Application of carbon-based nanomaterials in Alzheimer's disease
Mengyao
Bai
 ,
Xu
Shao
,
Xu
Shao
 ,
Chao
Wang
,
Juanxia
Wang
,
Xin
Wang
*,
Ping
Guan
* and
Xiaoling
Hu
*
,
Chao
Wang
,
Juanxia
Wang
,
Xin
Wang
*,
Ping
Guan
* and
Xiaoling
Hu
*
Department of Chemistry, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, 127 Youyi Road, Xi’an 710072, China. E-mail: xinwang@nwpu.edu.cn; guanping1113@nwpu.edu.cn; huxl@nwpu.edu.cn
First published on 11th November 2024
Abstract
Alzheimer's disease (AD) is a chronic, progressive neurodegenerative disorder marked by permanent impairment of brain function across the whole brain. This condition results in a progressive deterioration of cognitive function in patients and is frequently associated with psychological symptoms such as agitation and anxiety, imposing a significant burden on both patients and their families. Nanomaterials possess numerous distinctive physical and chemical features that render them extensively utilized. In the biomedical domain, nanomaterials can be utilized for disease prevention and therapy, including medication delivery systems, biosensors, and tissue engineering. This article explores the etiology and potential molecular processes of AD, as well as the application of carbon-based nanomaterials in the diagnosis and treatment of AD. Some of such nanomaterials are carbon quantum dots, carbon nanotubes, and graphene, among others. These materials possess distinctive physicochemical features that render them highly promising for applications in biosensing, drug delivery, neuroprotection, and photothermal treatment. In addition, this review explored various therapeutic approaches for AD in terms of reducing inflammation, preventing oxidative damage, and inhibiting Aβ aggregation. The advent of carbon nanomaterials in nanotechnology has facilitated the development of novel treatment approaches for Alzheimer's disease. These strategies provide promising approaches for early diagnosis, effective intervention and neuroprotection of the disease.
Wider impactThe diagnosis and treatment of Alzheimer's disease (AD) are pressing priorities in modern medical research. In recent years, nanomaterials have garnered significant attention owing to their distinctive advantage. An increasing number of academics have focused on the utilization of nanomaterials in the diagnosis and therapy of Alzheimer's disease, producing a series of latest findings. This review initially examines the pathophysiology of AD, subsequently categorizing carbon-based nanomaterials by multiple dimensions, followed by a detailed analysis and comparison of the benefits of carbon nanomaterials within each dimension. Consequently, we provide a summary and introduction, presenting our perspectives and recommendations. This review is anticipated to offer significant insights and guidance for the utilization of carbon nanomaterials in the diagnosis and treatment of Alzheimer's disease. |
1. Introduction
Alzheimer's disease (AD) is the predominant cause of dementia symptoms among the elderly worldwide. According to recent study, it is anticipated that by 2060,1 this currently incurable ailment will significantly impact over 13.8 million individuals, with this figure expected to rise in the ensuing years. In recent years, the incidence, prevalence, and mortality rates of Alzheimer's disease in China have escalated, resulting in a substantial economic burden on patients’ families, society, and the healthcare system overall.2Multiple studies have demonstrated that the aberrant buildup of beta-amyloid (Aβ) is the primary cause of the characteristic amyloid plaques found in the brains of individuals with AD.3,4 These plaques are intimately associated with neuronal damage and the deterioration of cognitive function.5,6 The buildup of Aβ can trigger an inflammatory response, exacerbating the abnormal phosphorylation of Tau. Furthermore, the incidence of neuronal damage and cellular apoptosis can lead to modifications in neurotransmitter levels, which in turn affect cognitive function. Tau has a role in preserving the stability of microtubules, essential for cellular structure and function.7–9 Tau proteins combine to create neurofibrillary tangles, which disrupt cellular transport and communication, initiating neurotoxicity that can ultimately result in cell death.6
Nanomaterials can be categorized into zero dimensional, one dimensional and two-dimensional forms. This review specifically examines the utilization of carbon-based nanomaterials, such as zero-dimensional carbon quantum dots,10 one-dimensional carbon nanotubes,11 and two-dimensional graphene,12 for the purpose of diagnosing and treating AD. Carbon dots possess substantial uses across various fields, including fluorescence detection13,14 and illness therapy,15–17 due to their diminutive size and exceptional biocompatibility. One-dimensional materials represented by carbon nanotubes (CNTS) are widely accepted in the field of biosensing due to their high specific surface area.18,19 Two-dimensional nanomaterials represented by graphene have advantages in drug delivery20,21 and biosensing22 due to their high electrical conductivity and surface modification.23 Carbon-based nanomaterials have shown considerable potential in biosensing, drug delivery, neuroprotection, and photothermal therapy. Scheme 1 illustrates several carbon-based nanomaterials employed in the diagnosis and treatment of Alzheimer's disease.
2. Pathogenesis of Alzheimer's disease
Alzheimer's disease (AD) is a neurodegenerative disease that leads to progressive degradation of brain structure and function, resulting in memory loss, cognitive decline, and other related symptoms.24–26 The precise mechanism by which Alzheimer's disease progresses remains incompletely understood, with numerous prominent theories, like the beta-amyloid (Aβ) theory and the tau hypothesis, being generally recognized.27 In addition to these two theories, there are several more possibilities, including the inflammatory theory,28 oxidative stress hypothesis,29 metal ion hypothesis,30 and others. The primary pathological characteristics of AD are the formation of plaques due to the deposition of β-amyloid (Aβ), the development of neurofibrillary tangles (NFT),31 the degeneration of neurons in the cerebral cortex and hippocampus, neuroinflammation, and the death of nerve cells.322.1. The beta-amyloid (Aβ) hypothesis
The accumulation of β-amyloid may be one of the early brain changes in patients with Alzheimer's disease (AD) or mild cognitive impairment (MCI) caused by dementia.33 Aβ is produced by β-amyloid precursor protein (APP) through a series of enzymatic reactions.34 First, APP is cleaved by β-secretase (BACE1) to produce the C99 intermediate, which is then further cleaved by γ-secretase, such as the γ-secretase complex, to release the Aβ peptide. Aβ peptides exist in various lengths, with Aβ40 and Aβ42 being the predominant types. Aβ42 has a higher propensity for aggregation compared to Aβ40 due to the presence of two hydrophobic amino acids at its terminus. The released Aβ peptide begins to aggregate in the brain, forming oligomers and fibrous structures. Aβ oligomers are recognized for their neurotoxicity, which is considered to be more detrimental than the toxicity associated with fibrous deposits.35 Over time, these Aβ fibrils aggregate in the brain, leading to the formation of deposits known as amyloid plaques. Predominantly found in the cerebral cortex and hippocampus—regions integral to memory and cognitive function—these plaques can have a significant impact on brain health.36 The deposition of Aβ may induce neurotoxicity through a variety of mechanisms. These include disrupting intercellular signaling, activating microglia to produce inflammatory responses, disrupting intracellular calcium balance, and triggering oxidative stress and mitochondrial dysfunction, among others.372.2. The hypothesis of Tau protein pathology
The primary localization of Tau protein is on the axon of neurons, where it functions to stabilize the shape of microtubules in healthy nerve cells.38 Microtubules are integral components of the cytoskeleton and play crucial roles in maintaining cell shape, facilitating cell division, and facilitating the transfer of materials inside the cell.39 AD involves an anomalous phosphorylation process of Tau, resulting in alterations to its structure and function. This alteration renders the Tau protein incapable of normal binding to microtubules, thereby compromising the integrity of microtubules.40 When Tau is phosphorylated in an abnormal way, it has a tendency to come together and create neurofibrillary tangles.41 These tangles are closely linked to problems with brain cells and a reduction in cognitive performance. Research has demonstrated that Tau aggregates may propagate throughout the brain, moving from one neuron to another in a manner akin to the spread of prions.42,43 This propagation has the potential to hasten the progression of neurodegeneration. Specific genetic variations, including as mutations in the MAPT gene responsible for encoding the Tau protein, have been linked to familial AD.44,45 These genetic variations have the potential to cause an aberrant structure of the Tau protein, hence increasing the likelihood of developing AD.41 Tau plays a crucial part in the pathogenesis of AD, making it a significant target for the advancement of novel treatments. Currently, several medications and therapies are being studied with the goal of inhibiting the aberrant process of phosphorylation, aggregation, or proliferation of Tau.46,47 Presently, several investigations are focused on the advancement of novel antibody treatments targeting the Tau protein. Using Roche's semorinemab,48 an anti-tau monoclonal antibody, as an illustration, despite its lack of efficacy in improving dementia symptoms during clinical trials, other research groups continue to investigate various antibody treatment approaches to attain more effective therapeutic outcomes.2.3. The hypothesis of neuroinflammation and oxidative stress
Inflammation serves as a common denominator connecting many disorders. Chronic inflammation is the primary underlying factor responsible for several severe health conditions and fatalities. It is associated with coronary heart disease,49 cancer,50 obesity,51 and AD.52,53 AD patients exhibit a persistent inflammatory reaction in their brain, maybe triggered by activated microglia and astrocytes. The cells generate inflammatory molecules in an effort to eliminate Aβ deposits, but an excessive inflammatory reaction can lead to further harm to nerve cells.54 Prolonged inflammation can result in compromised integrity of the blood–brain barrier (BBB), facilitating the infiltration of inflammatory cells and chemicals from the bloodstream into the brain, hence intensifying neuroinflammation.55 Inflammation can impact the production, release, and reabsorption of neurotransmitters, such as dopamine and glutamate. These alterations may be linked to the behavioral and cognitive symptoms observed in people with AD.28 Neuroinflammation has a multifaceted function in the development of AD, serving as both a result of the disease's pathological process and a factor that accelerates its advancement.Oxidative stress can activate microglia and trigger neuroinflammation. Excessive reactive oxygen species (ROS) can operate as signaling molecules that activate immune cells, leading to the initiation and amplification of inflammatory responses. Neuroinflammation, in turn, can potentially heighten oxidative stress. Activated microglia and other immune cells have the ability to generate more reactive oxygen species (ROS), which can worsen oxidative damage. In neurodegenerative disorders like Alzheimer's disease, there is a reciprocal relationship between oxidative stress and neuroinflammation, where they mutually exacerbate one other.56 Oxidative damage can result in the accumulation of proteins and impaired function of nerve cells, whereas neuroinflammation can worsen this damage. The tight association between oxidative stress and neuroinflammation makes them promising targets for therapeutic interventions in neurodegenerative disorders. Reducing oxidative stress or suppressing neuroinflammatory reactions may be beneficial in slowing or treating certain disorders.
