Evaluation of rare earth elements (REEs) in selected Nigerian coal fly ash: a prelude to extraction and waste management
Received
21st May 2024
, Accepted 8th December 2024
First published on 20th December 2024
Abstract
The rising need for rare earth elements (REEs) as critical materials for the development of clean energy technologies, as against the rapid depletion of virgin REE-bearing ores as well as their imbalance in geographical occurrence, calls for thorough search on secondary sources such as coal fly ash, given that the aluminosilicate mineral phase in the waste is enriched in REE particles. To support the geographical diversification of REE sources, there is a need for a comprehensive documentation of REE content and, by extension, the economic potential of fly ash derived from Nigeria's vast coal fields. Eight representative coal fly ash samples generated from coals from Nigeria's major coal belts were collected. Silica and alumina, with respective ranges of 38.1–44.5% and 14–15.98%, accounted for the bulk of the major elements in the samples. Total REE contents in the samples ranged from 874 ppm to 1127 ppm, while the cerium, yttrium, neodymium and lanthanum-dominated rare oxide totals were found to be in the range of 941–2145 ppm across the samples. The outlook coefficients (extractability indices) computed for the samples ranged between 0.8 and 1.3, with 0.7 as the benchmark. The range of percentage of critical REEs in the CFA samples was 28%–36%. This research has successfully explored the relative abundance and distribution of REEs in the studied fly ash samples, providing a theoretical lead for the basis of extraction and waste management.
Environmental significance
In Nigeria, there have been a series of studies concerning the use of coal combustion byproducts in several industrial domains. However, in spite of the growing fly ash wastes generated from industrial power plants in the country, little has been said about the need to put up strategies of mitigating the impact of this waste on the environment, in view of the fact that coal fly ash is often laden with harmful constituents. The authors believe that since the harmful fly ash waste is left on the landscape, one significant strategy of minimizing its impact would require extraction of valuable materials trapped in it. The evaluation of the rare earth contents, being a prerequisite for any potential extraction technology, has been therefore provided in the study. The environmental and economic implications for fly ash utilization in the country are highlighted in the study. The information provided can greatly support future fly ash management initiatives by the industries in Nigeria that utilize coal for power generation. The outcome of the study can also provide a guide for environmental regulation and tax authorities in the country.
|
1. Introduction
On the periodic table, a group of seventeen elements—namely: lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu), yttrium (Y) and scandium (Sc)—identified as the lanthanides, are referred to as rare earth elements (REEs). They possess unique physico-chemical characteristics that place them in the driver's seat for clean energy technologies, among other materials.1,2 In terms of activity, only group II (alkali earth elements) surpass REEs, the reason being that they have a vacant orbital electron layer of the 4f sublayer, which is necessary for the production of the various electronic energy levels needed for a wide range of applications in the domains of optics, electricity, metallurgy, magnetism, warfare armaments, petrochemicals, etc.3,4 REEs are said to serve as precursors for over 50 types of materials.5,6 Therefore, the gap between the demand and supply of these raw materials is expected to naturally widen. The scarcity is further complicated by the depletion of virgin REE ores due to extraction in the past century, by the associated costs of processing lean ores, and international geopolitics due to the imbalance in geographical spread of REE virgin ores. Fly ash is now being vigorously sought after as a potential alternative source of REEs, which is important to maintaining a sustainable supply chain.7 However, the research on the reuse of fly ash waste generated from Nigeria's huge coal reserves has not been viewed from the perspective of evaluating its rare earth content as a prelude to extraction, but has rather been tailored towards soil stabilisation in mines and the production of supplementary cementitious material (SCM) for civil engineering applications.8,9 Regarding the material's application in the domain of civil engineering within the Nigerian context, a number of recent studies have been reported, including the following; (i) a systematic survey of the use of coal fly ash in asphalt pavement and the construction of bricks, highway embarkments, and dams;10,11 (ii) extensive use of coal fly ash as a cementitious additive in concrete with a view to minimizing the environmental footprints associated with cement production and promoting resource conservation;12,13 and (iii) the effects of fly ash on the compressive strength and durability properties of lean concrete.14,15 As mentioned, the economic valuation and consequent extraction of REEs could potentially constitute an additional sustainable recycling and coal fly ash waste management strategy to guard against extreme environmental and health risks on the receiving environment.10 To this end, the main objective of the current study is to comprehensively document the rare earth content in selected coal fly ash samples sourced from Nigeria, which could form a theoretical basis for economic valuation and potential extraction.
2. Experimental
2.1 Sample collection and study area
Eight representative coal fly ash samples derived from Nigeria's coal belts were collected from UNICRANE power plants around Kogi State, Nigeria, and kept in airtight Falcon tubes prior to analyses. The ash samples are all products of fluidized bed combustion. Fig. 1 shows a detailed map of the coal sources from which fly ash samples were derived. An estimated proven 639 million metric tonnes of coal deposits are still buried in the bowels of the country, and there are over 22 commercial coal fields located in over 13 states in Nigeria. Details of coal mines and operators in the country can be found on the website of the Bureau of Public Enterprises.13
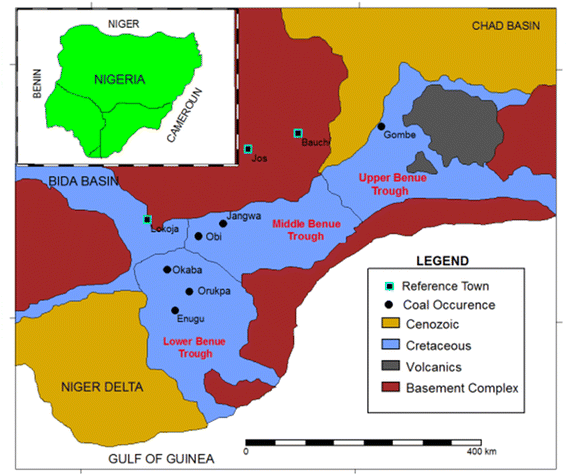 |
| | Fig. 1 Map showing the location of coal sources from which fly ash samples were derived [CC by 3.0 License].14 | |
2.2 Determination of major and rare element composition
The major and rare element compositions of the fly ash samples were determined with the use of (ICP-OES)-Agilent 5110 VDV, Agilent Technologies Inc., Santa Clara, CA, USA. The flux used for the fusion was dilithium tetraborate (Spectromelt A-10, EM Science, Gibbstown, NJ, USA). Standard operation procedure was followed. The specific parameters for measuring the samples were as follows: read time of 6 s, five replicates, RF power = 1.2 kW, axial viewing mode, and the following gas flows: nebulizer: 0.7 L min−1, plasma: 12 L min−1, auxiliary: 1 L min−1. The samples were diluted by a factor of 33.333 with a 2% HNO3 solution to ensure the pH was ≤2 for analysis. A continuing calibration verification (CCV) solution was also measured once every 24 samples to ensure the stability of the results. All solutions were made with analytical grade chemicals and fresh 18.2 MΩ ultrapure water (Milli-Q IQ 7000). The calibration solutions were prepared fresh from commercial standards. The REEs were prepared from Supelco TraceCERT Certified Reference Material (CRM) solution (Sigma-Aldrich product number 67349). Approximately 0.1 g of each sample was weighed into a PTFE vessel, after which 2 mL HNO3, 2 mL HF, and 6 mL HCl were added to the sample. The vessels were then closed and heated in a microwave (230 °C, ramp-up time 20 min, hold time 15 min), then allowed to cool before being moved to volumetric bottles and diluted to 100 mL total volume. For the analysis of REEs, the solutions were measured undiluted. For the analysis of major elements, the solutions were diluted by combining 200 μL of sample with 9800 μL of 2% HNO3 solution (dilution factor of 50). All values of the main constituent elements were converted from mg g−1 to weight percent.
2.3 Calculation of economic outlooks and percentages of critical elements in fly ash samples
The economic outlook for each coal fly ash sample was calculated on the basis of the Dai and Finkelman criteria, which utilize the formula in eqn (1). Eqn (1) may otherwise refer to the ratio of the relative amount of critical REEs in the total REEs to the relative amount of excessive REEs (Ce, Ho, Tm, Yb, and Lu).16 The percentage of critical elements per sample was computed using eqn (2).| |  | (1) |
| | 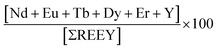 | (2) |
3. Results and discussion
3.1 Analysis of major elements
The extraction of REEs from coal fly ash is highly amenable to hydrometallurgy processes owing to its fine particle size. The lixiviants used in the mentioned extraction process engage in exchange reactions not only with REE ions but also with impurity ions such as aluminum ion (Al3+) and iron ion (Fe3+) as well as other minor ion impurities present.17–19 These impurities become enriched in the leachate, which decreases REE content in solution. The overall economic implication is an increase in leaching reagent consumption, cost, and/or possible decrease in the market value of the end product.19 It was therefore necessary to measure the amounts of impurity oxides present in the fly ash samples, which herein may be described as the principal constituents shown in Table 1.
Table 1 Chemical composition of major elements in the fly ash samples (wt%) measured with ICP-OES
| Elements |
BD-1 |
BD-2 |
BD-3 |
BD-4 |
BD-5 |
BD-6 |
BD-7 |
D-8 |
| Al2O3 |
14.000 |
15.680 |
16.440 |
16.46 |
16.080 |
16.140 |
15.980 |
15.740 |
| SiO2 |
41.000 |
41.600 |
38.10 |
40.2 |
43.500 |
37.400 |
44.500 |
43.300 |
| BaO |
0.130 |
0.116 |
0.112 |
0.109 |
0.113 |
0.117 |
0.115 |
0.122 |
| CaO |
0.923 |
0.810 |
0.875 |
0.620 |
1.000 |
0.890 |
0.880 |
0.880 |
| Fe2O3 |
4.340 |
4.510 |
4.550 |
4.600 |
4.650 |
4.390 |
5.000 |
4.890 |
| K2O |
0.480 |
0.470 |
0.500 |
0.412 |
0.393 |
0.416 |
0.450 |
0.403 |
| MgO |
2.380 |
2.180 |
2.460 |
2.400 |
2.380 |
2.360 |
2.320 |
2.320 |
| MnO |
0.950 |
0.920 |
0.930 |
1.040 |
1.010 |
0.890 |
0.950 |
0.930 |
| Na2O |
0.990 |
0.960 |
0.950 |
0.930 |
0.900 |
0.590 |
0.630 |
0.620 |
| PO2 |
1.220 |
1.170 |
1.150 |
1.180 |
1.180 |
1.110 |
1.100 |
1.210 |
| ZnO2 |
0.245 |
0.160 |
0.262 |
0.140 |
0.173 |
0.325 |
0.132 |
0.248 |
| Al2O3/SiO2 |
0.340 |
0.380 |
0.430 |
0.410 |
0.370 |
0.430 |
0.360 |
0.370 |
Within the given ranges, silica (37.4–44.5%) and alumina (14.04–15.98%) were respectively found as the principal constituents of the samples analyzed, along with iron oxide (4.34–5.00%) and other trace oxides. The significant percentages of SiO2, Al2O3, and iron oxide may be due to the presence of minerals such as mullite, quartzite and hematite in the primary coals.19 In addition, since the fly ash samples are products of fluidized bed combustion, in which typically, for the purpose of heat transfer, temperature gradients are minimized and combustion occurs at relatively low temperatures of 700–900 °C in the combustion bed boiler, sand floats with the fuel under high-velocity air jet.20 The high silica content (37.4–44.5%) in the fly ash samples may have also been derived from a combination of sand in the combustion boiler and in the primary coals. In addition, due to non-uniformity of fuel materials in terms of the quality of coal, biomass composition and moisture contents, no two fly ash samples can be said to have similar chemical characteristics, but they vary as follows: SiO2 (22–55.53%), alumina (0.1–50.98%), Fe2O3 (0.1–27.9%), MgO (0.1–7.10%), as traditional characteristics of ash derived from fluidized bed combustion.21–23 The results of the principal constituents in the sample analyzed fall within the mentioned ranges. Notable among the minor elements are oxides of magnesium and calcium. While MgO enrichment in the coal ash samples may be due to its presence in the primary coals, CaO may have been distributed as a result of its presence in the alkaline bed materials meant for SO2 capture with the ultimate aim of minimizing carbon footprints on the environment, as theoretically described by eqn (3) and (4).20,24
3.2 Analysis of rare earth elements
Table 2 presents a summary of REE content of the selected fly ash samples (D-1 to D-8). Studies on the composition and enrichment of REEs in coal combustion byproducts have gained global research interest in recent years—not just for the purpose of regional geological records but also for the potential economic benefits associated the elements.20 For this reason, the outcome of the analyses may be viewed, on one hand, via the lens of geochemistry, total rare earth elements, and total rare earths oxides; and on the other hand, from the perspective of economics of extraction and comparison with previous studies on fly ash from some other large coal-consuming nations.
Table 2 Rare earth content per sample (ppm)a
| Element (REEO conversion factor) |
D-1 |
D-2 |
D-3 |
D-4 |
D-5 |
D-6 |
D-7 |
D-8 |
| REE |
REO |
REE |
REO |
REE |
REO |
REE |
REO |
REE |
REO |
REE |
REO |
REE |
REO |
REE |
REO |
|
*: below detection limit; LREE: light rare earth elements; HREE: heavy rare earth elements; MREE: medium rare earth elements; REEY: rare earth elements and yttrium.
|
| La (1.17) |
133.000 |
156.000 |
125 |
146.0 |
124 |
145.100 |
131 |
153.300 |
126 |
14 |
140 |
164 |
142 |
166 |
144 |
168.5000 |
| Ce (1.17) |
291 |
340 |
276 |
323 |
273 |
319 |
288 |
336.960 |
286 |
335 |
308 |
360.400 |
316 |
370 |
317 |
371 |
| Pr (1.17) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
| Nd (1.16) |
115 |
1239 |
112 |
130 |
109 |
126 |
116 |
135 |
119 |
138 |
121 |
140.400 |
130 |
151 |
134 |
155.400 |
| Sm (1.16) |
79 |
92 |
74 |
89 |
72 |
84 |
74 |
86 |
76 |
88.200 |
81 |
1.350 |
86 |
99.800 |
88 |
102.100 |
| Eu (1.16) |
5 |
6 |
5 |
6 |
5 |
5.800 |
5 |
5.800 |
6 |
10 |
5 |
5.800 |
6 |
10 |
6 |
10 |
| Gd (1.15) |
70 |
81 |
65 |
75 |
64 |
74 |
67 |
77.1 |
71 |
81.7 |
73 |
84 |
130 |
150 |
134 |
154.100 |
| Tb (1.15) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
| Dy (1.15) |
2 |
26.600 |
18 |
21 |
22 |
25.300 |
30 |
35 |
31 |
35.700 |
32 |
37 |
130 |
150 |
134 |
154 |
| Ho (1.15) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
* |
* |
* |
|
* |
* |
| Er (1.14) |
3 |
35 |
28 |
32 |
29 |
33.100 |
30 |
34.200 |
31 |
35.300 |
32 |
36.500 |
33 |
37.600 |
34 |
38.800 |
| Tm (1.14) |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
| Yb (1.14) |
8.300 |
9.500 |
6.700 |
8 |
8.200 |
3.500 |
8.200 |
9.348 |
9.800 |
11.170 |
9.100 |
10.370 |
8.500 |
9.700 |
9 |
10.230 |
| Lu (1.14) |
2.800 |
3.200 |
2.600 |
3 |
2.700 |
3.080 |
2.800 |
3.190 |
3 |
3.420 |
3 |
3.260 |
3.100 |
3.530 |
3.300 |
3.760 |
| Sc (1.53) |
21.300 |
33 |
14.800 |
23 |
21.900 |
33.100 |
20.600 |
31.500 |
23.800 |
36.410 |
22.600 |
34.580 |
18.700 |
28.600 |
21.400 |
32.740 |
| Y (1.27) |
97.600 |
124 |
79.500 |
101 |
99.600 |
126.490 |
102 |
129.540 |
120 |
152.400 |
111 |
140.970 |
104 |
132.080 |
103 |
130.810 |
| ΣREEY/ΣREO |
877 |
2145.300 |
806.600 |
957 |
830.400 |
978.380 |
874.600 |
879 |
901.600 |
941.300 |
937 |
1018.630 |
1107.300 |
1308.310 |
1127.700 |
1331.440 |
|
C
out
|
0.810 |
|
0.840 |
|
0.933 |
|
0.850 |
|
1.030 |
|
0.940 |
|
0.740 |
|
1.300 |
|
| % critical |
28 |
|
34 |
|
32 |
|
32 |
|
34 |
|
32 |
|
36 |
|
36 |
|
| ΣLREE |
621 |
|
615 |
|
607 |
|
639 |
|
638 |
|
682 |
|
707 |
|
717 |
|
| ΣHREE |
134.700 |
|
134.800 |
|
141.500 |
|
173 |
|
84.600 |
|
257.790 |
|
278.600 |
|
283.300 |
|
| ΣMREE |
152 |
|
167 |
|
165 |
|
171 |
|
178 |
|
186 |
|
249 |
|
256 |
|
3.2.1 Geochemistry.
From the viewpoint of geochemistry, a decreasing trend in individual REE concentrations is often observed with increasing atomic number, and REEs with even atomic numbers tend to be more frequent than those with odd atomic numbers, in accordance with the Oddo–Harkins rule Ce > La > Nd > Pr > Sm > Gd > Dy > Er > Yb > Eu > Tb > Ho > Tm > Lu.25,26 While any existing Pr, Tb, Ho and Tm elements were below the detection limits, for each sample, we observed a trend in the occurrence of the elements detected in conformity with the Oddo–Harkins rule. For instance, in sample D-1, we observed that the trend conformed with the rule as follows: Ce (291) > La (133) > Nd (115) > Sm (79) > Gd (70). However, a deviation from this rule was observed in the cases of subsequent elements: Er > Yb > Eu > Tb > Ho > Tm > Lu did not conform with the rule as detected.27 We observed similar trends in samples D-2 to D-8. These findings do not seek to advance an argument that suggests the nullification of the absoluteness of the trend of occurrence of REEs as noted by the Oddo–Harkins rule. We therefore suppose that the observed deviation may be due to the following reasons: (i) the non-detection of some elements as a result of the technique used as well as other alterations which may have stemmed from several possible geochemical and anthropogenic processes, and (ii) the materials analyzed are combustion byproducts with complex lattice structures containing varying interfering substances.28–30 The anthropogenic processes herein mentioned influence the characteristics of secondary resources, such as the materials studied.31 To this end, it is important to mention that the Oddo–Harkins rule and its implications for absolute variation in the lanthanide series may be limited in secondary materials with complex lattice structures, such as coal fly ash.
3.2.2 Total rare earth elements.
Depending on the location, the average rare earth content in coal fly ash often reported is in the range of 100 to 404 ppm32–34 This range somewhat agrees with trends reported of the rare earth element content in United States – based coal fly ashes, which respectively averaged 591 ppm, 403 ppm, 337 ppm for the Appalachian, Illinois and Powder River basin coals.35 For the current study, it was generally observed that the total rare earth elements ranged from 806–1107, ppm which are well above the range noted by some previous researchers but below the 1213.6 to 1667.6 ppm sum of REEY in a report on Kentucky coal fly ash samples.36 In the current study, La, Ce and Nd would make significant contributions to the total REE value in the studied fly ash, based on their contents and market value.37 It is important to note that as the intensity of environmental awareness around mining of these elements from virgin ores increases, opportunities for recycling and sustainable practices, which not only help in addressing environmental issues but also strengthen supply chains, can be potentially identified from this study.38–40 In addition, the findings potentially provide an avenue for geographical diversification of rare earth element sources, which can further close the demand-and-supply gap.41 In summary, the findings from the studied fly ash suggest that the coal fly ash contains potentially upgradable REE concentrations.
3.2.3 Total rare earth oxides.
In terms of individual total rare earth oxides (TREO), we observed that samples D-1, D-6, D-7 and D-8 have contents above the benchmark of 1000 ppm required for potential economic extraction, while samples D-2, D-3, D-4 and D-5 have total rare earth contents below 1000 ppm.42 These values were however found to be close to the 1000 ppm potential profitable extraction benchmark and have potential for beneficiation.43,44 Overall, the samples were dominated by cerium, yttrium, neodymium, lanthanum, gadolinium, and samarium in the approximate order Ce > Nd > Y > La > Gd > Sm. The conversion of individual REE (∑REE) to REO (rare earth oxide) is based on two reasons: (1) the chemical reactions associated with release of heat during combustion are oxidation reactions, which convert the individual elements inherent in the coal into their respective oxides. This agrees with a synchrotron-based analysis conducted by Stuckman and co-authors,43 which reported that at microscale, Ce can undergo a transformation from Ce(III) to a mixture of Ce(III) and Ce(IV), and to 100% Ce(IV) oxide, indicating potential REE phase decomposition and oxidation in coal and coal combustion process. (2) Expressing REEs as oxides in an economic context, REEs are marketed as oxides.37 It is very important to note that the explanation of the results reported in oxides from above was only necessary as a basis for measuring the economic feasibility of the studied coal fly ash sample and as reference for literature comparison. The total REO values in the studied fly ash samples range between 879 ppm and 2145.30 ppm.
3.2.4 Percentage of critical elements versus outlook count.
Table 3 presents the outlook count data and percentage of critical elements as analyzed. Data obtained from Table 3 are pictorially shown in the succeeding Fig. 2, which provides a plot of percentage critical element versus outlook count. It was necessary to indicate Fig. 2 as a way of accessing the samples' percentage critical rare earth contents and economic outlooks for appropriate classification. From the figure, except for sample D-1, the percentages of critical REE content and outlook coefficients computed for the current study classify the samples as group 2, promising. The range of values of critical REEs measured in the samples agrees with the reports of researchers,45,46 which indicated that coal ash contains a higher fraction of critical REE (>30% of total REE) than virgin REE-bearing ores. Among the dominant REEs found in the samples analyzed, cerium, yttrium, neodymium, and lanthanum were found to be critical. An outlook coefficient ≥0.7 indicates that some samples can be used as secondary resources for rare earth element extraction.47
Table 3 Outlook coefficient and percentage of critical elements per sample
| Sample |
Outlook count |
% critical |
| D-1 |
0.800 |
28 |
| D-2 |
0.840 |
34 |
| D-3 |
0.930 |
32 |
| D-4 |
0.850 |
32 |
| D-5 |
1.040 |
34 |
| D-6 |
0.940 |
32 |
| D-7 |
0.740 |
36 |
| D-8 |
1.300 |
36 |
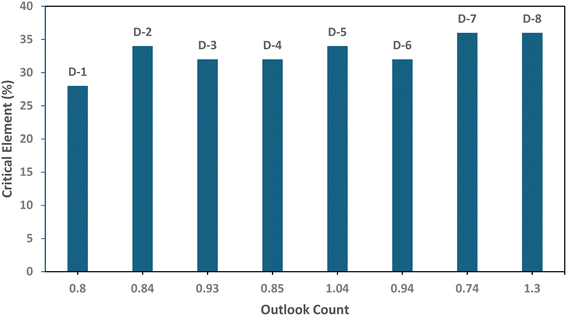 |
| | Fig. 2 A plot of percentage of critical elements versus outlook count. | |
3.2.5 Economic outlook.
Economic outlook relies on the Seredin and Dai criteria,48 which classified REEs as critical (Nd, Eu, Tb, Dy, Y and Er), uncritical (La, Pr, Sm dan Gd) and excessive (Ce, Ho, Tm, Yb and Lu). These criteria proposed a rapid method of evaluation of the economics of extraction of REEs from coal fly ash. Stemming from this classification, an outlook coefficient is defined by eqn (1), as earlier indicated in Section 2.3.25
Outlook coefficient is a measure of the extractability of critical REEs from fly ash. The minimum Cout index is 0.7. Thus, the higher the Cout, the higher the extractability of the critical REEs from coal fly ash. The Couts computed for all samples in the current work range from 0.74 to 1.04 and are above the minimum 0.7 count required for extractability, as suggested by Dai et al.49 All of the samples analyzed had favourable distributions with respect to content of critical REEs. These are widely known criteria on the industrial evaluation of coal fly ash, and yet another suggested classification of REE-laden coal fly ash on the basis of percentage total REE and outlook count is as follows: group 1, unpromising (total REE ≤ 26%; Cout ≤ 0.7); group 2, promising (30% ≤ total REE ≤ 51%; 0.7 ≤ Cout ≤ 1.9); and group 3, highly promising (total REE > 60%; Cout > 2.4).50 The percentage of critical elements per sample was computed using eqn (2). The range of percentage of critical REEs (Nd, Eu, Tb, Dy, Y, and Er) in the fly ashes was 28%–36% of the total and considerably higher than in conventional ores (typically less than 15%).51 Fly ash samples D-7 and D-8 had the highest extractable REE content, with 36% of the total REE. We suppose that this may be due to the higher calcium content in the samples, which promotes their solubility in the nitric acid used for the analyses. Sc, Nd, and Dy would account for the major contribution to the total REE value in fly ash, based on their contents and recent market prices.34
From the viewpoint of the potential economic and environmental implications for coal-fired plants in Nigeria, it is pertinent to note that approximately 0.15 short ton of fly ash is generated per short ton of coal. With Nigeria's production of 3335 short tons per annum,11,52,53 the total generation of fly ash is approximately 500.00 short tons per year. In considering potential extraction setups, the key environmental impact is that, as it is with virgin mineral development, the recovery of value from coal combustion byproduct requires haulage of feedstock to the processing facility location, as well as storage.54,55 Currently, these residues are stored in the landscape and waste management enterprises, with an average charge disposal (haulage) fee of N90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton (ninety thousand Nigerian Nairas).56 On this basis, the average cost per ton of disposal (haulage) of the solid waste per year may be approximately estimated as N45
000 per ton (ninety thousand Nigerian Nairas).56 On this basis, the average cost per ton of disposal (haulage) of the solid waste per year may be approximately estimated as N45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022
022![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (USD 30
500 (USD 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255.12). This indicates the unit cost of disposal per ton of coal fly ash generated per annum. Thus, any resource recovery that may be proposed would have potential economic and environmental implications for industries utilizing coal for power generation. In the overall view, this study shows that coal fly ash production has potential as a domestic supply of REEs, depending on the feasibility of development of extraction technologies.
255.12). This indicates the unit cost of disposal per ton of coal fly ash generated per annum. Thus, any resource recovery that may be proposed would have potential economic and environmental implications for industries utilizing coal for power generation. In the overall view, this study shows that coal fly ash production has potential as a domestic supply of REEs, depending on the feasibility of development of extraction technologies.
3.2.6 Comparison with REE contents in coal fly ashes of other regions.
Due to varying geological formations, no two coal sources and, by extension, fly ash, can be said to have similar characteristics. However, for the sake of future development, international collaboration and potential intercontinental investors, Table 4 provides a comparison of studied REE contents with those of some other coal-consuming nations. In addition, the typical REE contents in coal fly ash derived from Nigerian coals have not been comprehensively documented or compared with those of major coal-consuming countries for the purpose of potential upgrade and commercialization.35 The findings from the current research have now provided a theoretical basis for the mentioned documentation and comparison.
Table 4 Concentration (ppm) of Total REE (TREE) in the studied fly ash compared with those of some coal-consuming countries
| Fly ash sample |
Total REE |
Critical REE (%) |
Reference |
| D-1 |
877.000 |
28.000 |
Current study |
| D-2 |
806.000 |
34.000 |
Current study |
| D-3 |
830.000 |
32.000 |
Current study |
| D-4 |
874.000 |
32.000 |
Current study |
| D-5 |
901.000 |
34.000 |
Current study |
| D-6 |
937.000 |
32.000 |
Current study |
| D-7 |
1107.000 |
36.000 |
Current study |
| D-8 |
1127.000 |
36.000 |
Current study |
| Bhusawal coal-fired plant, India |
300–500 |
27.000 |
24 and 50 |
| Central appalachian fire clay, USA |
1668.000 |
37.000 |
54
|
| Illinois Basin, USA |
312.000 |
36.000 |
56
|
| Powder River Basin, USA |
283.000 |
33.000 |
56
|
4. Conclusions
In addressing the need for comprehensive documentation of rare earth contents in coal fly ash generated from Nigerian coals as well as the establishment of geographical diversification in support of a sustainable supply chain, this research has successfully explored the relative abundance and distribution of REEs in the studied fly ash, with TREO ranging 941–2145 ppm and outlook coefficients (extractability indices) ranging from 0.81 to 1.3 across the samples. The research has similarly highlighted the potential economic viability of the selected coal fly ash in terms of rare-earth content, forming a theoretical basis for extraction from the samples. The outcome of this research provides a vital reference for recycling coal combustion wastes in other parts of the globe.
Data availability
Raw data were generated at the research unit of Sustainable Chemistry, University of Oulu, Finland. Derived data supporting the findings of this study are available from the corresponding author J. P. on request.
Author contributions
Conceptualization: Theophilus I. Ojonimi. Data Curation: Janne Pesonen, Ferdinard Asuke, Aliyu Mohammed Ramalan, John Groppo. Formal Analysis: Theophilus I. Ojonimi. Funding Acquisition: Theophilus I. Ojonimi and Janne Pesonen. Investigation: Theophilus I. Ojonimi and Janne Pesonen. Methodology: Theophilus I. Ojonimi, Ferdinard Asuke and Aliyu Mohammed Ramalan. Project administration: Theophilus I. Ojonimi and Janne Pesonen. Software: Theophilus I. Ojonimi. Supervision: Ferdinard Asuke, Aliyu Mohammed Ramalan and Janne Pesonen. Writing – original draft: Theophilus I. Ojonimi. Writing – review and editing: Theophilus I. Ojonimi, Janne Pesonen and Ilemona Okeme.
Conflicts of interest
There are no competing interests to report.
Acknowledgements
The Nigerian Tertiary Education Trust Fund (TETFUND) supported the upkeep of the first author during his research visit at the research unit of Sustainable Chemistry, University of Oulu, Finland. The authors would like to thank laboratory technicians Mikko Häkkinen and Markus Väyrynen for the chemical analyses.
References
- K. K. Yadav, K. Dasgupta, D. K. Singh, M. Anitha, L. Varshney and H. Singh, Sep. Purif. Technol., 2015, 143, 115–124 CrossRef CAS.
- S. Pradhan, N. Swain, S. Prusty, R. K. Sahu and S. Mishra, Mater. Today: Proc., 2020, 30(2), 239–245, DOI:10.1016/j.matpr.2020.01.288.
- J. Zhang, Z. Wang, Q. Wu, Y. An, H. Jia and Y. Shen, Int. J. Environ. Res. Publ. Health, 2019, 16(20), 4052, DOI:10.3390/ijerph16204052.
- C. Liu, W. Liu, H. Huot, M. Guo and S. Zhu, Sci. Total Environ., 2022, 809, 152075, DOI:10.1016/j.scitotenv.2021.152075.
- A. M. Mowafy, World J. Microbiol. Biotechnol., 2020, 36(4), 61, DOI:10.1080/10643389.2020.1727718.
-
T. I. Ojonimi, T. P. Chanda, I. C. Okeme, F. Asuke and A. F. Mulaba-Bafubiandi, in Recovery of Values from Low-Grade and Complex Minerals: Development of Sustainable Processes, ed. E. Fosso-Kankeu, B. B. Mamba & A. A. Mulaba-mafubiandi, 2024, DOI:10.1080/00206814.2024.2384075.
-
E. Fosso, Rare Earth Elements: Sustainable Recovery, Processing, and Purification Athanasios, ed. K. Karamalidis and R. Eggert, 2024 Search PubMed.
- F. Althoey, W. S. Ansari, M. Sufian and A. F. Deifalla, Developments in the Built Environment, Built. Environ., 2023, 16, 100284, DOI:10.1016/j.dibe.2023.100284.
-
Coal Ash Rule: Environmental and Energy Law Program (EELP), accessed february 2024, https://eelp.law.harvard.edu/2023/08/coal-ash-rule/.
- D. O. Oyejobi, A. A. Firoozi, D. B. Fernández and S. Avudaiappan, Results Eng., 2024, 24, 102846, DOI:10.1016/j.rineng.2024.102846.
- S. E. Kelechi, M. Adamu, O. A. U. Uche, I. P. Okokpujie, Y. E. Ibrahim and I. I. Obianyo, Cogent Eng., 2022, 9, 1, DOI:10.1080/23311916.2022.2114201.
- O. J. Olatoyan, M. A. Kareem, A. U. Adebanjo, S. O. A. Olawale and K. T. Alao, Hybrid Mater., 2023, 4, 100076, DOI:10.1016/j.hybadv.2023.100076.
-
Coal production in Nigeria, accessed, february 2023, https://www.bpe.gov.ng/nigerian-coal-corporation.
- F. B. Fatoye and Y. B. Gideon, J. Environ. Earth Sci., 2013, 3(11), 1–9 Search PubMed.
- J. O. Ohwofasa, C. M. Ikumapayi and C. Arum, J. Appl. Sci. Environ. Manag., 2023, 27(11), 2597–2610 Search PubMed.
- S. Dai and R. B. Finkelman, Int. J. Coal Geol., 2018, 186, 155–164, DOI:10.1016/j.coal.2017.06.005.
- R. G. Silva, C. A. Morais and E. D. Oliveira, Miner. Eng., 2019, 134, 402–416, DOI:10.1007/s42461-020-00179-9.
- W. D. Judge and G. Azimi, Hydrometallurgy, 2020, 196, 105435, DOI:10.1016/j.hydromet.2020.105435.
- W. Lu, L. Ma, G. Huang, J. Li and H. Xu, Minerals, 2022, 12, 9–1089, DOI:10.3390/min12091089.
- K. Ohenoja, J. Pesonen, J. Yliniemi and M. Illikainen, Sustainability, 2020, 12, 2988, DOI:10.3390/su12072988.
- A. Valizadeh, N. Skoglund, F. Forsberg, H. Lycksam and M. Oham, Fuel, 2024, 358(A), 129702, DOI:10.1016/j.fuel.2021.122959.
- T. H. Huynh, V. H. Ha and M. T. Vu, Geosyst. Eng., 2022, 25(3–4), 150–164, DOI:10.1080/12269328.2022.2156399.
- A. Patel, P. Basu and B. Acharya, J. Environ. Chem. Eng., 2017, 5, 667–678, DOI:10.1016/j.jece.2016.12.047.
- I. C. Okeme, P. G. Martin, C. Jones, R. A. Crane, T. I. Ojonimi, K. Ignatyev, D. Megson-Smith and T. B. Scott, Spectrochim. Acta, Part B, 2021, 177, 105950, DOI:10.1016/j.sab.2020.105950.
- L. Wang, X. Han, T. Liang, O. Guo, J. Li, L. Dai and S. Ding, Chemosphere, 2019, 217, 851–857, DOI:10.1016/j.chemosphere.2018.11.060.
- L. Wang and T. Liang, Sci. Rep., 2015, 5(12483), 1–11, DOI:10.1038/srep12483.
-
M. B. Yeheyis, J. Q. Shang and E. K. Yanful, World of Coal Ash Conference, May 4-7 in Lexington, KY USA, http://www.flyashinfo/ Search PubMed.
- M. J. McCarthy, H. I. Yakub and L. J. Csetenyi, Constr. Build. Mater., 2022, 342, 127313, DOI:10.1016/j.conbuildmat.2022.127313.
- S. S. Alterary and N. H. Marei, J. King Saud Univ. Sci., 2021, 33(6), 101536, DOI:10.1016/j.jksus.2021.101536.
- O. T. Sandstrom, P. Lindgren, A. Lewerentz, A. Apler, C. Liljenstolp and T. Bejgarn, Earth Sci. Syst. Soc., 2024, 4, 10095, DOI:10.3389/esss.2024.10095.
- K. Zhang, X. Zhu and B. Yan, Chem. Geol., 2015, 412, 82–91, DOI:10.1016/j.chemgeo.2015.07.027.
- G. B. Abaka-wood, J. Addai-Mensah and W. Skinner, J. South. Afr. Inst. Min. Metall., 2022, 122(1), 21–28 CAS.
- M. Dardona, S. K. Mohanty, M. J. Allen and T. M. Dittrich, Sep. Purif. Technol., 2023, 314, 123532, DOI:10.1016/j.seppur.2023.123532.
- R. K. Targgart, J. C. Hower, G. S Dywer and H. Hsu-Kim, Environ. Sci. Technol., 2016, 50(11), 5919–5926 CrossRef PubMed.
- F. Firman and A. Haya, IOP Conf. Ser. Mater. Sci. Eng., 2021, 1125, 012003 CrossRef.
-
U. S. Geological Survey, Mineral Commodity Summaries, 2024, p. 212, DOI:10.3133/mcs2024.
- G. Gaustad, E. Williams and A. Leader, Resour. Conserv. Recycl., 2021, 167, 105213, DOI:10.1016/j.resconrec.2020.105213.
- R. K. Jyothi, T. Thenepalli, J. W. Ahn, P. K. Parhi, K. W. Chung and J. Lee, J. Clean. Prod., 2020, 247, 122048, DOI:10.1016/j.jclepro.2020.122048.
- Y. Fujita, S. K. McCall and D. Ginosar, MRS Bull., 2022, 47, 283–288, DOI:10.1557/s43577-022-00301-w.
- I. M. S. K. Ilankoon, N. P. Dushyantha, N. Mancheri, P. M. Edirisinghe, S. J. Neethling, N. P. Ratnayake, L. P. S. Rohitha, D. M. D. O. K. Dissanayake, H. M. R. Premasiri, A. M. K. B. Abeysinghe, P. G. R. Dharmaratne and N. M. Batapola, J. Clean. Prod., 2022, 332, 129932, DOI:10.1016/j.jclepro.2021.129932.
- Z. Adamczyk, J. Komorek, B. Białecka, J. Nowak and A. Klupa, Ore Geol. Rev., 2020, 124, 103638, DOI:10.1016/j.oregeorev.2020.103638.
- J. Pan, T. Nie, B. V. Hassas, M. Rezaee, Z. Wen and C. Zhou, Chemosphere, 2020, 248, 126112, DOI:10.1016/j.chemosphere.2020.126112.
- M. Y. Stuckman, C. L. Lopano and E. J. Granite, Int. J. Coal Geol., 2018, 195, 125–138, DOI:10.1016/j.coal.2018.06.001.
- R. K. Taggart, J. C. Hower and H. Hsu-Kim, Int. J. Coal Geol., 2018, 196, 106–114, DOI:10.1016/j.coal.2018.06.021.
- J. C. Hower, J. G. Groppo, H. Hsu-Kim and R. K. Taggart, Fuel, 2021, 289, 119990, DOI:10.1016/j.fuel.2020.119990.
- P. Sandeep, S. Maity, S. Mishra, D. K. Chaudhary, C. B. Dusane, A. S. Pillai and A. V. Kumar, J. Hazard. Mater. Adv., 2023, 10, 100257, DOI:10.1016/j.hazadv.2023.100257.
- H. Manurung, W. Rosita, I. M. Bendiyasa, A. Prasetya, F. Anggara, W. Astuti, D. R. Djuanda and H. T. B. M. Petrus, Med. J. Indones., 2020, 42, 35–42 Search PubMed . https://jurnalmetal.or.id/jmi/article/view/187.
- V. V. Seredin and S. Dai, Int. J. Coal Geol., 2012, 94, 67–93, DOI:10.1016/j.coal.2011.11.001.
- S. Dai, X. Yan, C. R. Ward, J. C. Hower, L. Zhao, X. Wang and R. B. Finkelman, Int. Geol. Rev., 2018, 60(5–6), 590–620, DOI:10.1080/00206814.2016.1197802.
- S. Mondal, A. Ghar, A. K. Satpati, P. Sinharoy, D. K. Singh, J. N. Sharma, T. Sreenivas and V. Kain, Hydrometallurgy, 2019, 185, 93–101, DOI:10.1016/j.hydromet.2019.02.005.
- W. Rosita, I. M. Bendiyasa, I. Perdana and F. Anggara, AIP Conf. Proc., 2020, 2223, 050004 Search PubMed , 1–7, https://tf.ugm.ac.id/publikasi/.
- W. Astuti, E. T. Wahyuni, A. Prasetya and I. M. Bendiyasa, Eur. J. Sustain. Dev., 2014, 3(3), 227, DOI:10.14207/ejsd.2014.v3n3p227.
- A. Ghosh, S. Dhiman, A. Gupta and R. Jain, Environments, 2023, 10(1), 8, DOI:10.3390/environments10010008.
- S. Longo, M. Cellura, L. Q. Luu, T. Q. Nguyen, R. Rincione and F. Guarino, Renewable Energy, 2024, 220, 11958, DOI:10.1016/j.renene.2023.119598.
-
U.S. National Technology Laboratory, Rare Earth Elements, 2016, https://www.netl.doe.gov/resource-sustainability/critical-minerals-and-materials, Accessed 7 November, 2024 Search PubMed.
- Z. Huang, M. Fan and H. Tian, J. Rare Earths, 2020, 38(2), 219–226, DOI:10.1016/j.jre.2019.05.004.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *b,
Ferdinard
Asuke
c,
Ramalan
Aliyu Mohammed
c,
Ilemona
Okeme
*b,
Ferdinard
Asuke
c,
Ramalan
Aliyu Mohammed
c,
Ilemona
Okeme
 d and
John
Groppo
e
d and
John
Groppo
e


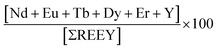
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton (ninety thousand Nigerian Nairas).56 On this basis, the average cost per ton of disposal (haulage) of the solid waste per year may be approximately estimated as N45
000 per ton (ninety thousand Nigerian Nairas).56 On this basis, the average cost per ton of disposal (haulage) of the solid waste per year may be approximately estimated as N45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022
022![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (USD 30
500 (USD 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255.12). This indicates the unit cost of disposal per ton of coal fly ash generated per annum. Thus, any resource recovery that may be proposed would have potential economic and environmental implications for industries utilizing coal for power generation. In the overall view, this study shows that coal fly ash production has potential as a domestic supply of REEs, depending on the feasibility of development of extraction technologies.
255.12). This indicates the unit cost of disposal per ton of coal fly ash generated per annum. Thus, any resource recovery that may be proposed would have potential economic and environmental implications for industries utilizing coal for power generation. In the overall view, this study shows that coal fly ash production has potential as a domestic supply of REEs, depending on the feasibility of development of extraction technologies.
