Open Access Article
Open Access ArticleInclusion of gold ions in tiara-like nickel hexanuclear nanoclusters†
Kana
Takemae‡
a,
Shiho
Tomihari‡
a,
Takumi
Naito
b,
Makito
Takagi
 b,
Tomomi
Shimazaki
b,
Tomomi
Shimazaki
 b,
Tokuhisa
Kawawaki
b,
Tokuhisa
Kawawaki
 *ac,
Masanori
Tachikawa
*ac,
Masanori
Tachikawa
 *b and
Yuichi
Negishi
*b and
Yuichi
Negishi
 *cd
*cd
aDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: kawawaki@rs.tus.ac.jp
bQuantum Chemistry Division, Yokohama City University, 22-2 Seto, Kanazawa-ku, Yokohama, 236-0027, Japan. E-mail: tachi@yokohama-cu.ac.jp
cResearch Institute for Science and Technology, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: yuichi.negishi.a8@tohoku.ac.jp
dInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai 980-8577, Japan
First published on 6th January 2025
Abstract
Tiara-like metal nanoclusters (TNCs) composed of group 10 transition metals and thiolates can easily change their number of polymerization and include various molecules or metal ions as guests within their ring structures. Therefore, they are expected to be applied in sensing, storage, and catalyst materials based on their selective inclusion characteristics. However, there are very few reports regarding the principles of selective inclusion for guest molecules/ions in TNCs. In this study, we focused on the nickel (Ni) hexanuclear TNC, Ni6(PET)12 (PET = 2-phenylethanethiolate), to clarify the kinds of metal ions that can be included in TNCs. As a result, we found that the gold (Au) ion can be selectively included in Ni6(PET)12 among various metal ions to form a stable structure. From various experiments and density functional theory calculations, we concluded that the main reasons for this are that [Ni6(PET)12]0 has (1) a pore large enough to include Au ions and (2) Ni and Au favour the formation of bonding orbitals based on charge transfer interactions. These insights are expected to lead to a better understanding of host–guest interactions in TNCs and provide clear design guidelines for forming various inclusion structures in the future.
Introduction
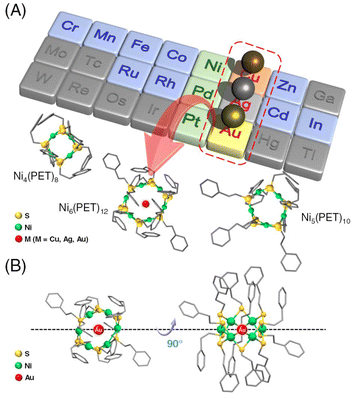
Multinuclear metal complexes, called metal nanoclusters (NCs), exhibit different physicochemical properties from those of bulk metals composed of the same metal elements.1,2 These metal NCs can be precisely synthesized as stable compounds using thiolate (SR) ligands.3–15 Furthermore, the obtained metal NCs are attracting much attention as highly functional materials in fields such as catalysis,1,16–21 bio-imaging,22–25 sensing,22,23 and optics.26,27 In particular, tiara-like metal NCs (TNCs), which are ring compounds containing metal ions, are known to exhibit high complexation ability with other metal ions and small organic molecules. Among them, [M(SR)2]n (M = Ni, Pd, and Pt) composed of group 10 transition metals and SR can easily change its degree of polymerization; thus TNCs with different numbers of atoms can be size-selectively obtained.28–37 In addition, [M(SR)2]n is more stable than other cyclic molecules such as crown ethers, cryptands, and cyclic dextrins because it has a relatively simple structure.These TNCs have been reported to exhibit inclusion properties, trapping a guest molecule in the ring as a host molecule.30,31,38–41 The inclusion properties of [M(SR)2]n (n = 8–12) have been reported, but the guest molecules were limited to iodine molecules and solvent molecules.30,31,38,39 Recently, it has been reported that a Pt hexanuclear TNC (Pt6(SR)12; SR = octanethiolate (C8) or dodecanethiolate (C12)) selectively included a monovalent Ag ion (Ag+) and exhibits excellent phosphorescence properties.40,41 However, much is still unknown about what kinds of TNCs can include metal ions and their selectivity. In particular, Ni TNCs (Nin(SR)2n) have attracted much attention in recent years because of their host–guest chemistry, catalytic activity in the reduction of 4-nitrophenol, and hydrogen and oxygen production from water.30,31,42–46 Therefore, when alloying by inclusion of different metal ions is achieved in Ni hexanuclear TNC (Ni6(SR)12) with pores of almost the same size as Pt6(SR)12,28,42–47 it will lead to further functionalization of the TNCs. In this study, we attempted to synthesize metal ion-included Ni6(PET)12 (PET = 2-phenylethanethiolate) for these reasons. In addition, we investigated the selective inclusion of metal ions in a series of PET-protected metal hexanuclear TNCs (M6(PET)12, M = Ni, Pd, and Pt) to elucidate the host–guest interactions of [M(SR)2]n (Fig. 1).
Experimental
The precursor TNCs, [Nin(PET)2n]0 (n = 4, 5, 6), were synthesized following previously reported methods,21 and the crude products were separated using thin-layer chromatography (TLC; Fig. S1†). The purity of the obtained [Ni6(PET)12]0 was confirmed using ultraviolet-visible absorption spectroscopy, matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS), Fourier transform infrared spectroscopy, and X-ray absorption fine structure analysis (Fig. 2, S2, and S3†).Screening for metal ion inclusion in Ni6(PET)12
Metal ion-included [Ni6(PET)12]0 was synthesized using the method for synthesizing Ag+-included Pt6(SR)12 (AgPt6(SR)12; SR = C8 or C12) reported by Konishi et al.40 and Yamamoto et al.41 with slight modification (Fig. S4†). An acetonitrile solution (2 mL) containing each metal ion precursor (Cr, Mn, Fe, Co, Cu, Zn, Ru, Rh, Ag, Cd, In, or Au ions; 1.0 equiv.) was added to a toluene solution (10 mL) containing Ni6(PET)12 (1.998 mg, 1 μmol) and the resulting solution was stirred for 10 min. The reaction solvent was then removed using a rotary evaporator, and the obtained product was extracted with dichloromethane (DCM). Metal ion-included Ni6(PET)12 was obtained by filtration through a Minisart® to remove the insoluble part. The obtained product was evaluated to screen for the metal ion-included Ni6(PET)12.Synthesis method for MNi6(PET)12 (M = Cu, Ag, and Au)
Tetrahydrofuran (THF) was selected as the reaction solvent due to the high solubility of both the [Ni6(PET)12]0 and metal salts (CuCl2, AgCl, and HAuCl4) in THF. [Ni6(PET)12]0 (1.998 mg, 1 μmol) was dissolved in THF (10 mL), followed by the addition of a THF solution (2 mL) of the metal salts (1 μmol). The mixture was stirred at 60 °C for 30 min. After the reaction, the solvent was removed using a rotary evaporator. The resulting crude product was extracted with DCM, followed by filtration through a Minisart® to remove the insoluble part. The crude product was further purified using TLC (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 3
hexane = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v). The first band from the top of the TLC plate was collected (Fig. S5†) and extracted with DCM to yield [MNi6(PET)12]+ (Fig. S6†).
4, v/v). The first band from the top of the TLC plate was collected (Fig. S5†) and extracted with DCM to yield [MNi6(PET)12]+ (Fig. S6†).
Results and discussion
Metal ion inclusion in Ni6(PET)12
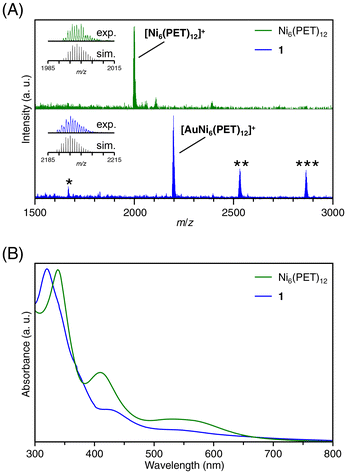
Transition metal ions from groups 6 to 13 (Cr, Mn, Fe, Co, Cu, Zn, Ru, Rh, Ag, Cd, In, and Au) were utilized to investigate the metal ion inclusion in the typical Ni TNC, [Ni6(PET)12]0. Each metal ion was added to [Ni6(PET)12]0, and changes in the optical absorption spectra and MALDI-MS spectra of the resulting products were examined. As a result, for many kinds of metal ions, the products obtained upon the addition of metal ions exhibited no significant change in spectral shape compared with [Ni6(PET)12]0, whereas significant changes occurred when Cu, Ag, and Au ions were added (Fig. 2 and S7†).The MALDI-MS spectra of [Ni6(PET)12]0 and the product (1) obtained after the addition of HAuCl4 and subsequent purification are presented in Fig. 2A (Table S1†). In the MS spectrum of 1, a peak was detected at a mass-to-charge ratio (m/z) of 2195, which differed from that of [Ni6(PET)12]0. By comparing the isotope pattern of 1 with that of the simulated Au ion-included Ni6(PET)12 ([AuNi6(PET)12]+) (Fig. 2A inset), it was found that 1 primarily contains [AuNi6(PET)12]+ as the main product. In addition, as byproducts, the existence of [AuNi7(PET)14]+ and [AuNi8(PET)16]+, in which the Au ion is doped into larger Ni TNCs (Ni7(PET)14 and Ni8(PET)16) and [Au2Ni3(PET)8]+, a double-crown structure alloy TNC,48 were also observed with small amounts. These results indicate that [Ni6(PET)12]0 has a relatively flexible structure in solution, allowing for the dissociation and polymerization of Ni(PET)2 units during the reaction. The formation of [AuNi6(PET)12]+ was also confirmed when Au(I) was used as a precursor (Fig. S7B†). Unfortunately, it was difficult to isolate [AuNi6(PET)12]+ with high purity for these reasons and thereby obtain a single crystal of [AuNi6(PET)12]+.
The existence of Au and Ni in 1 was also confirmed using inductively coupled plasma-MS. The optical absorption spectrum of [Ni6(PET)12]0 exhibited absorption peaks at around 340 nm and 410 nm (Fig. 2B), but for 1, peaks were observed at around 320 nm and 430 nm, and the shape of the absorption spectrum had changed from that of [Ni6(PET)12]0 (Fig. 2B). We further examined the electronic states of [Ni6(PET)12]0 and 1 using X-ray photoelectron spectroscopy (Fig. S8†). As a result, the peak of the Ni 2p spectrum was shifted to a slightly higher energy region in the spectrum of 1 than that of [Ni6(PET)12]0, indicating that the charge state of Ni became slightly positive by the inclusion of the Au ion into [Ni6(PET)12]0. In addition, it was found that the electronic state of the included Au was relatively close to that of a metal (Fig. S8†). Density functional theory (DFT) calculations conducted on Ag+-included Pt6(SR)12 ([AgPt6(SR)12]+) have also reported a similar tendency; the electronic state of the included Ag+ was relatively close to that of a metal.40 These results indicate that the included Au ion coordinates to the Ni6(PET)12 structure while receiving a partial electron from Ni or S. Fig. S9A† shows the time-dependent change in the optical absorption spectrum of 1 in THF solution at room temperature. The absorption spectrum did not change even after 24 h, indicating that 1 is a stable compound like [Ni6(PET)12]0 (Fig. S9B†). Due to this change in electronic structure, 1 exhibited superior activity for the hydrogen evolution reaction compared to Ni6(PET)12 (Fig. S10†). The doping properties of other group 11 metal ions (Cu, Ag and Au) in [Ni6(PET)12]0 were also investigated. Fig. S7† shows the MALDI-MS spectrum of the products obtained by adding CuCl2 to [Ni6(PET)12]0. The peak intensity at m/z = 1998 derived from Ni6(PET)12 decreases and a new peak attributed to Cu ion-doped Ni6(PET)12 ([CuNi6(PET)12]+) appeared at m/z = 2061 (Table S2†) by the addition of CuCl2. When AgCl was added to [Ni6(PET)12]0, the peak intensity of Ni6(PET)12 decreased, and a new peak was detected at m/z = 2105 that was attributed to Ag ion-doped Ni6(PET)12 ([AgNi6(PET)12]+; Fig. S7 and Table S2†). From these results, it can be considered that Cu and Ag ions can also be included in [Ni6(PET)12]0. However, it was difficult to stably extract these products in high purity.
Thus, it was found that [Ni6(PET)12]0 selectively includes Au, Ag, and Cu ions among the major metals from group 6 to group 13, and the resulting AuNi6(PET)12 exhibits higher stability than AgNi6(PET)12 and CuNi6(PET)12.
