Open Access Article
Open Access ArticlePhysicochemical analysis and toxicity of the Rainha River waters: conceptual design of a treatment plant
Gabriel G.
de Barros
a,
Anna
De Falco
 a,
Carlos Leonny R.
Fragoso
a,
Luis Fhernando Mendonça da
Silva
a,
Carlos Leonny R.
Fragoso
a,
Luis Fhernando Mendonça da
Silva
 a,
Adriana
Gioda
a,
Adriana
Gioda
 *a and
Roberto Bentes
de Carvalho
*a and
Roberto Bentes
de Carvalho
 b
b
aDepartment of Chemistry, Pontifical Catholic University of Rio de Janeiro, 22451-900 Rio de Janeiro – RJ, Brazil. E-mail: agioda@puc-rio.br
bDepartment of Chemistry Engineering and Materials, Pontifical Catholic University of Rio de Janeiro, 22451-900 Rio de Janeiro – RJ, Brazil
First published on 1st November 2024
Abstract
The current study, conducted over a year, involved a comprehensive analysis of water samples from the Rainha River. This river crosses the Pontifical Catholic University of Rio de Janeiro Campus to assess water quality and potential applications. The samples underwent rigorous physical–chemical tests, including metal concentrations, pH, turbidity and toxicity assessments. The water collected and analysed by the standards proposed by CONAMA was found to be below the limit of regulation, classified at class 1, and requiring only a simplified treatment to remove microorganisms and achieve potability. Toxicity tests using Saccharomyces cerevisiae were performed to examine biological effects, revealing no significant toxicity. The next step was to design a water treatment plant, following the viability, water studies and identification. The process involved designing a block diagram and, later, the process flow diagram (PFD). The processes consist of getting water, passing through microfiltration, decontaminating it with hypochlorite, and using adsorption methods to turn it into a potable and useable on campus, thereby ensuring a safe and sustainable water supply for the university community.
Environmental significanceThis study is environmentally significant as it not only assesses the current water quality of the Rainha River but also proposes practical solutions to ensure safe and sustainable water usage. By designing an effective treatment plant, the research contributes to the preservation of local water resources and promotes environmental sustainability on campus. |
Introduction
The scarcity of water is a global concern, with over half of the population suffering from water scarcity being expected until 2050. With salt water covering the majority of the Earth, it is unsuitable for consumption as fresh water.1 The Rainha River, a vital water resource for the Pontifical Catholic University of Rio de Janeiro Campus, is facing significant and immediate environmental threats. In Brazil, only 6% of rivers have good-quality water.2 The river's water quality has been compromised by pollution from urban runoff and other anthropogenic sources. This pressing situation urgently requires a comprehensive analysis and potential treatment to ensure the water is safe for use on campus. This study aims to understand the physicochemical characteristics and potential toxicity of the river's water, which is crucial for designing an effective treatment process that meets the required standards.3,4This study aims to conduct a detailed physicochemical analysis and toxicity assessment of the Rainha River water over a year to determine its suitability for consumption after treatment. Critical parameters such as metal concentrations, turbidity, conductivity, pH, and organic matter levels are evaluated to identify the main pollutants and their seasonal variations. Additionally, toxicity tests using Saccharomyces cerevisiae provide insight into the potential biological effects of the water, ensuring a comprehensive understanding of the water's quality and safety.5
Based on these findings, a conceptual design for a treatment plant is proposed, integrating advanced techniques like microfiltration and chemical disinfection and adsorption methods to improve the water quality.6 This study explores the effectiveness of these methods in removing contaminants and enhancing water quality while also comparing alternative adsorption methods, such as activated carbon, to improve the quality of treated water and encounter specific challenges like organic pollutants and taste and odour compounds. Even though some parameters are under legislation, those processes are necessary. The project's primary objective is to develop a sustainable and robust water treatment solution that meets regulatory standards and enhances the resilience of water management on campus.
Experimental part
Rio Rainha hydrographic basin
Hydrographic basins are geographic regions where water flows and moves in a specific direction. Water comes from the rain for rivers and their tributaries.7 The Rainha River has its source located in Ponta das Andorinhas, in the Tijuca Forest massif, RJ (22°58′48′′S 43°15′23′′ W), and is located in the hydrographic area belonging to the so-called Oceanic region, which covers neighborhoods such as Jacarepaguá, Gávea, Ipanema, Copacabana, Urca and Lagoa.8 The river that runs through the Gávea region flows into the Avenida Visconde de Albuquerque canal, close to Leblon Beach. Its location can be identified in the coordinates (22°58′43.1′′S 43°13′35.4′′W), as shown in Fig. 1. The samples were collected at three points: the source is at (22°58′43.8′′S 43°14′34.1′′ W), the inlet is at (22°58′48.0′′S 43°14′01.0′′ W) and the outlet is at (22°58′45.2′′S 43°13′51.8′′W).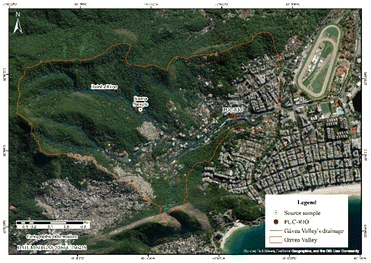 | ||
| Fig. 1 Flow from the Lagoa Rodrigo de Freitas Basin source in yellow and university campus in red.9 | ||
The samples were collected in autoclaved amber bottles once a month from May 2022 to April 2023.
ICP-MS characterisation
Elemental compositions were determined using inductively coupled plasma mass spectrometry (ICP-MS, NexION 300X, PerkinElmer, Massachusetts, USA). Four main interest elements for CONAMA (V, Cr, Cd, Pb) were detected. Calibration solutions (PerkinElmer 29, PerkinElmer 17 and PerkinElmer 12, 1000 μg L−1) were prepared in ultrapure water and acidified with bi-distilled nitric acid (HNO3, VETEC, Brazil). The analytical curve ranged from 1 to 1000 μg L−1, and Rh (40 μg L−1) was used as the internal standard in an acidified aqueous solution injected online.10,11 Operational conditions were optimised based on daily performance. All concentrations were checked against quality controls, and the coefficient of variation for this comparison was less than 10% on analysis of variance (ANOVA).Ionic chromatography characterisation
To eliminate insoluble material, samples and blank aliquots were filtered through 0.22 μm polyether sulfone membranes (Filtrilo, Paraná, Brazil). Water-soluble ions (Na+, Mg2+, Ca2+, Cl−, NO3−, SO42−) were determined using an ion chromatograph (ICS 5000, Thermo™ Scientific™ Dionex, Massachusetts, USA) equipped with a cation isocratic component, an anion gradient component and an AS-AP autosampler. Cations were analysed using a Dionex IonPac CS 12A column (Thermo™ Scientific™ Dionex, Massachusetts, USA) and a micro-membrane suppressor eluted with CH3SO2OH (18.0 mmol L−1). In contrast, a Dionex IonPac AS19 column (Thermo™ Scientific™ Dionex, Massachusetts, USA) eluted with KOH (3.0 mmol L−1) was used to analyse anions. The AS-AP temperature was set to 10 °C to minimise the loss of volatile species.12 Analytical curves were prepared for both anions and cations using standard solutions (Sigma-Aldrich, Missouri, USA) at a concentration of 1000 mg L−1. The analytical curves ranged from 0.20 to 40 mg L−1 for anions and 0.75 to 40 mg L−1 for cations. A calibration check with external standards was performed to ensure an accuracy of ±10% on analysis of variance (ANOVA).Cellular assays
Toxicity assay
The water samples were collected in autoclaved amber bottles filtered through a cut-off of 0.22 μm because the intention was to become free from other cells and other biological hazards and only pass chemical contamination. The assay was performed in a 96-well plate, allowing quintuplicates of the same conditions and triplicates of blanks from each condition to be performed. The toxicity study was conducted at 0.5 to 50% v/v concentrations (river samples and medium in the well). To achieve the desired concentration range for the toxicity assay, the river water samples were concentrated and then diluted with sterile water. Initially, samples were tested at concentrations between 0.5% and 50% v/v to evaluate potential toxicity. The culture medium was prepared in a concentrated form (2×), and dilutions were made using varying volumes of the samples and sterile water to maintain consistent conditions. However, the results did not indicate significant changes in yeast growth, as determined by Grubbs' statistical test. Subsequently, higher sample concentrations (25 to 90% v/v) were tested, with the culture medium prepared at a 10× concentration to ensure proper dilution. Control groups were included in all experiments: a positive control (C+) with no added river water and a negative control (C) in which yeast cells were exposed to 10% hydrogen peroxide to assess their sensitivity to toxic agents.15Physicochemical analysis
For the present study, pH, conductivity, colour, turbidity, and hardness (bicarbonate) were evaluated. The Hanna EdgepH and the Hanna EdgeEC were used for pH and conductivity measurement, respectively. Colour and turbidity parameters were analysed almost simultaneously with the SpectroquantNOVA60, using a pre-defined equipment method: code 32 for colour, code 77 for turbidity and titration for bicarbonate.Statistic treatment
After collecting absorbance values from the 96-well test with Saccharomyces cerevisiae, variance (ANOVA) was analysed to compare the means of three or more independent groups, determining if significant differences existed among them. ANOVA helped identify significant changes in cellular growth patterns over different months. The outliers were determined by Grubbs' test.16 Grubbs' test was employed to see if the parameters of the water changed significantly over time. The analyses assume a normal distribution of the data and calculate test statistics to determine if any value significantly deviates from the rest. Based on the sample size and a significance level of 0.05, the critical value was derived from the Student's t-distribution table.17Engineering design of a treatment plant
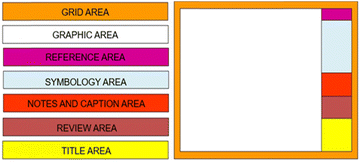
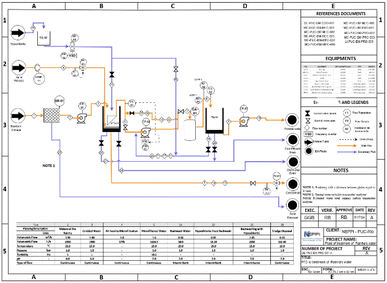
The block diagram is the first engineering document and consists of five big areas: the graphical area, where the process steps are represented in sequence, standard conventions; the inlet fluids are on the left and the outlet fluids on the right; the document area must include the documents that served as the basis for creating the block diagram; the symbology area, where the represents the different colours of input and output fluids, technologies and currents used are described; the note area, which contains additional explanations about specific parts of the process; and the header area, which provides the information about the client, executor, reviewer, approval, title and project number, according to the terminology of each company18,19 (Fig. 2).
Results and discussion
Physicochemical results
The graphs in Fig. 3 report the physical–chemical parameters obtained for this study. For instance, Fig. 3A illustrates the river's pH in the most natural state we can sample, revealing a nutrient increase along the river's path. This is particularly noticeable at the university's entrance and the campus's exit. Due to rainwater's acidic nature, the pH levels are lower during months with intense rainfall.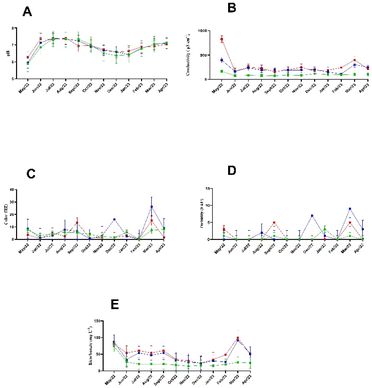 | ||
| Fig. 3 (A) pH; (B) conductivity; (C) colour; (D) turbidity; (E) bicarbonate for the Rainha's water from May 2022 to April 2023. The source is green, the inlet is blue, and the outlet is red. | ||
As per CONAMA, the pH range is 6.0–9.0, which aligns with legislation.21,22
Fig. 3B shows the conductivity levels of ion growth along the river's course. It remains relatively stable but peaks during specific months, which could correspond to increased ionic strength in the water. The elevated conductivity in water may influence yeast growth by altering osmotic conditions. Comparing these peaks to absorbance data allows significant associations to be identified; the higher conductivity correlates with lower growth rates due to osmotic stress.
Fig. 3C reports the watercolour data and highlights increased dissolved organic matter or metallic content, particularly during December and March. This aligns with periods of increased absorbance, indicating that colour, as an indirect measure of water quality, might impact the bioavailability of essential nutrients or toxins, which could be validated through additional ANOVA.
In Fig. 3D, turbidity is a parameter related to colour; due to the light scattering, we can see similar profiles between both. They show the correlations between rainfall and water flow disruptions. The data suggest that higher turbidity during the rainy season (November to March) is associated with elevated yeast growth, as the increased presence of particulate matter may offer more binding sites or alter light penetration, enhancing absorbance readings.
Fig. 3E shows that the waters from the river's source generally have a bicarbonate concentration lower than 50 mg L−1, in contrast to the exit and entry; greater variations are likely throughout the year due to the gradual accumulation of ions along the river. Comparing these trends with absorbance data through ANOVA could further elucidate bicarbonate's role in modulating yeast growth dynamics. March was the only month that showed a statistical difference.
Chemical composition
The results presented in Table 1 demonstrate that both elemental and ion concentrations in the river waters are significantly below the stringent limits set by CONAMA for class 1 waters.21,22 This indicates that the river waters analysed meet stringent regulatory standards for class 1 waters, underscoring their high quality and minimal pollution levels. In comparison to similar studies,23 the levels of contaminants such as chromium (Cr), cadmium (Cd), and lead (Pb) are significantly lower.| Annual average (mg L−1) | Standard deviation (mg L−1) | Limit of class 1 – CONAMA (mg L−1) | |
|---|---|---|---|
| V | 0.0009 | 0.0003 | 0.100 |
| Cr | 0.003 | 0.001 | 0.050 |
| Cd | 0.0004 | 0.0004 | 0.0010 |
| Pb | 0.01 | 0.02 | 0.0100 |
| Na+ | 14.2 | 14.4 | 200 |
| Mg2+ | 1.9 | 0.6 | N/A |
| Ca2+ | 2.9 | 1.5 | N/A |
| NO3− | 5.6 | 6.2 | 10 |
| Cl− | 23.4 | 5.5 | 250 |
| SO42− | 7.7 | 10.3 | 250 |
The low level of contaminants suggests it is less impacted by industrial effluents, which aligns with the more residential nature of its surroundings. That means achieving success in water treatment and conservation efforts and sustainable water management practices and providing reassurance about the water quality through filtration and adsorption.24 Magnesium and calcium contribute to water hardness.25,26 However, they have no specific limit in CONAMA. For this reason, the World Health Organization (WHO) recommends values of under 500 mg L−1 for Rainha's water.27 How the treatment plant was supposed to be built at the entrance of the campus (inlet) and distributed for the buildings was interesting to analyse how the water arrives on campus and to have a comparison with the origin (source) to see how the population around the river contributes to the quality water until it arrives on campus. The results of the inlet vs. the outlet on campus presented no significant statistical differences.28
Toxicity
The absorbance values and cellular growth in Fig. 4 suggest that acidic conditions closer to neutral pH may contribute to increased nutrient availability or altered cellular metabolism in S. cerevisiae. ANOVA results should reflect these seasonal pH variations as significant factors influencing growth.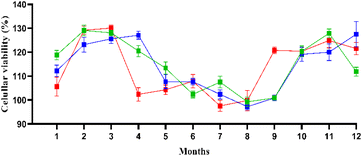 | ||
| Fig. 4 Cellular viability in 90% of toxicants (river water). The source is green, the inlet is blue, and the outlet is red. | ||
Fig. 4 also reveals seasonal trends. There was a general increase in cell numbers across all samplings. During periods of lower pH, yeast growth showed minor increases. This is consistent with the idea that mildly acidic conditions (closer to neutral) may provide nutrient solubility, increasing their availability to yeast cells. The correlation between pH and yeast cell viability suggests moderate pH levels can enhance yeast metabolism. The river's conductivity, indicative of ion concentration, is relatively stable, with occasional peaks during specific months, possibly due to increased ionic strength caused by runoff during rainfall. Elevated conductivity can create osmotic stress, which may impact the growth of S. cerevisiae. The yeast assay revealed that growth rates were lower during months of higher conductivity, suggesting osmotic pressure may inhibit yeast cell division.29 The correlation between conductivity and yeast growth highlights the importance of monitoring ionic content, as higher conductivity can stress cells, affecting their metabolic activities. During the year's colder months (May–Aug), this may indicate a reduced presence of organic matter during these periods. Additionally, during the rainier months (Sep–Mar), there was a marked increase in cell growth, colour and turbidity, which increased notably during the rainy season and are directly related to the presence of dissolved organic matter and particulates. The bioassay results showed that yeast growth was higher during these months, possibly due to the increased availability of organic material that serves as nutrients for the yeast. Turbidity and colour are often linked to the presence of organic pollutants or nutrients, which could provide binding sites or stimulate metabolic activity in yeast cells but do not represent a significant statistical difference between the source and inlet, except in April and September when the water was dammed because of the natural barrier with leaves, causing this statistical difference. This could be related to the higher levels of organic materials or nutrients originating from essential metals detected by elemental analyses during these wetter periods, as shown in Fig. 4. Importantly, the bicarbonate levels measured throughout the year generally remained low at the river's source.30 The entry and exit points near the campus exhibited a striking accumulation of ions. While bicarbonate levels were not directly linked to toxicity in the yeast assay, they likely buffered the pH, ensuring that the river remained within a range conducive to yeast growth. In all months, the water did not exhibit any toxicity, which means the chemicals and metals could not kill the yeast, providing further confidence in the safety of the water.
Plant treatment
The engineering design for the Rainha River treatment plant reflects a commitment to providing safe and potable water for campus use by integrating advanced water treatment technologies. The profile of water detected in the analysis of physical chemicals gives us a view of the water classified in class 1. According to the legislation, if you wish to transform this water into potable water, a simplified treatment is required; those possible solutions could be applied to filtration. The proposed treatment route utilises a combination of microfiltration, chemical disinfection with hypochlorite, and activated carbon adsorption to address the water quality challenges identified in the Rainha River. The first stage consists of microfiltration, critical for removing suspended particles, microorganisms, and other small contaminants. The membranes with a pore size of 0.22 μm ensure that biological contaminants such as bacteria and yeast are eliminated, improving the safety of the water for human consumption. After that, the chemical disinfection step ensures that any remaining, including viruses and residual bacteria, are effectively killed. Hypochlorite disinfection is a standard, cost-effective method. The main problem of the Rainha River water is the presence of organic pollutants, which contribute to undesirable taste, odour, and colour. Activated carbon adsorption is used to remove these organic contaminants, as it has a large surface area and a high adsorption capacity, making it particularly effective for removing organic compounds, taste, and odour compounds. It is more efficient than other methods, such as zeolites. Also, the efficiency of some residual industries like methyl orange (MO) removal by activated carbon can reach over 90% in optimal conditions, depending on factors like pH and adsorbent dosage. Its adsorption capacity is typically higher compared to many adsorbents due to its microporous structure.The adsorption stage ensures that the treated water is aesthetically and chemically suitable for consumption.31–33
The proposed route was chosen for its simplicity, cost-efficiency, compatibility with other treatment processes, and easy implementation. This integrated method ensures that the treated water meets all regulatory standards of CONAMA for potability and remains safe and appealing for consumption on campus. Integrating those advanced techniques is crucial for improving the overall quality of the treated water. This method is highly effective in environments like the Rainha River, where urban activities contribute organic and chemical pollutants. The proposed plant design was developed following standard engineering practices, beginning with creating a block diagram and eventually a process flow diagram (PFD). The block diagram outlines the sequence of treatment steps and ensures that all processes flow in the correct order. Each stage of the plant's design adheres to environmental and legislative requirements, ensuring compliance with Brazilian water quality regulations. The treatment process is designed to be adaptable and scalable, which means it can handle potential demand increases or water quality changes due to seasonal variations. This ensures that the treated water not only complies with legal standards but also improves its aesthetic qualities, making it suitable for daily consumption by the university community. The treatment plant is indicated in Fig. 5.34–36
Conclusions
The proposed treatment plant was specifically designed to improve the water quality of the Rainha River for use on campus. The process involves microfiltration and disinfection with hypochlorite, removing microorganisms and reducing turbidity. However, it was found these methods alone were not sufficient, so additional adsorption techniques were introduced to enhance the treatment process. Activated carbon adsorption was implemented because of the high surface area and robust adsorption capacity; it is particularly effective in eliminating organic contaminants, taste, and odour compounds that microfiltration alone may not adequately address. While zeolite is highly effective for removing specific contaminants like heavy metals and ammonium, it lacks the versatility of activated carbon in handling a broad spectrum of pollutants. Activated carbon can adsorb various contaminants, including the main contaminants such as organic matter, chlorine, and micro-pollutants, making it an ideal choice for addressing the diverse water quality challenges the Rainha River poses.Carbon adsorption is generally superior due to its versatility, compatibility with other water treatment processes in targeting various contaminants, and ease of regeneration.
Activated carbon can be regenerated multiple times through thermal or chemical processes, extending its lifespan and reducing operational costs. Zeolite, although regenerable, requires more complex chemical processes, which may involve acids or other harsh chemicals.
By incorporating an activated carbon stage, we can significantly improve the removal of dissolved organic matter, ultimately delivering higher-quality treated water. Combining basic treatment with adsorption methods can ensure Rainha's water is free from odour and organic matter and has turbidity lower than the 100 FAU stipulated by CONAMA legislation. This integrated approach not only guarantees better water quality for campus consumption but also improves the sustainability and resilience of the treatment system.
Data availability
The data used in this article are available in the following Google Drive folder: https://drive.google.com/drive/u/1/folders/1zm29xFpbLCXjBFDOAUJgRf1yNySux0_W.Author contributions
Gabriel G. De Barros: writing, sample collection, analysis, data processing. Anna De Falco: review and methodology development. Carlos Leonny R. Fragoso: ICP analysis. Luis Fhernando M. da Silva: review and ion chromatography analysis. Adriana Gioda: review. Roberto Bentes de Carvalho: review, engineering development plant.Conflicts of interest
There are no conflicts to declare.Acknowledgements
CAPES, FAPERJ, CNPq and the members of the LQA group.Notes and references
- G. Matta, P. Kumar, D. P. Uniyal and D. U. Joshi, ACS ES&T Water, 2022, 2, 667–689 Search PubMed.
- M. E. Cardim and G. Tunes, Apenas 6,5% dos rios brasileiros têm boa qualidade da água, aponta estudo, https://www.correiobraziliense.com.br/app/noticia/brasil/2019/03/23/interna-brasil,744836/apenas-6-5-dos-rios-brasileiros-tem-boa-qualidade-da-agua.shtml, accessed 11 May of 2022 Search PubMed.
- J. C. Azzolini, Contribuicao da poluicao fisica, quimica e bioquimica nas águas do rio do Peixe pelo afluente Rio Tigre, UFSC, 2002 Search PubMed.
- G. Matta, A. Kumar, A. Nayak and P. Kumar, Appl. Water Sci., 2022, 12, 33 CrossRef CAS.
- M. Safaa, M. E. Irzoqy and M. F. Haddad, J. Renewable Energy, 2021, 1, 7–13 Search PubMed.
- P. Schumann, M. Muschket, D. Dittmann, L. Rabe, T. Reemtsma, M. Jekel and A. S. Ruhl, Water Res., 2023, 235, 119861 CrossRef CAS PubMed.
- OECO, O que é uma Bacia Hidrográfica, https://www.oeco.org.br/dicionario-ambiental/29097-o-que-e-uma-bacia-hidrografica/, acessado 30 de julho de 2022 Search PubMed.
- Rio-Águas, Um manual dos rios, canais e corpos hídricos da cidade do Rio de Janeiro, Rio de Janeiro, 2020 Search PubMed.
- C. Castro, T. Pampolha, P. Campelo, L. Daemon, J. E. Xavier, J. De Andrade, D. Albuquerque and H. Filho, O que é uma Bacia Hidrogafica from site O ECO, INEA, 2022 Search PubMed.
- A. De Falco, Activity evaluation and toxicological profile of new potential “Metal Protein Attenuating Compounds” in biological models of Alzheimer’s disease, PUC-Rio, 2017 Search PubMed.
- E. P. S. Justo, M. F. C. Quijano, K. Beringui, T. D. Saint'Pierre and A. Gioda, J. Braz. Chem. Soc., 2020, 31, 1043–1054 CAS.
- A. C. S. Costa, ISSN 2502-3632 ISSN 2356-0304 J. Online Int. Nas. Vol. 7 No.1, Januari – Juni 2019 Univ. 17 Agustus 1945 Jakarta, 2017, 53, 1689–1699.
- A. Goffeau, B. G. Barrell, H. Bussey, R. W. Davis, B. Dujon, H. Feldmann, F. Galibert, J. D. Hoheisel, C. Jacq, M. Johnston, E. J. Louis, H. W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin and S. G. Oliver, Science, 1996, 274, 546–567 CrossRef CAS PubMed.
- K. F. de Araújo and F. F. dos, A utilização de probiótico causando infecção invasiva em humanos pelo Saccharomyces cerevisiae, UniCEUB, 2017 Search PubMed.
- A. De Falco, E. Santa-Helena, C. A. T. Toloza, J. M. S. Almeida, D. G. Larrude, F. V. P. Meirelles, C. R. Gioda, R. Q. Aucelio and A. Gioda, Fullerenes, Nanotubes Carbon Nanostruct., 2022, 30, 657–666 CrossRef CAS.
- Z. Campbell, A. Bray, A. Ritz and A. Groce, in 2018 1st International Conference on Data Intelligence and Security (ICDIS), IEEE, 2018, pp. 281–285 Search PubMed.
- I. A. Shah, I. Khan, S. A. Mir, O. Ozer, P. Mishra, S. Maqbool, I. A. Bhat and O. Bhat, Int. J. Agric. Stat. Sci., 2023, 19, 177 Search PubMed.
- C. A. Dias, Técnicas avançadas de instrumentação e controle de processos químicos, Thechinichal books livraria, Rio de Janeiro, 2nd edn, 2012 Search PubMed.
- International Organization for Standardization, ISO 106281997(E), 1997, p. 15 Search PubMed.
- N. Loureiro, M. Milani, Projeto de um Sistema de Tratamento para a água do Rio Rainha com tecnologia de microfiltração com membranas Mariana Milani Sumário [s.l.], Pontifice Universidade Católica-PUC-Rio, 2011 Search PubMed.
- Brasil, Resolução CONAMA N° 357, De 17 De Março De 2005*, https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf, accessed 11 May of 2022 Search PubMed.
- BRASIL, Resolução CONAMA No 430, DE 13 DE MAIO DE 2011, https://www.suape.pe.gov.br/images/publicacoes/CONAMA_n.430.2011.pdf, accessed 27 June of 2024 Search PubMed.
- V. Gupta, D. Kumar, A. Dwivedi, U. Vishwakarma, D. S. Malik, S. Paroha, N. Mohan and N. Gupta, Environ. Geochem. Health, 2023, 45, 1807–1818 CrossRef CAS.
- S. B. Pillai, in Handbook of Water and Used Water Purification, Springer International Publishing, Cham, 2024, pp. 99–120 Search PubMed.
- W. Liang, X. Wang, X. Zhang, L. Niu, J. Wang, X. Wang and X. Zhao, Sci. Total Environ., 2023, 858, 159554 CrossRef CAS PubMed.
- A. Nayak, G. Matta and D. P. Uniyal, Environ. Dev. Sustain., 2023, 25, 14229–14260 CrossRef.
- WHO, World Heal. Organ., 2009, vol. 5, pp. 1–10 Search PubMed.
- WHO, Hardness in Drinking-water, https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/hardness2003.pdf?sfvrsn=64c0da98_3, accessed 27 June of 2024 Search PubMed.
- J. Georgin, D. S. P. Franco, M. S. Manzar, L. Meili and N. El Messaoudi, Environ. Sci. Pollut. Res., 2024, 31, 24679–24712 CrossRef CAS.
- J. Guo, Y. Xie, A. Guan, W. Qi, X. Cao, J. Peng, H. Liu, X. Wu, C. Li, D. Wang and J. Qu, Environ. Pollut., 2023, 316, 120659 CrossRef CAS PubMed.
- R. EL Kaim Billah, A. Zaghloul, H. A. Ahsaine, A. BaQais, I. Khadoudi, N. El Messaoudi, M. Agunaou, A. Soufiane and R. Jugade, Int. J. Environ. Anal. Chem., 2022, 1–17 Search PubMed.
- Z. M. Şenol, H. Ertap, Y. Fernine and N. El Messaoudi, Polym. Bull., 2024, 81, 12795–12817 CrossRef.
- N. Jiang, R. Shang, S. G. J. Heijman and L. C. Rietveld, Water Res., 2018, 144, 145–161 CrossRef CAS.
- E. Pérez-Botella, S. Valencia and F. Rey, Chem. Rev., 2022, 122, 17647–17695 CrossRef.
- W. Qin, Y. Dong, H. Jiang, W. H. Loh, J. Imbrogno, T. M. Swenson, O. Garcia-Rodriguez and O. Lefebvre, Water Res., 2024, 252, 121180 CrossRef CAS.
- Z. M. Şenol, N. El Messaoudi, Z. Ciğeroglu, Y. Miyah, H. Arslanoğlu, N. Bağlam, E. S. Kazan-Kaya, P. Kaur and J. Georgin, Food Chem., 2024, 450, 139398 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |