Significant improvement in the conversion efficiency of black-dye-based dye-sensitized solar cells by cosensitization with organic dye†
Hironobu
Ozawa
,
Ryosuke
Shimizu
and
Hironori
Arakawa
*
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, 12-1, Ichigaya-Funagawara, Shinjuku, Tokyo, 162-0826, Japan. E-mail: h.arakawa@ci.kagu.tus.ac.jp; Fax: (+81)-3-5261-4631; Tel: (+81)-3-5228-8311
First published on 29th February 2012
Abstract
The conversion efficiency of black-dye-based dye-sensitized solar cell (DSC) was improved by cosensitization with organic dye (NKX-2553 or D131). This improved conversion efficiency was further enhanced by employing deoxycholic acid (DCA) as a coadsobent. The highest conversion efficiency, 11.6% under AM 1.5 irradiation (100 mW cm−2), was obtained in DSC with black dye and D131 in the presence of DCA.
Dye-sensitized solar cells (DSCs) have been extensively investigated over two decades because they are regarded as promising candidates as alternatives to silicon photovoltaic devices.1 Light-to-electrical energy conversion efficiency of DSCs has reached more than 11% by employing efficient ruthenium sensitizer, such as black dye {(TBA)3[Ru(Htcterpy)(NCS)3] (TBA = tetrabutylammonium, tcterpy = 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine), Fig. 1} or N719 {(TBA)2[cis-Ru(Hdcbpy)2(NCS)2] (dcbpy = 4,4′-dicarboxy-2,2′-bipyridine)}.2 Since the conversion efficiency of DSCs depends entirely on the sensitizing ability of dyes, extensive efforts have been made to develop highly efficient dyes for panchromatic sensitization. On the other hand, one of the other approaches to achieve panchromatic sensitization is by cosensitization using multiple dyes which have complementary absorption features. In most cases, the conversion efficiency of the DSC was not improved by such a cosensitization method, however, recently effective enhancement of the conversion efficiency has been achieved by cosensitization method using two kinds of organic dyes,3 Zn phthalocyanine (or Zn porphyrine) and organic dye,4 and ruthenium complex dye and organic dye.5 These studies clearly indicate that the combination of two dyes is essential to improve the conversion efficiency, although basic guidelines for the selection of dyes are still unclear. One of the important points required for the effective enhancement of the conversion efficiency by the cosensitization method is that each dye can suppress aggregation of the other dye at the TiO2 surface since dye aggregation at the TiO2 surface causes a decrease in the solar cell performance of the DSCs.6 In previous studies, effective enhancement of the conversion efficiency was achieved although a coadsorbent, such as deoxycholic acid (DCA), was not employed. Therefore, it is reported that each dye effectively suppresses the aggregation of the other dye at the TiO2 surface.3,4,5
 | ||
| Fig. 1 Structures of black dye, NKX-2553, D131, and DCA. | ||
On the other hand, continuous efforts have been made by our group to increase the conversion efficiency of black-dye-based DSCs, and more than 10.5% conversion efficiency has been achieved using DCA as a coadsorbent.7 In this communication, we report significant improvement of the conversion efficiency of black-dye-based DSC using an organic dye (NKX-25538 or D131,9Fig. 1) as a coadsorption dye. Moreover, we also report the effect of these coadsorption dyes on the suppression of black dye aggregation at the TiO2 surface. Conversion efficiency was further improved by the addition of DCA as a coadsorbent in these cosensitized DSCs.
Coadsorption of black dye and various kinds of organic dyes was carried out by immersing a TiO2 photoelectrode into the mixed dye solution. From preliminary investigations, NKX-2553 and D131 were found to be effective as a coadsorption dye for the black-dye-based DSC. The solar cell performances of cosensitized DSCs, together with those of DSCs with single dye, prepared under the optimized condition are summarized in Table 1. The conversion efficiency of black-dye-based DSC (10.0%) was improved to 10.3% by using NKX-2553 as a coadsorption dye (entry 7). This enhancement is mainly attributed to the large increase of Jsc value (from 20.61 to 22.11 mA cm−2). Since the total amount of dye adsorption was increased from 3.5 × 10−7 to 4.5 × 10−7 mol cm−2 by coadsorption with NKX-2553, this result is considered as a main reason for the improvement of Jsc value. IPCE spectra of black-dye-based and cosensitized DSCs are shown in Fig. 2. The maximum IPCE values between 650 and 800 nm were increased by cosensitization as well as those between 400 and 550 nm. NKX-2553 has almost no contribution to increase IPCE values under this region and the amount of black dye adsorption was decreased from 3.5 × 10−7 to 2.8 × 10−7 mol cm−2 by coadsorption. These results seem to imply that NKX-2553 suppressed black-dye aggregation at the TiO2 surface like a DCA. On the other hand, conversion efficiency was significantly improved by using D131 as a coadsorption dye, the same as previously reported.5a 11.1% conversion efficiency was achieved by the increase of Jsc value (from 20.61 to 23.23 mA cm−2, entry 9). Since the amount of D131 adsorption (3.3 × 10−7 mol cm−2) was twice as large as that of NKX-2553 (1.7 × 10−7 mol cm−2), the total amount of dye adsorption (6.1 × 10−7 mol cm−2) is larger than that of the DSC with black dye and NKX-2553 (4.5 × 10−7 mol cm−2). This larger total amount of dye adsorption is considered as a main reason for the observed higher Jsc value of the DSC with black dye and D131. The maximum IPCE values between 650 and 800 nm were also increased by cosensitization, suggesting that black-dye aggregation at the TiO2 surface is suppressed by the addition of D131. Such an efficacy of D131 on the suppression of black-dye aggregation at the TiO2 surface has been previously reported.5a The improvement of solar cell performance of black-dye-based DSC by cosensitization with an organic dye is mainly attributed to the increase of the total amount of dye adsorption. In addition, suppression of black-dye aggregation at the TiO2 surface also contributed to improve the solar cell performance to some extent.
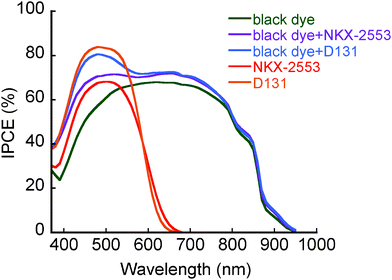 | ||
| Fig. 2 IPCE spectra of the DSC with black dye, NKX-2553, D131, black dye and NKX-2553, and black dye and D131. | ||
| Entry | Dye | J sc (mA cm−2) | V oc (V) | FF | η (%) | Amount of black dye (× 10−7 mol cm−2) | Amount of organic dye (× 10−7 mol cm−2) |
|---|---|---|---|---|---|---|---|
| a The electrolyte is an acetonitrile solution containing of 0.05 M I2, 0.1 M LiI, 0.6 M DMPImI, and 0.3 M TBP (TiO2 film thickness: 45 μm, active area: 0.26 cm2). Irradiation is carried out using a solar simulator (AM 1.5, 100 mW cm−2). b Solar cell performance of the DSC with an anti-reflection film and a black mask (active areas: 0.226 cm2). | |||||||
| 1 | Black dye | 20.61 | 0.682 | 0.711 | 10.00 | 3.5 | |
| 2 | Black dye + DCA | 21.27 | 0.697 | 0.711 | 10.57 | 2.7 | |
| 3 | NKX-2553 | 8.40 | 0.646 | 0.726 | 3.94 | 1.2 | |
| 4 | NKX-2553 + DCA | 8.06 | 0.667 | 0.741 | 3.98 | 0.5 | |
| 5 | D131 | 9.94 | 0.658 | 0.723 | 4.73 | 3.3 | |
| 6 | D131 + DCA | 9.53 | 0.678 | 0.741 | 4.79 | 1.9 | |
| 7 | Black dye + NKX-2553 | 22.11 | 0.657 | 0.710 | 10.32 | 2.8 | 1.7 |
| 8 | Black dye + NKX-2553 + DCA | 23.00 | 0.686 | 0.709 | 11.18 | 2.4 | 1.6 |
| 9 | Black dye + D131 | 23.23 | 0.676 | 0.706 | 11.08 | 2.8 | 3.3 |
| 10 | Black dye + D131 + DCA | 23.34 | 0.685 | 0.709 | 11.33 | 2.3 | 2.0 |
| 11 | Black dye + D131 + DCAb | 23.49 | 0.683 | 0.721 | 11.57 | ||