Electronic/geometrical structure of AuNi6(PET)12
We performed DFT calculations on [Ni6(PET)12]0 and [AuNi6(PET)12]+ to elucidate the reason why the Au ion can be stably included within [Ni6(PET)12]0. Fig. 3 shows the geometrical structure of [AuNi6(PET)12]+ obtained through structural optimization using DFT calculations. From the normal vibration analysis, it was found that [AuNi6(PET)12]+ is thermodynamically stable compared with [Ni6(PET)12]0. Similar calculations were also performed for [Ni6(PET)12]0 and [AuNi6(PET)12]+ in THF, the actual reaction solvent. The results implied that the inclusion of Au+ into [Ni6(PET)12]0 and [AuNi6(PET)12]+ causes a thermodynamical stability of 63.0 kcal mol−1 compared with Au+ + [Ni6(PET)12]0. We also performed DFT calculations on Ag and Cu with ion-included Ni6(PET)12 in THF (Table S3†). These calculations show that Ag+ and Cu+ are more stabilized by inclusion into Ni6(PET)12 (31.1 and 44.8 kcal mol−1 for [AgNi6(PET)12]+ and [CuNi6(PET)12]+, respectively). Thus, we confirmed that Au is most stabilized. From the Mulliken charge analysis, Ag+ and Cu+ tend to remain cationic, but Au+ is close to neutral due to the large charge transfer, suggesting that stronger interactions between Au and Ni6(PET)12. These calculations are consistent with the experimental results.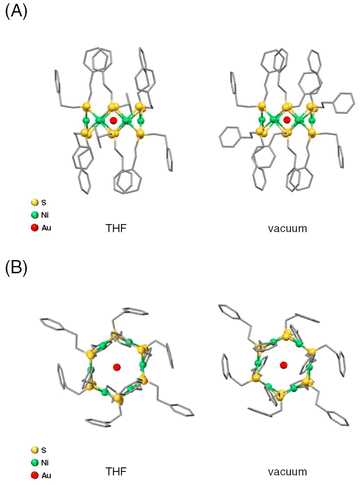 | ||
| Fig. 3 Optimized structures from (A) the side view and (B) the top view in THF and under vacuum for [AuNi6(PET)12]+ using DFT calculations. | ||
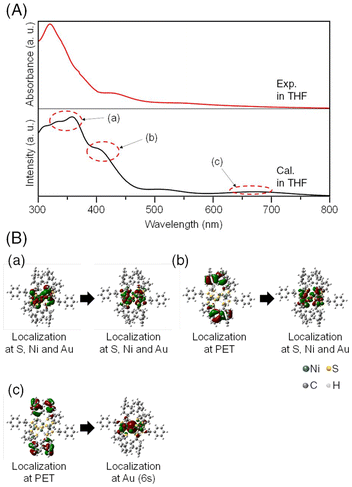
This stabilization is likely due to the hybridization of the orbitals between the Au ion and the Ni and S atoms in the ring structure. Accordingly, we calculated the electronic structures and optical absorption spectra of [Ni6(PET)12]0 and [AuNi6(PET)12]+ with the optimized structures. The calculated absorption spectrum of [Ni6(PET)12]0 showed several peaks corresponding to charge transfer (CT) transitions between the orbitals mainly consisting of S and Ni atoms (Fig. S11†). The peak at around 340 nm is mainly attributed to ligand-to-metal CT (LMCT) transitions and the peak at around 410 nm is mainly attributed to Ni d–d transitions. On the other hand, in the calculated absorption spectrum of [AuNi6(PET)12]+ (Fig. 4), the peaks due to d–d transitions specifically localized to Ni and Au were observed at around 340 nm. It was found that specific localization to Au also occurs in the LMCT transition at 420 nm. A new absorption band was also observed at approximately 660 nm, which is mainly attributed to the transition from the orbitals of the Ph group to those of Au 6s (Fig. 4). These new hybrid orbitals caused by the inclusion of the Au ion into [Ni6(PET)12]0 are considered to influence the interaction between the Ni and S atoms in the original [Ni6(PET)12]0, thereby causing slight changes in the energy of each orbital. Indeed, previous studies39–41 reported that when [M(SR)2]n includes solvent molecules or metal ions, changes occur in the M–S bond distance to maintain their structure. Therefore, it can be considered that the overall structural changes in the absorption spectrum due to the inclusion of the Au ion are also caused by the change in the bonding properties of the TNC framework. In fact, the Ni–S and Ni–Ni bond distances of [AuNi6(PET)12]+ in THF, which were estimated by DFT calculations, were shorter than those of [Ni6(PET)12]0, and the TNC structure shrank overall due to inclusion (Table S4†). Furthermore, we performed natural bond order analysis (Table S3†). We found that the bond order of Au–Ni is relatively high, while that of Au–S is small for [AuNi6(PET)12]+. Small bond orders on S are similarly observed for other metal ions from the calculations for [AgNi6(PET)12]+ and [CuNi6(PET)12]+. This suggests that the interaction with the Ni sites of Ni6(PET)12 plays an essential role in metal inclusion. From these results, we can consider that for [Ni6(PET)12]0, the Au ion is suitable for orbital hybridization and inducing stabilization without distorting the TNC structure. Considering the fact that a similar interpretation has also been obtained for [AgPt6(C12)12]+,40 it can be concluded that in the formation of metal ion-included TNCs composed of group 10 elements as hosts, the main driving force for inclusion is whether the guest group 11 elements can easily form bonding orbitals with the host TNCs.
Metal ion inclusion in M6(PET)12 (M = Ni, Pd, and Pt)
We also investigated whether the hexanuclear TNCs [M6(PET)12]0 (M = Ni, Pd, and Pt) can include metal ions as well (Fig. 5, Tables S5 and S6†). The MALDI-MS spectra suggested that for the Pt TNC ([Pt6(PET)12]0) and Pd TNC ([Pd6(PET)12]0), Cu and Ag ions were selectively included, whereas the Au ion was not included (Fig. 5). This result is consistent with the previous studies,40,41 in which the Ag ion was included in [Pt6(SR)12]0 (SR = C8 or C12), indicating that the difference in the kinds of SR ligands (PET vs. C8 vs. C12) affects only a little on the selectivity of the included metal ion in [M6(SR)12]0.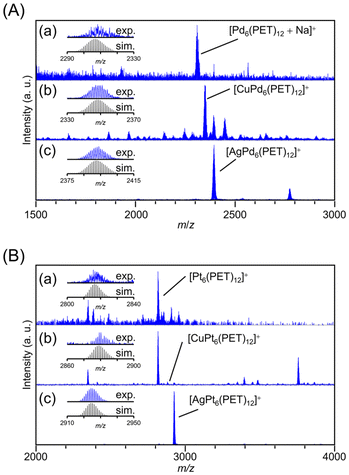 | ||
| Fig. 5 Positive-ion MALDI-mass spectra (a) before and after adding (b) Cu(I) and (c) Ag(I) salts into (A) Pd6(PET)12 and (B) Pt6(PET)12 solutions. The insets show the comparison of the isotope patterns between the experimental spectrum (blue) and the calculated one (black). Several peaks attributed to byproducts are assigned and summarized in Tables S5 and S6.† | ||
To deepen our understanding of the origin of the selectivity of metal ions, we next investigated the correlation between the ring spaces and the size of the included metal ions. The diagonal metal-to-metal distances are 6.34, 6.24, and 5.85 Å for [Pt6(SR)12]0, [Pd6(SR)12]0, and [Ni6(SR)12]0, respectively.33,40,49 From these values and the van der Waals radii49 of each metal ion, the pore sizes of [Pt6(SR)12]0, [Pd6(SR)12]0, and [Ni6(SR)12]0 have been estimated to be 2.84,40 2.98,33 and 2.59 Å,21 respectively. Here, the ionic diameters of Cu+, Ag+, and Au+ are 1.92, 2.52, and 2.74 Å, respectively.50 These results indicate that even though the pore of [Ni6(SR)12]0 is smaller than those of [Pt6(SR)12]0 and [Pd6(SR)12]0, the larger size group 11 metal ions, Au ions, were included in [Ni6(PET)12]0. These results indicate that the match between the size of the pore and the included metal ions is not sufficient to explain the selective inclusion of metal ions (Fig. S12†). It can be assumed that the ease of inclusion changes depending on the binding energy between the Au ions and the group 10 elements of the TNCs. In fact, previous studies reported40,41 that the six Pt–Ag bonds are not of equal length in [AgPt6(SR)12]+, but are biased, indicating that a relatively strong interaction (bonding) occurs between Ag+ and [Pt6(SR)12]0. Therefore, it can be presumed that the ease of constructing their bonding orbitals is the main driving force for inclusion, rather than the match between the size of the TNC pore and the metal ion to be included.
Finally, we show the result for the inclusion properties obtained using [Ni4(PET)8]0 or [Ni5(PET)10]0 as a precursor. In this case, only a small amount of Ni4(PET)8 or Ni5(PET)10 doped with group 11 elements was observed in the MALDI-MS spectra (Fig. S13–S15 and Table S7†). These results imply that the TNCs must have pores at least similar to or larger than the size of the group 11 element ions to cause inclusion. In addition, the introduction of Ag or Au ions resulted in peaks corresponding to [AuNin(PET)2n]+ (n = 6–8) and [AgNi6(PET)12]+ in the MALDI-MS spectra (Fig. S13–S15†), despite using high-purity [Ni4(PET)8]0 and [Ni5(PET)10]0 as precursors. This result confirms the above assumption that the dissociation and polymerization of Ni(SR)2 units in [Nin(SR)2n]0 occur relatively easily in solution, leading to the transformation into more thermodynamically stable TNC structures. For relatively small TNCs such as [M4(SR)8]0 (M = Ni, Pd, and Pt) and [Pd5(SR)10]0, there is also a possibility that doping with Au or Ag induces the formation of a double-crown shell structure.48,51–54 Indeed, in our study, when heterometal ions were introduced into [Ni4(PET)8]0, weak peaks corresponding to the double-crown shell structure were observed.
Based on the above results, it can be concluded that the primary factors for forming stable metal ion-included TNCs composed of group 10 elements as hosts are: (1) the TNCs have pores similar to or larger than the size of the included metal ions and (2) the formation of bonding orbitals between the included metal ions and the TNCs is promoted.
Conclusions
In this study, we aimed to understand the selectivity of the inclusion of metal ions within a ring structure of TNCs and the factors contributing to this process using SR-protected Ni, Pt, and Pd TNCs as hosts. As a result, we elucidated that [Ni6(PET)12]0 selectively includes Cu, Ag, and Au ions, which are group 11 elements, in solution. Notably, the Au ion-included Ni6(PET)12 ([AuNi6(PET)12]+) exhibited high stability. The various experimental and theoretical studies suggested that [Ni6(PET)12]0 has: (1) pores of sufficient size that can include Au ions and (2) Ni ions that facilitate the formation of bonding orbitals with Au ions. Furthermore, investigation of the ease of inclusion of various ions (Cu+, Ag+ and Au+) into [M6(SR)12]0 (M = Ni, Pd and Pt) suggested that the main driving force for the inclusion was not the compatibility between the size of the TNC pore and the metal ion, but the ease of constructing the bonding orbitals. These findings are expected to contribute to a better understanding of host–guest interactions in TNC systems and provide clear design guidelines for the formation of various inclusion complexes.Author contributions
T. Kawawaki and Y. Negishi designed the experiments and conducted the measurements with K. Takemae and S. Tomihari. T. Naito, M. Takagi, T. Shimazaki and M. Tachikawa performed the DFT calculations. T. Kawawaki, M. Tachikawa and Y. Negishi wrote the paper. All authors approved the final version of the manuscript.Data availability
All data generated in this study are provided in the manuscript and its ESI.†Relevant data are available from the corresponding authors (T. Kawawaki, M. Tachikawa and Y. Negishi) upon reasonable request.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Mr Tomoshige Okada, Sota Funaki and Yuki Iwamatsu (Tokyo University of Science) for their technical assistance. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers 22K19012, 23H00289, and 24K01459). Funding from the Takahashi Industrial and Economic Research Foundation, the Carbon Recycling Fund Institute, the Japan Gas Association, the Iwatani Naoji Foundation, the Ichimura Foundation for New Technology, the Suzuki Foundation, and the Japan Keirin Autorace Foundation is also gratefully acknowledged.References
- T. Kawawaki, A. Ebina, Y. Hosokawa, S. Ozaki, D. Suzuki, S. Hossain and Y. Negishi, Small, 2021, 17, 2005328 CrossRef CAS
.
- T. Kawawaki, Y. Imai, D. Suzuki, S. Kato, I. Kobayashi, T. Suzuki, R. Kaneko, S. Hossain and Y. Negishi, Chem. – Eur. J., 2020, 26, 16150–16193 CrossRef CAS
.
- S. Takano, S. Hasegawa, M. Suyama and T. Tsukuda, Acc. Chem. Res., 2018, 51, 3074–3083 CrossRef CAS
.
- R. L. Whetten, H.-C. Weissker, J. J. Pelayo, S. M. Mullins, X. López-Lozano and I. L. Garzón, Acc. Chem. Res., 2019, 52, 34–43 CrossRef CAS
.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed
.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS
.
- Z. Gan, N. Xia and Z. Wu, Acc. Chem. Res., 2018, 51, 2774–2783 CrossRef CAS
.
- Q. Tang, G. Hu, V. Fung and D.-E. Jiang, Acc. Chem. Res., 2018, 51, 2793–2802 CrossRef CAS PubMed
.
- B. Nieto-Ortega and T. Bürgi, Acc. Chem. Res., 2018, 51, 2811–2819 CrossRef CAS PubMed
.
- C. M. Aikens, Acc. Chem. Res., 2018, 51, 3065–3073 CrossRef CAS PubMed
.
- B. Bhattarai, Y. Zaker, A. Atnagulov, B. Yoon, U. Landman and T. P. Bigioni, Acc. Chem. Res., 2018, 51, 3104–3113 CrossRef CAS PubMed
.
- Y. Pei, P. Wang, Z. Ma and L. Xiong, Acc. Chem. Res., 2019, 52, 23–33 CrossRef CAS
.
- C. A. Hosier and C. J. Ackerson, J. Am. Chem. Soc., 2019, 141, 309–314 CrossRef CAS
.
- S. Chen, S. Wang, J. Zhong, Y. Song, J. Zhang, H. Sheng, Y. Pei and M. Zhu, Angew. Chem., Int. Ed., 2015, 54, 3145–3149 CrossRef CAS PubMed
.
- W. Du, S. Jin, L. Xiong, M. Chen, J. Zhang, X. Zou, Y. Pei, S. Wang and M. Zhu, J. Am. Chem. Soc., 2017, 139, 1618–1624 CrossRef CAS
.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Iwamatsu, S. Hossain and Y. Negishi, Nanoscale Horiz., 2021, 6, 409–448 RSC
.
- T. Kawawaki, Y. Kataoka, S. Ozaki, M. Kawachi, M. Hirata and Y. Negishi, Chem. Commun., 2021, 57, 417–440 RSC
.
- T. Kawawaki, Y. Mori, K. Wakamatsu, S. Ozaki, M. Kawachi, S. Hossain and Y. Negishi, J. Mater. Chem. A, 2020, 8, 16081–16113 RSC
.
- B. Kumar, T. Kawawaki, N. Shimizu, Y. Imai, D. Suzuki, S. Hossain, L. V. Nair and Y. Negishi, Nanoscale, 2020, 12, 9969–9979 RSC
.
- C. Garcia, V. Truttmann, I. Lopez, T. Haunold, C. Marini, C. Rameshan, E. Pittenauer, P. Kregsamer, K. Dobrezberger, M. Stöger-Pollach, N. Barrabés and G. Rupprechter, J. Phys. Chem. C, 2020, 124, 23626–23636 CrossRef CAS
.
- S. Funaki, T. Kawawaki, T. Okada, K. Takemae, S. Hossain, Y. Niihori, T. Naito, M. Takagi, T. Shimazaki, S. Kikkawa, S. Yamazoe, M. Tachikawa and Y. Negishi, Nanoscale, 2023, 15, 5201–5208 RSC
.
- T. Kawawaki, Y. Negishi and H. Kawasaki, Nanoscale Adv., 2020, 2, 17–36 RSC
.
- M.-M. Zhang, K. Li and S.-Q. Zang, Adv. Opt. Mater., 2020, 8, 1902152 CrossRef CAS
.
- X. Jiang, B. Du, Y. Huang and J. Zheng, Nano Today, 2018, 21, 106–125 CrossRef CAS PubMed
.
- F. Yu, Z. Cao, S. He, H. Xiang, G. Zhao, L. Yang and H. Liu, Chem. Commun., 2022, 58, 811–814 RSC
.
- X. Wang, B. Yin, L. Jiang, C. Yang, Y. Liu, G. Zou, S. Chen and M. Zhu, Science, 2023, 381, 784–790 CrossRef CAS
.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS
.
- Y. Pan, J. Chen, S. Gong and Z. Wang, Dalton Trans., 2018, 47, 11097–11103 RSC
.
- C. Tan, M. Jin, X. Ma, Q. Zhu, Y. Huang, Y. Wang, S. Hu, T. Sheng and X. Wu, Dalton Trans., 2012, 41, 8472–8476 RSC
.
- S. A. Ivanov, M. A. Kozee, W. A. Merrill, S. Agarwal and L. F. Dahl, J. Chem. Soc., Dalton Trans., 2002, 4105–4115 RSC
.
- C. Zhang, T. Matsumoto, M. Samoc, S. Petrie, S. Meng, T. C. Corkery, R. Stranger, J. Zhang, M. G. Humphrey and K. Tatsumi, Angew. Chem., Int. Ed., 2010, 49, 4209–4212 CrossRef CAS PubMed
.
- T. Imaoka, Y. Akanuma, N. Haruta, S. Tsuchiya, K. Ishihara, T. Okayasu, W.-J. Chun, M. Takahashi and K. Yamamoto, Nat. Commun., 2017, 8, 688 CrossRef PubMed
.
- J. Chen, L. Liu, L. Weng, Y. Lin, L. Liao, C. Wang, J. Yang and Z. Wu, Sci. Rep., 2015, 5, 16628 CrossRef CAS PubMed
.
- T. Okada, T. Kawawaki, K. Takemae, S. Tomihari, T. Kosaka, Y. Niihori and Y. Negishi, J. Phys. Chem. Lett., 2024, 15, 1539–1545 CrossRef CAS PubMed
.
- C. Zhang, S. Takada, M. Kölzer, T. Matsumoto and K. Tatsumi, Angew. Chem., Int. Ed., 2006, 45, 3768–3772 CrossRef CAS PubMed
.
- M. Kriege and G. Henkel, Z. Naturforsch., B: J. Chem. Sci., 1987, 42, 1121–1128 CrossRef CAS
.
- B.-K. Koo, E. Block, H. Kang, S. Liu and J. Zubieta, Polyhedron, 1988, 7, 1397–1399 CrossRef CAS
.
- Y. Yamashina, Y. Kataoka and Y. Ura, Eur. J. Inorg. Chem., 2014, 2014, 4073–4078 CrossRef CAS
.
- Y. Yamashina, Y. Kataoka and Y. Ura, Inorg. Chem., 2014, 53, 3558–3567 CrossRef CAS
.
- Y. Shichibu, K. Yoshida and K. Konishi, Inorg. Chem., 2016, 55, 9147–9149 CrossRef CAS PubMed
.
- Y. Akanuma, T. Imaoka, H. Sato and K. Yamamoto, Angew. Chem., Int. Ed., 2021, 60, 4551–4554 CrossRef CAS PubMed
.
- M. Zhu, S. Zhou, C. Yao, L. Liao and Z. Wu, Nanoscale, 2014, 6, 14195–14199 Search PubMed
.
- H. N. Kagalwala, E. Gottlieb, G. Li, T. Li, R. Jin and S. Bernhard, Inorg. Chem., 2013, 52, 9094–9101 CrossRef CAS PubMed
.
- R. Angamuthu and E. Bouwman, Phys. Chem. Chem. Phys., 2009, 11, 5578–5583 RSC
.
- D. R. Kauffman, D. Alfonso, D. N. Tafen, J. Lekse, C. Wang, X. Deng, J. Lee, H. Jang, J.-S. Lee, S. Kumar and C. Matranga, ACS Catal., 2016, 6, 1225–1234 CrossRef CAS
.
- K. S. Joya, L. Sinatra, L. G. AbdulHalim, C. P. Joshi, M. N. Hedhili, O. M. Bakr and I. Hussain, Nanoscale, 2016, 8, 9695–9703 RSC
.
- A. Datta, N. S. John, G. U. Kulkarni and S. K. Pati, J. Phys. Chem. A, 2005, 109, 11647–11649 Search PubMed
.
- H. Seong, Y. Jo, V. Efremov, Y. Kim, S. Park, S. M. Han, K. Chang, J. Park, W. Choi, W. Kim, C. H. Choi, J. S. Yoo and D. Lee, J. Am. Chem. Soc., 2023, 145, 2152–2160 Search PubMed
.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS
.
- R. D. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 925–946 CrossRef CAS
.
- J. Chen, L. Liu, X. Liu, L. Liao, S. Zhuang, S. Zhou, J. Yang and Z. Wu, Chem. – Eur. J., 2017, 23, 18187–18192 CrossRef CAS
.
- S. Hossain, Y. Imai, Y. Motohashi, Z. Chen, D. Suzuki, T. Suzuki, Y. Kataoka, M. Hirata, T. Ono, W. Kurashige, T. Kawawaki, T. Yamamoto and Y. Negishi, Mater. Horiz., 2020, 7, 796–803 RSC
.
- X. Liu, J. Yuan, J. Chen, J. Yang and Z. Wu, Part. Part. Syst. Charact., 2019, 36, 1900003 CrossRef
.
- X. Cheng, X. Sui, J. Xu, X. Liu, M. Chen and Y. Zhu, RSC Adv., 2021, 11, 32526–32532 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr04579c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |