Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthetic routes and fields of application of isohexides: comprehensive perspective of relevant industrial compounds
Daria
Armani
 *
*
Interdepartmental Centre for Industrial Agri-Food Research and Department of Civil, Chemistry, Environmental and Materials Engineering, University of Bologna, Via Umberto Terracini, 28, 40131 Bologna (BO), Italy. E-mail: daria.armani2@unibo.it
First published on 3rd March 2025
Abstract
Isohexides are a class of compounds that can be obtained from natural sources using simple processes, and have interesting properties. Among these, the intrinsic chirality of the rigid bicyclic structural cavity and the presence of two hydroxyl groups in positions 3 and 6, available for functional-group interconversion, appear the most interesting characteristics for real innovative applications. In this review, the strategies for the synthesis of isohexides from polysaccharides and biomasses of different complexities have been analyzed and the alternative processes to the traditional synthetic routes, in terms of sustainability, have been discussed. Secondly, the properties that make isosorbide the most suitable isohexide compound in terms of availability, stability and scalability for innovative production processes have been highlighted. The fields of application of these extremely interesting compounds have been investigated, proceeding with a discussion in which the areas of exploitation of isohexides are analyzed, in terms of their use as building blocks, chiral auxiliaries or constituents of chiral ionic liquids. Finally, the numerous applicative possibilities of isosorbide as a monomer or co-monomer of a wide range of polymeric materials of different natures, have been thoroughly investigated. This review represents a first investigation into the possible uses of isohexide-based compounds by analyzing the positive and negative aspects of their exploitation as ubiquitous alternatives to current oil-based intermediates. Certainly, future studies on the optimization of those production processes that occupy a large share of the chemical-product market will lead to the confirmation of the synthetic potential of compounds with an isohexidic structure.
Sustainability spotlightIsohexides can be used in production processes and synthetic transformations as sustainable alternatives to those currently employed, derived from crude oil. In fact, these compounds adhere to at least 7 of the 12 principles of Green Chemistry. In particular, isosorbide can be extracted from renewable feedstocks, preventing the wastage of precious resources and incorporating atom economy, it can be used as a benign solvent leading to less hazardous syntheses, and it can be employed in catalytic quantities or as a chiral auxiliary in processes that traditionally require toxic organocatalysts. These are bicyclic compounds that can be isolated from sugar wastes that do not compete with the agri-food supply chain, namely low-cost materials that are capable of undergoing even complex transformations with harsh reaction conditions and can be used to produce highly performing polymeric materials. The reader is therefore exposed to the real possibility of ubiquitous application of compounds with an isohexidic structure in very different but fundamental areas of the new sustainable transition. |
1. Introduction
In the United States alone, 400 million tons of sugar-based dry biomass are generated annually from the corn, soybean, flour, and sorghum production industries.1 The complete replacement of fossil fuels with biomass fuels is difficult to achieve due to the enormous scale of fuel consumption and the market demand. For these reasons, biomass is currently more effectively used in the conversion to chemical feedstocks and chemical precursors.2 However, the high market value of these products sometimes requires the use of expensive unit operations for pre-treatment, processing, and separation, completely ex novo compared to traditional industrial precursor plants.The growing interest in a circular economy and environmental sustainability has increased the search for renewable sources and their conversion into products, which include chemical reagents, energy resources and materials,3 to replace the enormous demand for fossil fuels (Fig. 1).
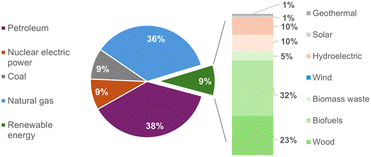 | ||
| Fig. 1 US primary energy consumption by energy source in 2023; only 9% is renewable.3 | ||
Given the abundance of these materials and the lack of competition with the agri-food sector, during the current century a market segment dedicated exclusively to the valorization of waste biomass was successfully established. Therefore, research is evolving towards the formulation of integrated processes that can lead to new products with high added-value, starting precisely from the mentioned biomasses.
Due to this promising background, many scientists from all over the world have dedicated their research to the extraction and separation of high-added-value components from wastes of different natures. This has opened new frontiers in different areas of the chemical industry, allowing components deriving from petrochemicals (used in already consolidated processes) to be replaced with interesting and more sustainable alternatives.1
In this context, agricultural raw materials come into play as a natural, renewable and sustainable resource to produce a large variety of biopolymers, biochemicals and bioenergy sources.4,5 For example, cellulose can be obtained mainly from agricultural residues and is one of the largest sources of organic raw materials in the world. It is in fact considered a promising resource that can be transformed into sustainable fuels and chemicals.6
Since the 1990s, there has been growing research on the isolation, synthesis and innovative use of numerous sugar-based products.7,8 As can be seen from the graph shown in Fig. 2, interest in this sector and the related scientific publications have witnessed an exceptional increase over the past decades.9
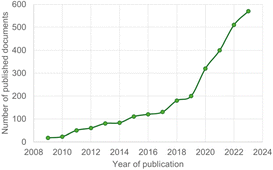 | ||
| Fig. 2 Number of documents published on biomass valorisation per year; data from cited Scopus database.9 | ||
Alcohols derived from C5 and C6 sugars obtained from cellulose or hemicelluloses are considered as sustainable building blocks and important synthons in organic synthesis.10 Among these, the hexitols are a class of polyols with six hydroxyl groups that can be obtained from the corresponding hexoses (Fig. 3).11
 | ||
| Fig. 3 Synthetic scheme to produce isohexide precursors starting from agro-food waste biomass.11 | ||
In fact, from plant-based biomasses rich in fibers (such as rice husk, wood pulp, banana peel, coffee husk, wheat straw, sisal fibers and sugarcane bagasse) it is possible to isolate cellulose microfibrils using recently consolidated processes.12
Crystalline structures that can be isolated from cellulose constitute some of the optimal precursors for the synthesis of hexose sugars from natural sources. The interconversion of functional groups starting from the OH present on the sugar-based carbon skeleton opens the door to different classes of compounds. Isohexides, for example, are compounds with a 6-carbon skeleton and 2 hydroxyl groups linked to different carbons, and are extremely interesting.
Therefore, the different configurations of the stereogenic centers define the structural divergences of the isohexidic spatial isomers and their further chemical modification. These differences will be highlighted throughout the manuscript and the important asymmetric reactivity of the two OH groups will be especially emphasized.
In this review, the synthetic strategies for isohexides will be thoroughly discussed, focusing the reader’s attention on the comparison between the processes traditionally used in industry or at the laboratory scale and the possible paths recently identified as greener alternatives.
Subsequently, the possible application of isohexides in the organic synthesis of discrete molecules, such as chiral auxiliaries and macromolecular materials with a polymeric structure, will be discussed, demonstrating the suitability of the use of compounds with an isohexidic structure in various industrial sectors.
1.1. Structure of isohexides
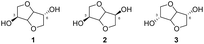
The term “isohexides” refers to a class of diol compounds whose structures are characterized by the presence of two tetrahydrofuran rings fused with a cis-type junction.Isosorbide (1,4:3,6-dianhydrosorbitol 1), isomannide (1,4:3,6-dianhydromannitol 2) and isoidide (1,4:3,6-dianhydroiditol 3), are three of the main isomers in this category of natural sugar-based compounds. They are distinguished by their different stereochemistries of the hydroxyl groups in position 3 and in position 6: the two OH groups of isosorbide 1 are endo–exo, while in isomannide 2 and in isoidide 3 they are oriented endo–endo and exo–exo, respectively.13 Their chemical structures are shown in Fig. 4.
The type of junction present between the two rings gives a concave vaulted structure to the isohexides, with a dihedral angle between the two tetrahydrofuran rings of about 120°. As can be observed in Fig. 5, in isosorbide 1, the concavity of the structure offers the possibility of forming intramolecular hydrogen bonds between the hydrogen of the hydroxyl group in position 3 and the oxygen of the adjacent ring.4 The two hydroxyl groups occupy different stereochemical surroundings; furthermore, we can deduce that they also show different degrees of reactivity.
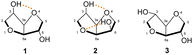 | ||
| Fig. 5 Three-dimensional structures of isohexides with possible hydrogen bonds shown.4 | ||
In isomannide, the hydroxyl groups have the same stereochemistry, thus making 2 a highly symmetric derivative with the possibility of forming two intramolecular hydrogen bonds, thanks to the endo arrangement of both hydroxyl groups facing the inside of the chiral cavity. In isoidide 3, no intramolecular hydrogen bond can occur due to the orientation of the two OH groups towards the outside of the cavity.
1.2. Properties of isohexides
Regardless of the endo or exo position of the hydroxyl groups, for the same carbon skeleton the reactivity of the two substituents in 3 and 6 is affected by the nearby chemical environment.The structural difference between the three isohexides can be also predicted from the wavelengths at which the maxima of the different peaks relating to the stretching of the O–H bonds can be observed via IR spectroscopy (Fig. 6).15,16
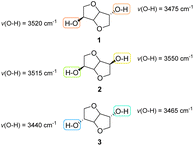 | ||
| Fig. 6 Open-source FT-IR simulation data for O–H stretching of isohexides.14 | ||
Even the literature 1H-NMR spectra15,16 of isohexides demonstrate the non-equivalence of the above-mentioned groups, if all other assumptions can be considered equal. The data and deductions shown in Table 1 have been obtained from theoretical simulations, while at the experimental level these differences are rarely observed through the most common techniques of characterization of organic compounds, performed under standard conditions.
| Compound | δ [ppm] | H | Multiplicity | J [Hz] |
|---|---|---|---|---|
| 1 | 3.65–3.95 | 8H | dd | 7.0, 5.9 |
| 3.81 | dd | 12.9, 6.8 | ||
| 3.87 | dd | 12.9, 4.7 | ||
| 3.88 | ddd | 6.8, 5.9, 4.7 | ||
| 3.65–4.00 | 8H | dd | 7.3, 6.7 | |
| 2 | 3.77 | dd | 10.4, 6.1 | |
| 3.90 | ddd | 6.7, 6.1, 5.5 | ||
| 3.93 | dd | 10.4, 5.5 | ||
| 3.66 | 2H | dd | 7.0, 3.8 | |
| 3 | 3.82–4.00 | 6H | ddd | 7.8, 6.8, 3.8 |
| 3.91 | dd | 10.5, 7.8 | ||
| 3.93 | dd | 10.5, 6.8 |
The structural difference between the three isomers clearly implies differences in their chemical–physical properties, such as their melting temperature (Table 2) and the reactivity of the hydroxyl groups.
| Compound | Melting temperature [°C] |
|---|---|
| 1 | 60–63 |
| 2 | 80–85 |
| 3 | 50–56 |
As discussed before, the difference in melting temperature ranges can be attributed to the presence or absence of intramolecular hydrogen bonds. Considering the steric effects and the establishment of hydrogen bonds, isomannide 2 with the two endo hydroxyl groups is certainly the least reactive isomer, and isoidide 3 with the two exo groups is more reactive than isosorbide 1. Therefore, it could be deduced that isoidide 3 is the most attractive isomer on the market, but, unfortunately, it is the rarest in nature and remains the most expensive for many industrial applications.
For the above-mentioned reasons, research attention in the field of isohexides derived from renewable sources and their applications is mainly directed towards the endo–exo isosorbide 1 and in recent years has witnessed a notable increase. The interest in isosorbide lies both in the possibility of selective derivatization of the OH groups and in the presence of the cavity-like structure. This cavity constitutes a true chiral confined system, within which molecules of appropriate size and stereochemistry can be hosted or converted, thus favouring enantioselection.
Meanwhile, the selective derivatization of the hydroxyl groups of isomannide 2 is possible only through the control of the stoichiometry of the reagents. Based on a statistical approach, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between isomannide and the derivatizing agent is usually employed, which often leads to the formation of a mixture of mono-derivatized product, disubstituted derivative and unreacted reagent.
1 ratio between isomannide and the derivatizing agent is usually employed, which often leads to the formation of a mixture of mono-derivatized product, disubstituted derivative and unreacted reagent.
On the other hand, the non-equivalence of the OH groups of isosorbide 1 enables its selective functionalization and hence the production of mono-substituted and non-symmetric composites with great industrial relevance.
2. Synthesis of isohexides
2.1. From biogenic polysaccharides
Polysaccharides are a class of macromolecules that are widespread in nature among animals, plants, microorganisms and algae. These polymeric materials are commonly characterized by high molecular weight and complex structural features that determine their biological functions.18The performance of these functions can be crucial for the survival, growth and health development of the organism that synthesizes them or that exploits their metabolism. In recent years, there have been increasing attempts to study natural biomaterials based on polysaccharides. The enhanced properties of polysaccharides have been extended to various applications, including biomedical engineering, human drug delivery, food packaging, biofuel isolation from biomasses, contaminated wastewater treatment and even textile fiber production.19
Due to their biodegradable and biocompatible characters, bioactive polysaccharides have been largely used in hexitol production processes, according to the synthetic scheme shown in Fig. 7.
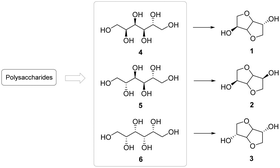 | ||
| Fig. 7 Scheme of isohexide production from hexitols.19 | ||
As previously mentioned in the Introduction (Section 1), hexitols are C6 polyols: organic compounds with various hydroxyl groups present in the structure with a skeleton composed of six carbon atoms. Therefore, starting from polysaccharides it is possible to isolate hexitols, thus obtaining the precursors for isohexides directly from sustainable sources.
In fact, isohexides have been known since 1880 and their synthesis commonly involved the dehydration of the corresponding hexitols (sorbitol, mannitol and iditol) in an acid environment and in the presence of a solvent.
 | ||
| Fig. 8 Isosorbide synthesis starting from sorbitol, using an acidic aqueous medium and traditional reaction conditions.20 | ||
The desire for the combination of Green Chemistry principles and industrial progress has led to the development of new production methods for this isohexidic intermediate. For example, in 2013 an innovative catalytic dehydration strategy of sorbitol 4 to isosorbide was proposed,21 which employed microwaves (MWs) as a heating method and Amberlyst 35 as an acid catalyst (Fig. 9).
 | ||
| Fig. 9 Isosorbide synthesis starting from sorbitol, using Amberlyst 35 in acidic aq. medium and a MW heating system.21 | ||
This research led to optimization of the reaction conditions, efficient recovery of energy and an increase in isosorbide yield. Following the principles of atomic efficiency and a good compromise between yield and purity of the product, this strategy is still nowadays the most widely used to produce isosorbide from hexitols.
 | ||
| Fig. 10 Isomannide synthesis starting from mannitol, using different solvent–catalyst systems and traditional heating.20 | ||
| Solvent/Catalyst | 2 yield [wt%] |
|---|---|
| Ethyl acetate/dioxane | 35 |
| Dichloroglycerol | 36 |
| Conc. hydrochloric acid | 25–40 |
The main disadvantages of these synthetic methods included the requirement of solvents, the commonly unsatisfactory yield achieved, the obtaining of a large amount of resinous distillation residues and the long reaction times often required, reaching over 85 hours for complete substrate-to-product conversion.
The issues, also highlighted in Section 1.1.1, exclude the possibility of preparing both isosorbide 1 and isomannide 2 on a large scale with these methods. In addition, the cost of the starting material (for example pure mannitol and sorbitol) is considerably high compared to the normal cost of the precursors used in the chemical industry to produce intermediates intended for commodities.
Subsequently, an innovative synthetic route for isohexides was proposed, where the authors obtained high yields of isomannide with a selective reaction, in the absence of solvent, by treating the crystalline hexitol with gaseous hydrogen halide (preferably HCl(g)), as shown Fig. 11. To date, these are the most used dehydration reaction conditions at the laboratory level to produce 1 and 2 from hexitols 4 and 5, respectively.
 | ||
| Fig. 11 Solvent-free isosorbide and isomannide synthesis starting from sorbitol and mannitol, using catalysts and gaseous hydrogen halide.20 | ||
The reaction conditions shown in Fig. 12 are analogous to those traditionally used. Therefore, an acidic medium, in which the acid functionalities catalyze the dehydration reaction and consequent intramolecular closure of the bicyclic structure, was successfully employed.
 | ||
| Fig. 12 Isoidide synthesis starting from iditol, using concentrate sulfuric acid and a traditional heating system.20 | ||
The authors were able to propose a reaction mechanism that involved the two steps shown in Fig. 13: a catalytic dehydrogenation–hydrogenation reaction and a hydride abstraction/re-addition step. The first step included the use of Ru/C as a catalyst in 2-propanol, while the hydride abstraction was performed with Pb(OAc)2 in acetic anhydride, leading to an overall isoidide purity with a value higher than 99%.
 | ||
| Fig. 13 Isoidide synthesis starting from isosorbide, using a two-step chemo-catalytic process.22 | ||
In fact, a few years earlier, the optimized conditions for the synthesis reaction of isoidide from isosorbide had been developed by the group of Le Nôtre et al.,15 highlighting the potential applications of epimerization reactions of isohexides.
As demonstrated by the survey discussed in Section 1, isohexide 1 has the greatest applicative possibilities and the most abundant availability in nature. Therefore, the discussion will focus on the industrial and laboratory perspectives of using isosorbide as a building block in the applied fields of organic synthesis.
2.2. Synthesis of isosorbide from raw biomasses
A more complex but more abundant sugar source than simple biogenic polysaccharides is constituted by cellulosic and lignocellulosic biomass. This type of biomass does not compete with the agri-food sector and often constitutes a waste volume that aggravates down-stream stages of industrial production processes of products such as paper, pulp, cellulose, etc.To date, studies have been carried out on the possibility of obtaining isosorbide by means of a one-pot synthesis starting from cellulose or lignocellulosic biomasses. The synthesis shown in Fig. 14 involved the following cascade: (i) the hydrolysis of cellulose or starch to glucose 7, (ii) the hydrogenation of glucose to sorbitol 4, (iii) the dehydration of sorbitol to sorbitan 8, and lastly (iv) the dehydration of sorbitan to isosorbide 1.
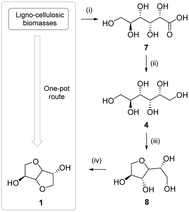 | ||
| Fig. 14 One-pot isosorbide synthesis starting from biomasses.13 | ||
The one-pot process required both an acidic environment and a hydrogenation catalyst. The acidity of the medium, the crystallinity and particle size of the cellulose, and the nature and therefore the origin of the raw materials, significantly influenced the yield of the isosorbide production process. The deactivation of the catalyst in the presence of lignin (present in most cellulosic raw material stocks) and its non-recyclability in an aqueous environment were considered the main problems of this proposed synthetic path.
In these studies, the possibility of formulating a sustainable and interesting way of designing a heterogeneous catalyst composed of a supported metal, designed to be stable in water and tolerant to the presence of lignin, was hypothesized. Carrying out this reaction in continuous flow using two alternating catalytic beds (suitable for the regeneration of the catalyst) could constitute an alternative solution for the synthesis of isosorbide from raw biomasses.
There was recent experimental evidence of a certain success for the synthesis of 1 starting from lignocellulosic biomass, which employed a heterogeneous ruthenium-based hydrogenation catalyst supported on carbon.24 Unfortunately, the reaction conditions shown in Table 4 led to the synthesis of isosorbide with low yields that do not exceed 65% by weight, a value that can be traced back to the phenomenon of deactivation of the hydrogenation catalyst, which significantly lowers the product yield.
Research in this area is moving towards the design of a heterogeneous catalyst composed of a transition metal supported on a carrier with acid moieties, stable in water and tolerant to the presence of the lignin contained in the starting material. In this way, it would be possible to overcome the difficulties encountered to date in undertaking a synthesis with a very complex matrix, such as lignocellulosic biomasses.
Certainly, the choice of synthesizing dianhydrous hexitols starting from biomass of plant origin represents the most complex strategy at the laboratory level, but it could constitute a scalable innovation process suitable for the industrial production of important intermediates such as isosorbide.
3. Isohexides as building blocks
In the chemical field, the term “building blocks” refers to a virtual molecular fragment or a real chemical compound that possesses reactive functional groups. Building blocks are used for the bottom-up assembly of molecular architectures such as nanoparticles, metal–organic structures, organic compounds with high molecular complexity and supra-molecular complexes.30The selective reactivity of the two hydroxyl groups, the chirality, the controlled stereochemistry and the performance of its derivatives, lead to isosorbide being a promising “green fine chemical”. In fact, isohexides in general are considered fine chemicals as they are produced in limited quantities and at a high market price and are therefore destined to be converted into value-added products.
3.1. Chiral intermediates based on isohexides
Dianhydrous hexitolic derivatives have been successfully employed in asymmetric catalysis.31 Asymmetric catalysis is the most widely used method for carrying out an asymmetric synthesis, i.e., the conversion of a prochiral substrate into a chiral product with preferential formation of one of the possible enantiomers.32 To perform a reaction within asymmetric catalysis, a chiral catalyst is commonly needed. Usually, a transition-metal complex coordinated by chiral ligands is employed and when it is formed of purely organic chiral ligands, the reaction is known as asymmetric organocatalysis.33The efficiency of an enantioselective synthesis process can be very high, but each stage of the synthesis influences the final product yield; therefore, an industrial design suitable for the purpose will be required. This approach is particularly important in the field of pharmaceutical synthesis, since different enantiomers or diastereomers of a molecule often have different biological activities.34
Among the many possible synthetic intermediates that can be produced from isohexides, chiral derivatives are of vital importance. Given the exceptional properties and advantages mentioned above, the mono- and disubstituted derivatives of isosorbide will be mainly discussed.
At first glance, some of the isosorbide derivatives worth mentioning are reported in Fig. 15.
 | ||
| Fig. 15 Industrially relevant derivatives of isosorbide.35 | ||
The synthesis of a wide spectrum of mono- and diesters, mono- and dinitrates, and mono- and ditosylates of isosorbide, such as compounds 9–13, were reported by Bhat et al.,36 expanding the chiral intermediates that can be obtained from biomass-derived isosorbide. For example, most of the amino derivatives of isosorbide are designed for the polymer field (e.g., polyurethanes and polyamides), as will be discussed in Section 3.3, but the role of isosorbide-based building blocks has attracted great curiosity, especially in the chemical industry for the asymmetric synthesis of enantiopure compounds.37
In fact, in recent years the call for a sustainable transition away from well-known polluting and hazardous reactions and an upgrade of the large-scale synthesis of intermediates, such as ligands and chiral auxiliaries, has found an outlet in asymmetric structures based on isosorbide derivatives.
For instance, Wu et al.38 developed a novel isosorbide-based dinitrile synthesis procedure, leading to the production of compounds with one extra carbon atom for each hydroxyl group through isosorbide epimerization under mild and base-catalyzed reaction conditions (Fig. 16). The author proposed a detailed reaction mechanism through a structural analysis of the possible dinitrile isomers via DRX analysis39 and DFT calculations,40 demonstrating that different characteristic structural parameters of the chiral cavity, such as dihedral angles and torsion angles, can vary over a wide range and modify the outcome of the reaction.
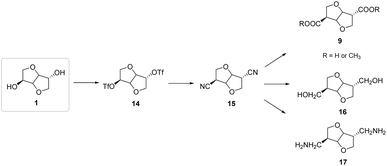 | ||
| Fig. 16 Synthesis of 1-carbon-extended isosorbide-based compounds through epimerization reactions.38 | ||
The concept of structural rigidity of the bicyclic cavity was overcome by the authors, who proposed the possibility of less conservation of torsion and were consequently able to demonstrate access to an interesting class of building blocks in which the substituent orientation effects the yield of the extended C2/C5 isohexidic compounds.38
Isohexidic derivatives have been used in asymmetric catalysis, as chiral metal ligands for the asymmetric reduction of alkenes, ketones and imines, and for the formation of C–C bonds via allylic substitution, alkylation and hydroformylation.31,41
They have also been employed as chiral auxiliaries in Diels–Alder reactions (Fig. 17), affording products with enantiomeric excesses of up to 99% for the desired product. The authors were able to fully demonstrate the efficiency of using isosorbide-based compounds as chiral auxiliaries, leading to comparable or even improved results with respect to the more traditional auxiliary compounds based on natural sources, such as camphor and menthol derivatives.