2.4. Metal ion hypothesis
The metal ion hypothesis is a significant theory in Alzheimer's disease (AD) research, positing that metal ions (such as copper, iron, and zinc) are crucial in the pathogenesis of AD.57 These metal ions are linked to the aberrant aggregation of beta-amyloid (Aβ) and Tau, potentially resulting in heightened oxidative stress, neuroinflammation, and neuronal injury.58 According to this idea, nanoparticles have been engineered to target metal ions in order to impede or halt the advancement of Alzheimer's disease. Researchers have engineered nanoparticles that chelate metal ions, which attach to them in the brain and diminish their interaction with Aβ and Tau proteins, thereby decelerating the degenerative process.59 Clioquinol serves as a metal ion chelator, and researchers from Tianjin University have created human serum albumin nanoparticles dcHGTNPs that incorporate both metal ion chelators and acetylcholinesterase inhibitors to enhance medication delivery and retention in the brain. In a murine model of Alzheimer's disease, this nanomedicine demonstrated the ability to mitigate neuronal morphological alterations, restore memory deficits, and decelerate the disease progression.603. Carbon-based nanomaterials for Alzheimer's disease diagnosis and therapy
Carbon-based nanomaterials have emerged as flexible diagnostic and therapeutic agents in the field of Alzheimer's disease (AD). Carbon quantum dots, which are zero-dimensional carbon nanomaterials, are highly regarded for their strong fluorescence.61 This makes them well-suited for many applications such as targeted treatment,62,63 photodynamic64 and photothermal therapy,65 immunology,66 and gene therapy.67 Their usefulness in cell imaging,68 bacterial imaging,69 and in vivo imaging70 is improved by their intrinsic stability. Furthermore, their ability to transfer drugs through cavities and their suitability for surface modification make them outstanding drug carriers.71,72 Carbon nanotubes, which are one-dimensional carbon nanomaterials, are ideal for transporting drugs because of their high aspect ratio and large surface area. Their ability to conduct electricity also makes them promising for use in biosensing, since they can greatly enhance the sensitivity of detecting AD biomarkers.73–75 Carbon nanomaterials in two dimensions, like graphene, are highly valued for their large specific surface area, making them ideal for the creation of sensors.76 Their capacity to be modified makes it easier for them to be combined with various biomolecules, which in turn increases their usefulness in the diagnosis and treatment of Alzheimer's disease.77,783.1. Zero-dimensional
The production of CQDs is generally divided into two main methods: top-down and bottom-up techniques. The top-down approach begins by using bigger carbon sources, such as commercially available carbon black, graphite, or carbon nanotubes. It utilizes physical or chemical methods to reduce or carve these materials into smaller quantum dots. The approach commonly employs techniques such as laser ablation,82 arc discharge, and chemical oxidation.83 The top-down technique provides a fast method for obtaining CQDs from readily available carbon sources, but it may also create faults that might possibly affect their optoelectronic properties. On the other hand, the bottom-up technique starts at the carbon level of molecules or atoms and carefully creates quantum dots via chemical synthesis. This methodology includes many synthesis technologies such as the solvent hot method,84 microwave-assisted synthesis,85 template-assisted synthesis,86 and chemical vapor deposition.87 The bottom-up approach provides greater precision in manipulating the size and structure of the quantum dots, resulting in more consistent and superior quality CQDs with customized attributes for particular uses. Every approach possesses unique benefits and is selected according to the specific characteristics required for the CQDs, including uniformity in size, surface functionality, and fluorescence effectiveness. These properties are crucial for ensuring optimal performance in different applications.
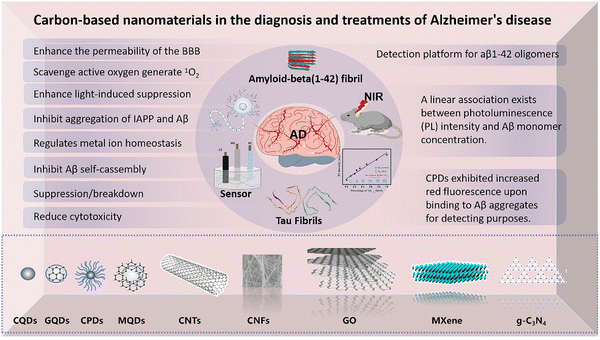
In order to mitigate the overall toxicity of Aβ, it is necessary to disrupt the normal process of Aβ molecules aggregating and forming bigger clusters. The amyloid aggregates possess a very robust self-assembled structure, which is distinguished by a dense arrangement of hydrogen bonds and hydrophobic contacts. Aβ mostly consists of β-lamellar secondary structures, and its stability presents a significant obstacle for its disintegration. In a work conducted in 2017, Chung using branched polyethylene imine (bPEI) to passivate CQDs.88 This passivation method involves the contact between bPEI and Aβ residues through electrostatic interactions, effectively preventing the aggregation of Aβ. The bPEI@CQDs exhibit visible light activity and biocompatibility, making them useful in breaking down Aβ aggregation and reducing Aβ-induced neurotoxicity (Fig. 1A). Significantly, exposure to sunshine greatly enhances the inhibitory impact of bPEI@CQDs on Aβ aggregation. In addition to this, Malishev et al. conducted a one-step carbonation procedure to create chiral carbon quantum dots (C-dots), utilizing either L-lysine or D-lysine as the only source of carbon.89 The study found that C-dots produced from L-lysine (L-Lys-C-dots) effectively prevented the transition of Aβ42 from a random coil to a β-sheet structure. In contrast, C-dots created from D-lysine (D-Lys-C-dots) had no effect on the conformation of Aβ42. L-Lys-C-dots were proposed as a means to control the aggregation of Aβ42 by disrupting its electrostatic contacts. This offers a new method to manipulate the aggregation of Aβ peptides in AD.
 | ||
| Fig. 1 (A) A schematic illustration of bPEI@CD capabilities in the inhibition of β-amyloid (Aβ) assembly and disaggregation of preformed fibrillar aggregates. Reproduced with permission.88 Copyright 2017, John Wiley and Sons. (B) A schematic illustration of bPEI@CD capabilities in the inhibition of β-amyloid (Aβ) assembly and disaggregation of preformed fibrillar aggregates. Reproduced with permission.90 Copyright 2024, The Royal Society of Chemistry. (C) Schematic representation for the synthesis of the yCDs and yCDs-Ce6 and illustration of the mechanism of the inhibitory effect on Aβ aggregation and microbial infection under PTT and PDT treatments. Reproduced with permission.91 Copyright 2022, American Chemical Society. (D) Schematic of the mechanism of CQD-RAW inhibition and clearance of Aβ. Reproduced with permission.92 Copyright 2023, Elsevier. | ||
Photodynamic therapy (PDT) and photothermal therapy (PTT) are two therapy methods that utilize the interaction between light and matter, showing promise in the treatment of AD.93 PDT employs a photosensitizer that, when exposed to precise wavelengths of light, produces reactive oxygen species (ROS), including singlet oxygen. These reactive oxygen species can engage with adjacent biological molecules, resulting in cellular harm or demise. PDT can inhibit the buildup of amyloid-beta protein (Aβ), alleviate neurotoxicity, and induce oxidative damage to pre-existing amyloid plaques in the treatment of AD.94 Shao et al. created a new photo-responsive carbon spot using solvent-free carbonization. CDs have demonstrated the ability to produce singlet oxygen, which can be utilized to oxidize amyloid.90 CDs impact the capacity for amyloid aggregation and depolymerization via hydrophobic interactions and photooxidation (Fig. 1B). PTT utilizes photothermal conversion agents to transform light energy into heat through light absorption.95 This process leads to a localized temperature rise, which in turn induces a thermotherapeutic impact on affected tissues. During the treatment of AD, PTT can utilize the process of thermal disaggregation to break down amyloid protein fibrils. This process helps to decrease the quantity and harmfulness of Aβ aggregates by utilizing thermal impacts.96 CDs preserved over 80% cell viability in PC12 and HT22 cell lines at doses up to 200 μg mL−1, demonstrating their exceptional biocompatibility with these cell lines. CDs exhibited no notable cytotoxicity towards cells in both light and non-light situations, indicating their excellent photostability. In animal models, histological evaluation of major organs post-CD injection revealed no substantial inflammation or structural damage, thereby further validating the biocompatibility of CDs. Flow cytometry and confocal laser scanning microscopy (CLSM) revealed that bEnd.3 cells could internalize CDs, with the uptake rate progressively increasing over time, suggesting the capacity of CDs to traverse the blood–brain barrier. Yan et al. utilized nanoassembly to mix a photosensitizer named chlorine e6 with yellow carbon nanodots (yCDs).91 These nanoassemblies have demonstrated efficacy in restricting the production of amyloid aggregates, decreasing cytotoxicity, managing microbial infections, and enhancing the capacity to traverse the blood–brain barrier (Fig. 1C). In a murine model, yCDs-Ce6 was evaluated for its capacity to traverse the blood–brain barrier (BBB) using a tail vein injection, subsequently followed by the irradiation of the mice's brains with near-infrared light. The findings indicated that the fluorescence signal of yCDs-Ce6 in murine brain tissue was markedly increased post-irradiation, suggesting that yCDs-Ce6 can efficiently traverse the blood–brain barrier under illuminated conditions. Moreover, in the APP/PS1 animal model, yCDs-Ce6 in conjunction with light therapy markedly diminished Aβ42 plaques in the brain. Immunofluorescence imaging of Aβ42 plaques in the hippocampus and cortical areas of mice demonstrated a significant reduction in both the quantity and size of Aβ42 plaques following treatment with yCDs-Ce6 and dual-wavelength laser. Furthermore, the analysis of fluorescence signals from principal organs revealed that yCDs-Ce6 was predominantly eliminated via the spleen and kidneys.
Liu et al. synthesized nitrogen-doped quantum dots (N-CDs) by mixing 1,2,3-phthalic acid with o-phenylene diamine.97 N-CDs possess a high concentration of amino, carboxyl, and hydroxyl groups on their surface, which offer several chemical sites for protein interactions. N-CDs have the ability to significantly decrease the clumping together of β-amyloid 1–42 (Aβ1–42) and mitigate the detrimental impacts of Aβ. Koppel et al. extracted carbon quantum dots (CQDs) from brown coal and demonstrated their ability to inhibit the development of Aβ amyloid peptide, indicating its potential as a therapeutic intervention for AD.98 Imbalance in the control of copper (Cu2+) levels in the body may have a role in the formation of Aβ aggregates, which can result in the development of harmful oligomers and fibrous clumps. This process initiates neuroinflammation and causes damage to neurons, ultimately leading to the development of Alzheimer's disease (AD).99 Ye et al. created nitrogen-doped carbon quantum dots (CQDs) and developed a nanosystem using CQDs that was coated with a macrophage membrane (RAW-M) in a novel manner.92 The nitrogen-containing functional groups on the surface of the CQD may efficiently capture and isolate excessive Cu2+ ions, therefore preventing the fast clustering of Aβ (Fig. 1D). In addition, the photothermal characteristics of the CQDs can cause the dissolution of fibril precipitates when exposed to near-infrared light (NIR). Both laboratory experiments (in vitro) and experiments conducted on living organisms (in vivo) have shown that this new nanosystem, when exposed to laser radiation, may greatly boost the capacity of pharmaceuticals to pass the blood–brain barrier (BBB). This breakthrough overcomes the limits of conventional treatments for AD. The nanosystem demonstrated significant efficacy in reducing Aβ deposition, alleviating neuroinflammation, and improving learning and memory impairments in an APP/PS1 mouse model, indicating its promise as a novel therapeutic approach for treating AD.
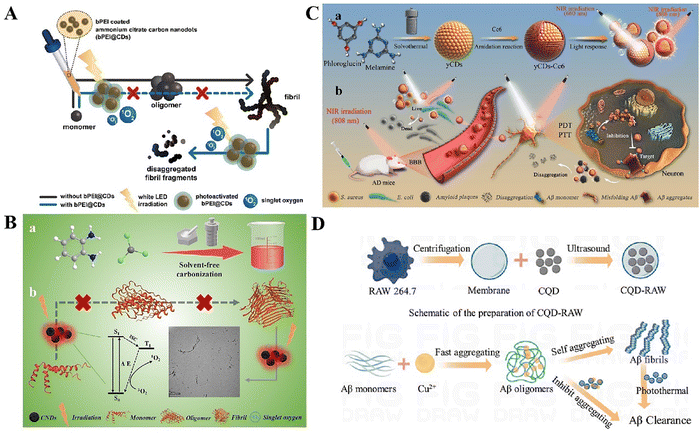
GQDs have potential in decreasing the buildup of amyloid plaques and alleviating their harmful impact on cells.107 The capacity of GQDs to traverse the blood–brain barrier (BBB) is of specific significance, given its potential use in addressing neurodegenerative conditions like AD. They have the ability to traverse the BBB either by passive diffusion or by using glucose transporters.100 Furthermore, GQDs, generated by molecules with the ability to traverse the BBB, show promise as an effective means of delivering drugs to the brain. Liu et al. discovered that the Aβ peptide binding to GQDs led to the creation of aggregates with dimensions varying from 100 to 900 nm.108 The aggregation process is primarily controlled by the mutual attraction of electrostatic forces, resulting in the formation of an interconnected network structure (Fig. 2A). Nevertheless, this configuration lacks stability and ultimately deteriorates entirely, thereby preventing any further accumulation of Aβ peptides. Xiao's research team conducted an experiment in which they successfully merged GQDs with the neuroprotective peptide glycine-proline-glutamate (GQDG).109 GQDs have the ability to inhibit the aggregation of Aβ peptides and provide protection to the nervous system. Tang et al. examined the capacity of GQDs to disrupt membrane-induced Aβ aggregation in three primary forms: monomer (Aβ-M), oligomeric (Aβ-O), and fibril (Aβ-F).110 GQDs have been found to efficiently bind with Aβ and mitigate peptide aggregation, as demonstrated by discrete molecular dynamics simulations. This study suggests that GQDs might be promising therapeutic agents for AD (Fig. 2B). GQDs have the ability to interfere with the membrane axis where β-amyloid (Aβ) collects, resulting in a decrease in the harmful effects associated with AD. Tak et al. employed a microwave-assisted one-pot technique to synthesize GQDs from Clitorina factories.111 The findings demonstrated a substantial inhibition of acetylcholinesterase (AChE) activity by GQDs, accompanied with elevated levels of glutathione and protein, and decreased levels of lipid peroxide and nitric oxide (Fig. 2C). In addition, research conducted using the Morris water maze showed that GQDs were more efficient in decreasing the time it took to reach the concealed platform compared to donepezil alone. The radial arm maze test assessed the impact of ctGQDs on the animals’ working memory, revealing that ctGQDs dramatically decreased the time required for the mice to locate the bait arm throughout a span of 7 days. These findings indicate that GQDs have the capacity to improve cognitive functions.
 | ||
| Fig. 2 (A) Schematic representation of the GQDs used for inhibiting the aggregation of Aβ1–42 peptides. Reproduced with permission.108 Copyright 2015, The Royal Society of Chemistry. (B) Interactions between Aβ-o with a graphene quantum dot. Reproduced with permission.110 Copyright 2022, Royal Society of Chemistry (RSC). (a) Initial structure of the simulation systems. A preformed Aβ tetramer was placed near the GQD. (b) Time evolution of the β-sheet content. Aβ-o: a preformed Aβ tetramer. Coaggregation: four Aβ-m with the GQDs. (c) Secondary structure propensities of Aβ after the simulations reached the steady state. (d) β-Sheet propensity of each Aβ residue in the absence and presence of the GQD. (e) Intra- and inter-peptide contact frequency maps for Ab peptides. (f) Changes in the contact frequency maps in the presence of GQDs compared with the control. (C) Schematic representation of graphene quantum dots (GQDs) synthesized from clitoris flowers using one-pot microwave-assisted green synthesis for the treatment of Alzheimer's disease. Reproduced with permission.111 Copyright 2020, American Chemical Society. (D) (a) Changes in the ratio between Aβ1–42 monomers and fibrils are shown. (b) Proposed mechanism for the different fluorescent behaviors of GQDs on Aβ1–42 monomers and fibrils. Reproduced with permission.112 Copyright 2022, The Royal Society of Chemistry. | ||
In the realm of amyloid detection, which is as critical as inhibiting amyloid aggregation for preventing amyloidogenesis, GQDs have shown dual functionality. Huang et al. used the fluorescence characteristics of graphene quantum dots (GQDs)130 to create a novel method for identifying individual β-amyloid (Aβ) peptide molecules.112 An accurate and dependable detection technique was developed, wherein the photoluminescence (PL) intensity of GQDs was directly correlated with the quantity of Aβ monomer. Conventional fluorescent dyes, such thioflaxanthin T (ThT), often need incubation with amyloid peptides (Fig. 2D). However, these dyes impede the aggregation process of amyloid peptides due to their inhibitory impact. Nevertheless, research conducted by Huang et al. showed that comparable rates of Aβ aggregation could be detected utilizing GQDs and ThT, even without the requirement of co-incubation with soluble amyloid peptide monomers. This discovery affirms the soundness of the detection approach based on graphene quantum dots (GQDs). Innocent et al. examined the effectiveness of platinum-doped carbon quantum dot (Pt@CQD) nanomaterials and their metal surface modifications (Co_Pt@CQDs, Fe_Pt@CQDs, and Mn_Pt@CQDs) in adsorbing nitrotyrosine (NTS), a biomarker associated with AD.113 The density functional theory (DFT) simulation findings indicate that the NTS_Mn_Pt@CQD system exhibits the highest performance in terms of NTS adsorption.
Graphene quantum dots exhibit significant potential for the detection of amyloid monomers and fibrils as a novel probe. They are highly effective in detecting and diagnosing degenerative illnesses and finding other structural anomalies. The application of GQD-based detection presents a viable avenue to improve our comprehension and treatment of protein misfolding and aggregation-related diseases, owing to its non-invasive and sensitive attributes.
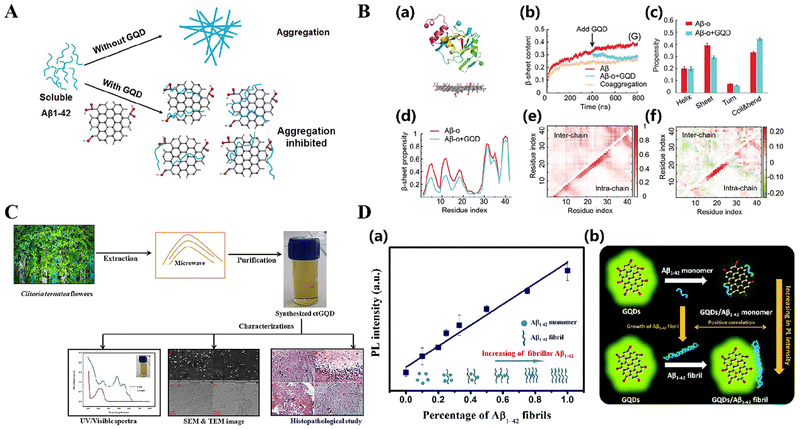
Epicatechin gallate (ECGC) is a natural polyphenolic compound sourced from green tea, noted for its substantial antioxidant and anti-inflammatory properties, and is a well-established inhibitor of amyloid beta (Aβ).122 Lin et al. initially devised a hydrothermal method for creating EGCG-derived carbon polymers (E-CPDs).123 The efficiency of E-75 CPDs in quickly depolymerizing Aβ fibrils surpasses that of any other known depolymerizing agents (Fig. 3A). The arrangement of aromatic compounds on the surface of E-CPDs, together with the bonding between hydroxyl and carboxyl groups and Aβ through hydrogen bonding, electrostatic interactions, and hydrophobic contacts, play a crucial role. The enhanced fluorescence emission observed upon binding of E-75 CPDs to Aβ fibrils demonstrates its significant potential as a fluorescent probe for Aβ (Fig. 3B).
 | ||
| Fig. 3 (A) Schematic illustration of the multifunctionality of E-CPDs. Reproduced with permission.123 Copyright 2023, Elsevier. (B) Fluorescence detection of Aβ40 aggregates by 75E-CPDs. Reproduced with permission.123 Copyright 2023, Elsevier. (a) Fluorescent spectra of (black) 50 μg mL−1 and (red) 100 μg mL−1 75E-CPDs. (b) Fluorescent spectra of 75E-CPDs upon mixing with different concentrations of Aβ40 aggregates. The concentration of 75E-CPDs was 50 μg mL−1. Excitation wavelength, 370 nm. Emission wavelength, 450nm. (C) Schematic representation of the synthesis of CPDs from o-phenylenediamine and nitric acid. Reproduced with permission.124 Copyright 2020, John Wiley and Sons. (D) Schematic representation of photosensitizer-doped carbonized polymer spots (PS-CPDs) inhibiting Aβ aggregation by photooxygenation. Reproduced with permission.125 Copyright 2023, American Chemical Society. | ||
Nitrogen-doped carbonized polymer dots (N-CPDs) are a newly developed group of fluorescent nanomaterials created using hydrothermal synthesis. Gao et al. successfully hindered the aggregation of Aβ monomers and decelerated the formation of Aβ fibrils using N-CPDs.124 This was achieved through a range of interactions, including as electrostatic forces, hydrogen bonding, and hydrophobic effects (Fig. 3C). Even in little quantities, N-CPDs significantly inhibit the aggregation of Aβ40, demonstrating its efficacy in altering the pathways that produce amyloid linked with AD. In the treatment area of cancer and neurological diseases such as AD, photooxygen scavenging drugs are used to specifically target and eliminate abnormal organization or misfolded protein clusters. An example of this is the treatment of AD, where Lin and colleagues developed carbonized polymer dots (MB-CPDs) doped with methylene blue,125 which may generate reactive oxygen species (ROS) when exposed to near-infrared light irradiation (Fig. 3D). The percentage of cells surviving exposure to MB-CPDs has been observed to increase significantly from 50% to a staggering 83%. This highlights the remarkable ability of this innovative therapeutic strategy to protect cells and remove light-generated oxygen.
While carbonized polymer spots that are doped with photosensitizers, like MB-CPDs, exhibit a high level of photooxygenation, their intrinsic photostability and potential dark toxicity may impose restrictions on their use in living organisms.125 The metabolism and removal of these photosensitizers in biological systems provide issues that need careful consideration. While CPDs has demonstrated the capacity to hinder Aβ aggregation, disassemble Aβ fibrils, and enhance fluorescence imaging, these capabilities may need more refining to enhance treatment efficacy and specificity. Currently, the use of CPDs in the treatment of AD is primarily in the experimental phase, and there is insufficient preclinical and clinical data to substantiate its safety and effectiveness.
This section focuses on the increasing function of zero-dimensional carbon-based nanomaterials, including as carbon dots, carbonized polymer dots, and quantum dots (QDs), in preventing the aggregation of β-amyloid (Aβ), breaking down existing fibrils, and reducing cytotoxicity. These materials adhere to Aβ by engaging in a sequence of interactions, such as electrostatic forces, hydrogen bonds, hydrophobic interactions, and chiral recognition, resulting in therapeutic benefits. Furthermore, these nanoparticles composed of carbon have optical characteristics, including the ability to emit fluorescence. This feature renders them promising for the imaging and diagnosis of Aβ plaques.
These findings lay the foundation for the development of new and creative treatment approaches for AD and emphasize the potential uses of carbon-based nanomaterials in medicine. The versatile properties of these nanomaterials, together with their capacity to engage with biological systems, signify a new model in the treatment and monitoring of neurodegenerative disorders such as AD.
3.2. One-dimensional carbon-based nanomaterials
One-dimensional carbon-based nanomaterials refer to carbon materials that possess a linear or fibrous structure along a single dimension. These materials frequently have notable electrical mobility, thermal conductivity, and mechanical strength. Nanomaterials with a one-dimensional structure are categorized based on their shape as nanorods (NRs), nanotubes (NTs), nanowires (NWs), self-assembling nanomaterials, and other variations. Due to their unique characteristics of large surface area and ability to conduct electricity, they have great promise for use in several biological applications, including medication delivery,130 biosensors,131 and gene therapy.132,133Ascorbic acid (AA) is essential for the physiological and pathological processes associated with brain illnesses. Nevertheless, the challenge of accurately identifying AA levels in the brain has impeded the comprehension of its exact importance. In order to tackle this issue, Zhang et al. developed a carbon nanotube (Q) fiber that functions as a small-scale sensor capable of detecting the concentration of AA in the brains of rats with AD with great sensitivity and specificity.137 This fiber enhances sensitivity and selectivity by facilitating the oxidation of AA at extremely low potentials, while efficiently mitigating interference from other constituents in the brain (Fig. 4A). Berberine is a versatile natural chemical that possesses antioxidant, anti-inflammatory, and antiplatelet properties. It has demonstrated potential as a treatment drug for AD. Nevertheless, the efficacy of these treatments can be hindered by inadequate absorption, restricted bioavailability, and challenges in crossing the blood–brain barrier (BBB). In order to address these constraints, Lohan et al. developed a novel approach for drug delivery by utilizing multi-walled carbon nanotubes (thermally fused) as carriers.138 This approach enhances the bioavailability and blood–brain barrier permeability of BRB. The results of the Morris water maze experiment indicated that BRB-MWCNTs treated animals showed better memory performance in the task of finding a hidden platform compared to the administration of the drug alone.
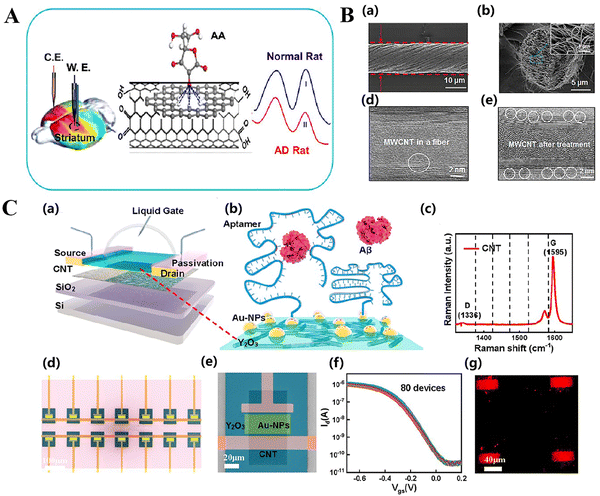 | ||
| Fig. 4 (A) Novel directional carbon nanotube (CNT) fibrils have been used as an accurate microsensor to measure the ratio of AA levels in the brain of Alzheimer's disease (AD) rats. Reproduced with permission.137 Copyright 2017, American Chemical Society. (B) SEM images of a CNF from a side (a) and end view (b). (c) STEM image of the MWCNT in a CNF. (d) STEM image of the MWCNT in a CNF after heating at 200 °C. Reproduced with permission.137 Copyright 2017, American Chemical Society. (C) Fabrication and characterization of the CNT FET biosensor. Reproduced with permission.139 Copyright 2022, American Chemical Society. (a) Schematic diagram of an aptamer-functionalized CNT FET biosensor for the detection of AD serum biomarkers. (b) Illustration of aptamer probe immobilization onto FG using a typical Au linker, followed by hybridization with Aβ peptides. (c) Raman image of sorted CNTs. (d) Scanning electron microscopy (SEM) image of the CNT FET biosensor array. The scale bar is 100 μm. (e) SEM image of the core reactive region of a biosensor. The scale bar is 20 μm. (f) Transfer curves of 80 CNT FETs (using a liquid gate) after the deposition of Au nanoparticles, Vds = −0.1 V. (g) Fluorescence image of a Cy5-labeled Aβ42 aptamer hybridized to Au-NPs immobilized on a CNT FG FET channel surface. | ||
Chen et al. created a biosensor by modifying a carbon-nanotube field-effect transistor (FET) with an oligonucleotide aptamer.139 This biosensor has the capability to identify β-amyloid (Aβ) present in serum, which is a crucial biomarker linked to AD (Fig. 4C). The sensor effectively gathered polypeptide targets and identified conformational changes with exceptional sensitivity in both phosphate buffer (PBS) and stock solution. It is able to detect as little as 50 atomoles (aM), which is rather astounding. The biosensor employs carbon nanotube field effect transistors (CNT FETs) that are functionalized with aptamers. This approach offers a cost-effective and fast detection technique suitable for clinical applications. It is appropriate for the early detection and widespread testing of AD.
Despite the great potential of CNTs in medical therapy, there are several unresolved inquiries concerning their nanotoxicological characteristics and their ecological ramifications. Additional research is required to examine the prolonged impacts of CNTs on human health and the environment, as their biodegradability is restricted.
Peng et al. have created a novel electrochemiluminescence (ECL) immunosensor that is intended for the precise and very sensitive identification of β-amyloid oligomers (Aβos), which are a crucial biomarker for AD.141 This immunosensor utilizes a PdPtB nano-strengthening agent and SiC@Au-PEDOT nanowires to improve the sensor's performance. The sensor functions under situations that do not necessitate external total reactants. The method utilizes a dual antibody sandwich approach to provide highly specific detection of Aβ oligomers. Under optimum circumstances, the ECL sensor has a remarkable linear response range spanning from 20 picomolar (pM) to 20 nanomolar (nM), and it has a detection limit of 10 pM. The aforementioned qualities indicate that the sensor has considerable promise for clinical applications, serving as a promising instrument for the timely detection and tracking of AD.
In the field of biomedicine, carbon nanowires possess several advantages including efficient drug delivery, multifunctional imaging properties, applications in photothermal therapy, antibacterial properties, and low toxicity after functionalization. These unique properties make carbon nanowires a promising biomedical material that offers high-resolution and sensitive diagnostic information while playing a crucial role in non-invasive therapies. However, the application of carbon nanowires also encounters challenges and disadvantages such as high production costs, concerns regarding biosafety and environmental impacts, complex functionalization methods, as well as technical difficulties in large-scale production and integration processes. These issues necessitate continuous research and technological innovation to enable diverse applications of carbon nanowires in the field of biomedicine. Although there is limited research on the utilization of carbon nanowires for Alzheimer's disease treatment and diagnosis at present, we anticipate that future researchers will recognize its exceptional properties and apply it within this domain.
3.3. Two-dimensional carbon-based nanomaterials
Carbon-based nanomaterials in the form of two-dimensional (2D) structures are considered to be at the forefront of material science. These structures can range in thickness from a single atomic layer to many layers of carbon atoms. They have a very small size in the direction that is perpendicular to the carbon plane, usually measured in nanometers. The distinctive electrical configuration, extraordinary mechanical characteristics, and remarkable chemical durability of these substances have attracted considerable interest in the scientific community.142In the field of AD detection and therapy, 2D carbon nanomaterials, such as graphene, provide several possibilities. Their large surface area and flexibility to change their surface chemistry make them well-suited for creating tailored drug delivery systems, which might improve the effectiveness of medical treatments.143 Moreover, using 2D carbon materials into biosensors is expected to enhance the identification of biomarkers associated with AD, increasing both sensitivity and specificity. This is essential for enabling timely and precise diagnoses.144
The utilization of 2D carbon nanomaterials in AD research and clinical treatment is a rapidly growing field with significant promise. It has the potential to greatly enhance our understanding and control of this complex neurological disorder. As our research efforts progress, these materials have the potential to stimulate significant advancements in diagnostic procedures, treatment tactics, and preventative measures for AD.
Graphene's possible neuroprotective benefits are particularly intriguing in the context of AD. Ongoing research is being conducted to investigate if graphene can shield neurons from degeneration linked to the illness, potentially by utilizing its antioxidant capabilities or by regulating biological processes. Li et al. have devised a novel heat treatment technique utilizing graphene oxide (GO), where Thioflavin T (TSH) is chemically linked to GO to create GO-TSH.147 This alteration allows GO to selectively attach to A beta (Aβ) aggregations. The GO-TSH-Aβ complex, when exposed to a high-intensity near-infrared (NIR) laser, produces heat in a specific area, causing the Aβ fibrils to break down. Li et al. have created a biosensor called graphene electrolyte-gated transistor (G-EGT) to detect biomarkers of AD.148 More precisely, the sensor is engineered to identify the extracellular Aβ42 (NDE-Aβ42) in serum, which is a crucial biomarker for AD (Fig. 5A). The sensor has exhibited an impressive sensitivity of 447 attograms per milliliter (ag mL−1) for detecting the Aβ42 peptide in phosphate-buffered saline (PBS), representing a noteworthy breakthrough in AD diagnosis (Fig. 5B). This method presents a promising non-intrusive technique for breaking down amyloid plaques linked to AD. Yang et al. conducted a study on the utilization of graphene and graphene oxide (GO) nanoparticles to successfully prevent the production of fibrils and eliminate pre-existing Aβ amyloid fibrils.149 Through the use of molecular dynamics simulations (Fig. 5C), researchers discovered that the powerful dispersion interactions between the graphene nanoparticles and the peptides, as well as the π–π stacking effect between the aromatic residues of Aβ peptides and the graphene surface, play a crucial role in destabilizing amyloid fibrils (Fig. 5D). Wang et al. proposed an exceptionally sensitive surface-enhanced Raman spectroscopy (SERS) technique for the detection of Aβ1–42 utilizing graphene oxide/gold nanoparticles (GO/AuNPs).150 Gold nanoparticles were coated on the graphene oxide surface using in situ reduction to create high-density hotspots for surface-enhanced Raman scattering. The detection limits for Aβ1–42 monomers and fibrils were 0.0232 ng mL−1 and 0.0192 ng mL−1, respectively. Furthermore, support vector machine (SVM) and one-dimensional convolutional neural network (1DCNN) methods were employed to differentiate samples based on varying fibril degrees. SERS has significant potential for label-free diagnosis and early detection of Alzheimer's disease, presenting intriguing opportunities for biological detection.
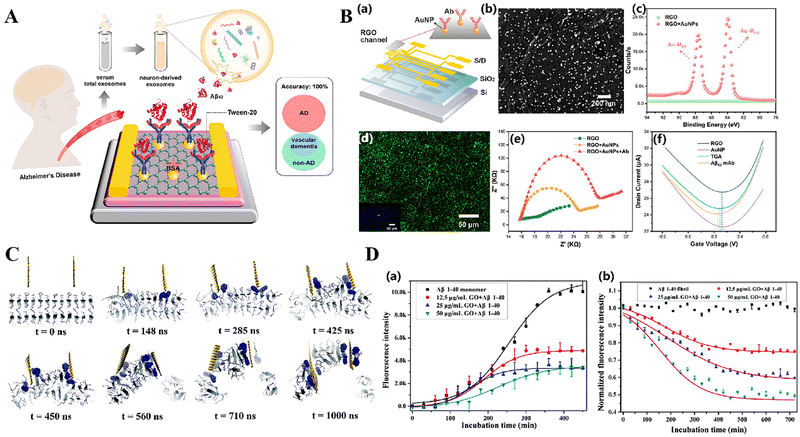 | ||
| Fig. 5 (A) Schematic illustration of the G-EGT biosensor for the detection of NDE-Aβ42 in Alzheimer's disease patients. Reproduced with permission.148 Copyright 2023, American Chemical Society. (B) Characterization of the G-EGT biosensor. Reproduced with permission.148 Copyright 2023, American Chemical Society. (a) Geometric diagram of the device. (b) SEM image of AuNPs deposited on the RGO surface. (c) XPS spectra of the Au element obtained after AuNP decoration. (d) Fluorescence microscopy image of the anti-Aβ42-functionalized device treated with the FITC-labeled anti-IgG. (e) EIS Nyquist plots of bare RGO, AuNP deposition, and specific Ab immobilization. (f) Id–Vg curves of the G-EGT biosensor in the stepwise modification. (C) Graphene nanosheet penetration and Aβ peptide extraction. Reproduced with permission.149 Copyright 2015, The Royal Society of Chemistry. The Aβ peptides (consisting of a total of 24 monomers) are shown in a cartoon representation, with the two aromatic phenylalanine residues shown in dark blue sticks. The graphene sheet is shown as an orange sheet. Extracted peptides are highlighted with their phenylalanine residues depicted in larger van der Waals spheres. (D) Kinetics of monomer fibrillation and fibril dissociation with or without GO. Reproduced with permission.149 Copyright 2015, The Royal Society of Chemistry. The dynamic processes of Aβ1–40 monomer fibrillation (a) and Aβ1–40 amyloid fibril dissociation (b) in the presence and absence of GO at different concentrations. The results indicate that GO nanosheets cannot only effectively inhibit the aggregation of Aβ monomers, but also dissociate and clear pre-formed Aβ amyloid fibrils. | ||
Nadiyeh et al. have presented a thorough overview of the latest progress in biosensing techniques that utilize graphene and its derivatives to detect biomarkers associated with Alzheimer's disease.144 Their research highlights the capability of these materials to improve the sensitivity, selectivity, and reliability of diagnostic instruments, therefore aiding in the early and precise identification of AD.
The studies and utilization of graphene and its derivatives in the scientific and therapeutic applications of AD is a rapidly developing and expanding area. As our understanding of these substances grows, it is anticipated that they will play an important role in the development of diagnostic tools, treatment approaches, and prevention measures for AD and other neurological disorders.
Diagnostic indicators 169 have been discovered in the exhaled breath of AD patients, namely in the form of volatile organic compounds (VOCs).154 Narender et al. examined the efficacy of titanium carbide MXenes, namely Ti3C2Tx and Ti2CTx, where Tx denotes oxygen or sulfur, as nanobiosensors for detecting biomarkers linked to AD.155 The calculations were performed using density functional theory (DFT) and first-principles approaches. The adsorption characteristics of these MXenes were assessed for three common volatile organic compounds (2,3-dimethylheptane, butylhydroxytoluene, and propionic acid) and four potentially obstructive air molecules (N2, O2, CO2, and H2O) (Fig. 6A). The thermodynamic investigations, employing the Langmuir adsorption model, confirmed the excellent adsorption capacity of Ti3C2O2 across a broad spectrum of BHT concentrations. These findings suggest that Ti3C2O2 is highly suitable as a nanobiosensor due to its exceptional sensitivity and selectivity in detecting volatile organic compounds (VOCs) linked to AD.
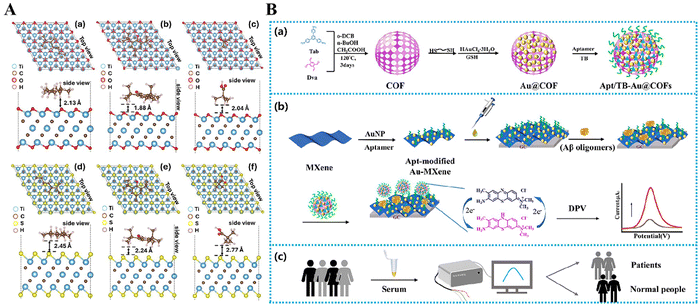 | ||
| Fig. 6 (A) (a)–(f) Relaxed configurations of three VOCs related to lung cancer biomarkers, (a) and (d) 2,3 dimethylheptane (23-DMH), (b) and (e) butylated hydroxytoluene (BHT), and (c) and (f) pivalic acid (PVA), on thick Ti3C2Tx MXenes (with Tx = O and S). All VOCs are shown to exhibit strong physisorption types of interactions. Reproduced with permission.155 Copyright 2024, American Chemical Society. (B) (a) Scheme of the synthesis process of the Apt/TB-Au@COFs. (b) Illustration of electrochemical determination of Aβ1–42 oligomers with the Au-MXene substrate and the Apt/TB-Au@COFs. (c) Clinical practical applications. Reproduced with permission.156 Copyright 2024, Elsevier. | ||
Lv et al. created an exceptionally responsive electrochemical biosensor to identify β-amyloid oligomers (specifically, Aβ1–42 oligomers), which serve as a biomarker for AD.156 This sensor utilizes a MXene substrate and a covalent organic framework (COF) probe to enhance the sensitivity and selectivity of detection using a twofold amplification approach. This development showcases the potential of Mxene-based materials in producing very sensitive biosensors for the swift and accurate identification of AD (Fig. 6B).
The current study on MXenes mostly revolves around evaluating their biocompatibility, biodegradability, and the potential long-term impacts they may have on human health and the environment. As the science progresses, it is expected that MXenes will play a crucial role in the diagnostic tools and treatment choices for AD and other neurological illnesses.
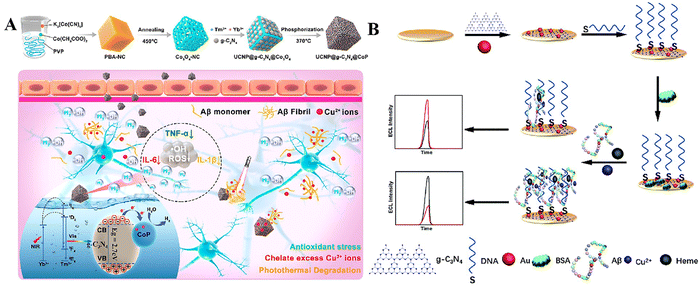 | ||
| Fig. 7 (A) U-CN/CoP synthesis and action mechanism. Reproduced with permission.157 Copyright 2023, American Chemical Society. (B) Schematic illustration of the competitive dual-mechanism-driven ECL biosensor for Aβ. Reproduced with permission.158 Copyright 2022, Royal Society of Chemistry (RSC). | ||
Chen et al. developed a sensor that utilizes a dual mechanism, combining a synergistic enhancing effect with a steric hindrance effect.158 Researchers have utilized g-C3N4 as the luminescence framework to achieve high-efficiency electrochemiluminescence (ECL) (Fig. 7B). They have effectively attached gold (Au) nanoparticles with sulfhydryl-modified aptamers onto the surface of a gold (Au) electrode in order to capture Aβ peptides. The ECL aptamer sensor is an advanced device particularly engineered to identify β-amyloid peptides (Aβ) that are linked to AD. The sensor has high sensitivity and possesses significant potential for field diagnostics.
When utilized in a regulated manner, the photocatalytic activity of g-C3N4 presents a new and innovative therapy approach for AD. Through the process of scavenging reactive oxygen species (ROS), g-C3N4 has the potential to shield neurons from oxidative damage, a crucial factor in the development of AD. This study highlights the potential of g-C3N4 as a photocatalytic agent in the creation of new treatment methods for AD. It serves as a foundation for further investigation into its therapeutic benefits and mechanisms of action in neurodegenerative illnesses.
3.4. Other carbon-based materials
Composite carbon-based materials are a category of versatile materials created by combining carbon-based materials with additional elements or compounds. These composites utilize the remarkable properties of carbon, such as its strong electrical conductivity, thermal conductivity, and chemical stability. Furthermore, they integrate the unique characteristics of the supplementary components or compounds, giving them a range of improved features. Composite carbon materials have several advantages and are suitable for a wide range of applications due to their production and utilization. Specifically, within the framework of Alzheimer's disease, these substances have demonstrated promise in both the identification and treatment aspects, hence aiding in the progress of the discipline.The foray of nanotechnology into the realm of diagnostics is groundbreaking. Chang et al. detail the creation of an advanced biosensor that utilizes the combined influence of graphene oxide (GO) and gold nanostars (GNSs) particularly for the identification of AD.159 This sensor confirms that miRNA-137 is a particular biomarker for AD. It does this by using a highly accurate and sensitive detection technique that relies on the binding of miRNA-137 to its complimentary sequence. Nair et al. conducted a study on the use of graphene-coated gold nanoellipsoids as plasmonic biosensors. They found that these biosensors had a high sensitivity to the fibrillation phase of amyloid beta protein, which is a significant biomarker for AD.160 The inclusion of nanomaterials enhances both the sensitivity and cost-effectiveness of these sensors, allowing scalable manufacture and widespread applications. Composites have demonstrated equivalent efficacy in the therapeutic domain. Composites tackle the complex pathophysiology of AD by employing a multi-faceted strategy that includes anti-inflammatory, anti-oxidative, and suppression of amyloid aggregation pathways. Wang et al. introduced a nano-formulation of Dauricine (Dau) enclosed in GO, which resulted in diverse therapeutic benefits on AD following nasal delivery.161 The combination of Dau's ability to regulate inflammation and oxidative stress, together with GO's capacity to block aberrant aggregation of amyloid, makes this nanoformulation very effective in preventing Alzheimer's disease in both laboratory and living organism settings. Composite materials have proven to be useful not only in providing relief from symptoms, but also in the areas of neuroprotection and regeneration. The work conducted by Fariba et al. introduced ultra-small particles composed of chitosan/graphene quantum dots (CS/GQDs) as prospective diagnostic agents for AD. These particles were chosen not only for their capacity to carry drugs, but also for their intrinsic therapeutic properties.162 The intranasal particles possess a notable capacity to traverse the blood–brain barrier, so enabling focused treatment for the brain. CS/GQD therapy in animal models of AD demonstrated a substantial enhancement in memory function, underscoring its neuroprotective efficacy. Composite nanomaterial technology has successfully breached the blood–brain barrier. Li et al. detailed the creation of biomimetic nanomedicine by the incorporation of aspirin curcumin ester (CA). The targeting capability of graphene oxide quantum dots (GOQDs) and RBC-MIC hybrid membrane was utilized to control the polarization of M1/M2 microglia in a mouse model of AD.163 Therefore, the cognitive impairments were enhanced. Composite materials enhance the drug's capacity to cross the blood–brain barrier via nanotechnology, which is essential for treating central nervous system disorders like AD. Both CS/GQD microparticles and biomimetic nanomedicals have shown this benefit.
The combined results of these investigations highlight the many possibilities of composite materials in the fields of diagnosis and treatment for AD. The advancement in composite material design offers a wide range of novel tactics for the early detection and effective treatment of AD, ranging from very sensitive biosensors to multifunctional nanotherapeutic agents. Table 1 delineates the many carbon-based nanomaterials utilized in the diagnosis and treatment of Alzheimer's disease. The convergence and innovation of material science and biotechnology are expanding the scope of composite material applications in AD therapy, providing hope to patients and caregivers.
| Name | Application | Methods | Size/nm | Effects | Ref. | |
|---|---|---|---|---|---|---|
| 0D | bPEI@CDs | Treatment | One-pot hydrothermal treatment | 4.4 ± 1.0 | Inhibit Aβ self-assembly, disassemble aggregates, enhance light-induced suppression, reduce cytotoxicity | 88 |
| L-Lys-C-dots | Treatment | Single-step carbonization | ∼4.0 | Inhibited Aβ42 random coil-to-β-sheet transformation, reduced cytotoxicity, affected membrane interactions | 89 | |
| D-Lys-C-dots | ||||||
| CDs | Treatment | Solvent-free carbonization | ∼6.0 | Inhibition of amyloid aggregation and produce 1O2 for photooxidation | 90 | |
| yCDs-Ce6 | Treatment | Coassembly through acylation reaction | 37.2 (yCDs-Ce6) | Inhibit Aβ self-assembly, disassemble aggregates, reduce cytotoxicity, prevent microbial growth, and enhance BBB permeability | 91 | |
| N-CDs | Treatment | One-pot solvothermal method | 2.2 | Inhibit Aβ self-assembly, disassemble aggregates, reduce cytotoxicity | 97 | |
| CQDs | Treatment | Controlled oxidation followed by filtration | 2.8 | Inhibit aggregation of IAPP and Aβ, mitigate toxicity, cross the BBB | 98 | |
| CQD-RAW | Treatment | Solvothermal synthesis | 5.0 | Regulates metal ion homeostasis, enhances BBB permeability, and photothermal properties | 92 | |
| GQDs | Treatment | Hydrothermal synthesis | 8.0 | Inhibit the aggregation of Aβ1–42 peptides, reduce Aβ1–42-induced cytotoxicity | 108 | |
| GQDs | Treatment | Modified Hummers' method | ∼18 | Inhibit aggregation and reduction of inflammation | 109 | |
| GQDs | Treatment | Purchase of hydroxylated GQDs | 11.4 ± 3.5 | Block the membrane axis of Aβ and reduce Aβ aggregation | 110 | |
| ctGQDs | Treatment | One-pot microwave-assisted green synthesis | 10 ± 1.3 | Reduce acetylcholinesterase activity, augment antioxidant capacity | 111 | |
| GQDs | Diagnosis | Oxidation cutting followed by synthesis | 5.1 ± 0.3 (lateral size), 2 nm ± 0.2 (height) | Photoluminescence detection of Aβ monomers | 112 | |
| E-CPDs | Treatment | Hydrothermal method | 1.9 ± 0.4 | Enhanced fluorescence, scavenged ROS and inhibitory effect | 123 | |
| CPDs | Treatment and diagnosis | One-pot hydrothermal method | 5.0 ± 1.6 | Red fluorescence and attenuated Aβ-induced toxicity | 124 | |
| MB-CPDs | Treatment | One-step hydrothermal method | 2.0–4.5 nm (average size 2.9 nm) | Inhibition of Aβ aggregation and generation of ROS | 125 | |
| N,P-MQDs | Diagnosis | One-pot hydrothermal method | 1–8 nm (average 2.93 nm) | Low detection limit | 127 | |
| BSA@MXene QDs | Diagnosis | Hydrothermal method | ∼2 | Excellent photophysical properties, stability and selective quenching | 128 | |
| C3N nanodots | Treatment | Hydrothermal synthesis | 4.5 ± 0.4 | Improved the behavioral defects of APP/PS1 AD mice | 164 | |
| 1D | Single-walled carbon nanotubes (SWCNTs) | Treatment | — | 0.8–1.2 nm in diameter and lengths of several microns | Avoid cytotoxicity through dose control | 136 |
| Single walled carbon nanotubes (SWNT) | Diagnosis | — | — | Specificity, low detection limit | 74 | |
| CNF | Diagnosis | Chemical vapor deposition (CVD) | ∼10 μm | Real-time quantitative detection | 137 | |
| BRB-C-MWCNT | Treatment | — | 125 ± 9–295 ± 5 nm | Enhances BBB permeability | 138 | |
| CNT FET biosensors | Diagnosis | — | — | Wide detection range | 139 | |
| SiC@Au-PEDOT nanowires | Diagnosis | — | 126 nm | The linear response ranges from 20 pM to 20 nM, and its detection limit is 10 pM | 141 | |
| 2D | GO-TSH | Treatment | — | — | Dissociate amyloid aggregation | 147 |
| G-EGT | Diagnosis | — | — | Sensitivity 447 μg mL−1 | 148 | |
| GO/AuNPs | Diagnosis | In situ reduction method | — | The detection limits for Aβ1–42 monomers and fibril were 0.0232 ng mL−1 and 0.0192 ng mL−1, respectively | 150 | |
| MXenes | Diagnosis | — | — | Exhaled breath analysis | 155 | |
| Apt/TB-Au@COFs | Diagnosis | — | — | The linear range was 0.01-180 pg mL−1, and the ultra-low detection limit was 4.27 fg mL−1 (S/N = 3) | 156 |
In summary, the many aspects of carbon-based nanomaterials provide several possibilities for increasing the diagnosis and treatment of Alzheimer's disease. Carbon-based nanomaterials, characterized by their diminutive dimensions, adjustable properties, extensive specific surface area, and plentiful surface-active groups, have been extensively investigated for nanomedicine applications, facilitating drug molecule penetration across the blood–brain barrier, disaggregation, and inhibition of amyloid proteins, thereby demonstrating significant potential in disease diagnosis. However, numerous problems must be addressed to enhance the therapeutic utilization of carbon-based nanomaterials in healthcare technologies. Targeting and in vivo metabolism are critical issues to address, while safety and toxicity remain primary concerns; a full study is necessary to ensure the biocompatibility and long-term safety of these materials. The advancement of scalable and repeatable synthetic methodologies is crucial for large-scale production and extensive clinical applications. Large-scale production techniques must be refined to guarantee the quality and uniformity of carbon-based nanomaterials in compliance with regulatory standards for clinical application. In the diagnosis and treatment of Alzheimer's disease (AD), we anticipate that more researchers will focus on the benefits of carbon-based nanomaterials, acknowledge the hurdles in their further applications, and surmount these limitations through novel approaches.
4. The challenge and prospect
The prevalence of Alzheimer's disease (AD) is increasing, resulting in a growing societal and economic impact. It is crucial to diagnose and treat the condition as soon as possible. Carbon-based nanomaterials have become promising platforms for both the diagnosis and treatment of AD. Nevertheless, there are certain obstacles that need to be overcome in order to pave the way for therapeutic applications. Research on biomarkers for Alzheimer's disease is advancing, with continuous efforts to discover and confirm new signs that might assist in detecting the disease at an early stage. Carbon-based materials, such as nanotubes and graphene, are highly appreciated for their unique physical, chemical, and biological compatibility. These candidates are regarded as promising for traversing the blood–brain barrier, facilitating precise medication administration, and providing neuroprotection. The promise of nanotechnology in treating Alzheimer's disease is emphasized by several research studies, specifically highlighting the advantages of carbon nanotubes (CNTs) and graphene (GDs) in penetrating the blood–brain barrier and providing tailored therapy. Carbon-based nanomaterials have the ability to improve medication delivery efficiency, reduce toxicity, and enhance therapeutic effectiveness.Carbon-based nanomaterials, although promising, have limits in their capacity to treat AD due to concerns about biosafety, biodegradability, and long-term consequences. Future research will focus on enhancing the physical and chemical characteristics of these nanomaterials and undertaking thorough safety and effectiveness assessments before they are used in clinical settings. Although several nanomedicines have shown promising anti-Alzheimer's disease benefits during the preclinical phase, the process of transitioning to clinical use is intricate and time-consuming.
In conclusion, despite the many challenges, it is anticipated that with further investigation into the pathogenesis of AD, coupled with advancements in nanotechnology and associated disciplines, nanotechnology will undoubtedly yield significant breakthroughs in targeted AD therapy.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported financially by the Key Research and Development Program of Shaanxi (2020GY-285, 2022GY-198 and 2023-YBGY-295).References
- 2024 Alzheimer's disease facts and figures, Alzheimer's Dementia, 2024, 205, 3708–3821 Search PubMed.
- G. Wang, China Alzheimer Report 2024, Journal of Diagnostics Concepts & Practice, 2024, 23(03), 219–256 Search PubMed.
- J. Hardy and D. Allsop, Amyloid deposition as the central event in the aetiology of Alzheimer's disease, Trends Pharmacol. Sci., 1991, 12, 383–388 CrossRef CAS.
- R. Coronel, et al., Role of Amyloid Precursor Protein (APP) and Its Derivatives in the Biology and Cell Fate Specification of Neural Stem Cells, Mol. Neurobiol., 2018, 55(9), 7107–7117 CrossRef CAS.
- S. Li, et al., Soluble Oligomers of Amyloid β Protein Facilitate Hippocampal Long-Term Depression by Disrupting Neuronal Glutamate Uptake, Neuron, 2009, 62(6), 788–801 CrossRef CAS.
- J. J. Palop and L. Mucke, Network abnormalities and interneuron dysfunction in Alzheimer disease, Nat. Rev. Neurosci., 2016, 17(12), 777–792 CrossRef CAS.
- R. L. Neve, et al., Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2, Mol. Brain Res., 1986, 1(3), 271–280 CrossRef CAS.
- P. Regan, et al., Physiological and Pathophysiological Implications of Synaptic Tau, Neuroscientist, 2016, 23(2), 137–151 CrossRef PubMed.
- P. Barbier, et al., Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects, Front. Aging Neurosci., 2019, 11, 204 CrossRef CAS.
- J. Kong, et al., Carbon Quantum Dots: Properties, Preparation, and Applications, Molecules, 2024, 29(9), 2002 CrossRef CAS PubMed.
- E.-J. Su, et al., Efficient Sorting of Semiconducting Single-Walled Carbon Nanotubes in Bio-Renewable Solvents Through Main-Chain Engineering of Conjugated Polymers, Small, 2024, 2403651 CrossRef CAS.
- H. Rasuli and R. Rasuli, Nanoparticle-decorated graphene/graphene oxide: synthesis, properties and applications, J. Mater. Sci., 2023, 58(7), 2971–2992 CrossRef CAS.
- S. Roy, Detection of fluoride ion by carbon dots-based fluorescent probes, J. Mol. Struct., 2025, 1319, 139465 CrossRef CAS.
- A. Selva Sharma and N. Y. Lee, Comprehensive review on fluorescent carbon dots and their applications in nucleic acid detection, nucleolus targeted imaging and gene delivery, Analyst, 2024, 149(16), 4095–4115 RSC.
- H. Yao, et al., Multifunctional Nanosystem Based on Ultrasmall Carbon Dots for the Treatment of Acute Kidney Injury, ACS Biomater. Sci. Eng., 2024, 10(8), 4970–4984 CrossRef CAS PubMed.
- V.-N. Nguyen, et al., Recent progress in organic carbon dot-based photosensitizers for photodynamic cancer therapy, Dyes Pigm., 2024, 230, 112359 CrossRef CAS.
- T. Bhattacharya, et al., Carbon Dots: Opportunities and Challenges in Cancer Therapy, Pharmaceutics, 2023, 15(3), 1019 CrossRef CAS PubMed.
- V. Schroeder, et al., Carbon Nanotube Chemical Sensors, Chem. Rev., 2019, 119(1), 599–663 CrossRef CAS.
- K. Luo, et al., Advances in carbon nanotube-based gas sensors: Exploring the path to the future, Coord. Chem. Rev., 2024, 518, 216049 CrossRef CAS.
- R. Yanikoglu, et al., Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate, Pharmaceutics, 2024, 16(6), 837 CrossRef CAS PubMed.
- R. Saharan, et al., Exploring graphene and its potential in delivery of drugs and biomolecules, J. Drug Delivery Sci. Technol., 2023, 84, 104446 CrossRef CAS.
- C. I. L. Justino, et al., Graphene based sensors and biosensors, TrAC, Trends Anal. Chem., 2017, 91, 53–66 CrossRef CAS.
- P.-J. Zhu, et al., Thermal Properties of Graphene and Graphene-Based Nanocomposites: A Review, ACS Appl. Nano Mater., 2024, 7(8), 8445–8463 CrossRef CAS.
- L. A. Rabin, et al., Subjective Cognitive Decline in Preclinical Alzheimer's Disease, Annu. Rev. Clin. Psychol., 2017, 13, 369–396 CrossRef.
- G. S. Zubenko, et al., A Collaborative Study of the Emergence and Clinical Features of the Major Depressive Syndrome of Alzheimer's Disease, Am. J. Psychiatry, 2003, 160(5), 857–866 CrossRef.
- M. Kalia, Dysphagia and aspiration pneumonia in patients with Alzheimer's disease, Metabolism, 2003, 52, 36 CrossRef CAS PubMed.
- S. A. Kent, et al., The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics, Acta Neuropathol., 2020, 140(4), 417–447 CrossRef CAS.
- P. Botella Lucena and M. T. Heneka, Inflammatory aspects of Alzheimer's disease, Acta Neuropathol., 2024, 148(1), 31 CrossRef.
- S. M. Firdous, et al., Oxidative stress–mediated neuroinflammation in Alzheimer’s disease, Naunyn-Schmiedeberg’s Arch. Pharmacol., 2024, 397, 8189–8209 CrossRef CAS.
- M. Babić Leko, et al., Metals in Alzheimer's Disease, Biomedicines, 2023, 1161 CrossRef PubMed.
- G. D. Rabinovici, et al., Testing and disclosures related to amyloid imaging and Alzheimer's disease: Common questions and fact sheet summary, Alzheimer's Dementia, 2016, 12(4), 510 CrossRef.
- C. Petrella, et al., Neuropeptides in Alzheimer's Disease: An Update, Curr. Alzheimer Res., 2019, 16(6), 544–558 CrossRef CAS PubMed.
- G. D. Rabinovici, et al., Testing and disclosures related to amyloid imaging and Alzheimer's disease: Common questions and fact sheet summary, Alzheimer's Dementia, 2016, 12(4), 510–515 CrossRef.
- O. Klementieva, et al., Pre-plaque conformational changes in Alzheimer's disease-linked Aβ and APP, Nat. Commun., 2017, 8(1), 14726 CrossRef CAS.
- S. J. C. Lee, et al., Towards an understanding of amyloid-β oligomers: characterization, toxicity mechanisms, and inhibitors, Chem. Soc. Rev., 2017, 46(2), 310–323 RSC.
- T.-P. V. Huynh and D. M. Holtzman, In Search of an Identity for Amyloid Plaques, Trends Neurosci., 2018, 41(8), 483–486 CrossRef CAS.
- S.-M. Wang, et al., Plasma oligomer beta-amyloid is associated with disease severity and cerebral amyloid deposition in Alzheimer's disease spectrum, Alzheimer's Res. Ther., 2024, 16(1), 55 CrossRef CAS.
- B. Nizynski, et al., Amyloidogenesis of Tau protein, Protein Sci., 2017, 26(11), 2126–2150 CrossRef CAS.
- D. Di Lorenzo, Tau Protein and Tauopathies: Exploring Tau Protein-Protein and Microtubule Interactions, Cross-Interactions and Therapeutic Strategies, ChemMedChem, 2024, e202400180 CrossRef CAS.
- A. Bejanin, et al., Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease, Brain, 2017, 140(12), 3286–3300 CrossRef.
- S. Muralidar, et al., Role of tau protein in Alzheimer's disease: The prime pathological player, Int. J. Biol. Macromol., 2020, 163, 1599–1617 CrossRef CAS PubMed.
- K. C. Gough and B. C. Maddison, Prion transmission: prion excretion and occurrence in the environment, Prion, 2010, 4(4), 275 CrossRef CAS PubMed.
- T. Kimura, et al., Novel aspects of the phosphorylation and structure of pathological tau: implications for tauopathy biomarkers, FEBS Open Bio, 2024, 14(2), 181–193 CrossRef CAS.
- E. Leveille, et al., Tau and MAPT genetics in tauopathies and synucleinopathies, Parkinsonism Relat. Disord., 2021, 90, 142–154 CrossRef CAS PubMed.
- M. I. Diamond, Travels with tau prions, Cytoskeleton, 2024, 81(1), 83–88 CrossRef CAS PubMed.
- C. L. Sayas, in Tau-based therapies for Alzheimer's disease: Promising novel neuroprotective approaches, Neuroprotection in Autism, Schizophrenia and Alzheimer's Disease, ed. I. Gozes, J. Levine, Academic Press, 2020, ch. 10, pp. 245–272 Search PubMed.
- R.-R. Lin, et al., Advancing the battle against Alzheimer's Disease: A focus ontargeting tau pathology by antisense oligonucleotide, Innovation Med., 2023, 1(2), 100020 CrossRef.
- C. Monteiro, et al., Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet, Neurology, 2023, 101(14), e1391–e1401 CrossRef CAS.
- L. Ferrucci and E. Fabbri, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nat. Rev. Cardiol., 2018, 15(9), 505–522 CrossRef CAS PubMed.
- G. Tezcan, et al., Resolution of chronic inflammation and cancer, Periodontology 2000, 2024, 1–21 Search PubMed.
- D. Artemniak-Wojtowicz, et al., Obesity and chronic inflammation crosslinking, Cent. Eur. J. Immunol., 2020, 45(4), 461–468 CrossRef CAS.
- C. Langworth-Green, et al., Chronic effects of inflammation on tauopathies, Lancet Neurol., 2023, 22(5), 430–442 CrossRef CAS PubMed.
- D. Zhao, et al., A dual-targeted multifunctional nanoformulation for potential prevention and therapy of Alzheimer’s disease, Innovation, 2021, 2(4), 100160 CAS.
- I. G. Onyango, et al., Neuroinflammation in Alzheimer’s Disease, Biomedicines, 2021, 9(5), 524 CrossRef CAS.
- S. Brandl and M. Reindl, Blood–Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models, Int. J. Mol. Sci., 2023, 24(16), 12699 CrossRef CAS PubMed.
- D. Vogrinc, et al., Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia, Antioxidants, 2023, 12(2), 316 CrossRef CAS PubMed.
- A. Abelein, Metal Binding of Alzheimer's Amyloid-β and Its Effect on Peptide Self-Assembly, Acc. Chem. Res., 2023, 56(19), 2653–2663 CrossRef CAS.
- L.-L. Chen, et al., The metal ion hypothesis of Alzheimer's disease and the anti-neuroinflammatory effect of metal chelators, Bioorg. Chem., 2023, 131, 106301 CrossRef CAS.
- A. García-García, et al., Therapy and diagnosis of Alzheimer's disease: from discrete metal complexes to metal–organic frameworks, J. Mater. Chem. B, 2023, 11(30), 7024–7040 RSC.
- H. Yang, et al., A Novel Targeted and High-Efficiency Nanosystem for Combinational Therapy for Alzheimer's Disease, Adv. Sci., 2020, 7(19), 1902906 CrossRef CAS.
- X. Tong, et al., Quantum/carbon dots-based fluorescent assays for enzyme activity, TrAC, Trends Anal. Chem., 2020, 131, 116008 CrossRef CAS.
- R. Lv, et al., Synthesis of Multi-Functional Carbon Quantum Dots for Targeted Antitumor Therapy, J. Fluoresc., 2021, 31(2), 339–348 CrossRef CAS PubMed.
- A. Prakash, et al., Development of folate-conjugated polypyrrole nanoparticles incorporated with nitrogen-doped carbon quantum dots for targeted bioimaging and photothermal therapy, Talanta, 2024, 278, 126528 CrossRef CAS PubMed.
- S. Jovanović, et al., Lights and Dots toward Therapy—Carbon-Based Quantum Dots as New Agents for Photodynamic Therapy, Pharmaceutics, 2023, 15(4), 1170 CrossRef PubMed.
- S. Wang, et al., Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy, Nanomaterials, 2024, 14(15), 1250 CrossRef CAS.
- L. Ren, et al., Applications and Immunological Effects of Quantum Dots on Respiratory System, Front. Immunol., 2022, 12, 795232 CrossRef.
- S. Ghosh, et al., Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy, Chem. Eng. J., 2019, 373, 468–484 CrossRef CAS.
- X. Fan, et al., Highly luminescent pH-responsive carbon quantum dots for cell imaging, Nanotechnology, 2022, 33(26), 265002 CrossRef CAS PubMed.
- X.-W. Fang, et al., Green Synthesis of Carbon Quantum Dots and Carbon Quantum Dot-Gold Nanoparticles for Applications in Bacterial Imaging and Catalytic Reduction of Aromatic Nitro Compounds, ACS Omega, 2024, 9(22), 23573–23583 CrossRef CAS PubMed.
- V. Singh, et al., Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C. elegans and BALB/c mice, J. Mater. Chem. B, 2018, 6(20), 3366–3371 RSC.
- P. Jana and A. Dev, Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer, Mater. Today Commun., 2022, 32, 104068 CrossRef CAS.
- H.-L. Yang, et al., Carbon quantum dots: Preparation, optical properties, and biomedical applications, Mater. Today Adv., 2023, 18, 100376 CrossRef CAS.
- S. Ranjbari, et al., Applications of carbon nanotube biosensors: Sensing the future, J. Drug Delivery Sci. Technol., 2024, 97, 105747 CrossRef CAS.
- A. N. Begum, et al., P2-255: ULTRASENSITIVE EARLY DIAGNOSIS OF ALZHEIMER'S DISEASE USING CARBON NANOTUBE-BASED BIOSENSOR ARRAYS, Alzheimer's Dementia, 2019, 15, P682–P682 Search PubMed.
- Y. Shen, Recent advances in Carbon Nanotube Based Field-Effect Transistor Biosensors for Established Biomarkers for the Diagnosis of Alzheimer’s Disease, ChemRxiv, 2023 DOI:10.26434/chemrxiv-2023-d9d92.
- S. Szunerits and R. Boukherroub, Graphene-based biosensors, Interface Focus, 2018, 8(3), 20160132 CrossRef.
- X. Sun, et al., Biosensors toward behavior detection in diagnosis of alzheimer’s disease, Front. Bioeng. Biotechnol., 2022, 10 DOI:10.3389/fbioe.2022.1031833.
- L. Tang, et al., Versatile carbon nanoplatforms for cancer treatment and diagnosis: strategies, applications and future perspectives, Theranostics, 2022, 12(5), 2290–2321 CrossRef CAS PubMed.
- R. de Boëver, et al., Carbon Dots for Carbon Dummies: The Quantum and The Molecular Questions Among Some Others, Chem. - Eur. J., 2022, 28(47), e202200748 CrossRef.
- Y. Liu, et al., Advances in carbon dots: from the perspective of traditional quantum dots, Mater. Chem. Front., 2020, 4(6), 1586–1613 RSC.
- C. Xia, et al., Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots, Adv. Sci., 2019, 6(23), 1901316 CrossRef CAS.
- L. Cui, et al., Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging, Mater. Today NANO, 2020, 12, 100091 CrossRef.
- H. Peng and J. Travas-Sejdic, Simple Aqueous Solution Route to Luminescent Carbogenic Dots from Carbohydrates, Chem. Mater., 2009, 21(23), 5563–5565 CrossRef CAS.
- J. Xu, et al., Synthesis of green-emitting carbon quantum dots with double carbon sources and their application as a fluorescent probe for selective detection of Cu2+ ions, RSC Adv., 2020, 10(5), 2536–2544 RSC.
- C. Ding, et al., One-step microwave synthesis of N,S co-doped carbon dots from 1,6-hexanediamine dihydrochloride for cell imaging and ion detection, Colloids Surf., B, 2020, 189, 110838 CrossRef CAS.
- R. Liu, et al., An Aqueous Route to Multicolor Photoluminescent Carbon Dots Using Silica Spheres as Carriers, Angew. Chem., Int. Ed., 2009, 48(25), 4598–4601 CrossRef CAS.
- L. Yan, et al., Synthesis of carbon quantum dots by chemical vapor deposition approach for use in polymer solar cell as the electrode buffer layer, Carbon, 2016, 109, 598–607 CrossRef CAS.
- Y. J. Chung, et al., Carbon Nanodot-Sensitized Modulation of Alzheimer’s beta-Amyloid Self-Assembly, Disassembly, and Toxicity, Small, 2017, 13(34), 1700983 CrossRef.
- R. Malishev, et al., Chiral modulation of amyloid beta fibrillation and cytotoxicity by enantiomeric carbon dots, Chem. Commun., 2018, 54(56), 7762–7765 RSC.
- X. Shao, et al., Novel photocatalytic carbon dots: efficiently inhibiting amyloid aggregation and quickly disaggregating amyloid aggregates, Nanoscale, 2024, 16(16), 8074–8089 RSC.
- C. Yan, et al., Multifunctional Carbon-Dot-Photosensitizer Nanoassemblies for Inhibiting Amyloid Aggregates, Suppressing Microbial Infection, and Overcoming the Blood-Brain Barrier, ACS Appl. Mater. Interfaces, 2022, 14(42), 47432–47444 CrossRef CAS.
- P. Ye, et al., Macrophage membrane-encapsulated nitrogen-doped carbon quantum dot nanosystem for targeted treatment of Alzheimer's disease: Regulating metal ion homeostasis and photothermal removal of beta-amyloid, J. Colloid Interface Sci., 2023, 650, 1749–1761 CrossRef CAS PubMed.
- W. Liu, et al., Photoresponsive materials for intensified modulation of Alzheimer's amyloid-β protein aggregation: A review, Acta Biomater., 2021, 123, 93–109 CrossRef CAS PubMed.
- Z. Ma, et al., Application of PEG-modified Copper-cysteamine in photodynamic therapy for Alzheimer's disease, Mater. Lett., 2022, 328, 133018 CrossRef CAS.
- B. Du, et al., Cooperative Strategies for Enhancing Performance of Photothermal Therapy (PTT) Agent: Optimizing Its Photothermal Conversion and Cell Internalization Ability, Small, 2017, 13(13), 1603275 CrossRef.
- Y. Liu, et al., Polydopamine/Ruthenium Nanoparticle Systems as Photothermal Therapy Reagents and Reactive Oxygen Species Scavengers for Alzheimer's Disease Treatment, ACS Appl. Nano Mater., 2023, 6(7), 5384–5393 CrossRef CAS.
- H. Liu, et al., Rational Design of Nitrogen-Doped Carbon Dots for Inhibiting beta-Amyloid Aggregation, Molecules, 2023, 28(3), 1451 CrossRef CAS PubMed.
- K. Koppel, et al., Elevated amyloidoses of human IAPP and amyloid beta by lipopolysaccharide and their mitigation by carbon quantum dots, Nanoscale, 2020, 12(23), 12317–12328 RSC.
- S. L. Sensi, et al., Copper and Zinc Dysregulation in Alzheimer's Disease, Trends Pharmacol. Sci., 2018, 39(12), 1049–1063 CrossRef CAS.
- S. A. Prabhu, et al., Graphene quantum dots synthesis and energy application: a review, Carbon Lett., 2021, 31(1), 1–12 CrossRef.
- H. Lu, et al., Graphene Quantum Dots for Optical Bioimaging, Small, 2019, 15(36), 1902136 CrossRef.
- J. S. Boruah and D. Chowdhury, Hybrid Oleic Acid-Graphene Quantum Dot Vesicles for Drug Delivery, ChemistrySelect, 2019, 4(14), 4347–4354 CrossRef CAS.
- R. Khan, et al., Recent Progress of Fluorescent Carbon Dots and Graphene Quantum Dots for Biosensors: Synthesis of Solution Methods and their Medical Applications, J. Fluoresc., 2024 DOI:10.1007/s10895-024-03809-3.
- P. A. Rasheed, et al., Graphene quantum dots for biosensing and bioimaging, RSC Adv., 2024, 14(23), 16001–16023 RSC.
- D. Iannazzo, et al., Graphene quantum dots for cancer targeted drug delivery, Int. J. Pharm., 2017, 518(1), 185–192 CrossRef CAS.
- J. Ruan, et al., Graphene Quantum Dots for Radiotherapy, ACS Appl. Mater. Interfaces, 2018, 10(17), 14342–14355 CrossRef CAS.
- S. Ghosh, et al., Graphene quantum dots as a potential diagnostic and therapeutic tool for the management of Alzheimer's disease, Carbon Lett., 2022, 32(6), 1381–1394 CrossRef.
- Y. Liu, et al., Graphene quantum dots for the inhibition of beta amyloid aggregation, Nanoscale, 2015, 7(45), 19060 RSC.
- S. Xiao, et al., Graphene quantum dots conjugated neuroprotective peptide improve learning and memory capability, Biomaterials, 2016, 106, 98–110 CrossRef CAS PubMed.
- H. Tang, et al., Graphene quantum dots obstruct the membrane axis of Alzheimer's amyloid beta, Phys. Chem. Chem. Phys., 2022, 24(1), 86–97 RSC.
- K. Tak, et al., Clitoria ternatea Mediated Synthesis of Graphene Quantum Dots for the Treatment of Alzheimer's Disease, ACS Chem. Neurosci., 2020, 11(22), 3741–3748 CrossRef CAS.
- H. Huang, et al., Graphene quantum dots for detecting monomeric amyloid peptides, Nanoscale, 2017, 9(16), 5044–5048 RSC.
- I. Benjamin, et al., Surface modification of transition metals (TM: Mn, Fe, Co) decorated Pt-doped carbon quantum dots (Pt@CQDs) nanostructure as nonenzymatic sensors for nitrotyrosine (a biomarker for Alzheimer): Perspective from density functional theory, Mater. Sci. Semicond. Process., 2024, 174, 108245 CrossRef CAS.
- C. Kang, et al., Aggregation and luminescence in carbonized polymer dots, Aggregate, 2022, 3(2), e169 CrossRef CAS.
- M. Lv, et al., Redox-responsive hyperbranched poly(amido amine) and polymer dots as a vaccine delivery system for cancer immunotherapy, J. Mater. Chem. B, 2017, 5(48), 9532–9545 RSC.
- G. Forte, et al., A nanosized photothermal responsive core-shell carbonized polymer dots based on poly(N-isopropylacrylamide) for light-triggered drug release, Colloids Surf., B, 2022, 217, 112628 CrossRef CAS PubMed.
- Y. Liu, et al., Noninvasive Brain Tumor Imaging Using Red Emissive Carbonized Polymer Dots across the Blood–Brain Barrier, ACS Omega, 2018, 3(7), 7888–7896 CrossRef CAS.
- T. Han, et al., Near-Infrared Carbonized Polymer Dots for NIR-II Bioimaging, Adv. Sci., 2022, 9(30), 2203474 CrossRef CAS.
- Q. Yang, et al., Luminescent Liquid Crystals Based on Carbonized Polymer Dots and Their Polarized Luminescence Application, ACS Appl. Mater. Interfaces, 2021, 13(22), 26522–26532 CrossRef CAS.
- C. Tan, et al., Sulfuric Acid Assisted Preparation of Red-Emitting Carbonized Polymer Dots and the Application of Bio-Imaging, Nanoscale Res. Lett., 2018, 13(1), 272 CrossRef PubMed.
- C. Dong, et al., Dynamic Thermosensitive Solid-State Photoluminescent Carbonized Polymer Dots as Temperature-Responsive Switches for Sensor Applications, ACS Appl. Nano Mater., 2020, 3(11), 10560–10564 CrossRef CAS.
- R.-z Nie, et al., A-type dimeric epigallocatechin-3-gallate (EGCG) is a more potent inhibitor against the formation of insulin amyloid fibril than EGCG monomer, Biochimie, 2016, 125, 204–212 CrossRef CAS PubMed.
- X. Lin, et al., Epigallocatechin gallate-derived carbonized polymer dots: A multifunctional scavenger targeting Alzheimer's beta-amyloid plaques, Acta Biomater., 2023, 157, 524–537 CrossRef CAS.
- W. Gao, et al., Nitrogen-Doped Carbonized Polymer Dots: A Potent Scavenger and Detector Targeting Alzheimer's beta-Amyloid Plaques, Small, 2020, 16(43), e2002804 CrossRef.
- X. Lin, et al., Methylene Blue-Doped Carbonized Polymer Dots: A Potent Photooxygenation Scavenger Targeting Alzheimer's beta-Amyloid, ACS Appl. Mater. Interfaces, 2023, 15(37), 44062–44074 CrossRef CAS.
- A. M. Amani, et al., Innovation applications of MXenes in biomedicine, Mater. Today Commun., 2024, 40, 109929 CrossRef CAS.
- Q. Guan, et al., Highly fluorescent Ti(3)C(2) MXene quantum dots for macrophage labeling and Cu(2+) ion sensing, Nanoscale, 2019, 11(30), 14123–14133 RSC.
- M. A. Al-Duais, et al., Bovine serum albumin functionalized blue emitting Ti(3) C(2) MXene quantum dots as a sensitive fluorescence probe for Fe(3+) ion detection and its toxicity analysis, Luminescence, 2022, 37(4), 633–641 CrossRef CAS.
- X. Yin, et al., C3N nanodots inhibits Aβ peptides aggregation pathogenic path in Alzheimer's disease, Nat. Commun., 2023, 14(1), 5718 CrossRef CAS.
- S. Khan, et al., Application of Carbon Nanotubes In Drug Delivery of Non-cancerous Diseases: A Review, Curr. Pharm. Des., 2021, 27(21), 2454–2467 CrossRef CAS.
- J. Ackermann, et al., Biosensing with Fluorescent Carbon Nanotubes, Angew. Chem., Int. Ed., 2022, 61(18), e202112372 CrossRef CAS PubMed.
- J. Shin, et al., One-dimensional nanomaterials for cancer therapy and diagnosis, Chem. Soc. Rev., 2023, 52(13), 4488–4514 RSC.
- K. Bates and K. Kostarelos, Carbon nanotubes as vectors for gene therapy: Past achievements, present challenges and future goals, Adv. Drug Delivery Rev., 2013, 65(15), 2023–2033 CrossRef CAS PubMed.
- A. Rode, et al., Carbon Nanotubes: Classification, Method of Preparation and Pharmaceutical Application, Curr. Drug Delivery, 2018, 15(5), 620–629 CrossRef CAS PubMed.
- M. Moghaddari, et al., Thermal conductivity and structuring of multiwalled carbon nanotubes based nanofluids, J. Mol. Liq., 2020, 307, 112977 CrossRef CAS.
- Z. Yang, et al., Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease, Nanomedicine, 2010, 6(3), 427–441 CrossRef CAS PubMed.
- L. Zhang, et al., Engineering Carbon Nanotube Fiber for Real-Time Quantification of Ascorbic Acid Levels in a Live Rat Model of Alzheimer's Disease, Anal. Chem., 2017, 89(3), 1831–1837 CrossRef CAS PubMed.
- S. Lohan, et al., Anti-Alzheimer's potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence, Int. J. Pharm., 2017, 530(1–2), 263–278 CrossRef CAS PubMed.
- H. Chen, et al., Aptamer-Functionalized Carbon Nanotube Field-Effect Transistor Biosensors for Alzheimer's Disease Serum Biomarker Detection, ACS Sens., 2022, 7(7), 2075–2083 CrossRef CAS PubMed.
- E. Garnett, et al., Introduction: 1D Nanomaterials/Nanowires, Chem. Rev., 2019, 119(15), 8955–8957 CrossRef CAS PubMed.
- L. Peng, et al., PdPtB Electrochemiluminescence Nanoenhancer and SiC@Au-PEDOT Nanowires-Based Detection of beta-Amyloid Oligomers in Alzheimer's Disease, ACS Appl. Mater. Interfaces, 2023, 15(51), 59189–59198 CrossRef CAS.
- D. Singh, et al., A Critical Appraisal of Functionalized 2-Dimensional Carbon-Based Nanomaterials for Drug Delivery Applications, Recent Pat. Nanotechnol., 2024, 18(4), 479–493 CrossRef CAS PubMed.
- A. M. L. Oliveira, et al., Graphene Oxide Thin Films with Drug Delivery Function, Nanomaterials, 2022, 1149 CrossRef CAS PubMed.
- N. Rouhi, et al., Recent progress in the graphene-based biosensing approaches for the detection of Alzheimer's biomarkers, J. Pharm. Biomed. Anal., 2023, 222, 115084 CrossRef CAS PubMed.
- C. Li, et al., The Development Trend of Graphene Derivatives, J. Electron. Mater., 2022, 51(8), 4107–4114 CrossRef CAS.
- M. Mahdavi, et al., Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems, J. Mater. Chem. B, 2016, 4(46), 7441–7451 RSC.
- M. Li, et al., Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer's disease, Adv. Mater., 2012, 24(13), 1722 CrossRef CAS PubMed.
- J. Li, et al., Nanosensor-Driven Detection of Neuron-Derived Exosomal Abeta(42) with Graphene Electrolyte-Gated Transistor for Alzheimer's Disease Diagnosis, Anal. Chem., 2023, 95(13), 5719–5728 CrossRef CAS.
- Z. Yang, et al., Destruction of amyloid fibrils by graphene through penetration and extraction of peptides, Nanoscale, 2015, 7(44), 18725 RSC.
- L. Wang, et al., Ultra-sensitive SERS detection of Aβ 1–42 for Alzheimer's disease using graphene oxide/gold nanohybrids, Vib. Spectrosc., 2023, 129, 103614 CrossRef CAS.
- B. Anasori, et al., Two-dimensional MXenes, MRS Bull., 2023, 48(3), 238–244 CrossRef CAS.
- W.-J. Zhang, et al., Dual (pH- and ROS-) Responsive Antibacterial MXene-Based Nanocarrier for Drug Delivery, Int. J. Mol. Sci., 2022, 14925 CrossRef CAS.
- B. Xu, et al., Latest advances in MXene biosensors, J. Phys.: Mater., 2020, 3(3), 031001 CAS.
- A. Tiele, et al., Breath-based non-invasive diagnosis of Alzheimer's disease: a pilot study, J. Breath Res., 2020, 14(2), 026003 CrossRef CAS PubMed.
- N. Kumar, et al., First-Principles Approach for Assessing the Detection of Alzheimer's Biomarkers Using Titanium Carbide MXenes, ACS Appl. Nano Mater., 2024, 7(7), 6873–6884 CrossRef CAS.
- Y. Lv, et al., Ultrasensitive electrochemical detection of amyloid-beta oligomers using double amplification strategy by MXene substrate and covalent organic framework-based probe, Talanta, 2024, 266, 125134 CrossRef CAS PubMed.
- K. Ge, et al., An NIR-Driven Upconversion/C3N4/CoP Photocatalyst for Efficient Hydrogen Production by Inhibiting Electron–Hole Pair Recombination for Alzheimer's Disease Therapy, ACS Nano, 2023, 17(3), 2222–2234 CrossRef CAS.
- Z. Chen, et al., A dual-mechanism-driven electrochemiluminescence aptasensor for sensitive detection of beta-amyloid peptides, Anal. Methods, 2022, 14(17), 1739–1746 RSC.
- W. Chang, et al., Graphene Oxide-Gold Star Construct on Triangular Electrodes for Alzheimer's Disease Identification, J. Anal. Methods Chem., 2021, 2021, 6661799 Search PubMed.
- R. V. Nair, et al., Orientation-Specific Plasmonic Biosensor for Alzheimer's Disease Detection Using Graphene-Wrapped Au Nano ellipsoids, Plasmonics, 2023, 19(2), 743–751 CrossRef.
- K. Wang, et al., Intranasal administration of dauricine loaded on graphene oxide: multi-target therapy for Alzheimer's disease, Drug Delivery, 2021, 28(1), 580–593 CrossRef CAS.
- F. Mohebichamkhorami, et al., Microfluidic Synthesis of Ultrasmall Chitosan/Graphene Quantum Dots Particles for Intranasal Delivery in Alzheimer's Disease Treatment, Small, 2023, 19(40), e2207626 CrossRef.
- Z. Li, et al., Aspirin curcumin ester loaded biomimetic nanodrug improves cognitive deficits in a mouse model of Alzheimer's disease by regulating M1/M2 microglial polarization, Mater. Today Adv., 2022, 16, 100321 CrossRef CAS.
- X. Yin, et al., C(3)N nanodots inhibits Abeta peptides aggregation pathogenic path in Alzheimer's disease, Nat. Commun., 2023, 14(1), 5718 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |