preceding the normal-sized counterparts’ potency by about 70%. In the haloperidol-induced PD in vivo model, the behavioral test results of the NP group showed improvements and enhanced exploration. Therefore, the Se-based nanoformulation is elicited as an effective anti-PD drug.145
Gao et al. developed combined genetic and antioxidant therapy using NIR-stimulated magnesium oxide (MgO)-based nanocomposites; MgOp@PPLP and both in vitro and in vivo assessment results demonstrated enhanced permeability to the BBB in addition to the exerted anti-inflammatory and antioxidant effects.146
PD-lesioned cell model by 1-methyl-4-phenylpyridinium (MPP+) was adopted to reveal the remedial effects of hexagonal boron nitride NPs (hBNs). MTT and LDH showed boosted cells’ viability and neural cell protection. Moreover, hBNs had aggravated defense against destructive oxidants, opposed the MPP+-induced cellular apoptosis and exerted neural cryoprotection.147
Cerium oxide nanoparticles (CeO2 NPs) are well-known anti-oxidant materials that resemble the effect of superoxide dismutase and catalase.148–150 Therefore, exploiting CeO2 NPs’ antioxidant properties was pertinent with PD treatment. Saccharomyces cerevisiae yeast model of PD was adopted to investigate the effect of CeO2 NPs. The α-syn expressing yeast cells showed enhanced viability with gradually increasing concentrations of CeO2 NPs up to 50 ng μL−1 due to the accumulation of α-syn in the plasma membrane instead of the cytoplasm, as shown by the cell lysate analysis. There was also observable diminishing of the mitochondrial impairment and ROS generation. This improvement was thought to be through the surface absorption of α-syn on CeO2 NPs, which constrained the α-syn fibrillation.151 Several shapes of ceria NPs were also investigated and the flower shape had the most powerful anti-oxidant activity.152
Although in vivo manganese (Mn) accumulation causes idiopathic PD-resembling symptoms,153 controversial results were reported in the literature. Based on Sharma and coworkers’ study, Mn NPs (30–40 nm) showed impairment of the cognition and motor ability of the rats after 8 days of administration. Additionally, definitive brain injuries were detected in multiple regions accompanied by BBB distortion, dissipated blood flow to the brain and cerebral edema.154 Furthermore, citrate-capped Mn2O3 NPs (C-n2O3 NPs) ameliorated the PD consequences and chelated the excess Mn, preventing subsequent neuro-damage.155 Wang et al. prepared chiral Mn2O3 NPs. The D-NPs showed enhanced α-syn fibrillation inhibition and anti-oxidant effect driven by an electromagnetic field.156 Additionally, using dopaminergic neural cells, MN9D, PD lesion was developed and then treated with NPs of different facets. It was observable the Mn3O4 nanorods with the (103) facet had high anti-oxidant capacity and diminishing of α-syn in the cerebrospinal fluid as elaborated by the biological assessments.157
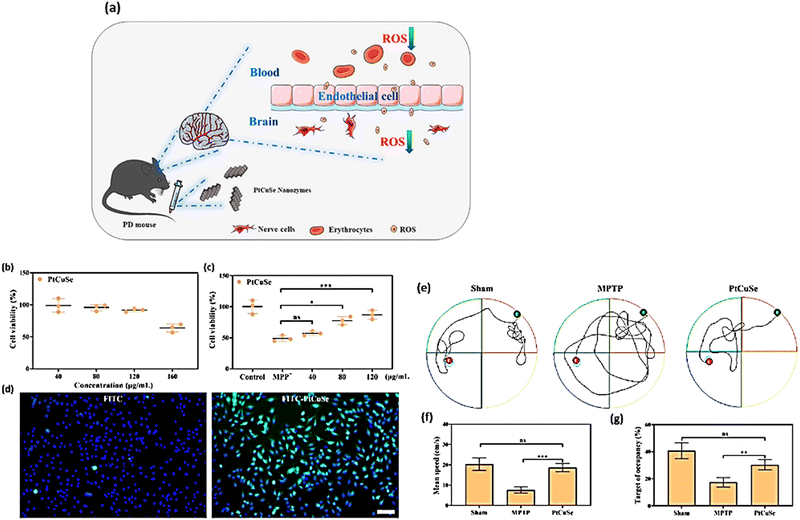 | ||
| Fig. 8 (a) Scheme of exploiting the antioxidant capability of the nanozyme PtCuSe. (b) In vitro cell viability of SH-SY5Y cells after the addition of PtCuSe. (c) In vitro cell viability of MPP−-treated SH-SY5Y cells after the addition of PtCuSe. (d) Cellular uptake of PtCuSe. (e) Behavioral assessment by the Morris water maze test, showing the path of mice. (f) and (g) The mean time and the relative mean time spent in the target quadrant. Reproduced from ref. 159. | ||
The experiment of chiral L/D-CuxCoyS SPs comparison between the two counterparts indicated that the D-SPs had preceding ability to prevent the formation of α-syn and disintegrate the already existing fibrils. This effect was attributed to the capability of D-SPs to generate ROS that exert its effect on α-syn.160 However, the study did not elucidate any off-target harmful effect of the generated ROS or any subsequent neutralizing cascades.
Li et al. formulated nano-bioconjugate/nanozyme of lactoferrin (Lf)-modified Au–Bi2Se3 nanodots (NDs) (Lf–Au–Bi2–Se3). The nanozyme Lf–Au–Bi2–Se3 had characteristic protective antioxidant activity that resembles that of SOD, CAT, POD and GPx, a group of enzymes that scavenge the ROS and normalize their level in the normal cells. The catalytic activity of Au–Bi2–Se3 is attributed to two reasons. First, the Au atom can alter the Se atoms within the lattice and replace it with Bi atoms representing defect points. The Bi defect points would enhance the electron transport, and consequently, the catalytic effect.161 Second, the Au atoms would act synergistically by allowing electron transport.162 Additionally, the LF-surface modification did not affect the catalytic activity of Au–Bi2–Se3. To assess the cellular internalization, bEnd.3 cells were used and Lf–Au–Bi2–Se3 was found to have great ability to penetrate the BBB. The Morris water maze in vivo model was adopted to assess the treatable effect of Lf–Au–Bi2–Se3 and the treated mice exhibited the best intellectual and physical improvement and confirmed the pivotal role of Lf in enhancing the transcytosis in the BBB. Additionally, high levels of tyrosine hydroxylase (TH), healed mitochondria, protected Nissel-positive cells and normalized ex vivo lipid peroxidation were confirmed in the Lf–Au–Bi2–Se3-treated group. The biosafety of Lf–Au–Bi2–Se3 was corroborated by the normally functioning main organs. The uptake of Lf–Au–Bi2–Se3 into the brain cells was found to be 2.67 times more than that of Au–Bi2–Se3, which further confirmed the ability of the nanorods to penetrate the BBB. Finally, the clearance of Lf–Au–Bi2–Se3 was found to be mainly through the urine, which is attributed to the enhanced renal infiltration of the tiny-sized nanorods.88
4. MNMs-based systems as theranostics for PD
Due to the continuous urge of early accurate detection and effective treatment of PD, researchers invested their efforts to develop theranostic platforms that achieve both goals. Novel multifunctional nanoparticles were designed for the real-time monitoring of the disease as well as use as targeted therapeutic agents like SPIONs. As mentioned earlier, SPIONs are biocompatible and biodegradable contrast agents exploited in MRI, and their tunable sizes and shapes allow manipulation to cross the BBB. Additionally, they can be green-synthesized, ensuring great biocompatibility.70 Moreover, they can remain circulating in the body allowing for better therapy and can be controlled using an external magnetic field.57,163 For instance, SPIONs-loaded liposomes were used as theranostic agents for image-guided drug delivery under glioma conditions; therefore, this formulation can be employed in PD.164Another study was conducted using resveratrol-Fe3O4-loaded liposomes for sustained drug release at the target site using an external magnetic field. This formulation was guided using MRI to ensure crossing the BBB and reaching the target site in PD rat models.165 Niu et al. investigated the use of magnetic nanoparticles in gene delivery, where they synthesized N-isopropylacrylamide-acrylic acid-functionalized Fe3O4 NPs to confer pH and temperature responsiveness. Then, they photo-immobilized nerve growth factor to the NPs along with short hairpin RNA for gene delivery in PD models; however, they did not assess the MRI properties of such NPs.166 Thus, this multifunctional system can be further implemented in MRI-guided drug and gene delivery in PD cases. Garcia-Pardo et al. also formulated DA-encapsulated iron nanoparticles made of iron metal nodes polymerized using bidentate ligands as bioinspired nanotheranostic agents. It displayed MRI properties, allowed for efficient DA delivery to PD animal models, and proved to be biosafe in vivo.167
Switchable nanoparticles for simultaneous drug/gene delivery and CT imaging were developed. The programmed drug delivery system (MBPCS) was composed of levodopa-quinone gold nanoparticles (GNPs) integrated with 2 derivatives of Zwitterionic poly-(carboxybetaine)-based curcumin, where the last was releasable via a cleavable link in the DA neurons of PD. B6 peptide was linked on the surface of the nanoparticles facilitating their penetration of the BBB. The intracellular internalization was adopted by using mazindol (MA) that binds preferentially to the DA neurons. Following the endosomal/lysosomal escape, the gene delivery phase starts by releasing α-syn gene (SNCA). The GNP and curcumin are then released because of MBPC degradation. GNP interacts with Fe3+ and quinone groups allowing the CT.106
5. MNMs and BBB penetration
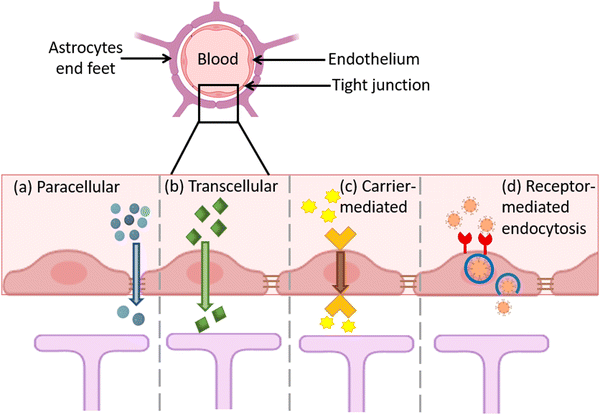
Generally, several BBB penetration mechanisms of the different classes of the nanomaterials were described and reviewed elsewhere.168 Concerning the MNMs, they can penetrate the BBB either by active or passive pathways. Receptor-mediated endo/transcytosis, adsorption-mediated endocytosis and carrier mediated transport are all examples of the active pathways. Furthermore, the passive pathway involves the passage of the hydrophilic small-sized NPs across the endothelial cells.168 (Fig. 9). Proteins as nerve growth factor (NGF),105,112 poly unsaturated fatty acids as docosahexaenoic acid (DHA)112 and PGM87 are all examples of surface functionalizing moieties allowing receptor-mediated endocytosis.168 Li et al. prepared Au–Bi2Se3 nanodots and functionalized the surface with lactoferrin (LF) to enable the active targeting of the BBB through receptor-driven transcytosis.88 The B6 peptide (CGHKAKGPRK) was used to design switchable AuNP-based theranostic formulations, where the penetration of the BBB was confirmed and the cellular internalization of SH-SY5Y cells was described to be through caveolae and clathrin-driven endocytosis.113 AuNCs (2.5 ± 1 nm) were investigated, and their penetration of the BBB was proven. The AuNCs were injected intraperitoneally in mice and after 6 h of 20 mg kg−1 injection, the AuNCs were detected by TEM in the SN sectors in addition to the neurons.96 Cheng et al. investigated the penetration of an iron-based nanoformulation through an in vitro model and found that the cellular penetration can be through 3 different pathways.135 Consequently, the MNMs were reported to penetrate the BBB through different mechanisms depending on the sizes and surface properties.6. Toxicity and biocompatibility of MNMs
Majorly, the axiomatic transportation of nanomaterials into clinical application was constrained by the inscrutable toxic effects on the CNS, specifically, the brain. Heavy metals have been previously reported to induce neurotoxicity that may resemble the PD-associated manifestation.169,170 Therefore, the toxicity of the nano-constructed counterparts would be of great importance and pertinent as many of these nanoparticles release metallic ions after in vivo administration. Additionally, the tiny size of the nanostructures permeates their smooth flow across the BBB and distribution within the brain parts remarkably, to the SP which has great importance due to the existence of dopaminergic neurons.6,171 PD is associated with other physiological alterations such as inflammation, α-syn aggregation and impaired anti-oxidant response. All these impairments were observable from the exposure to several MNMs.172–174 For instance, TiO2 NPs affect the hippocampus, cerebellum and substantia nigra.6,175 Certain specific pathways of the TiO2-induced toxicity have been illustrated in the literature proving detrimental effects. Wu and Xie conducted a study to investigate the in vitro and in vivo toxicity of TiO2 adopting PC12 cells and zebrafish embryos, respectively. The results corroborated the presence of neurotoxic effects due to the accumulation of TiO2 NPs and the generation of ROS. The degradation of the dopaminergic neurons was observable.176 TiO2 administration also caused electrolyte imbalance and activated inflammatory response by stimulating IL-6 and NF-κB,177,178 Moreover, TiO2 promoted cerebral stimulation of the oxidative stress-driven cellular damage by constraining the anti-oxidant factors179 and elevating the intra-mitochondrial ROS alone or with simultaneous exposure to AgNPs.180 From other studies, TiO2 caused the accumulation of α-syn that mitigates the DA in the brain, which is a substantial mechanism causing PD.172,181,182Despite the remedial effect of green-synthesized zinc oxide NPs (ZnO NPs),183 other studies reported their cerebral cytotoxicity.171,184 A study performed by Jin and coworkers compared the induced cytotoxic effects by several ZnO-based nanostructures. ZnO NPs, long ZnO nanorods (l-ZnO NRs) and short ZnO nanorods (s-ZnO NRs) were prepared and the in vitro assessment was performed using human neuroblastoma cells SH-SY5Y, revealing that l-ZnO NPs had the least toxicity (LD50 = 17 μg mL−1). Several concentrations of l-ZnO NPs were tested using an in vivo zebrafish larva model. From the investigation of the brain, l-ZnO NRs at high doses stimulated the ROD production that consequently, sequestered the motor ability and neuron development, disruption of dopaminergic neurons and cerebral apoptosis, coherently leading to PD-resembling manifestations.184 The toxicity of the Zn2+ ions from ZnO NPs was investigated through in vitro and in vivo assessments. The cell viability of the glial cells A172 was greatly decreased after 1 day of exposure and DNA damage was observable after only 3 h, as confirmed by the comet assay. The zebrafish embryos were exposed to gradient concentrations of ZnO NPs. After 96 hours post-fertilization (hpf), locomotor impairment was observed, suggesting the possible role of the released Zn2+. The cytotoxicity and genotoxicity were believed to be caused by enhanced ROS production. Additionally, the locomotor disorder was not elucidated explicitly but attributed to dopaminergic cells destruction, therefore affecting the muscles and nerves (Fig. 10).185Table 3 summarizes some PD-related toxicities associated with MNM exposure.
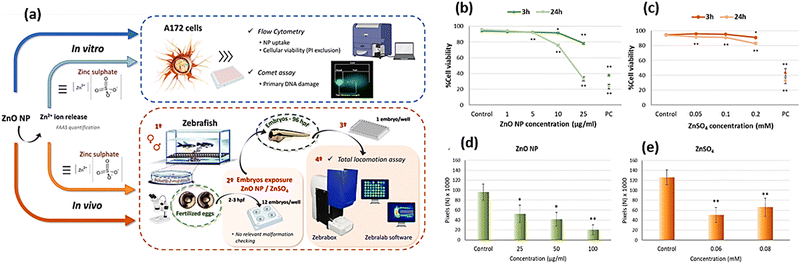 | ||
| Fig. 10 (a) Schematic of the prepared ZnO NPs and the effect of the released Zn2+ ions on A172 cells, along with the in vivo model using zebrafish embryos. (b) and (c) In vitro cell viability assessment of A172 cells compared to ZnSO4. (d) and (e) Locomotor impairment of zebrafish embryos after exposure to ZnO NPs and ZnSO4. Adapted from ref. 185 with permission from Elsevier, copyright 2024. | ||
| Type & size of NPs | Exposure duration | Biocompatibility & toxicity | Ref. |
|---|---|---|---|
| Fe3O4, about 10 nm | 24 h | Neuro-destructive effect through oxidative stress. Interaction between the iron and DA may induce neurons toxicity by generating toxic products. The aggregated α-syn can cause accretion of the iron level that leads to generation of OH destructive radicals. | 186–189 |
| Ag NPs, less than 100 nm | 28 days | Induced neuronal apoptosis and cellular degeneration known as dark neurons due to ROS production and exaggerated inflammatory response. | 190 |
| Co NPs, less than 100 nm | 24 h and 6 days | Destructive oxidative stress evidenced by the depletion of GSH, neurotoxicity due to calcium homeostasis and lipid peroxidation. Also, mitochondrial swelling and structures that resembles fibrosis were notable, leading to ferroptosis-like cell death. | 191 |
| CuO NPs, 50 nm | 60 and 120 h | α-syn aggregates, promote pertinent cellular cytotoxicity | 192 |
| CuS NPs, 77.89 nm | 24 h | Cytotoxic effect at low concentration, caused developmental neurotoxicity | 193 and 194 |
| NiO NPs, 50 nm | 24 h | Altered the fibrillation kinetics of α-syn, enhanced ROS production due to the alleviated defensive pathways and the enhanced inflammatory mediators’ expression | 195 |
| Long ZnO nanorods (l-ZnO NRs), diameter: 159.3 ± 17.9 nm and length: 1.1 ± 0.15 μm | 24–144 h post fertilization (hpf) | High doses stimulated the ROD production, sequestered the motor ability and neurons development, disruption of dopaminergic neurons and cerebral apoptosis leading to PD-resembling manifestations | 184 |
7. Challenges and future perspectives
The limitation of clinical improvement in the management of PD, from detection to treatment, is attributed to several gaps, including long-time of asymptomatic period, the absence of sharply definitive biomarkers, the heterogeneous nature of the disease, dearth of effective treatments and the resistance development. The use of MNMs represents a promising but complex avenue for addressing these issues. While NPs have shown potential in enhancing detection through sensitive biosensors and improving therapeutic delivery, challenges persist. These include ensuring biocompatibility and stability in biological systems, achieving precise targeting to diseased cells, and avoiding off-target effects.48 The encapsulation of the MNMs into emulsions would provide prolonged drug release. Moreover, the design of the carrier medium to be NIR-responsive would confer more specific release in the target cells/sites.111 The incorporation of the MNMs into biocompatible nanoshells196 or porous nanocarriers would add a protective shield from pre-mature in vivo degradation. Moreover, the surface modification of the MNMs with targeting ligands would enhance the on-site neuron-specific drug release.88,197 The exploiting of specific BBB-transporters would also enhance the cerebral drug delivery surface modification of the MNMs by ligands specific to these receptor, reviewed by Mhaske et al.198 Furthermore, scaling up from laboratory success to clinical application involves overcoming significant hurdles in manufacturing and regulatory approval. Concerning the MNMs, the design of a formulation for PD therapy or in vivo detection as imaging would require prolonged testing to ensure safety during the use and subsequent complete clearance from the body. Additionally, challenges, such as the limitations of animal model reciprocation, ethical issues surrounding human samples, and the ambiguity in providing clear mechanistic molecular pathways of aging and neurodegenerative diseases, particularly PD, are all insistent issues that need to be addressed.199,200 Future research should focus on refining nanoparticle technologies to enhance their specificity, reduce potential toxicity, and improve targeting mechanisms. Advancements in AI and ML could further aid in analyzing data from nanoparticle-based detection and therapies, leading to more personalized and effective treatment strategies. Addressing these challenges through innovative approaches in biomarker discovery, model development, and therapeutic applications will be crucial for advancing clinical outcomes and improving the quality of life for individuals with PD.Data availability
This review article does not contain original data. All data referenced in this article are from published studies and sources cited in the reference list. Additional information can be obtained from these sources as indicated.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their appreciation to the Science, Technology and Innovation Funding Authority (STDF), Egypt, for funding and supporting research related to this review through the funds (Capacity Building Fund, CB-22808 and STDF FLUG Call 1-Project ID 46715).References
- J. Zhu, Y. Cui, J. Zhang, R. Yan, D. Su, D. Zhao, A. Wang and T. Feng, Prevalence of Parkinson's disease and its trend from 1980 to 2023: A systematic review and meta-analysis, Lancet, 2023,(127), 1–26 Search PubMed.
- L. M. Shulman, Is there a connection between estrogen and Parkinson's disease?, Parkinsonism Relat. Disord., 2002, 8(5), 289–295 CrossRef PubMed.
- O. B. Tysnes and A. Storstein, Epidemiology of Parkinson's disease, J. Neural Transm., 2017, 124(8), 901–905, DOI:10.1007/s00702-017-1686-y.
- Organization WH. Neurological disorders: public health challenges. World Heal Organ. 2006.
- S. Y. Chen and S. T. Tsai, Epidemiology of Parkinson's disease, Lancet Neurol., 2006, 5(6), 525–535, DOI:10.1016/S1016-3190(10)60044-4.
- Z. Heidari, A. Mohammadipour, P. Haeri and A. Ebrahimzadeh-bideskan, The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra, Iran J. Basic Med. Sci., 2019, 22(7), 745, DOI:10.22038/ijbms.2019.33611.8018.
- P. Haeri, A. Mohammadipour, Z. Heidari and A. Ebrahimzadeh-bideskan, Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson's disease model of mice, Anat. Sci. Int., 2019, 94(1), 119–127, DOI:10.1007/s12565-018-0457-7.
- S. J. Cragg and M. E. Rice, DAncing past the DAT at a DA synapse, Trends Neurosci., 2004, 27(5), 270–277 Search PubMed.
- A. L. Bartels and K. L. Leenders, Parkinson's disease: The syndrome, the pathogenesis and pathophysiology, Cortex, 2009, 45, 915–921, DOI:10.1016/j.cortex.2008.11.010.
- P. L. Mcgeer, S. Itagaki, B. E. Boyes and E. G. Mcgeer, Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains, Neurology, 1988, 38(8), 1285 Search PubMed.
- F. Ahmadinejad, S. G. Møller, M. Hashemzadeh-Chaleshtori, G. Bidkhori and M. S. Jami, Molecular mechanisms behind free radical scavengers function against oxidative stress, Antioxidants, 2017, 6(3), 1–15, DOI:10.3390/antiox6030051.
- S. Franco-Iborra, T. Cuadros, A. Parent, J. Romero-Gimenez, M. Vila and C. Perier, Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson's disease, Cell Death Dis., 2018, 9(11), 1122, DOI:10.1038/s41419-018-1154-0.
- S. Chawla, D. Kalyane, V. Tambe, P. K. Deb, K. Kalia and R. K. Tekade, Evolving nanoformulation strategies for diagnosis and clinical interventions for Parkinson's disease, Drug Discovery Today, 2020, 25(2), 392–405, DOI:10.1016/j.drudis.2019.12.005.
- T. D. Kim, E. Choi, H. Rhim, S. R. Paik and C. H. Yang, α-Synuclein has structural and functional similarities to small heat shock proteins, Biochem. Biophys. Res. Commun., 2004, 4(324), 1352–1359, DOI:10.1016/j.bbrc.2004.09.208.
- K. Sode, S. Ochiai, N. Kobayashi and E. Usuzaka, Effect of Reparation of Repeat Sequences in the Human α-Synuclein on Fibrillation Ability, Int. J. Biol. Sci., 2007, 3(1), 1 CAS.
- M. I. Teixeira, C. M. Lopes, M. H. Amaral and P. C. Costa, Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases, Eur. J. Pharm. Biopharm., 2020, 149, 192–217, DOI:10.1016/j.ejpb.2020.01.005.
- F. Zambon, M. Cherubini and H. J. R. Fernandes, et al., Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson's iPSC-derived dopamine neurons, Hum. Mol. Genet., 2019, 28(12), 2001–2013, DOI:10.1093/hmg/ddz038.
- H. Braak, K. Del Tredici, U. Rüb, R. A. I. De Vos, E. N. H. Jansen Steur and E. Braak, Staging of brain pathology related to sporadic Parkinson's disease, Neurobiol. Aging, 2003, 24(2), 197–211, DOI:10.1016/S0197-4580(02)00065-9.
- B. Dubois and B. Pillon, Cognitive deficits in Parkinson's disease, J. Neurol., 1997, 244, 2–8 CrossRef CAS PubMed.
- L. V. Kalia and A. E. Lang, Parkinson's disease, Lancet, 2015, 29(389), 896–912 CrossRef PubMed.
- M. J. Armstrong and M. S. Okun, Diagnosis and Treatment of Parkinson Disease: A Review, JAMA, J. Am. Med. Assoc., 2020, 323(6), 548–560, DOI:10.1001/jama.2019.22360.
- K. R. Chaudhuri, C. Prieto-Jurcynska and Y. Naidu, et al., The nondeclaration of nonmotor symptoms of Parkinson's disease to health care professionals: An international study using the nonmotor symptoms questionnaire, Mov. Disord., 2010, 25(6), 704–709, DOI:10.1002/mds.22868.
- D. Berg, R. B. Postuma and C. H. Adler, et al., MDS research criteria for prodromal Parkinson's disease, Mov. Disord., 2015, 30(12), 1600–1611, DOI:10.1002/mds.26431.
- H. Hospital, Parkinson's disease and osteoporosis, Age Ageing, 2013, 42(2), 156–162, DOI:10.1093/ageing/afs161.
- C. T. Hong, H. H. Hu, L. Chan and C. H. Bai, Prevalent cerebrovascular and cardiovascular disease in people with Parkinson's disease: a meta-analysis, Clin. Epidemiol., 2018, 1147–1154, DOI:10.2147/CLEP.S163493.
- S. Ma, Q. Yang and W. Zhang, et al., Silver nanoclusters and carbon dots based light-addressable sensors for multichannel detections of dopamine and glutathione and its applications in probing of parkinson's diseases, Talanta, 2020, 219, 121290, DOI:10.1016/j.talanta.2020.121290.
- R. M. Meade, D. P. Fairlie and J. M. Mason, Alpha-synuclein structure and Parkinson's disease – lessons and emerging principles, Mol. Neurodegener., 2019, 14(1), 29, DOI:10.1186/s13024-019-0329-1.
- M. Gómez-Benito, N. Granado, P. García-Sanz, A. Michel, M. Dumoulin and R. Moratalla, Modeling Parkinson's Disease With the Alpha-Synuclein Protein, Front. Pharmacol., 2020, 11, 356, DOI:10.3389/fphar.2020.00356.
- E. Srinivasan, G. Chandrasekhar and P. Chandrasekar, et al., Alpha-Synuclein Aggregation in Parkinson's Disease, Front. Med., 2021, 8, DOI:10.3389/fmed.2021.736978.
- S. H. Mandala, T. J. Liu and C. M. Chen, et al., Enhanced Plasmonic Biosensor Utilizing Paired Antibody and Label-Free Fe3O4 Nanoparticles for Highly Sensitive and Selective Detection of Parkinson’s α-Synuclein in Serum, Biosensors, 2021, 11(10), 402, DOI:10.3390/bios11100402.
- E. D. Aminabad, A. Mobed, M. Hasanzadeh, M. A. Hosseinpour Feizi, R. Safaralizadeh and F. Seidi, Correction: Sensitive immunosensing of α-synuclein protein in human plasma samples using gold nanoparticles conjugated with graphene: an innovative immuno-platform towards early stage identification of Parkinson's disease using point of care (POC) analys, RSC Adv., 2022, 12(10), 5765, 10.1039/d2ra90011d.
- J. O. Watzlawik, X. Hou, D. Fricova, C. Ramnarine, S. K. Barodia, T. F. Gendron, M. G. Heckman, M. DeTure, J. Siuda, Z. K. Wszolek, C. R. Scherzer, O. A. Ross, G. Bu, D. W. Dickson, M. S. Goldberg, F. C. Fiesel and W. Springer, Sensitive ELISA-based detection method for the mitophagy marker p-S65-Ub in human cells, autopsy brain, and blood samples, Autophagy, 2021, 17(9), 2613–2628, DOI:10.1080/15548627.2020.1834712.
- H. H. Tsao, C. G. Huang and Y. R. Wu, Detection and assessment of alpha-synuclein in Parkinson disease, Neurochem. Int., 2022, 158, 105358, DOI:10.1016/j.neuint.2022.105358.
- A. M. Santos, A. Wong and L. M. C. Ferreira, et al., Multivariate optimization of a novel electrode film architecture containing gold nanoparticle-decorated activated charcoal for voltammetric determination of levodopa levels in pre-therapeutic phase of Parkinson's disease, Electrochim. Acta, 2021, 390, 138851, DOI:10.1016/j.electacta.2021.138851.
- A. Mobed, S. Razavi, A. Ahmadalipour, S. K. Shakouri and G. Koohkan, Biosensors in Parkinson's disease, Clin. Chim. Acta, 2021, 518, 51–58, DOI:10.1016/j.cca.2021.03.009.
- E. Samson and M. D. Noseworthy, A review of diagnostic imaging approaches to assessing Parkinson's disease, Brain Disord., 2022, 6, 100037, DOI:10.1016/j.dscb.2022.100037.
- E. Tolosa, A. Garrido, S. W. Scholz and W. Poewe, Challenges in the diagnosis of Parkinson's disease, Lancet Neurol., 2021, 20(5), 385–397, DOI:10.1016/S1474-4422(21)00030-2.
- N. Ghosh, K. Sinha and P. C. Sil, A review on the new age methodologies for early detection of Alzheimer's and Parkinson's disease, Basic Clin. Pharmacol. Toxicol., 2024, 134(5), 602–613, DOI:10.1111/bcpt.14003.
- P. K. Keserwani, S. Das and N. Sarkar, A comparative study: prediction of parkinson’s disease using machine learning, deep learning and nature inspired algorithm, Multimed Tools Appl., 2024, 83(27), 69393–69441, DOI:10.1007/s11042-024-18186-z.
- J. Wang, L. Xue and J. Jiang, et al., Diagnostic performance of artificial intelligence-assisted PET imaging for Parkinson's disease: a systematic review and meta-analysis, npj Digit Med., 2024, 7(1), 17, DOI:10.1038/s41746-024-01012-z.
- Y. Yang, Y. Yuan and G. Zhang, et al., Artificial intelligence-enabled detection and assessment of Parkinson's disease using nocturnal breathing signals, Nat. Med., 2022, 28(10), 2207–2215, DOI:10.1038/s41591-022-01932-x.
- L. Sigcha, L. Borzì and F. Amato, et al., Deep learning and wearable sensors for the diagnosis and monitoring of Parkinson's disease: A systematic review, Expert Syst Appl., 2023, 229, 120541, DOI:10.1016/j.eswa.2023.120541.
- C. Sotirakis, Z. Su and M. A. Brzezicki, et al., Identification of motor progression in Parkinson's disease using wearable sensors and machine learning, npj Parkinson's Dis., 2023, 9(1), 142, DOI:10.1038/s41531-023-00581-2.
- C. Moreau, T. Rouaud and D. Grabli, et al., Overview on wearable sensors for the management of Parkinson's disease, npj Parkinson's Dis., 2023, 9(1), 153, DOI:10.1038/s41531-023-00585-y.
- S. Dixit, K. Bohre and Y. Singh, et al., A Comprehensive Review on AI-Enabled Models for Parkinson’s Disease Diagnosis, Electronics, 2023, 12(4), 783, DOI:10.3390/electronics12040783.
- L. Perju-Dumbrava, M. Barsan and D. C. Leucuta, et al., Artificial intelligence applications and robotic systems in Parkinson's disease (Review), Exp. Ther. Med., 2022, 23(2), 153, DOI:10.3892/etm.2021.11076.
- C. Qin, J. Xia, Y. Wen, J. Wang and C. Zhong, A new immunofluorescence determination of Parkinson's disease biomarkers using silver nanoparticles, Alexandria Eng. J., 2025, 111, 404–414, DOI:10.1016/j.aej.2024.10.069.
- E. Scarpa, M. Cascione, A. Griego, P. Pellegrino, G. Moschetti and V. De Matteis, Gold and silver nanoparticles in Alzheimer's and Parkinson's diagnostics and treatments, Ibrain, 2023, 9(3), 298–315, DOI:10.1002/ibra.12126.
- Q. Du, Y. Liu, M. Fan, S. Wei, M. Ismail and M. Zheng, PEG length effect of peptide-functional liposome for blood brain barrier (BBB) penetration and brain targeting, J. Controlled Release, 2024, 372, 85–94, DOI:10.1016/j.jconrel.2024.06.005.
- J. Abujamai, R. Satar and S. A. Ansari, Designing and Formulation of Nanocarriers for “Alzheimer's and Parkinson's” Early Detection and Therapy, CNS Neurol. Disord. Drug. Targets, 2024, 23(10), 1251–1262, DOI:10.2174/0118715273297024240201055550.
- A. Parihar, K. Gaur and R. Khan, Chapter 10 – Rapid diagnostic assays for the detection of Alzheimer's and Parkinson's diseases, in Smart Diagnostics for Neurodegenerative Disorders, ed. A. Parihar, R. Khan and A. K. Srivastava, Academic Press, 2024, pp. 221–250 DOI:10.1016/B978-0-323-95539-3.00008-9.
- S. Chawla, D. Kalyane, V. Tambe, P. K. Deb, K. Kalia and R. K. Tekade, Evolving nanoformulation strategies for diagnosis and clinical interventions for Parkinson's disease, Drug Discovery Today, 2020, 25(2), 392–405, DOI:10.1016/j.drudis.2019.12.005.
- R. Thakur, A. K. Saini, R. Taliyan and N. Chaturvedi, Neurodegenerative diseases early detection and monitoring system for point-of-care applications, Microchem. J., 2025, 208, 112280, DOI:10.1016/j.microc.2024.112280.
- L. O. Orzari, L. R. G. e Silva, R. C. de Freitas, L. C. Brazaca and B. C. Janegitz, Lab-made disposable screen-printed electrochemical sensors and immunosensors modified with Pd nanoparticles for Parkinson's disease diagnostics, Microchim. Acta, 2024, 191(1), 76, DOI:10.1007/s00604-023-06158-3.
- J. H. Kim, Y. J. Suh and D. Park, et al., Technological advances in electrochemical biosensors for the detection of disease biomarkers, Biomed. Eng. Lett., 2021, 11(4), 309–334, DOI:10.1007/s13534-021-00204-w.
- R. Zhang, S. Wang and X. Huang, et al., Gold-nanourchin seeded single-walled carbon nanotube on voltammetry sensor for diagnosing neurogenerative Parkinson's disease, Anal. Chim. Acta, 2020, 1094, 142–150, DOI:10.1016/j.aca.2019.10.012.
- F. L. Zoey, M. Palanivel, P. Padmanabhan and B. Gulyás, Parkinson’s Disease: A Nanotheranostic Approach Targeting Alpha-Synuclein Aggregation, Front. Cell. Dev. Biol., 2021, 9, 707441, DOI:10.3389/fcell.2021.707441.
- B. Natarajan, P. Kannan and L. Guo, Metallic nanoparticles for visual sensing: Design, mechanism, and application, Chin. J. Struct. Chem., 2024, 43(9), 100349, DOI:10.1016/j.cjsc.2024.100349.
- M. N. Sonuç Karaboğa and M. K. Sezgintürk, A nano-composite based regenerative neuro biosensor sensitive to Parkinsonism-associated protein DJ-1/Park7 in cerebrospinal fluid and saliva, Bioelectrochemistry, 2021, 138, 107734, DOI:10.1016/j.bioelechem.2020.107734.
- H. Zhu, Z. Fohlerová, J. Pekárek, E. Basova and P. Neužil, Recent advances in lab-on-a-chip technologies for viral diagnosis, Biosens. Bioelectron., 2020, 153, 112041, DOI:10.1016/j.bios.2020.112041.
- S. Ge, G. Chen and J. Deng, et al., Multiplex signal amplification strategy-based early-stage diagnosis of Parkinson's disease on a SERS-enabled LoC system, Anal. Chim. Acta, 2023, 1247, 340890, DOI:10.1016/j.aca.2023.340890.
- X. Ren, Q. Zhang and J. Yang, et al., Dopamine Imaging in Living Cells and Retina by Surface-Enhanced Raman Scattering Based on Functionalized Gold Nanoparticles, Anal. Chem., 2021, 93(31), 10841–10849, DOI:10.1021/acs.analchem.1c01108.
- J. Hu, G. Gao and M. He, et al., Optimal route of gold nanoclusters administration in mice targeting Parkinson's disease, Nanomedicine, 2020, 15(6), 563–580, DOI:10.2217/nnm-2019-0268.
- H. Adam, S. C. B. Gopinath and H. Krishnan, et al., Cyclic and differential pulse voltammetric measurements on fibrils formation of alpha synuclein in Parkinson's disease by a gold interdigitated tetraelectrodes, Process Biochem., 2024, 136, 212–220, DOI:10.1016/j.procbio.2023.11.019.
- K. Nagaraj, P. Thangamuniyandi, G. Velmurugan, K. M. Alotaibi, K. Raja and B. K. Sharma, Green Synthesis of Eosin-Y Coated Silver Nanoparticles for Sensitive and Selective Fluorometric Detection of L-Dopa, J. Fluoresc., 2025, 1–12, DOI:10.1007/s10895-024-04116-7.
- I. Badillo-Ramírez, B. Landeros-Rivera, J. M. Saniger, J. Popp and D. Cialla-May, SERS-based detection of 5-S-cysteinyl-dopamine as a novel biomarker of Parkinson{’}s disease in artificial biofluids, Analyst, 2023, 148(8), 1848–1857, 10.1039/D3AN00027C.
- I. Badillo-Ramírez, J. M. Saniger, J. Popp and D. Cialla-May, SERS characterization of dopamine and in situ dopamine polymerization on silver nanoparticles, Phys. Chem. Chem. Phys., 2021, 23(21), 12158–12170, 10.1039/D1CP00966D.
- M. Rouhani and A. Soleymanpour, Preparation of Dawson heteropolyacid-embedded silver nanoparticles/graphene oxide nanocomposite thin film used to modify pencil graphite electrode as a sensor for trace electrochemical sensing of levodopa, Mater. Sci. Eng., C, 2020, 117, 111287, DOI:10.1016/j.msec.2020.111287.
- Z. Huang, H. Lu and H. Dong, et al., Fe3O4/Ni nanoparticles anchored nitrogen-doped porous carbon derived from core–shell MOF for simultaneous electrochemical detection of dopamine and 5-hydroxytryptamine, Talanta, 2025, 286, 127522, DOI:10.1016/j.talanta.2025.127522.
- M. Abdelmonem, E. L. Albert, M. A. Alhadad and C. A. Abdullah, Plant-Polyphenol-Mediated Synthesis of Magnetic Biocompatible Iron Oxide Nanoparticles for Diagnostic Imaging and Management of Neurodegenerative Diseases, Precis Nanomed., 2024, 7(1), 1233–1251, DOI:10.33218/001c.92424.
- L. An, Q. Tao and Y. Wu, et al., Synthesis of SPIO Nanoparticles and the Subsequent Applications in Stem Cell Labeling for Parkinson's Disease, Nanoscale Res. Lett., 2021, 16(1), 107, DOI:10.1186/s11671-021-03540-z.
- X. G. Liu, S. Lu and D. Q. Liu, et al., ScFv-conjugated superparamagnetic iron oxide nanoparticles for MRI-based diagnosis in transgenic mouse models of Parkinson's and Huntington's diseases, Brain Res., 2019, 1707, 141–153, DOI:10.1016/j.brainres.2018.11.034.
- S. Y. Yang, M. J. Chiu and C. H. Lin, et al., Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles, J. Nanobiotechnol., 2016, 14(1), 41, DOI:10.1186/s12951-016-0198-5.
- S. Luo, C. Ma, M. Q. Zhu, W. N. Ju, Y. Yang and X. Wang, Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases With Emphasis on Alzheimer's Disease, Front. Cell. Neurosci., 2020, 14, DOI:10.3389/fncel.2020.00021.
- Q. Chen, Y. Xue, Y. Huang, W. Guo, M. Wan and J. Shen, Mg-based micromotors for electrochemical detection of Parkinson's disease blood biomarkers, Sens. Actuators, B, 2024, 402, 135035, DOI:10.1016/j.snb.2023.135035.
- X. Zhang, M. Wu, S. C. B. Gopinath and Y. Chen, Dual-probe sandwich for Lewy body detection on nano-composite modified dielectric surface to determine Parkinson's disease, Sens Bio-Sens. Res., 2023, 42, 100599, DOI:10.1016/j.sbsr.2023.100599.
- A. Aziz, M. Asif and M. Azeem, et al., Self-stacking of exfoliated charged nanosheets of LDHs and graphene as biosensor with real-time tracking of dopamine from live cells, Anal. Chim. Acta, 2019, 1047, 197–207, DOI:10.1016/j.aca.2018.10.008.
- C. Y. Ge, M. M. Rahman and W. Zhang, et al., An Electrochemical Immunosensor Based on a Self-Assembled Monolayer Modified Electrode for Label-Free Detection of α-Synuclein, Sensors, 2020, 20(3), 617, DOI:10.3390/s20030617.
- G. M. Di Mari, M. Scuderi and G. Lanza, et al., Pain-Free Alpha-Synuclein Detection by Low-Cost Hierarchical Nanowire Based Electrode, Nanomaterials, 2024, 14(2), 170, DOI:10.3390/nano14020170.
- C. W. Chang, S. Y. Yang, C. C. Yang, C. W. Chang and Y. R. Wu, Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients With Parkinson's Disease, Front. Neurol., 2020, 10, DOI:10.3389/fneur.2019.01388.
- Q. Wu, R. Tan, X. Mi and Y. Tu, Electrochemiluminescent aptamer-sensor for alpha synuclein oligomer based on a metal–organic framework, Analyst, 2020, 145(6), 2159–2167, 10.1039/D0AN00169D.
- B. Obermeier, R. Daneman and R. M. Ransohoff, Review Development, maintenance and disruption of the blood-brain barrier, Nat. Med., 2013, 19(12), 1584–1596, DOI:10.1038/nm.3407.
- T. T. Zhang, W. Li, G. Meng, P. Wang and W. Liao, Strategies for transporting nanoparticles across the blood-brain barrier, Biomater. Sci., 2016, 4(2), 219–229, 10.1039/c5bm00383k.
- A. L. Merajoth, P. S. Pillai and T. Iype, Clinical Response of Levodopa Carbidopa Combination in Patients with Idiopathic Parkinsonism, J. Clin. Diagn. Res., 2016, 10(5), FC07, DOI:10.7860/JCDR/2016/16043.7886.
- O. Rascol, P. Payoux, F. Ory, J. J. Ferreira and C. Brefel-courbon, Montastruc J louis. Limitations of Current Parkinson's Disease Therapy, Ann. Neurol., 2003, 53(S3), S3–15, DOI:10.1002/ana.10513.Address.
- W. H. Oertel, Recent advances in treating Parkinson's disease, F1000Research, 2017, 6, 1–14, DOI:10.12688/f1000research.10100.1.
- N. Peranic, R. Barbir, C. R. Hall, T. A. Smith and M. A. Sani, Spectroscopic study of L-DOPA and dopamine binding on novel gold nanoparticles towards more efficient drug-delivery system for Parkinson's disease, Spectrochim. Acta, Part A, 2022, 5(268), 120707, DOI:10.1016/j.saa.2021.120707.
- L. Li, Y. Lu, X. Xu, X. Yang, L. Chen, C. Jiang, Y. Wang, W. Hu, X. Wei and Z. Yang, Catalytic-Enhanced Lactoferrin-Functionalized Au–Bi2Se3 Nanodots for Parkinson's Disease Therapy via Reactive Oxygen Attenuation and Mitochondrial Protection, Adv. Healthcare Mater., 2021, 10(13), 2100316 CrossRef CAS PubMed.
- E. da Silva Córneo, G. de Bem Silveira, R. Scussel, M. E. A. B. Correa, J. da Silva Abel, G. P. Luiz, P. E. Feuser, P. C. L. Silveira and R. A. Machado-de-Ávila, Effects of gold nanoparticles administration through behavioral and oxidative parameters in animal model of parkinson's disease, Colloids Surf., B, 2020, 196, 111302 CrossRef PubMed.
- J. Liu, D. Gao, D. Hu, S. Lan, Y. Liu and H. Zheng, Delivery of Biomimetic Liposomes via Meningeal Lymphatic Vessels Route for Targeted Therapy of Parkinson's Disease, Research, 2023, 6, 0030, DOI:10.34133/research.0030.
- G. Sharma, K. Wadhwa, S. Kumar, G. Singh and R. Pahwa, Revolutionizing Parkinson's treatment: Harnessing the potential of intranasal nanoemulsions for targeted therapy, Drug Delivery Transl. Res., 2025, 1–19 Search PubMed.
- K. Danz, J. Fleddermann and M. Koch, et al., Evaluation of the Transport and Binding of Dopamine-Loaded PLGA Nanoparticles for the Treatment of Parkinson’s Disease Using In Vitro Model Systems, Pharmaceutics, 2024, 16(5), 571 CrossRef CAS PubMed.
- G. Gao, M. Zhang, D. Gong, R. Chen, X. Hu and T. Sun, The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation, Nanoscale, 2017, 9(12), 4107–4113, 10.1039/c7nr00699c.
- L. Xiao, F. Wei, Y. Zhou, G. J. Anderson, D. M. Frazer and Y. C. Lim, Dihydrolipoic Acid − Gold Nanoclusters Regulate Microglial Polarization and Have the Potential To Alter Neurogenesis, Nano Lett., 2020, 20(1), 478–495, DOI:10.1021/acs.nanolett.9b04216.
- X. Ma, S. Lee and X. Fei, et al., Proteasome activity regulated by charged gold nanoclusters: Implications for neurodegenerative diseases, Nano Today, 2020, 35, 100933, DOI:10.1016/j.nantod.2020.100933.
- G. Gao, R. Chen, M. He, J. Li, L. Wang and T. Sun, Gold nanoclusters for Parkinson's disease treatment, Biomaterials, 2019, 194, 36–46, DOI:10.1016/j.biomaterials.2018.12.013.
- L. V. Nair, R. V. Nair, S. J. Shenoy, A. Thekkuveettil and R. S. Jayasree, Blood brain barrier permeable gold nanocluster for targeted brain imaging and therapy: An: in vitro and in vivo study, J. Mater. Chem. B, 2017, 5(42), 8314–8321, 10.1039/c7tb02247f.
- W. Wang, Y. Han, Y. Fan and Y. Wang, Effects of Gold Nanospheres and Nanocubes on Amyloid-β Peptide Fibrillation, Langmuir, 2019, 35(6), 2334–2342, DOI:10.1021/acs.langmuir.8b04006.
- M. Barthakur, P. Kalita and S. Mondal, Modulation of astrocytic membrane potential using citrate stabilized gold nanoparticle to control brain hyper-excitability, AIP Conf. Proc., 2020, 2259(1) DOI:10.1063/5.0015941.
- R. E. González-Reyes, M. O. Nava-Mesa, K. Vargas-Sánchez, D. Ariza-Salamanca and L. Mora-Muñoz, Involvement of astrocytes in Alzheimer's disease from a neuroinflammatory and oxidative stress perspective, Front. Mol. Neurosci., 2017, 10, 1–20, DOI:10.3389/fnmol.2017.00427.
- F. Talebpour and A. Ghahghaei, Effect of Green Synthesis of Gold Nanoparticles (AuNPs) from Hibiscus sabdariffa on the Aggregation of α-Lactalbumin, Int. J. Pept. Res. Ther., 2020, 26(4), 2297–2306, DOI:10.1007/s10989-020-10023-9.
- J. Xue, T. Liu and Y. Liu, et al., Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease – In vitro & In vivo model, J. Photochem. Photobiol., B, 2019, 200, 111635, DOI:10.1016/j.jphotobiol.2019.111635.
- B. Cicek, A. Hacimuftuoglu, Y. Yeni, M. Kuzucu, S. Genc, A. Cetin, E. Yavuz, B. Danısman, A. Levent, K. V. Ozdokur, M. Kantarcı, A. O. Docea, V. Siokas, K. Tsarouhas, M. D. Coleman, A. Tsatsakis and A. Taghizadehghalehjoughi, AuNPs with Cynara scolymus leaf extracts rescue arsenic-induced neurobehavioral deficits and hippocampal tissue toxicity in Balb/c mice through D1R and D2R activation, Environ. Toxicol. Pharmacol., 2024, 107, 104417 CrossRef CAS PubMed.
- L. Ling, Y. Jiang and Y. Liu, et al., Role of gold nanoparticle from Cinnamomum verum against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced mice model, J. Photochem. Photobiol., B, 2019, 201, 111657, DOI:10.1016/j.jphotobiol.2019.111657.
- K. Hu, X. Chen and W. Chen, et al., Neuroprotective effect of gold nanoparticles composites in Parkinson's disease model, Nanomedicine, 2018, 14(4), 1123–1136, DOI:10.1016/j.nano.2018.01.020.
- D. A. Gonzalez-Carter, Z. Y. Ong, C. M. McGilvery, I. E. Dunlop, D. T. Dexter and A. E. Porter, L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles, Nanomedicine, 2019, 1(15), 1–11 Search PubMed.
- L. Frkanec, R. Frkanec and A. Štimac, et al., Adamantane in Drug Delivery Systems and Surface Recognition, Molecules, 2017, 16(22), 297, DOI:10.3390/molecules22020297.
- J. Tomašić and I. Hršak, Peptidoglycan monomer originating from Brevibacterium divaricatum—its metabolism and biological activities in the host, Surface Structures of Microorganisms and Their Interaction with the Mammalian Host, 1987, pp. 113–121 Search PubMed.
- J. Xu, C. H. Tai and T. Y. Chen, Hsu S hui. An anti-inflammatory electroconductive hydrogel with self-healing property for the treatment of Parkinson’s disease, Chem. Eng. J., 2022, 446, 137180, DOI:10.1016/j.cej.2022.137180.
- J. Xu, T. Y. Chen, C. H. Tai and S. h Hsu, Bioactive self-healing hydrogel based on tannic acid modified gold nano-crosslinker as an injectable brain implant for treating Parkinson's disease, Biomater. Res., 2023, 27(1), 1–24, DOI:10.1186/s40824-023-00347-0.
- S. Li, J. Liu and G. Li, et al., Near-infrared light-responsive, pramipexole-loaded biodegradable PLGA microspheres for therapeutic use in Parkinson's disease, Eur. J. Pharm. Biopharm., 2019, 141, 1–11, DOI:10.1016/j.ejpb.2019.05.013.
- L. Liu, M. Li and M. Xu, et al., Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson's disease mice, Mater. Sci. Eng., C, 2020, 114, 111028, DOI:10.1016/j.msec.2020.111028.
- L. Liu, Y. Li and R. Liu, et al., Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment, Mater. Horiz., 2019, 6(9), 1923–1929, 10.1039/c9mh00482c.
- M. Trapani, A. Scala and P. G. Mineo, et al., Thiolated amphiphilic b -cyclodextrin-decorated gold colloids: Synthesis, supramolecular nanoassemblies and controlled release of dopamine, J. Mol. Liq., 2021, 336, 116880, DOI:10.1016/j.molliq.2021.116880.
- M. Khongkow, T. Yata and S. Boonrungsiman, Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood – brain barrier penetration, Sci. Rep., 2019, 1–9, DOI:10.1038/s41598-019-44569-6.
- Z. Y. Ong, S. Chen and E. Nabavi, et al., Multi-Branched Gold Nanoparticles with Intrinsic LAT-1 Targeting Capabilities for Selective Photothermal Therapy of Breast Cancer, ACS Appl. Mater. Interfaces, 2017, 15(9), 39259–39270, DOI:10.1021/acsami.7b14851.
- A. M. Khan, B. Korzeniowska and V. Gorshkov, et al., Silver nanoparticle-induced expression of proteins related to oxidative stress and neurodegeneration in an in vitro human blood-brain barrier model, Nanotoxicology, 2019, 13(2), 221–239, DOI:10.1080/17435390.2018.1540728.
- M. van der Zande, R. J. Vandebriel and E. Van Doren, et al., Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure, ACS Nano, 2012, 6(8), 7427–7442 CrossRef CAS PubMed.
- J. Skalska and L. Strużyńska, Toxic effects of silver nanoparticles in mammals – does a risk of neurotoxicity exist?, Folia Neuropathol., 2015, 53(4), 281–300, DOI:10.5114/fn.2015.56543.
- C. L. Huang, I. L. Hsiao, H. C. Lin, C. F. Wang, Y. J. Huang and C. Y. Chuang, Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells, Environ. Res., 2015, 136, 253–263, DOI:10.1016/j.envres.2014.11.006.
- M. Parveen, A. Kumar and M. S. Khan, et al., Comparative study of biogenically synthesized silver and gold nanoparticles of Acacia auriculiformis leaves and their efficacy against Alzheimer's and Parkinson's disease, Int. J. Biol. Macromol., 2022, 203, 292–301, DOI:10.1016/j.ijbiomac.2022.01.116.
- D. A. Gonzalez-carter, B. F. Leo and P. Ruenraroengsak, et al., Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H 2 S-synthesizing enzymes, Sci. Total Environ., 2017, 7(1), 42871, DOI:10.1038/srep42871.
- D. K. Shivanna, Datura Stramonium leaves extract Silver Nanoparticles regulates PINK1 gene in Parkinson's disease model of Drosophila melanogaster, Res Sq., 2022, 1–16 Search PubMed.
- R. E. Sardjono, F. Khoerunnisa, I. Musthopa, N. S. M. M. Akasum and R. Rachmawati, Synthesize, characterization, and anti-Parkinson activity of silver-Indonesian velvet beans (Mucuna pruriens) seed extract nanoparticles (AgMPn), J. Phys.: Conf. Ser., 2018, 1013(1), 012195 CrossRef.
- C. S. Silva, F. M. P. Tonelli and V. M. S. Delgado, et al., Nanoremediation and Antioxidant Potential of Biogenic Silver Nanoparticles Synthesized Using Leucena's Leaves, Stem, and Fruits, Int. J. Mol. Sci., 2024, 25(7), 3993, DOI:10.3390/ijms25073993.
- K. N. Mohamed Salam S, R. PV and C. J. NA, and MA A. Effect of Tualang Honey-Mediated Silver Nanoparticles on TNF-a level, Caspase-3 Activity and Hippocampal Morphology in Kainic Acid-Induced Neurodegeneration in Male Rats, IIUM Med. J. Malaysia, 2024, 23(4), 93 Search PubMed.
- N. A. Devi, P. Ravikumar, P. Devendran, R. Mohan, K. Ravichandran, M. Veeralakshmi, J. Yuvaloshini and M. M. Sundari, Efficacy of Catharanthus roseus leaf and flower extracts mediated Ag incorporated ZnO nanoparticles for enhanced antimicrobial and antioxidant abilities: a comparative analysis, Appl. Phys. A: Mater. Sci. Process., 2025, 131(2), 97 CrossRef CAS.
- K. Kaliappan, P. Nagarajan, J. Jayabalan, H. Pushparaj, S. Elumalai, B. Paramanathan, V. Manickam, H. T. Jang and G. Mani, Systematic antimicrobial, biofilm, free radical inhibition and tyrosinase inhibition assessments of efficient green silver nanoparticles from the aqueous root extract of Cyphostemma adenocaule (CA), RSC Pharm., 2025, 2(1), 147–162 RSC.
- S. Arulkumar and M. Sabesan, Rapid preparation process of antiparkinsonian drug Mucuna pruriens silver nanoparticle by bioreduction and their characterization, Pharmacogn. Res., 2010, 2(4), 233–236, DOI:10.4103/0974-8490.69112.
- C. Vissers, G. L. Ming and H. Song, Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders, Adv. Drug Delivery Rev., 2019, 1(148), 239–251, DOI:10.1016/j.addr.2019.02.007.
- S. Niu, L. K. Zhang and L. Zhang, et al., Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson's Disease Model, Theranostics, 2017, 7(2), 344, DOI:10.7150/thno.16562.
- G. Cheng, Z. Liu, Z. Yan, J. Wu, Z. Li, S. Gao, C. Zheng, S. Guo, Y. Pan, X. Chen and G. Lin, Minocycline nanoplatform penetrates the BBB and enables the targeted treatment of Parkinson's disease with cognitive impairment, J. Controlled Release, 2025, 377, 591–605 Search PubMed.
- R. A. Harris, Simulation study on the physicochemical properties of Fe 3 O 4 nanoparticles as drug delivery vehicles for dopamine replacement therapy of Parkinson's disease, Mater. Today Commun., 2022, 31, 103829, DOI:10.1016/j.mtcomm.2022.103829.
- Y. A. Khadrawy, E. N. Hosny and H. S. E. Mohamed, Assessment of the neuroprotective effect of green synthesized iron oxide nanoparticles capped with curcumin against a rat model of Parkinson's disease, Iran. J. Basic Med. Sci., 2024, 27(1), 81–89, DOI:10.22038/IJBMS.2023.73124.15892.
- G. Cheng, X. Liu and Y. Liu, et al., Ultrasmall Coordination Polymers for Alleviating ROS-Mediated Inflammatory and Realizing Neuroprotection against Parkinson’s Disease, Research, 2022, 2022 DOI:10.34133/2022/9781323.
- A. Moayeri, M. Darvishi and M. Amraei, Homing of Super Paramagnetic Iron Oxide Stem Cells by Magnetic Attraction in a Rat Model of Parkinson's Disease Homing of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) Labeled Adipose-Derived Stem Cells by Magnetic Attraction in a Rat Model of, Int. J. Nanomed., 2020, 1297–1308, DOI:10.2147/IJN.S238266.
- T. H. Chung, S. C. Hsu, S. H. Wu, J. K. Hsiao, C. P. Lin, M. Yao and D. M. Huang, Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson's disease, Nanoscale, 2018, 10(6), 2998–3007, 10.1039/C7NR06976F.
- D. Han, B. Zhang, L. Su and B. Yang, Attachment of streptavidin-modified superparamagnetic iron oxide nanoparticles to the PC-12 cell membrane, Biomed. Mater., 2020, 12(15), 045014 CrossRef PubMed.
- G. Prado-prone, P. Padilla, J. A. Garc and G. Gutie, Dopamine Released from TiO2 Semicrystalline Lattice Implants Attenuates Motor Symptoms in Rats Treated with 6 – Hydroxydopamine, ACS Omega, 2019, 4, 7953–7962, DOI:10.1021/acsomega.8b00626.
- M. Velázquez-Paniaguaa, A. M. Vázquez-Álvarezb, G. Valverde-Aguilarc and P. Vergara-Aragóna, Current treatments in Parkinson's including the proposal of an innovative dopamine microimplant, Rev. Med. Hosp. Gen., 2016, 79(2), 79–87, DOI:10.1016/j.hgmx.2015.10.006.
- V. N. Punitha, S. Vijayakumar, B. Sakthivel and P. K. Praseetha, Protection of neuronal cell lines, antimicrobial and photocatalytic behaviours of eco-friendly TiO2 nanoparticles, J. Environ. Chem. Eng., 2020, 8(5), 104343, DOI:10.1016/j.jece.2020.104343.
- P. C. Balaraju, J. B. Manchegowda, C. Kumar and S. S. Ahmed, Exploring the Therapeutic Potential of Selenium Nanoparticles in Central Nervous System Disorders: A Nanomedicine Approach, Int. J. Pharm. Invest., 2024, 14(4), 1025–1034, DOI:10.5530/ijpi.14.4.112.
- M. Shayganfard, Are Essential Trace Elements Effective in Modulation of Mental Disorders? Update and Perspectives, Biol. Trace Elem. Res., 2022, 200(3), 1032–1059, DOI:10.1007/s12011-021-02733-y.
- M. A. El-Ghazaly, N. Fadel, E. Rashed, A. El-Batal and S. Kenawy, Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats, Can. J. Physiol. Pharmacol., 2017, 95(2), 101–110 CrossRef CAS PubMed.
- L. O. El-Halaby, N. F. A. El-Magd, S. J. Almehmadi, A. A. El-Sayed, R. R. Khattab, S. El-Kalyoubi and S. M. Elfeky, Synthesis, In Vitro, In Vivo screening, and molecular docking of disubstituted aminothiazole derivatives and their selenium nanoparticles as potential antiparkinson agents, J. Mol. Struct., 2024, 1315, 138951 CrossRef CAS.
- Y. Gao, Y. Cheng, J. Chen, D. Lin, C. Liu, L. K. Zhang, L. Yin, R. Yang and Y. Q. Guan, NIR-Assisted MgO-Based Polydopamine Nanoparticles for Targeted Treatment of Parkinson's Disease through the Blood–Brain Barrier, Adv. Healthcare Mater., 2022, 11, 2201655 CrossRef CAS PubMed.
- R. Küçükdo, H. Türkez, M. E. Arslan, Ö. Ö. Tozlu and E. Sönmez, Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson's disease model against MPP + induced apoptosis, Metab. Brain Dis., 2020, 35, 947–957 CrossRef PubMed.
- J. Colon, N. Hsieh and A. Ferguson, et al., Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2, Nanomedicine, 2010, 6(5), 698–705, DOI:10.1016/j.nano.2010.01.010.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, The role of cerium redox state in the SOD mimetic activity of nanoceria, Biomaterials, 2008, 29(18), 2705–2709 CrossRef CAS PubMed.
- T. Pirmohamed, J. M. Dowding and S. Singh, et al., Nanoceria exhibit redox state-dependent catalase mimetic activity, Chem. Commun., 2010, 46(16), 2736–2738, 10.1039/b922024k.
- R. Ruotolo, G. De Giorgio, I. Minato, M. G. Bianchi, O. Bussolati and N. Marmiroli, Cerium oxide nanoparticles rescue α-synuclein-induced toxicity in a yeast model of parkinson's disease, Nanomaterials, 2020, 10(2), 235, DOI:10.3390/nano10020235.
- F. A. Tameh, Z. Jahani, S. Sedghiniya, M. A. Aghtaei, M. Abtahi, W. Xiang, M. Akbari, J. Soleimannejad and J. Janczak, Morphology-dependent multienzyme activity of nanoceria in antioxidant protection of MnCl2-treated PC-12 Cells, and the potential application for Parkinson's disease treatment, Inorg. Chem. Commun., 2024, 169, 113117 CrossRef.
- H. H. Dieter, T. A. Bayer and G. Multhaup, Environmental Copper and Manganese in the Pathophysiology of Neurologic Diseases (Alzheimer's Disease and Manganism), Acta Hydrochim. Hydrobiol., 2005, 33(1), 72–78, DOI:10.1002/aheh.200400556.
- A. Sharma, L. Feng, D. F. Muresanu, S. Sahib, Z. R. Tian, J. V. Lafuente, A. D. Buzoianu, A. Nozari, L. Wiklund and H. S. Sharma, Manganese nanoparticles induce blood-brain barrier disruption, cerebral blood flow reduction, edema formation and brain pathology associated with cognitive and motor dysfunctions, Nanomedicine and Neuroprotection in Brain Diseases, Elsevier, 2021, pp. 385–406 Search PubMed.
- A. Adhikari, M. Das and S. Mondal, et al., Manganese neurotoxicity: nano-oxide compensates for ion damage in mammals, Biomater. Sci., 2019, 7, 4491–4502, 10.1039/c9bm01039d.
- X. Wang, J. Zhao and W. Wang, et al., Electromagnetic field-enhanced chiral dimanganese trioxide nanoparticles mitigate Parkinson's disease, Sci. China: Chem., 2022, 65(10), 1911–1920 CrossRef CAS.
- Z. Xu, A. Qu, W. Wang, M. Lu, B. Shi, C. Chen, C. Hao, L. Xu, M. Sun, C. Xu and H. Kuang, Facet-Dependent Biodegradable Mn3O4 Nanoparticles for Ameliorating Parkinson's Disease, Adv. Healthcare Mater., 2021, 10(3), 2101316 CrossRef CAS PubMed.
- Y. Q. Liu, Y. Mao, E. Xu, H. Jia and S. Zhang, Nanozyme scavenging ROS for prevention of pathologic α-synuclein transmission in Parkinson's disease, Nano Today, 2021, 36, 101027, DOI:10.1016/j.nantod.2020.101027.
- H. Xu, X. Ding, L. Li, Q. Li and Z. Li, Tri-element nanozyme PtCuSe as an ingenious cascade catalytic machine for the amelioration of Parkinson's disease-like symptoms, Front. Bioeng. Biotechnol., 2023, 30(11), 1208693, DOI:10.3389/fbioe.2023.1208693.
- B. Shi, A. Qu and W. Wang, et al., Chiral Cu x Co y S Supraparticles Ameliorate Parkinson's Disease, CCS Chem., 2022, 14(4), 2440–2451, DOI:10.31635/ccschem.021.202101107.
- Y. Cheng, Y. Chang, Y. Feng, H. Jian, Z. Tang and H. Zhang, Deep-Level Defect Enhanced Photothermal Performance of Bismuth Sulfide–Gold Heterojunction Nanorods for Photothermal Therapy of Cancer Guided by Computed Tomography Imaging, Angew. Chem., Int. Ed., 2018, 57(1), 246–251, DOI:10.1002/anie.201710399.
- L. Xiao, A. Zhu, Q. Xu, Y. Chen, J. Xu and J. Weng, Colorimetric biosensor for detection of cancer biomarker by Au nanoparticle-decorated Bi2Se3 nanosheets, ACS Appl. Mater. Interfaces, 2017, 9(8), 6931–6940 CrossRef CAS PubMed.
- S. R. Ansari, J. Mahajan and A. Teleki, Iron oxide nanoparticles for treatment and diagnosis of chronic inflammatory diseases: A systematic review, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16(3), e1963, DOI:10.1002/wnan.1963.
- D. Calle, V. Negri, P. Ballesteros and S. Cerdán, Magnetoliposomes Loaded with Poly-Unsaturated Fatty Acids as Novel Theranostic Anti-Inflammatory Formulations, Theranostics, 2015, 5(5), 489–503, DOI:10.7150/thno.10069.
- M. Wang, L. Li and X. Zhang, et al., Magnetic Resveratrol Liposomes as a New Theranostic Platform for Magnetic Resonance Imaging Guided Parkinson's Disease Targeting Therapy, ACS Sustainable Chem. Eng., 2018, 6(12), 17124–17133, DOI:10.1021/acssuschemeng.8b04507.
- S. Niu, L. K. Zhang and L. Zhang, et al., Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson's Disease Model, Theranostics, 2017, 7(2), 344–356, DOI:10.7150/thno.16562.
- J. García-Pardo, F. Novio and F. Nador, et al., Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson's Disease, ACS Nano, 2021, 15(5), 8592–8609, DOI:10.1021/acsnano.1c00453.
- Y. Zhou, Z. Peng, E. S. Seven and R. M. Leblanc, Crossing the blood-brain barrier with nanoparticles, J. Controlled Release, 2018, 270, 290–303, DOI:10.1016/j.jconrel.2017.12.015.
- X. Wei, M. Cai and L. Jin, The Function of the Metals in Regulating Epigenetics During Parkinson's Disease, Front. Genet., 2021, 11, 616083, DOI:10.3389/fgene.2020.616083.
- S. Pyatha, H. Kim, D. Lee and K. Kim, Association between Heavy Metal Exposure and Parkinson's Disease: A Review of the Mechanisms Related to Oxidative Stress, Antioxidants, 2022, 11(12), 2467, DOI:10.3390/antiox11122467.
- F. Xiaoli, W. Junrong, L. Xuan, Z. Yanli, W. Limin, L. Jia and S. Longquan, Prenatal exposure to nanosized zinc oxide in rats: neurotoxicity and postnatal impaired learning and memory ability, Nanomedicine, 2017, 12(7), 777–795 Search PubMed.
- O. T. Phillipson, Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic, & circadian dysfunction in Parkinson's disease. An integrated strategy for management, Ageing Res. Rev., 2017, 1(40), 149–167, DOI:10.1016/j.arr.2017.09.006.
- D. N. Hauser and Te. G. Hastings, Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism, Neurobiol. Dis., 2013, 51, 35–42, DOI:10.1016/j.nbd.2012.10.011.Mitochondrial.
- A. Mohammadipour, H. Haghir and A. E. Bideskan, A link between nanoparticles and Parkinson's disease. Which nanoparticles are most harmful?, Rev. Environ. Health, 2020, 35(4), 545–556, DOI:10.1515/reveh-2020-0043.
- X. Valentini, P. Deneufbourg and P. Paci, et al., Morphological alterations induced by the exposure to TiO2 nanoparticles in primary cortical neuron cultures and in the brain of rats, Toxicol. Rep., 2018, 5, 878–889, DOI:10.1016/j.toxrep.2018.08.006.
- Q. Hu, F. Guo, F. Zhao and Z. Fu, Effects of titanium dioxide nanoparticles exposure on Parkinsonism in zebrafish larvae and PC12, Chmeosphere, 2017, 173, 373–379, DOI:10.1016/j.chemosphere.2017.01.063.
- R. Hu, X. Gong and Y. Duan, et al., Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO 2 nanoparticles, Biomaterials, 2010, 31, 8043–8050, DOI:10.1016/j.biomaterials.2010.07.011.
- I. Grissa, S. Guezguez, L. Ezzi and S. Chakroun, The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain, Environ. Sci. Pollut. Res., 2016, 23, 20205–20213, DOI:10.1007/s11356-016-7234-8.
- J. Wang, N. Li, L. Zheng, S. Wang and Y. Wang, P38-Nrf-2 Signaling Pathway of Oxidative Stress in Mice Caused by Nanoparticulate TiO2, Biol. Trace Elem. Res., 2011, 140, 186–197, DOI:10.1007/s12011-010-8687-0.
- L. Cristina, M. Pazin and M. F. Franco-bernardes, et al., A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs), J. Trace Elem. Med. Biol., 2018, 47, 63–69, DOI:10.1016/j.jtemb.2018.01.007.
- J. Wu and H. Xie, Effects of titanium dioxide nanoparticles on α -synuclein aggregation and the ubiquitin- proteasome system in dopaminergic neurons Effects of titanium dioxide nanoparticles on a -synuclein aggregation and the ubiquitin-proteasome system in dopaminergic ne, Artifi. Cells Nanomed. Biotechnol., 2016, 44, 690–694, DOI:10.3109/21691401.2014.980507.
- S. Mohammadi and M. Nikkhah, TiO2 Nanoparticles as Potential Promoting Agents of Fibrillation of α-Synuclein, a Parkinson's Disease-Related Protein, Natl. Inst. Genet. Eng. Biotechnol., 2017, 15(2), 87–94, DOI:10.15171/ijb.1519.
- J. K. Akintunde, T. I. Farai, M. R. Arogundade and J. T. Adeleke, Biogenic zinc-oxide nanoparticles of Moringa oleifera leaves abrogates rotenone induced neuroendocrine toxicity by regulation of oxidative stress and acetylcholinesterase activity, Biochem. Biophys. Rep., 2021, 26, 100999, DOI:10.1016/j.bbrep.2021.100999.
- M. Jin, N. Li, W. Sheng, X. Ji, X. Liang and B. Kong, Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson's disease-like symptoms induced by zinc oxide nanorods, Environ. Int., 2021, 146, 106179, DOI:10.1016/j.envint.2020.106179.
- N. Fernández-Bertólez, A. Alba-González and A. Touzani, et al., Toxicity of zinc oxide nanoparticles: Cellular and behavioural effects, Chemosphere, 2024, 363, 142993, DOI:10.1016/j.chemosphere.2024.142993.
- S. Ayton and P. Lei, Nigral Iron Elevation Is an Invariable Feature of Parkinson's Disease and Is a Sufficient Cause of Neurodegeneration, Biomed. Res., 2014, 2014 Search PubMed.
- D. J. Hare and K. L. Double, Iron and dopamine: a toxic couple, Brain, 2016, 1(139), 1026–1035, DOI:10.1093/brain/aww022.
- Z. Yarjanli, K. Ghaedi, A. Esmaeili, S. Rahgozar and A. Zarrabi, Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation, BMC Neurosci., 2017, 18, 1–12, DOI:10.1186/s12868-017-0369-9.
- S. Z. Imam, S. M. Lantz-mcpeak and E. Cuevas, et al., Iron Oxide Nanoparticles Induce Dopaminergic Damage: In vitro Pathways and In Vivo Imaging Reveals Mechanism of Neuronal Damage, Mol. Neurobiol., 2015, 913–926, DOI:10.1007/s12035-015-9259-2.
- F. Bagheri-Abassi, H. Alavi, A. Mohammadipour, F. Motejaded and A. Ebrahimzadeh-Bideskan, The effect of silver nanoparticles on apoptosis and dark neuron production in rat hippocampus, Iran. J. Basic Med. Sci., 2015, 18(7), 644–648 Search PubMed.
- G. Gupta, A. Gliga, J. Hedberg, I. Odnevall and W. Bengt, Cobalt nanoparticles trigger ferroptosis-like cell death (oxytosis) in neuronal cells: Potential implications for neurodegenerative disease, Fed. Am. Soc. Exp. Biol., 2020, 5262–5281, DOI:10.1096/fj.201902191RR.
- J. Lyu, X. Long and T. Xie, et al., Copper oxide nanoparticles promote α -synuclein oligomerization and underlying neurotoxicity as a model of Parkinson's disease, J. Mol. Liq., 2021, 323(323), 115051, DOI:10.1016/j.molliq.2020.115051.
- N. L. Botha, K. J. Cloete and Ž. Šmit, et al., Ionome mapping and amino acid metabolome profiling of Phaseolus vulgaris L. seeds imbibed with computationally informed phytoengineered copper sulphide nanoparticles, Discover Nano, 2024, 19(1), 8, DOI:10.1186/s11671-023-03953-y.
- M. Stern, N. Botha, K. J. Cloete, M. Maaza, S. Tan and G. Bicker, Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System, Int. J. Mol. Sci., 2024, 25(11), 5650, DOI:10.3390/ijms25115650.
- X. Li, Q. Li, Y. Zhang, Y. Bai, Y. Cao and Y. Yang, Nickel oxide nanoparticles increase a -synuclein amyloid formation and relevant overexpression of inflammatory mediators in microglia as a marker of Parkinson's disease, Arabian J. Chem., 2021, 14(10), 103380, DOI:10.1016/j.arabjc.2021.103380.
- C. Gao, F. Lyu and Y. Yin, Encapsulated metal nanoparticles for catalysis, Chem. Rev., 2020, 121(2), 834–881 Search PubMed.
- T. Boltman, N. R. S. Sibuyi, O. Ekpo and M. Meyer, Synthesis of chlorotoxin functionalized metallic nanoparticles and their in vitro evaluation of cytotoxic effects in nervous system cancer cell lines, Nano Express, 2024, 5(4), 045002, DOI:10.1088/2632-959X/ad80b0.
- A. Mhaske, S. Shukla, K. Ahirwar, K. K. Singh and R. Shukla, Receptor-Assisted Nanotherapeutics for Overcoming the Blood–Brain Barrier, Mol. Neurobiol., 2024, 61(11), 8702–8738, DOI:10.1007/s12035-024-04015-9.
- A. E. Lang, Clinical trials of disease-modifying therapies for neurodegenerative diseases: the challenges and the future, Nat. Med., 2010, 16(11), 1223–1226, DOI:10.1038/nm.2220.
- T. M. Dawson, T. E. Golde and C. Lagier-tourenne, Animal models of neurodegenerative diseases, Nat. Neurosci., 2018, 21(10), 1370–1379, DOI:10.1038/s41593-018-0236-8.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |