Strong upconverted circularly polarized emission from a chiral tetrahedral Yb/Eu cage†
Zhiwei
Yao
,
Ting
Gao
 ,
Pengfei
Yan
,
Pengfei
Yan
 ,
Yanyan
Zhou
* and
Hongfeng
Li
,
Yanyan
Zhou
* and
Hongfeng
Li
 *
*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science Heilongjiang University, 74 Xuefu Road, Harbin 150080, China. E-mail: lihongfeng@hlju.edu.cn; zhouyanyan@hlju.edu.cn
First published on 6th March 2025
Abstract
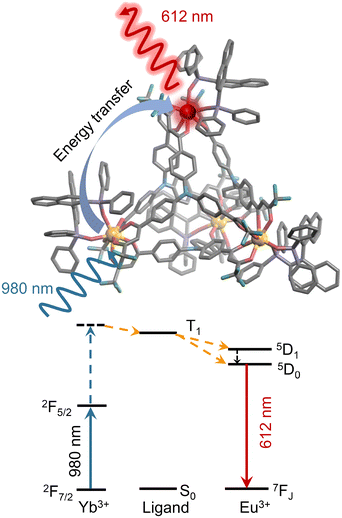
Upconverted circularly polarized luminescence (UC-CPL) materials have attracted significant attention due to their potential in optical sensing and bioimaging. However, achieving UC-CPL in lanthanide supramolecular systems remains a challenge due to the extended distances between lanthanide ions. Here, enantiopure tetrahedral cages, (Yb/Eu)4L4(R/S-BINAPO)4, are assembled using achiral C3-symmetric ligands, Ln(III) ions and chiral ancillary ligands. Upon 980 nm excitation, the heterometallic tetrahedral cages exhibit strong UC-CPL (glum = 0.22) and high ΦUC of 3.50 × 10−6. Moreover, through femtosecond transient absorption spectroscopy, we reveal that the ligand's triplet state (T1) serves as a critical mediator in the upconversion energy transfer process within the lanthanide complex, facilitating the efficient transfer of energy from the excited state of Yb3+ to the Eu3+ center via the mechanistic pathway Yb** → T1 → Eu*.
Introduction
Lanthanide complexes with upconverted circularly polarized luminescence (UC-CPL) offer notable advantages over other lanthanide-doped upconversion materials (such as inorganic upconversion nanoparticle materials,1,2 quantum dots,3,4etc.), including high biocompatibility, facile solution processing, and structural and functional tunability.5–7 These attributes make them promising candidates for diverse applications such as cellular imaging,8,9 remote cellular activation,10 and photodynamic therapy.11,12 Except for the two-photon absorption of the chromophore/ligand, the UCL in lanthanide complexes primarily depends on energy transfer between Ln3+ ions. To achieve efficient upconversion luminescence, various strategies have been employed to shorten the distance between Ln⋯Ln ions, including the use of bridging atoms,13–15 co-crystallization,16,17 and cluster formation.18–20Auzel has previously suggested that short distances between Ln3+ ions (typically less than 5 Å) are crucial for the CLU process.21 However, in the design of lanthanide supramolecular structures with specific functions, the use of larger-sized ligands often results in an increased distance between lanthanide ions, typically exceeding 5 Å. For example, in the simplest helicate, the distance between lanthanide ions usually ranges from 7 to 15 Å,22–28 whereas in more complex multinuclear tetrahedral and octahedral structures, the distance between most lanthanide ions is also at least over 8 Å.29,30 Such longer distances between lanthanide ions can adversely affect the upconversion energy transfer. As a result, finding a new energy transfer mechanism is particularly important. An important advantage of ligand involvement in the upconversion process is its ability to overcome the limitations imposed by Ln⋯Ln distances, making the preparation of structurally diverse lanthanide supramolecules with upconversion luminescence properties feasible, thereby broadening the potential application scope of upconversion-lanthanide complexes.
β-Diketonate ligands, widely recognized as excellent sensitizers for lanthanide luminescence,31,32 offer a promising avenue for investigating the role of triplet states in upconversion. Multitopic β-diketone ligands can form polynuclear structures such as helicates,33–35 tetrahedra,36 and octahedra.37 These diverse structures typically endow them with new properties, for instance, supramolecular chirality38 or host–guest recognition.39 We anticipate that a well-designed multitopic β-diketonate lanthanide complex will contribute to the study of triplet state involvement in upconversion due to the following advantages: (1) β-diketonate lanthanide complexes typically exhibit 100% intersystem crossing efficiency,40 which will increase the likelihood of triplet state participation in the upconversion process. (2) The relatively low triplet state energy level (T1 < 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) of aryl-containing β-diketones allows for energy transfer from the virtual energy level of Yb3+ (490 nm, 20
000 cm−1) of aryl-containing β-diketones allows for energy transfer from the virtual energy level of Yb3+ (490 nm, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 cm−1) generated by two-photon excitation (980 nm) to the T1 state.41 (3) The design of multitopic β-diketonates with long spacers can extend the distance between Ln3+ ions, thereby minimizing interference from Ln → Ln energy transfer and clarifying the participation of the T1 state.
480 cm−1) generated by two-photon excitation (980 nm) to the T1 state.41 (3) The design of multitopic β-diketonates with long spacers can extend the distance between Ln3+ ions, thereby minimizing interference from Ln → Ln energy transfer and clarifying the participation of the T1 state.
Based on these considerations, we designed and synthesized the chiral lanthanide heteropolynuclear tetrahedral cages Ln4L4(R/S-BINAPO)4 with upconverted circularly polarized luminescence properties. Upon excitation at 980 nm, the chiral tetrahedral cages (Yb/Eu)4L4(R/S-BINAPO)4 show excellent upconverted circularly polarized luminescence, with a maximum |glum| value of 0.22 at 595 nm. This obvious UC-CPL performance is driven by an upconversion energy transfer process mediated by the ligand triplet state (T1), promoting the potential of supramolecular cages in advanced luminescence applications (Scheme 1).
Results and discussion
Synthesis and characterization of the tetrahedral cages
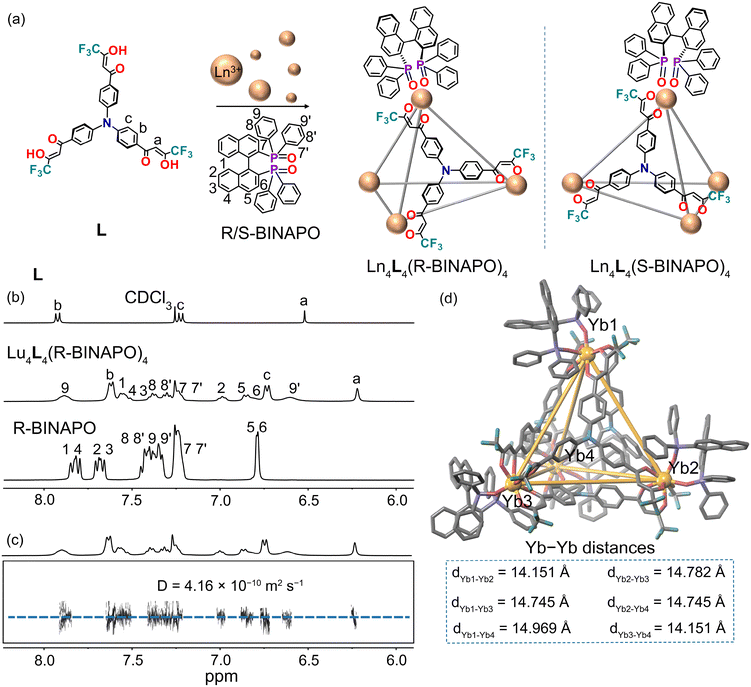
The preparation of the ligand L was conducted according to literature procedures.30 The tetrahedral cage Yb4L4(R-BINAPO)4 was prepared by the self-assembly of the ligand and R-BINAPO with YbCl3·6H2O salts in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CH3OH at room temperature. Owing to the low resolution of the 1H NMR spectra for Yb4L4(R-BINAPO)4 (Fig. S1†), the isostructural Lu(III) complex Lu4L4(R-BINAPO)4 was used as a surrogate for NMR analyses (Fig. S4 and S5;†1H–1H COSY and 1H–1H NOESY). As illustrated in Fig. 1b, the Lu4L4(R-BINAPO)4 complex exhibits a single set of broadened but distinguishable proton resonances. Compared to the free ligand L and the ancillary ligand R-BINAPO, the majority of the signals from the tetrahedral cage experience significant upfield shifts. The three distinct resonances corresponding to the tris(β-diketone) moiety exhibit broadening and relatively large shifts upon coordination with the metal center. Furthermore, no additional peaks associated with L were observed, suggesting the high diastereopurity of the self-assembled cages. The 31P and 19F NMR spectra further confirm the formation of a single species, as evidenced by the presence of a single, sharp singlet peak for the cage complex Lu4L4(R-BINAPO)4 (Fig. S6 and S7†). In 1H diffusion-ordered NMR spectroscopy (DOSY), the cage Lu4L4(R-BINAPO)4 displays one diffusion band at a diffusion coefficient of D = 4.16 × 10−10 m2 s−1 in line with the formation of a single species with a hydrodynamic diameter of 19 Å, estimated by the Stokes–Einstein equation (Fig. 1c). The dynamic diameter of the complex is in good agreement with that observed from the crystal structure of Yb4L4(R-BINAPO)4. The formation of Yb4L4(R-BINAPO)4 in solution was further confirmed by high-resolution electrospray ionization mass spectrometry (ESI-TOF-MS) (Fig. S8†). The spectrum exhibits molecular ions corresponding to the formula Yb4L4(BINAPO)4, and the observed isotopic distribution aligns with the theoretical profile for the [Yb4L4(BINAPO)4 + H]+ species.
1 ratio in CH3OH at room temperature. Owing to the low resolution of the 1H NMR spectra for Yb4L4(R-BINAPO)4 (Fig. S1†), the isostructural Lu(III) complex Lu4L4(R-BINAPO)4 was used as a surrogate for NMR analyses (Fig. S4 and S5;†1H–1H COSY and 1H–1H NOESY). As illustrated in Fig. 1b, the Lu4L4(R-BINAPO)4 complex exhibits a single set of broadened but distinguishable proton resonances. Compared to the free ligand L and the ancillary ligand R-BINAPO, the majority of the signals from the tetrahedral cage experience significant upfield shifts. The three distinct resonances corresponding to the tris(β-diketone) moiety exhibit broadening and relatively large shifts upon coordination with the metal center. Furthermore, no additional peaks associated with L were observed, suggesting the high diastereopurity of the self-assembled cages. The 31P and 19F NMR spectra further confirm the formation of a single species, as evidenced by the presence of a single, sharp singlet peak for the cage complex Lu4L4(R-BINAPO)4 (Fig. S6 and S7†). In 1H diffusion-ordered NMR spectroscopy (DOSY), the cage Lu4L4(R-BINAPO)4 displays one diffusion band at a diffusion coefficient of D = 4.16 × 10−10 m2 s−1 in line with the formation of a single species with a hydrodynamic diameter of 19 Å, estimated by the Stokes–Einstein equation (Fig. 1c). The dynamic diameter of the complex is in good agreement with that observed from the crystal structure of Yb4L4(R-BINAPO)4. The formation of Yb4L4(R-BINAPO)4 in solution was further confirmed by high-resolution electrospray ionization mass spectrometry (ESI-TOF-MS) (Fig. S8†). The spectrum exhibits molecular ions corresponding to the formula Yb4L4(BINAPO)4, and the observed isotopic distribution aligns with the theoretical profile for the [Yb4L4(BINAPO)4 + H]+ species.
To further determine the chemical structure and composition of tetrahedral Yb4L4(R-BINAPO)4, yellow square-shaped crystals were obtained by slow diffusion of hexane into its tetrahydrofuran solution at room temperature. X-ray diffraction analysis confirms that the complex Yb4L4(R-BINAPO)4 adopts the anticipated M4L4 tetrahedral architecture (Fig. 2d) and crystallizes in the chiral space group C2. This observation indicates that chirality from the R-BINAPO ligand is successfully transferred into the tetrahedral architecture. Within the cage, four Yb(III) ions are positioned at the vertices, while four ligands occupy the four faces. Each Yb center is coordinated by three β-diketonate moieties of the three ligands and one BINAPO ancillary ligand, forming a distorted triangular dodecahedral coordination geometry (Fig. S19†). In Yb4L4(R-BINAPO)4, the β-diketonate units helically twist each Yb(III) center in a left-handed, propeller-like manner, establishing a Λ configuration at each of the four vertices.
To investigate the upconversion luminescence properties of the heteropolynuclear tetrahedral cages (YbxEu4−xL4)(R/S-BINAPO)4 (x = 0.5–3.5) in solution, the (YbxEu4−xL4)(R/S-BINAPO)4 assemblies with different molar ratios of Yb3+ and Eu3+ ions were also synthesized by a similar synthesis method to that for (Yb4L4)(R-BINAPO)4. The heteropolynuclear tetrahedral cages (YbxEu4−xL4)(R-BINAPO)4 were characterized via ESI-TOF-MS and NMR spectra (Fig. S9–S18†).
Upconversion emission behavior of heterometallic tetrahedral cages (YbxEu4−xL4)(R/S-BINAPO)4
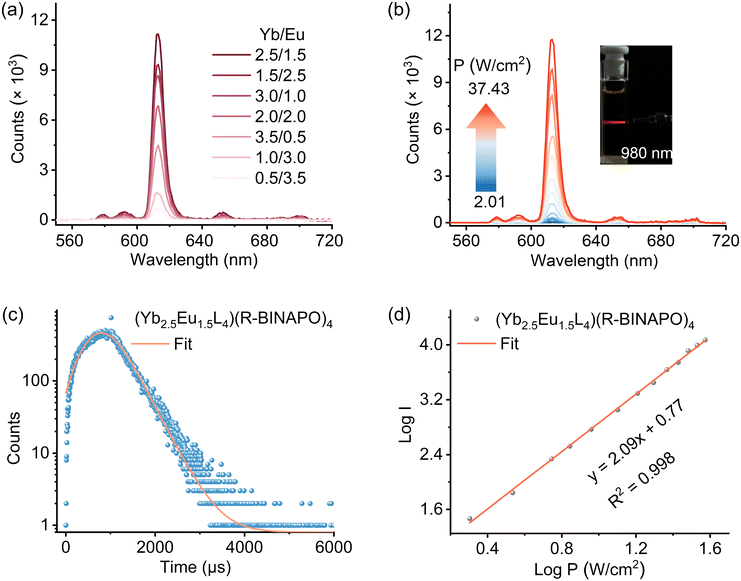
As shown in Fig. 2a, the upconversion luminescence properties of the tetrahedral cages were studied under 980 nm excitation at room temperature in CHCl3. The emission spectra of (YbxEu4−xL4)(R-BINAPO)4 (x = 0.5–3.5) all show five characteristic emission bands at 580, 594, 612, 650, and 702 nm, respectively, related to the 5D0 → 7FJ (J = 0–4) transitions. Among the seven assemblies of (YbxEu4−xL4)(R-BINAPO)4 (x = 0.5–3.5), the assembly of (Yb2.5Eu1.5L4)(R-BINAPO)4 exhibits the most intense upconversion luminescence. The higher molar ratio of Yb3+ ions in (YbxEu4−xL4)(R-BINAPO)4 may increase the energy transfer between Yb3+ ions, leading to a decrease in the energy transfer from Yb3+ to Eu3+,42,43 while a lower molar ratio of the sensitizer Yb3+ is usually insufficient to activate the Eu3+ ions.44,45To further investigate the upconversion luminescence process of the heteropolynuclear tetrahedral cages (YbxEu4−xL4)(R-BINAPO)4 (x = 0.5–3.5), the corresponding upconversion emission intensities of the tetrahedral cages were integrated as a function of 980 nm laser power density (P = 2.0–37.4 W cm−2) (Fig. 2b and S21–S24†). The slopes of logarithm I (I = integrated areas of luminescence intensity) versus logarithm P (laser power density, W cm−2) are related to the photon number to sensitize the 5D0 excited state of Eu3+.46 The slope values of (YbxEu4−xL4)(R/S-BINAPO)4 (x = 0.5–3.5) are all around 2.0 in CHCl3, strongly suggesting two-photon absorption characteristics of Eu3+ ion emission (Fig. 2d and S25–S28†). In addition, after being stored in a desiccator for 3 months, the upconversion emission intensity of (Yb2.5Eu1.5L4)(R-BINAPO)4 exhibits only a slight decrease (Fig. S29 and S30†), indicating its high structural stability and upconversion luminescence performance.
Then, the luminescence lifetime of (Yb2.5Eu1.5L4)(R-BINAPO)4 was studied upon 980 nm excitation (Fig. 2c). The decay time of the upconversion signal at 612 nm for (Yb2.5Eu1.5L4)(R-BINAPO)4 in CHCl3 was determined by fitting the decay curves to a single exponential function, which is consistent with the observations under 395 nm excitation (Fig. S31†). Therefore, this suggests that only a single species is present in the excited state of the complex. Using [(YbL)2Tb1] (Φref = 1.4 × 10−8) as the reference,16 the upconversion luminescence quantum yield (ΦUC) of the cage (Yb2.5Eu1.5L4)(R-BINAPO)4 was estimated to be 3.50 × 10−6 (P = 37.43 W cm−2, Fig. S37–40†). We propose that the relatively high upconversion luminescence quantum yield of the heteropolynuclear tetrahedral cage mainly arises from the incorporation of PO ancillary ligands, which not only enhances the rigidity of the tetrahedral skeleton but also prevents solvent molecules from entering the coordination sphere of the metal ions, thus inhibiting nonradiative transition.
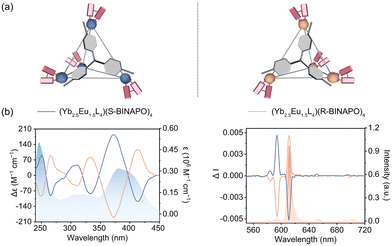
The chiroptical activity of (Yb2.5Eu1.5L4)(R/S-BINAPO)4 in CHCl3 was verified by circular dichroism (CD) spectroscopy (Fig. 3b left). The enantiomeric cages display mirror-image CD signals in the range of 250–500 nm, with a negative exciton couplet for the Λ-configurational (Yb2.5Eu1.5L4)(R-BINAPO)4 and a perfect mirror-image for the Δ-configurational (Yb2.5Eu1.5L4)(S-BINAPO)4. This correlation between the Δ/Λ configuration and the exciton couplet sign aligns with the empirical rule.47 The band in the 240–292 nm region is attributed to the π–π* transition of the chiral BINAPO. However, two distinct Cotton effects were observed within the absorption range (292–450 nm) of the achiral tris-β-diketone ligands. This result suggests that the chirality of R/S-BINAPO was effectively transferred to the achiral tris-β-diketone ligand, resulting in the formation of helical chirality in the tetrahedral cage.
Benefiting from the homochirality of the multimetallic tetrahedral cages (Yb2.5Eu1.5L4)(R/S-BINAPO)4, we explored their upconverted CPL properties. As expected, (Yb2.5Eu1.5L4)(R/S-BINAPO)4 exhibit upconverted CPL properties upon 980 nm excitation in CHCl3 (Fig. 3b right). A pair of heteropolynuclear tetrahedron cages show mirror-image CPL spectra; the two opposite major signals at 595 nm and 612 nm correspond to the transitions of 5D0 → 7F1 (ΔJ = 1) and 5D0 → 7F2 (ΔJ = 2), respectively. Despite the lower emission intensity of the 5D0 → 7F1 transition, its unique magnetic-dipole character results in a higher degree of circularly polarized emission compared to the 5D0 → 7F2 transition. The luminescence asymmetry factor (glum) is a key parameter for quantifying the magnitude of upconverted circularly polarized luminescence (UC-CPL). It is defined as glum = 2(IL − IR)/(IL + IR), where IL and IR represent the left- and right-polarized emission intensities, respectively, with the condition that −2 ≤ glum ≤ 2. Notably, the tetrahedral cages (Yb2.5Eu1.5L4)(R/S-BINAPO)4 exhibit a luminescence dissymmetry factor (glum) of up to 0.22 at the Eu3+ center under 980 nm excitation. Moreover, the heteropolynuclear tetrahedral cages (YbxEu4−xL4)(R/S-BINAPO)4 with other compositions also show favorable upconverted CPL properties, and their glum values are summarized in Table S2.†
Upconversion luminescence mechanism discussion
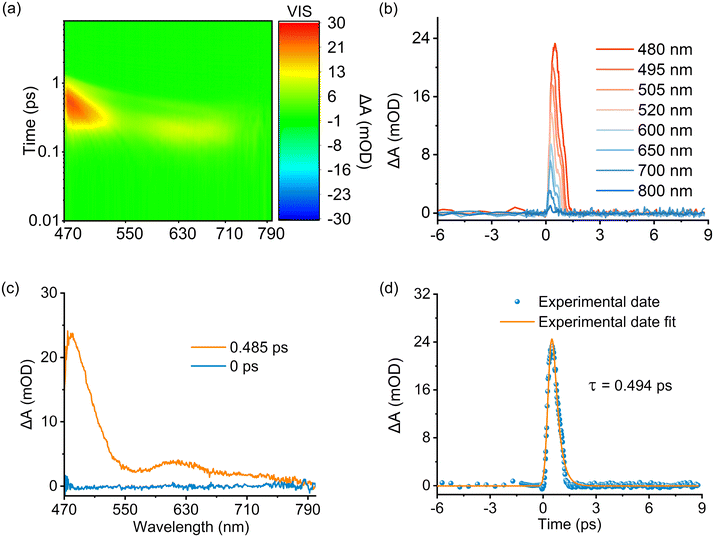
To date, four principal mechanisms underlying energy upconversion luminescence have been delineated: excited-state absorption (ESA),48 energy transfer (ETU),49,50 cooperative luminescence (CLU),51 and cooperative sensitized upconversion (CSU),52 despite the relatively limited research on upconversion luminescence in lanthanide complexes. Recently, Sun discovered a novel mechanism in which excited multimers participate in the upconversion cooperative sensitization process.53 Firstly, the emission spectrum of the homonuclear complex (Eu4L4)(R-BINAPO)4 was measured under 980 nm excitation, but no upconversion emission was observed, which rules out the pathway involving ligand two-photon absorption sensitization (Fig. S32†). Therefore, the two-photon UC luminescence from (Yb/Eu)4L4(R-BINAPO)4 most likely arises from the cooperative luminescence (CLU) or cooperative sensitization (CSU) of two excited Yb3+ ions. However, upon excitation of the homonuclear (Yb4L4)(R-BINAPO)4 at 980 nm, neither Yb two-photon upconversion luminescence (490 nm) nor ligand emission is detected in the visible spectral region (Fig. S33†). This result prompted us to speculate that the upconversion luminescence of (Yb/Eu)4L4(R-BINAPO)4 may arise from a bimetallic cooperative mechanism. Azel emphasized that a spatial distance of less than 5 Å between Ln3+ ions is crucial for the upconversion luminescence process.21 According to the crystal structure of the tetrahedral (Yb4L4)(R-BINAPO)4, the Ln⋯Ln distances range from 14.15 to 14.97 Å (Fig. 1d), suggesting that cooperative interactions between Yb*/Yb* should be difficult to realize. Additionally, the UC emission observed for the tetrahedral cage in solution rules out the possibility of a cooperative mechanism due to reduced Yb⋯Yb distances in the aggregated state. Therefore, considering the Ln⋯Ln distance and the absence of upconversion emission from Yb*/Yb* or the ligands in the visible spectral region, we hypothesize the presence of another energy transfer mechanism involving continuous absorption through Yb two-photon absorption (ESA), followed by energy transfer from the ligand's triplet state to the Eu3+ ion.To investigate the possible participation of the ligand triplet states (T1) in the upconversion (UC) process, we first determined the triplet energy level of the auxiliary ligand to be 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 cm−1 through phosphorescence spectroscopy (Fig. S34†). This value significantly exceeds the virtual state energy level of Yb3+ in two-photon absorption (20
666 cm−1 through phosphorescence spectroscopy (Fig. S34†). This value significantly exceeds the virtual state energy level of Yb3+ in two-photon absorption (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 cm−1, 490 nm), thereby ruling out the possibility of the auxiliary ligand participating in the upconversion process. Based on our previous work,54 the triplet energy level of ligand L is 19
408 cm−1, 490 nm), thereby ruling out the possibility of the auxiliary ligand participating in the upconversion process. Based on our previous work,54 the triplet energy level of ligand L is 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194 cm−1, indicating that ligand L fulfills the necessary conditions to participate in the upconversion process. However, under 980 nm excitation at 77 K, no emission associated with short- or long-lifetime fluorescence or phosphorescence was detected in the visible spectral region for (Yb2.5Gd1.5L4)(R-BINAPO)4 (Fig. S35†). This indicates that either the triplet states (T1) are not present or rapid energy transfer from the T1 state to the 2F5/2 state of Yb3+ ions occurs, leading to the quenching of ligand phosphorescence. Femtosecond transient absorption spectroscopy (fs-TAS) was then employed on (Yb4L4)(R-BINAPO)4 to investigate this ultrafast dynamic process. Under 980 nm femtosecond pulse excitation, a transient absorption band corresponding to the triplet state (T1) of the ligand was clearly observed in the 470–700 nm range (Fig. 4). This result unequivocally confirms the occurrence of an energy transfer process from Yb** to the ligand triplet state. Moreover, the lifetime of T1 was estimated to be 0.494 ps by fitting the kinetic curve of the TAS absorption (Fig. 4d). This value is notably shorter than the 2.7 ms lifetime of the ligand triplet state observed in (Gd4L4)(R-BINAPO)4 (Fig. S36†), suggesting an efficient and rapid energy transfer from the T1 state to the 2F5/2 state in the Eu/Yb heterometallic assembly. Furthermore, the rapid energy transfer rate from the T1 state to Eu(III) can be derived using eqn (1):
194 cm−1, indicating that ligand L fulfills the necessary conditions to participate in the upconversion process. However, under 980 nm excitation at 77 K, no emission associated with short- or long-lifetime fluorescence or phosphorescence was detected in the visible spectral region for (Yb2.5Gd1.5L4)(R-BINAPO)4 (Fig. S35†). This indicates that either the triplet states (T1) are not present or rapid energy transfer from the T1 state to the 2F5/2 state of Yb3+ ions occurs, leading to the quenching of ligand phosphorescence. Femtosecond transient absorption spectroscopy (fs-TAS) was then employed on (Yb4L4)(R-BINAPO)4 to investigate this ultrafast dynamic process. Under 980 nm femtosecond pulse excitation, a transient absorption band corresponding to the triplet state (T1) of the ligand was clearly observed in the 470–700 nm range (Fig. 4). This result unequivocally confirms the occurrence of an energy transfer process from Yb** to the ligand triplet state. Moreover, the lifetime of T1 was estimated to be 0.494 ps by fitting the kinetic curve of the TAS absorption (Fig. 4d). This value is notably shorter than the 2.7 ms lifetime of the ligand triplet state observed in (Gd4L4)(R-BINAPO)4 (Fig. S36†), suggesting an efficient and rapid energy transfer from the T1 state to the 2F5/2 state in the Eu/Yb heterometallic assembly. Furthermore, the rapid energy transfer rate from the T1 state to Eu(III) can be derived using eqn (1):
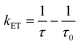 | (1) |
Conclusion
In summary, under 980 nm excitation, the chiral heterometallic tetrahedral cages (Yb2.5Eu1.5L4)(R/S-BINAPO)4 exhibit strong upconverted circularly polarized luminescence (UC-CPL) properties (|glum| = 0.22) and a high upconversion luminescence quantum yield of 3.50 × 10−6. This excellent UC-CPL performance is attributed to the rigid and saturated chiral environment created by the ancillary ligand R/S-BINAPO and the effective participation of the ligand triplet state in the upconversion luminescence process, where energy is transferred from Yb** through the triplet state (T1) of the ligand to the Eu3+ ion (Yb** → T1 → Eu*). This work expands the application of lanthanide supramolecules in the UC-CPL field.Author contributions
H. F. L. conceived and supervised the project. Z. W. Y. performed the experiments and analysed the data. Y. Y. Z. collected diffraction data and solved and refined the X-ray crystal structures. H. F. L., Y. Y. Z., T. G. and P. F. Y. reviewed and edited the paper. All authors contributed to the final draft of the paper.Data availability
The data that support the findings of this study, including X-ray crystallographic data for (Yb4L4)(R-BINAPO)4, are available in the ESI† of this article. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre as entry CCDC 2416911.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52273263, and 52203219) and the Scientific Research Project of Basic Scientific Research Operating Expenses of Colleges and Universities in Heilongjiang Province (2021-KYYWF-0029 and 2021-KYYWF-0041).References
- H. Zhang, H. Wei, L. Xu, Y. Li, Z. Song, D. Zhou, Q. Wang, Z. Long, Y. Yang, Y. Wen, J. Han, Y. Gao and J. Qiu, Ceram. Int., 2023, 49, 30436–30442 CrossRef CAS
.
- H. He, M. Cen, J. Wang, Y. Xu, J. Liu, W. Cai, D. Kong, K. Li, D. Luo, T. Cao and Y. J. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 53981–53989 CrossRef CAS PubMed
.
- J. Zhao and P. Xing, ChemPhotoChem, 2021, 6, e202100124 CrossRef
.
- B. Tang, Q. Wei, S. Wang, H. Liu, N. Mou, Q. Liu, Y. Wu, A. S. Portniagin, S. V. Kershaw, X. Gao, M. Li and A. L. Rogach, Small, 2024, 20, 2311639 CrossRef CAS PubMed
.
- E. G. Percástegui, T. K. Ronson and J. R. Nitschke, Chem. Rev., 2020, 120, 13480–13544 CrossRef PubMed
.
- E. G. Percástegui, Eur. J. Inorg. Chem., 2021, 2021, 4425–4438 CrossRef
.
- X.-Z. Li, C.-B. Tian and Q.-F. Sun, Chem. Rev., 2022, 122, 6374–6458 CrossRef CAS PubMed
.
- W. Ralph and N. Vasilis, Nat. Med., 2003, 9, 123–128 CrossRef PubMed
.
- Y. Kargar Gaz Kooh and N. Huebsch, Front. Mech. Eng., 2024, 10, 1481933 CrossRef
.
- X. Ai, L. Lyu, Y. Zhang, Y. Tang, J. Mu, F. Liu, Y. Zhou, Z. Zuo, G. Liu and B. Xing, Angew. Chem., Int. Ed., 2017, 56, 3031–3035 CrossRef CAS PubMed
.
- X. Zhang, F. Ai, T. Sun, F. Wang and G. Zhu, Inorg. Chem., 2016, 55, 3872–3880 CrossRef CAS PubMed
.
- G. Xu, C. Li, C. Chi, L. Wu, Y. Sun, J. Zhao, X.-H. Xia and S. Gou, Nat. Commun., 2022, 13, 3064 CrossRef CAS PubMed
.
- A. Nonat, C. F. Chan, T. Liu, C. Platas-Iglesias, Z. Liu, W.-T. Wong, W.-K. Wong, K.-L. Wong and L. J. Charbonnière, Nat. Commun., 2016, 7, 11978 CrossRef CAS PubMed
.
- L. Aboshyan-Sorgho, C. Besnard, P. Pattison, K. R. Kittilstved, A. Aebischer, J. C. G. Bünzli, A. Hauser and C. Piguet, Angew. Chem., Int. Ed., 2011, 50, 4108–4112 CrossRef CAS PubMed
.
- J. Wang, Y. Jiang, J. Y. Liu, H. B. Xu, Y. X. Zhang, X. Peng, M. Kurmoo, S. W. Ng and M. H. Zeng, Angew. Chem., Int. Ed., 2021, 60, 22368–22375 CrossRef CAS PubMed
.
- A. Nonat, S. Bahamyirou, A. Lecointre, F. Przybilla, Y. Mély, C. Platas-Iglesias, F. Camerel, O. Jeannin and L. J. Charbonnière, J. Am. Chem. Soc., 2019, 141, 1568–1576 CrossRef CAS PubMed
.
- G. Sun, Y. Xie, Y. Wang, G. A. Mandl, S. L. Maurizio, H. Zhang, X. Ottenwaelder, J. A. Capobianco and L. Sun, Angew. Chem., Int. Ed., 2023, 62, e202304591 CrossRef CAS PubMed
.
- D. A. Gálico, J. S. Ovens, F. A. Sigoli and M. Murugesu, ACS Nano, 2021, 15, 5580–5585 CrossRef PubMed
.
- R. C. Knighton, L. K. Soro, A. Lecointre, G. Pilet, A. Fateeva, L. Pontille, L. Francés-Soriano, N. Hildebrandt and L. J. Charbonnière, Chem. Commun., 2021, 57, 53–56 RSC
.
- S. P. K. Panguluri, E. Jourdain, P. Chakraborty, S. Klyatskaya, M. M. Kappes, A. M. Nonat, L. J. Charbonnière and M. Ruben, J. Am. Chem. Soc., 2024, 146, 13083–13092 CrossRef CAS PubMed
.
- F. Auzel and P. Goldner, Opt. Mater., 2001, 16, 93–103 CrossRef CAS
.
- K.-H. Yim, C.-T. Yeung, H.-Y. Wong and G.-L. Law, Inorg. Chem. Front., 2021, 8, 2952–2964 RSC
.
- J. K. Clegg, F. Li and L. F. Lindoy, Coord. Chem. Rev., 2022, 455, 214355 CrossRef CAS
.
- C.-T. Yeung, W. T. K. Chan, S.-C. Yan, K.-L. Yu, K.-H. Yim, W.-T. Wong and G.-L. Law, Chem. Commun., 2015, 51, 592–595 RSC
.
- R. Diego, M. Darawsheh, L. A. Barrios, A. Sadurní, J. García, P. Lloyd-Williams, S. J. Teat, O. Roubeau, D. Aguilà and G. Aromí, Dalton Trans., 2019, 48, 16844–16847 RSC
.
- X. Gao, L. Li, W. Sun and P. Chen, Dalton Trans., 2020, 49, 2843–2849 RSC
.
- R. Chen, Q.-Q. Yan, S.-J. Hu, X.-Q. Guo, L.-X. Cai, D.-N. Yan, L.-P. Zhou and Q.-F. Sun, Org. Chem. Front., 2021, 8, 2576–2582 RSC
.
- X. Wang, Q. Ma, S. Yin, T. Gao, P. Yan, Y. Zhou and H. Li, Dalton Trans., 2025, 54, 1343–1347 RSC
.
- C.-T. Yeung, K.-H. Yim, H.-Y. Wong, R. Pal, W.-S. Lo, S.-C. Yan, M. Yee-Man Wong, D. Yufit, D. E. Smiles, L. J. McCormick, S. J. Teat, D. K. Shuh, W.-T. Wong and G.-L. Law, Nat. Commun., 2017, 8, 1128 CrossRef PubMed
.
- Y. Zhou, H. Li, T. Zhu, T. Gao and P. Yan, J. Am. Chem. Soc., 2019, 141, 19634–19643 CrossRef CAS PubMed
.
- I. F. Costa, L. Blois, T. B. Paolini, I. P. Assunção, E. E. S. Teotonio, M. C. F. C. Felinto, R. T. Moura, Jr., R. L. Longo, W. M. Faustino, L. D. Carlos, O. L. Malta, A. N. C. Neto and H. F. Brito, Coord. Chem. Rev., 2024, 502, 215590 CrossRef CAS
.
- V. I. Vovna, V. V. Korochentsev, A. I. Cherednichenko and A. V. Shurygin, Russ. Chem. Bull., 2015, 8, 1701–1712 CrossRef
.
- Y. B. Tan, Y. Okayasu, S. Katao, Y. Nishikawa, F. Asanoma, M. Yamada, J. Yuasa and T. Kawai, J. Am. Chem. Soc., 2020, 142, 17653–17661 CrossRef CAS PubMed
.
- Y. Zhang, Y. Zhou, T. Gao, P. Yan and H. Li, Chem. Commun., 2020, 56, 13213–13216 RSC
.
- Y. Zhou, Y. Yao, Z. Cheng, T. Gao, H. Li and P. Yan, Inorg. Chem., 2020, 59, 12850–12857 CrossRef CAS PubMed
.
- W. Li, Y. Zhou, T. Gao, J. Li, S. Yin, W. Huang, Y. Li, Q. Ma, Z. Yao, P. Yan and H. Li, ACS Appl. Mater. Interfaces, 2022, 14, 55979–55988 CrossRef CAS PubMed
.
- T. Zhao, Y. F. Zhang, G. H. Wang, X. X. Wang, P. F. Feng and S. Q. Zang, Angew. Chem., Int. Ed., 2025, e202421426 CAS
.
- Q. Ma, S. Yin, Z. Song, T. Gao, P. Yan, Y. Zhou and H. Li, Inorg. Chem. Front., 2024, 11, 8093–8100 RSC
.
- Y. Yao, Y. Zhou, T. Zhu, T. Gao, H. Li and P. Yan, ACS Appl. Mater. Interfaces, 2020, 12, 15338–15347 CrossRef CAS PubMed
.
- S. Miyazaki, M. Gotanda, Y. Kitagawa, Y. Hasegawa, K. Miyata and K. Onda, J. Phys. Chem. Lett., 2024, 15, 10718–10724 CrossRef CAS PubMed
.
- F. J. Steemers, W. Verboom, D. N. Reinhoudt, E. B. van der Tol and J. W. Verhoeven, J. Am. Chem. Soc., 1995, 117, 9408–9414 CrossRef CAS
.
- B. Chen and F. Wang, Acc. Chem. Res., 2019, 53, 358–367 CrossRef PubMed
.
- S. Wen, D. Li, Y. Liu, C. Chen, F. Wang, J. Zhou, G. Bao, L. Zhang and D. Jin, J. Phys. Chem. Lett., 2022, 13, 5316–5323 CrossRef CAS PubMed
.
- C. Ma, X. Xu, F. Wang, Z. Zhou, D. Liu, J. Zhao, M. Guan, C. I. Lang and D. Jin, Nano Lett., 2017, 17, 2858–2864 CrossRef CAS PubMed
.
- Y. Wang, G. Sun, Q. Su, Y. Xie, F. Xing, H. Zhang and L. Sun, Chem. – Eur. J., 2024, 30, e202400911 CrossRef CAS PubMed
.
- B. Golesorkhi, A. Fürstenberg, H. Nozary and C. Piguet, Chem. Sci., 2019, 10, 6876–6885 RSC
.
-
G. Muller, in Luminescence oflanthanide ions in coordination compounds and nanomaterials, ed. A. de Bettencourt-Dias, Wiley, Hoboken, NJ, 2014, pp. 77–124 Search PubMed
.
- B. Golesorkhi, A. Fürstenberg, H. Nozary and C. Piguet, Chem. Sci., 2019, 10, 6876–6885 RSC
.
- I. Hyppänen, S. Lahtinen, T. Ääritalo, J. Mäkelä, J. Kankare and T. Soukka, ACS Photonics, 2014, 1, 394–397 CrossRef
.
- D. A. Gálico, J. S. Ovens, F. A. Sigoli and M. Murugesu, ACS Nano, 2021, 15, 5580–5585 CrossRef PubMed
.
- L. K. Soro, R. C. Knighton, F. Avecilla, W. Thor, F. Przybilla, O. Jeannin, D. Esteban-Gomez, C. Platas-Iglesias and L. J. Charbonnière, Adv. Opt. Mater., 2022, 11, 2202307 CrossRef
.
- R. C. Knighton, L. K. Soro, L. Francés-Soriano, A. Rodríguez-Rodríguez, G. Pilet, M. Lenertz, C. Platas-Iglesias, N. Hildebrandt and L. J. Charbonnière, Angew. Chem., Int. Ed., 2021, 61, e202113114 CrossRef PubMed
.
- X.-F. Duan, L.-P. Zhou, H.-R. Li, S.-J. Hu, W. Zheng, X. Xu, R. Zhang, X. Chen, X.-Q. Guo and Q.-F. Sun, J. Am. Chem. Soc., 2023, 145, 23121–23130 CrossRef CAS PubMed
.
- Y. Yao, Y. Zhou, T. Zhu, T. Gao, P. Yan and H. Li, ACS Appl. Mater. Interfaces, 2020, 12, 15338–15347 CrossRef CAS PubMed
.
- X.-F. Duan, H.-H. Ji, L.-P. Zhou, S.-J. Hu, J.-J. Fu, P.-F. Duan, X.-Q. Guo and Q.-F. Sun, CCS Chem., 2025 DOI:10.31635/ccschem.024.202405005
, Just Accepted.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis process, characterization, ESI-TOF-MS and 1H NMR spectra of the complexes. X-ray crystallographic data for (Yb4L4)(R-BINAPO)4. CCDC 2416911. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00219b |
| This journal is © The Royal Society of Chemistry 2025 |