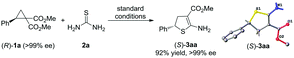Thiourea participation in [3+2] cycloaddition with donor–acceptor cyclopropanes: a domino process to 2-amino-dihydrothiophenes†
Ming-Sheng
Xie
 ,
Guo-Feng
Zhao
,
Tao
Qin
,
Yong-Bo
Suo
,
Gui-Rong
Qu
and
Hai-Ming
Guo
,
Guo-Feng
Zhao
,
Tao
Qin
,
Yong-Bo
Suo
,
Gui-Rong
Qu
and
Hai-Ming
Guo
 *
*
Henan Key Laboratory of Organic Functional Molecules and Drug Innovation, Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, China. E-mail: ghm@htu.edu.cn
First published on 7th January 2019
Abstract
The Yb(OTf)3-catalyzed [3+2] cycloaddition of donor–acceptor cyclopropanes with thiourea offers an efficient route to diverse 2-amino-4,5-dihydrothiophenes (up to 92% yield), in which optically active 2-amino-dihydrothiophenes can be produced from enantiomerically pure cyclopropanes. Thiourea, which is an odorless and cheap reagent, provides a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond, serves as an amino source, and functions as a decarbalkoxylation reagent in this reaction. Preliminary mechanistic studies demonstrate that the reaction undergoes a sequential [3+2] cycloaddition/deamination/decarboxylation process.
S double bond, serves as an amino source, and functions as a decarbalkoxylation reagent in this reaction. Preliminary mechanistic studies demonstrate that the reaction undergoes a sequential [3+2] cycloaddition/deamination/decarboxylation process.
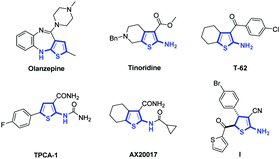
2-Aminothiophene is a special structural moiety present in many biologically active molecules.1 Examples of such molecules are shown in Fig. 1. Olanzapine is an atypical antipsychotic drug used for treating schizophrenia and bipolar disorder.2 Tinoridine is an anti-inflammatory drug that has potent antiperoxidative properties.3 T-62 is an allosteric enhancer of the adenosine A1 receptor, and TPCA-1 is a small-molecule IκB kinase β inhibitor.4 AX20017 has antituberculosis properties and has been identified as a specific inhibitor of protein kinase G.5 2-Amino-4,5-dihydrothiophene I exhibits antibacterial and antifungal properties.6 For most of these 2-aminothiophenes, which exhibit biological activities, it is found that an electron-withdrawing group (e.g., ester, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, or CN) is connected to the C3 position of the thiophenes. The most convenient method for preparing 2-aminothiophenes is the Gewald reaction, which involves the condensation of a ketone (or aldehyde) with activated nitrile and elemental sulfur.1,7 Although great achievements to construct 2-aminothiophenes have been made through the Gewald reaction, developing an alternative method to synthesize 2-aminothiophenes and their derivatives, which have an electron withdrawing group at the C3 position, is still highly desirable.8
O, or CN) is connected to the C3 position of the thiophenes. The most convenient method for preparing 2-aminothiophenes is the Gewald reaction, which involves the condensation of a ketone (or aldehyde) with activated nitrile and elemental sulfur.1,7 Although great achievements to construct 2-aminothiophenes have been made through the Gewald reaction, developing an alternative method to synthesize 2-aminothiophenes and their derivatives, which have an electron withdrawing group at the C3 position, is still highly desirable.8
Donor–acceptor (D–A) cyclopropanes are exceptionally useful three-carbon building blocks due to their synthetic utility and ease of preparation.9 In the presence of a Lewis acid, the normal [3+n] cycloaddition reactions of D–A cyclopropanes with various dipolarophiles, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, N
N, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N
O, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, C
C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, nitrones, heterocumulenes, and other dipolarophiles, have proven to be valuable tools for producing highly functionalized cyclic ring systems.10–19 However, the C
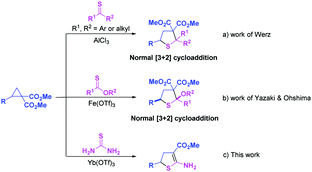
N, nitrones, heterocumulenes, and other dipolarophiles, have proven to be valuable tools for producing highly functionalized cyclic ring systems.10–19 However, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond has less been employed as a 2π component to react with D–A cyclopropanes.20,21 Very recently, the normal [3+2] cycloaddition of thioketones and D–A cyclopropanes has been published concurrently with the preparation of the present manuscript (Scheme 1a).20a Highly substituted tetrahydrothiophenes with two adjacent quaternary carbon atoms were generated in high yields using AlCl3 as a catalyst. Soon afterwards, a highly efficient Fe(OTf)3-promoted normal [3+2] cycloaddition of thionoesters with D–A cyclopropanes was developed for the synthesis of trans-configured tetrahydrothiophenes (Scheme 1b).21 As an odorless, cheap, and easy-to-handle sulfur source,22 thiourea has never previously been employed to react with D–A cyclopropanes. Herein, we report the Yb(OTf)3-catalyzed [3+2] cycloaddition of thiourea with D–A cyclopropanes to generate 2-amino-4,5-dihydrothiophene derivatives with only one ester group at the C3 position of thiophene (Scheme 1c).
S double bond has less been employed as a 2π component to react with D–A cyclopropanes.20,21 Very recently, the normal [3+2] cycloaddition of thioketones and D–A cyclopropanes has been published concurrently with the preparation of the present manuscript (Scheme 1a).20a Highly substituted tetrahydrothiophenes with two adjacent quaternary carbon atoms were generated in high yields using AlCl3 as a catalyst. Soon afterwards, a highly efficient Fe(OTf)3-promoted normal [3+2] cycloaddition of thionoesters with D–A cyclopropanes was developed for the synthesis of trans-configured tetrahydrothiophenes (Scheme 1b).21 As an odorless, cheap, and easy-to-handle sulfur source,22 thiourea has never previously been employed to react with D–A cyclopropanes. Herein, we report the Yb(OTf)3-catalyzed [3+2] cycloaddition of thiourea with D–A cyclopropanes to generate 2-amino-4,5-dihydrothiophene derivatives with only one ester group at the C3 position of thiophene (Scheme 1c).
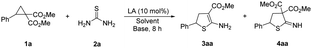
Initially, D–A cyclopropane 1a and thiourea 2a were selected as the model reactants (Table 1). When Cu(OTf)2 or Ni(OTf)2 was employed as a Lewis acid catalyst, the reaction did not occur (entries 1 and 2). When MgI2 was used, 2-amino-4,5-dihydrothiophene 3aa, which has only one ester group at the C3 position of dihydrothiophene, was obtained in 9% yield (entry 3). When the Lewis acid was changed to Yb(OTf)3, the yield of 3aa increased to 15% (entry 4). In the presence of Sc(OTf)3, only the cyclic imine 4aa, which has two ester groups at the C3 position of dihydrothiophene, was generated (entry 5). The solvents were then explored, and DCE is the optimal solvent (entries 4, 6 and 7). Increasing the temperature from rt to 90 °C resulted in an enhanced yield (entries 7 and 8). Several bases were then added, and the inorganic base Rb2CO3 delivered monoester 3aa in a better yield (entries 9–12). The cycloadduct 3aa can be afforded in 84% yield when 20 mol% of Yb(OTf)3 was employed (entry 13). When 1 equiv. of thiourea 2a was employed, the yield decreased (entry 14).
| Entry | LA | Solvent | T (°C) | Base | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 3aa | 4aa | |||||
| a Unless otherwise noted, the reaction conditions were: 1a (0.2 mmol), 2a (0.4 mmol), LA (10 mol%), solvent (3.0 mL), and base (20 mol%) at rt for 8 h. b Isolated yield. c Yb(OTf)3 (20 mol%). d 2a (0.2 mmol) was used. NR = no reaction. | ||||||
| 1 | Cu(OTf)2 | CH2Cl2 | rt | — | NR | |
| 2 | Ni(OTf)2 | CH2Cl2 | rt | — | NR | |
| 3 | MgI2 | CH2Cl2 | rt | — | 9 | — |
| 4 | Yb(OTf)3 | CH2Cl2 | rt | — | 15 | — |
| 5 | Sc(OTf)3 | CH2Cl2 | rt | — | — | 7 |
| 6 | Yb(OTf)3 | CHCl3 | rt | — | Trace | — |
| 7 | Yb(OTf)3 | DCE | rt | — | 29 | — |
| 8 | Yb(OTf)3 | DCE | 90 | — | 41 | — |
| 9 | Yb(OTf)3 | DCE | 90 | Cs2CO3 | 53 | — |
| 10 | Yb(OTf)3 | DCE | 90 | Na2CO3 | 61 | — |
| 11 | Yb(OTf)3 | DCE | 90 | Rb2CO3 | 65 | — |
| 12 | Yb(OTf)3 | DCE | 90 | Et3N | NR | |
| 13c | Yb(OTf)3 | DCE | 90 | Rb2CO3 | 84 | |
| 14d | Yb(OTf)3 | DCE | 90 | Rb2CO3 | 43 | |
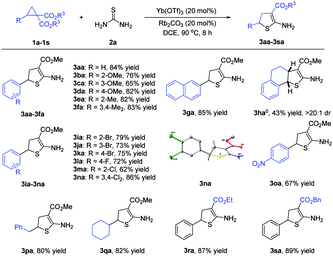
Under the optimized reaction conditions (Table 1, entry 13), the scope of D–A cyclopropanes was explored (Scheme 2). For cyclopropanes bearing electron-rich substituents at the aryl moieties, the adducts 3ba–3fa were produced in 65–83% yields. In the case of naphthalene-2-yl cyclopropane 1g and tetrahydronaphthalene-derived cyclopropane 1h, the adducts 3ga and 3ha could also be obtained. For the cyclopropanes with electron-withdrawing groups at the aryl moieties, the adducts 3ia–3oa were given in 62–86% yields. The structure of adduct 3na was determined by X-ray diffraction analysis. With respect to cyclopropanes with an alkyl group as the donor-substituent, the adducts 3pa and 3qa were afforded in 80–82% yields. In addition, D–A cyclopropanes with different ester groups were good reactants. It should be noted that the geminal diesters 4 were not observed in all of the cases.
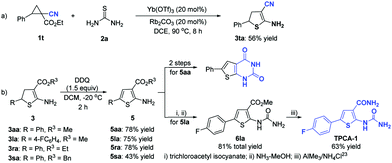
When ethyl 1-cyano-2-phenylcyclopropane-1-carboxylate 1t was reacted with thiourea 2a, the ester group was removed and the cyano group remained, giving the 2-amino-3-cyano-4,5-dihydrothiophene 3ta in 56% yield (Scheme 3a). Then, several 2-amino-4,5-dihydrothiophenes (3aa, 3la, 3ra, and 3sa) were selected as the representative substrates to react with DDQ, and the oxidation products, 2-aminothiophene derivatives (5aa, 5la, 5ra, and 5sa), were obtained in 43–78% yields (Scheme 3b). As for 2-aminothiophene 5aa, the corresponding ring-fused thienopyrimidinedione could be afforded in 2 steps.24 With 2-aminothiophene 5la as the reactant, the desired small-molecule IκB kinase β inhibitor TPCA-1 could be generated in 3 steps (Scheme 3b).23
 | ||
| Scheme 3 (a) Synthesis of 2-amino-3-cyano-4,5-dihydrothiophene; (b) transformation of 2-amino-4,5-dihydrothiophenes. | ||
Stereospecificity of the cycloaddition was explored using the enantiopure cyclopropane (R)-1a (>99% ee), and (S)-3aa was obtained in 92% yield and >99% ee (Scheme 4). The absolute configurations of (R)-1a and (S)-3aa were determined by X-ray analysis, and these configurations confirmed that an inversion at the stereogenic center was observed.