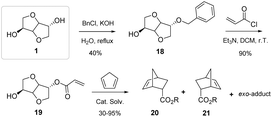 | ||
| Fig. 17 Diels–Alder reaction of an acrylate derived from exo-mono-benzylated isosorbide and cyclopentadiene.42 | ||
Isosorbide has also been employed as a starting material for the synthesis of many novel chiral amino alcohols and diamines, as shown in Fig. 18. These derivatives have been synthesized in moderate to good yields (43–75%), depending on the RNH2 reagents used. The above-mentioned study demonstrated the potential use of the obtained products as chiral ligands for asymmetric transfer hydrogenation (ATH) reactions of aromatic ketones catalyzed by ruthenium, providing chiral alcohols with enantiomeric excesses up to 91%.43
 | ||
| Fig. 18 Synthesis of some amino derivatives of isosorbide.43 | ||
The exploitation of biomass-based starting materials can also be applied in the case of isosorbide itself and not only for its derivatives,44 whose interconversion has appeared very attractive in recent years. In fact, the endo–exo spatial arrangement of the two hydroxyl groups in positions 3 and 6 of the isosorbide skeleton has been successfully converted to synthesize a wide range of organo-catalysts and chiral auxiliaries.45
Zullo et al.46 were able to synthesize an amino alcohol with an isohexide structure by subjecting isosorbide to various reactive steps, including the innovative selective acetylation of the endo–OH group through a protection performed by a biocatalyst in heterogeneous form (Fig. 19).46
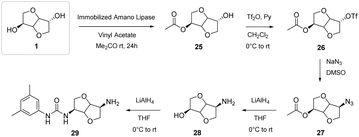 | ||
| Fig. 19 Synthesis of hydroxyurea from isosorbide, used as a chiral discrimination agent.46 | ||
Starting from the amino alcohol in question, they were able to successfully use this compound as a chiral solvating agent in the enantio-discrimination of complex aromatic structures, demonstrating the possibility of using the functionality of asymmetric induction from a chiral pool.47
Recently, the Mitsunobu reaction48 has also been successfully performed on isosorbide, leading to the synthesis of some bioactive intermediates with improved pharmacodynamic and pharmacokinetic properties.49
In fact, conjugates containing isohexidic moieties have been synthesized that confer improved solubility and permeability properties to the structure, compared to the use of bare scaffold compounds towards target compounds. Only a few of the possible examples of isosorbide-based compounds with high added-value in the field of enantioselective and asymmetric synthesis have been mentioned, but research has witnessed a large increase in possible chiral discriminants based on the strategies discussed in Section 3.1.
3.2. Chiral ionic liquids based on isohexides
The efficiency of using isosorbide and isomannide in the synthesis of chiral ionic liquids (CILs) has been widely demonstrated and discussed in the literature. CILs are a subset of ionic liquids (ILs), which are salts that are liquid in a temperature range close to room temperature.50In ionic liquids, the nature of the ions plays a fundamental role in their efficiency as solvents and the presence of a strong ionic interaction within these substances is reflected in their chemical–physical properties. Different ions impart different behavior in the final IL, but the most common characteristics include low vapor pressure, relative non-flammability, good thermal and mechanical properties and electrochemical stability.51 For the synthesis of high-value chemical products, such as fine chemicals and pharmaceuticals, the replacement of conventional processes with processes based on the use of an ionic liquid is interesting only if the following requirements are satisfied: the IL allows a high degree of recyclability, high yields and easy separation of products.
Studies carried out in recent years have led to the statement that not all ionic liquids are intrinsically green solvents, since some are considered extremely toxic. However, the ability to modify their properties through molecular design, due to their intrinsic versatility, and their correct use can contribute to the development of processes with significantly higher sustainability levels with respect with the twelve principles of the Green Chemistry.52
Chiral ionic liquids (CILs) have gained considerable potential in asymmetric synthesis, stereoselective polymerization, chiral chromatography and production of different liquid crystals.
In the past decades, the ammonium and imidazole salts of isosorbide have been studied thoroughly. As shown in Fig. 20, with simple methods it was possible to synthesize a new family of ammonium-based CILs, containing a chiral moiety due to the presence of the isosorbide structure and a hydroxyl functionality free for further modifications.53
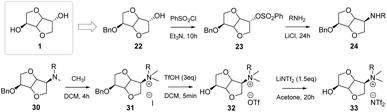 | ||
| Fig. 20 Example of synthesis of CILs from isosorbide endo-mono-benzoate.53 | ||
Chiral ammonium-based ionic liquids are just one example of possible CILs that are being widely studied.54 Chirality can also be imparted to ILs by the anion to form protic and aprotic ILs based on production needs and surely the range of new isosorbide-based ions will soon be expanded.
3.3. Isohexides in polymer science
Since isosorbide has been classified by the FDA as a GRAS (“Generally Recognized As Safe”) compound, it constitutes the most available dianhydro hexitol on the market. Therefore, isosorbide can be used as a greener alternative to petroleum-derived and oil-based chemicals in industrial polymerization processes. The molecular structure of isosorbide and the possibility of derivatization with appropriate functionalities provide excellent characteristics of compatibility with many commercial polymeric materials and additives for special uses.55 Indeed, it can be used in the synthesis of low-molecular-weight compounds that can act as plasticizers,56 stabilizers or compatibilizers and in the synthesis of thermosetting materials such as epoxy resins.57Since BPA is a compound that is recognized as toxic, it is sought to be replaced, especially in cases of use in the food packaging or biomedical industries. In fact, in recent years, alternative glycidyl ethers have been synthesized, such as those resulting from resorcinol or from simple sugars and carbohydrates of natural origin.
The use of isosorbide as a substitute for BPA fits perfectly into this context and Rose et al.13 were able to successfully employ 1 as an alternative diglycidyl ether for the preparation of epoxy resins (Fig. 21). With this synthetic strategy, it was possible to obtain epoxy resins with a behavior typical of a material with a high rubbery modulus thanks to the low molar mass and high percentage of functionalization of the formed oligomers.13,59 By the comparison of different studies, it emerged that the inclusion of isosorbide functionalities can lead to oligomers with a molar mass of 260–770 g mol−1 with an increased impact and tensile strength.
 | ||
| Fig. 21 Isosorbide as a substitute of BPA in the production of epoxy resins.13 | ||
The mentioned results were obtained despite the characteristic lower glass-transition temperature of diglycidyl ethers based on isosorbide compared to traditional epoxy resins that use BPA as a reagent.60
On an industrial scale, polyesters can be obtained from carboxylic acids or derivatives and diols using a two-stage polycondensation process that involves the release of a low-molecular-weight by-product. They can be either aromatic or aliphatic based on the nature of the monomers used in the production process.
Among the most common aromatic polyesters we can find poly(ethylene terephthalate) (PET), poly(butylene terephthalate) (PBT), poly(cyclohexylene dimethylene terephthalate) (PCT) and poly(ethylene naphthenate) (PEN), while among the aliphatic ones we can find poly(butylene succinate) (PBS), poly(ethylene succinate) (PES), poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), etc.61
Promising results have been obtained in the synthesis of a polyester based on the structure of PET, in which the ethylene glycol has been replaced with isosorbide. The polycondensation of isosorbide with terephthalic acid and its acid dichloride led to the production of poly(isosorbide terephthalate) (PIsT), as shown in Fig. 22.65
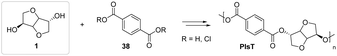 | ||
| Fig. 22 Isosorbide as a monomer for the synthesis of PET analogue PIsT.65 | ||
Given the versatility of the hydroxyl groups, 1 can also be converted into the respective diacids and/or diesters and used in the formulation of ester-based copolymers with innovative and interesting structures.66 Recently, Wu et al.67 were able to synthesize and thoroughly characterize a class of polyesters (shown in Fig. 23) that were completely based on the carbon skeleton of isosorbide, for both the diol and the diester components, named poly(isosorbide isosorbate) (PIsI).
 | ||
| Fig. 23 Fully isosorbide-based polyesters.67 | ||
This review has demonstrated the possibility of optimizing the atom economy of polyester production processes and the sustainable derivation of the raw materials used to obtain polymeric products, with characteristics comparable to those currently on the market for that specific commercial use.68,69
Still in the field of aliphatic polyesters, Wu et al.70 have been able to carry out a CALB-catalyzed enzymatic polymerization (EP) of diethyl succinate, 1,4-butanediol (1,4-BDO) and isosorbide, obtaining copolyesters of poly(butylenisosorbide-co-succinate) (PBIsS) in good yields. The polymeric materials produced by the authors (Fig. 24) showed a tunable temperature of glass transition and crystallinity in a wide range, which could make them suitable for new fields of applications, such as textile fibers or in engineering plastic materials.71,72
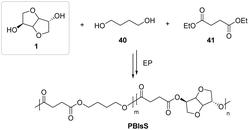 | ||
| Fig. 24 Fully aliphatic isosorbide-based copolyester via enzymatic polymerization using lipase.70 | ||
The most relevant scientific cases concerning the use of isosorbide derivatives in the innovative polymer industry will be discussed below, starting from aliphatic copolyesters based on 39, the methyl diester of isosorbide.
As shown in Fig. 25, the methyl diester of isosorbide has been successfully employed as one of the two diacid reagents in a polycondensation process that included the employment of dimethyl adipate and 1,4-BDO.73 The authors were able to fully demonstrate the production of novel systems with improved viscoelastic properties with an average molecular weight of approximately 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–47600 g mol−1, as well as surprising self-adhesion capabilities and consequent industrial weldability.
000–47600 g mol−1, as well as surprising self-adhesion capabilities and consequent industrial weldability.
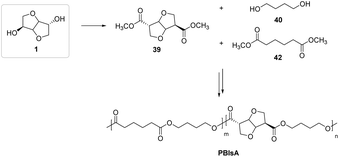 | ||
| Fig. 25 Fully aliphatic isosorbide-based copolyester via melt polycondensation process.73 | ||
Thiyagarajan et al.74 reported the synthesis of AB-type homo-polyesters based on exo-OH monomers of isohexides, with molecular weights comparable to those witnessed from the BB- or AA-type analogues.75 With the same molecular weight of 2500 g mol−1, the thermal properties of the produced samples were found to be suitable for application at higher temperatures. Poly(isoidide-co-isoidide dicarboxylic acid) (PIiI) and poly(isosorbide-co-isoidide dicarboxylic acid) (PIsI) were the references, and the samples proposed by the authors consisted of a set of six compositions of isohexides and their respective methylcarboxylates.74
The various bifunctional derivatives of isosorbide, isomannide and isoidide also have high application potential in the polymer industry.76 They were successfully included in the production of biobased polymers, solvents, fuel additives, nitrogenous-based plastics such as polyamides, polyimides and polyurethanes, and many others.13
Isohexidic functionalities were successfully inserted into the structures of partially biobased polyimide monomers by Thiyagarajan et al.78 The presence of isosorbide in the repeating chain resulted in polymeric samples exhibiting higher mechanical stiffness and thermal stability comparable to their petrochemical counterparts. In fact, the innovation resided in their excellent optical transparency and optical activity properties, attributable to the chiral cavities of isosorbide.79
Poly(ether imide)-based polymers (PEIs) that exploit the structure of isosorbide as a co-monomer to impart special properties to the produced materials have been successfully synthesized and fully characterized (Fig. 26).80 The authors were able to demonstrate that most of the samples derived from isosorbide possessed thermal properties (namely glass transition temperatures), tensile strengths and Young’s moduli comparable to those of petroleum-based poly(ether imide)s.
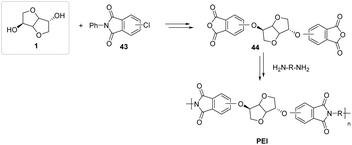 | ||
| Fig. 26 Poly(ether imide)s (PEIs) synthesized starting from isosorbide-derived dianhydrides.80 | ||
Polyurethanes have a very diversified field of application, but are commonly used to produce both flexible and rigid foams.82 An important structure–property correlation study of innovative polymers within the class of sustainable polyurethanes was carried out by the Kieber et al.83 The authors provided an interesting screening of the possibility of employing different stereochemical isomers of isohexides in the synthesis of polymeric materials with molecular weights in the range of 6–34 kDa.
The isosorbide-based PU in Fig. 27 showed the best results in terms of mechanical strength, higher density, reduced free volume and good thermal stability compared to the counterpart considered,84,85 based on biomass-derived diols86 of hydroxymethyl furfural (HMF).
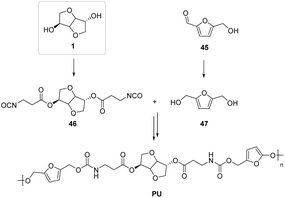 | ||
| Fig. 27 Polyurethane synthesis starting from biomass-derived monomers.83 | ||
Polyacetals are polymeric materials with simple bond repeating units (–CH2–O–) and are commonly used in the production of components for household appliances, gears, bearings and automotive parts, and components for biomedical devices.88 They are used as commodities and, given the large market demand, the possibility of replacing oil-based components with more sustainable solutions appears to be an excellent starting point for the green transition of the economy in the context of polyacetals.
Isosorbide-based diacetals were subjected to acid-catalyzed polycondensation (Fig. 28) under mild conditions. The authors were able to synthesize innovative materials with good yields, appearance and intrinsic characteristics, such as a molecular weight of 3200–27600 g mol−1 for the isolated product. This publication led several groups of researchers to investigate the possibility of using isosorbide in this field.89,90
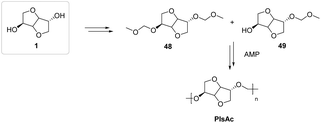 | ||
| Fig. 28 Isosorbide-based polyacetals synthesized through acetal metathesis polymerization (AMP).87 | ||
PAs are macromolecular materials characterized by an amide bond (–CO–NH–) in the main chain. In polymers, this bond is established by a polycondensation reaction of a –COOH terminal of a carboxylic acid and an –NH2 terminal of an amine.91 The amide bond can resonate between two structures and can assume a partial double-bond character, thus giving the bond a planar geometry, a certain rigidity and a non-negligible Debye dipole moment. Commonly, these characteristics allow macromolecules to be organized in trans-planar linear structures.
In addition, the spatial arrangement of the chains allows for effective overlap and the possibility of forming hydrogen bonds translates into a high degree of crystallinity that reaches values of up to 40–50%, and molecular weights of 104 g mol−1.92
Jasinska-Walc et al.93 were able to synthesize fully biobased polyamides from diamino-isosorbide and sebacic or brassylic acid, C10 and C13 aliphatic diacids derived from castor oil, as shown in Fig. 29. To date, all polyamides produced on an industrial scale from monomers derived from biomass have been based on monomers derived from castor oil.94 A problem that has not yet been addressed is the competition with the food supply chain for the use of building blocks of natural origin to produce polymeric commodities. The use of isohexides such as isosorbide could constitute the green breakthrough so sought after by many researchers in the sector of macromolecular products.95
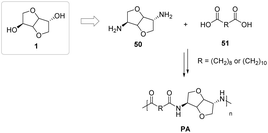 | ||
| Fig. 29 Polyamide synthesis starting from isosorbide-based diamine and aliphatic natural dicarboxylic acids.93 | ||
In Section 3, the current discussion on the sustainability of the monomer feedstock supply chain, and on the main potential application fields of isosorbide-based compounds in the polymeric materials industry, has been thoroughly highlighted.
4. Conclusions
In this review, a comprehensive survey of peculiar compounds with isohexidic structure was performed with a focus on isosorbide 1, a dianhydro hexitol with two endo–exo OH groups in positions 3 and 6, respectively. The main properties that distinguish isohexides from any other reactant class have been addressed in Section 1, with particular attention to the correlations between the molecular structure and properties of the final product. In Section 2, it was discussed how isosorbide can be successfully synthesized via common dehydration of hexitols or separated from vegetable waste biomasses that do not compete with the agri-food industry. Isosorbide’s interesting characteristics, which can be exploited for the replacement of different diol compounds commonly derived from the crude oil sector, have been emphasized starting from Section 3. The singular structure of isosorbide is based on two tetrahydrofuran rings fused with a cis-type junction and the spatial arrangement of the two hydroxyl substituents allows this isohexide to be used for its intrinsic chiral property as a chiral auxiliary and/or as a reactant.In the last part of the review, the reader can find in-depth bibliographic research with salient examples of uses of isosorbide in the synthesis of polymeric materials, with a high percentage of biobased components, thanks to its high reactivity and the possibility of forming intramolecular hydrogen bonds.
For instance, the use of isosorbide as a monomer, comonomer and precursor has been demonstrated in the production processes of different types of polymers, such as epoxy resins, polyesters, polyimides, polyurethanes, polyacetals, and polyamides with good to excellent yields and high average molecular weights.
Overall, this review has shown how and why isosorbide can be considered a compound of great industrial relevance in many chemical sectors. The reader will be able to recognize the potential exploitation of isosorbide in the field of asymmetric synthesis, the realization of chiral ionic liquids and the production of polymeric materials with high applicative potential. Following the author’s personal interpretation of the cited literature, the differences that can be encountered when isosorbide is used as a reactant or auxiliary diol, instead of the traditional diols deriving from the petrochemical chain, will be clarified.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The author would like to express her gratitude to Dr AP and Prof. AI for the training and valuable guidance provided during the author’s academic internship periods.References
- C. Briens, J. Piskorz and F. Berruti, Biomass valorization for fuel and chemicals production-A review, Int. J. Chem. React. Eng., 2008, 6(1) DOI:10.2202/1542-6580.1674
.
-
R. Homan. Determining the Feasibility of Selected Alternative Products Co-produced from Sugarcane, PhD dissertation, North-West University, South Africa, 2024 Search PubMed
.
-
U.S. Energy Information Administration, U.S. Energy Facts Explained [Internet]. Eia.Gov. [cited 2025 Feb 28], U.S. Energy Information Administration, 2024, Available from: https://www.eia.gov/energyexplained/us-energy-facts/ Search PubMed
.
- S. Pandey, B. S. Rajput and S. H. Chikkali, Refining plant oils and sugars to platform chemicals, monomers, and polymers, Green Chem., 2021, 23(12), 4255–4295 RSC
.
- P. Ning, G. Yang, L. Hu, J. Sun, L. Shi, Y. Zhou, Z. Wang and J. Yang, Recent advances in the valorization of plant biomass, Biotechnol. Biofuels, 2021, 14(1), 102 CrossRef PubMed
.
- X. Liu, X. Wang, S. Yao, Y. Jiang, J. Guan and X. Mu, Recent advances in the production of polyols from lignocellulosic biomass and biomass-derived compounds, RSC Adv., 2014, 4(90), 49501–49520 RSC
.
- A. Wisniewski, E. Skorupowa and J. Sokolowski, Dehydration of some hexitols, J. Carbohydr. Chem., 1991, 10(1), 77–90 CrossRef CAS
.
- M. Meghana and Y. Shastri, Sustainable valorization of sugar industry waste: Status, opportunities, and challenges, Bioresour. Technol., 2020, 303, 122929 CrossRef CAS
.
-
L. Ahsaini, A. Ait Benhamou, M. Mennani, Y. Abdellaoui, S. Sair, A. El Bouari and Z. Kassab, Advanced Development in Biomass Valorization for Biofuels, Value-Added Products and Energy Production, in Microbial Niche Nexus Sustaining Environmental Biological Wastewater and Water-Energy-Environment Nexus, Springer Nature Switzerland, Cham, 2025, pp. 1–35 Search PubMed
.
- E. Redina, O. Tkachenko and T. Salmi, Recent advances in C5 and C6 sugar alcohol synthesis by hydrogenation of monosaccharides and cellulose hydrolytic hydrogenation over non-noble metal catalysts, Molecules, 2022, 27(4), 1353 CrossRef CAS PubMed
.
- M. M. Rana and S. H. De la Hoz, Influence of ionic liquid (IL) treatment conditions in the regeneration of cellulose with different crystallinity, J. Mater. Res., 2023, 38(2), 328–336 CrossRef CAS
.
- L. Chopra, Extraction of cellulosic fibers from the natural resources: A short review, Mater. Today: Proc., 2022, 48, 1265–1270 CAS
.
- M. Rose and R. Palkovits, Isosorbide as a renewable platform chemical for versatile applications—quo vadis?, ChemSusChem, 2012, 5(1), 167–176 CrossRef CAS PubMed
.
- Simulate and predict IR spectra, 2025, [cited 2025 Feb 28], Available from: https://www.cheminfo.org/.
- J. Le Nôtre, J. van Haveren and D. S. van Es, Synthesis of isoidide through epimerization of isosorbide using ruthenium on carbon, ChemSusChem, 2013, 6(4), 693–700 CrossRef PubMed
.
- V. Jašek, J. Fučík, J. Krhut, L. Mravcova, S. Figalla and R. Přikryl, A study of isosorbide synthesis from sorbitol for material applications using isosorbide dimethacrylate for enhancement of bio-based resins, Polymers, 2023, 15(17), 3640 CrossRef PubMed
.
- Simulate and predict NMR spectra, 2025, [cited 2025 Feb 28], Available from: https://www.nmrdb.org/.
- Z. Wang, X. Zhou, Z. Shu, Y. Zheng, X. Hu, P. Zhang, H. Huang, L. Sheng, P. Zhang, Q. Wang and X. Wang, Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight, Int. J. Biol. Macromol., 2023, 244, 125360 CrossRef CAS PubMed
.
- S. Tunçer, Biopolysaccharides: properties and applications, Polysaccharides, 2021, 17, 95–134 Search PubMed
.
-
H. Salzburg, H. Meyborg and H. Ziemann, Process for the Preparation of 1, 4-3, 6-Dianhydro-Hexitols, US
Pat., No. 4408061A, 1983
.
- I. Polaert, M. C. Felix, M. Fornasero, S. Marcotte, J. C. Buvat and L. Estel, A greener process for isosorbide production: Kinetic study of the catalytic dehydration of pure sorbitol under microwave, Chem. Eng. J., 2013, 222, 228–239 CrossRef CAS
.
- J. Saska, S. Dutta, A. Kindler, S. J. Zuend and M. Mascal, Efficient and scalable production of isoidide from isosorbide, ACS Sustain. Chem. Eng., 2021, 9(34), 11565–11570 CrossRef CAS
.
- M. Reist, B. Testa, P. A. Carrupt, M. Jung and V. Schurig, Racemization, enantiomerization, diastereomerization, and epimerization: their meaning and pharmacological significance, Chirality, 1995, 7(6), 396–400 CrossRef CAS
.
- I. Bonnin, R. Mereau, T. Tassaing and K. D. Vigier, One-pot synthesis of isosorbide from cellulose or lignocellulosic biomass: a challenge?, Beilstein J. Org. Chem., 2020, 16(1), 1713–1721 CrossRef CAS
.
- G. Liang, C. Wu, L. He, J. Ming, H. Cheng, L. Zhuo and F. Zhao, Selective conversion of concentrated microcrystalline cellulose to isosorbide over Ru/C catalyst, Green Chem., 2011, 13(4), 839–842 RSC
.
- J. Keskiväli, S. Rautiainen, M. Heikkilä, T. T. Myllymäki, J. P. Karjalainen, K. Lagerblom, M. Kemell, M. Vehkamäki, K. Meinander and T. Repo, Isosorbide synthesis from cellulose with an efficient and recyclable ruthenium catalyst, Green Chem., 2017, 19(19), 4563–4570 RSC
.
- L. Chen, Y. Li, X. Zhang, Y. Liu, Q. Zhang, C. Wang and L. Ma, One-pot conversion of cellulose to liquid hydrocarbon efficiently catalyzed by Ru/C and boron phosphate in aqueous medium, Energy Procedia, 2019, 158, 160–166 CrossRef CAS
.
- R. Palkovits, K. Tajvidi, A. M. Ruppert and J. Procelewska, Heteropoly acids as efficient acid catalysts in the one-step conversion of cellulose to sugar alcohols, Chem. Commun., 2011, 47(1), 576–578 CAS
.
- B. Op de Beeck, J. Geboers, S. Van de Vyver, J. Van Lishout, J. Snelders, W. J. Huijgen, C. M. Courtin, P. A. Jacobs and B. F. Sels, Conversion of (ligno) cellulose feeds to isosorbide with heteropoly acids and Ru on carbon, ChemSusChem, 2013, 6(1), 199–208 CAS
.
- J. M. Fréchet, Dendrimers and other dendritic macromolecules: From building blocks to functional assemblies in nanoscience and nanotechnology, J. Polym. Sci., Part A: Polym. Chem., 2003, 41(23), 3713–3725 Search PubMed
.
- M. Kadraoui, T. Maunoury, Z. Derriche, S. Guillarme and C. Saluzzo, Isohexides as versatile scaffolds for asymmetric catalysis, Eur. J. Org. Chem., 2015, 2015(3), 441–457 CAS
.
- S. Masamune, W. Choy, J. S. Petersen and L. R. Sita, Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis, Angew. Chem., Int. Ed. Engl., 1985, 24(1), 1–30 CrossRef
.
- H. Pellissier, Asymmetric organocatalysis, Tetrahedron, 2007, 63(38), 9267–9331 CrossRef CAS
.
- J. H. Yu, Z. P. Yu, R. J. Capon and H. Zhang, Natural enantiomers: occurrence, biogenesis and biological properties, Molecules, 2022, 27(4), 1279 CrossRef CAS
.
- J. Wu, P. Eduard, S. Thiyagarajan, J. van Haveren, D. S. van Es, C. E. Koning, M. Lutz and C. Fonseca Guerra, Isohexide derivatives from renewable resources as chiral building blocks, ChemSusChem, 2011, 4(5), 599 CrossRef CAS PubMed
.
- N. S. Bhat, N. Vinod, K. Tarafder, M. K. Nayak, A. Jana, S.
S. Mal and S. Dutta, Efficient preparation of the esters of biomass-derived isohexides by base-catalyzed transesterification under solvent-free conditions, Ind. Eng. Chem. Res., 2023, 62(43), 17483–17492 CrossRef
.
- M. Janvier, S. Moebs-Sanchez and F. Popowycz, Nitrogen-functionalized isohexides in asymmetric induction, Chimia, 2016, 70(1–2), 77–83 CrossRef CAS PubMed
.
- J. Wu, S. Thiyagarajan, C. Fonseca Guerra, P. Eduard, M. Lutz, B. A. Noordover, C. E. Koning and D. S. Van Es, Isohexide dinitriles: A versatile family of renewable platform chemicals, ChemSusChem, 2017, 10(16), 3202–3211 CrossRef CAS PubMed
.
- A. Jesche, M. Fix, A. Kreyssig, W. R. Meier and P. C. Canfield, X-Ray diffraction on large single crystals using a powder diffractometer, Philos. Mag., 2016, 96(20), 2115–2124 CAS
.
- A. H. Mazurek, Ł. Szeleszczuk and D. M. Pisklak, Periodic DFT calculations—review of applications in the pharmaceutical sciences, Pharmaceutics, 2020, 12(5), 415 CrossRef CAS
.
- A. Loupy and D. A. Monteux, Isomannide and isosorbide as new chiral auxiliaries for the stereoselective synthesis of tertiary α-hydroxy acids, Tetrahedron, 2002, 58(8), 1541–1549 CrossRef CAS
.
- A. Loupy and D. Monteux, Asymmetric Diels-Alder: monobenzylated isosorbide and isomannide as highly effective chiral auxiliaries, Tetrahedron Lett., 1996, 37(39), 7023–7026 CrossRef CAS
.
- K. D. Huynh, H. Ibrahim, E. Kolodziej, M. Toffano and G. Vo-Thanh, Synthesis of a new class of ligands derived from isosorbide and their application to asymmetric reduction of aromatic ketones by transfer hydrogenation, New J. Chem., 2011, 35(11), 2622–2631 RSC
.
- S. M. Lait, D. A. Rankic and B. A. Keay, 1, 3-Aminoalcohols and their derivatives in asymmetric organic synthesis, Chem. Rev., 2007, 107(3), 767–796 CrossRef CAS PubMed
.
- S. M. Paek, M. Jeong, J. Jo, Y. M. Heo, Y. T. Han and H. Yun, Recent advances in substrate-controlled asymmetric induction derived from chiral pool α-amino acids for natural product synthesis, Molecules, 2016, 21(7), 951 CrossRef
.
- V. Zullo, A. Petri and A. Iuliano, An Efficient and Practical Chemoenzymatic Route to (3R, 3aR, 6R, 6aR)-Hexahydrofuro [3, 2-b] furan-6-amino-3-ol (6-Aminoisomannide) from Renewable Sources, SynOpen, 2021, 5(03), 161–166 CrossRef CAS
.
- F. Cefalì, A. Iuliano, F. Balzano, G. Uccello Barretta, V. Zullo and C. Baldassari, Isohexide-Based Tunable Chiral Platforms as Amide-and Thiourea-Chiral Solvating Agents for the NMR Enantiodiscrimination of Derivatized Amino Acids, Molecules, 2024, 29(6), 1307 CrossRef PubMed
.
- T. Y. But and P. H. Toy, The Mitsunobu reaction: origin, mechanism, improvements, and applications, Chem. – Asian J., 2007, 2(11), 1340–1355 CrossRef CAS PubMed
.
- A. Sidduri, M. J. Dresel and S. Knapp, Incorporation of an Isohexide Subunit Improves the Drug-like Properties of Bioactive Compounds, ACS Med. Chem. Lett., 2023, 14(2), 176–182 CrossRef CAS PubMed
.
- J. Ding and D. W. Armstrong, Chiral ionic liquids: synthesis and applications, Chirality, 2005, 17(5), 281–292 CrossRef CAS PubMed
.
- J. Flieger, J. Feder-Kubis and M. Tatarczak-Michalewska, Chiral ionic liquids: structural diversity, properties and applications in selected separation techniques, Int. J. Mol. Sci., 2020, 21(12), 4253 CrossRef CAS
.
- W. L. Wong and K. Y. Wong, Recent development in functionalized ionic liquids as reaction media and promoters, Can. J. Chem., 2012, 90(1), 1–6 CrossRef CAS
.
- O. Nguyen Van Buu and G. Vo-Thanh, Synthesis of novel chiral ammonium-based ionic liquids derived from isosorbide and their applications in an asymmetric aza diels-alder reaction, Lett. Org. Chem., 2007, 4(3), 158–167 CrossRef
.
- J. Pernak and J. Feder-Kubis, Synthesis and properties of chiral ammonium-based ionic liquids, Chem.– Eur. J., 2005, 11(15), 4441–4449 CrossRef CAS
.
- Y. Zhao, L. Guo, B. Fang and B. Liu, Recent Advances in the Research of Biobased Polymers and the Catalytic Application of Ionic Liquids in Their Synthesis, Ind. Eng. Chem. Res., 2024, 63(46), 19955–19971 CrossRef CAS
.
- D. Battegazzore, S. Bocchini, G. Nicola, E. Martini and A. Frache, Isosorbide, a green plasticizer for thermoplastic starch that does not retrogradate, Carbohydr. Polym., 2015, 119, 78–84 CrossRef CAS PubMed
.
- J. Hong, D. Radojčić, M. Ionescu, Z. S. Petrović and E. Eastwood, Advanced materials from corn: isosorbide-based epoxy resins, Polym. Chem., 2014, 5(18), 5360–5368 RSC
.
- F. L. Jin, X. Li and S. J. Park, Synthesis and application of epoxy resins: A review, J. Ind. Eng. Chem., 2015, 29, 1–11 CrossRef CAS
.
- M. Chrysanthos, J. Galy and J. P. Pascault, Preparation and properties of bio-based epoxy networks derived from isosorbide diglycidyl ether, Polymer, 2011, 52(16), 3611–3620 CrossRef CAS
.
- H. A. Patil, V. A. Maske and A. P. More, Synthesis and Characterization of Eco-Friendly Epoxy Resins and Novel Fillers for Enhanced Corrosion Protection of Mild Steel, Polym. Adv. Technol., 2024, 35(12), e70027 CrossRef CAS
.
- C. Vilela, A. F. Sousa, A. C. Fonseca, A. C. Serra, J. F. Coelho, C. S. Freire and A. J. Silvestre, The quest for sustainable polyesters–insights into the future, Polym. Chem., 2014, 5(9), 3119–3141 RSC
.
- D. Juais, A. F. Naves, C. Li, R. A. Gross and L. H. Catalani, Isosorbide polyesters from enzymatic catalysis, Macromolecules, 2010, 43(24), 10315–10319 CrossRef CAS
.
- J. M. Sadler, A. P. Nguyen, F. R. Toulan, J. P. Szabo, G. R. Palmese, C. Scheck, S. Lutgen and J. J. La Scala, Isosorbide-methacrylate as a bio-based low viscosity resin for high performance thermosetting applications, J. Mater. Chem. A, 2013, 1(40), 12579–12586 RSC
.
- J. J. Gallagher, M. A. Hillmyer and T. M. Reineke, Isosorbide-based polymethacrylates, ACS Sustainable Chem. Eng., 2015, 3(4), 662–667 CrossRef CAS
.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Polymers from renewable 1, 4: 3, 6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review, Prog. Polym. Sci., 2010, 35(5), 578–622 CrossRef CAS
.
- J. Wu, P. Eduard, S. Thiyagarajan, B. A. Noordover, D. S. van Es and C. E. Koning, Semi-Aromatic Polyesters Based on a Carbohydrate-Derived Rigid Diol for Engineering Plastics, ChemSusChem, 2015, 8(1), 67–72 CrossRef CAS PubMed
.
- J. Wu, P. Eduard, L. Jasinska-Walc, A. Rozanski, B. A. Noordover, D. S. Van Es and C. E. Koning, Fully isohexide-based polyesters: Synthesis, characterization, and structure–properties relations, Macromolecules, 2013, 46(2), 384–394 CrossRef CAS
.
- B. A. Noordover, V. G. van Staalduinen, R. Duchateau, C. E. Koning, R. A. van Benthem, M. Mak, A. Heise, A. E. Frissen and J. van Haveren, Co-and terpolyesters based on isosorbide and succinic acid for coating applications: synthesis and characterization, Biomacromolecules, 2006, 7(12), 3406–3416 CrossRef CAS
.
- Y. Zhang, Q. Lei, S. Zhang, L. Dai, B. Lyu and L. Liu, Biobased Unsaturated Polyester Thermosets from Castor Oil and Isosorbide with Life Cycle Assessment, ACS Sustainable Chem. Eng., 2024, 13(1), 374–385 CrossRef
.
- J. Wu, J. Qi, Y. Lin, Y. Chen, X. Zhang, R. Wu and H. Wang, Lipase-catalyzedfully
aliphatic copolyesters based on renewable isohexide isomers, ACS Sustainable Chem. Eng., 2021, 9(4), 1599–1612 CrossRef CAS
.
- L. Gustini, C. Lavilla, A. M. de Ilarduya, S. Muñoz-Guerra and C. E. Koning, Isohexide and Sorbitol-Derived, Enzymatically Synthesized Renewable Polyesters with Enhanced Tg, Biomacromolecules, 2016, 17(10), 3404–3416 CrossRef CAS PubMed
.
- S. Vijjamarri, M. Hull, E. Kolodka and G. Du, Renewable Isohexide-Based, Hydrolytically Degradable Poly (silyl ether) s with High Thermal Stability, ChemSusChem, 2018, 11(17), 2881–2888 CAS
.
- Y. Lin, M. Ye, X. Zhang, Y. Chen, Y. Chen, J. Wu and H. Wang, Biodegradable copolyesters based on a “soft” isohexide building block with tunable viscoelasticity and self-adhesiveness, Polym. Chem., 2022, 13(31), 4511–4523 RSC
.
- S. Thiyagarajan, J. Wu, R. J. Knoop, J. Van Haveren, M. Lutz and D. S. Van Es, Isohexide hydroxy esters: synthesis and application of a new class of biobased AB-type building blocks, RSC Adv., 2014, 4(89), 47937–47950 RSC
.
- J. Wu, P. Eduard, S. Thiyagarajan, L. Jasinska-Walc, A. Rozanski, C. F. Guerra, B. A. Noordover, J. Van Haveren, D. S. van Es and C. E. Koning, Semicrystalline polyesters based on a novel renewable building block, Macromolecules, 2012, 45(12), 5069–5080 CAS
.
- J. C. Morales-Huerta, A. Martinez de Ilarduya, S. León and S. Muñoz-Guerra, Isomannide-containing poly (butylene 2, 5-furandicarboxylate) copolyesters via ring opening polymerization, Macromolecules, 2018, 51(9), 3340–3350 CAS
.
-
R. G. Bryant, Polyimides, Encyclopedia of Polymer Science and Technology, 2002 Search PubMed
.
- S. Thiyagarajan, L. Gootjes, W. Vogelzang, J. van Haveren, M. Lutz and D. S. van Es, Renewable rigid diamines: efficient, stereospecific synthesis of high purity isohexide diamines, ChemSusChem, 2011, 4(12), 1823–1829 CAS
.
- X. Ji, Z. Wang, J. Yan and Z. Wang, Partially bio-based polyimides from isohexide-derived diamines, Polymer, 2015, 74, 38–45 CAS
.
- X. Ji, Z. Wang, Z. Wang and J. Yan, Bio-based poly (ether imide) s from isohexide-derived isomeric dianhydrides, Polymers, 2017, 9(11), 569 Search PubMed
.
- J. O. Akindoyo, M. H. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam and A. R. Yuvaraj, Polyurethane types, synthesis and applications–a review, RSC Adv., 2016, 6(115), 114453–114482 CAS
.
- T. A. Phung Hai, M. Tessman, N. Neelakantan, A. A. Samoylov, Y. Ito, B. S. Rajput, N. Pourahmady and M. D. Burkart, Renewable polyurethanes from sustainable biological precursors, Biomacromolecules, 2021, 22(5), 1770–1794 CAS
.
- R. J. Kieber, S. A. Silver and J. G. Kennemur, Stereochemical effects on the mechanical and viscoelastic properties of renewable polyurethanes derived from isohexides and hydroxy-methylfurfural, Polym. Chem., 2017, 8(33), 4822–4829 RSC
.
- O. Gómez-de-Miranda-Jiménez-de-Aberasturi, A. Centeno-Pedrazo, F. S. Prieto, R. Rodriguez Alonso, S. Medel, J. María Cuevas, L. G. Monsegue, S. De Wildeman, E. Benedetti, D. Klein and H. Henneken, The future of isosorbide as a fundamental constituent for polycarbonates and polyurethanes, Green Chem. Lett. Rev., 2021, 14(3), 534–544 CrossRef
.
- C. Díez-Poza, V. Sessini, D. Jaraba Cabrera, M. E. Mosquera and C. J. Whiteoak, Non-Isocyanate Polyurethanes (NIPUs) Incorporating Isomers of Abundant Sugar-Derived 1, 4: 3, 6-Dianhydrohexitols, ACSAppl. Polym. Mater., 2024, 6(23), 14695–14706 CrossRef
.
- V. Besse, R. Auvergne, S. Carlotti, G. Boutevin, B. Otazaghine, S. Caillol, J. P. Pascault and B. Boutevin, Synthesis of isosorbide-based polyurethanes: An isocyanate free method, React. Funct. Polym., 2013, 73(3), 588–594 CrossRef CAS
.
- B. S. Rajput, S. R. Gaikwad, S. K. Menon and S. H. Chikkali, Sustainable polyacetals from isohexides, Green Chem., 2014, 16(8), 3810–3818 RSC
.
- A. C. Renner, S. S. Thorat and M. P. Sibi, Synthesis of biobased polyacetals: a review, RSC Sustainability, 2024, 2(12), 3669–3703 RSC
.
- N. Hammami, N. Jarroux, M. Robitzer, M. Majdoub and J. P. Habas, Optimized synthesis according to one-step process of a biobased thermoplastic polyacetal derived from isosorbide, Polymers, 2016, 8(8), 294 CrossRef
.
- N. Hammami, M. Majdoub and J. P. Habas, Structure-properties relationships in isosorbide-based polyacetals: Influence of linear or cyclic architecture on polymer physicochemical properties, Eur. Polym. J., 2017, 93, 795–804 CrossRef CAS
.
- K. Marchildon, Polyamides–still strong after seventy years, Macromol. React. Eng., 2011, 5(1), 22–54 CrossRef CAS
.
- M. Winnacker and B. Rieger, Biobased polyamides: recent advances in basic and applied research, Macromol. Rapid Commun., 2016, 37(17), 1391–1413 CrossRef CAS PubMed
.
- L. Jasinska-Walc, D. Dudenko, A. Rozanski, S. Thiyagarajan, P. Sowinski, D. Van Es, J. Shu, M. R. Hansen and C. E. Koning, Structure and molecular dynamics in renewable polyamides from dideoxy–diamino isohexide, Macromolecules, 2012, 45(14), 5653–5666 CrossRef CAS
.
- J. Fan, W. Liu, L. Cai, T. Jiang and Z. Wang, Castor oil-based multi-functional monomers and their application in polyamide design, Ind. Crops Prod., 2023, 203, 117188–8 CrossRef CAS
.
- J. Wu, L. Jasinska-Walc, D. Dudenko, A. Rozanski, M. R. Hansen, D. Van Es and C. E. Koning, An investigation of polyamides based on isoidide-2, 5-dimethyleneamine as a green rigid building block with enhanced reactivity, Macromolecules, 2012, 45(23), 9333–9346 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |