Magnesium iodide-catalyzed synthesis of 2-substituted quinazolines using molecular oxygen and visible light†
T.
Yamaguchi
,
K.
Sakairi
,
E.
Yamaguchi
,
N.
Tada
and
A.
Itoh
*
Gifu Pharmaceutical University, 1-25-4 Daigaku-nishi, Gifu 501-1196, Japan. E-mail: itoha@gifu-pu.ac.jp; Fax: +81-58-230-8108; Tel: +81-58-230-8108
First published on 7th June 2016
Abstract
A novel and efficient approach for the synthesis of quinazolines by aerobic photooxidation with an iodine reagent at room temperature is reported. This method uses harmless visible light from compact fluorescent lamps and molecular oxygen as the sole oxidant without the need for a transition-metal catalyst or harsh reaction conditions.
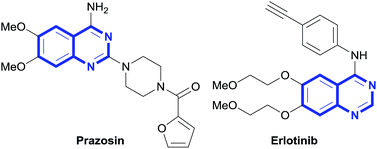
Nitrogen-containing heterocycles are a common and important structural motif both biologically and industrially.1 Of these heterocycles, quinazolines have been particularly studied for biological activity and have been reported to exhibit sedative, anticonvulsant, antitussive, hypotensive, and antidiabetic properties.2 Consequently, they have been used as sympatholytic drugs for the treatment of high blood pressure (Prazosin), non-small cell lung cancer, and pancreatic cancer (Erlotinib), as well as other conditions (Fig. 1).3
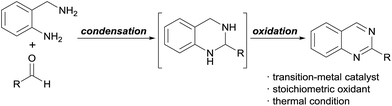
Consequently, many approaches for the efficient synthesis of quinazoline structures have been explored.4 One such strategy is the condensation of 2-aminobenzylamines and aldehydes followed by the oxidation of the intermediate (Scheme 1). However, these reactions require transition-metal catalysts, such as those based on Cu5 or Ir,6 or stoichiometric amounts or a large excess of oxidants, such as NaOCl,7 DDQ,8 or MnO2.9 Moreover, harsh reaction conditions are required. Thus, the development of more efficient and environmentally benign reactions is necessary to realize sustainable, green methods for quinazoline synthesis.
Recently, the use of molecular oxygen instead of other oxidants has received increasing attention because it is cheap and represents high atom economy. Thus, novel reactions using molecular oxygen in the presence of transition-metal catalysts or organocatalysts have been developed.10
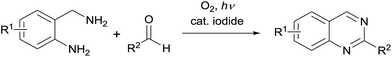
Our group has previously reported a catalytic cross-dehydrogenative coupling reaction between tertiary amines and nucleophiles using molecular oxygen and I2 under visible-light irradiation.11 This C–C bond forming reaction proceeded by oxidation of the amines into iminium ions, followed by addition of the nucleophiles without the need for transition-metal catalysts or thermal conditions. Therefore, we envisioned that a novel synthesis of quinazolines could be achieved by aerobic photooxidation in the presence of catalytic iodide (Scheme 2). Here we report an efficient synthetic approach for quinazolines under mild reaction conditions.
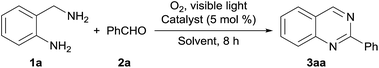
Table 1 shows the results of the optimization of the reaction conditions. As a result of solvent screening, 2-phenylquinazoline (3aa) is obtained in moderate yield by employing ethyl acetate as the solvent (entries 1–4). We then investigated the effect of the catalyst. This aerobic photooxidation proceeds with various iodide catalysts, and the yield increases to 88% when magnesium iodide is used (entries 5–8). The quantity of benzaldehyde required can be reduced to 1.0 eq. without decreasing the yield (entry 9). Magnesium iodide is essential for this oxidation system because the desired product is not formed without catalyst (entry 10).
| Entry | Catalyst | Solvent | Yieldb [%] |
|---|---|---|---|
| a Reaction conditions; 1a (0.3 mmol), 2a (0.45 mmol), and catalyst (5 mol%) in solvent (5 mL) under O2 atmosphere was stirred and irradiated with a fluorescent lamp at rt. b 1H NMR yield. c 2a (0.3 mmol) was used. | |||
| 1 | I2 | Hexane | 0 |
| 2 | I2 | EtOAc | 43 |
| 3 | I2 | CHCl3 | 3 |
| 4 | I2 | DMF | 5 |
| 5 | HI | EtOAc | 70 |
| 6 | NaI | EtOAc | 26 |
| 7 | MgI2 | EtOAc | 88 |
| 8 | Carbon tetraiodide | EtOAc | 22 |
| 9c | MgI2 | EtOAc | 89 |
| 10c | — | EtOAc | 0 |
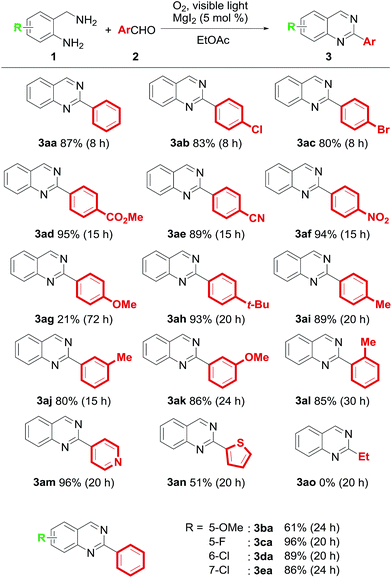
With the optimal reaction conditions established, we investigated the reaction of 1 with various substituted benzaldehydes (Table 2). The corresponding quinazolines are obtained in excellent yields when an electron-deficient benzaldehyde is employed (3aa–3af). In contrast, the yield dramatically decreases in the presence of an electron-donating group such as methoxy at the 4-position of the benzene ring (3ag). Reaction with 4-tert-butylbenzaldehyde or 4-methylbenzaldehyde generates the product in good yield (3ah–3ai). To confirm the effect of steric hindrance, meta- and ortho-substituted benzaldehydes were employed in this method. It was found that o- and m-tolualdehydes could be applied successfully. Moreover, 3-methoxybenzaldehyde is converted to the corresponding quinazoline in 86% yield (3aj–3al). This suggests that the electron density at the C2 position is a critical factor in this oxidation system. Other aldehydes such as 4-pyridinecarboxaldehyde and 2-thiophenecarboxaldehyde are oxidized to 3am and 3an, respectively. Unfortunately, no product is formed when an alkylaldehyde is used as the substrate (3ao). Furthermore, 4-, 5-, or 6-substituted 2-aminobenzylamines react with 2a, and the corresponding quinazolines are obtained in good to excellent yields (3ba–3ea).
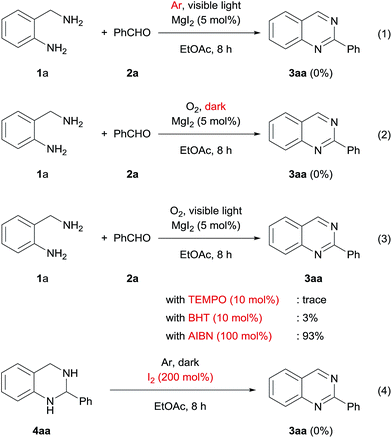
We then conducted several control experiments to investigate the reaction mechanism. The desired product was not formed in the absence of molecular oxygen and visible light irradiation (Scheme 3, eqn (1) and (2)). 2,2,6,6-Tetramethylpiperidine-1-oxyl and 2,6-di-tert-butyl-p-cresol, which are known to be radical scavengers, inhibit the generation of 3aa. On the other hand, 2,2′-azobis(isobutyronitrile), which is used to the free radical generator, does not affect the reaction (Scheme 3, eqn (3)). Thus, this oxidation system probably proceeds via a radical process. Next, we investigated whether 4aa is oxidized by iodine or another active species. When iodine (200 mol%) is employed as the oxidant in the dark under an argon atmosphere, the target product is not detected and dihydroquinazoline is formed in low yield (Scheme 3, eqn (4)). This result indicates that oxidation by iodine is not the major pathway in this reaction.
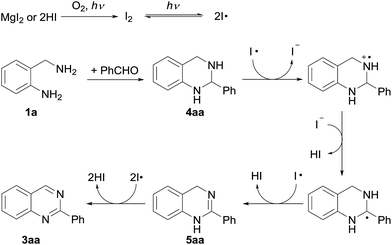
Scheme 4 shows a plausible mechanism for this reaction, which is postulated by considering all the above results. Initially, magnesium iodide is converted to iodine under irradiation by visible light in an O2 atmosphere, which is followed by homolysis of the iodine. 2-Phenyl-1,2,3,4-tetrahydroquinazoline (4aa), which is formed by condensation of 2-aminobenzylamine (1a) with benzaldehyde (2a), is converted to a benzyl radical species by single-electron transfer. The oxidation occurs again, and 2-phenyldihydroquinazoline is obtained. Subsequently, the desired product (3aa) is formed via oxidation of 5aa in the same manner. HI is reoxidized to I2 under aerobic photooxidative conditions; thus the catalytic cycle is completed.
Conclusions
In conclusion, we have developed an efficient synthesis of quinazolines under aerobic photooxidative conditions. This novel reaction is interesting as it uses catalytic magnesium iodide, molecular oxygen as the terminal oxidant, and visible light from a general-purpose fluorescent lamp. Further application and mechanistic study of this reaction are now in progress in our laboratory.Notes and references
- (a) R. Dua, S. Shrivastava, S. K. Sonwane and S. K. Srivastava, Adv. Biol. Res., 2011, 5, 120 CAS; (b) A. K. Lawrence and K. Gademann, Synthesis, 2008, 331 CAS.
- T. P. Selvam and P. V. Kumar, Res. Pharm., 2011, 1, 1 Search PubMed.
- (a) R. Gundla, R. Kazemi, R. Sanam, R. Muttineni, J. A. R. P. Sarma, R. Dayam and N. Neamati, J. Med. Chem., 2008, 51, 3367 CrossRef CAS; (b) J. F. Mendes da Silva, M. Walters, S. Al-Damluji and C. R. Ganellin, Bioorg. Med. Chem., 2008, 16, 7254 CrossRef CAS.
- (a) A. E. Wendlandt and S. S. Stahl, J. Am. Chem. Soc., 2014, 136, 506 CrossRef CAS; (b) Z. Chen, J. Chen, M. Liu, J. Ding, W. Gao, X. Huang and H. Wu, J. Org. Chem., 2013, 78, 11342 CrossRef CAS PubMed; (c) T. Vlaar, R. C. Cioc, P. Mampuys, B. U. W. Maes, R. V. A. Orru and E. Ruijter, Angew. Chem., Int. Ed., 2012, 51, 13058 CrossRef CAS; (d) S. Rachakonda, P. S. Pratap and M. V. B. Rao, Synthesis, 2012, 44, 2065 CrossRef CAS; (e) Y. Yan and Z. Wang, Chem. Commun., 2011, 47, 9513 RSC; (f) B. Han, C. Wang, R.-F. Han, W. Yu, X.-Y. Duan, R. Fang and X.-L. Yang, Chem. Commun., 2011, 47, 7818 RSC; (g) K. Karnakar, J. Shankar, S. Narayana Murthy, K. Ramesh and Y. V. D. Nageswar, Synlett, 2011, 8, 1089 Search PubMed; (h) J. Zhang, C. Yu, S. Wang, C. Wan and Z. Wang, Chem. Commun., 2010, 46, 5244 RSC; (i) J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS; (j) F. Portela-Cubillo, J. S. Scott and J. C. Walton, J. Org. Chem., 2009, 74, 4934 CrossRef CAS PubMed; (k) F. Portela-Cubillo, J. S. Scott and J. C. Walton, Chem. Commun., 2008, 2935 RSC; (l) S. Ferrini, F. Ponticelli and M. Taddei, Org. Lett., 2007, 9, 69 CrossRef CAS.
- B. Han, X.-L. Yang, C. Wang, Y.-W. Bai, T.-C. Pan, X. Chen and W. Yu, J. Org. Chem., 2012, 77, 1136 CrossRef CAS.
- J. Fang, J. Zhou and Z. Fang, RSC Adv., 2013, 3, 334 RSC.
- C. U. Maheswari, G. S. Kumar, M. Venkateshwar, R. A. Kumar, M. L. Kantam and K. R. Reddy, Adv. Synth. Catal., 2010, 352, 341 CrossRef CAS.
- J. J. Vanden Eynde, J. Godin, A. Mayence, A. Maquestiau and E. Anders, Synthesis, 1993, 867 CrossRef CAS.
- Y. Peng, Y. Zeng, G. Qiu, L. Cai and V. W. Pike, J. Heterocycl. Chem., 2010, 47, 1240 CrossRef CAS.
- For selected recent examples and reviews, see: (a) A. S.-K. Tsang, A. Kapat and F. Schoenebeck, J. Am. Chem. Soc., 2016, 138, 518 CrossRef CAS; (b) A. Bechtoldt, C. Tirler, K. Raghuvanshi, S. Warratz, C. Kornhaaβ and L. Ackermann, Angew. Chem., Int. Ed., 2016, 55, 264 CrossRef CAS; (c) A. Gonzalez-de-Castro and J. Xiao, J. Am. Chem. Soc., 2015, 137, 8206 CrossRef CAS; (d) Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045 CrossRef CAS PubMed; (e) J. Li, M. J. Lear, Y. Kawamoto, S. Umemiya, A. R. Wong, E. Kwon, I. Sato and Y. Hayashi, Angew. Chem., Int. Ed., 2015, 54, 12986 CrossRef CAS; (f) Z. Zhang, Y. Gao, Y. Liu, J. Li, H. Xie, H. Li and W. Wang, Org. Lett., 2015, 17, 5492 CrossRef CAS; (g) Y. Qin, L. Zhang, J. Lv, S. Luo and J.-P. Cheng, Org. Lett., 2015, 17, 1469 CrossRef CAS; (h) J. Liu, F. Liu, Y. Zhu, X. Ma and X. Jia, Org. Lett., 2015, 17, 1409 CrossRef CAS; (i) Q. Huang, X. Zhang, L. Qiu, J. Wu, H. Xiao, X. Zhang and S. Lin, Adv. Synth. Catal., 2015, 357, 3753 CrossRef CAS; (j) N. Bagi, J. Kaizer and G. Speier, RSC Adv., 2015, 5, 45983 RSC; (k) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS; (l) J. Piera and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2008, 47, 3506 CrossRef CAS.
- T. Nobuta, A. Fujiya, T. Yamaguchi, N. Tada, T. Miura and A. Itoh, RSC Adv., 2013, 3, 10189 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and characterization data. See DOI: 10.1039/c6ra04073j |
| This journal is © The Royal Society of Chemistry 2016 |