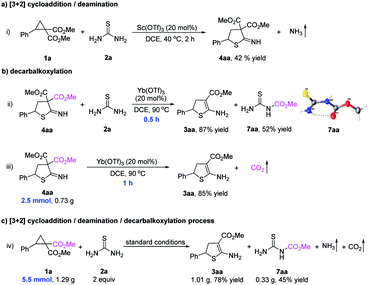
To understand the cycloaddition process, several control experiments were performed (Fig. 2). When Sc(OTf)3 was used as the catalyst, the reaction between cyclopropane 1a and thiourea 2a generated cycloadduct 4aa and released NH3 gas (Fig. 2a(i)). The released NH3 gas was detected by wet red litmus paper with blue color. When 1-methylthiourea 2b was used to react with cyclopropane 1a, NH3 or CH3NH2 could also be released (see ESI† for details). After that, geminal diester 4aa was then reacted with thiourea 2a in the presence of Yb(OTf)3, and the final product 3aa was formed in 87% yield within 0.5 h, indicating that the geminal diester 4aa might be an intermediate in the model reaction (Fig. 2b(ii)). Meanwhile, in the formation of monoester 3aa from geminal diester 4aa, an esterified thiourea 7aa was obtained (52% yield) and confirmed by X-ray diffraction analysis, which showed that thiourea 2a might function as a decarboxylation reagent (Fig. 2b(ii)). In the absence of thiourea 2a, geminal diester 4aa could also be converted into monoester 3aa with the release of CO2 gas, which was captured by 2-phenyloxirane (Fig. 2b(iii)). By comparing different reaction times (0.5 h vs. 1 h), the decarboxylation step proceeded faster in the presence of thiourea 2a (Fig. 2b(ii) and (iii)). Finally, the cycloaddition of D–A cyclopropane 1a with thiourea 2a produced the monoester 3aa (78% yield), esterified thiourea 7aa (45% yield), NH3 gas, CO2 gas, and a ring-opened triester 8aa (see ESI† for details) under the standard conditions (Fig. 2c(iv)). Formation of the esterified thiourea 7aa in a large proportion indicates that thiourea 2a participated in the decarboxylation reaction and was the main pathway during the decarboxylation step.
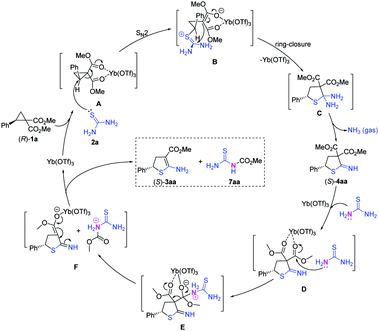
A plausible sequential mechanism of [3+2] cycloaddition/deamination/decarboxylation was proposed for this reaction on the basis of the stereospecificity experiments (Scheme 4) and preliminary mechanistic studies (Fig. 2), and this mechanism is depicted in Scheme 5. First, D–A cyclopropane (R)-1a is activated by Yb(OTf)3via coordination with the geminal diester moiety (A). The sulfur atom in thiourea 2a attacks the activated cyclopropane (R)-1a in an SN2 manner to produce the zwitterionic intermediate (B),11a–c which generates the cycloadduct 4,5-dihydrothiophene (C) through a ring-closure step. Because two amino groups are both connected at the C2 position in the dihydrothiophene (C), the dihydrothiophene (C) is unstable and produces the cyclic imine (S)-4aa along with a release of NH3 gas. The cyclic imine (S)-4aa is activated by Yb(OTf)3via coordination with the geminal diester moiety to enhance the positive charge at the carbonyl group (D). The nitrogen atom in another thiourea 2a attacks the carbonyl group and generates the tetrahedral intermediate (E).25 The crowded tetrahedral intermediate eliminates the protonated methyl carbamothioylcarbamate and generates the dihydrothiophene anion with a single ester group (F). Finally, the dihydrothiophene anion deprotonates the protonated methyl carbamothioylcarbamate, generating 2-amino-4,5-dihydrothiophene (S)-3aa and the esterified thiourea 7aa and releasing Yb(OTf)3.
In summary, thiourea, which is an odorless, cheap, and easy-to-handle sulfur source, was developed to react with D–A cyclopropanes to construct 2-amino-dihydrothiophenes. In this reaction, thiourea exhibited three functions: (1) providing a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond, (2) serving as an amino source for the 2-amino thiophenes, and (3) acting as a decarboxylation reagent. Through a Yb(OTf)3-catalyzed [3+2] cycloaddition/deamination/decarboxylation domino process, a range of D–A cyclopropanes could produce 2-amino-4,5-dihydrothiophenes in moderate to good yields (up to 92% yield).
S double bond, (2) serving as an amino source for the 2-amino thiophenes, and (3) acting as a decarboxylation reagent. Through a Yb(OTf)3-catalyzed [3+2] cycloaddition/deamination/decarboxylation domino process, a range of D–A cyclopropanes could produce 2-amino-4,5-dihydrothiophenes in moderate to good yields (up to 92% yield).
We are grateful for the financial support from the NSFC (No. 21472037 and 21672055), China Postdoctoral Science Foundation funded project (2016M592293 and 2018T110726), and the 111 Project (No. D17007).
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Huang and A. Dömling, Mol. Diversity, 2011, 15, 3 CrossRef CAS PubMed.
- H. Y. Meltzer and H. C. Fibiger, Neuropsychopharmacology, 1996, 14, 83 CrossRef CAS PubMed.
- P. D. Kalariya, P. N. Patel, P. Kavya, M. Sharma, P. Garg, R. Srinivas and M. V. N. K. Talluri, J. Mass Spectrom., 2015, 50, 1222 CrossRef CAS PubMed.
- (a) X. Li, D. Conklin, H.-L. Pan and J. C. Eisenach, J. Pharmacol. Exp. Ther., 2003, 305, 950 CrossRef CAS PubMed; (b) P. L. Podolin, J. F. Callahan, B. J. Bolognese, Y. H. Li, K. Carlson, T. G. Davis, G. W. Mellor, C. Evans and A. K. Roshak, J. Pharmacol. Exp. Ther., 2005, 312, 373 CrossRef CAS PubMed.
- A. Walburger, A. Koul, G. Ferrari, L. Nguyen, C. Prescianotto-Baschong, K. Huygen, B. Klebl, C. Thompson, G. Bacher and J. Pieters, Science, 2004, 304, 1800 CrossRef CAS PubMed.
- E. S. Darwish, Molecules, 2008, 13, 1066 CrossRef CAS PubMed.
- (a) K. Gewald, E. Schinke and H. Böttcher, Chem. Ber., 1966, 99, 94 CrossRef CAS; (b) R. W. Sabnis, D. W. Rangnekar and N. D. Sonawane, J. Heterocycl. Chem., 1999, 36, 333 CrossRef CAS; (c) H. Özbek, I. S. Veljkovic and H.-U. Reissig, Synlett, 2008, 3145 Search PubMed.
- P. Gopinath and S. Chandrasekaran, J. Org. Chem., 2011, 76, 700 CrossRef CAS PubMed.
- (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS PubMed; (b) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321 CrossRef CAS; (c) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051 RSC; (d) F. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912 RSC; (e) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed; (f) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804 RSC; (g) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC.
- Selected examples with C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C:
(a) D. B. England, T. D. O. Kuss, R. G. Keddy and M. A. Kerr, J. Org. Chem., 2001, 66, 4704 CrossRef CAS PubMed;
(b) F. de Nanteuil and J. Waser, Angew. Chem., Int. Ed., 2011, 50, 12075 CrossRef CAS PubMed;
(c) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167 CrossRef CAS PubMed;
(d) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed;
(e) F. de Nanteuil, E. Serrano, D. Perrotta and J. Waser, J. Am. Chem. Soc., 2014, 136, 6239 CrossRef CAS PubMed;
(f) S. Racine, F. de Nanteuil, E. Serrano and J. Waser, Angew. Chem., Int. Ed., 2014, 53, 8484 CrossRef CAS PubMed.
C:
(a) D. B. England, T. D. O. Kuss, R. G. Keddy and M. A. Kerr, J. Org. Chem., 2001, 66, 4704 CrossRef CAS PubMed;
(b) F. de Nanteuil and J. Waser, Angew. Chem., Int. Ed., 2011, 50, 12075 CrossRef CAS PubMed;
(c) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167 CrossRef CAS PubMed;
(d) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed;
(e) F. de Nanteuil, E. Serrano, D. Perrotta and J. Waser, J. Am. Chem. Soc., 2014, 136, 6239 CrossRef CAS PubMed;
(f) S. Racine, F. de Nanteuil, E. Serrano and J. Waser, Angew. Chem., Int. Ed., 2014, 53, 8484 CrossRef CAS PubMed. - Selected examples with C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O:
(a) P. D. Pohlhaus and J. S. Johnson, J. Am. Chem. Soc., 2005, 127, 16014 CrossRef CAS PubMed;
(b) P. D. Pohlhaus and J. S. Johnson, J. Org. Chem., 2005, 70, 1057 CrossRef CAS PubMed;
(c) P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li and J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 8642 CrossRef CAS PubMed;
(d) A. T. Parsons and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 3122 CrossRef CAS PubMed.
O:
(a) P. D. Pohlhaus and J. S. Johnson, J. Am. Chem. Soc., 2005, 127, 16014 CrossRef CAS PubMed;
(b) P. D. Pohlhaus and J. S. Johnson, J. Org. Chem., 2005, 70, 1057 CrossRef CAS PubMed;
(c) P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li and J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 8642 CrossRef CAS PubMed;
(d) A. T. Parsons and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 3122 CrossRef CAS PubMed. - Selected examples with C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N:
(a) C. A. Carson and M. A. Kerr, J. Org. Chem., 2005, 70, 8242 CrossRef CAS PubMed;
(b) S. K. Jackson, A. Karadeolian, A. B. Driega and M. A. Kerr, J. Am. Chem. Soc., 2008, 130, 4196 CrossRef CAS PubMed;
(c) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, J. Am. Chem. Soc., 2010, 132, 9688 CrossRef CAS PubMed;
(d) D.-C. Wang, M.-S. Xie, H.-M. Guo, G.-R. Qu, M.-C. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 14111 CrossRef CAS PubMed;
(e) J. Preindl, S. Chakrabarty and J. Waser, Chem. Sci., 2017, 8, 7112 RSC;
(f) M.-C. Zhang, D.-C. Wang, M.-S. Xie, G.-R. Qu, H.-M. Guo and S.-L. You, Chem, 2019, 5, 156 CrossRef.
N:
(a) C. A. Carson and M. A. Kerr, J. Org. Chem., 2005, 70, 8242 CrossRef CAS PubMed;
(b) S. K. Jackson, A. Karadeolian, A. B. Driega and M. A. Kerr, J. Am. Chem. Soc., 2008, 130, 4196 CrossRef CAS PubMed;
(c) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, J. Am. Chem. Soc., 2010, 132, 9688 CrossRef CAS PubMed;
(d) D.-C. Wang, M.-S. Xie, H.-M. Guo, G.-R. Qu, M.-C. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 14111 CrossRef CAS PubMed;
(e) J. Preindl, S. Chakrabarty and J. Waser, Chem. Sci., 2017, 8, 7112 RSC;
(f) M.-C. Zhang, D.-C. Wang, M.-S. Xie, G.-R. Qu, H.-M. Guo and S.-L. You, Chem, 2019, 5, 156 CrossRef. - With N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O:
(a) S. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 5964 CrossRef CAS PubMed With N
O:
(a) S. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 5964 CrossRef CAS PubMed With N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: ;
(b) V. S. Korotkov, O. V. Larionov, A. Hofmeister, J. Magull and A. de Meijere, J. Org. Chem., 2007, 72, 7504 CrossRef CAS PubMed.
N: ;
(b) V. S. Korotkov, O. V. Larionov, A. Hofmeister, J. Magull and A. de Meijere, J. Org. Chem., 2007, 72, 7504 CrossRef CAS PubMed. - Selected examples with C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C:
(a) V. K. Yadav and V. Sriramurthy, Angew. Chem., Int. Ed., 2004, 43, 2669 CrossRef CAS;
(b) S. Racine, B. Hegedüs, R. Scopelliti and J. Waser, Chem. – Eur. J., 2016, 22, 11997 CrossRef CAS PubMed.
C:
(a) V. K. Yadav and V. Sriramurthy, Angew. Chem., Int. Ed., 2004, 43, 2669 CrossRef CAS;
(b) S. Racine, B. Hegedüs, R. Scopelliti and J. Waser, Chem. – Eur. J., 2016, 22, 11997 CrossRef CAS PubMed. - Selected examples with C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N:
(a) M. Yu and B. L. Pagenkopf, Org. Lett., 2003, 5, 5099 CrossRef CAS PubMed;
(b) M. Yu and B. L. Pagenkopf, J. Am. Chem. Soc., 2003, 125, 8122 CrossRef CAS PubMed.
N:
(a) M. Yu and B. L. Pagenkopf, Org. Lett., 2003, 5, 5099 CrossRef CAS PubMed;
(b) M. Yu and B. L. Pagenkopf, J. Am. Chem. Soc., 2003, 125, 8122 CrossRef CAS PubMed. - Selected examples with nitrones: (a) I. S. Young and M. A. Kerr, Angew. Chem., Int. Ed., 2003, 42, 3023 CrossRef CAS PubMed; (b) M. P. Sibi, Z. Ma and C. P. Jasperse, J. Am. Chem. Soc., 2005, 127, 5764 CrossRef CAS PubMed; (c) Y.-B. Kang, X.-L. Sun and Y. Tang, Angew. Chem., Int. Ed., 2007, 46, 3918 CrossRef CAS PubMed; (d) P.-W. Xu, J.-K. Liu, L. Shen, Z.-Y. Cao, X.-L. Zhao, J. Yan and J. Zhou, Nat. Commun., 2017, 8, 1619 CrossRef PubMed.
- Selected examples with heterocumulenes: (a) C. Brückner and H.-U. Reissig, Angew. Chem., Int. Ed. Engl., 1985, 24, 588 CrossRef; (b) A. F. G. Goldberg, N. R. O’Connor, R. A. Craig II and B. M. Stoltz, Org. Lett., 2012, 14, 5314 CrossRef CAS; (c) Y. Sun, G. Yang, Z. Chai, X. Mu and J. Chai, Org. Biomol. Chem., 2013, 11, 7859 RSC; (d) D. Gladow and H.-U. Reissig, J. Org. Chem., 2014, 79, 4492 CrossRef CAS PubMed.
- Selected examples with other dipolarophiles: (a) T. P. Lebold, A. B. Leduc and M. A. Kerr, Org. Lett., 2009, 11, 3770 CrossRef CAS PubMed; (b) S.-W. Wang, W.-S. Guo, L.-R. Wen and M. Li, RSC Adv., 2015, 5, 47418 RSC; (c) H. Xu, J.-L. Hu, L. Wang, S. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006 CrossRef CAS PubMed; (d) Z. Su, S. Qian, S. Xue and C. Wang, Org. Biomol. Chem., 2017, 15, 7878 RSC.
- Selected examples with nucleophiles: (a) Y. Xia, X. H. Liu, H. F. Zheng, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2015, 54, 227 CrossRef CAS PubMed; (b) Y. Xia, L. L. Lin, F. Z. Chang, X. Fu, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2015, 54, 13748 CrossRef CAS PubMed; (c) Y. Xia, L. L. Lin, F. Z. Chang, Y. T. Liao, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2016, 55, 12228 CrossRef CAS PubMed.
- (a) A. U. Augustin, M. Sensse, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2017, 56, 14293 CrossRef CAS PubMed; (b) A. U. Augustin, M. Busse, P. G. Jones and D. B. Werz, Org. Lett., 2018, 20, 820 CrossRef CAS PubMed; (c) A. Kreft, P. G. Jones and D. B. Werz, Org. Lett., 2018, 20, 2059 CrossRef CAS PubMed.
- Y. Matsumoto, D. Nakatake, R. Yazaki and T. Ohshima, Chem. – Eur. J., 2018, 24, 6062 CrossRef CAS PubMed.
- (a) G.-p. Lu, F. Chen and C. Cai, J. Chem. Educ., 2017, 94, 244 CrossRef CAS; (b) J. Wang, Q.-Y. Zhang, M.-S. Xie, D.-C. Wang, G.-R. Qu and H.-M. Guo, Org. Lett., 2018, 20, 6578 CrossRef CAS PubMed.
- D. C. Cole, U.S. Pat. Appl., US20050038088 A1, 2005 Search PubMed.
- J. M. Salamoun, K. E. McQueeney, K. Patil, S. J. Geib, E. R. Sharlow, J. S. Lazo and P. Wipf, Org. Biomol. Chem., 2016, 14, 6398 RSC.
- D. H. Miles and B.-S. Huang, J. Org. Chem., 1976, 41, 208 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1849437 (3na), 1852130 ((R)-1a), 1852128 ((S)-3aa), 1852129 (7aa) and 1852131 (8aa). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc09595g |
| This journal is © The Royal Society of Chemistry 2019 |