Further investigation has been carried out to determine whether black-dye aggregation at the TiO2 surface is fully dispersed in cosensitized DSCs. Coadsorption of black dye and organic dye (NKX-2553 or D131) was carried out using the mixed dye solution containing of various concentrations of DCA. The solar cell performance of the DSC with black dye and NKX-2553 was significantly improved by the addition of DCA, and the conversion efficiency was enhanced from 10.3% to 11.2% (entry 8). The Jsc value was largely increased from 22.11 to 23.00 mA cm−2 by the addition of DCA even although the total amount of dye adsorption decreased from 4.5 × 10−7 to 4.0 × 10−7 mol cm−2. As shown in Fig. 3, maximum IPCE values increased over the whole visible range. These results indicate that additional DCA dispersed the remaining black-dye aggregation. Moreover, the Voc value also increased from 0.657 to 0.686 V by the addition of DCA, suggesting that the backward electron transfer from the conduction band of TiO2 is effectively prevented. This interpretation is supported by the fact that the electron lifetime in the TiO2 photoelectrode of DSC with black dye and NKX-2553 is significantly improved by the addition of DCA (Fig. S8†). On the other hand, slight enhancement of the solar cell performance was observed in the DSC with black dye and D131 by the addition of DCA (entry 10). Although the total amount of dye adsorption was decreased from 6.1 × 10−7 to 4.3 × 10−7 mol cm−2 by the addition of DCA, the Jsc value slightly increased. Therefore, additional DCA disrupted the remaining black-dye aggregation in the same way as DSC with black dye and NKX-2553. These results clearly indicate that black-dye aggregation at the TiO2 surface is not fully dispersed in the cosensitized DSCs. The solar cell performance of the DSC with black dye and NKX-2553 was largely improved by the addition of DCA compared to that of the DSC with black dye and D131, indicating that the effect of NKX-2553 on the suppression of black-dye aggregation is lower than that of D131. This difference probably comes from the difference of the molecular size between NKX-2553 and D131 (Fig. 1). Namely D131 can serve as a more efficient coadsorbent than NKX-2553 since the molecular size of D131 is much larger. On the other hand, the electron lifetime in the TiO2 photoelectrode of DSC with black dye and D131 was improved by the addition of DCA (Fig. S9†). This improvement is considered as one reason for the slight increase of Voc value. The highest conversion efficiency (11.6%) was obtained in the consensitized DSC with black dye and D131 in the presence of DCA equipped with an anti-reflecting film under AM 1.5 (100 mW cm−2) irradiation (active area: 0.226 cm2) (entry 11, Fig. S4†). The enhancement of the solar cell performances of the cosensitized DSCs by the addition of DCA is due to both suppression of black-dye aggregation and prevention of the backward electron transfer from the conduction band of TiO2.
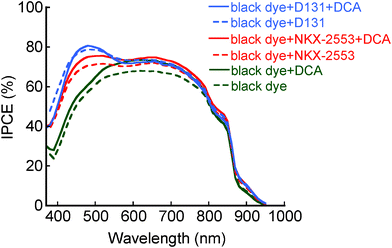 | ||
| Fig. 3 IPCE spectra of the DSC with black dye and NKX-2553, and black dye and D131 in the presence and absence of DCA. | ||
In conclusion, the conversion efficiency of the black-dye-based DSC was improved by cosensitization with NKX-2553 or D131. This study demonstrated that effects of NKX-2553 and D131 on the suppression of the black dye aggregation at the TiO2 surface are not sufficient and that the solar cell performances of the cosensitized DSCs were further improved by the addition of DCA which can disrupt the remaining dye aggregations and prevent the backward electron transfer from the conduction band of TiO2. 11.6% conversion efficiency was obtained in the cosensitized DSC with black dye and D131 in the presence of DCA.
Acknowledgements
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan. H. O. acknowledges a Grant-in-Aid for young scientist (B) (No. 22750057) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a research grant from Kurita Water and Environment Foundation (No. 23092).References
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS; (b) M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef; (c) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS.
- (a) M. K. Nazeeruddin, F. D. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS; (b) Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS; (c) F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphyry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 10720 CrossRef CAS; (d) C.-Y. Chen, M. Wang, J.-Y. Li, N. Pootrakulchote, L. Alibabaei, C. Ngocle, J.-D. Decoppet, J. Tsai, C. Grätzel, C.-G. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103 CrossRef CAS; (e) Y. Cao, Y. Bai, Q. Yu, Y. Cheng, S. Li, D. Shi, F. Gao and P. Wang, J. Phys. Chem. C, 2009, 113, 6290 CrossRef CAS; (f) F. Sauvage, J.-D. Decoppet, M. Zhang, S. M. Zakeeruddin, P. Comte, M. Nazeeruddin, P. Wang and M. Grätzel, J. Am. Chem. Soc., 2011, 133, 9304 CrossRef CAS.
- (a) D. Kuang, P. Walter, F. Nüesch, S. Kim, J. Ko, P. Comte, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, Langmuir, 2007, 23, 10906 CrossRef CAS; (b) J.-H. Yum, S.-R. Jang, P. Walter, T. Geiger, F. Nüesch, S. Kim, J. Ko, M. Grätzel and M. K. Nazeeruddin, Chem. Commun., 2007, 4680 RSC; (c) B. J. Song, H. M. Song, I. T. Choi, S. K. Kim, K. D. Seo, M. S. Kang, M. J. Lee, D. W. Cho, M. J. Ju and H. K. Kim, Chem.–Eur. J., 2011, 17, 11115 CrossRef CAS.
- (a) J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martinez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358 CrossRef CAS; (b) T. Bessho, S. M. Zakeeruddin, C.-Y. Yeh, E. W.-G. Diau and M. Grätzel, Angew. Chem., Int. Ed., 2010, 49, 6646 CrossRef CAS; (c) A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS.
- (a) R. Ogura, S. Nakane, M. Morooka, M. Orihashi, Y. Suzuki and K. Noda, Appl. Phys. Lett., 2009, 94, 073308 CrossRef; (b) S.-Q. Fan, C. Kim, B. Fang, K.-X. Liao, G.-J. Yang, C.-J. Li, J.-J. Kim and J. Ko, J. Phys. Chem. C, 2011, 115, 7747 CrossRef CAS.
- (a) M. K. Nazeeruddin, R. Humphry-Baker, M. Grätzel and B. A. Murrer, Chem. Commun., 1998, 719 RSC; (b) A. C. Khazraji, S. Hotchandani, S. Das and P. V. Kamat, J. Phys. Chem. B, 1999, 103, 4693 CrossRef CAS; (c) J. He, G. Benkö, F. Korodi, T. Polivka, R. Lomoth, B. Åkermark, L. Sun, A. Hagfeldt and V. Sundström, J. Am. Chem. Soc., 2002, 124, 4922 CrossRef CAS; (d) X. Ren, Q. Feng, G. Zhou, C.-H. Huang and Z.-S. Wang, J. Phys. Chem. C, 2010, 114, 7190 CrossRef CAS; (e) K. Hara, H. Sugihara, Y. Tachibana, A. Islam, M. Yanagida, K. Sayama and H. Arakawa, Langmuir, 2001, 17, 5992 CrossRef CAS; (f) K. Hara, Y. Dan-oh, C. Kasada, Y. Ohga, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, Langmuir, 2004, 20, 4205 CrossRef CAS.
- (a) Z.-S. Wang, T. Yamaguchi, H. Sugihara and H. Arakawa, Langmuir, 2005, 21, 4272 CrossRef CAS; (b) H. Ozawa, M. Awa, T. Ono and H. Arakawa, Chem.–Asian J., 2012, 7, 156 CrossRef CAS; (c) H. Ozawa, Y. Okuyama and H. Arakawa, Dalton Trans., 2012 Search PubMed , in press.
- (a) K. Hara, M. Kurashige, S. Ito, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, Chem. Commun., 2003, 252 RSC; (b) K. Hara, T. Sato, R. Katoh, A. Furube, T. Yoshihara, M. Murai, M. Kurashige, S. Ito, A. Shinpo, S. Suga and H. Arakawa, Adv. Funct. Mater., 2005, 15, 246 CrossRef CAS.
- (a) W. H. Howie, F. Claeyssens, H. Miura and L. M. Perter, J. Am. Chem. Soc., 2008, 130, 1367 CrossRef CAS; (b) T. Dentani, Y. Kubota, K. Funabiki, J. Jin, T. Yoshida, H. Minoura, H. Miura and M. Matsui, New J. Chem., 2009, 33, 93 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and figures S1–S9. See DOI: 10.1039/c2ra01257j |
| This journal is © The Royal Society of Chemistry 2012 |
