Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Benzothiophene based semi-bis-chalcone as a photo-luminescent chemosensor with real-time hydrazine sensing and DFT studies†
Nidhi H.
Oza
 ,
Dinkal
Kasundra
,
Dinkal
Kasundra
 ,
Amar G.
Deshmukh
,
Amar G.
Deshmukh
 ,
Niteen
Borane
,
Niteen
Borane
 ,
Rajamouli
Boddula
,
Rajamouli
Boddula
 and
Paresh N.
Patel
and
Paresh N.
Patel
 *
*
Laboratory of Bio-Organic Chemistry, Tarsadia Institute of Chemical Science (TICS), Uka Tarsadia University, Bardoli 394 350, Gujarat, India. E-mail: pareshn111@yahoo.com
First published on 31st October 2024
Abstract
Hydrazine is a very toxic chemical that poses a major threat to human health and the environment. As a further expansion of our ongoing research, this report validates the enhanced real-time hydrazine sensing using benzothiophene-based semi-bis-chalcone (SBC). Hypothesized SBC molecules that can be easily attacked by nucleophilic groups were synthesised via classical Claisen–Schmidt condensation. Two derivatives of novel SBC scaffolds were synthesised by the reaction of simple acetone with benzothiophene carbaldehydes. This reaction involved the use of KOH and pyrrolidine as catalysts, and they demonstrated two different processes in comparative studies. KOH worked as a speedy catalyst, while pyrrolidine was demonstrated to be a more efficient catalyst. The structures of the synthesised compounds were established by various spectral techniques. The optical properties of the prepared SBCs were studied in different solvent systems and demonstrated that methanol was the more suitable solvent. Density functional theory (DFT) calculations of both compounds in methanol were performed using the Gaussian software. Time-dependent density functional theory (TDDFT) calculations were performed to study the dynamic behaviour of electrons in both molecules and materials by considering their density as a function of time. Both DFT and TDDFT calculations were observed to have a good correlation with the experimental results. The obtained absorption and photoluminescence results and their theoretical correlation suggested that the prepared SBCs can be optimized for applications in optoelectronics, sensing, and bioimaging. As an improvement to our earlier protocol, more efficient real-time hydrazine sensing SBCs probes were established with prolonged π-conjugation. An exhaustive protocol with a working pH range, analyte selectivity, and real sample test was developed. The studied SBCs showed a broad working pH range and excellent hydrazine sensing selectivity. With these two included in our large library of photoresponsive molecules, we aim to construct a model device for hydrazine sensing in real life applications.
Environmental significanceAs per the journal scope, the present study highlights the synthesis of highly conjugated novel hetero-aryl substituted semi-bis-chalcone (SBC) scaffolds of the benzothiophene moiety. Structures of the prepared molecules were determined using various spectral techniques. As per our knowledge, this is the first report on a hetero-aryl substituted semi-bis-chalcone (SBC) of acetone. The photophysical properties of these conjugated scaffolds were investigated by dual spectroscopic techniques. They exhibited very effective photo-luminescent properties. DFT calculations of both compounds in methanol were performed using Gaussian software. TDDFT calculations were performed to study the dynamic behaviour of electrons in both molecules by considering their density as a function of time. The obtained results from all these studies suggested that the prepared SBCs can be optimized for their applications in optoelectronics, sensing, and bio-imaging. Therefore, these molecules were investigated for hydrazine sensing studies. Various solvent screening and titrations with different hydrazine concentrations were performed. The competitive selectivity of the prepared probe to different analytes and the pH dependence were also studied. A mechanistic study of the sensing reaction was also performed. These results prove that, compared to our earlier reports and other literature, these SBCs are highly selective and efficient sensors for hydrazine. |
1 Introduction
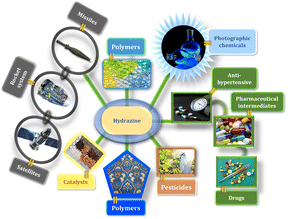
Hydrazine has considerable industrial uses due to its strong alkalinity, nucleophilicity and high reducing power.1 It has been used to produce pesticides, drugs, pharmaceutical intermediates, catalysts, textile dyes, plastic polymers, anti-hypertensive hydralazine and photographic compounds.2,3 Additionally, it has been used as a high-energy fuel propellant for rocket propulsion systems, missiles and satellites (Fig. 1).4–6However, one deadly consequence of hydrazine is environmental pollution.7,8 The effects of hydrazine on biological systems and numerous biochemical processes at the cellular level are also a major concern.9 Excessive exposure to this highly hazardous substance can cause skin irritation and damage to the liver, kidney, and central nervous system.10 As a result, its negative impacts create safety issues for its widespread usage in bulk production, transport and waste water disposal operations, which might result in major environmental contamination. The US Environmental Protection Agency (EPA) has since classified it as a potential carcinogen to living organisms and has advised a low threshold limit value (TLV) of 10 ppb hydrazine in drinking water.11,12
As a result, there has been a pressing need to develop efficient, sensitive and selective probes for monitoring hydrazine-contaminated environments. So far, hydrazine has been identified and quantified using a range of traditional techniques.13–15 In the last few years, numerous strategies have been established to sense hydrazine, including gas and high-performance liquid chromatography,16,17 atomic absorption spectroscopy,18 colorimetric19 and electrochemical analysis.20 These techniques all have significant inadequacies, such as awkward operation, complex sample preparation and the huge quantities of samples required. Due to its on-site detection and relevance to biological and environmental applications, spectroscopic determinations are more common and favoured among these approaches.21 However, to improve the operational convenience, cost-effectiveness, quick detection and high selectivity at low ppm level, efforts have been made in recent years to create novel fluorescent sensors.21–23
Fluorescent sensors are a modern analytic tool with better reliability, simplicity, operability and low-cost, among other advantages.24–26 Some of the most recent fluorescent sensors for hydrazine detection are based on three different reaction mechanisms: (i) hydrazine-assisted cleavage of protecting groups of levulinoyl ester or acetyl groups, 4-bromobutyrate; (ii) selective reaction with vinyl maleonitrile, barbituric acid; and (iii) hydrazine addition on an unsaturated system.27–30 Although a large number of hydrazine fluorescent sensors have been established,31–35 these methods usually limit their applicability due to their low selectivity, sensitivity, limited pH range, specific temperature, long detection period, as well as multistep synthesis. As a result, there is a need to develop simple fluorescent probes for the detection of hydrazine that will overcome all these limitations from the previously reported probes.36–40
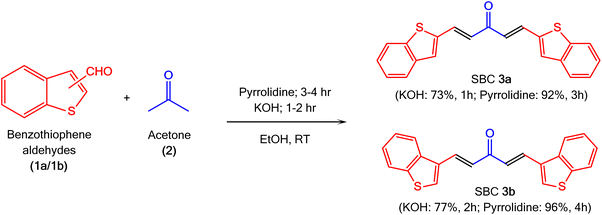
Herein, we report a one-step design strategy for the building of benzothiophene-treated SBCs as ratio-metric fluorescent sensors, which can detect hydrazine efficiently in real sample analysis. We have demonstrated two comparative methods catalysed by the cost-effective organic base pyrrolidine and inorganic base KOH. This hypothesized [2 + 3] Claisen–Schmidt condensation can be performed at room temperature (Scheme 1).
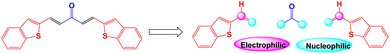
It starts via amphiphilic (i.e., 1C, 2C) acetone having a nucleophilic methyl carbon and an electrophilic carbonyl carbon. It follows the five-carbon-centre condensation with an electrophilic aldehyde (i.e., 1O, 3C) to form SBCs. The resultant product with a strong photoluminescent nature is used to develop an effective technique for the selective detection of hydrazine, and the results are presented here. These sensors are capable of providing the real time determination of hydrazine levels in the environment. The mechanistic investigation for the hydrazine detection study is also proven by spectral analysis and presented here.
2 Experiments
2.1 Synthesis of SBCs from benzothiophene aldehydes41
Benzothiophene aldehydes (1a/1b; 10 mmol) were dissolved in the least amount of ethanol (3 mL) as a solvent, and stirred to form a homogenous mixture in 10 mL RBF. Acetone (2; 5 mmol) was added dropwise to the stirred solution, and the resultant mixture was continually stirred at room temperature for 5 minutes. Later, a catalytic quantity of KOH or pyrrolidine solution in ethanol (4%, 2 mL) was added dropwise (Scheme 2). Finally, the resultant reaction mixture was stirred at room temperature for 1–2 h (for KOH)/3–4 h (for pyrrolidine). The completion of the reaction was monitored by thin layer chromatography (TLC) using ethyl acetate/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) as a solvent system. The product began to precipitate out in the reaction mixture as the reaction continued. After completion of the reaction, the precipitated product (3a/3b) was cooled and collected by filtration. The obtained residues were washed with cold ethanol, and purified by recrystallization through chloroform–methanol (2
3 v/v) as a solvent system. The product began to precipitate out in the reaction mixture as the reaction continued. After completion of the reaction, the precipitated product (3a/3b) was cooled and collected by filtration. The obtained residues were washed with cold ethanol, and purified by recrystallization through chloroform–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 8 mL).
1 v/v, 8 mL).
2.2 Photophysical properties of SBCs and their hydrazine detection study
The photophysical properties of both prepared SBCs were investigated by UV-visible absorption and fluorescence emission experiments using their 5 μM solution. To find the more appropriate solvent for the UV-visible absorption and fluorescence emission experiments, the solvents were screened with the same concentration in different HPLC grade solvents. Based on the Stokes shift values of both SBCs in different solvents, methanol was chosen for absorption-emission titrations of the hydrazine detection study with a fixed concentration of SBCs (5 μM) and varied concentration of hydrazine in methanol (0–8 μM). The operational solutions with different concentrations (2–8 μM) of hydrazine for detection studies were made by serial dilution in HPLC grade methanol.3 Results and discussion
3.1 Synthesis of novel SBCs derivatives
Two novel Semi-Bis-Chalcone molecules (3a/3b) were synthesized by Claisen–Schmidt condensation of acetone (2) and benzothiophene aldehydes (1a/1b) in EtOH at room temperature (Scheme 2). These derivatives were synthesized with two separate catalysts, i.e., pyrrolidine and KOH, to evaluate their influence on the product isolated yield and reaction time. These experimental results have clearly shown that pyrrolidine is an efficient substitute of KOH. The reactions were completed in a shorter reaction time for the KOH-catalysed experiments. However, there were significantly lower yields compared to the pyrrolidine-catalysed experiments. The lower isolated yield could be because of the significant solubility of the obtained chalcone in ethanolic KOH. These results show that pyrrolidine is an efficient catalyst for the reaction under milder conditions with a higher isolated yield in an extended reaction time. The prolonged reaction time could be because of the weak basic nature of pyrrolidine.3.2 Structure confirmation of the prepared SBC molecules by spectral studies
The structures of all of the newly prepared molecules were confirmed by different spectroscopic techniques, like NMR, IR and HRMS. All of the spectra and spectral data are given in the ESI.† In the 1H NMR spectrum of SBC 3a, two clear peaks as a doublet with a similar coupling constant of 15.5 Hz were observed at 7.965 and 6.905 δppm. These peaks confirmed the presence of two α and two β symmetric protons with trans geometry on the bis-α,β-unsaturated carbonyl system, respectively. One clearer singlet was observed at 7.574 δppm corresponding to two symmetric protons on the third position of the thiophene ring of the benzothiophene moieties. An additional two multiplets were observed at 7.794–7.841 and 7.386–7.417 δppm, corresponding to eight protons of the benzene ring of the benzothiophene moieties. The presence of the carbonyl group was further supported with the 13C NMR spectrum by the peak observed at 187.46 δppm, along with twelve symmetric carbons in the aromatic region of 122.58–140.22 δppm. The structure of SBC 3a was also confirmed by its HRMS spectrum. Here, the observed molecular weight (347.0716 gm per mole) perfectly matched with its calculated molecular weight (347.0712 gm per mole). The presence of a carbonyl group was additionally confirmed by FT-IR spectrum with a band at 1789 cm−1, along with a C–S stretching mode at 825 cm−1, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode at 1600 cm−1 and a C–H stretching at 3025 cm−1. Similarly, the structure of SBC 3b was also confirmed with 1H NMR, 13C NMR, HRMS and FT-IR spectral analyses.
C stretching mode at 1600 cm−1 and a C–H stretching at 3025 cm−1. Similarly, the structure of SBC 3b was also confirmed with 1H NMR, 13C NMR, HRMS and FT-IR spectral analyses.
3.3 Photophysical properties of the prepared SBCs
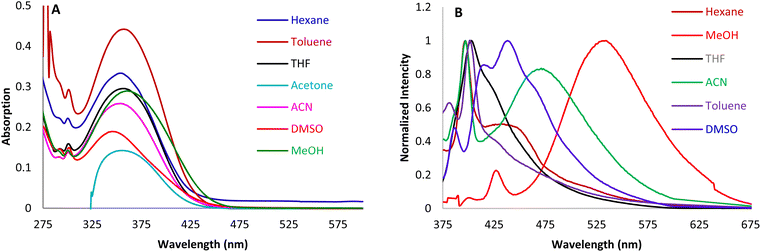
To study the hydrazine sensing ability of the synthesized novel Semi-Bis-Chalcone molecules, screening of both SBCs was performed. Based on the Stokes shift values of both SBCs in methanol (Table 1), SBC 3b with the highest Stokes shift was chosen for the absorption-emission titrations of the hydrazine detection study. The most suitable solvent for this investigation was selected by studying the photophysical properties of SBC 3a and 3b in different solvents with varied polarity from hexane to water (Fig. 2A and S7†). The main absorption peak at ∼360 nm was observed in all absorption spectra of SBC 3b in different solvents. Hexane and toluene, as less polar solvents, show the longer absorption peak (362 nm, ε = 3.51 × 104 cm−1 M−1). Conversely, methanol and DMSO as polar solvents show the shorter absorbance (340 nm, ε = 2.25 × 104 cm−1 M−1) of the SBC 3b. The photophysical characteristics of SBC 3a and 3b in different solvents with varied polarity were also studied by fluorescence spectra (Fig. 2B and S7†). As seen in the normalized fluorescence spectra, the emission maxima of SBC 3b gradually varied with the change of the solvent polarity from hexane to water. The emission peak positions show an obvious red shift from 427 nm in hexane to 537 nm (methanol), as anticipated for the increase in solvent polarity. Methanol shows better UV absorption and fluorescence emission properties of SBC 3b among all the studied solvents. Thus, all the hydrazine sensing studies were carried out in methanol (Fig. 3).| SBCs | Solvent | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Hexane | Toluene | ACN | THF | Acetone | Methanol | DMSO | ||
| 3a | 352 | 366 | 351 | 357 | 349 | 370 | 344 | λ abs (nm) |
| 430 | 405 | 473 | 407 | — | 532 | 433 | λ em (nm) | |
| 78 | 39 | 122 | 50 | — | 162 | 89 | Δλ (nm) | |
| 3.37 × 104 | 4.50 × 104 | 2.78 × 104 | 3.02 × 104 | 1.29 × 104 | 3.17 × 104 | 2.08 × 104 | ε (M−1 cm−1) | |
| 3b | 362 | 370 | 349 | 355 | 352 | 368 | 340 | λ abs (nm) |
| 427 | 398 | 465 | 408 | — | 537 | 436 | λ em (nm) | |
| 65 | 28 | 116 | 53 | — | 169 | 96 | Δλ (nm) | |
| 3.51 × 104 | 4.60 × 104 | 2.92 × 104 | 3.18 × 104 | 1.57 × 104 | 3.52 × 104 | 2.25 × 104 | ε (M−1 cm−1) | |
 | ||
| Fig. 2 (A) UV-vis absorption and (B) normalized fluorescence spectra of SBC 3b (5 μM) in various solvents. | ||
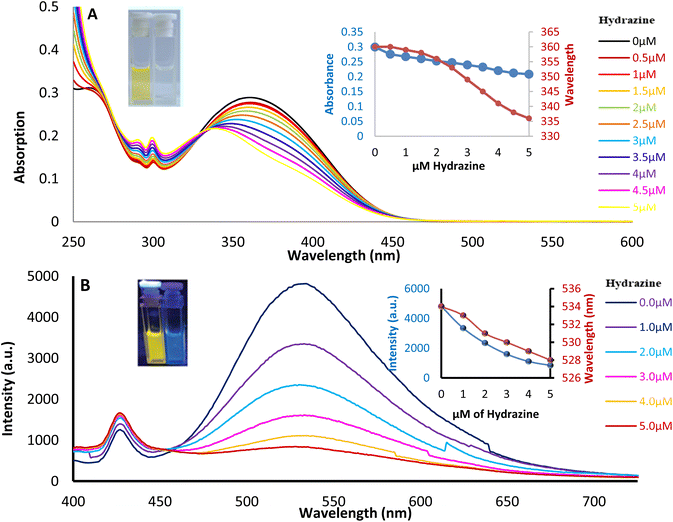 | ||
| Fig. 3 Hydrazine (0.0 to 5.0 μM) vs. SBC 3b (5.0 μM) titration by (A) UV-vis absorption and (B) fluorescence emission (λex = 358 nm) studies in methanol. | ||
3.4 Hydrazine sensing study by 3b
To determine the hydrazine sensing range of SBC 3a and 3b, the titration of hydrazine with varied concentration (0 to 5 μM) vs. SBC 3a/3b with fixed concentration (5 μM) was carried out via absorption and emission spectroscopy (Fig. 4a and S8†). The absorption maxima of SBC 3b at 368 nm gradually decreased with increasing hydrazine concentration and a major blue shift (368 to 335 nm) in the absorption maxima was also observed. The color also changed from yellow to colorless (graphical abstract). This shows that SBC 3b can be used as a sensor for hydrazine that can be observed by naked eye. The compound SBC 3b shows fluorescence emission at 537 nm, which is similar to the bright blue fluorescence. After the increase of the hydrazine concentration, a quenching in the fluorescence emission at 537 nm (λex = 368 nm) was observed. The detection limit of the studied molecule (5 μM) in terms of the minimum amount of hydrazine is 5 μM with 90% accuracy. Additionally, the fluorescence color before and after the titration changes from bright blue to almost zero emission. These titration experiments were accomplished within 2 minutes of each other, showing the sensitivity of the probe towards hydrazine. Overall, this investigation proves the efficacy of compound SBC 3b for the exposure of hydrazine even at very low concentrations.3.5 Interfering analytes of hydrazine sensing studies for SBC 3b
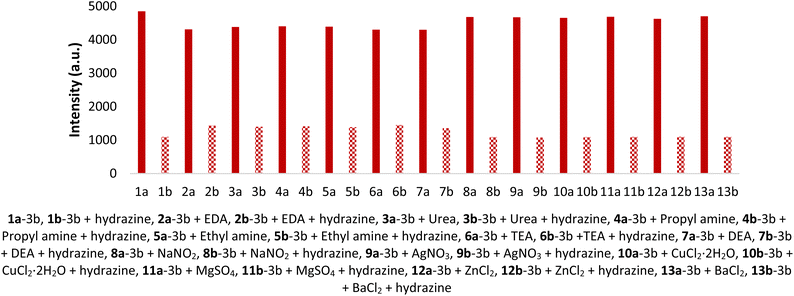
To check the discrimination and anti-interference of SBC 3b for its actual application, a competitive study for the preference of SBC 3b with other interfering chemicals was conducted. Initially, the emission studies at 537 nm were done with solutions of 5 μM SBC 3b and 5 μM of various interfering chemicals. The obtained results clearly showed the negligible effect of the interfering chemicals on the emission property of SBC 3b (Fig. 4, solid column). Later, 5 μM hydrazine was added to all of these solutions and the emission was studied at 537 nm. The observed results clearly showed very high quenching in the emission property of SBC 3b in the presence of interfering chemicals (Fig. 4, dotted column). This study demonstrated SBC 3b as an exceptional optical sensor for hydrazine with great selectivity and anti-interference in the presence of all of the studied interfering chemicals.3.6 Mechanistic exploration of the detection study by 3b
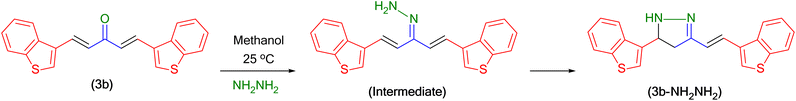
As per our earlier studies,28–30 to find the mechanistic track (Scheme 3) for the sensing of hydrazine, the resultant mixture of 3b and hydrazine in methanol after the emission study was injected into a GC-MS (Fig. S6†). The witnessed m/z value clearly ratified the formation of 1H-pyrazole (3b-NH2NH2). Similarly, it was also confirmed by NMR and FT-IR analysis, as discoursed in ESI.† The sensing mechanism possibly progressed through the unsteady intermediate imine formation by nucleophilic attachment on the carbonyl carbon, followed by dehydration.3.7 Effect of pH on the SBC 3b emission
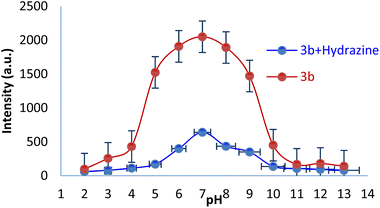
The pH value has a great influence on the chemosensing study. Therefore, a study involving the pH effect on the SBC 3b (Fig. 5) emission property was executed to find out the working pH range for 3b. The attained results confirmed that it has excellent sensing capability for hydrazine in the 5.0–10.0 pH range, which was observed in our earlier reports.28–303.8 The real time hydrazine sensing by SBC 3b in water and soil samples
The above studies show that 3b may have reasonable sensing capability for hydrazine in real water and soil samples. Therefore, the use of SBC 3b for sensing of hydrazine in various soils was also investigated.As a part of the 1st investigation, 1 g of clay soil/field soil/sandy soil was added to SBC 3b (5 μM) solution in methanol (5 mL). The fluorescence emission spectra of the supernatants were recorded (Fig. 6), which showed that there is no major influence from the different soil samples on the emission nature of 3b. This observation has shown that the chemicals in the different soils did not affect the emission nature of 3b. As a 2nd investigation, 1 g of clay soil/field soil/sandy soil was polluted with hydrazine (5 μM), and then it was added to the 3b (5 μM) solution in methanol (5 mL). Once again, the fluorescence emission spectra of the supernatants were recorded, and it showed a large decrease in the emission signal of SBC 3b.
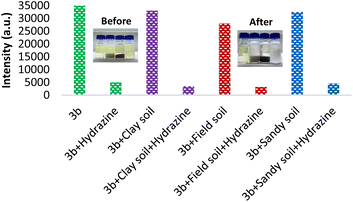 | ||
| Fig. 6 Emission intensity of SBC 3b (0.5 μM; 537 nm) after the addition of different soils in methanol with and without hydrazine. | ||
Observing all these experiments with the quick reaction time, high sensing capacity, anti-interference and excellent selectivity of SBC 3b, the sensing study of SBC 3b on hydrazine in real water samples was also performed. Potable water is known to have some level of minerals and dissolved organic substances, including Na+, Cu2+, Mg2+, Fe3+, and ClO−.
Therefore, SBC 3b was lastly examined for hydrazine sensing in various samples of water, i.e., tap water, river water and mineral water. Initially, the solution of SBC 3b (0.5 μM) in methanol was mixed with all three different water samples and the emission spectra were recorded (Fig. 7). The attained results have evidently shown that these water samples have not influenced the emission nature of 3b. A very low but substantial decrease in emission was present in the river water sample. This could be because of the presence of hydrazine or another similar analyte. However, after the addition of an equal amount of hydrazine (0.5 μM) in all of these water samples, there was a huge decrease in the emission intensity of SBC 3b. This shows the competence of the hydrazine sensing process in real time sample analysis.
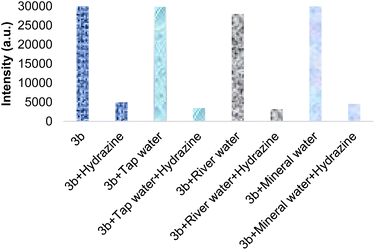 | ||
| Fig. 7 Emission intensity of SBC 3b (0.5 μM; 537 nm) after the addition of different water samples in methanol with and without hydrazine. | ||
3.9 DFT and TDDFT calculation
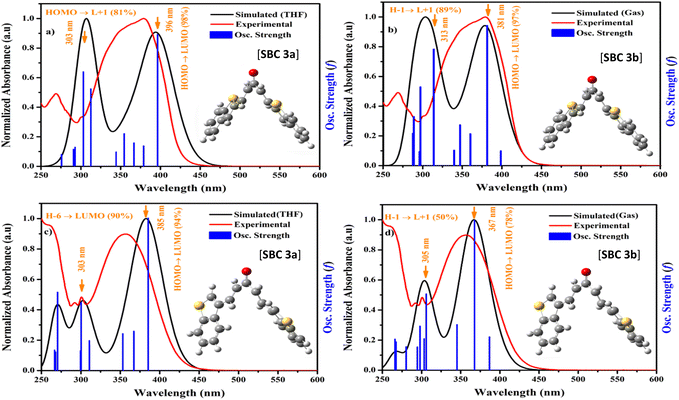
The obtained experimental analysis was confirmed further via density functional theory. Their excited energy levels were calculated via time-dependent density functional theory. Gaussian G09 with the B3LYP/6-31(G) base set was used to calculate the theoretical calculation by vertical excitation.42–44 The optimized structures of SBC 3a and 3b are noted in Table S1,† and with the respective atom labelling shown in Fig. S9.† The UV-absorption study of SBC 3a and 3b was calculated in the gas and solvent (methanol) phase, and compared with the experimental outcomes. In addition, we compared their oscillation strength and all the relative data incorporated in Fig. 8.SBC 3a in the solvent shows an absorption wavelength at 396 nm with 0.67 oscillation strength, and the HOMO → LUMO contribution is 98%. In the case of the gas phase, SBC 3a showed an absorption wavelength at 381 nm with 0.50 oscillation strength, and the HOMO → LUMO contribution is 97%. A second oscillation strength of 0.41 was found at 313 nm, and the H−1 → L+1 contribution is 89% (Table S2†). Both gas and solvent phases are matched with experimental analysis, although the gas simulation is better matched, as compared with the gas and solvent phase (Fig. 8).
SBC 3b in the solvent phase showed major oscillations at 0.39, 0.17 and 0.15 with absorption wavelengths at 385, 270 and 303 nm, and its contribution was found to be HOMO → LUMO (94%), H−6 → LUMO (90%) and H−1 → L+1 (77%), respectively. Meanwhile, the gas phase showed 367 nm absorption with 0.30 oscillation strength and a HOMO → LUMO contribution of 78%. The second highest oscillation is 0.12, and its H−1 → L+1 contribution was found to be 50% (Fig. 8c, d and Table S3†). SBC 3b in the solvent and gas phases nearly matched with the experimental outcomes (10–20 nm variation).
In addition, the measured bond angles and their dihedral angles of SBC 3a and 3b are tabulated in Table S1.† The bond angle and dihedral angles of both molecules both show a similar observation at the α,β-unsaturated carbonyl group. Due to the presence of the sulphur group attachment at different 2 and 3rd positions, there is a slight variation of the 3rd-degree bond angle. Overall, the molecule represented a ‘‘v’’ shape with a similar distribution towards the right and left side of the carbonyl centre. The frontier molecular orbitals of the SBC 3a and 3b were calculated to find the electron distribution in the molecule, and are represented in Fig. 8 (Fig. S10,† gas phase). The HOMO and LUMO of SBC 3a are located at −5.98 eV and −2.32 eV, respectively, and the calculated energy gap is 3.66 eV. In the case of SBC 3b, the HOMO and LUMO are −5.89 eV and −2.07 eV, respectively, and the energy gap is 3.81 eV. As compared to SBC 3a, 3b exhibited a higher energy difference of HOMO–LUMO (0.15 eV), which is expected due to the presence of the sulphur-containing 5-membered ring attachment at the 3rd carbon to the α,β-unsaturated carbonyl group. The detailed HOMO–LUMO levels are represented in Table S4.† The electron distribution on HOMO of SBC 3a is located mainly on benzo[b]thiophen, and LUMO is located mostly on the molecule including the carbonyl group. Meanwhile, the electron distribution of HOMO of SBC 3b is mostly located on the conjugated portion, and the LUMO is mainly distributed on the α,β-unsaturated carbonyl group. The presence of a position change of sulphur at 2 and 3 leads to a large difference in the electron distribution. Furthermore, the presence of sulphur in SBC 3b supressed the electrons on benzene, where the 5-membered ring is attached. The calculated singlet triplet energy levels are shown with the energy difference at approximately 1 eV, which is good for effective fluorescence emission behaviour. These outcomes indicate that the current synthesized molecules can efficiently produce fluorescence emission, which is used for the selective detection of hydrazine. Here, we have performed a basic asymptotic fraction functional (Tables S3 and S4†). However, the full range-separated functional may give better HOMO/LUMO and TDDFT energies (Fig. 9).45–47
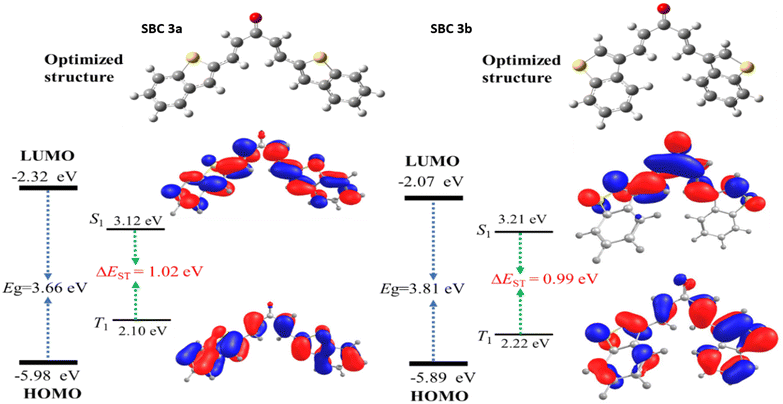 | ||
| Fig. 9 Calculated HOMO and LUMO energy levels for SBC 3a and 3b with their frontier orbitals and their singlet–triplet excited states. | ||
4 Conclusion
A straightforward Claisen–Schmidt condensation through two comparative protocols catalyzed by KOH/pyrrolidine with benzothiophene aldehydes and acetone yielded two different hydrazine-detecting molecules. The results have proven KOH as a more effective and pyrrolidine as a more efficient catalyst for the synthesis of these semi-bis-chalcone (SBCs) scaffolds. The structures of the synthesized compounds were confirmed by NMR, IR and HRMS analyses. Generally, chalcones are photoluminescent moieties; therefore, the photophysical properties of the prepared molecules were investigated by UV-visible and fluorescence analysis in various polar and non-polar solvents. Due to intramolecular charge-transfer (ICT), the prepared SBCs exhibit high fluorescence in both solution and solid states. The obtained results of the photophysical properties and their DFT-TDDFT theoretical correlation suggest that the prepared SBCs can be optimized for their applications in optoelectronics, sensing, and bio imaging. Looking at their photoluminescent nature, the selective benzothiophene 2-carbaldehyde SBC 3a molecule has been used for hydrazine detection with dual spectroscopies. In the presence of hydrazine, colorimetric and ratiometric fluorescence alterations for 3b were detected because they have an activated α,β-unsaturated carbonyl group that is highly reactive towards the nucleophilic hydrazine. The detailed sensing studies of this approach includes the selectivity, anti-interference capacity (pH, organic and inorganic), limit of detection, and solvent-dependent sensing efficiency. We believe that the outcome of these sensing methods will encourage the future design of real-time probes for hydrazine and a few other chemicals with diverse applications.Data availability
Additional data related to the main manuscript are available in the ESI.†Author contributions
Conceptualization of the work, funding acquisition, supervision, resources, writing – original draft and review – editing were performed by Paresh. Detailed investigation, data curation, formal analysis, methodology, validation and visualization were performed by Nidhi, Dinkal, Amar and Niteen. The computational work was performed and interpreted by B. Rajamouli.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge the financial support from GSBTM, Govt. of Gujarat (File No. GSBTM/JD(R&D)/626/22-23/00018349). The authors are also thankful to Aether Industry, Ltd for mass analysis.References
- S. P. Nikumbh, A. Raghunadh, V. N. Murthy, R. Jinkala, S. C. Joseph, Y. L. N. Murthy, B. Prasad and M. Pal, A greener approach towards double heteroarylation of N, O and S nucleophiles: synthesis of bioactive polynuclear fused N-heteroarenes, RSC Adv., 2015, 5, 74570–74574 RSC.
- S. Maheshwaran, W. H. Chen, S. L. Lin, M. Ghorbanie and A. T. Hoang, Electroanalytical overview: the electroanalytical sensing of hydrazine, Environ. Sci.: Adv., 2024, 3, 154–176 CAS.
- M. Devi, P. Kumar, R. Singh, L. Narayan, A. Kumar, J. Sindhu, S. Lal, K. Hussain and D. Singh, A comprehensive review on synthesis, biological profile and photophysical studies of heterocyclic compounds derived from 2, 3-diamino-1, 4-naphthoquinone, J. Mol. Struct., 2022, 1269, 133786 CrossRef CAS.
- P. N. Patel, D. H. Desai, N. C. Patel and A. G. Deshmukh, Efficient multicomponent processes for synthesis of novel poly-nuclear hetero aryl substituted terpyridine scaffolds: Single crystal XRD study, J. Mol. Struct., 2022, 1250, 131737 CrossRef CAS.
- C. Feng, M. Lv, J. Shao, H. Wu, W. Zhou, S. Qi, C. Deng, X. Chai, H. Yang, Q. Hu and C. He, Lattice Strain Engineering of Ni2P Enables Efficient Catalytic Hydrazine Oxidation-Assisted Hydrogen Production, Adv. Mater., 2023, 35(42), 2305598 CrossRef CAS.
- S. Ma, B. Yu, B. Y. Xia and Y. Bo, A pyridinic nitrogen-rich carbon paper for hydrazine oxidation-hybrid seawater electrolysis toward efficient H2 generation, Sci. China Mater., 2024, 67, 752761 Search PubMed.
- T. Vyas, H. Kumar, G. Nagpurea and A. Joshi, Fiber-optic thin film chemical sensor of 2, 4 dinitro-1-chlorobenzene and carbon quantum dots for the point-of-care detection of hydrazine in water samples, Environ. Sci.: Water Res. Technol., 2024, 10, 1481–1491 RSC.
- J. Ma, X. Kong, M. Zhao, Z. Jiao, X. Zhang, H. Xie and Z. Zhang, A water-soluble red-emitting fluorescence probe for detecting hazardous hydrazine in environmental waters and bio-systems, Sci. Total Environ., 2024, 944, 173810 CrossRef CAS.
- G. Alberti, C. Zanoni, V. Losi, L. R. Magnaghi and R. Biesuz, current trends in polymer based sensors, Chemosensors, 2021, 9, 108 CrossRef CAS.
- M. F. Maitz, Applications of synthetic polymers in clinical medicine, Biosurf. Biotribol., 2015, 1(3), 161–176 CrossRef.
- E. Kandemir, M. Özkütük, B. Aydınera, N. Seferoğluc, H. Erer and Z. Seferoğlu, Novel fluorescent coumarin-thiazole based sensors for selective determination of cyanide in aqueous media, J. Mol. Struct., 2022, 1249, 131593 CrossRef CAS.
- U. Krishnan, S. Manickam and S. K. Iyer, Turn-off fluorescence of imidazole-based sensor probe for mercury ions, Sens. Diagn., 2024, 3, 87–94 RSC.
- C. Zhuang, W. Zhang, C. Sheng, W. Zhang, C. Xing and Z. Miao, Chalcone: a privileged structure in medicinal chemistry, Chem. Rev., 2017, 117(12), 7762–7810 CrossRef CAS.
- Y. Fu, D. Liu, H. Zeng, X. Ren, B. Song, D. Hu and X. Gan, New chalcone derivatives: synthesis, antiviral activity and mechanism of action, RSC Adv., 2020, 10, 24483–24490 RSC.
- P. N. Patel, K. D. Patel and H. S. Patel, Synthesis and biological study of novel 5-{(4-(6, 7-dihydrothieno-[3, 2-c] pyridin-5 (4H)-ylsulfonyl) phenylamino)-methyl} quinolin-8-ol and its metal complexes, Chin. Chem. Lett., 2011, 22(11), 1297–1300 CrossRef CAS.
- W. Sijia, W. Enting and Y. Yuan, Detection of furan levels in select Chinese foods by solid phase microextraction-gas chromatography/mass spectrometry method and dietary exposure estimation of furan in the Chinese population, Food Chem. Toxicol., 2014, 64, 34–40 CrossRef.
- A. Torbica, D. Horvat, D. Zivancev, M. Belović, G. Šimić, D. Magdić, N. Đukić and K. Dvojković, Prediction of the genetic similarity of wheat and wheat quality by reversed-phase high-performance liquid chromatography and lab-on-chip methods, Acta Aliment., 2017, 46, 137–144 CAS.
- S. Seidi and L. Alavi, Novel and rapid deep eutectic solvent (DES) homogeneous liquid-liquid Microextraction (HLLME) with flame atomic absorption spectrometry (FAAS) detection for the determination of copper in vegetables, Anal. Lett., 2019, 52, 2092–2106 CrossRef CAS.
- X. Luo and L. T. Lim, Curcumin-loaded electrospun nonwoven as a colorimetric indicator for volatile amines, LWT–Food Sci. Technol., 2020, 128, 109493 CrossRef CAS.
- A. M. Razdari, M. G. Varnamkhasti, Z. Izadi, S. Rostami, A. A. Ensafi, M. Siadat and E. Losson, Detection of sulfadimethoxine in meat samples using a novel electrochemical biosensor as a rapid analysis method, J. Food Compos. Anal., 2019, 82, 103252 CrossRef.
- B. Mohan, N. M. Kunhumon and S. Shanmugaraju, Fluorescence sensing and bioimaging of Cu (II) ions using amino-1, 8-naphthalimide-based small-molecule chemosensors, Sens. Diagn., 2023, 2, 11581175 Search PubMed.
- S. Singh, N. Kumari, B. K. Kanungo and M. Baral, Hydroxypyridinone based chelators: a molecular tool for fluorescence sensing and sensitization, Sens. Diagn., 2024, 3, 968986 RSC.
- L. Liu, M. Xing, Y. Han, X. Zhang, P. Li, D. Cao, S. Zhao, L. Ma and Z. Liu, Sensing for hydrazine of a pyrene chalcone derivative with acryloyl terminal group, Spectrochim. Acta, Part A, 2022, 264, 120272 CrossRef CAS PubMed.
- L. Yan, S. Zhang, Y. Xie, X. Mu and J. Zhu, Recent progress in the development of fluorescent probes for the detection of hydrazine, Crit. Rev. Anal. Chem., 2022, 52, 210–229 CrossRef CAS.
- B. Shi, Y. Chai, P. Qin, X. X. Zhao, W. Li, Y. M. Zhang, T. B. Wei, Q. Lin, H. Yao and W. J. Qu, Detection of aliphatic aldehydes by a pillar[5]arene-based fluorescent supramolecular polymer with vaporchromic behavior, Chem.–Asian J., 2022, 17, e202101421 CrossRef CAS PubMed.
- H. Xing, Y. Yu, J. Liu, P. Qin, J. W. Y. Lam, B. Shi, G. Xie and B. Z. Tang, A Discrete platinum(II) metallacycle harvesting triplet excitons for solution-processed deep-red organic light-emitting diodes, Adv. Opt. Mater., 2022, 10, 2101925 CrossRef CAS.
- E. N. Okolo, D. I. Ugwu, B. E. Ezema, J. C. Ndefo, F. U. Eze, C. G. Ezema, J. A. Ezugwu and O. T. Ujam, New chalcone derivatives as potential antimicrobial and antioxidant agent, Sci. Rep., 2021, 11, 21781 CrossRef CAS.
- P. N. Patel and A. Chadha Synthesis, single crystal structure and spectroscopic aspects of Benzo [b] thiophene-3-carbaldehyde based chalcones, J. Chem. Crystallogr., 2016, 46, 245–251 CrossRef CAS.
- S. Tandel, N. C. Patel, S. Kanvah and P. N. Patel, An efficient protocol for the synthesis of novel hetero-aryl chalcone: A versatile synthon for several heterocyclic scaffolds and sensors, J. Mol. Struct., 2022, 1269, 133808 CrossRef CAS.
- S. N. Tandel, A. G. Deshmukh, B. U. Rana and P. N. Patel, Studies of novel benzofuran based chalcone scaffolds: A dual spectroscopic approach as selective hydrazine sensor, Chem. Phys. Lett., 2023, 817, 140426 CrossRef CAS.
- S. Halder, B. Nayak, B. Bhattacharjee, A. Ramesh and G. Das, Insight into the aggregation prospective of Schiff base AIE gens enabling an efficient hydrazine sensor in their aggregated state, J. Mater. Chem. C, 2021, 9, 8596–8605 RSC.
- Y. Jung, I. G. Ju, Y. H. Choe, Y. Kim, S. Park, Y. M. Hyun, M. S. Oh and D. Kim, Hydrazine exposé: the next-generation fluorescent probe, ACS Sens., 2019, 4, 441–449 CrossRef CAS PubMed.
- X. Y. Zhang, Y. S. Yang, W. Wang, Q. C. Jiao and H. L. Zhu, Fluorescent sensors for the detection of hydrazine in environmental and biological systems: Recent advances and future prospects, Coord. Chem. Rev., 2020, 417, 213367 CrossRef CAS.
- Y. Zhang, Z. Wang, J. Song, M. Li, Y. Yang, X. Xu, H. Xu and S. Wang, A simple camphor based AIE fluorescent probe for highly specific and sensitive detection of hydrazine and its application in living cells, Anal. Methods, 2019, 11, 3958–3965 RSC.
- G. Wu, X. Tang, W. Ji, K. Lai and Q. Tong, A turn-on fluorescent probe based on coumarin-anhydride for highly sensitive detection of hydrazine in the aqueous solution and gas states, Methods Appl. Fluoresc., 2017, 5(1), 015001 CrossRef PubMed.
- R. Maji, A. K. Mahapatra, K. Maiti, S. Mondal, S. S. Ali, P. Sahoo, S. Mandal, M. R. Uddin, S. Goswami, C. K. Quah and H. K. Fun, A highly sensitive fluorescent probe for detection of hydrazine in gas and solution phases based on the Gabriel mechanism and its bioimaging, RSC Adv., 2016, 6, 70855–70862 RSC.
- W. Chen, W. Liu, X. J. Liu, Y. Q. Kuang, R. Q. Yu and J. H. Jiang, A novel fluorescent probe for sensitive detection and imaging of hydrazine in living cells, Talanta, 2017, 162, 225–231 CrossRef CAS.
- Q. Fang, L. Yang, H. Xiong, S. Han, Y. Zhang, J. Wang, W. Chen and X. Song, Coumarinocoumarin-based fluorescent probe for the sensitive and selective detection of hydrazine in living cells and zebra fish, Chin. Chem. Lett., 2020, 31, 129–132 CrossRef CAS.
- W. Zhang, F. Huo, T. Liu and C. Yin, Ratiometric fluorescence probe for hydrazine vapor detection and biological imaging, J. Mater. Chem. B, 2018, 6, 8085–8089 RSC.
- X. Jin, C. Liu, X. Wang, H. Huang, X. Zhang and H. Zhu, A flavone-based ESIPT fluorescent sensor for detection of N2H4 in aqueous solution and gas state and its imaging in living cells, Sens. Actuators, B, 2015, 216, 141–149 CrossRef CAS.
- S. N. Tandel, P. Mistry and P. N. Patel, Novel chalcone scaffolds of benzothiophene as an efficient real time hydrazine sensor: Synthesis and single crystal XRD studies, J. Mol. Struct., 2023, 1274, 134495 CrossRef CAS.
- L. Zhu, L. Du, G. Caoa and Z. Cai, AI-guided electro-decomposition of persistent organic pollutants: a long-awaited vision becoming reality?, Environ. Sci.: Adv., 2023, 2, 1302–1305 CAS.
- G. R. Jenness and M. K. Shukla, What can Blyholder teach us about PFAS degradation on metal surfaces?, Environ. Sci.: Adv., 2024, 3, 383–401 CAS.
- M. Fischer, Density functional theory study of hydrophobic zeolites for the removal of triclosan from aqueous solution, Environ. Sci.: Adv., 2023, 2, 1082–1098 CAS.
- M. E. Foster and B. M. Wong, Nonempirically Tuned Range-Separated DFT Accurately Predicts Both Fundamental and Excitation Gaps in DNA and RNA Nucleobases, J. Chem. Theory Comput., 2012, 8, 2682–2687 CrossRef CAS PubMed.
- B. M. Wong and T. H. Hsieh, Optoelectronic and Excitonic Properties of Oligoacenes: Substantial Improvements from Range-Separated Time-Dependent Density Functional Theory, J. Chem. Theory Comput., 2010, 6, 3704–3712 CrossRef CAS.
- B. M. Wong, M. Piacenzab and F. D. Sala, Absorption and fluorescence properties of oligothiophene biomarkers from long-range-corrected time-dependent density functional theory, Phys. Chem. Chem. Phys., 2009, 11, 4498–4508 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00306c |
| This journal is © The Royal Society of Chemistry 2025 |